- The Department of Anesthesiology, Ningbo Medical Center Lihuili Hospital, Ningbo, China
Background: Ketamine was developed as an anesthetic. Esketamine is the isolated S-enantiomer of racemic ketamine. They provide new avenues for the treatment of depression, especially treatment-resistant depression. Considering differences in the pharmacokinetics and hormonal status of ketamine in patients of different genders, sex-based differences in esketamine adverse drug events (ADE) may also be observed. This study presents data mining and safety analysis of adverse events of ketamine and esketamine between genders, promoting the individualization of clinical practice.
Methods: Adverse drug reactions to ketamine and esketamine reported between the first quarter of 2004 and the second quarter of 2023 in the U.S. Food and Drug Administration on Adverse Event Reporting System (FAERS) were extracted. Thereafter, the reporting odds ratio (ROR) with 95% confidence interval (CI) was calculated.
Results: A total of 2907 female reports and 1634 male reports on esketamine were included in the analysis. ROR mining showed that completed suicide, decreased therapeutic product effects, urinary retention, and hypertension were common in men. Additionally, 552 female and 653 male ketamine reports were recorded. ROR mining revealed that toxicity to various agents, bradycardia, cystitis and agitation, were more likely to occur in men, whereas women were more likely to develop suicidal ideation, increased transaminase levels, sclerosing cholangitis, and sterile pyuria.
Conclusion: The adverse events of esketamine and ketamine differ across genders, which should be considered in clinical practice to provide individualized treatment.
1 Introduction
Ketamine was originally developed as an anesthetic and approved by the Food and Drug Administration (FDA) in 1970. Esketamine, a novel antidepressant that is the isolated S-enantiomer of racemic ketamine, was approved for treatment in March 2019. Esketamine’s efficacy and safety in treatment-resistant depression were evaluated in three 4-week, placebo-controlled, parallel-group studies and one longer-term randomized withdrawal study. To mitigate the risk of serious adverse outcomes resulting from sedation, dissociation, and abuse and misuse, while providing access to this effective treatment for treatment-resistant depression, the FDA approved esketamine with a Risk Evaluation and Mitigation Strategy (REMS) (Kim et al., 2019). Esketamine has good efficacy and tolerability even in more difficult-to-treat populations, such as those with comorbid substance use (Chiappini et al., 2023). A real-world study compared the antidepressant effects and tolerability of ketamine and esketamine, with data that seems to favor the nasal spray formulation in terms of Treatment Emergent Side Effects. The results indicate that, ketamine showed a higher short-term antidepressant effect, whereas esketamine exhibited lower side effects. Both were generally well tolerated (d'Andrea et al., 2024). In a phase 3b trial involving patients with treatment-resistant depression, esketamine was superior to quetiapine with respect to remission at week 8. The most common adverse reactions in the esketamine group include dizziness, nausea, drowsiness, etc (Reif et al., 2023). Ketamine is safe as an anesthetic, analgesic, and antidepressant. When ketamine is used as an entertainment drug, it can lead to serious consequences such as cognitive impairment, mental illness, bladder and nasal mucosal damage, and so on (Cheng et al., 2018). Treatment-emergent adverse events with ketamine and esketamine in major depression may be categorized as psychiatric (e.g., dissociation, psychotomimetic), neurologic/cognitive, hemodynamic, genitourinary, and abuse liability (Short et al., 2018). There is currently no research on gender differences between ketamine and esketamine. Considering the differences in the pharmacokinetics and hormonal status of ketamine in patients of different genders (Zarate et al., 2012; Chen et al., 2020), differences in esketamine regarding adverse drug events (ADE) based on sex may also be observed. Therefore, this study aimed to analyze differences in the ADE of esketamine and ketamine based on gender. Relevant data were extracted from the U.S. Food and Drug Administration Adverse Event Reporting System (FAERS). Signal mining and evaluation of the differences in ADE of esketamine and ketamine between genders were conducted based on data-mining algorithm and statistical testing (Park et al., 2020). Drug vigilance and safety monitoring were also performed. This study aimed to provide decision support for patients of different genders in the treatment plan and provide a reference for clinically rational medication.
2 Materials and methods
The data for this study were sourced from the FAERS database, which is publicly available since 2004 (updated quarterly). This study extracted all ASCII data packages from the first quarter of 2004 to the second quarter of 2023 and imported them into SAS9.4 software for data cleaning and analysis. Then, according to CASEID, FDA_ DT and PRIMARYID remove duplicate information. The FAERS database includes spontaneous safety reports and post-market clinical research reports related to medications used both in the US and overseas. The latest version of the Medical Dictionary for Regulatory Activities (MedDRA) was used to correct the preferred term (PTs) names in the FAERS database, as PT from MedDRA was used to record adverse reactions in the FAERS database. The MedDRA dictionary is updated annually in March and September, and each update involves adjustments to the PT hierarchy and changes in the system organ class (SOC). Therefore, SOC and PT were obtained from the latest version of the MedDRA. Next, with “ESKETAMINE,” “SPRAVATO,” “KETAMINE,” and “KETALAR” as keywords, the database was further defined with “PS (primary suspect drug)” to exclude non-primary suspected drugs.
The disproportionality method mainly includes frequency and Bayesian methods (Jokinen et al., 2018). Frequency methods include reporting odds ratio (ROR) and medicines and healthcare products regulatory agency (MHRA). MHRA evaluates adverse event based on three indicators: proportional reporting ratio (PRR), X2, and number of reports. Bayesian methods include the Bayesian confidence propagation neural network (BCPNN) and multi-item gamma Poisson shrinker (MGPS) methods. In this study, these four methods were adopted for signal mining (Li et al., 2023). The detailed contents of the four algorithms are provided in the Supplementary File S1. Signals that meet three or more algorithms are considered potential ADE signals related to ketamine or esketamine. The frequency method is simple and sensitive, but has low stability and high false positives (Chen et al., 2022). When the number of reports is reduced, Bayesian method can effectively avoid false positives, but the calculation is complex and the signal detection time is relatively delayed (He et al., 2021; Li et al., 2023). Multiple algorithms can be used to mine high-quality signals, providing a basis for clinical drug safety and further research.
To identify gender differences in ADEs, the obtained potential signals were further analyzed at the PT levels and classified into different SOCs to better outline the signals. The gender data in the 2 × 2 contingency table along with a modified ROR signal mining method was employed as shown in Table 1 (Li et al., 2023).
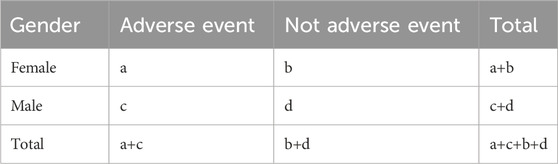
Table 1. A 2 × 2 contingency table for disproportionality analysis of the gender difference in ADEs.
ROR=(a/c)/(b/d), 95% confidence interval (CI) =
3 Results
3.1 General characteristics
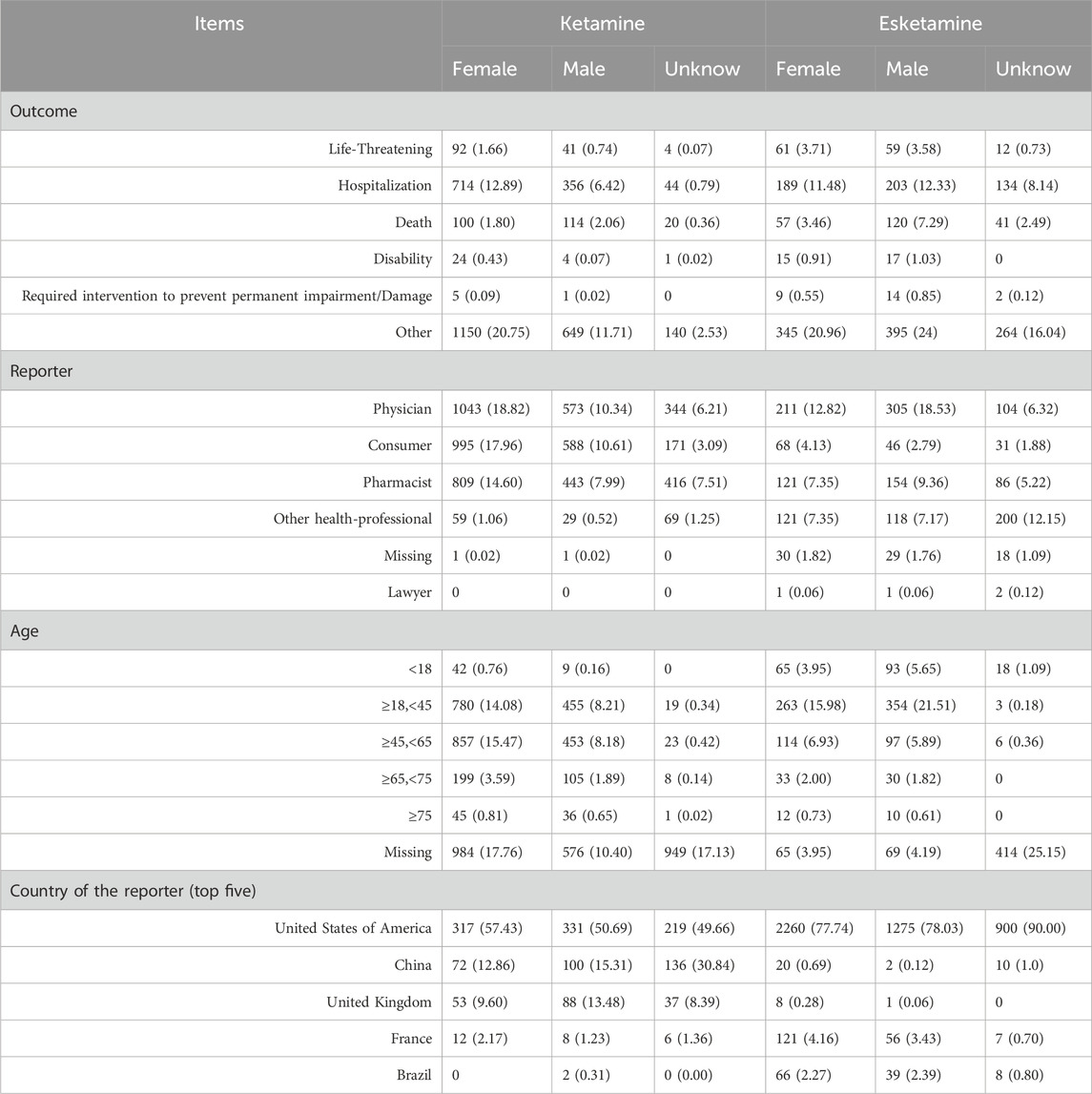
Based on the extracted basic information from ADE reports, a total of 5541 reports for esketamine were obtained, including 2907 reports for women, 1634 reports for men, and 1000 reports for unknown sex. The total number of reports for ketamine ADE was 1646, with 552 reports for women, 653 reports for men, and 441 reports for unknown sex. The clinical outcomes, age distribution, reporting population of patients and country of the reporter are shown in Table 2. More deaths were reported in the men. The country with the most reports is mainly the United States of America.
3.2 Identification of potential ADE signals
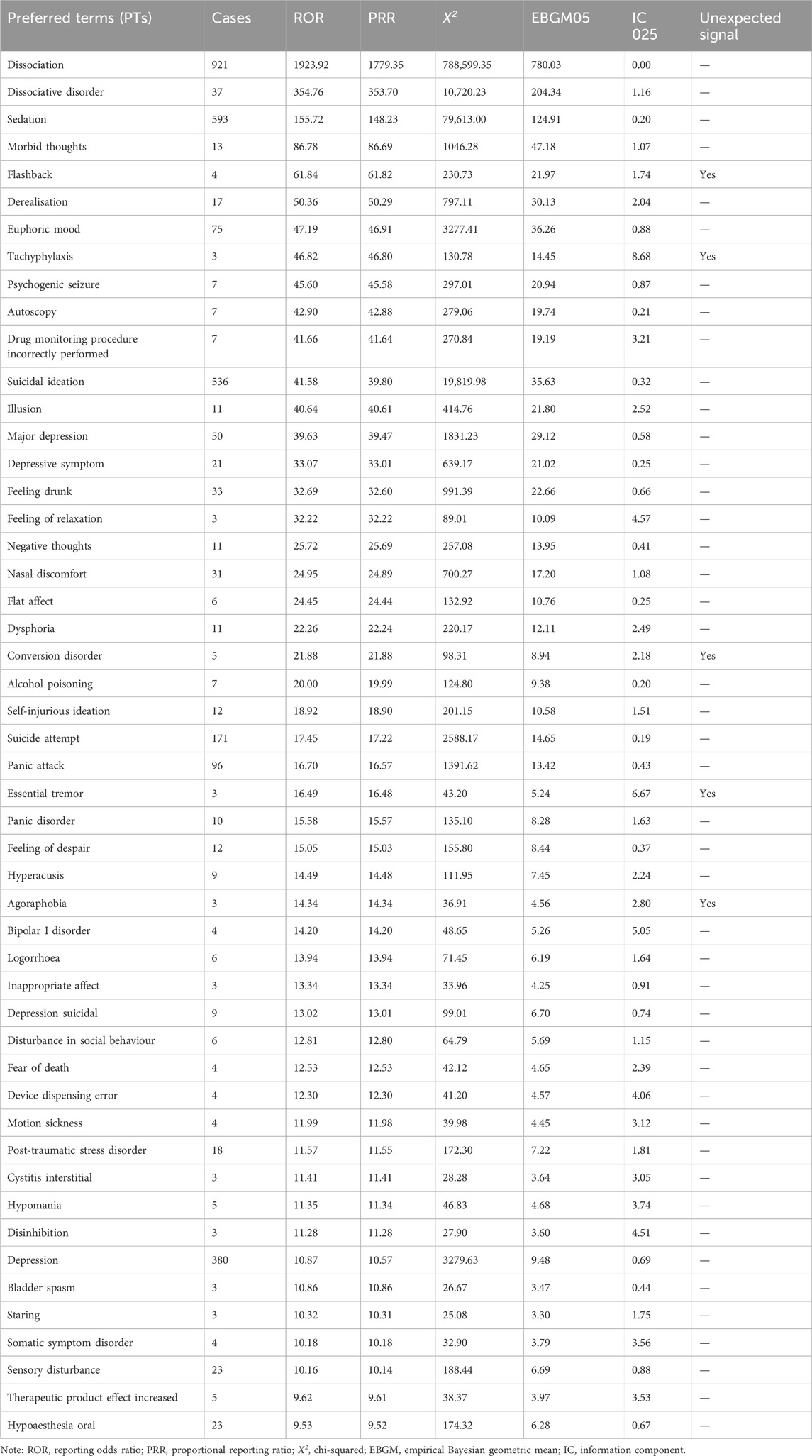
Through signal mining of esketamine, 161 PT signals reached the threshold. Excluding signals unrelated to drugs or disease progression, such as product issues, social circumstances, and surgical and medical procedures, 151 PT signals were ultimately obtained from 13 system organ classes (SOCs), as shown in Supplementary Table S1. The top three SOC level reports were for psychiatric disorders (n = 3916), nervous system disorders (n = 1990), and general disorders and administration site conditions (n = 1699). At the PT level, the most frequent reports were dissociation, sedation, and suicidal ideation, consistent with the warning items in drug instructions. The top 50 ADEs related to esketamine, in terms of ROR values, are shown in Table 3. This study discovered new potential ADE signals with clinical value such as flashbacks, tachyphylaxis, conversion disorders, essential tremors, and agoraphobia. These signals are not mentioned in the drug label.
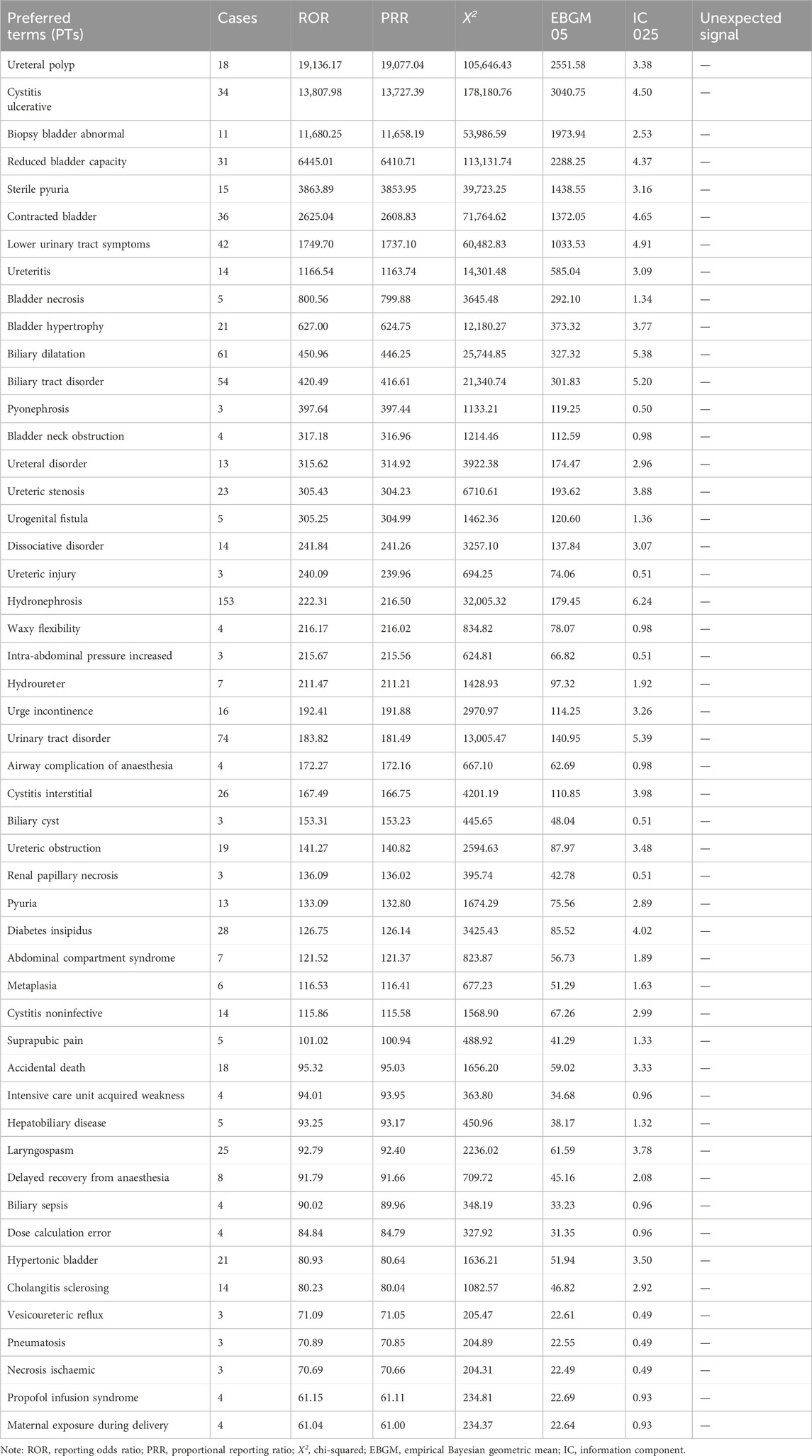
Ketamine yielded 221 PT signals. Excluding signals unrelated to the drug or disease progression, 215 PT signals were mapped to 16 SOCs, as shown in Supplementary Table S2. The top three frequencies of SOC level reports were psychiatric disorders (n = 1018); renal and urinary disorders (n = 1990); and injury, poisoning, and procedural complications (n = 639). At the PT level, the most frequent reports were drug abuse, off-label use, and hydronephrosis, consistent with the symptoms of drug abuse. The top 50 PTs of ADEs related to ketamine in terms of ROR values are shown in Table 4.
3.3 Signal detection results
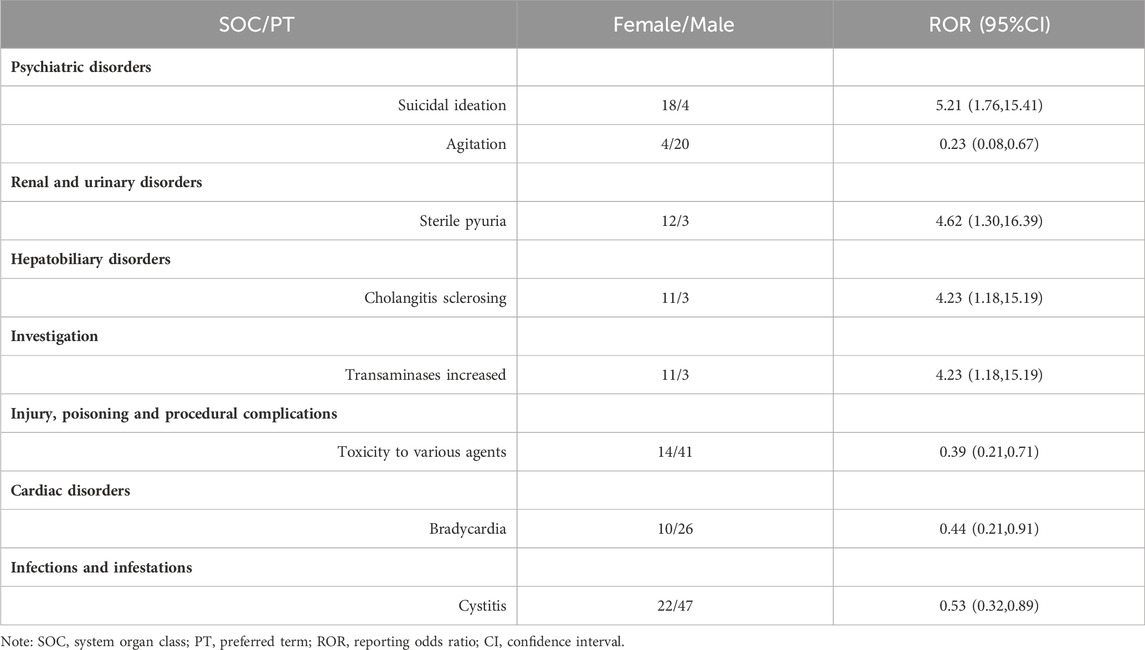
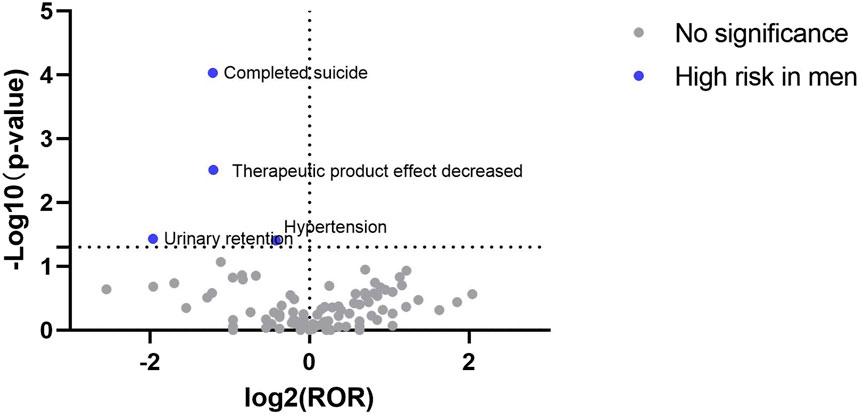
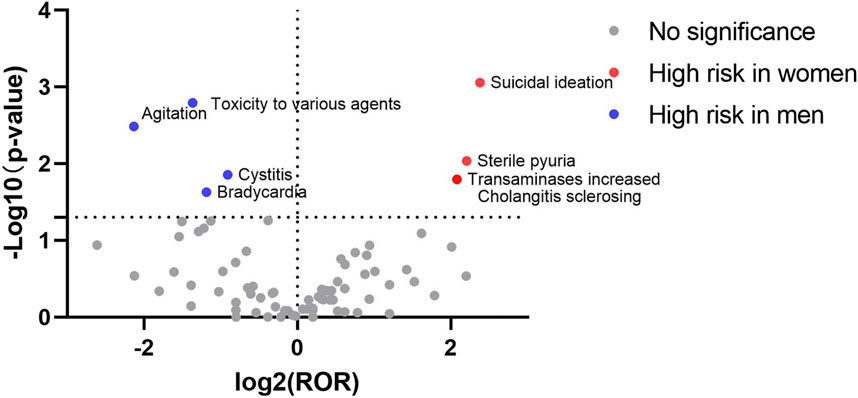
At the PT level, the signal detection results for esketamine showed that the high-risk male signals included completed suicide, decreased therapeutic product effect, urinary retention, and hypertension (Table 5). The signal detection results for ketamine revealed that toxicity to various agents, bradycardia, cystitis, and agitation, were more likely to occur in men, whereas women were more likely to develop suicidal ideation, increased transaminase levels, sclerosing cholangitis, and sterile pyuria (Table 6).
3.4 Visualization of signal results
The signal results were visualized to analyze the gender differences in the ADE signal mining results between esketamine and ketamine. A “volcano plot” was constructed to visualize the signals. The vertical axis of the volcano plot is marked with the log10p-value and the horizontal axis is marked with the log2ROR value. The results are shown in Figures 1, 2, where each point represents an adverse event. The red dots represent potential ADE signals for female patients and the blue dots represent potential ADE signals for male patients. ADE signals with significant log2ROR and log10p values are shown in Figures 1, 2.
4 Discussion
The FAERS database has been publicly available since 2004 with quarterly updates and a significant amount of data. This can effectively support post-market safety risk monitoring and drug analysis. By mining ADE data from the FAERS database, this study revealed that men receiving esketamine had a higher risk of completed suicide, decreased therapeutic product effect, urinary retention, and hypertension. Furthermore, ketamine ADE reports indicated that males were at a higher risk of toxicity to various agents, bradycardia, cystitis, and agitation, whereas females were more likely to develop suicidal ideation, increased transaminase levels, sclerosing cholangitis, and sterile pyuria.
Through comprehensive pharmacovigilance analysis, 151 PT positive signals were associated with esketamine (Supplementary Table S1), and 215 PT positive signals were associated with ketamine (Supplementary Table S2), almost covering the information provided in the instruction manual. This indicates that the data mining strategy used in this study is feasible for identifying potential ADE signals. In addition, this study found some important ADEs that were not mentioned in the esketamine instruction manual, including flashbacks, tachyphylaxis, conversion disorders, essential tremors, and agoraphobia. The PT signals were strong. Randomized controlled trials are the gold standard for determining therapeutic effects, but have some shortcomings in evaluating ADE, especially in identifying gender differences in the occurrence of ADE. Recent research findings have indicated a significant correlation between disproportionality analyses and risk estimation in clinical studies (Maciá-Martínez et al., 2016; Khouri et al., 2021; Li et al., 2023). Therefore, gender differences in ADE should be explored through post-market safety monitoring of drugs, thereby providing decision-making support for personalized medication guidance and improving the level of rational clinical medication.
4.1 Drug abuse and addiction
Ketamine is easily used for entertainment because of its dissociative properties, causing concerns regarding drug abuse and the possibility of addiction. A recent study has found that euphoria, relaxation, and drunkenness are adverse reactions associated with the use of esketamine (Jiang et al., 2023), consistent with the ADE signals identified in this study, and have high ROR values. Similarly, ketamine has become a popular recreational drug owing to its psychotropic effects, including a dream-like state (Van Amsterdam and Van Den Brink, 2022). These psychotropic effects might increase the risk of drug abuse. However, in our study, there were no sex differences in these psychotropic effects. Christian et al. suggested that ketamine reinforces initial self-administration but does not induce synaptic plasticity, which is typically observed with addictive drugs in mice (Simmler et al., 2022). The number of RCTs showing that sub-anesthetic esketamine may not be related to drug abuse (Chiappini et al., 2023; Reif et al., 2023). But some studies have shown that esketamine, (S)-enantiomer of ketamine, has the potential for drug abuse (Nguyen et al., 2023). Esketamine can produce euphoric mood, dissociation, feeling drunk, hallucinations, and other mental states and perceptual changes, which may be abused by some people for entertainment or mental stimulation (Gastaldon et al., 2021). Other reasons for this concern are that some patients have developed drug dependence on ketamine or other substances, as well as recognition of the diversion and misuse of prescription drugs (Kim et al., 2019). The FDA added a REMS to manage possible risks while ensuring its benefits. However, due to the lack of detailed information on drug abuse populations in the FAERS database, which collects ADE information through spontaneous reporting, it is impossible to determine the actual situation of the ADE of concern. In contrast, an increasing number of studies have shown that another (R)-enantiomer, arketamine, has greater efficacy and long-lasting antidepressant-like effects than esketamine (Yang et al., 2015). Importantly, in some animal and human studies, the side effects of arketamine, namely, its psychiatric effects and the risk of abuse, were smaller than those of ketamine and esketamine (Chang et al., 2019; Zhang et al., 2022). Drug signal monitoring, crucial for human health, leverages algorithms to enhance safety detection and uncover risks, yet faces challenges with data quality and false positives. Enhanced by standardization, global collaboration, and regulatory backing, its effectiveness in worldwide drug safety surveillance can be amplified.
4.2 Psychiatric and nervous system
This study found a high rate of suicide. Suicide attempts are high-risk signals in men who use esketamine. However, suicidal ideation was a high-risk signal for women, and agitation was a high-risk signal for men with ketamine, consistent with previous research results (Gastaldon et al., 2021). The side effects of antidepressant-dose ketamine, including confusion/agitation, are tolerable and limited to treatment period (Yavi et al., 2022). In the treatment of bipolar depression, the risk of mood switches is an important safety concern. Some literatures suggest that ketamine and esketamine have potential in the treatment of bipolar depression and psychological symptoms have not worsened (Gałuszko-Wȩgielnik et al., 2023; Martinotti et al., 2023). According to systematic reviews and meta-analyses, ketamine and/or esketamine can quickly reduce suicidal ideation (Wang et al., 2021; Chen et al., 2023). However, there is a lack of evidence to prove whether ketamine or esketamine can more persistently reduce suicidal ideation and the decrease in suicide completion rate after 6 weeks (Phillips et al., 2020). Another study has shown that after 24 h and/or 25 days of administration, there was no significant reduction in suicidal ideation compared to placebo (Canuso et al., 2019). A recent study suggested that esketamine could affect brain plasticity and neural networks, thereby influencing mental health during long-term use (Jiang et al., 2023). Several different explanations for the growing trend on overall suicidality and suicidal attempts in patients treated with ketamine and its enantiomers have been described, such as unknown baseline features, comorbidity and its severity, concomitant use of other substances, withdrawal symptom, dissociation, feeling drunk, delayed psychiatric reactions, and ineffectiveness in treating TRD (Schatzberg, 2019; Beck et al., 2020; Gastaldon et al., 2021). However, regardless of the reason, clinicians should be vigilant of such serious complications and conduct research on the long-term effects and risks. Unfortunately, due to too many confounding factors, our research results cannot be strongly compared with clinical trial results. Among the ADEs in esketamine, psychological and neurological symptoms are the most frequent. These datas are consistent with RCT research and real-world data, for example, effects on the psychological and nervous systems are reported more frequently in the elderly population treated with esketamine (d'Andrea et al., 2023). At present, no gender differences in neurological symptoms have been found.
4.3 Cardiovascular system
Ketamine can activate the sympathetic nervous system and produce brief cardiovascular stimulation, leading to increased blood pressure and heart rate. The heart is directly inhibited when used at large doses. Usually, after cessation of drug infusion, the cardiovascular inhibitory effect is more pronounced, resulting in a decreased cardiac output (Szarmach et al., 2019). This study detected ADEs related to hypertension and bradycardia, which were all reported as high-risk signals in males, consistent with previous research (Singh et al., 2016; Jones et al., 2022; Nikayin et al., 2022). Therefore, particular attention should be paid to the cardiovascular system conditions of patients receiving ketamine and esketamine, such as heart rate and blood pressure, particularly in hypertensive patients with poor blood pressure control and high-dose drug use.
4.4 Hepatobiliary and urinary systems
Ketamine-related abdominal organ damage mainly manifests in the urinary (Chan et al., 2022) and hepatobiliary systems (Cotter et al., 2021). It is primarily metabolized in the liver after entering the human body. Its decomposition products and original compounds are metabolized by the kidneys and eliminated from the body through the urine. In ketamine abuse, drug concentration increases and stimulates the liver, gallbladder, and urinary system for extended periods, leading to abdominal organ damage. AEs associated with the urinary system in males included urinary retention and cystitis, whereas sterile pyuria, increased transaminase levels, and sclerosing cholangitis were identified as high-risk signals in females, consistent with previous studies (Wise et al., 2015). In this study, the high-risk signals for abdominal organ damage were mainly reported with ketamine, which may be related to its susceptibility to abuse and long-term and repeated use (Cotter et al., 2021). Another study suggested that female ketamine users self-reported significantly greater levels of severity of urinary discomfort than did male users (Chen et al., 2014). In the present study, both male and female patients experienced urinary system damage; however, their symptoms differed. This may be related to the hormonal changes and physiological anatomical structures in women. Regular screening, and liver and kidney function follow-up should be conducted in high-risk populations. These results will enable individualized recommendations for ketamine abusers, including different screening and treatment methods for men and women.
4.5 Others
The therapeutic product effect decreased, and toxicity to various agents was found to be a high-risk signal for males. Clinicians should be vigilant of male users.
5 Conclusion
This study conducted signal mining using the FAERS database and performed an exploratory analysis of the gender differences in ketamine and esketamine ADE signals. These results can assist healthcare professionals in developing personalized treatment plans based on sex-based differences. Adequate measures to reduce the occurrence of ADE are crucial to improve medication safety.
Nevertheless, this study still has certain limitations. Firstly, the reports of this study mostly come from the United States, and cannot accurately reflect the ADE in different population. Signal mining models may overfit training data, resulting in insufficient generalization ability in new data or actual situations. Secondly, when analyzing ADE signals, there may be uncontrollable confounding factors such as age, gender, underlying disease, lifestyle, and combination therapy that affect the accuracy of the results. Thirdly, in the spontaneous reporting system, it is inevitable that there will be duplication, underreporting, omission, and inaccuracy in the report, which may lead to biased research results. Fourthly, social determinants such as socioeconomic status, education level, and availability of medical resources may affect the occurrence and reporting of ADE. Therefore, the gender differences in ADE signals generated by data mining require further evaluation, validation, and subsequent research. This study provides ideas for future signal mining based on patient risk factors.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
XY: Writing–original draft, Methodology, Data curation. DC: Writing–review and editing, Methodology, Project administration, Validation.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We are grateful to all those who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1329436/full#supplementary-material
Abbreviations
ADE, adverse drug event; FAERS, Food and Drug Administration on Adverse Event Reporting System; ROR, reporting odds ratio; CI, confidence interval; FDA, Food and Drug Administration; MedDRA, medical dictionary for regulatory activities; PT, preferred term; SOC, system organ class; PRR, proportional reporting ratio; BCPNN, Bayesian confidence propagation neural network; MGPS, multi-item gamma Poisson shrinker; REMS, Risk Evaluation and Mitigation Strategy; MHRA, medicines and healthcare products regulatory agency; RCT, Randomized Controlled Trial.
References
Beck, K., Hindley, G., Borgan, F., Ginestet, C., Mccutcheon, R., Brugger, S., et al. (2020). Association of ketamine with psychiatric symptoms and implications for its therapeutic use and for understanding schizophrenia: a systematic review and meta-analysis. JAMA Netw. Open 3, e204693. doi:10.1001/jamanetworkopen.2020.4693
Canuso, C. M., Singh, J. B., Fedgchin, M., Alphs, L., Lane, R., Lim, P., et al. (2019). Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Focus Am Psychiatr. Publ. 17, 55–65. doi:10.1176/appi.focus.17105
Chan, E. O. T., Chan, V. W. S., Tang, T. S. T., Cheung, V., Wong, M. C. S., Yee, C. H., et al. (2022). Systematic review and meta-analysis of ketamine-associated uropathy. Hong Kong Med. J. 28, 466–474. doi:10.12809/hkmj209194
Chang, L., Zhang, K., Pu, Y., Qu, Y., Wang, S. M., Xiong, Z., et al. (2019). Comparison of antidepressant and side effects in mice after intranasal administration of (R,S)-ketamine, (R)-ketamine, and (S)-ketamine. Pharmacol. Biochem. Behav. 181, 53–59. doi:10.1016/j.pbb.2019.04.008
Chen, B. K., Luna, V. M., Lagamma, C. T., Xu, X., Deng, S.-X., Suckow, R. F., et al. (2020). Sex-specific neurobiological actions of prophylactic (R,S)-ketamine, (2R,6R)-hydroxynorketamine, and (2S,6S)-hydroxynorketamine. Neuropsychopharmacology 45, 1545–1556. doi:10.1038/s41386-020-0714-z
Chen, C. C., Zhou, N., Hu, N., Feng, J. G., and Wang, X. B. (2023). Acute effects of intravenous sub-anesthetic doses of ketamine and intranasal inhaled esketamine on suicidal ideation: a systematic review and meta-analysis. Neuropsychiatr. Dis. Treat. 19, 587–599. doi:10.2147/ndt.S401032
Chen, J. J., Huo, X. C., Wang, S. X., Wang, F., and Zhao, Q. (2022). Data mining for adverse drug reaction signals of daptomycin based on real-world data: a disproportionality analysis of the US Food and Drug Administration adverse event reporting system. Int. J. Clin. Pharm. 44, 1351–1360. doi:10.1007/s11096-022-01472-x
Chen, W. Y., Huang, M. C., and Lin, S. K. (2014). Gender differences in subjective discontinuation symptoms associated with ketamine use. Subst. Abuse Treat. Prev. Policy 9, 39. doi:10.1186/1747-597x-9-39
Cheng, W. J., Chen, C. H., Chen, C. K., Huang, M. C., Pietrzak, R. H., Krystal, J. H., et al. (2018). Similar psychotic and cognitive profile between ketamine dependence with persistent psychosis and schizophrenia. Schizophr. Res. 199, 313–318. doi:10.1016/j.schres.2018.02.049
Chiappini, S., D'Andrea, G., de Filippis, S., di Nicola, M., Andriola, I., Bassetti, R., et al. (2023). Esketamine in treatment-resistant depression patients comorbid with substance-use disorder: a viewpoint on its safety and effectiveness in a subsample of patients from the REAL-ESK study. Eur. Neuropsychopharmacol. 74, 15–21. doi:10.1016/j.euroneuro.2023.04.011
Cotter, S., Wong, J., Gada, N., Gill, R., Jones, S. C., Chai, G., et al. (2021). Repeated or continuous medically supervised ketamine administration associated with hepatobiliary adverse events: a retrospective case series. Drug Saf. 44, 1365–1374. doi:10.1007/s40264-021-01120-9
D'Andrea, G., Chiappini, S., Mcintyre, R. S., Stefanelli, G., Carullo, R., Andriola, I., et al. (2023). Investigating the effectiveness and tolerability of intranasal esketamine among older adults with treatment-resistant depression (TRD): a post-hoc analysis from the REAL-ESK study group. Am. J. Geriatr. Psychiatry 31, 1032–1041. doi:10.1016/j.jagp.2023.06.016
D'Andrea, G., Pettorruso, M., di Lorenzo, G., Rhee, T. G., Chiappini, S., Carullo, R., et al. (2024). The rapid antidepressant effectiveness of repeated dose of intravenous ketamine and intranasal esketamine: a post-hoc analysis of pooled real-world data. J. Affect Disord. 348, 314–322. doi:10.1016/j.jad.2023.12.038
Gałuszko-Wȩgielnik, M., Jakuszkowiak-Wojten, K., Wiglusz, M. S., Cubała, W. J., and Pastuszak, M. (2023). Central nervous system-related safety and tolerability of add-on ketamine to standard of care treatment in treatment-resistant psychotic depression in patients with major depressive disorder and bipolar disorder. Front. Neurosci. 17, 1214972. doi:10.3389/fnins.2023.1214972
Gastaldon, C., Raschi, E., Kane, J. M., Barbui, C., and Schoretsanitis, G. (2021). Post-marketing safety concerns with esketamine: a disproportionality analysis of spontaneous reports submitted to the FDA adverse event reporting system. Psychother. Psychosom. 90, 41–48. doi:10.1159/000510703
He, B. J., Zhang, M. X., and Zhan, S. Y. (2021). Prescription sequence symmetry analysis in pharmacoepidemiology: a systematic review. Zhonghua Liu Xing Bing Xue Za Zhi 42, 1641–1649. doi:10.3760/cma.j.cn112338-20201208-01386
Jiang, Y., du, Z., Shen, Y., Zhou, Q., and Zhu, H. (2023). The correlation of Esketamine with specific adverse events: a deep dive into the FAERS database. Eur. Arch. Psychiatry Clin. Neurosci. doi:10.1007/s00406-023-01732-5
Jokinen, J. D., Lievano, F., Scarazzini, L., and Truffa, M. (2018). An alternative to disproportionality: a frequency-based method for pharmacovigilance data mining. Ther. Innov. Regul. Sci. 52, 294–299. doi:10.1177/2168479017728986
Jones, R. R., Freeman, M. P., Kornstein, S. G., Cooper, K., Daly, E. J., Canuso, C. M., et al. (2022). Efficacy and safety of esketamine nasal spray by sex in patients with treatment-resistant depression: findings from short-term randomized, controlled trials. Arch. Womens Ment. Health 25, 313–326. doi:10.1007/s00737-021-01185-6
Khouri, C., Petit, C., Tod, M., Lepelley, M., Revol, B., Roustit, M., et al. (2021). Adverse drug reaction risks obtained from meta-analyses and pharmacovigilance disproportionality analyses are correlated in most cases. J. Clin. Epidemiol. 134, 14–21. doi:10.1016/j.jclinepi.2021.01.015
Kim, J., Farchione, T., Potter, A., Chen, Q., and Temple, R. (2019). Esketamine for treatment-resistant depression - first FDA-approved antidepressant in a new class. N. Engl. J. Med. 381, 1–4. doi:10.1056/NEJMp1903305
Li, Z., Zou, W., Yuan, J., Zhong, Y., and Fu, Z. (2023). Gender differences in adverse events related to Osimertinib: a real-world pharmacovigilance analysis of FDA adverse event reporting system. Expert Opin. Drug Saf. 23, 763–770. doi:10.1080/14740338.2023.2243220
Maciá-MartíNEZ, M. A., de Abajo, F. J., Roberts, G., Slattery, J., Thakrar, B., and Wisniewski, A. F. (2016). An empirical approach to explore the relationship between measures of disproportionate reporting and relative risks from analytical studies. Drug Saf. 39, 29–43. doi:10.1007/s40264-015-0351-3
Martinotti, G., Dell'Osso, B., di Lorenzo, G., Maina, G., Bertolino, A., Clerici, M., et al. (2023). Treating bipolar depression with esketamine: safety and effectiveness data from a naturalistic multicentric study on esketamine in bipolar versus unipolar treatment-resistant depression. Bipolar Disord. 25, 233–244. doi:10.1111/bdi.13296
Nguyen, T. M. L., Defaix, C., Mendez-David, I., Tritschler, L., Etting, I., Alvarez, J. C., et al. (2023). Intranasal (R, S)-ketamine delivery induces sustained antidepressant effects associated with changes in cortical balance of excitatory/inhibitory synaptic activity. Neuropharmacology 225, 109357. doi:10.1016/j.neuropharm.2022.109357
Nikayin, S., Murphy, E., Krystal, J. H., and Wilkinson, S. T. (2022). Long-term safety of ketamine and esketamine in treatment of depression. Expert Opin. Drug Saf. 21, 777–787. doi:10.1080/14740338.2022.2066651
Park, G., Jung, H., Heo, S. J., and Jung, I. (2020). Comparison of data mining methods for the signal detection of adverse drug events with a hierarchical structure in postmarketing surveillance. Life (Basel) 10, 138. doi:10.3390/life10080138
Phillips, J. L., Norris, S., Talbot, J., Hatchard, T., Ortiz, A., Birmingham, M., et al. (2020). Single and repeated ketamine infusions for reduction of suicidal ideation in treatment-resistant depression. Neuropsychopharmacology 45, 606–612. doi:10.1038/s41386-019-0570-x
Reif, A., Bitter, I., Buyze, J., Cebulla, K., Frey, R., Fu, D. J., et al. (2023). Esketamine nasal spray versus quetiapine for treatment-resistant depression. N. Engl. J. Med. 389, 1298–1309. doi:10.1056/NEJMoa2304145
Schatzberg, A. F. (2019). A word to the wise about intranasal esketamine. Am. J. Psychiatry 176, 422–424. doi:10.1176/appi.ajp.2019.19040423
Short, B., Fong, J., Galvez, V., Shelker, W., and Loo, C. K. (2018). Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry 5, 65–78. doi:10.1016/s2215-0366(17)30272-9
Simmler, L. D., Li, Y., Hadjas, L. C., Hiver, A., van Zessen, R., and LüSCHER, C. (2022). Dual action of ketamine confines addiction liability. Nature 608, 368–373. doi:10.1038/s41586-022-04993-7
Singh, J. B., Fedgchin, M., Daly, E., XI, L., Melman, C., de Bruecker, G., et al. (2016). Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol. Psychiatry 80, 424–431. doi:10.1016/j.biopsych.2015.10.018
Szarmach, J., Cubała, W. J., Włodarczyk, A., and Wiglusz, M. S. (2019). Short-term ketamine administration in treatment-resistant depression: focus on cardiovascular safety. Psychiatr. Danub 31, 585–590.
van Amsterdam, J., and van Den Brink, W. (2022). Harm related to recreational ketamine use and its relevance for the clinical use of ketamine. A systematic review and comparison study. Expert Opin. Drug Saf. 21, 83–94. doi:10.1080/14740338.2021.1949454
Wang, S. M., Kim, N. Y., Na, H. R., Lim, H. K., Woo, Y. S., Pae, C. U., et al. (2021). Rapid onset of intranasal esketamine in patients with treatment resistant depression and major depression with suicide ideation: a meta-analysis. Clin. Psychopharmacol. Neurosci. 19, 341–354. doi:10.9758/cpn.2021.19.2.341
Wise, G. J., Longo, D. L., and Schlegel, P. N. (2015). Sterile pyuria. N. Engl. J. Med. 372, 1048–1054. doi:10.1056/NEJMra1410052
Yang, C., Shirayama, Y., Zhang, J. C., Ren, Q., Yao, W., Ma, M., et al. (2015). R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl. Psychiatry 5, e632. doi:10.1038/tp.2015.136
Yavi, M., Lee, H., Henter, I. D., Park, L. T., and Zarate, C. A. (2022). Ketamine treatment for depression: a review. Discov. Ment. Health 2, 9. doi:10.1007/s44192-022-00012-3
Zarate, C. A., Brutsche, N., Laje, G., Luckenbaugh, D. A., Venkata, S. L., Ramamoorthy, A., et al. (2012). Relationship of ketamine's plasma metabolites with response, diagnosis, and side effects in major depression. Biol. Psychiatry 72, 331–338. doi:10.1016/j.biopsych.2012.03.004
Keywords: esketamine, ketamine, gender difference, signal mining, adverse drug events
Citation: Yang X and Chen D (2024) Comparing the adverse effects of ketamine and esketamine between genders using FAERS data. Front. Pharmacol. 15:1329436. doi: 10.3389/fphar.2024.1329436
Received: 05 January 2024; Accepted: 11 June 2024;
Published: 12 July 2024.
Edited by:
Christos Kontogiorgis, Democritus University of Thrace, GreeceReviewed by:
Kenji Hashimoto, Chiba University, JapanBreno Souza-Marques, Federal University of Bahia (UFBA), Brazil
Giacomo d'Andrea, University of Studies G d’Annunzio Chieti and Pescara, Italy
Copyright © 2024 Yang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongdong Chen, chen_dd163@163.com
 Xinxia Yang
Xinxia Yang Dongdong Chen*
Dongdong Chen*