
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 22 January 2024
Sec. Pharmacology of Anti-Cancer Drugs
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1293039
Background: Platinum-based dual-drug first-line chemotherapy is commonly employed in the treatment of patients with advanced non-small cell lung cancer (NSCLC), although its clinical efficacy is limited. Bevacizumab can antagonize vascular endothelial cell growth factor (VEGF), which inhibit tumor angiogenesis and prevent tumor invasion and development. However, a comprehensive meta-analysis evaluating the effectiveness and safety of combining bevacizumab with platinum-based chemotherapy in advanced NSCLC patients is lacking.
Methods: Randomized controlled trials (RCTs) investigating the combination therapy of bevacizumab and platinum-based chemotherapy for treating advanced NSCLC were searched across six databases. Data on objective response rate (ORR), disease control rate (DCR), 1-year survival rate, 2-year survival rate, 3-year survival rate, VEGF levels, and side effects were synthesized. Relative risk degree (RR) along with 95% confidence interval (CI) was used as statistical analysis measures for binary outcomes while continuous variables were analyzed using mean difference (MD) along with 95% CI. Heterogeneity was evaluated by Chi-squared and I2 tests. If there was heterogeneity, subgroup analysis was performed. Sensitivity analysis of the main outcome measures and assessment of publication bias were also performed.
Results: According to our screening criteria, a total of Forty-nine RCTs were included, involving data from 4268 patients. The results of this analysis showed that compared with platinum-containing chemotherapy alone, bevacizumab combined with platinum-containing chemotherapy significantly improved ORR (RR [95% CI], 1.53 [1.44, 1.63], p < 0.00001), DCR (RR [95% CI], 1.24 [1.19, 1.29], p < 0.0001), 1-year survival rate (RR [95% CI], 1.34 [1.15, 1.57], p = 0.0003), 2-year survival rate (RR [95% CI], 2.16 [1.35, 3.43], p = 0.001), 3-year survival rate (RR [95% CI], 2.00 [1.21, 3.30], p = 0.007). In addition, bevacizumab with platinum-containing chemotherapy observably decreased the VEGF levels (RR [95% CI], −67.35 [−91.46, −43.25], p < 0.00001).
Conclusion: Combination therapy involving bevacizumab demonstrated improved antitumor effects compared to chemotherapy alone in terms of ORR, DCR, 1-year survival rate, 2-year survival rate, 3-year survival rate, and VEGF levels without an increased incidence of adverse reactions. These analyses’ results can provide clinicians guidance when selecting appropriate treatments for patients diagnosed with advanced non-small cell lung cancer.
Lung cancer is the most common malignancy worldwide and has the highest mortality rate of all cancers. It is estimated that by 2030, it will cause 2 million deaths a year worldwide (Mattiuzzi and Lippi, 2019). Lung cancer can be classified into small-cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). NSCLC, as a group of histological subtypes, accounts for 80 percent of total lung cancer diagnoses (Thai et al., 2021). In the early stage of non-small cell lung cancer, there is a lack, leading to most diagnosed at an advanced stage and missing the opportunity for radical surgery. In the past 2 decades, the emergence of targeted therapy and the application of immunotherapy have made substantial progress in the treatment of NSCLC. Antibodies to CTLA-4, PD-1, PD-L1 and other immune checkpoints have been approved by the US FDA for the treatment of a variety of tumors, including non-small cell lung cancer (Le et al., 2017). It has been included in the latest global cancer treatment guidelines (Planchard et al., 2018). Nevertheless, the existence of drug resistance still makes its accessibility and effectiveness limited (Herbst et al., 2018). Platinum-based dual-drug first-line chemotherapy is commonly used for advanced NSCLC treatment; however, patients exhibit low sensitivity to this approach with an overall effective rate ranging from 25.0% to 35.0% (Chino et al., 2016). Therefore, it has become imperative to identify an efficient comprehensive treatment method for advanced NSCLC from traditional chemotherapy, targeted and immunotherapy have emerged as novel treatment modalities.
The formation of blood vessels plays an important role in the growth and metastasis of tumors, and vascular endothelial growth factor (VEGF) is named for its ability to promote endothelial cell proliferation and lumen formation, and its overexpression is associated with poor prognosis in patients with non-small cell lung cancer (Niki et al., 2017). Bevacizumab, a monoclonal antibody with high affinity towards VEGF, is frequently employed in the management of ovarian cancer, advanced metastatic colorectal cancer, and other diseases (Fan et al., 2019). By competitively binding with by tumor tissues, bevacizumab inhibits angiogenesis within these tissues while preventing tumor cell proliferation. Additionally, bevacizumab improves blood vessel function, microenvironment and enhances drug concentration within cancer tissues thereby achieving favorable therapeutic effects (Zheng et al., 2018). Clinical studies have demonstrated that bevacizumab confers significant survival benefits in patients with advanced NSCLC (Yang et al., 2018). There have been similar meta-analyses of bevacizumab combined with chemotherapy in the treatment of non-small cell lung cancer, but some of them only involved overall survival (OS), progression-free survival (PFS) and side effects (Botrel et al., 2011), and some lacked further verification regarding disease control rate (DCR) which serves as an important indicator (Zhou et al., 2021). The objective of this systematic review and meta-analysis was to comprehensively evaluate the efficacy and safety of bevacizumab and platinum-containing chemotherapy in the treatment of advanced NSCLC by including more recent studies.
We followed the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) checklist for systematic reviews and meta-analyses (Liberati et al., 2009). We conducted a systematic search for randomized controlled trials investigating the combination of bevacizumab and platinum-containing chemotherapy in patients with advanced NSCLC published in six databases from January 2018 to April 2023, including PubMed, Web of Science, the Cochrane Library, Wan Fang Database, Chinese VIP Information (VIP) and China National Knowledge Infrastructure (CNKI).
The strategy of combining subject words and free words is adopted to search. In the Chinese database, the following words are used in a combination manner: “Bei fazhu,” “feixiao xibao feiai” and “hualiao”; for the English databases, the text terms we use include “Bevacizumab,” “Carcinoma, Non-Small-Cell Lung” and “Drug Therapy”
The inclusion criteria were as follows: 1) study design: randomized randomized controlled trials (RCTs). 2) population: pathologically, cytologically or histologically confirmed to have advanced NSCLC. 3) intervention: the control group was treated with platinum-containing chemotherapy and correspondingly, the experimental group was treated with bevacizumab on the basis of the control group. 4) outcome: objective response rate, disease control rate, year survival rate, progression-free survival, overall survival, VEGF levels and treatment-related aside effects.
The exclusion criteria were as follows: 1) non-RCTs including case reports, reviews, animal or cell studies and studies without a control group. 2) The interventions were not bevacizumab and platinum-containing chemotherapy. 3) ambiguous results of statistical methods and research indicators, difficult to extract outcome indicators data, and multiple publications. 4) The patient had small cell lung cancer or early-stage NSCLC.
Data extraction was carried out independently by two reviewers (Guang Su Han and Chen Lu Li) and differences on study eligibility were resolved by consensus. The key information extracted by the evaluators is as follows: the author, year of publication, the number of participants, intervention details, the TNM stage of NSCLC and relevant outcomes.
Two researchers (Guang Su Han and Chen Lu Li) independently evaluated the quality of the included literature using the Cochrane risk of bias tool. The following six criteria were evaluated: 1) random sequence generation and allocation concealment; 2) blinding of participants and personnel; 3) blinding of outcome assessment; 4) incomplete outcome data; 5) selective reporting; 6) other bias. Differences arising in the process of quality evaluation were resolved through mutual consultation and discussion.
In this study, Review Manager (ver. 5.3) and STATA (ver.14) software were used for statistical analysis. RR and 95%CI were used as effect analysis statistics to treat binary outcomes. Continuous variables were analyzed by MD and 95%CI. p < 0.05 means the difference is statistically significant. Heterogeneity was evaluated by Chi-squared and I2 tests. If p > 0.1 and I2 < 50%, indicating that there was no statistical heterogeneity among the studies, and a fixed effect model was used for meta-analysis; If the heterogeneity was moderate or severe (p ≤ 0.1 or I2 ≥ 50%), the factors that might lead to heterogeneity were analyzed by subgroup analysis. If there was heterogeneity between the two studies and the clinical difference was not statistically significant, we choose a random-effect model. Then we performed sensitivity analyses to account for the effect of changing the study mode on the results of the pooled analysis. Since more than 10 studies were included, publication bias was identified using Begg’s tests and p-value > 0.05 was considered as no publication bias.
734 literatures that meet the screening requirements were initially searched from the database, including 191 repeated records (Figure 1). After that, 330 studies were included by reviewing the titles and abstracts of the remaining 543 studies and removing 213 reviews, animal or cell experiments, case reports, and other inconsistent literature. Finally, studies with no control group or platinum-containing chemotherapy regimen were excluded (n = 281), and 49 RCTs were selected in this systematic analysis (Dang et al., 2018; Ding and Zhu, 2018; Duan et al., 2018; Luo and Liu, 2018; Gui, 2019; Ma, 2019; Chen, 2020; Duan, 2020; Han, 2020; Li, 2020; Liao, 2020; Chang et al., 2021; Dai et al., 2021; Du, 2021; Fan, 2021; Huang and Du, 2021; Mao, 2021; Pan and Xia, 2021; Chen et al., 2022a; Liu et al., 2022a; Chen et al., 2022b; Liu et al., 2022b; He, 2022; Li et al., 2022; Liu, 2022; Ning et al., 2022; Shi, 2022; Song et al., 2019; Sun et al., 2019; Xu, 2019; Zhai et al., 2019; Wang et al., 2020a; Wang et al., 2020b; Song et al., 2020; Wang and Qian, 2020; Wu, 2020; Zhang, 2020; Zhang and Wang, 2020; Song, 2021; Wang, 2021; Wang and Zheng, 2021; Yuan, 2021; Tian and Wang, 2022; Wu et al., 2022; Yang, 2022; Ye et al., 2022; Zeng and Li, 2022; Sun, 2023; Zhou and Rong, 2023).
A total of forty-nine studies were included in the evaluation, and 4268 patients were studied. All RCTs originated from China. The year of publication was between 2018 and 2023. The characteristics of patients in the selected studies include age, gender, clinical stage, chemotherapy regimen, and treatment indicators, which are summarized in Table 1.
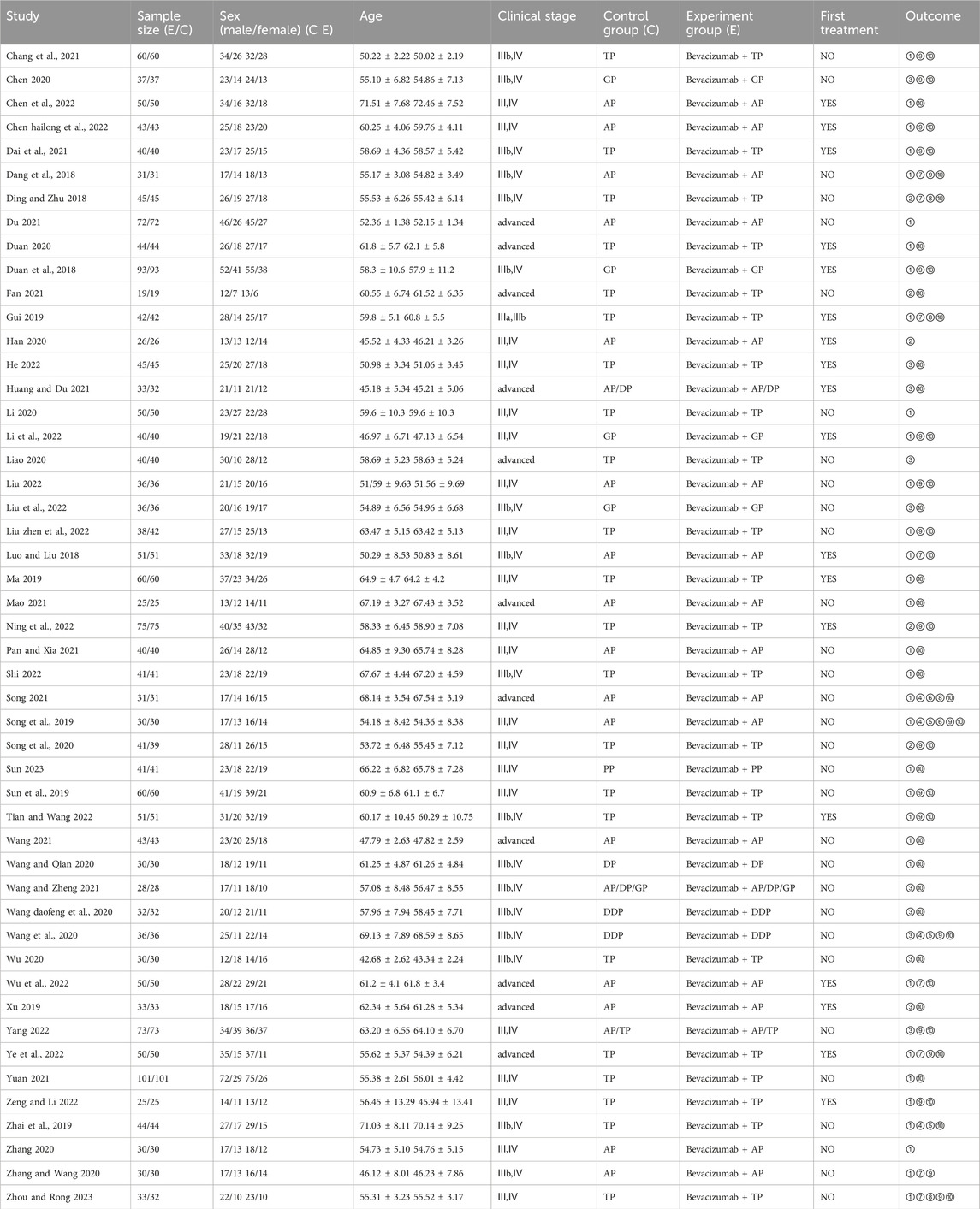
TABLE 1. Characteristics of RCTs included in the study. (GP, Gemcitabine; AP, Pemetrexed; TP/DDP/PP, Taxol; DP, Docetaxel; ①Efficacy (RECIST); ②Efficacy (WHO); ③Efficacy (Not mentioned); ④1-year survival; ⑤2-year survival; ⑥3-year survival; ⑦Progression-free survival; ⑧Overall survival; ⑨VEGF; ⑩Adverse reaction).
The assessment of the bias risk of these 49 RCTs was shown in Figure 2. All of these studies described specific random sequence generation. However, the detailed reporting of allocation, concealment, and blinding of outcome assessment had not been addressed in any studies. As for performance bias, it was mentioned in only two studies. The results of each study had been faithfully reported, so we consider all studies to be free of reporting bias (Figures 2, 3).
Forty-nine studies reported short-term efficacy of bevacizumab in combination with platinum-containing chemotherapy for advanced NSCLC. As there was no heterogeneity (χ2 = 41.20, p = 0. 75, I2 = 0%; Z = 13.57, p < 0.00001), we chose a fixed effects model for the analysis. As shown in Figure 4, the objective response rate (ORR) was higher in the experimental group than in the control group (RR [95% CI], 1.53 [1.44, 1.63], p < 0.00001). For the disease control rate (DCR), the test of heterogeneity (χ2 = 97.96, p < 0.0001, I2 = 51%; Z = 9.99, p < 0.00001) suggested that there was significant heterogeneity, so the random models were employed to combine effect sizes. Compared with platinum-based chemotherapy alone, the addition of bevacizumab had a better effect in terms of DCR (RR [95% CI], 1.24 [1.19, 1.29], p < 0.0001) (Figure 5). We then performed regression and subgroup analyses, which showed no significant differences in evaluation criteria, chemotherapy agents, and first treatment (Supplementary Figures S1, S2).
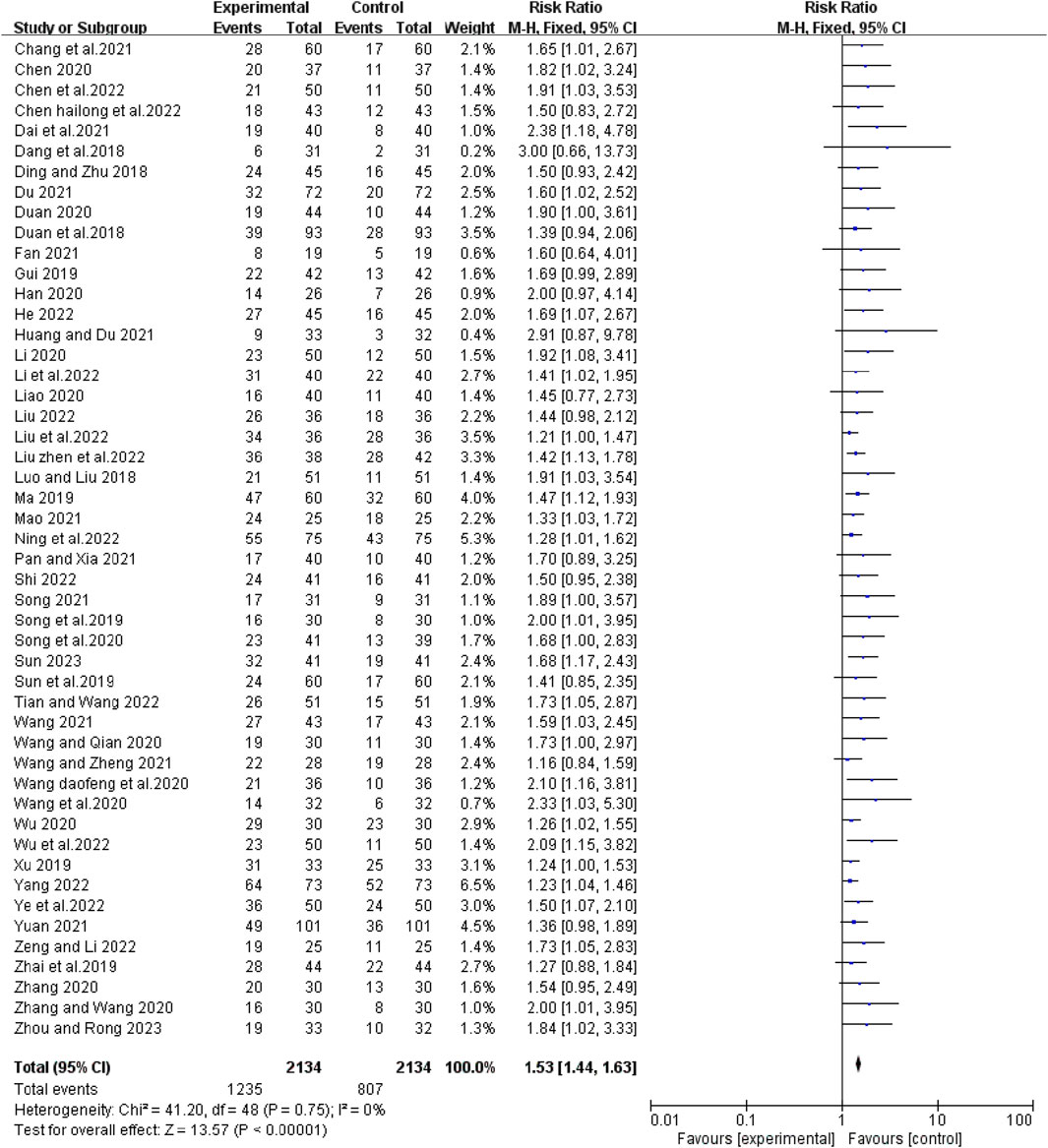
FIGURE 4. The pooled effects of bevacizumab combined with platinum-containing chemotherapy on objective response rate.
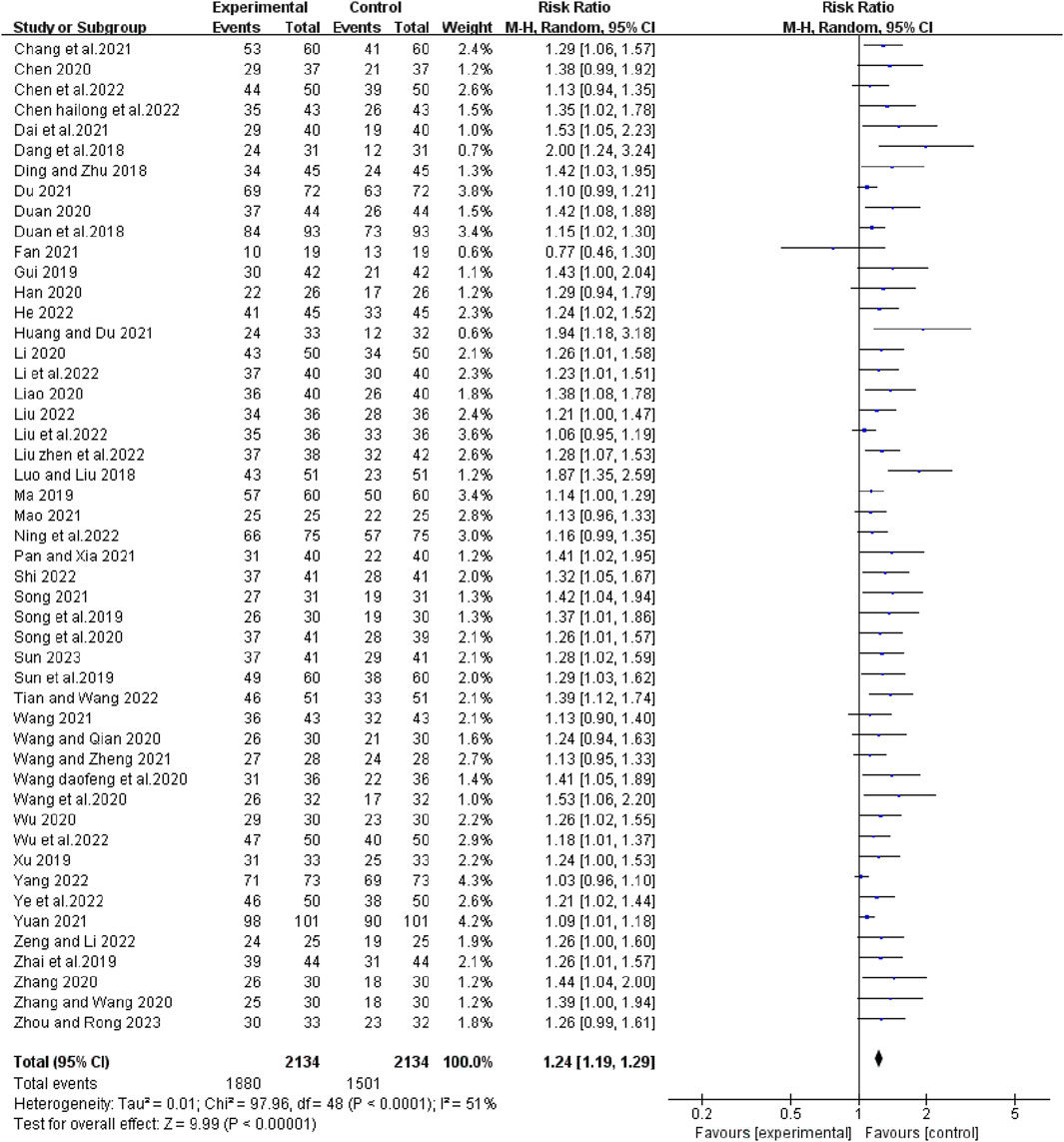
FIGURE 5. The pooled effects of bevacizumab combined with platinum-containing chemotherapy on disease control rate.
The heterogeneity test suggested that there was no statistical heterogeneity among the studies, so the fixed effects model was selected. Four articles reported 1-year survival rate (χ2 = 1.00, p = 0.80, I2 = 0%; Z = 3.65, p = 0.0003), which showed that bevacizumab plus platinum-based chemotherapy was superior to chemotherapy alone (RR [95% CI], 1.34 [1.15, 1.57], p = 0.0003) (Figure 6). Three studies reported 2-year survival (χ2 = 0.09, p = 0.95, I2 = 0%; Z = 3.24, p = 0.001), which was superior with bevacizumab plus platinum-based chemotherapy (RR [95% CI], 2.16 [1.35, 3.43], p = 0.001) (Figure 7). Two studies reported 3-year survival (χ2 = 1.34, p = 0.25, I2 = 26%; Z = 2.72, p = 0.007), which was also significantly higher in the combination group (RR [95% CI], 2.00 [1.21, 3.30], p = 0.007) (Figure 8).
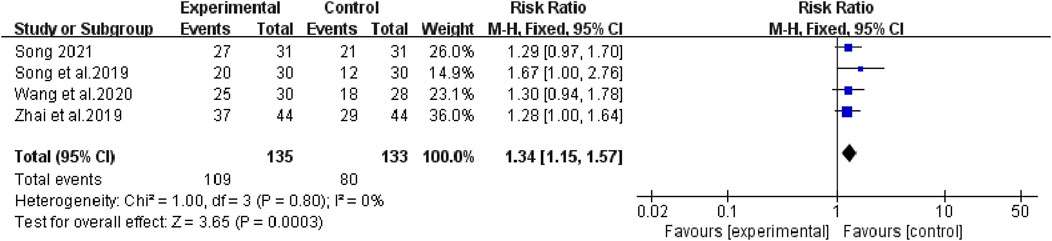
FIGURE 6. The pooled effects of bevacizumab combined with platinum-containing chemotherapy on 1-year survival rate.
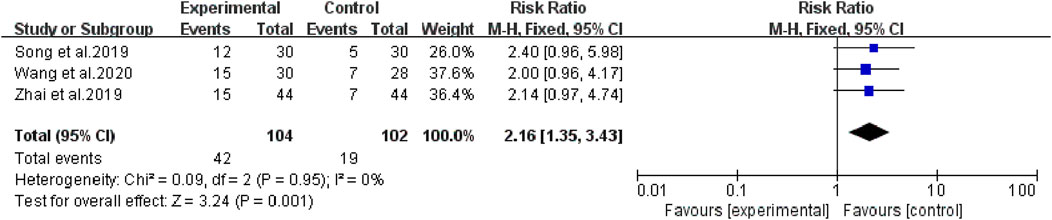
FIGURE 7. The pooled effects of bevacizumab combined with platinum-containing chemotherapy on 2-year survival rate.
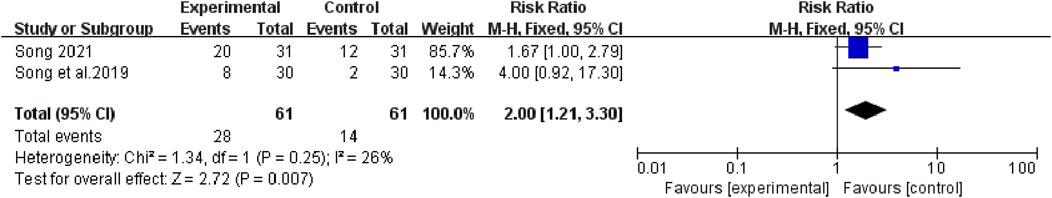
FIGURE 8. The pooled effects of bevacizumab combined with platinum-containing chemotherapy on 3-year survival rate.
Sixteen articles reported changes in serum VEGF levels before and after chemotherapy. The results of heterogeneity test showed that there was statistical heterogeneity among the studies (χ2 = 362.35, p < 0.00001, I2 = 96%; Z = 5.48, p < 0.00001), so a random effects model was chosen to combine effect sizes. The results of the analysis showed that the combination treatment group reduced VEGF levels better than the control group (RR [95% CI], −67.35 [−91.46, −43.25], p < 0.00001) (Figure 9).
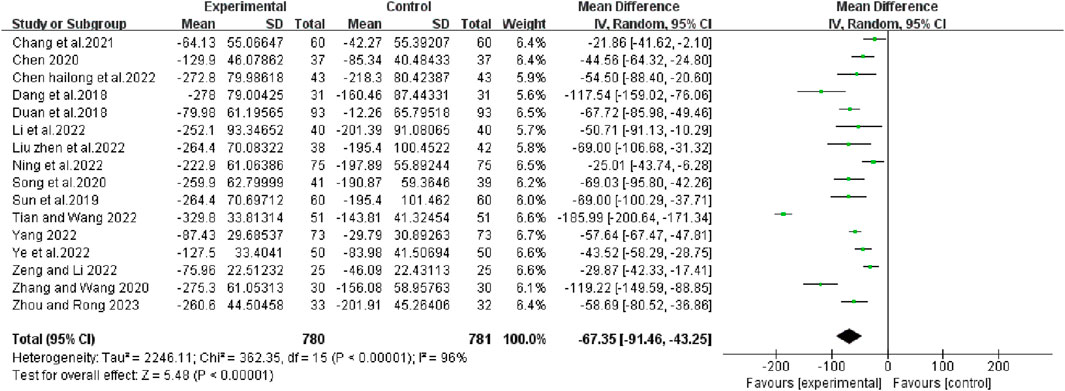
FIGURE 9. The pooled effects of bevacizumab combined with platinum-containing chemotherapy on VEGF leave.
The adverse reactions of chemotherapy involved in this study mainly included gastrointestinal reactions, bone marrow suppression, liver and kidney injury, and hematological toxicity. The results of Meta-analysis showed that there was no significant difference in the incidence of gastrointestinal reactions (RR [95% CI], 0.96 [0.88, 1.05], p = 0.38), myelosuppression (RR [95% CI], 0.95 [0.84, 1.09],p = 0.48), liver and kidney dysfunction (RR [95% CI], 0.92 [0.70, 1.20], p = 0.52) and hematologic toxicity (thrombocytopenia: RR [95% CI], 0.88 [0.72, 1.07], p = 0.20; leukopenia: RR [95% CI], 0.95 [0.81, 1.11], p = 0.49; hemoglobin reduction: RR [95% CI], 0.89 [0.71, 1.12], p = 0.31) between the experimental group and the control group (Supplementary Figures S3–S8).
Begg’s test was used to analyze the publication bias of ORR and DCR, and the results showed that publication bias may have a certain impact on the objective response rate and disease control rate (ORR: p = 0.000; DCR: p = 0.000) (Figure 10). Subsequently, sensitivity analysis was performed on outcome indicators such as objective response rate and disease control rate, and the results showed that the change of effect model had no significant effect on the combined results (Figure 11).
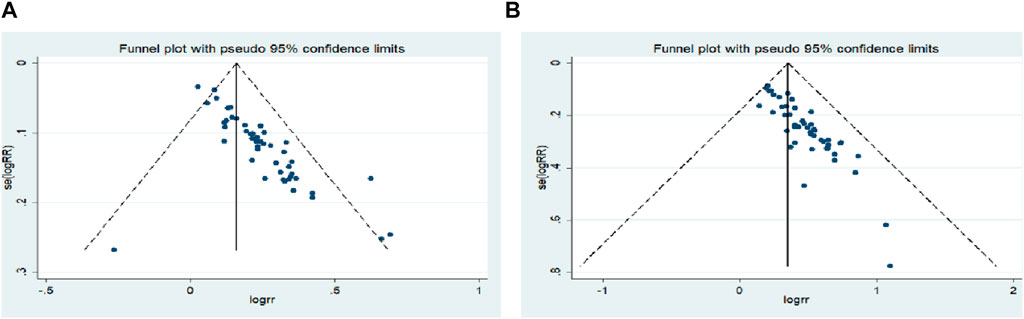
FIGURE 10. Begg`s regression analyses for publication bias: (A) Objective response rate; (B) Disease control rate.
Despite major advances in molecular targeted therapy and immunotherapy in the past few years, platinum regimens remain the most active combination in clinical practice (Watanabe et al., 2017), Combination therapy has gained prominence in clinical practice. This systematic review and meta-analysis aim to assess the efficacy and safety of combining bevacizumab with platinum-containing chemotherapy in advanced NSCLC. The findings demonstrate superior objective response rate, disease control rate, 1/2/3-year survival rates, as well as a greater reduction in VEGF levels when compared to platinum-based chemotherapy alone. Furthermore, the combination therapy does not augment the adverse effects associated with chemotherapy alone while ensuring its safety is maintained.
Response Evaluation Criteria in Solid Tumors (RECIST) reflects the therapeutic effect of clinical tumors by detecting changes in tumor burden, including tumor shrinkage (objective response) and disease progression. ORR was evaluated as complete response (CR) and partial response (PR). DCR included CR or PR in all patients and stable disease (SD) in patients with progressive disease (PD) at the time of chemotherapy (Eisenhauer et al., 2009).
The 49 studies included in this meta-analysis reported ORR and DCR. The results of statistical analysis proved that bevacizumab combined with platinum-based chemotherapy had a significant advantage in improving ORR (RR 1.53) and DCR (RR 1.24) compared with platinum-based chemotherapy alone. The results of the ORR analysis are consistent with those of previous studies (Zhou et al., 2021). However, the significant improvement in DCR outcomes with the bevacizumab-containing therapy in our analysis is inconsistent with this finding. The study did not show significant outcomes with DCR, and the authors suggest that one of the studies may have influenced the results, acknowledging that the limited number of included RCTS limits the positive findings. Then subgroup analysis was performed according to the different chemotherapy drugs and whether they received anti-tumor treatment for the first time. Subgroup analyses showed no statistically significant differences between groups, indicating a significant benefit of bevacizumab-containing chemotherapy in improving DCR regardless of differences in the use of platinum-based chemotherapy agents or whether patients had received prior anticancer therapy.
For patients with advanced cancer, the annual survival rate is an important parameter in evaluating prognosis. In our analysis, the results of 1-year/2-year/3-year survival rates demonstrated a significant improvement in survival time for patients with advanced NSCLC when bevacizumab was combined with platinum compared to chemotherapy alone. However, due to limited literature included in of the evidence is relatively low, necessitating further data analysis to substantiate this conclusion in future studies.
Chemotherapy commonly used in clinical practice for NSCLC patients usually leads to serious adverse reactions, such as gastrointestinal reactions, bone marrow suppression, liver and kidney damage, etc. (Mangal et al., 2017) The results of our analysis show that the addition of bevacizumab to platinum-based chemotherapy does not increase the incidence of side effects of chemotherapy and confirm that bevacizumab has an acceptable safety profile, even when combined with different chemotherapy regimens. This conclusion is consistent with the results of the previous phase 4 study (Crinò et al., 2010).
Currently, extensive research on molecular targeted therapy for malignant tumors is being conducted in clinical practice, leading to of various targeted therapy drugs that have significantly improved the efficacy and control of malignant tumors. Studies have shown that neovascularization is closely related to the formation, development and prognosis of tumors. In the process of solid tumor growth, a variety of vascular growth factors are usually produced to promote the formation of new blood vessels. Among them, VEGF is considered to be the key factor inducing tumor angiogenesis. A number of studies have confirmed that VEGF is highly expressed in lung cancer, gastric cancer, intravascular sarcoma, gastrointestinal tumors and gynecological tumors (Lu et al., 2019). Blocking VEGF signaling can restore the vascular system to a more normal state, and improve the permeability of drugs in the tumor through the decrease of intercellular fluid pressure and the increase of tumor oxygenation, thereby enhancing the efficacy of chemotherapy (Jain, 2005). Bevacizumab can competitively bind to VEGF released by cancer tissues to impede angiogenesis within these tissues and inhibit tumor cell proliferation. Our findings suggest that bevacizumab combined with platinum-based chemotherapy can effectively reduce serum VEGF levels in patients with advanced NSCLC, which is consistent with the results of previous meta-analysis.
In conclusion, this meta-analysis showed that the combination of bevacizumab significantly improved the efficacy of chemotherapy and prolonged survival in patients with advanced non-small-cell lung cancer, without causing an increase in serious adverse effects.
All the studies included in this Meta-analysis were Chinese, which may lack sufficient representativeness and generalization across different countries. Further studies on different populations are needed to verify the generalizability of our findings. In addition, the description of distribution concealment and blinding was missing in most of the literature, resulting in a reduction in the quality of the included studies. The diversity of chemotherapeutic drugs also affected the results to a certain extent.
Bevacizumab can enhance drug efficacy and improve the prognosis. In patients with advanced NSCLC, the efficacy of bevacizumab combined with platinum-based chemotherapy is better than that of single chemotherapy, and it does not increase the incidence of side effects of chemotherapy. These findings provide support for clinical treatment, suggesting that bevacizumab combination therapy in the treatment of advanced NSCLC has a significant benefit, with a modest safety profile. Further follow-up in the real world is needed to clarify its long-term benefits.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
GH: Writing–original draft, Writing–review and editing. CL: Writing–review and editing. PY: Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This article was supported by the National Natural Science Foundation of China (No. 82174457).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1293039/full#supplementary-material
Botrel, T. E., Clark, O., Clark, L., Paladini, L., Faleiros, E., and Pegoretti, B. (2011). Efficacy of bevacizumab (Bev) plus chemotherapy (CT) compared to CT alone in previously untreated locally advanced or metastatic non-small cell lung cancer (NSCLC): systematic review and meta-analysis. Lung cancer (Amsterdam, Neth. 74 (1), 89–97. doi:10.1016/j.lungcan.2011.01.028
Chang, X., Pang, H., and Liu, X. (2021). Efficacy of bevacizumab combined with paclitaxel and cisplatin chemotherapy in the treatment of non-small cell lung cancer. Chin J Cancer Clin. Rehabilitation 28 (11), 1357–1360. doi:10.13455/j.cnki.cjcor.2021.11.20
Chen, H., Xie, C., Zhong, Q., et al. (2022b). Effect of bevacizumab combined with chemotherapy on serum VEGF and bFGF levels in patients with advanced non-squamous carcinoma and non-small cell lung cancer. J. Drug Eval. 19 (16), 996–999. doi:10.19939/j.cnki.1672-2809.2022.16.11
Chen, M., Li, L., Liao, X., et al. (2022a). Effect of bevacizumab combined with pemetrexed plus carboplatin neoadjuvant chemotherapy on immune and cognitive function in elderly patients with non-small cell lung cancer. Big Dr. 7 (11), 26–29.
Chen, W. (2020). Efficacy of bevacizumab combined with GP chemotherapy in patients with advanced non-small cell lung cancer. Chin. Med. J. 32 (8), 13–15. doi:10.3969/j.issn.1672-0369.2020.08.005
Chino, H., Amano, Y., Yamauchi, Y., Matsuda, J., Takeda, N., Tanaka, G., et al. (2016). Cardiogenic syncope possibly related to bevacizumab-containing combination chemotherapy for advanced non-small cell lung cancer. J. Thorac. Dis. 8 (9), 2646–2650. doi:10.21037/jtd.2016.08.96
Crinò, L., Dansin, E., Garrido, P., Griesinger, F., Laskin, J., Pavlakis, N., et al. (2010). Safety and efficacy of first-line bevacizumab-based therapy in advanced non-squamous non-small-cell lung cancer (SAiL, MO19390): a phase 4 study. Lancet. Oncol. 11 (8), 733–740. doi:10.1016/S1470-2045(10)70151-0
Dai, L., Wang, X., Yang, W., et al. (2021). Effects of bevacizumab combined with TP chemotherapy regimen on immune function, quality of life and serum tumor markers in patients with advanced non-squamous carcinoma and non-small cell lung cancer. Adv. Mod. Biomed. 21 (11), 2174–2178. doi:10.13241/j.cnki.pmb.2021.11.038
Dang, M., Xi, Y., Yan, L., et al. (2018). Efficacy and safety analysis of bevacizumab combined with PC chemotherapy in the treatment of non-small cell lung cancer. Henan Med. Res. 27 (7), 1199–1201. doi:10.3969/j.issn.1004-437X.2018.07.016
Ding, Q., and Zhu, S. (2018). Effect of bevacizumab combined with PC chemotherapy on disease control rate and survival cycle of patients with non-small cell lung cancer. J. Med. Forum 39 (6), 63–65.
Du, W. (2021). Clinical study of bevacizumab in the treatment of advanced EGFR mutated non-small cell lung cancer. Health Must Read. (26), 30.
Duan, H. (2020). Clinical study of Bevacizumab combined with TP chemotherapy in the treatment of advanced non-squamous non-small cell lung cancer. Gt. Health 129 (17), 131.
Duan, J., Yang, Z., Liu, D., and Shi, Y. (2018). Clinical efficacy of bevacizumab combined with gemcitabine and cisplatin combination chemotherapy in the treatment of advanced non-small cell lung cancer. J. buon 23 (5), 1402–1406.
Eisenhauer, E. A., Therasse, P., Bogaerts, J., Schwartz, L. H., Sargent, D., Ford, R., et al. (2009). New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45 (2), 228–247. doi:10.1016/j.ejca.2008.10.026
Fan, C. (2021). Efficacy of bevacizumab combined with chemotherapy in the treatment of advanced non-small cell lung cancer. Electron. J. Clin. Med. Literature 8 (30), 21–23.
Fan, H., Yuan, J., Wu, J., Jia, Y. X., Ma, Y. H., and Li, X. Y. (2019). Influence of polymorphisms of VEGFR2 on clinical outcomes and safety of advanced non-small-cell lung cancer treated by first line Bevacizumab plus chemotherapy regimens. Chin. J. Med. 99 (2), 99–104. doi:10.3760/cma.j.issn.0376-2491.2019.02.005
Gui, Y. (2019). Efficacy of bevacizumab combined with paclitaxel and carboplatin in the treatment of advanced non-small cell lung cancer. Chin. J. Health Eng. 18 (02), 299–301. (in Chinese). doi:10.19937/j.issn.1671-4199.2019.02.054
Han, X. (2020). Application of bevacizumab as an anti-VEGFR pathway drug in the clinical treatment of lung cancer. Med. Food Ther. Health 92 (6), 95.
He, J. (2022). Efficacy of bevacizumab combined with chemotherapy in patients with advanced non-small cell lung cancer (NSCLC). China Sci. Technol. periodical database Med. (5).
Herbst, R. S., Morgensztern, D., and Boshoff, C. (2018). The biology and management of non-small cell lung cancer. Nature 553 (7689), 446–454. doi:10.1038/nature25183
Huang, G., and Du, Z. (2021). To investigate the clinical effect of bevacizumab combined with chemotherapy in the treatment of advanced non-small cell lung cancer. Health Must Read. (20), 20.
Jain, R. K. (2005). Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Sci. (New York, N.Y.) 307 (5706), 58–62. doi:10.1126/science.1104819
Le, D. T., Durham, J. N., Smith, K. N., Wang, H., Bartlett, B. R., Aulakh, L. K., et al. (2017). Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357 (6349), 409–413. doi:10.1126/science.aan6733
Li, J., Zhang, Q., Zhang, L., et al. (2022). Effect and safety of Bevacizumab injection on immune function of patients with non-small cell lung cancer. Chin. J. Med. Educ. 45 (10), 918–921. doi:10.3760/cma.J.c.n115455-20210111-00060
Li, Z. X. (2020). Effect of bevacizumab combined with paclitaxel and cisplatin in the treatment of non-small cell lung cancer and analysis of the effect on microRNA-99a and miRNA-145. Chin. Pract. Med. 15 (6), 99–100. doi:10.14163/j.cnki.11-5547/r.2020.06.045
Liao, X. (2020). Efficacy of bevacizumab combined with paclitaxel plus cisplatin (TP) in the treatment of advanced non-squamous carcinoma non-small cell lung cancer patients. Health Must-Read (12), 23.
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gøtzsche, P. C., Ioannidis, J. P. A., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann. Intern Med. 151 (4), W65–W94. doi:10.7326/0003-4819-151-4-200908180-00136
Liu, X., Liu, L., and Chen, L. (2022a). Clinical observation of bevacizumab combined with gemcitabine and cisplatin in the treatment of advanced non-small cell lung cancer. Chin. J. Pract. Med. 49 (20), 85–88. doi:10.3760/cma.J.c.n115689-20220717-03127
Liu, Z. (2022). Effect of bevacizumab combined with pemetrexed and cisplatin on vascular endothelial cell growth factor in patients with advanced non-small cell lung cancer. Reflexology Rehabilitation Med. 3 (11), 155–157.
Liu, Z., Deng, L., and Cao, X. (2022b). Clinical efficacy of bevacizumab combined with paclitaxel liposome and cisplatin in patients with non-squamous non-small cell lung cancer. J. Clin. Ration. drug use 15 (6).
Lu, M., Zhou, H., and Zhou, X. G. (2019). Short-term efficacy and toxicity of combination chemotherapy with bevacizumab or angioendostatin in the treatment of locally advanced wild-type non-small cell lung cancer. Chin. J. Tumor Biotherapy 26 (4), 67–71. doi:10.3872/j.issn.1007-385x.2019.04.009
Luo, H., and Liu, K. (2018). Effect of bevacizumab combined with chemotherapy on the expression of vascular endothelial growth factor in advanced non-small cell lung cancer. J. Clin. Pulmonol. 23 (2), 220–224. doi:10.3969/j.issn.1009-6663.2018.02.007
Ma, C. (2019). Efficacy of bevacizumab combined with paclitaxel and cisplatin in the treatment of non-small cell lung cancer and its effect on microRNA-99a and miRNA-145. J. Clin. Pulmonol. 24 (4), 632–636. doi:10.3969/j.issn.1009-6663.2019.04.012
Mangal, S., Gao, W., Li, T., and Zhou, Q. T. (2017). Pulmonary delivery of nanoparticle chemotherapy for the treatment of lung cancers: challenges and opportunities. Acta Pharmacol. Sin. 38 (6), 782–797. doi:10.1038/aps.2017.34
Mao, H. (2021). Efficacy of chemotherapy combined with targeted drugs in advanced non-small cell lung cancer patients. Chin. Sci. Technol. J. Database (full text) Med. Health (7).
Mattiuzzi, C., and Lippi, G. (2019). Current cancer epidemiology. J. Epidemiol. Glob. health 9 (4), 217–222. doi:10.2991/jegh.k.191008.001
Niki, M., Yokoi, T., Kurata, T., and Nomura, S. (2017). New prognostic biomarkers and therapeutic effect of bevacizumab for patients with non-small-cell lung cancer. Lung Cancer (Auckl) 8, 91–99. doi:10.2147/LCTT.S138887
Ning, S., Lin, C., Luo, M., et al. (2022). Efficacy of bevacizumab combined with paclitaxel and carboplatin in patients with advanced non-small cell lung cancer. Chin. J. Med. Sci. 34 (23), 39–42. doi:10.3969/j.issn.1672-0369.2022.23.012
Pan, E., and Xia, Q. (2021). Effect of bevacizumab combined with chemotherapy in the treatment of non-small cell lung cancer and its effect on T cell subsets and immune function. Chin. Med. Rev. 18 (29), 118–121.
Planchard, D., Popat, S., Kerr, K., Novello, S., Smit, E. F., Faivre-Finn, C., et al. (2018). Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 29 (Suppl. 4), iv192–iv237. doi:10.1093/annonc/mdy275
Shi, L. (2022). Effect of bevacizumab combined with chemotherapy in the treatment of advanced non-small cell lung cancer. Clin. Med. 42 (8), 103–105. doi:10.19528/j.iSSN.1003-3548.2022.08.040
Song, C., Gao, Z., Lu, G., et al. (2019). Effects of anti-VEGF targeted drugs combined with chemotherapy on the incidence, survival time and laboratory indexes of brain metastases in patients with NSCLC. And Clin. Pract. Med. 22 (2), 119–122. doi:10.14053/j.cnki.ppcr.201902002
Song, l., Han, Y., Wei, L., et al. (2020). Effect of bevacizumab combined with paclitaxel/carboplatin regimen on related cell growth factors and tumor markers in patients with advanced non-squamous non-small cell lung cancer. And Clin. Pract. Med. 23 (6). doi:10.14053/j.cnki.ppcr.202006008
Song, Z. J. (2021). Clinical effect of bevacizumab combined with pemetrexed plus cisplatin in advanced non-squamous non-small cell lung cancer. Chin. foreign Med. Res. 19 (16), 46–48. doi:10.14033/j.cnki.cfmr.2021.16.016
Sun, L., Zhang, J., Ge, X., et al. (2019). Clinical study of bevacizumab combined with TP chemotherapy in the treatment of advanced non-squamous carcinoma non-small cell lung cancer. J. Clin. Exp. Med. 18 (15), 1631–1634. doi:10.3969/j.issn.1671-4695.2019.15.019
Sun, Y. J. (2023). Efficacy of bevacizumab combined with PP in the treatment of non-small cell lung cancer. Diet. Health care (8), 53–56.
Thai, A. A., Solomon, B. J., Sequist, L. V., Gainor, J. F., and Heist, R. S. (2021). Lung cancer. Lancet 398 (10299), 535–554. doi:10.1016/S0140-6736(21)00312-3
Tian, S., and Wang, J. (2022). Efficacy of bevacizumab combined with comprehensive nursing in patients with advanced non-squamous carcinoma and non-small cell lung cancer. Mod. Med. Health Res. Electron. Ed. 6 (18), 123–125.
Wang, D., Fu, J., and Fang, S. (2020a). Effect of bevacizumab combined with chemotherapy on disease control rate and serum T cell subsets in patients with stage III b/IV non-squamous non-small cell lung cancer. J. Clin. lung 25 (8) doi:10.3969/j.issn.1009-6663.2020.08.022
Wang, D., Fu, J., and Fang, S. (2020b). Efficacy of bevacizumab combined with DDP chemotherapy in patients with non-small cell lung cancer. J. Pract. Clin. Med. 24 (22). doi:10.7619/jcmp.202022002
Wang, H., and Qian, H. (2020). Clinical observation of bevacizumab combined with chemotherapy in the treatment of advanced non-squamous non-small cell lung cancer. J. Clin. Ration. Drug Use 13 (1). doi:10.15887/j.cnki.13-1389/r.2020.01.030
Wang, H. L. (2021). Clinical efficacy of bevacizumab combined with chemotherapy in patients with advanced non-small cell lung cancer (NSCLC). Kang Yi (14), 239. doi:10.12332/j.issn.2095-6525.2021.14.227
Wang, X., and Zheng, J. (2021). Clinical efficacy of bevacizumab combined with chemotherapy in patients with advanced non-small cell lung cancer (NSCLC). Med. Food Ther. Health 19 (23), 94–96.
Watanabe, S. I., Nakagawa, K., Suzuki, K., Takamochi, K., Ito, H., Okami, J., et al. (2017). Neoadjuvant and adjuvant therapy for Stage III non-small cell lung cancer. Jpn. J. Clin. Oncol. 47 (12), 1112–1118. doi:10.1093/jjco/hyx147
Wu, S. Y. (2020). Efficacy of bevacizumab, paclitaxel and cisplatin (TP) chemotherapy in the treatment of advanced non-squamous carcinoma non-small cell lung cancer patients. Chin. J. Health Care 38 (10). doi:10.14033/j.cnki.cfmr.2022.09.007
Wu, Z., Huang, J., Wu, Y., et al. (2022). Clinical effect of bevacizumab combined with chemotherapy in the treatment of advanced non-small cell lung cancer. Med. Res. both A. T. home abroad 20 (9). doi:10.14033/j.cnki.cfmr.2022.09.007
Xu, J. (2019). Analysis of the efficacy and safety of bevacizumab in the treatment of advanced non-small cell lung cancer. Chin. J. Pract. Med. 14 (34), 116–118. doi:10.14163/j.cnki.11-5547/r.2019.34.062
Yang, H., Wang, C., Wang, X., et al. (2018). Efficacy of bevacizumab in the treatment of elderly non-small cell lung cancer with brain metastases. Southwest Natl. Def. Med. 28 (7), 663–665.
Yang, H. J. (2022). Effect of bevacizumab combined with chemotherapy on the therapeutic efficacy and tumor markers of non-small cell lung cancer. China Prescr. Drugs 20 (10).
Ye, F., He, S., and Wei, X. M. (2022). Clinical efficacy of TP regimen combined with bevacizumab in the treatment of advanced non-small cell lung cancer and its effect on the expression of programmed T lymphocyte death receptor 1 and cytotoxic T lymphocyte associated antigen 4. Heilongjiang Med. Sci. 46 (19), 2323–2326. doi:10.3969/j.issn.1004-5775.2022.19.005
Yuan, T. (2021). Effect of chemotherapy with bevacizumab combined with TP regimen in patients with advanced non-small cell lung cancer. Chin. Minkang Med. 33 (16), 13–15. doi:10.3969/j.issn.1672-0369.2021.16.006
Zeng, H., and Li, X. (2022). Efficacy of bevacizumab targeted therapy combined with TC chemotherapy in the treatment of advanced non-small cell lung cancer. Chin. J. drug abuse Prev. 28 (3), 308–312. doi:10.15900/j.cnki.zylf1995.2022.03.008
Zhai, Z., Yang, Y., and Ren, Z. (2019). Effects of bevacizumab combined with TC chemotherapy on serum VEGF and bFGF levels and quality of life in elderly patients with advanced NSCLC. Mod. Oncol. Med. 27 (9), 1541–1545. doi:10.3969/j.issn.1672-4992.2019.09.018
Zhang, L. (2020). Efficacy of bevacizumab in the treatment of non-small cell lung cancer. Big Dr. 5 (24), 56–58.
Zhang, Q., and Wang, W. Y. (2020). Efficacy of bevacizumab combined with PC chemotherapy in advanced non-squamous non-small cell lung cancer. Henan Med. Res. 29 (13), 2416–2417. (in Chinese). doi:10.3969/j.issn.1004-437X.2020.13.056
Zheng, X., Wang, H., Zhang, G., and Yan, X. (2018). Efficacy and safety of second-line bevacizumab combined with chemotherapy in the treatment of advanced non-squamous non-small cell lung cancer. Chin. J. Lung Cancer 21 (7), 513–518. doi:10.3779/j.issn.1009-3419.2018.07.02
Zhou, Y., He, M., Li, R., Peng, Y., Li, F., Li, S., et al. (2021). The safety and effectiveness of bevacizumab in the treatment of nonsquamous non-small-cell lung cancer: a meta-analysis of randomized controlled trials. BioMed Res. Int. 2021, 5537899. doi:10.1155/2021/5537899
Keywords: bevacizumab, non-small cell lung cancer, chemotherapy, meta-analysis, systematic review
Citation: Han G, Li C and Yi P (2024) The efficacy of bevacizumab combined with platinum-containing chemotherapy in the treatment of advanced non-small cell lung cancer in China: a systematic review and meta-analysis of randomized clinical trials. Front. Pharmacol. 15:1293039. doi: 10.3389/fphar.2024.1293039
Received: 12 September 2023; Accepted: 04 January 2024;
Published: 22 January 2024.
Edited by:
Qingbin Cui, University of Toledo College of Medicine and Life Sciences, United StatesReviewed by:
Ran Wang, Anhui Medical University, ChinaCopyright © 2024 Han, Li and Yi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Yi, eWlwaW5nQHRqaC50am11LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.