- 1College of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2State Key Laboratory of Southwestern Chinese Medicine Resources, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3College of Ethnomedicine, Chengdu University of Traditional Chinese Medicine, Chengdu, China
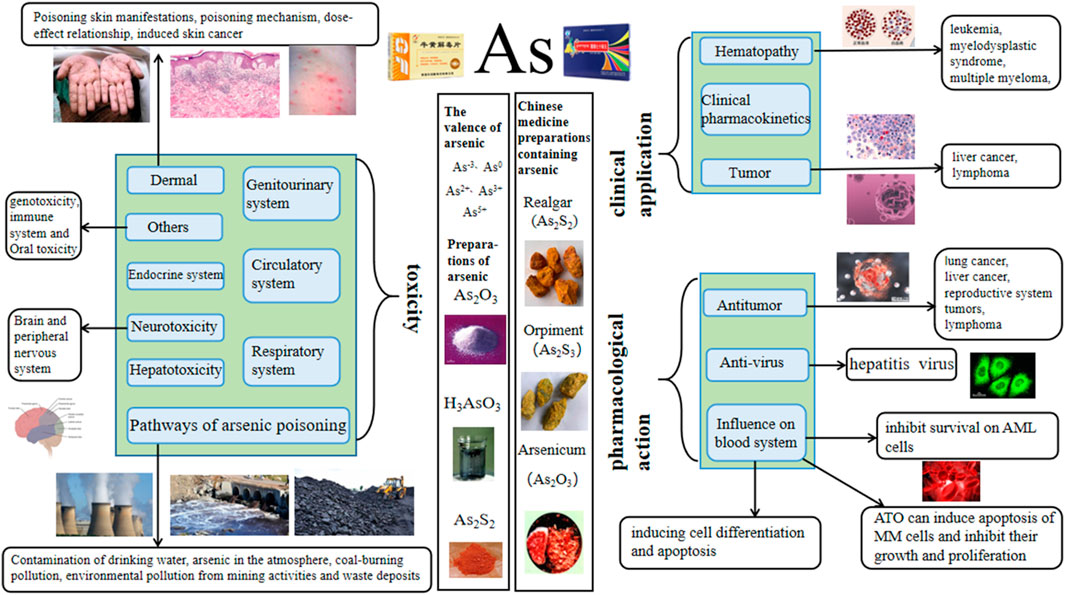
Introduction: The toxicity of arsenic is widely recognized globally, mainly harming human health by polluting water, soil, and food. However, its formulations can also be used for the clinical treatment of diseases such as leukemia and tumors. Arsenic has been used as a drug in China for over 2,400 years, with examples such as the arsenic-containing drug realgar mentioned in Shennong’s Herbal Classic. We have reviewed references on arsenic over the past thirty years and found that research has mainly focused on clinical, pharmacological, and toxicological aspects.
Results and Discussion: The finding showed that in clinical practice, arsenic trioxide is mainly used in combination with all-trans retinoic acid (ATRA) at a dose of 10 mg/d for the treatment of acute promyelocytic leukemia (APL); realgar can be used to treat acute promyelocytic leukemia, myelodysplastic syndrome, and lymphoma. In terms of pharmacology, arsenic mainly exerts anti-tumor effects. The dosage range of the action is 0.01–80 μmol/L, and the concentration of arsenic in most studies does not exceed 20 μmol/L. The pharmacological effects of realgar include antiviral activity, inhibition of overactivated lactate dehydrogenase, and resistance to malaria parasites. In terms of toxicity, arsenic is toxic to multiple systems in a dose-dependent manner. For example, 5 μmol/L sodium arsenite can induce liver oxidative damage and promote the expression of pro-inflammatory factors, and 15 μmol/L sodium arsenite induces myocardial injury; when the concentration is higher, it is more likely to cause toxic damage.
1 Introduction
Arsenic, commonly known as pi, is located in the fourth cycle and the VA group in the periodic table of elements. Its atomic number is 33, and the element symbol is As. It is a toxic metalloid element. It has an atomic weight of 74.92159. It is located between phosphorus and antimony, and its physical and chemical properties are similar to those of phosphorus. It can form alloys and is easy to covalently combine with carbon, hydrogen, and oxygen. Arsenic exists in inorganic form, organic form, and different oxidation states. It is widely distributed in nature. It not only exists in the crust, groundwater, soil, seawater, river water, atmosphere, rock strata, and coal seams (Wu, 2002; Jessica, 2020) but also in air and food at low levels. Arsenic mainly exists in the form of sulfide in nature, accompanied by a small amount of natural arsenic and metal arsenide (Pan et al., 2013). Arsenic has many different valence states. The arsenic element present in nature mainly has five valence states: As3−, As0, As2+, As3+, and As5+. Arsenic in different valence states has varying levels of toxicity. In general, inorganic arsenic is more toxic than organic arsenic, and trivalent arsenic is more toxic than pentavalent arsenic (Zhang et al., 2021a). The toxicity mechanisms of inorganic arsenic include inducing oxidative damage (Kitchin et al., 2010), activating mitosis and inducing genetic toxicity damage, interfering with methyl transfer to DNA (Kundu et al., 2011), and perturbing histones (Chervona et al., 2012).
The toxicity of arsenic has always been a concern, and it is considered a first-class carcinogen in humans. In recent years, a large number of epidemiological and toxicological studies have proven that it is directly related to the incidence of skin cancer, bladder cancer, kidney cancer, lung cancer, and gastric cancer (Tchounwou et al., 2004; Palma-Lara et al., 2020). Individuals highly exposed to arsenic may experience acute, subacute, or chronic poisoning symptoms, which are characterized by skin damage, cardiovascular symptoms, and multiple organ failure (Nurchi et al., 2020). In addition, it can cause reproductive and developmental toxicity, neurotoxicity, and immunotoxicity and induce various cancers in humans. Inorganic arsenic compounds are also carcinogenic to experimental animals. Arsenic exposure is positively correlated with the incidence rate of human kidney cancer, liver cancer, and prostate cancer (Xu et al., 2014). However, the clear mechanism of arsenic carcinogenesis has not yet been confirmed. Possible mechanisms include changes in DNA repair, DNA methylation oxidative stress, and genotoxicity, (Kuivenhoven et al., 2023).
Among the various forms of arsenic, ATO (As2O3) is the most widely used. As2O3 is a kind of slightly acidic amphoteric oxide, which is a white frost-like powder or crystal that is slightly soluble in water to generate arsenous acid. It is soluble in acid and alkali, and its amorphous solid is insoluble in ethanol. As2O3 has a relative molecular weight of 197.84, a melting point of 315°C, a boiling point of 457.2°C, and a saturated vapor pressure of 13.33 kPa (Hao et al., 2020). It is easy to sublimate when heated. The severe toxicity and narrow treatment window of As2O3 limit its frequency and scope of clinical use. However, when made into appropriate formulations and administered correctly, it has unique effects on various diseases such as asthma, syphilis, and ulcers, as well as difficult conditions such as leukemia and malignant tumors. As2O3 has been used as a drug for more than 2000 years (Rao et al., 2013). As early as the 1930s, Western scholars applied it to treat leukemia, but the results were unsatisfactory. Until the 1970s, Chinese scholars developed ‘Ailing Yihao’ with arsenic as the main component, which was applied to a variety of blood diseases. It exerts remarkable effects on acute promyelocytic leukemia (APL) (Chen et al., 2011), and arsenic has dual pharmacological effects of inducing differentiation and apoptosis of leukemia cells (Zhu et al., 2012). The therapeutic effect of As2O3 is time-dependent and dose-dependent. A small amount of As2O3 does not work for a short time, but a large amount of As2O3 can easily cause toxic side effects. Therefore, maintaining a stable and effective blood arsenic concentration for a long time and increasing and reducing toxicity on this basis (Sönksen et al., 2022) are urgent challenges that need to be addressed. Additionally, attention should be paid to gastrointestinal reactions, cardiac toxicity, kidney toxicity, and other adverse reactions during treatment. Arsenic is also often used to treat APL. Many studies have shown that all-trans retinoic acid (ATRA) combined with arsenic can achieve good results. In clinical practice, the combination of ATRA and arsenic can improve the overall effective rate of the treatment and reduce the recurrence rate.
In traditional Chinese medicine, arsenic-containing products include realgar (As2S2), orpiment (As2S3) and Pi-Shi (As2O3). Among them, the polypill of traditional Chinese medicine containing realgar is widely used. According to the 2020 edition of the Chinese Pharmacopoeia, 37 kinds of traditional Chinese patent medicines and simple preparations contain realgar. In Shennong’s Herbal Classic of Materia Medica, realgar is recorded as follows: ‘golden stone, pungent, and warm, can control cold and heat, rat fistula, sore, gangrene, and dead muscle, and kill white insect poison.’ Therefore, realgar has a history of over a thousand years as a medicinal herb in China. In the Pharmacopoeia of the People’s Republic of China 2020 (Commission, 2020), it is recorded as a pungent, warm, and toxic agent that is beneficial to the liver and large intestine, and it has the effects of detoxifying and killing insects, eliminating dampness and phlegm, and treating malaria. It is mainly used for treating carbuncles, boils, snake and insect bites, abdominal pain, convulsions, and malaria. Orpiment is a monoclinic crystal system mineral mainly composed of arsenic trisulfide; it is highly toxic, mild in nature, and spicy in taste. It is beneficial to the liver and large intestine, and it has the effects of detoxification, insect disinfestation, dampness and phlegm elimination, and malaria treatment. It is mainly used to treat carbuncle and swelling, snake and insect bites, abdominal pain caused by parasites, convulsions, epilepsy, and malaria. In nature, realgar and orpiment often co-exist (Cao et al., 2011), so people call it Yuanyang Stone. Pi-Shi (derived from Arsenolite, Arsenopyrite, Realgar, or Orpiment) has a pungent taste, causes high fever, and exhibits high toxicity. It is beneficial for the lungs and liver, and it has the effects of removing carrion for external use and relieving asthma for internal use. The refined product obtained from the sublimation of Pi-Shi is arsenic trioxide. It is one of the oldest poisons, and it has been used as a drug for more than 2,400 years (Jiang et al., 2006). After entering the human body, it can destroy some cell respiratory enzymes (Yuan, 2010), making the tissue cells unable to obtain oxygen and die. It can also strongly stimulate the gastrointestinal mucosa, damage the liver, and, in serious cases, lead to death due to respiratory and circulatory failure. Chinese medicine prescriptions record that it can treat cold phlegm, asthma, malaria, dysentery, syphilis, hemorrhoids, scrofula, chancre, tinea, ulcers, and persistent carrion.
This article reviews the clinical application, pharmacological effects, and toxicity of arsenic to avoid its toxicity, enhance its therapeutic effect, and provide a theoretical basis for the clinical medication and development of arsenic agents.
2 Clinical applications of arsenic and its preparations
Arsenic is commonly used in clinical applications to treat hematological diseases, bone marrow, coronary heart disease, urogenital diseases, immune diseases, skin diseases or skin injuries, asthma, dental diseases, and tumors. Among them, the treatment of tumors and hematological diseases is its main application pathway, and the most documented one is for the treatment of APL. Among all forms of arsenic, ATO is the most commonly used.
2.1 Hematology
2.1.1 Leukemia
APL is a unique subtype of acute myeloid leukemia (AML), accounting for approximately 10%–15% of AML cases. Amadori et al. (2005) reported that good tolerability has been found in clinical studies of arsenic trioxide, and its development reveals its widespread applicability in patients with hematological malignancies. Arsenic-containing traditional Chinese medicine, such as realgar, can be applied in treating APL in clinical practice. In the study by Guan et al. (2002), after 20 patients with APL (resistant to ATRA) were treated with realgar (3.0–3.75 g/day), the total effective rate (complete response (CR) + partial response (PR)) was 90%, and the time required to reach CR was 31–60 days. ATO has therapeutic effects on APL, involving the central nervous system. After multiple ineffective treatments, a 34-year-old patient with APL was treated with ATO monotherapy for 25 days. The patient’s neurological performance improved, bone marrow molecules were completely relieved, and mother cells in cerebrospinal fluid were significantly reduced, indicating the efficacy of ATO in treating APL recurrence involving the central nervous system (Zappasodi et al., 2011).
ATO or arsenious acid monotherapy has therapeutic effects on APL. Ascorbic acid (AA) can expand the treatment range of ATO and improve the clinical results of ATO monotherapy (Yedjou et al., 2008). Mathews et al. (2010) reported 72 patients with APL who were treated with a single dose of ATO, with a CR rate of 86.1%. The estimated 5-year event-free survival rate, disease-free survival (DFS) rate, and overall survival (OS) rate were 69%, 80%, and 74.2%, respectively. Ghavamzadeh et al., 2011) reported 197 patients with APL who were treated with a single dose of 0.15 mg/kg ATO, with a CR rate of 85.8% and a 5-year DFS of 66.7%. Shen et al. (1997) reported 10 cases of patients with APL, with a dose of 10 mg/day ATO. After treatment, the CR rate was 90%, and the required duration of ATO treatment to obtain CR was 28–44 days (median of 38 days). Jiang et al. (2008) reported 153 patients with APL, with a dose of 10 mg/day ATO. The CR rate was 80.21% (77/96) in the ATRA group and 92.98% (53/57) in the arsenite group.
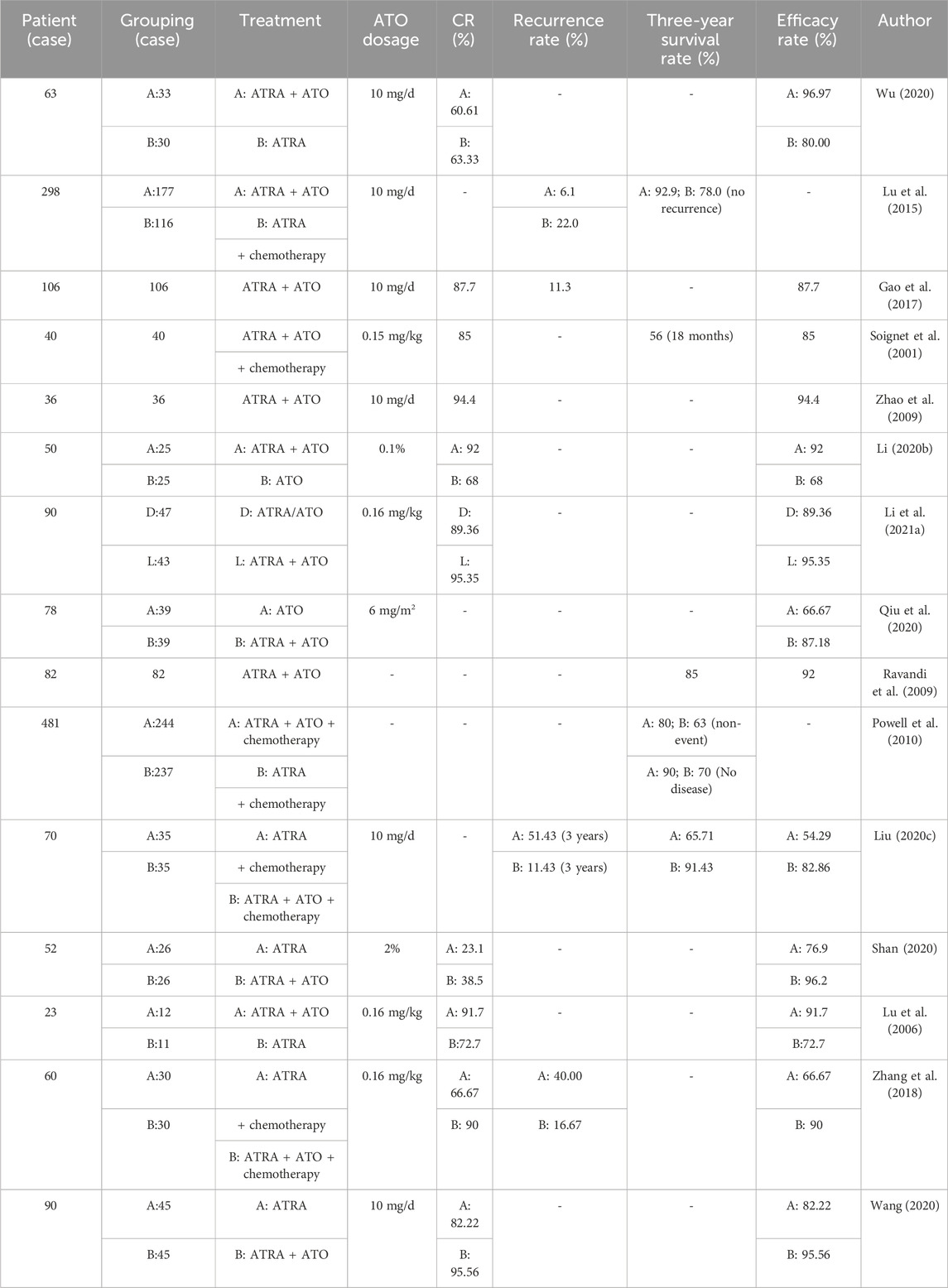
Clinical studies have shown that ATRA combined with ATO is effective in the treatment of APL, and it is superior to using ATRA or ATO alone (Table 1).
Except for retinoic acid, ATO can also be used with other drugs to treat APL, such as HHT, IDA (Chen et al., 2018), and Fu Fang Qing Dai Pian (including realgar) (Qu et al., 2018).
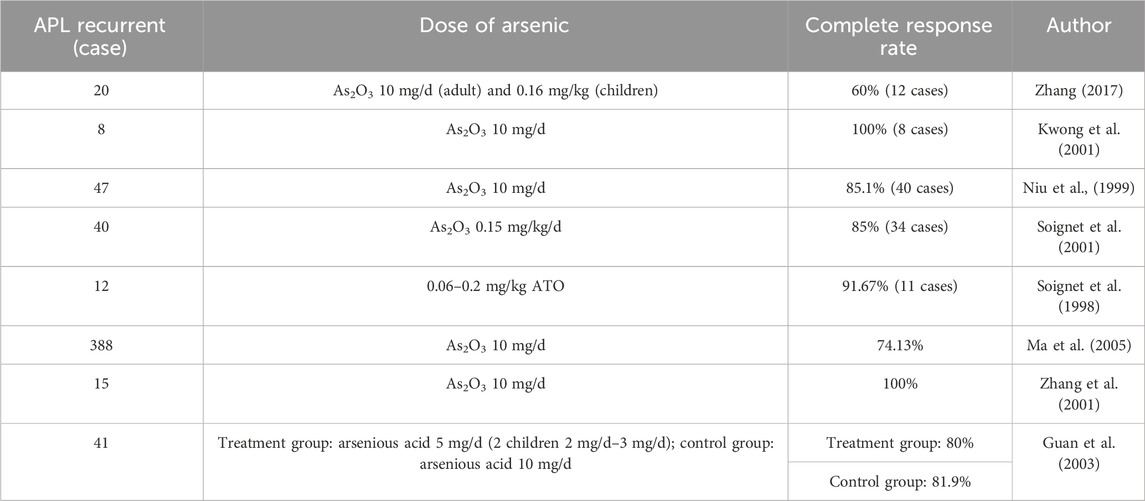
The treatment of patients with recurrent APL via arsenic is also relatively safe and effective (Table 2).
However, the high cost of ATO treatment for APL is one of the reasons that hinder its clinical application (Zhu et al., 2016). Oral arsenic not only demonstrates clinical efficacy comparable with intravenous preparations but also demonstrates better safety, improved quality of life, and reduced patient medical costs, which promote its clinical application (Zhu et al., 2019). Zhu et al. (2014) reported that 20 non-high-risk APL volunteers were treated with oral arsenic (realgar and indigo) and ATRA, and they achieved CR after a median time of 29.5 days, indicating that the use of these two oral molecularly targeted drugs without chemotherapy is also effective, convenient, and economical. Ravandi et al. (2020) observed 12 patients with advanced hematological malignancies who received oral ORH-2014 (a novel oral ATO formulation), and their results showed that 15 mg of ORH-2014 is safe and bioavailable compared with an approved dose (0.15 mg/kg) of intravenous As2O3.
2.1.2 Myelodysplastic syndrome
Myelodysplastic syndrome (MDS) is a clonal disease and a common hematological malignancy. It is a pathological or ineffective hematopoietic disease caused by the abnormal proliferation of bone marrow hematopoietic stem cells (Li et al., 2015a). ATO has a good effect on the treatment of MDS. The combination of ATO and thalidomide is a feasible treatment for MDS (Lengfelder et al., 2012), and ATO is an important drug that plays a major therapeutic role. For example, in 70 patients with MDS in the hematology department of Yangguang Ronghe Hospital (Zhu, 2020), thalidomide alone achieved a total effective rate of only 51.4%, whereas the combination of ATO and thalidomide achieved a total effective rate of 68.6%. In addition, the combination of azacitidine and ATO for the treatment of elderly MDS (Xie et al., 2022), which involves continuous treatment with azacitidine (75 mg/m2, subcutaneous injection) for 7 days and intravenous infusion of ATO (7 mg/m2, intravenous infusion), is more effective than using azacitidine alone, achieving a total effective rate of 76.92%, whereas azacitidine alone only achieves a total effective rate of 50.00%. Arsenic acid can inhibit myeloid clones, accelerate cell apoptosis (Emadi et al., 2010), and have significant clinical efficacy in treating MDS. The combination of ATO, cytarabine, aclacinomycin, and granulocyte colony-stimulating factor regimens can effectively treat medium-to-high-risk MDSs (Li, 2017), induce apoptosis of abnormal cloned cells, prolong patient survival, and reduce adverse reactions. Lu et al. (2019) used decitabine combined with ATO induction therapy to treat 39 patients with medium-to-high-risk MDS. Among them, 26 patients achieved clinical response, including 7 cases of CR.
Arsenic-containing traditional Chinese medicine is also a feasible path for treating MDS. Zhou et al. (2020) and others believed that arsenic-containing traditional Chinese medicine could treat MDS by regulating abnormal hypomethylation. The arsenic-containing compound Qinghuang powder is also a safe and effective method for treating MDS, as its daily dose can be adjusted without increasing clinical toxicity (Zhu et al., 2017).
2.1.3 Multiple myeloma
Clinical research on arsenic compounds, especially ATO, has clinical activity in patients with recurrent/refractory multiple myeloma (MM) (Berenson et al., 2006). However, if ATO is used improperly (dosage or treatment method is inappropriate) in the treatment of MM, it can be poisonous (Rousselot et al., 2004).
Treating MM with ATO alone is an option in clinical practice. Hussein et al. (2004) reported the treatment of 24 MM patients with 0.25 mg/kg/d As2O3, with a response rate (RR) of 43%. The adverse reactions were mild, and patients can tolerate the treatment of As2O3 at this dose. Tian et al. (2006) observed the therapeutic effect of ATO on 14 patients with MM. The total effective rate was 64%, and the adverse reactions were relatively mild. Moreover, the combination of ATO and vitamin C has a certain therapeutic effect on MM (Bahlis et al., 2002; Li et al., 2015b).
2.2 Tumor (solid tumor)
2.2.1 Liver cancer
Treating liver cancer with arsenic alone has certain clinical value. Zhu et al. (2003) reported 17 patients with liver cancer who were not suitable for surgery and underwent continuous regional chemotherapy with ATO (20 mg/day using a micropump). The results showed that six patients had a tumor volume reduction of more than 50% (PR = 35.2%), no new lesions appeared, eight patients had a reduction of 10%–49% (effective rate of 41.1%), one patient remained unchanged, and two patients had a tumor volume increase of more than 25%. Fu et al. (2006) observed 25 patients with advanced primary liver cancer who received a single daily dose of ATO injection (10 mg) via the intravenous drip for 10 days as a cycle; the treatment was repeated for 2 weeks intermittently. The results showed 4 cases of partial remission (PR), 17 cases of no change (NC), and 4 cases of progressive disease (PD), with a remission rate of 16.0%. The main adverse reactions were gastrointestinal reactions, bone marrow suppression, and liver function changes.
In addition to using arsenic alone for treatment, it can be combined with other drugs for treatment, such as 5-fluorouracil (Chen et al., 2008), sorafenib (Wu et al., 2019), and chemotherapy drugs (Liu et al., 2006).
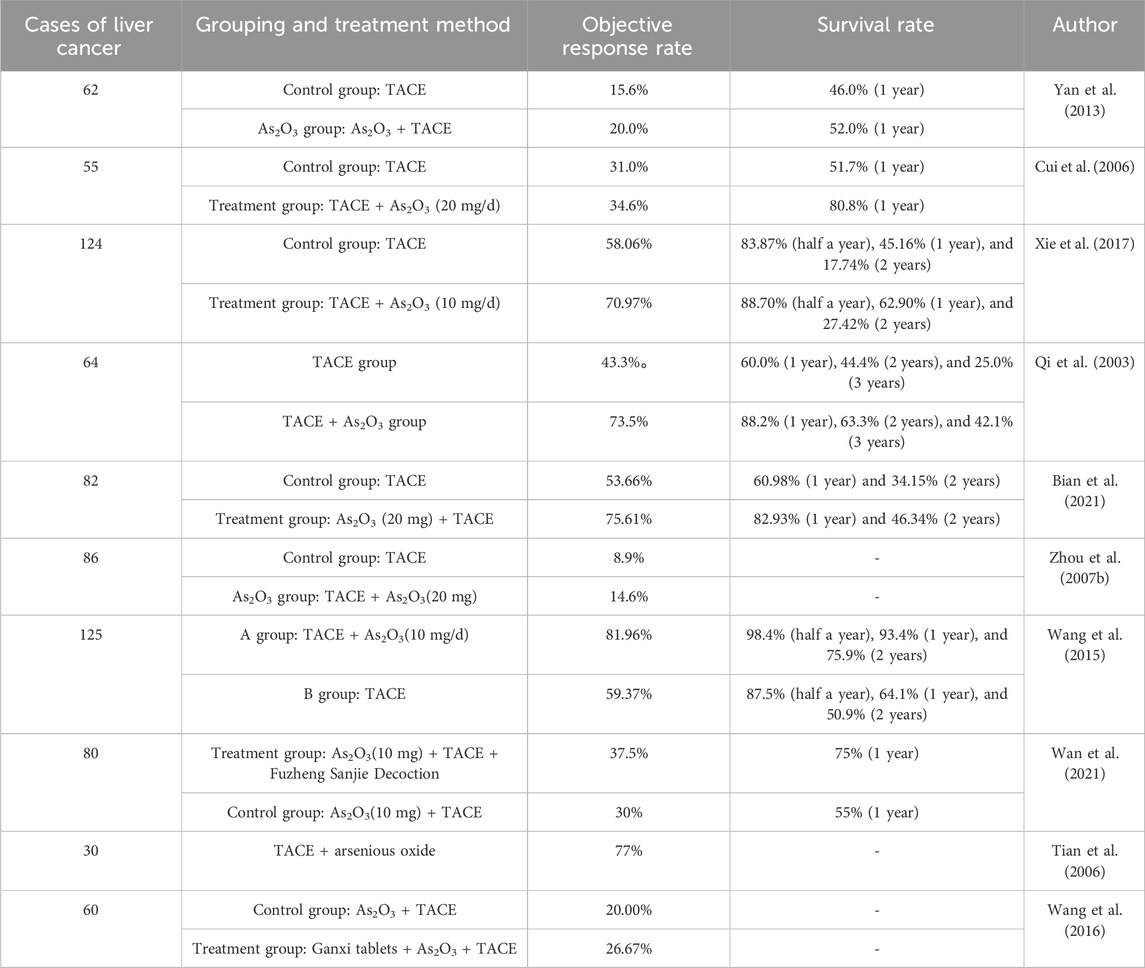
The combination of arsenic trioxide/arsenite and transcatheter arterial chemoembolization (TACE) has a certain therapeutic effect on liver cancer (Table 3).
2.2.2 Lymphoma
When ATO is applied to lymphoma, intravenous injection of 0.25 mg/m2 combined with AA can lead to disease progression, but dosage changes can have therapeutic effects. Ravandi et al. (2003) reported a 29-year-old female patient with refractory Burkitt-like lymphoma who had a high lactate dehydrogenase (LDH) value. As2O3 (intravenous injection of 0.25 mg/m2 per day for 2 h) and AA (oral administration of 500 mg twice a day) were administered, followed by prednisone treatment. As a result of the side effects and further development of the disease, treatment was discontinued. However, due to its ability to reduce serum LDH, attempts were made to change the dosage of As2O3 to obtain a safe and effective treatment. Michel et al. (2003) applied As2O3 to two patients with cutaneous T-cell lymphoma, and the results showed that the first patient achieved PR, whereas the second patient remained stable. Huang et al. (2007) subsequently observed 23 patients with relapsed and refractory malignant lymphoma who were treated with As2O3. The As2O3 injection was slowly administered intravenously at a dose of 10 mg/m2. Among the 21 evaluable cases, they reported 3 cases with complete remission (CR) (14.3%), 10 cases with PR (47.6%), 5 cases with stable disease (SD) (23.8%), and 3 cases with PDs (14.3%). The response rate (RR) was 61.9%, indicating that As2O3 has a good therapeutic effect on relapsed and refractory malignant lymphoma and is worthy of further clinical trials.
2.3 Clinical pharmacokinetics of arsenic
When ATO is used to treat APL, its content in serum and urine gradually decreases, but it is also sufficient to treat APL. Ghiuzeli et al. (2022) observed the metabolism of ATO in 25 patients with APL and measured inorganic As (iAs), monomethylarsenic acid (MMA), and dimethylarsenic acid (DMA). After the administration of ATO, the total plasma iAs increased, followed by a rapid decrease, reaching a low level within 4–6 h. Fukai et al. (2006) studied Japanese patients with relapsed APL who received daily treatment with 0.08 mg/kg ATO for 35 days to achieve CR. Serum and urine samples were collected on the fourth and fifth days of ATO consolidation treatment, and the test results showed that arsenic in the serum was sufficient to exert therapeutic effects on APL cells. Guo et al. (2021) suggested that infusion methods and combination therapy may affect arsenic metabolism. In 305 patients with APL treated with ATO, the distribution trend of arsenic in plasma was as follows: DMAV > AsIII > MMAV > AsV during continuous slow infusion and DMAV > MMAV > AsIII > AsV during routine infusion.
Arsenic-containing traditional Chinese medicine has non-active ingredients that cannot be absorbed, and the degree of absorption of arsenic in different proportions of compound arsenic also varies. The main components HgS and As2S2 of cinnabar and realgar are inactive ingredients that cannot be absorbed by the body (Ye et al., 2005). Liu et al. (2016) studied the proportion relationship between indigo naturalis and realgar compound, and they found that an increase in the proportion of indigo naturalis can promote the absorption of arsenic in rats.
3 Pharmacological effects of arsenic
Among all the pharmacological effects of arsenic, anti-tumor is the most important and dose-dependent, with a dosage range of 0.01–80 μmol/L, and the concentration of arsenic does not exceed 20 μmol/L in most studies.
3.1 Anti-tumor (solid tumor)
Cancer is one of the most common public health issues worldwide (Zhang et al., 2019a). Arsenic and its preparations can inhibit tumor growth by regulating inflammatory signaling pathways, and they are used for the treatment of various solid tumors. ATO can inhibit the growth of many different types of cancer cells and promote apoptosis (Murgo, 2001).
3.1.1 Lung cancer
Lung cancer is the main cause of cancer death (Sung et al., 2021). Smoking or exposure to second-hand smoke, chronic obstructive pulmonary disease, occupational exposure (asbestos, cadmium, silicon, diesel waste, nickel, coal smoke, and coal smoke ash), history of radon exposure, and family history of first-degree relatives with lung cancer are related to the occurrence of lung cancer (Malhotra et al., 2016; Turner et al., 2020; Fan et al., 2022). Arsenic-containing traditional Chinese medicine orpiment is a monoclinic ore that is mainly composed of arsenic trisulfide and is highly toxic. Liu et al. (2008) conducted pharmacological and toxicological studies on orpiment, and they reported that it can effectively treat certain malignant tumors and external skin diseases.
In vitro, arsenic compounds have been shown to have cytotoxic effects, induce tumor cell apoptosis, inhibit tumor cells, and affect tumor angiogenesis in a dose-dependent manner. Dong et al. (2007) found that after treating human lung cancer cell lines with high concentrations of As2O3, the proliferation inhibition rate of cancer cells can reach over 80%. Chen et al. (2020) used As2O3 (0, 2.5, 5, 10, 15, 20, and 25 μmol/mL) to intervene in the human large-cell lung cancer NCI-H460 cell line, verifying that ATO may inhibit proliferation, block the cell cycle, and induce the apoptosis of NCI-H460 cells by downregulating the expression of Bcl-2, CDK1, and c-Myc proteins; upregulating Bax protein expression; and activating caspase-9 and caspase-3 proteases. Liang et al. (2020) treated lung adenocarcinoma A549 cells with different concentrations of ATO (1, 3, and 6 μmol/L) for 12, 24, and 48 h, indicating that ATO can induce apoptosis in lung adenocarcinoma A549 cells by downregulating the level of cell cycle genes, upregulating the content of apoptosis-related factors, and reducing MAPK/ERK signaling. In animal experiments, ATO can also inhibit tumor growth. Chen et al. (2021a) treated 5- to 6-week-old BALB/c-nu male nude mice with As2O3 (2.5 and 5.0 mg/kg) and implanted 125 I particles to construct a lung cancer transplant tumor model. The results showed that both the As2O3 (2.5 and 5.0 mg/kg) treatment group and the combination treatment group could significantly inhibit tumor growth in mice, indicating that As2O3 and its combination with 125 I particle implantation could inhibit tumor growth in lung cancer transplant tumor mice.
3.1.2 Liver cancer
Liver cancer is one of the most common malignant tumors in clinical practice and has the highest mortality rate. Among numerous treatment options, ATO has always played an important role as a broad-spectrum anti-cancer drug (Wu et al., 2006). In recent years, domestic and international research has confirmed its positive role in inhibiting the growth of solid tumor cells and promoting cell apoptosis (Zhao et al., 2015a; Shen et al., 2017). Given that the liver is a detoxifying organ, the local concentration of ATO is high, which is conducive to its anti-liver cancer effect. Its anti-liver cancer effect is mainly achieved by inducing the apoptosis of liver cancer cells (Hu et al., 2003).
Arsenic can inhibit tumor cell proliferation and induce cell apoptosis. In vitro, ATO can act on multiple targets and induce apoptosis in liver cancer cells, and ATO only inhibits liver cancer cells without affecting normal liver cells (Zhao, 2016). Gao et al. (2010) demonstrated that As2O3 can activate the JNKs/AP-1 cell apoptosis pathway by inducing growth inhibition and DNA damage-induced protein 45α (GADD45α) expression in the human liver cancer cell line HepG2. However, under the same arsenic exposure conditions, GADD45α was not induced to be expressed in normal human liver cells HL7702 and LO2.
In the liver cancer transplant tumor model, administration of ATO can promote tumor cell apoptosis (Chen et al., 2000; Li et al., 2001) and inhibit tumor growth (Wang et al., 2020a; Duan et al., 2021).
3.1.3 Reproductive system tumors
Breast cancer is a malignant tumor with the highest incidence rate among women. In developed countries such as Europe and the United States, breast cancer accounts for 25%–30% of female malignant tumors (Siegel et al., 2019). Liu et al. (2020b) treated the human breast cancer cell line MCF7 with arsenic trioxide to reduce the release of adenosine diphosphate (ADP) from platelets, thereby inhibiting platelet aggregation induced by MCF7. Triple-negative breast cancer mostly shows invasive growth with a high degree of malignancy. Research on its treatment still lacks breakthrough progress (Cserni et al., 2021). The expression of promyelocytic leukemia (PML) protein in breast cancer tissue is significantly higher than that in adjacent tissue and normal breast tissue, and it has potential anti-cancer ability (Gamell et al., 2014). Zhang et al. (2021a) proved that As2O3 can enhance the inhibitory effect of PML on the proliferation, invasion, and migration of triple-negative breast cancer cells to treat triple-negative breast cancer. Ji et al. (2021) treated the human triple-negative breast cancer cell line MDA-MB-231 with 2, 4, 6, 8, 10, and 12 μmol/L As2O3; with the increase in concentration, they found that ATO can downregulate the expression of macrophage colony-stimulating factor 1 (CSF1) and inhibit the chemotaxis of the triple-negative breast cancer cell line MDA-MB-231 to human monocyte line U937.
ATO can promote apoptosis of breast cancer cells (Chen et al., 2003), inhibit the growth of breast cancer (Kozono et al., 2018), and have a cytotoxic effect on breast cancer cells (Karasulu et al., 2004). Liao et al. (2009) has shown that abnormalities in the Hedgehog pathway are closely related to the occurrence and development of some tumors. The combination of arsenic disulfide and itraconazole has a synergistic effect, jointly downregulating the expression of SMO and Glil proteins in the Hedgehog signaling pathway to achieve anti-tumor effects (Wang et al., 2021b).
ATO can be used as an alternative chemotherapy for ovarian cancer, inducing apoptosis of ovarian cancer cells (Uslu et al., 2000), inhibiting drug resistance in tumor cells (Du et al., 2001), and directly acting on tumor cells. In addition, As2O3 can induce cell apoptosis by inhibiting the activity of glutathione peroxidase (GPX) and increasing the production of H2O2 in mitochondria (Dai et al., 1999). As2O3 can inhibit microtubule depolymerization and regulate key regulatory proteins of cell cycle G2-M phase transformation so that ovarian cancer, cervical cancer, and breast cancer cells can stagnate in the G2/M phase and then induce apoptosis (Ling et al., 2002). For ovarian cancer drug-resistant cell lines, As2O3 can still play an inhibitory role. Huang et al. (2002) showed that the human ovarian cancer-resistant cell line 3AO/cDDP has cisplatin resistance. As2O3 has a significant inhibitory effect on the growth of human ovarian cancer cell lines 3AO and 3AO/cDDP, and the inhibitory effect of As2O3 on the growth of 3AO/cDDP cells is not significant compared with 3AO cells, so As2O3 is equally effective on 3AO/cDDP cells as it is on 3AO cells.
3.1.4 Lymphoma
Lymphoma is divided into Hodgkin’s lymphoma and non-Hodgkin’s lymphoma (Katabi et al., 2017). Some arsenic compounds, such as arsenic trioxide, realgar, and sodium arsenate, have significant effects in treating lymphoma. As2O3 can inhibit the proliferation of DLBCL and Jurkat lymphoma cells in a concentration- and time-dependent manner (Wang et al., 2013). Lo et al. (2014) found that As2O3 induces apoptosis in MCL lymphocytes in a time- and dose-dependent manner while inhibiting cyclin D1. Gao et al. (2009) found that As2O3 at eight different concentrations (0.1, 0.3, 0.5, 0.6, 0.8, 1.0, 2.0, and 3.0 mol/L) has an inhibitory effect on EL4 cell proliferation. Yoon et al. (2016) implanted lymphoma Daudi cells subcutaneously into nude mice; after 14 days, the mice were injected with 3.5 and 7.0 mg/kg sodium arsenate. The results showed that the tumor volume in the treatment group was smaller than that in the control group, indicating that sodium arsenate can inhibit the growth of lymphoma cells. Wang et al. (2012) found that the combination of As2O3 and ethacrynic acid can induce apoptosis in lymphoma cells. The two synergistically activate the c-Jun-NH2-terminal kinase and reduce the expression of Mcl-1 protein, leading to a decrease in mitochondrial transmembrane potential and the release of cytochrome c. Subsequently, caspase-3 and caspase-9 are activated, inducing apoptosis in the cells.
3.2 Influence on the blood system
3.2.1 Influence on leukemia (hematological tumors)
ATO, whose mechanism in treating leukemia is related to inducing cell differentiation and apoptosis, is a multi-target and multi-pathway anti-tumor drug (Wu et al., 2017). In leukemia, ATO is most commonly used in the treatment of APL, and it is mainly used in AML. AML is one of the most common hematological malignancies in clinical practice (Xiao et al., 2020a). ATO can inhibit survival and induce apoptosis on AML cells, and STAT3 inhibitor Stattic can enhance this effect (Wu et al., 2021a). The mechanism may be related to the increase in ROS caused by the inhibition of Nrf2 and Nrf2 downstream gene expression (Nrf2 enhances the drug resistance of AML cells (Sekeres et al., 2012)). The synergistic application of ATO with decitabine (Gore et al., 2010; Lo-Coco et al., 2015) or rapamycin (Xiao et al., 2020a) can inhibit AML cells. Low concentrations of ATO combined with NK cells can inhibit the proliferation of the AML KG1a cell line (Wang et al., 2021a).
APL is a special subtype of AML characterized by the infinite proliferation of tumor cells in the body’s hematopoietic system (Abedin et al., 2016), which inhibits the production of normal cells (Zheng, 2018). It has unique cytogenetic changes. ATO has a dose-dependent dual effect on APL cells, triggering cell apoptosis at high doses and inducing partial cell differentiation at low doses (Zhou et al., 2007a; Zheng et al., 2008; Chen et al., 2021b). In addition, ATO can also be rapidly converted into trivalent methylation metabolites in the liver through methyltransferase (AS3MT) and redistributed to the blood of patients with APL receiving ATO treatment (Maimaitiyiming et al., 2020). The combination of ATO with tanshinone IIA (Wang et al., 2020b) or Puerariae Radix Flavones (Chen et al., 2021c) can induce apoptosis of promyelocytes. The adverse prognostic factors for treating APL may include additional gene mutations, including FLT3-ITD (Shen et al., 2015). ATRA combined with ATO can improve the prognosis of patients with APL by inhibiting the FLT3-ITD signaling pathway (Wang et al., 2017). In addition, the synergistic treatment of ATRA and arsenic significantly weakens the inhibitory effect of FLT3-ITD on ATRA, restores the degradation of PML-RARα induced by ATRA, promotes recombination of PML nucleosomes, activates P53, and eradicates APL (Esnault et al., 2019).
3.2.2 Multiple myeloma
ATO can induce apoptosis in MM cells and inhibit their growth and proliferation. Mathas et al. (2003) used in vitro cultured myeloma cells to show that ATO can inhibit the activity of NF-κB induced by tumor necrosis factor α and prevent NF-κB from upregulating IL-6 and other genes, thereby reducing IL-6 concentration, eliminating IL-6-mediated proliferation of MM cells, and weakening their ability to resist apoptosis. However, when Rousselot et al. (2004) treated 10 patients with advanced MM with arsenic trioxide, they found that ATO as a single agent does not produce any significant response. When used in combination with melarsoprol, ATO can inhibit the activity and growth of plasma cells in the bone marrow or blood of patients with MM, induce apoptosis, and ultimately lead to myeloma cell death (Estrov et al., 1999). Bahlis et al. (2002) demonstrated that As2O3 inhibits growth and induces apoptosis in drug-resistant MM cells in vitro. The addition of vitamin C can enhance the activity of As2O3. The combination of ATO and vitamin C has a good therapeutic effect on patients with MM (Fu et al., 2020). It can downregulate the expression of matrix metalloproteinase MMP-13 [involved in the occurrence and development of MM (Lin et al., 2016)] and nuclear factor NF-κB-p65 protein and upregulate the expression of CCAAT enhancer-binding protein C/EBPα, with a good prognosis. Arsenic acid can increase the production of superoxide and inhibit the redox system, leading to a large accumulation of superoxide in the body, directly damaging mitochondria, and inducing cell apoptosis through the internal mitochondrial apoptosis pathway (Sánchez et al., 2008). It can also reduce the secretion of IL-6 by preventing MM cells from adhering to bone marrow stromal cells, slow down the progression of MM, and weaken the anti-apoptotic effect mediated by IL-6 (Ravandi, 2004). Hu et al. (2021a) confirmed that ATO can inhibit the proliferation and promote the apoptosis of MM cells by inhibiting the activation of the PI3K/AKT pathway.
3.3 Antivirus
Arsenic has anti-viral effects in the form of ATO or As2S2. Low concentrations of ATO can effectively inhibit the replication of hepatitis C virus in sub-gene replicon cells carrying hepatitis C virus, and ATO affects interferon against hepatitis C virus, making it a potential anti-hepatitis C virus drug (Hwang et al., 2004). The main component of realgar is As2S2, with a dosage of 100–200 mg each time. As2S2 has slight toxicity to the human body, but it has a strong inactivation effect on viral proteases (Ding, 2012), thereby playing an anti-viral role. Moreover, realgar has an inactivating effect on various proteases in human hosts, such as DNA-linking enzymes, DNA primer enzymes, DNA polymerase, and RNA reverse transcriptase, making it a full-spectrum anti-viral drug (Ren, 2004).
4 Toxicity of arsenic and its preparations
Arsenic is widely found in soil, rock, and water (Li et al., 2016) and may contaminate drinking water, air, food, and industrial products. Among various arsenic exposure pathways, arsenic poisoning occurs mainly through drinking arsenic-contaminated water and coal combustion (Zhang et al., 2020a). Arsenic poisoning is a serious threat to human health, causing skin toxicity, blood toxicity, eye toxicity, and genito-urinary toxicity, of which carcinogenic toxicity is the most common. Statistical data (P. et al., 2013) showed that China is gradually becoming one of the countries with the greatest impact and highest incidence of endemic arsenicosis. Costa (2019) concluded that knockdown of H3.3 alone induces carcinogenesis, and the replacement of functional H3.3 proteins by increased H3.1 proteins may be one of the mechanisms of arsenic carcinogenesis.
4.1 Pathways of arsenic poisoning
Arsenic is susceptible to environmental contamination, causing arsenic poisoning in humans after contaminating food, water, air, and soil (Hughes et al., 2011). Environmental contamination by arsenic remains a major public health problem in many countries worldwide (Bolt, 2012; Bolt, 2013b), the most important of which are drinking water contamination (groundwater contamination) (Rahaman et al., 2021), atmospheric arsenic contamination, coal combustion-type contamination, environmental contamination from mining activities, and waste deposits (Bolt, 2013a).
Of all the modes of arsenic exposure, infection by ingestion of contaminated groundwater is most prevalent (Caussy, 2003) (more than 200 million people are estimated to be at risk of arsenic exposure (Bundschuh et al., 2013)). In recent years, the main causes of serious groundwater contamination include industrial wastewater and pesticide and fertilizer discharges that have led to arsenic entering natural water bodies (Shakoor et al., 2017). In China, 19 provinces have groundwater arsenic concentrations exceeding 10 μg/L; in some areas, more than 50% of the water has arsenic concentrations exceeding 50 μg/L, with a maximum of 2,330 μg/L (Jia et al., 2018). Arsenic contamination of groundwater is also prevalent in many countries, such as Bangladesh, Central Europe, Chile, Mexico, and the United States, where the arsenic in the water exceeds the WHO guidance value of 10 μg/L. As early as 2001, groundwater wells in Vietnam had arsenic levels exceeding 3,000 μg/L (Christen, 2001).
Arsenic can cause cardiovascular disease (CVD), skin cancer, respiratory cancer, and other diseases after it is ingested or inhaled in the atmosphere (Welch et al., 1982; Engel et al., 1994; Yu et al., 2006). The European Union (EU) has a limit of 6 ng/m3 for atmospheric arsenic. Zhang et al. (2020b) found that air arsenic concentrations in 2005 were highest in Chile and eastern China, with mean values of 8.34 and 5.63 ng/m3, respectively. By 2015, as a result of the rapid growth of the Indian coal quantity, India’s average atmospheric concentrations of arsenic (4.57 ng/m3) were higher than those of eastern China (4.38 ng/m3). The combined effect of arsenic in the atmosphere and groundwater may significantly increase the risk of cancer. Coal burning and copper smelting are the main sources of arsenic in atmospheric PM2.5 (Jiang et al., 2015), which can cause serious air pollution (Liu et al., 2020a). He et al. (2019) detected the arsenic content in PM2.5 in Baoding, China, and found that the indoor and outdoor concentrations were 3.7–36.8 and 9.6–59.5 ng/m3, respectively. The average arsenic concentration exceeded 6 ng/m3, indicating serious atmospheric arsenic pollution.
4.2 Dermal toxicity
The WHO defines arsenicosis as a chronic health disorder that, if sustained intake of arsenic in excess of a safe dose for more than 6 months, patients usually present with skin lesions characterized by melanosis, keratosis pilaris (Tian et al., 2020), palmoplantar keratosis (Patel et al., 2021), dermatitis, hyper- and hypopigmentation of the skin (Chandra et al., 2022), verrucous hyperplasia, skin cancers, and thickening of the skin (Liu et al., 2001); in severe cases, even squamous cell carcinomas and basal cell carcinomas may develop (Zhou, Q. et al., 2018). Das et al. (2008) concluded that skin damage is generally the initial symptom of arsenic poisoning and is the basis for diagnosis. Therefore, in Taiwan, skin pigmentation and hyperkeratotic skin damage are common indicators of arsenic exposure (Mead, 2005). Although skin lesions can occur in any part of the body, they are more common in non-exposed areas such as the trunk, buttocks, and thighs and appear as raindrops or extensive florid black or tan spots (Yang, 2007).
Long-term use of herbs containing large amounts of arsenic can lead to chronic arsenic poisoning, and skin lesions are the most common and earliest manifestation in patients with arsenic poisoning. The skin manifestations of patients with arsenic poisoning are detailed in Table 4.
A close relationship exists between the concentration of arsenic exposure and skin damage, with the degree of skin damage gradually increasing as the concentration of arsenic exposure increases (Guo et al., 2010; Li et al., 2021a).
Most cases of arsenic-induced skin cancer are caused by poisoning from the administration of arsenic-containing drugs. The induction of skin cancer by arsenic mixtures used for medicinal purposes was reported as early as 1888 (Furst, 1984). Minkowitz (1964) reported a case of arsenic poisoning from the administration of Fowler’s solution, which caused a variety of characteristic keratoconus and neoplastic lesions, including primary cutaneous epidermoid carcinoma and papillary epidermoid carcinoma of the left posterior region of the laryngeal ring. Siefring et al. (2018) reported a case of 46-year-old Vietnamese men who developed extensive, multiple, and concurrent cutaneous squamous cell carcinoma in non-sun-exposed skin areas after taking arsenic-based traditional medicine for chronic plaque psoriasis. Additionally, arsenic-induced skin cancer starts from basal germinal cells (Chang et al., 1998; Matsui et al., 1999).
4.3 Genito-urinary toxicity
Arsenic can cause damage to the reproductive system; according to statistics, China has more than 3 million people with high arsenic exposure (Zhang, 2015). Long-term exposure to arsenic has certain health hazards (Zhang et al., 2021b). Research suggests that arsenic compounds are a class of environmental estrogens (EEs) (Stoica et al., 2000), which can affect the human endocrine system and nervous system development and interfere with reproductive function.
4.3.1 Effects on germ cells
Studies have found that arsenic above a certain dose has serious effects on male sexual function, such as reduction in testicular weight and alterations in steroidogenesis and spermatogenesis (Machado-Neves, 2022). Arsenic causes a decrease in sperm count (Zhang et al., 2003; Sanghamitra et al., 2008; Zeng et al., 2019) and sperm deformities (Shalat et al., 1996; Zhang et al., 1996; Gao et al., 2019) in rats and mice. Arsenic can also interfere with spermatogenesis by inhibiting the Ddx3y gene (Luo et al., 2020). Other studies (Dai et al., 2020) found that arsenic may indirectly alter spermatogenesis and development by affecting the expression of VEGF and VEGFR2, testicular mesenchymal cells, and blood vessels, which in turn may collectively affect male fertility.
4.3.2 Effects on embryos and offspring
During development, chronic exposure to arsenic and its compounds can easily cross the placenta and accumulate in the embryo’s brain (Qu et al., 2020), enter the fetus through the blood–placenta barrier, and affect the embryo’s development, leading to congenital malformations. In severe cases, miscarriage and stillbirth can occur (Kang, 2004). Thus, arsenic has embryotoxicity. Robinson et al. (2011) showed that As has a dose-dependent effect on gene expression in mouse embryos and has potential embryotoxic effects. Mirkes et al. (1992) found that acute in vitro exposure of embryos to sodium arsenite (50 μmol/L) on day 10 of gestation in rats was embryotoxic, as evidenced by decreased growth and abnormal development. Rodríguez et al. (2002) showed that inorganic arsenic has embryotoxic and teratogenic effects on experimental animals. Li et al. (2009) exposed zebrafish embryos to high concentrations (0.5–10 mmol/L) of arsenite pollutants, and they detected reduced survival of embryos in the arsenic-treated group and abnormalities such as delayed hatching, growth retardation, and morphological changes. Colomina et al. (1997) administered 10 mg/kg sodium arsenite via gavage to mice on days 15–18 of gestation and observed significant delays in ear opening and eye-opening times in the offspring of mice exposed to sodium arsenite after natural delivery. Chen et al. (2023) found inverse associations between prenatal arsenic exposure and the neurodevelopment of children at 2 years old, even at low exposure levels.
4.3.3 Effects on estrogen receptors
ATO has an effect on the estrogen receptor (ER). Chen et al. (2002) showed through in vitro tests with breast cancer cells that high doses of As2O3 reduce cell survival, whereas low doses inhibit ERα gene expression, which is restored 24 h after the removal of arsenide. Da Silva et al. (2017) found that mice injected subcutaneously with ATO for 5 consecutive weeks had downregulated levels of AR expression in mesenchymal and epididymal epithelial cells. Rosenblatt et al. (2009) found that ATO downregulates AR expression levels and inhibits AR transcriptional activity in prostate cell lines (PC3, LNCap, and LAPC4 cells).
4.3.4 Effects on the urinary system
Arsenic that enters the human body is mainly excreted in the urine, which inevitably affects the urinary system. Ghosh et al. (2008) have shown that humans, rhesus monkeys, rabbits, rats, hamsters, mice, dogs, and cows excrete MMA and DMA in their urine; studies in Taiwan, Bangladesh, and China found that low levels of DMA cause MMA to accumulate in the body, and elevated concentrations of MMA increase the risk of arsenic exposure-related diseases such as bladder cancer (Tseng et al., 2005; Abhyankar et al., 2012; Lin et al., 2018).
Arsenic exposure causes bladder cancer and produces significant renal pathological changes. Müller et al. (2007) studied the epidemiology and tumor toxicology of collectives exposed to arsenic orally, and they found that uroepithelial and renal cancers may be a consequence of chronic (oral) arsenic ingestion. Fukushima et al. (2005) found that organoarsenic dicarboxylic acid is carcinogenic to the bladder of rats in the multi-organ carcinogenesis bioassay model (DMBDD model) and carcinogenicity test. A controlled study (Ferreccio et al., 1998) in Chile found an association between bladder cancer and arsenic exposure. In an epidemiological survey of 42 villages near staghorn mines in the United States, Lamm et al. (2005) found that residents with arsenic levels in drinking water above 400 μg·L−1 have a significantly increased risk of bladder cancer. Arsenic enters the organism, causing renal injury and renal dysfunction. Zhong et al. (2018) found that sub-acute arsenic poisoning causes a decline in renal function by studying cases of patients with sub-acute arsenic poisoning in Ganzhou People’s Hospital.
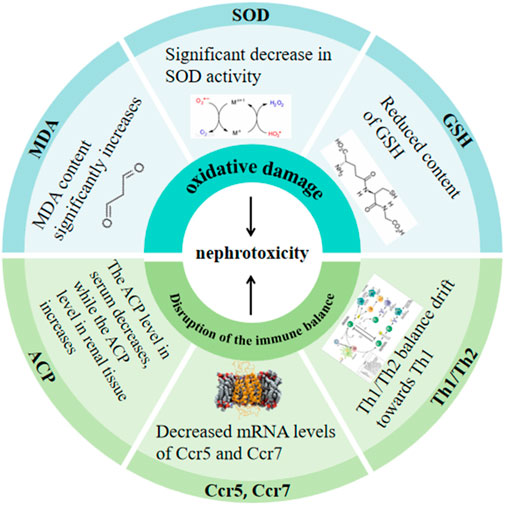
Arsenic can induce nephrotoxicity through oxidative damage and disruption of the immune balance within the kidney. Details are presented in Figure 1 (Li et al., 1997; Sun et al., 1998; Li et al., 2020a; Xu et al., 2020; Li et al., 2021b).
4.4 Neurotoxicity
Arsenic is neurotoxic, and long-term arsenic exposure can be observed in the central nervous system’s inhibitory symptoms, including headache, drowsiness, irritability, memory loss, convulsions, coma, and peripheral neuritis accompanied by muscle weakness (Kang, 2004). The conditions of severe cases can lead to death (Smith et al., 2000). Moreover, when the peripheral nervous system is impaired, peripheral neuritis and vegetative nerve disorders are common signs and symptoms (Zhao et al., 2010). Arsenic-induced neurotoxicity consists of oxidative stress, apoptosis, thiamine deficiency, and decreased acetylcholinesterase activity (Mochizuki, 2019). Arsenic-induced neuropathy can occur in acute and chronic poisoning, but it is more common in acute arsenic poisoning. Acute arsenic poisoning often presents with peripheral neuropathy within a few weeks (usually 1–3 weeks) after poisoning (You, 2000), and Ramírez-Campos et al. (1998) reported a case of peripheral neuropathy in only 3 days. Kuang (2004) reported a case of paralysis of respiratory muscles in a patient with sub-acute arsenic poisoning 15 days after poisoning.
4.4.1 Toxicity to the central nervous system
The toxicity of arsenic to the central nervous system is caused by multiple mechanisms (Garza-Lombó et al., 2019). For example, it can cause damage such as oxidative damage (Chattopadhyay et al., 2002; Perveen et al., 2017; Pan et al., 2018; Zhou et al., 2018) to brain tissue, disruption of neurotransmitter metabolizing enzymes in brain tissue (Zhang et al., 2015), disruption of the cholinergic system (Tyler et al., 2014), and pathological changes in hippocampal tissue in the brain (Qin et al., 2017).
Arsenic exposure can damage hippocampal tissues. A previous study reported (Zhao et al., 2013) that arsenic can cross the blood–brain barrier, thereby damaging the basal ganglia, hippocampus, and cerebral cortex. In a study by Zhang et al. (2020c), in a rat model of arsenic poisoning induced by NaAsO2, the hippocampal tissues of rats in the model group developed severe pathological lesions, such as neuronal degeneration, hemorrhage in part of the hippocampal tissues, disorganization of the structural arrangement of the hippocampal tissues, and glioblast hyperplasia. Yang et al. (2021a) proved that the metabolites of realgar in rats are iAs, MMA, and DMA; MMA and DMA can accumulate in brain tissue through the blood–brain barrier, which can cause a decrease in the ability to recognize new things, resulting in the damage of hippocampal neuronal cells. Sheng et al. (2021) used the mouse hippocampal neuronal cell line HT-22 as a subject and found that arsenic can affect the expression of ERK, JNK, p38, PI3K/Akt, and Nrf2/HO-1 proteins and promote the apoptosis of hippocampal neurons.
Arsenic affects intelligence and reduces learning memory in mice and rats. Calderón et al. (2001) found a significant decrease in children’s verbal intelligence quotient (IQ) with increasing levels of urinary arsenic, and children with high levels of urinary arsenic have lower prolonged memory scores and verbal abstraction scores than children with low levels of urinary arsenic. Wasserman et al. (2004) and Wasserman et al. (2007) found a negative correlation between reduced intelligence and drinking water arsenic levels (water arsenic concentrations of 117.8 ± 145.2 and 120.1 ± 134.4 μg/L, respectively) in 10- and 6-year-old children from arsenic-exposed drinking water in Araihazar district, Bangladesh. Zhang et al. (2019b) found that arsenic exposure leads to a decrease in learning and memory ability in mice, and Sun et al. (2017) reported that chronic arsenic exposure results in a significant decrease in learning and memory ability, accompanied by an upregulation of the expression of GRP78, PERK, ATF4, and CHOP proteins in the hippocampal cells of rats. Krüger et al. (2006) found that sodium arsenite significantly inhibits LTP action in hippocampal brain slices of adult rats (two to four months) and young rats (14–21 days), thereby inhibiting learning and memory. Luo et al. (2009) showed that drinking water exposure to arsenic (2.72, 13.6, and 68 mg/L) for 3 months affects spatial memory, hippocampal ultrastructure, and expression of n-methyl-d-aspartate receptor genes in rats.
4.4.2 Effects on the peripheral nervous system
Arsenic toxicity to the peripheral nervous system has been reported in many clinical settings. For example, Xu et al. (2008) reported that 104 workers overdosed on arsenic after a pipeline leak at a copper smelting plant; at 17 days after admission to the hospital, many of the subjects (45, 43.3%) developed peripheral neuropathy, with 25 of them (24.0%) experiencing decreased motor and sensory nerve conduction velocities. Valappil et al. (2019) reported a 9-year-old girl with sub-acute sensory-motor peripheral neuropathy for 2 weeks, starting 3 weeks after taking a locally produced conventional drug for obesity; the patient had high serum arsenic levels, and nerve conduction on admission showed axonal sensory motor neuropathy with slow conduction. Gherardi et al. (1990) reported a young woman with sleeping sickness who was treated with melarsoprol (an organic arsenic compound); On the 38th day after administration of the drug, she had massive distal Wöhler degeneration of the peripheral nerves and abnormalities of the dorsal ganglion and spinal cord. Xu (2018) divided 186 cases of patients with PNOCAP (a peripheral neuropathy caused by long-term exposure of patients to arsenic) into two groups according to the level of arsenide concentrations in the patient’s body, namely, the low-arsenic group (the value of arsenic <30 μg/g) and the high-arsenic group (the value of arsenic >30 μg/g); 146 patients (78.49%) showed the phenomenon of numbness of the limbs, and the motor nerve conduction velocity of the high-arsenic group was lower than that of the low-arsenic group. Arsenic affects peripheral nerve mechanical nociception in rats. Lai et al. (2020) administered arsenic (25 and 100 mg·L−1 sodium arsenite) to rats via drinking water; at the 20th week of poisoning, compared with the control group, the mean mechanical pain thresholds of the two groups decreased by 38% and 54%, respectively, and the expression levels of myelin basic protein of nerve fibers and the inflammatory factors IFN-γ and TNF-α were elevated.
4.5 Hepatotoxicity
The liver, as a metabolic organ, is the main site of As2O3 metabolism, and retention of high concentrations during its metabolism is highly likely to cause liver injury (Acosta et al., 1985). After acute or chronic arsenic exposure, toxicities such as inflammation, genotoxicity, mutagenicity, and carcinogenic effects may occur (Mandal et al., 2002), among which arsenic damage to liver cells is one of the most serious types of arsenic toxicity. Arsenic is often used in the treatment of APL, and hepatic adverse effects have been observed during treatment. For example, Mathews et al. (2006) reported that As2O3 alone causes hepatotoxic reactions when used in the treatment of patients with newly diagnosed APL, with a 38.2% incidence of hepatotoxicity in 76 patients. Hao et al. (2013) showed that approximately 24.4% of APL patients treated with As2O3 develop liver injury, most of which is mild and moderate, and APL is mainly characterized by elevated alanine aminotransferase (ALT), alanine transaminase (AST), and gamma-glutamyl transpeptidase (GGT). Grade 3–4 liver injury was also observed in 25%–63% of patients with APL treated with As2O3 in combination with ATTRA by Burnett et al. (2015). A previous study reported (Nishikawa et al., 2002) that the oral administration of DMA has a promotional effect on hepatocellular carcinoma (HCC) in rats, causing a significant increase in the level of hepatic 8-oxodG and inducing an increase in P-450 synthesis. In addition, Miao et al. (2011) found that 600 mg·kg−1 realgar (arsenic-containing traditional Chinese medicines) can cause partial liver cell edema in mice.
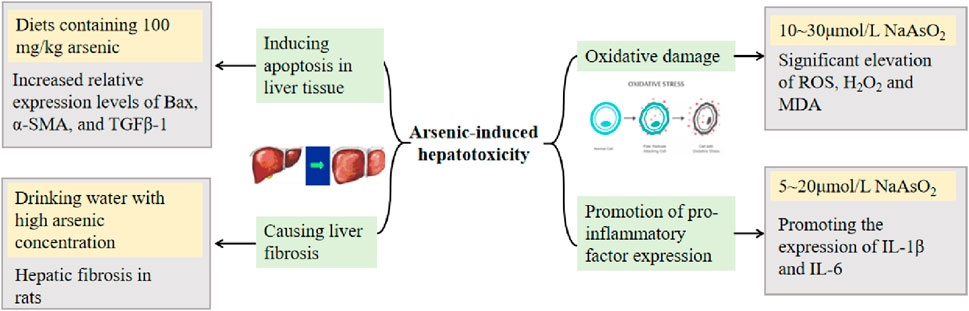
The potential mechanisms of arsenic-induced hepatotoxicity include oxidative damage, promotion of pro-inflammatory factor expression, induction of hepatocyte apoptosis, and liver fibrosis (Renu et al., 2021). Arsenic-induced oxidative damage is associated with ROS formation in a time-dependent manner (Santra et al., 2022). The mechanism of arsenic-induced apoptosis may be related to the activation of p38, which increases the level of p-p38 protein expression (Hu et al., 2020a).
Many studies on the mechanism of arsenic toxicity have been reported through animal experiments. Chi et al. (2019) found that arsenic interferes with gene expression, including the p53 signaling pathway, upregulates many oncogenes highly expressed in HCC, and downregulates a number of oncogenes, suggesting an increased risk for the development of HCC after injection of arsenious acid into routinely reared normal mice and mice in which antibiotics were used to disrupt intestinal flora. Li et al. (2015b) induced hepatic injury in mice by gavage administration of As2O3; mice showed a decrease in total antioxidant capacity (T-AOC) and glutathione (GSH) levels, an increase in MDA content, a significant increase in serum ALT and AST, and a significant elevation of hepatocyte cellular structure and morphological changes in liver histopathological tests compared with those of blank group mice. Liu et al. (2018) showed that As2O3 causes a significant increase in the percentage of apoptotic cells in the liver of male Hy-line chickens. In addition, arsenic affects the metabolism of Ca and Mg in the liver, causing liver damage in rats and affecting the ability to repair the damage. Specific concentrations and toxicity mechanisms are detailed in Figure 2 (Yan et al., 2009; Hu et al., 2021b; Deng et al., 2021; Zeng et al., 2022; Zhao et al., 2022).
4.6 Circulatory system toxicity
CVD poses a serious risk to human health; increased mortality from CVD is associated with exposure to high levels of arsenic, and As exposure is associated with atherosclerosis (Wang et al., 2002; Wang et al., 2010). The molecular mechanism of arsenic-induced cardiovascular toxicity is not fully understood, but epigenetic changes, increased platelet aggregation, and increased oxidative stress are all related to it (Kononenko et al., 2021).
Arsenic can directly damage the capillaries and act on the vasoconstrictor center, paralyzing the smooth muscle of the vascular wall, increasing permeability, reducing blood volume, and aggravating organ damage. The interaction of arsenic with arachidonic acid biotransformation (Bunderson et al., 2004), affecting vasoconstriction and diastole, may be one of the mechanisms responsible for cardiovascular toxicity. Through a review of the literature, Stea et al. (2014) discovered an association between low-level arsenic exposure and CVD and its complications. Ochoa-Martínez Á et al. (2019) evaluated prognostic CVD biomarkers in Mexican women exposed to iAs in drinking water and found that the most sensitive CVD biomarkers are plasma atherosclerotic index (AIP), plasma asymmetric dimethylarginine (ADMA), and adipocyte fatty acid-binding protein (FABP4), which are associated with arsenic exposure.
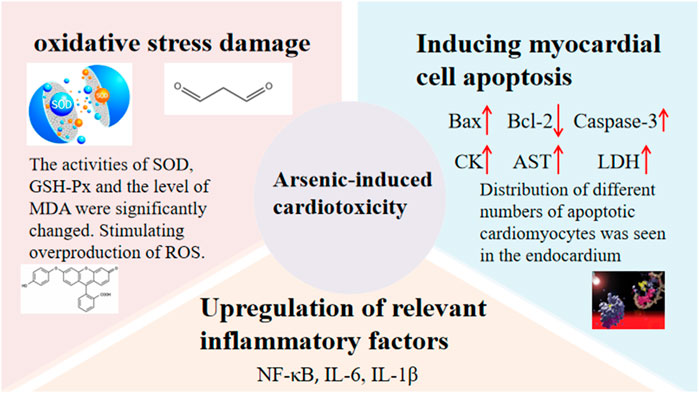
Cardiotoxicity has also been found to be significant when ATO is used therapeutically (Zhang, F. et al., 2018), and cardiotoxicity is mainly of two types: (sub)acute cardiotoxicity and chronic cardiotoxicity. ATO has multiple mechanisms of inducing cardiotoxicity, such as oxidative stress damage, upregulation of relevant inflammatory factors, and induction of myocardial damage (Haybar et al., 2019; Vineetha et al., 2019). Details are shown in Figure 3 (Saad et al., 2006; Han et al., 2010; Zhao et al., 2015b; Li et al., 2020c; Yang et al., 2021b; Zhao et al., 2021b; Yang et al., 2021c; Xu et al., 2021).
In addition, arsenic exposure causes anemia, leukopenia, lymphocytosis, and thrombocytopenia (Li et al., 2000; Islam et al., 2004; Lee et al., 2004; Wang et al., 2009a; Mahmud et al., 2009; Parvez et al., 2017; Sun et al., 2019).
4.7 Effects on the endocrine system
Experimental evidence by Tseng et al. (2002) demonstrated that chronic long-term exposure to arsenic can induce the development of diabetes (Rahaman et al., 2021), especially type 2 diabetes. Overall, urinary arsenic levels may be higher in the type 2 diabetic population than in the general population, and urinary arsenic levels are associated with glycosylated hemoglobin levels in the type 2 diabetic population (Feseke et al., 2015). Lai et al. (1994) detected excessive arsenic exposure in drinking well water in a highly endemic area of Taiwan with crow’s foot disease, and the prevalence of diabetes mellitus in these villages was higher than that of the general population of Taiwan by a factor of approximately two; their research group was the first to determine a relationship between arsenic exposure and diabetes mellitus. By adopting a case-series approach, Xiao et al. (2020b) found that the average urinary arsenic level in the type 2 diabetic population was 38.81 μg/L, which was higher than the average urinary arsenic level in the general population of 13.72 μg/L (Tian et al., 2015). Through clinical observation, Hu et al. (2020b) found that low arsenic levels in maternal blood may promote the onset and development of maternal gestational diabetes mellitus (GDM). Navas-Acien et al. (2006) found that arsenic may affect the development of diabetes through other mechanisms such as oxidative stress, inflammation, or apoptosis; all non-specific mechanisms have been implicated in the development of type 2 diabetes. A study involving 45 pre-diabetic and 65 diabetic patients in New York City (Wu et al., 2021b) demonstrated that high MMA (MMA%) and low DMA (DMA%), i.e., lower ability to methylate arsenic, in urine are associated with poor glycemic control and diabetes. In addition, arsenic causes an increase in blood glucose levels and disorders of glucose metabolism in the body (Wang et al., 2009b; Xie et al., 2020; Dai et al., 2021).
4.8 Respiratory toxicity
Chronic arsenic exposure can cause lung cancer (Ng et al., 2003). The mechanism of arsenic carcinogenesis may be related to ERs, oxidative stress, high levels of EGFR proteins, and disruption of this balance between pro- and anti-apoptotic factors.
Arsenic is considered an endocrine disruptor with estrogen-like effects, capable of interfering with the expression of ER; the abnormal expression of ER is closely related to the development of lung cancer (He et al., 2015). Cheng et al. (2019) persistently stained the human bronchial epithelial cell line 16-HBE with 2.5 μM of sodium arsenite. The results indicated that sodium arsenite induced the malignant transformation of the human bronchial epithelial cell line 16-HBE, and ERs may be involved in arsenic-induced malignant transformation of cells. Arsenic may induce lung carcinogenesis through oxidative stress (Zhao et al., 2021a) and can cause cells to proliferate abnormally and eventually become malignant by upsetting this balance between pro- and anti-apoptotic factors (Zhu et al., 2020; Chen et al., 2021c).
Environmental arsenic exposure or misuse of arsenic-containing medications can lead to cancers of the respiratory system. Buechley (1963) found that lung cancer mortality is directly proportional to the amount of arsenic in the environment. Half of the miners in Sachsen died of lung cancer (Hueper, 1956). A meta-analysis of five articles using a fixed-effects model was conducted by Chen et al. (2014). The results of their analysis showed that the exposed group was approximately 1.37 times more likely to develop lung cancer relative to the non-exposed group. Thus, arsenic exposure may cause a high incidence of lung cancer and may be a high-risk factor for the development of lung cancer. Mabuchi et al. (1979) documented a case of lung cancer due to exposure to inorganic arsenic pesticides. Robson et al. (1963) reported a 45-year-old woman who had been taking arsenic-containing Fowler’s solution for intermittent bullous pemphigoid for more than 15 years, and an examination showed undifferentiated carcinoma in the right main bronchus. Lee et al. (2005) reported a case of a 53-year-old lifelong non-smoker with chronic asthma who was treated with arsenic-containing herbal medicines for 10 years as a child, and the patient was diagnosed with Bowen’s disease and extensive stage small cell carcinoma of lung (SCLC) at 10 and 47 years after arsenic exposure, respectively.
4.9 Other toxicity
Arsenic’s effects on chromosomes have been extensively studied, with either trivalent or pentavalent arsenic causing chromosomal aberrations and increasing sister chromatid monosome exchange (An et al., 1999; Nuta et al., 2021). Arsenic has been tested in vitro and in vivo for genotoxicity (Patlolla et al., 2012; Zheng et al., 2020; Qian et al., 2021). Genotoxicity and proteotoxicity, which arsenic can cause, may be interrelated and together contribute to proteinopathies (Wysocki et al., 2023).
Arsenic is also toxic to the immune system (Giles et al., 2022). Arsenic accumulation inhibits lymphocyte proliferation, migration, and activation; induces T-cell apoptosis; and suppresses T-cell activity by increasing oxidative stress and decreasing IL-2 secretion (Huang et al., 2019).
In addition, arsenic increases pressure within the pulp cavity, destroying capillaries and causing bleeding (Luo et al., 2004).
5 Discussion
In clinical practice, ATO is usually administered intravenously at a dose of 10 mg/day for the treatment of hematological diseases, including leukemia, MDS, and MM. When ATO is used in combination with ATRA in the treatment of APL, it can achieve good therapeutic effects. In addition, it is often used for the treatment of solid tumors, such as liver cancer and lymphoma cancer. Among arsenic-containing traditional Chinese medicine, realgar is widely used in clinical practice and can be used to treat APL, MDS, and lymphoma. However, clinical pharmacokinetic research is relatively scarce, and further research is needed.
In terms of pharmacology, arsenic is most commonly used for anti-tumor functions because it can inhibit the growth of solid tumor cells, promote cell apoptosis, resist tumor angiogenesis, promote partial differentiation, degrade certain specific fusion proteins, downregulate the expression of the Bcl-2 protein, downregulate mitochondrial transmembrane potential ∆Ψm, and activate cysteine protease caspases. In addition to its anti-tumor effects, arsenic can fight against viruses and asthma. However, research on the pharmacokinetics of arsenic remains limited. The pharmacological effects of arsenic are dose-dependent, ranging from 0.01 μmol/L to 80 μmol/L, with arsenic concentrations not exceeding 20 μmol/L in most studies.
Arsenic not only has anti-tumor effects but also has carcinogenic and teratogenic toxicity. It can cause environmental pollution, such as coal burning, arsenic poisoning, and drinking water poisoning. Skin damage, such as rash or dermatitis, is usually the primary manifestation and sign of arsenic poisoning. In severe cases, it can evolve into skin cancer, and dosage plays a crucial role. The pathways of arsenic poisoning mainly include ingestion of contaminated groundwater, arsenic pollution in the atmosphere, and coal-fired pollution. For example, Waalkes et al. (2003) demonstrated that C3H mice exposed to high levels of inorganic arsenic (42.5 and 85 ppm) can develop HCC, lung cancer, liver tumors, and ovarian tumors. The important mechanisms of arsenic carcinogenesis are methylation metabolism and oxidative damage. The toxicity of arsenic is also dose-dependent. Within the effective concentration range of arsenic (less than 20 μmol/L), it can cause toxic damage. For example, 5 μmol/L sodium arsenite can induce liver oxidative damage and promote the expression of pro-inflammatory factors; 10 and 20 μmol/L sodium arsenite can promote the proliferation of human lung cancer A549 cells, and 15 μmol/L sodium arsenite can induce myocardial damage. When the concentration is high, it is likely to cause toxic damage. For example, sodium arsenite is embryotoxic to rats at 50 μmol/L; when the concentration of arsenic reaches 600 μmol/L, it causes obvious toxic damage to astrocytes (ACs) in the brain (Feng et al., 2019). Therefore, when applying it, a concentration of 20 μmol/L should not be exceeded, and the treatment concentration can be adjusted according to the patient’s physical condition.
It is necessary for the government to regularly monitor the concentration of arsenic. First, the government should control the concentration of arsenic in groundwater to not exceed 10 μg/L to prevent arsenic poisoning caused by ingestion of groundwater, as well as to prevent contamination of crops such as rice. For high-arsenic water exceeding this concentration, low-arsenic water can be mixed to achieve an acceptable arsenic concentration or used for laundry and other purposes. Second, the government should also limit the amount of arsenic in the atmosphere of residential areas to below 3 ng/m3, mainly by reducing coal pollution (implementing mandatory arsenic removal treatment for high arsenic coal). Third, the management of arsenic-containing pesticides and pharmaceuticals must be strengthened. For example, the packaging of arsenic-containing pesticides must be dyed red to prevent confusion with food. Arsenic-containing drugs, such as realgar, should be tested for arsenic content to control drug standards. For the general public, it is necessary to understand the dangers of high arsenic exposure and the sources of arsenic exposure, including crops, distinguish the use of low-arsenic water from high-arsenic water, and avoid ingesting high-arsenic water. In addition, people should also be aware of the signs of arsenic poisoning in order to seek timely medical attention.
6 Conclusion
As is well known, arsenic is a toxic metalloid element and a global pollutant. In the past 30 years, most studies and reports on arsenic research have focused on toxicity in various aspects, with relatively less research on pharmacology and clinical applications. Exposure to arsenic can cause various types of toxicity, including circulatory system toxicity, urogenital system toxicity, endocrine system toxicity, skin toxicity, neurotoxicity, hepatotoxicity, and respiratory system toxicity. Initially, arsenic exposure is mainly manifested as skin toxicity and causes significant toxicity to the liver, kidney, and nervous system. The main mechanisms by which arsenic induces liver and kidney toxicity are oxidative damage, disruption of immune balance in the kidneys, promotion of pro-inflammatory factor expression, induction of liver cell apoptosis, and initiation of liver fibrosis. The limited clinical application of arsenic is mainly due to its high toxicity and numerous adverse reactions, making it difficult to find a suitable dosage. Additionally, the dosage should be tailored to the individual’s physical condition. In previous studies, 10 mg/d was a commonly used dose for arsenic trioxide, and all-trans retinoic acid was a commonly used combination drug. However, there is still an urgent need for more research on arsenic in clinical pharmacokinetics to ensure the safety of clinical applications. The pharmacological effects of arsenic are dose-dependent and have been extensively studied in anti-tumor studies, with inhibitory effects on various solid tumors. However, there is still limited research on the pharmacokinetics of arsenic.
Therefore, attention should be paid to environmental arsenic pollution, prevention of arsenic poisoning, and risk assessment of arsenic. In addition, attention should also be paid to the relationship among dose, time, pharmacological effects, and toxicity. According to the theory of traditional Chinese medicine, future research should focus on combining medications to enhance efficacy and reduce toxicity.
Author contributions
YY: writing–original draft and writing–review and editing. YL: writing–original draft. RL: writing–original draft. ZW: writing–review and editing, data curation, methodology, and supervision.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Natural Science Foundation of Sichuan Province (2023NSFSC0654).
Acknowledgments
The authors would like to thank all authors for their contributions to this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abedin, S., and Altman, J. K. (2016). Acute promyelocytic leukemia: preventing early complications and late toxicities. Hematol. Am. Soc. Hematol. Educ. Program 2016 (1), 10–15. doi:10.1182/asheducation-2016.1.10
Abhyankar, L. N., Jones, M. R., Guallar, E., and Navas-Acien, A. (2012). Arsenic exposure and hypertension: a systematic review. Environ. health Perspect. 120 (4), 494–500. doi:10.1289/ehp.1103988
Acosta, D., Sorensen, E. M., Anuforo, D. C., Mitchell, D. B., Ramos, K., Santone, K. S., et al. (1985). An in vitro approach to the study of target organ toxicity of drugs and chemicals. vitro Cell. Dev. Biol. J. Tissue Cult. Assoc. 21 (9), 495–504. doi:10.1007/BF02620841
Amadori, S., Fenaux, P., Ludwig, H., O’Dwyer, M., and Sanz, M. (2005). Use of arsenic trioxide in haematological malignancies: insight into the clinical development of a novel agent. Curr. Med. Res. Opin. 21 (3), 403–411. doi:10.1185/030079904X20349
An, Y., and Gao, Z. L. (1999). Progress in molecular mechanisms of arsenic carcinogenesis. J. Int. Oncol. (06), 344–348.
Bahlis, N. J., McCafferty-Grad, J., Jordan-McMurry, I., Neil, J., Reis, I., Kharfan-Dabaja, M., et al. (2002). Feasibility and correlates of arsenic trioxide combined with ascorbic acid-mediated depletion of intracellular glutathione for the treatment of relapsed/refractory multiple myeloma. Clin. cancer Res. official J. Am. Assoc. Cancer Res. 8 (12), 3658–3668. doi:10.1038/sj.bmt.1702850
Bartolomé, B., Córdoba, S., Nieto, S., Fernández-Herrera, J., and García-Díez, A. (1999). Acute arsenic poisoning: clinical and histopathological features. Br. J. dermatology 141 (6), 1106–1109. doi:10.1046/j.1365-2133.1999.03213.x
Berenson, J. R., and Yeh, H. S. (2006). Arsenic compounds in the treatment of multiple myeloma: a new role for a historical remedy. Clin. lymphoma & myeloma 7 (3), 192–198. doi:10.3816/CLM.2006.n.058
Bian, X. S., Wang, Z. Z., and Song, Z. X. (2021). Clinical efficacy and safety of arsenic trioxide in interventional treatment of advanced primary liver cancer with transcatheter hepatic arterial chemoembolization. J. Clin. Exp. Med. 20 (07), 705–708. doi:10.3969/j.issn.1671-4695.2021.07.010
Bolt, H. M. (2012). Arsenic: an ancient toxicant of continuous public health impact, from Iceman Ötzi until now. Archives Toxicol. 86 (6), 825–830. doi:10.1007/s00204-012-0866-7
Bolt, H. M. (2013a). Current developments in toxicological research on arsenic. EXCLI J. 12, 64–74. doi:10.17877/DE290R-5506
Bolt, H. M. (2013b). Current research trends on arsenic toxicology. Archives Toxicol. 87 (6), 925–926. doi:10.1007/s00204-013-1073-x
Buechley, R. W. (1963). EPIDEMIOLOGICAL CONSEQUENCES OF AN ARSENIC-LUNG CANCER THEORY. Am. J. public health nation’s health 53 (8), 1229–1232. doi:10.2105/ajph.53.8.1229
Bunderson, M., Brooks, D. M., Walker, D. L., Rosenfeld, M. E., Coffin, J. D., and Beall, H. D. (2004). Arsenic exposure exacerbates atherosclerotic plaque formation and increases nitrotyrosine and leukotriene biosynthesis. Toxicol. Appl. Pharmacol. 201 (1), 32–39. doi:10.1016/j.taap.2004.04.008
Bundschuh, J., Bhattacharya, P., Nath, B., Naidu, R., Ng, J., Guilherme, L. R., et al. (2013). Arsenic ecotoxicology: the interface between geosphere, hydrosphere and biosphere. J. Hazard. Mater. 262, 883–886. doi:10.1016/j.jhazmat.2013.08.019
Burnett, A. K., Russell, N. H., Hills, R. K., Bowen, D., Kell, J., Knapper, S., et al. (2015). Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet. Oncol. 16 (13), 1295–1305. doi:10.1016/S1470-2045(15)00193-X
Calderón, J., Navarro, M. E., Jimenez-Capdeville, M. E., Santos-Diaz, M. A., Golden, A., Rodriguez-Leyva, I., et al. (2001). Exposure to arsenic and lead and neuropsychological development in Mexican children. Environ. Res. 85 (2), 69–76. doi:10.1006/enrs.2000.4106
Cao, M. Y., Gong, J., Gao, A., Guo, L., Zhao, T., Lu, F., et al. (2011). Overview of pharmacological research on orpiment. J. Liaoning Univ. Traditional 13 (03), 54–56. doi:10.13194/j.jlunivtcm.2011.03.56.caomy.059
Caussy, D. (2003). Case studies of the impact of understanding bioavailability: arsenic. Ecotoxicol. Environ. Saf. 56 (1), 164–173. doi:10.1016/s0147-6513(03)00059-9
Chandra, A., and Shah, K. A. (2022). Chronic arsenic poisoning. N. Engl. J. Med. 387 (15), 1414. doi:10.1056/NEJMicm2203290
Chang, C. H., Tsai, R. K., Chen, G. S., Yu, H. S., and Chai, C. Y. (1998). Expression of bcl-2, p53 and Ki-67 in arsenical skin cancers. J. Cutan. pathology 25 (9), 457–462. doi:10.1111/j.1600-0560.1998.tb01775.x
Chattopadhyay, S., Bhaumik, S., Nag Chaudhury, A., and Das Gupta, S. (2002). Arsenic induced changes in growth development and apoptosis in neonatal and adult brain cells in vivo and in tissue culture. Toxicol. Lett. 128 (1-3), 73–84. doi:10.1016/s0378-4274(01)00535-5
Chen, B. L., Sun, G. F., and Xie, H. F. (2014). Meta analysis of lung cancer due to arsenic exposure. Bull. Dis. Control Prev. 29 (01), 10–12. doi:10.13215/j.cnki.jbyfkztb.1311021
Chen, C. N., Pan, Q. Z., Huang, H. X., Lin, Y. X., Su, M. J., Liang, S. J., et al. (2008). Treatment of advanced hepatocellular carcinoma with combination regimen of subcutaneous implant of 5-fluorouracil sustained-release plus intravenous infusion of arsenous acid. J. Mod. Oncol. (02), 243–245.
Chen, D., Lu, Y., Pei, R. Z., Lou, Y. J., Wang, J. H., Ye, P. P., et al. (2018). Outcome of acute promyelocytic leukemia treated with low dose homoharringtonine,alltrans retinoic acid and arsenic trioxide. Chin. J. Clin. Pharmacol. Ther. 23 (05), 561–569. doi:10.12092/j.issn.1009-2501.2018.05.014
Chen, G. C., Guan, L. S., Hu, W. L., and Wang, Z. Y. (2002). Functional repression of estrogen receptor a by arsenic trioxide in human breast cancer cells. Anticancer Res. 22 (2a), 633–638. doi:10.1016/j.crci.2007.01.005
Chen, H., Qin, S. K., Pan, Q. S., Chen, H. Y., and Ma, J. (2000). Antitumor effect of arsenic trioxide on mice experimental liver cancer. Chin. J. Hepatology 8 (01), 27–29. doi:10.3760/j.issn:1007-3418.2000.01.008
Chen, H., Zhang, H., Wang, X., Wu, Y., Zhang, Y., Chen, S., et al. (2023). Prenatal arsenic exposure, arsenic metabolism and neurocognitive development of 2-year-old children in low-arsenic areas. Environ. Int. 174, 107918. doi:10.1016/j.envint.2023.107918
Chen, Q., Cheng, H. R., Huang, H., Yang, J., Feng, W. J., and Wen, W. H. (2021a). Effects of arsenic and its metabolites on cIAP1、cIAP2 and XIAP genes in A549 cells. J. Dali Univ. 6 (10), 76–81.
Chen, S. J., Zhou, G. B., Zhang, X. W., Mao, J. H., de Thé, H., and Chen, Z. (2011). From an old remedy to a magic bullet: molecular mechanisms underlying the therapeutic effects of arsenic in fighting leukemia. Blood 117 (24), 6425–6437. doi:10.1182/blood-2010-11-283598
Chen, T., Cai, Y. Y., and Shen, Q. (2021b). Experimental study of Puerariae Radix Flavones combined with arsenic trioxide inducing apoptosis of NB4-R1 cells in vitro. Guid. J. Traditional Chin. Med. Pharm. 27 (03), 40–45. doi:10.13862/j.cnki.cn43-1446/r.2021.03.010
Chen, X., and Wu, C. Y. (2003). Effect of arsenic trioxide on the heterologous graft model for human breast infiltrating duct carcinoma in nude mice. Chin. J. Exp. Surg. (08), 52–53.
Chen, Z. H., Lei, G. Y., Han, L., Li, S. X., and Xu, Z. J. (2020). Experimental study on arsenic trioxide inhibiting proliferation and promoting apoptosis of human lung cancer cell line NCI-H460. Shaanxi Med. J. 49 (10), 1219–1227. doi:10.3969/j.issn.1000-7377.2020.10.005
Chen, Z. H., Lei, G. Y., Han, L., Li, S. X., and Xu, Z. J. (2021c). Arsenic trioxide combined with 125I seed implantation inhibits tumor growth in lung cancer xenograft in mice. J. Gansu Sci. 33 (02), 38–43. doi:10.16468/j.cnki.issn1004-0366.2021.02.007
Cheng, Y. L., Che, W. J., Wang, J. T., Zhang, H., Yang, M. P., Xu, W. L., et al. (2019). Inhibition effect of fulvestrant on the sodium arsenite-induced malignant transformation of human bronchial epithelial cells. Mod. Prev. Med. 46 (22), 4170–4174.
Chervona, Y., Hall, M. N., Arita, A., Wu, F., Sun, H., Tseng, H. C., et al. (2012). Associations between arsenic exposure and global posttranslational histone modifications among adults in Bangladesh. Cancer Epidemiol. biomarkers Prev. a Publ. Am. Assoc. Cancer Res. cosponsored by Am. Soc. Prev. Oncol. 21 (12), 2252–2260. doi:10.1158/1055-9965.EPI-12-0833
Chi, L., Xue, J., Tu, P., Lai, Y., Ru, H., and Lu, K. (2019). Gut microbiome disruption altered the biotransformation and liver toxicity of arsenic in mice. Archives Toxicol. 93 (1), 25–35. doi:10.1007/s00204-018-2332-7
Christen, K. (2001). The arsenic threat worsens. Environ. Sci. Technol. 35 (13), 286A–291a. doi:10.1021/es012394f
Colomina, M. T., Albina, M. L., Domingo, J. L., and Corbella, J. (1997). Influence of maternal stress on the effects of prenatal exposure to methylmercury and arsenic on postnatal development and behavior in mice: a preliminary evaluation. Physiology Behav. 61 (3), 455–459. doi:10.1016/s0031-9384(96)00462-3
Commission, C. P. (2020). Pharmacopoeia of the People’s Republic of China 2020. Beijing: China Medical Science Press.
Costa, M. (2019). Review of arsenic toxicity, speciation and polyadenylation of canonical histones. Toxicol. Appl. Pharmacol. 375, 1–4. doi:10.1016/j.taap.2019.05.006
Cserni, G., Quinn, C. M., Foschini, M. P., Bianchi, S., Callagy, G., Chmielik, E., et al. (2021). Triple-negative breast cancer histological subtypes with a favourable prognosis. Cancers 13 (22), 5694. doi:10.3390/cancers13225694
Cui, S. Z., Wu, Y. B., Wang, J. K., Tang, Y. Q., Shang, R. J., Wang, B., et al. (2006). Clinical research of arsenic trioxide(As2O3) continuous regional hepatic arterial infusion in the treatment of primary hepatic carcinoma. Chin. J. Biomed. Eng. (05), 433–436. doi:10.3760/cma.j.issn.1674-1927.2006.05.015
Dai, J., Weinberg, R. S., Waxman, S., and Jing, Y. (1999). Malignant cells can be sensitized to undergo growth inhibition and apoptosis by arsenic trioxide through modulation of the glutathione redox system. Blood 93 (1), 268–277. doi:10.1182/blood.v93.1.268
Dai, L. L., Yu, Q., Chen, Z. B., Zhou, Y. Z., and Shen, X. B. (2021). Role of GSK-3β in arsenic-induced glucose metabolism disorders. J. Zunyi Med. Univ. 44 (01), 36–41. doi:10.14169/j.cnki.zunyixuebao.2021.0007
Dai, Y. P., and Gao, X. Q. (2020). Expression and significance of VEGF and VEGFR2 in the testis of rats with chronic arsenic poisoning. Chin. J. Anat. 43 (03), 186–176. doi:10.3969/j.issn.1001-1633.2020.03.002
Das, N. K., and Sengupta, S. R. (2008). Arsenicosis: diagnosis and treatment. Indian J. dermatology, Venereol. leprology 74 (6), 571–581. doi:10.4103/0378-6323.45098
Da Silva, R. F., Borges, C. D. S., de Almeida Lamas, C., Cagnon, V. H. A., and de Grava Kempinas, W. (2017). Arsenic trioxide exposure impairs testicular morphology in adult male mice and consequent fetus viability. J. Toxicol. Environ. health. Part A 80 (19-21), 1166–1179. doi:10.1080/15287394.2017.1376405
Deng, B., Ren, Q. X., and Li, S. G. (2021). Arsenic induces hepatocyte toxicity through NF-κB/miR-21/PDCD4 pathway. J. Shihezi Univ. Sci. 39 (05), 631–638. doi:10.13880/j.cnki.65-1174/n.2021.22.039
Ding, X. K. (2012). New congnition of antiviral pharmacological action in cinnabar and realgar. Chin. J. Clin. Ration. Drug Use 5 (19), 8–9. doi:10.15887/j.cnki.13-1389/r.2012.19.021
Dong, X., Dong, J., and Peng, G. (2007). Growth-inhibiting and apoptosis-inducing effects of Tanshinone II A on human gastric carcinoma cells. J. Huazhong Univ. Sci. Technol. Med. Sci. = Hua zhong ke ji da xue xue bao. Yi xue Ying De wen ban = Huazhong keji daxue xuebao. Yixue Yingdewen ban 27 (6), 706–709. doi:10.1007/s11596-007-0623-y
Du, Y. H., and Ho, P. C. (2001). Arsenic compounds induce cytotoxicity and apoptosis in cisplatin-sensitive and -resistant gynecological cancer cell lines. Cancer Chemother. Pharmacol. 47 (6), 481–490. doi:10.1007/s002800100278
Duan, X., Zhao, G., Han, X., Ren, J., Li, H., Chen, P., et al. (2021). Arsenic trioxide-loaded CalliSpheres: in vitro study of drug release and antitumor activity, and in vivo study of pharmacokinetics, treatment efficacy and safety in liver cancer. Oncol. Rep. 46 (1), 124. doi:10.3892/or.2021.8075
Emadi, A., and Gore, S. D. (2010). Arsenic trioxide - an old drug rediscovered. Blood Rev. 24 (4-5), 191–199. doi:10.1016/j.blre.2010.04.001
Engel, R. R., and Smith, A. H. (1994). Arsenic in drinking water and mortality from vascular disease: an ecologic analysis in 30 counties in the United States. Archives Environ. health 49 (5), 418–427. doi:10.1080/00039896.1994.9954996
Esnault, C., Rahmé, R., Rice, K. L., Berthier, C., Gaillard, C., Quentin, S., et al. (2019). FLT3-ITD impedes retinoic acid, but not arsenic, responses in murine acute promyelocytic leukemias. Blood 133 (13), 1495–1506. doi:10.1182/blood-2018-07-866095
Estrov, Z., Manna, S. K., Harris, D., Van, Q., Estey, E. H., Kantarjian, H. M., et al. (1999). Phenylarsine oxide blocks interleukin-1β–induced activation of the nuclear transcription factor NF-κB, inhibits proliferation, and induces apoptosis of acute myelogenous leukemia cells. Blood 94 (8), 2844–2853. doi:10.1182/blood.v94.8.2844.420k43_2844_2853
Fan, Y., Jiang, Y., Li, X., Li, X., Li, Y., Wu, H., et al. (2022). Burden of lung cancer attributable to occupational carcinogens from 1990 to 2019 and projections until 2044 in China. Cancers 14 (16), 3883. doi:10.3390/cancers14163883
Feng, R., Jiao, X. X., Gao, L. Y., Huo, T. G., Liu, J. Y., and Jiang, H. (2019). Effects of arsenic on protein of EAAT1,EAAT2 and xCT in astrocytes. Lishizhen Med. Materia Medica Res. 30 (12), 2825–2829.
Ferreccio, C., González Psych, C., Milosavjlevic Stat, V., Marshall Gredis, G., and Sancha, A. M. (1998). Lung cancer and arsenic exposure in drinking water: a case-control study in northern Chile. Cad. saude publica 14, 193–198. Suppl 3. doi:10.1590/s0102-311x1998000700021
Feseke, S. K., St-Laurent, J., Anassour-Sidi, E., Ayotte, P., Bouchard, M., and Levallois, P. (2015). Arsenic exposure and type 2 diabetes: results from the 2007-2009 Canadian Health Measures Survey. Health Promot. chronic Dis. Prev. Can. Res. policy Pract. 35 (4), 63–72. doi:10.24095/hpcdp.35.4.01
Fu, J., and Shi, S. Z. (2006). Clinical study of arsenic trioxide in the treatment of advanced primary liver cancer. Chin. J. Clin. Oncol. Rehabilitation (03), 232–233. doi:10.13455/j.cnki.cjcor.2006.03.016
Fu, M. T., Gou, L., Liu, S. S., and Li, S. S. (2020). The effect of arsenic trioxide combined with vitamin C on the expression of MMP-13, NF-κB-p65, C/EBPα and prognosis in patients with multiple myeloma. Chin. J. Gerontology 40 (15), 3186–3189. doi:10.3969/j.issn.1005-9202.2020.15.016
Fukai, Y., Hirata, M., Ueno, M., Ichikawa, N., Kobayashi, H., Saitoh, H., et al. (2006). Clinical pharmacokinetic study of arsenic trioxide in an acute promyelocytic leukemia (APL) patient: speciation of arsenic metabolites in serum and urine. Biol. Pharm. Bull. 29 (5), 1022–1027. doi:10.1248/bpb.29.1022
Fukushima, S., Morimura, K., Wanibuchi, H., Kinoshita, A., and Salim, E. I. (2005). Current and emerging challenges in toxicopathology: carcinogenic threshold of phenobarbital and proof of arsenic carcinogenicity using rat medium-term bioassays for carcinogens. Toxicol. Appl. Pharmacol. 207 (2 Suppl. l), 225–229. doi:10.1016/j.taap.2005.01.037
Furst, A. (1984). Mechanism of action of nickel as a carcinogen: needed information. IARC Sci. Publ. (53), 245–252.
Gamell, C., Jan Paul, P., Haupt, Y., and Haupt, S. (2014). PML tumour suppression and beyond: therapeutic implications. FEBS Lett. 588 (16), 2653–2662. doi:10.1016/j.febslet.2014.02.007
Gao, J. Y., and Yi, H. L. (2019). Antagonistic effects of breakfast cereals on arsenic-induced reproductive toxicity in male mice. Food Sci. 40 (23), 183–188. doi:10.7506/spkx1002-6630-20181110-120
Gao, M., Dong, W., Hu, M., Yu, M., Guo, L., Qian, L., et al. (2010). GADD45alpha mediates arsenite-induced cell apoptotic effect in human hepatoma cells via JNKs/AP-1-dependent pathway. J. Cell. Biochem. 109 (6), 1264–1273. doi:10.1002/jcb.22509
Gao, N., Wang, X. X., Sun, J. R., Yu, W. Z., and Li, X. Z. (2017). Clinical impact of galectin-3 in newly diagnosed t (15;17)(q22;q21)/PML-RARa acute promyelocytic leukemia treated with all-trans retinoic acid and arsenic trioxide-based regimens. Ann. Hematol. 96 (5), 711–718. doi:10.1007/s00277-017-2948-3
Gao, Y., Cai, Q. Q., Li, S., Bu, Q., Liao, H., Zhou, Y., et al. (2009). Effects of arsenic trioxide under different administration ways on T-cell lymphoma xenografts in nude mice: in vivo and in vitro experiments. Ai zheng = Aizheng = Chin. J. cancer 28 (2), 127–131. doi:10.3321/j.issn:1000-467X.2009.02.009
Garza-Lombó, C., Pappa, A., Panayiotidis, M. I., Gonsebatt, M. E., and Franco, R. (2019). Arsenic-induced neurotoxicity: a mechanistic appraisal. J. Biol. Inorg. Chem. JBIC a Publ. Soc. Biol. Inorg. Chem. 24 (8), 1305–1316. doi:10.1007/s00775-019-01740-8
Ghavamzadeh, A., Alimoghaddam, K., Rostami, S., Ghaffari, S. H., Jahani, M., Iravani, M., et al. (2011). Phase II study of single-agent arsenic trioxide for the front-line therapy of acute promyelocytic leukemia. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 29 (20), 2753–2757. doi:10.1200/JCO.2010.32.2107
Gherardi, R. K., Chariot, P., Vanderstigel, M., Malapert, D., Verroust, J., Astier, A., et al. (1990). Organic arsenic-induced Guillain-Barré-like syndrome due to melarsoprol: a clinical, electrophysiological, and pathological study. Muscle & nerve 13 (7), 637–645. doi:10.1002/mus.880130713
Ghiuzeli, C. M., Stýblo, M., Saunders, J., Calabro, A., Budman, D., Allen, S., et al. (2022). The pharmacokinetics of therapeutic arsenic trioxide in acute promyelocytic leukemia patients. Leukemia lymphoma 63 (3), 653–663. doi:10.1080/10428194.2021.1978084
Ghosh, P., Banerjee, M., Giri, A. K., and Ray, K. (2008). Toxicogenomics of arsenic: classical ideas and recent advances. Mutat. Res. 659 (3), 293–301. doi:10.1016/j.mrrev.2008.06.003
Giles, B. H., and Mann, K. K. (2022). Arsenic as an immunotoxicant. Toxicol. Appl. Pharmacol. 454, 116248. doi:10.1016/j.taap.2022.116248
Gore, S. D., Gojo, I., Sekeres, M. A., Morris, L., Devetten, M., Jamieson, K., et al. (2010). Single cycle of arsenic trioxide-based consolidation chemotherapy spares anthracycline exposure in the primary management of acute promyelocytic leukemia. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 28 (6), 1047–1053. doi:10.1200/JCO.2009.25.5158
Guan, E. G., Cheng, X. J., and Yu, X. F. (2003). SMALL DOSE ARSENOUS ACID IN TREATING RECURRENT PROMYELOCYTIC LEUKEMIA. J. Shandong Med. Coll. (03), 181–183. doi:10.3969/j.issn.1674-0947.2003.03.006
Guan, J. P., Yuan, W., Yang, H., Song, C. L., and Wang, M. C. (2002). Clinical study of realgar in the treatment of retinoic acid resistant Acute promyelocytic leukemia. J. Mod. Oncol. (04), 281–282. doi:10.3969/j.issn.1672-4992.2002.04.023
Guo, M., Zhou, J., Fan, S., Li, L., Chen, H., Lin, L., et al. (2021). Characteristics and clinical influence factors of arsenic species in plasma and their role of arsenic species as predictors for clinical efficacy in acute promyelocytic leukemia (APL) patients treated with arsenic trioxide. Expert Rev. Clin. Pharmacol. 14 (4), 503–512. doi:10.1080/17512433.2021.1893940
Guo, Z. W., and Xia, Y. J. (2010). Advance in research on dermal toxicity of arsenic. J. Environ. Health 27 (10), 937–939.
Han, Y. H., Moon, H. J., You, B. R., Kim, S. Z., Kim, S. H., and Park, W. H. (2010). Effects of arsenic trioxide on cell death, reactive oxygen species and glutathione levels in different cell types. Int. J. Mol. Med. 25 (1), 121–128. doi:10.3892/ijmm_00000321
Hao, L., Zhao, J., Wang, X., Wang, H., Wang, H., and Xu, G. (2013). Hepatotoxicity from arsenic trioxide for pediatric acute promyelocytic leukemia. J. Pediatr. hematology/oncology 35 (2), e67–e70. doi:10.1097/MPH.0b013e31827e91bc
Hao, Y., and Jiang, H. (2020). Application of arsenic poisoning test in investigating and solving cases. Sichuan Chem. Ind. 23 (05), 31–33.
Haybar, H., Shahrabi, S., Rezaeeyan, H., Jodat, H., and Saki, N. (2019). Strategies to inhibit arsenic trioxide-induced cardiotoxicity in acute promyelocytic leukemia. J. Cell. physiology 234 (9), 14500–14506. doi:10.1002/jcp.28292
He, J., and Song, X. (2015). Advances in association of estrogen and human non-small cell lung cancer. Chin. J. Lung Cancer 18 (05), 315–320. doi:10.3779/j.issn.1009-3419.2015.05.10
He, K. Q., Yuan, C. G., Yin, L. Q., Zhang, K. G., Xu, P. Y., Xie, J. J., et al. (2019). A comparative study on arsenic fractions in indoor/outdoor particulate matters: a case in Baoding, China. Environ. Monit. Assess. 191 (8), 528. doi:10.1007/s10661-019-7643-5
Hu, D. Q., Chen, Y., and Wu, H. Y. (2003). Progress in the study of anticancer effects of arsenic trioxide. Pharm. Biotechnol. (01), 62–64. doi:10.19526/j.cnki.1005-8915.2003.01.019
Hu, G. F., Dong, K., Lu, J. F., Wang, Y., and Gao, W. (2021a). Effects of artesunate combined with arsenious acid on proliferation and apoptosis of multiple myeloma cells via PI3K/AKT signaling pathway. J. Exp. Hematol. 29 (06), 1819–1824. doi:10.19746/j.cnki.issn.1009-2137.2021.06.022
Hu, H., Xu, F. P., Ding, A. L., Liu, L. Y., Peng, J., and Dong, X. D. (2020a). The transfer of arsenic from mother to newborn and its relationship with pregnancy complications. Chongqing Med. 49 (14), 2321–2325. doi:10.3969/j.issn.1671-8348.2020.14.017
Hu, Q., Chen, J., and Guo, H. (2021b). Effects of zerumbone on the expression of Bax and Bcl-2 protein in liver tissue and liver fibrosis in rats with coal-burning arsenism. Chin. J. Control Endemic Dis. 36 (04), 301–303.
Hu, Y. H., Li, T., Luan, J., Pan, Y. M., Zhou, Y. R., Song, G. L., et al. (2020b). Systematic evaluation of arsenic and p38 MAPK signaling pathway. J. Shihezi Univ. Sci. 38 (06), 753–760. doi:10.13880/j.cnki.65-1174/n.2020.22.031
Huang, H. W., Lee, C. H., and Yu, H. S. (2019). Arsenic-induced carcinogenesis and immune dysregulation. Int. J. Environ. Res. public health 16 (15), 2746. doi:10.3390/ijerph16152746
Huang, S. G., Kong, B. H., Ma, Y. Y., and Jiang, S. (2002). Impact of arsenic trioxide on proliferation and metastasis of drug-resistant human ovarian carcinoma cell line. Chin. J. Cancer 21 (08), 863–867.
Huang, Y., Qin, S. K., Wang, L., Yang, N. R., Chen, Y. X., Qian, J., et al. (2007). Clinical study of arsenic trioxide for patients with malignant lymphoma. Chin. Clin. Oncol. (08), 598–600. doi:10.3969/j.issn.1009-0460.2007.08.010
Hueper, W. C. (1956). A quest into the environmental causes of cancer of the lung. Public health Monogr. 36, 1–54.
Hughes, M. F., Beck, B. D., Chen, Y., Lewis, A. S., and Thomas, D. J. (2011). Arsenic exposure and toxicology: a historical perspective. Toxicol. Sci. official J. Soc. Toxicol. 123 (2), 305–332. doi:10.1093/toxsci/kfr184
Hussein, M. A., Saleh, M., Ravandi, F., Mason, J., Rifkin, R. M., and Ellison, R. (2004). Phase 2 study of arsenic trioxide in patients with relapsed or refractory multiple myeloma. Br. J. Haematol. 125 (4), 470–476. doi:10.1111/j.1365-2141.2004.04941.x
Hwang, D. R., Tsai, Y. C., Lee, J. C., Huang, K. K., Lin, R. K., Ho, C. H., et al. (2004). Inhibition of hepatitis C virus replication by arsenic trioxide. Antimicrob. agents Chemother. 48 (8), 2876–2882. doi:10.1128/AAC.48.8.2876-2882.2004
Islam, L. N., Nabi, A. H., Rahman, M. M., Khan, M. A., and Kazi, A. I. (2004). Association of clinical complications with nutritional status and the prevalence of leukopenia among arsenic patients in Bangladesh. Int. J. Environ. Res. public health 1 (2), 74–82. doi:10.3390/ijerph2004020074
Ji, Q. L., and Ding, J. X. (2021). Effects of arsenic trioxide on triple negative breast cancer cell line MDA-MB-231 educating monocytes. Med. Equip. 34 (05), 15–16+19. doi:10.3969/j.issn.1002-2376.2021.05.005
Jia, Y., Xi, B., Jiang, Y., Guo, H., Yang, Y., Lian, X., et al. (2018). Distribution, formation and human-induced evolution of geogenic contaminated groundwater in China: a review. Sci. total Environ. 643, 967–993. doi:10.1016/j.scitotenv.2018.06.201
Jiang, H. L., Wang, Z. S., Wu, X. F., Guo, M., and Gu, Y. Y. (2015). Pollution Characteristics,Sources and control of arsenic in PM2.5 in China. J. Environ. Eng. Technol. 5 (06), 464–470. doi:10.3969/j.issn.1674-991X.2015.06.073
Jiang, M. D., and Li, S. L. (2006). Advances in antihepatocellular-carcinoma of arsenic trioxide. Int. J. Dig. Dis. (01), 26–28.
Jiang, Y., and Sun, Y. J. (2008). Clinical study on the treatment of acute promyelocytic leukemia with arsenious acid. J. Liaoning Univ. Traditional Chin. Med. (03), 38–39. doi:10.13194/j.jlunivtcm.2008.03.40.jiangy.092
Kang, J. Q., and Jin, Y. (2004). Study progress of adverse effects of arsenic on health. J. Hyg. Res. 33 (03), 372–376. doi:10.3969/j.issn.1000-8020.2004.03.039
Karasulu, H. Y., Karabulut, B., Kantarci, G., Ozgüney, I., Sezgin, C., Sanli, U. A., et al. (2004). Preparation of arsenic trioxide-loaded microemulsion and its enhanced cytotoxicity on MCF-7 breast carcinoma cell line. Drug Deliv. 11 (6), 345–350. doi:10.1080/10717540490494096
Katabi, N., and Lewis, J. S. (2017). Update from the 4th edition of the world health organization classification of head and neck tumours: what is new in the 2017 WHO blue book for tumors and tumor-like lesions of the neck and lymph nodes. Head neck pathology 11 (1), 48–54. doi:10.1007/s12105-017-0796-z
Kitchin, K. T., and Conolly, R. (2010). Arsenic-induced carcinogenesis--oxidative stress as a possible mode of action and future research needs for more biologically based risk assessment. Chem. Res. Toxicol. 23 (2), 327–335. doi:10.1021/tx900343d
Kononenko, M., and Frishman, W. H. (2021). Association between arsenic exposure and cardiovascular disease. Cardiol. Rev. 29 (4), 217–221. doi:10.1097/CRD.0000000000000357
Kozono, S., Lin, Y. M., Seo, H. S., Pinch, B., Lian, X., Qiu, C., et al. (2018). Arsenic targets Pin1 and cooperates with retinoic acid to inhibit cancer-driving pathways and tumor-initiating cells. Nat. Commun. 9 (1), 3069. doi:10.1038/s41467-018-05402-2
Krüger, K., Binding, N., Straub, H., and Musshoff, U. (2006). Effects of arsenite on long-term potentiation in hippocampal slices from young and adult rats. Toxicol. Lett. 165 (2), 167–173. doi:10.1016/j.toxlet.2006.03.005
Kuang, X. F. (2004). Clinical analysis of 32 cases of subacute arsenic toxic peripheral neuropathy. Occup. Health (11), 33–34. doi:10.3969/j.issn.1004-1257.2004.11.026
Kuivenhoven, M., and Mason, K. (2023). Arsenic toxicity, StatPearls. Treasure Island: StatPearls Publishing; Copyright © 2023, StatPearls Publishing LLC, Place: Published.
Kundu, M., Ghosh, P., Mitra, S., Das, J. K., Sau, T. J., Banerjee, S., et al. (2011). Precancerous and non-cancer disease endpoints of chronic arsenic exposure: the level of chromosomal damage and XRCC3 T241M polymorphism. Mutat. Res. 706 (1-2), 7–12. doi:10.1016/j.mrfmmm.2010.10.004
Kwong, Y. L., Au, W. Y., Chim, C. S., Pang, A., Suen, C., and Liang, R. (2001). Arsenic trioxide- and idarubicin-induced remissions in relapsed acute promyelocytic leukaemia: clinicopathological and molecular features of a pilot study. Am. J. Hematol. 66 (4), 274–279. doi:10.1002/ajh.1057
Lai, M. S., Hsueh, Y. M., Chen, C. J., Shyu, M. P., Chen, S. Y., Kuo, T. L., et al. (1994). Ingested inorganic arsenic and prevalence of diabetes mellitus. Am. J. Epidemiol. 139 (5), 484–492. doi:10.1093/oxfordjournals.aje.a117031
Lai, Y., He, Q. C., and Duan, Y. Y. (2020). Effect of chronic arsenic exposure on peripheral nerve mechanical algesthesia in rats and potential protection of mecobalamin. J. Environ. Occup. Med. 37 (03), 260–266. doi:10.13213/j.cnki.jeom.2020.19741
Lamm, S. H., Kruse, M. B. J. H., and Journal, E. R. A. A. I. (2005). Arsenic ingestion and bladder cancer mortality—what do the dose-response relationships. Suggest About Mechanism? 11, 433–450. doi:10.1080/10807030590925678
Lee, J. J., Kim, Y. K., Cho, S. H., Park, K. S., Chung, I. J., Cho, D., et al. (2004). Hemolytic anemia as a sequela of arsenic intoxication following long-term ingestion of traditional Chinese medicine. J. Korean Med. Sci. 19 (1), 127–129. doi:10.3346/jkms.2004.19.1.127
Lee, L., and Bebb, G. (2005). A case of Bowen’s disease and small-cell lung carcinoma: long-term consequences of chronic arsenic exposure in Chinese traditional medicine. Environ. health Perspect. 113 (2), 207–210. doi:10.1289/ehp.7200
Lengfelder, E., Hofmann, W. K., and Nowak, D. (2012). Impact of arsenic trioxide in the treatment of acute promyelocytic leukemia. Leukemia 26 (3), 433–442. doi:10.1038/leu.2011.245
Li, D., Lu, C., Wang, J., Hu, W., Cao, Z., Sun, D., et al. (2009). Developmental mechanisms of arsenite toxicity in zebrafish (Danio rerio) embryos. Aquat. Toxicol. Amst. Neth. 91 (3), 229–237. doi:10.1016/j.aquatox.2008.11.007
Li, F. J., Sun, G. F., Lv, X. Q., and Zhao, M. (1997). Experimental studies on influence of arsenic on GSH content in blood and tissues of mice Chinese. J. Public Health (06), 370–371. doi:10.1007/BF02943147
Li, G. D., Cheng, Z. Y., Wang, F. Y., Xie, X. L., Zhang, A. H., and Xie, N. (2015a). Secondary myelodysplastic syndrome induced by chronic lead poisoning: one case report. J. New Med. 46 (06), 414–416. doi:10.3969/g.issn.0253-9802.2015.06.016
Li, G. M., Li, Q., and Wang, X. G. (2000). The effect of realgar on the morphology of blood and bone marrow cells in mice. Pharmacol. Clin. Chin. Materia Medica (05), 25.
Li, H. J., and Yan, X. H. (2021). The combined induction therapy of all trans retinoic acid and arsenic trioxide in the first treatment of acute promyelocytic leukemia patients. Henan Med. Res. 30 (03), 477–479. doi:10.3969/j.issn.1004-437X.2021.03.036
Li, J. J. (2020c). Observation on the therapeutic effect and nursing care of arsenic trioxide combined with all trans retinoic acid in the treatment of acute promyelocytic leukemia. Mod. Nurse 27 (09), 70–72. doi:10.19792/j.cnki.1006-6411.2020.26.028
Li, J. Y., Li, X., Li, M., Wang, S. W., Liu, Q. T., Huang, Y. C., et al. (2020a). Inorganic arsenic exposure suppresses immunomodulatory effect of renal CD4+ T lymphocytes in mice. Chin. J. Cell. Mol. Immunol. 36 (10), 871–876. doi:10.13423/j.cnki.cjcmi.009076
Li, J. Y., Xu, H. L., Wang, Z., and Duan, X. X. (2021a). Effects of chronic arsenic exposure on renal Th1/Th2 transcription factors and cytokines expression in mice. J. Community Med. 19 (05), 280–283. doi:10.19790/j.cnki.JCM.2021.05.05
Li, R. H., Han, M. S., Hu, X., and Ma, Y. Q. (2020b). Effects of subchronic arsenic exposure on cardiomyocyte apoptosis of mice. J. Shanxi Agric. Sci. 48 (06), 923–926.
Li, S., Ding, Y., Niu, Q., Xu, S., Pang, L., Ma, R., et al. (2015b). Lutein has a protective effect on hepatotoxicity induced by arsenic via Nrf2 signaling. BioMed Res. Int. 2015, 315205. doi:10.1155/2015/315205
Li, S. M. (2017). Clinical trial of arsenic trioxide in the treatment of patients with middle and high risk myelodysplastic syndrome. Chin. J. Clin. Pharmacol. 33 (23), 2364–2367. doi:10.13699/j.cnki.1001-6821.2017.23.011
Li, S. S., Chen, J. Q., Peng, M. H., Lu, Y. F., Li, J. Q., Qiu, Q. M., et al. (2001). The effect of arsenic trioxide on liver cancer cell lines BEL-7404, SMMC-7721, and liver cancer xenograft models in nude mice. Chin. J. Exp. Surg. (04), 85.
Li, W. B., Lang, M., Dilinaer, M., Yuan, C. J., Chen, P. D., and Xie, H. F. (2021b). Study on skin injury induced by sodium arsenite in adult SD rats. J. Med. Res. 50 (04), 55–58.
Li, X., and Sun, W. J. (2015). The clinical activity of arsenic trioxide, ascorbic acid, ifosfamide and prednisone combination therapy in patients with relapsed and refractory multiple myeloma. OncoTargets Ther. 8, 775–781. doi:10.2147/OTT.S81022
Li, Y., Ye, F., Wang, A., Wang, D., Yang, B., Zheng, Q., et al. (2016). Chronic arsenic poisoning probably caused by arsenic-based pesticides: findings from an investigation study of a household. Int. J. Environ. Res. public health 13 (1), 133. doi:10.3390/ijerph13010133
Liang, Y., Cheng, G., and Huang, D. G. (2020). Arsenous acid in the induction of apoptosis of lung adenocarcinoma A549 cells and its effects on MAPK/ERK signaling pathway. Chin. J. Surg. Oncol. 12 (01), 13–17.
Liao, X., Siu, M. K., Au, C. W., Wong, E. S., Chan, H. Y., Ip, P. P., et al. (2009). Aberrant activation of hedgehog signaling pathway in ovarian cancers: effect on prognosis, cell invasion and differentiation. Carcinogenesis 30 (1), 131–140. doi:10.1093/carcin/bgn230
Lin, T., Zhang, Q. G., Wu, H. Y., Zhang, L. Y., Dong, H. B., and Ou, Y. J. (2016). Expression of NF-κB/p65 associated with the efficacy of bortezomib in multiple myeloma. J. Southeast Univ. Sci. Ed. 35 (01), 41–45. doi:10.3969/j.issn.1671-6264.2016.01.009
Lin, Y. C., Chen, W. J., Huang, C. Y., Shiue, H. S., Su, C. T., Ao, P. L., et al. (2018). Polymorphisms of arsenic (+3 oxidation state) methyltransferase and arsenic methylation capacity affect the risk of bladder cancer. Toxicol. Sci. official J. Soc. Toxicol. 164 (1), 328–338. doi:10.1093/toxsci/kfy087
Ling, Y. H., Jiang, J. D., Holland, J. F., and Perez-Soler, R. (2002). Arsenic trioxide produces polymerization of microtubules and mitotic arrest before apoptosis in human tumor cell lines. Mol. Pharmacol. 62 (3), 529–538. doi:10.1124/mol.62.3.529
Liu, D. H., Hua, Y., Li, S., and Luo, Y. H. (2006). Clinical observation on the interventional treatment of liver cancer with arsenious acid injection combined with chemotherapy drugs. Mod. Med. J. China (07), 59–60.
Liu, J., Lu, Y., Wu, Q., Goyer, R. A., and Waalkes, M. P. (2008). Mineral arsenicals in traditional medicines: orpiment, realgar, and arsenolite. J. Pharmacol. Exp. Ther. 326 (2), 363–368. doi:10.1124/jpet.108.139543
Liu, J., Zhao, H., Wang, Y., Shao, Y., Li, J., and Xing, M. (2018). Alterations of antioxidant indexes and inflammatory cytokine expression aggravated hepatocellular apoptosis through mitochondrial and death receptor-dependent pathways in Gallus gallus exposed to arsenic and copper. Environ. Sci. Pollut. Res. Int. 25 (16), 15462–15473. doi:10.1007/s11356-018-1757-0
Liu, J. X., Wang, S. S., Dong, C. Y., Fu, Q. H., and Yan, C. H. (2020a). Research progress on sources, health risks of children’s arsenic exposure. J. Environ. Health 37 (03), 265–269. doi:10.16241/j.cnki.1001-5914.2020.03.020
Liu, M. (2020b). Clinical analysis of sequential treatment of adult acute promyelocytic leukemia with chemotherapy, all-trans retinoic acid and arsenic. Guide China Med. 18 (28), 79–80. doi:10.15912/j.cnki.gocm.2020.28.033
Liu, S. H., Sun, B. F., Wang, Q. L., Zou, Z. L., Ma, L., Wang, W. J., et al. (2021). Correlation between arsenic methylation metabolism and skin and liver damage in patients with coal-burning arsenism. Chin. J. Public Health 37 (12), 1788–1792. doi:10.11847/zgggws1133524
Liu, Y. Q., Chen, Y. X., Zhang, T. S., Dai, L. H., Zhu, J. L., and Zhang, S. (2001). A retrospective survey on occupational arsenic poisoning. Chin. J. Industrial Med. (05), 300–302.
Liu, Y. W., Wang, D. L., Huang, P., Wang, X. Q., Yu, J., and Huang, H. P. (2016). ICP-MS study on the effect of different combinations of indigo naturalis and realgar on arsenic absorption in rats. J. Chin. Med. Mater. 39 (04), 902–904.
Liu, Z. P., Zhang, D. X., Pan, J., and Zhang, Y. S. (2020c). The inhibitory effect of arsenic trioxide on MCF7-induced platelet aggregation. J. Harbin Med. Univ. 54 (02), 130–132+137.
Lo, R. K., and Kwong, Y. L. (2014). Arsenic trioxide suppressed mantle cell lymphoma by downregulation of cyclin D1. Ann. Hematol. 93 (2), 255–265. doi:10.1007/s00277-013-1866-2
Lo-Coco, F., and Cicconi, L. (2015). Outpatient oral treatment for acute promyelocytic leukemia. N. Engl. J. Med. 372 (9), 884–885. doi:10.1056/NEJMc1500125
Lu, A. D., Zhang, L. P., Liu, G. L., and Wang, B. (2006). Clinical study on induction therapy of arsenic trioxide in combination with all-transretinoic acid for childhood acute promyelocytic leukemia. Chin. J. Pract. Pediatr. (02), 93–95.
Lu, X. Y., Wu, X. M., Wu, W. Z., Li, B. Z., Lin, Y., Zhang, X. L., et al. (2019). Decitabine combined with arsenious acid in the treatment of patients with higher-risk myelodysplastic syndromes and chronic myelomonocytic leukemia. Chin. J. Pract. Intern. Med. 39 (05), 452–455.
Lu, Y., and Jin, J. (2015). “Clinical observation on the treatment of 177 cases of acute promyelocytic leukemia with all-trans retinoic acid combined with arsenic trioxide,” in 2015 Zhejiang Provincial Hematology Academic Annual Meeting, Hangzhou, Zhejiang Province, China, September 2015, 192.
Luo, J. H., Qiu, Z. Q., Shu, W. Q., Zhang, Y. Y., Zhang, L., and Chen, J. A. (2009). Effects of arsenic exposure from drinking water on spatial memory, ultra-structures and NMDAR gene expression of hippocampus in rats. Toxicol. Lett. 184 (2), 121–125. doi:10.1016/j.toxlet.2008.10.029
Luo, J. M., Long, X., Yang, X. W., Cheng, Y., and Li, X. D. (2004). Clinical study of mandibular bone necrosis after using an arsenical endodontic preparation. J. Clin. Stomatology (11), 676–677.
Luo, Y. Z., Wang, L., Guo, J. X., Chen, J. M., Sun, Z. Y., Li, X. Y., et al. (2020). Effects of arsenic on expression of Ddx3y gene and protein in rat testis. J. Environ. Health 37 (03), 216–218. doi:10.16241/j.cnki.1001-5914.2020.03.007
Ma, J., Zhan, Z. M., Liang, H., Gong, T. J., Tang, Q. H., Ren, S., et al. (2005). Effect of arsenic trioxide on the treatment of relapsed acute promyelocytic leukemia. Oncol. Prog. (02), 98–101. doi:10.3969/j.issn.1672-1535.2005.02.003
Mabuchi, K., Lilienfeld, A. M., and Snell, L. M. (1979). Lung cancer among pesticide workers exposed to inorganic arsenicals. Archives Environ. health 34 (5), 312–320. doi:10.1080/00039896.1979.10667423
Machado-Neves, M. (2022). Arsenic exposure and its implications in male fertility. Anim. Reprod. 19 (4), e20220119. doi:10.1590/1984-3143-AR2022-0119
Mahmud, H., Föller, M., and Lang, F. (2009). Arsenic-induced suicidal erythrocyte death. Archives Toxicol. 83 (2), 107–113. doi:10.1007/s00204-008-0338-2
Maimaitiyiming, Y., Zhu, H. H., Yang, C., and Naranmandura, H. (2020). Biotransformation of arsenic trioxide by AS3MT favors eradication of acute promyelocytic leukemia: revealing the hidden facts. Drug metab. Rev. 52 (3), 425–437. doi:10.1080/03602532.2020.1791173
Malhotra, J., Malvezzi, M., Negri, E., La Vecchia, C., and Boffetta, P. (2016). Risk factors for lung cancer worldwide. Eur. Respir. J. 48 (3), 889–902. doi:10.1183/13993003.00359-2016
Mandal, B. K., and Suzuki, K. T. (2002). Arsenic round the world: a review. Talanta 58 (1), 201–235. doi:10.1016/s0039-9140(02)00268-0
Mathas, S., Lietz, A., Janz, M., Hinz, M., Jundt, F., Scheidereit, C., et al. (2003). Inhibition of NF-kappaB essentially contributes to arsenic-induced apoptosis. Blood 102 (3), 1028–1034. doi:10.1182/blood-2002-04-1154
Mathews, V., Desire, S., George, B., Lakshmi, K. M., Rao, J. G., Viswabandya, A., et al. (2006). Hepatotoxicity profile of single agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia, its impact on clinical outcome and the effect of genetic polymorphisms on the incidence of hepatotoxicity. Leukemia 20 (5), 881–883. doi:10.1038/sj.leu.2404165
Mathews, V., George, B., Chendamarai, E., Lakshmi, K. M., Desire, S., Balasubramanian, P., et al. (2010). Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: long-term follow-up data. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 28 (24), 3866–3871. doi:10.1200/JCO.2010.28.5031
Matsui, M., Nishigori, C., Toyokuni, S., Takada, J., Akaboshi, M., Ishikawa, M., et al. (1999). The role of oxidative DNA damage in human arsenic carcinogenesis: detection of 8-hydroxy-2’-deoxyguanosine in arsenic-related Bowen’s disease. J. investigative dermatology 113 (1), 26–31. doi:10.1046/j.1523-1747.1999.00630.x
Mead, M. N. (2005). Arsenic: in search of an antidote to a global poison. Environ. health Perspect. 113 (6), A378–A386. doi:10.1289/ehp.113-a378
Miao, J. W., Liu, X. L., Liang, S. X., Yan, J. W., Wu, Q., Liu, J., et al. (2011). Comparative study on the acute toxicity of niuhuang jiedu tablets, realgar, and other arsenides. J. Toxicol. 25 (03), 217–221. doi:10.16421/j.cnki.1002-3127.2011.03.015
Michel, L., Dupuy, A., Jean-Louis, F., Sors, A., Poupon, J., Viguier, M., et al. (2003). Arsenic trioxide induces apoptosis of cutaneous T cell lymphoma cells: evidence for a partially caspase-independent pathway and potentiation by ascorbic acid (vitamin C). J. investigative dermatology 121 (4), 881–893. doi:10.1046/j.1523-1747.2003.12479.x
Minkowitz, S. (1964). MULTIPLE CARCINOMATA FOLLOWING INGESTION OF MEDICINAL ARSENIC. Ann. Intern. Med. 61, 296–299. doi:10.7326/0003-4819-61-2-296
Mirkes, P. E., and Cornel, L. (1992). A comparison of sodium arsenite- and hyperthermia-induced stress responses and abnormal development in cultured postimplantation rat embryos. Teratology 46 (3), 251–259. doi:10.1002/tera.1420460308
Mochizuki, H. (2019). Arsenic neurotoxicity in humans. Int. J. Mol. Sci. 20 (14), 3418. doi:10.3390/ijms20143418
Müller, M., Böcher, A., and Buchter, A. (2007). Induction of urothelial carcinoma due to chronic arsenic ingestion? A occupational medicine-toxicological excursion. Der Urol. Ausg. A 46 (5), 511–512. doi:10.1007/s00120-006-1264-7
Murgo, A. J. (2001). Clinical trials of arsenic trioxide in hematologic and solid tumors: overview of the national cancer institute cooperative research and development studies. Oncol. 6, 22–28. Suppl 2. doi:10.1634/theoncologist.6-suppl_2-22
Navas-Acien, A., Silbergeld, E. K., Streeter, R. A., Clark, J. M., Burke, T. A., and Guallar, E. (2006). Arsenic exposure and type 2 diabetes: a systematic review of the experimental and epidemiological evidence. Environ. health Perspect. 114 (5), 641–648. doi:10.1289/ehp.8551
Ng, J. C., Wang, J., and Shraim, A. (2003). A global health problem caused by arsenic from natural sources. Chemosphere 52 (9), 1353–1359. doi:10.1016/S0045-6535(03)00470-3
Nishikawa, T., Wanibuchi, H., Ogawa, M., Kinoshita, A., Morimura, K., Hiroi, T., et al. (2002). Promoting effects of monomethylarsonic acid, dimethylarsinic acid and trimethylarsine oxide on induction of rat liver preneoplastic glutathione S-transferase placental form positive foci: a possible reactive oxygen species mechanism. Int. J. cancer 100 (2), 136–139. doi:10.1002/ijc.10471
Niu, C., Yan, H., Yu, T., Sun, H. P., Liu, J. X., Li, X. S., et al. (1999). Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood 94 (10), 3315–3324. doi:10.1182/blood.v94.10.3315.422k16_3315_3324
Nurchi, V. M., Djordjevic, A. B., Crisponi, G., Alexander, J., Bjørklund, G., and Aaseth, J. (2020). Arsenic toxicity: molecular targets and therapeutic agents. Biomolecules 10 (2), 235. doi:10.3390/biom10020235
Nuta, O., Bouffler, S., Lloyd, D., Ainsbury, E., Sepai, O., and Rothkamm, K. (2021). Investigating the impact of long term exposure to chemical agents on the chromosomal radiosensitivity using human lymphoblastoid GM1899A cells. Sci. Rep. 11 (1), 12616. doi:10.1038/s41598-021-91957-y
Ochoa-Martínez Á, C., Ruiz-Vera, T., Almendarez-Reyna, C. I., Zarazúa, S., Carrizales-Yáñez, L., and Pérez-Maldonado, I. N. (2019). Impact of arsenic exposure on clinical biomarkers indicative of cardiovascular disease risk in Mexican women. Ecotoxicol. Environ. Saf. 169, 678–686. doi:10.1016/j.ecoenv.2018.11.088
Palma-Lara, I., Martínez-Castillo, M., Quintana-Pérez, J. C., Arellano-Mendoza, M. G., Tamay-Cach, F., Valenzuela-Limón, O. L., et al. (2020). Arsenic exposure: a public health problem leading to several cancers. Regul. Toxicol. Pharmacol. RTP 110, 104539. doi:10.1016/j.yrtph.2019.104539
Pan, D. R., and Xing, H. S. (2018). Clinical experimental study on the degree of lipid peroxidation damage in the brain of mice caused by chronic fluorosis and arsenic poisoning. Chin. J. Control Endemic Dis. 33 (04), 388+390.
Pan, Y. H., and Bai, L. S. (2013). Exploring the role and harm of arsenic from its distribution, structure and characteristics. Stud. Trace Elem. Health 30 (05), 71–73.
Parvez, F., Medina, S., Santella, R. M., Islam, T., Lauer, F. T., Alam, N., et al. (2017). Arsenic exposures alter clinical indicators of anemia in a male population of smokers and non-smokers in Bangladesh. Toxicol. Appl. Pharmacol. 331, 62–68. doi:10.1016/j.taap.2017.05.014
Patel, K., Gin, A., and Scardamaglia, L. (2021). Palmoplantar keratosis caused by arsenic toxicity. Med. J. Aust. 214 (6), 258. doi:10.5694/mja2.50965
Patlolla, A. K., Todorov, T. I., Tchounwou, P. B., van der Voet, G., and Centeno, J. A. (2012). Arsenic-induced biochemical and genotoxic effects and distribution in tissues of Sprague-Dawley rats. Microchem. J. devoted Appl. Microtech. all branches Sci. 105, 101–107. doi:10.1016/j.microc.2012.08.013
Perveen, H., Dash, M., Khatun, S., Maity, M., Islam, S. S., and Chattopadhyay, S. (2017). Electrozymographic evaluation of the attenuation of arsenic induced degradation of hepatic SOD, catalase in an in vitro assay system by pectic polysaccharides of Momordica charantia in combination with curcumin. Biochem. biophysics Rep. 11, 64–71. doi:10.1016/j.bbrep.2017.06.002
Powell, B. L., Moser, B., Stock, W., Gallagher, R. E., Willman, C. L., Stone, R. M., et al. (2010). Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: north American Leukemia Intergroup Study C9710. Blood 116 (19), 3751–3757. doi:10.1182/blood-2010-02-269621
P, W., Yuan, Z. C., Zhang, H. T., and Huang, C. Q. (2013). Analysis of arsenic content detection results in drinking water of rural residents in Qianguo, Jilin Province. Chin. J. Control Endemic Dis. 28 (03), 215–216.
Qi, X. J., Zhao, H. D., Gao, W. B., and Yin, L. W. (2003). Combined percutaneous arsenic trioxide injection with transcatheter hepatic arterial chemoembolization for primary liver cancer. J. Chin. Oncol. (03), 147–149.
Qian, Q., Wang, Z. Y., Bian, Q., Liu, D. Y., Li, W., Dong, J., et al. (2021). Comparative study on the genotoxicity of realgar and arsenic methylated metabolites. Pharmacol. Clin. Chin. Materia Medica 37 (02), 65–68. doi:10.13412/j.cnki.zyyl.2021.02.007
Qian, X. C., Shi, S. Y., and Yuan, C. Y. (1965a). Clinical studies on dermatitis medicamentosa: Ⅸ. Arsenic dermatitis accompanied with pancreatitis. Fudan Univ. J. Med. Sci. (03), 281–282.
Qian, X. C., Zhou, M. H., and Yang, G. L. (1965b). Clinical studies on dermatitis medicamentosa: Ⅲ. Arsenic Dermatitis-Report 20 Cases Fudan Univ. J. Med. Sci. (02), 189–192.
Qin, W. J., Guan, D. F., Xing, G. Q., Shi, H. J., Lv, H. L., and Jiang, Y. F. (2017). Impact of NaAsO2 poisoning on rat hippocampal neurons and the kidney function. Chin. General Pract. 20 (26), 3246–3250.
Qiu, R. P., Zhu, T., Zhang, H. H., and Li, N. (2020). Effect analysis of all trans retinoic acid combined with arsenic trioxide in the treatment of acute promyelocytic leukemia. Chin. J. Thrombosis Hemostasis 26 (06), 1000–1001. doi:10.3969/j.issn.1009-6213.2020.06.043
Qu, M., Liang, H., Zhang, T., and Cui, X. H. (2018). Effect of compound huangdai tablet on acute promyelocytic leukemia. Chin. Archives Traditional Chin. Med. 36 (07), 1683–1686. doi:10.13193/j.issn.1673-7717.2018.07.039
Qu, T. J., Sun, Y. F., Liu, G. F., Gu, S. Y., and He, Z. S. (2020). Research progression of microRNAs involved in regulation of arsenic toxicity. Chin. J. Industrial Med. 33 (06), 512–515. doi:10.13631/j.cnki.zggyyx.2020.06.012
Rahaman, M. S., Rahman, M. M., Mise, N., Sikder, M. T., Ichihara, G., Uddin, M. K., et al. (2021). Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environ. Pollut. (Barking, Essex 1987) 289, 117940. doi:10.1016/j.envpol.2021.117940
Ramírez-Campos, J., Ramos-Peek, J., Martínez-Barros, M., Zamora-Peralta, M., and Martínez-Cerrato, J. (1998). Peripheral neuropathy caused by acute arsenic poisoning. Gac. medica Mex. 134 (2), 241–246.
Rao, Y., Li, R. H., and Zhang, D. Q. (2013). Transforming toxins into drugs: discovery of the therapeutic effect of arsenic trioxide on acute promyelocytic leukemia. Sci. Sin. 43 (08), 700–707. doi:10.1360/zc2013-43-8-700
Ravandi, F. (2004). Arsenic trioxide: expanding roles for an ancient drug? Leukemia 18 (9), 1457–1459. doi:10.1038/sj.leu.2403419
Ravandi, F., Estey, E., Jones, D., Faderl, S., O’Brien, S., Fiorentino, J., et al. (2009). Effective treatment of acute promyelocytic leukemia with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab ozogamicin. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 27 (4), 504–510. doi:10.1200/JCO.2008.18.6130
Ravandi, F., Koumenis, I., Johri, A., Tallman, M., Roboz, G. J., Strickland, S., et al. (2020). Oral arsenic trioxide ORH-2014 pharmacokinetic and safety profile in patients with advanced hematologic disorders. Haematologica 105 (6), 1567–1574. doi:10.3324/haematol.2019.229583
Ravandi, F., and van Besien, K. (2003). Clinical activity of arsenic trioxide in Burkitt-like lymphoma. Leukemia 17 (1), 271–272. doi:10.1038/sj.leu.2402735
Ren, Z. Y. (2004). Staged treatment of hepatitis B virus liver disease. Henan Tradit. Chin. Med. (07), 58–59.
Renu, K., Chakraborty, R., Myakala, H., Koti, R., Famurewa, A. C., Madhyastha, H., et al. (2021). Molecular mechanism of heavy metals (lead, chromium, arsenic, mercury, nickel and cadmium) - induced hepatotoxicity - a review. Chemosphere 271, 129735. doi:10.1016/j.chemosphere.2021.129735
Robinson, J. F., Yu, X., Moreira, E. G., Hong, S., and Faustman, E. M. (2011). Arsenic- and cadmium-induced toxicogenomic response in mouse embryos undergoing neurulation. Toxicol. Appl. Pharmacol. 250 (2), 117–129. doi:10.1016/j.taap.2010.09.018
Robson, A. O., and Jelliffe, A. M. (1963). Medicinal arsenic poisoning and lung cancer. Br. Med. J. 2 (5351), 207–209. doi:10.1136/bmj.2.5351.207
Rodríguez, V. M., Carrizales, L., Mendoza, M. S., Fajardo, O. R., and Giordano, M. (2002). Effects of sodium arsenite exposure on development and behavior in the rat. Neurotoxicology Teratol. 24 (6), 743–750. doi:10.1016/s0892-0362(02)00313-6
Rosenblatt, A. E., and Burnstein, K. L. (2009). Inhibition of androgen receptor transcriptional activity as a novel mechanism of action of arsenic. Mol. Endocrinol. Baltim. Md. 23 (3), 412–421. doi:10.1210/me.2008-0235
Rousselot, P., Larghero, J., Arnulf, B., Poupon, J., Royer, B., Tibi, A., et al. (2004). A clinical and pharmacological study of arsenic trioxide in advanced multiple myeloma patients. Leukemia 18 (9), 1518–1521. doi:10.1038/sj.leu.2403424
Saad, S. Y., Alkharfy, K. M., and Arafah, M. M. (2006). Cardiotoxic effects of arsenic trioxide/imatinib mesilate combination in rats. J. Pharm. Pharmacol. 58 (4), 567–573. doi:10.1211/jpp.58.4.0017
Sánchez, Y., Amrán, D., Fernández, C., de Blas, E., and Aller, P. (2008). Genistein selectively potentiates arsenic trioxide-induced apoptosis in human leukemia cells via reactive oxygen species generation and activation of reactive oxygen species-inducible protein kinases (p38-MAPK, AMPK). Int. J. cancer 123 (5), 1205–1214. doi:10.1002/ijc.23639
Sanghamitra, S., Hazra, J., Upadhyay, S. N., Singh, R. K., and Amal, R. C. (2008). Arsenic induced toxicity on testicular tissue of mice. Indian J. physiology Pharmacol. 52 (1), 84–90.
Santra, A., Bishnu, D., Santra, S., Ghatak, S., Mukherjee, P. S., Dhali, G. K., et al. (2022). Arsenic-induced injury of mouse hepatocytes through lysosome and mitochondria: an in vitro study. Int. J. hepatology 2022, 1546297. doi:10.1155/2022/1546297
Sekeres, M. A., and Steensma, D. P. (2012). Boulevard of broken dreams: drug approval for older adults with acute myeloid leukemia. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 30 (33), 4061–4063. doi:10.1200/JCO.2012.44.2962
Shakoor, M. B., Nawaz, R., Hussain, F., Raza, M., Ali, S., Rizwan, M., et al. (2017). Human health implications, risk assessment and remediation of As-contaminated water: a critical review. Sci. total Environ. 601-602, 756–769. doi:10.1016/j.scitotenv.2017.05.223
Shalat, S. L., Walker, D. B., and Finnell, R. H. (1996). Role of arsenic as a reproductive toxin with particular attention to neural tube defects. J. Toxicol. Environ. health 48 (3), 253–272. doi:10.1080/009841096161320
Shan, X. (2020). Study on the effect of arsenic trioxide injection on acute promyelocytic leukemia. Contemp. Med. 26 (03), 59–61. doi:10.3969/j.issn.1009-4393.2020.03.024
Shen, L., Zhang, G., Lou, Z., Xu, G., and Zhang, G. (2017). Cryptotanshinone enhances the effect of Arsenic trioxide in treating liver cancer cell by inducing apoptosis through downregulating phosphorylated- STAT3 in vitro and in vivo. BMC complementary Altern. Med. 17 (1), 106. doi:10.1186/s12906-016-1548-4
Shen, Y., Fu, Y. K., Zhu, Y. M., Lou, Y. J., Gu, Z. H., Shi, J. Y., et al. (2015). Mutations of epigenetic modifier genes as a poor prognostic factor in acute promyelocytic leukemia under treatment with all-trans retinoic acid and arsenic trioxide. EBioMedicine 2 (6), 563–571. doi:10.1016/j.ebiom.2015.04.006
Shen, Z. X., Chen, G. Q., Ni, J. H., Li, X. S., Xiong, S. M., Qiu, Q. Y., et al. (1997). Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood 89 (9), 3354–3360. doi:10.1182/blood.v89.9.3354
Sheng, Z. Y., Liu, J. Y., Wang, D., Liu, Y., Ma, X. Z., Huo, T. G., et al. (2021). Study on compatible detoxification mechanism of arsenic and bilirubin based on MAPK,PI3K and Nrf2 signaling pathways in neurons. Chin. Archives Traditional Chin. Med. 39 (07), 151–276. doi:10.13193/j.issn.1673-7717.2021.07.039
Siefring, M. L., Lu, D., States, J. C., and Van Hoang, M. (2018). Rapid onset of multiple concurrent squamous cell carcinomas associated with the use of an arsenic-containing traditional medicine for chronic plaque psoriasis. BMJ case Rep. 2018, bcr2017222645. doi:10.1136/bcr-2017-222645
Siegel, R. L., Miller, K. D., and Jemal, A. (2019). Cancer statistics, 2019. CA a cancer J. Clin. 69 (1), 7–34. doi:10.3322/caac.21551
Smith, A. H., Lingas, E. O., and Rahman, M. (2000). Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull. World Health Organ. 78 (9), 1093–1103.
Soignet, S. L., Frankel, S. R., Douer, D., Tallman, M. S., Kantarjian, H., Calleja, E., et al. (2001). United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 19 (18), 3852–3860. doi:10.1200/JCO.2001.19.18.3852
Soignet, S. L., Maslak, P., Wang, Z. G., Jhanwar, S., Calleja, E., Dardashti, L. J., et al. (1998). Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N. Engl. J. Med. 339 (19), 1341–1348. doi:10.1056/NEJM199811053391901
Sönksen, M., Kerl, K., and Bunzen, H. (2022). Current status and future prospects of nanomedicine for arsenic trioxide delivery to solid tumors. Med. Res. Rev. 42 (1), 374–398. doi:10.1002/med.21844
Spilchuk, V., and Thompson, A. (2019). Chronic arsenic poisoning from traditional Chinese medicine. CMAJ Can. Med. Assoc. J. = J. de l’Association medicale Can. 191 (15), E424. doi:10.1503/cmaj.181176
Stea, F., Bianchi, F., Cori, L., and Sicari, R. (2014). Cardiovascular effects of arsenic: clinical and epidemiological findings. Environ. Sci. Pollut. Res. Int. 21 (1), 244–251. doi:10.1007/s11356-013-2113-z
Stoica, A., Pentecost, E., and Martin, M. B. (2000). Effects of arsenite on estrogen receptor-alpha expression and activity in MCF-7 breast cancer cells. Endocrinology 141 (10), 3595–3602. doi:10.1210/endo.141.10.7704
Sun, G. F., Li, F. J., Li, G. X., and Ding, G. Y. (1998). The exploration for the mechanism of arsenic on kidney toxicity in mice. Chin. J. Endemiology (05), 10–12.
Sun, H., Yang, Y., Shao, H., Sun, W., Gu, M., Wang, H., et al. (2017). Sodium arsenite-induced learning and memory impairment is associated with endoplasmic reticulum stress-mediated apoptosis in rat Hippocampus. Front. Mol. Neurosci. 10, 286. doi:10.3389/fnmol.2017.00286
Sun, Y. H., Wang, J., Ma, X. Y., and Teng, L. (2019). The influence factors of the adverse reactions of acute promyelocytic leukemia in patients received arsenic trioxide (ATO) chemotherapy in initial treatment. Chin. J. Hosp. Pharm. 39 (01), 72–100.
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA a cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Tay, C. H. (1974). Cutaneous manifestations of arsenic poisoning due to certain Chinese herbal medicine. Australas. J. dermatology 15 (3), 121–131. doi:10.1111/j.1440-0960.1974.tb00546.x
Tchounwou, P. B., Centeno, J. A., and Patlolla, A. K. (2004). Arsenic toxicity, mutagenesis, and carcinogenesis--a health risk assessment and management approach. Mol. Cell. Biochem. 255 (1-2), 47–55. doi:10.1023/b:mcbi.0000007260.32981.b9
Teng, Y. (2020). Forensic identification of a case of death from chronic arsenic poisoning. Shandong Chem. Ind. 49 (19), 118. doi:10.19319/j.cnki.issn.1008-021x.2020.19.049
Tian, C. T., Han, L. Y., and Li, Y. (2006). Clinical study of transcatheter arterial chemoem-bolization combined with arsenious oxide injection on liver cancer in middle and advanced stage. Mod. J. Integr. Traditional Chin. West. Med. (18), 2463–2464. doi:10.3969/j.issn.1008-8849.2006.18.011
Tian, H. Y., Zhang, Y. J., Zheng, X. M., Wei, M. Z., Wang, K., Zhang, Y. L., et al. (2015). Investigation of arsenic levels in blood and urine of general population in Liaoning province. J. Environ. Health 32 (12), 1083–1086. doi:10.16241/j.cnki.1001-5914.2015.12.012
Tian, W., Wang, T. S., Fang, Y., Wu, H. T., Zheng, G. Q., Guo, K. R., et al. (2020). Aberrant lncRNA profiles are associated with chronic benzene poisoning and acute myelocytic leukemia. J. Occup. Environ. Med. 62 (7), e308–e317. doi:10.1097/JOM.0000000000001875
Tian, X. Q., and Lv, R. L. (2006). Clinical study on the treatment of refractory multiple myeloma with arsenic trioxide. J. Yan’an Univ. Sci. Ed. (03), 13.
Tseng, C. H., Huang, Y. K., Huang, Y. L., Chung, C. J., Yang, M. H., Chen, C. J., et al. (2005). Arsenic exposure, urinary arsenic speciation, and peripheral vascular disease in Blackfoot disease-hyperendemic villages in Taiwan. Toxicol. Appl. Pharmacol. 206 (3), 299–308. doi:10.1016/j.taap.2004.11.022
Tseng, C. H., Tseng, C. P., Chiou, H. Y., Hsueh, Y. M., Chong, C. K., and Chen, C. J. (2002). Epidemiologic evidence of diabetogenic effect of arsenic. Toxicol. Lett. 133 (1), 69–76. doi:10.1016/s0378-4274(02)00085-1
Turner, M. C., Andersen, Z. J., Baccarelli, A., Diver, W. R., Gapstur, S. M., Pope, C. A., et al. (2020). Outdoor air pollution and cancer: an overview of the current evidence and public health recommendations. CA a cancer J. Clin. 70, 460–479. doi:10.3322/caac.21632
Tyler, C. R., and Allan, A. M. (2014). The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: a review. Curr. Environ. health Rep. 1 (2), 132–147. doi:10.1007/s40572-014-0012-1
Uslu, R., Sanli, U. A., Sezgin, C., Karabulut, B., Terzioglu, E., Omay, S. B., et al. (2000). Arsenic trioxide-mediated cytotoxicity and apoptosis in prostate and ovarian carcinoma cell lines. Clin. cancer Res. official J. Am. Assoc. Cancer Res. 6 (12), 4957–4964. doi:10.1093/carcin/21.12.2293
Valappil, A. V., and Mammen, A. (2019). Subacute arsenic neuropathy: clinical and electrophysiological observations. J. Neurosci. rural Pract. 10 (3), 529–532. doi:10.1055/s-0039-1695693
Vineetha, V. P., and Raghu, K. G. (2019). An overview on arsenic trioxide-induced cardiotoxicity. Cardiovasc. Toxicol. 19 (2), 105–119. doi:10.1007/s12012-018-09504-7
Waalkes, M. P., Ward, J. M., Liu, J., and Diwan, B. A. (2003). Transplacental carcinogenicity of inorganic arsenic in the drinking water: induction of hepatic, ovarian, pulmonary, and adrenal tumors in mice. Toxicol. Appl. Pharmacol. 186 (1), 7–17. doi:10.1016/s0041-008x(02)00022-4
Wan, Q. Q., Guo, C. X., Shi, L. H., Wu, S. B., and Wan, X. Y. (2021). Clinical research of fuzheng sanjie decoction and arsenic trioxide combined with TACE in the treatment of advanced primary hepatocellular carcinoma. Clin. J. Traditional Chin. Med. 33 (03), 502–507. doi:10.16448/j.cjtcm.2021.0325
Wang, C. H., Chen, C. L., Hsiao, C. K., Chiang, F. T., Hsu, L. I., Chiou, H. Y., et al. (2010). Arsenic-induced QT dispersion is associated with atherosclerotic diseases and predicts long-term cardiovascular mortality in subjects with previous exposure to arsenic: a 17-Year follow-up study. Cardiovasc. Toxicol. 10 (1), 17–26. doi:10.1007/s12012-009-9059-x
Wang, C. H., Jeng, J. S., Yip, P. K., Chen, C. L., Hsu, L. I., Hsueh, Y. M., et al. (2002). Biological gradient between long-term arsenic exposure and carotid atherosclerosis. Circulation 105 (15), 1804–1809. doi:10.1161/01.cir.0000015862.64816.b2
Wang, D., Zhang, Y. Z., Chen, J., You, H. L., Ding, Y. J., Wei, L. L., et al. (2020a). Effects of tanshinone ⅡA combined with arsenic trioxide on apoptosis and autophagy of human leukemia cell line NB4 in vitro and its mechanism. Henan Med. Res. 29 (23), 4231–4235. doi:10.3969/j.issn.1004-437X.2020.23.003
Wang, H., Liu, Y., Wang, X., Liu, D., Sun, Z., Wang, C., et al. (2015). Randomized clinical control study of locoregional therapy combined with arsenic trioxide for the treatment of hepatocellular carcinoma. Cancer 121 (17), 2917–2925. doi:10.1002/cncr.29456
Wang, J. P., Wang, S. L., Lin, Q., Zhang, L., Huang, D., and Ng, J. C. (2009a). Association of arsenic and kidney dysfunction in people with diabetes and validation of its effects in rats. Environ. Int. 35 (3), 507–511. doi:10.1016/j.envint.2008.07.015
Wang, L., Ding, S. Q., Li, H. W., Du, S. H., Chen, C., Liu, Y. Y., et al. (2021a). Effects of arsenic disulfide combined with itraconazole on proliferation and apoptosis and hedgehog pathway of diffuse large B-cell lymphoma cells. J. Exp. Hematol. 29 (05), 1504–1509. doi:10.19746/j.cnki.issn.1009-2137.2021.05.020
Wang, L., Liu, X., Li, X., Lv, X., Lu, K., Chen, N., et al. (2013). Arsenic disulfide induces apoptosis of human diffuse large B cell lymphoma cells involving Bax cleavage. Oncol. Rep. 30 (5), 2427–2434. doi:10.3892/or.2013.2729
Wang, L., F, W., Duan, H. P., and Xu, W. D. (2009b). Clinical study on patients with TTP-HUS syndrome caused by occupational acute arsine poisoning. J. Clin. Hematol. 22 (11), 612–614.
Wang, L. N., Tang, Y. L., Zhang, Y. C., Zhang, Z. H., Liu, X. J., Ke, Z. Y., et al. (2017). Arsenic trioxide and all-trans-retinoic acid selectively exert synergistic cytotoxicity against FLT3-ITD AML cells via co-inhibition of FLT3 signaling pathways. Leukemia lymphoma 58 (10), 2426–2438. doi:10.1080/10428194.2017.1289522
Wang, Q. M., Wang, R. R., Zhang, D., Pan, M. Q., Pan, B., Deng, X. S., et al. (2016). Clinical study on treating primary hepatic carcinoma with syndrome of stagnatioin of liver qi and spleen deficiency with combination of oral Ganxi Tablets and arsenic trioxide bia transcatheter arterial chemoembolization. China J. Traditional Chin. Med. Pharm. 31 (03), 1121–1125.
Wang, R., Liu, C., Xia, L., Zhao, G., Gabrilove, J., Waxman, S., et al. (2012). Ethacrynic acid and a derivative enhance apoptosis in arsenic trioxide-treated myeloid leukemia and lymphoma cells: the role of glutathione S-transferase p1-1. Clin. cancer Res. official J. Am. Assoc. Cancer Res. 18 (24), 6690–6701. doi:10.1158/1078-0432.CCR-12-0770
Wang, W. H., Duan, X. H., Li, H., Li, F. Y., Ju, S. G., Wang, M. Z., et al. (2020b). Effect of arsenic trioxide-loaded CalliSpheres beads in the treatment of rabbits with VX2 liver tumor. J. Clin. Hepatology 36 (12), 2730–2734.
Wang, W. W. (2020). Effect of combined induction of retinoic acid and arsenite on the prolonged sleep time of newly diagnosed acute promyelocytic leukemia. World J. Sleep Med. 7 (07), 1140–1141.
Wang, Y. J., Cao, W., Zhou, Y. S., Yang, R., and Niu, X. Q. (2021b). Role of NKG2D ligands in killing KG1a cells treated with arsenic trioxide by NK cells. Chin. J. Immunol. 37 (03), 268–272. doi:10.3969/j.issn.1000-484X.2021.03.003
Wasserman, G. A., Liu, X., Parvez, F., Ahsan, H., Factor-Litvak, P., Kline, J., et al. (2007). Water arsenic exposure and intellectual function in 6-year-old children in Araihazar, Bangladesh. Environ. health Perspect. 115 (2), 285–289. doi:10.1289/ehp.9501
Wasserman, G. A., Liu, X., Parvez, F., Ahsan, H., Factor-Litvak, P., van Geen, A., et al. (2004). Water arsenic exposure and children’s intellectual function in Araihazar, Bangladesh. Environ. health Perspect. 112 (13), 1329–1333. doi:10.1289/ehp.6964
Welch, K., Higgins, I., Oh, M., and Burchfiel, C. (1982). Arsenic exposure, smoking, and respiratory cancer in copper smelter workers. Archives Environ. health 37 (6), 325–335. doi:10.1080/00039896.1982.10667586
Wu, C. X., Zhao, Y., Zhang, T., Zhang, J., Yu, W. F., Bai, H., et al. (2021a). Synergistic killing effects of Stattic and arsenic trioxide on acute myeloid leukemia THP1 cell line and underlying mechanism. J. Xi’an Jiaot. Univ. Sci. 42 (01), 53–58. doi:10.7652/jdyxb202101010
Wu, D. D., Shi, J. J., Zhang, Q., and Liu, L. Y. (2019). Clinical observation of patients with advanced primary liver cancer treated by sorafenib combined with arsenic trioxide. J. Mod. Oncol. 27 (15), 2728–2731. doi:10.3969/j.issn.1672-4992.2019.15.025
Wu, F., Chen, Y., Navas-Acien, A., Garabedian, M. L., Coates, J., and Newman, J. D. (2021b). Arsenic exposure, arsenic metabolism, and glycemia: results from a clinical population in New York city. Int. J. Environ. Res. public health 18 (7), 3749. doi:10.3390/ijerph18073749
Wu, H. X., and Yu, Z. J. (2006). Mechanism and clinical research progress of arsenic trioxide in the treatment of malignant tumors of the liver and gallbladder system. J. Nant. Univ. Sci. (02), 154–156. doi:10.3969/j.issn.1674-7887.2006.02.037
Wu, J. W., Xiao, W. T., Zeng, Y. J., Fan, J. X., Ye, Y. B., Li, Y. M., et al. (2017). Synergistic killing effects of arsenic trioxide combined with itraconazole on KG1a cells. J. Exp. Hematol. 25 (04), 1003–1010. doi:10.7534/j.issn.1009-2137.2017.04.008
Wu, S. H. (2002). Research progress on the effects of arsenic on health. Foreign Med. Sci. Medgeogr. (04), 145–160. doi:10.3969/j.issn.1001-8883.2002.04.001
Wu, X. H. (2020). Study on the application value of arsenic acid in the treatment of acute promyelocytic leukemia. Med. Diet Health 18 (19), 79–80.
Wysocki, R., Rodrigues, J. I., Litwin, I., and Tamás, M. J. (2023). Mechanisms of genotoxicity and proteotoxicity induced by the metalloids arsenic and antimony. Cell. Mol. life Sci. CMLS 80 (11), 342. doi:10.1007/s00018-023-04992-5
Xiao, C., Weng, F., Liu, J. Y., Xiao, B. X., Wang, X. G., Liu, H. X., et al. (2020a). Relationships between urine arsenic levels and hemoglobin A1C in type 2 diabetic patients exposed to arsenic. Carcinogenesis,Teratogenesis Mutagen. 32 (02), 87–91+97.
Xiao, P. L., Guo, S. L., Wang, S. L., Liu, S. Z., Peng, L., Wang, W. L., et al. (2020b). Effects of arsenic trioxide combined with rapamycin on proliferation,apoptosis and autophagy of acute myeloid leukemia THP-1 cells. J. Xinxiang Med. Univ. 37 (09), 818–823. doi:10.7683/xxyxyxb.2020.09.003
Xie, H. F., Ren, D. Y., and Sun, G. F. (2020). Expression difference of ZAG and PGC-1α genes between arsenism model and diabetes model SD rats. J. Environ. Health 37 (08), 692–696. doi:10.16241/j.cnki.1001-5914.2020.08.009
Xie, P., Yu, X., Li, G., and Gu, L. (2017). A clinical study of interventional chemoembolization therapy combined with intravenous injection of arsenic trioxide in the treatment of primary hepatic carcinoma. Pract. J. Clin. Med. 14 (02), 69–72. doi:10.3969/j.issn.1672-6170.2017.02.023
Xie, W. C., Cheng, S. Q., Qu, H., Liu, X. S., and Liang, Z. W. (2022). The efficacy of 5-azacytidine combined with arsenic trioxide in the treatment of refractory anemia type MDS. Chin. J. Gerontology 42 (01), 74–77. doi:10.3969/j.issn.1005-9202.2022.01.021
Xu, H. L., Xu, G. W., Mao, Y. P., Dou, W. J., Wang, Z., and Duan, X. X. (2020). Effects of acute arsenic exposure on renal immune function in mice. J. Shenyang Med. Coll. 22 (05), 486–490. doi:10.16753/j.cnki.1008-2344.2020.05.025
Xu, H. L., Zhang, X. S., Tan, H. Y., Ding, J., Xie, J. H., and Zhang, Y. (2021). Effects of sodium tanshinoneⅡA sulfonate injection on H9c2 cardiomyocyte injury induced by sodium arsenite. J. China Med. Univ. 50 (04), 332–335.
Xu, M. (2018). Clinical study on patients with occupational chronic arsenide poisoning and peripheral neuropathy. Chin. J. Control Endemic Dis. 33 (01), 32+34.
Xu, Y., Wang, Y., Zheng, Q., Li, B., Li, X., Jin, Y., et al. (2008). Clinical manifestations and arsenic methylation after a rare subacute arsenic poisoning accident. Toxicol. Sci. official J. Soc. Toxicol. 103 (2), 278–284. doi:10.1093/toxsci/kfn041
Xu, Y. Y., Wang, Q., Fu, X. P., Song, Y., Mu, Y. C., and Meng, D. (2014). Research progress of the arsenic exposure in food. Chin. Agric. Sci. Bull. 30 (34), 187–192. doi:10.11924/j.issn.1000-6850.2014-1270
Yan, C., Wu, J., Liu, F. R., and Zhang, L. S. (2009). Model establishment of liver injury and fibrosis in rats exposed to oral arsenic solution. World Chin. J. Dig. 17 (09), 862–866. doi:10.11569/wcjd.v17.i9.862
Yan, T. H., Xu, W. D., Gu, W. L., and Yu, J. Y. (2013). Clinical observation of arsenic trioxide combined with transcatheter arterial chemoembolization treatment of advanced primary liver cancer. Chin. J. Clin. Oncol. Rehabilitation 20 (12), 1377–1380. doi:10.13455/j.cnki.cjcor.2013.12.04
Yang, Q. (2007). The toxic effects of arsenic and its research progress. J. Med. Pest Control (01), 66–67. doi:10.3969/j.issn.1003-6245.2007.01.044
Yang, X. H., Wang, W. W., Liu, Z. J., Zhang, R., Liang, T. Y., Peng, X. Q., et al. (2021a). Study on the effect of total flavnoids of fructus choerospondiatis on myocardial injury in rats induced by arsenic-trioxide. J. North Pharm. 18 (06), 8–12.
Yang, Y., Song, S., Liu, Y. L., and Dong, L. (2021b). The association of Sema 3A/4A with arsenic-induced cardiotoxicity in mice. Chin. Prev. Med. 22 (04), 257–263. doi:10.16506/j.1009-6639.2021.04.004
Yang, Z., Liu, J. Y., Huo, T. G., Sun, G. F., and Jiang, H. (2021c). Effect of arsenic in realgar on rat ability in novel objects recognition test. Chin. J. Exp. Traditional Med. Formulae 27 (09), 63–69. doi:10.13422/j.cnki.syfjx.20210991
Ye, Z. G., Wang, Z. M., Wang, Y. S., Wang, J. H., Liang, G. G., Du, G. Y., et al. (2005). Pharmacological characteristics and safety evaluation of cinnabar and realgar in Angong Niuhuang wan. J. Med. Res. (09), 35. doi:10.3969/j.issn.1673-548X.2005.09.017
Yedjou, C. G., Brown, E., Rogers, C., and Tchounwou, P. B. (2008). ASCORBIC ACID - MODULATION OF ARSENIC TRIOXIDE TOXICITY: IMPLICATION FOR THE CLINICAL TREATMENT OF ACUTE PROMYELOCYTIC LEUKEMIA. Metal ions Biol. Med. Proc. Int. Symposium Metal Ions Biol. Med. held = Les ions metalliques en Biol. en Med. Symposium Int. sur les ions metalliques 10, 413–418.
Yoon, J. S., Hwang, D. W., Kim, E. S., Kim, J. S., Kim, S., Chung, H. J., et al. (2016). Anti-tumoral effect of arsenic compound, sodium metaarsenite (KML001), in non-Hodgkin’s lymphoma: an in vitro and in vivo study. Investig. new drugs 34 (1), 1–14. doi:10.1007/s10637-015-0301-z
You, Q. C. (2000). Industrial environment and peripheral nervous injury. Chin. J. Tissue Eng. Res. (12), 1779–1781. doi:10.3321/j.issn:1673-8225.2000.12.007
Yu, H. S., Liao, W. T., and Chai, C. Y. (2006). Arsenic carcinogenesis in the skin. J. Biomed. Sci. 13 (5), 657–666. doi:10.1007/s11373-006-9092-8
Yuan, J. (2010). Research progress in the chemical composition and medicinal use of arsenic. Henan Chem. Ind. 27 (04), 88. doi:10.14173/j.cnki.hnhg.2010.04.046
Zappasodi, P., Rossi, M., Ambaglio, I., Bernasconi, P., Corso, A., Lazzarino, M., et al. (2011). Clinical efficacy of arsenic trioxide in a patient with acute promyelocytic leukemia with recurrent central nervous system involvement. Ann. Hematol. 90 (5), 595–597. doi:10.1007/s00277-010-1046-6
Zeng, Q., Yi, H., Huang, L., An, Q., and Wang, H. (2019). Long-term arsenite exposure induces testicular toxicity by redox imbalance, G2/M cell arrest and apoptosis in mice. Toxicology 411, 122–132. doi:10.1016/j.tox.2018.09.010
Zeng, Q., Zhai, C. T., Zhao, G. Y., Wu, N., Yang, L. Y., and Mi, L. H. (2022). Chronic arsenic exposure oxidative damage,cell apoptosis and inflammatory response in the liver of mice. J. Shanxi Univ. Chin. Med. 23 (05), 415–420. doi:10.19763/j.cnki.2096-7403.2022.05.05
Zhang, A. J. (2015). Research progress on the pathogenesis of endemic arsenic poisoning. all Health 9 (07), 278–279.
Zhang, C., Xiao, B. Y., Ma, L., Jiang, P., and Liu, K. T. (1996). The effect of fluoride-arsenic to development and rates of sperm malformation and micronucleus on rats. J. Xinjiang Med. Univ. (04), 257–260.
Zhang, F., Zhang, C. M., Li, S., Wang, K. K., Guo, B. B., Fu, Y., et al. (2018). Low dosage of arsenic trioxide inhibits vasculogenic mimicry in hepatoblastoma without cell apoptosis. Mol. Med. Rep. 17 (1), 1573–1582. doi:10.3892/mmr.2017.8046
Zhang, H. Q., and Yang, Y. J. (2018). Clinical study on arsenic trioxide combined with all-trans rtinoic acid in the treatment of acute promyelocytic leukemia. China Mod. Dr. 56 (25), 105–108.
Zhang, J., Ma, Y., Zheng, Y. J., Wu, J., Zhu, Y. L., and Hu, T. (2015). Effects of sodium arsenite exposure on learning and memory of rats. Mod. Prev. Med. 42 (01), 114–116+146.
Zhang, L., Gao, Y., Wu, S., Zhang, S., Smith, K. R., Yao, X., et al. (2020a). Global impact of atmospheric arsenic on health risk: 2005 to 2015. Proc. Natl. Acad. Sci. U. S. A. 117 (25), 13975–13982. doi:10.1073/pnas.2002580117
Zhang, M., and Yang, X. (2019a). Research progress on mechanism of inflammatory signaling pathway-driven cancer induced by arsenide. J. Environ. Occup. Med. 36 (11), 1071–1078. doi:10.13213/j.cnki.jeom.2019.19142
Zhang, P. (2017). On arsenic trioxide in the clinical treatment of acute promyelocytic leukemia. Leukemia Res. Rep. 7, 29–32. doi:10.1016/j.lrr.2017.03.001
Zhang, R. Y., Chen, C. Z., Cheng, S. Q., and Xia, Y. Y. (2019b). Effect and mechanism of cyclooxygenase-2 on sodium arsenic-induced microglia activation in mice. Chin. J. Public Health 35 (07), 847–850. doi:10.11847/zgggws1124161
Zhang, W., Qi, L. J., Ning, J. Y., Gao, S., and Li, G. J. (2021a). Health hazard assessment of arsenic. J. Toxicol. 35 (05), 367–372+378. doi:10.16421/j.cnki.1002-3127.2021.05.002
Zhang, W. J., Zhang, Z. Y., Lou, S. T., Li, D. H., and Wang, L. X. (2021b). Arsenic trioxide inhibits the proliferation,invasion and migration of triple-negative breast cancer cells through promyelocytic leukemia gene. J. Basic Clin. Oncol. 34 (06), 461–466.
Zhang, X. H., Zhou, H. R., Song, S. J., Qiao, Z. H., Yang, L. H., and Hu, Y. (2001). The impact of arsenic trioxide or all-trans retinoic acid treatment on coagulopathy in acute promyelocytic leukemia. Chin. J. Intern. Med. (12), 40–44.
Zhang, Y., Chen, L., Xie, C., Yang, X. X., Luo, J., and Luo, X. R. (2020b). Effects of ganoderma lucidum triterpenes on hippocampal histomorphology and Ach,ChAT content in arsenism rats. J. Guizhou Med. Univ. 45 (07), 781–785. doi:10.19367/j.cnki.2096-8388.2020.07.006
Zhang, Y., Chen, L., Xie, C., Yang, X. X., Luo, J., and Luo, X. R. (2020c). Effects of ganoderma lucidum triterpenoids on oxidative stress injury in brain tissues of rats with arsenism. J. Guizhou Med. Univ. 45 (03), 277–280. doi:10.19367/j.cnki.1000-2707.2020.03.006
Zhang, Y., Liang, H., Sheng, S. Q., Li, R. Q., Wang, X. L., Wang, Z. Q., et al. (2003). EFFECT OF ARSENIC (As2O3) OF PHARMACOLOGICAL CONCENTRATION ON THE MORPHOLOGICAL STRUCTURE CHANGES OF TESTIS AND SPERM PRODUCTION IN RATS. J. Clin. Med. Pract. (01), 53–56. doi:10.3969/j.issn.1672-2353.2003.01.016
Zhao, C. W. (2016). Analysis of clinical application effect of arsenic trioxide in the treatment of liver cancer patients. World Latest Med. Inf. 16 (71), 145–146. doi:10.3969/j.issn.1671-3141.2016.71.109
Zhao, J. W., Hua, C. F., Shang, P. P., Xie, F. W., and Li, X. (2021a). Induction of oxidative stress by arsenious acid As(Ⅲ) in A549 cells. Carcinogenesis,Teratogenesis Mutagen. 33 (01), 28–31. doi:10.3969/j.issn.1004-616x.2021.01.006
Zhao, M., Shi, W. Y., Zhu, H., Li, L., Ma, K. T., and Si, J. Q. (2010). Researach progress on effects of arsenic poisoning and substance P in nerve injury. Prog. Mod. Biomed. 10 (08), 1598–1600. doi:10.13241/j.cnki.pmb.2010.08.033
Zhao, S., Dong, D. D., Duan, X. X., Liu, D., and Li, B. (2013). Effects of acute arsenic exposure on Nrf2 pathway in mice cerebral cortex. J. Environ. Health 30 (10), 849–852. doi:10.16241/j.cnki.1001-5914.2013.10.016
Zhao, W. H., and Hu, L. H. (2009). Clinical research on the curative effect of combination chemotherapy of all-trans retinoic acid and arsenic trioxide on acute promyelocytic leukemia. J. Inn. Mong. Minzu Univ. Sci. 24 (01), 85–87. doi:10.3969/j.issn.1671-0185.2009.01.025
Zhao, X., Shi, Y. Q., Yan, C. C., Feng, P. F., Wang, X., Zhang, R., et al. (2015a). Up-regulation of miR-21 and miR-23a Contributes to As2 O3 -induced hERG Channel Deficiency. Basic & Clin. Pharmacol. Toxicol. 116 (6), 516–523. doi:10.1111/bcpt.12348
Zhao, Y. Y., Yu, L., Liu, B. L., He, X. J., and Zhang, B. Y. (2015b). Downregulation of P-gp, Ras and p-ERK1/2 contributes to the arsenic trioxide-induced reduction in drug resistance towards doxorubicin in gastric cancer cell lines. Mol. Med. Rep. 12 (5), 7335–7343. doi:10.3892/mmr.2015.4367
Zhao, Z. F., Wang, H. F., Zhang, J. P., Han, X., and Chu, L. (2021b). Protective effect of crocetin on myocardial injury induced by arsenic trioxide in rats. Chin. J. New Drugs Clin. Remedies 40 (01), 58–63. doi:10.14109/j.cnki.xyylc.2021.01.12
Zhao, Z. Y., Wang, Z. R., Fang, X. Y., Wang, t., Luo, Z. X., and Xie, T. T. (2022). The mechanism of injury indued by sodium arsenite on mouse hepatocytes AML12. J. Guizhou Med. Univ. 47 (01), 1–6. doi:10.19367/j.cnki.2096-8388.2022.01.001
Zheng, F. Y. (2018). Clinical efficacy of dual induction chemotherapy with retinoic acid and arsenite in the treatment of acute promyelocytic leukemia. Strait Pharm. J. 30 (07), 150–151.
Zheng, N., Xue, L. Y., Wang, X. N., Liu, Y., and Li, J. (2021). A case of severe rash caused by arsenic trioxide and literature review. J. Med. Theory Pract. 34 (22), 3968–3965. doi:10.19381/j.issn.1001-7585.2021.22.049
Zheng, P. Z., Zhao, C. J., and Fan, H. Y. (2008). Combined effects of retinoic acid and arsenic trioxide in acute promyelocytic leukemia. Fujian Med. J. (05), 68–69. doi:10.3969/j.issn.1002-2600.2008.05.043
Zheng, Y. Y., Xie, J., Gan, Y. J., Liang, D. Y., and Li, X. J. (2020). Micronucleus test of arsenic exposure in vitro and in vivo. Hainan Med. J. 31 (08), 953–955.
Zhong, Z. Z., Liao, X. M., Xiao, X. L., and Xiao, S. H. (2018). Exploration of the relationship between clinical manifestations and urinary arsenic levels in patients with subacute arsenic poisoning. Chin. J. Control Endemic Dis. 33 (03), 287+289.
Zhou, G. B., Zhang, J., Wang, Z. Y., Chen, S. J., and Chen, Z. (2007a). Treatment of acute promyelocytic leukaemia with all-trans retinoic acid and arsenic trioxide: a paradigm of synergistic molecular targeting therapy. Philosophical Trans. R. Soc. Lond. Ser. B, Biol. Sci. 362 (1482), 959–971. doi:10.1098/rstb.2007.2026
Zhou, H. F., Yang, M., Wang, Y., Tan, L. J., and Zhang, J. L. (2018). Effect of sub-acute arsenic exposure on cerebral oxidative stress and Fos/Jun expression in rats. Chin. J. Public Health 34 (11), 1523–1526. doi:10.11847/zgggws1117062
Zhou, Q., and Xi, S. (2018). A review on arsenic carcinogenesis: epidemiology, metabolism, genotoxicity and epigenetic changes. Regul. Toxicol. Pharmacol. RTP 99, 78–88. doi:10.1016/j.yrtph.2018.09.010
Zhou, Q. B., Zhu, Q. Z., Wang, H. Z., Wang, D. X., Liu, Z. T., Xu, Y. G., et al. (2020). Traditional Chinese medicine containing arsenic treated MDS patients effectively through regulating aberrant hypomethylation. Evidence-based complementary Altern. Med. eCAM 2020, 7469809. doi:10.1155/2020/7469809
Zhou, Z. T., Lin, X. J., Cui, B. K., and Li, G. H. (2007b). Clinical study of arsenic trioxide-containing regimen in the treatment of middle or advanced hepatocellular carcinoma by TACE. Chin. J. Cancer Prev. Treat. (14), 1094–1103. doi:10.16073/j.cnki.cjcpt.2007.14.015
Zhu, A. L., Liu, L. X., Piao, D. X., and Jiang, H. C. (2003). Continuous regional chemotherapy using arsenic trioxide for primary hepatic carcinoma. Chin. J. Hepatobiliary Surg. (04), 16–17. doi:10.3760/cma.j.issn.1007-8118.2003.04.005
Zhu, C. X. (2020). Investigate the effect of arsenic trioxide combined with thalidomide in the treatment of myelodysplastic syndrome. China & Foreign Med. Treat. 39 (13), 99–101. doi:10.16662/j.cnki.1674-0742.2020.13.099
Zhu, H. H., Hu, J., and Gu, X. F. (2016). Arsenic as traditional Chinese medicine provides new hope for overcoming high treatment costs of acute promyelocytic leukemia. J. Glob. Oncol. 2 (6), 442–443. doi:10.1200/JGO.2016.005405
Zhu, H. H., Hu, J., Lo-Coco, F., and Jin, J. (2019). The simpler, the better: oral arsenic for acute promyelocytic leukemia. Blood 134 (7), 597–605. doi:10.1182/blood.2019000760
Zhu, H. H., and Huang, X. J. (2014). Oral arsenic and retinoic acid for non-high-risk acute promyelocytic leukemia. N. Engl. J. Med. 371 (23), 2239–2241. doi:10.1056/NEJMc1412035
Zhu, H. M., and Li, J. M. (2012). Progress on the treatment of acute promyelocytic leukemia with arsenic trioxide. Pharm. Clin. Res. 20 (04), 277–284. doi:10.13664/j.cnki.pcr.2012.04.013
Zhu, Q., Deng, Z., Zhu, S., Zhao, P., Wang, M., and Hu, X. (2017). Study on the clinical safe and effective methods of arsenic-containing compound-qinghuang powder in the treatment of myelodysplastic syndrome. Evidence-based complementary Altern. Med. eCAM 2017, 2095682. doi:10.1155/2017/2095682
Keywords: arsenic, arsenic-containing medicinal materials, arsenic-containing preparations, clinical application, pharmacology, toxicity
Citation: Yang Y, Li Y, Li R and Wang Z (2024) Research progress on arsenic, arsenic-containing medicinal materials, and arsenic-containing preparations: clinical application, pharmacological effects, and toxicity. Front. Pharmacol. 15:1338725. doi: 10.3389/fphar.2024.1338725
Received: 15 November 2023; Accepted: 06 February 2024;
Published: 01 March 2024.
Edited by:
Jianqiang Xu, Dalian University of Technology, ChinaReviewed by:
Vineeta Dixit, Nilamber Pitamber University, IndiaSze Kwan Lam, The University of Hong Kong, Hong Kong SAR, China
Copyright © 2024 Yang, Li, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhang Wang, d2FuZ3poYW5nY3FjZEBjZHV0Y20uZWR1LmNu
 Yichu Yang1,2
Yichu Yang1,2 Zhang Wang
Zhang Wang