- 1Department of Pharmacy, The 940th Hospital of Joint Logistic Support Force of PLA, Lanzhou, China
- 2School of Medicine, Tibet University, Lhasa, China
- 3School of Pharmacy, Gansu University of Chinese Medicine, Lanzhou, Gansu, China
Ligustrum lucidum W.T. Aiton is an outstanding herb with the homology of medicine and food. Its ripe fruits are traditionally used as an important tonic for kidneys and liver in China. Ligustrum lucidum W.T. Aiton is rich in nutritional components and a variety of bioactive ingredients. A total of 206 compounds have been isolated and identified, they mainly include flavonoids, phenylpropanoids, iridoid glycosides, and triterpenoids. These compounds exert anti-osteoporosis, anti-tumor, liver protective, antioxidant, anti-inflammatory, and immunomodulatory effects. Ligustrum lucidum W.T. Aiton has been traditionally used to treat many complex diseases, including osteoporotic bone pain, rheumatic bone, cancer, related aging symptoms, and so on. In the 2020 Edition of Chinese Pharmacopoeia, there are more than 100 prescriptions containing L. lucidum W.T. Aiton. Among them, some classical preparations including Er Zhi Wan and Zhenqi fuzheng formula, are used in the treatment of various cancers with good therapeutic effects. Additionally, L. lucidum W.T. Aiton has also many excellent applications for functional food, ornamental plants, bioindicator of air pollution, algicidal agents, and feed additives. Ligustrum lucidum W.T. Aiton has rich plant resources. However, the application potential of it has not been fully exploited. We hope that this paper provides a theoretical basis for the high-value and high-connotation development of L. lucidum W.T. Aiton in the future.
1 Introduction
Ligustrum lucidum W.T. Aiton (L. lucidum), also known as glossy privet, is a popular species of flowering plant that belongs to the olive family Oleaceae. It has been cultivated and distributed in most areas of China and many parts of the world since ancient times, and has rich plant resources (Liu et al., 2019). With the prosperous development of the social economy and the persistent improvement of people’s living standards, it has become increasingly popular globally that preventing ailments via diet is an effective avenue to ensure the health of people in recent decades (Duque-Buitrago et al., 2023). Medicine food homology (MFH) materials just cater to the demand. The theory of MFH is long-standing in China, which systematically elaborates on how to combine foods and drugs for health and medical properties (Gong et al., 2020). It means that traditional Chinese medicine (TCM) and food occur simultaneously. MFH combines the virtue of drug and food perfectly and thus can be utilized both for drug and food. Besides the effect on the prevention and treatment of ailments, MFH materials also usually possess many other nutritional values and healthcare functions (Lerner and Benzvi, 2021). To ensure the safe application, the China Food and Drug Administration (CFDA) has authorized specific provisions on MFH items. Ligustrum lucidum is not only a unique herbal remedy for tonifying the liver and kidney in TCM, but also a significant MFH material released by CFDA. In other words, L. lucidum has been applied as both food and medicine in numerous conventional medical systems throughout history.
As a good MFH material, it is broadly recognized that L. lucidum possesses the function of health preservation and prolonging the life span through invigorating the liver and kidney deficiency, and is also widely used in healthy food. Additionally, L. lucidum is one of the most popular green dietary supplements utilized by tumor patients all over the world. It is also consumed as herbal tea in Korea and India. It is noteworthy that L. lucidum also possesses many unique properties, such as being a health promoter utilized by animals, a general bioindicator of air pollution environments, the treatment of eutrophic water, and an outstanding ornamental plant, and is widely used in food, livestock husbandry, gardens, and other fields.
In conclusion, as a kind of potential treasure trove for medicine, functional foods, feed additives, and other industries, L. lucidum has extremely high exploitation value. In the present study, we used the VOS-viewer to analyze the co-occurrence of links between keywords of the publications related to L. lucidum in the Web of Science, which is a total of 219 pieces of relevant literature up to April 2024 (Figure 1). This review not only focuses on the phytochemicals and pharmacological properties of L. lucidum, but also for the first time reviews the application of L. lucidum in terms of economics based on its translational applications. We hope that this paper provides a theoretical basis for high-value and high-connotation development of L. lucidum in the future.
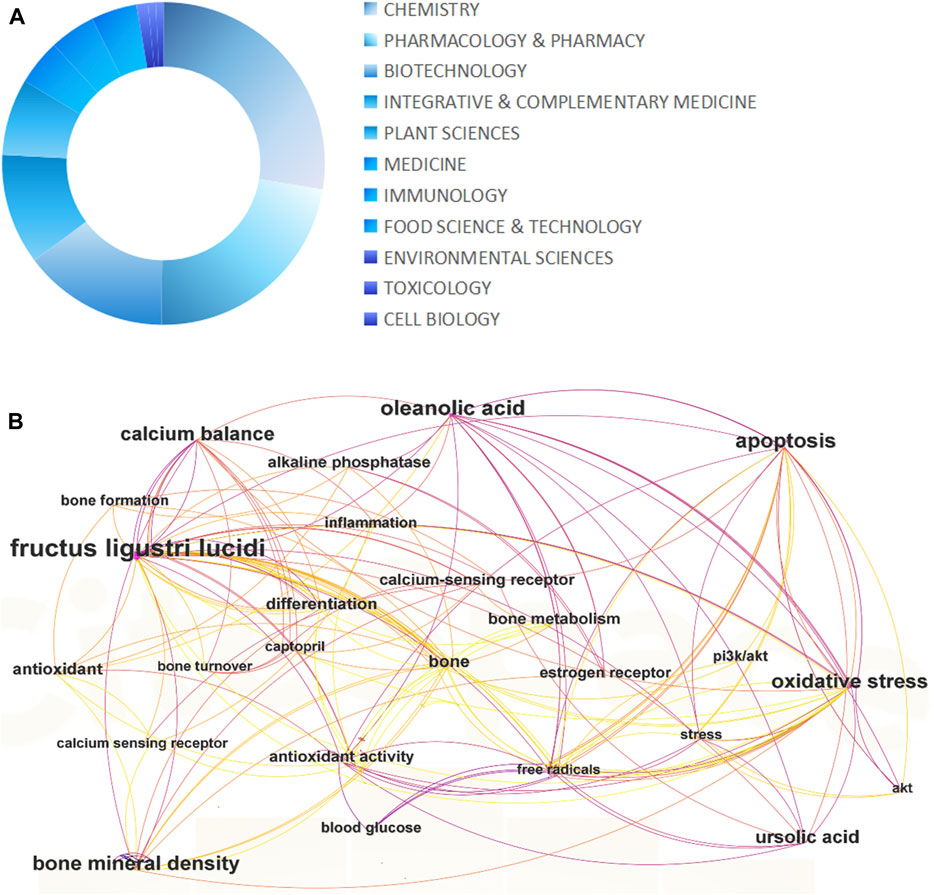
Figure 1. Number of articles (>5 articles) about different research interests of Ligustrum lucidum W.T. Aiton as of April 2024, based on the Web of Science (A), analysis of the co-occurrence links between keywords that occur at least five times in the selected articles (B).
2 Botanical description
Ligustrum lucidum W.T. Aiton (also named “Nvzhenzi” in China and “Dang-gwang-na-mu” in Korea), belongs to the Oleaceae family (Jang et al., 2020). It was recorded in the Compendium of Materia Medica that L. lucidum kept verdant rather than withering even in the depth of winter, seemed to have the virtue of a chaste woman. And thus, the name of Nv Zhen was obtained since ancient times in China. According to “The Plant List,” L. lucidum is the only accepted name for this MFH plant, with other 18 synonyms. The synonyms with the highest confidence levels include Esquirolia sinensis H. Lév., Ligustrum esquirolii H. Lév., Ligustrum magnoliifolium Dippel, Ligustrum roxburghii Blume, Ligustrum wallichii Vis., Olea chinensis Sweet, Olea clavata G. Don, Phillyrea paniculata Roxb., Phillyrea terminalis B. Heyne ex Wall., and Visiania paniculata (Roxb.) DC. (https://mpns.science.kew.org/).
As shown in Figure 2, L. lucidum is a dramatic evergreen arbor tree, usually utilized as an ornamental tree for landscape. The hairless leaves are opposite, generally oval to oblong-oval or broadly elliptic, base rounded, thinly coriaceous, glossy dark green. The flowers are creamy or white and have a heavy fragrance. The inflorescences are broadly pyramid to cone in shape, 100–250 mm long and 200 mm wide. Young fruits are usually obovoid or ovoid in shape, and green in color. Ripe fruits are in large clusters of shiny purplish-black berries, which are 6–8 mm in diameter, endocarps thickly papery. Seeds are oval, 5–7 mm long, and 3 mm in diameter, typically one or two per fruit (Urcelay et al., 2019).

Figure 2. The whole plant (A), leaf (B), flower (C), fresh ripe fruit (D), desiccative ripe fruit (E), and wine-processed product (F) of Ligustrum lucidum W.T. Aiton.
Ligustrum lucidum is mainly native to the southern half of China and Korea, whereas it was subsequently introduced for use as a garden and landscape ornamental plant in many other countries worldwide as well, such as Spain, Argentina, Italy, South Africa, Japan, Australia, the southern United States, and so on. In the late 19th century (Meiji Era), L. lucidum was first naturalized for use as an urban greening species in Japan. Whereas in Argentina, this herbal plant has been cultivated extensively since 1900 as hedging (Ren et al., 2020) L. lucidum is suitable for occurring in hot, full-sun and humid climates, where it grows fast, reaching 15 m or more of height. In China, this folk plant is distributed in at least 17 provinces, including Zhejiang, Jiangsu, Hunan, Sichuan, Anhui, Fujian, Gansu, Guangdong, Guangxi, Guizhou, Hainan, Henan, Hubei, Jiangxi, Shaanxi, Xizang, Yunnan (https://eol.org/pages/487035/articles#cite_note-12). As a consequence, there are abundant wild resources of L. lucidum worldwide.
3 Nutritional value and chemical composition
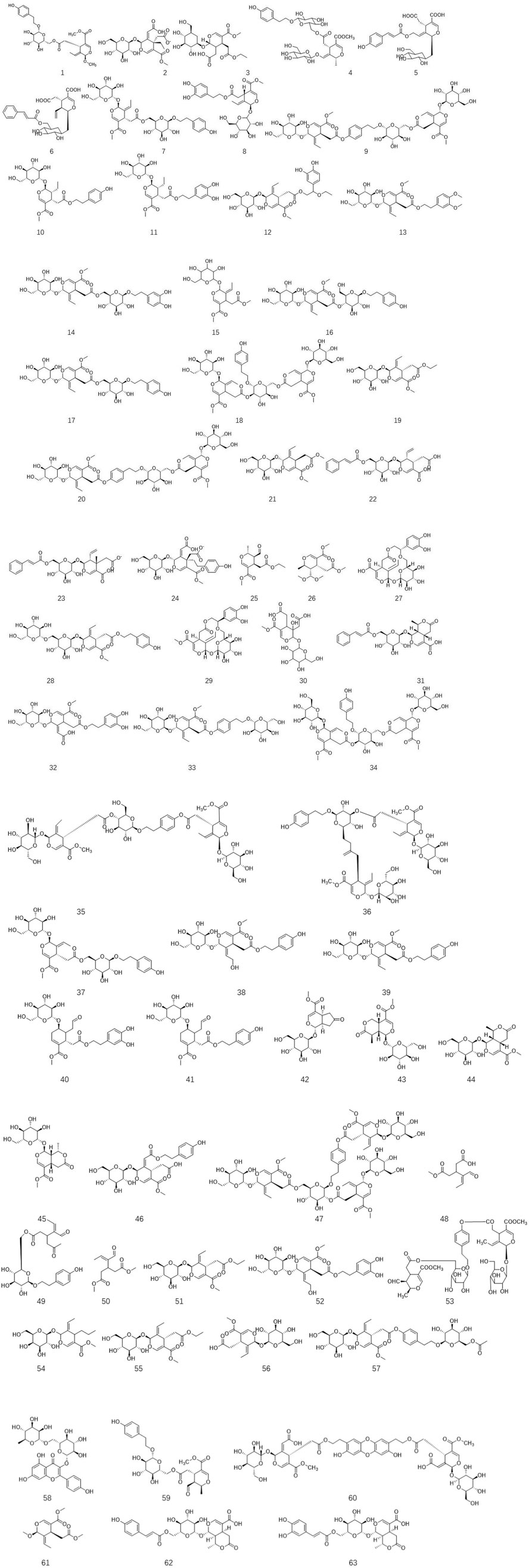
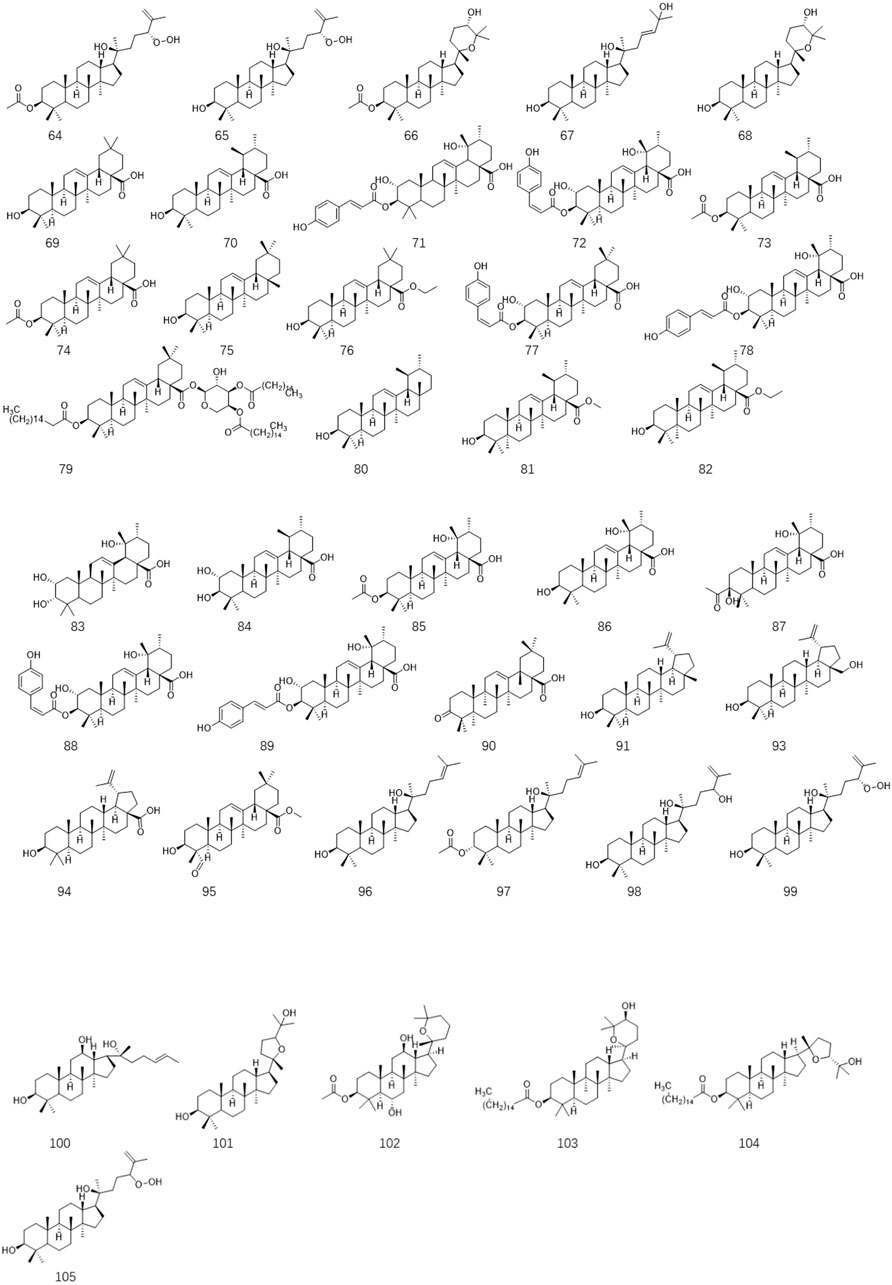
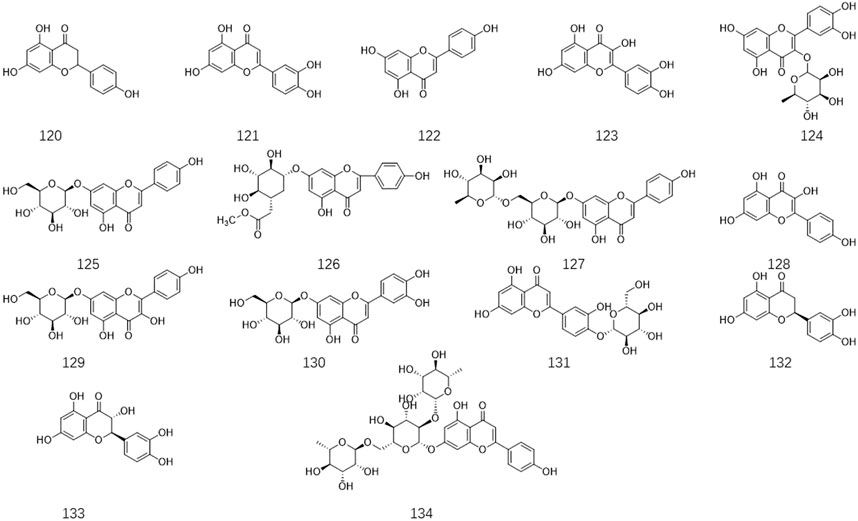
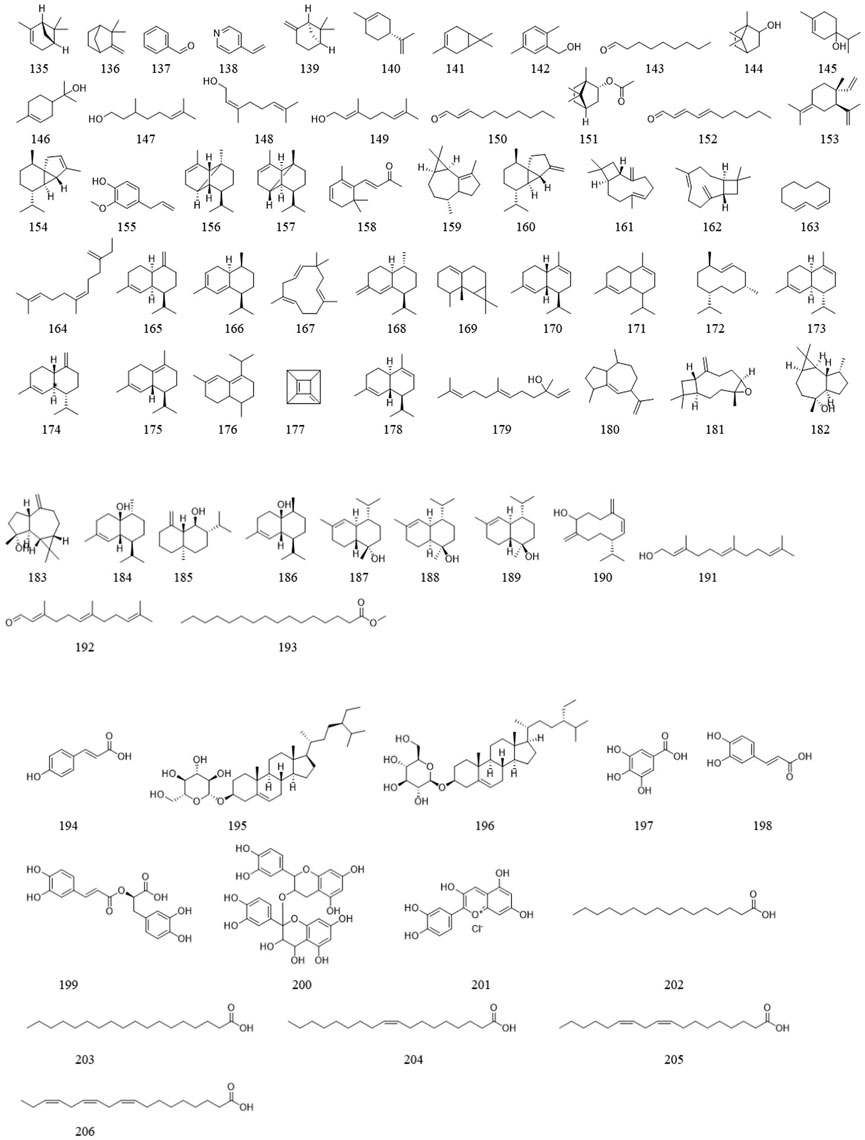
Ligustrum lucidum is a good medicine food homology plant. Accumulating evidence has demonstrated that the components of L. lucidum are divided into nutritional components and bioactive components including iridoids (1–63), triterpenoids (64–105), phenylethanoid glycosides (106–119), flavonoids (120–134), volatile components (135–193) and polysaccharides. These chemical ingredients are summarized in Supplementary Table S1 and their structures are shown in Figures 3–7.
3.1 Nutritional components
The nutritional compounds of L. lucidum vary with geographic location, processing method, harvest time, and even extraction method (Li et al., 2021). The moisture content in L. lucidum is 40.3 g/kg, and the ash and solid content is 38.4 and 510.6 g/kg, respectively (Zhang et al., 2011). With the deepening of the research, it has been confirmed that the polysaccharides contained in the raw and processing products are different, and the polysaccharide content of the processing product has more advantages, which is about 8.7041–9.516 mg/g. Ligustrum lucidum polysaccharide is mainly composed of fucose, glucose, arabinose, and rhamnose with a molar ratio of 1.80:4.58:2.55:1.91 (Cao et al., 2019). The content of protein in L. lucidum is 80.3 g/kg, and the content of free amino acid is 2.9 mg/kg. Researchers identified about 21 amino acids (8 essential amino acids) in L. lucidum, with four major amino acids being aspartic acid, glutamic acid, alanine, and arginine (Zhao et al., 2016). In addition, it is rich in minerals including Potassium (K), Sodium (Na), Calcium (Ca), Magnesium (Mg), Phosphor (P), Zinc (Zn), and Iron (Fe) (Li et al., 2021). Hence, L. lucidum has considerable medical and nutritional value.
3.2 Iridoids
Iridoids, belonging to cyclopentane pyran monoterpenes, are acetal derivatives of iridodial. They usually react with sugar to form glycosides due to the unstable character of their C1-OH group. Based on the integrity of the cyclopentane unit, this large class of compounds has been divided into two types including iridoid glycosides and secoiridoid glycosides (Wang et al., 2020). Iridoids, especially secoiridoid glycosides, are widely prevalent in the plant kingdom, such as Caprifoliaceae, Nymphaeaceae, Gentianaceae, and Oleaceae. In secoiridoids, the C7–C8 in the parent nucleus of these active compounds is often broken to form a cleavage ring. Ligustrum lucidum is enriched with secoiridoids (Zhang et al., 2013). Up till now, over sixty-three secoiridoids have been isolated and purified from the leaves and fruits of L. lucidum. As described in Figure 2, all secoiridoids identified from L. lucidum have featured a β configuration of H-5. In 2013, the first 1-OCH3 substituent secoiridoid obtained from the plant kingdom, namely, ligulucidumoside A 1, has been isolated and elucidated by spectroscopic methods and chemical analysis from the fruit of L. lucidum (Zhang et al., 2013). In 2018, three new secoiridoids, including nuezhenelenoliciside 4, isojaslanceoside B 5, and 6′-O-trans-cinnamoyl-secologanoside 6, have been obtained and elucidated by comprehensive spectroscopic analysis. Among them, four featured a rare rearrangement product of secoiridoids, which existed the cleavage of a chemical bond between C-1 and O-2, then the reformation of a new iridoid ring between C-8 and O-2 (Qiu et al., 2018).
As a major class of chemical components in L. lucidum, these compounds have various biological activity. They have been demonstrated to prevent and treat many types of ailments such as pain, inflammation, hyperlipidemic, and hepatotoxicity as well as vision improvement effects (Liu H. et al., 2020; Wu et al., 2021). Specnuezhenide 7, one of the most abundant components and its content used as the criteria to evaluate the quality of this crude herb, has been proven to possess strong biological effects, including regulating immunity, antivirus, anti-oxidation, and hepatoprotective activities. In 2001, He et al. first isolated lucidumosides C 12 and D 13, and 12 exhibited strong antioxidant effects (IC50 = 9.3 mM), which might be related to the number of phenolic hydroxyl groups (He et al., 2001). Two new secoiridoid glucosides, namely, iso-oleonuezhenide 18 and methyloleoside seven-ethyl ester 19 were isolated and assigned from the fruits of L. lucidum. This research has demonstrated that 18 induced the phosphorylation of ERK and CREB in primary cortical neurons in a dose-dependent and time-dependent manner, indicating its potential effect on neurons (Fu et al., 2010). Three new secoiridoid glycosides, ligulucisides A-C 22–24 as well as two new secoiridoids including liguluciridoids A 25 and B 26 were identified from the fruit of L. lucidum in 2018. Among these five compounds, 22, 24, and 25 exhibited a better effect on anti-influenza A virus with the 50% inhibitory concentration (IC50) values of 16.5, 13.1, and 18.5 µM, respectively, compared to ribavirin (IC50 22.6 µM) (Pang et al., 2018).
3.3 Triterpenoids
Several investigations on L. lucidum have suggested that triterpenoids are another major component that has been isolated from this species. Until now, forty-two triterpenoids have been separated and characterized based on spectroscopic and chemical analysis from the fruits of L. lucidum. In 2008, five new dammarane triterpenes including 3β-acetyl-20S,24R-dammarane-25-ene-24-hydroperoxy-20-ol 64, 20S,24R-dammarane-25-ene-24-hydroperoxy-3β, 20-diol 65, 3β-acetyl-20S, 25-epoxydammarane-24a-ol 66, 20S,25-epoxydammarane-3β, 24a-diol 67 and 20S-dammarane-23-ene-3β,20,25-triol 68 were isolated from L. lucidum for the first time (Xu et al., 2008). Oleanolic acid 69 and ursolic acid 70 are a pair of isomers that are obtained to be the ubiquitous triterpenoids in various medical plants. 69 and 70 illustrated from L. lucidum are the main effective constituents in this well-respected medicinal herb, which were used as the chemical markers for quality evaluation of L. lucidum preparations in the 2015 Chinese Pharmacopoeia (Cao et al., 2018b). Due to the thrilling reputation of these two compounds in bioactive efficacy, systematic pharmacology explorations of them have been carried out by different research groups. Accumulating evidence has indicated that these two compounds have exerted anti-aging, anti-inflammatory, antidiabetic, antioxidative, antitumor, antimutagenic, and anti-osteoporosis properties in in-vivo and in-vitro experimental studies (Zhang et al., 2007; Gao et al., 2009; Wu et al., 2010; Zhao et al., 2017; Cao et al., 2018a). Compounds 3-O-cis- p-coumaroyl maslinic acid (71) and 3-O-trans-p-coumaroyl maslinic acid (72) are two isomeric pentacyclic triterpenes found in L. lucidum, and 72 has specifically suppressed γ-secretase and decreased amyloid-beta levels, which suggesting used as a promising candidate for Alzheimer’s disease (AD) treatment (Luo et al., 2020). However, the bioactive properties of other triterpenoids remain unclear and need to be further explored. The structures of these compounds are shown in Figure 4.
3.4 Phenylethanoid glycosides
Phenylethanoid glycosides are typically presented in various plant kingdoms, which are essential phenolic components with anti-inflammatory, antibacterial, anti-tumor, neuro-protective, anti-viral, and other pharmacological activities. Therefore, phenylethanoid glycoside has captured considerable concern from scholars. At present, a total of fourteen phenylethanoid glycosides have been isolated and elucidated from the fruit and leaves of L. lucidum with unambiguous structures and significant bioactive characteristics. These compounds are shown in Supplementary Table S1 and Figure 5. Among the phenylethanoid glycosides, salidroside 106 is the characteristic chemical with broad pharmacological application prospect from L. lucidum and has generally been considered to be the quality control marker in wine-steamed L. lucidum, which should be quantified based on Chinese Pharmacopeia. Verbascoside 107 and Echinacoside 108 are the most frequently reported chemical components and have been proven to possess strong biological effects, such as anti-inflammatory, anti-hyperglycemic, anti-osteoporotic, liver protection, and anti-AD.
3.5 Flavonoids
Currently, fifteen flavonoids have been isolated and identified from the fruit, leaves, and flowers (Figure 6). There is much evidence to suggest that significant amounts of flavonoids are present in L. lucidum extracts (Jeong et al., 2016). It is noteworthy that six flavonoids were obtained from the flowers of L. lucidum for the first time in 2011, such as naringenin 120, luteolin 121, apigenin 122, quercetin 123, rutin 124, and apigenin-7-O-glucoside 125 (Long et al., 2011). Previous research has indicated that ligustroflavone 134 is a natural flavonoid glycoside with excellent pharmacological properties, including anti-inflammatory, anti-fibrosis, antioxidant, anti-complementary, and anti-osteoporosis effects (Feng et al., 2019; Kang et al., 2021).
3.6 Polysaccharides
Plant polysaccharides have become a popular topic of research owing to their wide range of biological properties like antioxidant, immunomodulatory, and anti-tumor effects (Mukherjee et al., 2022). Research has uncovered a heteropolysaccharide extract from the fruits of L. lucidum, and the monosaccharide compositions of this plant’s polysaccharides are fucose, glucose, arabinose, and rhamnose (molar ratio of 1.80: 4.58: 2.55: 1.91) (Wang et al., 2003). Subsequently, Yin et al. obtained water-soluble polysaccharide extract with a good anticoagulant effect in vitro in the flowers of L. lucidum, which is composed of L-rhamnose, L-arabinose, D-xylose, D-glucose, and D-galactose in a molar ratio of 3.16: 2.46: 1.00: 7.27: 4.22 (Yin et al., 2017).
3.7 Others
In addition to the bioactive ingredients mentioned above, volatile components (135–193) and other compounds (194–206) have been extracted from L. lucidum. Relevant research has confirmed that possess certain antioxidant and antibacterial activities (Gao et al., 2022).
4 Biological and pharmacological activities
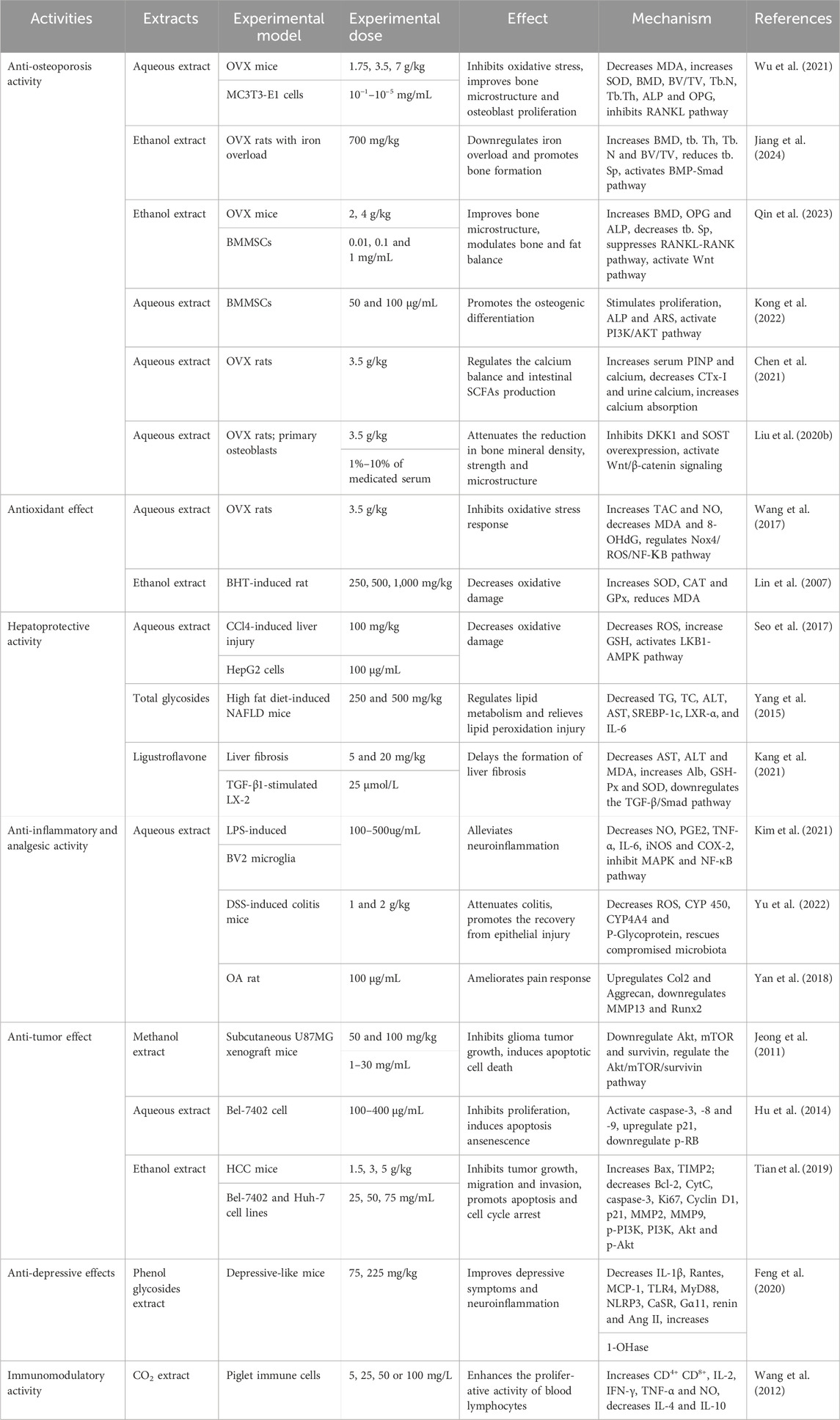
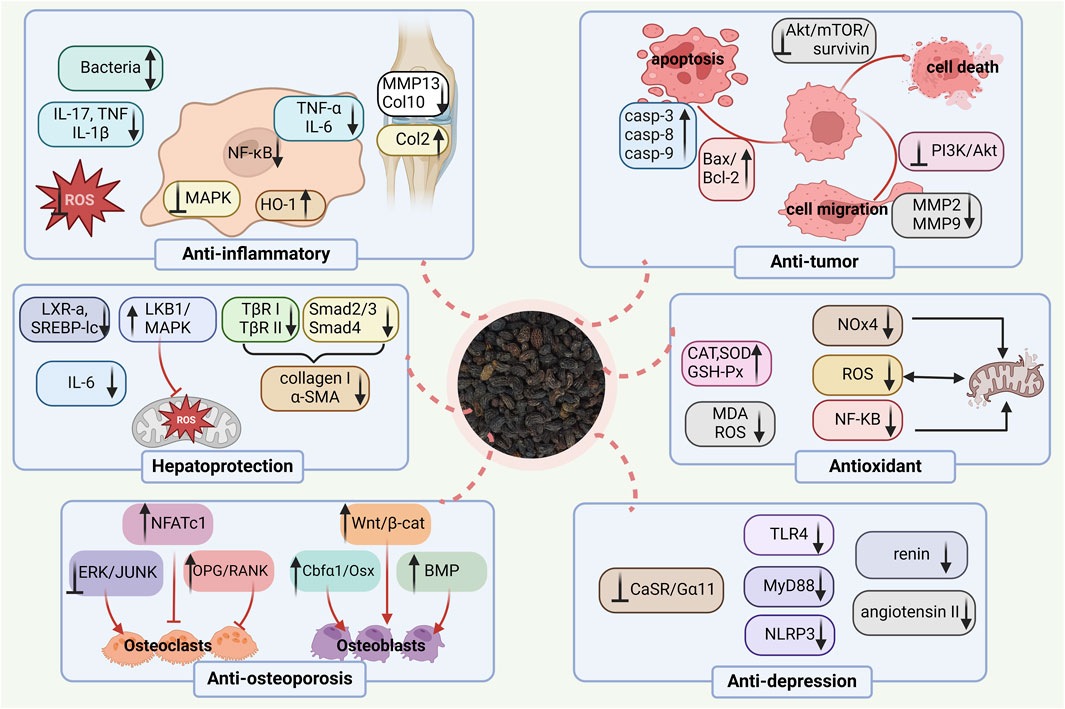
Herbal medicine, as the fundamental and vital part of many traditional medicines, has been gradually accepted for the prevention and treatment of different ailments worldwide, because of its multilevel function characteristics and remarkable efficacy with fewer adverse effects. Ligustrum lucidum is reputed to exert many strong pharmacological activities in vivo and in vitro, such as anti-osteoporosis, anti-fatigue, anti-oxidant, anti-tumor, anti-aging, anti-inflammatory, and so on. The pharmacological effects of Ligustrum lucidum have been summarized in Table 1 and Figure 8.
4.1 Anti-osteoporotic activity
Osteoporosis, a refractory disease, becomes a kind of universal occurrence in older people worldwide, which can lead to decreased life functioning and quality of life to a considerable extent. The prevalence of this skeletal ailment has posed severe public health and economic challenges to our society (Ensrud and Crandall, 2024). Ligustrum lucidum has traditionally been used for the treatment of bone diseases in TCM for thousands of years. As one of the liver and kidney-tonifying herbs and consistency in the TCM theory of “kidney governing bones,” L. lucidum is especially applied to prevent and treat postmenopausal osteoporosis (PMOP) (Chen et al., 2021; Qin et al., 2023). It also exerts anti-osteoporotic effects in diabetes-induced osteoporosis (Feng et al., 2019), senile osteoporosis (Li et al., 2019), and oxidative stress-related osteoporosis (Wu et al., 2021). Ligustrum lucidum has been reported to downregulate iron overload and promote bone formation in ovariectomized (OVX) rat models through the BMP-Smad pathway after continuous administration for almost 2 months (Jiang et al., 2024).
Appropriate control of short-chain fatty acids (SCFAs) production and calcium metabolism is a new avenue in the prevention of osteoporosis (Lucas et al., 2018). In 2021, Chen et al. assessed the effects of L. lucidum aqueous extract on SCFAs production, calcium balance, and bone homeostasis in the model of OVX rats (Chen et al., 2021). The extract (3.5 g/kg) was daily and orally administered to ovariectomized rats for 14 weeks. Compared with the control group that received appropriate amounts of saline alone. The level of calcium-sensing receptor (CaSR) expression was decreased, and surprisingly, the extract effectively increased SCFAs levels and calcium absorption. In addition, the extract has been shown to suppress the decline of the bone microstructure, bone strength, and various bone material properties. Thus, the aqueous extract may preserve bone quality via prevention against calcium loss and regulation of the SCFAs production in ovariectomized rats. Additionally, L. lucidum ethanol extract and its two primary compounds, ursolic acid and oleanolic acid, significantly inhibit osteoclast differentiation and bone resorption of RAW264.7 murine monocyte/macrophage cells through receptor activator of nuclear factor КB ligand (NF-КB) signaling pathways (Xu et al., 2016). However, this study had apparent limitations one dose of the extract was applied, and thus the information on the dose-dependent activity was limited.
4.2 Antioxidative activity
As can be seen from the above, antioxidation is the underlying mechanism of L. lucidum in treating many diseases. For example, oxidative stress is considered to play a contributory role in the imbalance between bone resorption and formation, and thus this has received increasing prominence for the elucidation of post-menopausal osteoporosis. In ovariectomized rats, oral administration of L. lucidum aqueous extract (3.5 g/kg, i. g.) prevented the accumulation of free radicals and NF-КB activation, and thus decreased oxidative damage and improved bone microstructure and mineral density, which indicated the correlation between antioxidation and anti-osteoporotic effects (Wang et al., 2017). However, a single-dose study was included in this trial thus necessitating the dose-dependent study. In 2007, Lin et al. investigated the antioxidant activities of L. lucidum ethanol extract and its effects on butylated hydroxytoluene (BHT)-induced oxidative stress in Male Wistar rats. The result revealed that the extract could effectively inhibit the acute BHT-deduced oxidative stress through the decline in serum glutamic pyruvic transaminase, glutamic oxaloacetic transaminase, alkaline phosphatase, and lactate dehydrogenase as well as the upregulation of antioxidant enzymes in lung, liver and kidney (Lin et al., 2007). However, no positive control was included in this trial.
4.3 Liver protective activity
For centuries, L. lucidum has been commonly used as a traditional health food to nourish and detoxify the liver worldwide including in China, Korea, and so on (Yao et al., 2013). According to Seo’s study, oral administration of 100 mg/kg L lucidum aqueous extract dramatically attenuated the activities of alanine aminotransferase and oxidative stress in the liver, showing a protective effect on CCl4-stimulated liver injury in C57BL/6 male mice. Meanwhile, in vitro experiments showed that the extract (100 μg/mL) inhibited arachidonic acid + iron-induced ROS generation, GSH depletion, and mitochondrial dysfunction via the AMPK pathway in HepG2 cells (Seo et al., 2017). However, these investigations lacked appropriate positive controls. CCl4 is one of the most common xenobiotics for induced-liver injury and the injury can be strongly attacked by Ligustroflavone (5 mg/kg and 20 mg/kg, i. p.). An in vitro study (25 μmol/L) found that the fundamental mechanism of antifibrotic effects was through down-regulating the TGF-β/Smad signaling pathway in the human hepatic stellate cell line (LX-2) (Kang et al., 2021). However, this study was conducted without the use of a positive control.
4.4 Anti-inflammatory and analgesic activity
It was reported that the use of L. lucidum water extract possessed an analgesic effect on the osteoarthritis (OA) rat model. A related study established the rat OA model through intra-articular (IA) injection of mono-iodoacetate. After IA administration (100 μg/mL), the extract effectively ameliorated joint pain response through increasing heat pain sensitivity and thresholds of mechanical allodynia as well as spontaneous activity, demonstrating the analgesic function of L. lucidum (Yan et al., 2018). Nevertheless, the major shortage of this study was only conducted with a single dose.
4.5 Anti-tumor activity
The wide application of health-strengthening herbs in cancer treatment has given rise to growing research interests all over the world. Ligustrum lucidum has been demonstrated to exert its characteristic antitumor potential. One study showed that L. lucidum extract inhibited glioma tumor growth in vivo and induced glioma cell death in vitro, the underlying mechanism involved in the regulation of the Akt/mammalian target of rapamycin (mTOR)/survivin pathway. Therefore, L. lucidum might serve as a potential drug candidate for malignant human gliomas (Jeong et al., 2011). Yet the study did not illustrate the major active component of the extracts inducing suppression of glioma tumor growth. Hu et al. found that L. lucidum aqueous extract suppressed the proliferation of human hepatocellular carcinoma Bel-7402 cells in a dose-dependent and time-dependent manner (50–800 μg/mL for 24 h, 48 h, and 72 h). The extract also had a pro-apoptotic ability in the Bel-7402 cells, by causing huge and flat morphologic cellular change, and blocking the G0/G1 cell cycle, accompanied by sensitization of caspases. To further illustrate the mitochondrial transformation in apoptosis induced by the aqueous extract, it was demonstrated that L. lucidum contributed to the Bel-7402 cell apoptosis and cell senescence through upregulation of p21 and downregulation of RB phosphorylation (Hu et al., 2014). In addition, Tian et al. confirmed that the ethanol extract of L. lucidum leaves possessed excellent anti-tumor effects on hepatocellular carcinoma (HCC) in vitro and in vivo. The results showed that the extract effectively promoted the apoptosis of Bel-7402 and Huh-7 cells by regulating the activity of caspase-3, as well as the expressions of B cell lymphoma 2 (Bcl-2), Bcl-2 associated X (Bax) and Cytochrome-C (CytC). It was demonstrated that the extract had a markedly inhibitory effect on cell migration and invasion. In addition, it strongly decreased the expressions of matrix metalloproteinase2 (MMP2) and MMP9 and enhanced the expression of tissue inhibitors of metalloproteinases two in Bel-7402 and Huh-7 cells. The ethanol extract of L. lucidum leaves mediated by the suppression of Phosphoinositide 3-kinase (PI3K)/Akt pathway was closely associated with DNA de-methylation of PTEN. In further in vivo studies, the extract suppressed the tumor growth of hepatocellular carcinoma (Tian et al., 2019). However, further researches are needed to determine which components are involved in L. lucidum-induced antitumor activities.
4.6 Anti-depressive effects
Depression is the most common of the severe neuropsychiatric illness and has been identified as the critical cause of disability worldwide (Beurel et al., 2020). Inflammatory processes in the central nervous system are associated with the pathogenesis of depression (Leng et al., 2018). Of noted, the phenol glycosides from L. lucidum (75 mg/kg and 225 mg/kg, i. g.) were elucidated to have beneficial ameliorative effects on lipopolysaccharide (LPS)-triggered depressive-like performance in mice, which might be dependent on repressing neuroinflammation in the hypothalamus that was mediated by abrogation the activation of microglia and the release of inflammatory cytokines by regulation on f toll-like receptor-4 (TLR4) signaling pathway (Feng et al., 2020). Nonetheless, the study lacked positive control.
4.7 Immunomodulatory activity
The CO2 supercritical extract from L. lucidum (5, 25, 50, and 100 mg/L) was found to promote immune responses through stimulating lymphocyte proliferation as well as upregulation of the lymphocyte populations of CD4+ CD8− and CD4+ CD8+. Furthermore, the extract regulated the expression of Th1- and Th2-related cytokines, enhanced the levels of IFN-γ, IL-2, and TNF-α in helper T-cells (Th)1, decreased the release of IL-4 and IL-10 in Th2, and stimulated the secretion of NO (Wang et al., 2012).
5 Applications
5.1 Medicine field
Ligustrum lucidum is listed as a “Top grade” drug in Shen Nong’s Herbal (Dong Han Dynasty, A.D. 25–220). According to TCM, women, winter, and quiet characters belong to yin in China. From the name of L. lucidum, we can understand how the ancients interpreted its hallmarks and nature. Traditionally, the leaves and fruits are obtained, dried, and used in clinical practice for several purposes, such as preventing aging and hair graying, improving vision, alleviating the soreness and weakness of the waist and knees, and strengthening human energy in its native and introduced areas. The theoretical basis of TCM is kidney domains bone and produces marrow, as a general tonic herb, the dried mature fruits are documented in the version of 2020 Chinese pharmacopeia, possessing the effects to invigorate muscles and bones, supplement the kidney and liver, nourish yin, and clear vision. In traditional Japanese and Swedish medicine, L. lucidum has been used to treat osteoporosis (Wegiel and Persson, 2010). It has also been commonly applied in traditional Korean medicine to detoxify the liver and kidneys (Kim et al., 2021).
5.1.1 Herb pairs application
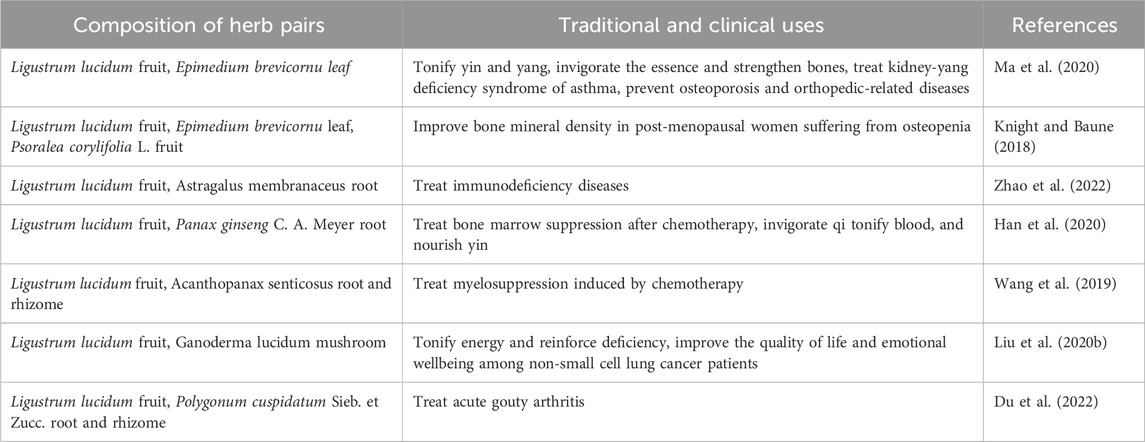
Herbal formulae are the predominant pattern of traditional clinical practice. Herb pairs are the most essential unit and the simplest form of TCM prescription. As a bridge between a single herb and a complex formulation, herb pairs amass the experience of veteran TCM doctors, greatly interpret the traits of the “gregarious utilization” of TCM, and perfectly embody the connotation of holistic therapeutic theory and syndrome differentiation treatment (Wang et al., 2022). Herb drugs usually consist of a unique combination of two or three relatively fixed herbs, which embody a centralized representative of herbal compatibility according to their characteristics, such as strengthening treatment function and attenuating virulence through potentially synergistic herb interactions compared to single herb (Han et al., 2020). Ligustrum lucidum not only can treat diseases with single medicine but also can be widely used to treatment of immunodeficiency, bone marrow suppression, and cancer when it is combined with other drugs. The herb pairs compatibility of L. lucidum are summarized in Table 2.
5.1.2 Prescription application
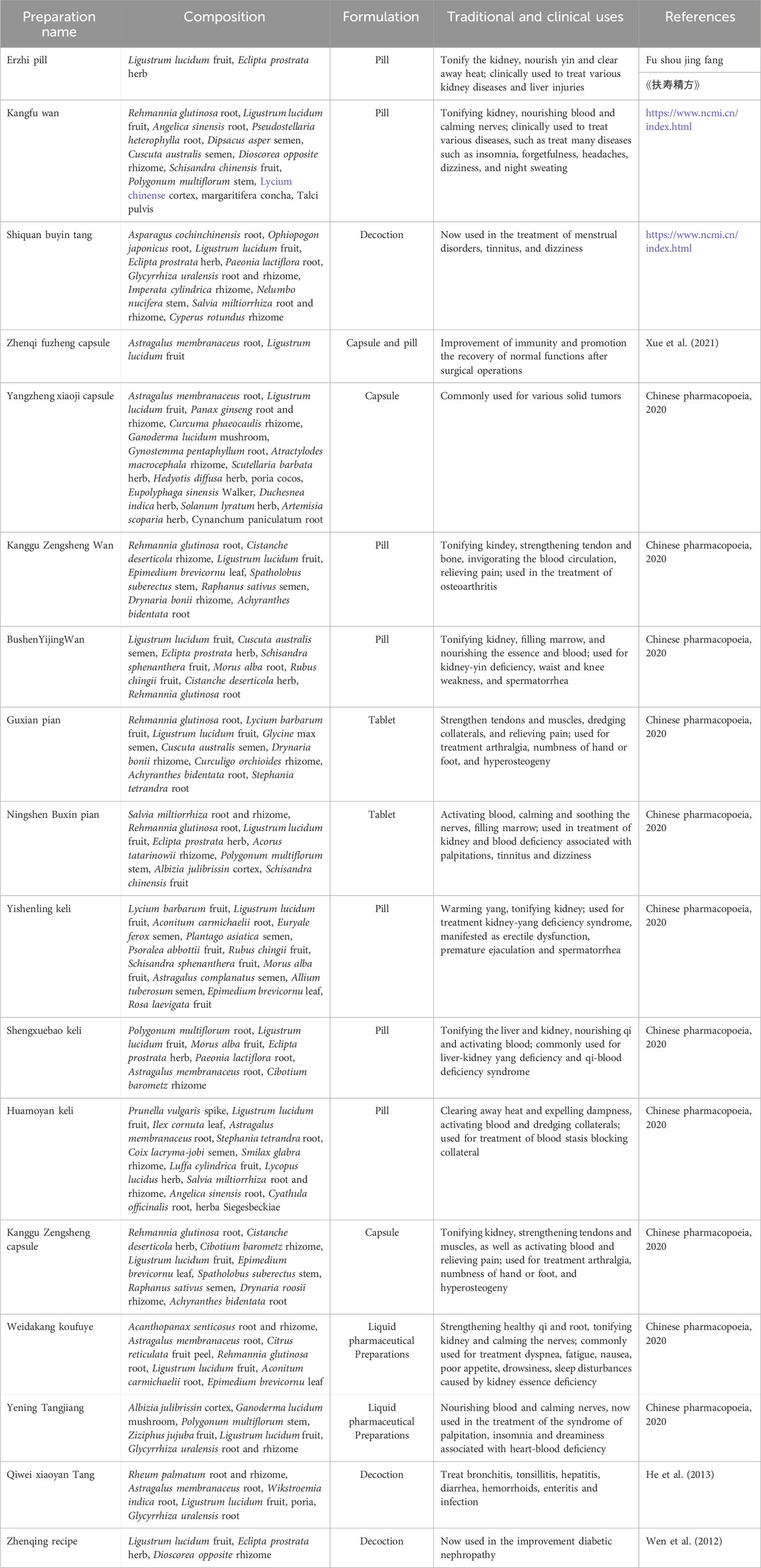
There are hundreds of L. lucidum-related prescriptions in the clinic, as shown in Table 3. Erzhi Pill (EZP), a classic TCM prescription used for the treatment of Xiaoke disease and turbid urine, was initially documented in Fu Shou Jing Fang by Wu MinJi in China during the Ming Dynasty (A.D.1530). EZP is a simple combination of only two medicinal herbs, which consists of equal amounts (at a ratio 1:1) of Ligustri Lucidi and Ecliptae Herba. This formulation has been used to prevent and treat various kidney diseases by tonifying the body’s essential fluid, enhancing tendons and bones, and arresting hemorrhage. Nowadays, EZP is the most frequently mentioned as an anti-aging, hepatoprotective, promoting hematopoietic, and anti-oxidant agent for menopausal symptoms in clinical application in Taiwan and China (Chen et al., 2011; Cheng et al., 2011; Xu et al., 2012). EZP has shown marked effects in the treatment of diabetic cardiomyopathy (Peng et al., 2022), benign prostatic hyperplasia (Tao et al., 2022), liver injury (Zhao et al., 2018), AD (Xie et al., 2021), osteoporosis (Zhong et al., 2021), rheumatoid arthritis (RA) (Li et al., 2020), fatty liver (Huang et al., 2020), and aging-associated symptoms (Feng et al., 2021).
Another famous folk formula is the Zhenqi Fuzheng formula (ZQFZ), composed of Ligustri Lucidi Fructus and Astragali Radix (1:2, w/w). According to the qi-blood theory of TCM, qi is the commander of blood, and blood is the mother of qi. Qi and blood are mutually dependent. Qi deficiency or blood-deficiency may cause all kinds of diseases. ZQFZ is the role of supplementing-Qi and enriching blood and is usually utilized clinically to improve immunity, increase leukocytes, protect bone marrow and adrenal cortex (Shi et al., 2011; Miao et al., 2018). Complementary and alternative medicine has gradually gained popularity in the world over the last decades. As an adjuvant therapy, TCM preparation decreases dramatically adverse effects induced by surgery, radiotherapy, and chemotherapy, and acts synergistically with them. ZQFZ is also used in clinics to treat a variety of cancers, and is widely used together with or after these therapies to promote the recovery of body functions, prolong the survival time of cancer patients, and improve their quality of life. Accumulation research indicated that the curative effect is remarkable (Li et al., 2020; Meng et al., 2022).
Moreover, there are some undocumented L. lucidum-related formulae frequently utilized by traditional Chinese doctors like Qiwei Xiaoyan Tang (He et al., 2013), Shuangdi Shouzhen Tablet (Fang et al., 2021), Zhenqing recipe (Wen et al., 2012; Song et al., 2020) and so on.
5.2 Food application
As a compelling tonic herb, the fruit of L. lucidum is not just used in treatment for ailments but also as health food owing to its excellent liver tonic, kidney tonic, and hair blackening (Guo et al., 2008).
5.2.1 Functional food
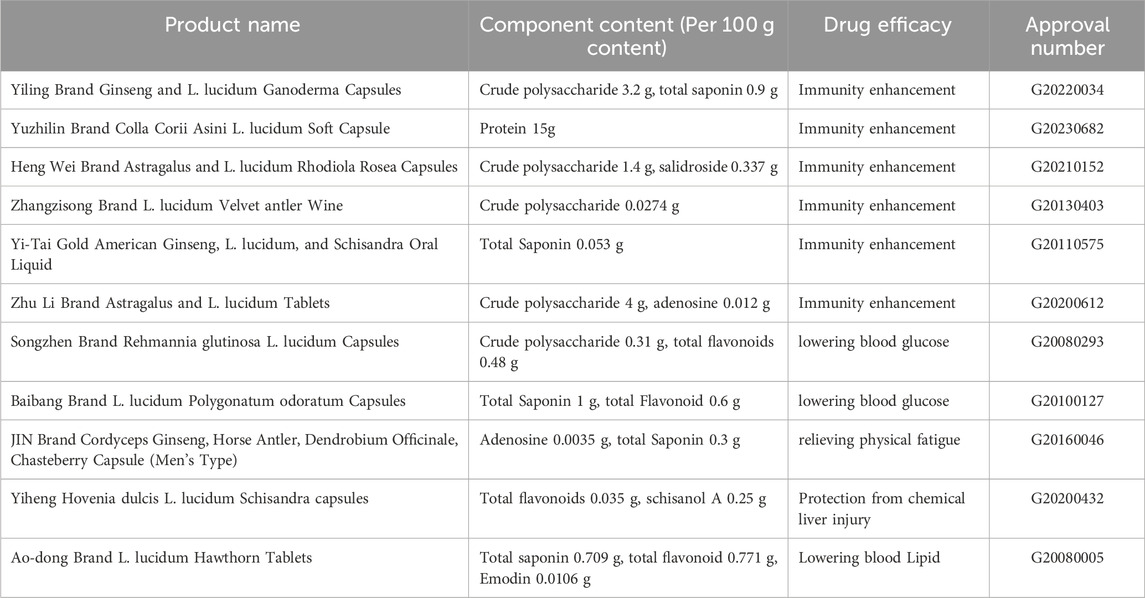
Based on its rich efficacy and high safety profile, L. lucidum has been made into a variety of functional foods, mostly with immune regulation, liver function protection, anti-fatigue, and hypoglycemic effects. The products mainly include tablets, capsules, granules oral liquid, wine, and tea preparation, as shown in Table 4 (http://ypzsx.gsxt.gov.cn/specialfood/#/food). They are mostly in traditional preparations and need to be further innovated.
5.2.2 Snack foods
With the improvement of public health awareness, increased attention has been focused on the development of L. lucidum. To improve the utilization mechanism of L. lucidum, the food industry tries to combine L. lucidum polysaccharides or extracts with food ingredients to make various types of food rich in bioactive compounds, such as beverages, yogurt, fruit tea, wine, preserves, and fruits vinegar.
5.3 Animal feed additives
One major problem faced by the livestock and poultry industry is a loss of productivity because of ailments. Utilization of in-feed antibiotics is one solution to overcome infections and improve the health status of animals (Gao et al., 2017). Nevertheless, antibiotic residue in foodstuffs due to the administration of antibiotics in feed is considered an important reason for the rapid spread of antimicrobial resistance in humans. In light of this, seeking alternatives for in-feed antibiotics has become imperative. Among the possible alternatives to antibiotics, herbal medicine has attracted enormous interest in recent years given their beneficial effects on immune function, animal growth, and metabolism (Wang et al., 2021). There is a very long history of TCM which has been characterized as antimicrobials and antiseptics. Results from the current study have demonstrated that administration of L. lucidum aqueous extract in broilers significantly alleviated malondialdehyde concentration, elevated glutathione reductase activity, and enhanced antibody titers against Newcastle disease virus as well as spleen lymphocyte proliferation of broilers, indicating the potential value of L. lucidum as antibiotics’ substitute in the poultry industry (Ma et al., 2009). Furthermore, in laying hens, L. lucidum is shown to attenuate mortality, cracked-egg rate, and blood serum levels of triglycerides, cholesterol, low-density lipoprotein cholesterol, and alanine aminotransferase, enhance blood serum levels of high-density lipoprotein cholesterol, which suggested the tremendous potential of L. lucidum as the alternatives to antibiotics in poultry farming (Li et al., 2017). It was reported that the addition of L. lucidum into the diet at morning feed strongly improved blood antioxidant function, nutrient digestibility, and slaughtered body weight in sheep (Qiao et al., 2012).
5.4 Garden field
Ligustrum lucidum is a dramatic evergreen arbor tree, usually utilized as an ornamental tree for shade, and shelter, especially for hedging purposes, which can be conducted to a specific size and shape through regular pruning. This plant is regarded as an outstanding landscaping plant because it gives dense shade, grows rapidly even in poor soils, resists wind, pests, and air pollution and its flowers are attractive and heavily fragrant. Furthermore, L. lucidum is known for its health benefits, and it has been recognized as a potentially suitable plant for use as the general bioindicator of urban air pollution environments (Wei et al., 2018; Graziani et al., 2019).
5.5 Other application
Extensive eutrophication has led to the bloom of toxic cyanobacteria in major lakes in China. Microcystis aeruginosa (MA), as the most important kind of toxic cyanobacteria, is a vital threat to human and environmental health. Ligustrum lucidum has a long fruiting season which is from July to May of next year, and a large number of the fruits are unpicked almost every year. One study evaluated the effects of L. lucidum on the growth inhibition, cell integrity, and algicidal properties of MA. The result indicated that L. lucidum could be an excellent algicidal agent in the treatment of eutrophic water, ascribing to its strong inhibition on the growth of MA in the acute time. Consequently, utilization of the forgotten fruit may be an attractive, environmentally friendly, and cost-efficient option for cyanobacteria inhibition on eutrophic water (Varela et al., 2023; Zheng et al., 2024).
6 Safety
Although L. lucidum has been used as a critical tonic in different traditional medical systems worldwide for centuries. Until up to now, data on the systematic safety evaluation of this herb is still insufficient, toxicological experiments were seldom reported.
More recently, researchers have paid growing attention to respiratory allergies because they can cause illness and disability, as well as affect the quality of life (Vizuet-de-Rueda et al., 2022). Respiratory allergies influence humans in different parts of the world, leading to wide morbidity (Suanno et al., 2021). There is plenty of evidence that L. lucidum-derived pollen is one of the most prominent inhalant allergens contributing to respiratory allergic diseases worldwide given the ornamental plant introduced extensively (Mani et al., 2015). It is claimed that the pollen from L. lucidum causes wheezing, asthma, and eczema (Robledo-Retana, 2020). Additionally, in 2011, Dong et al. demonstrated that within 30 days after orally administering supercritical CO2 extract of L. lucidum fruit (at the dose of 0.75, 2.25, 3.75 g/kg) the behavior, hemogram, and blood component of the tested rats did not alter, nor caused any signs of genetic toxicity and subacute toxicity (Dong et al., 2011).
7 Conclusion and outlooks
Medicinal plants have long served as a natural treasure of bioactive components used to prevent and treat ailments. As an excellent medicinal plant with the homology of medicine and food, L. lucidum is rich in nutrients and active ingredients and, consequently, has a variety of biological properties such as anti-osteoporosis, antioxidant, liver protection, anti-inflammatory, and analgesic activity, as well as the effect on the central nervous system and glucose metabolism, especially excellent anti-tumor activity. The above researches partially shed light on the interacting underlying mechanism between L. lucidum and organisms and provides the theoretical and practical basis for part traditional applications. It is suggested that the following areas should be taken into consideration in future research.
With modern research tools applied, much in-depth study has been performed in L. lucidum for many years. Nonetheless, modern pharmacological research has been concentrated on the anti-osteoporotic and hepatoprotective activity with relatively weak applications in antioxidant and immunomodulatory activity. The molecular mechanisms of action, signaling pathways, and targets remain largely unknown. The active ingredients responsible for some activities of L. lucidum are far from clear, which strongly impeded the application of this herb. Therefore, the pharmacological mechanism of action of the bioactive components in L. lucidum should be studied in depth to provide a theoretical basis for their application in health products.
Ligustrum lucidum is typically applied in multi-herb formulation or integrated with conventional drugs in long-standing clinical applications, resulting in significant therapeutic benefits. However, most of the modern pharmacological and toxicological research has been generally performed on single L. lucidum or its main compositions, which critically ignored the effects induced by medicine interaction. According to the traditional applications, it is vital to explore the synergistic efficacy of this herb with drug use and enhance clinical efficacy and safety.
As a traditional medicine and food plant, toxicological studies and the detailed bioavailability, distribution, metabolism, and excretion of its main compositions in the body are still insufficient, thereby limiting its wider applications. Most toxicological research has focused on herbal medicine toxicity on healthy organisms, ignoring possible existing transformations in herb properties triggered by different pathological conditions. Therefore, further research on the toxicology and pharmacokinetics of L. lucidum should be strengthened. Nowadays, L. lucidum has limitations in the development and application of healthcare products. To ensure the long-term sustainable development prospects of the herb, further study should be closely linked to develop new products, and then continue to promote the development of L. lucidum in medicine, food, and health products, to achieve new values and connotations of L. lucidum.
Author contributions
LC: Conceptualization, Writing–original draft. DH: Software, Writing–review and editing. LJ: Data curation, Investigation, Writing–review and editing. JY: Data curation, Formal Analysis, Writing–review and editing. XS: Data curation, Resources, Validation, Writing–review and editing. RW: Funding acquisition, Investigation, Writing–original draft. WL: Project administration, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Gansu Provincial Science and Technology Project (23ZDFA013-5), China; Fund Project of the 940th Hospital (No. 2021yxky043, No. 2023YXKY016), China; Innovation Team Project of Lanzhou Science and Technology Bureau (No. 2023-2-62), China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1330732/full#supplementary-material
References
Aoki, S., Honda, Y., Kikuchi, T., Miura, T., Sugawara, R., Yaoita, Y., et al. (2012). Six new secoiridoids from the dried fruits of Ligustrum lucidum. Chem. Pharm. Bull. (Tokyo) 60 (2), 251–256. doi:10.1248/cpb.60.251
Beurel, E., Toups, M., and Nemeroff, C. B. (2020). The bidirectional relationship of depression and inflammation: double trouble. Neuron 107 (2), 234–256. doi:10.1016/j.neuron.2020.06.002
Cao, R., Deng, C., and Li, L. L. (2019). Changes of polyphenols and polysaccharides in fructusligustrum lucidum before and after processing. J. Shaanxi Univ. Chin. Med. 42 (2), 39–49. doi:10.13424/j.cnki.jsctcm.2019.04.010
Cao, S., Tian, X. L., Yu, W. X., Zhou, L. P., Dong, X. L., Favus, M. J., et al. (2018a). Oleanolic acid and ursolic acid improve bone properties and calcium balance and modulate vitamin D metabolism in aged female rats. Front. Pharmacol. 9, 1435. doi:10.3389/fphar.2018.01435
Cao, S., Wastney, M. E., Lachcik, P. J., Xiao, H. H., Weaver, C. M., and Wong, M. S. (2018b). Both oleanolic acid and a mixture of oleanolic and ursolic acids mimic the effects of fructus ligustri lucidi on bone properties and circulating 1,25-dihydroxycholecalciferol in ovariectomized rats. J. Nutr. 148 (12), 1895–1902. doi:10.1093/jn/nxy242
Chen, B., Wei, J., Zhu, R., Zhang, H., Xia, B., Liu, Y., et al. (2021). Fructus Ligustri Lucidi aqueous extract promotes calcium balance and short-chain fatty acids production in ovariectomized rats. J. Ethnopharmacol. 279, 114348. doi:10.1016/j.jep.2021.114348
Chen, H. Y., Lin, Y. H., Wu, J. C., Chen, Y. C., Yang, S. H., Chen, J. L., et al. (2011). Prescription patterns of Chinese herbal products for menopausal syndrome: analysis of a nationwide prescription database. J. Ethnopharmacol. 137 (3), 1261–1266. doi:10.1016/j.jep.2011.07.053
Chen, L. L., Verpoorte, R., Yen, H. R., Peng, W. H., Cheng, Y. C., Chao, J., et al. (2018). Effects of processing adjuvants on traditional Chinese herbs. J. Food Drug Anal. 26 (2S), S96–S114. doi:10.1016/j.jfda.2018.02.004
Chen, Q. f., Yang, L. j., Zhang, G. l., and Wang, F. (2013). Bioactivity-guided Isolation of antiosteoporotic compounds from Ligustrum lucidum. Phytother. Res. 27 (7), 973–979. doi:10.1002/ptr.4820
Chen, S. J., Du, K. Z., Li, J., and Chang, Y. X. (2020). A chitosan solution-based vortex-forced matrix solid phase dispersion method for the extraction and determination of four bioactive constituents from Ligustri Lucidi Fructus by high performance liquid chromatography. J. Chromatogr. A 1609, 460509. doi:10.1016/j.chroma.2019.460509
Cheng, M., Wang, Q., Fan, Y., Liu, X., Wang, L., Xie, R., et al. (2011). A traditional Chinese herbal preparation, Er-Zhi-Wan, prevent ovariectomy-induced osteoporosis in rats. J. Ethnopharmacol. 138 (2), 279–285. doi:10.1016/j.jep.2011.09.030
Du, K., Wang, W. D., Han, B., Wang, Y. F., and Wang, Z. P. (2022). Mechanism and experimental verification of "Polygoni Cuspidati Rhizoma et Radix-Ligustri Lucidi Fructus" combination in treatment of acute gouty arthritis based on network pharmacology. Zhongguo zhongyao zazhi 47 (6), 1677–1686. doi:10.19540/j.cnki.cjcmm.20211103.402
Duque-Buitrago, L. F., Tornero-Martínez, A., Loera-Castañeda, V., and Mora-Escobedo, R. (2023). Use of food and food-derived products in the treatment of gastritis: a systematic review. Crit. Rev. Food Sci. Nutr. 63 (22), 5771–5782. doi:10.1080/10408398.2021.2024131
Eberhardt, T. L., Catallo, W. J., and Shupe, T. F. (2010). Hydrothermal transformation of Chinese privet seed biomass to gas-phase and semi-volatile products. Bioresour. Technol. 101 (11), 4198–4204. doi:10.1016/j.biortech.2010.01.064
Ensrud, K. E., and Crandall, C. J. (2024). Osteoporosis. Ann. Intern. Med. 177 (1), ITC1–ITC16. doi:10.7326/aitc202401160
Fang, Y., Liu, X., and Su, J. (2021). Network pharmacology analysis of traditional Chinese medicine formula shuang di Shou zhen tablets treating nonexudative age-related macular degeneration. Evid. Based Complement. Altern. Med. 2021, 6657521. doi:10.1155/2021/6657521
Feng, J., Feng, Z. Y., Wang, J. M., and Cui, Y. (2011). Study on the triterpenoids from the fruits of ligustrum lucidum. J. Chin. Med. Mater. 34 (10), 1540–1544. doi:10.13863/j.issn1001-4454.2011.10.032
Feng, L., Gao, M., Zhai, Y., Li, X., Wang, Y., Xie, T., et al. (2021). A novel strategy based on targeted cellular metabolomics for quantitatively evaluating anti-aging effect and screening effective extracts of Erzhi Wan. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1178, 122857. doi:10.1016/j.jchromb.2021.122857
Feng, R., Ding, F., Mi, X.-H., Liu, S.-F., Jiang, A.-L., Liu, B.-H., et al. (2019). Protective effects of ligustroflavone, an active compound from ligustrum lucidum, on diabetes-induced osteoporosis in mice: a potential candidate as calcium-sensing receptor antagonist. Am. J. Chin. Med. 47 (02), 457–476. doi:10.1142/s0192415x1950023x
Feng, R., He, M. C., Li, Q., Liang, X. Q., Tang, D. Z., Zhang, J. L., et al. (2020). Phenol glycosides extract of Fructus Ligustri Lucidi attenuated depressive-like behaviors by suppressing neuroinflammation in hypothalamus of mice. Phytother. Res. 34 (12), 3273–3286. doi:10.1002/ptr.6777
Fu, G., Ip, F. C., Pang, H., and Ip, N. Y. (2010). New secoiridoid glucosides from Ligustrum lucidum induce ERK and CREB phosphorylation in cultured cortical neurons. Planta Med. 76 (10), 998–1003. doi:10.1055/s-0029-1240869
Gao, D., Li, Q., Li, Y., Liu, Z., Fan, Y., Liu, Z., et al. (2009). Antidiabetic and antioxidant effects of oleanolic acid from Ligustrum lucidum Ait in alloxan-induced diabetic rats. Phytother. Res. 23 (9), 1257–1262. doi:10.1002/ptr.2603
Gao, H. X., Liang, H. Y., Chen, N., Shi, B., and Zeng, W. C. (2022). Potential of phenolic compounds in Ligustrum robustum (Rxob.) Blume as antioxidant and lipase inhibitors: multi-spectroscopic methods and molecular docking. J. Food Sci. 87 (2), 651–663. doi:10.1111/1750-3841.16020
Gao, P., Ma, C., Sun, Z., Wang, L., Huang, S., Su, X., et al. (2017). Feed-additive probiotics accelerate yet antibiotics delay intestinal microbiota maturation in broiler chicken. Microbiome 5 (1), 91. doi:10.1186/s40168-017-0315-1
Gong, X., Ji, M., Xu, J., Zhang, C., and Li, M. (2020). Hypoglycemic effects of bioactive ingredients from medicine food homology and medicinal health food species used in China. Crit. Rev. Food Sci. Nutr. 60 (14), 2303–2326. doi:10.1080/10408398.2019.1634517
Graziani, N. S., Tames, M. F., Mateos, A. C., Silva, J. A., Ramos, S., Homem, V., et al. (2019). Estimation of urban POP and emerging SVOC levels employing Ligustrum lucidum leaves. Atmos. Pollut. Res. 10 (5), 1524–1530. doi:10.1016/j.apr.2019.04.010
Guo, D. J., Cheng, H. L., Chan, S. W., and Yu, P. H. (2008). Antioxidative activities and the total phenolic contents of tonic Chinese medicinal herbs. Inflammopharmacology 16 (5), 201–207. doi:10.1007/s10787-008-8016-9
Han, J., Dai, M., Zhao, Y., Cai, E., Zhang, L., Jia, X., et al. (2020). Compatibility effects of ginseng and Ligustrum lucidum Ait herb pair on hematopoietic recovery in mice with cyclophosphamide-induced myelosuppression and its material basis. J. Ginseng Res. 44 (2), 291–299. doi:10.1016/j.jgr.2019.01.001
He, F., Chen, L., Liu, Q., Wang, X., Li, J., and Yu, J. (2018). Preparative separation of phenylethanoid and secoiridoid glycosides from ligustri lucidi fructus by high-speed counter-current chromatography coupled with ultrahigh pressure extraction. Molecules 23 (12), 3353. doi:10.3390/molecules23123353
He, X. Y., Liu, Q. C., Peng, W., Huang, Y. L., and Wu, C. J. (2013). Bioactivities and serum pharmacochemistry of Qi-Wei-Xiao-Yan-Tang. Pharm. Biol. 51 (5), 629–634. doi:10.3109/13880209.2012.761243
He, Z. D., Pph, B., Chan, T. W., Dong, H., Xu, H. X., Lau, C. P., et al. (2001). Antioxidative glucosides from the fruits of Ligustrum lucidum. Chem. Pharm. Bull. (Tokyo) 49 (6), 780–784. doi:10.1248/cpb.49.780
Hu, B., Du, Q. I. N., Deng, S., An, H.-M., Pan, C.-F., Shen, K.-P., et al. (2014). Ligustrum lucidum Ait. fruit extract induces apoptosis and cell senescence in human hepatocellular carcinoma cells through upregulation of p21. Oncol. Rep. 32 (3), 1037–1042. doi:10.3892/or.2014.3312
Huang, S., Mu, F., Li, F., Wang, W., Chen, H., Lei, L., et al. (2020). A network-based approach to explore the mechanism and bioactive compounds of erzhi pill against metabolic dysfunction-associated fatty liver disease. J. Diabetes Res. 2020, 7867245. doi:10.1155/2020/7867245
Huang, X. P., Ke, Y., Feng, K., Li, H. J., and Liu, H. M. (2011). Chemical consituents of petroleum ether extract from fruits of ligustrum lucidum. Chin. Pharm. J. 46 (13), 984–987.
Jang, J. E., Oh, S. H., Choi, M. J., Lee, J. H., Chung, G. Y., and Choi, H. J. (2020). A taxonomic revision of ligustrum (Oleaceae) in Korea. J. Asia-Pacific Biodivers. 13 (3), 406–429. doi:10.1016/j.japb.2020.03.016
Jeong, J. C., Kim, J. W., Kwon, C. H., Kim, T. H., and Kim, Y. K. (2011). Fructus ligustri lucidi extracts induce human glioma cell death through regulation of Akt/mTOR pathway in vitro and reduce glioma tumor growth in U87MG xenograft mouse model. Phytother. Res. 25 (3), 429–434. doi:10.1002/ptr.3265
Jeong, S. C., Tulasi, R., and Koyyalamudi, S. R. (2016). Antioxidant capacities of hot water extracts and endopolysaccharides of selected Chinese medicinal fruits. Cancers (Basel) 8 (3), 33. doi:10.3390/cancers8030033
Jiang, J., Zhao, B., Xiao, J., Shi, L., Shang, W., Shu, Y., et al. (2024). Exploring the boost of steaming with wine on Ligustri Lucidi Fructus in treating postmenopausal osteoporosis based on superior “multi-component structure” and iron/bone metabolism coregulation. Phytomedicine 123, 155275. doi:10.1016/j.phymed.2023.155275
Kang, R., Tian, W., Cao, W., Sun, Y., Zhang, H.-N., Feng, Y.-D., et al. (2021). Ligustroflavone ameliorates CCl4-induced liver fibrosis through down-regulating the TGF-β/Smad signaling pathway. Chin. J. Nat. Med. 19 (3), 170–180. doi:10.1016/s1875-5364(21)60018-3
Kikuchi, M., and Kakuda, R. (1999). Studies on the constituents of Ligustrum species. XIX. Structures of iridoid glucosides from the leaves of Ligustrum lucidum AIT. Yakugaku Zasshi 119 (6), 444–450. doi:10.1248/yakushi1947.119.6_444
Kim, Y. J., Park, S. Y., Koh, Y. J., and Lee, J. H. (2021). Anti-neuroinflammatory effects and mechanism of action of fructus ligustri lucidi extract in BV2 microglia. Plants (Basel) 10 (4), 688. doi:10.3390/plants10040688
Knight, M. J., and Baune, B. T. (2018). Cognitive dysfunction in major depressive disorder. Curr. Opin. Psychiatry 31 (1), 26–31. doi:10.1097/YCO.0000000000000378
Kong, Y., Ma, X., Zhang, X., Wu, L., Chen, D., Su, B., et al. (2022). The potential mechanism of Fructus Ligustri Lucidi promoting osteogenetic differentiation of bone marrow mesenchymal stem cells based on network pharmacology, molecular docking and experimental identification. Bioengineered 13 (4), 10640–10653. doi:10.1080/21655979.2022.2065753
Leng, L., Zhuang, K., Liu, Z., Huang, C., Gao, Y., Chen, G., et al. (2018). Menin deficiency leads to depressive-like behaviors in mice by modulating astrocyte-mediated neuroinflammation. Neuron 100 (3), 551–563. doi:10.1016/j.neuron.2018.08.031
Lerner, A., and Benzvi, C. (2021). Let food Be thy medicine": gluten and potential role in neurodegeneration. Cells 10 (4), 756. doi:10.3390/cells10040756
Li, H., Yao, W., Liu, Q., Xu, J., Bao, B., Shan, M., et al. (2017). Application of UHPLC-ESI-Q-TOF-MS to identify multiple constituents in processed products of the herbal medicine ligustri lucidi fructus. Molecules 22 (5), 689. doi:10.3390/molecules22050689
Li, L., Chen, B. B., Zhu, R. Y., Li, R., Tian, Y. M., Liu, C. Y., et al. (2019). Fructus Ligustri Lucidi preserves bone quality through the regulation of gut microbiota diversity, oxidative stress, TMAO and Sirt6 levels in aging mice. Aging (Albany NY) 11 (21), 9348–9368. doi:10.18632/aging.102376
Li, M. Y., Liu, X. M., Deng, S., Yang, H. J., Li, X. K., and Ma, L. (2021). Analysis of inorganic elements in Ligustrum officinale herbs. Lishizhen Med. Mater. Med. Res. 32 (2), 302–305. doi:10.3969/j.issn.1008-0805.2021.02.12
Li, X., Lu, X., Fan, D., Li, L., Lu, C., Tan, Y., et al. (2020). Synergistic effects of erzhi pill combined with methotrexate on osteoblasts mediated via the wnt1/LRP5/β-catenin signaling pathway in collagen-induced arthritis rats. Front. Pharmacol. 11, 228. doi:10.3389/fphar.2020.00228
Lin, H. M., Yen, F. L., Ng, L. T., and Lin, C. C. (2007). Protective effects of Ligustrum lucidum fruit extract on acute butylated hydroxytoluene-induced oxidative stress in rats. J. Ethnopharmacol. 111 (1), 129–136. doi:10.1016/j.jep.2006.11.004
Liu, H., Guo, Y., Zhu, R., Wang, L., Chen, B., Tian, Y., et al. (2020a). Fructus Ligustri Lucidi preserves bone quality through induction of canonical Wnt/β-catenin signaling pathway in ovariectomized rats. Phytotherapy Res. 35 (1), 424–441. doi:10.1002/ptr.6817
Liu, H., Xiong, H., Xue, X., Liu, M. N., Wang, H. L., Liu, W., et al. (2019). Study on quality characteristics of ligustri lucidi fructus in national resource survey and consideration on standards of ligustri lucidi fructus in Chinese Pharmacopoeia. Zhongguo zhongyao zazhi 44 (1), 68–76. doi:10.19540/j.cnki.cjcmm.20181025.004
Liu, J., Mao, J. J., Li, S. Q., and Lin, H. (2020b). Preliminary efficacy and safety of reishi and privet formula on quality of life among non-small cell lung cancer patients undergoing chemotherapy: a randomized placebo-controlled trial. Integr. Cancer Ther. 19, 1534735420944491. doi:10.1177/1534735420944491
Liu, J., Liu, Z. Y., Wang, L. L., He, H., Mu, H. L., Sun, W. J., et al. (2021). Bioactivity-guided isolation of immunomodulatory compounds from the fruits of Ligustrum lucidum. J. Ethnopharmacol. 28 (274), 114079. doi:10.1016/j.jep.2021.114079
Long, F., Deng, L., and Chen, Y. (2011). Study on the chemical constituents in the flowers of Ligustrum lucium. Huaxi Yaoxue Zazhi 26 (2), 97–100. doi:10.13375/j.cnki.wcjps.2011.02.008
Lucas, S., Omata, Y., Hofmann, J., Bottcher, M., Iljazovic, A., Sarter, K., et al. (2018). Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 9 (1), 55. doi:10.1038/s41467-017-02490-4
Luo, W., Ip, F. C. F., Fu, G., Cheung, K., Tian, Y., Hu, Y., et al. (2020). A pentacyclic triterpene from ligustrum lucidum targets gamma-secretase. ACS Chem. Neurosci. 11 (18), 2827–2835. doi:10.1021/acschemneuro.0c00389
Ma, D., Shan, A., Li, J., Zhao, Y., and Guo, X. (2009). Influence of an aqueous extract of Ligustrum lucidum and an ethanol extract of Schisandra chinensis on parameters of antioxidative metabolism and spleen lymphocyte proliferation of broilers. Arch. Anim. Nutr. 63 (1), 66–74. doi:10.1080/17450390802611578
Ma, Z., Tang, X., Gao, Y., Wang, H., Yu, P., and Liu, R. (2020). Combined extracts of epimedii folium and ligustri lucidi fructus with budesonide attenuate airway remodeling in the asthmatic rats by regulating apoptosis and autophagy. Evid. Based Complement. Altern. Med. 2020, 2319409. doi:10.1155/2020/2319409
Mani, B. M., Huerta-Ocampo, J. A., Garcia-Sanchez, J. R., Barrera-Pacheco, A., de la Rosa, A. P., and Teran, L. M. (2015). Identification of Ligustrum lucidum pollen allergens using a proteomics approach. Biochem. Biophys. Res. Commun. 468 (4), 788–792. doi:10.1016/j.bbrc.2015.11.033
Meng, W. Q., Li, Z. P., Zhang, Y. T., Yang, A. H., Wang, Y. Z., Zhou, Y. L., et al. (2022). ZhenQi FuZheng formula inhibits the growth of colorectal tumors by modulating intestinal microflora-mediated immune function. Aging (Albany NY) 14 (11), 4769–4785. doi:10.18632/aging.204111
Miao, M., Cao, L., Bai, M., and Chen, G. (2018). Effect of Zhen Qi Fu Zheng granules on the bone marrow depression model induced by Zidorf. Saudi J. Biol. Sci. 25 (2), 220–225. doi:10.1016/j.sjbs.2017.10.002
Mukherjee, S., Jana, S., Khawas, S., Kicuntod, J., Marschall, M., Ray, B., et al. (2022). Synthesis, molecular features and biological activities of modified plant polysaccharides. Carbohydr. Polym. 289, 119299. doi:10.1016/j.carbpol.2022.119299
Pang, X., Zhao, J. Y., Yu, H. Y., Yu, L. Y., Wang, T., Zhang, Y., et al. (2018). Secoiridoid analogues from the fruits of Ligustrum lucidum and their inhibitory activities against influenza A virus. Bioorg Med. Chem. Lett. 28 (9), 1516–1519. doi:10.1016/j.bmcl.2018.03.080
Peng, M., Xia, T., Zhong, Y., Zhao, M., Yue, Y., Liang, L., et al. (2022). Integrative pharmacology reveals the mechanisms of Erzhi Pill, a traditional Chinese formulation, against diabetic cardiomyopathy. J. Ethnopharmacol. 296, 115474. doi:10.1016/j.jep.2022.115474
Qiao, G. H., Zhou, X. H., Li, Y., Zhang, H. S., Li, J. H., Wang, C. M., et al. (2012). Effect of several supplemental Chinese herbs additives on rumen fermentation, antioxidant function and nutrient digestibility in sheep. J. Anim. Physiol. Anim. Nutr. Berl. 96 (5), 930–938. doi:10.1111/j.1439-0396.2011.01211.x
Qin, X., Wei, Q., An, R., Yang, Y., Cai, M., Han, X., et al. (2023). Regulation of bone and fat balance by Fructus Ligustri Lucidi in ovariectomized mice. Pharm. Biol. 61 (1), 391–403. doi:10.1080/13880209.2023.2168019
Qiu, Z. C., Zhao, X. X., Wu, Q. C., Fu, J. W., Dai, Y., Wong, M. S., et al. (2018). New secoiridoids from the fruits of Ligustrum lucidum. J. Asian Nat. Prod. Res. 20 (5), 431–438. doi:10.1080/10286020.2018.1454438
Ren, J. L., Zhang, A. H., Kong, L., Han, Y., Yan, G. L., Sun, H., et al. (2020). Analytical strategies for the discovery and validation of quality-markers of traditional Chinese medicine. Phytomedicine 67, 153165. doi:10.1016/j.phymed.2019.153165
Robledo-Retana, T., Mani, B. M., and Teran, L. M. (2020). Ligustrum pollen: new insights into allergic disease. World Allergy Organ J. 13 (2), 100104. doi:10.1016/j.waojou.2020.100104
Seo, H. L., Baek, S. Y., Lee, E. H., Lee, J. H., Lee, S. G., Kim, K. Y., et al. (2017). Liqustri lucidi Fructus inhibits hepatic injury and functions as an antioxidant by activation of AMP-activated protein kinase in vivo and in vitro. Chem. Biol. Interact. 262, 57–68. doi:10.1016/j.cbi.2016.11.031
Shi, Y. K., Cui, F., Hu, F. D., Bi, Y. Y., Ma, Y. F., and Feng, S. L. (2011). Quantification of six bioactive compounds in Zhenqi Fuzheng preparation by high-performance liquid chromatography coupled with diode array detector and evaporative light scattering detector. J. Pharm. Analysis 1 (1), 20–25. doi:10.1016/s2095-1779(11)70004-1
Song, D., Yin, L., Wang, C., and Wen, X. (2020). Zhenqing recipe attenuates non-alcoholic fatty liver disease by regulating the SIK1/CRTC2 signaling in experimental diabetic rats. BMC Complement. Med. Ther. 20 (1), 27. doi:10.1186/s12906-019-2811-2
Suanno, C., Aloisi, I., Fernandez-Gonzalez, D., and Del Duca, S. (2021). Pollen forecasting and its relevance in pollen allergen avoidance. Environ. Res. 200, 111150. doi:10.1016/j.envres.2021.111150
Tao, R., Liu, E., Zhao, X., Han, L., Yu, B., Mao, H., et al. (2022). Combination of Ligustri Lucidi Fructus with Ecliptae Herba and their phytoestrogen or phytoandrogen like active pharmaceutical ingredients alleviate oestrogen/testosterone-induced benign prostatic hyperplasia through regulating steroid 5-α-reductase. Phytomedicine 102, 154169. doi:10.1016/j.phymed.2022.154169
Tian, G., Chen, J., Luo, Y., Yang, J., Gao, T., and Shi, J. (2019). Ethanol extract of Ligustrum lucidum Ait. leaves suppressed hepatocellular carcinoma in vitro and in vivo. Cancer Cell Int. 19, 246. doi:10.1186/s12935-019-0960-5
Urcelay, C., Longo, S., Geml, J., and Tecco, P. A. (2019). Can arbuscular mycorrhizal fungi from non-invaded montane ecosystems facilitate the growth of alien trees? Mycorrhiza 29 (1), 39–49. doi:10.1007/s00572-018-0874-4
Varela, Z., Martínez-Abaigar, J., Tomás-Las-Heras, R., Fernández, J. Á., Del-Castillo-Alonso, M.-Á., and Núñez-Olivera, E. (2023). Tree physiological variables as a proxy of heavy metal and platinum group elements pollution in urban areas. Biology 12 (9), 1180. doi:10.3390/biology12091180
Vizuet-de-Rueda, J. C., Montero-Vargas, J. M., Galvan-Morales, M. A., Porras-Gutierrez-de-Velasco, R., and Teran, L. M. (2022). Current insights on the impact of proteomics in respiratory allergies. Int. J. Mol. Sci. 23 (10), 5703. doi:10.3390/ijms23105703
Wang, C., Gao, H., Cai, E., Zhang, L., Zheng, X., Zhang, S., et al. (2019). Protective effects of Acanthopanax senticosus - ligustrum lucidum combination on bone marrow suppression induced by chemotherapy in mice. Biomed. Pharmacother. 109, 2062–2069. doi:10.1016/j.biopha.2018.11.071
Wang, C., Gong, X., Bo, A., Zhang, L., Zhang, M., Zang, E., et al. (2020). Iridoids: research advances in their phytochemistry, biological activities, and pharmacokinetics. Molecules 25 (2), 287. doi:10.3390/molecules25020287
Wang, J., Cao, Y., Feng, X., Li, T., Bi, Y., Zhang, T., et al. (2022). Study on the synergistic and attenuating mechanism of the combination of Epimedium and Ligustri lucidi fructus based on pharmacokinetics. J. Sep. Sci. 45, 3382–3392. doi:10.1002/jssc.202200336
Wang, J., Shan, A., Liu, T., Zhang, C., and Zhang, Z. (2012). In vitro immunomodulatory effects of an oleanolic acid-enriched extract of Ligustrum lucidum fruit (Ligustrum lucidum supercritical CO2 extract) on piglet immunocytes. Int. Immunopharmacol. 14 (4), 758–763. doi:10.1016/j.intimp.2012.10.006
Wang, L., Ma, R., Guo, Y., Sun, J., Liu, H., Zhu, R., et al. (2017). Antioxidant effect of fructus ligustri lucidi aqueous extract in ovariectomized rats is mediated through nox4-ROS-NF-κb pathway. Front. Pharmacol. 8, 266. doi:10.3389/fphar.2017.00266
Wang, Q., Yu, H., Zong, J., He, P., and Fang, Y. (2003). Determination of the composition of Chinese ligustrum lucidum polysaccharide by capillary zone electrophoresis with amperometric detection. J. Pharm. Biomed. Analysis 31 (3), 473–480. doi:10.1016/s0731-7085(02)00714-8
Wang, X. J., Ding, L. M., Wei, H. Y., Jiang, C. X., Yan, Q., Hu, C. S., et al. (2021). Astragalus membranaceus root supplementation improves average daily gain, rumen fermentation, serum immunity and antioxidant indices of Tibetan sheep. Animal 15 (1), 100061. doi:10.1016/j.animal.2020.100061
Wegiel, B., and Persson, J. L. (2010). Effect of a novel botanical agent Drynol Cibotin on human osteoblast cells and implications for osteoporosis: promotion of cell growth, calcium uptake and collagen production. Phytother. Res. 24, S139–S147. Suppl 2. doi:10.1002/ptr.3026
Wei, P. L., Gu, H., Liu, J., and Wang, Z. (2018). Development of fangjiomics for systems elucidation of synergistic mechanism underlying combination therapy. Comput. Struct. Biotechnol. J. 16, 565–572. doi:10.1016/j.csbj.2018.10.015
Wen, X., Zeng, Y., Liu, L., Zhang, H., Xu, W., Li, N., et al. (2012). Zhenqing recipe alleviates diabetic nephropathy in experimental type 2 diabetic rats through suppression of SREBP-1c. J. Ethnopharmacol. 142 (1), 144–150. doi:10.1016/j.jep.2012.04.028
Wu, C. R., Hseu, Y. C., Lien, J. C., Lin, L. W., Lin, Y. T., and Ching, H. (2010). Triterpenoid contents and anti-inflammatory properties of the methanol extracts of ligustrum species leaves. Molecules 16 (1), 1–15. doi:10.3390/molecules16010001
Wu, Y., Hu, Y., Zhao, Z., Xu, L., Chen, Y., Liu, T., et al. (2021). Protective effects of water extract of fructus ligustri lucidi against oxidative stress-related osteoporosis in vivo and in vitro. Veterinary Sci. 8 (9), 198. doi:10.3390/vetsci8090198
Xia, E. Q., Yu, Y. Y., Xu, X. R., Deng, G. F., Guo, Y. J., and Li, H. B. (2012). Ultrasound-assisted extraction of oleanolic acid and ursolic acid from Ligustrum lucidum Ait. Ultrason. Sonochem 19 (4), 772–776. doi:10.1016/j.ultsonch.2011.11.014
Xiao, X., Gao, Y. M., Xu, S. M., Li, S. S., Wu, C. R., and Yan, S. K. (2019). GC-MS studies on the volatile oil composition of different forms of Ligustrum officinale. J. Chin. Med. Mater. 42 (10), 2280–2283. doi:10.13863/j.issn1001-4454.2019.10.014
Xie, Y., Yan, B., Hou, M., Zhou, M., Liu, C., Sun, M., et al. (2021). Erzhi pills ameliorate cognitive dysfunction and alter proteomic hippocampus profiles induced by d-galactose and Aβ1-40 injection in ovariectomized Alzheimer's disease model rats. Pharm. Biol. 59 (1), 1402–1414. doi:10.1080/13880209.2021.1990353
Xu, D., Lyu, Y., Chen, X., Zhu, X., Feng, J., and Xu, Y. (2016). Fructus Ligustri Lucidi ethanol extract inhibits osteoclastogenesis in RAW264.7 cells via the RANKL signaling pathway. Mol. Med. Rep. 14 (5), 4767–4774. doi:10.3892/mmr.2016.5849
Xu, H., Su, Z. R., Huang, W., Choi, R. C., Zheng, Y. Z., Lau, D. T., et al. (2012). Er Zhi Wan, an ancient herbal decoction for woman menopausal syndrome, activates the estrogenic response in cultured MCF-7 cells: an evaluation of compatibility in defining the optimized preparation method. J. Ethnopharmacol. 143 (1), 109–115. doi:10.1016/j.jep.2012.06.009
Xu, X. H., Yang, N. Y., Qian, S. H., Xie, N., and Duan, J. A. (2008). Dammarane triterpenes from Ligustrum lucidum. J. Asian Nat. Prod. Res. 10 (1-2), 33–37. doi:10.1080/10286020701273833
Xue, Z., Xu, L., Shang, Z., Shi, X., Ye, M., and Qiao, X. (2021). Discovery of minor quality evaluation marker compounds for Chinese patent medicine products using a two-leveled metabolomics strategy. J. Chromatogr. A 1652, 462354. doi:10.1016/j.chroma.2021.462354
Yan, B., Zhou, L., Wang, C., Wang, R., Yan, L., Yu, L., et al. (2018). Intra-articular injection of fructus ligustri lucidi extract attenuates pain behavior and cartilage degeneration in mono-iodoacetate induced osteoarthritic rats. Front. Pharmacol. 9, 1360. doi:10.3389/fphar.2018.01360
Yang, N., Zhang, Y., and Guo, J. (2015). Preventive effect of total glycosides from Ligustri Lucidi Fructus against nonalcoholic fatty liver in mice. Z. für Naturforsch. C 70 (9-10), 237–241. doi:10.1515/znc-2015-4161
Yao, W., He, M., Jiang, Y., Zhang, L., Ding, A., and Hu, Y. (2013). Integrated LC/MS and GC/MS metabolomics data for the evaluation of protection function of fructus ligustri lucidi on mouse liver. Chromatographia 76 (17-18), 1171–1179. doi:10.1007/s10337-013-2519-2
Yin, Z., Zhang, W., Zhang, J., and Kang, W. (2017). Isolation, purification, structural analysis and coagulatory activity of water-soluble polysaccharides from Ligustrum lucidum Ait flowers. Chem. Cent. J. 11 (1), 98. doi:10.1186/s13065-017-0332-y
Yu, W., Sun, S., Zhang, K., Li, H., Xin, M., Liu, Y., et al. (2022). Fructus ligustri lucidi suppresses inflammation and restores the microbiome profile in murine colitis models. Phytomedicine 106, 154438. doi:10.1016/j.phymed.2022.154438
Zhang, P., Li, H., Chen, D., Ni, J., Kang, Y., and Wang, S. (2007). Oleanolic acid induces apoptosis in human leukemia cells through caspase activation and poly(ADP-ribose) polymerase cleavage. Acta Biochim. Biophys. Sin. (Shanghai) 39 (10), 803–809. doi:10.1111/j.1745-7270.2007.00335.x
Zhang, X. B., Gao, P. G., Zhang, X. R., and Xu, X. Q. (2011). Analysis on nutritional components of Ligustrum lucidum. J. Yangzhou Univ. 32 (2), 86–88. doi:10.16872/j.cnki.1671-4652.2011.02.018
Zhang, Y., He, Y., Liu, C., Liu, C., and Li, S. (2018). Screening and isolation of potential neuraminidase inhibitors from leaves of Ligustrum lucidum Ait. based on ultrafiltration, LC/MS, and online extraction-separation methods. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1083, 102–109. doi:10.1016/j.jchromb.2018.03.006
Zhang, Y., Liu, L., Gao, J., Wu, C., Han, L., Liu, E., et al. (2013). New secoiridoids from the fruits of Ligustrum lucidum Ait with triglyceride accumulation inhibitory effects. Fitoterapia 91, 107–112. doi:10.1016/j.fitote.2013.08.022
Zhao, H., Liu, J., Song, L., Liu, Z., Han, G., Yuan, D., et al. (2017). Oleanolic acid rejuvenates testicular function through attenuating germ cell DNA damage and apoptosis via deactivation of NF-κB, p53 and p38 signalling pathways. J. Pharm. Pharmacol. 69 (3), 295–304. doi:10.1111/jphp.12668
Zhao, H. M., Zhang, X. Y., Lu, X. Y., Yu, S. R., Wang, X., Zou, Y., et al. (2018). Erzhi Pill® protected experimental liver injury against apoptosis via the PI3K/Akt/Raptor/Rictor pathway. Front. Pharmacol. 9, 283. doi:10.3389/fphar.2018.00283
Zhao, Y., Xu, Y., Hou, Y. Y., Tang, G. S., Zhao, T. Q., and Guo, S. (2016). Content determination of 21 kinds of amino acids in ligustri lucidi fructus by RP-HPLC. Chin. J. Exp. Traditional Med. Formulae 22 (14), 79–83. doi:10.13422/j.cnki.syfjx.2016140079
Zhao, Z. H., Bi, J. H., Ye, S. J., Huang, Y. K., Min, T., Wang, H. X., et al. (2022). Mechanism of astragalus-ligustrum lucidum in the treatment of immunodeficiency diseases based on network pharmacology and molecular docking. Sci. Technol. Food Industry 43 (3), 374–383. doi:10.13386/j.issn1002-0306.2021060024
Zheng, J., Qu, X., Hou, R., Tang, X., Xu, Z., Huang, Z., et al. (2024). A comparative study of air pollution tolerance capabilities of four tree species in Xi’an city, China. Int. J. Environ. Sci. Technol. 21 (1), 665–674. doi:10.1007/s13762-023-04970-1
Zhong, Z., Li, Y., Chen, Y., Chen, W., Li, S., Lv, X., et al. (2021). Predicting and exploring the mechanisms of erzhi pill in prevention and treatment of osteoporosis based on network pharmacology and zebrafish experiments. Drug Des. Devel Ther. 15, 817–827. doi:10.2147/DDDT.S293455
Glossary
Keywords: Ligustrum lucidum W.T. aiton, homology of medicine and food, nutritional value and chemical composition, pharmacological activity, applications
Citation: Chen L, Huang D, Jiang L, Yang J, Shi X, Wang R and Li W (2024) A review of botany, phytochemistry, pharmacology, and applications of the herb with the homology of medicine and food: Ligustrum lucidum W.T. Aiton. Front. Pharmacol. 15:1330732. doi: 10.3389/fphar.2024.1330732
Received: 31 October 2023; Accepted: 14 May 2024;
Published: 12 June 2024.
Edited by:
Wei Peng, Chengdu University of Traditional Chinese Medicine, ChinaReviewed by:
Dandan Tang, Sichuan College of Traditional Chinese Medicine, ChinaRuolan Li, Chengdu University of Traditional Chinese Medicine, China
Copyright © 2024 Chen, Huang, Jiang, Yang, Shi, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Wang, bHVja2NobHAzNjlAMTYzLmNvbQ==; Wenbin Li, eWZjczIwMDJAMTYzLmNvbQ==
 Liping Chen1
Liping Chen1 Lin Jiang
Lin Jiang