- Laiko General Hospital, Medical School, National and Kapodistrian University of Athens, Athens, Greece
Non alcoholic fatty disease (NAFLD) is the most common chronic liver disease that is managed in the liver departments. It seems that the prevalence of the disease is rising worldwide and as it has the same pathogenetic pathways with metabolic syndrome, treatments that target components of the metabolic syndrome seem promising for the therapy of NAFLD as well. In this review we discuss the evolving role of semaglutide, which is a glucagon-like peptide-1 receptor agonist (GLP-1 RA) that has been already approved for the treatment of type II diabetes mellitus (T2DM) and obesity.
Introduction
Although many trials worldwide have been conducted to find an effective and safe treatment, the recommendation for patients with nonalcoholic fatty liver disease (NAFLD), which is an entity with rising prevalence (Vernon et al., 2011; Younossi et al., 2016; Mundi et al., 2020), still constitutes of exercise and dietary modifications (Vilar-Gomez et al., 2015; Kenneally et al., 2017; Romero-Gómez et al., 2017; Hallsworth and Adams, 2019). The goal for these patients is to prevent the evolution of NAFLD to its advanced form of nonalcoholic steatohepatitis (NASH) which is mainly related to the development of cirrhosis and hepatocellular carcinoma.
Semaglutide, which is a human glucagon-like peptide-1 receptor agonist (GLP-1 RA), seems an attractive therapeutic option for these patients. After binding to its ligand, the GLP-1 receptor activates intracellular signaling pathways that have multiple effects. It is known that semaglutide exerts beneficial effects on parameters of metabolic syndrome, which is directly associated with NAFLD (Eslam et al., 2020; Nauck and Quast, 2021; Gofton et al., 2023; Machado and Cortez-Pinto, 2023). It lowers glucose levels by stimulating glucose-dependent insulin secretion and by reducing fasting and postprandial glucagon (Pyke et al., 2014; Knudsen and Lau, 2019). It also reduces energy intake by affecting appetite (Blundell et al., 2017; Friedrichsen et al., 2021; Gibbons et al., 2021) and inhibits gastric emptying causing weight loss (Brierley et al., 2021; Drucker, 2022). The prevalence of NAFLD in the general population is 30% (Younossi et al., 2023) but in obese patients it is estimated that it ranges from 60% to 95% (Godoy-Matos et al., 2020). Several studies have shown that weight loss is associated with NAFLD resolution (Vilar-Gomez et al., 2015; European Association for the Study of the Liver EASLEuropean Association for the Study of Diabetes EASDEuropean Association for the Study of Obesity EASO, 2016) and semaglutide has already been approved for the management of obesity (Wilding et al., 2021). Moreover, semaglutide reduces cardiovascular risk by decreasing blood pressure, postprandial lipid levels and inflammation (Marso et al., 2016; Rakipovski et al., 2018; Weghuber et al., 2022; Kosiborod et al., 2023).
Nevertheless, apart from the weight loss related benefit in the liver, it has been shown that semaglutide also has antioxidative effects, while it reduces mitochondrial damage, which is considered to play a central role in the pathogenesis and progression of NAFLD (Paradies et al., 2014; Niu et al., 2022). Additionally, data from animal models support the anti-inflammatory effects of semaglutide by inhibition of upregulation of pro-inflammatory factors such as tumor necrosis factor-A and interleukin −6 and by down-regulating the expression of inflammatory factors such as arachidonic acid (Niu et al., 2022). Furthermore, semaglutide reduces lipogenesis and lipid deposition and increases beta-oxidation. All these mechanisms of action are supposed to act beneficially in the improvement of liver histology and NAFLD resolution (Pontes-da-Silva et al., 2022).
So far there are a few literature data regarding the role of semaglutide in NAFLD, mainly from studies concerning type II diabetes mellitus (T2DM) or obesity. In this review, we will focus on the new data concerning the use of semaglutide in NAFLD/NASH patients, i.e., in combination with other medication against NAFLD, as well as its weekly and per os administration.
Semaglutide in combination therapy
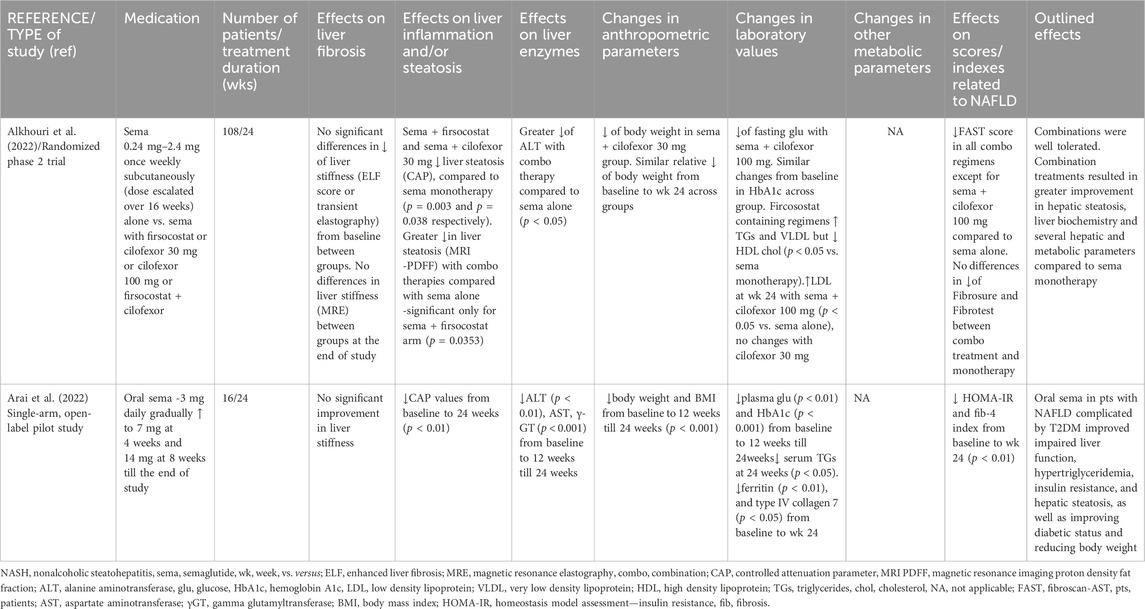
The safety and tolerability of subcutaneous semaglutide alone or in combination with cilofexor (a nonsteroidal farnesoid X receptor agonist) and/or firsocostat (an acetyl-CoA carboxylase inhibitor) in NASH patients with mild-to moderate fibrosis (F2-F3) on biopsy or fat fraction ≥10% on magnetic resonance imaging proton density fat fraction (MRI-PDFF) and liver stiffness ≥7 kPa on transient elastography were evaluated in a phase II open-label, randomized trial by Alkhouri et al. (2022). Patients were treated with semaglutide alone 0.24 mg–2.4 mg once weekly (dose escalated over 16 weeks) or combined with cilofexor 30 mg/day or cilofexor 100 mg/day or firsocostat 20 mg/day or cilofexor 30 mg and firsocostat 20 mg for 24 weeks. Notably, although weight loss was observed in all groups compared to baseline, only the combination of semaglutide plus cilofecor 30 mg achieved greater weight loss compared to semaglutide alone (p<0.05). Liver steatosis (evaluated by MRI-PDFF) was decreased to a greater grade with all combination treatments compared with semaglutide alone, but the improvement was statistically significant only in the semaglutide plus firsocostat arm (−11% vs. −8% in semaglutide alone, p = 0.035). Nevertheless, in a sensitivity analysis, excluding patients with imaging data at least 1 month after the last dose, the difference in steatosis between semaglutide alone and semaglutide plus cilofexor plus firsocostat was also significant (−8.6% vs. −12.6%, p = 0.008). Interestingly, a greater proportion of patients under combination treatment, compared to semaglutide alone, achieved a relative reduction in MRI-PDFF of ≥50% compared to baseline (58.8%–76.2% vs 38.9%, respectively, always p > 0.05). Treatment with semaglutide plus firsocostat and semaglutide plus cilofexor 30 mg significantly reduced liver steatosis assessed by the controlled attenuation parameter (CAP), compared to semaglutide monotherapy (p = 0.003 and 0.038, respectively). However, it should be mentioned that all these results regarding liver steatosis were not adjusted with weight loss. Finally, no differences in liver stiffness measured by magnetic resonance elastography (MRE) were observed between groups at the end of study Table 1.
Weekly administration of semaglutide
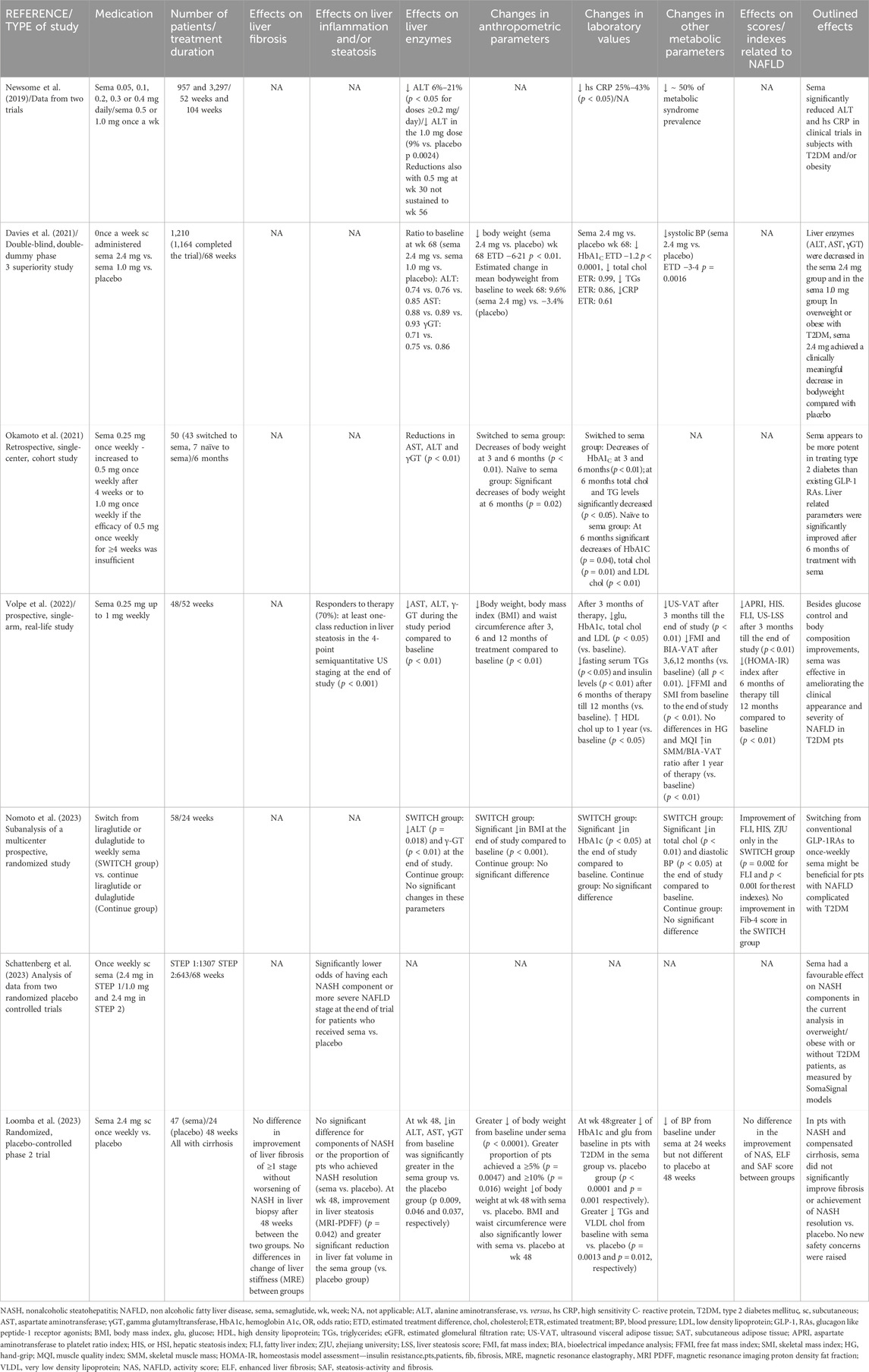
Newsome et al. (2019) conducted a post hoc analysis using data from two randomized, double-blind trials regarding the effects of semaglutide on alanine aminotransferase (ALT) and high sensitivity C reactive protein (hs CRP) in patients who were at risk for NAFLD. The authors analyzed data from a 104-weeks cardiovascular outcomes trial in T2DM patients with hemoglobin A1c (HbA1c) ≥7% (semaglutide 0.5 or 1.0 mg/week) and a 52-weeks weight management trial in obese patients without T2DM (semaglutide 0.05–0.4 mg/day). Elevated baseline ALT was recorded in 41% (1,325/3,268) in cardiovascular outcomes-trial subjects, but at the end-of-treatment a statistically significant reduction in ALT was seen in the 1.0 mg dose of semaglutide (p = 0.0024). However, after adjustment for change in body weight, treatment ratios vs. placebo for ALT and hsCRP were not significant, indicating that these improvements were associated with weight loss Table 2.
Another double-blind, phase III study (Davies et al., 2021) assessed the efficacy and safety of once a week subcutaneously administered semaglutide for weight management in overweight or obese T2DM adults. In total, 1,210 patients were allocated (1:1:1) to receive semaglutide (2.4 mg or 1.0 mg per week) or placebo for 68 weeks. Liver enzymes-ALT, aspartate aminotransferase (AST), gamma glutamyltransferase (γGT)- were evaluated at baseline and week 68. Although no statistical analysis was provided, the authors noted that these liver biochemical parameters -usually increased in the context of NAFLD—reduced from baseline in the semaglutide 2.4 mg/week group as well as in the semaglutide 1.0 mg/week group to a greater extent, compared to placebo group. Thus, the mean ratios to baseline at week 68 for semaglutide 2.4 and 1.0 mg/week vs. placebo were lower for ALT (0.74 and 0.76 vs. 0.85), AST (0.88 and 0.89 vs. 0.93) and γGT (0.71 and 0.75 vs. 0.86). Interestingly, estimated change in mean body weight from baseline to week 68 was –7% with semaglutide 1 mg/week and –9.6% with semaglutide 2.4 mg/week vs. –3.4% with placebo. However, these changes in body weight were not considered for adjustment in liver biochemistry improvement.
A retrospective cohort study (Okamoto et al., 2021) evaluated patients with T2DM who were switched to or initiated on weekly semaglutide because of obesity or poor diabetes mellitus control while treated with other anti-diabetic medications. Forty-three patients were switched to semaglutide (group A) and seven were naïve to semaglutide (group B). Aminotransferases (AST and ALT) and γGT were reduced 6 months after treatment with semaglutide (significant changes in the first group: p = 0.01 for AST and ALT, p < 0.01 for γGT), compared to the baseline. Interestingly, total cholesterol, triglycerides and uric acid also decreased (statistically significant changes for total cholesterol in both groups, while for triglycerides and uric acid only in the first group). Significant reductions were also noted in ΗbA1c (p < 0.01 and p = 0.04 for the two groups, respectively), as well as in body weight (p<0.01 in the first group and p = 0.02 in the second one), compared to the baseline. Interestingly, there was no significant correlation between HbA1c and changes in body weight, but similar analysis was not performed regarding aminotransferases reduction.
Another study from Volpe et al. (2022) evaluated the effectiveness of weekly subcutaneous semaglutide add on to metformin in patients with T2DM eligible for glucagon like peptide 1 receptor agonists (GLP-1 RAs). Forty-eight patients received gradually increasing doses of semaglutide (starting from 0.25 mg up to 1 mg per week). Body mass index (BMI) and waist circumference were decreased after three, six and 12 months of treatment compared to baseline (p<0.01) (e.g., mean loss of body weight was 7.4% after 3 months, 9.2% after 6 months and 10.3% at 1 year). Regarding liver biochemistry, AST, ALT, and γ-GT decreased significantly during the study period (p < 0.01 compared to baseline). The aspartate aminotransferase to platelet ratio index (APRI) was also significantly reduced after 3 months till the end of study (p < 0.01 compared to baseline). Seventy percent of patients -who were defined as responders to therapy-achieved at least one-class reduction in liver steatosis in the 4-point semiquantitative ultrasound (US) staging at the end of study (p < 0.001). No adjustment to the weight loss was performed. In the remaining 30% of non-responders, no change in the steatosis grading was found. However, no differences were found between responders and non-responders regarding BMI, HOMA-IR and liver enzymes from baseline to the end of the study.
Interestingly, the fat mass index (FMI) and vascular adipose tissue evaluated by bioelectrical impedance analysis (BIA-VAT) decreased significantly at each observation time after three, six and 12 months compared to baseline (all p < 0.01) (Volpe et al., 2022). Although reduction in the skeletal mass index (SMI) was observed, the handgrip (HG) and muscle quality index (MQI) -both indicative of muscular functional status-were not significantly different at the end of study, compared to baseline. In addition, changes in the skeletal muscle mass (SMM)/BIA-VAT ratio progressively increased, reaching significantly higher values than at baseline after 1 year of therapy (p < 0.01) (Volpe et al., 2022).
A recently published study from Japan (Nomoto et al., 2023) constituted a sub analysis of a multicenter prospective, randomized study, which compared the efficacy of switching from liraglutide or dulaglutide to once weekly semaglutide on glycemic control in adults with T2DM (SWITCH group) compared to continuing current GLP1RAs (Continue group) for 24 weeks. Semaglutide was started at a dose of 0.25 mg and after at least 4 weeks, the dose was increased to 0.5–1.0 mg weekly. A significant reduction was found in ALT (p = 0.018) and γ-GT (<0.01), but without considering confounding factors. No changes in the aforementioned parameters were detected in the Continue group. Fatty liver index (FLI), which was the main outcome of the analysis, improved only in the SWITCH group (p = 0.002) but not in the Continue group. Switching to semaglutide did not improve liver fibrosis as assessed by FIB-4 index. Patients in whom dulaglutide was changed to semaglutide showed larger improvements in FLI than those who changed from liraglutide. Both switch strategies (from liraglutide to semaglutide and from dulaglutide to semaglutide) resulted in significant reductions in HbA1c and BMI but no significant differences regarding the extent of the reduction was detected between the subgroups.
In another trial that was presented in an abstract form in the last European Association for the Study of the Liver (EASL) Congress (Schattenberg et al., 2023), SomaSignal tests were applied to proteomics data that were derived from two studies-STEP1 and STEP2-that investigated the effect of weekly subcutaneous semaglutide for 68 weeks on weight loss in overweight or obese patients with (STEP2) or without (STEP1) T2DM, compared to placebo. A targeted proteomics signature derived from patients with histologically proven NASH was developed with the NASH Clinical Research Network (SomaSignal tests) to find the relation between the presence and severity of NASH components and changes over time. Proteomics data were available for 1,307/1961 patients from STEP 1 and 643/1,210 patients from STEP 2. At baseline, 43% of patients in STEP 1 had steatosis whereas the prevalence of the other components was 5% or less. In STEP 2, 72% of patients exhibited steatosis, 15% had NASH and 12% had NASH with fibrosis. The odds of having each NASH histological component were significantly lower at the end of trial for patients who received semaglutide compared to placebo (e.g., in STEP 2 study for semaglutide 1.0 mg/week: 0.25 for steatosis and 0.52 for inflammation). Also, semaglutide was associated with significantly lower odds of having a more severe NAFLD stage after treatment compared to placebo, but nor further data were provided.
Weekly semaglutide in cirrhosis
In a double-blind, placebo-controlled phase II trial by Loomba et al. (2023), 71 patients (75% with diabetes mellitus) from 38 centres in Europe and the United States with biopsy-confirmed cirrhosis caused by NASH and BMI of ≥27 kg/m2 were randomly assigned to receive either once-weekly subcutaneous semaglutide 2.4 mg (n = 47) or placebo (n = 24). The primary endpoint was the proportion of patients with an improvement in liver fibrosis of one stage or more without worsening of NASH in liver biopsy after 48 weeks. At the end of study, although in the placebo group a higher proportion of patients met the primary end point compared to the semaglutide group, this difference was not significant (29% vs. 11%, p = 0.087). There was also no difference between groups in the proportion of patients who achieved NASH resolution (p = 0.29), as well as regarding the components of NASH (steatosis, lobular inflammation, hepatocyte ballooning). However, at week 48, improvement in liver steatosis assessed by MRI-PDFF was greater in the semaglutide group than in the placebo group (p = 0.042) and reduction in liver fat volume was also significantly greater in the semaglutide group than in the placebo group. Concerning liver enzymes, at week 48, reductions in ALT, AST and γGT levels from baseline were significantly greater in the semaglutide group vs. the placebo group (p = 0.009, 0.046 and 0.037, respectively). A greater reduction in body weight from baseline was found under semaglutide, compared to placebo group (p<0.01). Also, a greater proportion of patients achieved a ≥5% (p = 0.0047) and ≥10% (p = 0.016) loss of body weight at week 48 with semaglutide, compared to placebo. BMI and waist circumference were also significantly lower with semaglutide, compared to placebo at week 48. However, only baseline BMI was considered as confounding factor for the evaluation of beneficial impact of semaglutide on this cohort Table 2.
Finally, eighty nine percent of patients in the semaglutide group vs. 79% in the placebo group reported adverse events-most of them being nausea (45% vs. 17%), diarrhea (19% vs. 8%) and vomiting (17% vs. 0%). Serious adverse events were reported in 3% and 8% respectively, while no changes in hepatic and renal function and no decompensating events or deaths were noted (Loomba et al., 2023).
Orally semaglutide
The efficacy and safety of oral semaglutide in patients with NAFLD and T2DM was assessed in a single-arm, open-label pilot study (Arai et al., 2022). Sixteen patients were started on oral semaglutide at a dose of 3 mg daily, which was gradually increased to 7 mg at 4 weeks and 14 mg at 8 weeks till the end of the study at 24th week. Body weight, AST, HbA1c, γ-GT, ALT and plasma glucose decreased significantly from baseline to 12 weeks (p<0.001 for the first four parameters, p < 0.01 for the last two) and these changes remained until the end of the study. Levels of the HOMA-IR and serum triglyceride were also significantly reduced at 24 weeks (p < 0.01 and p < 0.05, respectively). Moreover, CAP values decreased from baseline to 24 weeks (p < 0.01). Interestingly, changes in body weight were significantly correlated with those in ALT (r = 0.52, p < 0.05) and CAP (r = 0.72, p < 0.01). Platelet count increased from baseline to 12 weeks (p < 0.05), and it was maintained at 24 weeks (p < 0.01). Notably, levels of the fibrosis-4 index, ferritin, and type IV collagen 7 were significantly decreased from baseline to week 24 (p < 0.01 for the first two parameters and p < 0.05 for the last one). However, the liver stiffness measurement was not significantly improved. Most adverse events were mild to moderate gastrointestinal disorders whereas no severe adverse events or deaths were detected Table 1.
Orally semaglutide in cirrhosis
A multicenter, open-label, parallel-group trial (Bækdal et al., 2018) investigated whether hepatic impairment affects the pharmacokinetics, safety, and tolerability of oral semaglutide. Child-Pugh classification was used to categorize patients into four groups: normal hepatic function (n = 24) and mild (n = 12), moderate (n = 12), or severe (n = 8) hepatic impairment. Mild impairment was referred to Child-Pugh class A (5–6 points), moderate impairment to Child-Pugh class B (7–9 points) and severe impairment to Child-Pugh class C (10–15 points). The patients received once-daily oral semaglutide (5 mg for 5 days and then 10 mg for the next 5 days). Semaglutide plasma concentrations were measured during dosing and for up to 21 days post-last dose. Area under the semaglutide plasma concentration–time curve from 0 to 24 h after the 10th dose (AUC 0–24h,Day10)-which was the primary end point-as well as maximum semaglutide concentration after the 10th dose (Cmax,Day10) were similar across groups. Also, time to maximum semaglutide concentration (tmax,Day10) and half-life (t1/2,Day10) were not affected by hepatic impairment. Semaglutide was found to be safe in patients with hepatic impairment. Interestingly, 14.3% of patients reported headache, 8.9% dyspepsia, 7.1% vomiting, 7.1% decreased appetite and 5.4% diarrhea. The authors concluded that no dose adjustment of oral semaglutide is warranted in subjects with hepatic impairment.
Discussion
Semaglutide, which is a GLP1-RA available in subcutaneous and oral forms, is supposed to exert beneficial effects on NAFLD by numerous mechanisms of action rendering it a promising treatment for the disease (Cigrovski Berkovic et al., 2022). It is known that there is a dose dependent response between weight loss and the magnitude of histological improvement in patients with NAFLD (Godoy-Matos et al., 2020), but apart from the weight loss, semaglutide seems to also benefit liver through anti-inflammatory and antioxidative actions (Niu et al., 2022; Lee and Kim, 2023). The direct hepatic lipid metabolism-modulating properties of GLP1-RAs have also been studied in cell culture models of NAFLD (Petrovic et al., 2023).
Several published studies have evaluated the role of semagutide in patients with NAFLD/NASH, in which semaglutide has been given once a day subcutaneously (Newsome et al., 2019; Flint et al., 2021). However, few studies have evaluated its administration in combination with ‘NAFLD specific drugs’ or its weekly subcutaneous and oral administration.
Many trials are ongoing concerning combination treatments in NAFLD/NASH giving the potential of synergistic effects of the used medicines. ‘NAFLD specific drugs’ are part of the studied regimens. Based on the available literature data (Alkhouri et al., 2022), in which semaglutide was administered either alone or combined with cilofexor and/or firsocostat in patients with NASH and mild to moderate fibrosis, semaglutide combined with firsocostat resulted in greater improvement in hepatic steatosis compared to semaglutide monotherapy estimated by MRI-PDFF and CAP, whereas the combination of semaglutide with cilofexor 30 mg improved hepatic steatosis only measured by CAP. Although no differences in liver fibrosis (evaluated by MRE) were found between groups at the end of study, FAST score, which incorporates liver steatosis and stiffness, was reduced with all combination treatments except for semaglutide plus cilofexor 100 mg. It is well-established that the stage of liver fibrosis is the strongest predictor for development of metabolic-associated comorbidities and liver-related mortality in patients with NASH (Ekstedt et al., 2015; Dulai et al., 2017; Leung et al., 2017). However, no powerful data regarding fibrosis improvement from semaglutide alone or in combination therapy emerged from this study (Alkhouri et al., 2022). Nevertheless, this may be attributed to the short duration of follow up, which was only 6 months. It is of interest that similar weight loss was observed across the study groups indicating that the greater improvements in aminotrasferases, liver fat and FAST with combination therapies were not mediated solely by the loss of body weight and support the complementary actions of farnesoid X receptor agonists and acetyl-coenzyme A carboxylase inhibitors with semaglutide. Regarding the safety of semaglutide, the severity of most of the adverse events were grade 1 or 2, similar across the groups, and thus no drug-drug interactions were clinically observed. However, it should be mentioned that although 108 patients were included in total, each group had a small number of patients, and no placebo control group was incorporated in this study. Additionally, patients with cirrhosis were excluded, but it would be interesting to be enrolled in studies of combination treatment, as data are lacking. Further results from ongoing studies on combination treatments (NCT04971785, NCT05016882, NCT04639414) are awaited.
Very few studies in the literature have evaluated the subcutaneous administration of semaglutide once a week in patients with NAFLD (Newsome et al., 2019; Davies et al., 2021; Okamoto et al., 2021; Volpe et al., 2022; Loomba et al., 2023; Nomoto et al., 2023; Schattenberg et al., 2023) (Table 2). All relevant studies (Newsome et al., 2019; Davies et al., 2021; Okamoto et al., 2021; Volpe et al., 2022; Loomba et al., 2023; Nomoto et al., 2023; Schattenberg et al., 2023) enrolled patients with T2DM with or without obesity. Notably, only three of these studies (Davies et al., 2021; Loomba et al., 2023; Schattenberg et al., 2023) included placebo group-the first one (Davies et al., 2021) evaluated a large cohort of patients with NAFLD (n = 1,210) and with a relatively long duration of follow up (68 weeks). However, only two trials (Loomba et al., 2023; Schattenberg et al., 2023) included patients with histologically proven NASH- the first one (Loomba et al., 2023) regarding cirrhotic patients. Based on the current literature data, weekly semaglutide was found to reduce liver enzymes (Newsome et al., 2019; Davies et al., 2021; Okamoto et al., 2021; Volpe et al., 2022; Loomba et al., 2023; Nomoto et al., 2023) and to achieve loss of body weight (Davies et al., 2021; Okamoto et al., 2021; Volpe et al., 2022; Loomba et al., 2023; Nomoto et al., 2023). The latter is very important, since weight loss has been associated with improvement of metabolic profile (Wilding, 2014) and the risk of cardiovascular disease, which is the leading cause of morbidity and mortality in patients with NAFLD (Targher et al., 2016). Interestingly, these beneficial effects of semaglutide once a week were also confirmed in two trials, in which patients were switched from other GLP1-RAs to weekly subcutaneous semaglutide (Okamoto et al., 2021; Nomoto et al., 2023), indicating that semaglutide may be the GLP1-RAs of choice in patients with NAFLD. Regarding the study by Volpe et al (Volpe et al., 2022), where 10% body weight reduction was observed, it is worth mentioning that at least 10% of body weight loss is required to see NASH resolution (Vilar-Gomez et al., 2015) and this may be the driving factor behind the benefits of semaglutide. Importantly, only one study (Newsome et al., 2019) evaluated the impact of BMI reduction on ALT improvement indicating that liver biochemistry changes under weekly semaglutide administration were associated with weight loss. Thus, further studies are needed to elucidate further this association. As may be expected, weekly administration of semaglutide has been associated with improvement of liver steatosis based on US assessment (Volpe et al., 2022). However, it is known that US has several limitations in this setting including its low sensitivity, particularly in obese individuals or when <30% of liver parenchyma has steatosis (Ferraioli and Monteiro, 2019). Regarding the impact of administration of weekly semaglutide on severity of histological lesions in the liver, this has been assessed only in NASH-associated compensated cirrhosis (Loomba et al., 2023), with no significant improvement in liver fibrosis or resolution of NASH. Probably the short duration of the study (48 weeks) and the presence of baseline cirrhosis prevented the observation of any benefit on histological lesions, and thus, more data are needed to clarify better this issue.
Semaglutide is the only GLP-1RA that has been approved in an oral form, that is something very important reinforcing the compliance of the patients compared to the injectable forms. In the only available study (Arai et al., 2022), daily oral semaglutide in patients with NAFLD and T2DM given for 24 weeks improved parameters of metabolic syndrome, as well as liver steatosis evaluated by CAP. Body weight and BMI reduction was seen from baseline to week 12 till the end of study. These data taken together with the results from studies on weekly semaglutide support the belief that apart from liver specific benefits of semaglutide, weight loss may be the main motivating factor that benefits liver biochemistry and steatosis. However, no improvement was detected in liver fibrosis estimated by transient elastography and fibrosis markers. The small duration of treatment probably did not allow for changes in liver fibrosis to occur. Interestingly, no severe adverse events or deaths were reported. Larger and with long duration trials, regarding the effect of oral semaglutide on the histological lesions of patients with NAFLD/NASH are needed.
It is worth mentioning that a groundbreaking double-blind, placebo-controlled phase three trial study was recently published (Harrison et al., 2023), that pointed out the safety and liver specific benefits of resmetirom on NAFLD, which is the first drug expected to be approved for the disease. Notably, many of these patients were also taking GLP1-RAs including semaglutide in combination with resmetirom during the study, but no separate data were provided. Nevertheless, future studies concerning the co-administration of resmetirom with semaglutide in patients with NAFLD are needed to elucidate if the combination offers an additional benefit, compared to resmetirom or semaglutide alone. In conclusion, semaglutide seems to exert favourable effects on parameters of metabolic syndrome and is a safe drug even in advanced stages of hepatic impairment (Bækdal et al., 2018; Jensen et al., 2018). Several studies regarding its role in NAFLD showed an improvement of liver steatosis. However, improvement in liver fibrosis constitutes a more difficult target and data for its role in preventing the complications of hepatic impairment are still lacking from the literature.
Author contributions
EC: Writing—review and editing. EK: Writing—original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alkhouri, N., Herring, R., Kabler, H., Kayali, Z., Hassanein, T., Kohli, A., et al. (2022). Safety and efficacy of combination therapy with semaglutide, cilofexor and firsocostat in patients with non-alcoholic steatohepatitis: a randomised, open label phase II trial. J. Hepatol. 77, 607–618. doi:10.1016/j.jhep.2022.04.003
Arai, T., Atsukawa, M., Tsubota, A., Ono, H., Kawano, T., Yoshida, Y., et al. (2022). Efficacy and safety of oral semaglutide in patients with non-alcoholic fatty liver disease complicated by type 2 diabetes mellitus: a pilot study. JGH Open 6 (7), 503–511. doi:10.1002/jgh3.12780
Bækdal, T. A., Thomsen, M., Kupcova, V., Hansen, C. W., and Anderson, T. W. (2018). Pharmacokinetics, safety, and tolerability of oral semaglutide in subjects with hepatic impairment. J. Clin. Pharmacol. 58 (10), 1314–1323. doi:10.1002/jcph.1131
Blundell, J., Finlayson, G., Axelsen, M. B., Flint, A., Gibbons, C., Kvist, T., et al. (2017). Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes. Metab. 19, 1242–1251. doi:10.1111/dom.12932
Brierley, D. I., Holt, M. K., Singh, A., de Araujo, A., McDougle, M., Vergara, M., et al. (2021). Central and peripheral GLP-1 systems independently suppress eating. Trapp. S. Nat. Metab. 3 (2), 258–273. doi:10.1038/s42255-021-00344-4
Cigrovski Berkovic, M., Rezic, T., Bilic-Curcic, I., and Mrzljak, A. (2022). Semaglutide might be a key for breaking the vicious cycle of metabolically associated fatty liver disease spectrum? World J. Clin. Cases 10 (20), 6759–6768. doi:10.12998/wjcc.v10.i20.6759
Davies, M., Færch, L., Jeppesen, O. K., Pakseresht, A., Pedersen, S. D., Perreault, L., et al. (2021). Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 397 (10278), 971–984. doi:10.1016/S0140-6736(21)00213-0
Drucker, D. J. (2022). GLP-1 physiology informs the pharmacotherapy of obesity. Mol. Metab. 57, 101351. doi:10.1016/j.molmet.2021.101351
Dulai, P. S., Singh, S., Patel, J., Soni, M., Prokop, L. J., Younossi, Z., et al. (2017). Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology 65 (5), 1557–1565. doi:10.1002/hep.29085
Ekstedt, M., Hagström, H., Nasr, P., Fredrikson, M., Stål, P., Kechagias, S., et al. (2015). Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 61 (5), 1547–1554. doi:10.1002/hep.27368
Eslam, M., Sanyal, A. J., and George, J.International Consensus Panel (2020). MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 158 (7), 1999–2014.e1. doi:10.1053/j.gastro.2019.11.312
European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), and European Association for the Study of Obesity (EASO) (2016). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 64 (6), 1388–1402. doi:10.1016/j.jhep.2015.11.004
Ferraioli, G., and Monteiro, L. B. S. (2019). Ultrasound-based techniques for the diagnosis of liver steatosis. World J. Gastroenterol. 25 (40), 6053–6062. doi:10.3748/wjg.v25.i40.6053
Flint, A., Andersen, G., Hockings, P., Johansson, L., Morsing, A., Sundby Palle, M., et al. (2021). Randomised clinical trial: semaglutide versus placebo reduced liver steatosis but not liver stiffness in subjects with non-alcoholic fatty liver disease assessed by magnetic resonance imaging. Aliment. Pharmacol. Ther. 54, 1150–1161. doi:10.1111/apt.16608
Friedrichsen, M., Breitschaft, A., Tadayon, S., Wizert, A., and Skovgaard, D. (2021). The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating, and gastric emptying in adults with obesity. Diabetes Obes. Metab. 23 (3), 754–762. doi:10.1111/dom.14280
Gibbons, C., Blundell, J., Tetens Hoff, S., Dahl, K., Bauer, R., and Baekdal, T. (2021). Effects of oral semaglutide on energy intake, food preference, appetite, control of eating and body weight in subjects with type 2 diabetes. Diabetes Obes. Metab. 23 (2), 581–588. doi:10.1111/dom.14255
Godoy-Matos, A. F., Silva Júnior, W. S., and Valerio, C. M. (2020). NAFLD as a continuum: from obesity to metabolic syndrome and diabetes. Diabetol. Metab. Syndr. 12, 60. doi:10.1186/s13098-020-00570-y
Gofton, C., Upendran, Y., Zheng, M. H., and George, J. (2023). MAFLD: how is it different from NAFLD? Clin. Mol. Hepatol. 29 (Suppl. l), S17–S31. doi:10.3350/cmh.2022.0367
Hallsworth, K., and Adams, L. A. (2019). Lifestyle modification in NAFLD/NASH: facts and figures. JHEP Rep. 1 (6), 468–479. doi:10.1016/j.jhepr.2019.10.008
Harrison, S. A., Taub, R., Neff, G. W., Lucas, K. J., Labriola, D., Moussa, S. E., et al. (2023). Resmetirom for nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled phase 3 trial. Nat. Med. 29 (11), 2919–2928. doi:10.1038/s41591-023-02603-1
Jensen, L., Kupcova, V., Arold, G., Pettersson, J., and Hjerpsted, J. B. (2018). Pharmacokinetics and tolerability of semaglutide in people with hepatic impairment. Diabetes, Obes. Metabolism 20 (4), 998–1005. doi:10.1111/dom.13186
Kenneally, S., Sier, J. H., and Moore, J. B. (2017). Efficacy of dietary and physical activity intervention in non-alcoholic fatty liver disease: a systematic review. BMJ Open Gastroenterol. 4, e000139. doi:10.1136/bmjgast-2017-000139
Knudsen, L. B., and Lau, J. (2019). The discovery and development of liraglutide and semaglutide. Front. Endocrinol. (Lausanne) 10, 155. doi:10.3389/fendo.2019.00155
Kosiborod, M. N., Bhatta, M., Davies, M., Deanfield, J. E., Garvey, W. T., Khalid, U., et al. (2023). Semaglutide improves cardiometabolic risk factors in adults with overweight or obesity: STEP 1 and 4 exploratory analyses. Diabetes Obes. Metab. 25 (2), 468–478. doi:10.1111/dom.14890
Lee, H. A., and Kim, H. Y. (2023). Therapeutic mechanisms and clinical effects of glucagon-like peptide 1 receptor agonists in nonalcoholic fatty liver disease. Int. J. Mol. Sci. 24 (11), 9324. doi:10.3390/ijms24119324
Leung, J. C., Loong, T. C., Wei, J. L., Wong, G. L., Chan, A. W., Choi, P. C., et al. (2017). Histological severity and clinical outcomes of nonalcoholic fatty liver disease in nonobese patients. Hepatology 65 (1), 54–64. doi:10.1002/hep.28697
Loomba, R., Abdelmalek, M. F., Armstrong, M. J., Jara, M., Kjær, M. S., Krarup, N., et al. (2023). Semaglutide 2·4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: a randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol. Hepatol. 8 (6), 511–522. doi:10.1016/S2468-1253(23)00068-7
Machado, M. V., and Cortez-Pinto, H. (2023). NAFLD, MAFLD and obesity: brothers in arms? Nat. Rev. Gastroenterol. Hepatol. 20 (2), 67–68. doi:10.1038/s41575-022-00717-4
Marso, S. P., Bain, S. C., Consoli, A., Eliaschewitz, F. G., Jodar, E., Leiter, L. A., et al. (2016). Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375, 1834–1844. doi:10.1056/NEJMoa1607141
Mundi, M. S., Velapati, S., Patel, J., Kellogg, T. A., Abu Dayyeh, B. K., and Hurt, R. T. (2020). Evolution of NAFLD and its management. Nutr. Clin. Pract. 35, 72–84. doi:10.1002/ncp.10449
Nauck, M. A., and Quast, D. R. (2021). Cardiovascular safety and benefits of semaglutide in patients with type 2 diabetes: findings from SUSTAIN 6 and PIONEER 6. Front. Endocrinol. (Lausanne) 12, 645566. doi:10.3389/fendo.2021.645566
Newsome, P., Francque, S., Harrison, S., Ratziu, V., Van Gaal, L., Calanna, S., et al. (2019). Effect of semaglutide on liver enzymes and markers of inflammation in subjects with type 2 diabetes and/or obesity. Aliment. Pharmacol. Ther. 50, 193–203. doi:10.1111/apt.15316
Niu, S., Chen, S., Chen, X., Ren, Q., Yue, L., Pan, X., et al. (2022). Semaglutide ameliorates metabolism and hepatic outcomes in an NAFLD mouse model. Front. Endocrinol. (Lausanne) 13, 1046130. doi:10.3389/fendo.2022.1046130
Nomoto, H., Takahashi, Y., Takano, Y., Yokoyama, H., Tsuchida, K., Nagai, S., et al. (2023). Effect of switching to once-weekly semaglutide on non-alcoholic fatty liver disease:the SWITCH-SEMA 1 subanalysis. Pharmaceutics 15 (8), 2163. doi:10.3390/pharmaceutics15082163
Okamoto, A., Yokokawa, H., Nagamine, T., Fukuda, H., Hisaoka, T., and Naito, T. (2021). Efficacy and safety of semaglutide in glycemic control, body weight management, lipid profiles and other biomarkers among obese type 2 diabetes patients initiated or switched to semaglutide from other GLP-1 receptor agonists. J. Diabetes and Metabolic Disord. 20, 2121–2128. doi:10.1007/s40200-021-00899-9
Paradies, G., Paradies, V., Ruggiero, F. M., and Petrosillo, G. (2014). Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J. Gastroenterol. 20 (39), 14205–14218. doi:10.3748/wjg.v20.i39.14205
Petrovic, A., Igrec, D., Rozac, K., Bojanic, K., Kuna, L., Kolaric, T. O., et al. (2023). The role of GLP1-RAs in direct modulation of lipid metabolism in hepatic tissue as determined using in vitro models of NAFLD. Curr. Issues Mol. Biol. 45 (6), 4544–4556. doi:10.3390/cimb45060288
Pontes-da-Silva, R. M., de Souza Marinho, T., de Macedo Cardoso, L. E., Mandarim-de-Lacerda, C. A., and Aguila, M. B. (2022). Obese mice weight loss role on nonalcoholic fatty liver disease and endoplasmic reticulum stress treated by a GLP-1 receptor agonist. Int. J. Obes. (Lond) 46, 21–29. doi:10.1038/s41366-021-00955-7
Pyke, C., Heller, R. S., Kirk, R. K., Ørskov, C., Reedtz-Runge, S., Kaastrup, P., et al. (2014). GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 155, 1280–1290. doi:10.1210/en.2013-1934
Rakipovski, G., Rollin, B., Nøhr, J., Klewe, I., Frederiksen, K. S., Augustin, R., et al. (2018). The GLP-1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE-/- and LDLr-/- mice by a Mechanism that includes inflammatory pathways. JACC Basic Transl. Sci. 3, 844–857. doi:10.1016/j.jacbts.2018.09.004
Romero-Gómez, M., Zelber-Sagi, S., and Trenell, M. (2017). Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 67, 829–846. doi:10.1016/j.jhep.2017.05.016
Schattenberg, J. M., Grønbæk, H., Kliers, I., Ladelund, S., Long, M., Nygård, S. B., et al. (2023). Prevalence of, and effect of semaglutide on, features of non-alcoholic steatohepatitis in patients with obesity with and without type 2 diabetes: analysis of data from two randomised placebo-controlled trials using SomaSignal tests EASL Congress. J. Hepatology.
Targher, G., Byrne, C. D., Lonardo, A., Zoppini, G., and Barbui, C. (2016). Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J. Hepatol. 65 (3), 589–600. doi:10.1016/j.jhep.2016.05.013
Vernon, G., Baranova, A., and Younossi, Z. M. (2011). Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 34, 274–285. doi:10.1111/j.1365-2036.2011.04724.x
Vilar-Gomez, E., Martinez-Perez, Y., Calzadilla-Bertot, L., Torres-Gonzalez, A., Gra-Oramas, B., Gonzalez-Fabian, L., et al. (2015). Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 149, 367–378. doi:10.1053/j.gastro.2015.04.005
Volpe, S., Lisco, G., Fanelli, M., Racaniello, D., Colaianni, V., Triggiani, D., et al. (2022). Once-weekly subcutaneous semaglutide improves fatty liver disease in patients with type 2 diabetes: a 52-week prospective real-life study. Nutrients 14, 4673. doi:10.3390/nu14214673
Weghuber, D., Barrett, T., Barrientos-Pérez, M., Gies, I., Hesse, D., Jeppesen, O. K., et al. (2022). Once-weekly semaglutide in adolescents with obesity. N. Engl. J. Med. 387 (24), 2245–2257. doi:10.1056/NEJMoa2208601
Wilding, J. P. (2014). The importance of weight management in type 2 diabetes mellitus. Int. J. Clin. Pract. 68 (6), 682–691. doi:10.1111/ijcp.12384
Wilding, J. P. H., Batterham, R. L., Calanna, S., Davies, M., Van Gaal, L. F., Lingvay, I., et al. (2021). Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 384 (11), 989–1002. doi:10.1056/NEJMoa2032183
Younossi, Z. M., Golabi, P., Paik, J. M., Henry, A., Van Dongen, C., and Henry, L. (2023). The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology 77 (4), 1335–1347. doi:10.1097/HEP.0000000000000004
Keywords: NAFLD, NASH, semaglutide combination, weekly semaglutide, semaglutide orally
Citation: Koureta E and Cholongitas E (2024) Evolving role of semaglutide in NAFLD: in combination, weekly and oral administration. Front. Pharmacol. 15:1343587. doi: 10.3389/fphar.2024.1343587
Received: 24 November 2023; Accepted: 13 February 2024;
Published: 23 February 2024.
Edited by:
Hosui Atsushi, Osaka Rosai Hospital, JapanReviewed by:
Rory Cunningham, Ochre Bio, United StatesJulia Lischka, Paracelsus Medical University, Austria
Copyright © 2024 Koureta and Cholongitas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Evangelos Cholongitas, Y2hvbG9uZ2l0YXNAeWFob28uZ3I=
 Evgenia Koureta
Evgenia Koureta Evangelos Cholongitas
Evangelos Cholongitas