
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 27 March 2024
Sec. Obstetric and Pediatric Pharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1325381
This article is part of the Research Topic Women in Obstetric and Pediatric Pharmacology: 2023 View all 6 articles
 Giovanna Esposito1*†
Giovanna Esposito1*† Anna Cantarutti2,3†
Anna Cantarutti2,3† Angela Lupattelli4
Angela Lupattelli4 Matteo Franchi2,3
Matteo Franchi2,3 Giovanni Corrao2,3
Giovanni Corrao2,3 Fabio Parazzini1
Fabio Parazzini1Background: Preterm birth may affect maternal mental health. We explored the relationship between preterm birth and the risk of initiating antidepressant use during the year after birth.
Methods: We conducted a population-based investigation using regional healthcare utilization databases. The exposure considered was preterm birth. The outcome was having at least one prescription for antidepressant medications during the year after birth. We used a log-binomial regression model including terms for maternal age at birth, nationality, educational level, parity, modality of conception, modality of delivery, use of other psychotropic drugs, and diabetes to estimate relative risk (RR) and 95% confidence intervals (CI) for the association between preterm birth and the initiation of antidepressant use. In addition, the absolute risk differences (ARD) were also computed according to the timing of birth.
Results: The cohort included 727,701 deliveries between 2010 and 2020 in Lombardy, Northern Italy. Out of these, 6,522 (0.9%) women had at least one prescription for antidepressant drugs during the year after birth. Preterm births were related to a 38% increased risk of initiation of antidepressant use during the year after birth (adjusted RR = 1.38; 95% CI: 1.25–1.52) for moderate to late preterm and to 83% (adjusted RR = 1.83; 95% CI: 1.46–2.28) for extremely and very preterm. Excluding women with only one antidepressant prescription, the association was consistent (adjusted RR = 1.41, 95%CI: 1.23–1.61 for moderate to late preterm and adjusted RR = 1.81, 95% CI: 1.31–2.49 for extremely and very preterm). Also, excluding women who used other psychotropics, the association remained consistent (adjusted RR = 1.39, 95%CI: 1.26–1.54 and adjusted RR = 1.91, 95% CI: 1.53–2.38, respectively for moderate to late and extremely and very preterm).
Conclusion: Women who delivered preterm may have an excess risk of initiation of antidepressant consumption during the first year after birth.
Postpartum mental disorders, most commonly unipolar depression and anxiety, are important clinical and public health concerns. They can negatively affect women’s somatic and psychiatric health and ability to work; at worst, these disorders are associated with an increased risk of suicide (Chin et al., 2022). In addition, infants of mothers with postpartum depression have a greater risk for adverse developmental outcomes and poorer mother-child bonding (Parsons et al., 2012; Hoffman et al., 2017).
Mothers can experience mental and emotional disorders of various nature and severity in the postpartum period. The emotional and hormonal changes that accompany childbirth can affect mothers’ mood. More than one in three women experience the so-called “maternity blues”, and more rarely, severe and prolonged depression (Rezaie-Keikhaie et al., 2020; Tosto et al., 2023). The prevalence of postpartum depression is around 15%–20%, with some variability across populations (Zhao and Zhang, 2020; Liu et al., 2022). The depressive state, typically within the first year since childbirth (Hacking, 2013), comprises a wide range of symptoms such as difficulty bonding with the child, fear of not being a good mother, feelings of worthlessness, shame, guilt or inadequacy, and thoughts of harming oneself or the infant. Postpartum depression often co-exists with anxiety disorders, adding substantial burden to women’s mental health (Falah-Hassani et al., 2017). Postpartum psychosis, a life-threatening psychiatric emergency including acute mania or depression with psychosis, is infrequent and affects 1–2 women per 1,000 (Perry et al., 2021).
Antidepressant pharmacotherapy is indicated in situations characterized by moderate to severe severity or inadequate response to initial psychotherapy (Hendrick, 2006). Selective serotonin reuptake inhibitors are the mainstay of pharmacological treatment for perinatal depression, with an international prescribing prevalence of 4.7% in the first year postpartum (Molenaar et al., 2020). The management of antidepressant treatment in the postpartum period, particularly during the breastfeeding period, is a complex issue. The potential adverse effects of poorly managed peripartum maternal depression on both maternal and infant health must be balanced against the potential risks of drug exposure to the infant through breast milk. However, there is evidence that most antidepressants are not contraindicated during breastfeeding (Gentile, 2005; Kendall-Tackett and Hale, 2010; Stewart and Vigod, 2019). Even to date, many European countries lack consistent and up-to-date clinical practice guidelines for the pharmacological treatment of peripartum depression (Kittel-Schneider et al., 2022).
Environmental and socioeconomic factors, as well as psychiatric and obstetric conditions such as depression during pregnancy, history of depression, caesarean section, and gestational diabetes, play a crucial role in the incidence of postpartum depression (Ghaedrahmati et al., 2017; Zhao and Zhang, 2020; Liu et al., 2022). The role of preterm birth, the leading cause of neonatal deaths worldwide, has been previously explored, providing some evidence for an increased risk for postpartum depression, but results remain inconsistent (Vigod et al., 2010). Given the relevant impact of mental illness on mothers, infants, and families and the implications of preterm birth, it is of utmost importance to further investigate the association between the two conditions.
We conducted a population-based cohort study using the regional healthcare utilization databases in the Lombardy region of Italy to evaluate the potential association between preterm birth and maternal initiation of antidepressant use during the postpartum year, taking into account potential confounders, such as socio-demographic features and selected maternal clinical factors.
Data for the study were obtained from the regional health databases of Lombardy, which collect a wide range of information on services provided to beneficiaries of the National Health Service (NHS), a tax-funded universal healthcare system that provides free or subsidized health services to all Italian citizens and residents, ensuring equal access for the entire population. The databases include the registry of diagnosis at discharge from public or private hospitals, the outpatient drug prescriptions reimbursed by the NHS registry, and the outpatient services registry. In addition, a specific form for the birth event, the certificate of delivery assistance (CedAP), reports information about maternal characteristics, pregnancy, and delivery. We linked the above databases using the unique identification code, which identified each selected unit across all databases, through a deterministic record linkage. For privacy issues, each identification code is automatically anonymized. This record linkage process offers the opportunity to design investigations including very large, unselected birth cohort populations and to generate real-world evidence on several fields of public health, including the birth event.
Deliveries that occurred in the region from 1st January 2010 to 1st January 2020 were identified. We excluded (1) deliveries that did not match a hospitalization related to childbirth, (2) deliveries of women that the NHS did not cover in the year before and after the birth, (3) deliveries of women aged less than 15 or more than 55, (4) deliveries before the 22nd or after the 42nd week of gestation, (5) stillbirths, (6) multiple deliveries, (7) deliveries of children with congenital malformations, and (8) deliveries of women who were not free of depression or anxiety in the year before the conception or during pregnancy (at least one of following events, i.e., having an inpatient and/or outpatient diagnosis of depression and/or anxiety and/or at least one prescription with antidepressant medications) (Supplementary Table S1). A one-year wash-out period before conception seems a reasonable choice. Given that depression usually requires long-term treatment or access to specialized care, it is reasonable to assume that a woman is free of depression if she has not accessed such care during this period. This timeframe is a compromise between the various criteria used to exclude women with current depression in randomized clinical trials of antidepressant treatment for postpartum depression, ranging from 6 months to 2 years (Brown et al., 2021).
The exposure considered was preterm birth, defined as any birth before 37 completed weeks of pregnancy (Lawn et al., 2010). This information was available in CedAP database. We categorized preterm birth as binary (i.e., yes if preterm before 37 complete weeks and no if at term) and on the basis of gestational weeks according to World Health Organization (i.e., extremely and very preterm before 32 weeks, moderate to late preterm between 32 and 36 weeks) (WHO, 2023). Extremely preterm (i.e., <28 gestational weeks) was not evaluated separately since they represent less than 5% of all births before 37 weeks.
Information on the outcome of interest was obtained from the outpatient drug prescription registry. The outcome was the initiation of antidepressant use during the year after birth (time of follow-up), defined as having filled at least a prescription for antidepressant medications (ATC code: N06A). We also considered the year after birth, splitting the time in the first 6 months (early) and 7–12 months (later). The reference population included all women who did not start taking antidepressants during the year after giving birth.
Information on covariates that were used for confounding adjustment was obtained from the CedAP registry and the outpatient drug prescriptions registry. We considered several baseline maternal characteristics that may affect initiation with antidepressants during the year postpartum and prematurity as sufficient set of confounders: demographic variables [i.e., maternal age at birth (<30, 30–34, 35–39, >39 years), nationality (i.e., Italian or not based on birthplace), educational level (i.e., university, high school, middle or primary school)], pregnancy covariates [i.e., parity (nulliparous or multiparous women), mode of conception (natural or medically assisted)], and clinical variables (i.e., diabetes, defined as at least one prescription of an antidiabetic drug in the year before and during pregnancy considering both gestational and pre-existing diabetes, and prescription of other psychotropics in the same period). Missing data (regarding educational level and modality of delivery) was random and minimal, at less than 0.3%. Therefore, births with missing data were excluded from analyses.
We computed the initiation of antidepressant use rate per 100 according to selected maternal characteristics (i.e., age, nationality, educational level, parity, and diabetes) and information related to birth (i.e., mode of conception, mode and timing of delivery). Chi-squared test and the test for trend were used to compare initiators of antidepressant use and no-initiators among the selected covariates, appropriately.
The relative risk (RR) estimates of antidepressant initiation with 95% confidence interval (CI) were computed. The use of the robust variance estimator to account for correlations within women with multiple pregnancies did not change the CI considerably in the unadjusted analyses, so correlation structures were omitted from all analyses. Results are presented according to two levels of adjustment. The first unadjusted analysis examined the association between each covariate and the outcome. Further, we assessed the RR of antidepressant initiation according to gestational age at birth, considering all covariates as confounders. In addition, the absolute risk differences (ARD) with 95%CI were computed.
We conducted several sensitivity and subgroup analyses to evaluate the effect of outcome misclassification and effect modification by several covariates. (i) We redefined outcome as having filled at least 2 prescriptions of antidepressants; women who had only one antidepressant prescription in the postpartum year were excluded. (ii) We also considered the timing of initiation of antidepressant use, i.e., early initiation in the first 6 months after delivery and late initiation in the second 6 months after delivery. (iii) To ensure a more accurate assessment of women’s mental health before pregnancy, we only included women with at least 4 years of health coverage prior to conception. Consequently, we excluded women who had at least an inpatient or outpatient diagnosis of depression or anxiety and/or a prescription filled for antidepressant medications. Extending the wash-out period to 4 years allowed us to include a larger sample of women who were unlikely to be suffering from depression or anxiety at the time of conception. It should be noted that we could not exclude the possibility that a small number of these women should be considered as having depression/anxiety that was not in complete remission. According to the American Psychological Association (American Psychological Association, 2013), remission is defined as an extended period without significant symptoms (often 2 months). However, the line between remission and complete freedom from depression remains unclear, as there are no standardized timeframe thresholds to separate the two states (Furukawa et al., 2008; de Zwart et al., 2019). In addition, (iv) we performed a subgroup analysis among those deliveries that did not record any prescription of other psychotropics (ATC: N03A and N05A) in the year before conception and during pregnancy. Finally, (v) we carried out stratified analyses by maternal age, nationality, educational level, parity, and mode of conception.
We performed analysis using the Statistical Analysis System Software (version 9.4; SAS Institute, Cary, NC, USA).
During the study period 2010–2020, we identified 727,701 deliveries (see Supplementary Figure S1 for the definition of the cohort), of which 689,261 (94.7%) were at term and 38,440 (5.3%) preterm. During the year after birth, 526 women with preterm birth (1.37 per 100 births) and 5,996 (0.87 per 100 births) with term birth had at least one filled antidepressant prescription. In Supplementary Material (Supplementary Table S2; Supplementary Figure S2), we reported patterns of prescribed antidepressants and other psychotropics according to preterm birth status. The average number of antidepressant prescriptions during the year after pregnancy was around 2.6 for both women who gave birth prematurely and those who gave birth at term (p = 0.782); 279 of women who initiated antidepressant had more than one concomitant prescription for different antidepressants. Women who started taking antidepressants were more likely to use other psychotropic drugs than women who did not start taking antidepressants (about 10% versus 1%).
Table 1 provides the rate per 100 and the crude RR of antidepressant initiation according to selected covariates. The initiation rate per 100 deliveries increased with the increase of age, ranging from 0.78 for women aged less than 30 to 1.18 for women aged more than 39 (p-trend <0.001), and the timing of birth. In the latter case, it ranged from 0.87 in term deliveries to 1.83 in very preterm ones (p-trend<0.001). Conversely, the rate was lower among not Italian women (p < 0.001) and in multiparous ones (p < 0.001). Accordingly, the risk was increased for increasing age (RR = 1.52, 95% CI: 1.39–1.66 for women aged 39 years or more versus women aged less than 30 years), lower educational level (RR = 1.20, 95% CI: 1.13–1.28 for women who attended middle/primary school versus women who attended university), medically assisted conception (RR = 1.29, 95% CI: 1.14–1.48), cesarean section (RR = 1.39, 95% CI: 1.32–1.47), and preterm birth (RR = 1.50, 95% CI: 1.37–1.66 for births between 32 and 36 weeks and RR = 2.10, 95% CI: 1.69–2.62 for deliveries before the 32 complete weeks). The risk was decreased in not Italian women (RR = 0.65, 95% CI: 0.61–0.69). No difference emerged for parity (RR = 0.97, 95% CI: 0.92–1.02) and diabetes (RR = 1.17, 95% CI: 0.97–1.40).
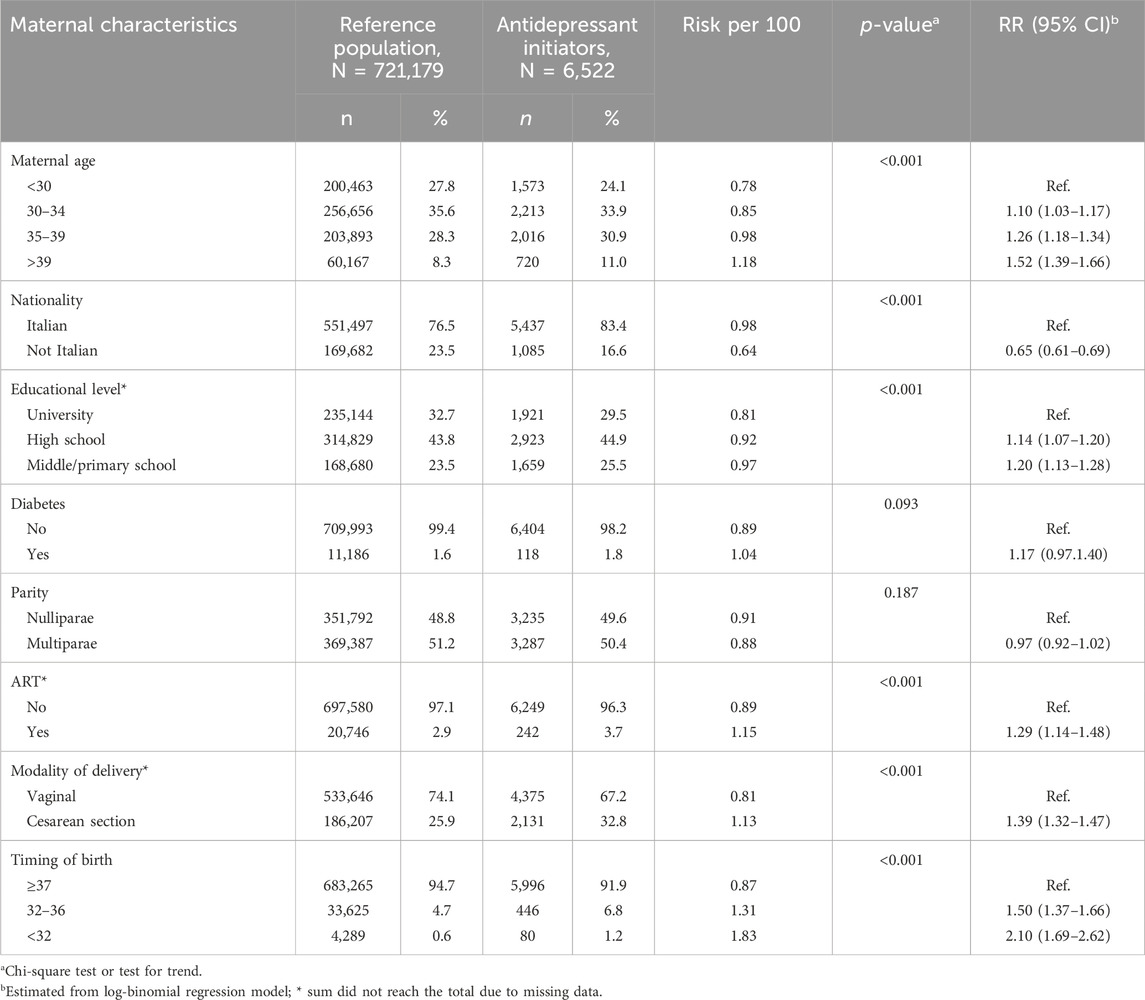
Table 1. Selected maternal characteristics according to initiation of antidepressant use in the year after birth. Lombardy, Italy, 2010–2020.
Table 2 shows the RR of antidepressant initiation according to the timing of birth, adjusted for selected confounders. Having moderate to late preterm birth was associated with a 38% increased risk of maternal antidepressant initiation during the year after birth (adjusted RR = 1.38; 95% CI: 1.25–1.52), relative to term birth. For extremely and very preterm births, there was a 83% increased risk (adjusted RR = 1.83; 1.46–2.28). In absolute terms, the ARD was 0.34% (95% CI: 0.22%–0.46%) for moderate to late preterm births and 0.85% (95%CI: 0.45%–1.24%).
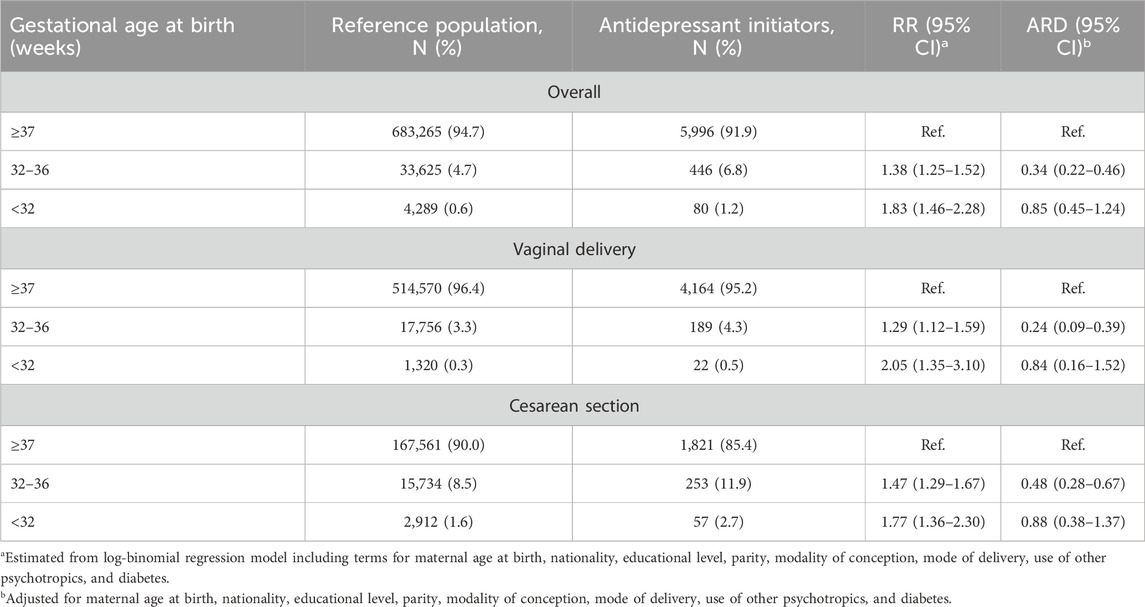
Table 2. Relative risk (RR) and absolute risk difference (ARD) and corresponding 95% confidence interval (CI) of initiation of antidepressant use in the year after the birth according to gestational age at birth. Lombardy, Italy, 2010–2020.
The results were confirmed in the sensitivity analysis, where we changed the outcome definition to requiring women to have filled at least 2 prescriptions within the year after childbirth (adjusted RR = 1.41, 95% CI: 1.23–1.61 in moderate to late preterm birth and adjusted RR = 1.81, 95% CI: 1.31–2.49 in extremely and very preterm births). In addition, when we divided the postpartum year into early and late, we observed a slightly weaker association with antidepressant initiation in later postpartum. For early antidepressant initiation, the adjusted RR was 1.43 (95% CI: 1.25–1.64) for moderate to late preterm births and 1.92 (95% CI: 1.42–2.61) for extreme and very preterm births. For later antidepressant initiation, the adjusted RR was 1.34 (95% CI: 1.16–1.54) and 1.75 (95% CI: 1.26–2.43) for moderate to late and for extremely and very preterm births, respectively.
When we excluded women without health coverage within the 4 years before the conceptions (N = 80,801) and those with at least (i) an inpatient and/or outpatient diagnosis of depression and/or anxiety, and/or (ii) at least one prescription with antidepressant medications (N = 24,017), the association between preterm birth and initiation of antidepressant use was consistent (adjusted RR = 1.37, 95% CI: 1.22–1.53 in moderate to late preterm birth and adjusted RR = 1.94, 95% CI: 1.50–2.50 in extremely and very preterm births). Therefore, we also estimated that only 3.3% of the women we defined as free of depression could be considered as having depression/anxiety in remission because they had been treated for or diagnosed with it in the 3 years prior to the year before conception. However, the main results were confirmed in sensitivity analyses where we changed the depression-free definition to women without antidepressant prescriptions and/or inpatient/outpatient diagnosis of depression/anxiety during the 4 years before conception.
In addition, considering the subgroup of those deliveries that did not record any prescription of other psychotropics in the year before the conception and during pregnancy, the association remained consistent, the adjusted RR was 1.39 (95%CI: 1.26–1.54) for moderate to late preterm births and 1.91 (95% CI: 1.53–2.38) for extremely and very preterm births.
There was no evidence of effect modification by the considered covariates. Indeed, results were consistent in the subgroup analyses performed (Figure 1).
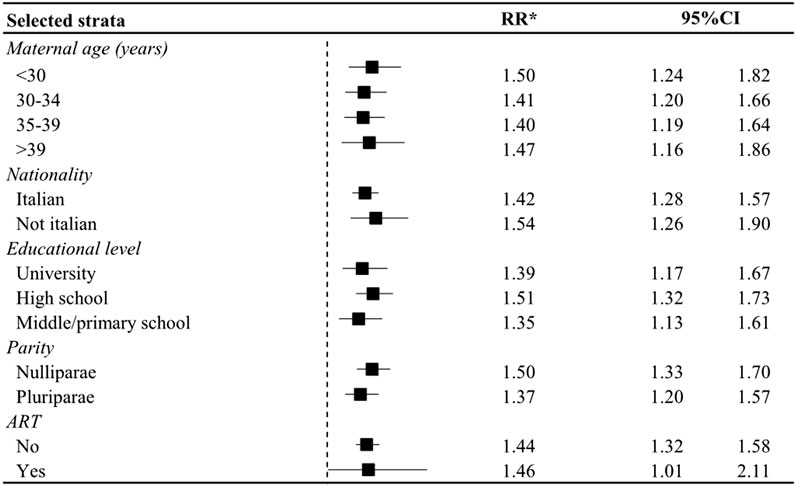
Figure 1. Relative Risk (RR) and corresponding 95% confidence interval (CI) of initiation of antidepressant use in the year after the birth in women who had a preterm birth in strata of selective covariates. Lombardy, Italy, 2010–2020. * Estimated from log-binomial regression model including terms for maternal age at birth, nationality, educational attainment, parity, modality of conception, mode of delivery, use of other psychotropics, and diabetes; unless the variable was the stratification factor.
Our study found that among mothers free of depression for at least a year before conception, the risk of initiating antidepressant treatment during the first year after childbirth was 0.9%; mothers who experienced a moderate to late preterm birth had an additional risk of 0.3%, and those who experienced an extremely and very preterm birth had an additional risk of 0.9%. These estimates remained consistent with a more conservative outcome definition, requiring at least two filled prescriptions for antidepressant in the postpartum year.
Antidepressants, such as selective serotonin reuptake inhibitors, are the mainstay of pharmacological treatment for perinatal depression; fluvoxamine, paroxetine, and sertraline are preferred in breastfeeding women, leading to the lowest serum medication levels in infants (Langan and Goodbred, 2016). According to a study conducted in the USA, about half of women diagnosed with postpartum depression are on antidepressant treatment (Vu and Shaya, 2017). It is worth noting that treatment approaches for postpartum depression vary from region to region. For example, European countries tend to have lower levels of antidepressant use after childbirth than the USA (Molenaar et al., 2020). This variation between geographical areas may be influenced by cultural norms, differences in healthcare systems, and the availability and accessibility of alternative interventions. However, postpartum depression is not treated solely with antidepressants; the American Psychiatric Association and the National Institute for Health and Care Excellence recommend evidence-based treatments, including cognitive behavioural therapy and interpersonal psychotherapy, and suggest that non-medication psychotherapy should be considered as a potential first-line treatment for women with mild to moderate depression who are pregnant or breastfeeding (Gelenberg et al., 2010; NICE, 2020). It is reasonable to assume that in countries where the use of antidepressants is low, other options are considered. Having a preterm birth represents a stressful experience that could lead to depressive symptoms. According to a systematic review (Vigod et al., 2010), mothers of preterm infants were at high risk of depression in the immediate postpartum period in comparison to mothers of at term infants. In addition, mothers of very preterm birth have higher levels of depressive symptoms throughout the first postpartum year, with a limited reduction in symptoms after hospital discharge. However, not all the studies included provided consistent evidence. In light of these findings, it is imperative to emphasize the importance of implementing targeted interventions and support for mothers of preterm infants in the immediate postpartum period. These interventions may include providing comprehensive psychological support, facilitating access to mental health services, offering educational resources on coping strategies, and establishing community-based support groups tailored to the specific needs of preterm mothers. In addition, healthcare providers should prioritize regular screening for postpartum depression in this vulnerable population to ensure timely identification and intervention.
One plausible explanation of the relationship between preterm birth and the initiation of antidepressant after birth is the enhanced stress with increasing prematurity; the neonatal comorbidity, the length of hospitalization, and the difficulties in the management of the newborn are inversely proportional to gestational age (Vigod et al., 2010). Moreover, as premature infants may need to spend time in intensive care units, mothers of these babies experienced a limited opportunity to bond immediately after birth, which can hinder adequate attachment between mother and infant and affect maternal mood (Vigod et al., 2010). A study from Wales including parents with an infant admitted to a neonatal intensive care unit reported a dose-response relationship between the level of prematurity and depressive symptoms; there were higher depression scores in mothers of infants born before the 33rd week than in mothers of infants born at 33–35 weeks’ gestation and mothers of term infants (Carter et al., 2005). Our findings aligned with this hypothesis; the risk of maternal mental illness was higher in the subset of births before the 32nd week compared to the subset of births between 32 and 36 weeks. However, evidence was not fully consistent, a large prospective study conducted in 15 countries around the world found no association between the duration of parent-infant closeness during the first weeks of hospitalization and parental depressive symptoms (Lehtonen et al., 2022).
The temperament of preterm babies is less regulated and shows less attention and more signs of activity than babies born at term (Montirosso et al., 2016; Cassiano et al., 2020). The relationship between perceived difficult temperament in infants by mothers and maternal-infant attachment has been investigated as the mediating role of maternal health (Tolja et al., 2020; Davies et al., 2021; Li, 2022). Poor infant engagement and orientation, typical of preterm babies, has been shown to negatively affect the way in which a mother feels about her infant (Beebe et al., 2012). In a survey study of over 250 mothers of healthy term newborns during the first postpartum month, an association of difficult infant temperament with maternal postpartum anxiety and depressive symptoms emerged early in the postpartum period, independently of their known contributors to postpartum mood (Britton, 2011). The cause-and-effect relationship between maternal perinatal mood disorders and difficult infant behaviour could be considered bidirectional. As infant temperamental difficulty predisposes to altered maternal mood, poor mental health has been reported to impact the mother’s capacity for, and quality of, bonding and interaction with her child (Binda et al., 2019). This aspect represents a priority for public health since attachment between mother and newborn at the beginning of life significantly impacts a child’s wellbeing in later years, his emotional, social, and cognitive development.
Evidence suggests that sleep disturbance during pregnancy may increase the risk of postpartum depression (Maghami et al., 2021; Li et al., 2023). As sleep problems tend to last longer in mothers of preterm babies than in mothers of term babies (Toda Miyano et al., 2022), sleep disturbance may be another reason for the association between maternal depression and preterm birth. A preliminary study to assess the feasibility of a prospective, comparative, longitudinal study of the sleep and psychosocial health of parents of preterm and term infants during the first year after birth found that parents in the preterm group had a lower median total sleep time than parents in the term group, and that mothers in the preterm group tended to sleep more during the day than mothers in the term group (Marthinsen et al., 2022). This study has several strengths. First, this was a very large population-based cohort study covering all regional residents and a span of over 10 years. Second, the availability of high-quality integrated individual data from healthcare utilization databases on outpatient and inpatient services provided by the NHS and the record linkage process offers the opportunity to trace and evaluate the complete care pathway of women included. Our study also has several potential limitations. Most importantly, confounding variables, such as lifestyle factors (e.g., smoking, alcohol use, obesity), are unknown in administrative databases. This could result in residual confounding. Moreover, redeeming a prescription does not necessarily imply that the woman took the medication; we have no information available in this study to further examine this possibility (Corrao and Mancia, 2015). On the other hand, using data from outpatient drug prescriptions reimbursed by the NHS registry, we underestimated the actual use of antidepressants and the prevalence of postpartum depression. One reason could be that some drugs were prescribed outside the NHS system. However, there is no reason to believe that this proportion differed between at term and preterm births. The study may potentially undercount depression also using just pharmaceutical prescriptions. A systematic review and meta-analysis including 29 cohort studies and 4 case-control studies from different countries of the world reported a prevalence of postpartum depression of 14% (ranging from 5% to 26%); the presence of disease was assessed using the Edinburgh Postpartum Depression Scale in all included studies (Liu et al., 2022). In addition, the use of antidepressants in the years after childbirth can be a proxy for postpartum depression but also for other mental illnesses such as anxiety or even bulimia nervosa. Importantly, we must consider that women may opt not to take antidepressants specifically because they are pregnant or trying to conceive. Understanding that untreated depression during pregnancy carries risks for preterm birth (Jahan et al., 2021), we conducted a sensitivity analysis including only women with no signs of depression or anxiety for at least 4 years before conception, and our findings remained consistent.
In conclusion, our study sheds light on the relationship between the experience of preterm birth and the subsequent development of new-onset mental illness in the postpartum period, focusing on a population of healthy women free of depression/anxiety condition at least 1 year before the conception. These findings highlight the urgent need for evidence-based approaches to improve care and not neglect mental health in high risk childbirth conditions. Given the association we observed between the experience of preterm birth and antidepressant initiation, it is clear that strategies to ensure a healthy and full-term pregnancy are not only an important step in promoting maternal and fetal wellbeing during pregnancy, but also play a crucial role in reducing the risk of postpartum maternal mental illness. Looking forward, we might consider how longitudinal analysis could be crucial in deepening our understanding of the long-term effects of preterm birth on maternal mental health. Examining the persistence and development of depressive symptoms over time could provide valuable insights into the dynamic nature of this relationship. In addition, a longitudinal approach could help to identify any risk or protective factors that influence maternal mental health in the long term after preterm birth, thereby informing more effective intervention and support strategies.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
This study followed the principles of the Declaration of Helsinki. Data used in this study were anonymized before their use. According to Italian law, studies based entirely on registry data are exempt from IRB authorization and informed consent.
GE: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Writing–original draft. AC: Data curation, Formal Analysis, Investigation, Methodology, Software, Writing–review and editing. AL: Writing–review and editing. MF: Writing–review and editing. GC: Supervision, Writing–review and editing. FP: Conceptualization, Supervision, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by a research grant from the Italian Ministry of Education, University and Research (“PRIN” 2017, project 2017728JPK). The authors also acknowledge support from the Department of Clinical Sciences and Community Health, University of Milan through the APC initiative. GC received research support from the European Community (EC), the Italian Agency of Drugs (AIFA), and the Italian Ministry for University and Research (MIUR).
GC took part in a variety of projects that were funded by pharmaceutical companies (i.e., Novartis, GSK, Roche, AMGEN and BMS). He also received honoraria as a member of the advisory board to Roche.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1325381/full#supplementary-material
American Psychological Association (2013). Diagnostic and statistical manual of mental disorders. 5th edn. Arlington, VA: American Psychiatric Publishing.
Beebe, B., Lachmann, F., Jaffe, J., Markese, S., Buck, K. A., Chen, H. N., et al. (2012). Maternal postpartum depressive symptoms and 4-month mother-infant interaction. Psychoanal. Psychol. 29 (4), 383–407. doi:10.1037/a0029387
Binda, V., Figueroa-Leigh, F., and Olhaberry, M. (2019). Antenatal and postnatal depressive symptoms: association with quality of mother-infant interaction. Infant Behav. Dev. 57, 101386. doi:10.1016/j.infbeh.2019.101386
Britton, J. R. (2011). Infant temperament and maternal anxiety and depressed mood in the early postpartum period. Women Health 51 (1), 55–71. doi:10.1080/03630242.2011.540741
Brown, J. V. E., Wilson, C. A., Ayre, K., Robertson, L., South, E., Molyneaux, E., et al. (2021). Antidepressant treatment for postnatal depression. Cochrane Database Syst. Rev. 2 (2), CD013560. doi:10.1002/14651858.CD013560.pub2
Carter, J. D., Mulder, R. T., Bartram, A. F., and Darlow, B. A. (2005). Infants in a neonatal intensive care unit: parental response. Arch. Dis. Child. Fetal Neonatal 90 (2), F109–F113. doi:10.1136/adc.2003.031641
Cassiano, R. G. M., Provenzi, L., Linhares, M. B. M., Gaspardo, C. M., and Montirosso, R. (2020). Does preterm birth affect child temperament? A meta-analytic study. Infant Behav. Dev. 58, 101417. doi:10.1016/j.infbeh.2019.101417
Chin, K., Wendt, A., Bennett, I. M., and Bhat, A. (2022). Suicide and maternal mortality. Curr. Psychiatry Rep. 24 (4), 239–275. doi:10.1007/s11920-022-01334-3
Corrao, G., and Mancia, G. (2015). Generating evidence from computerized healthcare utilization databases. Hypertension 65 (3), 490–498. doi:10.1161/HYPERTENSIONAHA.114.04858
Davies, S. M., Silverio, S. A., Christiansen, P., and Fallon, V. (2021). Maternal-infant bonding and perceptions of infant temperament: the mediating role of maternal mental health. J. Affect. Disord. 282, 1323–1329. doi:10.1016/j.jad.2021.01.023
de Zwart, P. L., Jeronimus, B. F., and de Jonge, P. (2019). Empirical evidence for definitions of episode, remission, recovery, relapse and recurrence in depression: a systematic review. Epidemiol. Psychiatr. Sci. 28 (5), 544–562. doi:10.1017/S2045796018000227
Falah-Hassani, K., Shiri, R., and Dennis, C. L. (2017). The prevalence of antenatal and postnatal co-morbid anxiety and depression: a meta-analysis. Psychol. Med. 47 (12), 2041–2053. doi:10.1017/S0033291717000617
Furukawa, T. A., Fujita, A., Harai, H., Yoshimura, R., Kitamura, T., and Takahashi, K. (2008). Definitions of recovery and outcomes of major depression: results from a 10-year follow-up. Acta Psychiatr. Scand. 117 (1), 35–40. doi:10.1111/j.1600-0447.2007.01119.x
Gelenberg, A. J., Freeman, M. P., Markowitz, J. C., Rosenbaum, J. F., Thase, M. E., Trivedi, M. H., et al. (2010). American Psychiatric Association practice guidelines for the treatment of patients with major depressive disorder. Am. J. Psychiatry 167 (Suppl. 10), 9–118.
Gentile, S. (2005). The safety of newer antidepressants in pregnancy and breastfeeding. Drug Saf. 28, 137–152. doi:10.2165/00002018-200528020-00005
Ghaedrahmati, M., Kazemi, A., Kheirabadi, G., Ebrahimi, A., and Bahrami, M. (2017). Postpartum depression risk factors: a narrative review. J. Educ. Health Promot 6, 60. doi:10.4103/jehp.jehp_9_16
Hacking, I. (2013). DSM-5: diagnostic and statistical manual of mental disorders. Fifth Edition. American Psychiatric Association Lond Review of Books.
Hendrick, V. (2006). Psychiatric disorders in pregnancy and the postpartum: principles and treatment. Totowa: Humana Press.
Hoffman, C., Dunn, D. M., and Njoroge, W. F. M. (2017). Impact of postpartum mental illness upon infant development. Curr. Psychiatry Rep. 19 (12), 100. doi:10.1007/s11920-017-0857-8
Jahan, N., Went, T. R., Sultan, W., Sapkota, A., Khurshid, H., Qureshi, I. A., et al. (2021). Untreated depression during pregnancy and its effect on pregnancy outcomes: a systematic review. Cureus 13 (8), e17251. doi:10.7759/cureus.17251
Kendall-Tackett, K., and Hale, T. W. (2010). The use of antidepressants in pregnant and breastfeeding women: a review of recent studies. J. Hum. Lactation 26 (2), 187–195. doi:10.1177/0890334409342071
Kittel-Schneider, S., Felice, E., Buhagiar, R., Lambregtse-van den Berg, M., Wilson, C. A., Banjac Baljak, V., et al. (2022). Treatment of peripartum depression with antidepressants and other psychotropic medications: a synthesis of clinical practice guidelines in europe. Int. J. Environ. Res. Public Health 19 (4), 1973. doi:10.3390/ijerph19041973
Langan, R., and Goodbred, A. J. (2016). Identification and management of peripartum depression. Am. Fam. Physician 93 (10), 852–858.
Lawn, J. E., Gravett, M. G., Nunes, T. M., Rubens, C. E., Stanton, C., and Group, G. R. (2010). Global report on preterm birth and stillbirth (1 of 7): definitions, description of the burden and opportunities to improve data. BMC Pregnancy Childbirth 10 (Suppl. 1), S1. doi:10.1186/1471-2393-10-S1-S1
Lehtonen, L., Lillieskold, S., De Coen, K., Toome, L., Gimeno, A., Caballero, S., et al. (2022). Parent-infant closeness after preterm birth and depressive symptoms: a longitudinal study. Front. Psychol. 13, 906531. doi:10.3389/fpsyg.2022.906531
Li, H. (2022). Maternal-infant attachment and its relationships with postpartum depression, anxiety, affective instability, stress, and social support in a Canadian community sample. Psychiatr. Q. 94, 9–22. doi:10.1007/s11126-022-10011-w
Li, H., Li, H., Zhong, J., Wu, Q., Shen, L., Tao, Z., et al. (2023). Association between sleep disorders during pregnancy and risk of postpartum depression: a systematic review and meta-analysis. Arch. Womens Ment. Health 26 (2), 259–267. doi:10.1007/s00737-023-01295-3
Liu, X., Wang, S., and Wang, G. (2022). Prevalence and risk factors of postpartum depression in women: a systematic review and meta-analysis. J. Clin. Nurs. 31 (19-20), 2665–2677. doi:10.1111/jocn.16121
Maghami, M., Shariatpanahi, S. P., Habibi, D., Heidari-Beni, M., Badihian, N., Hosseini, M., et al. (2021). Sleep disorders during pregnancy and postpartum depression: a systematic review and meta-analysis. Int. J. Dev. Neurosci. 81 (6), 469–478. doi:10.1002/jdn.10118
Marthinsen, G. N., Helseth, S., Smastuen, M., Bjorvatn, B., Bandlien, S. M., and Fegran, L. (2022). Sleep patterns and psychosocial health of parents of preterm and full-born infants: a prospective, comparative, longitudinal feasibility study. Bmc Pregnancy Childbirth 22 (1), 546. doi:10.1186/s12884-022-04862-1
Molenaar, N. M., Bais, B., Lambregtse-van den Berg, M. P., Mulder, C. L., Howell, E. A., Fox, N. S., et al. (2020). The international prevalence of antidepressant use before, during, and after pregnancy: a systematic review and meta-analysis of timing, type of prescriptions and geographical variability. J. Affect Disord. 264, 82–89. doi:10.1016/j.jad.2019.12.014
Montirosso, R., Provenzi, L., Fumagalli, M., Sirgiovanni, I., Giorda, R., Pozzoli, U., et al. (2016). Serotonin transporter gene (SLC6A4) methylation associates with neonatal intensive care unit stay and 3-month-old temperament in preterm infants. Child. Dev. 87 (1), 38–48. doi:10.1111/cdev.12492
National Institute for Health and Care Excellence (2020). Antenatal and postnatal mental health: clinical management and service guidance. NICE. Available at: https://www.nice.org.uk/guidance/cg192/chapter/1-Recommendations#principles-of-care-in-pregnancy-and-the-postnatal-period-2.
Parsons, C. E., Young, K. S., Rochat, T. J., Kringelbach, M. L., and Stein, A. (2012). Postnatal depression and its effects on child development: a review of evidence from low- and middle-income countries. Br. Med. Bull. 101, 57–79. doi:10.1093/bmb/ldr047
Perry, A., Gordon-Smith, K., Jones, L., and Jones, I. (2021). Phenomenology, epidemiology and aetiology of postpartum psychosis: a review. Brain Sci. 11 (1), 47. doi:10.3390/brainsci11010047
Rezaie-Keikhaie, K., Arbabshastan, M. E., Rafiemanesh, H., Amirshahi, M., Ostadkelayeh, S. M., and Arbabisarjou, A. (2020). Systematic review and meta-analysis of the prevalence of the maternity blues in the postpartum period. Jognn-Journal Obstetric Gynecol. Neonatal Nurs. 49 (2), 127–136. doi:10.1016/j.jogn.2020.01.001
Stewart, D. E., and Vigod, S. N. (2019). Postpartum depression: pathophysiology, treatment, and emerging therapeutics. Annu. Rev. Med. 70, 183–196. doi:10.1146/annurev-med-041217-011106
Toda Miyano, M., Yasuda, H., and Takada, S. (2022). Longitudinal changes and features of sleep patterns of mothers with preterm infants during the early postpartum period. Kobe J. Med. Sci. 68 (1), E11–E22.
Tolja, R., Rados, S. N., and Andelinovic, M. (2020). The role of maternal mental health, infant temperament, and couple's relationship quality for mother-infant bonding. J. Reproductive Infant Psychol. 38 (4), 395–407. doi:10.1080/02646838.2020.1733503
Tosto, V., Ceccobelli, M., Lucarini, E., Tortorella, A., Gerli, S., Parazzini, F., et al. (2023). Maternity blues: a narrative review. J. Pers. Med. 13 (1), 154. doi:10.3390/jpm13010154
Vigod, S. N., Villegas, L., Dennis, C. L., and Ross, L. E. (2010). Prevalence and risk factors for postpartum depression among women with preterm and low-birth-weight infants: a systematic review. BJOG 117 (5), 540–550. doi:10.1111/j.1471-0528.2009.02493.x
Vu, H., and Shaya, F. T. (2017). Predicting factors of depression, antidepressant use and positive response to antidepressants in perinatal and postpartum women. Clin. Pract. Epidemiol. Ment. Health 13, 49–60. doi:10.2174/1745017901713010049
WHO (2023). News-room. Available at https://www.who.int/news-room/fact-sheets/detail/preterm-birth.
Keywords: preterm, antidepressant, depression, maternal mental health, postpartum
Citation: Esposito G, Cantarutti A, Lupattelli A, Franchi M, Corrao G and Parazzini F (2024) Does preterm birth increase the initiation of antidepressant use during the postpartum? A population-based investigation. Front. Pharmacol. 15:1325381. doi: 10.3389/fphar.2024.1325381
Received: 21 October 2023; Accepted: 11 March 2024;
Published: 27 March 2024.
Edited by:
Silvia Visentin, University of Padua, ItalyReviewed by:
Anna-Sophie Rommel, Icahn School of Medicine at Mount Sinai, United StatesCopyright © 2024 Esposito, Cantarutti, Lupattelli, Franchi, Corrao and Parazzini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giovanna Esposito, Z2lvdmFubmEuZXNwb3NpdG9AdW5pbWkuaXQ=
†ORCID: Giovanna Esposito, orcid.org/0000-0001-7894-4456; Anna Cantarutti, orcid.org/0000-0002-3700-780X
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.