
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 31 May 2024
Sec. Ethnopharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1334474
Purpose: Current pharmacological treatments for Ulcerative Colitis (UC) have limitations. Therefore, it is important to elucidate any available alternative or complementary treatment, and Chinese herbal medicine shows the potential for such treatment. As a traditional Chinese herbal medicine, Danshen-related preparations have been reported to be beneficial for UC by improving coagulation function and inhibiting inflammatory responses. In spite of this, the credibility and safety of this practice are incomplete. Therefore, in order to investigate whether Danshen preparation (DSP) is effective and safe in the treatment of UC, we conducted a systematic review and meta-analysis.
Methods: PubMed, Embase, Cochrane Library, Web of Science, China National Knowledge Infrastructure, Wanfang Database and CQVIP Database were searched for this review.The main observation indexes were the effect of DSP combined with mesalazine or DSP on the effective rate, platelet count (PLT), mean platelet volume (MPV) and C-reactive protein (CRP) of UC. The Cochrane risk of bias tool was used to assess the risk of bias. The selected studies were evaluated for quality and data processing using RevMan5.4 and Stata17.0 software.
Results: A total of 37 studies were included. Among them, 26 clinical trials with 2426 patients were included and 11 animal experimental studies involving 208 animals were included. Meta-analysis results showed that compared with mesalazine alone, combined use of DSP can clearly improve the clinical effective rate (RR 0.86%, 95% CI:0.83–0.88, p < 0.00001) of UC. Furthermore it improved blood coagulation function by decreasing serum PLT and increasing MPV levels, and controlled inflammatory responses by reducing serum CRP, TNF-α, IL-6, and IL-8 levels in patients.
Conclusion: Combining DSP with mesalazine for UC can enhance clinical efficacy. However, caution should be exercised in interpreting the results of this review due to its flaws, such as allocation concealment and uncertainty resulting from the blinding of the study.
Systematic Review Registration: http://www.crd.york.ac.uk/PROSPERO/myprospero.php, identifier PROSPERO: CRD42022293287
Ulcerative colitis (UC) is an inflammatory bowel disease primarily affecting the rectum and colon mucosa, characterized by abdominal pain, diarrhea, and mucopurulent stools as its main symptoms. UC poses serious health and safety risks to patients, significantly impacting their quality of life and increasing mortality (Ungaro et al., 2017). Classified by the World Health Organization as one of the most challenging diseases to treat, UC is difficult to cure and prone to recurrence and cancer. While primarily found in Western countries, its incidence is on the rise in developing nations (Ramos and Papadakis, 2019). There is no sex predilection in UC, with the peak age of onset occurring between 30 and 40 years old (Ungaro et al., 2017). However, its exact etiology remains not fully understood. Conventional anti-inflammatory agents like sulfasalazine and mesalazine have a long history of use, alongside bioimmunosuppressive agents, steroids, and microbiome therapies, which have also shown efficacy (Plichta et al., 2019). Although numerous drugs are available for UC treatment, not all patients respond to these medications, and some carry significant adverse effects. Therefore, efforts to enhance UC treatment efficacy are imperative.The traditional Chinese medicinal herb Danshen, with a history of over 2,000 years, consists of the dried root and rhizome of Salvia miltiorrhiza Bge. Danshen is primarily produced in Hebei, Shanxi, Shandong, and other regions of China. The 2020 edition of the Chinese Pharmacopoeia records that Danshen can activate blood circulation, remove blood stasis, clear heat from the blood, and tranquilize the mind (National Pharmacopoeia Commission, 2020). Consequently, it has traditionally been used to treat various conditions such as cerebrovascular hemorrhage, edema, malignancies, menstrual abnormalities, miscarriages, and cardiovascular diseases (Nwafor et al., 2021). Modern studies have revealed that Danshen exhibits a wide range of complex pharmacological effects. Its chemical components mainly include diterpenoids, triterpenoids, phenolic acids, flavonoids, nitrogenous compounds, lactones, and polysaccharides (Mei et al., 2019; Li et al., 2020; Wan et al., 2020). Among these, diterpenoids and phenolic acids are the primary active components of Danshen. Danshen demonstrates pharmacological effects such as anti-tumor, anti-inflammatory, immunomodulatory, anti-fibrosis, and cardiovascular benefits (Shan et al., 2021).
Due to the diverse structures and extensive pharmacological effects of the active components of Danshen, many scholars have initiated studies on Danshen preparations (Li et al., 2022; Shen et al., 2022). These preparations include Danshen acid B dry powder (Lu et al., 2022), Danshen injection (Wang et al., 2017), Tanshinone capsules (Fu et al., 2022), Sodium Tanshinone IIA Sulfonate (Zhou et al., 2021), and others, which have been widely used in treating various diseases. The anti-inflammatory effects of Danshen extracts and related preparations have been extensively investigated (Yuan et al., 2021). Studies have revealed that Danshen may play a therapeutic role in UC by inhibiting platelet activation (Zhang, 2017), improving blood hypercoagulability (Liu et al., 2011a), antagonizing oxidative damage (Fu et al., 2023), enhancing intestinal flora (Wang et al., 2018a), and regulating inflammatory factors (Min et al., 2020; Peng et al., 2021a). In recent years, Danshen preparations have been widely utilized as adjuvants in UC treatment, with numerous animal and clinical trials demonstrating the reliable efficacy of Danshen in treating ulcerative colitis (Liu et al., 2019; Liu et al., 2020; Wu et al., 2021). However, there remains a lack of reliable evidence-based data to evaluate the efficacy and safety of Danshen. In this study, we comprehensively collected published literature and utilized meta-analysis to systematically evaluate the efficacy and safety of Danshen combined with mesalazine or used alone in UC treatment, employing a large, objective sample size, aiming to provide higher-level evidence for clinical decision-making.
Based on the Cochrane Handbook for Systematic Reviews of Interventions, the meta-analysis and systematic review were performed according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) (Page et al., 2021) and PRISMA-CHM 2020 (PRISMA Extension for Chinese Herbal Medicines 2020) guidelines.
The systematic review and meta-analysis has been registered (Registered with PROSPERO: CRD42022293287, available online at http://www.crd.york.ac.uk/PROSPERO/myprospero.php.
We used “Dan Shen” and “Ulcerative Colitis” as search terms to search various literature and electronic databases for randomized controlled trials from January 2000 to May 2023. These sources included PubMed, Embase, Web of Science, CNKI, Wanfang Database and CQVIP Database.
In particular, medical subject headings (MeSH) terms with free words were employed in English databases. The relevant terms were as follows: ulcerative colitis [MeSH], UC, ulcer colonitis, colitis gravis; DanShen [MeSH], Tan Seng, Chinese Salvia. Chinese databases were searched according to the aforementioned search terms in Chinese. The detailed search strategies containing more search terms are provided in Supplementary Material S1.
We also searched the International Clinical Trial Registry (http://ClinicalTrials.gov/and the Chinese Clinical Trial Registry (Http://www.chictr.org.cn/index.aspx.
We review the inclusion criteria: 1) Participants: had a diagnosis of UC, regardless of gender or ethnicity and all animal models with UC; 2) Intervention: DSP combined with mesalazine was used in the clinical study, and DSP was used in the animal experiment, the amount of herbal medicine in DSP conforms to the standard stipulated by Chinese Pharmacopoeia (2020 edition); 3) Control group: mesalazine alone, same solvent, no intervention, etc.; 4) Study design: randomized controlled trials; 5) Outcomes: effective rate, PLT, MPV and CRP were the primary outcomes, TNF-α, IL-6 and IL-8 were the secondary outcomes; 6) Language: Chinese and English.
We review the exclusion criteria: 1) Duplicate publication; 2) Study design: traditional systematic reviews, case reports, guidelines, recommendations, etc.; 3) in vitro studies, etc.; 4)Not an original full research paper (e.g., conference proceedings, review, abstracts); 5) Control group: other medications.
The selection of material was first screened by 2 reviewers who read the title and abstract of the article independently. The two reviewers then read the full text separately to determine whether the final article should be included. At each stage, the two reviewers would independently record the results of the screening. In the event of disagreement, the two reviewers would negotiate a settlement or a third reviewer would be consulted. The integration process is shown as PRISMA flow chart (Figure 1).
A data collection table was designed in advance, with two reviewers independently extracting and collecting data from the articles involved, including the following contents: 1) Basic information: title, author and year; 2) Baseline data: sample size, age, sex, species, etc.; 3) Intervention information: Figure 15 Traditional Chinese medicine preparation, control intervention medication, intervention time; 4) Outcome measures: effective rate, PLT, MPV, CRP, TNF-α, IL-6 and IL-8. For continuous data (TNF-α, IL-6, IL-8), the mean and the standard deviation of each intervention group were extracted. For dichotomous data (e.g., effective rate), the numbers experiencing the outcome and the total numbers for each intervention group were collected. If a trial contains more than two intervention groups, interventions that meet the eligibility criteria will be included in the review (Higgins and Green, 2011).
Two reviewers assessed the quality of the included clinical studies using Cochrane risk of bias tool (RoB-2 tool). The RoB-2 tool contains 5 entries based on six types of bias: 1) the randomization process; 2) deviations from intended interventions; 3) missing outcome data; 4) measurement of the outcome; and 5) selection of the reported result. The judgments are expressed simply as “low,” “high” or “some concerns” of bias (Sterne et al., 2019).
The methodological quality of included animal studies was evaluated on the basis of the SYRCLE’s RoB tool. The SYRCLE’s RoB tool for animal experiments involves 10 entries based on six types of bias: 1) Sequence generation (selection bias); 2) Baseline characteristics (selection bias); 3) Allocation concealment (selection bias); 4) Random housing (performance bias); 5) Blinding (performance bias); 6) Random outcome assessment (detection bias); 7) Blinding (detection bias); 8) Incomplete outcome data (attrition bias); 9) Selective outcome reporting (reporting bias); (10) Other sources of bias (other). The results of the evaluation are “yes,”“no” and “unclear,” representing “low risk of bias,”“high risk of bias” and “insufficient details have been reported to assess the risk of bias properly” (Hooijmans et al., 2014).
Two reviewers independently assessed the risk of bias. If there is a difference in assessment results, two reviewers should consult or consult with a third party.
This systematic review used RevMan 5.4 (developed by the Cochrane Collaboration international, United Kingdom) software and Random-effects (DerSimonian and Laird) method to analyze the data. The details are as follows: 1) Outcome measures: The standardized mean difference (SMD) was considered for continuous data (e.g., TNF-α, IL-6), while the pooled effect size was expressed as risk ratio (RR) for dichotomous data. 2) Statistically significant: The confidence interval (CI) was established at 95%, and p-value <0.05 was considered to be statistically significant. 3) Between-study heterogeneity: The chi-squared test with a significance level of α = 0.1 was used as statistical measure of heterogeneity, I2 > 50% represented a substantial heterogeneity. 4) Subgroup analyses: According to the intervention duration (<1 month, 1 month, >1 month), composition (DanShen, tanshinone), species (rat, mice) and DanShen application dose, to explore the potential sources of heterogeneity and the influence of various factors on the combined effect size. 5) Sensitivity analysis: Following each document’s exclusion, the new results were re-analyzed and compared with the original results to determine if the results remained stable.
In the case of meaningful outcome indicators where at least 10 studies were included, a funnel plot was used to detect publication bias qualitatively. Begg’s test (Begg and Mazumdar, 1994) and Egger’s test (Egger et al., 1997) were used to evaluate potential publication bias quantitatively.
After retrieval, a total of 322 studies were included, 128 of which were deleted due to duplication. After reading the titles and abstracts of the 194 studies, 142 of them did not meet the requirements and were therefore excluded. The reasons for exclusion were as follows: 1) 1 study was case report; 2) 14 studies were experimental study; 3) 79 studies were not Danshen or UC or mesalazine; 4) 48 other studies. The full text of 52 studies was read with reference to inclusion and exclusion criteria for inclusion in the systematic review, 15 studies were excluded for lack of sufficient data. Eventually, 37 studies (Chen et al., 2005; Liu (2011); Xin et al., 2015; Xu, 2015; Zhang et al., 2015; Deng et al., 2016; Li, 2016; Xiong and Wang, 2016; Liang et al., 2017; Yang and Wang, 2017; Zhou et al., 2017; He, 2018a; He, 2018b; Wang et al., 2018b; Chen et al., 2018; Guo et al., 2018; Liu et al., 2018; Zhu, 2018; Du, 2019; Li, 2019; Li and Tian, 2019; Sun, 2019; Zhu, 2019; Wang, 2020a; Liu et al., 2020; Wang, 2020b; Xu, 2020; Zhang, 2020; Peng et al., 2021b; Wu et al., 2021; Su et al., 2021; Zhao, 2021; Zhu et al., 2021; Fan et al., 2022; Wang, 2022; Zhou, 2022; Zhu et al., 2022) were included in China formed eligible for systematic review, and PRISM flow chart is shown in Figure 1.
The main characteristics of the included clinical studies are shown in Table 1, and the animal experimental studies are shown in Table 2. Studies reported specific outcomes in Table 3. All the 37 studies were randomized controlled trials. Among them, 26 were clinical trials and 11 were animal experiments. The clinical studies involved a total of 2426 participants, 1213in the intervention group and 1213 in the control group. Participants in all studies were both male and female and were from China. Participants were 18–70 years of age in 25 studies, and age was not reported in one study. Participants were recruited from the inpatient or outpatient departments of their respective institutions and all met internationally accepted diagnostic criteria for UC (Winther et al., 1998). In all studies, DSP included two chemical components, namely, DanShen and tanshinone.The treatment strategy for the intervention group was DSP combined with mesalazine and for the control group was mesalazine alone. The duration of treatment ranged from 7 days to 2 months.
A total of 218 animals were included in animal experimental studies studies. All animals in the experimental group was 110 and that in the control group was 108. Animal species including rat and mice were included in meta-analysis. The weight of rats ranged from 200 to 220 g in all studies and that of mice ranged from 15 to 25 g, one study did not report the weight of animals. There were two types of animal models in these studies, namely, TNBS-induced UC in rats and DSS-mediated UC in mice.
All studies mentioned random grouping. The quality of the included literature was evaluated according to the Risk of Bias Table recommended by the Cochrane Manual (ROB-2 tool) and SYRCLE’s RoB tool. The specific results are shown in Figure 2 and Table 4.

Figure 2. Risk of bias assessment of included clinical studies for this review (A) Randomization process; (B) Deviations from intended intervention; (C) Missing outcome data; (D) Measurement of the outcome; (E) Selection of the reported result; (F) Overall.
The 37 included studies were evaluated using the Cochrane Collaboration tool, with two independent reviewers assessing the quality of each study and a third reviewer resolving disagreements.
Twenty-two clinical studies (Liu (2011); Xin et al., 2015; Xu, 2015; Deng et al., 2016; Liang et al., 2017; Yang and Wang, 2017; He, 2018a; He, 2018b; Wang et al., 2018b; Chen et al., 2018; Liu et al., 2018; Zhu, 2018; Du, 2019; Li, 2019; Sun, 2019; Zhu, 2019; Wang, 2020a; Wang, 2020b; Xu, 2020; Zhang, 2020; Zhao, 2021; Wang, 2022) reported effective rate, heterogeneity test results were p = 0.08 and I2 = 31%, indicating that there was no obvious heterogeneity among the results. Using a fixed-effect model, the analysis showed statistically significant levels of efficiency (N = 2072, RR 0.86, 95% CI:0.83–0.88, p < 0.00001), indicating that the combination of DSP and mesalazine was clinically more effective than mesalazine alone Figure 3.
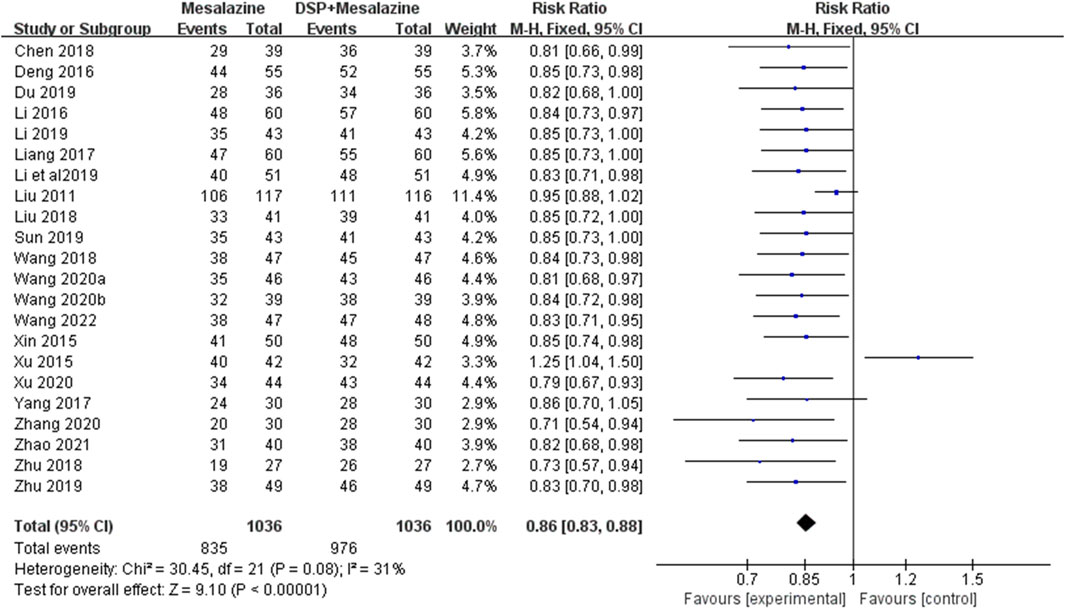
Figure 3. A meta-analysis of clinical efficacy rate in clinical studies of DSP + mesalazine in the treatment of UC.
Seventeen clinical studies (Liu (2011); Xu, 2015; Zhang et al., 2015; Deng et al., 2016; Li, 2016; Xiong and Wang, 2016; Liang et al., 2017; He, 2018a; He, 2018b; Wang et al., 2018b; Liu et al., 2018; Du, 2019; Zhu, 2019; Wang, 2020a; Wang, 2020b; Zhao, 2021; Wang, 2022) reported PLT. No significant heterogeneity (p = 0.12, I2 = 29%) was seen between the same tests and the analysis showed that PLT levels (N = 1712, SMD -37.82%, 95% CI: −41.65 to −34.00, p < 0.00001) were statistically significant, indicating that PLT decreased better in the experimental group than in the control group after treatment (Figure 4).
Eleven clinical studies (Zhang et al., 2015; Deng et al., 2016; Li, 2016; Xiong and Wang, 2016; Liang et al., 2017; He, 2018a; He, 2018b; Liu et al., 2018; Du, 2019; Wang, 2020b; Wang, 2022) reported MPV. As a result of the obvious heterogeneity (p < 0.00001, I2 = 100%) between the same tests, a random effect model was adopted. The analysis showed a statistically significant increase MPV levels (N = 1005, SMD 2.00%, 95% CI:0.76 to 3.24, p = 0.002), suggesting that the combination of DSP and mesalazine was superior to Mesalazine alone after treatment (Figure 5). Subgroup analysis showed that the effects of different time of administration (p = 0.08, I2 = 60.7%) and different DSP components (p = 0.53, I2 = 0%) on CRP levels were not significant, while the effects of different doses (p = 0.03, I2 = 71.3%) of administration were opposite (Table 5).
Nine clinical studies (Xin et al., 2015; Xu, 2015; Zhang et al., 2015; Xiong and Wang, 2016; Yang and Wang, 2017; He, 2018a; He, 2018b; Sun, 2019; Zhang, 2020; Wang, 2022) reported CRP, there was significant heterogeneity among different tests (p < 0.00001, I2 = 83%). Therefore a random effects model was adopted. Statistically significant reduction in CRP levels (N = 718, SMD −4.47, 95% CI: −5.28 to −3.67, p < 0.00001) was observed after treatment with DSP and mesalazine, showing that their combination was superior to mesalazine alone (Figure 6). In none of the subgroups mentioned above, different timing of medication different components of DSP and different administration doses on CRP levels (Table 5).
Fifeen clinical studies (Deng et al., 2016; Li, 2016; Xiong and Wang, 2016; Liang et al., 2017; Wang et al., 2018b; Chen et al., 2018; Zhu, 2018; Du, 2019; Li, 2019; Li and Tian, 2019; Zhu, 2019; Wang, 2020a; Xu, 2020; Zhang, 2020; Wang, 2022) reported TNF-α. The random-effects model was chosen because of significant heterogeneity (p < 0.00001, I2 = 99%) between tests. The difference in TNF-α levels (N = 1365, SMD −41.30, 95%CI: −52.03 to −30.58, p < 0.00001) after treatment was statistically significant, implying that in the control group was not as good as that in the experimental group after treatment (Figure 7). Subgroup analysis showed significant effects of different chemical composition (p = 0.001, I2 = 90.1%), administration time (p < 0.0001, I2 = 97.6%) and administration dose (p < 0.00001, I2 = 94.2%) on TNF-α levels (Table 5).
Ten animal experimental studies (Chen et al., 2005; Zhou et al., 2017; Guo et al., 2018; Peng et al., 2021b; Wu et al., 2021; Su et al., 2021; Zhu et al., 2021; Fan et al., 2022; Zhou, 2022; Zhu et al., 2022) reported TNF-α. The random-effects model was chosen because of significant heterogeneity (p < 0.00001, I2 = 99%) between tests. The pooled effect sizes indicated that DSP could significantly decrease levels compared with the control group (SMD −37.64, 95%CI: −41.01 to −34.27, p < 0.00001) (Figure 8).Subgroup analysis showed significant effects of different species (p < 0.00001, I2 = 99.7%), administration time (p < 0.0001, I2 = 94.7%) and administration dose (p < 0.00001, I2 = 99.4%) on TNF-α levels (Table 5).
Eleven clinical studies (Deng et al., 2016; Xiong and Wang, 2016; Liang et al., 2017; Wang et al., 2018b; Chen et al., 2018; Zhu, 2018; Li, 2019; Li and Tian, 2019; Xu, 2020; Zhang, 2020; Wang, 2022) reported IL-6. We used a random effects model due to significant heterogeneity between the tests. The analysis showed a statistically significant decrease in IL-6 levels (N = 983, SMD −23.89, 95% CI: −31.09 to −16.69, p < 0.00001), suggesting that a better decrease occurred in the experimental group compared with the control group (Figure 9). Subgroup analysis showed that different administration time (p < 0.00001, I2 = 99%) and administration dose (p = 0.04, I2 = 70.0%) of DSP had significant effect on IL-6 level, but different components (p = 0.62, I2 = 0%) had no significant effect (Table 5).
Nine animal experimental studies (Zhou et al., 2017; Guo et al., 2018; Liu et al., 2020; Peng et al., 2021b; Wu et al., 2021; Su et al., 2021; Zhu et al., 2021; Zhou, 2022; Zhu et al., 2022) reported IL-6. The random-effects model was chosen because of significant heterogeneity (p < 0.00001, I2 = 99%) between tests. The pooled effect sizes indicated that DSP could significantly decrease IL-6 levels compared with the control group (SMD −60.88, 95% CI: −65.10 to −56.67, p < 0.00001) (Figure 10). Subgroup analysis showed that there were significant differences in administration time (p < 0.00001, I2 = 97.3%) and dose (p < 0.00001, I2 = 97.6%) had a significant effect on IL-6 levels. Subgroup analysis showed no significant differences among species (p = 0.10, I2 = 63.6%) (Table 5).
Twelve clinical studies (Deng et al., 2016; Li, 2016; Liang et al., 2017; Wang et al., 2018b; Zhu, 2018; Du, 2019; Li and Tian, 2019; Zhu, 2019; Wang, 2020a; Xu, 2020; Zhang, 2020; Wang, 2022) reported IL-8. A random effects model was used because of significant heterogeneity (p < 0.00001, I2 = 99%) between the tests. The results of the analysis (N = 1131, SMD −206.08, 95% CI: −238.04 to −174.13, p < 0.00001) showed that IL-8 levels was statistically significant, indicating that the experimental group had better IL-8 levels than the control group after treatment (Figure 11). Subgroup analysis showed that different chemical components (p < 0.00001, I2 = 99%) of DSP significantly affected the level of IL-8, while different administration time (p = 0.99, I2 = 0%) and species (p = 0.48, I2 = 0%) had opposite effect (Table5).
A total of 14 clinical studies (Zhang et al., 2015; Deng et al., 2016; Liang et al., 2017; Wang et al., 2018b; Zhu, 2018; Du, 2019; Li, 2019; Li and Tian, 2019; Sun, 2019; Zhu, 2019; Wang, 2020a; Xu, 2020; Zhang, 2020; Zhao, 2021; Wang, 2022) reported adverse events, including onek (Zhao, 2021) in which no significant adverse events were found in either group during the study. One of the 13 study reports (Zhang, 2020) did not indicate the specific number of adverse events, and the heterogeneity among studies was low (p = 0.47, I2 = 0%). Random effects model was helpful to analyze adverse reactions. There was no significant difference between the Mesalazine combined DSP group and the Mesalazine single drug group (RR = 0.96, 95% CI = 0.64 to 1.43, p = 0.83; Figure 12). Our results showed that nausea, abdominal pain, diarrhea, and pruritus were the most common adverse events. These adverse reactions were mild and could be relieved spontaneously or after symptomatic treatment. No serious adverse events or deaths were reported in the included studies. In general, TCM is relatively safe as an adjunct to western medicine. See the Supplementary Material S2 for details.
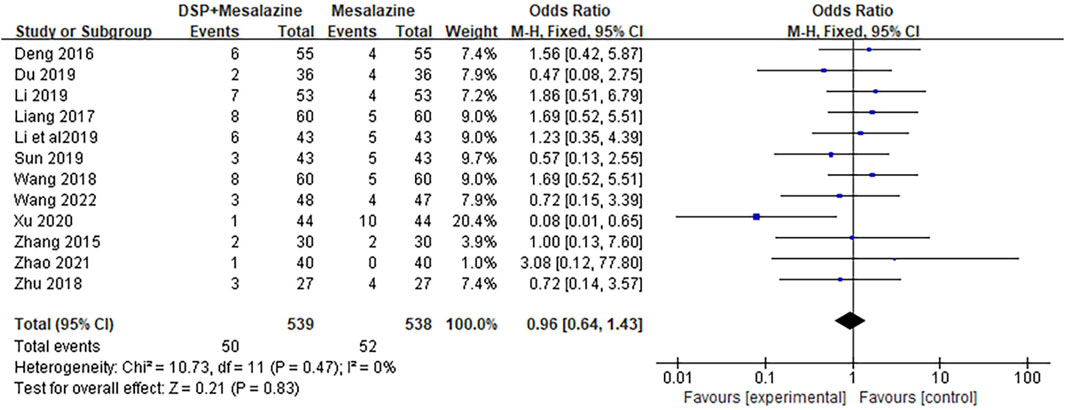
Figure 12. Forest plot of adverse events in clinical studies of DSP + mesalazine in the treatment of UC.
Sensitivity analysis of the above indexes was carried out using the elimination word is repeated twice, with the effects and p-value changes observed after exclusion of the included studies in turn. The results showed no significant changes in the effect sizes of the prognostic indicators TNF-α, IL-6, IL-8, effective rate, PLT and MPV after the exclusion of all included studies, confirming the stability and reliability of the meta-analysis.
Based on the sensitivity analysis, the difference in meta-analysis results excluding other studies did not significantly change for the prognostic indicator CRP. However, after excluding Zhang’s (2015) study, the heterogeneity (p = 0.17, I2 = 32%, N = 658, SMD −4.88, 95% CI: −5.32 to −5.44, p < 0.00001) changed significantly.
Funnel plot for evaluating publication bias of 10 or more articles. Therefore, the validity of PLT, effective rate, MPV, TNF-α, IL-6 and IL-8 met the requirements (Figures 13, 14).
(1) effective rate: Through the observation of funnel plot, it was found that the effect of DSP combined with mesalazine on the effective rate may be asymmetric. While the result of Egger’s test was statistically significant. However, Egger’s test (P = 0.079) and Begg’s test (P = 0.248) found no obvious publication bias.
(2) MPV: Funnel plot observation showed that the effect of DSP combined with mesalazine on MPV levels may be asymmetric. Whereas Egger’s test (P = 0.185) and Begg’s test (P = 0.029) found obvious publication bias.
(3) PLT: In visual inspection of funnel plots, we found that DSP combined with mesalazine had symmetric effects on PLT levels, whereas Egger’s test (P = 0.533) and Begg’s test (P = 0.053) did not indicate significant publication bias.
(4) TNF-α: Visual inspection of funnel plots from clinical studies indicated probable symmetrical for the efficacy of DSP combined with mesalazine on TNF-α levels, however, Egger’s test (P = 0.001) and Begg’s test (P = 0.018) showed significant publication bias.
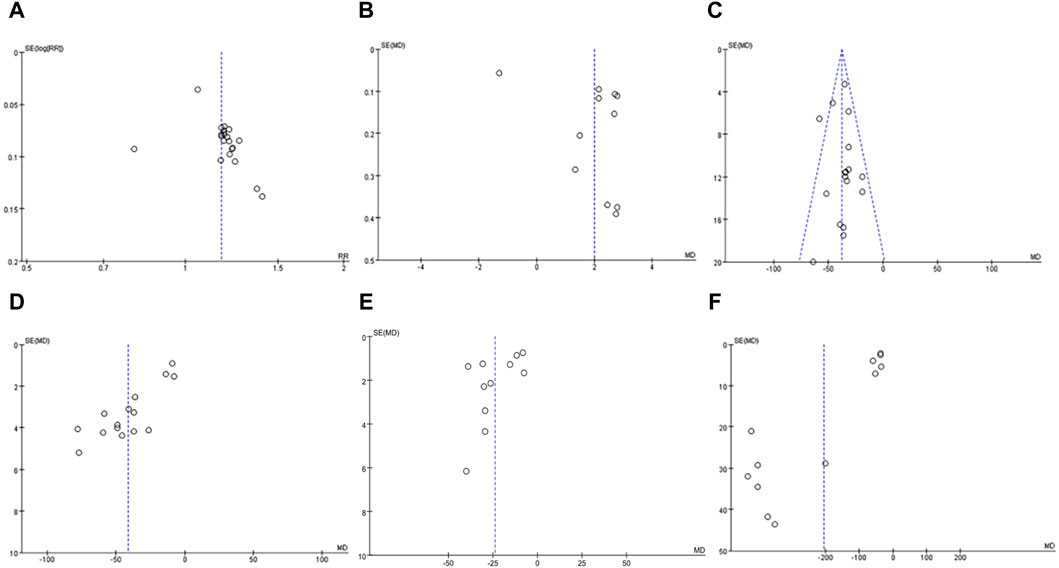
Figure 13. In clinical studies funnel plot of effects of DSP + mesalazine on (A) effective rate; (B) MPV; (C) PLT; (D) TNF-α; (E) IL-6; (F) IL-8.

Figure 15. The mechanism of colitis progression and the effect of Danshen against colitis. This figure was drawn in Figdraw.
Visual inspection of funnel plots from animal studies indicated probable symmetrical for the efficacy of DSP on TNF-α levels, however, Egger’s test (p = 0.008) and Begg’s test (p = 0.049) showed significant publication bias.
(5) IL-6: By observing funnel plot, it was found that the effect of DSP combined with mesalazine on IL-6 levels might be symmetrical, nevertheless, Egger’s test (P = 0.095) and Begg’s test (P = 0.35) demonstrated no significant publication bias.
(6) IL-8: After observation of funnel plot, it was found that the effect of DSP combined with mesalazine on IL-8 level was asymmetric, and this result was supported by Egger’s test (P = 0.000) and Begg’s test (P = 0.011).
A current systematic review and meta-analysis were conducted to evaluate the coagulation function and anti-inflammatory activity of DSP when combined with mesalazine for UC treatment. The results of clinical studies indicate that the combination of mesalazine with DSP significantly improved the clinical efficacy of UC compared to mesalazine alone, a finding supported by animal experiments. Furthermore, it improved coagulation function by reducing PLT levels in patients’ serum and increasing MPV levels, helping control the inflammatory response by reducing CRP, TNF-α, IL-6, and IL-8 levels in patients’ serum. Interestingly, sensitivity analysis, which excluded one study for each stage and response rate, showed that the results for PLT, MPV, TNF-α, IL-6, and IL-8 did not significantly change, whereas Zhang’s study (Zhang et al., 2015) significantly altered the results for CRP. This illustrates that the 95% CI of the results after excluding these studies one by one is less variable, with more overlap, and the results of the meta-analysis are relatively stable, except for CRP.
In recent years, the incidence of UC has stabilized in developed countries such as Europe and the United States, but it is rapidly increasing in several emerging countries in Asia, South America, and the Middle East, posing significant risks to human life (Ha, 2020). Modern medicine remains uncertain about the pathogenesis of UC. Most scholars believe that the pathogenesis of UC is complex and diverse, with research being conducted in the directions of genetics, environment, intestinal microecology, and immune response. As research has progressed, a correlation has been found between UC and coagulation. During the active stage of UC, the body’s blood viscosity increases, coagulation function becomes imbalanced, and the body enters a hypercoagulable state, leading to microthrombosis and impaired microcirculation. This, in turn, results in ischemic necrosis and ulcer formation in the intestinal mucosa, further aggravating the degree of intestinal lesions. Studies have shown that the release of inflammatory factors after platelet activation can promote inflammatory cell infiltration, and inflammatory substances can activate the fibrinolytic-coagulant cascade reaction, resulting in coagulation dysfunction, thus creating a vicious cycle (Polińska et al., 2011; Purnak and Yuksel, 2015; Xu et al., 2017). Danshen is a traditional Chinese medicine known for its ability to promote blood circulation and resolve blood stasis. Pharmacological studies (Dong et al., 2016; Shan et al., 2021) have revealed that Danshen not only improves blood rheology and coagulation but also exhibits anti-inflammatory effects.
Researchers (Tekelioglu et al., 2014; Matowicka, 2016; Gawrońska et al., 2017) have discovered that the number, shape and function of PLT in the serum of UC patients are altered, and that these changes are mainly due to the highly activated state of PLT in the patients’ blood circulation. Meanwhile, most scholars (Lu et al., 2014; Zheng et al., 2018; Zhang et al., 2020) have found a positive correlation between PLT and clinical stage and disease severity of UC, which was also supported by this study. Yüksel’s study (Yüksel et al., 2009) found that MPV was negatively correlated with disease activity in UC, which was consistent with the results of this study. It also examined for the first time the value of MPV in discriminating between active and remitting UC, and concluded that the overall accuracy of MPV in predicting active UC was higher than that of CRP and ESR, implying that MPV is the best indicator for evaluating UC activity. Modern research has shown that the duration of treatment has an effect on the outcome, so it is important to choose the appropriate duration for patient. In this meta-analysis, DSP were available for three different durations (<1 month, 1 month, >1 month), with no significant differences between different treatment courses (p = 0.08). Chemical components of DanShen have been widely used in the treatment of UC, such as tanshinone, but there are no relevant studies on the differences in efficacy of different chemical components for treatment. In this meta-analysis, there was no significant difference in the relationship between DanShen and tanshinone at the MPV levels (p = 0.53). However, three different administration doses (injection ≥20 mL, injection <20 mL, oral medication 1 g), and the difference was statistically significant (p = 0.03).
In our meta-analysis, we discovered that the treatment group was able to significantly reduce PLT and elevate MPV levels, further confirming that DSP combined with mesalazine improved UC by altering intestinal coagulation function. Based on the above findings, we found a crucial problem in the numerous clinical trials, most if not all of which focused on the efficacy of DSP without considering other factors, such as duration of treatment, chemical composition, administered dose, etc. Hence, attention should be paid to the following two aspects in the future clinical trials: Firstly, the conclusion that duration has an effect on the efficacy of DSP needs to be substantiated by more prospective evidence. Secondly, it is necessary to compare the effects of different chemical components in DSP to determine which chemical component is more effective. Thirdly, different administered doses may yield varying levels of bioactive compounds, potentially impacting efficacy.
CRP is an acute phase protein synthesized by hepatocytes in response to inflammatory stimuli such as microbial invasion or tissue damage, which can be used as a marker to evaluate the activity of inflammatory bowel diseases (Sands, 2915). Experimental study (Wang et al., 2022) found that CRP levels were positively correlated with the severity of active UC, which is consistent with the findings of the present study. In our meta-analysis, the difference in CRP levels before and after treatment was statistically significant (p < 0.00001), while no significant difference was found in the relationship between course of treatment (p = 0.21), chemical composition (p = 0.91) and administered dose (p = 0.19) in CRP levels in the subgroup analysis. DSP combined with mesalazine significantly reduced CRP levels, implying that the combination suppressed inflammation and improved UC by inhibiting the expression of CRP.
TNF-α is one of the most important pro-inflammatory cytokines and participates in vasodilation, oedema formation and leukocyte adhesion to epithelial cells through the expression adhesion molecules. It regulates blood clotting, contributes to inflammation at sites of oxidative stress and indirectly leads to fever (Zelová and Hošek, 2013; Zhang et al., 2024) Overexpression of IL-6 causes disturbances in the body’s internal environment, affecting electrolyte secretion in the intestinal epithelium and increasing its permeability, which in turn allows neutrophils to escape and infiltrate into inflammatory site (Li et al., 2010). IL-8 is a pro-inflammatory factor produced by monocytes and macrophages, capable of recruiting monocytes and granulocytes to participate in the progression of local inflammation in the intestine (Chapuy et al., 2020). Experimental studies (Kanda et al., 2020; Li et al., 2021; Ge et al., 2022) have demonstrated an increase in colonic tissue TNF-α, IL-6, and IL-8 during the active phase of UC, a fact that was positively correlated with the disease activity of UC, as confirmed by this study.
In this meta-analysis, DSP in combination with mesalazine was found to suppress inflammation and improve UC symptoms by inhibiting TNF-α, IL-6 and IL-8 expression in the treatment group. However, the comparative efficacy of different chemical components on inflammatory factors in UC patients is still unclear. According to our study, DanShen was more effective in improving IL-8 and TNF-α levels compared to tanshinone. On the other hand, there was no apparent difference in IL-6 levels (p = 0.62) before and after treatment with various components. These contradictory results may be due to the small sample size in the subgroup analysis. For example, only one study was examined in the IL-8 tanshinone group. In order to further clarify the effects of the chemical composition of DSP on the anti-inflammatory capacity of UC treatment, a larger sample size is necessary. Similarly, there was no measurable difference in IL-8 levels (p = 0.99) before and after treatment with different courses of treatment. Treatment duration of 1 month produced the greatest improvement in IL-6 and TNF-α levels compared to both >1 month and <1 month. Again, the reason is that more samples are required to establish the effect of DSP treatment duration on the ability to alleviate inflammation in UC.
Meta-analysis of animal experiments also confirmed this view. It was found that there were significant differences in the levels of TNF-α between different species, different drug time and administration dose. The study found that there were statistically significant differences in IL-6 levels between the time and dose of each drug. However, there was no significant difference in IL-6 level among different species (p = 0.1). This may be due to the insufficient sample size.
Based on the results of the meta-analysis, the combination of DSP and mesalazine demonstrated superior efficacy in treating UC compared to mesalazine alone. However, our systematic review and meta-analysis encountered several limitations, including small sample sizes in most included studies, lack of description regarding blinding, and poor methodological quality. Additionally, due to the short intervention duration of up to 2 months, there was insufficient time to evaluate the efficacy and complications of DSP combined with mesalazine throughout the disease course. Moreover, follow-up after treatment was not reported in the included studies. Heterogeneity among the studies may have influenced the assessment of treatment efficacy in the meta-analysis. Furthermore, adverse events were not well-reported in some studies of DSP combined with mesalazine for UC, necessitating a conservative assessment of their safety. These factors could potentially lead to biased results.
The meta-analysis in this systematic review demonstrated that DSP and mesalazine were effective in the treatment of UC by reducing PLT, TNF-α, IL-6, IL-8, CRP, and elevating MPV levels, thereby improving intestinal coagulation function and inflammation levels. Nevertheless, there are some limitations that need to be addressed in the future. Larger sample sizes of randomised controlled trials, higher methodological quality and longer intervention times, and clinical trials involving more countries will help validate the efficacy and safety of DSP in combination with mesalazine in the treatment of UC.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
WZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. PX: Conceptualization, Investigation, Methodology, Project administration, Validation, Visualization, Writing–original draft, Writing–review and editing. JL: Formal Analysis, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. HH: Data curation, Formal Analysis, Investigation, Software, Supervision, Writing–review and editing. LS: Data curation, Investigation, Methodology, Project administration, Writing–original draft. XL: Conceptualization, Data curation, Project administration, Validation, Writing–original draft, Writing–review and editing. BJ: Conceptualization, Data curation, Funding acquisition, Project administration, Validation, Writing–original draft, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 82074327).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1334474/full#supplementary-material
DSP, DanShen preparation; DSS, dextran sodium sulfate; UC, Ulcerative Colitis; PLT, platelet count; MPV, mean platelet volume; CRP, C-reactive protein; TNBS, 2, 4, 6-trinitrobenzene sulfonic acid; TNF-α, Tumor necrosis factor-α; IL, Interleukin; CT, conventional treatment; 95% CI, 95% confidence interval; RCTs, randomized controlled trials; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses; Mesh, Medical subject headings; SMD, standardized mean difference; RR, risk ratio.
Begg, C. B., and Mazumdar, M. (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics 50 (4), 1088–1101. doi:10.2307/2533446
Chapuy, L., Bsat, M., Rubio, M., Sarkizova, S., Therrien, A., Bouin, M., et al. (2020). IL-12 and mucosal CD14+ monocyte-like cells induce IL-8 in colonic memory CD4+ T cells of patients with ulcerative colitis but not crohn's disease. J. Crohns Colitis 14 (1), 79–95. doi:10.1093/ecco-jcc/jjz115
Chen, X. X., Liang, S. H., and Ma, F. Q. (2018). Effects of DanShen injection combined with mesalazine on serum HIF-1α and inflammatory cytokines levels in patients with mild to moderate active ulcerative colitis. Shanghai J. Tradit. Chin. Med. 52 (07), 55–58.
Chen, Z. W., Xu, H. Y., and Feng, Z. C. (2005). Effect of Danshensu on cytokines in rats with ulcerative colitis. Chin Archives Trad Chin Med (02), 299–304. doi:10.13193/j.archtcm.2005.02.108.chenzhw.048
Deng, W. J., Ma, Y. C., and Ma, L. L. (2016). Mesalazine in combination with Danshen injection for treatment of ulcerative colitis: curative efficacy and effect on inflammatory factors and coagulation parameters. World Chin. J. Dig. 24 (03), 462–466. doi:10.11569/wcjd.v24.i3.462
Dong, C. K., Ma, B. X., and Wang, Y. Z. (2016). Experimental research progress of DanShen injection in improving hemorheology and coagulation function. Henan Tradit. Chin. Med. 36 (02), 355–357.
Du, X. T. (2019). Clinical analysis of Mesalazine combined with DanShen injection in the treatment of ulcerative colitis. China Health Nutr. 29 (33), 218.
Egger, M., Davey, S. G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315 (7109), 629–634. doi:10.1136/bmj.315.7109.629
Fan, L. M., Zhang, Y. Q., Chen, Y. P., Chen, L. L., Xu, W. H., Nan, L. H., et al. (2022). Cryptotanshinone ameliorates dextran sulfate sodium-induced murine acute and chronic ulcerative colitis via suppressing STAT3 activation and Th17 cell differentiation. Int. Immunopharmacol. 108, 108894. Epub 2022 May 31. PMID: 35729830. doi:10.1016/j.intimp.2022.108894
Fu, Q., Huang, M. Y., Tang, L., Zheng, Q., Huang, F. J., Zhou, X., et al. (2022). Tanshinone capsules combined with prednisone for facial seborrheic dermatitis: a systematic review and meta-analysis of randomized clinical trials. Front. Med. 9, 816419. doi:10.3389/fmed.2022.816419
Fu, Y. P., Peng, X., Zhang, C. W., Jiang, Q. X., Li, X. Y., Paulsen, B. S., et al. (2023). Salvia miltiorrhiza polysaccharide and its related metabolite 5-methoxyindole-3-carboxaldehyde ameliorate experimental colitis by regulating Nrf2/Keap1 signaling pathway. Carbohydr. Polym. 306, 120626. doi:10.1016/j.carbpol.2023.120626
Gawrońska, B., Matowicka, K. J., Kralisz, M., and Kemona, H. (2017). Markers of inflammation and influence of nitric oxide on platelet activation in the course of ulcerative colitis. Oncotarget 8 (40), 68108–68114. doi:10.18632/oncotarget.19202
Ge, W., Li, S. P., Zhao, H. M., Liu, D. Y., Cheng, S. M., Wang, H. Y., et al. (2022). Effect of Sishen pill on inflammatory dendritic cells in ulcerative colitis mice with deficiency of spleen and kidney Yang. Chin. Arch. Tradit. Chin. Med., 1–14.
Guo, Y., Wu, X., Wu, Q., Lu, Y., Shi, J., and ChenDihydrotanshinone, X. I. (2018). Dihydrotanshinone I, a natural product, ameliorates DSS-induced experimental ulcerative colitis in mice. Toxicol. Appl. Pharmacol. 344, 35–45. Epub 2018 Feb 27. PMID: 29496522. doi:10.1016/j.taap.2018.02.018
Ha, C. (2020). Epidemiology and pathogenesis of ulcerative colitis. Gastroenterol. Clin. North Am. 49 (4), 643–654. doi:10.1016/j.gtc.2020.07.005
He, H. L. (2018b). Effect of tanshinone combined with mesalazine on coagulation function and tissue TF and TFPI expression in patients with ulcerative colitis. Research of Integrated Tradi. Chin. West Med. 10 (05), 225–228. doi:10.3969/j.issn.1674-4616.2018.05.001
Higgins, J. P. T., and Green, S. (2011). Cochrane Handbook for systematic reviews of interventions version 5.1.0 (updated march 2011). Available at: http://training.cochrane.org/handbook.
Hooijmans, C. R., Rovers, M. M., de Vries, R. B., Leenaars, M., RitskesHoitinga, M., and Langendam, M. W. (2014). SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 14, 43. doi:10.1186/1471-2288-14-43
Kanda, T., Ueda, N., Ikebuchi, Y., Hashiguchi, K., Nakao, K., Isomoto, H., et al. (2020). IL-8 and LYPD8 expression levels are associated with the inflammatory response in the colon of patients with ulcerative colitis. Biomed. Rep. 12 (4), 193–198. doi:10.3892/br.2020.1280
Li, Q. M., and Tian, Z. Y. (2019). Clinical study of tanshinone -A injection combined with mesalazine enteric-coated tablet in treatment of ulcerative colitis. Chin. J. Clin. Pharmacol. 35 (14), 1425–1427.
Li, S. C., Yin, P. H., Chen, T., Chen, C., Li, W. S., and Wang, J. (2021). HuaChan element on the role and mechanism of ulcerative colitis rats. Chin. J. Clin. Pharmacol. (21), 2924–2928.
Li, S. Q., Zhang, S. N., Zhou, L. H., Zhang, F. L., and Liu, W. Y. (2020). Research progress of phenolic acids in Salvia miltiorrhiza and their degradation and transformation in aqueous solution. J. Liaoning Univ. Tradit. Chin. Med. 22 (04), 109–117. doi:10.13194/j.issn.1673-842x.2020.04.028
Li, W. Z., Zhao, F., Yang, J. Y., Pan, J. Y., and Qu, H. B. (2022). Development of a comprehensive method based on quantitative 1H NMR for quality evaluation of Traditional Chinese Medicine injection: a case study of Danshen Injection. J. Pharm. Pharmacol. 74 (7), 1006–1016. doi:10.1093/jpp/rgac034
Li, X. (2016). Clinical efficacy of mesalazine combined with DanShen injection in the treatment of ulcerative colitis and its effect on inflammatory factors and coagulation indicators. J Med Theory Pract 29 (12), 1591–1592.
Li, X. P. (2019). Effect of DanShen powder in the treatment of ulcerative colitis and its effect on Hcy and inflammatory factor levels in patients. Clin Res. Pract 4 (02), 26–27.
Li, Y., de Haar, C., Chen, M., Deuring, J., Gerrits, M. M., Smits, R., et al. (2010). Disease-related expression of the IL6/STAT3/SOCS3 signalling pathway in ulcerative colitis and ulcerative colitis-related carcinogenesis. Gut 59 (2), 227–235. doi:10.1136/gut.2009.184176
Liang, T., Tang, H. B., and Chen, H. J. (2017). Effect of mesalazine and DanShen injection on inflammatory factors and coagulation index of UC patients. Chin. J. Integr. Tradit. West Med. Dig. 25 (01), 27–30.
Liu, C., Jiang, W. J., Hou, Y. T., et al. (2018). Effect of tanshinone capsule combined with mesalazine on hypercoagulability in patients with active ulcerative colitis and its clinical effect evaluation. Chin. J. Crit. Care Med. Electron Ed. 38 (z1), 60.
Liu, C., Yang, J., Zhu, F. C., Li, X. L., and Ma, J. (2019). Efficacy evaluation of salvia miltiorrhiza injection combined with lactic acid bacteria capsule in treatment of ulcerative colitis. Hebei Med. J. 41 (13), 2012–2014+2018.
Liu, W., Liu, Y., Zhang, C. Y., Zhang, X., He, L., and Li, L. (2011). Effect of salvia miltiorrhiza powder injection for injection on bleeding and coagulation in patients with ulcerative colitis. Chin. Arch. Tradit. Chin. Med. 29 (10), 2304–2305. doi:10.13193/j.archtcm.2011.10.154.liuw.045
Liu, X. H., Zhang, L. C., and Ge, W. (2020). Therapeutic effect of tanshinone -A on ulcerative colitis in rats. Chin J Clin. Pharm 36 (16), 2422–2424. doi:10.13699/j.cnki.1001-6821.2020.16.012
Lu, P., Li, J. W., Liu, C. X., Yang, J., Peng, H., Xue, Z. F., et al. (2022). Salvianolic acid B dry powder inhaler for the treatment of idiopathic pulmonary fibrosis. Asian J. Pharm. Sci. 17 (3), 447–461. doi:10.1016/j.ajps.2022.04.004
Lu, Y. L., Shen, H., Zhu, Q. P., and Zhang, L. (2014). Clinical effect of qingchang huashui prescription on ESR,PLT and D-dimer of ulcerative colitis. Chin. J. Exp. Tradit. Med. Formulae 20 (8), 199–202. doi:10.13422/j.cnki.syfjx.2014080199
Matowicka, K. J. (2016). Markers of inflammation, activation of blood platelets and coagulation disorders in inflammatory bowel diseases. Postepy Hig. Med. Dosw (Online) 70, 305–312. doi:10.5604/17322693.1199305
Mei, X. D., Cao, Y. F., Che, Y. Y., Li, J., Shang, J. P., Zhao, W. Y., et al. (2019). Danshen: a phytochemical and pharmacological overview. Chin. J. Nat. Med. 17 (01), 59–80. doi:10.1016/S1875-5364(19)30010-X
Min, X., Zeng, X., Zhao, W., Han, Z., Wang, Y., Han, Y., et al. (2020). Cryptotanshinone protects dextran sulfate sodium-induced experimental ulcerative colitis in mice by inhibiting intestinal inflammation. Phytother. Res. 34 (10), 2639–2648. Epub 2020 Apr 17. PMID: 32302031. doi:10.1002/ptr.6693
National Pharmacopoeia Commission (2020). Pharmacopoeia of the people's Republic of China. China Med. Sci. Tech. Press 77.
Nwafor, E. O., Lu, P., Li, J. W., Zhang, Q. Q., Qi, D., Liu, Z., et al. (2021). Traditional Chinese medicine of Salvia miltiorrhiza Bunge: a review of phytochemistry, pharmacology and pharmacokinetics. Tradit Med Res. 6 (4), 35–25. doi:10.53388/tmr20201027204
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. Mar 29;372:n71. doi:10.1136/bmj.n71
Peng, K. Y., Gu, J. F., Su, S. L., Zhu, Y., Guo, J. M., Qian, D. W., et al. (2021b). Salvia miltiorrhiza stems and leaves total phenolic acids combination with tanshinone protect against DSS-induced ulcerative colitis through inhibiting TLR4/PI3K/AKT/mTOR signaling pathway in mice. J. Ethnopharmacol. 264, 113052. Epub 2020 Jun 11. PMID: 32535239. doi:10.1016/j.jep.2020.113052
Peng, K. Y., Gu, J. F., Su, S. L., Zhu, Y., Guo, J. M., Qian, D. W., et al. (2021a). Salvia miltiorrhiza stems and leaves total phenolic acids combination with tanshinone protect against DSS-induced ulcerative colitis through inhibiting TLR4/PI3K/AKT/mTOR signaling pathway in mice. J. Ethnopharmacol. 264, 113052. doi:10.1016/j.jep.2020.113052
Plichta, D. R., Graham, D. B., Subramanian, S., and Xavier, R. J. (2019). Therapeutic opportunities in inflammatory bowel disease: mechanistic dissection of host-microbiome relationships. Cell 178 (5), 1041–1056. PMID: 31442399; PMCID: PMC6778965. doi:10.1016/j.cell.2019.07.045
Polińska, B., Matowicka-Karna, J., and Kemona, H. (2011). Assessment of the influence of the inflammatory process on the activation of blood platelets and morphological parameters in patients with ulcerative colitis (colitis ulcerosa). Folia Histochem Cytobiol. 49 (1), 119–124. doi:10.5603/fhc.2011.0017
Purnak, T., and Yuksel, O. (2015). Overview of venous thrombosis in inflammatory bowel disease. Inflamm. Bowel Dis. 21 (5), 1195–1203. doi:10.1097/MIB.0000000000000274
Ramos, G. P., and Papadakis, K. A. (2019). Mechanisms of disease: inflammatory bowel diseases. Mayo Clin. Proc. 94 (1), 155–165. doi:10.1016/j.mayocp.2018.09.013
Sands, B. E. (2915). Biomarkers of inflammation in inflammatory bowel disease. Gastroenterology 149 (5), 1275–1285. doi:10.1053/j.gastro.2015.07.003
Shan, X. X., Hong, B. Z., Liu, J., Wang, G. K., Chen, W. D., Yu, N. J., et al. (2021). Research progress on pharmacological action of chemical components of Dan Shen and clinical application and predictive analysis of quality markers. China J. Chin. Mater Med. 46 (21), 5496–5511. doi:10.19540/j.cnki.cjcmm.20210630.203
Shen, Q., Wang, H., Quan, B., Sun, X., Wu, G., Huang, D., et al. (2022). Rapid quantification of bioactive compounds in Salvia miltiorrhiza Bunge derived decoction pieces, dripping pill, injection, and tablets by polarity-switching UPLC-MS/MS. Front. Chem. 10, 964744. doi:10.3389/fchem.2022.964744
Sterne, J. A. C., Savovi´c, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898. doi:10.1136/bmj.l4898
Su, L., Su, Y., An, Z., Zhang, P., Yue, Q., Zhao, C., et al. (2021). Fermentation products of Danshen relieved dextran sulfate sodium-induced experimental ulcerative colitis in mice. Sci. Rep. 11 (1), 16210. PMID: 34376708; PMCID: PMC8355158. doi:10.1038/s41598-021-94594-7
Sun, S. F. (2019). Clinical analysis of mesalazine combined with DanShen injection in treatment of ulcerative colitis. Guide China Med (04), 60–61. doi:10.15912/j.cnki.gocm.2019.04.051
Tekelioglu, Y., Uzun, H., and Sisman, G. (2014). Activated platelets in patients suffering from inflammatory bowel disease. Bratisl. Lek. Listy 115 (2), 83–85. doi:10.4149/bll_2014_018
Ungaro, R., Mehandru, S., Allen, P. B., Peyrin-Biroulet, L., and Colombel, J. F. (2017). Ulcerative colitis. Lancet 389 (10080), 1756–1770. doi:10.1016/S0140-6736(16)32126-2
Wan, X. H., Wang, Y. L., Zhou, C. Z., Guo, H., Ma, S., and Wang, L. Z. (2020). Advances in chemical constituents and pharmacological effects of Salvia miltiorrhiza. Chin. Tradit. Herb. Drugs 51 (03), 788–798. doi:10.7501/j.issn.0253-2670.2020.03.032
Wang, K., Yang, Q., Ma, Q., Wang, B., Wan, Z., Chen, M., et al. (2018a). Protective effects of salvianolic acid A against dextran sodium sulfate-induced acute colitis in rats. Nutrients 10 (6), 791. doi:10.3390/nu10060791
Wang, L., Luo, R. J., Li, N., Jiang, S. S., Xu, B. J., and Liu, Y. D. (2022). Effects of Kuijie Prescription on the clinical efficacy and serum inflammatory factor CRP, TNF-α in patients with ulcerative colitis. Chin. Arch. Tradit. Chin. Med. 40 (04), 89–92. doi:10.13193/j.issn.1673-7717.2022.04.018
Wang, L. Y., Yu, J. H., Fordjour, P. A., Xing, X. X., Gao, H., Li, Y. Y., et al. (2017). Danshen injection prevents heart failure by attenuating post-infarct remodeling. J. Ethnopharmacol. 205, 22–32. doi:10.1016/j.jep.2017.04.027
Wang, W. J. (2022). Clinical effect of mesalazine + salvia miltiorrhiza injection in the treatment of ulcerative colitis. Med And Health 11, 154–157.
Wang, W. W., Wei, S. C., and Xing, W. T. (2018b). Effects of DanShen injection on D-dimer, PT, FIB and PLT in subjects with ulcerative colitis. J. Clin. Exp. Med. 17 (08), 834–838. doi:10.3969/j.issn.1671-4695.2018.08.016
Wang, X. J. (2020a). Clinical effect of salvia miltiorrhiza injection combined with mesalazine in the treatment of ulcerative colitis. Med And Health 11, 93–94.
Wang, X. M. (2020b). Effect of mesalazine combined with DanShen injection on ulcerative colitis. Chin. Pract. Med. (03), 135–137. doi:10.14163/j.cnki.11-5547/r.2020.03.06
Winther, K. V., Føgh, P., Thomsen, O. Ø., and Brynskov, J. (1998). Inflammatory bowel disease (ulcerative colitis and Crohn's disease): diagnostic criteria and differential diagnosis. Drugs Today (Barc) 34 (11), 935–942. doi:10.1358/dot.1998.34.11.487477
Wu, X. Q., Huang, W. F., Kong, D. S., Zhou, W. K., and Fan, Z. M. (2021). To investigate the therapeutic effect of tanshinone ⅡA on ulcerative colitis and its mechanism based on bioinformatics. Chin. Pharm. Bull. 37 (12), 1750–1756. doi:10.3969/j.issn.1001-1978.2021.12.020
Xin, K. B., Zhang, J. L., Yang, J., Dian, X. P., Wei, H. N., et al. (2015). Clinical efficacy of tanshinone ⅡA sodium sulfonate injection in the treatment of ulcerative colitis. Prog. Mod. Biomed. (34), 6701–6703 + 6623. doi:10.13241/j.cnki.pmb.2015.34.025
Xiong, D. S., and Wang, Y. B. (2016). Effects of mesalazine combined with tanshinone on inflammatory factors and hypercoagulability in patients with ulcerative colitis. J. Hainan Med. Univ. 22 (22), 2718–2720. doi:10.13210/j.cnki.jhmu.20160814.017
Xu, D. Y. (2020). Application of Melazine combined with DanShen injection in the treatment of ulcerative colitis. Chin. Community Dr. 36 (20), 76–77. doi:10.3969/j.issn.1007-614x.2020.20.043
Xu, F., Chen, L., and Tu, B. (2017). Association of coagulation disorder and inflammatory bowel disease. Mod. J. Integr. Tradit. Chin. West Med. 26 (22), 2506–2508. doi:10.3969/j.issn.1008-8849.2017.22.039
Xu, Y. F. (2015). Effect of tanshinone capsule combined with mesalazine on hypercoagulability in patients with active ulcerative colitis and its clinical efficacy analysis. Chin. J. Integr. Tradit. West Med. Dig. 23 (04), 245–249. doi:10.3969/j.issn.1671-038X.2015.04.06
Yang, L. N., and Wang, J. (2017). DanShen injection combined beauty salad oxazine clinical observation on treatment of ulcerative colitis. Clin. Med. 5 (4), 117–119. doi:10.19528/j.issn.1003-3548.2017.04.055
Yuan, J., Du, H., Wan, M. X., Li, Z., Zhang, Y. X., Li, D. K., et al. (2021). Research progress on effective components of Salvia miltiorrhiza and anti-inflammatory pharmacological effects of salvia miltiorrhiza preparations. J. Drug Eval. 44 (11), 2322–2332. doi:10.7501/j.issn.1674-6376.2021.11.005
Yüksel, O., Helvaci, K., Başar, O., Köklü, S., Caner, S., Helvaci, N., et al. (2009). An overlooked indicator of disease activity in ulcerative colitis: mean platelet volume. Platelets 20 (4), 277–281. doi:10.1080/09537100902856781
Zelová, H., and Hošek, J. (2013). TNF-αsignalling and inflammation: interactions between old acquaintances. Inflamm. Res. 62 (7), 641–651. doi:10.1007/s00011-013-0633-0
Zhang, H., Zhang, M., Wang, J. L., et al. (2015). Effect analysis of mesalazine combined with tanshinone capsule on active ulcerative colitis. Prog. Mod. Biomed. 15 (34), 6704–6707. doi:10.13241/j.cnki.pmb.2015.34.026
Zhang, H. L. (2017). Application of salvianolate in the treatment of ulcerative colitis. Chin. J. Mod. Drug Appl. 11 (21), 115–116. doi:10.14164/j.cnki.cn11-5581/r.2017.21.063
Zhang, J. (2020). Effects of tanshinone ⅡA combined with mesalazine on inflammatory factors and MHC-Ⅱ levels of colonic mucosa in patients with ulcerative colitis. Chin. Community Dr. 36 (33), 95–96+99. doi:10.3969/j.issn.1007-614x.2020.33.046
Zhang, T. H., Shen, H., and Zhu, L. (2020). A study on the relationship between colitis and blood hypercoagulability. China J. Tradit. Chin. Med. Pharm. 35 (08), 4156–4158.
Zhao, L. (2021). Effect of tanshinone capsule combined with mesalazine on coagulation function and oxidative stress in patients with ulcerative colitis. Heilongjiang Med. Pharm. 44 (03), 160–161.
Zheng, K., Shen, H., Gu, P. Q., Zhang, S. S., Zhao, W. X., and Wang, C. J. (2018). Effect of qingchang huashi prescription on platelet of mild and moderate ulcerative colitis. Lishizhen Med. Mater Med. Res. 29 (03), 624–625. doi:10.3969/j.issn.1008-0805.2018.03.036
Zhou, H. F., Zhao, Y., Peng, W. H., Han, W. B., Wang, Z. C., Ren, X. X., et al. (2021). Effect of sodium tanshinone IIA sulfonate injection on blood lipid in patients with coronary heart disease: a systematic review and meta-analysis of randomized clinical trials. Front. Cardiovasc Med. 8, 770746. doi:10.3389/fcvm.2021.770746
Zhou, W. K. (2022) To explore the mechanism of salvia miltiorrhiza in the treatment of ulcerative colitis based on network pharmacology. Nanjing, Jiangsu, China: Nanjing University of TCM. doi:10.27253/d.cnki.gnjzu.2021.000468
Zhou, Y., Li, A., and Zhu, J. (2017). Tanshinone IIA alleviates DSS-induced acute colitis in mice by regulating neutrophil activity. J Toxicol. 31 (02), 84–88. doi:10.16421/j.cnki.1002-3127.2017.02.002
Zhu, G., Wu, X., Jiang, S., Wang, Y., Kong, D., Zhao, Y., et al. (2022). The application of omics techniques to evaluate the effects of Tanshinone IIA on dextran sodium sulfate induced ulcerative colitis. Mol. Omics 18 (7), 666–676. PMID: 35670211. doi:10.1039/d2mo00074a
Zhu, H., Tong, S., Cui, Y., Wang, X., and Wang, M. (2021). Tanshinol alleviates ulcerative colitis by promoting the expression of VLDLR. Drug Dev. Res. 82 (8), 1258–1268. Epub 2021 Jun 18. PMID: 34145621; PMCID: PMC9290650. doi:10.1002/ddr.21840
Zhu, H. Y. (2018). Effect of Mesalazine combined with DanShen injection on inflammatory factors in patients with ulcerative colitis. Contemp. Med. 24 (01), 100–101. doi:10.3969/j.issn.1009-4393.2018.01.051
Keywords: Danshen, Danshen preparation, mesalazine, ulcerative colitis, meta-analysis, systematic review
Citation: Zhang W, Xiong P, Liu J, Hu H, Song L, Liu X and Jia B (2024) A systematic review and meta-analysis of Danshen combined with mesalazine for the treatment of ulcerative colitis. Front. Pharmacol. 15:1334474. doi: 10.3389/fphar.2024.1334474
Received: 09 November 2023; Accepted: 22 April 2024;
Published: 31 May 2024.
Edited by:
William Chi-Shing Tai, Hong Kong Polytechnic University, Hong Kong SAR, ChinaReviewed by:
Rehan Khan, Rutgers University, Newark, United StatesCopyright © 2024 Zhang, Xiong, Liu, Hu, Song, Liu and Jia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinglong Liu, bGl1eGluZ2xvbmdjZHV0Y21AMTI2LmNvbQ==; Bo Jia, SmlhYm9jaGVuZ2R1dGNtQDE2My5jb20=
‡ORCID: Bo Jia, https://orcid.org/0000-0002-8161-843x
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.