
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 21 June 2024
Sec. Pharmacology of Anti-Cancer Drugs
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1337179
 Zhi-Hai Wu1,2†
Zhi-Hai Wu1,2† Hai-Feng Zhang3
Hai-Feng Zhang3 Jun-Yan Li4†
Jun-Yan Li4† Yi-Rui Diao5
Yi-Rui Diao5 Man-Jing Huang6
Man-Jing Huang6 Dong-Yang Gao2
Dong-Yang Gao2 Chang-Hao Liang7*
Chang-Hao Liang7* Zhi-Qiang Luo1*
Zhi-Qiang Luo1*Background: The effectiveness and safety of using Brucea javanica oil (BJO) in combination with Transarterial Chemoembolization (TACE) for liver cancer treatment are subjects of debate. This study aims to assess the comparative effectiveness and safety of BJO-assisted TACE versus TACE alone and quantifies the differences between these two treatment methods.
Methods: A systematic search was conducted in multiple databases including PubMed, Cochrane, CNKI, and Wanfang, until 1 July 2023. Meta-analysis was conducted, and the results were presented as mean difference (MD), risk ratio (RR), and 95% confidence intervals (CI).
Results: The search yielded 11 RCTs, with a combined sample size of 1054 patients. Meta-analysis revealed that BJO-assisted TACE exhibited superior outcomes compared to standalone TACE. Specific data revealed that BJO-assisted TACE improves clinical benefit rate by 22% [RR = 1.22, 95% CI (1.15, 1.30)], increases the number of people with improved quality of life by 32%, resulting in an average score improvement of 9.53 points [RR = 1.32, 95% CI (1.22, 1.43); MD = 9.53, 95% CI (6.95, 12.10)]. Furthermore, AFP improvement rate improved significantly by approximately 134% [RR = 2.34, 95% CI (1.58, 3.46)], accompanied by notable improvements in liver function indicators, with an average reduction of 27.19 U/L in AST [MD = −27.19, 95% CI (−40.36, −14.02)], 20.77 U/L in ALT [MD = −20.77, 95% CI (−39.46, −2.08)], 12.17 μmol/L in TBIL [MD = −12.17, 95% CI (−19.38, −4.97)], and a decrease of 43.72 pg/mL in VEGF [MD = −43.72, 95% CI (−63.29, −24.15)]. Most importantly, there was a 29% reduction in the occurrence of adverse reactions [RR = 0.71, 95% CI (0.60, 0.84)].
Conclusion: These findings indicate that BJO-assisted TACE may be considered as a potentially beneficial treatment option for liver cancer patients when compared to standalone TACE. It appears to contribute to improved treatment outcomes, enhanced quality of life, and potentially reduced adverse reactions, suggesting it warrants further investigation as a promising approach for liver cancer treatment.
Systematic Review Registration: identifier CRD42023428948
Liver cancer, a prevalent and consequential malignancy worldwide, exhibits a persistent upward trajectory in terms of mortality rates, consistently holding the third position (Wei et al., 2016; Nio et al., 2017; Chen et al., 2020). Liver cancer’s insidious onset often means that symptoms do not appear until the disease has reached intermediate stages, rendering surgical removal less effective (Nio et al., 2017; Zheng et al., 2018). Transarterial chemoembolization (TACE) serves to impede tumor growth and progression as per the Barcelona Clinic Liver Cancer system (Lacaze and Scotté, 2015). After TACE, tissue hypoxia may facilitate the recurrence of liver cancer (Llovet et al., 1999; Chang et al., 2020). To combat this situation, sorafenib is typically used, but it can lead to gastrointestinal discomfort and other adverse reactions (Colagrande et al., 2015; Keating, 2017; Llovet et al., 2021). Currently, we are working diligently to find more suitable drugs to assist in TACE treatment for liver cancer patients.
Traditional Chinese Medicine (TCM) has a long history of successful utilization in the treatment of cancer tumors (So et al., 2019; Xiang et al., 2019). Recent studies have shed light on the remarkable anti-tumor properties of various components derived from specific TCM formulas (Liu et al., 2019; Vitelli Storelli et al., 2019; Zhang et al., 2019). Among these components, Brucea javanica oil (BJO) has exhibited notable abilities in inhibiting tumor cells (Ren et al., 2011; Pan et al., 2018; Tan et al., 2019; Xie et al., 2019; Fan et al., 2020; Li et al., 2021). Meta-analyses have indicated the potential of BJO as a complementary treatment for various cancers, particularly those affecting the digestive system (Jin et al., 2015; Chen et al., 2022). Additionally, numerous studies have reported its clinical efficacy in assisting TACE for liver cancer (J. Chen et al., 2022; Jin et al., 2015). However, there is currently no evidence-based medicine to demonstrate the efficacy and safety of BJO-assisted TACE in the treatment of liver cancer. Therefore, it is imperative to conduct a meta-analysis to evaluate the effectiveness and safety of BJO-assisted TACE versus standalone TACE in liver cancer patients. This analysis will provide clinicians with essential insights to find more appropriate, efficient, and secure drug regimens for adjuvant TACE treatment in liver cancer patients.
The present systematic review and meta-analysis was conducted in adherence with the PRISMA guidelines and Cochrane Handbook for Systematic Reviews (Moher et al., 2009; Cumpston et al., 2019; Shariati et al., 2023). The study was registered with PROSPERO under the number CRD42023428948. Code and data for this study is publicly available.
A comprehensive search for relevant studies was conducted in various databases including PubMed, Cochrane Library, Wanfang Data Knowledge Service Platform (Wanfang), and China National Knowledge Infrastructure (CNKI). The search period encompassed the inception of these libraries up to 1 July 2023, with no restrictions on source or language. The following keywords (subject in CNKI and Wanfang) were used for retrieval: 1) ‘liver cancer’; 2) ‘brucea javanica oil’. Subsequently, the two groups were connected using the term ‘AND’. For retrieval, the following keywords (MeSH in PubMed and Cochrane Library) and free words were employed: 1) Liver neoplasm, hepatic neoplasms, hepatic neoplasm, cancer of liver, hepatocellular cancer, hepatocellular cancers, hepatic cancer, hepatic cancers, liver cancer, liver cancers, cancer of the liver; 2) Brucea javanica oil, rhus javanica, java brucea, brucea amarissima. Subsequently, the heading terms and free words within each group were connected using the term ‘or’. The two groups were connected using the term ‘and’. Furthermore, a meticulous review of the relevant literature in the included studies was conducted to ensure the identification of all potential studies.
Study design: Randomized controlled trials (RCTs); Patient: All patients clinically diagnosed with liver cancer were not limited by age, region, gender, race, or other factors; However, it was ensured that there were no statistically significant differences in age, gender, and liver function grading between the experimental and control groups; Intervention: The control group received TACE as the intervention, while the experimental group received a combination of TACE and BJO; Type of comparison: The experimental group (BJO-assisted TACE) was compared with the control group (TACE alone); Outcomes: Including one of these outcomes: clinical benefit rate (CBR), quality of life (Karnofsky) (Yates et al., 1980; Clancey, 1995), aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TBIL), alpha fetoprotein (AFP) or vascular endothelial growth factor (VEGF).
All reviews, letters, case reports, conference summaries or records, systematic reviews, scientific and technological achievements and meta-analyses; All animal studies; The outcome data could not be extracted, nor could they be calculated according to the graphs in the article, or the studies obtained by contacting the authors; The experimental group’s intervention included additional types of medicine in addition to BJO and TACE, while the control group’s intervention included additional types of medicine in addition to TACE; No treatment courses recorded.
Two reviewers (Zhi-Hai Wu and Hai-feng Zhang) extracted independently from the full text of the studies that met the screening criteria. After re-checking with Endnote X9 for Windows (Thomson Reuters, United States) literature management software, the preliminary screening was completed by reading the titles and abstracts, and the full text of potential studies was read to determine whether to include them. If necessary, the authors of the original study can be contacted by email or phone to obtain information of critical importance. All information was independently extracted into a Microsoft Excel spreadsheet, including, if any, country of origin, first author, year of publication, study type, a sample size of patients included, interventions, outcome measures, and outcome data were extracted into a standardized form. Results are checked back-to-back and any discrepancies can be resolved by referring to the original study or consulting a third reviewer (Zhi-qiang Luo).
Two independent reviewers evaluated the quality of each enrolled study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0). The assessment covered six key domains: (i) randomization process, (ii) concealment of allocation, (iii) blinding of participants and personnel, (iv) blinding of outcome assessment, (v) handling of incomplete outcome data, and (vi) reporting of outcomes in a non-biased manner. The grading of recommendations assessment, development, and evaluation (GRADE) approach was used to determine the quality of the evidence (Guyatt et al., 2011). Each result is assigned a certainty level (high, moderate, low, or very low) based on the risk of bias, publication bias, indirectness, imprecision, inconsistency, large effect, plausible confounding, and dose–response gradient.
Mean difference (MD) and Risk Ratio (RR) with corresponding 95% confidence intervals (CI) were calculated and reported. Heterogeneity between studies was evaluated using Cochrane Q statistic and I2 statistic. I2 > 50%, along with a p-value <0.10 was treated as an indication for substantial heterogeneity. We used a random effects model in all analyses regardless of heterogeneity measures as evidence has shown more robust effect estimates compared with fixed effect models (Tufanaru et al., 2015). Sensitivity analysis was performed by removing one study at a time to confirm the robustness of outcomes (Higgins et al., 2003). Publication bias was assessed using a funnel plot when the number of included studies exceeded ten.
The initial search strategy yielded a total of 343 records. Subsequent removal of duplicates resulted in the identification of 231 unique records. Of these, 220 studies were excluded based on the predetermined inclusion and exclusion criteria. For example, 53 studies were found to be ineligible as they did not meet the criteria of having the control group intervention as TACE alone and the experimental group intervention as BJO-assisted TACE. Following this rigorous selection process, a total of 11 eligible studies were included in the final analysis (Ding and al., 2008; Fu and al., 2016; Huang and al., 2017; Jia and al., 2003; Keyoumu and al., 2017; T. Li and al., 2012; W. Z. Li and Feng, 2006; Y. Li and al., 2016; Lu and al., 2005; Tu and al., 2014; Y. M. Wei and al., 2009). The visual representation of the literature retrieval process was depicted in Figure 1.
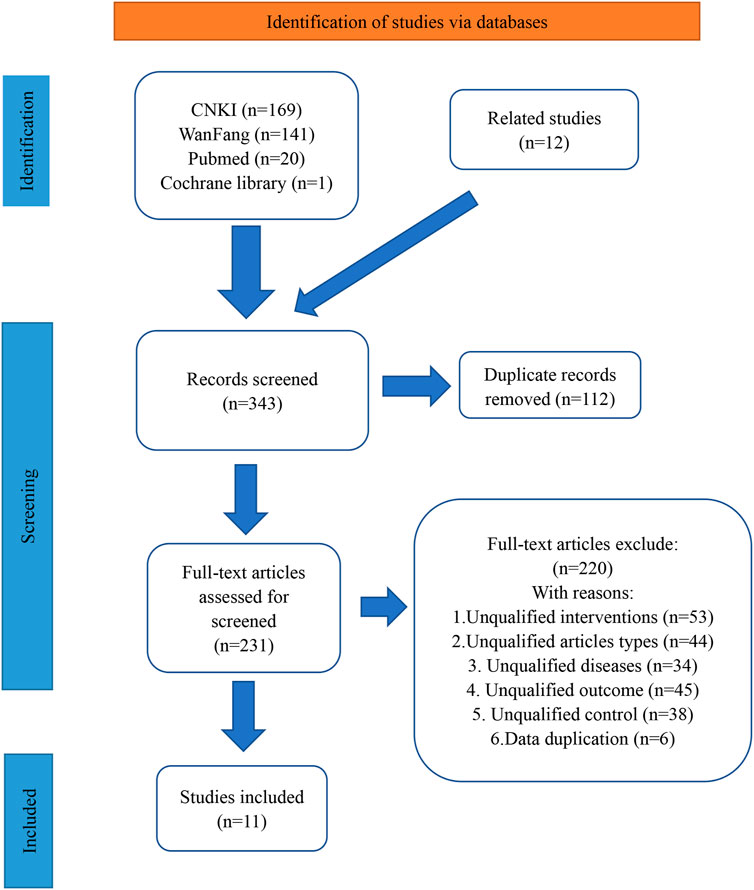
Figure 1. The literature retrieval process Abbreviations: CNKI, China National Knowledge Infrastructure. Annotations: Wanfang represents Wanfang Data Knowledge Service Platform
This study included 11 RCTs conducted within the geographical boundaries of China during the period spanning from 2003 to 2017 (Ding and al., 2008; Fu and al., 2016; Huang and al., 2017; Jia and al., 2003; Keyoumu and al., 2017; T. Li and al., 2012; W. Z. Li and Feng, 2006; Y. Li and al., 2016; Lu and al., 2005; Tu and al., 2014; Y. M. Wei and al., 2009). A total of 1054 patients were included in these trials, with 526 patients assigned to the experimental group and 528 to the control group. The duration of interventions ranged from 6 to 60 days across the various studies. Each study incorporated a minimum of two outcome indicators for comparative analysis. For further details regarding the characteristics of these studies, refer to Table 1.
Table 2 presents the bias risk of 11 studies (Ding and al., 2008; Fu and al., 2016; Huang and al., 2017; Jia and al., 2003; Keyoumu and al., 2017; T. Li and al., 2012; W. Z. Li and Feng, 2006; Y. Li and al., 2016; Lu and al., 2005; Tu and al., 2014; Y. M. Wei and al., 2009). All of these studies employed randomization, but only one study explicitly described the method for concealing the allocation, while the others provided insufficient details regarding blinding procedures. Among the studies included, there were no instances of missing outcome data or selective reporting, resulting in an overall low risk assessment.
All studies compared the clinical benefit rate (CBR) between patients receiving BJO-assisted TACE treatment and those receiving TACE treatment alone (Ding and al., 2008; Fu and al., 2016; Huang and al., 2017; Jia and al., 2003; Keyoumu and al., 2017; T. Li and al., 2012; W. Z. Li and Feng, 2006; Y. Li and al., 2016; Lu and al., 2005; Tu and al., 2014; Y. M. Wei and al., 2009). The meta-analysis results demonstrate a significantly higher CBR in patients who received BJO-assisted TACE treatment compared to those who received TACE treatment alone [RR = 1.22, 95% CI (1.15, 1.30), p < 0.001; I2 = 68%, p < 0.001] (Figure 2A). Sensitivity analysis confirmed the stability of these findings, as there were no significant changes in the results (Supplementary Figure S1). The funnel plot displayed in Supplementary Figure S2 exhibited a cluster of circles in the upper narrow region, suggesting a larger sample size, reduced variance, minimal standard error, and the generation of reliable outcomes.
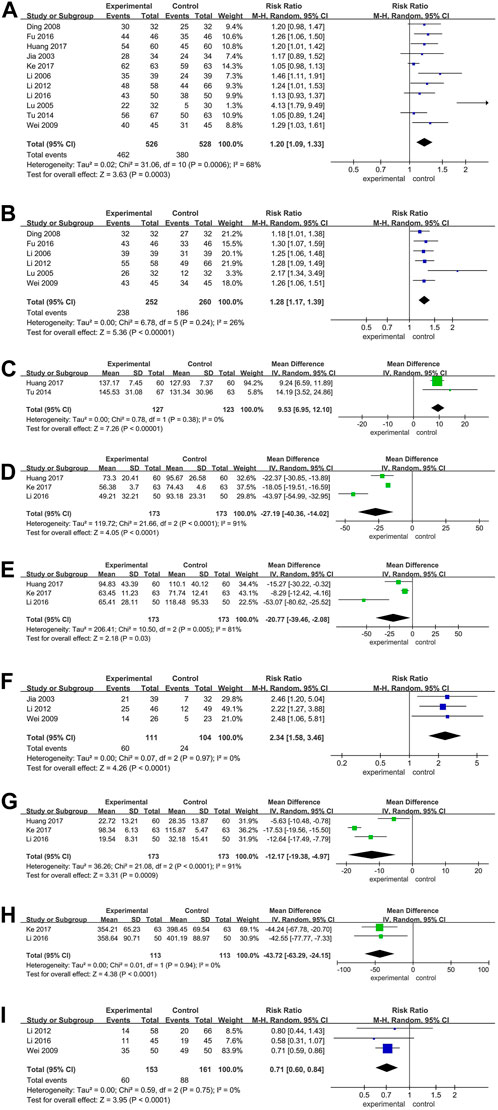
Figure 2. (A) Forest plot of the CBR; (B) Forest plot of the quality-of-life evaluation (dichotomous data); (C) Forest plot of the quality-of-life evaluation (continuous data); (D) Forest plot of the AST; (E) Forest plot of the ALT; (F) Forest plot of the AFP; (G) Forest plot of the TBIL; (H) Forest plot of the VEGF; (I) Forest plot of the adverse reaction. Annotations: In the plot, the 95% confidence interval for each study is represented by the horizontal line and the point estimate is represented by a square. The size of the square is representative of the weight that each study has in the overall effect size estimate. The confidence interval for the overall effect is indicated by a diamond shape at the bottom of the plot. P < 0.05 was considered significant.
Eight studies compared the quality of Life in patients receiving BJO-assisted TACE treatment and those receiving TACE treatment (Ding and al., 2008; Fu and al., 2016; Huang and al., 2017; T. Li and al., 2012; W. Z. Li and Feng, 2006; Lu and al., 2005; Tu and al., 2014; Y. M. Wei and al., 2009). Among these studies, six used binary data (Ding and al., 2008; Fu and al., 2016; T. Li and al., 2012; W. Z. Li and Feng, 2006; Lu and al., 2005; Y. M. Wei and al., 2009), while the remaining two utilized continuous data (Huang and al., 2017; Tu and al., 2014). Separate analyses were conducted for each type of data. The results of the meta-analysis revealed that, in comparison to patients receiving TACE treatment alone, patients who received TACE treatment with BJO assistance showed a significant increase in both the number of patients experiencing improved quality of life and the average quality of life scores [RR = 1.32, 95% CI (1.22, 1.43), p < 0.00001; I2 = 26%, p = 0.24; MD = 9.53, 95% CI (6.95, 12.10), p < 0.00001; I2 = 0%, p = 0.38] (Figures 2B, C).
Three studies compared patients receiving BJO-assisted TACE treatment with those receiving TACE treatment alone in terms of AST levels (Huang and al., 2017; Keyoumu and al., 2017; Y. Li and al., 2016). The meta-analysis results demonstrated that patients receiving BJO-assisted TACE treatment had significantly lower AST levels than those undergoing TACE treatment alone [MD = −27.19, 95% CI (−40.36, −14.02), p < 0.001; I2 = 91%, p < 0.001] (Figure 2D). Sensitivity analysis did not yield significant alterations in the results (Supplementary Figure S3).
Three studies compared the ALT levels of patients receiving BJO-assisted TACE treatment with those receiving TACE treatment (Huang and al., 2017; Keyoumu and al., 2017; Y. Li and al., 2016). The meta-analysis results showed that patients receiving BJO-assisted TACE treatment had significantly lower AST levels than patients receiving TACE treatment [MD = −20.77, 95% CI (−39.46, −2.08), p < 0.05; I2 = 81%, p < 0.01] (Figure 2E). Sensitivity analysis did not yield significant changes in the results (Supplementary Figure S4).
Three studies compared the levels of AFP in patients receiving BJO-assisted TACE treatment with those receiving TACE treatment (Jia and al., 2003; T. Li and al., 2012; Y. M. Wei and al., 2009). The meta-analysis results indicated that patients receiving BJO-assisted TACE treatment had significantly lower AFP levels compared to those receiving TACE treatment [RR = 2.34, 95% CI (1.58, 3.46), p < 0.001; I2 = 0%, p = 0.97] (Figure 2F).
Three studies compared the TBIL levels in patients receiving BJO-assisted TACE treatment to those receiving TACE treatment (Huang and al., 2017; Keyoumu and al., 2017; Y. Li and al., 2016). The meta-analysis results revealed a significant decrease in TBIL levels in patients receiving BJO-assisted TACE treatment compared to those receiving TACE treatment [MD = −12.17, 95% CI (−19.38, −4.97), p < 0.001; I2 = 91%, p < 0.001] (Figure 2G). Sensitivity analysis did not produce any significant changes in the results (Supplementary Figure S5).
Two studies compared the levels of TBIL in patients who received BJO-assisted TACE treatment with those who received TACE treatment alone (Keyoumu and al., 2017; Y. Li and al., 2016). The results of a meta-analysis revealed that the VEGF levels in patients who received BJO-assisted TACE treatment were significantly lower than in patients who received TACE treatment alone [MD = −43.72, 95% CI (−63.29, −24.15), p < 0.001; I2 = 0%, p = 0.94] (Figure 2H).
Two studies compared the adverse reactions in patients who received BJO-assisted TACE treatment and those who received TACE treatment (T. Li and al., 2012; Y. Li and al., 2016). The results of a meta-analysis showed that the adverse reactions in patients who received BJO-assisted TACE treatment were significantly lower than those in patients who received TACE treatment [RR = 0.71, 95% CI (0.60, 0.84), p < 0.001; I2 = 0%, p = 0.75] (Figure 2I).
The GRADE assessment of overall evidence quality ranges from very low to moderate. Specifically, one piece of evidence is of very low quality, three pieces are of low quality, and four pieces are of moderate quality (Table 3).
This study aimed at assessing the effectiveness and safety of BJO-assisted TACE versus TACE for liver cancer patients. Our findings highlighted the notable superiority of BJO-assisted TACE over standalone TACE, particularly in terms of improving the clinical benefit rate (CBR), quality of life, alpha-fetoprotein (AFP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TBIL), and vascular endothelial growth factor (VEGF) levels in liver cancer patients. Additionally, there was a reduction in the occurrence of adverse reactions associated with TACE treatment (Chang et al., 2020).
This systematic review only considered randomized controlled trials of Grade Ⅰ rating, making its overall findings highly reliable. However, the GRADE assessment revealed a low overall quality of evidence, comprising one very low-quality and three low-quality sources of evidence. This is primarily due to the presence of a high risk of bias in the included studies. To address this issue, we require more relevant high-quality clinical research in the future.
The main components of BJO are Brusatol and Bruceine D. These components primarily exert anticancer effects by inhibiting proliferation and promoting apoptosis. Research has found that Brusatol inhibits the progression of liver cancer by weakening STAT3-driven metastasis in liver cancer cells through altering the levels of epithelial–mesenchymal transition related proteins (Lee et al., 2020). By modulating the PI3K/Akt/mTOR signaling pathway, Brusatol effectively inhibits proliferation, induces apoptosis, and thereby suppresses tumor invasion and migration in liver cancer. This pathway plays a crucial role in signal transduction as well as biological processes such as apoptosis, proliferation, metabolism, and angiogenesis (Ye et al., 2018). The anticancer effect of Brusatol is at least partially mediated by the activation of miRNA-29b expression, inducing p53 and further activating the mitochondrial apoptotic pathway (Wang et al., 2021). Bruceine D is capable of inhibiting the proliferation of liver cancer cells and promoting apoptosis by downregulating the expression of β-catenin and jagged 1 (Cheng et al., 2017). Another study indicates that Bruceine D exerts its anti-liver cancer activity by modulating the expression of miR-95 (Xiao et al., 2014). Therefore, the efficacy of BJO-assisted TACE in the treatment of liver cancer may be achieved through the aforementioned pharmacological mechanisms.
Although BJO shows potential as an adjunct in liver cancer therapy, it is crucial to consider potential limitations and adverse effects. Firstly, the precise dosage and administration regimen of BJO for optimal therapeutic effects are not well-established, which could lead to variability in treatment outcomes and potential toxicity. Additionally, the safety profile of BJO, including its potential interactions with other medications or therapies, requires further investigation. Moreover, while studies have highlighted the anticancer effects of Brusatol and Bruceine D, their specific mechanisms of action and potential side effects in the context of liver cancer treatment need to be elucidated further (Fan et al., 2020). Lastly, the long-term effects of BJO treatment on liver function and overall patient outcomes warrant careful monitoring and investigation.
This study represents the first evaluation of the efficacy and safety of BJO-assisted TACE compared to standalone TACE in the treatment of liver cancer with a quantification of the differences between them. This novel finding provides a more suitable drug option for liver cancer patients, improving treatment outcomes and reducing the risk of adverse reactions associated with TACE. The research expands the treatment options available to clinicians when dealing with liver cancer patients, while also introducing new perspectives in the field of liver cancer treatment and broadening the applications of BJO medications.
Limitations identified in this study are as follows: Firstly, the absence of clinical guidance pertaining to the optimal dosage and duration of BJO-assisted TACE for the treatment of liver cancer. Secondly, the included studies were predominantly conducted in China, thereby limiting the generalizability of the findings to populations in other geographical regions. Thirdly, although the meta-analysis incorporated randomized clinical trials, which are considered the most dependable study design in medical research, the quality and methodological biases inherent in each individual study may have varied, potentially influencing the pooled results. Finally, the data sources utilized were restricted to Pubmed, Cochrane, CNKI, and Wanfang databases, possibly leading to the exclusion of relevant studies. To comprehensively explore the impact of BJO-assisted TACE on patients with liver cancer and establish comprehensive clinical guidelines for adjunctive medication, future research endeavors should encompass a greater number of high-quality studies while simultaneously endeavoring to minimize the impact of confounding factors, such as clinical variations, methodological disparities, and statistical discrepancies.
This study suggests potential benefits of BJO-assisted TACE treatment compared to standalone TACE in liver cancer patients, potentially reducing the incidence of adverse reactions associated with TACE. This novel finding introduces promising pharmacological alternatives for TACE combination therapy, offering clinicians additional treatment options for managing liver cancer patients.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Z-HW: Data curation, Formal Analysis, Writing–original draft, Writing–review and editing. H-FZ: Supervision, Conceptualization, Formal analysis, Writing–original draft. J-YL: Data curation, Methodology. SoftwareWriting–original draft. Y-RD: Resources, Visualization, Writing–original draft. M-JH: Validation, Investigation, Writing–original draft. DG: Methodology, Project administration, Validation, Investigation, Writing–review and editing. C-HL: Conceptualization, Writing–original draft. Z-QL: Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by the Fundamental Research Funds for the Central public welfare research institutes of China Academy of Chinese Medical Sciences (No. ZZ16-YQ-044 and No. ZZXT202207).
We are grateful to all the studies that made data publicly available. The corresponding author would like to thank all the co-workers for collecting, managing, and maintaining the data used in this analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1337179/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Sensitivity analysis of the CBR.
SUPPLEMENTARY FIGURE S2 | Funnel plot of the CBR. Abbreviations: RR, risk ratio; SE, standard error.
SUPPLEMENTARY FIGURE S3 | Sensitivity analysis of the AST.
SUPPLEMENTARY FIGURE S4 | Sensitivity analysis of the ALT.
SUPPLEMENTARY FIGURE S5 | Sensitivity analysis of the TBIL.
AFP, alpha-fetoprotein; ALT, alanine aminotransferase; BJO, brucea javanica oil; CBR, clinical benefit rate; PRISMA, preferred reporting items for systematic reviews and meta-analyses; RCTs, relevant randomized controlled trials; TACE, transarterial chemoembolization; VEGF, vascular endothelial growth factor.
Chang, Y., Jeong, S. W., Young Jang, J., and Jae Kim, Y. (2020). Recent updates of transarterial chemoembolilzation in hepatocellular carcinoma. Int. J. Mol. Sci. 21 (21), 8165. doi:10.3390/ijms21218165
Chen, J., Chen, S., Yang, X., Wang, S., and Wu, W. (2022). Efficacy and safety of Brucea javanica oil emulsion injection as adjuvant therapy for cancer: an overview of systematic reviews and meta-analyses. Phytomedicine 102, 154141. doi:10.1016/j.phymed.2022.154141
Chen, Z., Xie, H., Hu, M., Huang, T., Hu, Y., Sang, N., et al. (2020). Recent progress in treatment of hepatocellular carcinoma. Am. J. Cancer Res. 10 (9), 2993–3036. doi:10.1097/00001622-199008000-00012
Cheng, Z., Yuan, X., Qu, Y., Li, X., Wu, G., Li, C., et al. (2017). Bruceine D inhibits hepatocellular carcinoma growth by targeting β-catenin/jagged1 pathways. Cancer Lett. 403, 195–205. doi:10.1016/j.canlet.2017.06.014
Colagrande, S., Regini, F., Taliani, G. G., Nardi, C., and Inghilesi, A. L. (2015). Advanced hepatocellular carcinoma and sorafenib: diagnosis, indications, clinical and radiological follow-up. World J. Hepatol. 7 (8), 1041–1053. doi:10.4254/wjh.v7.i8.1041
Cumpston, M., Li, T., Page, M. J., Chandler, J., Welch, V. A., Higgins, J. P., et al. (2019). Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 10 (10), Ed000142. doi:10.1002/14651858.Ed000142
Ding, X. M. (2008). Observation on the therapeutic effect of TACE combined with Brucea javanica oil emulsion injection in the treatment of primary liver cancer. Chin. J. Misdiagnostics 8 (14), 3314–3315. doi:10.3969/j.issn.1009-6647.2008.14.027
Fan, J., Ren, D., Wang, J., Liu, X., Zhang, H., Wu, M., et al. (2020). Bruceine D induces lung cancer cell apoptosis and autophagy via the ROS/MAPK signaling pathway in vitro and in vivo. Cell Death Dis. 11 (2), 126. doi:10.1038/s41419-020-2317-3
Fu, J. (2016). Curative effect of Brucea Javanica oil emulsion combined with cisplatin on hepatocellular ascites. Shaanxi J. Traditional Chin. Med. 37 (2). doi:10.3969/j.issn.1000-7369.2016.02.001
Guyatt, G. H., Oxman, A. D., Vist, G., Kunz, R., Brozek, J., Alonso-Coello, P., et al. (2011). GRADE guidelines: 4. Rating the quality of evidence--study limitations (risk of bias). J. Clin. Epidemiol. 64 (4), 407–415. doi:10.1016/j.jclinepi.2010.07.017
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Huang, J. N., (2017). Clinical effect of Brucea javanica oil soft capsule combined with transhepatic arterial chemoembolization for advanced liver cancer. 39(6), 106–109.
Jia, Y. S. (2003). Clinical analysis of Brucea javanica oil emulsion combined with interventional therapy for primary liver cancer. China J. Chin. Materia Medica 28 (7), 100–101. doi:10.3321/j.issn:1001-5302.2003.07.031
Jin, W., Han, H., Zhou, S., Wang, Y., Dong, T., and Zhao, C. (2015). Therapeutic efficacy of brucea javanica oil emulsion (BJOE) combined with transcatheter hepatic arterial chemoembolization (TACE) in patients with primary liver cancer. Int. J. Clin. Exp. Med. 8 (10), 18954–18962. (Published October 15, 2015)
Keating, G. M. (2017). Sorafenib: a review in hepatocellular carcinoma. Target Oncol. 12 (2), 243–253. doi:10.1007/s11523-017-0484-7
Keyoumu, A. (2017). Clinical effect of Brucea javanica oil emulsion assisted transcatheter arterial chemoembolization for hepatocellular carcinoma and its effect on plasma endotoxin and vascular endothelial growth factor levels. Clin. J. Med. Officers 45 (8), 851–853.
Lacaze, L., and Scotté, M. (2015). Surgical treatment of intra hepatic recurrence of hepatocellular carcinoma. World J. Hepatol. 7 (13), 1755–1760. doi:10.4254/wjh.v7.i13.1755
Lee, J. H., Mohan, C. D., Deivasigamani, A., Jung, Y. Y., Rangappa, S., Basappa, S., et al. (2020). Brusatol suppresses STAT3-driven metastasis by downregulating epithelial-mesenchymal transition in hepatocellular carcinoma. J. Adv. Res. 26, 83–94. doi:10.1016/j.jare.2020.07.004
Li, K. W., Liang, Y. Y., Wang, Q., Li, Y., Zhou, S. J., Wei, H. C., et al. (2021). Brucea javanica: a review on anticancer of its pharmacological properties and clinical researches. Phytomedicine 86, 153560. doi:10.1016/j.phymed.2021.153560
Li, T. (2012). Clinical observation on 124 cases of hepatocellular carcinoma treated with Brucea oil emulsion combined with hepatic artery intubation. China J. Mod. Med. 22 (13), 91–94. doi:10.3969/j.issn.1005-8982.2012.13.023
Li, W. Z., and Feng, R. Z. (2006). Clinical observation of Brucea javanica oil emulsion injection combined with TACE in the treatment of primary liver cancer. J. Chin. Med. Mater. 29 (6), 632–633. doi:10.3321/j.issn:1001-4454.2006.06.047
Li, Y. (2016). Clinical efficacy of Brucea javanica oil oral emulsion combined with hepatic artery embolization chemotherapy in the treatment of hepatocellular carcinoma and its effect on plasma endotoxin and vascular endothelial growth factor levels. Shaanxi Med. J. 45 (7), 892–893+896. doi:10.3969/j.issn.1000-7377.2016.07.51
Liu, C., Yang, S., Wang, K., Bao, X., Liu, Y., Zhou, S., et al. (2019). Alkaloids from traditional Chinese medicine against hepatocellular carcinoma. Biomed. Pharmacother. 120, 109543. doi:10.1016/j.biopha.2019.109543
Llovet, J. M., Brú, C., and Bruix, J. (1999). Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin. Liver Dis. 19 (3), 329–338. doi:10.1055/s-2007-1007122
Llovet, J. M., Kelley, R. K., Villanueva, A., Singal, A. G., Pikarsky, E., Roayaie, S., et al. (2021). Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 7 (1), 6. doi:10.1038/s41572-020-00240-3
Lu, Y. T. (2005). Clinical effect of Brucea javanica oil emulsion injection on advanced liver cancer. Mod. Prev. Med. 32 (7), 845–846. doi:10.3969/j.issn.1003-8507.2005.07.076
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G., (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339, b2535. doi:10.1136/bmj.b2535
Nio, K., Yamashita, T., and Kaneko, S. (2017). The evolving concept of liver cancer stem cells. Mol. Cancer 16 (1), 4. doi:10.1186/s12943-016-0572-9
Pan, P., Yang, B. X., and Ge, X. L. (2018). Brucea javanica seed oil enhances the radiosensitivity of esophageal cancer by inhibiting hypoxia-inducible factor 1α, in vitro and in vivo. Oncol. Lett. 15 (3), 3870–3875. doi:10.3892/ol.2018.7779
Ren, D., Villeneuve, N. F., Jiang, T., Wu, T., Lau, A., Toppin, H. A., et al. (2011). Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc. Natl. Acad. Sci. U. S. A. 108 (4), 1433–1438. doi:10.1073/pnas.1014275108
Shariati, S., Behroozian, T., Kennedy, S., Caini, S., Herst, P. M., Zhang, L., et al. (2023). Mepitel film for the prevention and treatment of acute radiation dermatitis in breast cancer: a systematic review and meta-analysis of randomized controlled trials. Support Care Cancer 31 (9), 524. doi:10.1007/s00520-023-07982-2
So, T. H., Chan, S. K., Lee, V. H., Chen, B. Z., Kong, F. M., and Lao, L. X. (2019). Chinese medicine in cancer treatment - how is it practised in the east and the west? Clin. Oncol. R. Coll. Radiol. 31 (8), 578–588. doi:10.1016/j.clon.2019.05.016
Tan, B., Huang, Y., Lan, L., Zhang, B., Ye, L., Yan, W., et al. (2019). Bruceine D induces apoptosis in human non-small cell lung cancer cells through regulating JNK pathway. Biomed. Pharmacother. 117, 109089. doi:10.1016/j.biopha.2019.109089
Tu, Y. H. (2014). Clinical application of Brucea javanica oil soft capsules combined with hepatic artery cannula in the treatment of hepatocellular carcinoma. Chin. J. Clin. Oncol. Rehabilitation 21 (6), 691–693.
Tufanaru, C., Munn, Z., Stephenson, M., and Aromataris, E. (2015). Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int. J. Evid. Based Healthc. 13 (3), 196–207. doi:10.1097/xeb.0000000000000065
Vitelli Storelli, F., Molina, A. J., Zamora-Ros, R., Fernández-Villa, T., Roussou, V., Romaguera, D., et al. (2019). Flavonoids and the risk of gastric cancer: an exploratory case-control study in the MCC-Spain study. Nutrients 11 (5), 967. doi:10.3390/nu11050967
Wang, T., Dou, Y., Lin, G., Li, Q., Nie, J., Chen, B., et al. (2021). The anti-hepatocellular carcinoma effect of Brucea javanica oil in ascitic tumor-bearing mice: the detection of brusatol and its role. Biomed. Pharmacother. 134, 111122. doi:10.1016/j.biopha.2020.111122
Wei, D., Zeng, Y., Xing, X., Liu, H., Lin, M., Han, X., et al. (2016). Proteome differences between hepatitis B virus genotype-B- and genotype-C-induced hepatocellular carcinoma revealed by iTRAQ-based quantitative proteomics. J. Proteome Res. 15 (2), 487–498. doi:10.1021/acs.jproteome.5b00838
Wei, Y. M. (2009). Treatment of 45 cases of primary liver cancer with Brucea javanica oil emulsion combined with TACE. Chin. Med. Mod. Distance Educ. China 7 (11), 129–130. doi:10.3969/j.issn.1672-2779.2009.11.088
Xiang, Y., Guo, Z., Zhu, P., Chen, J., and Huang, Y. (2019). Traditional Chinese medicine as a cancer treatment: modern perspectives of ancient but advanced science. Cancer Med. 8 (5), 1958–1975. doi:10.1002/cam4.2108
Xiao, Z., Ching Chow, S., Han Li, C., Chun Tang, S., Tsui, S. K., Lin, Z., et al. (2014). Role of microRNA-95 in the anticancer activity of Brucein D in hepatocellular carcinoma. Eur. J. Pharmacol. 728, 141–150. doi:10.1016/j.ejphar.2014.02.002
Xie, J. H., Lai, Z. Q., Zheng, X. H., Xian, Y. F., Li, Q., Ip, S. P., et al. (2019). Apoptosis induced by bruceine D in human non-small-cell lung cancer cells involves mitochondrial ROS-mediated death signaling. Int. J. Mol. Med. 44 (6), 2015–2026. doi:10.3892/ijmm.2019.4363
Yates, J. W., Chalmer, B., and McKegney, F. P. (1980). Evaluation of patients with advanced cancer using the Karnofsky performance status. Cancer 45 (8), 2220–2224. doi:10.1002/1097-0142(19800415)45:8<2220::aid-cncr2820450835>3.0.co;2-q
Ye, R., Dai, N., He, Q., Guo, P., Xiang, Y., Zhang, Q., et al. (2018). Comprehensive anti-tumor effect of Brusatol through inhibition of cell viability and promotion of apoptosis caused by autophagy via the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Biomed. Pharmacother. 105, 962–973. doi:10.1016/j.biopha.2018.06.065
Zhang, M., Zhang, Y., Zhang, L., and Tian, Q. (2019). Mushroom polysaccharide lentinan for treating different types of cancers: a review of 12 years clinical studies in China. Prog. Mol. Biol. Transl. Sci. 163, 297–328. doi:10.1016/bs.pmbts.2019.02.013
Keywords: liver cancer, transarterial chemoembolization (TACE), brucea javanica oil (BJO), efficacy, meta-analysis
Citation: Wu Z-H, Zhang H-F, Li J-Y, Diao Y-R, Huang M-J, Gao D-Y, Liang C-H and Luo Z-Q (2024) Effectiveness and safety of brucea javanica oil assisted TACE versus TACE in the treatment of liver cancer: a systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 15:1337179. doi: 10.3389/fphar.2024.1337179
Received: 12 January 2023; Accepted: 06 March 2024;
Published: 21 June 2024.
Edited by:
Sumera Zaib, University of Central Punjab, PakistanReviewed by:
Yun K. Tam, Sinoveda Canada Inc., CanadaCopyright © 2024 Wu, Zhang, Li, Diao, Huang, Gao, Liang and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi-Qiang Luo, bHVvemhpcWlhbmdAbnJjLmFjLmNu; Chang-Hao Liang, Y2hhbmdoYW9saWFuZ0BidWNtLmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.