- 1Department of Pharmacy, The First Affiliated Hospital, Guangzhou Medical University, Guangzhou, China
- 2School of Pharmaceutical Science, Guangzhou Medical University, Guangzhou, China
- 3National Clinical Research Center for Respiratory Disease, State Key Laboratory of Respiratory Disease, The First Affiliated Hospital, Guangzhou Institute of Respiratory Health, Guangzhou Medical University, Guangzhou, China
Background: The clinical characteristics and risk factors of infusion reactions (IRs) are inadequately described in clinical practice due to underreported cases. In the present study, we reported the current status of IRs based on an in-hospital pharmacovigilance database of a tertiary care hospital.
Methods: Our study conducted a retrospective analysis of drug-induced IRs recorded at an in-hospital pharmacovigilance center between January 2015 to December 2019. The descriptive statistical analysis encompassed main causative agents, clinical manifestations, organ/system involvement and outcome. The severity of IRs was assessed with reference to the CTCAE version 5.0 criteria and we investigated risk factors associated with severe IRs.
Results: During the study period, a total of 505 cases of inpatient drug-induced IRs were detected, of which 79.2% (400 cases) were classified as general IRs and 20.8% (105 cases) were categorized as severe IRs. The primary drugs responsible for these reactions were antibiotics (23%, 116 cases), with piperacillin sodium—sulbactam sodium being the most prevalent, followed by antineoplastic agents (18.4%, 93 cases) and traditional Chinese medicine injections (TCMIs) (12.9%, 65 cases). The administration of cefoperazone - sulbactam, mannatide, Shenqi Fuzheng, elemene, and diterpene ginkgolides meglumine resulted in a higher incidence of critical IRs. Among all cases of IRs, 43.2%, 41.2%, and 23.4% showed signs and symptoms of circulation, skin mucosa, and respiratory organs/systems, respectively. 9.1% of cases experienced systemic damage, while 7.1% and 5.9% of cases reported neurological and gastrointestinal related adverse reactions, respectively. The multivariate analysis revealed that alcohol consumption (OR = 2.389%, 95% CI 1.141–5.002, p = 0.021), age over 65 (OR = 1.814%, 95% CI 1.052–3.127, p = 0.032) and the utilization of contrast media (OR = 4.072%, 95% CI 1.903–8.713, p < 0.001) were identified as risk factors for the development of severe IRs.
Conclusion: Understanding the clinical characteristics of IRs helps to implement effective pharmaceutical monitoring and appropriate preventive measures for susceptible populations with risk factors.
1 Introduction
Intravenous infusion is a prevalent therapeutic modality in medical practice. Infusion reactions (IRs) encompass a spectrum of adverse events that occur during or following the administration of pharmacologically active substances or biologically active agents. These reactions may manifest either immediately during the infusion process or within a few hours to days post-administration. (Roselló et al., 2017). IRs were previously defined as unpredictable, also unrelated to dose and pharmacological activity of the drug, generally, they would be relieved or reversed when the treatment is terminated (Edwards and Aronson, 2000; Baldo, 2013). The two types of IRs can be classified as anaphylaxis, which is mediated by immunoglobulin E (IgE) antibody responses, and IgE non-dependent reactions (Joerger, 2012). Both types exhibit similar clinical symptoms that commonly affect the skin mucosa, respiratory system, circulatory system, gastrointestinal organs, and may pose life-threatening risks (Roselló et al., 2017).
A survey conducted by the Portuguese Pharmacovigilance System revealed that drug-induced anaphylaxis accounted for approximately 6% of all adverse drug reactions (ADRs), with antibiotics being reported as the most common causative agents, followed by acetaminophen and antineoplastics. Furthermore, contrast medium emerged as a significant contributor to allergic events (Ribeiro-Vaz et al., 2013). According to the latest research from the US Food and Drug Administration Adverse Event Reporting System, major agents associated with anaphylaxis include antibiotics, monoclonal antibodies, and non-steroidal anti-inflammatory drugs, while antibiotics, radiocontrast agents, and intraoperative drugs were linked to fatal allergic reactions (Yu et al., 2021). Additionally, the field of allergic reactions focuses on recognizing and exploring risk factors. Numerous studies have demonstrated a significant association between advanced age, coexisting asthma, and underlying cardiovascular disease with the occurrence of severe or fatal anaphylaxis (Turner et al., 2017; Kim et al., 2018; Worm et al., 2018; Pflipsen and Vega Colon, 2020). The evaluation of an in-hospital pharmacovigilance database from a tertiary care hospital in Korea shown that the use of iodine-containing contrast agents and neuromuscular blocking agents were regarded as potential risk factors for the development of anaphylactic shock (Park et al., 2017). Given that allergic reactions are a subset of IRs, it is plausible to postulate the presence of similar drug triggers and risk factors in IRs, thereby necessitating an exploration for reliable causative drugs and risk factors.
Successive reports have documented the occurrence of pegloticase, vancomycin, and intravenous iron-related IRs (Baraf et al., 2014; Alvarez-Arango et al., 2021; Stojanovic et al., 2021). The clinical features description and risk causes cognition of IRs, however, have received limited attention. The objective of this retrospective analysis of real-world data is to provide a comprehensive report on the current status of IRs, including the main culprit agents, clinical symptoms, organ/system involvement, outcome and regression. Additionally, it aims to evaluate the risk factors related to severe IRs. Understanding the clinical characteristics of IRs can help with active drug monitoring and adequate preventive measures for vulnerable groups with risk factors.
2 Methods
2.1 Clinical data
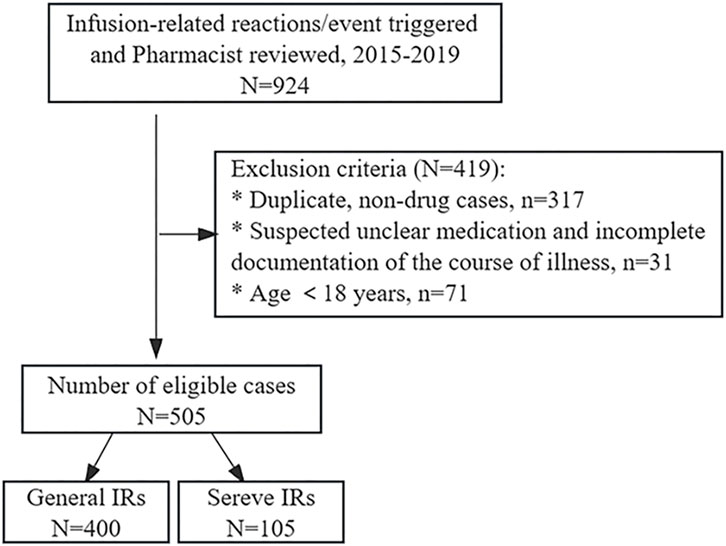
This retrospective study was based on the in-hospital pharmacovigilance system, setting triggers for infusion-related reactions/events triggers, and spanned the period from January 2015 to December 2019, with a total of 924 cases reviewed by pharmacists eligible for inclusion. Repeat reports and non-drug-induced adverse reactions, such as events related to blood transfusions and transfusion ports, were excluded. In addition, cases aged <18 years, suspected unknown drugs, and incomplete disease course were excluded (Figure 1). The patient’s demographic and clinical data, including age, gender, history of drug-related allergies, smoking status, alcohol consumption history, co-morbidities, initial suspected medication, anti-allergic pretreatment regimen, eosinophil count, clinical presentations, outcome and regression of IRs were systematically recorded. The study was reviewed and approved by the First Affiliated Hospital of Guangzhou Medical University ethics committee (No. ES-2023-146-01).
2.2 Assessment of IRs
The assessment was conducted in accordance with the National Cancer Institute common terminology criteria for adverse events (CTCAE, version 5.0). Our study protocol defines the classification of IRs as follows: Grade 1 refers to transient and mild reactions that do not necessitate interruption of the infusion, such as reducing the infusion dose or slowing down the infusion rate, and do not require any specific treatment. Grade 2 requires therapy or suspension of infusion, but can be rapidly alleviated with symptomatic treatment (e.g., antihistamines, glucocorticoids, intravenous fluids, supportive care) and prophylactic medication for less than 24 h. Grade 3 represents serious or medically important, yet not immediately life-threatening, characterized by prolonged symptom duration, poor response to symptomatic treatment, and/or relapse after initial improvement. Grade 4 indicates life-threatening and deserving urgent treatment. Grade 5 denotes death connected with an adverse event. Of these, grades 1 and 2 are categorized as general IRs, while grades 3, 4, and 5 are recognized as severe IRs.
2.3 Statistical analysis
The count data were statistically described by frequency and compared using Pearson’s chi-square test and Fisher’s exact test for univariate analysis. Variables with p-values <0.2 were screened, and logistic regression analysis was performed to determine risk factors relevant to severe IRs, with severity of IRs as the dependent variable. One-way ANOVA was employed to compare the difference in eosinophil count between groups. Statistical analyses were performed using SPSS 26.0 software (IBM, Armonk, NY, USA, RRID: SCR_016479), considering p-values <0.05 as statistically significant.
3 Results
3.1 Demographic and clinical characteristics of drug-induced IRs
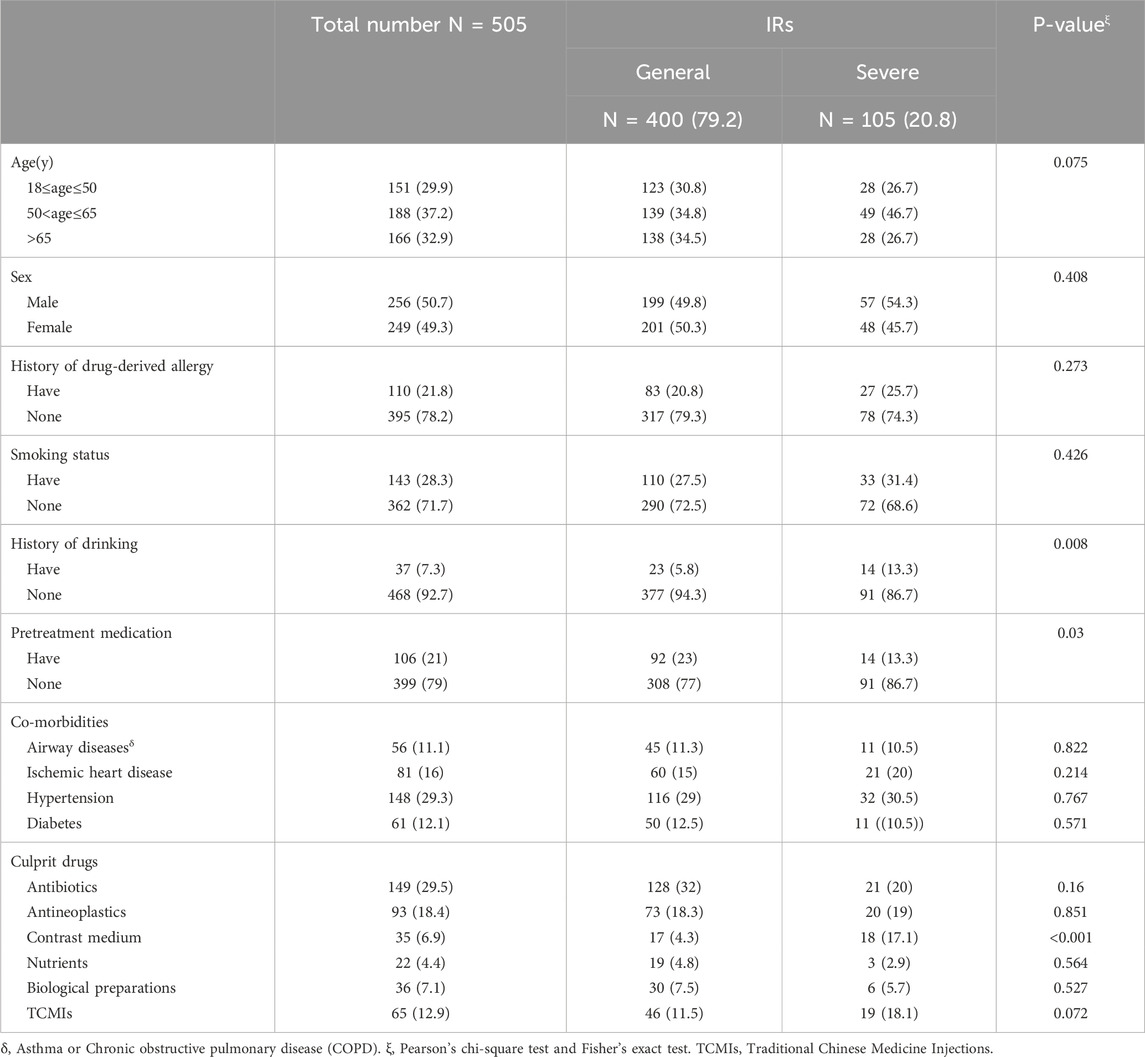
A total of 505 cases of IRs were retrieved. The severity of IRs was evaluated based on CTCAE version 5.0, of which 400 cases (79.2%) classified as mild and 105 cases (20.8%) categorized as severe. Of these patients, 151 cases (29.9%) were aged between 18 and 50 years, while 188 cases (37.2%) fell within the age range of 50–65 years. Additionally, 166 patients (32.9%) were aged over 65. Adverse reactions resulting from intravenous drug infusion were predominantly observed in patients aged 50 and above. The gender composition of male and female patients was similar, with a history of drug-induced allergies reported in 110 cases (21.8%). The majority of combined diseases were hypertensive in nature. Anti-allergic premedication was administered to 106 cases (21%), comprising 92 cases of general IRs and 14 cases of severe IRs (Table 1).
3.2 The main causative agents of IRs
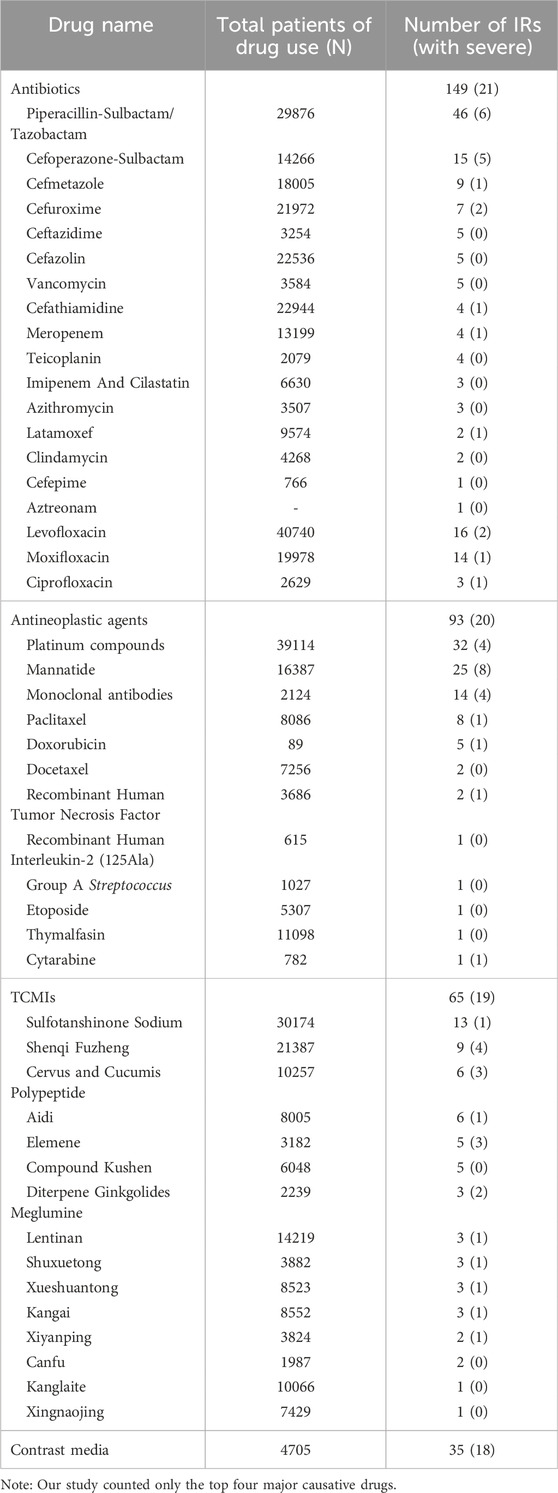
Antibiotics (116/23%) were the primary causative agents for in-hospital IRs. Among the antibiotics, piperacillin sodium-sulbactam sodium was the most frequently implicated, while cefoperazone sodium-sulbactam sodium exhibited the highest propensity for severe IRs. The second most often reported antineoplastic agents (93/18.4%) were predominantly characterized by the high prevalence of platinum compounds and mannatide, with mannatide displaying the highest number of instances resulting in severe IRs. Subsequently, Traditional Chinese Medicine Injections (TCMIs) were observed in 65 cases (12.9%), of which Sulfotanshinone sodium was the principal trigger for IRs. However, severe IRs were mainly caused by Shenqi Fuzheng, elemene and diterpene ginkgolides meglumine. Furthermore, more than half of the severe IRs were attributed to intravenous infusion of contrast media. The main causative agents of IRs may be associated with the overall frequency of utilization. However, there does not appear to exist a correlation between the frequency of utilization and the severity of IRs (Table 2).
3.3 Organ/system involvement and major clinical signs of IRs
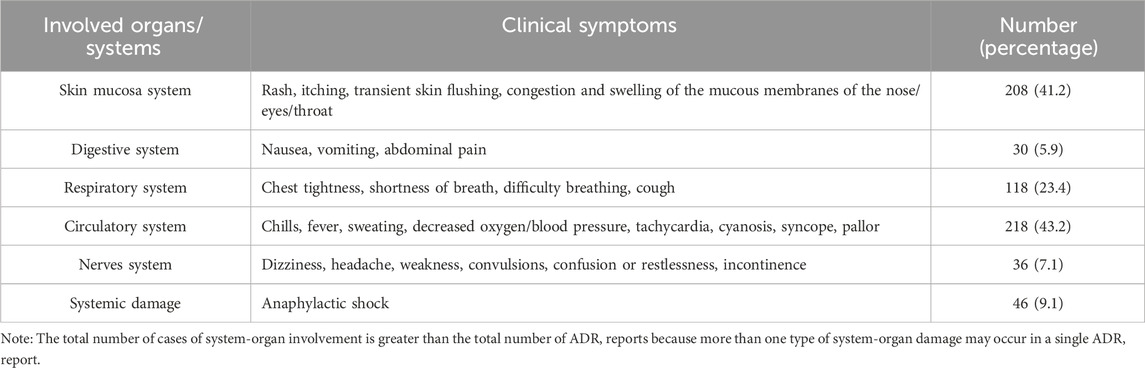
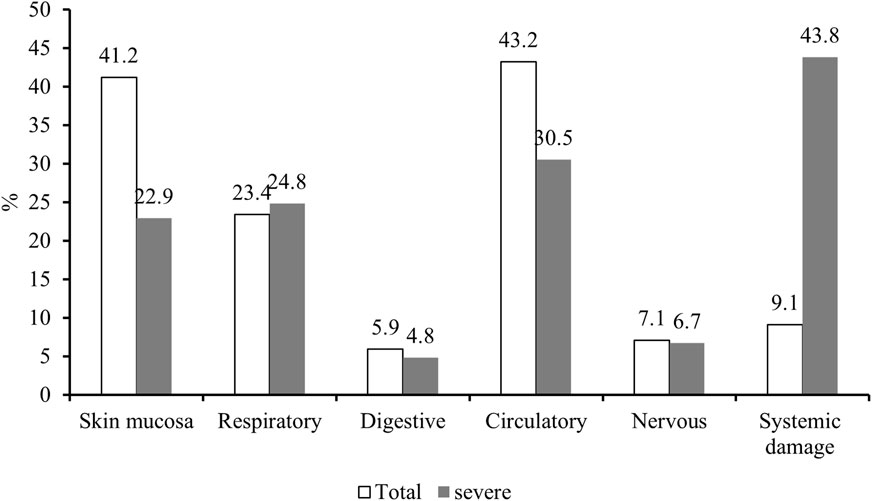
Among the 505 cases included in this retrospective analysis, 43.2%, 41.2%, and 23.4% experienced signs and symptoms of circulatory, cutaneous mucosal, and respiratory, respectively. In addition, systemic damage was reported in 9.1% of cases. While neurological and gastrointestinal adverse reactions occurred in 7.1% and 5.9% of cases, respectively. Detailed clinical manifestations are provided in Table 3.
The details of both overall and severe IRs involving various organ systems are presented in Figure 2, revealing a notable predominance of severe IRs affecting the respiratory system and significantly heightened systemic damage compared to the overall incidence of IRs.
The administration of antibiotics was predominantly associated with skin and mucosal adverse reactions, while being less correlated with circulatory events. Conversely, the use of antineoplastic agents primarily exhibited relevance to respiratory events. Moreover, the application of contrast agents often accompanied by systemic damage and less related to respiratory and circulatory events. In contrast, the side effects linked to the administration of nutritional drugs and herbal injections showed the opposite pattern to that of antibiotics (Figure 3).
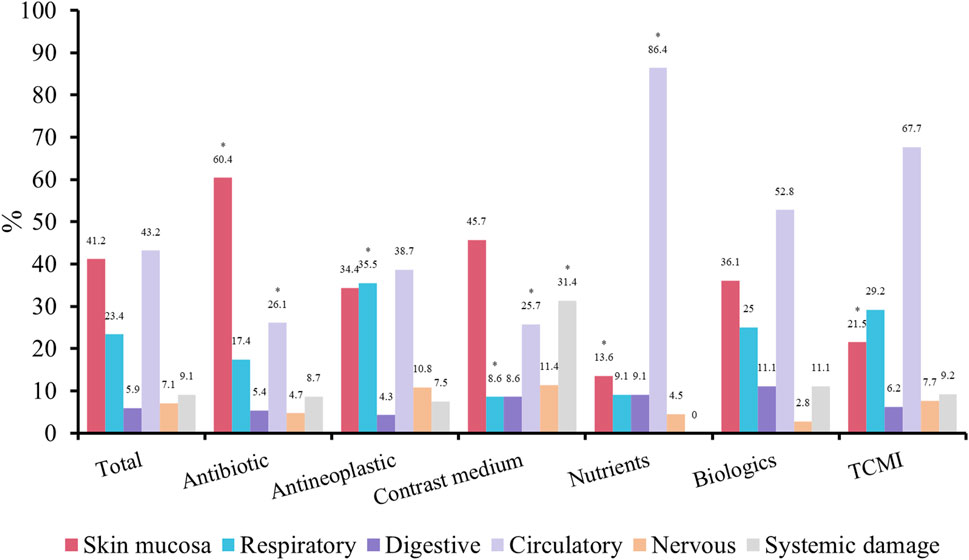
FIGURE 3. Symptoms and signs of IRs. *p < 0.05 compare with total patients. TCMI, Traditional Chinese medicine injection.
3.4 Outcome and regression
General IRs are typically transient and mild, and do not necessitate treatment discontinuation or complete resolution of signs and symptoms in all cases following interruption of infusion or management. Conversely, severe IRs often manifest as life-threatening events subsequent to intravenous drug administration, demanding immediate therapeutic intervention. Most of these cases were resolved through a series of clinical pathway interventions, including discontinuation of the suspected drug infusion, administration of intravenous fluids, anti-allergy medications (such as corticosteroids/antihistamines), and adjunctive support. However, a minority of cases exhibited unsatisfactory management outcomes with prolonged symptom duration and even two adverse event-related deaths (Table 4).
3.5 Risk factors for severe IRs
A total of 105 cases of severe IRs were reported. There was a significant association between intravenous administration of contrast media and an elevated risk of systemic damage. In addition, the administration of antineoplastic agents was found to induce severe IRs specifically affecting the respiratory system. Among these cases, cefoperazone-sulbactam, mannatide, Shenqi Fuzheng, elemene, and diterpene ginkgolides meglumine were relatively more serious IRs triggered by antibiotics, antineoplastic drugs, and TCMIs, respectively.
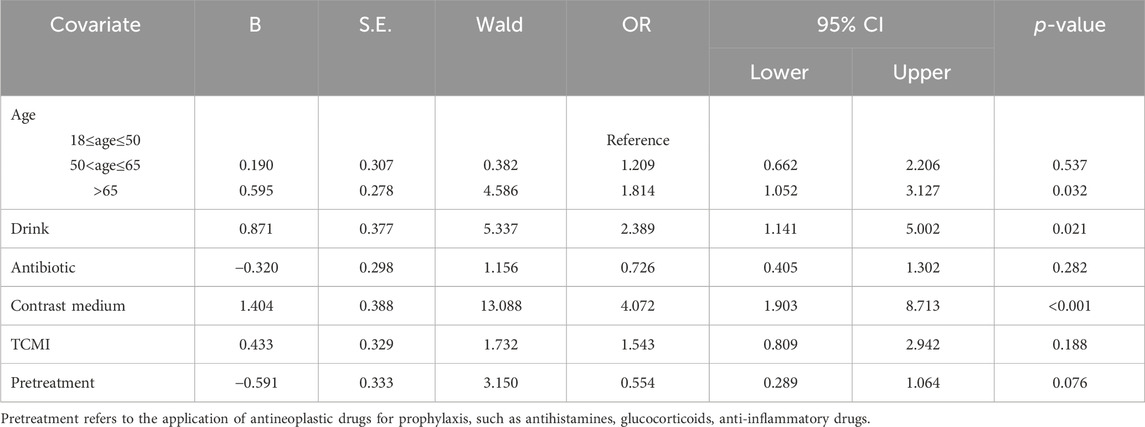
The factors influencing severe IRs were further investigated through logistic regression analysis, aiming to identify the risk factors associated with serious IRs. Univariate analysis was conducted to screen for variables with a significance level of p < 0.2, including age, alcohol consumption, use of antibiotics, contrast agents and TCMIs, as well as whether or not premedication was administered. Multivariate analysis confirmed that alcohol consumption (OR = 2.389%, 95% CI 1.141–5.002, p = 0.021), age over 65 (OR = 1.814%, 95% CI 1.052–3.127, p = 0.032) and contrast media use (OR = 4.072%, 95% CI 1.903–8.713, p < 0.001) were substantial risk factors associated with the development of serious IRs (Table 5).
3.6 Changes in eosinophil count
The number of cases that fulfilled the criteria for testing serum eosinophil counts at, before, and after the onset of the IR was 106. Multiple measurements were averaged to compare the differences between these three time points. However, no statistically significant differences were observed (Figure 4).
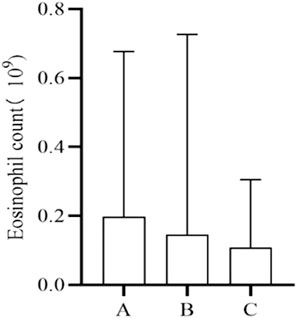
FIGURE 4. Changes in eosinophil count. A, Baseline level before IRs. B, Time level of IRs. C, Level after IRs.
4 Discussion
Our study retrospectively reported the current status of IRs based on domestic real-world data from hospital pharmacovigilance centre. Studies derived from domestic and foreign pharmacovigilance databases have consistently found the primary causative agents responsible for allergic reactions, which align with the characteristics of drugs that triggered IRs in this investigation (Ribeiro-Vaz et al., 2013; Wang et al., 2017). A majority of previous studies have demonstrated that β-lactam antibiotics were the culprit drugs of anaphylaxis (Renaudin et al., 2013; Ribeiro-Vaz et al., 2013; Turner et al., 2017), and one study suggested that piperacillin, a penicillin derivative, was the most frequently reported drug (Park et al., 2021). The current study indicated that antibiotics were the most common causative agents of IRs, with piperacillin sodium-sulbactam sodium being recorded in the greatest frequency, revealing parallels between the profiles of the drugs triggering IRs and the prevalent drugs formerly described as allergic reactions. Therefore, clinical use of medications should be alert to and prevent the possibility of IRs induced by intravenous infusion of allergy-prone drugs.
The idea that the skin mucosa is the organ/system most significantly affected by ADRs to antibiotics is consistently supported by a large number of studies (Diaz and Ciurea, 2012; Zhao et al., 2019; Jourdan et al., 2020). Therefore, meticulous attention was given to possible skin and mucosal side effects such as rash, pruritus, and flushing during intravenous infusion. Meanwhile, piperacillin, levofloxacin, cefoperazone and moxifloxacin were reported mostly antibiotics in present study. Notably, cefoperazone was the culprit of severe IRs, which need to be strengthened to prevent and monitor the drugs that cause frequent and serious ADRs.
Although platinum compounds were the primary cause of IRs among antineoplastic agents, mannatide induced the highest number of severe IRs. This may be attributed to adherence to clinical dosing principles or institutional policies, where routine administration of classical antineoplastic agents such as platinum compounds, paclitaxel, docetaxel, and monoclonal antibodies significantly mitigated the risk of IRs associated with chemotherapy drugs. The available evidence suggests that a combination of prophylactic strategies, including antihistamines, H2 antagonists, leukotriene receptor antagonists, corticosteroids, and other reasonable interventions, can effectively reduce the incidence and severity of chemotherapy drug-induced IRs while enhancing safety (ALMuhizi et al., 2022). Mannatide, a glycopeptide compound distinct from conventional anti-tumour drugs. A study on the pro-inflammatory response and chemotaxis of mannatide demonstrated its potential impact on the severity of IgE-mediated diseases, including allergic reactions (Żelechowska et al., 2021). The findings of this study caution that mannatide was susceptible to severe IRs, and this product has been linked to ADRs such as hypersensitivity responses, chest tightness and dyspnea. Therefore, it is recommended to use under close physician supervision and with resuscitative measures.
TCMIs are an extension and development of traditional Chinese medicine, boasting a rich history spanning over 70 years. As of 2017, the sale of TCMI products has been authorized for a total of 134 generic names from 224 reputable manufacturers (Li et al., 2018). The utilization of TCMIs, however, has resulted in a growing number of documented IRs (Li et al., 2019). The results of this study demonstrated that TCMIs are important triggers of IRs, often involving the circulatory system. This suggests that TCMIs are becoming more prevalent in clinical use, however, continued attention to ADRs is warranted, common clinical manifestations include chills, fever, sweating, reduced blood oxygen levels/pressure, tachycardia, cyanosis, syncope and pallor.
According to a study conducted between 2014 and 2019 on the safety of TCMIs, Shenmai, Xiangdan, Danshen, Shengmai, Huangqi, and Xuebijing injection exhibited a higher proportion of severe ADRs compared to the average (Huang et al., 2021). The findings are inconsistent with current research, which reported a higher incidence of ADRs associated with Shenqi Fuzheng, Elemene, and diterpene ginkgolides meglumine injection. The variation in culprit drugs was inferred to reflect the diversity in in-hospital drug utilization patterns among tertiary medical centers. Furthermore, there are discrepancies in the drug characteristics summary between individual hospitals and provincial municipalities. Therefore, it is imperative to implement targeted drug monitoring during the clinical application of Shenqi Fuzheng, elemene, and diterpene ginkgolides meglumine herbal injections to mitigate the occurrence of severe IRs.
Previous studies have primarily focused on the risk factors of individual drug-induced IRs, such as infliximab (Duron et al., 2015; Wang et al., 2019) and cetuximab (Touma et al., 2014). The present study investigated the risk factors of severe IRs, and established alcohol consumption, age over 65 and the application of contrast media as risk factors for severe IRs. The reports stated that drinking may interfere with the absorption, distribution, metabolism and excretion of drugs, thereby increase the likelihood of ADR (Weathermon and Crabb, 1999; Castle et al., 2016). To prevent alcohol-drug interactions, it is recommended to avoid consuming alcohol while taking drugs that interact with it. Drug use varies by age. Polypharmacy is very common in the elderly, which may contribute to mainly the growth in the severity of IRs in older individuals (Jerschow et al., 2014). Among pathogenic medications, the use of contrast agents was found to increase the vulnerability to critical IRs, which may be attributed to their inherent properties. The p-value for univariate analysis of anti-allergic pretreatment was 0.03, while for multivariate analysis it approached 0.05, suggesting a potential protective effect against drug-induced severe IRs. However, due to limitations in sample size, this difference was not statistically significant. Moreover, several studies have identified comorbidities such as asthma, chronic obstructive pulmonary disease (COPD), and cardiovascular disorders as significant risk factors for severe allergic reactions (Simons et al., 2011; Turner et al., 2017). In this study, airway disease (asthma/COPD), hypertension, diabetes mellitus, and ischemic heart disease were assessed as co-morbidities increasing the risk of severe IRs, however, these differences did not reach statistical significance. Adverse event-related deaths occurred in 2 cases, mainly owning to patients suffering from an underlying disease that did not progress optimistically progressing to cardiac arrest, with the corresponding medication considered as a secondary factor.
Serum eosinophilia can arise from various drug reactions, including but not limited to NSAIDs, antibiotics, and anticonvulsants (Gottlieb et al., 2022; Hama et al., 2022; Awad et al., 2023). We endeavored to assess alterations in eosinophil counts following drug-induced IRs, unfortunately, no statistically significant differences were observed when comparing eosinophil counts before, during, and after the onset of IRs. This analysis may be attributed to the fact that the causative drugs of IRs are not among the primary agents known to induce serum eosinophilia.
This study has several limitations. Firstly, it was a retrospective analysis, which inevitably introduces bias and confounding factors. Additionally, accurately tracking the time interval between drug infusion and onset of adverse reactions posed challenges. Moreover, the rate of infusion may be considered an influential factor in IRs. To overcome these constraints and provide a more precise evaluation of severe IR risk factors, large-scale prospective studies are warranted.
In conclusion, the present study retrospectively reported an update on IRs based on domestic real-world data from hospital pharmacovigilance center and demonstrated antibiotics, antineoplastic agents and TCMIs as the prime culprit drugs in the tertiary care center, with a relatively high number of drugs triggering serious IRs including cefoperazone and sulbactam, mannatide, Shenqi Fuzheng, elemene and diterpene ginkgolides meglumine. The occurrence of IRs may be associated with the total number of medications administered. However, no such correlation seems to exist in terms of severity. In addition, alcohol consumption, age over 65 and the use of contrast media were risk factors of serious IRs. Therefore, reaching a comprehensive understanding of the clinical characteristics of IRs will facilitate active pharmacovigilance and the implementation of appropriate preventive measures for susceptible groups with risk factors.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the First Affiliated Hospital of Guangzhou Medical University ethics committee (No. ES-2023-146-01). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
WY: Conceptualization, Data curation, Writing–original draft, Writing–review and editing. BW: Conceptualization, Investigation, Writing–review and editing. GW: Data curation, Writing–review and editing. ZW: Investigation, Writing–review and editing. XK: Writing–review and editing, Data curation. YW: Data curation, Writing–review and editing. XM: Investigation, Writing–review and editing. XO: Data curation, Writing–review and editing. LW: Supervision, Writing–review and editing, Conceptualization. PY: Conceptualization, Project administration, Supervision, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by 2022 Special Fund for Hospital Pharmaceutical Research of Guangdong Province Hospital Association (No. YXKY202218), Guangzhou Municipal Science and Technology Bureau Joint Project (No. 202102010186), Guangzhou Municipal Science and Technology Bureau Minsheng Science and Technology Research Plan Project (No. 201803010063), Guangzhou Municipal Science and Technology Bureau Project- 2024 Municipal Schools (Institutes) and Enterprises Joint Grants (No. SL 2024A03J01351).
Acknowledgments
We are grateful to patients from the First Affiliated Hospital of Guangzhou Medical University who participated in this study, as well as all healthcare workers involved in the diagnosis and treatment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Almuhizi, F., De Las Vecillas Sanchez, L., Gilbert, L., Copaescu, A. M., and Isabwe, G. A. C. (2022). Premedication protocols to prevent hypersensitivity reactions to chemotherapy: a literature review. Clin. Rev. Allergy Immunol. 62 (3), 534–547. doi:10.1007/s12016-022-08932-2
Alvarez-Arango, S., Ogunwole, S. M., Sequist, T. D., Burk, C. M., and Blumenthal, K. G. (2021). Vancomycin infusion reaction - moving beyond "red man syndrome. N. Engl. J. Med. 384 (14), 1283–1286. doi:10.1056/NEJMp2031891
Awad, A., Goh, M. S., and Trubiano, J. A. (2023). Drug reaction with eosinophilia and systemic symptoms: a systematic review. J. Allergy Clin. Immunol. Pract. 11, 1856–1868. doi:10.1016/j.jaip.2023.02.035
Baldo, B. A. (2013). Adverse events to monoclonal antibodies used for cancer therapy: focus on hypersensitivity responses. Oncoimmunology 2 (10), e26333. doi:10.4161/onci.26333
Baraf, H. S., Yood, R. A., Ottery, F. D., Sundy, J. S., and Becker, M. A. (2014). Infusion-related reactions with pegloticase, a recombinant uricase for the treatment of chronic gout refractory to conventional therapy. J. Clin. Rheumatol. 20 (8), 427–432. doi:10.1097/rhu.0000000000000200
Castle, I. J., Dong, C., Haughwout, S. P., and White, A. M. (2016). Emergency department visits for adverse drug reactions involving alcohol: United States, 2005 to 2011. Alcohol Clin. Exp. Res. 40 (9), 1913–1925. doi:10.1111/acer.13167
Diaz, L., and Ciurea, A. M. (2012). Cutaneous and systemic adverse reactions to antibiotics. Dermatol Ther. 25 (1), 12–22. doi:10.1111/j.1529-8019.2012.01494.x
Duron, C., Goutte, M., Pereira, B., Bommelaer, G., and Buisson, A. (2015). Factors influencing acute infusion reactions in inflammatory bowel disease patients treated with infliximab in the era of scheduled maintenance therapy. Eur. J. Gastroenterol. Hepatol. 27 (6), 705–711. doi:10.1097/meg.0000000000000354
Edwards, I. R., and Aronson, J. K. (2000). Adverse drug reactions: definitions, diagnosis, and management. Lancet 356 (9237), 1255–1259. doi:10.1016/s0140-6736(00)02799-9
Gottlieb, M., Figlewicz, M. R., Rabah, W., Buddan, D., and Long, B. (2022). Drug reaction with eosinophilia and systemic symptoms: an emergency medicine focused review. Am. J. Emerg. Med. 56, 1–6. doi:10.1016/j.ajem.2022.03.024
Hama, N., Abe, R., Gibson, A., and Phillips, E. J. (2022). Drug-induced hypersensitivity syndrome (DIHS)/Drug reaction with eosinophilia and systemic symptoms (DRESS): clinical features and pathogenesis. J. Allergy Clin. Immunol. Pract. 10 (5), 1155–1167.e5. e1155. doi:10.1016/j.jaip.2022.02.004
Huang, R., Cai, Y., Yang, L., Shangguan, X., Ghose, B., and Tang, S. (2021). Safety of traditional Chinese medicine injection based on spontaneous reporting system from 2014 to 2019 in Hubei Province, China. Sci. Rep. 11 (1), 8875. doi:10.1038/s41598-021-88339-9
Jerschow, E., Lin, R. Y., Scaperotti, M. M., and McGinn, A. P. (2014). Fatal anaphylaxis in the United States, 1999-2010: temporal patterns and demographic associations. J. Allergy Clin. Immunol. 134 (6), 1318–1328. e1317. doi:10.1016/j.jaci.2014.08.018
Joerger, M. (2012). Prevention and handling of acute allergic and infusion reactions in oncology. Ann. Oncol. 23, x313–x319. Suppl 10. doi:10.1093/annonc/mds314
Jourdan, A., Sangha, B., Kim, E., Nawaz, S., Malik, V., Vij, R., et al. (2020). Antibiotic hypersensitivity and adverse reactions: management and implications in clinical practice. Allergy Asthma Clin. Immunol. 16, 6. doi:10.1186/s13223-020-0402-x
Kim, S. Y., Kim, M. H., and Cho, Y. J. (2018). Different clinical features of anaphylaxis according to cause and risk factors for severe reactions. Allergol. Int. 67 (1), 96–102. doi:10.1016/j.alit.2017.05.005
Li, H., Deng, J., Deng, L., Ren, X., and Xia, J. (2019). Safety profile of traditional Chinese herbal injection: an analysis of a spontaneous reporting system in China. Pharmacoepidemiol Drug Saf. 28 (7), 1002–1013. doi:10.1002/pds.4805
Li, H., Wang, S., Yue, Z., Ren, X., and Xia, J. (2018). Traditional Chinese herbal injection: current status and future perspectives. Fitoterapia 129, 249–256. doi:10.1016/j.fitote.2018.07.009
Park, C. S., Yang, M. S., Kang, D. Y., Park, H. J., Park, S. Y., Nam, Y. H., et al. (2021). Risk factors of beta-lactam anaphylaxis in Korea: a 6-year multicenter retrospective adult case-control study. World Allergy Organ J. 14 (9), 100580. doi:10.1016/j.waojou.2021.100580
Park, H. K., Kang, M. G., Yang, M. S., Jung, J. W., Cho, S. H., and Kang, H. R. (2017). Epidemiology of drug-induced anaphylaxis in a tertiary hospital in Korea. Allergol. Int. 66 (4), 557–562. doi:10.1016/j.alit.2017.02.008
Pflipsen, M. C., and Vega Colon, K. M. (2020). Anaphylaxis: recognition and management. Am. Fam. Physician 102 (6), 355–362.
Renaudin, J. M., Beaudouin, E., Ponvert, C., Demoly, P., and Moneret-Vautrin, D. A. (2013). Severe drug-induced anaphylaxis: analysis of 333 cases recorded by the Allergy Vigilance Network from 2002 to 2010. Allergy 68 (7), 929–937. doi:10.1111/all.12168
Ribeiro-Vaz, I., Marques, J., Demoly, P., Polónia, J., and Gomes, E. R. (2013). Drug-induced anaphylaxis: a decade review of reporting to the Portuguese Pharmacovigilance Authority. Eur. J. Clin. Pharmacol. 69 (3), 673–681. doi:10.1007/s00228-012-1376-5
Roselló, S., Blasco, I., García Fabregat, L., Cervantes, A., and Jordan, K. (2017). Management of infusion reactions to systemic anticancer therapy: ESMO Clinical Practice Guidelines. Ann. Oncol. 28, iv100–iv118. suppl_4. doi:10.1093/annonc/mdx216
Simons, F. E., Ardusso, L. R., Bilò, M. B., El-Gamal, Y. M., Ledford, D. K., Ring, J., et al. (2011). World allergy organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ J. 4 (2), 13–37. doi:10.1097/WOX.0b013e318211496c
Stojanovic, S., Graudins, L. V., Aung, A. K., Grannell, L., Hew, M., and Zubrinich, C. (2021). Safety of intravenous iron following infusion reactions. J. Allergy Clin. Immunol. Pract. 9 (4), 1660–1666. doi:10.1016/j.jaip.2020.11.028
Touma, W., Koro, S. S., Ley, J., Wildes, T. M., Michel, L., Tao, Y., et al. (2014). Risk factors for and pre-medications to prevent cetuximab-induced infusion reactions in patients with squamous cell carcinoma of the head and neck. Oral Oncol. 50 (9), 895–900. doi:10.1016/j.oraloncology.2014.06.017
Turner, P. J., Jerschow, E., Umasunthar, T., Lin, R., Campbell, D. E., and Boyle, R. J. (2017). Fatal anaphylaxis: mortality rate and risk factors. J. Allergy Clin. Immunol. Pract. 5 (5), 1169–1178. doi:10.1016/j.jaip.2017.06.031
Wang, T., Ma, X., Xing, Y., Sun, S., Zhang, H., Stürmer, T., et al. (2017). Use of epinephrine in patients with drug-induced anaphylaxis: an analysis of the Beijing pharmacovigilance database. Int. Arch. Allergy Immunol. 173 (1), 51–60. doi:10.1159/000475498
Wang, X., Cao, J., Wang, H., and Ye, C. (2019). Risk factors associated with infusion reactions to infliximab in Chinese patients with inflammatory bowel disease: a large single-center study. Med. Sci. Monit. 25, 2257–2264. doi:10.12659/msm.913152
Weathermon, R., and Crabb, D. W. (1999). Alcohol and medication interactions. Alcohol Res. Health 23 (1), 40–54.
Worm, M., Francuzik, W., Renaudin, J. M., Bilo, M. B., Cardona, V., Scherer Hofmeier, K., et al. (2018). Factors increasing the risk for a severe reaction in anaphylaxis: an analysis of data from the European Anaphylaxis Registry. Allergy 73 (6), 1322–1330. doi:10.1111/all.13380
Yu, R. J., Krantz, M. S., Phillips, E. J., and Stone, C. A. (2021). Emerging causes of drug-induced anaphylaxis: a review of anaphylaxis-associated reports in the fda adverse event reporting system (faers). J. Allergy Clin. Immunol. Pract. 9 (2), 819–829. e812. doi:10.1016/j.jaip.2020.09.021
Żelechowska, P., Brzezińska-Błaszczyk, E., Różalska, S., Agier, J., and Kozłowska, E. (2021). Mannan activates tissue native and IgE-sensitized mast cells to proinflammatory response and chemotaxis in TLR4-dependent manner. J. Leukoc. Biol. 109 (5), 931–942. doi:10.1002/jlb.4a0720-452r
Keywords: infusion reactions, clinical characteristics, risk factors, pharmacovigilance, tertiary hospital
Citation: Yin W, Wen B, Wang G, Wang Z, Kong X, Wu Y, Meng X, Ou X, Wei L and Yu P (2024) Clinical characteristics and risk factors analysis of 505 cases of infusion reactions in a tertiary hospital. Front. Pharmacol. 15:1292347. doi: 10.3389/fphar.2024.1292347
Received: 11 September 2023; Accepted: 25 January 2024;
Published: 06 February 2024.
Edited by:
Ahmet Akici, School of Medicine, Marmara University, TürkiyeReviewed by:
Emma Monique Tillman, Indiana University, United StatesEleonora Lai, University Hospital and University of Cagliari, , Italy
Copyright © 2024 Yin, Wen, Wang, Wang, Kong, Wu, Meng, Ou, Wei and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pengjiu Yu, eXBqNzI1QDE2My5jb20=; Li Wei, cnVua2luZ29uZUAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Weiwei Yin
Weiwei Yin Bingqin Wen1†
Bingqin Wen1† Guoan Wang
Guoan Wang Xuetao Kong
Xuetao Kong Yaozhou Wu
Yaozhou Wu Pengjiu Yu
Pengjiu Yu