- 1College of Traditional Chinese Medicine, Shandong University of Traditional Chinese Medicine, Jinan, China
- 2College of Rehabilitation Medical, Shandong University of Traditional Chinese Medicine, Jinan, China
- 3Research Department of Shandong University of Traditional Chinese Medicine, Jinan, China
Pulmonary fibrosis (PF) is a chronic and progressive disease characterized by fibrosis and interstitial pneumonia. It has similar clinical symptoms to “Fei Bi” and “Fei Wei” as described in the traditional Chinese medicine (TCM) classic Jingui Yaolue written by Zhang Zhongjing in the Han Dynasty. This study explored the potential of Maimendong Decoction (MMDD). MMDD consists of Ophiopogon japonicus (L.f) (ophiopogonis), Pinellia ternata (Thunb.) Breit. (pinellia), Panax ginseng C. A. Mey. (ginseng), Glycyrrhiza uralensis Fisch. (glycyrrhiza), Zizi phus jujuba Mill. (jujuba), and Oryza sativa L. (oryza sativa), with the function of nourishing the lung and stomach, and reducing the effect of reverse qi. It has been used clinically for over two thousand years to treat conditions like “Fei Bi” and “Fei Wei”. Previous research suggests that MMDD and its individual herbal extracts have anti-fibrotic effects. The main focus of MMDD in treating PF is to reduce inflammatory cytokines, inhibit pro-fibrotic factors and oxidative stress, promote differentiation and homing of bone marrow mesenchymal stem cells, and enhance cell autophagy activity. This review summarized the clinical applications, mechanisms, and pharmacological effects of MMDD in treating PF based on existing clinical applications and experimental research. It also discussed current issues and prospects, aiming to provide a reference for further research on the mechanism of PF, drug development, and clinical trials.
1 Introduction
Pulmonary fibrosis (PF) is a chronic and irreversible lung condition classified under interstitial lung diseases. It involves the scarring and thickening of lung tissue. Various triggers, including environmental factors, occupational exposure, medications, autoimmune diseases, and genetic factors, can cause PF. Common symptoms experienced by PF patients include shortness of breath, persistent dry cough, wheezing, sputum production, chest tightness, and chest pain (Mathai and Schwartz, 2019; Liu et al., 2022). In recent years, the incidence of PF is more than 100,000 people each year, and it has been rising sharply (Pan et al., 2020). Among them, idiopathic PF, in particular, has garnered public attention due to its increasing prevalence and incidence worldwide (Wijsenbeek, 2020; Maher et al., 2021). The annual incidence of idiopathic PF reaches as high as 17.4 people per 100,000 (Zhao et al., 2020), with an average survival after diagnosis of only 2.8 years. The mortality rate exceeds that of most tumors (Viale, 2020), and complications are common. PF patients also face a significantly higher risk of lung cancer compared to the general population, further impacting their quality of life and burdening society (Tzouvelekis et al., 2020). Consequently, prevention and treatment have become urgent priorities within the medical community. Currently, Western medicine primarily employs glucocorticoids (Wiertz et al., 2018), nintedanib, pirfenidone, and other drugs to slow down disease progression (Richeldi et al., 2020). However, these treatments cannot offer a complete cure, often carry significant side effects, are expensive, and lack widespread acceptance among the general public (Cottin et al., 2023).
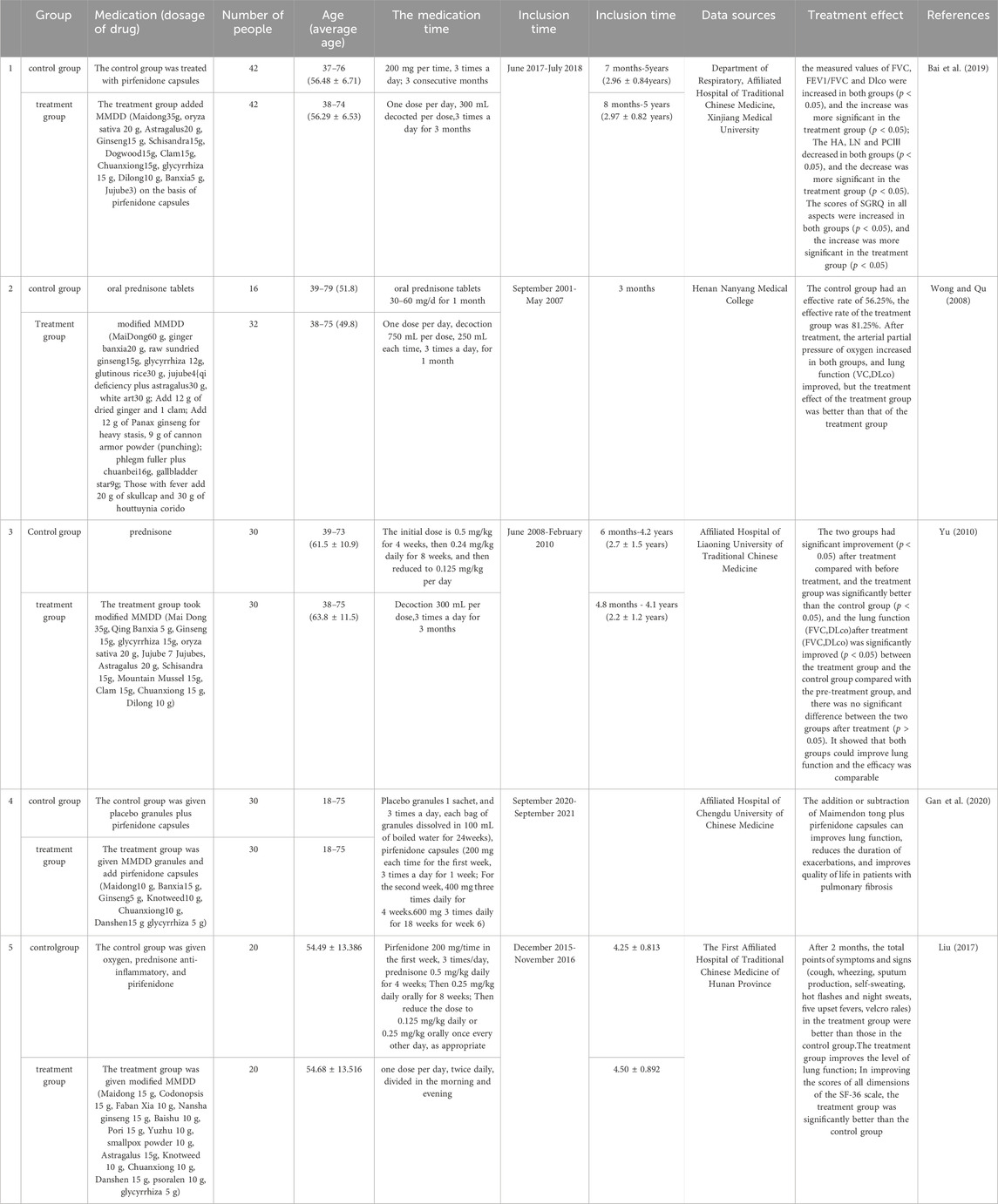
In recent years, with the deepening of Chinese medicine research, the study found that Chinese medicine in the treatment of PF in a significant effect, and minimizing side effects (Guo et al., 2019). TCM categorizes PF as a manifestation of “Fei Bi” and “Fei Wei,” as documented in the Jingui Yaolue: “Cunkou pulse rapid, who coughs and has turbid saliva in the mouth. Teacher said: for the disease of Atrophy of the lung lobes.” According to this perspective, the lungs are closely connected to the spleen and kidneys. Exogenous etiological factors, seven emotions, sexual overindulgence and mistreatment contribute to the development of PF. The pathogenesis is characterized by deficiencies in healthy qi, yin deficiency leading to hyperactivity of fire, lung yin deficiency pattern, phlegm and blood stasis obstructing the lung. Consequently, the syndrome is categorized as originating from deficiency with superficial excess. Clinical treatment can tonify lung qi, resolve phlegm, promote blood circulation, and remove blood stasis (Zang et al., 2017; Cheng et al., 2020; Li et al., 2022). Maimendong Decoction (MMDD), a classic prescription from the Jingui Yaolue, consists of Ophiopogon japonicus (L.f) (ophiopogonis), Pinellia ternata (Thunb.) Breit. (pinellia), Panax ginseng C. A. Mey. (ginseng), Glycyrrhiza uralensis Fisch. (glycyrrhiza), Oryza sativa L. (oryza sativa), and Zizi phus jujuba Mill. (jujuba) (Figure 1). It has the effect to nourish and supplement lung and stomach, and reduce the effect of reverse qi. It has long been used to treat cough, lung cancer, PF, and other respiratory diseases (Yulong and Chunshui, 2017). Due to the influence of various factors like environment, genetics, and lifestyle, the prevalence of PF continues to rise annually. Consequently, there is an urgent need to identify effective therapeutic solutions (Bhavana et al., 2021). While MMDD has been widely employed in PF treatment, its precise mechanism remains unclear. Therefore, this systematic review was aimed to provide a comprehensive analysis of clinical application, mechanism, pharmacological action, and effective material basis of MMDD in treating PF, enabling a deeper understanding of its pharmacological mechanism and prescription characteristics. This review will serve as a reference for the clinical development of new drug treatments for PF.
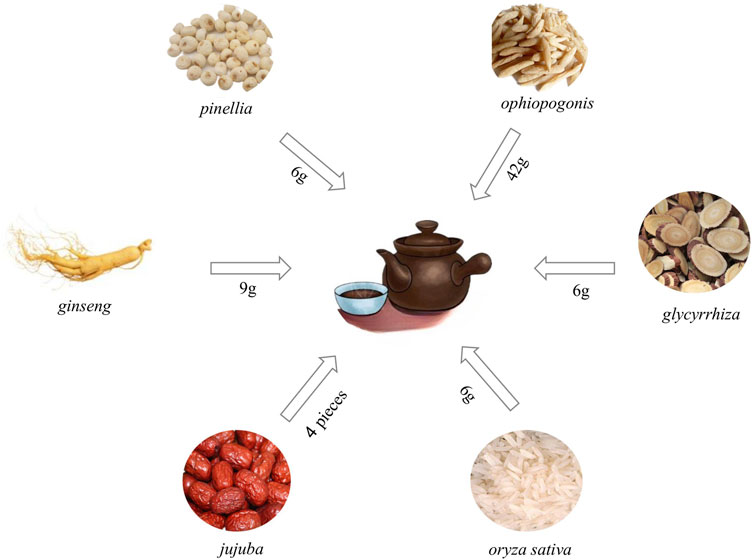
Figure 1. Composition and dosage of Maimendong Dection (Maimendong Dection composed of ophiopogonis, pinellia, ginseng, glycyrrhiza, jujuba and oryza sativa).
2 Methodology
Keywords, including Maimendong, Maimendong Decoction, Pulmonary Fibrosis, Idiopathic Pulmonary Fibrosis, Idiopathic Diffuse Interstitial Pulmonary Fibrosis, Fibrosis, lung, were used to search related literature and data through digital resources and paper-based materials. The earliest available documents were published in 1968, while the latest was published in April 2024. These documents discussed the clinical randomized controlled trials of MMDD in the treatment of PF and the mechanism of MMDD in the treatment of PF. Most of the literature research was conducted through the following eight online scientific databases: PubMed, Web of Science, Google Scholar, SciFinder, CNKI, Baidu Scholar, China Science and Technology Journal Database, Wan Fang Data Knowledge Service Platform. The review also included results from Ph.D. theses, M. Sc. Dissertations.
3 Clinical applications of MMDD in the treatment of PF
Modern studies have demonstrated that modified MMDD or its combination with Western medicine can effectively treat PF with fewer side effects. It has been considered as an effective treatment for PF. For instance, Bai et al. (2019) conducted a study with 84 PF patients divided into control and treatment groups. The control group received pirfenidone capsules, while the treatment group received pirfenidone capsules along with MMDD. The researchers found that both groups experienced reduced TCM syndrome scores after treatment. Additionally, the forced vital capacity (FVC), carbon monoxide diffusion volume (DLCO), and forced expiratory volume occupancy lung capacity ratios increased, while levels of hyaluronic acid (HA), laminin (LN), and type Ⅲ precollagen (PCⅢ) decreased. Scores on the George Respiratory Questionnaire (SGRQ) increased in all aspects, indicating significant improvements in the treatment group compared to the control group. These findings suggest that the therapeutic effects of combining pirfenidone with MMDD were superior to pirfenidone capsules alone. Wong and Qu (2008) conducted a clinical observation involving 48 PF patients to compare the efficacy of MMDD and glucocorticoids. Pulmonary function and arterial oxygen partial pressure data showed better indicators for the MMDD group than the glucocorticoid group. Furthermore, the total effective treatment rate was 81.25% in the MMDD group, while it was 56.25% in the glucocorticoid group, indicating that MMDD was more effective than glucocorticoids in treating PF. Yu (2010) randomly assigned 60 PF patients to a control group and a treatment group. The control group received prednisone, while the treatment group received modified MMDD (consisting of ophiopogonis, pinellia, ginseng, Baked glycyrrhiza, oryza sativa, jujuba, Astragali radix, Schisandrae Chinensis Fructus, Cornus Officinalis, Gecko, Chuanxiong Rhizoma, and Pheretima) orally for 3 months in addition to prednisone. The study measured the FVC and DLCO of the patients before and after treatment, and scored improvements in symptoms such as cough, sputum volume, sleep, fatigue, and number of breaths per minute. The nimodipine score showed that the MMDD plus prednisone group had better scores than the prednisone group, indicating that the combination of MMDD and Western medicine was more effective than Western medicine alone. Gan et al. (2020) intervened with modified MMDD and pirfenidone capsules in PF patients, while the control group received placebo and pirfenidone capsules. The study evaluated the efficacy and safety of modified MMDD and pirfenidone capsules by observing average changes in lung capacity, the number of acute exacerbations, baseline changes, liver and kidney function, and other laboratory tests through a double-blind, randomized trial. The researchers initially determined that MMDD and pirfenidone capsules could improve lung function, reduce the duration of acute exacerbation, and enhance the quality of life in patients. Liu (2017) studied the qi and yin deficiency syndrome in PF patients. The control group received oxygen inhalation, anti-inflammatory treatment with prednisone, and pirfenidone, while the treatment group received MMDD in addition to the control group’s treatment. After 2 months, the treatment group showed better total scores for symptoms and signs (cough, asthma, expectoration, spontaneous sweating, hot flashes, night sweats, five upset heat, and velcro rales) compared to the control group. The treatment group also exhibited improved lung function levels and significantly higher scores in each dimension of the short form 36 health survey questionnaire (SF-36) scale, indicating that MMDD had a therapeutic effect on qi and yin deficiency syndrome in PF patients. The effects were superior to the conventional Western medicine treatment alone. These clinical studies provide evidence that MMDD has significant therapeutic effects on PF (Table 1). Moreover, MMDD, either used alone or in combination with Western medicine, demonstrates better efficacy and fewer side effects compared to Western medicine alone. Therefore, MMDD holds promise as a future treatment modality for PF.
However, from the existing research, it is found that MMDD is mainly used in China, Japan, South Korea and other countries. It has not been found whether MMDD has been approved for use in Western countries. Therefore, in the future, the benefits of traditional Chinese medicine can be popularized and promoted, so that traditional Chinese medicine can play a role in the health of all mankind.
4 Mechanism of MMDD in the treatment of PF
4.1 Reduction of inflammatory cytokine levels by MMDD
PF is characterized by inflammatory injury, with inflammation playing a crucial role throughout its progression. Interleukins (ILs) and other inflammatory mediators have been confirmed to be involved in PF, reflecting its degree of inflammation, prognosis, and severity assessment (She et al., 2021). Tumor necrosis factor-α (TNF-α) activity and the presence of inflammatory cells are pro-inflammatory factors that stimulate chemotaxis of inflammatory cells and promote local inflammatory responses (Tang et al., 2011). Therefore, reducing levels of inflammatory cytokines is an effective strategy for treating PF. IL-10 exerts anti-inflammatory effects by inhibiting TNF-α production at the transcriptional level (Islam et al., 2022). Studies have shown that MMDD can reduce alveolar inflammation and PF in rats, potentially through the decrease in IL-10 expression and inhibition of lung TNF-α, transforming growth factor-β1 (TGF-β1), platelet-derived factor (PDGF), and connective tissue growth factor (CTGF) (Du, 2015). Huang et al. (2020) found demonstrated that the combination of MMDD and Shenling Baizhu Powder can significantly reduce the protein content and gene expression of high mobility group protein 1 (HMGB1), IL-6, PDGF, and K-Ras in lung tissue. They also found a positive correlation between HMGB1 and PDGF, suggesting that TCM treatment for silicosis fibrosis involves the HMGB1/PDGF/Ras signaling pathway. Li et al. (2009) observed that MMDD can significantly reduce alveolitis caused by pingyangmycin in rats, increase the expression of IL-10 in lung tissue, and inhibit the overexpression of TNF-α. He et al. (2024) found that MMDD could improve the body weight and reduce the inflammatory response of PF model mice, and the possible mechanism was that MMDD inhibited M2 macrophage polarization, released profibrotic factors, and inhibited PF in rats.
4.2 Inhibition of profibrotic factor expression by MMDD
TGF-β is a cytokine that contributes to the development of PF and plays a role throughout its progression (Ghatak et al., 2017). It promotes the activation and differentiation of inflammatory cytokines in the early stage and the division and proliferation of fibroblasts in the later stage, ultimately leading to collagen synthesis and ECM (ECM) deposition, resulting in thickening of the alveolar wall and PF(Stewart et al., 2018). Therefore, inhibiting the expression of TGF-β and its related factors can slow down PF progression. Yuan et al. (2023) observed improved lung function in patients with silicosis-induced fibrosis following intervention with miR-200 bagomir. This intervention alleviated protein concentration, total white blood cell count, and TGF-β1 content in bronchoalveolar lavage fluid. Liu et al. (2022) investigated the effects of modified MMDD on mice with bleomycin-induced PF. They found that the modified MMDD group exhibited reduced mortality and lung coefficient compared to the model group. Pathological changes such as inflammatory cell infiltration, severe alveolar structure destruction, and collagen deposition in lung tissue were also reduced. Moreover, the protein and mRNA expression of TGF-β1, type I collagen (COL1A), smooth muscle actin (α-SMA), phosphorylated (p)- Phosphoinositide 3-kinase (PI3K), p-protein kinase B (AKT), and mTOR were decreased, indicating that modified MMDD could effectively improve bleomycin-induced PF. The mechanism may involve the regulation of the PI3K/AKT/mTOR signaling pathway, inhibition of epithelial mesenchymal transition, and reduction of ECM deposition. Zhang et al. (2017) administered MMDD to rats with PF and found that it significantly inhibited the expression of Smad3 and TGF-β1 protein in the lung tissue of the model group while increased the expression of Smad7 protein. These results suggest that MMDD may improve the pathological state of PF in rats by regulating the expression of TGF-β1, Smad3, and Smad7 proteins. Liu et al. (2018) proposed that MMDD alleviated alveolar inflammation and fibrosis by inhibiting the protein expression of TGF-β1, matrix metalloproteinase (MMP-9), and matrix metalloproteinase tissue inhibitor 1 (TIMP-1) in lung tissue, thereby regulating abnormal ECM metabolism. Wang (2017) suggested that reducing the expression of TGF-β1 and ColI may alleviate lung tissue damage, alveolar inflammation, and collagen fiber formation induced by bleomycin, subsequently reducing the development of PF. Xu et al. (2024) found that mice in the model group had elevated lung coefficients, a large number of inflammatory cell infiltration and collagen fiber deposition in lung tissues, and elevated lung fiber scores, and after administration of MMDD, mice had reduced lung coefficients, decreased inflammatory cell infiltration and collagen fiber deposition in lung tissues, and reduced lung fiber scores, and Collagen I, TGF-β1, p-STAT3, PD-1, PD-L1, and IL-17A decreased under expression, suggesting that maitake soup retarded the process of PF by reducing ECM deposition, and the mechanism may be related to with the inhibition of STAT3/PD-1/PD-L1 immunoregulatory signaling pathway.
4.3 Inhibition of oxidative stress damage by MMDD
Exposure of lung tissue to high oxygen concentrations can lead to oxidative stress damage. When the body’s production of reactive oxygen species (ROS) is excessive or antioxidant capacity is insufficient, ROS accumulation induces oxidative stress, resulting in lung tissue damage and remodeling. Hence, inhibiting oxidative stress damage is crucial for preventing and alleviating PF(Fois et al., 2018). Chen (2020) induced two mouse models of PF using trans-resveratrol (TR) and bleomycin, respectively, and treated both groups with MMDD. The results showed significant differences in the treatment effects between the two models. The TR model mice exhibited higher weight and lung coefficient, while the bleomycin model showed better pathological changes. The activity levels of superoxide dismutase (SOD) and glutathione peroxidase (GSH-PX) increased in the TR model but decreased in the bleomycin model. Additionally, the decrease in malondialdehyde (MDA) in lung tissue was significantly higher in the TR model. The levels of IL-13 and IL-4 decreased to varying degrees, with IL-13 decreasing more in the bleomycin model and IL-4 decreasing more in the TR model. TNF-α content increased in the TR model but showed the opposite trend in the bleomycin model, demonstrating that MMDD had a therapeutic effect on both PF models, potentially through oxidative stress regulation. Zhang et al. (2012) explored the intervention mechanism during the formation stage of PF and found that MMDD treatment resulted in higher SOD activity and lower MDA content in serum and bronchoalveolar lavage fluid of rats. This indicated that MMDD had an inhibitory effect on the decrease of SOD activity and increase of MDA content in lung tissue and serum of model rats. Liu et al. (2019) induced fibrosis in human embryonic lung epithelial cells (MRC5 cells) using TGFβ1 and then intervened with MMDD-containing serum and the peroxisome proliferator-activated receptor (PPARγ) antagonist GW9662. They observed that MMDD-containing serum reduced the increase of soluble collagen induced by TGFβ1 stimulation, increased PPARγ activity, and inhibited intracellular ROS damage. Conversely, the PPARγ antagonist GW9662 inhibited PPARγ activity induced by MMDD-containing serum. Animal experiments showed increased PPARγ expression in lung tissue and decreased soluble collagen content, suggesting that the mechanism of MMDD in treating PF may be related to the synthesis of fibrogenic factors and collagen (Liu et al., 2019). It was found that excessive absorption of molybdenum in humans may cause PF, and its mechanism of action is related to inhibition of the SLC 7A 11/GSH/GPX 4 axis leading to iron death, increased CAV-1 expression, and activation of the Wnt/β-linker pathway (Lipner et al., 2022).
4.4 Promotion of the differentiation and homing of bone marrow mesenchymal stem cells (BM-MSCs) by MMDD
Bone marrow has the ability to generate fibroblasts that promote PF, but it also produces mesenchymal stem cel ls (MSCs) that possess anti-fibrotic properties. Derived from bone marrow, BM-MSCs are pluripotent stem cells with self-proliferation and multidirectional differentiation potential (Friedenstein et al., 1968). These cells can play a role in preventing fibrosis through their homing and differentiation abilities (Zhao et al., 2019). Lei (2018) observed lung inflammatory responses and collagen fiber expression in rat lung tissues, finding that MMDD effectively treated PF by reducing lung inflammatory infiltration and collagen fiber deposition. This effect was associated with the mobilization and differentiation of BMSCs, as well as the expression of aquaporin-5 (AQP-5), Surfactant protein C (SP-C), and their mRNA. Liu et al. (2018) used a similar method and discovered that MMDD promotes the expression of CD44 and CD90 positive cells in peripheral blood through BMSC cell mobilization, which might be one of the mechanisms of action. Liu (2019) found that MMDD or MMDD-containing serum medium combined with serum-free small airway epithelial cell culture medium can induce BMSCs to differentiate into type Ⅱ alveolar epithelial cells (AEC2s) by activating the Wnt/β-catenin signaling pathway. This indicates that the mechanism behind MMDD’s treatment of PF is related to the Wnt/β-catenin signaling pathway. He (2019) investigated whether MMDD-treated BMSCs could improve PF tissue repair through the Stromal cell-derived factor-1 (SDF-1)/C-X-C chemokine receptor type 4 (CXCR4) axis. The results showed that the MMDD group significantly increased the mRNA and protein expression of SDF-1, while the addition of AMD3100 to block the combination of SDF-1 and CXCR4 inhibited the improvement effect. Compared to the BMSCs group, the MMDD-BMSCs group exhibited reduced PF area, decreased inflammatory cell infiltration, and increased homing of BMSCs. This suggests that MMDD enhances the directional homing of BMSCs to injured lung tissue, promotes tissue regeneration, and reduces fibrosis by increasing the number of homing BMSCs. These effects may be associated with the SDF-1/CXCR4 axis. Liu et al. (2019) observed the effect of MMDD-containing serum on the differentiation of BMSCs into alveolar epithelial cells in rats. The results indicated that the protein expression of AQP-5 and green fluorescent protein (GFP) -BMSC cell SP-C was significantly higher in the MMDD group compared to the model group, suggesting that MMDD can promote the differentiation of GFP-BMSCs into alveolar epithelial cells, which may be one of the mechanisms behind its treatment of PF. Shen (Shen et al., 2020) found that MMDD could alleviate the pathological process of PF to some extent, improve lung function in rats with PF, reduce ECM deposition, and inhibit the transformation of fibroblasts into myofibroblasts. They speculate that MMDD treated PF mainly by reducing the inflammatory reaction in lung tissue of fibrotic rats, thereby reducing ECM collagen content, and increasing the secretion of active SP-C by AECⅡ to the alveolar surface.
4.5 Enhancement of autophagy activity by MMDD
Autophagy is an adaptive cellular process that helps protect against various forms of stress, such as nutrient deficiency, growth factor deprivation, infection, and hypoxia, thereby preventing cell damage (Hu et al., 2022). Studies have shown that autophagy can delay the progression of PF(Shi et al., 2021). Zhao et al. (Zhao et al., 2021) treated mice with interstitial pneumonia using different concentrations of MMDD and found that compared to the model group, the lung tissue wet/dry weight ratio (W/D) decreased and the alveolar fluid clearance rate increased in each dose group of MMDD. The high and middle dose groups exhibited clearer alveolar structures, reduced collagen protein levels, and improved PF, while the low dose group showed evident PF and collagen deposition. The expression of α-SMA, p-PI3K, p-AKT, p62, and p-mTOR proteins in type Ⅱ alveolar epithelial cells decreased in each dose group, while the expression of light chain 3 (LC3)-Ⅱ protein increased. These findings suggest that MMDD has a therapeutic effect on interstitial pneumonia mice, with the therapeutic effect being closely related to the drug dosage. The mechanism behind this effect involves the PI3K/AKT/mTOR pathway and the enhancement of autophagy activity in lung tissue cells. Based on network pharmacology and experimental verification, Wang et al. (2022) found that MMDD treatment of PF had lower binding energy with β-sitosterol, stigmasterol, kaempferol, protopine, betulinic acid, quercetin compounds and targets AKT1, GAPDH, STAT3, MAPK3, indicating that these compounds have potential inhibition of pulmonary fibrosis activity; in vitro experiments showed that MMDD could promote the expression of AKT1, p-AKT protein and downregulate the expression of VFGF protein.
In summary, MMDD exerts anti-PF effects by reducing levels of inflammatory cytokines, inhibiting the expression of profibrotic factors, inhibiting oxidative stress damage, promoting the differentiation and homing of BMSCs, and enhancing autophagy activity (Figure 2). However, there are only a few studies related to the treatment of PF using MMDD, primarily focusing on in vivo experiments. In the future, more emphasis will be placed on cell experiments to explore additional mechanisms of action of MMDD in the treatment of PF.

Figure 2. Mechanism of Maimendong Dection in the treatment of pulmonary fibrosis (the mechanism of MMDD in the treatment of Pulmonary fibrosis is mainly focused on reducing the level of inflammatory cytokines, inhibiting the expression of pro-fibrotic factors, inhibiting oxidative stress injury, promoting the differentiation and homing of bone marrow mesenchymal stem cells, and enhancing cell autophagy activity).
5 Pharmacological study of MMDD in PF treatment
5.1 Ophiopogonis
Ophiopogonis is the dried root of liliaceae Ophiopogon japonicus. It has a sweet and slightly bitter taste, as well as a slightly cold nature. It is commonly used to treat cough (Chen et al., 2016), tumor (Liu et al., 2023), diabetes and its complications (Mao et al., 2020), cardiovascular and cerebrovascular diseases (Yang et al., 2020), and other ailments. Modern pharmacological studies have discovered various beneficial effects of ophiopogonis, including anti-heart failure and anti-tumor properties, regulation of gastrointestinal smooth muscle and intestinal flora, protection of the nervous system, improvement of liver and lung injuries, anti-inflammatory and antioxidant effects, hypoglycemic and anti-aging properties, as well as immune regulation (Shen et al., 2020). The main chemical components of ophiopogonis include steroidal saponins, polysaccharides, amino acids, and high isoflavone compounds (Wang et al., 2021; Zha et al., 2022; Lei et al., 2024). Additionally, ophiopogonis and its extracts have demonstrated protective effects against lung injury (Wang et al., 2020). Studies have shown that ophiopogonin D, an active ingredient in ophiopogonis, plays a role in treating PF by promoting PINK1/Parkin-dependent mitophagy in lung tissue and improving mitochondrial function (Peng et al., 2023). Furthermore, ophiopogonin C can reduce collagen deposition and the accumulation of inflammatory cells and fibroblasts in mice with PF, decrease the levels of pro-fibrotic cytokine TGF-β1, and increase SOD activity in lung tissue. These effects ultimately alleviate mortality and fibrosis levels in mice (Fu et al., 2022).
5.2 Pinellia
Pinellia is the dried tuber of Pinellia ternata, a plant belonging to the Araceae family. It possesses spicy and warm properties, but it is also toxic. Pinellia is often used in the treatment of peptic ulcers (Zhu et al., 2021), sleep disorders (Lin et al., 2019), hypertension, and other diseases (Jiang et al., 2021). Modern pharmacological studies have revealed that it has anti-inflammatory, antitumor, sedative, hypnotic, antitussive, and expectorant effects; the main chemical components include alkaloids, organic acids, amino acids, flavonoids, volatile oils, sterols, polysaccharides, and other compounds (Ting et al., 2023). Studies have demonstrated that raw pinellia can alleviate inflammatory infiltration, restore alveolar structure, reduce collagen deposition, and decrease hydroxyproline content in lung tissue of mice with PF(He et al., 2022). These effects may be related to peptides and organic acids present in pinellia decoction, which provides direction for further investigation into the pharmacodynamic molecules of pinellia (Xue et al., 2021). Flavonoids baicalin and baicalein, found in pinellia, exhibit therapeutic effects on PF(Ting et al., 2023). Baicalein has been shown to inhibit TGF-β1-induced lung fibroblast differentiation by downregulating miR-2 and connective tissue growth factor (CTGF), thereby reducing the production of type I collagen (ColI) in lung tissue (Cui et al., 2018; Sun et al., 2020). On the other hand, baicalin inhibits the TGF-β1-induced extracellular signal-regulated kinase 1/2 (ERK1/2) signaling pathway, lung fibroblast and fibroblast proliferation, and reduces Ca2+ concentration, thus achieving an anti-PF effect (Huang et al., 2016; Zhao et al., 2020).
5.3 Ginseng
Ginseng refers to the dried root and rhizome of the Araliaceae plant ginseng. It possesses a sweet and slightly bitter taste and has a slightly warm nature. It is commonly used in the treatment of diabetes (Juan et al., 2023), cardiovascular disease (Lanchun et al., 2022), breast cancer (Hamidian et al., 2023). Pharmacological studies have identified several beneficial effects of ginseng, including antioxidant, anti-inflammatory, antibacterial, anti-cardiovascular, and anti-diabetic properties. The main active components of ginseng are ginsenosides, volatile oil, and polysaccharides (Ratan et al., 2021). Ginsenosides, in particular, play a key role in its therapeutic effects against PF. They can downregulate the expression of TGF-β1, Smad2, Smad3, MMP-2, MMP-9, and metalloproteinase-1 inhibitors, while upregulating the expression of Smad7 protein. These effects contribute to the anti-PF effects of ginseng (Yang et al., 2019). Ginsenoside Rg3, a steroidal saponin found in ginseng, can improve lung function by reducing collagen deposition and the expression of mesenchymal markers in lung tissue (Lee et al., 2020). Deng et al. (2023) found that ginsenoside Rg1 has been shown to slow down the progression of PF by inhibiting the expression of cysteine aspartate protease-1, thus inhibiting cell pyroptosis, a novel inflammatory cell death mode. Additionally, ginsenoside AD-1 exhibits anti-PF effects by improving apoptosis and inhibiting the expression of TGF-β1, α-SMA, Sirtuin 3 (Sirt3), and other proteins (Su et al., 2021).
5.4 Glycyrrhiza
Glycyrrhiza refers to the dried root and rhizome of the sweet and flat legume glycyrrhiza plant. It is commonly used in the treatment of lung cancer (Rasha et al., 2022), ulcerative colitis (Lu et al., 2022), depression (Cao et al., 2020) and other diseases. glycyrrhiza contains various chemical constituents, including flavonoids, saponins, alkaloids, amino acids, coumarins, and polysaccharides, these extracted and isolated compounds have demonstrated diverse pharmacological effects, such as anti-tumor, anti-viral, anti-bacterial, anti-inflammatory, and immune regulatory activities (Yang et al., 2017; Dheeraj et al., 2022). Research has shown that glycyrrhiza and some of its extracts possess anti-PF effects (Ghorashi et al., 2016). One of its saponin components, glycyrrhizic acid, has been found to inhibit the increase of TGF-β1, IL-17, and p-Smad2 expression in mice with PF, thus exerting anti-fibrotic effects by suppressing the expression of transforming growth factor and inflammatory factors (Li et al., 2018; James et al., 2022). Another compound, licochalcone A, a phenolic chalcone derived from glycyrrhiza significantly inhibits TGF-β-induced transformation of fibroblast MRC-5 cells and reduces the expression of α-α-SMA and fibronectin (FN) in lung tissue. This indicates that Licochalcone A achieves anti-PF effects by blocking the TGF-β/Smad signaling pathway and inhibiting fibroblast transformation (Fu et al., 2019). Isoliquiritigenin, a flavonoid component of glycyrrhiza extract (Miyamura et al., 2021), has been observed to significantly inhibit the proliferation, migration, and morphological changes of A549 cells induced by TGF-β1. It increases E-cadherin levels while reducing the expression of N-cadherin, Vimentin, α-SMA, FN, EMT-related transcription factors, and phosphorylated Erk1/2. These effects help maintain cell epithelioid morphology and reduce the production of lung fibroblasts (Cai et al., 2022).
5.5 Jujuba
Jujuba, the dry and mature fruit of the Rhamnaceae plant jujube, is widely used in clinical practice for treating various diseases such as cardiovascular diseases (Zahra et al., 2019), liver cancer (Guo et al., 2016), and rectal cancer (Ruan et al., 2023). It possesses a sweet and warm nature, and its chemical components consist of triterpenoids, saponins, flavonoids, alkaloids, glycosides, nucleosides, vitamins, steroids, and other compounds (Liu et al., 2015). Jujuba exhibits pharmacological effects including antibacterial, antioxidant, sedative, hepatoprotective, antihyperglycemic, and antihyperlipidemic activities (Zahra et al., 2019). Flavonoids are one of the key chemical components found in jujube (Ren et al., 2022), and quercetin belongs to flavonoids (Baby et al., 2021). Studies have demonstrated that fibroblast senescence contributes to the pathogenesis of PF. Quercetin has been found to reverse the resistance to death ligand-induced apoptosis by promoting the expression of FasL receptor and caveolin-1, inhibiting AKT activation, restoring the sensitivity of senescent fibroblasts to pro-apoptotic stimulation, and reducing bleomycin-induced PF in elderly individuals (Hohmann et al., 2019). Moreover, quercetin can improve PF by inhibiting the SphK1/S1P signal transduction (Zhang et al., 2018). Another compound present in jujuba, ursolic acid, which is a terpenoid, also exhibits an anti-PF effect. Ursolic acid inhibits CASP3 to alleviate inflammation, thereby slowing down the occurrence of PF(Ma et al., 2019). Chen and Tian (2019) discovered that ursolic acid significantly reduces macrophage infiltration and lymphocyte exudation in the alveolar cavity and alveolar septum, thereby reducing PF. This effect may be attributed to the inhibition of the Toll-like receptor 4 (TLR4)/nuclear factor-kappaB (NF-κb) signaling pathway and the subsequent release of inflammatory factors.
The study found that MMDD contains flavonoids, saponins, alkaloids, terpenoids, phenylpropanoids and other compounds, among which flavonoids mainly come from ophiopogonis, glycyrrhiza, and jujuba; Terpenoids are mainly derived from ginseng, glycyrrhiza, jujuba, and oryza sativa; steroid compounds are mainly derived from ophiopogonis; phenylpropanoid compounds are mainly from pinellia, oryza sativa, glycyrrhiza and jujuba (Yang et al., 2020). The active ingredients in MMDD for treating PF encompass flavonoids, saponins, terpenoids, and alkaloids (Ren et al., 2022). These include ophiopogonin C, ophiopogonin D, baicalin, baicalein, ginsenoside Rg3, ginsenoside Rg1, glycyrrhizic acid, licochalcone A, isoliquiritigenin, ursolic acid, oleanolic acid, and quercetin (Table 2), primarily sourced from ophiopogonis, pinellia, ginseng, glycyrrhiza and jujuba. Currently, there has been no relevant research on using oryza sativa for PF treatment. Therefore, further investigation is required to determine whether the chemical components and extracts of oryza sativa possess therapeutic effects for PF.
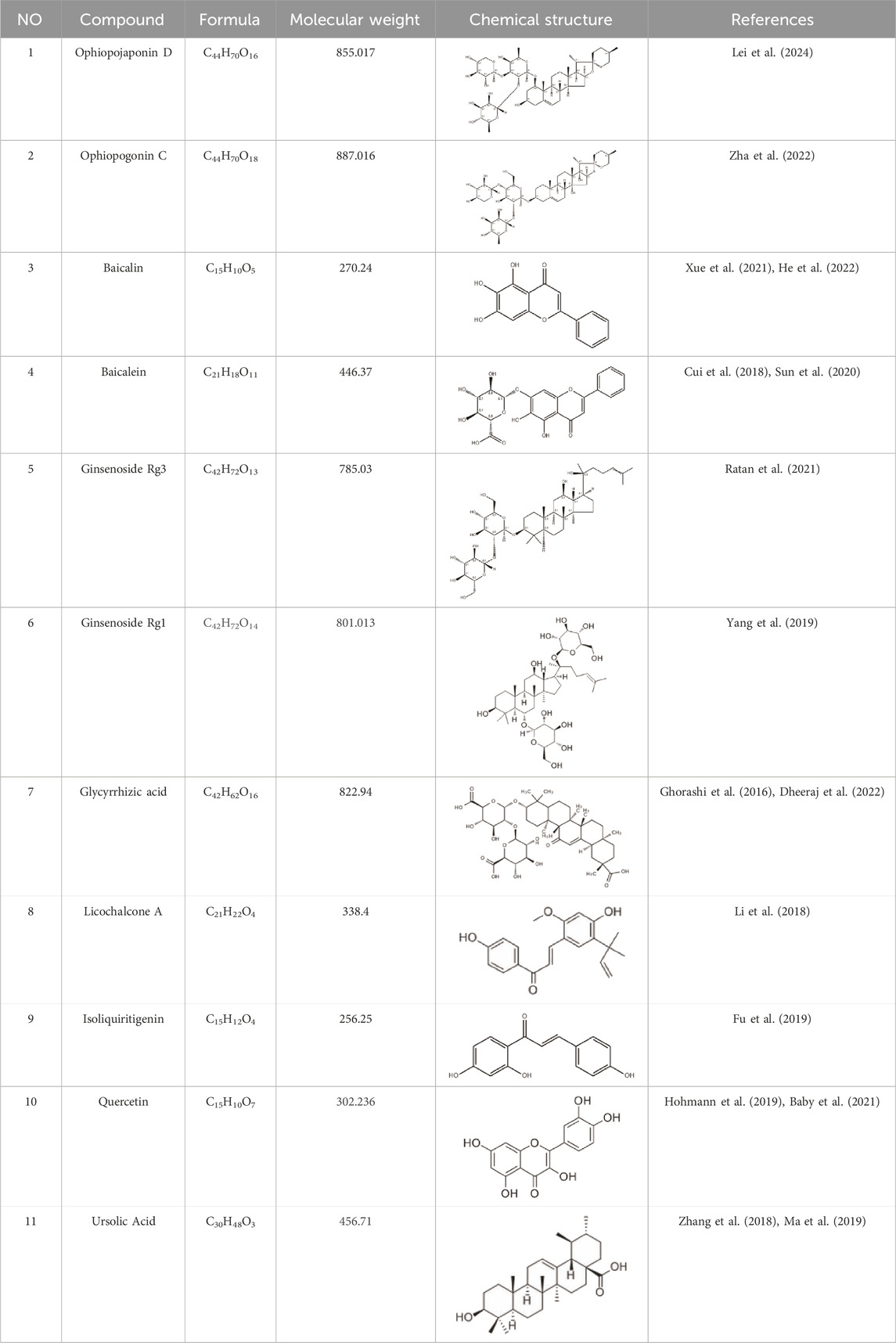
Table 2. Effective chemical constituents of Maimendong Decoction in the treatment of pulmonary fibrosis.
6 Summary and outlook
The treatment of PF in TCM is primarily focused on addressing deficiency, phlegm, and blood stasis. A representative prescription for PF treatment is MMDD, where each medicine in the prescription plays a complementary role. MMDD has shown significant effectiveness in treating PF, with its mechanism confirmed to involve reducing inflammatory cytokine levels, inhibiting pro-fibrotic factor expression, suppressing oxidative stress injury, promoting differentiation and homing of BM-MSCs, and enhancing autophagy activity. While many scholars have confirmed the efficacy of MMDD in alleviating PF, there are still some areas that require improvement. Firstly, although significant therapeutic effects have been observed in vitro and animal experiments, clinical studies are limited. Therefore, future research should focus on increasing clinical investigations. Secondly, the animal models used for PF induction need refinement. Currently, bleomycin and TR-induced models are commonly used, but there are variations in treatment efficacy among different drug-induced models, which may not accurately reflect the symptoms of clinical PF patients. This discrepancy is a common limitation in disease modeling. Thirdly, current research primarily focuses on understanding the mechanisms of PF without thoroughly exploring the corresponding targets of the disease. Further research can delve into studying the potential targets of MMDD’s components for treating PF. Fourth, there has been currently no research on using oryza sativa for treating PF, and it remains unknown whether oryza sativa and its extracts contain important components for PF treatment. Identifying more chemical components of MMDD in treating PF will be crucial for informing clinical approaches. Fifth, In recent years, with the wide application of traditional Chinese medicine, traditional Chinese medicine is shoddy, and the problem of false chaos begins to appear, and the quality of traditional Chinese medicine is uneven, which leads to the failure of the patient to achieve the expected effect after taking the medicine, and may produce certain side effects, so in order to achieve good efficacy, relevant departments should improve the quality evaluation system of traditional Chinese medicine, formulate relevant evaluation standards, and reduce the occurrence of safety problems of traditional Chinese medicine. Lastly, Most clinical studies have shown that MMDD has a significant effect in the treatment of pulmonary fibrosis, but it does not mention its adverse reactions, because some traditional Chinese medicine will lead to liver injury in the treatment of diseases, such as Pinellia has hepatotoxicity, and the material basis and mechanism of hepatotoxicity are not yet clear, so the clinical use of MMDD should not only consider the severity of the patient‘s condition, but also consider the hepatotoxicity of Pinellia. It is necessary to select the appropriate dose of Pinellia according to the severity of the patient‘s condition to avoid liver injury.
Although the single herb in MMDD contains anti-pulmonary fibrosis chemicals, there is no research on the main active ingredients of MMDD in the treatment of PF. Some scholars speculate that β-sitosterol, stigmasterol, kaempferol, fumarine, mairin, quercetin, and ginsenosides may be the components of MMDD to inhibit PF, but there is no relevant mass spectrometry detection, so metabolomic analysis of MMDD can be carried out in the future to find the main active ingredients of MMDD in the treatment of PF. Due to the complex mechanism of PF and the characteristics of multi-component and multi-target of traditional Chinese medicine, biotechnology such as network pharmacology and metabolomics can be used to clarify the active ingredients and corresponding disease targets of MMDD in the treatment of PF in the future, so as to provide a reference for the development and clinical treatment of traditional Chinese medicine.
Author contributions
QL: Writing-original draft. XW: Investigation, Writing-review and editing. GZ: Investigation, Writing-review and editing. HY: Investigation, Writing-review and editing. TM: Funding acquisition, Writing-review and editing. NW: Funding acquisition, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Baby, J., Devan, A. R., Kumar, A. R., Gorantla, J. N., Nair, B., Aishwarya, T. S., et al. (2021). Cogent role of flavonoids as key orchestrators of chemoprevention of hepatocellular carcinoma: a review. J. Food Biochem. 45 (7), e13761. doi:10.1111/jfbc.13761
Bai, W. M., Wang, B., and Liao, C. Y. (2019). Effect of Maimendong decoction on carbon monoxide diffusion capacity, serum HA level and TCM syndrome score in patients with idiopathic pulmonary fibrosis. J. Sichuan Tradit. Chin. 37 (8), 92–95.
Bhavana, K., Puneet, K., and Soumya, S. (2021). Pulmonary fibrosis in COVID-19 recovered patients: problem and potential management. Indian J. Crit. care Med. peer-reviewed 25 (2), 242–244. doi:10.5005/jp-journals-10071-23733
Cai, F. L., Wang, M. F., Cheng, X. Q., Yuan, L. Y., He, J. J., W, H. W., et al. (2022). Effect and mechanism of isoglycyrrhizin on in vitro pulmonary fibrosis model. Her. Med. 41 (02), 167–174. doi:10.3870/j.issn.1004-0781.2022.02.005
Cao, Z. Y., Liu, Y. Z., Li, J. M., Ruan, Y. M., Yan, W. J., Zhong, S. Y., et al. (2020). Glycyrrhizic acid as an adjunctive treatment for depression through anti-inflammation: a randomized placebo-controlled clinical trial. J. Affect Disord. 265, 247–254. doi:10.1016/j.jad.2020.01.048
Chen, M. H., Chen, X. J., Wang, M., Lin, L. G., and Wang, Y. T. (2016). Ophiopogon japonicus--A phytochemical, ethnomedicinal and pharmacological review. J. Ethnopharmacol. 181, 193–213. doi:10.1016/j.jep.2016.01.037
Chen, Y. W. (2020). A comparative study of two mouse models of pulmonary fibrosis based on the idea of "square evidence correspondence. J. Beijing Univ. Tradit. Chin. Med., 115.
Chen, Z. J., and Tian, D. (2019). Intervention effect and mechanism of ursolic acid on bleomycin-induced pulmonary fibrosis in rats. Hainan Med. J. 30 (06), 681–684. doi:10.3969/j.issn.1003-6350.2019.06.001
Cheng, J. M., Wu, X., Zhu, J. Y., He, D. C., and Liu, X. W. (2020). From the traditional Chinese medicine ’ lung arthralgia, lung flaccidity ’ to explore connective tissue disease-related pulmonary interstitial fibrosis. Lishizhen Med. Mat. Med. Res. 31 (3), 668–669. doi:10.3969/j.issn.1008-0805.2020.03.053
Cottin, V., Bonniaud, P., Cadranel, J., Crestani, B., Jouneau, S., Marchand-Adam, S., et al. (2023). French practical guidelines for the diagnosis and management of idiopathic pulmonary fibrosis - 2021 update. Full-length version. Respir. Med. Res. 83, 100948. doi:10.1016/j.resmer.2022.100948
Cui, X., Sun, X., Lu, F., and Jiang, X. (2018). Baicalein represses TGF-β1-induced fibroblast differentiation through the inhibition of miR-21. Toxicol. Appl. Pharmacol. 358, 35–42. doi:10.1016/j.taap.2018.09.007
Deng, H. Z., Chen, K., Li, P., and Zhu, Q. H. (2023). Pulmonary fibrosis inhibition of pulmonary fibrosis in rats by the regulation of the AMPK/NLRP3 pathway mediated by cell pyrozosis by the Ginsenoside Rg1. Chin Tradit Herb. Drugs. 54 (03), 841–848. doi:10.12360/CPB202204034
Dheeraj, B., Mohmmad, R., Kant, A. R. K., Deepak, K., Kumar, C. S., Singh, R. V., et al. (2022). Revisiting liquorice (Glycyrrhiza glabra L.) as anti-inflammatory, antivirals and immunomodulators: potential pharmacological applications with mechanistic insight. Phytomedicine Plus 2 (1), 100206. doi:10.1016/j.phyplu.2021.100206
Du, G. M. (2015). The effects of Maimendong decoction on Lung、Intestine histomorphology and TNF-α etc. In pulmonary fibrosis rats. Henan J. Tradit. Chin. Med. 57.
Fois, A. G., Paliogiannis, P., Sotgia, S., Mangoni, A. A., Zinellu, E., Pirina, P., et al. (2018). Evaluation of oxidative stress biomarkers in idiopathic pulmonary fibrosis and therapeutic applications: a systematic review. Respir. Res. 19 (1), 51. doi:10.1186/s12931-018-0754-7
Friedenstein, A. J., Petrakova, K. V., Kurolesova, A. I., and Frolova, G. P. (1968). Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 6 (2), 230–247. doi:10.1097/00007890-196803000-00009
Fu, X., Li, T., and Yao, Q. (2022). The effect of ophiopogonin C in ameliorating radiation-induced pulmonary fibrosis in C57bl/6 mice: an update study. Front. Oncol. 12, 811183. doi:10.3389/fonc.2022.811183
Fu, Y., Wu, F., and Chen, S. Q. (2019). Licochalcone A inhibits pulmonary fibrosis in mice by regulating the TGF-β/Smad signaling pathway. Chin. J. Exp. Tradit. Med. Formulae 25 (04), 94–100. doi:10.13422/j.cnki.syfjx.20190402
Gan, W., Huang, Q., Xiao, G., Luo, Y., Wang, J., Zhang, C., et al. (2020). Modified Maimendong decoction in the treatment of patients with idiopathic pulmonary fibrosis: study protocol for a randomized controlled trial. Med. Baltim. 99 (49), e23460. doi:10.1097/MD.0000000000023460
Ghatak, S., Hascall, V. C., Markwald, R. R., Feghali-Bostwick, C., Artlett, C. M., Gooz, M., et al. (2017). Transforming growth factor β1 (TGFβ1)-induced CD44V6-NOX4 signaling in pathogenesis of idiopathic pulmonary fibrosis. J. Biol. Chem. 292 (25), 10490–10519. doi:10.1074/jbc.M116.752469
Ghorashi, M., Rezaee, M. A., Rezaie, M. J., Mohammadi, M., Jalili, A., and Rahmani, M. R. (2016). The attenuating effect of aqueous extract of licorice on bleomycin-induced pulmonary fibrosis in mice. Food Agr. Immunol. 28 (1), 67–77. doi:10.1080/09540105.2016.1203294
Guo, J., Li, B., Wu, W., Wang, Z., Wang, F., and Guo, T. (2019). Chinese herbal medicines compared with N-acetylcysteine for the treatment of idiopathic pulmonary fibrosis: a systematic review of randomized controlled trials. Evid. Based Complement. Altern. Med. 2019, 5170638. doi:10.1155/2019/5170638
Guo, Y., Ni, Y., and Kokot, S. (2016). Evaluation of chemical components and properties of the jujube fruit using near infrared spectroscopy and chemometrics. Spectrochimica Acta Part A Mol. Biomol. Spectrosc. 153, 79–86. doi:10.1016/j.saa.2015.08.006
Hamidian, M., Foroughinia, F., Yousefi, M., Haghighat, S., and Haem, E. (2023). Effects of Panax ginseng on health-related quality of life in patients with non-metastatic breast cancer: a randomized, double-blind, placebo-controlled clinical trial ginseng for hrqol in breast cancer. Nutr. Cancer. 75 (6), 1429–1437. doi:10.1080/01635581.2023.2181735
He, J. (2019). “Effect of Mai Mendong decoction on the directional homing of BMSCs in pulmonary fibrosis rats via SDF-1/CXCR4 axis,” (Nanning, China: Guangxi Univ Chin Med), 89. Master’s thesis.
He, S. S., Li, Y., Zhang, B. B., Wei, S. Q., Shen, M. M., Lin, J. Y., et al. (2022). In vitro screening and in vivo evaluation of anti-pulmonary fibrosis effects of different half-summer cannon products. J. Beijing Univ. Tradit. Chin. Med. 45 (03), 275–283. doi:10.3969/j.issn.1006-2157.2022.03.011
He, S. S., Shen, M. M., Lan, Z., Fang, Z., and Yu, L. (2024). Corrigendum to "Maimendong decoction regulates M2 macrophage polarization to suppress pulmonary fibrosis via PI3K/Akt/FOXO3a signalling pathway-mediated fibroblast activation" [J. Ethnopharmacol. 319 117308]. J. Ethnopharmacol. 323, 117719. doi:10.1016/j.jep.2024.117719
Hohmann, M. S., Habiel, D. M., Coelho, A. L., Verri, W. J., and Hogaboam, C. M. (2019). Quercetin enhances ligand-induced apoptosis in senescent idiopathic pulmonary fibrosis fibroblasts and reduces lung fibrosis in vivo. Am. J. Respir. Cell Mol. Biol. 60 (1), 28–40. doi:10.1165/rcmb.2017-0289OC
Hu, Q., Zhang, C. X., and Ke, S. R. (2022). Mechanism of autophagy regulating epithelial-mesenchymal transformation in pulmonary fibrosis. J. Pract. Med. 38 (01), 38–44. doi:10.3969/j.issn.1006-5725.2022.01.008
Huang, G., G, C. J., He, G. Z., Y, Y. G., F, Y. C., and Q, W. K. (2020). Effects of Samling Baishu Mixed Maimendong decoction on HMGB1 and PDGF in rat lung histiocytes with siliproconiosis fibrosis (lung-spleen deficiency type). World Sci. Technol-Mod Tradit. Chin. Med. 22 (4), 1285–1291. doi:10.11842/wst.20190102007
Huang, X., He, Y., Chen, Y., Wu, P., Gui, D., Cai, H., et al. (2016). Baicalin attenuates bleomycin-induced pulmonary fibrosis via adenosine A2a receptor related TGF-β1-induced ERK1/2 signaling pathway. BMC Pulm. Med. 16 (1), 132. doi:10.1186/s12890-016-0294-1
Islam, H., Jackson, G. S., Yoon, J., Cabral-Santos, C., Lira, F. S., Mui, A. L., et al. (2022). Sex differences in IL-10’s anti-inflammatory function: greater STAT3 activation and stronger inhibition of TNF-α production in male blood leukocytes ex vivo. Am. J. Physiol. Cell Physiol. 322 (6), C1095–C1104. doi:10.1152/ajpcell.00091.2022
James, A., Gunasekaran, N., Krishnan, R., Arunachalam, P., and Mahalingam, R. (2022). Anti-fibrotic activity of licorice extract in comparison with colchicine on areca nut-induced fibroblasts: an in vitro study. J. Oral Maxillofac. Pathol. 26 (2), 173–178. doi:10.4103/jomfp.jomfp_110_21
Jiang, Y. H., Zhang, P., Tao, Y., Liu, Y., Cao, G., Zhou, L., et al. (2021). Banxia Baizhu Tianma decoction attenuates obesity-related hypertension. J. Ethnopharmacol. 266, 113453. doi:10.1016/j.jep.2020.113453
Juan, Z., Jinping, B., Jia, L., Hongbo, M., and Haitao, Y. (2023). Ginsenoside RG1-induced mesenchymal stem cells alleviate diabetic cardiomyopathy through secreting exosomal circNOTCH1 to promote macrophage M2 polarization. Phytotherapy Res. PTR 38, 1745–1760. doi:10.1002/ptr.8018
Lanchun, L., Jun, H., Qiyuan, M., Chao, L., Haoqiang, H., Xiaoshan, H., et al. (2022). Functional compounds of ginseng and ginseng-containing medicine for treating cardiovascular diseases. Front. Pharmacol. 13, 1034870. doi:10.3389/fphar.2022.1034870
Lee, A., Yun, E., Chang, W., and Kim, J. (2020). Ginsenoside Rg3 protects against iE-DAP-induced endothelial-to-mesenchymal transition by regulating the miR-139-5p-NF-κB axis. J. Ginseng Res. 44 (2), 300–307. doi:10.1016/j.jgr.2019.01.003
Lei, F., Heinrich, M., Reich, E., and Weckerle, C. (2024). Quality variation of maidong (Ophiopogon japonicus and Liriope spicata) – a HPTLC-based approach. J. Pharm. Biomed. 241, 115990. doi:10.1016/j.jpba.2024.115990
Lei, N. N. (2018). “Effects of Mai Mendong decoction on BMSCs mobilization and differentiation in rats with pulmonary fibrosis model,” (Nanning, China: Guangxi Univ Chin Med.), 99. Master’s thesis.
Li, L. M., Wang, H., Cao, B., and Wang, N. (2018). Mechanism of nebulized inhalation of glycyrrhizic acid to alleviate bleomycin-induced pulmonary fibrosis in mice. China J. Mod. Med. 28 (13), 1–8. doi:10.3969/j.issn.1005-8982.2018.13.001
Li, N., Shao, M. Y., Wang, Q., Li, J. K., Bi, Q., and Yu, H. B. (2022). Chinese medicine of idiopathic pulmonary fibrosis:an overview of systematic reviews. World Sci. Technol-Mod Tradit. Chin. Med. 24 (8), 2898–2913.
Li, R. Q., Song, J. P., Zhang, R., Li, W., Tian, L., and M, Y. J. (2009). The effect of Maimendong Decoction on the expression of TNF-α and IL-10 in lung tissue in the early stage of pulmonary fibrosis model. Tradit. Chin. Med. 22 (9), 10–13. doi:10.3969/j.issn.1001-6910.2009.09.005
Lin, S., Nie, B., Yao, G., Yang, H., Ye, R., and Yuan, Z. (2019). Pinellia ternata (Thunb.) Makino Preparation promotes sleep by increasing REM sleep. Nat. Prod. Res. 33 (22), 3326–3329. doi:10.1080/14786419.2018.1474466
Lipner, E. M., Crooks, J. L., French, J., Strong, M., Nick, J. A., and Prevots, D. R. (2022). Nontuberculous mycobacterial infection and environmental molybdenum in persons with cystic fibrosis: a case-control study in Colorado. J. Expo. Sci. Environ. Epidemiol. 32 (2), 289–294. doi:10.1038/s41370-021-00360-2
Liu, B., Hu, X. G., Zhao, L. M., Liu, Z. D., Zhang, X. J., and Liu, S. (2019). HStudy on the inhibition of cell oxidative damage in idiopathic pulmonary interstitial fibrosis by PPARγ. China J. Tradit. Chin. Med. Pharm. 34 (11), 5136–5140.
Liu, B., Hu, X. G., Zhao, L. M., D, L. Z., Zhang, Z. J., and Liu, S. H. (2019). Effects of Maimendon decoction on PPARγ expression and soluble collagen content in lung tissues of mice with idiopathic pulmonary interstitial fibrosis. J. Tradit. Chin. Med. 60 (12), 1067–1070. doi:10.13288/j.11-2166/r.2019.12.016
Liu, G. Y., Budinger, G., and Dematte, J. E. (2022). Advances in the management of idiopathic pulmonary fibrosis and progressive pulmonary fibrosis. BMJ 377, e066354. doi:10.1136/bmj-2021-066354
Liu, Q., Lu, J. J., Hong, H. J., Yang, Q., Wang, Y., and Chen, X. J. (2023). Ophiopogon japonicus and its active compounds: a review of potential anticancer effects and underlying mechanisms. Phytomedicine 113, 154718. doi:10.1016/j.phymed.2023.154718
Liu, R., He, J., Liang, Z. Y., Liu, X. F., Hou, T. B., and Wu, J. J. (2018). Effects of Maimendong decoction on the expression of lung tissue transformation growth factor β1, matrix metalloproteinase 9 and matrix metalloproteinase tissue inhibitor 1 in rats with pulmonary fibrosis model. Chung Kuo Ch.’uan K'o I Hsueh 21 (29), 3590–3596. doi:10.12114/j.issn.1007-9572.2018.00.103
Liu, R., Lei, N. N., Wu, J. J., Ye, C. D., and N, C. S. (2019). Effects of Maimendon decoction on differentiation of rat bone marrow mesenchymal stem cells to alveolar epithelial cells. Tianjin J. Tradit. Chin. Med. 36 (02), 171–175. doi:10.11656/j.issn.1672-1519.2019.02.20
Liu, R., Lei, N. N., Wu, J. J., D, Y. C., and Q, M. Y. (2018). Effect of Maimendon decoction on BMSCs mobilization in rats with pulmonary fibrosis model. Shanxi J. Tradit. Chin. Med. 34 (03), 52–54. doi:10.3969/j.issn.1000-7156.2018.03.021
Liu, S. J., Tang, Z. S., Cui, C. L., B, L. H., N, L. Y., Zhang, Y., et al. (2015). Research progress on chemical composition of jujube. J. Yunnan Univ. Chin. 38 (03), 96–100. doi:10.19288/j.cnki.issn.1000-2723.2015.03.027
Liu, S. S., Sheng, C. R., Du, Q. H., Ren, Q. J., Chen, Y. W., Zong, C. Z., et al. (2022). Regulation of PI3K/AKT/mTOR pathway in mice with idiopathic pulmonary fibrosis. Prog. Mod. Biomed. 22 (17), 3214–3219+3224. doi:10.13241/j.cnki.pmb.2022.17.003
Liu, X. (2017). Observation on the efficacy of addition and subtraction of Maimendong decoction in the treatment of idiopathic pulmonary fibrosis qi and yin deficiency syndrome. Hunan J. Tradit. Chin. Med., 48.
Liu, X. F. (2019). “Effect of Mai Mendong decoction on the differentiation of bone marrow mesenchymal stem cells to alveolar epithelial cells induced by the classical Wnt signaling pathway,” (Nanning, China: Guangxi Univ Chin Med), 78. Master’s thesis.
Lu, P. D., Yuan, M. C., Quan, X. P., Chen, J. F., and Zhao, Y. H. (2022). Preclinical studies of licorice in ulcerative colitis: a systematic review with meta-analysis and network pharmacology. J. Ethnopharmacol. 296, 115444. doi:10.1016/j.jep.2022.115444
Ma, X., Liu, A., Liu, W., Wang, Z., Chang, N., Li, S., et al. (2019). Analyze and identify peiminine target EGFR improve lung function and alleviate pulmonary fibrosis to prevent exacerbation of chronic obstructive pulmonary disease by phosphoproteomics analysis. Front. Pharmacol. 10, 737. doi:10.3389/fphar.2019.00737
Maher, T. M., Bendstrup, E., Dron, L., Langley, J., Smith, G., Khalid, J. M., et al. (2021). Global incidence and prevalence of idiopathic pulmonary fibrosis. Respir. Res. 22 (1), 197. doi:10.1186/s12931-021-01791-z
Mao, D., Tian, X. Y., Mao, D., Hung, S. W., Wang, C. C., Lau, C., et al. (2020). A polysaccharide extract from the medicinal plant Maidong inhibits the IKK-NF-κB pathway and IL-1β-induced islet inflammation and increases insulin secretion. J. Biol. Chem. 295 (36), 12573–12587. doi:10.1074/jbc.RA120.014357
Mathai, S. K., and Schwartz, D. A. (2019). Translational research in pulmonary fibrosis. Transl. Res. 209, 1–13. doi:10.1016/j.trsl.2019.02.001
Miyamura, Y., Hitomi, S., Omiya, Y., Ujihara, I., Kokabu, S., Morimoto, Y., et al. (2021). Isoliquiritigenin, an active ingredient of Glycyrrhiza, elicits antinociceptive effects via inhibition of Na(v) channels. Naunyn Schmiedeb. Arch. Pharmacol. 394 (5), 967–980. doi:10.1007/s00210-020-02030-w
Pan, L., Lu, Y., Li, Z., Tan, Y., Yang, H., Ruan, P., et al. (2020). Ginkgo biloba extract EGb761 attenuates bleomycin-induced experimental pulmonary fibrosis in mice by regulating the balance of M1/M2 macrophages and nuclear factor kappa B (NF-κB)-Mediated cellular apoptosis. Med. Sci. Monit. 26, e922634. doi:10.12659/MSM.922634
Peng, W. P., Xu, Y., Zhou, Y. H., Wu, J. J., Peng, G. Q., Gu, Y. Y., et al. (2023). Mechanism of ophiopogonin D in treatment of pulmonary fibrosis based on network pharmacology and experimental verification. Chin. Pharmacol. Bull. 39 (08), 1557–1565. doi:10.12360/CPB202204034
Rasha, I., Nafis, R. A. G. G., Nikhat, M., Mohammad, H., and Husain, M. (2022). Integrated network pharmacology and experimental analysis unveil multi-targeted effect of 18α-glycyrrhetinic acid against non-small cell lung cancer. Front. Pharmacol. 13, 1018974. doi:10.3389/fphar.2022.1018974
Ratan, Z. A., Haidere, M. F., Hong, Y. H., Park, S. H., Lee, J. O., Lee, J., et al. (2021). Pharmacological potential of ginseng and its major component ginsenosides. J. Ginseng Res. 45 (2), 199–210. doi:10.1016/j.jgr.2020.02.004
Ren, J. N., Fan, W. J., Li, T., Lei, P., Zhan, X. J., Dong, P. Z., et al. (2022). Research progress on chemical composition and treatment of pulmonary fibrosis of Maimendon decoction. J. Liaoning Univ. Tradit. Chin. Med. 24 (06), 155–159. doi:10.13194/j.issn.1673-842x.2022.06.034
Ren, W., Ma, Y., Liu, D., Liang, P., Du, J., Yang, S., et al. (2022). Chemical composition analysis, antioxidant activity, and target cell-based screening of the potential active components in jujube and its fermented product. J. Food Sci. 87 (2), 664–685. doi:10.1111/1750-3841.16022
Richeldi, L., Fernandez, P. E., Costabel, U., Albera, C., Lederer, D. J., Flaherty, K. R., et al. (2020). Pamrevlumab, an anti-connective tissue growth factor therapy, for idiopathic pulmonary fibrosis (PRAISE): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 8 (1), 25–33. doi:10.1016/S2213-2600(19)30262-0
Ruan, J., Li, H., Lu, M., Hao, M., Sun, F., Yu, H., et al. (2023). Bioactive triterpenes of jujube in the prevention of colorectal cancer and their molecular mechanism research. Phytomedicine 110, 154639. doi:10.1016/j.phymed.2022.154639
She, Y. X., Yu, Q. Y., and Tang, X. X. (2021). Role of interleukins in the pathogenesis of pulmonary fibrosis. Cell Death Discov. 7 (1), 52. doi:10.1038/s41420-021-00437-9
Shen, M., Nan, Y., Zhang, L., Di, L., He, S., Li, Y., et al. (2020). Maimendong decoction improves pulmonary function in rats with idiopathic pulmonary fibrosis by inhibiting endoplasmic reticulum stress in AECIIs. Front. Pharmacol. 11, 1262. doi:10.3389/fphar.2020.01262
Shi, L., Han, Q., Hong, Y., Li, W., Gong, G., Cui, J., et al. (2021). Inhibition of miR-199a-5p rejuvenates aged mesenchymal stem cells derived from patients with idiopathic pulmonary fibrosis and improves their therapeutic efficacy in experimental pulmonary fibrosis. Stem Cell Res. Ther. 12 (1), 147. doi:10.1186/s13287-021-02215-x
Stewart, A. G., Thomas, B., and Koff, J. (2018). TGF-β: master regulator of inflammation and fibrosis. Respirology 23 (12), 1096–1097. doi:10.1111/resp.13415
Su, G. Y., Ke, A. G., Li, T., Chen, Y., Zhou, Y. W., Jia, J. F., et al. (2021). Anti-pulmonary fibrosis effect and mechanism of AD-1 ginsenoside. Chin. J. Pharmacol. Toxicol. 35 (10), 748–749. doi:10.3867/j.issn.1000-3002.2021.10.051
Sun, X., Cui, X., Chen, X., and Jiang, X. (2020). Baicalein alleviated TGF β1-induced type I collagen production in lung fibroblasts via downregulation of connective tissue growth factor. Biomed. Pharmacother. 131, 110744. doi:10.1016/j.biopha.2020.110744
Tang, X., O’Reilly, A., Asano, M., Merrill, J. C., Yokoyama, K. K., and Amar, S. (2011). p53 peptide prevents LITAF-induced TNF-alpha-mediated mouse lung lesions and endotoxic shock. Curr. Mol. Med. 11 (6), 439–452. doi:10.2174/156652411796268731
Ting, Z., Jing, W., Xu, W., Kai, Y., Qiao, Z., Changli, W., et al. (2023). A review of the research progress on Pinellia ternata (Thunb.) Breit.: botany, traditional uses, phytochemistry, pharmacology, toxicity and quality control. Heliyon 9 (11), e22153. doi:10.1016/j.heliyon.2023.e22153
Tzouvelekis, A., Karampitsakos, T., Gomatou, G., Bouros, E., Tzilas, V., Manali, E., et al. (2020). Lung cancer in patients with Idiopathic Pulmonary Fibrosis. A retrospective multicenter study in Greece. Pharmacol. Ther. 60, 101880. doi:10.1016/j.pupt.2019.101880
Viale, P. H. (2020). The American cancer society’s facts & figures: 2020 edition. J. Adv. Pract. Oncol. 11 (2), 135–136. doi:10.6004/jadpro.2020.11.2.1
Wang, L., Qin, Y., Wang, Y., Liu, B., and Zhou, Y. (2021). A pair of homoisoflavonoid analogues (6-aldehydo-isoophiopogonanone A/6-aldehydo-isoophiopogonanone B) from Ophiopogon japonicus as a tyrosinase inhibitor: inhibitory activity, conformational change and mechanism. Eur. Food Res. Technol.(prepublish) 248, 553–565. doi:10.1007/s00217-021-03902-y
Wang, Y. (2017). Study on the mechanism of bleomycin-induced pulmonary fibrosis in rats. Henan J. Tradit. Chin. Med. 61.
Wang, Y., Li, D., Song, L., and Ding, H. (2020). Ophiopogonin D attenuates PM2.5-induced inflammation via suppressing the AMPK/NF-κB pathway in mouse pulmonary epithelial cells. Exp. Ther. Med. 20 (6), 139. doi:10.3892/etm.2020.9268
Wang, Y. M., Xu, M., Luo, M. Z., Su, M., and Li, M. Y. (2022). Preliminary study on mechanism of Maimendong decoction in treatment pulmonary fibrosis based on network pharmacology and experimental verification. World Sci. Technol-Mod Tradit. Chin. Med. 24 (10), 3932–3940. doi:10.11842/wst.20211014009
Wiertz, I. A., Wuyts, W. A., van Moorsel, C., Vorselaars, A., van Es, H. W., van Oosterhout, M., et al. (2018). Unfavourable outcome of glucocorticoid treatment in suspected idiopathic pulmonary fibrosis. Respirology 23 (3), 311–317. doi:10.1111/resp.13230
Wijsenbeek, M. (2020). Progress in the treatment of pulmonary fibrosis. Lancet Respir. Med. 8 (5), 424–425. doi:10.1016/S2213-2600(20)30062-X
Wong, H., and Qu, Z. P. (2008). Clinical observation of 32 cases of idiopathic pulmonary interstitial fibrosis treated with Maimendon tong. Forum Tradi Chin. Med. (1), 6–7. doi:10.3969/j.issn.1002-1078.2008.01.004
Xu, M. Z., Liu, C. G., Gong, L. L., Chen, H. H., Wang, D., and Zhu, Q. J. (2024). Effect of Ophiopogonis Root Decoction on a mouse model of idiopathic pulmonary fibrosis based on PD-1/PD-L1 signaling pathway. Chin. Tradit. Pat. Med. 46 (02), 437–443. doi:10.3969/j.issn.1001-1528.2024.02.014
Xue, F., Yu, H. L., Liu, R., Wu, H., Zhang, Y. B., Liu, D. F., et al. (2021). Antagonistic respiratory tract inflammation in mice and component analysis of effect sites in different parts of half-summer decoction. China J. Chin. Mater Med. 46 (22), 5912–5921. doi:10.19540/j.cnki.cjcmm.20210812.401
Yang, L., Chen, P. P., Luo, M., Shi, W. L., Hou, D. S., Gao, Y., et al. (2019). Inhibitory effects of total ginsenoside on bleomycin-induced pulmonary fibrosis in mice. Biomed. Pharmacother. 114, 108851. doi:10.1016/j.biopha.2019.108851
Yang, L., Zhang, C., Chen, J., Zhang, S., Pan, G., Xin, Y., et al. (2020). Shenmai injection suppresses multidrug resistance in MCF-7/ADR cells through the MAPK/NF-κB signalling pathway. Pharm. Biol. 58 (1), 276–285. doi:10.1080/13880209.2020.1742167
Yang, L., Zhu, Z., Qi, Z., Fan, X., Qian, D., Zhang, J., et al. (2020). Comparative analysis of the chemical consistency between the traditional and mixed decoction of Maimendong decoction by ultra-performance liquid chromatography coupled to quadrupole with time-of-flight mass spectrometry (UPLC-QTOF-MS)-Based chemical profiling approach. J. Chromatogr. Sci. 58 (6), 549–561. doi:10.1093/chromsci/bmz104
Yang, R., Yuan, B. C., Ma, Y. S., Zhou, S., and Liu, Y. (2017). The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm. Biol. 55 (1), 5–18. doi:10.1080/13880209.2016.1225775
Yu, L. (2010). Effects of flavored Maimendon decoction on lung function and quality of life in patients with idiopathic pulmonary fibrosis. Liaoning:Liaoning J. Tradit. Chin. Med. Liao Ning, 35.
Yuan, C. F., Li, H., Xiong, Y. L., Guo, Z. Y., F, W., R, C. R., et al. (2023). Inhibition of miR-200b on silicariosis in mice. Acta Univ. Med. anhui. 58 (08), 1313–1316. doi:10.19405/j.cnki.issn1000-1492.2023.08.011
Yulong, Z., and Chunshui, D. (2017). Curative effect of Maimendong decoction in treating advanced lung cancer with Qi-Yin deficiency cough. Pak. J. Pharm. Sci. 30 (1 Suppl. l), 309–311.
Zahra, S., Sara, N., Sadegh, A. M., Mahin, R., Ahmad, E. S., and Amirhossein, S. (2019). Therapeutic effects of Ziziphus jujuba Mill. fruit in traditional and modern medicine: a review. Med. Chem. Shariqah (United Arab. Emir.) 15.
Zang, N. Z., Pang, L. J., Li, P., Liu, C., Zhao, Z. X., Hua, Z., et al. (2017). Meta-analysis of clinical effect of collaterals disease theory of traditional Chinese medicine in treating idiopathic pulmonary fibrosis. China J. Tradit. Chin. Med. Pharm. 32 (7), 3170–3174.
Zha, X., Li, G., Zhang, L., Chen, Q., and Xia, Q. (2022). Identification of active compounds in Ophiopogonis Radix from different geographical origins by UPLC-Q/TOF-MS combined with GC-MS approaches. Open Life Sci. 17 (1), 865–880. doi:10.1515/biol-2022-0096
Zhang, M. Y., Han, Y. S., Hou, Z. T., and Li, D. D. (2017). Effects of Maimendon decoction on TGF-β1, Smad3 and Smad7 protein expression in rats with pulmonary fibrosis. J. Qiqihar Med. Univ. 38 (24), 2856–2857. doi:10.3969/j.issn.1002-1256.2017.24.002
Zhang, R., Song, J. P., Li, R. Q., W, L., Xie, Z. L., and Zhang, T. F. (2012). Effects of Maimendon decoction on the formation stage of pulmonary fibrosis rats. Chin. Arch. Tradit. Chin. Med. 30 (09), 2022–2024. doi:10.13193/j.archtcm.2012.09.104.zhangr.047
Zhang, X., Cai, Y., Zhang, W., and Chen, X. (2018). Quercetin ameliorates pulmonary fibrosis by inhibiting SphK1/S1P signaling. Biochem. Cell Biol. 96 (6), 742–751. doi:10.1139/bcb-2017-0302
Zhao, F., Liu, W., Yue, S., Yang, L., Hua, Q., Zhou, Y., et al. (2019). Pretreatment with G-CSF could enhance the antifibrotic effect of BM-MSCs on pulmonary fibrosis. Stem Cells Int. 2019, 1726743. doi:10.1155/2019/1726743
Zhao, H., Li, C., Li, L., Liu, J., Gao, Y., Mu, K., et al. (2020). Baicalin alleviates bleomycin-induced pulmonary fibrosis and fibroblast proliferation in rats via the PI3K/AKT signaling pathway. Mol. Med. Rep. 21 (6), 2321–2334. doi:10.3892/mmr.2020.11046
Zhao, X., Kwan, J., Yip, K., Liu, P. P., and Liu, F. F. (2020). Targeting metabolic dysregulation for fibrosis therapy. Nat. Rev. Drug Discov. 19 (1), 57–75. doi:10.1038/s41573-019-0040-5
Zhao, Y. H., Chang, S., Shi, R., and Zhang, H. (2021). Study on the mechanism of Maimendon decoction on autophagy and lung water clearance of type II. alveolar epithelial cells in mice with interstitial pneumonia. J. Zhejiang ChinMedical Univ. 45 (02), 116–123. doi:10.16466/j.issn1005-5509.2021.02.003
Keywords: pulmonary fibrosis, Maimendong decoction, classical prescription of traditional Chinese medicine, mechanism, pharmacological effects
Citation: Lao Q, Wang X, Zhu G, Yuan H, Ma T and Wang N (2024) A Chinese classical prescription Maimendong decoction in treatment of pulmonary fibrosis: an overview. Front. Pharmacol. 15:1329743. doi: 10.3389/fphar.2024.1329743
Received: 02 November 2023; Accepted: 11 April 2024;
Published: 09 May 2024.
Edited by:
Adolfo Andrade-Cetto, National Autonomous University of Mexico, MexicoReviewed by:
Chenghai Liu, Shuguang Hospital, Shanghai University of Traditional Chinese Medicine, ChinaFan Yang, Jiangxi Agricultural University, China
Rolf Teschke, Hospital Hanau, Germany
Copyright © 2024 Lao, Wang, Zhu, Yuan, Ma and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting Ma, bWFzdGVybXQ3N0BzaW5hLmNvbQ==; Ning Wang, enl3bjE5OTJAMTYzLmNvbQ==
 Qiurong Lao
Qiurong Lao Xianbin Wang1
Xianbin Wang1 Haochen Yuan
Haochen Yuan