- 1Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, China
- 2TCM Regulating Metabolic Diseases Key Laboratory of Sichuan Province, Chengdu, Sichuan, China
- 3Department of Endocrinology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
Type 2 diabetes presents a significant global health burden and is frequently linked to serious clinical complications, including diabetic cardiomyopathy, nephropathy, and retinopathy. Astragalus polysaccharide (APS), extracted from Astragalus membranaceus, exhibits various biochemical and physiological effects. In recent years, a growing number of researchers have investigated the role of APS in glucose control and the treatment of diabetes and its complications in various diabetes models, positioning APS as a promising candidate for diabetes therapy. This review surveys the literature on APS from several databases over the past 20 years, detailing its mechanisms of action in preventing and treating diabetes mellitus. The findings indicate that APS can address diabetes by enhancing insulin resistance, modulating the immune system, protecting islet cells, and improving the intestinal microbiota. APS demonstrates positive pharmacological value and clinical potential in managing diabetic complications, including diabetic retinopathy, nephropathy, cardiomyopathy, cognitive dysfunction, wound healing, and more. However, further research is necessary to explore APS’s bioavailability, optimal dosage, and additional clinical evidence.
1 Introduction
As of 2021, the global diabetic population reached 529 million, with diabetes standing as a leading cause of blindness, renal failure, heart attacks, stroke, and lower limb amputation (GBD, 2021 Diabetes Collaborators, 2023). While glucose-lowering therapies remain fundamental in diabetes management, the pursuit of novel therapeutics has gained momentum in research and development (Nauck et al., 2021). Herbal medicine emerges as a promising avenue, offering the potential for diversified diabetes treatment and improved patient quality of life (Tian et al., 2019; Ai et al., 2020; Zhang et al., 2022). Its efficacy stems from various mechanisms, including inhibition of α-glucosidase and α-amylase to reduce carbohydrate digestion and absorption, protection of pancreatic β-cells, enhancement of insulin sensitivity, promotion of gluconeogenesis and glycogen storage in the liver and muscle, antioxidant defense against organ damage, and attenuation of tissue inflammation to shield impaired tissues (Sun et al., 2021).
Polysaccharides are prominent constituents of herbal plants, and in recent decades, polysaccharides isolated from various herbs have exhibited a range of biological activities, including antitumor, antioxidant, hypoglycemic, antiradical, antiviral, hypolipidemic, and immunomodulatory effects. Polysaccharides from herbs such as Astragalus membranaceus, Angelica sinensis, Cordyceps sinensis, and Ophiopogon japonicus possess antidiabetic properties (Zeng et al., 2018).
A. membranaceus, initially documented in the Shennong Ben Cao Jing (Classic of the Divine Husbandman’s Materia Medica), refers to the dried root of the leguminous plants A. membranaceus Bge. var. mongholicus (Bge.) Hsiao and A. membranaceus (Fisch.) Bge (Chinese Pharmacopoeia Commission, 2020) and can be applied in the treatment of conditions such as weakness, wounds, anemia, fever, allergies, chronic fatigue, loss of appetite, uterine hemorrhage, and uterine prolapse (Kim et al., 2003). Studies have verified the immunomodulatory, antioxidant, anti-inflammatory, and anti-tumor properties of A. membranaceus, leading to its widespread use in various diseases like cardiovascular diseases (Su et al., 2021), diabetes mellitus (DM) (Tian et al., 2016), cancers (Sheik et al., 2021), respiratory diseases (Yang C.-G. et al., 2023), and neurological disorders (Xia et al., 2020).
Astragalus polysaccharides (APS) can be classified into heteropolysaccharides, glucans, neutral polysaccharides, and acid polysaccharides, with their monosaccharide composition varying based on the astragalus source and polysaccharide molecular weight (Tang and Huang, 2022). The complex chemical structures of individual APS make their isolation and characterization challenging, resulting in limited knowledge of APS composition. To date, 30 polysaccharides have been isolated and identified from A. membranaceus using extraction methods like water extraction, microwave extraction, enzyme extraction, and alkaline water extraction (Guo et al., 2018). Wang et al. utilized various techniques to analyze APS structure and composition, revealing rhamnose, galacturonic acid, glucose, galactose, and arabinose as components, with Glc as the primary constituent. Gas chromatography indicated eight main glycosidic bond types, with 1,4-glucose predominating, and NMR spectroscopy confirmed the α-configuration of isohead hydrogen (Wang et al., 2017). Yan et al. obtained APS by alcohol precipitation of astragalus extract followed by Sevage deproteinization, yielding three APS fractions (APS-1, APS-2, and APS-3) via DEAE-resin column chromatography (Yan et al., 2012).
Numerous studies have highlighted the potential of APS in treating Type 1 diabetes mellitus (T1DM), Type 2 diabetes mellitus (T2DM), and diabetic complications such as diabetic retinopathy (DR), diabetic nephropathy (DN), diabetic cardiovascular disease, and diabetic cognitive dysfunction, through various pathways (Wu et al., 2007; Dun et al., 2016; Sun S. et al., 2019; Meng et al., 2020; Ma, 2022). However, the broad scope of APS’s effects necessitates a detailed description to fully comprehend its role in diabetes. Therefore, this review summarizes APS’s role and underlying molecular mechanisms in diabetes and its related complications.
2 Methods
Studies related to APS’s anti-diabetic effects were identified through major scientific databases (PubMed, Web of Science, Embase, Google Scholar, and China National Knowledge Infrastructure) over the last 20 years. Some articles were also discovered through citation tracing or by visiting journal websites. Keywords used during the search included APS, astragalus membrane, astragalus montana, antidiabetic, hypoglycemic, hypolipidemic, mechanism, insulin sensitivity, and insulin resistance.
3 Effects of APS in DM
DM is a chronic disease characterized by hyperglycemia (Mathis et al., 2001). T1DM is a chronic, immune-mediated disease characterized by the destruction of insulin-producing β-cells in the pancreas (Norris et al., 2020), while T2DM is primarily characterized by insulin resistance and impaired insulin secretion (DeFronzo et al., 2015).
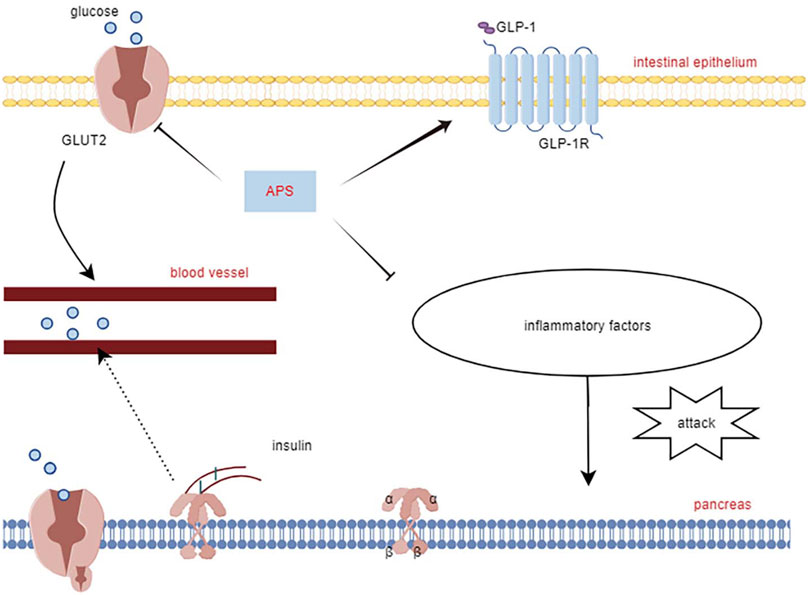
APS improves both T1DM and T2DM through different molecular mechanisms. In streptozotocin combined with a high-fat diet (HFD)-induced diabetic rats, APS (700 mg/kg, orally) for 8 weeks significantly reduced fasting plasma glucose, random blood glucose, glycated hemoglobin, and homeostatic model assessment of insulin tolerance (Zou et al., 2009). In another study in diabetic rats with streptozotocin/HFD, APS (400 mg/kg, orally) treatment for 5 weeks significantly reduced random blood glucose and improved insulin sensitivity in diabetic rats (Wu et al., 2005). In KKAy mice, APS improved hyperglycemia and systemic insulin sensitivity and reduced hepatic triglyceride and free fatty acid content (Mao et al., 2007). Furthermore, in Goto Kakizaki (GK) rats, APS (500 mg/kg, orally) for 8 weeks resulted in reduced body weight, area under the curve of postprandial blood glucose, and total cholesterol, triglycerides, and low-density lipoprotein cholesterol levels (Wei et al., 2018). In a study using APS (200 mg/kg, orally) to prevent the onset of diabetes in non-obese diabetes (NOD) mice, it was found that APS-administered NOD mice had a lower incidence of T1DM than the controls (Chen et al., 2008). The molecular mechanism of APS’s hypoglycemic effect involves its action on insulin-sensitive organs such as the liver, skeletal muscle, adipose tissue, and pancreas (Figure 2).
3.1 Improvement of insulin resistance by APS
Insulin resistance is characterized by an insulin-mediated defect in glucose metabolism control, particularly in the muscles, adipose tissues, and liver (Roden and Shulman, 2019; James et al., 2021). The insulin-sensitizing effect of APS has been extensively documented (Mao et al., 2009; Zhang et al., 2015; Sun J. et al., 2019). Adipose tissue is a major insulin target, and impaired glucose uptake in adipose tissue is linked to insulin resistance (Abel et al., 2001). The use of mouse 3T3-L1 preadipocytes is common in studying the insulin-sensitizing activity of hypoglycemic compounds. Zhang et al. (2018) used 3T3-L1 preadipocytes and APS (0.1 μg/mL) for intervention, demonstrating that APS increased preadipocyte proliferation in a dose-dependent manner, increased mRNA and protein content of glucose transporter protein 4, enhanced tyrosine phosphorylation of insulin receptor substrate 1 and phospho-Akt content, and increased AMP-activated protein kinase (AMPK) content. Ke et al. (2017) found that APS (1 μg/mL) suppressed miR-721 expression and increased PPAR-γ expression, promoting glucose uptake and enhancing insulin sensitivity in 3T3-L1 adipocytes in a dose- and time-dependent manner, through the miR-721-PPAR-γ-PI3K/AKT-GLUT4 signaling pathway.
The liver plays a central role in glucose synthesis and metabolism and is a major target organ for insulin resistance (Rodríguez-Gutiérrez et al., 2018). Wei et al. (2018) used a T2DM rat model established with GK rats and administered APS (500 mg/kg/day, orally) for 8 weeks. The results showed that APS attenuated insulin resistance in T2DM by upregulating or maintaining hepatic miR-203a-3p expression levels and by decreasing GRP78, CHOP, pJNK1, and cysteine asparaginase-12 protein expression levels. GU et al. (2015) administered APS (700 mg/kg, orally) for 8 weeks in GK rats and found that APS elevated hepatic PPARα, FGF21, and SIRT1 expression levels, reduced chronic inflammation, and partially attenuated hepatic steatosis, inhibiting aberrant glucose-lipid metabolism and insulin resistance.
Skeletal muscle, constituting 40%–50% of total body mass, is the primary tissue responsible for insulin-dependent glucose utilization (Sinacore and Gulve, 1993). Myostatin, a growth factor secreted by skeletal muscle, plays a pivotal role in regulating insulin signaling and insulin resistance (Guo et al., 2009). Liu M. et al. (2013) employed an HFD to induce diabetes in KKAy mice and treated them with APS (700 mg/kg, orally) for 8 weeks. The results revealed that APS treatment reduced myostatin expression and malondialdehyde production in skeletal muscle of non-insulin-dependent diabetic KKAy mice, ameliorated hyperglycemia, hyperlipidemia, and insulin resistance. In vitro studies using saturated acid palmitate-induced C2C12 cells showed that APS treatment (200 μg/mL) reduced reactive oxygen species (ROS) overproduction, extracellular signal-regulated kinase activation, and nuclear factor kappa B function, partially restored impaired glucose uptake, improved insulin sensitivity, and reduced myostatin expression in skeletal muscle. Glucose processing in skeletal muscle is regulated by the AMPK signaling pathway. Zou et al. (2009) intervened in HFD combined with streptozotocin-induced diabetic rats using APS (700 mg/kg, intragastric) and found that APS alleviated glucose toxicity by increasing hepatic glycogen synthesis and skeletal muscle glucose translocation through AMPK activation in T2DM rats. Cellular assays further demonstrated that APS treatment (400 μg/mL) significantly increased glucose uptake in L6 myotubes in a time- and concentration-dependent manner, promoted AMPK activation mediated by Ca2+/calmodulin-dependent protein kinase β or liver kinase B1 (Liu J. et al., 2013). Additionally, Liu et al. (2010) treated 12-week-old diabetic KKAy mice with APS (700 mg/kg) for 8 weeks and found that APS-treated diabetic mice showed partial restoration of insulin-induced phosphorylation of protein kinase B Ser-473 and glucose transporter protein 4 translocation in skeletal muscle. Wu et al. (2005) used APS (400 mg/kg, orally for 5 weeks) in HFD combined with streptozotocin-treated diabetic rats and observed that APS decreased the protein level and activity of protein tyrosine phosphatase 1B in the muscle of type 2 diabetic rats, increased insulin-induced tyrosine phosphorylation of insulin receptor β-subunits and insulin receptor substrate-1. Collectively, these studies demonstrate that APS can improve insulin resistance in adipose tissue, liver, and skeletal muscle through various pathways, highlighting its potential as an insulin sensitizer for type 2 diabetes treatment (Figure 1).
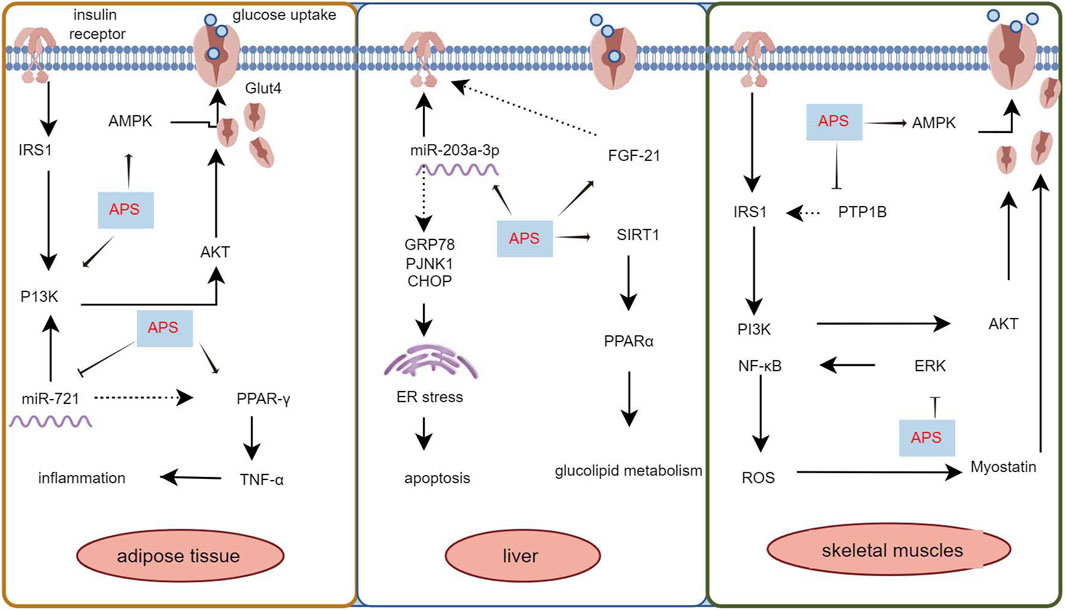
Figure 1. Mechanism of APS to improve insulin resistance in adipose tissue, liver and skeletal muscle.
3.2 Improvement of pancreatic islet cell function by APS
Impaired insulin secretion by pancreatic β-cells is a core pathomechanism of T2DM, where disease progression hampers insulin secretion’s ability to maintain glucose homeostasis, leading to hyperglycemia (Weyer et al., 1999; DeFronzo et al., 2015). Pancreatic β-cells are crucial in maintaining glucose metabolism balance. APS has been shown to improve pancreatic β-cell number and function in diabetic rats through various protective mechanisms (Cui et al., 2016; Tang et al., 2017; Yang Z.-M. et al., 2021).
Yang Z.-M. et al. (2021) reported that APS treatment reversed the decreased glucagon-like peptide-1 and its receptors expression levels in the pancreas of T2DM diabetic rats, along with increased expression of glucose transporter 2, promoting restoration of insulin secretion levels by affecting the STR/GLP-1/GLP-1R pathway in the enteropancreatic axis of T2DM rats. Deng et al. (2021) treated MIN6 cells (mouse pancreatic β-cell line) with APS (50, 100, and 200 μg/mL) after high glucose (HG) with palmitic acid treatment. APS-treated MIN6 cells exhibited higher viability, increased insulin secretion and pancreatic and duodenal homeobox 1 expression, and reduced apoptosis, reversing the effects of HG/palmitic acid on MIN6 cells. Additionally, chronic low-grade inflammation plays a crucial role in the development of metabolic disorders and diabetes mellitus. Excessive release of cytokines can severely damage pancreatic islet cells (Greenfield and Campbell, 2006). APS suppresses interleukin (IL)-1β protein production and the expression of several pro-inflammatory genes (e.g., iNOS, IL-1β, IL-6, MCP-1, and CD11c), which may also contribute to APS’s protective mechanism for islet cells (Lu et al., 2013). This underscores APS’s ability to safeguard pancreatic islets by reducing inflammatory factors, inhibiting islet β-cell apoptosis, and promoting insulin secretion (Figure 2).
3.3 Effects of APS in immunomodulation
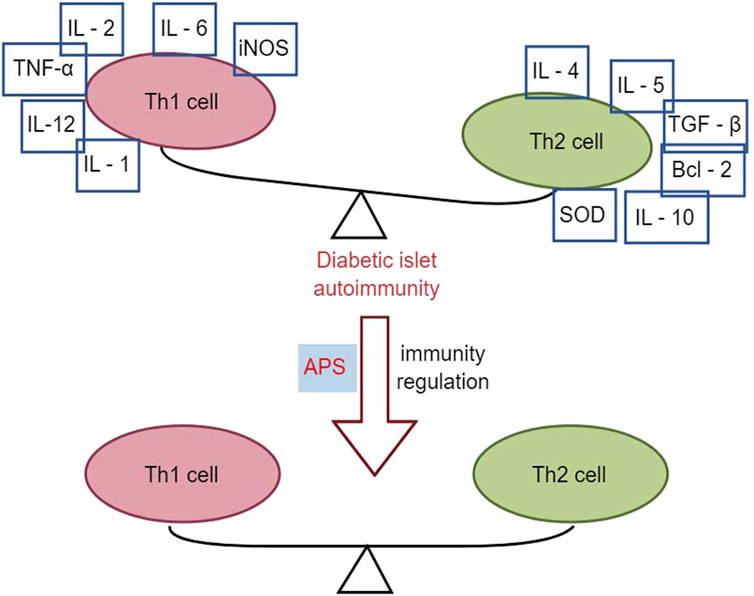
APS has immunomodulatory effects on various immune cells, inhibiting over-activation of the immune system, and is widely used as an immunomodulator in clinical practice (Li et al., 2022). T1DM is an autoimmune disease mediated by T cells that destroy insulin-producing beta cells in the pancreatic islets (Marfil-Garza et al., 2021). It is associated with an imbalance between T helper 1 (Th1) and T helper 2 (Th2) subpopulations of helper T-lymphocytes and their cytokines. Th1 cytokines, such as interferon-gamma (IFN-γ), promote islet inflammation and DM, whereas Th2 cytokines, such as IL-4, protect pancreatic islet β-cells from damage (Roep, 2003).
Research has demonstrated that intervention with APS (2.0 mg/kg p. o.) in NOD mice for 10 weeks resulted in reduced infiltration of pancreatic islets with CD4+ T-lymphocytes, lower spleen T-lymphocyte CD4+/CD8+ ratios, and decreased gene expression of Th1-type cytokines in the pancreas compared to control NOD mice. This led to a decrease in the conversion of Th1-type cytokines to Th2 cytokines, including IL-4, IL-5, IL-10, transforming growth factor (TGF), B-cell lymphoma-2 (Bcl-2), and superoxide dismutase (SOD), thereby altering the autoimmune response and delaying or preventing the development of T1DM in NOD mice (Chen et al., 2008). Furthermore, another study using APS (2 g/kg p. o.) to intervene in NOD mice for 2 months found that early application of APS pre-immunization significantly downregulated the expression levels of Fas and iNOS genes in the pancreatic islets of NOD mice. Simultaneously, it upregulated the expression levels of Bcl-2 and SOD genes in the pancreatic islets, correcting the immune imbalance of oxidative or apoptotic death in NOD mice (Chen et al., 2007). In multiple low-dose streptozotocin (MLD-streptozotocin)-induced diabetic mice treated with APS (100, 200, and 400 mg/kg i. p.) for 15 or 30 days, serum insulin concentration was upregulated, the β-cell mass increased, the percentage of apoptotic β-cells decreased, the Th1/Th2 cytokine ratio was downregulated, and peroxisome proliferator-activated receptor γ gene expression was upregulated, suggesting that APS could act through immunomodulation of the Th1/Th2 cytokine ratio (Li et al., 2007). Zhou et al. (2011) demonstrated that APS (100 mg/kg, 400 mg/kg p. o.) intervention in streptozotocin-induced diabetic mice for 15 days reduced pancreatic islet β-cell damage by modulating galactoglucan-1 (gal-1) and down-regulating the Th1 response, leading to apoptosis of CD8 T cells. Overall, APS increased total β-cell mass in T1DM mice by reducing apoptosis of pancreatic β-cells and protecting regenerating β-cells from damage through immunomodulation (Figure 3).
3.4 Effects of APS in gut microorganisms
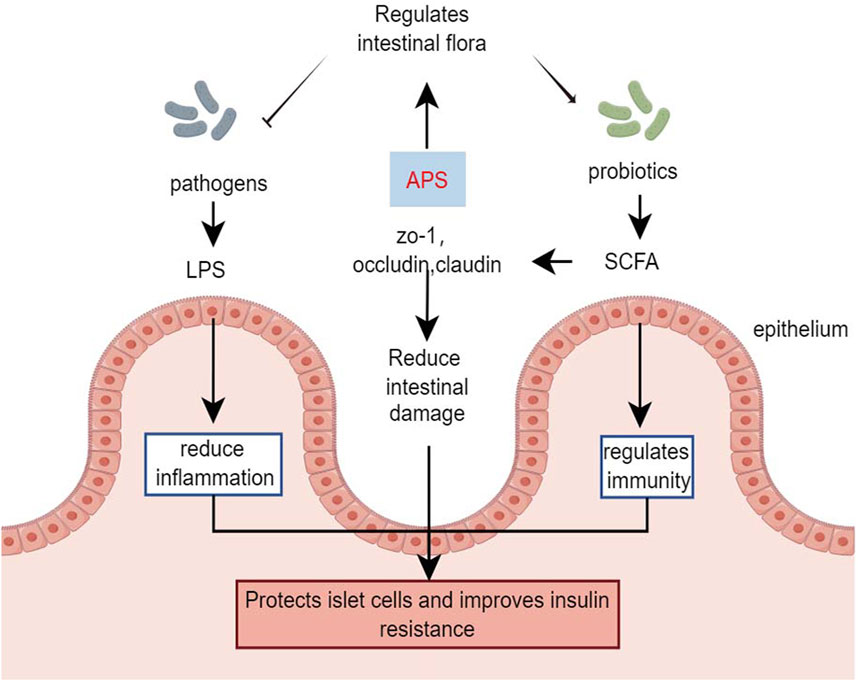
The gut microbiota plays a significant role in metabolism and immune regulation, acting as an endocrine organ. Dysbiosis of the gut microbiota and disruption of the intestinal barrier can lead to organ damage in patients with diabetes (Yang G. et al., 2021).
Chen et al. (2022) demonstrated that in HFD combined with streptozotocin-induced diabetic mice, APS intervention (400 mg/kg/day, orally) for 6 weeks strongly inhibited the potential pathogen Shigella and promoted the growth of beneficial bacteria such as Eubacterium rectale and Lactobacillus species. Additionally, APS repaired intestinal microbiota, remodeled specific intestinal barrier damage, reduced lipopolysaccharide and systemic inflammation, and improved metabolic parameters in T2DM mice (Chen et al., 2022). Yang B. et al. (2023) treated streptozotocin-induced type 1 diabetes mice with APS-1 (200 mg/kg, orally) for 8 weeks, showing that APS-1 modulated the expression of zona occludens 1, occludin, and claudin-1, improving intestinal barrier function. APS-1 also restored the relative abundance of Trichoderma reesei, Lactobacillus reesei, and Faecalibaculum, inhibiting inflammatory responses, protecting pancreatic islet cells, reducing blood glucose, and improving insulin resistance by increasing the relative abundance of intestinal flora (Yang B. et al., 2023). The effects of APS on gut microbes were further demonstrated in db/db mice, where APS treatment (600 mg/kg, orally) for 16 days increased the production of fecal short-chain fatty acids. This improvement in short-chain fatty acids production enhanced the expression of G-protein-coupled receptors 41/43 and tight junction proteins (occudin and zona occludens 1) restored the diabetic community, improved gut integrity, and alleviated diabetes symptoms in db/db mice (Song et al., 2022).
Butyrate-producing bacteria are crucial for human health, providing energy to the intestinal epithelium, maintaining intestinal bacterial balance, and regulating host cellular responses (Louis et al., 2010). However, a clinical study involving in vitro fermentation of fresh feces from healthy donors and patients with T2DM found that after 48 h of fermentation with APS, the organic acid profile of APS fermentation was more influenced by individual differences in gut microbiota than in the healthy and T2DM groups (Xu et al., 2023). Animal studies have indicated that APS improves the profile of intestinal flora and restores the intestinal barrier in diabetic mice. However, the role of APS in regulating the intestinal flora of individuals with diabetes remains controversial and requires further research (Figure 4).
4 Effects of APS on diabetes complications
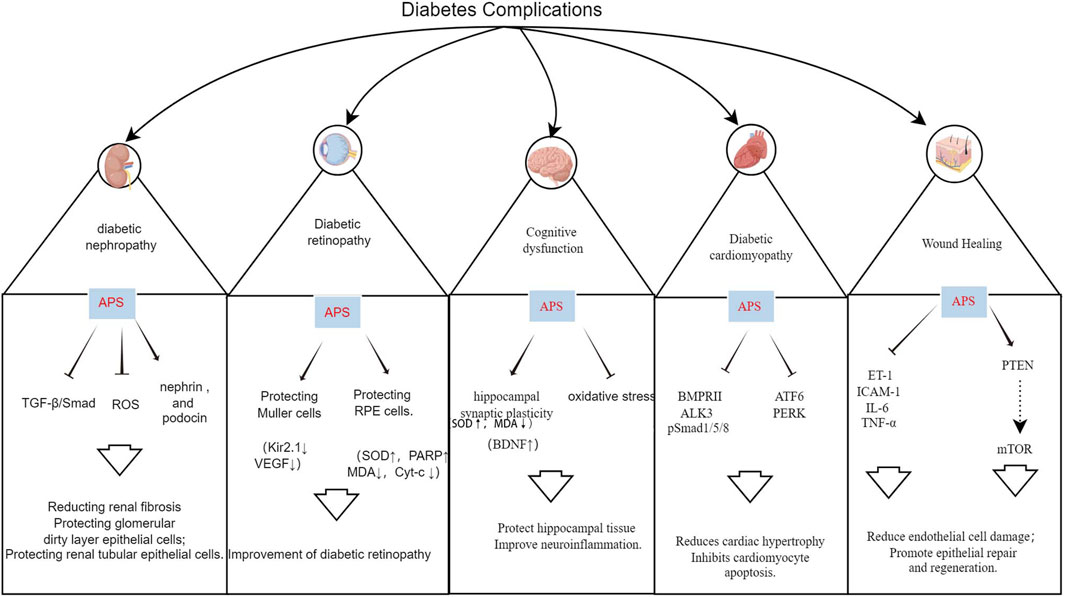
Hyperglycemia is a primary cause of diabetes-related morbidity and mortality, leading to various vascular complications. Oxidative stress and inflammation are key factors in these complications. APS has shown promise in ameliorating these complications through various pathways (Figure 5; Supplementary Table S1).
4.1 APS and DN
DN is characterized by persistent albuminuria and progressive decline in renal function, affecting up to 50% of patients with diabetes, and is a leading cause of end-stage renal disease (ESRD), associated with increased cardiovascular morbidity and mortality (Selby and Taal, 2020). APS has a protective effect on DN, delaying its development through podocyte repair, reduction of tumor necrosis factor, inhibition of renal tubular epithelial cell apoptosis, and improvement of oxidative stress (Li et al., 2011; 2018; Guo et al., 2018; Meng et al., 2020).
TGF-β1, a key member of the TGF-β family, plays a central role in the TGF-β/Smad signaling pathway, promoting fibrosis. Its overexpression stimulates the growth of renal fibroblasts, increases extracellular matrix content, and leads to renal sclerosis. Controlling TGF-β1 expression is beneficial for ameliorating diabetic-induced renal damage, making it a significant target in the treatment of DN (Voelker et al., 2017). APS attenuates renal lesions by down-regulating TGF-β1 protein content and mRNA overexpression in the kidneys of diabetic rats (Lai et al., 2002). Li et al. (2018) demonstrated that APS (200 and 400 mg/kg, orally) inhibited the renal TGF-β1/Smads signaling pathway in diabetic rats after 8 weeks of intervention, significantly reducing fasting glucose, blood creatinine, and urea nitrogen. Meng et al. (2020) also showed that different doses of APS (25, 50, and 100 mg/kg, orally) reduced kidney weight, 24-h urinary microalbumin, blood urea nitrogen, creatinine, collagens III and IV, transforming growth factor-β3, α smooth muscle actin, and Smad3 levels in HFD combined with streptozotocin-induced diabetic rats after 8 weeks of intervention. APS protects the kidney from interstitial fibrosis by suppressing the TGF-β/Smad signaling pathway and reducing extracellular matrix formation.
Epithelial cell, or podocyte, injury in the glomerular basement membrane is a critical factor in the formation of DN proteinuria (Barutta et al., 2022). Li et al. (2011) administered APS (400 mg/kg/day, orally) to streptozotocin-induced diabetic rats for 8 weeks. The results showed significant reductions in blood glucose, blood urea nitrogen, blood creatinine, and total 24-h urinary protein. Renal pathological changes were attenuated, and the expression of the major podocyte-specific proteins nephrin and podocin was increased in the rats with APS intervention, suggesting that APS’s preventive effect on DN may be related to the maintenance of podocyte integrity (Li et al., 2011).
Early in the development of DN, renal tubular cells undergo apoptosis and oxidative damage. Apoptosis of renal tubular epithelial cells further promotes interstitial fibrosis and atrophy, while the overproduction of ROS is a primary initiator of diabetic complications and a key factor in cellular damage (De Nicola et al., 2014). Guo et al. (2018) demonstrated that human renal tubular epithelial cells (HK-2) treated with APS showed increased survival, decreased apoptosis, and reduced ROS content. This indicates that APS could promote HG-induced proliferation and inhibit apoptosis and transdifferentiation of HK-2 cells (Guo et al., 2018).
In clinical use, APS has been developed as APS injection and APS fFlush, with clinical studies confirming that APS flush can treat DN, with lipid-lowering effects and a reduction in the urinary albumin excretion rate (Cao and Zhang, 2007). APS injection can reduce early activated T-lymphocytes, improve cellular immune function, and enhance the body’s immune level in older patients with DN after 3 weeks of treatment (Deng et al., 2014). However, further research via controlled, large-scale clinical trials is still required.
4.2 APS and DR
DR is a significant ocular complication of diabetes and is the leading cause of blindness and visual impairment in individuals with diabetes worldwide. The prevalence of DR is expected to increase annually (Teo et al., 2021; Tan and Wong, 2023). Li et al. (2008) demonstrated that administering APS (700 mg/kg p. o.) to streptozotocin combined with HFD-induced diabetic rats for 8 weeks led to a decrease in the expression of Kir2.1 protein in retinal Muller cells at an early stage, thereby protecting the Muller cells and reducing the incidence of DR. Ke et al. (2010) cultured second-generation glial fibrillary acid protein-positive Müller cells for 3 days using 400 μg/mL APS and 20 mmol/L glucose in a normal medium, resulting in a significant reduction in the expression of vascular endothelial growth factor in Müller cells in the high-glucose APS group. This suggests that APS prevents and treats DR by reducing the expression of vascular endothelial growth factor in Müller cells (Ke et al., 2010). Additionally, a study demonstrated that administering APS solution (700 mg/kg p. o.) for 8 weeks to high-fat-fed KKAy mice reduced blood glucose levels, enhanced insulin sensitivity, and attenuated the expression of TNF-α, thereby ameliorating retinopathy in diabetic KKAy mice (Wu et al., 2007).
The retinal pigment epithelium is a crucial target for DR initiation after hyperglycemia, and microRNAs can inhibit the expression of target genes by directly targeting the 3′ untranslated regions of genes at the post-transcriptional level (Shukla et al., 2011). Cellular experiments demonstrated that APS could increase cleaved-ATF6; Bax; p-PERK; p-IRE-1; Bcl-2; cleaved caspase-12, -9, -3; and unleaved SOD and PARP levels; it could also decrease malondialdehyde and mitochondrial Cyt-c levels, ameliorate HG-induced oxidative stress, mitochondrial damage, endoplasmic reticulum stress and apoptosis, and alleviate the metabolic memory of HG-treated retinal pigment epithelium cells. These findings suggest that APS has a potential therapeutic role in the development of DR (Liu et al., 2019; Peng et al., 2020).
4.3 APS and diabetic cardiomyopathy (DCM)
Cardiovascular events are the leading cause of death in patients, and DCM is a pathological condition caused by DM. DCM is initially characterized by myocardial fibrosis and associated diastolic dysfunction, followed by the manifestation of systolic dysfunction and ultimately clinical heart failure (HF) (Jia et al., 2018). DCM results from the interaction of several factors, including hyperinsulinemia, hyperglycemia, oxidative stress, abnormal fatty acid metabolism, and cardiac autonomic neuropathy (Acar et al., 2011; Dillmann, 2019).
Cardiac hypertrophy is a major feature of DCM, with HG inducing cardiac hypertrophy, and multiple signaling pathways involved in this process. Bone morphogenetic protein 10, a cardiac peptide growth factor, plays a specific role in cardiac hypertrophy and is considered an influential target for its treatment (Sun et al., 2023). Sun et al. (2023) demonstrated that APS had a potent anti-hypertrophic effect on HG-stimulated H9c2 cardiomyocytes and streptozotocin-induced DCM rats. In animal experiments, APS (0.5, 1, 2 g/kg p. o.) administered to streptozotocin-induced diabetic rats for 16 weeks reduced the expression of BMPRII, ALK3, and p-Smad1/5/8, alleviated cardiac hypertrophy, and improved cardiac function by inhibiting the activation of the bone morphogenetic protein 10 pathway in a dose-dependent manner (Sun et al., 2023).
Apoptosis is considered an important contributor to DCM, leading to cardiac cell loss, reduced cardiac contractility, and ultimately cardiac remodeling (Cai et al., 2006). Sun et al. showed that in vivo, APS (1 g/kg p. o.) intervention in streptozotocin-induced T1DM rats for 16 weeks downregulated the protein expression of activating transcription factor 6 and protein kinase RNA-like ER kinase. In vitro, APS inhibited HG-induced apoptosis in H9C2 cells and reduced the expression of ATF6 and PERK-related proteins in the endoplasmic reticulum stress pathway. These findings confirmed that APS enhanced cardiac function and alleviated myocardial apoptosis in diabetic conditions through ex vivo and in vivo studies (Sun et al., 2017; Sun et al., 2019 S.). In conclusion, APS can protect the myocardium via its anti-hypertrophic and anti-apoptotic effects on cardiomyocytes.
4.4 APS and cognitive dysfunction
Cognitive impairment and dementia, including Alzheimer’s disease, are increasingly recognized as common complications and comorbidities of both T1DM and T2DM, with individuals with diabetes having a 2.4–1.25 times higher risk of cognitive impairment compared to the general population (Biessels et al., 2020). Mechanistic studies provide various pathophysiological clues, including dysmetabolic disorders, brain insulin resistance, vascular endothelial dysfunction, accumulation of glycosylation end products, neurodegeneration, and inflammation (Ehtewish et al., 2022). Several studies have demonstrated that APS can significantly reduce the latency period of locomotion navigation experiments, decrease the dwell time of space exploration experiments, and improve performance in water maze experiments in diabetic rats (Dun et al., 2016; Li et al., 2017; Yang, 2017). Yang (2017) administered APS (60 mg/kg p. o.) to rats fed a high-fat and high-sugar diet and observed potential improvement in hippocampal synaptic plasticity through increased expression of brain-derived neurotrophic factor in the hippocampus, thus enhancing learning and memory functions. In interventions by Dun et al. (2016); Li et al. (2017) in streptozotocin-induced Wistar rats for 8 weeks using varying doses of APS (200, 400, and 800 mg/kg p. o.), APS was found to enhance glucose-lipid metabolism, insulin resistance, and antioxidant capacity in diabetic rats. Moreover, APS elevated hippocampal tissue SOD activity and reduced malondialdehyde content, suggesting potential protection against diabetic-induced brain damage via anti-oxidative stress and anti-apoptotic effects on hippocampal tissue (Dun et al., 2016; Li et al., 2017).
Alzheimer’s disease is considered a metabolic disorder, with metabolic disturbances contributing directly to Alzheimer’s disease through various pathways, including synaptic disconnection, neuronal loss, accumulation of amyloid-β, and hyperphosphorylation of tau protein (de la Monte, 2014). Epidemiological data strongly suggest an association between type 2 diabetes and an increased risk of dementia (Hamzé et al., 2022). After administration of APS (700 mg/kg p. o.) for 4 weeks to intervene in HFD combined with streptozotocin-injected APP/PS1 double transgenic mice, Huang et al. (2017) observed reduced insulin resistance and hepatic triglyceride levels induced by metabolic stress, decreased astrocytosis and microglial activation in the vicinity of plaques, and alleviation of metabolic stress-induced diabetes and subsequent neuroinflammation, thereby improving behavior in these transgenic mice.
4.5 APS and wound healing in diabetic
Diabetic foot ulcers are a significant concern, affecting 15% of individuals with diabetes and posing a serious threat to their quality of life, often leading to lower limb amputations (Okonkwo and DiPietro, 2017; Yue et al., 2022; McDermott et al., 2023). The complex pathogenesis of these ulcers involves inflammation, angiogenesis, and extracellular matrix remodeling, which impede proper wound healing in patients with diabetes (Chang and Nguyen, 2021). The literature highlights the crucial role of APS in managing diabetic wound healing.
Phosphatase and tensin homolog (PTEN) are variably expressed in diabetic patients, and their downregulation can delay wound healing in those with diabetic foot ulcers (Xu et al., 2020). Ma’s study used APS (50, 100, or 200 mg/kg p. o.) in streptozotocin-induced diabetic rats with skin wounds. The results demonstrated that APS reduced endothelial damage by inhibiting the release of inflammatory mediators such as ET-1, ICAM-1, IL-6, and TNF-alpha levels. Moreover, APS upregulated PTEN and suppressed the mTOR pathway activation, facilitating wound healing in diabetic rats (Ma, 2022). Additionally, the combination of APS with different drug carriers for novel wound management materials is a promising avenue of research. In a diabetic rat model, APS-loaded tissue-engineered scaffolds enhanced periwound cutaneous blood flow, increased endocrine expression, and boosted microvessel density in regenerating skin tissues, leading to improved wound healing in a dose-dependent manner (Yang et al., 2015; Ma, 2022). Another in vivo animal experiment reported that nanofibrous membranes loaded with APS and astragaloside IV curbed wound inflammation, promoted collagen fiber deposition, regenerative epithelial repair, and significantly accelerated wound healing in diabetic rats (Yue et al., 2022). These studies underscore the potential of APS as a therapeutic agent in promoting diabetic wound healing. However, clinical trials investigating the efficacy of APS in treating diabetic foot ulcers are currently lacking.
5 Conclusion and directions
DM and its complications are major chronic non-communicable diseases that significantly impact quality of life. While current clinical treatments can effectively control symptoms and slow disease progression, they often fail to prevent multi-organ damage and functional failure. Therefore, developing new effective treatments for diabetes is crucial for improving patients’ quality of life, leading to a focus on novel molecular drugs targeting diabetic complications.
APS, a natural plant extract, shows promise in hypoglycemia and treating diabetic complications. In vitro studies and animal models have demonstrated APS’s effectiveness in treating DM and its complications, including DR, DN, DCM, diabetic cognitive dysfunction, and diabetic wound healing. However, these studies often focus on initial assessments of pharmacodynamic effects, necessitating further investigation into dose-effect relationships and toxic side effects. While studies indicate that polysaccharides from Chinese herbal medicine, like APS, have significant hypoglycemic activity without toxic side effects or adverse reactions, there is a lack of research specifically on APS’s toxic side effects and adverse reactions. Therefore, studies on its safety and toxicity are necessary before considering it for human studies. The complex chemical structure of polysaccharides, including APS, limits our understanding of their exact composition and structure, hindering clinical studies and resulting in a lack of high-quality clinical trials. Thus, future research should focus on conducting more clinical studies based on thorough structural characterization, along with pharmacokinetic and pharmacotoxicity studies. Furthermore, exploring the exact mechanism, potential molecular targets, pharmacokinetics, pharmacodynamics, and side effects of APS on diabetes mellitus and its complications through clinical trials is crucial.
Author contributions
SL: Writing–original draft, Writing–review and editing. LW: Writing–review and editing, Writing–original draft. ZZ: Supervision, Writing–review and editing. YL: Supervision, Writing–review and editing. YY: Supervision, Writing–review and editing. XF: Writing–review and editing. HX: Writing–review and editing. HG: Writing–review and editing. CX: Conceptualization, Supervision, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The research was supported by innovation team and talents cultivation program of National Administration of Traditional Chinese Medicine (ZYYCXTD-C-202209), Study on the effect of Shenqi Compound series on cardiovascular benefit in diabetes mellitus based on macrovascular protective effect, Sichuan Provincial Department of Science and Technology (2022ZDZX0022), and and the Talent Support Project of the National Administration of Traditional Chinese Medicine: Qihuang Scholar (National Chinese Medicine Letter 2022 No. 6).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1339406/full#supplementary-material
References
Abel, E. D., Peroni, O., Kim, J. K., Kim, Y. B., Boss, O., Hadro, E., et al. (2001). Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 409, 729–733. doi:10.1038/35055575
Acar, E., Ural, D., Bildirici, U., Sahin, T., and Yılmaz, I. (2011). Diabetic cardiomyopathy. Anadolu Kardiyol. Derg. 11, 732–737. doi:10.5152/akd.2011.196
Ai, X., Yu, P., Hou, Y., Song, X., Luo, J., Li, N., et al. (2020). A review of traditional Chinese medicine on treatment of diabetic retinopathy and involved mechanisms. Biomed. Pharmacother. 132, 110852. doi:10.1016/j.biopha.2020.110852
Barutta, F., Bellini, S., and Gruden, G. (2022). Mechanisms of podocyte injury and implications for diabetic nephropathy. Clin. Sci. (Lond) 136, 493–520. doi:10.1042/CS20210625
Biessels, G. J., Nobili, F., Teunissen, C. E., Simó, R., and Scheltens, P. (2020). Understanding multifactorial brain changes in type 2 diabetes: a biomarker perspective. Lancet Neurol. 19, 699–710. doi:10.1016/S1474-4422(20)30139-3
Cai, L., Wang, Y., Zhou, G., Chen, T., Song, Y., Li, X., et al. (2006). Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J. Am. Coll. Cardiol. 48, 1688–1697. doi:10.1016/j.jacc.2006.07.022
Cao, W., and Zhang, Y. (2007). Clinical observation on the treatment of diabetic nephropathy with astragalus polysaccharide punch. Hubei J. Traditional Chin. Med., 12–13.
Chang, M., and Nguyen, T. T. (2021). Strategy for treatment of infected diabetic foot ulcers. Acc. Chem. Res. 54, 1080–1093. doi:10.1021/acs.accounts.0c00864
Chen, W., Li, Y.-M., and Yu, M.-H. (2008). Astragalus polysaccharides: an effective treatment for diabetes prevention in NOD mice. Exp. Clin. Endocrinol. Diabetes 116, 468–474. doi:10.1055/s-2008-1058081
Chen, W., Yu, M., and Li, Yi. (2007). Effects of astragalus polysaccharides on ultrastructure and oxidation/apoptosis related cytokines′gene expression of non-obese diabetic mice′s islets. Fudan Univ. J. Med. Sci. 34, 269–272.
Chen, X., Chen, C., and Fu, X. (2022). Hypoglycemic effect of the polysaccharides from Astragalus membranaceus on type 2 diabetic mice based on the “gut microbiota–mucosal barrier”. Food Funct. 13, 10121–10133. doi:10.1039/D2FO02300H
Chinese Pharmacopoeia Commission (2020). “Pharmacopoeia of the people’s Republic of China,” in Pharmacopoeia of the people’s Republic of China (Beijing: China Medical Science Press), 315.
Cui, K., Zhang, S., Jiang, X., and Xie, W. (2016). Novel synergic antidiabetic effects of Astragalus polysaccharides combined with Crataegus flavonoids via improvement of islet function and liver metabolism. Mol. Med. Rep. 13, 4737–4744. doi:10.3892/mmr.2016.5140
de la Monte, S. M. (2014). Type 3 Diabetes is Sporadic Alzheimer’s disease: Mini-Review. Eur Neuropsychopharmacol 24, 1954–1960. doi:10.1016/j.euroneuro.2014.06.008
DeFronzo, R. A., Ferrannini, E., Groop, L., Henry, R. R., Herman, W. H., Holst, J. J., et al. (2015). Type 2 diabetes mellitus. Nat. Rev. Dis. Prim. 1, 15019. doi:10.1038/nrdp.2015.19
Deng, H., Lin, K., Li, Y., Zhi, X., and Wu, W. (2014). Effects of astragalus polysaccharide on TNF-α, IL-6 and immune function in elderly patients with early diabetic nephropathy. J. Chin. Med. Mater. 37, 713–716. doi:10.13863/j.issn1001-4454.2014.04.048
Deng, S., Yang, L., Ma, K., and Bian, W. (2021). Astragalus polysaccharide improve the proliferation and insulin secretion of mouse pancreatic β cells induced by high glucose and palmitic acid partially through promoting miR-136-5p and miR-149-5p expression. Bioengineered 12, 9872–9884. doi:10.1080/21655979.2021.1996314
De Nicola, L., Gabbai, F. B., Liberti, M. E., Sagliocca, A., Conte, G., and Minutolo, R. (2014). Sodium/glucose cotransporter 2 inhibitors and prevention of diabetic nephropathy: targeting the renal tubule in diabetes. Am. J. Kidney Dis. 64, 16–24. doi:10.1053/j.ajkd.2014.02.010
Dillmann, W. H. (2019). Diabetic Cardiomyopathy: what is it and can it be fixed? Circ. Res. 124, 1160–1162. doi:10.1161/CIRCRESAHA.118.314665
Dun, C., Liu, J., Qiu, F., Wu, X., Wang, Y., Zhao, Y., et al. (2016). Effects of Astragalus polysaccharides on memory impairment in a diabetic rat model. Neuropsychiatr. Dis. Treat. 12, 1617–1621. doi:10.2147/NDT.S106123
Ehtewish, H., Arredouani, A., and El-Agnaf, O. (2022). Diagnostic, prognostic, and mechanistic biomarkers of diabetes mellitus-associated cognitive decline. Int. J. Mol. Sci. 23, 6144. doi:10.3390/ijms23116144
GBD 2021 Diabetes Collaborators (2023). Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 402, 203–234. doi:10.1016/S0140-6736(23)01301-6
Greenfield, J. R., and Campbell, L. V. (2006). Relationship between inflammation, insulin resistance and type 2 diabetes: “cause or effect”. Curr. Diabetes Rev. 2, 195–211. doi:10.2174/157339906776818532
Gu, C., Zeng, Y., Tang, Z., Wang, C., He, Y., Feng, X., et al. (2015). Astragalus polysaccharides affect insulin resistance by regulating the hepatic SIRT1-PGC-1α/PPARα-FGF21 signaling pathway in male Sprague Dawley rats undergoing catch-up growth. Mol. Med. Rep. 12, 6451–6460. doi:10.3892/mmr.2015.4245
Guo, T., Jou, W., Chanturiya, T., Portas, J., Gavrilova, O., and McPherron, A. C. (2009). Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS One 4, e4937. doi:10.1371/journal.pone.0004937
Guo, X., Kang, L., Ren, M., and Tan, J. (2018). Effect of astragalus polysaccharides on apoptosis,transdifferentiation and ROS content in renal tubular epithelial cells of diabetic nephropathy. Chin. J. Immunol. 34, 388–392. doi:10.3969/j.issn.1000-484X.2018.03.014
Hamzé, R., Delangre, E., Tolu, S., Moreau, M., Janel, N., Bailbé, D., et al. (2022). Type 2 Diabetes Mellitus and Alzheimer’s Disease: Shared Molecular Mechanisms and Potential Common Therapeutic Targets. Int J Mol Sci. 23, 15287. doi:10.3390/ijms232315287
Huang, Y.-C., Tsay, H.-J., Lu, M.-K., Lin, C.-H., Yeh, C.-W., Liu, H.-K., et al. (2017). Astragalus membranaceus-polysaccharides ameliorates obesity, hepatic steatosis, neuroinflammation and cognition impairment without affecting amyloid deposition in metabolically stressed APPswe/PS1dE9 mice. Int. J. Mol. Sci. 18, 2746. doi:10.3390/ijms18122746
James, D. E., Stöckli, J., and Birnbaum, M. J. (2021). The aetiology and molecular landscape of insulin resistance. Nat. Rev. Mol. Cell Biol. 22, 751–771. doi:10.1038/s41580-021-00390-6
Jia, G., Hill, M. A., and Sowers, J. R. (2018). Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ. Res. 122, 624–638. doi:10.1161/CIRCRESAHA.117.311586
Ke, B., Ke, X., Wan, X., Yang, Y., Huang, Y., Qin, J., et al. (2017). Astragalus polysaccharides attenuates TNF-α-induced insulin resistance via suppression of miR-721 and activation of PPAR-γ and PI3K/AKT in 3T3-L1 adipocytes. Am. J. Transl. Res. 9, 2195–2206.
Ke, M., Dai, B., and Wang, L. (2010). The effect of astragalus polysaccharide on the expression of VEGF in Müller cell cultured in high level of glucose. FASEB J. 24, 641.1. doi:10.1096/fasebj.24.1_supplement.641.1
Kim, C., Ha, H., Kim, J. S., Kim, Y. T., Kwon, S.-C., and Park, S. W. (2003). Induction of growth hormone by the roots of Astragalus membranaceus in pituitary cell culture. Arch. Pharm. Res. 26, 34–39. doi:10.1007/BF03179928
Lai, Y., Yu, M., Zhu, Q., Yang, H., Zhang, Z., and Yang, X. (2002). Effect of Astragalus polysoccharidde on TGF-β1 in renal tissue of diabetic rats. Fudan Univ. J. Med. Sci., 255–258.
Li, C., Liu, Y., Zhang, Y., Li, J., and Lai, J. (2022). Astragalus polysaccharide: a review of its immunomodulatory effect. Arch. Pharm. Res. 45, 367–389. doi:10.1007/s12272-022-01393-3
Li, C., Wang, Y., Qu, J., Zhang, X., Mao, S., and Nie, K. (2018). Effects of Astragalus polysaccharide on renal TGF-β1/Smads signalling pathway in diabetic rats. Chin. Pharmacol. Bull. 34, 512–516. doi:10.3969/j.issn.1001-1978.2018.04.015
Li, N., Li, H., and Liu, D. (2017). Effect of Astragalus Polysacharin on the cognitive function of diabetic rats. Chin. J. Gerontology 37, 2098–2100. doi:10.3969/j.issn.1005-9202.2017.09.007
Li, R.-J., Qiu, S.-D., Chen, H.-X., Tian, H., and Wang, H.-X. (2007). The immunotherapeutic effects of Astragalus polysaccharide in type 1 diabetic mice. Biol. Pharm. Bull. 30, 470–476. doi:10.1248/bpb.30.470
Li, Y., Ke, M., and Zhang, F. (2008). Effects of Astragalus polysaccharide on the ExpressionofKir2. 1 of muller cells of retinain STZ-induced diabetes mellitus rats. Wuhan, Hubei, China: Medical Journal of Wuhan University, 177–180.
Li, Z., Zhang, Y., Liu, Y., Lu, H., Li, Y., and Zhang, Y. (2011). Effect of astragalus polysaccharin on expression of nephrin and podocin in podocytes of early diabetic nephropathy rats. Chin. J. Pathophysiol. 27, 1772–1776. doi:10.3969/j.issn.1000-4718.2011.09.021
Liu, J., Zhang, J., Lu, J., Zhang, D., Li, K., Su, K., et al. (2013a). Astragalus polysaccharide stimulates glucose uptake in L6 myotubes through AMPK activation and AS160/TBC1D4 phosphorylation. Acta Pharmacol. Sin. 34, 137–145. doi:10.1038/aps.2012.133
Liu, M., Qin, J., Hao, Y., Liu, M., Luo, J., Luo, T., et al. (2013b). Astragalus polysaccharide suppresses skeletal muscle myostatin expression in diabetes: involvement of ROS-ERK and NF-κB pathways. Oxid. Med. Cell Longev. 2013, 782497. doi:10.1155/2013/782497
Liu, M., Wu, K., Mao, X., Wu, Y., and Ouyang, J. (2010). Astragalus polysaccharide improves insulin sensitivity in KKAy mice: regulation of PKB/GLUT4 signaling in skeletal muscle. J. Ethnopharmacol. 127, 32–37. doi:10.1016/j.jep.2009.09.055
Liu, P., Peng, Q.-H., Tong, P., and Li, W.-J. (2019). Astragalus polysaccharides suppresses high glucose-induced metabolic memory in retinal pigment epithelial cells through inhibiting mitochondrial dysfunction-induced apoptosis by regulating miR-195. Mol. Med. 25, 21. doi:10.1186/s10020-019-0088-z
Louis, P., Young, P., Holtrop, G., and Flint, H. J. (2010). Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ. Microbiol. 12, 304–314. doi:10.1111/j.1462-2920.2009.02066.x
Lu, J., Chen, X., Zhang, Y., Xu, J., Zhang, L., Li, Z., et al. (2013). Astragalus polysaccharide induces anti-inflammatory effects dependent on AMPK activity in palmitate-treated RAW264.7 cells. Int. J. Mol. Med. 31, 1463–1470. doi:10.3892/ijmm.2013.1335
Ma, L. (2022). Astragalus polysaccharides promote wound healing in diabetic rats by upregulating PETN and inhibiting the mTOR pathway. Comput. Math. Methods Med. 2022, 3459102. doi:10.1155/2022/3459102
Mao, X., Wu, Y., Wu, K., Liu, M., Zhang, J., Zou, F., et al. (2007). Astragalus polysaccharide reduces hepatic endoplasmic reticulum stress and restores glucose homeostasis in a diabetic KKAy mouse model. Acta Pharmacol. Sin. 28, 1947–1956. doi:10.1111/j.1745-7254.2007.00674.x
Mao, X., Yu, F., Wang, N., Wu, Y., Zou, F., Wu, K., et al. (2009). Hypoglycemic effect of polysaccharide enriched extract of Astragalus membranaceus in diet induced insulin resistant C57BL/6J mice and its potential mechanism. Phytomedicine 16, 416–425. doi:10.1016/j.phymed.2008.12.011
Marfil-Garza, B. A., Hefler, J., Bermudez De Leon, M., Pawlick, R., Dadheech, N., and Shapiro, A. M. J. (2021). Progress in translational regulatory T cell therapies for type 1 diabetes and islet transplantation. Endocr. Rev. 42, 198–218. doi:10.1210/endrev/bnaa028
Mathis, D., Vence, L., and Benoist, C. (2001). beta-Cell death during progression to diabetes. Nature 414, 792–798. doi:10.1038/414792a
McDermott, K., Fang, M., Boulton, A. J. M., Selvin, E., and Hicks, C. W. (2023). Etiology, epidemiology, and disparities in the burden of diabetic foot ulcers. Diabetes Care 46, 209–221. doi:10.2337/dci22-0043
Meng, X., Wei, M., Wang, D., Qu, X., Zhang, K., Zhang, N., et al. (2020). Astragalus polysaccharides protect renal function and affect the TGF-β/Smad signaling pathway in streptozotocin-induced diabetic rats. J. Int. Med. Res. 48, 0300060520903612. doi:10.1177/0300060520903612
Nauck, M. A., Wefers, J., and Meier, J. J. (2021). Treatment of type 2 diabetes: challenges, hopes, and anticipated successes. Lancet Diabetes Endocrinol. 9, 525–544. doi:10.1016/S2213-8587(21)00113-3
Norris, J. M., Johnson, R. K., and Stene, L. C. (2020). Type 1 diabetes—early life origins and changing epidemiology. Lancet Diabetes Endocrinol. 8, 226–238. doi:10.1016/S2213-8587(19)30412-7
Okonkwo, U. A., and DiPietro, L. A. (2017). Diabetes and wound angiogenesis. Int. J. Mol. Sci. 18, 1419. doi:10.3390/ijms18071419
Peng, Q.-H., Tong, P., Gu, L.-M., and Li, W.-J. (2020). Astragalus polysaccharide attenuates metabolic memory-triggered ER stress and apoptosis via regulation of miR-204/SIRT1 axis in retinal pigment epithelial cells. Biosci. Rep. 40, BSR20192121. doi:10.1042/BSR20192121
Roden, M., and Shulman, G. I. (2019). The integrative biology of type 2 diabetes. Nature 576, 51–60. doi:10.1038/s41586-019-1797-8
Rodríguez-Gutiérrez, R., Salcido-Montenegro, A., and González-González, J. G. (2018). Early clinical expressions of insulin resistance: the real enemy to look for. Diabetes Ther. 9, 435–438. doi:10.1007/s13300-017-0348-2
Roep, B. O. (2003). The role of T-cells in the pathogenesis of Type 1 diabetes: from cause to cure. Diabetologia 46, 305–321. doi:10.1007/s00125-003-1089-5
Selby, N. M., and Taal, M. W. (2020). An updated overview of diabetic nephropathy: diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes. Metab. 22 (1), 3–15. doi:10.1111/dom.14007
Sheik, A., Kim, K., Varaprasad, G. L., Lee, H., Kim, S., Kim, E., et al. (2021). The anti-cancerous activity of adaptogenic herb Astragalus membranaceus. Phytomedicine 91, 153698. doi:10.1016/j.phymed.2021.153698
Shukla, G. C., Singh, J., and Barik, S. (2011). MicroRNAs: processing, maturation, target recognition and regulatory functions. Mol. Cell Pharmacol. 3, 83–92.
Sinacore, D. R., and Gulve, E. A. (1993). The role of skeletal muscle in glucose transport, glucose homeostasis, and insulin resistance: implications for physical therapy. Phys. Ther. 73, 878–891. doi:10.1093/ptj/73.12.878
Song, Q., Cheng, S. W., Li, D., Cheng, H., Lai, Y. S., Han, Q., et al. (2022). Gut microbiota mediated hypoglycemic effect of Astragalus membranaceus polysaccharides in db/db mice. Front. Pharmacol. 13, 1043527. doi:10.3389/fphar.2022.1043527
Su, H.-F., Shaker, S., Kuang, Y., Zhang, M., Ye, M., and Qiao, X. (2021). Phytochemistry and cardiovascular protective effects of huang-qi (astragali radix). Med. Res. Rev. 41, 1999–2038. doi:10.1002/med.21785
Sun, J., Liu, Y., Yu, J., Wu, J., Gao, W., Ran, L., et al. (2019a). APS could potentially activate hepatic insulin signaling in HFD-induced IR mice. J. Mol. Endocrinol. 63, 77–91. doi:10.1530/JME-19-0035
Sun, J., Ren, J., Hu, X., Hou, Y., and Yang, Y. (2021). Therapeutic effects of Chinese herbal medicines and their extracts on diabetes. Biomed. Pharmacother. 142, 111977. doi:10.1016/j.biopha.2021.111977
Sun, S., Yang, S., An, N., Wang, G., Xu, Q., Liu, J., et al. (2019b). Astragalus polysaccharides inhibits cardiomyocyte apoptosis during diabetic cardiomyopathy via the endoplasmic reticulum stress pathway. J. Ethnopharmacol. 238, 111857. doi:10.1016/j.jep.2019.111857
Sun, S., Yang, S., Dai, M., Jia, X., Wang, Q., Zhang, Z., et al. (2017). The effect of Astragalus polysaccharides on attenuation of diabetic cardiomyopathy through inhibiting the extrinsic and intrinsic apoptotic pathways in high glucose -stimulated H9C2 cells. BMC Complement. Altern. Med. 17, 310. doi:10.1186/s12906-017-1828-7
Sun, S., Yang, S., Zhang, N., Yu, C., Liu, J., Feng, W., et al. (2023). Astragalus polysaccharides alleviates cardiac hypertrophy in diabetic cardiomyopathy via inhibiting the BMP10-mediated signaling pathway. Phytomedicine 109, 154543. doi:10.1016/j.phymed.2022.154543
Tan, T.-E., and Wong, T. Y. (2023). Diabetic retinopathy: looking forward to 2030. Front. Endocrinol. (Lausanne) 13, 1077669. doi:10.3389/fendo.2022.1077669
Tang, S., Yang, Z., Chen, W., Yuan, Q., Chen, S., and Li, H. (2017). Astragalus polysaccharide protects pancreatic β-cells to improve type 2 diabetes mellitus in rats. Acad. J. Nav. Med. Univ. 38, 482–487. doi:10.16781/j.0258-879x.2017.04.0482
Tang, Z., and Huang, G. (2022). Extraction, structure, and activity of polysaccharide from Radix astragali. Biomed Pharmacother 150, 113015. doi:10.1016/j.biopha.2022.113015
Teo, Z. L., Tham, Y.-C., Yu, M., Chee, M. L., Rim, T. H., Cheung, N., et al. (2021). Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology 128, 1580–1591. doi:10.1016/j.ophtha.2021.04.027
Tian, H., Lu, J., He, H., Zhang, L., Dong, Y., Yao, H., et al. (2016). The effect of Astragalus as an adjuvant treatment in type 2 diabetes mellitus: a (preliminary) meta-analysis. J. Ethnopharmacol. 191, 206–215. doi:10.1016/j.jep.2016.05.062
Tian, J., Jin, D., Bao, Q., Ding, Q., Zhang, H., Gao, Z., et al. (2019). Evidence and potential mechanisms of traditional Chinese medicine for the treatment of type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes. Metab. 21, 1801–1816. doi:10.1111/dom.13760
Voelker, J., Berg, P. H., Sheetz, M., Duffin, K., Shen, T., Moser, B., et al. (2017). Anti-TGF-β1 antibody therapy in patients with diabetic nephropathy. J. Am. Soc. Nephrol. 28, 953–962. doi:10.1681/ASN.2015111230
Wang, Y., Wang, Z., An, J., Wan, Y., Jin, H., Ma, S., et al. (2017). Study on standardization method of Astragalus polysaccharide reference substance. Chinese Traditional and Herbal Drugs 48, 4897–4903.
Wei, Z., Weng, S., Wang, L., and Mao, Z. (2018). Mechanism of Astragalus polysaccharides in attenuating insulin resistance in Rats with type 2 diabetes mellitus via the regulation of liver microRNA-203a-3p. Mol. Med. Rep. 17, 1617–1624. doi:10.3892/mmr.2017.8084
Weyer, C., Bogardus, C., Mott, D. M., and Pratley, R. E. (1999). The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J. Clin. Invest. 104, 787–794. doi:10.1172/JCI7231
Wu, B., Wang, B., and Ouyang, J. (2007). Effect of astragalus polysaccharide on TNF-α expression in the retina of genetically diabetic kkay mice. Lishizhen Med. Materia Medica Res. 18, 1031–1032.
Wu, Y., Ou-Yang, J., Wu, K., Wang, Y., Zhou, Y., and Wen, C. (2005). Hypoglycemic effect of Astragalus polysaccharide and its effect on PTP1B. Acta Pharmacol. Sin. 26, 345–352. doi:10.1111/j.1745-7254.2005.00062.x
Xia, M.-L., Xie, X.-H., Ding, J.-H., Du, R.-H., and Hu, G. (2020). Astragaloside IV inhibits astrocyte senescence: implication in Parkinson’s disease. J. Neuroinflammation 17, 105. doi:10.1186/s12974-020-01791-8
Xu, J., Wang, R., Liu, W., Yin, Z., Wu, J., Yu, X., et al. (2023). The specificity of ten non-digestible carbohydrates to enhance butyrate-producing bacteria and butyrate production in vitro fermentation. Food Sci. Hum. Wellness 12, 2344–2354. doi:10.1016/j.fshw.2023.03.038
Xu, Y., Yu, T., He, L., Ouyang, L., Qu, Y., Zhou, J., et al. (2020). Inhibition of miRNA-152-3p enhances diabetic wound repair via upregulation of PTEN. Aging (Albany NY) 12, 14978–14989. doi:10.18632/aging.103557
Yan, J., Yi, Y., Wu, X., He, G., Liang, J., Liu, X., et al. (2012). The isolation, molecular size and monosaccharides compositions of polysaccharides from Astragalus. Food Science and Technology 37, 278–283. doi:10.13684/j.cnki.spkj.2012.12.069
Yang, B., Xiong, Z., Lin, M., Yang, Y., Chen, Y., Zeng, J., et al. (2023a). Astragalus polysaccharides alleviate type 1 diabetes via modulating gut microbiota in mice. Int. J. Biol. Macromol. 234, 123767. doi:10.1016/j.ijbiomac.2023.123767
Yang, C.-G., Mao, X.-L., Wu, J.-F., An, X., Cao, J.-J., Zhang, X.-Y., et al. (2023b). Amelioration of lung fibrosis by total flavonoids of Astragalus via inflammatory modulation and epithelium regeneration. Am. J. Chin. Med. 51, 373–389. doi:10.1142/S0192415X23500192
Yang, G., Wei, J., Liu, P., Zhang, Q., Tian, Y., Hou, G., et al. (2021a). Role of the gut microbiota in type 2 diabetes and related diseases. Metabolism 117, 154712. doi:10.1016/j.metabol.2021.154712
Yang, T. (2017). Improving learning and memory of the high fat-high sugar diet rats with astragalus polysaccharides. Clin. J. Chin. Med. 9, 4–7.
Yang, Y., Wang, F., Yin, D., Fang, Z., and Huang, L. (2015). Astragulus polysaccharide-loaded fibrous mats promote the restoration of microcirculation in/around skin wounds to accelerate wound healing in a diabetic rat model. Colloids Surfaces B Biointerfaces 136, 111–118. doi:10.1016/j.colsurfb.2015.09.006
Yang, Z.-M., Wang, Y., and Chen, S.-Y. (2021b). Astragalus polysaccharide alleviates type 2 diabetic rats by reversing the glucose transporters and sweet taste receptors/GLP-1/GLP-1 receptor signaling pathways in the intestine-pancreatic axis. J. Funct. Foods 76, 104310. doi:10.1016/j.jff.2020.104310
Yue, Y., Liu, X., Pang, L., Liu, Y., Lin, Y., Xiang, T., et al. (2022). Astragalus polysaccharides/PVA nanofiber membranes containing astragaloside IV-loaded liposomes and their potential use for wound healing. Evid. Based Complement. Altern. Med. 2022, 9716271. doi:10.1155/2022/9716271
Zhang, K., Pugliese, M., Pugliese, A., and Passantino, A. (2015). Biological active ingredients of traditional Chinese herb Astragalus membranaceus on treatment of diabetes: a systematic review. Mini Rev. Med. Chem. 15, 315–329. doi:10.2174/1389557515666150227113431
Zhang, L., Miao, R., Yu, T., Wei, R., Tian, F., Huang, Y., et al. (2022). Comparative effectiveness of traditional Chinese medicine and angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and sodium glucose cotransporter inhibitors in patients with diabetic kidney disease: a systematic review and network meta-analysis. Pharmacol. Res. 177, 106111. doi:10.1016/j.phrs.2022.106111
Zhang, R., Qin, X., Zhang, T., Li, Q., Zhang, J., and Zhao, J. (2018). Astragalus polysaccharide improves insulin sensitivity via AMPK activation in 3T3-L1 adipocytes. Molecules 23, 2711. doi:10.3390/molecules23102711
Zhou, X., Xu, Y., Yang, G., and Li, F. (2011). Increased galectin-1 expression in muscle of Astragalus polysaccharide-treated Type 1 diabetic mice. J. Nat. Med. 65, 500–507. doi:10.1007/s11418-011-0527-9
Keywords: astragalus polysaccharide, diabetes mellitus, insulin resistance, immunomodulation, diabetes complications
Citation: Liu S, Wang L, Zhang Z, Leng Y, Yang Y, Fu X, Xie H, Gao H and Xie C (2024) The potential of astragalus polysaccharide for treating diabetes and its action mechanism. Front. Pharmacol. 15:1339406. doi: 10.3389/fphar.2024.1339406
Received: 16 November 2023; Accepted: 26 March 2024;
Published: 10 April 2024.
Edited by:
Zhanyong Wang, Shenyang Agricultural University, ChinaReviewed by:
Adolfo Andrade-Cetto, National Autonomous University of Mexico, MexicoLei Chen, Guangdong Ocean University, China
Copyright © 2024 Liu, Wang, Zhang, Leng, Yang, Fu, Xie, Gao and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunguang Xie, eGllY2dAY2R1dGNtLmVkdS5jbg==
 Shiyu Liu
Shiyu Liu Luyao Wang
Luyao Wang Zehua Zhang
Zehua Zhang YuLin Leng1
YuLin Leng1 Yan Yang
Yan Yang Xiaoxu Fu
Xiaoxu Fu Hongyan Xie
Hongyan Xie Chunguang Xie
Chunguang Xie