- 1Department of Pharmacy, Children’s Hospital of Chongqing Medical University, National Clinical Research Center for Child Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing Key Laboratory of Pediatric Metabolism and Inflammatory Diseases, Chongqing, China
- 2Department of Pharmacy, People’s Hospital of Chongqing Liangjiang New Area, Chongqing, China
- 3Department of hematology, Children’s Hospital of Chongqing Medical University, Chongqing, China
Background: Voriconazole (VRZ) is involved in a variety of drug‒drug interactions (DDIs), but few studies have reported adverse events (AEs) associated with the DDIs of VRZ. The primary goal of this study was to analyse the potential risk factors for AEs caused by DDIs between VRZ and other drugs via the OpenVigil FDA platform and to provide a reference for preventing VRZ DDIs and monitoring clinically related adverse drug events.
Methods: A retrospective pharmacovigilance study was conducted to investigate the AEs related to DDIs between VRZ and four categories of drugs: proton pump inhibitors (PPIs), non-steroidal anti-inflammatory drugs (NSAIDs), immunosuppressants, and other antibacterial drugs. AE information for the target drugs from the first quarter of 2004 to the third quarter of 2022 was downloaded from the OpenVigil FDA data platform. Four frequency statistical models—the reporting ratio method, Ω shrinkage measure model, combination risk ratio model, and the chi-square statistics model—were used to analyse the AEs related to DDIs and evaluate the correlation and influence of sex and age between the drug(s) and the target AEs detected.
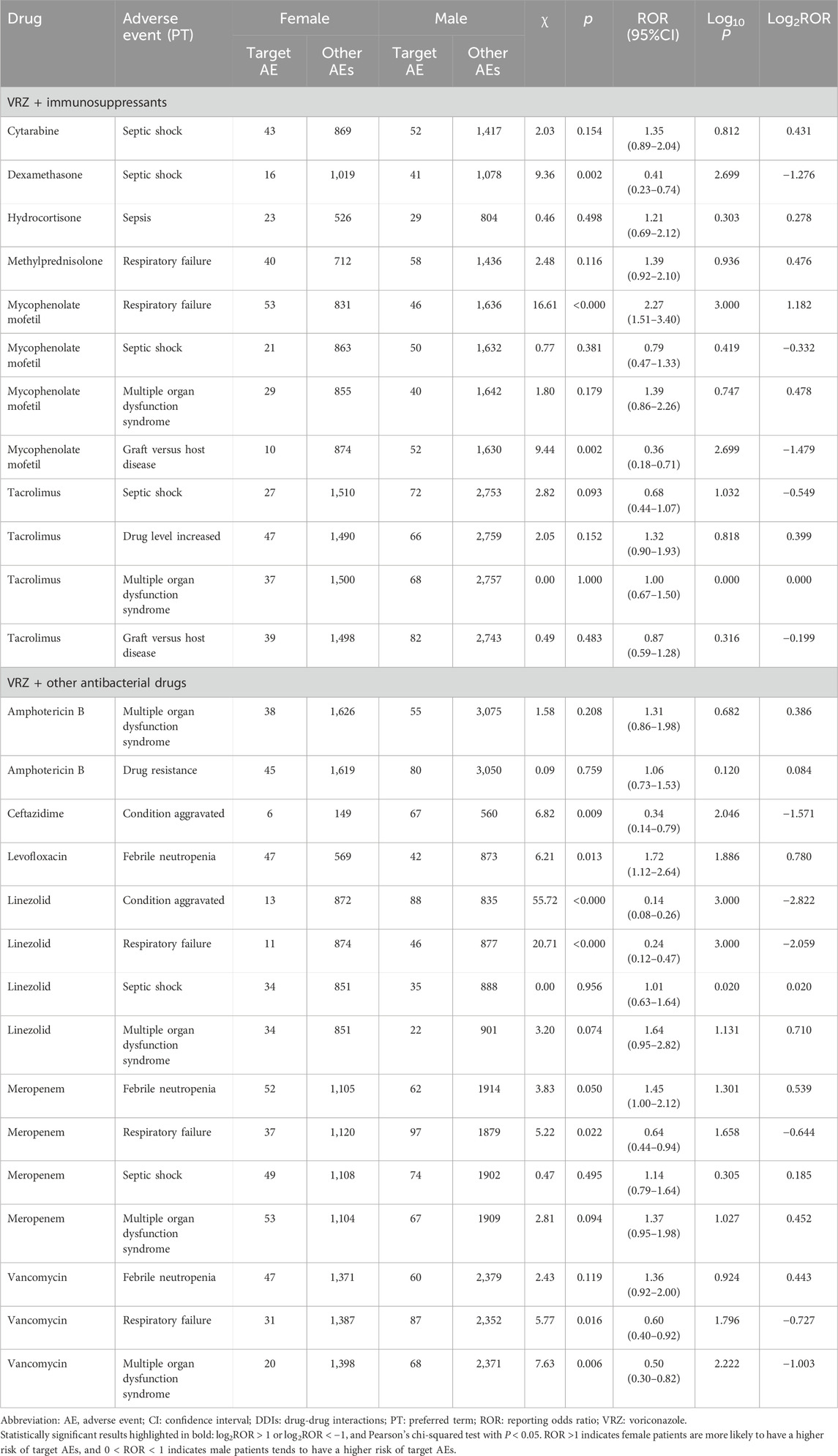
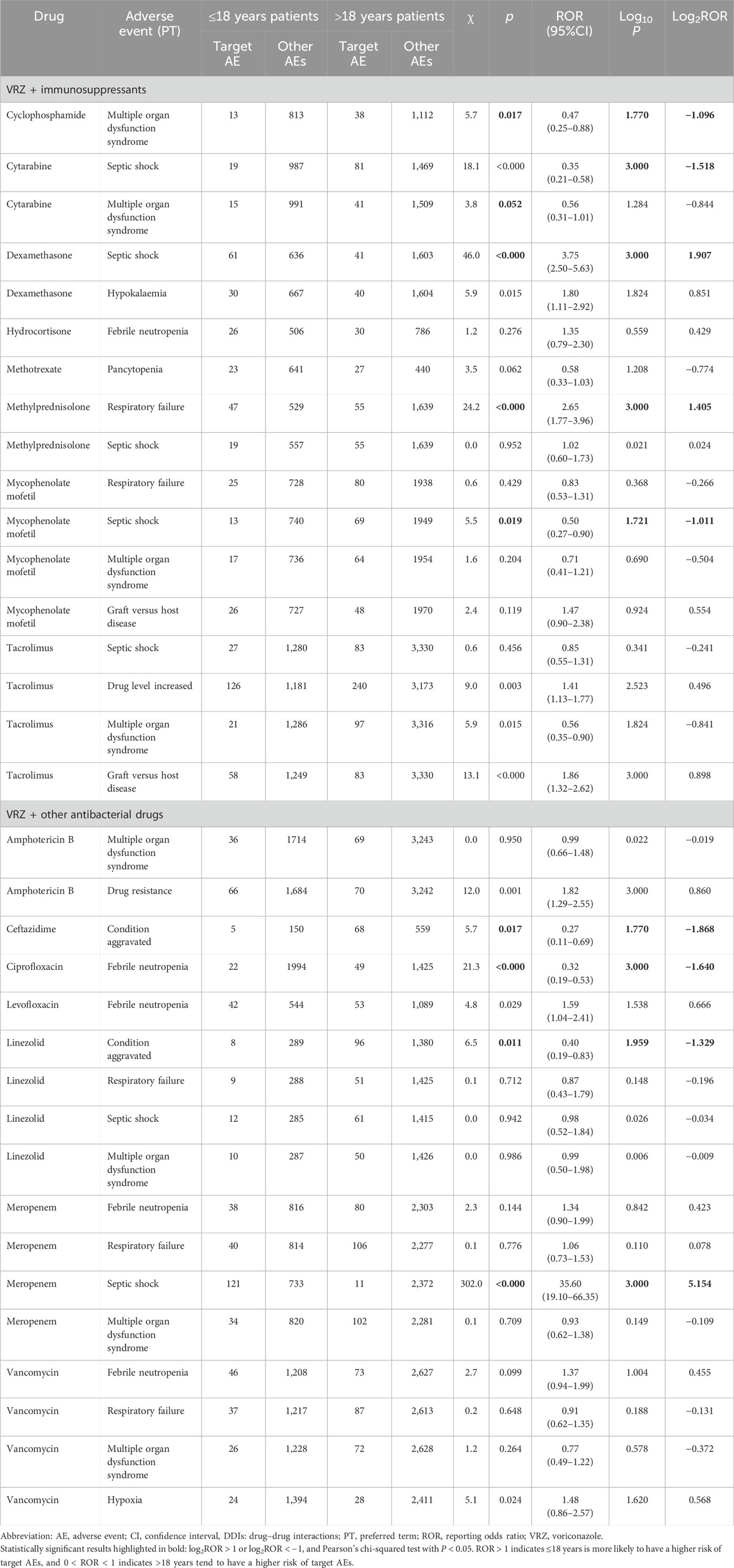
Results: A total of 38 drugs were included, with 262 AEs detected by at least one of the four models and 48 AEs detected by all four models. Some 77 detected AEs were significantly positively correlated with DDIs and were related to higher reporting rates of AEs than when used alone. Graft-versus-host disease was the AE that had the strongest correlation with the drug interaction between VRZ and immunosuppressants (tacrolimus, mycophenolate mofetil, cyclophosphamide, and cyclosporine), and multiple organ dysfunction syndrome was correlated with VRZ in combination with other antibacterial drugs (linezolid, meropenem, cefepime, and vancomycin). Significant sex and age differences in the target AEs were detected for five and nine target drugs, respectively. For VRZ in combination with linezolid, aggravated conditions and respiratory failure should be given more attention in male patients, and mycophenolate mofetil and respiratory failure in female patients. When conditions are aggravated, febrile neutropenia and septic shock should be of particular concern in patients over 18 years of age who use VRZ in combination with ceftazidime, ciprofloxacin, or cytarabine. In patients aged under 18, septic shock should be considered when VRZ is used in combination with meropenem and dexamethasone.
Conclusion: AEs related to DDIs should receive more attention when VRZ is used in combination with PPIs (renal impairment), NSAIDs (constipation and renal failure), immunosuppressants (graft versus host disease, septic shock) and other antibacterial drugs (multiple organ dysfunction syndrome, febrile neutropenia, and respiratory failure). Considering the influence of sex and age differences in VRZ DDIs, these factors need to be considered when assessing the risk of AEs in patients receiving VRZ and other drugs.
1 Background
Voriconazole (VRZ) is a synthetic second-generation broad-spectrum triazole agent that is recommended for first-line treatment and the prevention of a variety of invasive fungal diseases such as invasive aspergillosis, oesophageal candidiasis, and severe infections caused by Scedosporium apiospermum and Fusarium spp. (Lee et al., 2021; Pfizer, 2020). VRE is highly prone to drug‒drug interactions (DDIs), mainly related to cytochrome P450 (CYP450) enzymes, as many commonly used prescription drugs are also metabolized through these enzymes (Job et al., 2016). Many additional factors, such as plasma concentration, age, and other complications, may also affect the DDI of VRZ (Pfizer, 2020; Tian et al., 2021). Therefore, health professionals should be more cautious when prescribing VRZ because of the high risk of DDIs.
A number of studies have shown that DDIs are the primary cause of adverse events (AEs) and are considered one of the most serious global health concerns (Kantor et al., 2015). In clinical practice, DDIs often occur in patients with complications and concomitant medications (Prybys et al., 2002). Previous studies have reported that with increasing rates of concomitant medications, the risk of AEs increased from 13% for two drugs to 58% for five drugs (Kantor et al., 2015; Prybys et al., 2002). Rodrigues and Oliveira (2016) reported that the majority of fungal infection patients suffer from other diseases and need to be treated with other drugs, which is more likely to lead to DDIs. The guidelines recommend that the efficacy and safety of VRZ should be closely monitored when it is used in combination with proton pump inhibitors (PPIs) (e.g., omeprazole, aesomeprazole, pantoprazole, rabeprazole, and lansoprazole), immunosuppressant agents (e.g., glucocorticoids, cyclosporine, and tacrolimus), and other antibacterial drugs (e.g., erythromycin, azithromycin, and clarithromycin) which may lead to unexpected toxicity or decreased therapeutic efficacy due to potential DDIs (Chen et al., 2018). Therefore, identifying potential DDIs between VRZ and commonly prescribed drugs is of great clinical importance and may help to reduce the risk of AEs.
Existing research has revealed a variety of DDIs between VRZ and other drugs, but the occurrence of interaction-related AEs of VRZ is rarely reported (Groll et al., 2017). A publicly available FDA AE Reporting System (FAERS) database was used as an efficient tool for identifying DDIs of VRZ. The US FDA Open Data Project (OpenVigil FDA) platform is a novel web-based pharmacovigilance analysis tool that uses the OpenFDA online interface to access the drug-event dataset from FAERS; it can provide disproportionality analyses to estimate rare or new adverse drug reactions and check arbitrary combinations of two drugs for unknown DDI signals (Meng et al., 2022; Böhm et al., 2016; Böhm et al., 2021). Thus, the primary goal of this study was to analyse the potential risk factors for AEs caused by DDIs between VRZ and the drugs usually used in combination via the OpenVigil FDA platform and to provide a reference for preventing VRZ DDIs and monitoring clinically related adverse drug events.
2 Methods
2.1 Study design
A cross-sectional and retrospective pharmacovigilance study was conducted to investigate AEs related to drug interactions between VRZ and other drugs usually used in combination with VRZ and to search for possible DDIs. On the basis of the relevant literature and guidelines (Job et al., 2016; Chen et al., 2018; Thomas et al., 2009; Dong et al., 2024; Idoate et al., 2019; Kim et al., 2010), the current situation of the concomitant use of VRZ, and discussion with clinical experts, the four most concerning and widely prescribed drug classes that may affect drug safety and are commonly used in combination with VRE in the clinic—PPIs, non-steroidal anti-inflammatory drugs (NSAIDs), immunosuppressants, and other antibacterial drugs—were included in this analysis.
2.2 Data sources and selection criteria
Relevant data from the first quarter of 2004 to the third quarter of 2022 were downloaded from the OpenVigil FDA data platform (https://openvigil.pharmacology.uni-kiel.de/openvigilfda.php). If there were more than three reports of AEs related to VRZ and the target drug interaction (Noguchi and Teramachi, 2020), the AE information of the target drug used alone or in combination with VRZ was further extracted for AE interaction analysis. AEs recorded in the OpenVigil FDA database were coded according to the preferred term (PT) by the International Harmonized Conference on Human Drug Registration Technology (ICH) in the Medical Dictionary for Regulatory Activities Terminology (MedDRA) (Wong et al., 2015).
2.3 Statistical analysis
Descriptive analyses were used to summarize the data of the AE reports collected from the OpenVigil FDA platform, and the number (%) was used for qualitative variables. A P value of less than 0.05 was considered statistically significant, and all the statistical analyses were performed on a personal computer with the SPSS for Windows statistical package (version 22.0). Image processing for correlation analysis and influencing factor analysis of DDIs was performed with R 4.2.2 software.
2.3.1 Statistical models and criteria for the detection of adverse events
Four frequency statistical models of signal mining were used to calculate the threshold and detect potential AEs related to DDIs (Noguchi and Teramachi, 2020): the reporting ratio method (Böhm et al., 2021), Ω shrinkage measure model (Norén et al., 2008), combination risk ratio model (Susuta and Takahashi, 2014), and the chi-square statistics model (Gosho et al., 2017). The related parameters and algorithms of these models are shown in Supplementary Tables S1–3, and a brief description is provided below.
(1) Reporting ratio method: this method was used to compare the frequency of AEs between drugs used alone and those used in combination. If the observed rate of AEs for the combination was greater than the expected rate (the sum of the occurrence frequency when each drug was used separately) —that is, if the percentage difference (R_diff) was <0—positive safety signals for the DDI of the two drugs were detected.
(2) Ω shrinkage measurement model: this model calculates the logarithm of the ratio between the actual observation value and the expected value of the target AE reports when the drug is used in combination. When Ω > 0.25, positive safety signals for the DDI of the two drugs were detected.
(3) Combination risk ratio model: this model evaluates probabilities of drug interactions by calculating the comprehensive risk of the target AE—that is, to evaluate the ratio of the proportional reporting ratio (PRR) —when two drugs are used together (PRR) and the ratio of the PRR when one of the two drugs is used alone (PRR1, PRR2). In cases where both drugs were used and the target AE occurred ≥3, a combination risk ratio >2, PRR>2, and χ2 > 4, positive safety signals for the DDI of the two drugs were detected (Böhm et al., 2016).
(4) Chi-square statistics model: this model uses chi-square statistics to estimate the discrepancy between the observed and expected values of target AEs with two drugs used together. When χ > 2, the positive safety signals for the DDI of the two drugs were detected.
2.3.2 Correlation analysis
The relative reporting ratio (RRR) was used to quantitatively measure the correlation between the drug(s) and the target AEs, with positive signals of DDIs detected by the above models; the greater the RRR, the greater the correlation between the drug and the target AE (Glotzbecker et al., 2012). The RRR was calculated as follows:
where a and b represent the same parameters as in the combination risk ratio model, and the related parameters are shown in Supplementary Table S1. Delta_RRR is the difference between the RRRE value when two drugs are used together and the mean RRR1 and RRR2 when two drugs are used alone. The Delta_RRR was calculated as follows:
The ratio of Delta_RRR to the mean Delta_RRR of all AEs (Delta_RRRall), defined as RRR_diff, was used to evaluate the difference in the correlation degree of AEs between two drugs used together or alone (Böhm et al., 2016). The RRR_diff was calculated as follows:
The RRR_diff values of related adverse drug events with interaction signals detected by the above four models were calculated to evaluate the degree of correlation difference. When RRR_diff >0.75, the correlation between the target AE and the combined use of two drugs is greater than that of two single drugs, which indicates a significant positive correlation and leads to an increased risk of target AEs when the drug is used in combination (Böhm et al., 2016). When RRR_diff < −0.75, the correlation between the target AE and the two drugs used alone is greater than when two drugs are used together, which indicates a significant negative correlation, and the risk of target AEs is greater when two drugs are used alone.
2.3.3 Influencing factors analysis
The DDIs related to adverse drug events with positive interaction signals detected by at least one of the above four models and an RRR_diff value >0.75 were included to evaluate the influence of sex and age. A two-by-two contingency table was used to calculate the reporting odds ratio (ROR) values and 95% confidence intervals (CIs). Related parameters are shown in Supplementary Table S4, and the algorithms were as follows:
Criteria for inclusion in the statistical analysis were a > 5, c > 5, a+c>50. Pearson’s chi-square test was used to compare the differences in the risk of target AEs between female and male patients and between ≤18- and >18-year-old patients. The standards for statistical significance were as follows: a Pearson’s chi-square test with P < 0.05 (−log10 P value >1.301) and a log2ROR > 1 indicates that female or ≤18-year-old patients are more likely to have a greater risk of target AEs, and P < 0.05 (−log10 P value > 1.301) and a log2ROR < −1 indicate that male or >18-year-old patients tend to have a greater risk of target AEs (Yu et al., 2016).
3 Results
3.1 General results
There were no AE reports about piperacillin sodium, sulbactam sodium, cefoperazone sodium, sulbactam sodium, cefathiamidine, cefotiam, or ceftizoxime in combination with VRZ recorded in the OpenVigil FDA platform. The z number of AE reports about amoxicillin, clavulanate potassium, cefadroxil, cefaclor, and cefixime combined with VRZ was less than three (Böhm et al., 2016). Thus, 38 drugs commonly used in combination with VRZ were included in this study for the detection of AEs related to DDIs. The 38 are:
(1) PPIs: aesomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole.
(2) NSAIDs: acetaminophen, aspirin, celecoxib, and ibuprofen.
(3) Immunosuppressants: azathioprine, cytarabine, cyclosporine, cyclophosphamide, dexamethasone, hydrocortisone, methylprednisolone, methotrexate, mycophenolate mofetil, tacrolimus, and tocilizumab.
(4) Other antibacterial drugs: amphotericin B, azithromycin, caspofungin, cefazolin, cefepime, cefpodoxime proxetil, ceftazidime, ceftriaxone, cefuroxime, clarithromycin, ciprofloxacin, imipenem, imipenem and cilastatin sodium, levofloxacin, linezolid, meropenem, sulfamethoxazole, and vancomycin.
A total of 15,234,431 AE reports were recorded in the FAERS database on the OpenVigil FDA platform as of 30 September 2022, of which 4,563, 2,392, 20,964, and 25,583 AE reports were recorded when VRE was used in combination with PPIs, NSAIDs, immunosuppressants, and other antibacterial drugs, respectively. The flow chart is shown in Figure 1.
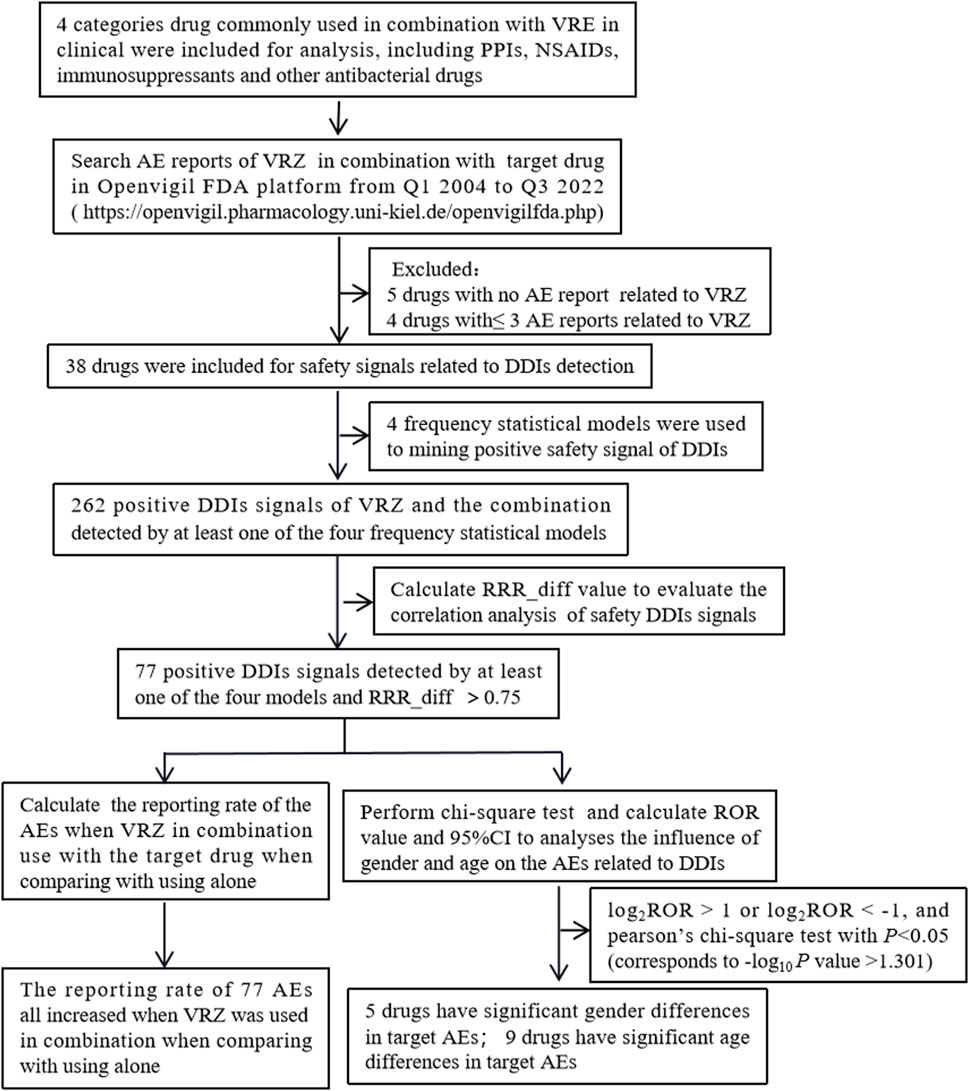
Figure 1. Flow chart of the inclusion of adverse events (Abbreviations: AE, adverse event; DDIs, drug‒drug interactions; RRR, relative reporting ratio; VRZ, voriconazole).
3.2 VRZ adverse events related to DDIs
The numbers of AE types and positive DDI AEs detected by at least one of the four frequency statistical models when VRZ was used in combination with the 38 drugs and the sex and age distributions of the related patients are presented in Table 1. A total of 262 AEs were detected by at least one of the four frequency statistical models when VRZ and 38 drugs were co-administered. For a specific positive signal result, the more models that detected it, the more reliable the result. Among the 262 AEs, 48 were detected by all four models (Table 2). The most common AEs related to DDIs were anaemia (10.4% (5/48)) and condition aggravated (10.4% (5/48)), followed by constipation (8.3% (4/48)). The results of the other 214 positive AEs related to DDIs of VRZ and the target drug detected by at least one of the above four models are shown in Supplementary Table S5.
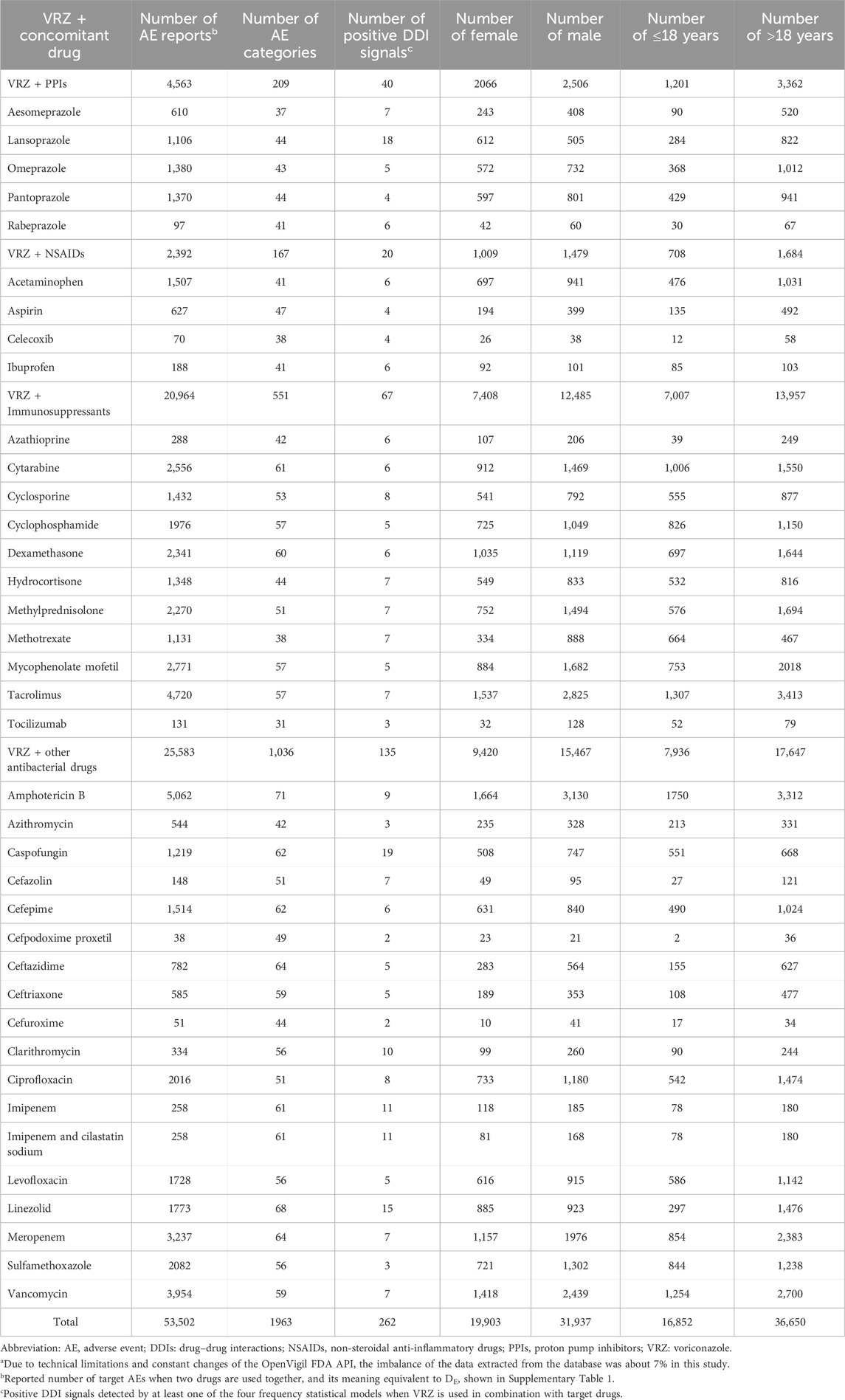
Table 1. Number of AE categories, positive safety signals and the gender, age distribution of the AEs when VRZ used in combination with target drugsa.
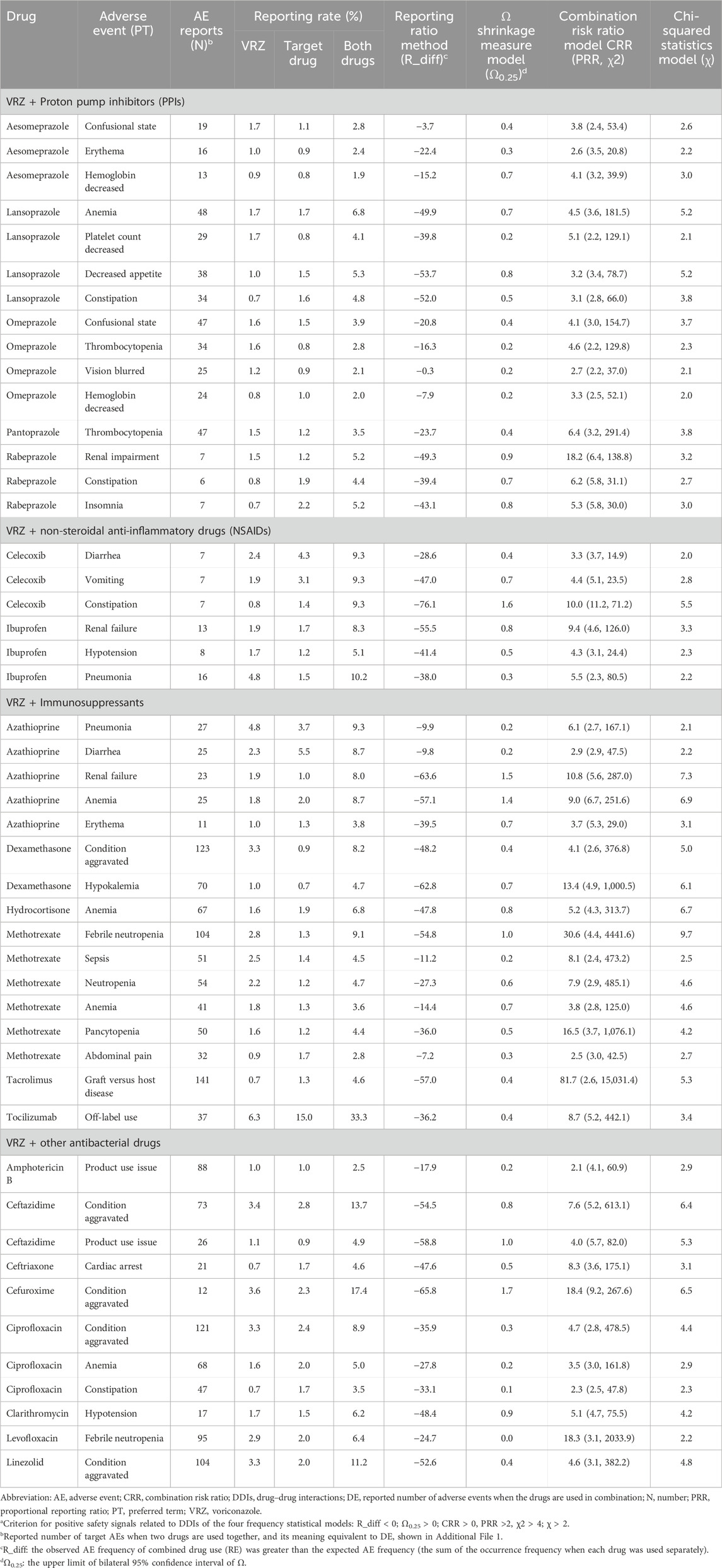
Table 2. Positive safety signals related to DDIs detected by all four frequency statistical models when VRZ in combined use with target drugs (n = 48)a.
3.3 Correlation analysis
The correlation analysis results between the target drug and the target AEs with positive DDI signals are shown in Figure 2A. Some 77 (29.4%) target AEs were significantly positively correlated with DDIs when VRZ was used in combination, leading to an increased risk of AEs (Table 3). The reporting rates and four frequency statistical model distributions of these 77 AEs are presented in Figures 2B–E. The reporting rate of all 77 AEs significantly positively correlated with DDIs increased when VRZ was used in combination with the target drug compared with when VRZ was used alone; close attention should thus be given to these target AEs. For VRZ in combination with tacrolimus, mycophenolate mofetil, or cyclophosphamide, graft-versus-host disease should be of particular concern because this AE had the strongest correlation with the drug interaction, and multiple organ dysfunction syndrome deserves great attention when VRZ is used in combination with meropenem, cefepime, or mycophenolate mofetil.
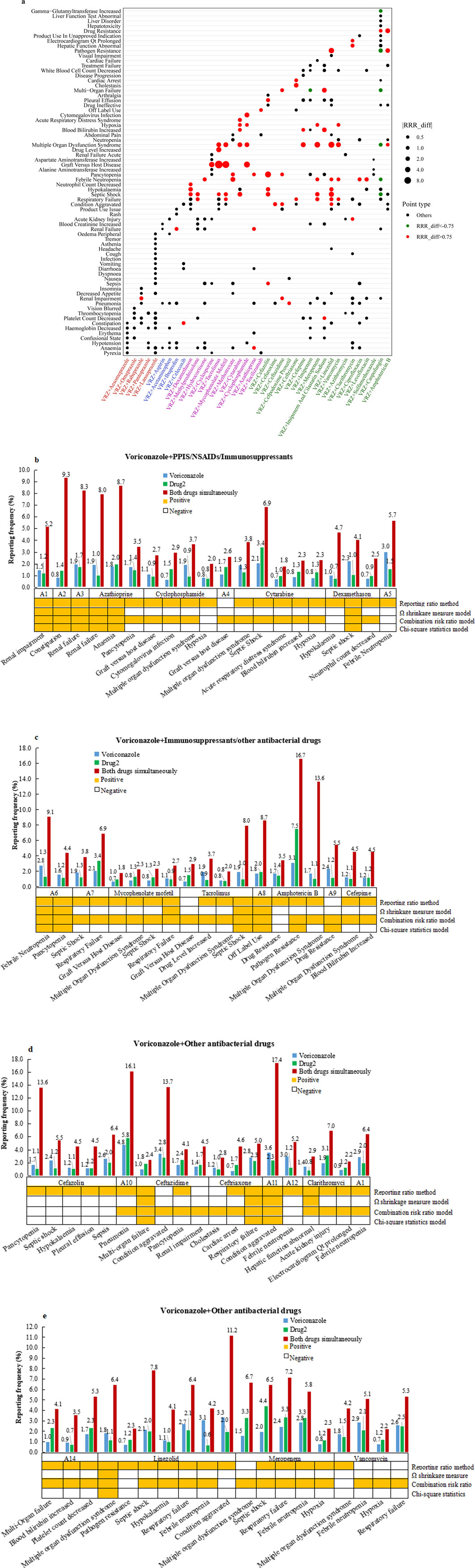
Figure 2. Correlation analysis results between drug(s) and target AEs with positive DDI signals. (A) Bubble chart depicting correlation analysis results of the 262 adverse events. Red spots represent RRR_diff values >0.75, indicating that the significant positive correlation between the target AE and the combined use of the two drugs was greater than that of the two single drugs. Green spots represent RRR_diff values < −0.75 and indicate a significant negative correlation between the target AE and the two drugs used alone when more than two drugs are used together. The larger the bubble, the greater the absolute value of the RRR_diff value, indicating a greater correlation between the drug(s) and the target AE. (B-E): Reporting rates of 77 adverse events positively correlated with DDIs when VRE in combination with target drugs was compared with when VRE alone was used. The orange square under the bar chart indicates a positive safety signal related to the DDI calculated by the corresponding model. (Abbreviations: A1: rabeprazole; A2: celecoxib; A3: ibuprofen; A4: cyclosporine; A5: hydrocortisone; A6: methotrexate; A7: methylprednisolone; A8: tocilizumab; A9: caspofungin; A10: cefpodoxime proxetil; A11: cefuroxime; A12: ciprofloxacin; A13: levofloxacin; A14: imipenem and cilastatin sodium).
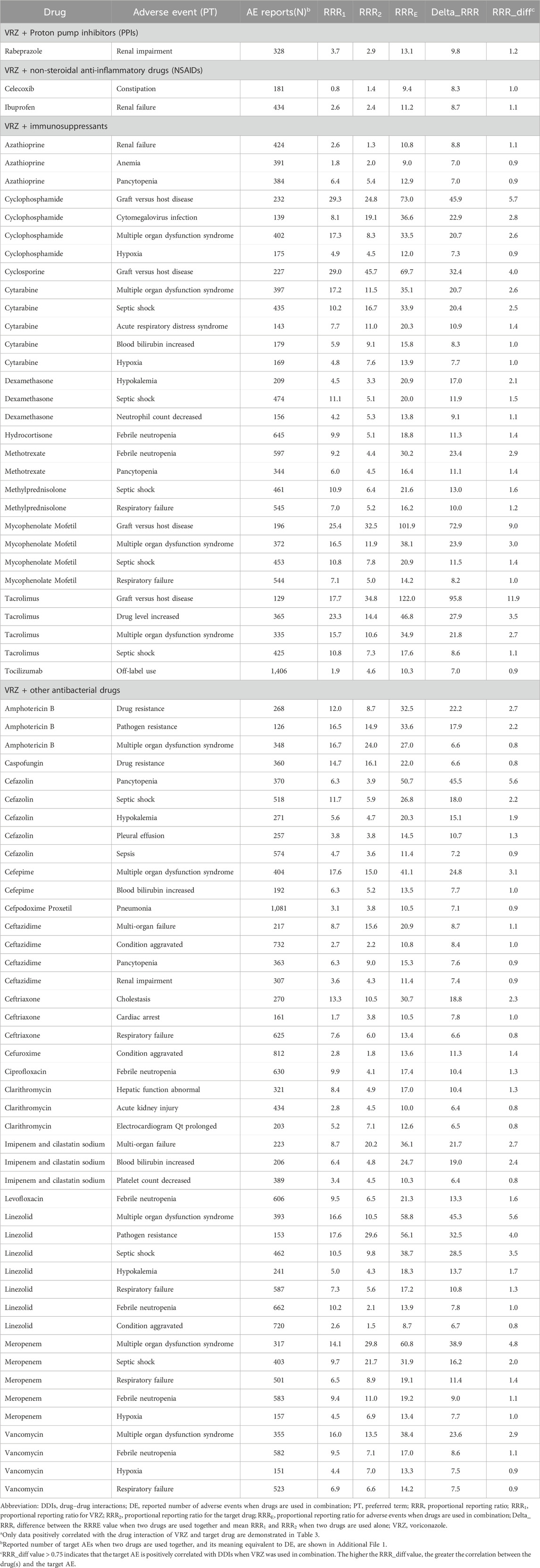
Table 3. Results of target AEs with positive correlation with DDIs when VRZ was used in combination with the target druga (n = 77).
On the other hand, according to the statistical analysis results of this study, we did not find a significant correlation with target AEs related to DDIs between VRZ and aesomeprazole, lansoprazole, omeprazole, pantoprazole, acetaminophen, aspirin, azithromycin, imipenem, or sulfamethoxazole. Seven (2.7%) target AEs were negatively correlated with DDIs when VRZ was used in combination, so the risk of the target AEs might thus be greater when the two drugs are used alone than when VRZ is used in combination.
3.4 Influence of sex and age on the AEs related to DDIs
The 77 AEs positively correlated with DDIs were included to evaluate the influence of sex and age; 27 target AEs in the sex group and 34 in the age group met the inclusion criteria for statistical analysis (Tables 4 and 5, Figures 3A and B). Five drugs had significant sex differences in target AEs related to DDIs. Male patients might be more susceptible than female patients to these target AEs (6/27 vs. 1/27), and close attention should be given to disease aggravation and respiratory failure when VRZ is used in combination with linezolid. For female patients, respiratory failure should be considered first when VRZ is used in combination with mycophenolate mofetil.
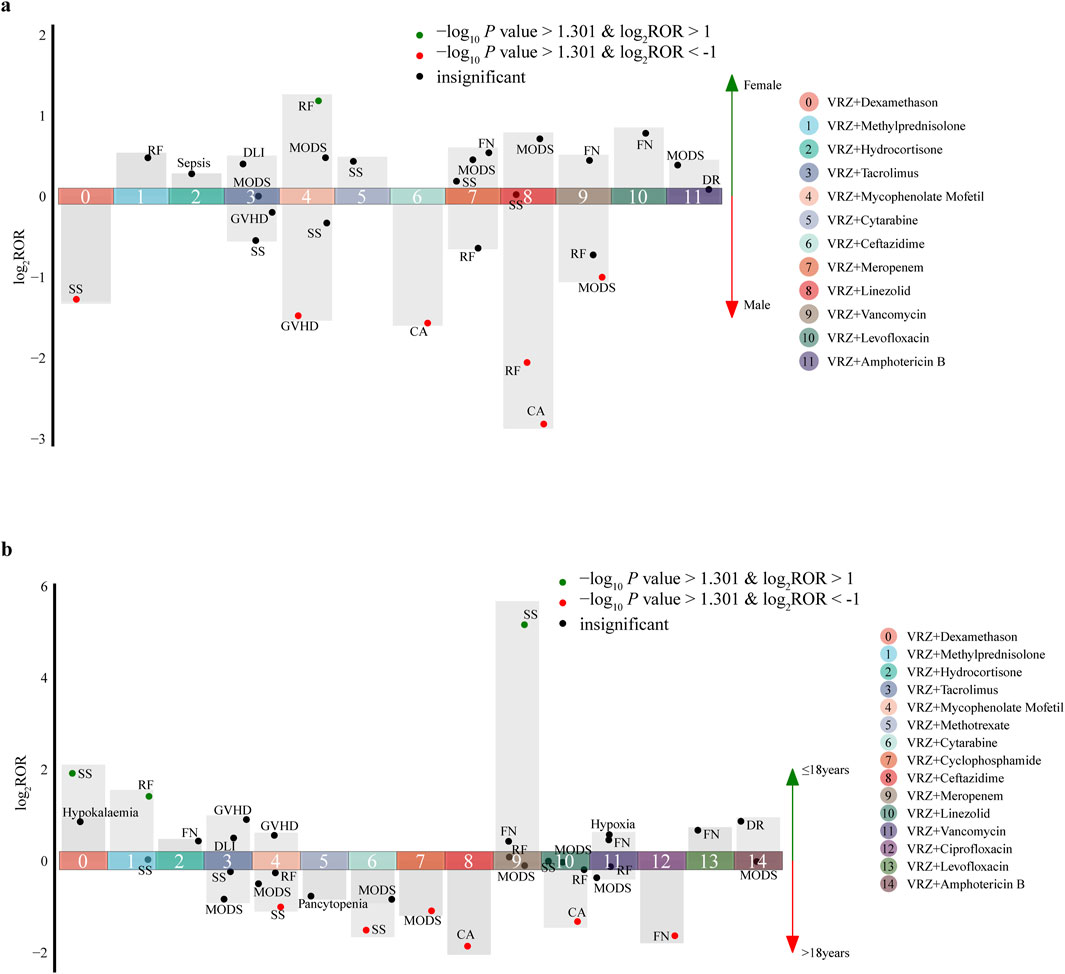
Figure 3. Influence of sex and age on the incidence of AEs related to drug‒drug interactions (DDIs). (A) sex; (B) age. Each spot represents a specific AE related to DDIs. Green spots represent AEs more frequently associated with female (or ≤18 years) patients; red spots represent AEs more frequently associated with male (or >18 years) patients. (Abbreviations: CA, condition aggravated; DLI, drug level increased; DR, drug resistance; FN, febrile neutropenia; GVHD, graft-versus-host disease; MODS, multiple organ dysfunction syndrome; RF, respiratory failure; SS, septic shock).
For age groups, nine drugs had significant age differences in target AEs related to DDIs. Compared with ≤18-year-old patients, >18-year-old patients tended to have detected AEs (6/34 vs. 3/34), and more attention should be given to disease aggravation, febrile neutropenia and septic shock when VRZ is used in combination with ceftazidime, ciprofloxacin, and cytarabine, respectively. For ≤18-year-old patients, septic shock should be considered when VRZ is used in combination with meropenem and dexamethasone. Moreover, on the basis of the statistical analysis results of this study, we did not find a significant difference in sex and age for the AEs related to DDIs associated with the PPIs and NSAIDs included in this study.
4 Discussion
To our knowledge, this is the first pharmacovigilance study to comprehensively assess the DDIs between VRZ and 38 drugs commonly prescribed in clinical practice. Signal mining via FAERS could provide a reference for safety monitoring when VRZ is combined with other drugs in clinical practice and could serve as a starting point for further analysis. Owing to the voluntary nature of FAERS reporting, underreporting, overreporting, or missing information was unavoidable (Meng et al., 2022; Allegra et al., 2020), and due to the technical limitations and the constant changes of the OpenVigil FDA API, there might also be some imbalance in the data extracted from the database. During our analysis, we found that the imbalance of the data was approximately 7%. For a specific positive signal result, the more models that detected it, the more reliable the result; therefore, to ensure the consistency and reliability of the drug interaction results, four models were used to analyse the data simultaneously. The reporting ratio method and combined risk ratio model were used to compare the related parameters when drugs were used in combination and alone and then to evaluate the influence of DDI (Norén et al., 2008). The Ω shrinkage measure model and chi-square statistics model were used to compare the observation values and the expected values of the related parameters. The false-positive rate and sensitivity of random fluctuations for DDI results could be effectively controlled with the use of a chi-square statistics model and an Ω shrinkage measure model, respectively (Gosho et al., 2017). Overall, real-world research from a database is more feasible than drug clinical trials for drug interaction studies because clinical trials needs to consider stricter entry criteria, more limited patients, and greater risk. In this study, potential AEs related to DDIs of VRZ were detected, which can provide a reference for safety monitoring when VRZ is used in combination with other drugs in clinical practice.
Voriconazole in various drug combinations has the potential for multiple adverse drug–drug interactions; describing drug interactions in detail in the literature is often difficult because of large differences in how reactions are defined and the severity of reactions between individuals. In addition, although VRZ interactions between drugs are theoretically recognizable, all of these interactions may not necessarily be clinically significant (Glotzbecker et al., 2012). Therefore, this study focused on the AEs that had the strongest correlation with the drug interaction and its influence.
Age and sex may influence VRZ levels and play crucial roles in the occurrence of AEs (Yu et al., 2016). One study focused on sex differences in adverse drug events using FAERS and detected sex differences in AEs (i.e., alopecia, amnesia, and urticaria) (Yu et al., 2016). Our analysis revealed that sex and age might influence the occurrence of some adverse reactions, especially when VRZ is combined with some immunosuppressants or other antibacterial drugs. For example, when VRZ is combined with mycophenolate mofetil, the risk of respiratory failure might be greater in female patients, but the risk of graft-versus-host disease might be greater in male patients, and the risk of septic shock might be greater in >18-year-old patients. Sex and age could influence VRZ trough concentrations, and the sex effect on drug concentrations may be due to sex differences in CYP-mediated metabolism, the influence of sex hormones on drug absorption, and differences in fat percentage with respect to body composition (Allegra et al., 2020); sex differences could also be the result of different doses/kg of body weight. Thus, the recommended weight-based VRZ dosing should also consider a patient’s sex to avoid underexposure, especially in women. In addition, when oral administration is not prescribed by body weight, BMI plays a predictive role, and a lower BMI value predicts higher drug concentrations (Shao et al., 2017).
In terms of age, one study on healthy volunteers revealed that the maximum VRZ concentration and area under the curve were greater in elderly male subjects and in women than in younger men, as increasing age was a predictive factor of a higher VRZ trough via the intravenous route. Decreased metabolic clearance in elderly individuals could strongly influence the drug concentration, especially given that VRZ is a renally excreted drug; therefore, drug-impaired renal function should be judged in clinical practice by examining creatinine levels (Allegra et al., 2020).
In this study, we found that VRZ in combination with cyclosporine, tacrolimus, mycophenolate mofetil, and cyclophosphamide could lead to a significant increase in the probability of graft-versus-host disease, with a strong correlation with DDIs; these results are consistent with those of Groll et al. (2017). The modulation of cytochrome P450 3A4 and drug transporters such as P-gp may alter the blood levels of both antimicrobial agents and immunosuppressants, and the use of antimicrobial agents can interfere with the metabolism of immunosuppressants, which may put patients at risk of developing severe AEs due to unwanted increases or decreases in the serum levels of immunosuppressive agents (Shao et al., 2017; Bhagat et al., 2021). Therefore, interactions between immunosuppressants and antimicrobial agents can cause non-infectious complications like graft-vs.-host-disease (GVHD) flares (Champion et al., 2006). The appropriate dosing and delivery of antimicrobial agents in immunosuppressed patients with organ dysfunction is a major therapeutic challenge. It is desirable, from a clinical perspective, to avoid unnecessarily high exposure to immunosuppressants (Thomas et al., 2009; Dong et al., 2024). A previous study reported that VRZ treatment led to a dramatic increase in tacrolimus concentration, which required discontinuation despite the manufacturer’s guidelines recommending a one-third reduction in tacrolimus dosage. Therefore, the drug dose needs to be adjusted on the basis of the results of therapeutic drug monitoring to ensure that patients avoid serious AEs when VRZ is utilized in combination with immunosuppressants (Beata et al., 2017).
A previous study demonstrated that many antibiotics, including imipenem, cefepime, ceftazidime, vancomycin, and levofloxacin, are unlikely to cause DDIs because they are primarily eliminated in their unchanged form via glomerular filtration (Job et al., 2016). However, we found that the occurrence of adverse reactions was strongly related to DDIs when VRZ was used in combination with other antibiotics. For example, the frequency of multiple organ dysfunction syndrome might be approximately four times greater when VRZ is combined with linezolid or meropenem and is strongly correlated with DDIs. When linezolid combined with VRZ treatment increases VRZ clearance to between 250% and 700% and serum antifungal concentrations decrease clinically, the effectiveness of antifungal therapy is lost in 80% of cases (Idoate et al., 2019). Therefore, the combination of linezolid and voriconazole is not recommended if no other clinical alternative exists, and VRZ pharmacokinetic monitoring is recommended to ensure the effectiveness of antifungal treatment.
A combination of VRZ and glucocorticoids can prevent invasive fungal infections, but the concomitant administration of glucocorticoids and VRZ might be challenging due to the high propensity for DDIs (Gergis et al., 2010). In this study, septic shock and respiratory failure should receive close attention when VRZ is combined with methylprednisolone. The reason could be that VRZ markedly increases the plasma concentrations of dexamethasone and methylprednisolone, leading to AEs when dexamethasone or methylprednisolone is used in combination with VRZ. Therefore, the dose of dexamethasone or methylprednisolone should be reduced to maintain approximately similar exposures, and close attention should be given to the symptoms of patients when glucocorticoids and voriconazole are used in combination in the clinic (Li M. et al., 2018).
Both VRZ and PPIs are metabolized primarily by CYP2C19, and the VRZ concentration increases with the administration of PPIs (Tian et al., 2021), possibly increasing the risk of adverse drug reactions. To our knowledge, there are few reports regarding the interaction of VRZ with PPIs, except for omeprazole, such as lansoprazole, pantoprazole, and rabeprazole (Tian et al., 2021). Our study found that VRZ in combination with rabeprazole could lead to a significant increase in the probability of renal impairment, with a strong correlation with DDIs. VRZ-induced renal impairment has been reported by Turner et al. (2015). Although we did not find a significant correlation with target AEs related to DDIs between VRZ and omeprazole, all four models indicated that a confused state, thrombocytopenia, blurred vision, and decreased haemoglobin were positive safety indicators, and the reporting rates of those AEs were increased by three, two, two, and two times in concurrent use with VRZ, respectively. Dosage adjustments are recommended to prevent drug interactions from occurring when VRZ is used in combination with PPIs (Beata et al., 2017).
Our analysis found that the reporting rates of constipation and renal failure increased significantly when VRZ was used in combination with celecoxib and ibuprofen, respectively, and that both rates were strongly correlated with DDI. These results might be explained by increased exposure to ibuprofen or celecoxib because the inhibition of CYP2C9 by VRZ may lead to an increased risk of impaired renal function or constipation, respectively (Li N. et al., 2018). The AEs and toxicity associated with NSAIDs should be closely monitored when taken in combination with VRZ, and a reduced dose of ibuprofen or celecoxib should be considered to reduce the risk of DDIs when used in combination with VRZ, especially when the initial dose is high (Li N. et al., 2018; Chen et al., 2022).
In this study, although 29.4% of the target AEs were positively correlated with DDIs when VRZ was used in combination, the target AEs (i.e., septic shock, multiple organ dysfunction syndrome, multiorgan failure, increased amma-glutamyltransferase activity, and pathogen resistance) were negatively correlated with DDIs when VRZ was combined with caspofungin. A previous retrospective study of signal detection for adverse drug reactions based on databases in Japan and the United States also revealed that the proportional reporting ratios of neutropenia, haemorrhagic cystitis, and alopecia tended to be reduced when VRZ was combined with cyclophosphamide (Zhao et al., 2021), but the strength of the correlation between adverse drug effects and DDIs was unclear. Different patients have different physiological and pathological statuses, and the related mechanism deserves further study, which may be related to the large inter- and individual variability in VRZ metabolism (Kim et al., 2010).
5 Limitations
This study had several limitations. First, the data presented in the OpenVigil FDA platform were incomplete and lacked detailed patient information, especially for patients with complications and concomitant medications; this limited our ability to further assess the patient’s disease status and the severity of the AEs. Second, this study evaluated only the interaction between two drugs; multidrug interactions should also be studied in the future, and further extraction of the original reports is necessary to obtain more information. Additionally, the mechanism of DDIs could not be assessed in this study because of factors such as a lack of information on drug doses and laboratory values; therefore, further studies are needed to confirm the AEs detected in this study.
6 Conclusion
Voriconazole, an inhibitor of CYP3A4, can interact with many drugs, which may result in changes in the activity of the drug and cause serious AEs. An understanding of VRZ drug‒drug interactions (DDIs) and therapeutic drug monitoring is important for providing effective antifungal therapy. Health professionals should be more cautious when VRZ is used concomitantly with other drugs due to the risk of DDIs. More important AEs related to DDIs should receive more attention when VRZ is used in combination with PPIs (renal impairment), NSAIDs (constipation and renal failure), immunosuppressants (graft versus host disease, septic shock) and other antibacterial drugs (multiple organ dysfunction syndrome, febrile neutropenia, and respiratory failure). The influence of sex and age differences in VRZ DDIs also needs to be considered during the risk assessment of AEs in clinical therapy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
B-NH: Formal Analysis, Project administration, Writing–original draft. LS (2nd author): Formal Analysis, Project administration, Writing–original draft. J-WX: Data curation, Writing–review and editing. N-GY: Data curation, Writing–review and editing. M-LA: Data curation, Writing–review and editing. Y-TJ: Conceptualization, Methodology, Writing–review and editing. LS (7th author): Conceptualization, Methodology, Writing–review and editing.
Funding
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the Chongqing Science and Health Joint Medical Research Project (project number: 2024QNXM012 and 2023GDRC014) and is gratefully acknowledged.
Acknowledgments
The authors thank Wei Zhao for additional assistance with the full-text review of papers and data extraction.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1292163/full#supplementary-material
References
Allegra, S., De Francia, S., De Nicolò, A., Cusato, J., Avataneo, V., Manca, A., et al. (2020). Effect of gender and age on voriconazole trough concentrations in Italian adult patients. Eur. J. Drug Metab. Pharmacokinet. 45, 405–412. doi:10.1007/s13318-019-00603-6
Beata, S., Donata, U. K., Jarosław, D., Tomasz, W., and Anna, W. H. (2017). Influence of CYP2C19*2/*17 genotype on adverse drug reactions of voriconazole in patients after allo-HSCT: a four-case report. J. Cancer Res. Clin. Oncol. 143, 1103–1106. doi:10.1007/s00432-017-2357-y
Bhagat, V., Pandit, R. A., Ambapurkar, S., Sengar, M., and Kulkarni, A. P. (2021). Drug interactions between antimicrobial and immunosuppressive agents in solid organ transplant recipients. Indian J. Crit. Care Med. 25, 67–76. doi:10.5005/jp-journals-10071-23439
Böhm, R., Bulin, C., Waetzig, V., Cascorbi, I., Klein, H. J., and Herdegen, T. (2021). Pharmacovigilance-based drug repurposing: the search for inverse signals via OpenVigil identifies putative drugs against viral respiratory infections. Br. J. Clin. Pharmacol. 87, 4421–4431. doi:10.1111/bcp.14868
Böhm, R., von Hehn, L., Herdegen, T., Klein, H. J., Bruhn, O., Petri, H., et al. (2016). OpenVigil FDA - inspection of U.S. American adverse drug events pharmacovigilance data and novel clinical applications. PLoS One 11, e0157753. doi:10.1371/journal.pone.0157753
Champion, L., Stern, M., Israël-Biet, D., Mamzer-Bruneel, M. F., Peraldi, M. N., Kreis, H., et al. (2006). Brief communication: sirolimus associated pneumonitis: 24 cases in renal transplant recipients. Ann. Intern Med. 144, 505–509. doi:10.7326/0003-4819-144-7-200604040-00009
Chen, J., Wu, Y., He, Y., Feng, X., Ren, Y., and Liu, S. (2022). Combined effect of CYP2C19 genetic polymorphisms and C-reactive protein on voriconazole exposure and dosing in immunocompromised children. Front. Pediatr. 10, 846411. doi:10.3389/fped.2022.846411
Chen, K., Zhang, X., Ke, X., Du, G., Yang, K., and Zhai, S. (2018). Individualized medication of voriconazole: a practice guideline of the division of therapeutic drug monitoring, Chinese pharmacological society. Ther. Drug Monit. 40, 663–674. doi:10.1097/FTD.0000000000000561
Dong, L., Zhuang, X., Yang, T., Yan, K., and Cai, Y. (2024). A physiologically based pharmacokinetic model of voriconazole in human CNS--Integrating time-dependent inhibition of CYP3A4, genetic polymorphisms of CYP2C19 and possible transporter mechanisms. Int. J. Antimicrob. Agents 19, 107310. doi:10.1016/j.ijantimicag.2024.107310
Gergis, U., Markey, K., Greene, J., Kharfan-Dabaja, M., Field, T., Wetzstein, G., et al. (2010). Voriconazole provides effective prophylaxis for invasive fungal infection in patients receiving glucocorticoid therapy for GVHD. Bone Marrow Transpl. 45, 662–667. doi:10.1038/bmt.2009.210
Glotzbecker, B., Duncan, C., Alyea, E. 3rd, Campbell, B., and Soiffer, R. (2012). Important drug interactions in hematopoietic stem cell transplantation: what every physician should know. Biol. Blood Marrow Transpl. 18, 989–1006. doi:10.1016/j.bbmt.2011.11.029
Gosho, M., Maruo, K., Tada, K., and Hirakawa, A. (2017). Utilization of chi-square statistics for screening adverse drug-drug interactions in spontaneous reporting systems. Eur. J. Clin. Pharmacol. 73, 779–786. doi:10.1007/s00228-017-2233-3
Groll, A. H., Townsend, R., Desai, A., Azie, N., Jones, M., Engelhardt, M., et al. (2017). Drug-drug interactions between triazole antifungal agents used to treat invasive aspergillosis and immunosuppressants metabolized by cytochrome P450 3A4. Transpl. Infect. Dis. 19, 19. doi:10.1111/tid.12751
Idoate, A., Leache, L., and Aldaz, A. (2019). 4CPS-058 Avoid simultaneus prescription between linezolid and voriconazole: pharmacokinetic study. Eur. J. Hosp. Pharm. 26, A94–A95.
Job, K. M., Olson, J., Stockmann, C., Constance, J. E., Enioutina, E. Y., Rower, J. E., et al. (2016). Pharmacodynamic studies of voriconazole: informing the clinical management of invasive fungal infections. Expert Rev. Anti Infect. Ther. 4 (8), 731–746. doi:10.1080/14787210.2016.1207526
Kantor, E. D., Rehm, C. D., Haas, I. S., Chan, A. T., and Giovannucci, E. L. (2015). Trends in prescription drug use among adults in the United States from 1999-2012. JAMA 314, 1818–1831. doi:10.1001/jama.2015.13766
Kim, A. M., Tingen, C. M., and Woodruff, T. K. (2010). Sex bias in trials and treatment must end. Nature 465, 688–689. doi:10.1038/465688a
Lee, J., Ng, P., Hamandi, B., Husain, S., Lefebvre, M. J., and Battistella, M. (2021). Effect of therapeutic drug monitoring and cytochrome P450 2C19 genotyping on clinical outcomes of voriconazole: a systematic review. Ann. Pharmacother. 55 (4), 509–529. doi:10.1177/1060028020948174
Li, M., Zhu, L., Chen, L., Li, N., and Qi, F. (2018a). Assessment of drug-drug interactions between voriconazole and glucocorticoids. J. Chemother. 30, 296–303. doi:10.1080/1120009X.2018.1506693
Li, N., Zhu, L., Qi, F., Li, M., Xu, G., and Ge, T. (2018b). Prediction of the effect of voriconazole on the pharmacokinetics of non-steroidal anti-inflammatory drugs. J. Chemother. 30, 240–246. doi:10.1080/1120009X.2018.1500197
Meng, L., Huang, J., Qiu, F., Shan, X., Chen, L., Sun, S., et al. (2022). Peripheral neuropathy during concomitant administration of proteasome inhibitors and factor xa inhibitors: identifying the likelihood of drug-drug interactions. Front. Pharmacol. 13, 757415. doi:10.3389/fphar.2022.757415
Noguchi, Y., and Teramachi, H. (2020). Comparison of signal detection algorithms based on frequency statistical model for drug-drug interaction using spontaneous reporting systems. Pharm. Res. 37, 86. doi:10.1007/s11095-020-02801-3
Norén, G. N., Sundberg, R., Bate, A., and Edwards, I. R. (2008). A statistical methodology for drug-drug interaction surveillance. Statistics Med. 27, 3057–3070. doi:10.1002/sim.3247
Pfizer (2020). VFEND® IV (VRE) for injection, VFEND®Tablets, VFEND® for oral suspension. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021266s032lbl.pdf (Accessed July 21, 2020).
Prybys, K. M., Melville, K. A., and Hanna, J. R. (2002). Polypharmacy in the elderly: clinical challenges in emergency practice. Part 1: overview, etiology, and drug interactions. Emerg. Med. Rep. 23, 145–151.
Rodrigues, M. C., and Oliveira, Cd (2016). Drug-drug interactions and adverse drug reactions in polypharmacy among older adults: an integrative review. Rev. Lat. Am. Enferm. 24, e2800. doi:10.1590/1518-8345.1316.2800
Shao, B., Ma, Y., Li, Q., Wang, Y., Zhu, Z., Zhao, H., et al. (2017). Effects of cytochrome P450 3A4 and non-genetic factors on initial voriconazole serum trough concentrations in hematological patients with different cytochrome P450 2C19 genotypes. Xenobiotica 47, 1121–1129. doi:10.1080/00498254.2016.1271960
Susuta, Y., and Takahashi, Y. (2014). 4. Safety risk evaluation methodology in detecting the medicine concomitant use risk which might cause critical drug rash. Jpn. J. Pharmacoepidemiol 19, 39–49. doi:10.3820/jjpe.19.39
Thomas, L. D., and Miller, G. G.AST Infectious Diseases Community of Practice (2009). Interactions between anti-infective agents and immunosuppressants. Am. J. Transpl. 9 (9), S263–S266. doi:10.1111/j.1600-6143.2009.02918.x
Tian, X., Zhang, C., Qin, Z., Wang, D., Yang, J., and Zhang, X. (2021). Impact of CYP2C19 phenotype and drug-drug interactions on voriconazole concentration in pediatric patients. Antimicrob. Agents Chemother. 17 (9), e0020721. doi:10.1128/AAC.00207-21
Turner, R. B., Martello, J. L., and Malhotra, A. (2015). Worsening renal function in patients with baseline renal impairment treated with intravenous voriconazole: a systematic review. Int. J. Antimicrob. Agents 46, 362–366. doi:10.1016/j.ijantimicag.2015.05.023
Wong, C. K., Ho, S. S., Saini, B., Hibbs, D. E., and Fois, R. A. (2015). Standardisation of the FAERS database: a systematic approach to manually recoding drug name variants. Pharmacoepidemiol. drug Saf. 24, 731–737. doi:10.1002/pds.3805
Yu, Y., Chen, J., Li, D., Wang, L., Wang, W., and Liu, H. (2016). Systematic analysis of adverse event reports for sex differences in adverse drug events. Sci. Rep. 6, 24955. doi:10.1038/srep24955
Keywords: voriconazole, PPIs, NSAIDs, immunosuppressants, drug–drug interaction, safety, FAERS
Citation: Huo B-N, Shu L, Xiao J-W, Yin N-G, Ai M-L, Jia Y-T and Song L (2024) Clinical drug interactions between voriconazole and 38 other drugs: a retrospective analysis of adverse events. Front. Pharmacol. 15:1292163. doi: 10.3389/fphar.2024.1292163
Received: 08 October 2023; Accepted: 02 September 2024;
Published: 30 September 2024.
Edited by:
Li Ang, First Affiliated Hospital of Zhengzhou University, ChinaReviewed by:
Paulo Arturo Caceres Guido, Garrahan Hospital, ArgentinaTayyaba Iftikhar, Shenzhen University, China
Copyright © 2024 Huo, Shu, Xiao, Yin, Ai, Jia and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun-Tao Jia, amlheXVudGFvbWFpbEBob3NwaXRhbC5jcW11LmVkdS5jbg==; Lin Song, c29uZ2xpbkBob3NwaXRhbC5jcW11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Ben-Nian Huo
Ben-Nian Huo Ling Shu2†
Ling Shu2† Yun-Tao Jia
Yun-Tao Jia Lin Song
Lin Song