- 1Department of Geriatric Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Hubei Key Laboratory of Genetics and Molecular Mechanisms of Cardiological Disorders, Wuhan, China
- 3Department of Rehabilitation Medicine, Zhongda Hospital, Southeast University, Nanjing, China
- 4Division of Cardiology and Department of Internal Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Ginsenoside Rg3, a compound derived from Panax ginseng C. A. Mey., is increasingly recognized for its wide range of pharmacological effects. Under the worldwide healthcare challenges posed by heart diseases, Rg3 stands out as a key subject in modern research on Chinese herbal medicine, offering a novel approach to therapy. Mental illnesses are significant contributors to global disease mortality, and there is a well-established correlation between cardiac and psychiatric conditions. This connection is primarily due to dysfunctions in the sympathetic-adrenomedullary system (SAM), the hypothalamic-pituitary-adrenal axis, inflammation, oxidative stress, and brain-derived neurotrophic factor impairment. This review provides an in-depth analysis of Rg3’s therapeutic benefits and its pharmacological actions in treating cardiac and mental health disorders respectively. Highlighting its potential for the management of these conditions, Rg3 emerges as a promising, multifunctional therapeutic agent.
1 Introduction
Despite extensive research over the years, both heart diseases and mental disorders continue to exert significant pressure on global healthcare systems. Heart diseases maintain their status as the leading cause of death globally (Liu and Miao, 2022; Lupisella et al., 2022). The pharmacological landscape for heart diseases is diverse, encompassing a range of drugs. However, these treatments largely fail to restore cardiac function fundamentally and are frequently associated with side effects.
At the same time, mental illness significantly contributes to the global disease burden. A recent meta-analysis reveals that 14.3% of global deaths annually, equivalent to roughly eight million fatalities, are linked to mental disorders (Walker et al., 2015). Individuals with severe mental illnesses, such as schizophrenia, bipolar disorder, and major depressive disorder (MDD), face a mortality rate of two to three times higher than the average population. The elevated rate corresponds to a reduced life expectancy of 10–25 years (Correll et al., 2015). However, issues associated with treatment discontinuation and ineffectiveness are prevalent.
Up to now, numerous studies have established a strong link between cardiac diseases and psychiatric conditions. It is frequently observed that individuals with cardiac ailments often experience psychiatric disturbances (Piepenburg et al., 2019). Inversely, those with mental disorders appear to have a higher risk of developing heart diseases (Hagi et al., 2021). Several biological mechanisms are suggested to clarify the association between mental disorders and cardiac events. Mental disorders are linked to dysfunctions in the sympathetic-adrenomedullary system (SAM), and the hypothalamic-pituitary-adrenal (HPA) axis, as well as to inflammation, oxidative stress, and impairments in the brain-derived neurotrophic factor (BDNF) system. All these physiological processes play significant roles in the onset and progression of cardiac diseases.
Recently, the field of Chinese herbal medicine has captured the interest of the scientific community, owing to its extensive range of pharmacological properties and a lower incidence of adverse side effects. Particularly, the effects of ginsenoside Rg3 (Rg3) in cardiac and mental diseases have gained more and more attention.
Consequently, in this review, we provide a detailed investigation of the therapeutic effects and pharmacological action of Rg3 in addressing heart and mental disorders (Table 1). Based on the association between heart and mental disorders, we aim to provide prospects on the potential effects and mechanisms of Rg3 in the comorbid conditions.
2 Origin and structure of Rg3
Rg3 is a constituent of ginsenosides extracted from Panax ginseng C. A. Mey. (Nakhjavani et al., 2020). Key ginsenoside constituents, specifically Rb1, Rb2, and Rd, possess the capacity for enzymatic transformation into Rg3 (Lee H. et al., 2020). Rg3 is categorized into two distinct stereoisomers based on its unique spatial configurations at the C20 position: 20(R)-Rg3, as illustrated in Figure 1, and 20(S)-Rg3, depicted in Figure 2. Research findings have underscored the pivotal role of Rg3 across diverse domains, encompassing its involvement in anti-aging mechanisms, anticancer properties, bone development, cellular differentiation, neuroprotection, and cardiac function (Lee et al., 2019; Zarneshan et al., 2022).
3 The relationships between heart diseases and mental disorders
3.1 Mental disorders induce the occurrence of heart diseases
Recent meta-analytic studies have shown that mental illness is a high risk for cardiac events (Zarneshan et al., 2022). In addition, a Mendelian randomization analysis revealed a significant genetic association between MDD and coronary artery disease (CAD). Specifically, the analysis indicated that for each one-unit increase in the natural logarithm of odds of MDD, the odds ratio for CAD was 1.16 (95% confidence interval: 1.05 to 1.29; p = 0.0047) (Tang et al., 2020). Recent observational research has indicated a correlation between genetic predispositions for schizophrenia and notable changes in cardiac structure. These alterations have been found to potentially aggravate cardiac health outcomes (Pillinger et al., 2023). Mental health disorders, such as MDD, anxiety, and stress-related conditions, may contribute to behaviors including smoking, inactivity, and drinking adversely affecting cardiac function.
3.2 Heart diseases promote the development of mental disorders
Clinically, a subset of patients with heart diseases commonly experience co-occurring mental disorders, specifically anxiety and depression. Multiple meta-analyses have demonstrated that individuals diagnosed with heart failure (HF) are at an elevated risk of experiencing depression (Hagi et al., 2021). Certain drugs used in managing heart diseases, including beta-blockers, have the potential to initiate or aggravate symptoms associated with anxiety and depression (Bornand et al., 2022; Carnovale et al., 2023). Additionally, managing heart conditions, including HF, entails a prolonged treatment course that can result in significant financial strain for patients and create a disparity between hospitalization needs and employment stability. This significantly heightens the risk of developing mental disorders in individuals with heart diseases.
3.3 The major mechanisms linking heart diseases and mental disorders
3.3.1 Inflammation
Inflammatory processes are associated with the initiation and progression of mental disorders (Ben-Azu et al., 2022a; Ben-Azu et al., 2023). Mental disorders may enhance the expression of pro-inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-ɑ), which are associated with endothelial dysfunction and metabolic alterations (Vaccarino et al., 2018; Misiak et al., 2022; Tsioufis et al., 2022; Chen et al., 2023). Inflammation, vascular dysfunction, and metabolic abnormalities frequently contribute to the pathogenesis of heart diseases. Furthermore, inflammatory processes within heart tissue can result in elevated blood levels of pro-inflammatory cytokines and other acute-phase reactants (Hartupee and Mann, 2017). Inflammatory factors may lead to the upregulation of indoleamine 2,3-dioxygenase (IDO), diverting tryptophan (TRP) into the kynurenine (KYN) pathway, potentially reducing serotonin (5-HT) synthesis and contributing to the onset of mental disorders, including depression. 5-HT functions as a neurotransmitter in the central nervous system, a blood factor, and a neurohormone that regulates the function of various peripheral organs (De Deurwaerdere and Di Giovanni, 2020). A deficiency in 5-HT increases susceptibility to social defeat stress and impairs responses to antidepressants (Sachs et al., 2015). Additionally, pro-inflammatory cytokines are associated with a marked decrease in both BDNF gene and protein expression (Zhang et al., 2014). BDNF is crucial for the plasticity of glutamatergic and gamma aminobutyric acid (GABA)ergic synapses and is intimately linked to severe mental illnesses (Colucci-D'Amato et al., 2020). Interestingly, animal studies have demonstrated that in post-myocardial infarction, BDNF expression is upregulated through neuronal signaling originating from the heart. This upregulation serves to shield the myocardium from ischemic damage, thereby exerting a protective effect against cardiac remodeling (Cannavo et al., 2023). Furthermore, studies have noted lower BDNF levels in patients with HF when compared to healthy controls (Xie et al., 2023; Wang et al., 2024). BDNF plays a crucial role in supporting the survival of endothelial cells during the development of the cardiovascular system (Kermani and Hempstead, 2019).
3.3.2 HPA axis
The HPA axis serves as a critical component of the neuroendocrine system, orchestrating responses to both internal and external stressors. Various mental disorders have been shown to trigger the activation of the HPA axis (Sur and Lee, 2022b; Emudainohwo et al., 2023; Menke, 2024). This activation leads to an upsurge in cortisol synthesis within the adrenal cortex, along with enhanced production of adrenaline and noradrenaline in the adrenal medulla (Ugwu et al., 2022). Hypercortisolemia, commonly observed in mental disorders, can lead to escalated steroid production, elevated blood pressure, and an increase in visceral fat (García-Eguren et al., 2019; Favero et al., 2021; Teng et al., 2021). These changes significantly heighten the risk of heart diseases. Elevated cortisol levels following stress are directly linked to the hypertrophy of cardiomyocytes and cardiac remodeling (Opinion et al., 2023). Additionally, plasma cortisol concentrations have been identified as an independent risk factor for cardiac events and mortality (Crawford et al., 2019; Kim et al., 2022). Furthermore, the stimulation of the HPA axis results in increased aldosterone levels. Studies have shown that stress-related aldosterone activity has been linked to hypertension, myocardial necrosis, and fibrosis. Additionally, a rise in aldosterone has been associated with increased insulin resistance, oxidative stress, and pro-inflammatory responses (Bothou et al., 2020; Tsai et al., 2021; Campana et al., 2022). In chronic HF, elevated serum cortisol levels have been identified as an independent predictor of increased mortality risk (Güder et al., 2007). Moreover, increased cortisol levels may exacerbate the brain’s vulnerability to oxidative stress, potentially leading to detrimental effects on neurobehavioral health. Stress-induced cortisol secretion may lower brain 5-HT and BDNF function, potentially leading to the onset of depressive symptoms and anxiety (Bhagwagar et al., 2002; Motta et al., 2021).
3.3.3 SAM
Negative psychological states can activate the SAM system, resulting in elevated catecholamine levels. Catecholamines, including epinephrine, norepinephrine, and dopamine, are tyrosine-derived hormones and neurotransmitters primarily synthesized in the adrenal medulla, sympathetic nerves, and brain. Elevated catecholamine levels can induce vasoconstriction, leading to cardiac injury, HF, myocardial ischemia, and necrosis (Szatko et al., 2023). Persistent catecholamine elevation may cause myocardial calcium overload in cytosolic and mitochondrial compartments, trigger oxidative stress, increase mitochondrial permeability, and cell death (Szatko et al., 2023). In patients with HF, there is an activation of the neuroendocrine systems, particularly the sympathetic nervous system and the renin-angiotensin-aldosterone system, leading to elevated levels of neurohormones such as catecholamines (Manolis et al., 2023). Dopamine, an important catecholamine, plays a crucial role in regulating various mental and physical functions, including anxiety, fear, attention deficit hyperactivity disorder (ADHD), and schizophrenia (Jayanti et al., 2023). Additionally, norepinephrine, another key catecholamine, acts as a neurotransmitter and is essential in mediating physiological and behavioral responses to stress (Schmidt et al., 2019).
4 Pharmacological action of Rg3 in heart diseases
4.1 HF
HF is a complicated condition that leads to aggressive hazards to human health. The common therapeutic regimens for HF patients predominantly involve diuretics, vasoactive drugs, and other pharmaceutical approaches (Truby and Rogers, 2020). Medications such as angiotensin-converting enzyme inhibitors (ACEIs), beta-blockers, mineralocorticoid receptor antagonists, and angiotensin receptor-neprilysin inhibitors can partially alleviate HF symptoms. However, these treatments are often insufficient in significantly reducing rehospitalization rates and mortality, even when patients adhere to established guidelines (Lai et al., 2022). Moreover, traditional medication can lead to adverse effects, including hypotension, hypokalemia, and renal function impairment (Hubers and Brown, 2016; Marciniak and Serebruany, 2019; Rossello et al., 2022). Consequently, there is a pressing need to explore new therapeutic agents to enhance survival rates and improve the quality of life for HF patients. Rg3 has emerged as a promising treatment option in HF, offering protective benefits such as promoting cardiomyocyte relaxation, enhancing mitochondrial structure and function, regulating metabolism, reducing cardiac fibrosis, and preventing cell apoptosis.
Cardiac sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA2a), a crucial protein in the Ca2+ cycle of cardiomyocytes, is involved in Ca2+ reuptake into the cytoplasm and subsequent transport to the endoplasmic reticulum (ER), ultimately promoting cardiomyocytes relaxation (Liu et al., 2021; Chen et al., 2022). SUMO binds to certain lysine sites on SERCA2a, forming the SERCA2a/SUMO complex. Notably, HF patients and mice exhibited significantly reduced levels of SERCA2a SUMOylation (Mendler et al., 2016). However, Rg3 treatment could increase the SUMOylation of SERCA2a, further increase intracellular Ca2+ cycle protein levels, suppress ER stress and prevent reaction oxygen species (ROS) generation, thus improving cardiac function and inhibiting cardiomyocyte hypertrophy in transverse aortic constriction (TAC) induced HF mice (Liu et al., 2021).
Moreover, Rg3 could improve disordered mitochondrial ultrastructure, functions such as ATP production and spare respiratory capacity, and regulate glucose uptake, and myocardial insulin resistance (Ni et al., 2022a). Rg3 regulated glucose uptake and myocardial insulin resistance through the activation of insulin receptor substrate (IRS)-phosphoinositide 3 kinase (PI3K)-protein kinase B (Akt) signaling pathway (Ni et al., 2022a). Several pieces of experimental evidence suggested that the decoupling of glucose oxidation to glycolysis may be the cause of unaltered or reduced pyruvate oxidation in mitochondria in HF (Pound et al., 2009; Bertero and Maack, 2018; Fillmore et al., 2018). Pyruvate dehydrogenase complex (PDHc) plays a pivotal role in regulating mitochondrial pyruvate metabolism with dihydrolipoamide dehydrogenase (DLD), serving as a crucial part of PDHc (Staretz-Chacham et al., 2021; Kim et al., 2023). P300 and tat-interacting protein 60 (TIP60) are recognized as 2-hydroxyisobutyryltransferases that could regulate the activity of PDHc (Sabari et al., 2017; Huang et al., 2018). In HF, the 2-hydroxyisobutylation of DLD was significantly upregulated, resulting from the downregulation of PDHc activity. However, Rg3 can lower the 2-hydroxyisobutylation levels of DLD and maintain the PDHc activity by suppressing the acyltransferase activity of P300, further regulating pyruvate metabolism and sustaining glucose homeostasis in cardiac tissue, consequently, improving cardiac function (Ni et al., 2022b).
Another study demonstrated that in HF mice, Rg3 administration increased the expression of aminoacylase-1 (ACY1) and inhibited cardiac fibrosis, thereby, ameliorating heart function through the ACY1-mediated transforming growth factor-β1 (TGF-β1)/Smad3 pathway. In this study, metoprolol served as the positive control. Both Rg3 and metoprolol significantly enhanced cardiac function. Notably, the impact of Rg3 at high dosage on HF was found to be comparable to that of metoprolol. Furthermore, in murine cardiac fibroblasts, the intervention of angiotensin II (AngII) resulted in an upregulation of collagen 1, collagen 3, ɑ-smooth muscle actin, tissue inhibitor of metalloproteinases 1, and the TGF-β1/Smad3 signaling pathway, which could be reversed in case of overexpressing ACY1 and Rg3 administration (Lai et al., 2022). Additionally, Baoyuan decoction, a mixture of several Chinese herbs, consists of Astragalus membranaceus (Fisch.) Bunge, Glycyrrhiza uralensis Fisch., Cinnamomum cassia Presl and P. ginseng C. A. Mey. (Wang et al., 2020). A study demonstrated that its active component Rg3 effectively suppressed cardiomyocyte apoptosis via angiotensin type 1 receptor (AT1)-cardiac ankyrin repeat protein (CARP) signaling pathway (Wang et al., 2020). CARP, a downstream protein of AT1 can accelerate apoptosis by activating the P53-mitochondrial apoptotic pathway (Shen et al., 2015). In another study, Rg3 could be an Unc51-like-kinase 1 (ULK1) regulator to facilitate FUN14 domain-containing protein 1 (FUNDC1)-mediated mitophagy, thus restoring mitochondria homeostasis and energy metabolism in HF (Wang et al., 2023). Additionally, trimetazidine was selected as the positive control. The findings indicated that the efficacy of trimetazidine was equivalent to that of a medium dose of Rg3 in rats with HF. Furthermore, a high dose of Rg3 exhibited the most pronounced therapeutic effectiveness (Wang et al., 2023).
4.2 Myocardial infarction (MI) and myocardial ischemia-reperfusion injury (MIRI)
MI and MIRI are global health issues characterized by high incidence and mortality rates across different countries. The pathophysiological basis of MI is closely related to mitochondria dysfunction, the depletion of endogenous antioxidants, and lipid peroxidation (Wang and Kang, 2021; Li D. et al., 2023; Cai et al., 2023). Following an acute MI event, clinical intervention typically involves thrombolysis, percutaneous coronary intervention (PCI), and coronary artery bypass grafting (Doenst et al., 2019; Mackman et al., 2020; Sabatine and Braunwald, 2021). These therapeutic strategies are effective in restoring blood flow, alleviating pain, and minimizing myocardial damage. Although these treatments have contributed to a substantial decrease in mortality rates, a subset of patients may experience complications, including hemorrhage and MIRI (Reed et al., 2017; McCarthy et al., 2018; Doenst et al., 2019). It has been observed that an incidence of 10%–25% of recurrent acute MI and a hospital death rate of 6%–14% among MIRI patients following PCI therapy (Wu et al., 2019). In MIRI, several medications, including antiplatelet drugs may extend survival times. However, a significant proportion of these treatments are associated with adverse effects such as bleeding and suboptimal targeting efficiency (Zhang et al., 2022). Additionally, some studies suggest that conventional antiplatelet medications do not markedly improve clinical symptoms (Li et al., 2021). Thus, combining with traditional Chinese medicine may be a promising therapeutic method under virtue of fewer adverse reactions (Tu et al., 2020).
A few studies in animals have proved that Rg3 exerted protective action on MI by promoting mitophagy, and inhibiting apoptosis, myocardial fibrosis as well as inflammation. A study showed that in isoproterenol (ISO)-induced MI mice, Rg3 pretreatment could decrease ROS content in the myocardium, promote autophagy via the AMP-activated protein kinase (AMPK)/acetyl CoA carboxylase (ACC) signal pathway, and inhibit apoptosis (Sun et al., 2020). Another study displayed that in MI rats, Rg3 downregulated the levels of pro-inflammatory cytokines in serum and cardiac tissue such as TNF-α, interleukin-1β (IL-1β), and IL-6 and increased the levels of anti-inflammatory cytokine interleukin-10 (IL-10). The anti-inflammation mechanism of Rg3 is related to the increased expression levels of sirtuin 1 (SIRT1) and decreased expression of p-P65 (Tu et al., 2020). P65 is a crucial part of the nuclear factor κB (NF-κB). SIRT1-deacetylated P65 inhibits NF-κB activation by impeding its nuclear translocation and further restrains the transcription of TNF-α, IL-6, and other inflammatory genes downstream of NF-κB, thereby mitigating the inflammatory response (Chen et al., 2016; Tu et al., 2020). A study showed in MI mice and TGFβ1-stimulated primary cardiac fibroblasts (CFs), Rg3 suppressed CF proliferation along with collagen deposition by inactivation of transforming growth factor beta receptor 1 (TGFBR1)/Smads signaling dose-dependently (Xu et al., 2023). TGFBR1 overexpression partially abolished Rg3’s inhibition on Smad2/Smad3 activation, CFs growth, together with collagen production. In this study, captopril was employed as the positive control. The results indicated that Rg3 improved cardiac function in a dose-dependent manner, with the high dose of Rg3 demonstrating effects comparable to those of captopril (Xu et al., 2023).
In addition, Rg3 exerted a beneficial role in MIRI via inhibiting inflammation, apoptosis, oxidative stress, and attenuating cardiac fibrosis. In MIRI rat models, the use of Rg3 downregulated significantly the levels of inflammatory cytokines in plasma by inhibiting inhibitor of kappa B alpha (IκBα)/NF-κB signal pathway and further improved cardiac function (Zhang et al., 2016; Li et al., 2020). Moreover, Rg3 ameliorated myocardial collagen deposition via the blockage of the TGF-β/Smad signaling pathway and exerted an anti-apoptotic effect via the Akt/endothelial nitric oxide synthase (eNOS) signaling pathway and the B cell lymphoma-2 (Bcl-2)/Bcl2-associated X protein (Bax) pathway (Wang Y. et al., 2015; Li et al., 2020). Hypoxia/reoxygenation (H/R) is a typical approach to generate MIRI injury in cardiocytes (Du et al., 2023). Rg3 administration strongly targeted FoxO3a to decrease the ROS content via the SIRT1/peroxisome proliferators-activated receptor γ coactivator-1α (PGC1-α)/nuclear factor erythroid 2-related factor (Nrf) pathway, and inhibit inflammation through IκBα/NF-κB signal pathway in H9C2 cells induced by H/R (Li et al., 2020).
4.3 Cardiotoxicity
Cardiotoxicity is an essential consideration in evaluating whether drugs can be marketed during preclinical trials and is a major reason for treatment withdrawal even after approval (Coelho et al., 2017). Even though some drugs have been used, cardiotoxicity confines their use in clinical practice. Nowadays, a good chunk of drugs, mostly anti-cancer medicine, usually lead to cardiotoxicity. Cancer therapy-induced cardiotoxicity significantly impacts patients mortality, long-term prognosis, and overall quality of life. Anthracyclines play an essential role in cancer therapy, including doxorubicin (DOX), epirubicin, daunorubicin, actinomycin, and valrubicin, have been widely applied to treat multiple types of cancer (Sawicki et al., 2021; Qu et al., 2022). However, their clinical application has been constrained by diverse adverse actions, including cardiotoxicity (Qu et al., 2022). DOX-induced cardiotoxicity involves a complex mechanism, including excessive ROS production, alterations in cell membrane integrity, and apoptosis (Upadhyay et al., 2020; Rawat et al., 2021; Tai et al., 2023). Standard treatments for cardiotoxicity encompass ACEIs, angiotensin receptor blockers (ARBs), beta-blockers, and calcium channel blockers. Research has indicated that ACEI/ARB and beta-blockers may have a positive impact on reducing the long-term health risks associated with anthracycline-induced cardiac dysfunction (Livi et al., 2021). However, previous studies have yielded inconsistent conclusions, possibly related to tumor subtypes and staging. Consequently, there is a need for further investigation into novel medications and complementary therapeutic approaches. A substantial array of natural medicines plays a significant role in mitigating drug-induced cardiotoxicity (Qu et al., 2022).
Traditional Chinese medicine has gained increasing attention for the treatment of various diseases. The protective effects of Rg3 on drug-induced cardiotoxicity have been proved in experiments. Rg3 demonstrated a remarkable ability to inhibit the upregulation of ROS and malondialdehyde, in the meantime, promote superoxide dismutase (SOD), and restore the balance of SOD/glutathione peroxidase in DOX-treated cardiac microvascular endothelial cells and rats through activating the Nrf2/antioxidant response element (ARE) and PI3K/Akt pathway (Wang X. et al., 2015). Furthermore, Rg3 could enhance endothelial function, thus playing a protective impact on cardiac function (Geng et al., 2020).
Another study showed the administration of Rg3 could enhance cardiac function in a dose-dependent manner. Specifically, in cardiotoxicity induced by microcystin, Rg3 acted by downregulating miR-128–3p and upregulating the expression of double minute 4 protein (MDM4), thereby mitigating oxidative stress and reducing cell apoptosis (Zhou and Xia, 2023).
4.4 Diabetic cardiomyopathy (DCM)
DCM is a pathophysiological condition induced by diabetes, potentially leading to HF. The diminished performance of the diabetic heart is attributed to multiple factors, including hyperglycemia, elevated fatty acids, and inflammatory cytokines (Dillmann, 2019). Current therapeutic options for DCM primarily comprise sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide-1 receptor agonists, metformin, thiazolidinediones, and dipeptidyl peptidase 4 inhibitors. While clinical trials have confirmed their effectiveness in ameliorating cardiac dysfunction, their ability to fully cure or substantially improve the prognosis of DCM remains limited. Consequently, there is an urgent need to discover novel treatments specifically targeting DCM. A recent research highlights the potential of Rg3 in DCM management (Zhang et al., 2023). Rg3 appears to protect against DCM by modulating glucose and lipid metabolism, achieved through direct binding to peroxisome proliferator-activated receptor γ (PPAR-γ) and stimulating the adiponectin pathway. Additionally, Rg3 reduces proinflammatory cytokines and mitigates mitochondrial dysfunction. In this study, metformin served as a positive control, and varying doses of Rg3 were evaluated in DCM mice models. The findings indicated that high doses of Rg3, similar to metformin, effectively improved body weight, blood glucose, body fat, and serum lipid levels. However, lower doses of Rg3 did not exhibit these benefits. Moreover, high-dose Rg3 significantly decreased serum creatine kinase (CK), creatine kinase-MB (CK-MB), and lactate dehydrogenase (LDH) levels in diabetic mice, indicative of reduced cardiac dysfunction. In contrast, metformin and low-dose Rg3 only partially improved CK and LDH levels. Therefore, high-dose Rg3 demonstrated more comprehensive systemic effects than metformin.
5 Pharmacological action of Rg3 in mental disorders
5.1 MDD
MDD, commonly referred to as depression, is a long-lasting, recurring, and possibly life-threatening psychiatric illness that impacts up to 20% of the global population (Nabavi et al., 2017). It is usually characterized by diminished self-esteem, cognitive and emotional impairments, reduced energy levels, as well as unexplained pain. Depression has been recognized as one of the primary contributors to the global burden of disease (Akpınar and Karadağ, 2022). The management of depression has become a critical issue for humanity. Primary drugs include serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, serotonin modulators, and atypical antidepressants (Chin et al., 2022). Meta-analyses indicate that the efficacy of existing antidepressants is observed in only about half to one-third of patients with depression (Kennedy et al., 2009; Jensen et al., 2023). Moreover, a significant number of patients experience recurrent episodes of depression. Consequently, there is an urgent need to explore and develop new antidepressant medications. Several investigations have demonstrated that Rg3 exhibits a therapeutic effect on depression.
Rg3 could alleviate depression-like behaviors such as anorexia, anhedonia, and decreased social exploration (Kang et al., 2017; You et al., 2017; Zhang et al., 2017). The exact mechanism refers to inhibiting inflammation cytokines such as IL-1β and IL-6, restoring the balance of TRP-KYN metabolism, enhancing cell proliferation, and suppressing apoptosis. In MDD patients and animal models of depression, the TRP-KYN metabolic pathway is over-activated (Parrott et al., 2016). The metabolic processing of neurotoxic KYN initiates depressive-like behavior after peripheral immune activation. However, Rg3 administration could decrease the level of KYN (Kang et al., 2017). Moreover, one extensively supported hypothesis regarding depression is the neurotrophic hypothesis, positing that the pathogenesis of depression is associated with impaired functioning of the BDNF system within the brain (Colucci-D'Amato et al., 2020). BDNF is crucial for neural signal transduction and the facilitation of neuronal plasticity, achieved through its specific binding and subsequent activation of tropomyosin-related kinase B (TrkB) receptors (Moya-Alvarado et al., 2022; Pahlavani, 2023). In the chronic mild stress mouse model, Rg3 markedly enhanced the expression of BDNF and the phosphorylation of cyclic adenosine monophosphate response element binding protein (CREB), thereby mitigating depressive symptoms (You et al., 2017). Meanwhile, fluoxetine was employed as a positive control, revealing that to achieve a comparable antidepressant effect to that of fluoxetine, a higher dosage of Rg3 is necessary (You et al., 2017). Additionally, it was observed that chronic stress exposure markedly decreased body weight. While fluoxetine was effective in mitigating this weight loss, Rg3 exhibited no significant impact on body weight.
5.2 Anxiety disorders
Anxiety disorders constitute the most prevalent category of mental illness, typically originating before or during early adulthood. Key characteristics encompass excessive fear and anxiety or avoidance of perceived threats that are persistent and impairing (Penninx et al., 2021). In adults, anxiety prevention has been evaluated in a few trials of selective or indicated prevention (Deady et al., 2017). Effective treatments for anxiety disorders are available, which not only alleviate symptoms of anxiety but also enhance overall quality of life and functioning. Both pharmacotherapy and psychotherapy are regarded as primary approaches to managing anxiety disorders. Studies have demonstrated that routine medications are mildly to moderately effective in treating these disorders, although there is a noted variability in response rates (Penninx et al., 2021). Chinese herbs have shown therapeutic effects on anxiety including Rg3.
Rg3 has shown a beneficial role in GABAA receptor-related anxiety (Lee et al., 2013). GABAA receptors consist of three subunits (α1, β1, γ2). The GABAA receptor is responsible for fast inhibitory synaptic transmission. The principal physiological and pharmacological functions of GABAA receptors encompass the mitigation of anxiety symptoms in patients. Additionally, the γ2 subunit of the GABAA receptor is pivotal in the control and management of human epilepsy (Lee et al., 2013). In a study, Rg3 exhibited enhancing effects on the GABA-induced inward current (IGABA) with γ2 subunit in a dependent manner. When the γ2 subunit expression ratio was raised, the degree of the Rg3-induced activation of the GABAA receptor increased (Lee et al., 2013).
5.3 Post-traumatic stress disorder (PTSD)
PTSD is a severe psychiatric condition linked to substantial distress and impaired functional capacity (Bryant et al., 2023). Psychological therapies are established as the primary treatment modality for PTSD (Bisson and Olff, 2021). Numerous systematic reviews have consistently affirmed the efficacy of these therapies in addressing PTSD symptoms (Bisson et al., 2013). Nevertheless, the effectiveness of psychological interventions may fluctuate based on individual factors, including the intensity of PTSD. Consequently, pharmacotherapy plays a crucial role in the symptom management of PTSD patients. While common pharmacological treatments have shown encouraging outcomes in diminishing the severity of PTSD symptoms, their overall efficacy remains moderate, and they may precipitate adverse reactions. Therefore, there is an imperative need for the exploration and development of innovative therapeutic strategies.
Rg3 has been reported to have a role in improving fear memory and spatial memory (Sur and Lee, 2022a). The HPA axis and monoamine imbalance in the medial prefrontal cortex and hippocampus contribute to the pathogenesis of PTSD. However, Rg3 administration could be involved in regulating the HPA axis and BDNF-TrkB pathway (Sur and Lee, 2022a). In rats administered with Rg3, a significant decrease in serum corticosterone and adrenocorticotropic hormone levels was observed, alongside a marked increase in BDNF, TrkB, catecholamine, and 5-HT concentrations. The study utilized paroxetine hydrochloride as a positive control. Compared to paroxetine hydrochloride, Rg3 demonstrated superior effects in behavioral tests. Notably, a dosage of 50 mg/kg of Rg3 was effective in elevating 5-HT levels, while significant differences were observed in the response to paroxetine hydrochloride treatment.
5.4 ADHD
ADHD is a mental disorder that typically emerges in childhood and is characterized by developmentally inappropriate and impairing inattention, motor hyperactivity, and impulsivity, with difficulties often continuing into adulthood. Medication and behavioral therapies are widely recognized and frequently utilized treatments for ADHD, demonstrating substantial efficacy and notable rates of symptom remission. Furthermore, a recent meta-analysis has revealed a significant preference for herbal medicine as an effective treatment option for ADHD (Dutta et al., 2022). An open-label pilot study demonstrated that the combination of omega-3 and Korean red ginseng may improve ADHD symptoms and cognitive functions including attention, memory, and executive function in children with ADHD (Lee J. et al., 2020). Additionally, YY162, consisting of Rg3 extracted from P. ginseng C. A. Mey. and Ginkgo biloba L. (Ben-Azu et al., 2022b; Asiwe et al., 2023), exerted protective activity on ADHD through modulating oxidative metabolism and the BDNF/TrkB pathway (Nam et al., 2014). Yet, the mechanism of Rg3 on ADHD requires further research and exploration.
6 Rg3 in the coexistence of cardiac diseases and psychiatric disorders
Current investigations into the therapeutic effects and mechanisms of Rg3, especially concerning the comorbidity of heart and mental disorders, are still in their early stages. Nowadays, there is substantial basic research supporting Rg3’s efficacy in treating heart or mental disorders separately, based on their relationship, Rg3 may show potential effects in treating their comorbidity (Figure 3).
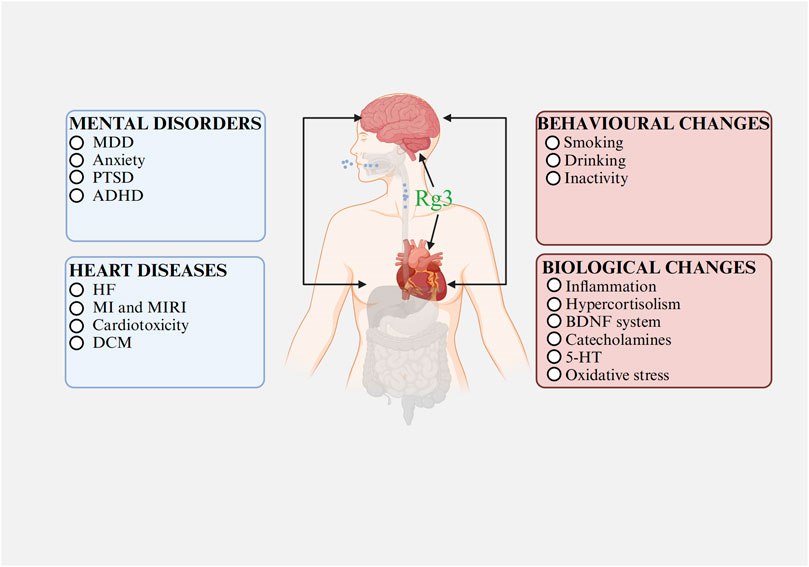
FIGURE 3. The relationships between heart diseases and mental disorders Abbreviations: MDD, major depressive disorder; PTSD, post-traumatic stress disorder; ADHD, attention deficit hyperactivity disorder; HF, heart failure; MI, myocardial infarction; MIRI, myocardial ischemia-reperfusion injury; DCM, diabetic cardiomyopathy; BDNF, brain-derived neurotrophic factor; 5-HT, serotonin. Image created with BioRender.com, with permission.
Rg3 might play a dual preventive role, potentially arresting the progression or aiding the recovery of mental disorders when used in cardiac disease treatments. Its potential mechanisms might involve modulating inflammation and oxidative stress. In cardiac disease management, Rg3 chiefly functions by reducing inflammation and curtailing oxidative stress. Anti-inflammatory action is linked to the regulation of TRP/KYN metabolism, enhancing 5-HT and BDNF levels, and thereby alleviating mental disorders (Kuo et al., 2021; Fellendorf et al., 2022; Gong et al., 2023). MDD is characterized by reduced antioxidant concentrations in plasma (Bhatt et al., 2020). Oxidative stress, characterized by the overproduction of ROS and the depletion of antioxidative defenses, leads to pro-inflammatory signaling and induces cellular apoptosis (Bhatt et al., 2020). Counteracting oxidative stress can therefore diminish inflammation, reduce cellular death, and improve mental health conditions.
Furthermore, in the treatment of mental disorders, Rg3 may also enhance cardiac function. For mental conditions, Rg3 works by suppressing inflammation and HPA axis activity, reducing oxidative stress, boosting BDNF levels, and lowering catecholamine levels. Inflammation and oxidative stress are known to worsen myocardial fibrosis and impair cardiac function. Elevated cortisol levels can directly lead to hypertrophy of cardiomyocytes and cardiac remodeling (Braukyliene et al., 2022). A normal BDNF system is essential for cardiovascular development, while high catecholamine levels can cause vasoconstriction, contributing to cardiac injury, pathological remodeling, heart failure, myocardial ischemia, and necrosis (Du et al., 2021; Agorrody et al., 2022; Tsai et al., 2022). Thus, the mechanism through which Rg3 enhances cardiac function while ameliorating mental disorders may involve modulating inflammation, catecholamine, the BDNF system, the HPA axis, and oxidative stress.
7 Adverse reactions of Rg3
Few studies have shown the side effects of Rg3. Existing clinical investigations of Rg3 at various dosages have not revealed any harmful reaction. The sole adverse observation demonstrated increased but reversible kidney weight in dogs that received 60 mg/kg 20(S)-Rg3 (Gao et al., 2020; Nakhjavani et al., 2020). However, given that Rg3 is extracted from ginseng, high doses of ginseng (15 g/d) will lead to ginseng abuse syndrome. Most symptoms encompass headache, dizziness, breast pain, nausea, asthma, and so on (Siegel, 1979; Deng et al., 2023). Although Rg3 offers a broad spectrum of clinical applications in cancer patients, it should not be abused. Taking the recommended dose of Rg3 will not cause serious adverse reactions. Due to the multiple ingredients of ginseng, the adverse effects of Rg3 are not completely equal to ginseng and need to be explored.
8 Conclusion and perspectives
Recent studies have provided increasing evidence of the broad pharmacological effects of Rg3 in treating heart diseases and mental disorders through a variety of signaling pathways and influencing changes at the transcriptional level (Figure 4; Figure 5).
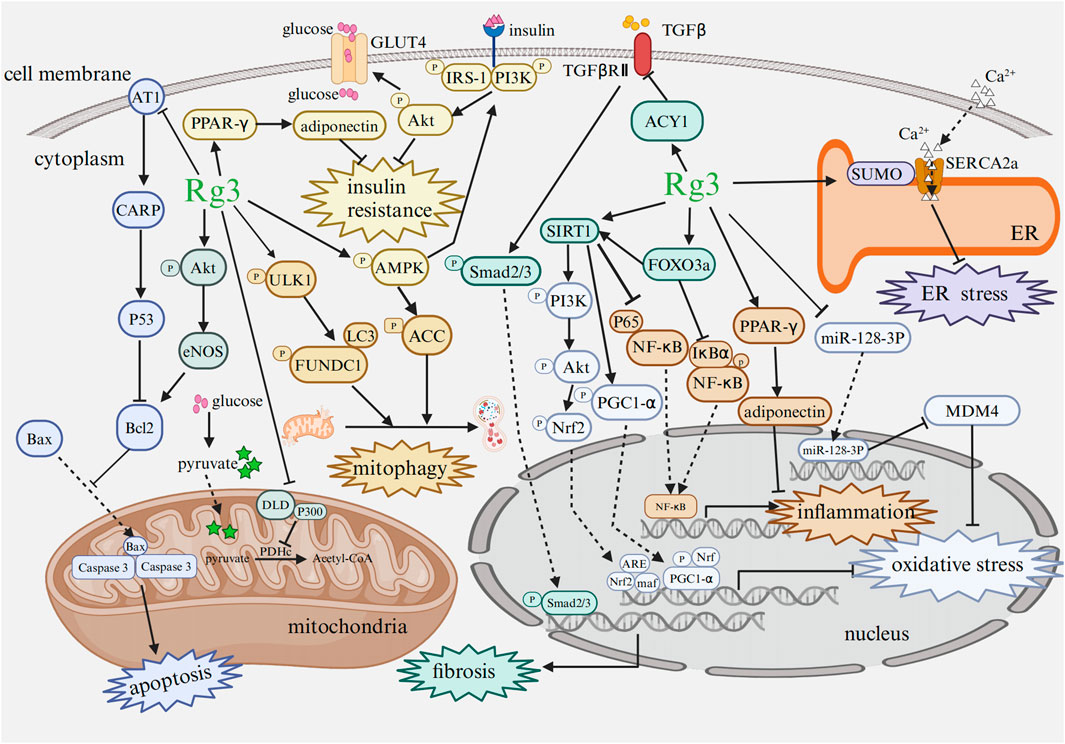
FIGURE 4. Mechanism of Rg3 on cardiac protection Abbreviations: AT1, angiotensin type 1 receptor; CARP, cardiac ankyrin repeat protein; Bcl2, B cell lymphoma-2; Bax, Bcl2-associated X protein; Akt, protein kinase B; eNOS, endothelial nitric oxide synthase; DLD, dihydrolipoamide dehydrogenase; GLUT4, glucose transporter 4; PDHc, pyruvate dehydrogenase complex; ULK1, Unc51-like-kinase 1; FUNDC1, FUN14 domain-containing protein 1; LC3, microtubule-associated protein 1 light chain 3; PI3K, phosphoinositide 3 kinase; IRS, insulin receptor substrate; AMPK, AMP-activated protein kinase; ACC, acetyl CoA carboxylase; Nrf, nuclear factor erythroid 2-related factor; PGC1-α, peroxisome proliferators-activated receptor γ coactivator-1α; PPAR-γ, peroxisome proliferator-activated receptor γ; NF-κB, nuclear factor κB; IκBα, inhibitor of kappa B alpha; ARE, antioxidant response element; maf, musculoaponeurotic fibrosarcoma oncogene homolog; SIRT1, sirtuin 1; FOXO3a, forkhead box O3a; ER, endoplasmic reticulum; SERCA2a, sarcoplasmic/endoplasmic reticulum Ca2+-ATPase; MDM4, double minute 4 protein. Image created with BioRender.com, with permission.
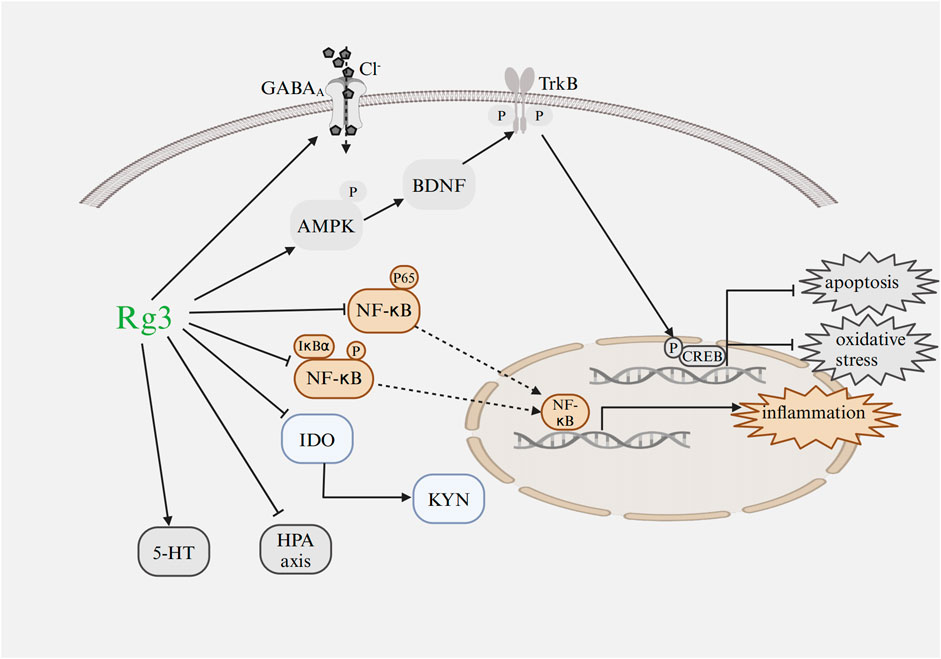
FIGURE 5. Mechanism of Rg3 on mental disorders Abbreviations: GABA, gamma aminobutyric acid; IDO, indoleamine 2,3-dioxygenase; NF-κB, nuclear factor κB; IκBα, inhibitor of kappa B alpha; KYN, kynurenine; HPA, hypothalamic-pituitary-adrenal; CREB, cyclic adenosine monophosphate response element binding protein; TrkB, tropomyosin-related kinase B; BDNF, brain-derived neurotrophic factor; 5-HT, serotonin; AMPK, AMP-activated protein kinase. Image created with BioRender.com, with permission.
However, the majority of studies primarily concentrate on in vitro cell or animal models, with less emphasis on clinical research. The shenyi capsule, comprising Rg3 monomer, is currently employed in the clinical treatment of cancer patients. Clinical studies have demonstrated that shenyi capsules mitigate the toxicity of platinum-based chemotherapy in non-small-cell lung cancer (NSCLC), including a reduction in platelet toxicity (Xu et al., 2020). Additionally, in a double-blind, randomized, crossover study involving 23 participants, oral administration of Rg3-enriched Korean red ginseng at a dose of 400 mg/day for 7 days demonstrated a decrease in aortic stiffness and central blood pressure (Jovanovski et al., 2014). These findings suggest that Rg3 supplementation may help reduce risk factors for heart diseases such as platelet abnormality, vascular stiffness, and hypertension. However, a clinical study was conducted to recruit NSCLC patients with normal cardiac function. The study showed the treatment group with exclusive administration of Rg3 and the control group with placebo treatment exhibited comparable cardiac functions before and after the therapy (Li D.-R. et al., 2023). Furthermore, an open-label pilot study indicated that a combination of omega-3 and Korean red ginseng, which is rich in Rg3, could potentially alleviate symptoms of ADHD (Lee J. et al., 2020).
However, to date, there is no research exploring the exclusive or combined clinical use of Rg3 for treating heart diseases or mental disorders or the comorbidity of both in humans. The limited clinical research on Rg3 may be attributed to several factors. First, existing studies indicate that a higher concentration of Rg3 is necessary to attain similar therapeutic outcomes as routine drugs (You et al., 2017). Then, the utilization of Rg3 in the clinic is restrictive due to low bioavailability and poor intestinal absorption capability with a percentage of approximately 10% (Xiong et al., 2023). Moreover, common methods for preparing Rg3, such as heat treatment, acid-base treatment, and biological transformation (Fu, 2019; Siddiqi et al., 2020), are hindered by the low natural concentration of Rg3 in ginseng plants (Yan et al., 2019). This results in the inability of most methods to achieve large-scale production of Rg3. Finally, there is a necessity to increase awareness and understanding of Rg3’s therapeutic potential in mental disorders and its possible synergistic use in cases of mental disorders co-occurring with heart diseases.
Given these potential reasons, clinical practitioners and researchers are urged to pursue further studies on Rg3. Currently, Rg3 is primarily administered orally. However, novel administration methods, such as catheter-based endocardial or intramyocardial injections, should be explored to enhance its clinical utility. Polyethylene glycol (PEG) is commonly utilized as a hydrophilic block in drug delivery systems for its resistance to protein adsorption and minimal toxicity (Li et al., 2020). Conversely, polypropylene sulfide (PPS) is favored as the hydrophobic block due to its pronounced hydrophobic properties. This PEG-b-PPS amphiphilic block copolymer demonstrates potential as a ROS-responsive nanovesicle, specifically designed for efficient drug delivery (Li et al., 2020). The combination of PEG and PPS offers a promising platform for enhancing the therapeutic effects of Rg3 (Li et al., 2020). Additionally, the development of Rg3-loaded nanoparticles with specific targeting abilities could enable non-invasive, tissue-specific drug delivery (Li et al., 2020). Furthermore, there is also a pressing need for extensive, well-controlled clinical trials to more accurately determine Rg3’s benefits on cardiac and mental health. Regarding Rg3, there exist two variants: 20(R)-Rg3 and 20(S)-Rg3. The distinct impacts of these variants on cardiac and mental health remain to be elucidated. Given that these two epimers have already shown varied anticancer effects, their potential differential influence on heart diseases and mental disorders presents a research opportunity.
In summary, we have reviewed recent findings that illustrate the important role of Rg3 as a novel therapeutic agent in heart diseases and mental disorders. More clinical trials are motivated to be investigated for Rg3 in heart and mental diseases, especially in patients with the coexistence of cardiac diseases and psychiatric disorders.
Author contributions
LS: Writing–original draft. JL: Writing–review and editing. XW: Writing–review and editing. XX: Writing–review and editing. LT: Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agorrody, G., Peclat, T. R., Peluso, G., Gonano, L. A., Santos, L., van Schooten, W., et al. (2022). Benefits in cardiac function by CD38 suppression: improvement in NAD(+) levels, exercise capacity, heart rate variability and protection against catecholamine-induced ventricular arrhythmias. J. Mol. Cell. Cardiol. 166, 11–22. doi:10.1016/j.yjmcc.2022.01.008
Akpınar, Ş., and Karadağ, M. G. (2022). Is vitamin D important in anxiety or depression? What is the truth? Curr. Nutr. Rep. 11 (4), 675–681. doi:10.1007/s13668-022-00441-0
Asiwe, J. N., Ekene, E. N., Agbugba, L. C., Moke, E. G., Akintade, A. V., Ben-Azu, B., et al. (2023). Ginkgo biloba supplement abates lead-induced endothelial and testicular dysfunction in Wistar rats via up-regulation of Bcl-2 protein expression, pituitary-testicular hormones and down-regulation of oxido-inflammatory reactions. J. Trace. Elem. Med. Biol. 79, 127216. doi:10.1016/j.jtemb.2023.127216
Ben-Azu, B., Adebayo, O. G., Jarikre, T. A., Oyovwi, M. O., Edje, K. E., Omogbiya, I. A., et al. (2022a). Taurine, an essential β-amino acid insulates against ketamine-induced experimental psychosis by enhancement of cholinergic neurotransmission, inhibition of oxidative/nitrergic imbalances, and suppression of COX-2/iNOS immunoreactions in mice. Metab. Brain. Dis. 37 (8), 2807–2826. doi:10.1007/s11011-022-01075-5
Ben-Azu, B., Adebayo, O. G., Wopara, I., Aduema, W., Onyeleonu, I., Umoren, E. B., et al. (2022b). Lead acetate induces hippocampal pyramidal neuron degeneration in mice via up-regulation of executioner caspase-3, oxido-inflammatory stress expression and decreased BDNF and cholinergic activity: reversal effects of Gingko biloba supplement. J. Trace. Elem. Med. Biol. 71, 126919. doi:10.1016/j.jtemb.2021.126919
Ben-Azu, B., Uruaka, C. I., Ajayi, A. M., Jarikre, T. A., Nwangwa, K. E., Chilaka, K. C., et al. (2023). Reversal and preventive pleiotropic mechanisms involved in the antipsychotic-like effect of taurine, an essential β-amino acid in ketamine-induced experimental schizophrenia in mice. Neurochem. Res. 48 (3), 816–829. doi:10.1007/s11064-022-03808-5
Bertero, E., and Maack, C. (2018). Metabolic remodelling in heart failure. Nat. Rev. Cardiol. 15 (8), 457–470. doi:10.1038/s41569-018-0044-6
Bhagwagar, Z., Hafizi, S., and Cowen, P. J. (2002). Cortisol modulation of 5-HT-mediated growth hormone release in recovered depressed patients. J. Affect. Disord. 72 (3), 249–255. doi:10.1016/s0165-0327(01)00467-0
Bhatt, S., Nagappa, A. N., and Patil, C. R. (2020). Role of oxidative stress in depression. Drug Discov. Today. 25 (7), 1270–1276. doi:10.1016/j.drudis.2020.05.001
Bisson, J. I., and Olff, M. (2021). Prevention and treatment of PTSD: the current evidence base. Eur. J. Psychotraumatol. 12 (1), 1824381. doi:10.1080/20008198.2020.1824381
Bisson, J. I., Roberts, N. P., Andrew, M., Cooper, R., and Lewis, C. (2013). Psychological therapies for chronic post-traumatic stress disorder (PTSD) in adults. Cochrane Database Syst. Rev. 2013 (12), CD003388. doi:10.1002/14651858.CD003388.pub4
Bornand, D., Reinau, D., Jick, S. S., and Meier, C. R. (2022). β-blockers and the risk of depression: a matched case-control study. Drug Saf. 45 (2), 181–189. doi:10.1007/s40264-021-01140-5
Bothou, C., Beuschlein, F., and Spyroglou, A. (2020). Links between aldosterone excess and metabolic complications: a comprehensive review. Diabetes Metab. 46 (1), 1–7. doi:10.1016/j.diabet.2019.02.003
Braukyliene, R., Aldujeli, A., Haq, A., Maciulevicius, L., Jankauskaite, D., Jurenas, M., et al. (2022). Impact of mineralocorticoid receptor gene NR3C2 on the prediction of functional classification of left ventricular remodeling and arrhythmia after acute myocardial infarction. Int. J. Environ. Res. Public Health 20 (1), 12. doi:10.3390/ijerph20010012
Bryant, R. A., McFarlane, A. C., Silove, D., O'Donnell, M. L., Forbes, D., and Creamer, M. (2023). The lingering impact of resolved PTSD on subsequent functioning. Focus (Am. Psychiatr. Publ.) 21 (3), 290–295. doi:10.1176/appi.focus.23021016
Cai, S., Zhao, M., Zhou, B., Yoshii, A., Bugg, D., Villet, O., et al. (2023). Mitochondrial dysfunction in macrophages promotes inflammation and suppresses repair after myocardial infarction. J. Clin. Invest. 133 (4), e159498. doi:10.1172/jci159498
Campana, P., Palaia, M. E., Conte, M., Cante, T., Petraglia, L., Femminella, G. D., et al. (2022). The elderly at risk: aldosterone as modulator of the immune response to SARS-CoV-2 infection. Geroscience 44 (2), 567–572. doi:10.1007/s11357-021-00481-4
Cannavo, A., Jun, S., Rengo, G., Marzano, F., Agrimi, J., Liccardo, D., et al. (2023). β3AR-dependent brain-derived neurotrophic factor (BDNF) generation limits chronic postischemic heart failure. Circ. Res. 132 (7), 867–881. doi:10.1161/circresaha.122.321583
Carnovale, C., Perrotta, C., Baldelli, S., Cattaneo, D., Montrasio, C., Barbieri, S. S., et al. (2023). Antihypertensive drugs and brain function: mechanisms underlying therapeutically beneficial and harmful neuropsychiatric effects. Cardiovasc. Res. 119 (3), 647–667. doi:10.1093/cvr/cvac110
Chen, G. D., Yu, W. D., and Chen, X. P. (2016). Sirt1 activator represses the transcription of TNF-α in THP-1 cells of a sepsis model via deacetylation of H4K16. Mol. Med. Rep. 14 (6), 5544–5550. doi:10.3892/mmr.2016.5942
Chen, J., Liu, Y., Pan, D., Xu, T., Luo, Y., Wu, W., et al. (2022). Estrogen inhibits endoplasmic reticulum stress and ameliorates myocardial ischemia/reperfusion injury in rats by upregulating SERCA2a. Cell Commun. Signal. 20 (1), 38–16. doi:10.1186/s12964-022-00842-2
Chen, M. H., Hsu, J. W., Huang, K. L., Tsai, S. J., Tu, P. C., and Bai, Y. M. (2023). Inflammatory cytokines in and cognitive function of adolescents with first-episode schizophrenia, bipolar disorder, or major depressive disorder. CNS Spectr. 28 (1), 70–77. doi:10.1017/S1092852921000857
Chin, T., Huyghebaert, T., Svrcek, C., and Oluboka, O. (2022). Individualized antidepressant therapy in patients with major depressive disorder: novel evidence-informed decision support tool. Can. Fam. Physician 68 (11), 807–814. doi:10.46747/cfp.6811807
Coelho, A. R., Martins, T. R., Couto, R., Deus, C., Pereira, C. V., Simoes, R. F., et al. (2017). Berberine-induced cardioprotection and sirt3 modulation in doxorubicin-treated H9c2 cardiomyoblasts. Biochim. Biophys. Acta Mol. Basis Dis. 1863 (11), 2904–2923. doi:10.1016/j.bbadis.2017.07.030
Colucci-D'Amato, L., Speranza, L., and Volpicelli, F. (2020). Neurotrophic factor BDNF, physiological functions and therapeutic potential in depression, neurodegeneration and brain cancer. Int. J. Mol. Sci. 21 (20), 7777. doi:10.3390/ijms21207777
Correll, C. U., Detraux, J., De Lepeleire, J., and De Hert, M. (2015). Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry 14 (2), 119–136. doi:10.1002/wps.20204
Crawford, A. A., Soderberg, S., Kirschbaum, C., Murphy, L., Eliasson, M., Ebrahim, S., et al. (2019). Morning plasma cortisol as a cardiovascular risk factor: findings from prospective cohort and mendelian randomization studies. Eur. J. Endocrinol. 181 (4), 429–438. doi:10.1530/eje-19-0161
Deady, M., Choi, I., Calvo, R. A., Glozier, N., Christensen, H., and Harvey, S. B. (2017). eHealth interventions for the prevention of depression and anxiety in the general population: a systematic review and meta-analysis. BMC Psychiatry 17 (1), 310–314. doi:10.1186/s12888-017-1473-1
De Deurwaerdere, P., and Di Giovanni, G. (2020). Serotonin in health and disease. Int. J. Mol. Sci. 21 (10), 3500. doi:10.3390/ijms21103500
Deng, W., Liu, H., Guo, L., Liu, Y., and Ma, Z. (2023). Panax ginseng abuse exhibits a pro-inflammatory effect by activating the NF-κB pathway. Food Sci. Nutr. 11 (5), 2130–2140. doi:10.1002/fsn3.3011
Dillmann, W. H. (2019). Diabetic cardiomyopathy. Circ. Res. 124 (8), 1160–1162. doi:10.1161/circresaha.118.314665
Doenst, T., Haverich, A., Serruys, P., Bonow, R. O., Kappetein, P., Falk, V., et al. (2019). PCI and CABG for treating stable coronary artery disease: JACC review topic of the week. J. Am. Coll. Cardiol. 73 (8), 964–976. doi:10.1016/j.jacc.2018.11.053
Du, Y., Demillard, L. J., and Ren, J. (2021). Catecholamine-induced cardiotoxicity: a critical element in the pathophysiology of stroke-induced heart injury. Life Sci. 287, 120106. doi:10.1016/j.lfs.2021.120106
Du, Y., Li, J., Cai, C., Gong, F., Zhou, G., Liu, F., et al. (2023). Plantamajoside alleviates hypoxia-reoxygenation injury through integrin-linked kinase/c-Src/Akt and the mitochondrial apoptosis signaling pathways in H9c2 myocardial cells. BMC Complement. Med. Ther. 23 (1), 64. doi:10.1186/s12906-023-03880-6
Dutta, T., Anand, U., Mitra, S. S., Ghorai, M., Jha, N. K., Shaikh, N. K., et al. (2022). Phytotherapy for attention deficit hyperactivity disorder (ADHD): a systematic review and meta-analysis. Front. Pharmacol. 13, 827411. doi:10.3389/fphar.2022.827411
Emudainohwo, J. O. T., Ben-Azu, B., Adebayo, O. G., Aduema, W., Uruaka, C., Ajayi, A. M., et al. (2023). Normalization of HPA axis, cholinergic neurotransmission, and inhibiting brain oxidative and inflammatory dynamics Are Associated with the adaptogenic-like effect of rutin against psychosocial defeat stress. J. Mol. Neurosci. 73 (1), 60–75. doi:10.1007/s12031-022-02084-w
Favero, V., Cremaschi, A., Falchetti, A., Gaudio, A., Gennari, L., Scillitani, A., et al. (2021). Management and medical therapy of mild hypercortisolism. Int. J. Mol. Sci. 22 (21), 11521. doi:10.3390/ijms222111521
Fellendorf, F. T., Bonkat, N., Dalkner, N., Schönthaler, E. M. D., Manchia, M., Fuchs, D., et al. (2022). Indoleamine 2,3-dioxygenase (Ido)-activity in severe psychiatric disorders: a systemic review. Curr. Top. Med. Chem. 22 (25), 2107–2118. doi:10.2174/1568026622666220718155616
Fillmore, N., Levasseur, J. L., Fukushima, A., Wagg, C. S., Wang, W., Dyck, J. R. B., et al. (2018). Uncoupling of glycolysis from glucose oxidation accompanies the development of heart failure with preserved ejection fraction. Mol. Med. 24 (1), 3. doi:10.1186/s10020-018-0005-x
Fu, Y. (2019). Biotransformation of ginsenoside Rb1 to Gyp-XVII and minor ginsenoside Rg3 by endophytic bacterium Flavobacterium sp. GE 32 isolated from Panax ginseng. Lett. Appl. Microbiol. 68 (2), 134–141. doi:10.1111/lam.13090
Gao, Y., Wang, G., Wang, T., Li, G., Lin, J., Sun, L., et al. (2020). A 26-week 20(S)-ginsenoside Rg3 oral toxicity study in Beagle dogs. Regul. Toxicol. Pharmacol. 110, 104522. doi:10.1016/j.yrtph.2019.104522
García-Eguren, G., Giró, O., Romero, M. D. M., Grasa, M., and Hanzu, F. A. (2019). Chronic hypercortisolism causes more persistent visceral adiposity than HFD-induced obesity. J. Endocrinol. 242 (2), 65–77. doi:10.1530/joe-19-0168
Geng, J., Fu, W., Yu, X., Lu, Z., Liu, Y., Sun, M., et al. (2020). Ginsenoside Rg3 alleviates ox-LDL induced endothelial dysfunction and prevents atherosclerosis in ApoE(-/-) mice by regulating PPARγ/FAK signaling pathway. Front. Pharmacol. 11, 500. doi:10.3389/fphar.2020.00500
Gong, X., Chang, R., Zou, J., Tan, S., and Huang, Z. (2023). The role and mechanism of tryptophan - kynurenine metabolic pathway in depression. Rev. Neurosci. 34 (3), 313–324. doi:10.1515/revneuro-2022-0047
Güder, G., Bauersachs, J., Frantz, S., Weismann, D., Allolio, B., Ertl, G., et al. (2007). Complementary and incremental mortality risk prediction by cortisol and aldosterone in chronic heart failure. Circulation 115 (13), 1754–1761. doi:10.1161/circulationaha.106.653964
Hagi, K., Nosaka, T., Dickinson, D., Lindenmayer, J. P., Lee, J., Friedman, J., et al. (2021). Association between cardiovascular risk factors and cognitive impairment in people with schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry 78 (5), 510–518. doi:10.1001/jamapsychiatry.2021.0015
Hartupee, J., and Mann, D. L. (2017). Neurohormonal activation in heart failure with reduced ejection fraction. Nat. Rev. Cardiol. 14 (1), 30–38. doi:10.1038/nrcardio.2016.163
Huang, H., Luo, Z., Qi, S., Huang, J., Xu, P., Wang, X., et al. (2018). Landscape of the regulatory elements for lysine 2-hydroxyisobutyrylation pathway. Cell Res. 28 (1), 111–125. doi:10.1038/cr.2017.149
Hubers, S. A., and Brown, N. J. (2016). Combined angiotensin receptor antagonism and neprilysin inhibition. Circulation 133 (11), 1115–1124. doi:10.1161/circulationaha.115.018622
Jayanti, S., Dalla Verde, C., Tiribelli, C., and Gazzin, S. (2023). Inflammation, dopaminergic brain and bilirubin. Int. J. Mol. Sci. 24 (14), 11478. doi:10.3390/ijms241411478
Jensen, K. H. R., Dam, V. H., Ganz, M., Fisher, P. M., Ip, C. T., Sankar, A., et al. (2023). Deep phenotyping towards precision psychiatry of first-episode depression-the brain drugs-depression cohort. BMC Psychiatry 23 (1), 151. doi:10.1186/s12888-023-04618-x
Jovanovski, E., Bateman, E. A., Bhardwaj, J., Fairgrieve, C., Mucalo, I., Jenkins, A. L., et al. (2014). Effect of Rg3-enriched Korean red ginseng (Panax ginseng) on arterial stiffness and blood pressure in healthy individuals: a randomized controlled trial. J. Am. Soc. Hypertens. 8 (8), 537–541. doi:10.1016/j.jash.2014.04.004
Kang, A., Xie, T., Zhu, D., Shan, J., Di, L., and Zheng, X. (2017). Suppressive effect of ginsenoside Rg3 against lipopolysaccharide-induced depression-like behavior and neuroinflammation in mice. J. Agric. Food Chem. 65 (32), 6861–6869. doi:10.1021/acs.jafc.7b02386
Kennedy, S. H., Andersen, H. F., and Thase, M. E. (2009). Escitalopram in the treatment of major depressive disorder: a meta-analysis. Curr. Med. Res. Opin. 25 (1), 161–175. doi:10.1185/03007990802622726
Kermani, P., and Hempstead, B. (2019). BDNF actions in the cardiovascular system: roles in development, adulthood and response to injury. Front. Physiol. 10, 455. doi:10.3389/fphys.2019.00455
Kim, J., Yun, K. S., Cho, A., Kim, D. H., Lee, Y. K., Choi, M. J., et al. (2022). High cortisol levels are associated with oxidative stress and mortality in maintenance hemodialysis patients. BMC Nephrol. 23 (1), 98. doi:10.1186/s12882-022-02722-w
Kim, M. J., Lee, H., Chanda, D., Thoudam, T., Kang, H. J., Harris, R. A., et al. (2023). The role of pyruvate metabolism in mitochondrial quality control and inflammation. Mol. Cells 46 (5), 259–267. doi:10.14348/molcells.2023.2128
Kuo, C. Y., Lin, C. H., and Lane, H. Y. (2021). Molecular basis of late-life depression. Int. J. Mol. Sci. 22 (14), 7421. doi:10.3390/ijms22147421
Lai, Q., Liu, F. M., Rao, W. L., Yuan, G. Y., Fan, Z. Y., Zhang, L., et al. (2022). Aminoacylase-1 plays a key role in myocardial fibrosis and the therapeutic effects of 20(S)-ginsenoside Rg3 in mouse heart failure. Acta Pharmacol. Sin. 43 (8), 2003–2015. doi:10.1038/s41401-021-00830-1
Lee, B. H., Kim, H. J., Chung, L., and Nah, S. Y. (2013). Ginsenoside Rg₃ regulates GABAA receptor channel activity: involvement of interaction with the γ₂ subunit. Eur. J. Pharmacol. 705 (1-3), 119–125. doi:10.1016/j.ejphar.2013.02.040
Lee, H., Hong, Y., Tran, Q., Cho, H., Kim, M., Kim, C., et al. (2019). A new role for the ginsenoside Rg3 in antiaging via mitochondria function in ultraviolet-irradiated human dermal fibroblasts. J. Ginseng Res. 43 (3), 431–441. doi:10.1016/j.jgr.2018.07.003
Lee, H., Kong, G., Tran, Q., Kim, C., Park, J., and Park, J. (2020a). Relationship between ginsenoside Rg3 and metabolic syndrome. Front. Pharmacol. 11, 130. doi:10.3389/fphar.2020.00130
Lee, J., Lee, A., Kim, J. H., Shin, Y. M., Kim, S. J., Cho, W. D., et al. (2020b). Effect of omega-3 and Korean red ginseng on children with attention deficit hyperactivity disorder: an open-label pilot study. Clin. Psychopharmacol. Neurosci. 18 (1), 75–80. doi:10.9758/cpn.2020.18.1.75
Li, D., Zhang, G., Wang, Z., Guo, J., Liu, Y., Lu, Y., et al. (2023b). Idebenone attenuates ferroptosis by inhibiting excessive autophagy via the ROS-AMPK-mTOR pathway to preserve cardiac function after myocardial infarction. Eur. J. Pharmacol. 943, 175569. doi:10.1016/j.ejphar.2023.175569
Li, D.-R., Hou, W., Hua, B.-J., Zhang, P.-T., Xiong, L., Liu, H., et al. (2023a). Shenyi capsule prolongs postoperative survival of patients with nonsmall cell lung cancer: a multicenter, randomized, controlled trial. World J. Traditional Chin. Med. 9 (3), 314–321. doi:10.4103/2311-8571.382023
Li, L., Wang, Y., Guo, R., Li, S., Ni, J., Gao, S., et al. (2020). Ginsenoside Rg3-loaded, reactive oxygen species-responsive polymeric nanoparticles for alleviating myocardial ischemia-reperfusion injury. J. Control. Release. 317, 259–272. doi:10.1016/j.jconrel.2019.11.032
Li, Y., Li, Y., Li, B., Liu, Y., Zhang, J., Kuang, W., et al. (2021). Antiplatelet therapy with integrated traditional Chinese and western medicine for use in myocardial ischemia-reperfusion injury: a review of clinical applications and mechanisms. Evid. Based Complement. Altern. Med. 2021, 7409094. doi:10.1155/2021/7409094
Liu, Y., and Miao, J. (2022). An emerging role of defective copper metabolism in heart disease. Nutrients 14 (3), 700. doi:10.3390/nu14030700
Liu, Z., Bian, X., Gao, W., Su, J., Ma, C., Xiao, X., et al. (2021). Rg3 promotes the SUMOylation of SERCA2a and corrects cardiac dysfunction in heart failure. Pharmacol. Res. 172, 105843. doi:10.1016/j.phrs.2021.105843
Livi, L., Barletta, G., Martella, F., Saieva, C., Desideri, I., Bacci, C., et al. (2021). Cardioprotective strategy for patients with nonmetastatic breast cancer who are receiving an anthracycline-based chemotherapy: a randomized clinical trial. JAMA Oncol. 7 (10), 1544–1549. doi:10.1001/jamaoncol.2021.3395
Lupisella, J. A., Shirude, P. S., Wurtz, N. R., and Garcia, R. A. (2022). Formyl peptide receptor 2 and heart disease. Semin. Immunol. 59, 101602. doi:10.1016/j.smim.2022.101602
Mackman, N., Bergmeier, W., Stouffer, G. A., and Weitz, J. I. (2020). Therapeutic strategies for thrombosis: new targets and approaches. Nat. Rev. Drug Discov. 19 (5), 333–352. doi:10.1038/s41573-020-0061-0
Manolis, A. A., Manolis, T. A., and Manolis, A. S. (2023). Neurohumoral activation in heart failure. Int. J. Mol. Sci. 24 (20), 15472. doi:10.3390/ijms242015472
Marciniak, T. A., and Serebruany, V. (2019). Ranolazine, ACE inhibitors, and angiotensin receptor blockers. Am. J. Med. 132 (12), e844–e845. doi:10.1016/j.amjmed.2019.02.032
McCarthy, C. P., Vaduganathan, M., McCarthy, K. J., Januzzi, J. L., Bhatt, D. L., and McEvoy, J. W. (2018). Left ventricular thrombus after acute myocardial infarction: screening, prevention, and treatment. JAMA Cardiol. 3 (7), 642–649. doi:10.1001/jamacardio.2018.1086
Mendler, L., Braun, T., and Muller, S. (2016). The ubiquitin-like SUMO system and heart function: from development to disease. Circ. Res. 118 (1), 132–144. doi:10.1161/CIRCRESAHA.115.307730
Menke, A. (2024). The HPA axis as target for depression. Curr. Neuropharmacol. 22 (5), 904–915. doi:10.2174/1570159x21666230811141557
Misiak, B., Wójta-Kempa, M., Samochowiec, J., Schiweck, C., Aichholzer, M., Reif, A., et al. (2022). Peripheral blood inflammatory markers in patients with attention deficit/hyperactivity disorder (ADHD): a systematic review and meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 118, 110581. doi:10.1016/j.pnpbp.2022.110581
Motta, J. R., Jung, I., Azzolin, V. F., Teixeira, C. F., Braun, L. E., De Oliveira Nerys, D. A., et al. (2021). Avocado oil (Persea americana) protects SH-SY5Y cells against cytotoxicity triggered by cortisol by the modulation of BDNF, oxidative stress, and apoptosis molecules. J. Food Biochem. 45 (2), e13596. doi:10.1111/jfbc.13596
Moya-Alvarado, G., Guerra, M. V., Tiburcio, R., Bravo, E., and Bronfman, F. C. (2022). The Rab11-regulated endocytic pathway and BDNF/TrkB signaling: roles in plasticity changes and neurodegenerative diseases. Neurobiol. Dis. 171, 105796. doi:10.1016/j.nbd.2022.105796
Nabavi, S. M., Daglia, M., Braidy, N., and Nabavi, S. F. (2017). Natural products, micronutrients, and nutraceuticals for the treatment of depression: a short review. Nutr. Neurosci. 20 (3), 180–194. doi:10.1080/1028415X.2015.1103461
Nakhjavani, M., Smith, E., Townsend, A. R., Price, T. J., and Hardingham, J. E. (2020). Anti-angiogenic properties of ginsenoside Rg3. Molecules 25 (21), 4905. doi:10.3390/molecules25214905
Nam, Y., Shin, E. J., Shin, S. W., Lim, Y. K., Jung, J. H., Lee, J. H., et al. (2014). YY162 prevents ADHD-like behavioral side effects and cytotoxicity induced by Aroclor1254 via interactive signaling between antioxidant potential, BDNF/TrkB, DAT and NET. Food Chem. Toxicol. 65, 280–292. doi:10.1016/j.fct.2013.12.046
Ni, J., Liu, Z., Jiang, M., Li, L., Deng, J., Wang, X., et al. (2022a). Ginsenoside Rg3 ameliorates myocardial glucose metabolism and insulin resistance via activating the AMPK signaling pathway. J. Ginseng Res. 46 (2), 235–247. doi:10.1016/j.jgr.2021.06.001
Ni, J., Zhang, H., Wang, X., Liu, Z., Nie, T., Li, L., et al. (2022b). Rg3 regulates myocardial pyruvate metabolism via P300-mediated dihydrolipoamide dehydrogenase 2-hydroxyisobutyrylation in TAC-induced cardiac hypertrophy. Cell Death Dis. 13 (12), 1073. doi:10.1038/s41419-022-05516-y
Opinion, A. G. R., Vanhomwegen, M., De Boeck, G., and Aerts, J. (2023). Long-term stress induced cortisol downregulation, growth reduction and cardiac remodeling in Atlantic salmon. J. Exp. Biol. 226 (22), jeb246504. doi:10.1242/jeb.246504
Pahlavani, H. A. (2023). Exercise therapy to prevent and treat Alzheimer's disease. Front. Aging Neurosci. 15, 1243869. doi:10.3389/fnagi.2023.1243869
Parrott, J. M., Redus, L., Santana-Coelho, D., Morales, J., Gao, X., and O'Connor, J. C. (2016). Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl. Psychiatry 6 (10), e918. doi:10.1038/tp.2016.200
Penninx, B. W., Pine, D. S., Holmes, E. A., and Reif, A. (2021). Anxiety disorders. Lancet 397 (10277), 914–927. doi:10.1016/s0140-6736(21)00359-7
Piepenburg, S. M., Faller, H., Stork, S., Ertl, G., and Angermann, C. E. (2019). Symptom patterns and clinical outcomes in women versus men with systolic heart failure and depression. Clin. Res. Cardiol. 108 (3), 244–253. doi:10.1007/s00392-018-1348-6
Pillinger, T., Osimo, E. F., de Marvao, A., Shah, M., Francis, C., Huang, J., et al. (2023). Effect of polygenic risk for schizophrenia on cardiac structure and function: a UK biobank observational study. Lancet Psychiatry 10 (2), 98–107. doi:10.1016/S2215-0366(22)00403-5
Pound, K. M., Sorokina, N., Ballal, K., Berkich, D. A., Fasano, M., Lanoue, K. F., et al. (2009). Substrate-enzyme competition attenuates upregulated anaplerotic flux through malic enzyme in hypertrophied rat heart and restores triacylglyceride content: attenuating upregulated anaplerosis in hypertrophy. Circ. Res. 104 (6), 805–812. doi:10.1161/circresaha.108.189951
Qu, P. R., Jiang, Z. L., Song, P. P., Liu, L. C., Xiang, M., and Wang, J. (2022). Saponins and their derivatives: potential candidates to alleviate anthracycline-induced cardiotoxicity and multidrug resistance. Pharmacol. Res. 182, 106352. doi:10.1016/j.phrs.2022.106352
Rawat, P. S., Jaiswal, A., Khurana, A., Bhatti, J. S., and Navik, U. (2021). Doxorubicin-induced cardiotoxicity: an update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 139, 111708. doi:10.1016/j.biopha.2021.111708
Reed, G. W., Rossi, J. E., and Cannon, C. P. (2017). Acute myocardial infarction. Lancet 389 (10065), 197–210. doi:10.1016/s0140-6736(16)30677-8
Rossello, X., Menon, V., and Vranckx, P. (2022). Acetazolamide for patients with acute decompensated heart failure with volume overload. Eur. Heart J. Acute Cardiovasc. Care 11 (9), 712–713. doi:10.1093/ehjacc/zuac108
Sabari, B. R., Zhang, D., Allis, C. D., and Zhao, Y. (2017). Metabolic regulation of gene expression through histone acylations. Nat. Rev. Mol. Cell Biol. 18 (2), 90–101. doi:10.1038/nrm.2016.140
Sabatine, M. S., and Braunwald, E. (2021). Thrombolysis in myocardial infarction (TIMI) study group: JACC focus seminar 2/8. J. Am. Coll. Cardiol. 77 (22), 2822–2845. doi:10.1016/j.jacc.2021.01.060
Sachs, B. D., Ni, J. R., and Caron, M. G. (2015). Brain 5-HT deficiency increases stress vulnerability and impairs antidepressant responses following psychosocial stress. Proc. Natl. Acad. Sci. U. S. A. 112 (8), 2557–2562. doi:10.1073/pnas.1416866112
Sawicki, K. T., Sala, V., Prever, L., Hirsch, E., Ardehali, H., and Ghigo, A. (2021). Preventing and treating anthracycline cardiotoxicity: new insights. Annu. Rev. Pharmacol. Toxicol. 61, 309–332. doi:10.1146/annurev-pharmtox-030620-104842
Schmidt, K. T., Makhijani, V. H., Boyt, K. M., Cogan, E. S., Pati, D., Pina, M. M., et al. (2019). Stress-induced alterations of norepinephrine release in the bed nucleus of the stria terminalis of mice. ACS Chem. Neurosci. 10 (4), 1908–1914. doi:10.1021/acschemneuro.8b00265
Shen, L., Chen, C., Wei, X., Li, X., Luo, G., Zhang, J., et al. (2015). Overexpression of ankyrin repeat domain 1 enhances cardiomyocyte apoptosis by promoting p53 activation and mitochondrial dysfunction in rodents. Clin. Sci. (Lond) 128 (10), 665–678. doi:10.1042/CS20140586
Siddiqi, M. Z., Srinivasan, S., Park, H. Y., and Im, W. T. (2020). Exploration and characterization of novel glycoside hydrolases from the whole genome of lactobacillus ginsenosidimutans and enriched production of minor ginsenoside Rg3(S) by a recombinant enzymatic process. Biomolecules 10 (2), 288. doi:10.3390/biom10020288
Siegel, R. K. (1979). Ginseng abuse syndrome. problems with the panacea. Jama 241 (15), 1614–1615. doi:10.1001/jama.241.15.1614
Staretz-Chacham, O., Pode-Shakked, B., Kristal, E., Abraham, S. Y., Porper, K., Wormser, O., et al. (2021). The effects of a ketogenic diet on patients with dihydrolipoamide dehydrogenase deficiency. Nutrients 13 (10), 3523. doi:10.3390/nu13103523
Sun, G. Z., Meng, F. J., Cai, H. Q., Diao, X. B., Zhang, B., and Bai, X. P. (2020). Ginsenoside Rg3 protects heart against isoproterenol-induced myocardial infarction by activating AMPK mediated autophagy. Cardiovasc. Diagn. Ther. 10 (2), 153–160. doi:10.21037/cdt.2020.01.02
Sur, B., and Lee, B. (2022a). Ginsenoside Rg3 modulates spatial memory and fear memory extinction by the HPA axis and BDNF-TrkB pathway in a rat post-traumatic stress disorder. J. Nat. Med. 76 (4), 821–831. doi:10.1007/s11418-022-01636-z
Sur, B., and Lee, B. (2022b). Myricetin inhibited fear and anxiety-like behaviors by HPA axis regulation and activation of the BDNF-ERK signaling pathway in posttraumatic stress disorder rats. Evid. Based Complement. Altern. Med. 2022, 8320256. doi:10.1155/2022/8320256
Szatko, A., Glinicki, P., and Gietka-Czernel, M. (2023). Pheochromocytoma/paraganglioma-associated cardiomyopathy. Front. Endocrinol. (Lausanne) 14, 1204851. doi:10.3389/fendo.2023.1204851
Tai, P., Chen, X., Jia, G., Chen, G., Gong, L., Cheng, Y., et al. (2023). WGX50 mitigates doxorubicin-induced cardiotoxicity through inhibition of mitochondrial ROS and ferroptosis. J. Transl. Med. 21 (1), 823. doi:10.1186/s12967-023-04715-1
Tang, B., Yuan, S., Xiong, Y., He, Q., and Larsson, S. C. (2020). Major depressive disorder and cardiometabolic diseases: a bidirectional mendelian randomisation study. Diabetologia 63 (7), 1305–1311. doi:10.1007/s00125-020-05131-6
Teng, T., Shively, C. A., Li, X., Jiang, X., Neigh, G. N., Yin, B., et al. (2021). Chronic unpredictable mild stress produces depressive-like behavior, hypercortisolemia, and metabolic dysfunction in adolescent cynomolgus monkeys. Transl. Psychiatry 11 (1), 9. doi:10.1038/s41398-020-01132-6
Truby, L. K., and Rogers, J. G. (2020). Advanced heart failure: epidemiology, diagnosis, and therapeutic approaches. JACC Heart. Fail. 8 (7), 523–536. doi:10.1016/j.jchf.2020.01.014
Tsai, C. H., Pan, C. T., Chang, Y. Y., Peng, S. Y., Lee, P. C., Liao, C. W., et al. (2021). Aldosterone excess induced mitochondria decrease and dysfunction via mineralocorticoid receptor and oxidative stress in vitro and in vivo. Biomedicines 9 (8), 946. doi:10.3390/biomedicines9080946
Tsai, C. K., Chen, B. H., Chen, H. H., Hsieh, R. J., Lee, J. C., Chu, Y. T., et al. (2022). Low-dose propranolol prevents functional decline in catecholamine-induced acute heart failure in rats. Toxics 10 (5), 238. doi:10.3390/toxics10050238
Tsioufis, P., Theofilis, P., Tsioufis, K., and Tousoulis, D. (2022). The impact of cytokines in coronary atherosclerotic plaque: current therapeutic approaches. Int. J. Mol. Sci. 23 (24), 15937. doi:10.3390/ijms232415937
Tu, C., Wan, B., and Zeng, Y. (2020). Ginsenoside Rg3 alleviates inflammation in a rat model of myocardial infarction via the SIRT1/NF-κB pathway. Exp. Ther. Med. 20 (6), 238. doi:10.3892/etm.2020.9368
Ugwu, P. I., Ben-Azu, B., Ugwu, S. U., Uruaka, C. I., Nworgu, C. C., Okorie, P. O., et al. (2022). Preventive putative mechanisms involved in the psychopathologies of mice passively coping with psychosocial defeat stress by quercetin. Brain Res. Bull. 183, 127–141. doi:10.1016/j.brainresbull.2022.03.004
Upadhyay, S., Mantha, A. K., and Dhiman, M. (2020). Glycyrrhiza glabra (Licorice) root extract attenuates doxorubicin-induced cardiotoxicity via alleviating oxidative stress and stabilising the cardiac health in H9c2 cardiomyocytes. J. Ethnopharmacol. 258, 112690. doi:10.1016/j.jep.2020.112690
Vaccarino, V., Sullivan, S., Hammadah, M., Wilmot, K., Al Mheid, I., Ramadan, R., et al. (2018). Mental stress-induced-myocardial ischemia in young patients with recent myocardial infarction: sex differences and mechanisms. Circulation 137 (8), 794–805. doi:10.1161/CIRCULATIONAHA.117.030849
Walker, E. R., McGee, R. E., and Druss, B. G. (2015). Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry 72 (4), 334–341. doi:10.1001/jamapsychiatry.2014.2502
Wang, L., Lu, Z., Teng, Y., Pan, W., Li, Y., Su, S., et al. (2024). Cognitive impairment is associated with BDNF-TrkB signaling mediating synaptic damage and reduction of amino acid neurotransmitters in heart failure. Faseb J. 38 (1), e23351. doi:10.1096/fj.202301699RR
Wang, X., Chen, L., Wang, T., Jiang, X., Zhang, H., Li, P., et al. (2015a). Ginsenoside Rg3 antagonizes adriamycin-induced cardiotoxicity by improving endothelial dysfunction from oxidative stress via upregulating the Nrf2-ARE pathway through the activation of akt. Phytomedicine 22 (10), 875–884. doi:10.1016/j.phymed.2015.06.010
Wang, X., Ling, G., Wei, Y., Li, W., Zhang, Y., Tan, N., et al. (2023). Activation of ULK1 to trigger FUNDC1-mediated mitophagy in heart failure: effect of ginsenoside Rg3 intervention. Phytomedicine 120, 155042. doi:10.1016/j.phymed.2023.155042
Wang, X., Meng, H., Wang, Q., Shao, M., Lu, W., Chen, X., et al. (2020). Baoyuan decoction ameliorates apoptosis via AT1-CARP signaling pathway in H9C2 cells and heart failure post-acute myocardial infarction rats. J. Ethnopharmacol. 252, 112536. doi:10.1016/j.jep.2019.112536
Wang, X. D., and Kang, S. (2021). Ferroptosis in myocardial infarction: not a marker but a maker. Open Biol. 11 (4), 200367. doi:10.1098/rsob.200367
Wang, Y., Hu, Z., Sun, B., Xu, J., Jiang, J., and Luo, M. (2015b). Ginsenoside Rg3 attenuates myocardial ischemia/reperfusion injury via Akt/endothelial nitric oxide synthase signaling and the B-cell lymphoma/B-cell lymphoma-associated X protein pathway. Mol. Med. Rep. 11 (6), 4518–4524. doi:10.3892/mmr.2015.3336
Wu, J., Hall, M., Dondo, T. B., Wilkinson, C., Ludman, P., DeBelder, M., et al. (2019). Association between time of hospitalization with acute myocardial infarction and in-hospital mortality. Eur. Heart. J. 40 (15), 1214–1221. doi:10.1093/eurheartj/ehy835
Xie, C., Zhan, Y., Wu, Y., Zhang, Z., Xiang, Y., Wang, L., et al. (2023). Expression and clinical significance of serum sST2, BDNF, CTnI, and BUN/Cr in patients with heart failure. Altern. Ther. Health Med. 29 (1), 176–181.
Xiong, J., Yuan, H., Fei, S., Yang, S., You, M., and Liu, L. (2023). The preventive role of the red gingeng ginsenoside Rg3 in the treatment of lung tumorigenesis induced by benzo(a)pyrene. Sci. Rep. 13 (1), 4528. doi:10.1038/s41598-023-31710-9
Xu, H., Miao, H., Chen, G., Zhang, G., Hua, Y., Wu, Y., et al. (2023). 20(S)-ginsenoside Rg3 exerts anti-fibrotic effect after myocardial infarction by alleviation of fibroblasts proliferation and collagen deposition through TGFBR1 signaling pathways. J. Ginseng Res. 47 (6), 743–754. doi:10.1016/j.jgr.2023.06.007
Xu, Y., Peng, W., Han, D., Wang, Z., Gu, C., Feng, F., et al. (2020). Combined treatment of non-small-cell lung cancer using shenyi capsule and platinum-based chemotherapy: a meta-analysis and systematic review. Evid. Based Complement. Altern. Med. 2020, 3957193. doi:10.1155/2020/3957193
Yan, H., Jin, H., Fu, Y., Yin, Z., and Yin, C. (2019). Production of rare ginsenosides Rg3 and Rh2 by endophytic bacteria from Panax ginseng. J. Agric. Food Chem. 67 (31), 8493–8499. doi:10.1021/acs.jafc.9b03159
You, Z., Yao, Q., Shen, J., Gu, Z., Xu, H., Wu, Z., et al. (2017). Antidepressant-like effects of ginsenoside Rg3 in mice via activation of the hippocampal BDNF signaling cascade. J. Nat. Med. 71 (2), 367–379. doi:10.1007/s11418-016-1066-1
Zarneshan, S. N., Fakhri, S., and Khan, H. (2022). Targeting Akt/CREB/BDNF signaling pathway by ginsenosides in neurodegenerative diseases: a mechanistic approach. Pharmacol. Res. 177, 106099. doi:10.1016/j.phrs.2022.106099
Zhang, C., Yu, H., Ye, J., Tong, H., Wang, M., and Sun, G. (2023). Ginsenoside Rg3 protects against diabetic cardiomyopathy and promotes adiponectin signaling via activation of PPAR-γ. Int. J. Mol. Sci. 24 (23), 16736. doi:10.3390/ijms242316736
Zhang, H., Zhou, Z., Chen, Z., Zhong, Z., and Li, Z. (2017). Ginsenoside Rg3 exerts anti-depressive effect on an NMDA-treated cell model and a chronic mild stress animal model. J. Pharmacol. Sci. 134 (1), 45–54. doi:10.1016/j.jphs.2017.03.007
Zhang, J. C., Wu, J., Fujita, Y., Yao, W., Ren, Q., Yang, C., et al. (2014). Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int. J. Neuropsychopharmacol. 18 (4), pyu077. doi:10.1093/ijnp/pyu077
Zhang, L. P., Jiang, Y. C., Yu, X. F., Xu, H. L., Li, M., Zhao, X. Z., et al. (2016). Ginsenoside Rg3 improves cardiac function after myocardial ischemia/reperfusion via attenuating apoptosis and inflammation. Evid. Based Complement. Altern. Med. 2016, 6967853. doi:10.1155/2016/6967853
Zhang, Z., Dalan, R., Hu, Z., Wang, J. W., Chew, N. W., Poh, K. K., et al. (2022). Reactive oxygen species scavenging nanomedicine for the treatment of ischemic heart disease. Adv. Mat. 34 (35), e2202169. doi:10.1002/adma.202202169
Zhou, X., and Xia, X. (2023). Ginsenoside Rg3 improves microcystin-induced cardiotoxicity through the miR-128-3p/MDM4 axis. Drug Chem. Toxicol. 2023, 1–11. doi:10.1080/01480545.2023.2251716
Glossary
Keywords: ginsenoside Rg3, heart diseases, mental disorders, heart failure, depression
Citation: Shi L, Luo J, Wei X, Xu X and Tu L (2024) The protective role of ginsenoside Rg3 in heart diseases and mental disorders. Front. Pharmacol. 15:1327033. doi: 10.3389/fphar.2024.1327033
Received: 24 October 2023; Accepted: 07 February 2024;
Published: 26 February 2024.
Edited by:
Yusof Kamisah, Faculty of Medicine Universiti Kebangaan Malaysia, MalaysiaReviewed by:
Kang Liu, China Pharmaceutical University, ChinaBenneth Ben-Azu, Delta State University, Nigeria
Copyright © 2024 Shi, Luo, Wei, Xu and Tu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Tu, MjgzNjcxMjQ2MUBxcS5jb20=
 Lili Shi
Lili Shi Jinlan Luo1,2
Jinlan Luo1,2 Xiupan Wei
Xiupan Wei