
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 02 February 2024
Sec. Pharmacology of Anti-Cancer Drugs
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1337910
This article is part of the Research TopicInnovative Approaches to Overcome Resistance and Toxicities of Anti-Cancer DrugsView all 21 articles
 Mohamed A. A. Orabi1*
Mohamed A. A. Orabi1* Mohamed E. Abouelela2*
Mohamed E. Abouelela2* Faten M. M. Darwish3
Faten M. M. Darwish3 Mohamed S. A. Abdelkader4
Mohamed S. A. Abdelkader4 Bakheet E. M. Elsadek5
Bakheet E. M. Elsadek5 Ahmed Abdullah Al Awadh6
Ahmed Abdullah Al Awadh6 Mohammed Merae Alshahrani6
Mohammed Merae Alshahrani6 Abdulaziz Hassan Alhasaniah6
Abdulaziz Hassan Alhasaniah6 Nayef Aldabaan7
Nayef Aldabaan7 Reda A. Abdelhamid8
Reda A. Abdelhamid8Hepatocellular carcinoma (HCC) is a prevalent cancer worldwide. Late-stage detection, ineffective treatments, and tumor recurrence contribute to the low survival rate of the HCC. Conventional chemotherapeutic drugs, like doxorubicin (DOX), are associated with severe side effects, limited effectiveness, and tumor resistance. To improve therapeutic outcomes and minimize these drawbacks, combination therapy with natural drugs is being researched. Herein, we assessed the antitumor efficacy of Ceiba pentandra ethyl acetate extract alone and in combination with DOX against diethylnitrosamine (DENA)-induced HCC in rats. Our in vivo study significantly revealed improvement in the liver-function biochemical markers (ALT, AST, GGT, and ALP), the tumor marker (AFP-L3), and the histopathological features of the treated groups. A UHPLC-Q-TOF-MS/MS analysis of the Ceiba pentandra ethyl acetate extract enabled the identification of fifty phytomolecules. Among these are the dietary flavonoids known to have anticancer, anti-inflammatory, and antioxidant qualities: protocatechuic acid, procyanidin B2, epicatechin, rutin, quercitrin, quercetin, kaempferol, naringenin, and apigenin. Our findings highlight C. pentandra as an affordable source of phytochemicals with possible chemosensitizing effects, which could be an intriguing candidate for the development of liver cancer therapy, particularly in combination with chemotherapeutic drugs.
Hepatocellular carcinoma (HCC) is a multistage, widely occurring disease; in Egypt, it is the sixth most common in women and the second most common in men (Rashed et al., 2020). The prevalence of risk factors is linked to environmental, dietary lifestyle factors as aflatoxins, alcohol (Omar et al., 2013) and pathologic factors like diabetes and viral hepatitis (McGlynn and London, 2005). Numerous studies indicate that these risk factors may work in concert or separately to affect the chance of developing liver cancer (McGlynn and London, 2005; Rashed et al., 2020).
Herbal medicines are being used more and more as anticancer therapies since they are readily available and have fewer adverse effects than conventional drugs. Several natural compounds, including resveratrol, curcumin, quercetin, silibinin, silymarin, n-trans-feruloyl octopamine, lycopene, gallic acid, berberine emodin, and phloretin, have shown promise in treating HCC and other hepatic toxicities (Rawat et al., 2018; Mandlik and Mandlik, 2021).
The natural products studied for their impact on HCC appear to have a variety of mechanisms to attain their effects, including promoting apoptosis and cell cycle arrest, exhibiting antioxidant and anti-inflammatory properties, boosting the immunity, suppressing angiogenesis, preventing cancer cell invasion, and detoxifying liver carcinogens (Mandlik and Mandlik, 2021).
Ceiba pentandra (L.) Gaertn. (Bombacaceae) trees are planted for horticultural purposes in many countries. Its aerial parts, leaves, stems, and stem-barks showed anti-inflammatory, hepatoprotective, antioxidant, hypoglycemic, hypolipidemic, bactericidal, and anticancer properties in pharmacological studies (Lim, 2012; Refaat et al., 2014; Abouelela et al., 2019). In terms of phytochemistry, it is distinguished by the presence of anthocyanins, steroids, triterpenes, sesquiterpenes, and other polyphenols, some of which are featured in our earlier work (Abouelela et al., 2018; Abouelela et al., 2020).
Among Ceiba species, C. pentandra is the most studied for its cytotoxic and antitumor effects. The bark extracts increased the mean survival time of tumor-bearing mice in Ehrlich ascites carcinoma (EAC, liquid tumor) and Dalton’s ascites lymphoma (solid tumor) models (Kumar et al., 2016). It also showed a potent cytotoxic effect on mouse melanoma (B16-F10) and EAC cell lines (Kumar et al., 2016). In a study on angiogenesis, the leaf extract drastically reduced the number of tubes that human umbilical vein endothelial cells could generate (Nam et al., 2003). The formation of reactive oxygen species (ROS) associated the oxidative stress has been shown to enhance tumor growth. This supports the importance of antioxidants to safeguard cells from oxidative stress and prevent DNA damage, while their anticancer properties can inhibit the growth and proliferation of cancer cells (Kola et al., 2022). This was demonstrated in our previous study, where C. pentandra ethyl acetate (EtOAc) extract significantly reduced methotrexate-induced kidney damage in rats, which was attributed to the involvement of the association of antioxidant and anti-inflammatory, and antiapoptotic mechanisms (Abouelela et al., 2020).
One of the most widely used approaches in the treatment of unresectable HCC in recent decades has been the use of standard chemotherapeutic drugs like doxorubicin (DOX) (Lahoti et al., 2012). Nevertheless, DOX’s low efficacy—likely a consequence of drug resistance—as well as its undisputed involvement in the onset of numerous dose-related side effects that ultimately lead to inadequate antitumor outcomes—have limited its clinical applicability (Patel et al., 2010). As a result, increasing both the therapeutic effectiveness and the DOX index is regarded as an unmet medical need.
Significant evidence suggests that co-therapy with drugs from natural source is an accepted attempt in cancer therapy, as it can attain improved therapeutic effects than an individual drug or modality while also reducing side effects and drug resistance (Capone et al., 2014). Therefore, research into the use of chemosensitizing phytochemicals in conjunction with approved chemotherapeutic drugs is being conducted as a substitute and superior method of managing and treating cancer (Wagner, 2011).
The current study aimed to explore the likely use of C. pentandra EtOAc faction as a therapeutic agent and/or a co-therapy with DOX for HCC based on an in vivo assessment of the extract’s efficacy in inhibiting tumor growth in chemically induced HCC in a rat model. Consequently, we have analyzed the EtOAc extract by an ultra-high-performance liquid chromatography-electrospray ionization-quadrupole-time of flight-mass spectrometer (UHPLC/Q-TOF/MS/MS) to explore the compounds accountable for the antitumor activity. Molecular ions and their fragment ions (MS/MS data) were detected during the LC/MS profiling of secondary metabolites. These were then located in databases and compared with existing literature to ensure accurate identification (Dunn et al., 2013).
Aerial parts (2 kg), involving leaves and tender young branches, of C. pentandra were gathered from the garden of the Faculty of Agriculture, University of Assiut, Egypt. The air-dried plant material was extracted by soaking in MeOH/H2O (8:2, v/v, 5 L × 3) at normal temperature. The aqueous methanolic extract was dried at ≤ 45°C under vacuum until reached a constant weight (250 g). The obtained extract was partitioned between water/methylene chloride (MC), EtOAc, and n-butanol, successively, and afford the corresponding MC (52 g), EtOAc (28 g), n-butanol (19 g), and water fractions (14a g), respectively (Abouelela et al., 2018).
The experiment was conducted using healthy male Wistar rats (14–15 weeks old, weighing 140–150 g) obtained from Assiut University, Egypt. The rats were housed in controlled conditions with standard diet and water access. The rats were randomly divided into a control group (n = 8) receiving carboxymethyl cellulose (CMC) and an experimental group (n = 32) administered diethylnitrosamine (100 mg/L) in their water ad libitum for 8 weeks, the latter was further subdivided one-month post-diethylnitrosamine (DENA). The subgroups included a DENA control group (n = 8), a DENA+DOX group (n = 8) receiving weekly intravenous doxorubicin (4 × 2.5 mg/kg, I.V.), a DENA+Extract group (n = 8) with daily oral plant extract (400 mg/kg) gavages, and a DENA+DOX+Extract group (n = 8) receiving daily oral treatments of both DOX+Extract concurrently for 4 weeks (Atwa et al., 2021).
A week afterward the last treatment, all animals were submitted to cervical decapitation under isoflurane anaesthesia. For preparing the serum, blood samples were taken from all rats via the retro-orbital vein plexus. After autopsy, the rat livers were excised, cleansed of adhering connective tissues and fat, then cleaned in ice-cold isotonic saline, and finally classified into three divisions. The first was stored in 10% neutral buffered formalin solution and examined histopathologically, while the other two portions were immediately flash-frozen in liquid N2 and kept separately at −70°C for following biochemical assays (Di Stefano et al., 2008; Mansour et al., 2019).
Non-enzymatic liver functions, such as albumin and total bilirubin, were estimated using colorimetric assay kits according to the manufacturer’s instructions. Serum ALT, AST, GGT, ALP levels were estimated using the procedures and kits outlined by the manufacturers (Elitech diagnostic Co., France). Moreover, serum AFP-L3, a specific tumour marker for HCC, was estimated using a rat-specific ELISA assay kit following the manufacturer’s protocol (Elabscience Biotechnology Co., Ltd. Wuhan, China) (Fathy et al., 2017).
For histopathological inspection, liver tissues were embedded in paraffin, fixed in 10% formalin, and consistently stained with haematoxylin and eosin (H&E) stain. After cleansing in a sterile tap water, the tissues were dehydrated using serial dilutions of methanol, ethanol, and absolute ethanol. In a hot air oven, samples were cleaned in xylene and embedded in paraffin for 24 h at 56°C. Paraffin beeswax tissue blocks were used for making tissue slices at four microns using a sled microtome. A glass slide was used to hold tissue samples, which were then deparaffinized and stained with H&E stain for examination under a light microscope (Culling, 2013).
Utilizing the version 5 of the GraphPad Prism (Graph Pad Software, San Diego, United States), data statistical analysis was accomplished. Analysis of variance (ANOVA) and Tukey’s t-test were considered to compare the results. p < 0.05 was the accepted level of significance, and the data were graphically represented as mean SD.
Methanol (CH3OH) and acetonitrile (CH3CN) of HPLC quality (Merck, Germany), formic acid (Fisher, United States). Culture plastic dishes and plates (96-well) were bought from Becton Dickinson (Franklin Lakes, NJ, United States).
UHPLC ExionLC™ AC system, involving dual high-pressure gradient pump, vacuum degasser, autosampler, column oven (SCIEX, United States), coupled with AB SCEIX Triple-TOF ™ 5600+ mass spectrometer (AB SCIEX, United States, with ESI source), and KQ-300DB-type CNC ultrasonic instrument (Kunshan Ultrasonic Instrument Co., Ltd.). BSA224S-CW-type electronic balance (Sedolis Scientific Instrument Company). LG16-W-type high-speed centrifuge (Beijing Jingli Centrifuge Co., Ltd.).
A sample of the EtOAc fraction (2 mg) was dissolved in 5 mL of dissolution system [deionized H2O/CH3OH/CH3CN (5:2.5:2.5, v/v)] to obtain 0.4 μg/μL working solution. The solution was vortexed for 2 min followed by ultrasonication for 10 min and centrifuge for 5 min at 1792 g. An aliquot (10 μL) was injected for an LC analysis system along with a 10 μL dissolution system as a control.
The UHPLC-Q-TOF-MS/MS analysis was conducted according to chromatographic conditions by Abdelhafez, et al. (2018). Briefly, 10 μL of the sample was injected into the UHPLC ExionLC™ AC system, with pre-column in-line filter disk (0.5 µm × 3.0 mm) (Phenomenex, United States), Xbridge C18 column (2.1 mm × 50 mm, 3.5 µm) (Waters, United States), at 40°C. The mobile phase is composed of deionized H2O (A) and CH3CN (B) each containing 0.1% formic acid. The gradient elution started with 10% B, which linearly increased to 90% B within 20 min and remained isocratic for 5 min before linearly decreasing back to 10% B for the following 3 min. The mobile phase was then equilibrated for 10 min between injections. The flow rate was set at 0.3 mL/min.
The ESI-Q-TOF-MS in positive ion mode was used in the following conditions: The ESI ion source voltage was 5–500 V, the ion source temperature was 550°C, the cracking voltage was 80 V, and the collision energy was 35 eV, respectively. The collision energy spread was ±15 eV, the atomizing gas was nitrogen, the nebulizer gas was 45 kPa, the heater gas was 45 kPa, and the curtain gas was 25 kPa. The primary mass spectrometer has a scan range of mass-to-charge ratio (m/z) 50 to 1,000 for ESIMS. Information dependent acquisition sets the peaks with a response value exceeding 200 cps for secondary mass spectrometry. The sub-ion scan range was m/z of 50–1,000, and dynamic background subtraction was turned on and ions tolerance was 10 ppm with the exclusion of former target ions with 3 repeats and 3 s and exclusion of isotopes within 2.0 Da (Abdelhafez et al., 2018).
The data acquisition software used for processing raw data is Analyst TF 1.7.1 software (AB SCEIX, United States). Data processing software systems: Peakview Ver. 2.2, and Markerview Ver. 1.3 (AB SCEIX, United States) was used for peaks extraction from total ion chromatogram with a signal-to-noise ratio >3 (non-targeted analysis). They were also used for peak filtering based on comparing the peak intensity of plant sample to blank (intensity ratio >3), and for removing isotopic peaks. Accurate mass and composition for the precursor and fragment ions were analyzed using Peakview software integrated with the instrument. The first and second-order spectra were compared with accurate mass and fragmentation patterns of metabolites in online databases: mass bank; a dictionary of natural products; metlin databases; mzCloud; human metabolome database (HMDB); PubChem; competitive fragmentation modeling for components identification (CFM-ID version 2.0), combined with the literature on previously isolated compounds from the family, to determine the structures of the detected compounds.
Despite the advances made recently in HCC treatment, there has been limited success in improving the survival rates of people with HCC (Ganesan and Kulik, 2023). This is partly due to the lack of effective treatment options, the recurrence of tumors and the tendency to identify the disease at an advanced stage (Han et al., 2023). Therefore, the object of the current study was to evaluate the in vivo anticancer efficacy of C. pentandra extract, either alone or in combination with the conventional DOX, against diethylnitrosamine (DENA)-induced HCC in rats.
Our results revealed that animals in DENA group exhibited obvious impaired liver functions including substantial hypoalbuminemia (p < 0.01), together with elevated serum activities of alanine transaminase (ALT) (p < 0.001), aspartate aminotransferase (AST) (p < 0.001), gamma-glutamyl transferase (GGT) (p < 0.001) and alkaline phosphatase (ALP) (p < 0.001), and total serum bilirubin level (p < 0.001), in comparison to the healthy control group—see Table 1. DENA-induced changes in serum indicators of liver function may be due to peroxidation of hepatocyte membranes, resulting in increased release of AST, GGT, ALT, ALP, and total bilirubin from compromised liver tissues as has been previously demonstrated in various models of DENA-induced hepatocellular deterioration (Bansal et al., 2005; El-Hawary et al., 2015).
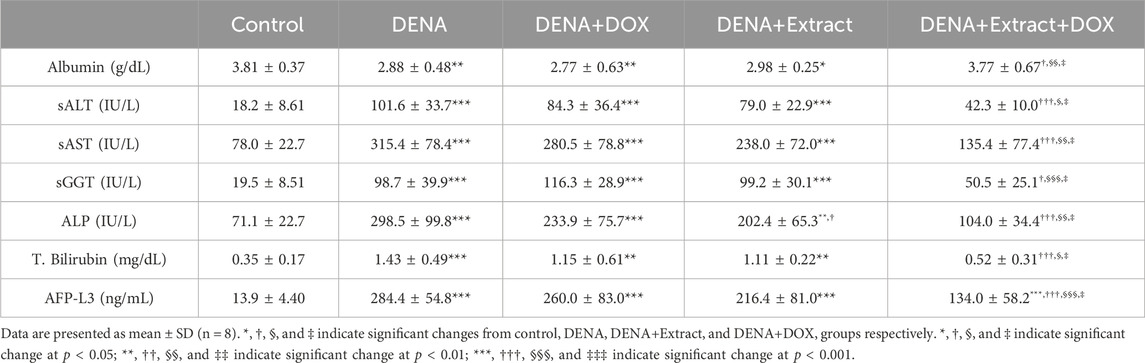
TABLE 1. Effect of tested C. pentandra extract on liver function tests and tumor markers of rat models.
The present research noted that although the administration of either C. pentandra extract or DOX is moderately effective in restoration of these impaired indicators to their respective normal ranges, the administration of C. pentandra alone has a comparatively stronger effect than the standard chemotherapeutic doxorubicin against chemically induced HCC in a rat model. Meanwhile, a positive outcome emerged in the group that received a combined treatment of C. pentandra extract and DOX (DENA+Extract+DOX), leading to a noticeable enhancement in the overall estimated liver function indices as illustrated in Table 1. The animals treated with the combined regimen (DENA+Extract+DOX) displayed a significant rise in serum albumin levels (p < 0.05). Additionally, they exhibited noteworthy reductions in serum ALT (p < 0.001), AST (p < 0.001), GGT (p < 0.05), ALP (p < 0.001), and total bilirubin levels (p < 0.001). Remarkably, the combination therapy outperformed individual treatments in the (DENA+Extract) and (DENA+DOX) groups. This superiority was evident through a significant surge in serum albumin levels (p < 0.05 and p < 0.01, respectively), coupled with considerable reduction in serum ALT (p < 0.05 and p < 0.05, respectively), AST (p < 0.05 and p < 0.01, respectively), GGT (p < 0.05 and p < 0.001, respectively), ALP (p < 0.05 and p < 0.01, respectively), and total bilirubin levels (p < 0.05 and p < 0.05, respectively).
The results of the assessments of the isoform L-3 of alpha-fetoprotein (AFP-L3), an HCC tumour marker, provided additional evidence that supports the superiority of C. pentandra extract and DOX combination over both C. pentandra extract and DOX individual administration. Several investigators have recently used this marker for the timely recognition and observing of treatment response, as well the HCC relapse, and/or malignance invasion, as well as surrogate markers of the liver clinicopathological changeability with suitable sensitivity and specificity (Lagana et al., 2013; Jain, 2014; Wang et al., 2014).
According to our findings, the serum AFP-L3 level in the DENA group was significantly higher than in the normal healthy control group (p < 0.001) (Table 1). Although each of C. pentandra extract and DOX alone failed to significantly reduce the DENA-induced elevation in AFP-L3 levels, their co-administration efficiently reduced the elevated AFP-L3 levels, approximately 52.8% (p < 0.001), 38.0% (p < 0.05), and 48.4% (p < 0.001) decreases in comparison to the DENA, DENA+Extract, and DENA+DOX groups, respectively.
The liver tissues histological investigation (Figures 1A–E) offered strong proof for the combined C. pentandra extract and DOX’s valuable effects in fighting DENA-induced HCC. The representative photomicrographs of liver sections obtained from animals in the DENA group revealed HCC in which carcinoma cells showed large vesicular nuclei with more than one nucleoli, mitotic figure, and intracytoplasmic eosinophilic hyaline bodies (Figure 1B). Analogous histological findings have been previously documented in published DENA-induced rat models (Afzal et al., 2012; Golla et al., 2013).
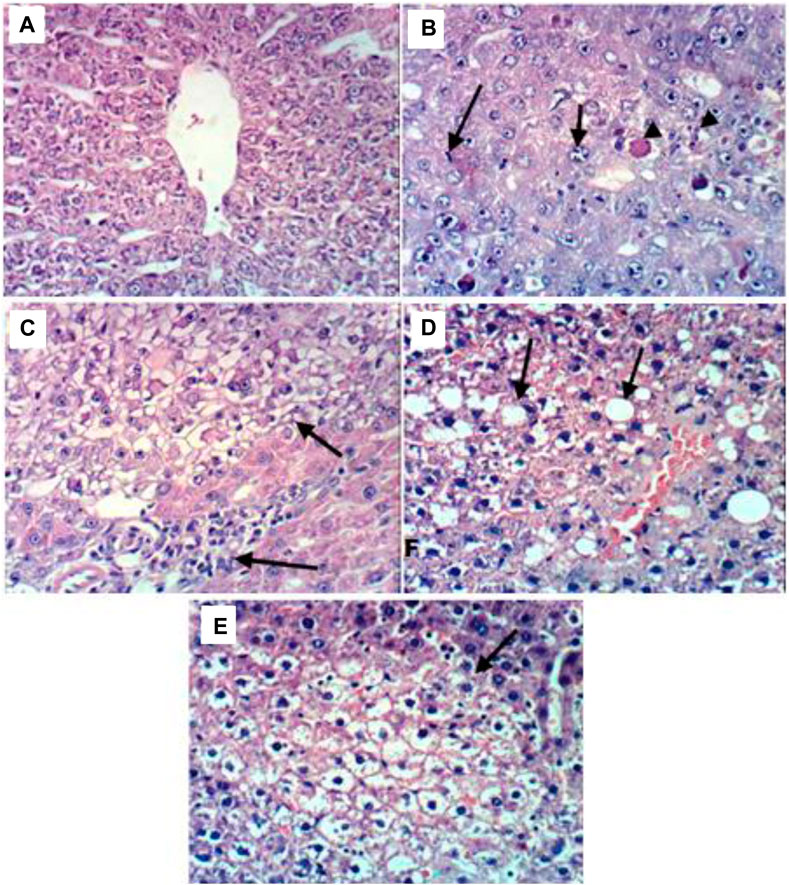
FIGURE 1. Representative photomicrographs of liver sections from different groups. (A) Liver tissues of the control group showing the normal histological structure of hepatic lobule; (B) Liver tissues of the DENA group revealing HCC in which carcinoma cells showed large vesicular nuclei with more than one nucleoli, mitotic figure, and intracytoplasmic eosinophilic hyaline bodies; (C) Liver tissues of the DENA+extract group showing relative improvement in the histopathological picture as the examined sections revealed only small focal clear hepatocytes; (D) Liver tissues of the DENA+DOX group showing severe histopathological alterations confined as HCC with destruction in the normal trabecular structure of the liver and proliferation of oval cells with mitotic figures; (E) Liver tissues of the DENA+extract+DOX group showing marked improvement in the histopathological picture with steatosis and vacuolation of hepatocytes, dilatation and congestion of hepatic sinusoids.
Meanwhile, the rat’s liver administered the combination of C. pentandra extract and DOX, on the other hand, showed relative enhancement in the histopathological features. The investigated sectors showed steatosis and hepatocytes vacuolation, dilatation, and hepatic sinusoidal congestion (Figure 1E). These findings definitely imply the tumor-defeating ability of the mix involving both C. pentandra extract and DOX against DENA-induced HCC. Meantime, livers of rats treated with DOX alone showed severe histopathological changes confined as HCC with normal trabecular destruction of the liver structure and propagation of oval cells with mitotic figures (Figure 1D). However, the livers of the rats administered C. pentandra EtOAc extract alone showed relative improvement in the histopathological features as the examined sectors showed only small focal clear hepatocytes (Figure 1C).
Worthwhile, throughout the experiment, healthy control group rats stayed vital, and conscious, and achieved a significant increase of the body weight as compared to the DENA-drinking animals, which appeared sluggish with an insensible increase in the body weight. As shown in Figure 2, all DENA-drinking rats suffered weight loss during the exposure before treatment, which is probably due to the initial tumor burden or the tumor metastasis. Similar findings were previously seen in the same animal model (Bansal et al., 2005; Elsadek et al., 2017). Groups treated with extracts or therapy displayed stable weight trends. The DENA+DOX and DENA+Extract+DOX groups experienced initial sharp weight declines, followed by gradual recovery, while the DENA drinking group had a slight dip. This result represents respectable evidence of the superiority of the ethyl acetate extract of C. pentandra over the conventional DOX regarding the systemic toxicity and tolerability. Overall, this data visually illustrates the impact of different treatments on animal body weight, aiding researchers in concluding their effects.
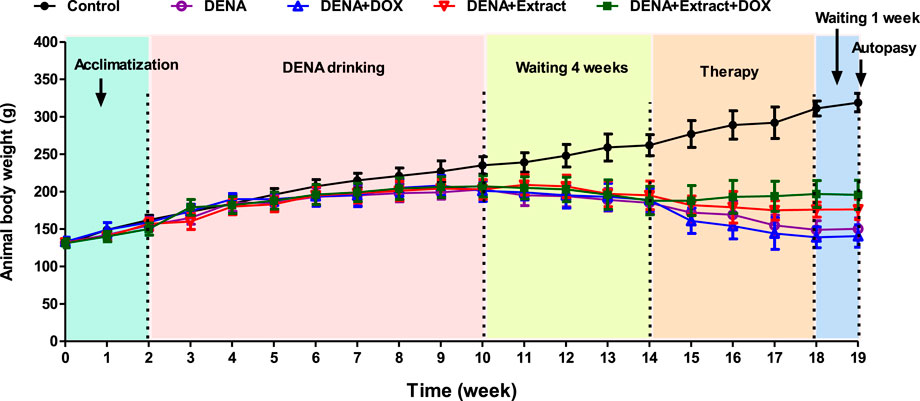
FIGURE 2. Illustration of the changes in the animal’s body weights in different groups during the experiment.
In consistence with our findings, C. pentandra bark extract has shown a protective effect on CCl4-induced liver damage and oxidative stress (Ellappan et al., 2022), and a similar protective effect against paracetamol-induced hepatotoxicity in rats in previous studies (Bairwa et al., 2010). However, our investigation today is more comprehensive.
To explore the phytochemical constituents corresponding to this promising antitumor activity, a UHPLC-Q-TOF-MS/MS analysis of the C. pentandra EtOAc extract was conducted.
Following optimizing the chromatographic and mass spectroscopic conditions, a UHPLC-Q-TOF-MS/MS (positive ion mode) investigation was conducted. The total ion current (TIC) map of the compounds of the EtOAc extract was produced (Figure 3). The marker view 1.3 software’s non-targeted screening revealed that several different chemicals are represented by each chromatographic peak. Based on Q-TOF-HRESIMS measured ions mass (at < 1.0 × 10−5 deviation) combined with the isotope abundance ratio, the molecular formula for each common peak of the compounds was accurately determined. The peak was accurately located by molecular ion (m/z) value search regardless of the retention time drifts due to chromatographic parameters or instrument equipment changes, which ensures good reproducibility of the metabolite’s fingerprint.
Structural analysis of the components was attained by two-stage mass spectrometry to obtain the accurate element composition and characteristic fragment ions for each compound. The resulting data were then matched with the calculated and reported high-resolution mass of known compounds, and fragmentation patterns for the given classes of compounds, in connection with the structural information given by the online databases and the secondary mass spectrum (MS2) analysis of the previously isolated compounds available in literature (Refaat et al., 2013).
Fifty components (Table 2; Figure 4) were identified by analysis of the mass results. The retention time, the high-resolution mass of the molecular ion, chemical formula, and MS2 fragments of the identified chemical constituent are shown in Table 2. The compounds were arranged according to their retention time. The identified phytoconstituents are four amino acids (1, 2, 4, and 5), nine amino acids derivatives (14, 16, 23, 24, 28, 35, 38, 46, and 47), fourteen flavonoids (11, 12, 19, 21, 22, 27, 29, 30, 32, 36, 42, 43, 44, and 45), eight flavanolignans (17, 18, 20, 26, 31, 34, 40, and 41), two procyanidins (7 and 13), five coumarins (8, 10, 33, 37, and 39), two phenolic acids (6 and 9), and six miscellaneous compounds (3, 15, 25, and 48–50). The coumarin aesculetin and the flavonoids apigenin, astragalin, and rutin are reported in the plant for the first time.
The occurrence and progression of liver cancer are well-known to be multi-gene, and multi-stage processes (Li et al., 2023a). Herbal extracts or natural substances extracted from them have been used to treat liver cancer patients in recent years. For example, curcumin’s several pharmacologic properties against HCC make it a useful treatment for the disease. In preclinical models of liver disease, silymarin, a flavonolignan complex found in milk thistle seeds, has been demonstrated to have hepatoprotective properties.
In our preceding phytochemical isolation from C. pentandra has evidenced the occurrence of large amount of flavonolignans in addition to flavonoids, phenylpropanoids (Abouelela et al., 2020). Pharmacological research of extracts from various morphological parts of C. pentandra have also demonstrated anti-inflammatory, hepatoprotective, antioxidant, hypoglycaemic, hypolipidemic, and anticancer (Lim, 2012; Refaat et al., 2014; Abouelela et al., 2019). In our in vivo study of C. pentandra, the ethyl acetate fraction has emerged as the most promising nephroprotective part of the extract.
To explore further health benefits about C. pentandra, in vivo anticancer efficacy of C. pentandra ethyl acetate extract, alone or in combination with the conventional DOX, against diethylnitrosamine (DENA)-induced HCC in rats was investigated. The results showed that the DENA-induced changes in serum indicators of liver function were relatively restored to their respective normal ranges by the administration of either C. pentandra extract or DOX. Although, the administration of C. pentandra alone had a comparatively stronger effect than DOX against chemically induced HCC in a rat model. Also, the rats treated with the ethyl acetate extracts displayed stable weight trends during the study, whereas the rats treated with DOX experienced sharp weight declines, which is a common side effect associated with DOX (Colombo et al., 1989). The combination of C. pentandra extract and DOX outperformed the individual treatments in the DENA+extract and DENA+DOX groups, leading to a noticeable enhancement in the overall estimated liver function indices. The DENA+DOX and DENA+Extract+DOX groups experienced initial sharp weight drops, followed by gradual recovery, while the DENA drinking group had a slight decline.
Furthermore, co-administration of C. pentandra extract and DOX effectively decreased the raised AFP-L3 levels, despite the fact that neither substance by itself was able to appreciably lower the DENA-induced rise in levels of the AFP-L3 HCC tumour marker. The liver tissues histological investigation offered strong proof for the combined C. pentandra extract and DOX’s valuable effects in fighting DENA-induced HCC.
In order to investigate the compounds responsible for the antitumor properties, we have consequently analyzed the EtOAc fraction using an UHPLC/Q-TOF/MS/MS. The mass analysis results (Table 1) highlighted the occurrence of large number of plant polyphenols, including fourteen flavonoids (11, 12, 19, 21, 22, 27, 29, 30, 32, 36, 42, 43, 44, and 45), eight flavanolignans (17, 18, 20, 26, 31, 34, 40, and 41), two procyanidins (7 and 13), five coumarins (8, 10, 33, 37, and 39), two phenolic acids (6 and 9).
Plant polyphenols demonstrate protective effects against oxidative stress, inflammation, ageing, and other pathophysiological processes in relation to liver disorders (Cha and DeMatteo, 2005; Wu et al., 2012; Sangineto et al., 2020; Ruiz-Manriquez et al., 2022). The protective effects of various polyphenols on liver cancer are caused by numerous molecular mechanisms involving modulation of lipid metabolism, glucose metabolism, mitochondrial metabolism, oxidative stress, and other metabolic reactions (Li et al., 2023a).
Hepatocellular carcinoma-preventive properties of flavonoids including those identified in the EtOAc fraction of C. pentandra via several intracellular signaling pathways have been explained in various reports (Xia et al., 2013; García et al., 2018). Among the reported antitumor molecular mechanisms of some of the identified compounds are as follows:
(i). Apigenin (45, Figure 4) has been shown to exhibit anticancer properties in various malignancies, including the HCC cell line (BEL-7402/ADM) through the miR-101/Nrf2 pathway (Gao et al., 2017)]. Hesperidin and apigenin strengthened the cytotoxic effect of DOX on the HepG2 cell line (Korga et al., 2019). It also retrieved the cytotoxicity of natural killer cells via repairing the linkage between cancer cells and NK cells and inhibiting the generation of regulatory T cells (Tregs) (Li et al., 2023b). Apigenin enhanced HIF-1α expressing HCC natural killer cytotoxicity through increasing the cluster of differentiation 95 (CD95)/CD95 ligand (CD95L) interaction (Lee and Cho, 2022).
(ii). Catechin (11, Figure 4) exhibited proliferation-inhibitory and pro-apoptosis effects on the human HepG2 cell line (Monga and Sharma, 2013). Catechin also induced cell apoptosis which was associated with inhibiting the expression of B-cell lymphoma 2 (Bcl-2) as well as elevating the expression of Bcl-2-associated X protein (BAX) and caspase-3 in a concentration-dependent manner (Liao et al., 2015).
(iii). Isovitexin [21 apigenin-6-C-glucoside, Figure 4], suppressed the stemness of human HCC (SK-Hep-1) cells. It has revealed suppression of sphere and colony formation, decreased CD44+ cell populations, and decreased ATP binding cassette subfamily G member 2 (ABCG2), aldehyde dehydrogenase 1 family member A1 (ALDH1), and NANOG mRNA levels while increasing miR-34a levels (Xu et al., 2020).
(iv). Quercetin (30, Figure 4) has been shown to have anti-tumor effects on HCC through various mechanisms. One study found that quercetin can reverse multidrug resistance (MDR) of HCC cells by down-regulating the expression of mdr1, multidrug resistance-associated protein (MRP), glutathione-S-transferase-π (GST-π), and H-ras, while also down-regulating P-glycoprotein expression (Wei et al., 2012). Another study found that quercetin inhibits the migration ability of HCC cells by inhibiting the expression of transcriptional co-suppressor, C-terminal binding protein 1 (CtBP1), and up-regulating the expression of epithelial adhesion molecule E-cadherin, a tumor suppressor protein (Tang et al., 2018). Additionally, quercetin has been shown to inhibit the proliferation of glycolysis-addicted HCC cells by reducing hexokinase 2 and the Akt-mTOR pathway (Wu et al., 2019). Another study found that quercetin can inhibit the growth of transplanted HCC in nude mice by reducing Inositol trisphosphate (IP3) production and Bax protein expression (Huang and Zhang, 2001). Finally, quercetin-3-O-glucoside has been shown to induce human DNA topoisomerase II inhibition, cell cycle arrest, and apoptosis in HCC cells (Sudan and Rupasinghe, 2014)
(v). A study investigated the kaempferol (36, Figure 3) anti-migratory and anti-invasive effects on HCC cells (Ju et al., 2021) has been realized that kaempferol reduced these effects of HCC cells by targeting the matrix metalloproteinase-9 (MMP-9) protein kinase B (AKT) pathways. In addition, kaempferol lowered the MMP-9 protein expression and activities and suppressed the phosphorylation of the Akt expression. Another study showed that kaempferol can sensitize liver cancer cells toward the effect of sorafenib for the management of advanced HCC (Nair et al., 2020). Furthermore, kaempferol effectively inhibited cellular proliferation and migration, and enhanced cell cycle arrest, autophagy, apoptosis, and mediated chemosensitivity with 5-fluorouracil in HCC cells (Kannan et al., 2019)
(vi). Naringenin (42, Figure 4) has been found to reduce HCC cell viability, block epithelial-mesenchymal-transition, and inhibit sphere creation, cell migration and invasion. It also downregulated stemness-associated transcription factors and diminished hypoxia-inducible factor-1 (HIF-1) activity (Kang et al., 2019). Furthermore, naringenin substantially boosted the sensitivity of HCC cells to medicines, and hampered the growth of the HCC tumor, and metastasis of HCC cells to the lung. Naringenin silenced Wnt/β-catenin signaling by provoking β-catenin degradation and stopping its nuclear translocation. Upregulation of glycogen synthase kinase 3β (GSK3β) looked to be crucial for naringenin’s repressing effect in the signaling pathway (Kang et al., 2019)
(vii). A study performed by Wang et al. observed that eriodictyol (43, Figure 4) induced concentration-dependent selective cytotoxicity against the Hep-G2 cell line. Eriodictyol has also induced morphological changes, apoptosis-related chromatin condensation, and nuclear fragmentation. Furthermore, it also encouraged G2/M cell cycle arrest, downregulated Bcl-2 protein, and upregulated BAX and poly-ADP ribose polymerase (PARP) in these cells (Wang et al., 2016).
(viii). Protocatechuic acid (6, Figure 4) induced cell death in HepG2 cells through a c-Jun N-terminal kinase-dependent mechanism (Yip et al., 2006; Yip et al., 2006).
(ix). Kumar et al. developed a suitable delivery system for umbelliferone β-D-galactopyranoside (UFG) targeting the enhancement of its therapeutic efficacy against DENA-induced HCC in Wistar rats. The anticancer potential of these nanoparticles was able to manage DENA-induced reactive oxygen species generation, mitochondrial dysfunction, proinflammatory cytokines alteration, and induction of apoptosis (Kumar et al., 2017).
(x). Aesculetin (10, Figure 4) has shown promising anticancer activity against various organ cancers including breast, lung, and liver (Zhang et al., 2021). A study that investigated the anticancer properties of aesculetin against gastric cancer demonstrated that aesculetin lowered cellular proliferation in a time and dose-dependent manner, suppressed the clonogenic tendency of cancer cells, and encouraged apoptosis. The study also revealed that aesculetin blocked the phosphatidylinositol-3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway in gastric cancer cells (Zhang et al., 2021).
Collectively, previous studies on the impact of identified components on the therapy of HCC provide credence to our experimental results. Hence, it is obvious that C. pentandra EtOAc extract is rich in promising phytoconstituents with known antioxidant, anti-inflammatory, and antitumor properties which may integrate to afford hepatoprotective activity and/or anti-HCC activity in combination with chemotherapeutics such as doxorubicin.
Our study demonstrates that the EtOAc fraction of C. pentandra hydromethanolic extract has a relatively respectable activity against chemically induced HCC in rat model. Moreover, it strongly suggests that the combinatorial use of C. pentandra extract and DOX produced undoubted in vivo antitumor activity against this type of cancer, as proven herein through estimation of diverse molecular, biochemical, and histological parameters. In addition, fifty phytomolecules have been identified from the same fraction based on UHPLCQ-TOF-MS/MS analysis. Numerous anti-cancer effects of flavonoids have been shown, including immune-modulating, anti-neoplastic, and drug-sensitizing capabilities. Among them, the dietary phenolic acid, protocatechuic acid (6), flavonoids, catechin (11), isovitexin (21), quercetin (30), kaempferol (36), naringenin (42), eriodictyol (43), and apigenin (45), and coumarin, aesculetin (10), have been shown to fight cancer by various mechanisms (Liu-Smith and Meyskens, 2016; Lotha and Sivasubramanian, 2018; Hazafa et al., 2019; Ezzati et al., 2020), which agree with our discoveries.
Overall, despite the lack of a specific molecular mechanism study in this research, our findings highlight that C. pentandra, which is rich in flavonoids and the related promising compounds flavolignans, coumarins, and phenolic acids, could be a hopeful candidate for the development of liver cancer therapies in combination with doxorubicin. This will spur further preclinical and clinical research on this phytomolecules-rich plant as an inexpensive cancer care strategy when considering tailored, preventative, and predictive treatment.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The animal study protocol was approved by Ethical Committee of the Faculty of Pharmacy, Al-Azhar University, Assiut, Egypt (protocol code AZ/AS/PHREC/27/2023, date of approval 23/4/2023). The study was conducted in accordance with the local legislation and institutional requirements.
MO: Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Writing–original draft, Writing–review and editing. MEA: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Writing–original draft, Writing–review and editing. FD: Data curation, Supervision, Visualization, Writing–original draft. MSA: Methodology, Supervision, Validation, Visualization, Writing–original draft. BE: Conceptualization, Data curation, Formal Analysis, Investigation, Validation, Writing–original draft, Writing–review and editing. AAA: Data curation, Methodology, Resources, Software, Validation, Writing–original draft. MMA: Formal Analysis, Methodology, Resources, Software, Validation, Visualization, Writing–original draft. AHA: Data curation, Formal Analysis, Resources, Software, Visualization, Writing–original draft. NA: Data curation, Formal Analysis, Resources, Software, Validation, Visualization, Writing–original draft. RA: Conceptualization, Data curation, Investigation, Supervision, Writing–original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by Deanship of Scientific research, Najran University, Saudi Arabia, under the National Research Priority funding Program (Grant number. NU/NRP/MRC/12/1).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1337910/full#supplementary-material
Abdelhafez, O. H., Fawzy, M. A., Fahim, J. R., Desoukey, S. Y., Krischke, M., Mueller, M. J., et al. (2018). Hepatoprotective potential of Malvaviscus arboreus against carbon tetrachloride-induced liver injury in rats. PloS one 13 (8), e0202362. doi:10.1371/journal.pone.0202362
Abouelela, M. E. (2016). “Pharmacognostical study of Ceiba pentandra (L.) Gaertn var. Pentandra,” in Family bombacaceae cultivated in Egypt (Egypt: Assiut University).
Abouelela, M. E., Abdelhamid, R. A., Orabi, M. A. A., and Darwish, F. M. M. (2019). Taxonomy, phytochemistry, and therapeutic potentials of the genus Ceiba (bombacaceae): a review. Saudi. J. Med. Pharm. Sci. 5 (7), 666–682. doi:10.21276/sjmps.2019.5.7.17
Abouelela, M. E., Orabi, M. A., Abdelhamid, R. A., Abdelkader, M. S., and Darwish, F. M. (2018). Chemical and cytotoxic investigation of non-polar extract from Ceiba pentandra (L.) Gaertn.: a study supported by computer based screening. J. Appl. Pharm. Sci. 8 (07), 057–064. doi:10.7324/japs.2021.1101112
Abouelela, M. E., Orabi, M. A., Abdelhamid, R. A., Abdelkader, M. S., Madkor, H. R., Darwish, F. M., et al. (2020). Ethyl acetate extract of Ceiba pentandra (L.) Gaertn. reduces methotrexate-induced renal damage in rats via antioxidant, anti-inflammatory, and antiapoptotic actions. J. Tradit. Complement. Med. 10 (5), 478–486. doi:10.1016/j.jtcme.2019.08.006
Afzal, M., Kazmi, I., Gupta, G., Rahman, M., Kimothi, V., and Anwar, F. (2012). Preventive effect of Metformin against N-nitrosodiethylamine-initiated hepatocellular carcinoma in rats. Saudi Pharm. J. 20 (4), 365–370. doi:10.1016/j.jsps.2012.05.012
Allen, F. R. (2016). “Competitive fragmentation modeling of mass spectra for metabolite identification,”. Doctor of Philosophy (Alberta: University of Alberta).
Atwa, G., Omran, G., Abd Elbaky, A., and Okda, T. (2021). The antitumour effect of galangin and luteolin with doxorubicin on chemically induced hepatocellular carcinoma in rats. Contemp. Oncology/Współczesna Onkol. 25 (3), 174–184. doi:10.5114/wo.2021.110048
Bairwa, N. K., Sethiya, N. K., and Mishra, S. (2010). Protective effect of stem bark of Ceiba pentandra linn. against paracetamol-induced hepatotoxicity in rats. Pharmacogn. Res. 2 (1), 26–30. doi:10.4103/0974-8490.60584
Bansal, A. K., Bansal, M., Soni, G., and Bhatnagar, D. (2005). Protective role of Vitamin E pre-treatment on N-nitrosodiethylamine induced oxidative stress in rat liver. Chem. Biol. Interact. 156 (2), 101–111. doi:10.1016/j.cbi.2005.08.001
Capone, F., Guerriero, E., Sorice, A., Colonna, G., Storti, G., Pagliuca, J., et al. (2014). Synergistic antitumor effect of Doxorubicin and tacrolimus (FK506) on hepatocellular carcinoma cell lines. Sci. World J. 2014, 450390. doi:10.1155/2014/450390
Cha, C., and DeMatteo, R. P. (2005). Molecular mechanisms in hepatocellular carcinoma development. Best. Pract. Res. Clin. Gastroenterol. 19 (1), 25–37. doi:10.1016/j.bpg.2004.11.005
Clifford, M. N., and Knight, S. (2004). The cinnamoyl–amino acid conjugates of green robusta coffee beans. Food Chem. 87 (3), 457–463. doi:10.1016/j.foodchem.2003.12.020
Colombo, T., Donelli, M., Urso, R., Dallarda, S., Bartosek, I., and Guaitani, A. (1989). Doxorubicin toxicity and pharmacokinetics in old and young rats. Exp. Gerontol. 24 (2), 159–171. doi:10.1016/0531-5565(89)90026-0
Culling, C. F. A. (2013). Handbook of histopathological and histochemical techniques: including museum techniques. United Kingdom: Butterworth-Heinemann.
Cuyckens, F., and Claeys, M. (2004). Mass spectrometry in the structural analysis of flavonoids. J. Mass Spectrom. 39 (1), 1–15. doi:10.1002/jms.585
Di Stefano, G., Fiume, L., Baglioni, M., Bolondi, L., Chieco, P., Kratz, F., et al. (2008). Efficacy of doxorubicin coupled to lactosaminated albumin on rat hepatocellular carcinomas evaluated by ultrasound imaging. Dig. Liver. Dis. 40 (4), 278–284. doi:10.1016/j.dld.2007.10.008
Dunn, W. B., Erban, A., Weber, R. J., Creek, D. J., Brown, M., Breitling, R., et al. (2013). Mass appeal: metabolite identification in mass spectrometry-focused untargeted metabolomics. Metabolomics 9 (1), 44–66. doi:10.1007/s11306-012-0434-4
El-Hawary, S. S., Mohammed, R., AbouZid, S. F., Rateb, M. E., and Sayed, A. M. (2015). Cytotoxicity of Solanum nigrum L green fruits on breast (MCF-7) and liver (HepG-2) cancer cell lines. Pharma Innov. J. 3 (11), 87–89.
Ellappan, T., Ramar, M., Manikrishnan, R., Melepuram, S. G., Balaji, P., Sekar, V. K., et al. (2022). Protective effect of pentandra (L) Gaertn on CCl4-induced oxidative stress and liver damage in rats. Pharmacol. Res. - Mod. Chin. Med. 5, 100196. doi:10.1016/j.prmcm.2022.100196
Elsadek, B., Mansour, A., Saleem, T., Warnecke, A., and Kratz, F. (2017). The antitumor activity of a lactosaminated albumin conjugate of doxorubicin in a chemically induced hepatocellular carcinoma rat model compared to sorafenib. Dig. Liver Dis. 49 (2), 213–222. doi:10.1016/j.dld.2016.10.003
El-Sayed, M. A., Al-Gendy, A. A., Hamdan, D. I., and El-Shazly, A. M. (2017). Phytoconstituents, LC-ESI-MS profile, antioxidant and antimicrobial activities of Citrus x limon L. Burm. F. Cultivar variegated pink lemon. J. Pharm. Sci. Res. 9 (4), 375–391.
Ezzati, M., Yousefi, B., Velaei, K., and Safa, A. (2020). A review on anti-cancer properties of Quercetin in breast cancer. Life Sci. 248, 117463. doi:10.1016/j.lfs.2020.117463
Fang, T., Wang, Y., Ma, Y., Su, W., Bai, Y., and Zhao, P. (2006). A rapid LC/MS/MS quantitation assay for naringin and its two metabolites in rats plasma. J. Pharm. Biomed. Anal. 40 (2), 454–459. doi:10.1016/j.jpba.2005.07.031
Fathy, A. H., Bashandy, M. A., Bashandy, S. A., Mansour, A. M., and Elsadek, B. (2017). Sequential analysis and staging of a diethylnitrosamine-induced hepatocellular carcinoma in male Wistar albino rat model. Can. J. physiology Pharmacol. 95 (12), 1462–1472. doi:10.1139/cjpp-2017-0413
Feng, X., Liu, Y., Wang, X., and Di, X. (2014). Analysis of linarin and its metabolites in rat urine by LC–MS/MS. Chromatographia 77 (7-8), 571–579. doi:10.1007/s10337-014-2641-9
Ganesan, P., and Kulik, L. M. (2023). Hepatocellular carcinoma: new developments. Clin. liver Dis. 27 (1), 85–102. doi:10.1016/j.cld.2022.08.004
Gao, A.-M., Zhang, X.-Y., and Ke, Z.-P. (2017). Apigenin sensitizes BEL-7402/ADM cells to doxorubicin through inhibiting miR-101/Nrf2 pathway. Oncotarget 8 (47), 82085–82091. doi:10.18632/oncotarget.18294
García, E. R., Gutierrez, E. A., Melo, F. C. S. A., Novaes, R. D., and Gonçalves, R. V. (2018). Flavonoids effects on hepatocellular carcinoma in murine models: a systematic review. Evid. Based Complement. Altern. Med. 2018, 6328970. doi:10.1155/2018/6328970
Golla, K., Bhaskar, C., Ahmed, F., and Kondapi, A. K. (2013). A target-specific oral formulation of doxorubicin-protein nanoparticles: efficacy and safety in hepatocellular cancer. J. Cancer 4 (8), 644–652. doi:10.7150/jca.7093
Gu, L., Kelm, M. A., Hammerstone, J. F., Zhang, Z., Beecher, G., Holden, J., et al. (2003). Liquid chromatographic/electrospray ionization mass spectrometric studies of proanthocyanidins in foods. J. Mass Spectrom. 38 (12), 1272–1280. doi:10.1002/jms.541
Gu, W.-Y., Li, N., Leung, E.L.-H., Zhou, H., Luo, G.-A., Liu, L., et al. (2015). Metabolites software-assisted flavonoid hunting in plants using ultra-high performance liquid chromatography-quadrupole-time of flight mass spectrometry. Molecules 20 (3), 3955–3971. doi:10.3390/molecules20033955
Gyobin, G. (2016). LC-MS based targeted isolation of cyclopeptide alkaloids and triterpenoids from ziziphus jujuba roots with development of dereplication methods. Seoul: Seoul National University.
Han, R., Li, J., Hony, J., Xiao, Z., Yao, M., Liang, S., et al. (2023). CAXII inhibitors: potential sensitizers for immune checkpoint inhibitors in HCC treatment. Front. Immunol. 14, 1052657. doi:10.3389/fimmu.2023.1052657
Hazafa, A., Rehman, K.-U., Jahan, N., and Jabeen, Z. (2019). The role of polyphenol (flavonoids) compounds in the treatment of cancer cells. Nutr. cancer 72, 386–397. doi:10.1080/01635581.2019.1637006
He, Y., Li, Z., Wang, W., Sooranna, S., Shi, Y., Chen, Y., et al. (2018). Chemical profiles and simultaneous quantification of aurantii fructus by use of HPLC-Q-TOF-MS combined with GC-MS and HPLC methods. Molecules 23 (9), 2189–2206. doi:10.3390/molecules23092189
Hokkanen, J., Mattila, S., Jaakola, L., Pirttila, A. M., and Tolonen, A. (2009). Identification of phenolic compounds from lingonberry (Vaccinium vitis-idaea L.), bilberry (Vaccinium myrtillus L.) and hybrid bilberry (Vaccinium x intermedium ruthe L.) leaves. J. Agric. Food Chem. 57 (20), 9437–9447. doi:10.1021/jf9022542
Huang, X., and Zhang, J. (2001). Effect and mechanism of Querecetin treatment of transplanted hepatic cancer in nude mice. Chin. J. General Surg. 17 (7), 663–667.
Jain, D. (2014). Tissue diagnosis of hepatocellular carcinoma. J. Clin. Exp. Hepatol. 4, S67–S73. doi:10.1016/j.jceh.2014.03.047
Ju, P. C., Ho, Y. C., Chen, P. N., Lee, H. L., Lai, S. Y., Yang, S. F., et al. (2021). Kaempferol inhibits the cell migration of human hepatocellular carcinoma cells by suppressing MMP-9 and Akt signaling. Environ. Toxicol. 36 (10), 1981–1989. doi:10.1002/tox.23316
Kang, Q., Gong, J., Wang, M., Wang, Q., Chen, F., and Cheng, K.-W. (2019). 6-C-(E-Phenylethenyl)Naringenin attenuates the stemness of hepatocellular carcinoma cells by suppressing wnt/β-catenin signaling. J. Agric. Food Chem. 67 (50), 13939–13947. doi:10.1021/acs.jafc.9b05733
Kannan, M., Jayamohan, S., Mohanakumar, A. K., Moorthy, R. K., Purushothama, K. M., and Arockiam, A. J. V. (2019). Bcl-2/BCL2L12 mediated apoptosis and cell cycle arrest induced by Kaempferol through the suppression of PI3K/AKT signaling pathway in Hepatocellular carcinoma. J. Adv. Appl. Sci. Res. 2 (1), 20–37. doi:10.46947/joaasr21A201999
Kim, S., Thiessen, P. A., Bolton, E. E., Chen, J., Fu, G., Gindulyte, A., et al. (2015). PubChem substance and compound databases. Nucleic acids Res. 44 (1), D1202–D1213. doi:10.1093/nar/gkv951
Kola, P., Metowogo, K., Manjula, S., Katawa, G., Elkhenany, H., Mruthunjaya, K., et al. (2022). Ethnopharmacological evaluation of antioxidant, anti-angiogenic, and anti-inflammatory activity of some traditional medicinal plants used for treatment of cancer in Togo/Africa. J. Ethnopharmacol. 283, 114673. doi:10.1016/j.jep.2021.114673
Korga, A., Ostrowska, M., Jozefczyk, A., Iwan, M., Wojcik, R., Zgorka, G., et al. (2019). Apigenin and hesperidin augment the toxic effect of doxorubicin against HepG2 cells. BMC Pharmacol. Toxicol. 20, 22–13. doi:10.1186/s40360-019-0301-2
Kumar, R., Kumar, N., Ramalingayya, G. V., Setty, M. M., and Pai, K. S. R. (2016). Evaluation of Ceiba pentandra (L.) gaertner bark extracts for in vitro cytotoxicity on cancer cells and in vivo antitumor activity in solid and liquid tumor models. Cytotechnology 68 (5), 1909–1923. doi:10.1007/s10616-016-0002-2
Kumar, V., Bhatt, P. C., Rahman, M., Kaithwas, G., Choudhry, H., Al-Abbasi, F. A., et al. (2017). Fabrication, optimization, and characterization of umbelliferone β-D-galactopyranoside-loaded PLGA nanoparticles in treatment of hepatocellular carcinoma: in vitro and in vivo studies. Int. J. Nanomed. 12, 6747–6758. doi:10.2147/IJN.S136629
Lagana, S. M., Salomao, M., Bao, F., Moreira, R. K., Lefkowitch, J. H., and Remotti, H. E. (2013). Utility of an immunohistochemical panel consisting of glypican-3, heat-shock protein-70, and glutamine synthetase in the distinction of low-grade hepatocellular carcinoma from hepatocellular adenoma. Appl. Immunohistochem. Mol. Morphol. 21 (2), 170–176. doi:10.1097/PAI.0b013e31825d527f
Lahoti, T. S., Patel, D., Thekkemadom, V., Beckett, R., and D Ray, S. (2012). Doxorubicin-induced in vivo nephrotoxicity involves oxidative stress-mediated multiple pro-and anti-apoptotic signaling pathways. Curr. Neurovascular Res. 9 (4), 282–295. doi:10.2174/156720212803530636
Lee, H. H., and Cho, H. (2022). Apigenin increases natural killer cytotoxicity to human hepatocellular carcinoma expressing HIF-1α through high interaction of CD95/CD95L. J. Microbiol. Biotechnol. 32 (4), 397–404. doi:10.4014/jmb.2201.01010
Li, S., Li, H., He, Q., and Yang, X. (2023b). Potential of compounds originating from the nature to act in hepatocellular carcinoma therapy by targeting the tumor immunosuppressive microenvironment: a review. Molecules 28 (1), 195. doi:10.3390/molecules28010195
Li, S., Yin, S., Ding, H., Shao, Y., Zhou, S., Pu, W., et al. (2023a). Polyphenols as potential metabolism mechanisms regulators in liver protection and liver cancer prevention. Cell Prolif. 56 (1), e13346. doi:10.1111/cpr.13346
Liao, C.-Y., Lee, C.-C., Tsai, C.-C., Hsueh, C.-W., Wang, C.-C., Chen, I., et al. (2015). Novel investigations of flavonoids as chemopreventive agents for hepatocellular carcinoma. Biomed. Res. Int. 2015, 840542–840626. doi:10.1155/2015/840542
Lim, T. K. (2012). “Ceiba pentandra,” in Edible medicinal and non-medicinal plants (Berlin, Germany: Springer), 540–549.
Lin, Y., Xu, W., Huang, M., Xu, W., Li, H., Ye, M., et al. (2015). Qualitative and quantitative analysis of phenolic acids, flavonoids and iridoid glycosides in yinhua kanggan tablet by UPLC-QqQ-MS/MS. Molecules 20 (7), 12209–12228. doi:10.3390/molecules200712209
Liu-Smith, F., and Meyskens, F. L. (2016). Molecular mechanisms of flavonoids in melanin synthesis and the potential for the prevention and treatment of melanoma. Mol. Nutr. Food Res. 60 (6), 1264–1274. doi:10.1002/mnfr.201500822
Locatelli, M., Travaglia, F., Giovannelli, L., Coïsson, J. D., Bordiga, M., Pattarino, F., et al. (2013). Clovamide and phenolics from cocoa beans (Theobroma cacao L.) inhibit lipid peroxidation in liposomal systems. Food Res. Int. 50 (1), 129–134. doi:10.1016/j.foodres.2012.10.008
Lotha, R., and Sivasubramanian, A. (2018). Flavonoids nutraceuticals in prevention and treatment of cancer: a review. Asian J. Pharm. Clin. Res. 11, 42–47. doi:10.22159/ajpcr.2018.v11i1.23410
Maldini, M., Montoro, P., Piacente, S., and Pizza, C. (2009). ESI-MS, ESI-MS/MS fingerprint and LC-ESI-MS analysis of proathocyanidins from Bursera simaruba sarg bark. Nat. Prod. Commun. 4 (12), 1934578X0900401. doi:10.1177/1934578x0900401212
Mandlik, D. S., and Mandlik, S. K. (2021). Herbal and natural dietary products: upcoming therapeutic approach for prevention and treatment of hepatocellular carcinoma. Nutr. cancer 73 (11-12), 2130–2154. doi:10.1080/01635581.2020.1834591
Mansour, D. F., Abdallah, H. M., Ibrahim, B. M., Hegazy, R. R., Esmail, R. S., and Abdel-Salam, L. O. (2019). The carcinogenic agent diethylnitrosamine induces early oxidative stress, inflammation and proliferation in rat liver, stomach and colon: protective effect of ginger extract. Asian Pac. J. cancer Prev. APJCP 20 (8), 2551–2561. doi:10.31557/APJCP.2019.20.8.2551
March, R. E., Lewars, E. G., Stadey, C. J., Miao, X.-S., Zhao, X., and Metcalfe, C. D. (2006). A comparison of flavonoid glycosides by electrospray tandem mass spectrometry. Int. J. Mass Spectrom. 248 (1-2), 61–85. doi:10.1016/j.ijms.2005.09.011
Masike, K., Khoza, B. S., Steenkamp, P. A., Smit, E., Dubery, I. A., and Madala, N. E. (2017). A metabolomics-guided exploration of the phytochemical constituents of vernonia fastigiata with the aid of pressurized hot water extraction and liquid chromatography-mass spectrometry. Molecules 22 (8), 1200–1215. doi:10.3390/molecules22081200
McGlynn, K. A., and London, W. T. (2005). Epidemiology and natural history of hepatocellular carcinoma. Best. Pract. Res. Clin. Gastroenterol. 19 (1), 3–23. doi:10.1016/j.bpg.2004.10.004
Monga, J., and Sharma, M. (2013). (+)-Catechin produces antiproliferative effects in human hepatocellular carcinoma cells and inhibits tumor growth in vivo. Ann. Oncol. 24, iv45. doi:10.1093/annonc/mdt203.30
Moqbel, H., El Hawary, S. S. E. D., Sokkar, N. M., El Boghdady, N., and El Halawany, A. M. (2018). HPLC-ESI-MS/MS characterization of phenolics in prunus amygdalus, cultivar “umm alfahm” and its antioxidant and hepatoprotective activity. J. Food Meas. Charact. 12 (2), 808–819. doi:10.1007/s11694-017-9695-y
Nair, B., Anto, R. J., Sabitha, M., and Nath, L. R. (2020). Kaempferol-mediated sensitization enhances chemotherapeutic efficacy of sorafenib against hepatocellular carcinoma: an in silico and in vitro approach. Adv. Pharm. Bull. 10 (3), 472–476. doi:10.34172/apb.2020.058
Nam, N. H., Kim, H. M., Bae, K. H., and Ahn, B. Z. (2003). Inhibitory effects of Vietnamese medicinal plants on tube-like formation of human umbilical venous cells. Phytother. Res. 17 (2), 107–111. doi:10.1002/ptr.934
Narváez-Cuenca, C.-E., Vincken, J.-P., and Gruppen, H. (2012). Identification and quantification of (dihydro) hydroxycinnamic acids and their conjugates in potato by UHPLC–DAD–ESI-MSn. Food Chem. 130 (3), 730–738. doi:10.1016/j.foodchem.2011.04.050
Omar, A., Abou-Alfa, G. K., Khairy, A., and Omar, H. (2013). Risk factors for developing hepatocellular carcinoma in Egypt. Chin. Clin. Oncol. 2 (4), 43–51. doi:10.3978/j.issn.2304-3865.2013.11.07
Patel, N., Joseph, C., Corcoran, G. B., and Ray, S. D. (2010). Silymarin modulates doxorubicin-induced oxidative stress, Bcl-xL and p53 expression while preventing apoptotic and necrotic cell death in the liver. Toxicol. Appl. Pharmacol. 245 (2), 143–152. doi:10.1016/j.taap.2010.02.002
Peña-Morán, O. A., Villarreal, M. L., Álvarez-Berber, L., Meneses-Acosta, A., and Rodríguez-López, V. (2016). Cytotoxicity, post-treatment recovery, and selectivity analysis of naturally occurring podophyllotoxins from Bursera fagaroides var. Fagaroides on breast cancer cell lines. Molecules 21 (8), 1013–1028. doi:10.3390/molecules21081013
Rashed, W. M., Kandeil, M. A. M., Mahmoud, M. O., and Ezzat, S. (2020). Hepatocellular Carcinoma (HCC) in Egypt: a comprehensive overview. J. Egypt. Natl. Cancer Inst. 32 (1), 5–11. doi:10.1186/s43046-020-0016-x
Rawat, D., Shrivastava, S., Naik, R. A., Chhonker, S. K., Mehrotra, A., and Koiri, R. K. (2018). An overview of natural plant products in the treatment of hepatocellular carcinoma. Anti-Cancer Agents Med. Chem. 18 (13), 1838–1859. doi:10.2174/1871520618666180604085612
Refaat, J., Desoky, S. Y., Ramadan, M. A., and Kamel, M. S. (2013). Bombacaceae: a phytochemical review. Pharm. Biol. 51 (1), 100–130. doi:10.3109/13880209.2012.698286
Refaat, J., Desoky, S. Y., Ramadan, M. A., and Kamel, M. S. (2014). Bombacaceae between the ethnomedical uses and pharmacological evidences: a review. Nat. Prod. J. 4 (4), 254–270. doi:10.2174/2210315504666141125003412
Ruiz-Manriquez, L. M., Carrasco-Morales, O., Sanchez, Z. E. A., Osorio-Perez, S. M., Estrada-Meza, C., Pathak, S., et al. (2022). MicroRNA-mediated regulation of key signaling pathways in hepatocellular carcinoma: a mechanistic insight. Front. Genet. 13, 910733. doi:10.3389/fgene.2022.910733
Sangineto, M., Villani, R., Cavallone, F., Romano, A., Loizzi, D., and Serviddio, G. (2020). Lipid metabolism in development and progression of hepatocellular carcinoma. Cancers 12 (6), 1419. doi:10.3390/cancers12061419
Smith, C. A., O'Maille, G., Want, E. J., Qin, C., Trauger, S. A., Brandon, T. R., et al. (2005). METLIN: a metabolite mass spectral database. Ther. Drug Monit. 27 (6), 747–751. doi:10.1097/01.ftd.0000179845.53213.39
Sudan, S., and Rupasinghe, H. V. (2014). Quercetin-3-O-glucoside induces human DNA topoisomerase II inhibition, cell cycle arrest and apoptosis in hepatocellular carcinoma cells. Anticancer Res. 34 (4), 1691–1699.
Tang, J.-Z., Bian, L., Xiao, S., Zhang, X., Wang, X., and Yang, Y. (2018). Inhibitory effecT of quercetin on protein expression CtBP1 and migration of hepatocellular carcinoma cell SMMC-7721. Comput. Oper. Res. 2018, 1–5. doi:10.31487/j.COR.2018.02.001
Tine, Y., Renucci, F., Costa, J., Wélé, A., and Paolini, J. (2017). A method for LC-MS/MS profiling of coumarins in Zanthoxylum zanthoxyloides (lam.) B. Zepernich and timler extracts and essential oils. Molecules 22 (1), 174–186. doi:10.3390/molecules22010174
Tsimogiannis, D., Samiotaki, M., Panayotou, G., and Oreopoulou, V. (2007). Characterization of flavonoid subgroups and hydroxy substitution by HPLC-MS/MS. Molecules 12 (3), 593–606. doi:10.3390/12030593
Wagner, H., and Ulrich-Merzenich, G. (2011). Synergy research: approaching a new generation of phytopharmaceuticals. Fitoterapia 82 (1), 97–110. doi:10.1016/j.phymed.2008.12.018
Wang, F., Wang, Y.-H., Wang, J.-J., Xu, H.-L., and Wang, C.-M. (2016). Eriodictyol-induced anti-cancer and apoptotic effects in human hepatocellular carcinoma cells are associated with cell cycle arrest and modulation of apoptosis-related proteins. Bangladesh J. Pharmacol. 11 (2), 285–291. doi:10.3329/bjp.v11i2.25549
Wang, N. Y., Wang, C., Li, W., Wang, G. J., Cui, G. Z., He, H., et al. (2014). Prognostic value of serum AFP, AFP-L3, and GP73 in monitoring short-term treatment response and recurrence of hepatocellular carcinoma after radiofrequency ablation. Asian pac. J. Cancer Prev. 15 (4), 1539–1544. doi:10.7314/APJCP.2014.15.4.1539
Wei, Y., Zhang, H., and Liang, G. (2012). The reverse effect of quercetin on multidrug resistance of human hepatocellular carcinoma. Tianjing Med. J. 40, 1022–1025.
Won, J., Lee, K., Park, S., Kim, S.-J., Yun, S.-Y., Kang, M.-A., et al. (2004). Derivatives of hydroxyphenyl, a method for preparing thereof and their pharmaceutical composition. Google Patents.
Wu, H., Pan, L., Gao, C., Xu, H., Li, Y., Zhang, L., et al. (2019). Quercetin inhibits the proliferation of glycolysis-addicted HCC cells by reducing hexokinase 2 and Akt-mTOR pathway. Molecules 24 (10), 1993. doi:10.3390/molecules24101993
Wu, S.-D., Ma, Y.-S., Fang, Y., Liu, L.-L., Fu, D., and Shen, X.-Z. (2012). Role of the microenvironment in hepatocellular carcinoma development and progression. Cancer Treat. Rev. 38 (3), 218–225. doi:10.1016/j.ctrv.2011.06.010
Wungsintaweekul, B., Umehara, K., Miyase, T., and Noguchi, H. (2011). Estrogenic and anti-estrogenic compounds from the Thai medicinal plant, Smilax corbularia (Smilacaceae). Phytochemistry 72 (6), 495–502. doi:10.1016/j.phytochem.2010.12.018
Xia, J., Gao, J., Inagaki, Y., Kokudo, N., Nakata, M., and Tang, W. (2013). Flavonoids as potential anti-hepatocellular carcinoma agents: recent approaches using HepG2 cell line. Drug Discov. Ther. 7 (1), 1–8. doi:10.5582/ddt.2013.v7.1.1
Xu, C., Cao, X., Cao, X., Liu, L., Qiu, Y., Li, X., et al. (2020). Isovitexin inhibits stemness and induces apoptosis in hepatocellular carcinoma SK-Hep-1 spheroids by upregulating miR-34a expression. Anti-Cancer Agents Med. Chem. 20 (14), 1654–1663. doi:10.2174/1871520620666200424123139
Xu, S., Shang, M.-Y., Liu, G.-X., Xu, F., Wang, X., Shou, C.-C., et al. (2013). Chemical constituents from the rhizomes of Smilax glabra and their antimicrobial activity. Molecules 18 (5), 5265–5287. doi:10.3390/molecules18055265
Yip, E. C. H., Chan, A. S. L., Pang, H., Tam, Y. K., and Wong, Y. H. (2006). Protocatechuic acid induces cell death in HepG2 hepatocellular carcinoma cells through a c-Jun N-terminal kinase-dependent mechanism. Cell Biol. Toxicol. 22, 293–302. doi:10.1007/s10565-006-0082-4
Zhang, J., Feng, M., and Guan, W. (2021). Naturally occurring aesculetin coumarin exerts antiproliferative effects in gastric cancer cells mediated via apoptotic cell death, cell cycle arrest and targeting PI3K/AKT/M-TOR signalling pathway. Acta Biochim. Pol. 68 (1), 109–113. doi:10.18388/abp.2020_5463
Zhang, J., Yang, J., Duan, J., Liang, Z., Zhang, L., Huo, Y., et al. (2005). Quantitative and qualitative analysis of flavonoids in leaves of Adinandra nitida by high performance liquid chromatography with UV and electrospray ionization tandem mass spectrometry detection. Anal. Chim. Acta 532 (1), 97–104. doi:10.1016/j.aca.2004.10.042
Keywords: Ceiba pentandra, antitumor, in vivo, AFP-l3, hepatocellular carcinoma, doxorubicin, UHPLC-Q-ToF-MS/MS, dietary flavonoids
Citation: Orabi MAA, Abouelela ME, Darwish FMM, Abdelkader MSA, Elsadek BEM, Al Awadh AA, Alshahrani MM, Alhasaniah AH, Aldabaan N and Abdelhamid RA (2024) Ceiba pentandra ethyl acetate extract improves doxorubicin antitumor outcomes against chemically induced liver cancer in rat model: a study supported by UHPLC-Q-TOF-MS/MS identification of the bioactive phytomolecules. Front. Pharmacol. 15:1337910. doi: 10.3389/fphar.2024.1337910
Received: 13 November 2023; Accepted: 19 January 2024;
Published: 02 February 2024.
Edited by:
Sandeep Kumar Dash, University of Gour Banga, IndiaReviewed by:
Yaser Karem, Assiut University, EgyptCopyright © 2024 Orabi, Abouelela, Darwish, Abdelkader, Elsadek, Al Awadh, Alshahrani, Alhasaniah, Aldabaan and Abdelhamid. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohamed A. A. Orabi, bW9oYW1lZG9yYWJpQGF6aGFyLmVkdS5lZw==; Mohamed E. Abouelela, bV9hYm91ZWxlbGFAYXpoYXIuZWR1LmVn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.