
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 23 January 2024
Sec. Ethnopharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1290888
This article is part of the Research Topic Reviews in Ethnopharmacology: 2023 View all 31 articles
 Shun Tang1†
Shun Tang1† Minmin Wang1†
Minmin Wang1† Yuhui Peng1
Yuhui Peng1 Yuanjing Liang1
Yuanjing Liang1 Jiarong Lei1
Jiarong Lei1 Qiu Tao1
Qiu Tao1 Tianqi Ming1
Tianqi Ming1 Yanqiao Shen1
Yanqiao Shen1 Chuantao Zhang2
Chuantao Zhang2 Jinlin Guo3*
Jinlin Guo3* Haibo Xu1*
Haibo Xu1*Armeniacae semen amarum—seeds of Prunus armeniaca L. (Rosaceae) (ASA), also known as Kuxingren in Chinese, is a traditional Chinese herbal drug commonly used for lung disease and intestinal disorders. It has long been used to treat coughs and asthma, as well as to lubricate the colon and reduce constipation. ASA refers to the dried ripe seed of diverse species of Rosaceae and contains a variety of phytochemical components, including glycosides, organic acids, amino acids, flavonoids, terpenes, phytosterols, phenylpropanoids, and other components. Extensive data shows that ASA exhibits various pharmacological activities, such as anticancer activity, anti-oxidation, antimicrobial activity, anti-inflammation, protection of cardiovascular, neural, respiratory and digestive systems, antidiabetic effects, and protection of the liver and kidney, and other activities. In clinical practice, ASA can be used as a single drug or in combination with other traditional Chinese medicines, forming ASA-containing formulas, to treat various afflictions. However, it is important to consider the potential adverse reactions and pharmacokinetic properties of ASA during its clinical use. Overall, with various bioactive components, diversified pharmacological actions and potent efficacies, ASA is a promising drug that merits in-depth study on its functional mechanisms to facilitate its clinical application.
Armeniacae semen amarum—seeds of Prunus armeniaca L. (Rosaceae) (ASA), also known as bitter almond or apricot kernel and Kuxingren in Chinese, is a widely used traditional Chinese herbal drug. It is renowned for its effectiveness in treating lung and intestinal diseases (Wei et al., 2023). In traditional Chinese medicine, it is commonly prescribed for relieving cough and asthma, as well as moisturizing the intestine to alleviate constipation (Gao et al., 2014). Modern studies have shown that ASA has a diverse range of pharmacological effects, including alleviating cough and resolving phlegm, as well as immunomodulation and anti-inflammatory properties (Ma et al., 2021; Zhao Y. et al., 2022). Meanwhile, both clinical and animal experiments have demonstrated that the effective components and prescriptions of ASA have significant therapeutic effects on respiratory diseases (Si and Zhang, 2021; Wang et al., 2023).
ASA is composed of various chemical components including glycosides, organic acids, amino acids, flavonoids, terpenes, phytosterols, phenylpropanoids, and other substances. The abundance of these active components makes ASA a valuable subject for research and application. Amygdalin, as the main active ingredient in ASA, has been found to have beneficial effects in relieving cough and asthma, as well as exhibiting anti-inflammatory and anti-fibrotic properties, which makes it a promising candidate for the treatment of respiratory diseases, with significant potential for disease management (Wang et al., 2021). Numerous studies have demonstrated the positive effects of ASA and its active ingredients on various respiratory conditions, including cough, asthma, chronic obstructive pulmonary disease (COPD), pulmonary heart disease, and lung function injury. Moreover, recent research has also suggested its potential role in treating COVID-19 (Luo et al., 2020; Zhou et al., 2020). Furthermore, ASA can be combined with other treatments to enhance its efficacy (Li et al., 2021; Noureen et al., 2022).
Although considerable studies have been performed on the ASA (Wei et al., 2023), there is still a lack of comprehensive and in-depth review of ASA. Herein, we conducted a comprehensive literature search using online databases such as PubMed, Web of Science, China National Knowledge Infrastructure (CNKI), and Google Scholar, with the keywords including ASA, its bioactive components, or ASA-containing formulas, up to December 2023. Then, we systematically summarize and highlight the botanical features and traditional uses, phytochemical components, pharmacological activities, clinical applications, toxicological effects including adverse reactions and detoxification methods, and pharmacokinetic characteristics of ASA, attempting to lay a foundation for the in-depth basic research on ASA and expanding its application in the clinical settings.
ASA, as defined in the 2020 edition of Chinese Pharmacopoeia, refers to the dried ripe seeds of various species of Rosaceae, namely, P. armeniaca L.var.ansu Maxim., Prunus sibirica L., Prunus mandshurica (Maxim.) Koehne, or P. armeniaca L.
It is recommended to harvest fully ripe fruits in the summer and extract their seeds by removing the pulp and core shell. The seeds should then be dried under the Sun. ASA, which contains cyanogenic components (Kovacikova et al., 2019), is known to have beneficial properties and minor toxicity. In traditional Chinese medicine, it is believed that ASA affects the lung and large intestine meridian. The Chinese Pharmacopoeia 2020 states that ASA has therapeutic effects such as lowering Qi, relieving cough and asthma, moisturizing the intestine, and relaxing the bowels (Wei et al., 2023) (Figure 1).
ASA was first documented in Shennong’s Herbal (Shen Nong Ben Cao Jing). It has a sweet taste and warm nature, primarily used for alleviating coughs caused by Qi. However, according to Miscellaneous Records of Famous Physicians (Ming Yi Bie Lu), ASA is described as having a bitter and toxic taste, commonly used to treat distress below the heart, abdominal fullness and distention, and occasionally headaches (Xue et al., 2022). The essentials of Materia Medica (Ben Cao Bei Yao) states that ASA is bitter in taste and warm in nature, with the ability to dissipate cold and alleviate irritable heat and shortness of breath. The Compendium of Materia Medica (Ben Cao Gang Mu) further indicates that ASA has various effects such as dispersing and reducing energy, relieving muscle and dispelling wind, reducing the Qi and moistening dryness, eliminating food stagnation, and treating injuries. Additionally, ASA has been found to have the potential of treating sores and repelling insects due to its toxicity. The book Materia Medica Companion (Ben Cao Meng Quan) describes its properties in further detail. However, it is important to note that ASA should not be used in conjunction with Astragali radix—roots of Astragalus mongholicus Bunge (Fabaceae), Scutellariae radix—roots of Scutellaria baicalensis Georgi (Lamiaceae), and Puerariae lobatae radix—roots of Pueraria lobata Ohwi (Fabaceae). ASA is commonly used for coughs with phlegm, constipation, and insect bites. It is worth mentioning that the treatment for constipation varies depending on whether it is related to Qi or blood deficiency. ASA is used for addressing Qi deficiency, while Persicae semen—seeds of Prunus persica (L.) Batsch (Rosaceae) is employed to promote blood circulation. In cases of Qi deficiency and a floating pulse, a combination of ASA and Citri reticulatae pericarpium—epicarps of Citrus reticulata Blanco (Rutaceae) is recommended. On the other hand, combining P. semen with C. reticulatae pericarpium is advised for addressing blood deficiency and a sinking pulse (Du and Yu, 2023).
Numerous studies have shown that ASA contains a variety of bioactive components and nutrients including glycosides, organic acids, amino acids, flavonoids, terpenes, phytosterols, phenylpropanoids, and other compounds. This section presents a compilation of literature on the chemical composition of ASA, providing detailed information on 170 major chemical components that have been isolated from it (Table 1). Furthermore, we have depicted the chemical structures of the main active components found in ASA (Figure 2).
The glycosides found in ASA primarily consist of cyanogenic glycosides, which serve as both its main toxic components and its primary pharmacologically active ingredients. The principal glycoside in ASA is amygdalin (1). It is important to note that consuming a large amount of amygdalin within a short period of time may lead to cyanide poisoning. This occurs due to the hydrolysis of amygdalin by β-D-glucosidase, leading to the production of benzaldehyde and hydrocyanic acid, which can cause respiratory depression (Song and Xu, 2014). Pharmacological studies have demonstrated that amygdalin exhibits significant anti-tumor activity, as well as antinociceptive and antiphlogistic effects, making it a promising candidate for various applications (Park et al., 2005; Hwang et al., 2008; Figurová et al., 2021; Guo et al., 2023; Zhang et al., 2023). In addition, another cyanogenic glycoside called neoamygdalin (2) has been isolated and identified from ASA. Neoamygdalin is an epimorphous isoform of amygdalin and shows great potential in the treatment of cough and asthma (Xu et al., 2017). Besides, mass spectrometry analysis has revealed the presence of amygdalin metabolites and its glycosides in ASA extracts, including prunasin (3), mandelic acid-β-glucopyranoside (5), mandelic acid-β-gentiobioside (6), mandelic acid amide-β-glucopyranoside (7), mandelic acid amide-β-gentiobioside (8), and benzyl-β-gentiobioside (9). Furthermore, ASA methanol extracts also contain propyl-β-gentiobioside (4), adenosine (10) and cytarabine (11) (Chen Y. et al., 2022). The information of these glycosides is listed in Table 1, and the chemical structures were drawn by ChemDraw 20.0 and presented in Figure 2.
Currently, a total of 39 organic acids have been isolated and identified in ASA. Among them, (12–27) are fatty acids, accounting for approximately 50% of ASA (Jin et al., 2018), which can be divided into saturated fatty acids (12–17), monounsaturated fatty acids (18–23), and polyunsaturated fatty acids (24–27). Notably, unsaturated fatty acids such as oleic acid (20), linoleic acid (24), and linolenic acid (25) are essential for the human body as they cannot be synthesized internally and must be obtained from food (Spector and Kim, 2015). Pharmacological studies have demonstrated that unsaturated fatty acids possess various beneficial effects such as regulation of thrombosis, immune modulation, and anti-fibrosis (Khosla and Fungwe, 2001; Vangaveti et al., 2016; Turolo et al., 2021), making them of significant medicinal value. In addition, ASA contains a range of phenolic acids (28–36), which have antibacterial, anti-inflammatory, anti-oxidation and other pharmacological effects (Bak et al., 2013; Thakare et al., 2017). Furthermore, mandelic acid (42), a metabolite of amygdalin, has been investigated for its antimicrobial activity and low vaginal irritation, particularly in the context of urinary tract infections and vaginal trichomoniasis (Xia et al., 2020). Other organic acids, including fumaric acid (47), malic acid (48), citric acid (49), and gluconic acid (50), have also been isolated and identified from ASA. Information of these organic acids is listed in Table 1. The chemical structures were drawn by ChemDraw 20.0 and shown in Figure 2.
Protein is a crucial component of human cells and tissues. The human body contains numerous proteins with diverse functions, all of which are formed through the dehydration and condensation of amino acids. The protein content in ASA is more than 20%, and the content of important amino acids is reasonable and sufficient (Li et al., 2004). Currently, 18 amino acids (51–68) have been isolated and identified from ASA, among which leucine (54), isoleucine (55), phenylalanine (56), tryptophan (57), threonine (58), methionine (64), valine (65) and lysine (66) are essential amino acids, while histidine (67) is also an essential amino acid for infant growth. These amino acids are summarized in Table 1, and their chemical structures were drawn by ChemDraw 20.0 and presented in Figure 2.
Flavonoids have various physiological effects such as antioxidant, anti-inflammatory, and improvement of cardiovascular function (Feng et al., 2016; Shen et al., 2022). However, the content of flavonoids in ASA is 14.81 mg/100 g, less than 2‰ (Tanwar et al., 2018). Until now, 43 flavonoids (69–112) have been isolated and characterized from ASA, among which catechin (69), epicatechin (70), rutin trihydrate (85), apigenin-7-glucoside (86), luteolin 7-xyloside (91), apigenin (92), tricetin 3′-xyloside (93) are flavanols. Dimethoxyflavone (71), acetylgenistin (72), daidzein (73), genistein (74) and neobavaisoflavone (75) are isoflavones. Bavachinin (76), naringenin hexoside (77), procyanidin dimer (78) and isoliquiritigenin (98) are dihydroflavonoids. Phloridzin (79) and naringenin (87) are dihydrochalcones. Compounds (80–84, 88–90, 94–97, 112) are flavanols. Additionally, 12 anthocyanins (99–111) have been extracted from ASA skins, which belong to flavonoids as well (Qin et al., 2019; Cecarini et al., 2022). These flavonoids are summarized in Table 1, and their chemical structures were drawn using ChemDraw 20.0 and presented in Figure 2.
Terpenoids, which consist of isoprene as the fundamental structural unit, are commonly found in Chinese herbal medicine and exhibit various pharmacological effects such as antioxidant, antimalarial, antibacterial, anti-inflammatory, and anti-cancer properties (Atriya et al., 2023). Currently, 20 terpenoids have been isolated and identified from ASA. These include 13 monoterpenoids (113–125), 3 sesquiterpenoids (126–128), two diterpenoids (trans-geranylgeraniol (129) and phytol (130)), and squalene (131), which belongs to the triterpenoid group. Moreover, amarogentin (132), a schizocyclic iridoterpenoid, has also been isolated from the aqueous extract of ASA. These terpenoids are summarized in Table 1, and their chemical structures were drawn by ChemDraw 20.0 and presented in Figure 2.
The basic structure of sterols consists of cyclopentane polyhydrophenanthrene and a hydroxyl group. Phytosterols, a type of sterols, are commonly found in various parts of plants such as roots, stems, leaves, fruits, and seeds. Pharmacological studies have demonstrated the beneficial physiological effects of phytosterols, including their ability to prevent cardiovascular diseases, inhibit tumor growth, promote metabolism, and regulate hormone levels (Bakrim et al., 2022; Nattagh-Eshtivani et al., 2022). The total phytosterol content in different varieties of ASA ranges from 215.7 to 973.6 mg/100 g of bitter apricot kernel oil (Rudzińska et al., 2017). So far, researchers have isolated and identified 10 phytosterols (133–142) from ASA. In addition, Rudzińska Magdalena et al. analyzed the composition of ASA fat oil using TLC and capillary GLC methods, which revealed the presence of major phytosterols such as cholesterol (134), campesterol (135), gramisterol (136), Δ5-avenasterol (137), Δ7-stigmasterol (138), Δ7-avenasterol (139), β-sitosterol (140), citrostadienol (141), and 24-methylene-cycloartanol (142). These physterols are summarized in Table 1. The corresponding chemical structures were drawn using ChemDraw 20.0 and presented in Figure 2.
The basic structural unit of phenylpropanoids consists of a benzene ring and three branched carbons (C6-C3). Until now, 16 phenylpropanoids have been successfully isolated and identified from ASA, among which (143–151, 155–158) are phenylpropanoic acids, coumarin (152) and psoralen (153) are coumarins, and schisandrin (154) is lignan. Besides, chlorogenic acid (144), 5-feruloylquinic acid (148) and dicaffeoylquinic acid (151) are polyphenols with significant anti-oxidant activity and free radical scavenging activity (Iwai et al., 2004; Cao et al., 2010; Park et al., 2015). These phenylpropanoids are summarized in Table 1, and their chemical structures were drawn by ChemDraw 20.0 and presented in Figure 2 as well.
Besides the chemical constituents mentioned above, other components have also been investigated and summarized in Table 1, and the corresponding chemical structures are drawn by ChemDraw 20.0 in Figure 2. In brief, trehalose (159) and sucrose (160) are saccharides, berberine (161) and tetrahydropalmatine (162) are alkaloids, amygdalin amide (163), mandelamide (164), N-methoxy-N-methylbenzamide (165) and nicotinamide (166) are amide compounds. Furthermore, the compounds (167–170) are the main ingredients in ASA volatile oil.
ASA exhibits a wide range of pharmacological activities and effects due to its abundance of chemical components and active substances. These include anticancer activity (breast carcinoma, prostatic cancer, hepatocellular carcinoma, lung cancer, renal cell carcinoma, bladder cancer and other cancers), anti-oxidant activity, antimicrobial activity, anti-inflammation activity, cardiovascular protection, neuroprotection, respiratory protection, digestive system protection, antidiabetic, liver and kidney protection, skin protection and other pharmacological activities (Figure 3). The following is a detailed introduction to the pharmacological effects of ASA.
In recent years, the overall incidence and mortality of cancer are still on the rise. Despite advances in various comprehensive therapies, the mortality rate of advanced malignant tumors remains high (Chen L. et al., 2021; Zhao et al., 2022a; Ming et al., 2022). ASA is rich in a variety of phytochemical ingredients, and amygdalin is one of its main active ingredients. Amygdalin is a phytochemical ingredient that has been extensively studied for its therapeutic effects on various types of cancers, including breast cancer, prostate cancer, hepatocellular carcinoma, renal cell carcinoma, lung cancer, bladder cancer, and others. Numerous studies have demonstrated the therapeutic potential of different ASA extracts and amygdalin. The therapeutic mechanism of ASA primarily involves inhibiting cancer cell adhesion, migration, and proliferation, as well as blocking the cell cycle, inducing cell oxidative damage and apoptosis, and regulating autophagy. However, it is important to note that the current research on the anticancer activity of ASA is mostly limited to in vitro cell studies, with fewer in vivo studies and a lack of clinical trials. Therefore, further investigation is needed to fully explore ASA as a potential alternative therapy for cancer. The effects of ASA and amygdalin on different types of cancer and their action mechanisms are summarized in Table 2 and Figure 4.
Breast cancer is the most prevalent gynecological malignant tumor worldwide. The cure rate for patients diagnosed with early-stage breast cancer can reach 80%. However, treating patients in the advanced stages poses significant challenges (Zannetti, 2023). Conventional chemotherapy, radiotherapy, and targeted drug treatment are commonly used to treat breast cancer. Unfortunately, many patients develop drug resistance, experience cancer recurrence, and develop secondary diseases. In vitro studies, amygdalin, found in ASA, shows suppressive effects on various breast cancer cell lines including Hs578T, MCF-7, MDA-MB-231, SK-BR-3, and T47D cells, by inhibiting cancerous proliferation and migration, and inducing apoptosis, autophagy and oxidative stress.
Amygdalin impedes cell adhesion and migration by regulating integrin protein expression, which are cell adhesion molecules consisting of α and β subunits. Integrins facilitate the interaction between cancer cells and components of the extracellular matrix, thus influencing cell adhesion and eventually leading to cancer cell metastasis (Hoshino et al., 2015). In Hs578T breast cancer cells, amygdalin demonstrated a dose-dependent inhibition of cell adhesion, and it was observed that this inhibitory effect could potentially be attributed to the downregulation of integrin α5 protein expression (Lee and Moon, 2016). A decrease in mRNA levels of integrin αV/β3 and integrin α5 was observed in both MDA-MB-231 and MCF-7 cell lines, leading to the adhesion of cancer cells to fibronectin and collagen in the extracellular matrix. This decrease has an impact on the migration and metastasis of cancer cells. Notably, amygdalin shows a stronger inhibitory effect on integrin αV/β3 in MDA-MB-231 cells. Additionally, there were distinct variations in mRNA levels of integrin β1, β2, and β4 between the two cell lines. In MCF7 cells, integrin β1 and β4 levels increased, while integrin β2 levels decreased. Conversely, in MDA-MB-231 cells, the opposite trend was observed (Mosayyebi et al., 2021). The impact of amygdalin on cell adhesion and its effect on integrin protein expression have been extensively studied. However, the specific impact on different heterodimers is still not fully understood. A study conducted on MCF-7 cells showed that after 24 h and 48 h of amygdalin treatment, the IC50 values were determined to be 200.6 and 197 μg/mL, respectively. Additionally, Microarray Hybridization revealed that amygdalin can downregulate 19 out of 32 DNA replication-related genes, including MCM3, MCM6, MCM4, PCNA, and FEN1. This suggests that amygdalin may inhibit the proliferation of breast cancer cells by affecting DNA replication (Albogami and Alnefaie, 2021).
Apoptosis has long been recognized as a significant mechanism for preventing tumor development. The inhibitory effect of apoptosis is determined by the expression of Bcl-2 and Bax proteins (Czabotar et al., 2014). Studies have shown that amygdalin, at concentrations of 10 and 20 mg/mL, effectively suppresses the expression of Bcl-2 protein and enhances the expression of Bax in SK-BR-3 and MCF-7 cell lines (Moradipoodeh et al., 2020). This indicates that amygdalin can inhibit apoptosis in breast cancer cells. The human epidermal receptor 2 (HER2) is closely associated with breast cancer development and apoptosis (Shi et al., 2022). Molecular docking studies have revealed that amygdalin forms hydrogen bonds and hydrophobic interactions with Bcl-2 and the active site amino acids of HER2 in HER2-overexpressing SK-BR-3 cells. However, the binding ability of amygdalin to the active site amino acids of HER2 is weaker compared to lapatinib, a HER2 tyrosine kinase inhibitor. The metabolites of amygdalin, such as benzaldehyde, mandelonitrile, and cyanide, also bind to Bcl-2, although their binding affinity is weaker compared to amygdalin (Moradipoodeh et al., 2019). Another study found that amygdalin can diminish the apoptosis of Hs578T breast cancer cells by activating the p38 MAPK signaling pathway and regulating the expression of Bcl-2 family and Caspase family proteins (Lee and Moon, 2016). Furthermore, when MCF-7 breast cancer cells and MCF-10A normal cells were treated with 50 μM amygdalin and 1 mg/mL ASA extract, it was observed that the activities of proteasomes 20S and 26S, Cathepsin B, and cathepsin L in MCF-7 cells were inhibited. Additionally, the expressions of p53, p27, and Bax were increased, indicating that amygdalin and ASA extract may promote apoptosis and regulate the autophagy cascade (Cecarini et al., 2022). Moreover, amygdalin can induce oxidative stress in breast cancer cells by increasing GSH activity and reducing MDA and oxidized glutathione levels, thereby exerting anti-cancer effects (Abboud et al., 2019).
Prostatic cancer is the most common type of cancer in men, with approximately 40% of patients eventually developing other metastatic diseases. Therefore, it is crucial to investigate the potential of natural chemical components found in plants as alternative therapies for prostate cancer treatment (Martínez-Piñeiro et al., 2003). Amygdalin has demonstrated anti-prostate cancer activity in LNCaP, DU-145, and PC3 cells. Its primary mechanisms involve inhibiting cell adhesion, migration and metastasis, and inducing apoptosis and cell cycle arrest, attributed to its downregulation of integrin α6 and Bcl-2, while upregulation of integrin α2, Bax and caspase-3, as well as inhibition of CDK1-cyclin B axis and the AKT-mTOR pathway.
A study demonstrated that treating DU-145 prostate cancer cells with 10 mg/mL amygdalin for 24 h inhibited their adhesion, chemotaxis, and migration. This inhibition was attributed to the downregulation of integrin α2 and the upregulation of α6. Integrin α2 plays a critical role in cell adhesion, which in turn regulates cell invasion and metastasis. However, a decrease in adhesion of PC3 cells was observed only after 2 weeks of amygdalin treatment, with no impact on their chemotaxis and migration abilities. Further experiments involving the knockout of integrins α2, α6, and β1 revealed distinct changes in the adhesion, chemotaxis, and migration abilities of DU-145 and PC3 cells (Mani et al., 2020). In conclusion, the effects of amygdalin on cell adhesion, migration, and metastasis are influenced by the epigenetics of tumor cells, and each cell line may have a specific set of receptors. Amygdalin has shown potential anticancer activities by influencing the cell cycle. In a 2-week study, amygdalin administration resulted in the prolongation of the G0/G1 phase and the shortening of the S phase and G2/M phase in LNCaP, DU-145, and PC3 cells. Additionally, it inhibited the expression of cell cycle regulatory proteins, including CDK1, CDK2, CDK4, cyclin A, cyclin B and cyclin D3, as well as the AKT-mTOR signaling cascade (Makarević et al., 2016). Furthermore, amygdalin has been found to enhance cell apoptosis by increasing caspase-3 enzyme activity and Bax protein expression, while decreasing Bcl-2 protein expression (Chang et al., 2006).
Hepatocellular carcinoma is a prevalent type of cancer. A study involving 148 hepatocellular carcinoma patients found that 75 of them died within 22 months. Cirrhosis developed in 77% of the patients, and the 1-year and 3-year survival rates were 70.8% and 47.6% respectively (Wongjarupong et al., 2021). After administering ASA treatment, there was a significant increase in the proportion of early apoptosis, late apoptosis, and necrosis cells in HepG2 hepatocellular carcinoma. This effect was positively correlated with the upregulation of p53, Caspase-3, and Bcl-2 activities, as well as the downregulation of Bax. It is worth noting that the pro-apoptotic effect of amygdalin is enhanced with the addition of zinc (El-Desouky et al., 2020). Sorafenib, a commonly used targeted drug for liver cancer treatment, often leads to severe side effects and drug resistance in patients (Zheng et al., 2014). Experiments have demonstrated that 2.6 mg/mL amygdalin alone or in combination with sorafenib can induce cell cycle arrest in HepG2 cells and trigger autophagy and apoptosis. These results align with the upregulation of AMPK, HMGB1, beclin-1, and ATG5 mRNA levels, as well as the downregulation of mTOR and Bcl-2 levels. Unlike sorafenib, amygdalin can increase GSH level, reduce MDA level, and exhibit strong DPPH free radical scavenging ability (El-Sewedy et al., 2023). These findings suggest that amygdalin holds significant potential for the treatment of hepatocellular carcinoma.
The therapeutic effects of ASA extract on liver cancer have been demonstrated in vivo. When liver cancer is induced by 2,2′-Bis (hydroxymethyl)butyric (DMBA), ASA methanol-water extract and amygdalin have been shown to significantly increase the levels of SOD, CAT, GSH, and TAC, while inhibiting MDA levels. These effects contribute to the anti-oxidant properties of ASA, which are crucial in protecting the liver from oxidative damage. Additionally, ASA has been found to downregulate the mRNA levels of Bcl-2 and beclin-1, reduce TNF-α and VEGF contents, and downregulate PCNA protein expression in mouse liver tissues (Hosny et al., 2021). These findings indicate that ASA can inhibit inflammation through apoptosis, autophagy, angiogenesis, and proliferation pathways, thereby exerting anti-cancer effects.
Lung cancer is a prevalent and deadly malignant tumor that often metastasizes to various organs including the brain, bone, liver, and kidney. Current treatments primarily focus on primary lung cancer, leading to a poor prognosis for metastatic patients (Yin et al., 2021). However, in highly metastatic non-small cell lung cancer cell lines H1299/M and PA/M, amygdalin at concentrations of 2.5 and 5 mg/mL significantly inhibits cell proliferation, migration, and invasion. The inhibition rates of cell proliferation decreased by 15.6% and 25.1% respectively under these concentrations. Amygdalin achieves its function by reducing the levels of integrin β1 and β4, while upregulating the level of E-cadherin (Qian et al., 2015). This not only affects tumor cell adhesion but also activates FAK, β-catenin, and the downstream AKT-mTOR signaling pathway to mediate cell proliferation, adhesion, and metastasis. Additionally, amygdalin effectively promotes cancer cell apoptosis in A549 and PC9 cancer cells in vitro, as well as in A549 cell xenograft mice. This is achieved by inhibiting the NF-κB signaling pathway through increased protein expression of NF-κB-1 and further altering the expression of apoptosis-related proteins Bax, Bcl-2, cytochrome C, caspase 9, caspase 3, and PARP (Lin et al., 2022). In conclusion, amygdalin shows promising potential for treating lung cancer and may serve as a potential NF-κB-1 agonist.
Renal cell carcinoma, which accounts for 80% of all kidney cancers, is a common type of urinary tract tumor. In the United States, there are approximately 64,000 new cases and 14,000 deaths associated with renal cell carcinoma each year (Singh, 2021). Amygdalin has demonstrated anti-renal cell carcinoma activity in vitro, specifically in Caki-1, KTC-26, and A498 cells. This activity is attributed to the regulation of integrin α and β protein expressions, leading to the inhibition of adhesion and migration. Additionally, amygdalin inhibits CDK/cyclin complexes, thereby arresting the cell cycle.
Amygdalin at a concentration of 10 mg/mL has been found to inhibit the adhesion, chemotaxis and migration of Caki-1, KTC-26 and A498 cells, due to the downregulation of integrins α5 and α6 levels. Furthermore, the expression changes of other integrin subtypes in these cells vary, suggesting that the integrin profile may be specific to each cell line (Juengel et al., 2016a). Additionally, amygdalin induces cell cycle arrest by increasing the number of cells in the G0/G1 phase of Caki-1 and A498 cells, and in the S phase of KTC-26 cells, which may be attributed to the diminishment of CDK, CDK2, CDK4, cyclin A, cyclin B and cyclin D protein expressions (Juengel et al., 2016b). Notably, amygdalin may impact cancer cell differentiation by regulating N-cadherin and E-cadherin, potentially influencing the prognosis of the cancer. However, further research is necessary to investigate the specific impact of cadherin on cell differentiation in renal cell carcinoma.
Bladder cancer is a prevalent form of cancer that affects the urinary system, leading to significant morbidity and mortality. A key symptom of bladder cancer is painless hematuria. As the disease progresses, patients may experience urinary retention, poor urination, and urinary tract obstruction (Xiang et al., 2021). In recent studies, amygdalin has shown promise in inhibiting the adhesion of bladder cancer cells (UMUC-3, TCCSUP, and RT112) by potentially affecting integrin expression. However, the specific integrin profile in different cell lines appears to play a more significant role. Furthermore, amygdalin has been observed to impede the migration of UMUC-3 and RT112 cells, while paradoxically increasing the migration of TCCSUP cells (Makarević et al., 2014b). It is important to note that although amygdalin can inhibit cancer cell adhesion, prolonged exposure to certain cancer cells may promote the migration of non-adherent cells. Additionally, amygdalin has demonstrated inhibitory effects on the growth and proliferation of UMUC-3, TCCSUP, and RT112 cancer cells. This is primarily achieved by causing cell cycle delay and arresting cells in the G0/G1 phase, possibly through the downregulation of CDK2 and cyclin A protein expression (Makarević et al., 2014a).
In addition to its therapeutic potential for the above cancer types, ASA has also shown suppression of cervical cancer, pancreatic cancer and blood cancer, mainly based on the effects of amygdalin. Both in vivo and in vitro studies have demonstrated that amygdalin has positive therapeutic effects on cervical cancer. The main mechanism of amygdalin’s therapeutic effect is inhibition of cell growth and promotion of apoptosis (Chen et al., 2013). Furthermore, research has shown that the methanol aqueous extract of ASA and amygdalin can promote apoptosis in PANC-1 pancreatic cancer cells (Aamazadeh et al., 2020). Additionally, a separate study found that the ethyl acetate extract of ASA has an inhibitory effect on NALM-6 acute B lymphoid leukemia cells and KG-1 myeloid leukemia cells (Mosadegh Manshadi et al., 2019).
The anti-oxidant activity of ASA primarily involves the elimination of lipid peroxidation, reduction of reactive oxygen species (ROS) accumulation, and enhancement of anti-oxidant enzyme activity, and the main functional substances are polyphenols (Table 3). Malondialdehyde (MDA) is a crucial marker for LPO resulting from the oxidation of polyunsaturated fatty acids (Liu et al., 2018). A study with the ethanol-induced rat liver injury and oxidative stress model has demonstrated that consumption of ASA significantly decreases LDH content in serum, MDA level in red blood cells, brain, kidney and heart of rats while increasing the content of anti-oxidant enzymes such as superoxide dismutase (SOD) and glutathione S-transferase (GST) in the liver (Yurt and Celik, 2011). This indicates that ASA can prevent liver injury by increasing the activity of anti-oxidant enzymes and inhibiting lipid peroxides to resist oxidative stress. Mahboub, H.H. et al. have also reported that ASA consumption significantly enhances the overall anti-oxidant capacity within cyprinus carpio, which may be attributed to the upregulation of anti-oxidant enzymes. When 10 g/kg ASA was added to the basic diet for continuous feeding over a period of 60 days, the total anti-oxidant capacity (TAC), glutathione (GSH), and SOD contents in liver tissue were increased from 16.66 ng/mg to 58.33 ng/mg, 30.33 mmol/g to 66.33 mmol/g, and 14 to 48 U/mg respectively, meanwhile, SOD, GPX, and GSS mRNA levels in spleen were also intensified (Mahboub et al., 2022).
In addition, the anti-oxidant capacity of ASA is positively correlated with the total phenolic content in the extract. Phenolic compounds have the ability to scavenge free radicals and participate in redox reactions to protect cells from oxidative damage (Desmarchelier et al., 2005). Qin, F. et al. extracted ASA with 50% ethanol and found that the extract had a total phenolic content of 874.49 ± 6.75 mg GAE (gallic acid equivalent)/100 g fresh weight. This extract demonstrated excellent free radical scavenging ability in free radical scavenging assays. The extract showed significantly stronger total reducing activity, 2′-Azinobis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS) free radical scavenging activity, and H2O2 scavenging activity compared to ascorbic acid. However, its 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging ability, hydroxide ion, and peroxy ion were comparable to that of ascorbic acid (Qin et al., 2019). However, Yiğit, D. et al. extracted ASA with methanol and water, the total phenolic content was 0.4 and 0.5 μg GAE/mL, respectively, while the DPPH free radical scavenging activity was poor at the concentration of 100–300 μg/mL, indicating that the total phenolic content of ASA is a key factor affecting its anti-oxidant capacity (Yiğit et al., 2009). Furthermore, the variety and origin of ASA also play important roles in determining the total phenolic content. Among the five varieties of ASA in Poland, the “Somo” variety had the highest total phenolic content of 1.22 mM GAE/L, and this variety showed the best anti-oxidant activity according to the ferric reducing anti-oxidant power (FRAP) test. Similarly, ASA from five different regions of Pakistan exhibited significant differences in anti-oxidant activity after extraction with n-hexane. The ASA from Badoghur had a total phenol content of 5,005 mg GAE/100 g dry weight, which was significantly higher than that of other origins. Additionally, ASA from Badoghur showed the smallest half maximal inhibitory concentration (IC50) value in total anti-oxidant capability, hydrogen peroxide scavenging, DPPH, and FRAP experiments, indicating the strongest anti-oxidant activity (Tareen et al., 2021).
Moreover, recent studies have revealed that ASA contains other components, besides phenols, that have anti-oxidant capacity. One such component is a neutral polysaccharide called AP-1, which was extracted and isolated from ASA. AP-1 exhibited a maximum inhibition rate of 87.74% for DPPH radical scavenging activity at a concentration of 10 mg/mL, which is slightly lower than that of vitamin C. However, ABTS assay revealed that AP-1 has comparable free radical scavenging ability and hydroxyl radicals to vitamin C (Peng et al., 2023). Furthermore, amygdalin also demonstrated anti-oxidant capacity by inhibiting ROS accumulation and activating anti-oxidant enzyme activities such as catalase (CAT) and SOD in RAW264.7 cells (Trang et al., 2022).
A growing number of experimental studies have demonstrated the broad spectrum of antibacterial activity exhibited by ASA. Different extracts of ASA have varying degrees of antibacterial activity, as outlined in Table 4. Among these extracts, ASA volatile oil stands out for its extensive antibacterial activity, which is likely attributed to its main component, benzaldehyde. This component has been widely utilized in cosmetics due to its antibacterial, antiseptic, and stabilizing effects (Rodrigues and de Carvalho, 2022). ASA volatile oil exhibits excellent antibacterial activity against Gram-positive bacteria such as Staphylococcus aureus, Staphylococcus epidermidis and methicillin-resistant S. aureus as well as Gram-negative bacteria including Escherichia coli, Pseudomonas aeruginosa, P. aeruginosa D24, Salmonella typhimurium and Shigella sonnei. Complete growth inhibition was observed with a minimum inhibitory concentration (MIC) ranging from 250 to 500 μg/mL. Furthermore, the ASA essential oil also displayed certain antibacterial activity against several other clinical pathogenic bacteria (Lee et al., 2014). Additionally, ASA volatile oil exhibited a significant inhibitory effect on Listeria monocytogenes in solid medium, micro-atmospheric medium, liquid medium and beef slices (Wang et al., 2020). Listeria monocytogenes is an intracellular parasite, primarily transmitted through food, and severe poisoning can result in blood and brain infections (Stevens et al., 2006). Studies have indicated that polyphenols can bind to bacterial cell membrane, disrupt bacterial cell membrane proteins, induce bacterial metabolic disorders, and ultimately inhibit bacterial growth or kill bacteria (Messaoudene et al., 2022). ASA is rich in polyphenols, which exhibit significant antibacterial activity against both Gram-negative bacteria (E. coli and Acetobacter aceti) and Gram-positive bacteria (S. aureus, Bacillus subtilis and Bacillus cereus). The inhibitory zone ranges from 13.0 to 18.6 mm and MIC between 31.25 and 250 μg/mL (Qin et al., 2019). However, ASA demonstrates a stronger antibacterial effect against Gram-positive bacteria. This could be attributed to the outer membrane permeability barrier of Gram-negative bacteria cell wall, which limits the interaction between antibacterial agents and their targets within bacterial cells. Moreover, both aqueous and alcoholic extracts of ASA display significant antibacterial activity against E. coli and S. aureus with inhibitory diameters ranging from 13 to 15 mm and MIC values of 0.312–0.625 mg/mL (Yiğit et al., 2009). However, the ASA base oil exhibits poor antibacterial activity, consistent with previous findings that the fatty acids in ASA lack antibacterial properties (Moola et al., 2022).
Millions of people worldwide are affected by superficial fungal infections, the most common skin disease caused by dermatophytes that parasitize on the surface layer of the stratum corneum. Microsporum canis and Microsporum are often implicated in these infections. The clinical symptoms of dermatophytosis are generally mild, and active lesions typically heal within 6–8 weeks. ASA volatile oil has demonstrated significant antibacterial activity against keratinophilic fungi, completely inhibiting their growth at a concentration of 100 μg/mL (Ibrahim and Abd El-Salam, 2015). In addition, among various ASA extracts, volatile oil exhibited notable inhibitory effects on Malassezia furfur and Candida albicans, with MIC of 250 and 1,000 μg/mL (Lee et al., 2014), respectively. However, ASA polyphenols only showed moderate inhibition against candida, while base oil displayed poor inhibitory activity (Yiğit et al., 2009; Moola et al., 2022). Furthermore, ASA volatile oil exhibited inhibitory effect on 19 plant pathogenic fungi, suggesting its potential as a plant and agricultural fungicide (Geng et al., 2016).
ASA is known to contain antibacterial substances such as volatile oil and polyphenols, which contribute to its excellent antibacterial potential. While there have been numerous studies on the antibacterial activity of ASA, few have explored its underlying mechanism. Mahboub, H.H. et al. suggested that the antibacterial effect of ASA might be attributed to immune enhancement (Mahboub et al., 2022), while Mikoshiba, S. et al. proposed that metabolism could play a vital role (Mikoshiba et al., 2006). However, these studies are still limited, and further research is necessary to fully understand the antibacterial mechanism of ASA.
The main substance exerting anti-inflammation effect in ASA may be amygdalin, which can inhibit the abnormal activation of TGF-β1/Smad signaling pathway and TLR4/NF-κB signaling pathway (Figure 5, Table 5). It was found that intraperitoneal injection of 4 mg/kg amygdalin significantly alleviate bleomycin-induced neutrophil inflammatory infiltration in mouse lung tissues and reduced the number of macrophages and neutrophils in BALF, which are precursors of immune defense. The underlying mechanism may be the inhibition of TGF-β1/Smad signaling pathway (Jiao et al., 2023). In addition, amygdalin can directly hamper the expression of cytokines to exert anti-inflammatory effect. In the model of intraplantar injection of formalin, 1 mg/kg amygdalin significantly inhibited TNF-α and IL-1β mRNA levels in rat paw skins, which was comparable to that of indomethacin (Hwang et al., 2008). Besides, amygdalin can regulate the expression of inflammation-related enzymes and play an indirect anti-inflammatory role. Cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) are involved in the inflammatory response and induce the production of inflammatory mediators prostaglandin E2 (PGE2) and NO, respectively (Chang et al., 2005). In LPS-stimulated BV2 cell model, treatment with 10 or 100 μg/mL amygdalin and 0.1 or 1 mg/mL ASA aqueous extract can significantly downregulate COX-2 and iNOS mRNA levels, and the contents of PGE2 and NO (Chang et al., 2005; Yang et al., 2007). Furthermore, in a model of HUVEC injury induced by PM2.5, amygdalin at concentrations of 2.5, 5, and 10 μg/mL has been shown to diminish the levels of COX-2, IL-6, TNF-α, and IL-1β, while promoting apoptosis of damaged cells via impeding aberrant activation of TLR4/NF-κB signaling pathway (Wang et al., 2022). Moreover, it has been discovered that oral administration of 15 mg/kg amygdalin can restore Th1/Th2 immune imbalance to alleviate airway inflammation in an ovalbumin-induced asthma mice model (Cui et al., 2023). However, further studies are needed to determine whether other components of ASA have anti-oxidant effects.
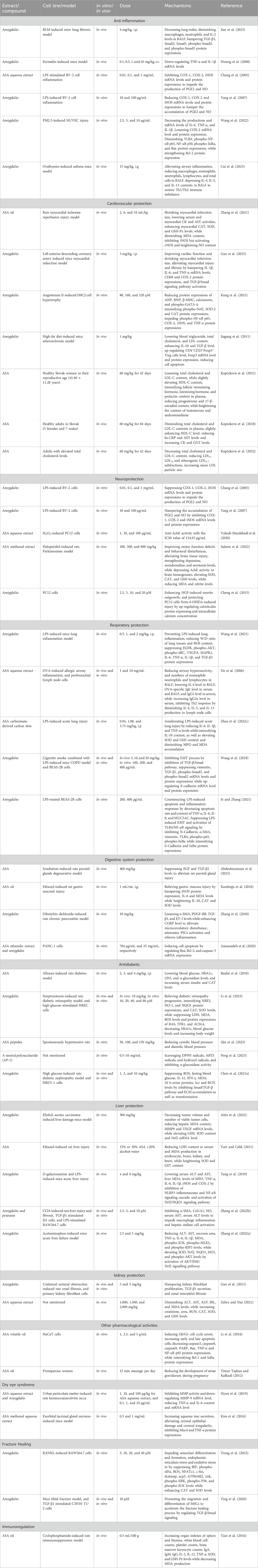
TABLE 5. Anti-inflammation, cardiovascular protection, neuroprotection, respiratory and digestive system protection, antidiabetic, liver and kidney protection and other pharmacological activities of ASA.
The latest evidence indicates that cardiovascular disease is responsible for 31% of global deaths. It has been established that adopting a healthy diet is crucial in reducing the risk of cardiovascular diseases (Dikariyanto et al., 2021). Cardiovascular diseases encompass various heart and vascular conditions such as coronary heart disease, hypertension, heart failure, peripheral vascular disease, cerebrovascular disease, vascular disease, and rheumatic heart disease. ASA, which is rich in unsaturated fatty acids, has been proven to effectively lower biochemical and arterial markers associated with cardiovascular risk (de Oliveira et al., 2017). Moreover, ASA is abundant in anthocyanins, flavonoids, and phenolic acids, with concentrations of up to 118.17 mg/100 g, 113.66 mg/L, and 91.42 mg/100 mL, respectively (Qin et al., 2019). These substances have also demonstrated positive effects on cardiovascular diseases (Perez-Vizcaino and Duarte, 2010; Blesso, 2019; Potì et al., 2019; Mattioli et al., 2020). Therefore, ASA exhibits significant potential and advantages in the treatment of cardiovascular system diseases, mainly due to the functions of unsaturated fatty acids, polyphenols, flavonoids, and amygdalin.
Currently, ASA and its active ingredients have been shown to contribute to cardiovascular health in both in vivo experiments and clinical studies (Table 5). In a rat myocardial ischemia-reperfusion injury model, it was observed that continuous treatment with 2, 6, and 10 mL/kg of ASA oil for 2 weeks resulted in a significant reduction in the myocardial infarction area of rats. Additionally, the activities of serum creatine kinase and aspartate aminotransferase increased, leading to an increased production of ATP. This increase in ATP production provides sufficient energy for the physiological needs of the heart. Moreover, supplementation with ASA oil also demonstrated a significant increase in the activity of antioxidant enzymes such as myocardial CAT, SOD, and glutathione peroxidase. This increase in anti-oxidant enzyme activity enhances the anti-oxidant defense system while reducing the content of MDA and inhibiting lipid peroxidation. Ultimately, these effects provide a protective effect against myocardial ischemia-reperfusion injury in cardiomyocytes (Zhang et al., 2011). In recent years, there has been increasing attention on amygdalin, the main component of ASA. It has been demonstrated in vitro that amygdalin can effectively inhibit Ang II-induced cardiomyocyte hypertrophy, reduce inflammatory response, and exhibit anti-oxidant activity when treating H9C2 cells induced by Ang II at concentrations of 80, 160, and 320 μM. These effects of amygdalin are primarily achieved through the reduction of atrial natriuretic peptide, B-type natriuretic peptide, and β-MHC, which are related to cardiac hypertrophy. Additionally, amygdalin inhibits the expression of inflammatory markers such as TNF-α, iNOS, COX-2, and phospho-NF-κB protein. Furthermore, amygdalin increases the expression of Nrf2, CAT, SOD-2, and GPX-4, which are proteins related to oxidative stress (Kung et al., 2021). Both in vitro and in vivo studies have also indicated that amygdalin can alleviate atherosclerosis. This effect may be attributed to its inhibition of the inflammatory response, enhancement of immune regulatory function in regulatory T cells, or inhibition of the TLR4/NF-κB and Bcl-2/Bax signaling pathways (Jiagang et al., 2011; Wang et al., 2022).
The benefits of ASA for cardiovascular disease have been extensively studied due to its various components and proven efficacy. ASA has been shown to exert cardiovascular protective effects by reducing cholesterol levels, particularly low-density lipoprotein cholesterol (LDL-C) (Kopčeková et al., 2021). Clinical research reports have demonstrated that after 6 consecutive weeks of taking 60 mg/kg ASA, volunteers experienced a significant decrease in serum LDL-C levels. It is important to note that elevated levels of LDL-C can contribute to the development of cardiovascular atherosclerosis and the blockage of blood vessels by causing excessive fat absorption in extrahepatic cell tissues (Siri-Tarino et al., 2010). In another clinical study, it was observed that after 12 weeks of taking 60 mg/kg ASA, total cholesterol levels decreased by 8.64% and LDL-C levels decreased by 21.2%. Additionally, there was a slight increase in high-density lipoprotein cholesterol (HDL-C) levels, along with an increase in C-reactive protein and serum creatine kinase levels (Kopčeková et al., 2018). Importantly, studies have shown that for every 1% reduction in LDL-C, the risk of coronary heart disease is reduced by up to 3% (Brown and Goldstein, 2006). This indicates that consuming ASA can significantly reduce the risk of cardiovascular disease. Further investigation revealed that after a 6-week administration of ASA to 21 individuals with normal cholesterol levels and 13 patients with high cholesterol levels, there was no significant change observed in the total cholesterol content and average LDL-C levels of the normal individuals. Similarly, the average total cholesterol content and average LDL-C levels of the patients with HDL-C levels also did not exhibit a significant change. However, a reduction in density cholesterol levels was observed, and the LDL3–7 subfractions were only detected in one individual (Kopčeková et al., 2022). It is important to note that the LDL3–7 subfractions, which are part of very low-density lipoproteins, have smaller particle sizes compared to LDL1 and LDL2, and are associated with a higher risk of atherosclerosis (Qiao et al., 2022). In simpler terms, the intake of ASA can modify the lipoprotein profile of individuals with hypercholesterolemia by primarily reducing low-density lipoprotein levels, without negatively affecting lipid metabolism in healthy individuals.
In summary, ASA exerts cardiovascular protection mainly by reducing LDL levels, inhibiting oxidative stress and regulating immunity, which strongly supports the use of ASA in the management of cardiovascular diseases.
Alzheimer’s disease and Parkinson’s disease are two common neurodegenerative diseases characterized by neuronal damage and behavioral dysfunction. The pathological processes involved in these diseases include immune inflammation, oxidative stress, and mitochondrial dysfunction (Chen W. et al., 2022). Phytochemicals with anti-oxidant properties are known to have the potential to provide neuroprotection (Chakraborty et al., 2022). ASA, abundant in flavonoids, polyphenols, and other anti-oxidative compounds, shows promising potential for treating neurodegenerative diseases by suppressing inflammation, oxidative stress and acetylcholinesterase (AchE) activity (Table 5).
Microglia, immune effector cells in the central nervous system, play a role in releasing inflammatory mediators that contribute to neurotoxicity and the development of neurodegenerative diseases (Simpson and Oliver, 2020). Studies have demonstrated that ASA extract can inhibit COX-2 and iNOS mRNA levels in BV2 cells stimulated by LPS. This inhibition leads to a reduction in the synthesis of PGE2 and the production of NO, thereby suppressing immune and inflammatory responses and exerting a neuroprotective effect (Chang et al., 2005; Yang et al., 2007). AchE, present in neurons, serves as an indicator of neuronal damage (Olasehinde and Olaniran, 2022). In vitro studies, ASA water extract exhibits significant anticholinesterase activity with an IC50 of 134.93 μg/mL. Additionally, treatment with 100 μg/mL ASA water extract demonstrates a favorable neuroprotective effect against H2O2-induced damage to PC12 neuron cells, resulting in a cell survival rate of 70.71%. In comparison, PC12 cells treated with 400 μM hydrogen peroxide exhibit a survival rate of less than 40% (Vahedi-Mazdabadi et al., 2020).
It has been demonstrated in vivo studies that the methanol extract of ASA at concentrations of 100, 300, and 800 mg/kg has a protective effect on haloperidol-induced Parkinson’s disease model. Behavioral analysis has shown that ASA treatment improves motor activity, motor coordination, and exploratory activities in rats. It also reduces depression, anxiety, and convulsive seizures, accompanied by a decrease in dopamine, 5-hydroxytryptamine, and norepinephrine neurotransmitter levels. Additionally, there is a significant increase and decrease in AchE levels. Furthermore, behavioral improvement and brain function recovery are positively correlated with increased anti-oxidant enzyme activity in the body (Saleem et al., 2022). Moreover, amygdalin also shows potential neuroprotective effects, possibly due to its induction of calreticulin protein expression, which plays a vital role in the survival, differentiation, and regulation of neurons (Cheng et al., 2015).
Respiratory system diseases are diverse and common, affecting the trachea, bronchi, and lungs. Some prevalent conditions in this category include asthma, COVID-19, acute lung injury, and chronic obstructive pneumonia (Tavares et al., 2020). ASA, an important Chinese herbal medicine, is used to treat cough and has various functions such as enhancing lung function, relieving constipation, and promoting intestinal peristalsis. According to traditional Chinese medicine, bitter purgation helps disperse and move lung Qi, thereby eliminating phlegm (Gao et al., 2011). Pharmacological studies have shown that amygdalin, an effective component of ASA, is hydrolyzed to hydrocyanic acid and benzaldehyde in the body after oral administration, thereby relieving cough, asthma and other respiratory system diseases (Figure 6).
The COVID-19 pandemic, caused by the 2019 novel coronavirus, is spreading globally. It is characterized by symptoms such as fever, dry cough, and fatigue, which can lead to severe respiratory failure and even death. Additionally, patients may experience muscle aches and diarrhea, and in severe cases, they may develop acute respiratory distress syndrome, septic shock, or succumb to the disease (Du et al., 2021). Through network pharmacology and molecular docking, it was found that stigmasterol, sitosterol, sholesterol, (6Z,10E,14E,18E)-2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene, oestrone, diisooctyl succinate, 11,14-eicosadienoic acid, and amygdalin are suggested to be the nine key active ingredients for the treatment of COVID-19. Moreover, IL6, SRC, MAPK1, MAPK3, VEGFA, EGFR, HRAS, and CASP3 are identified as potential core targets for ASA treatment. It has been demonstrated that a therapeutic potential of amygdalin in vivo experiments. Moreover, The administration of 0.5–2 mg/kg of amygdalin has been shown to regulate the PI3K-AKT signaling pathway, VEGF signaling pathway, and MAPK signaling pathway, resulting in significant inhibition of EGFR, phospho-AKT, phospho-SRC, VEGFA, MAPK1, IL-6, IL-1β, and TNF-α protein expressions (Wang et al., 2021). However, more research is required to support the use of ASA in the treatment of COVID-19.
Allergic asthma, which is the most common type of asthma, is characterized by chronic airway inflammation involving T lymphocytes, mast cells, eosinophils, and other cells (Possa et al., 2013). Several studies have demonstrated that ASA aqueous extract shows promising therapeutic effects in both an ovalbumin-induced allergic airway inflammation model in vivo and lymph node primary cells in vitro. This therapeutic effect of ASA is attributed to a reduction in IL-4 and IL-5 levels (Do et al., 2006). IL-4 is responsible for the transformation of regulatory T cells into helper T cells, while IL-5 regulates the growth, differentiation, and activation of eosinophils (Jin et al., 2019). However, further research is necessary to determine whether ASA exhibits similar therapeutic effects on other types of asthma and to investigate the underlying molecular mechanisms involved.
Acute lung injury (ALI) is a severe medical condition associated with significant morbidity and mortality. It is characterized by damage to the alveolar epithelial cells and pulmonary capillary endothelial cells, resulting from non-cardiogenic factors (Tang et al., 2023). The clinical manifestations of ALI include dyspnea and intractable hypoxemia, which can progress to severe respiratory disorders. ALI is characterized by the infiltration of a large number of neutrophils into lung tissue, leading to the release of inflammatory cytokines and damage to pulmonary endothelial and epithelial cells. LPS, also known as endotoxin, is a major component of the outer membrane of Gram-negative microorganisms and is highly pathogenic (Liu et al., 2020). The ASA carbon nano-material has demonstrated its ability to inhibit the release of IL-6, IL-1β, and TNF-α inflammatory mediators in rat serum. Moreover, it has been shown to reduce the increase of neutrophils in the blood. Additionally, it exhibits a decrease in the chemotaxis of neutrophils to inflammatory sites and inhibits the injury and aggravation of LPS to lung tissue. These findings suggest that ASA carbon nano-material shows promising potential as a candidate treatment for ALI (Zhao Y. et al., 2022).
In addition, amygdalin may also have therapeutic effects on chronic obstructive pulmonary disease (COPD) (Sun et al., 2020). COPD is characterized by airway remodeling, which involves epithelial-mesenchymal transition (EMT). Recent studies have shown that amygdalin, administered at doses of 5, 10, and 20 mg/kg, has a protective effect on the EMT process in COPD mice induced by cigarette smoke. These findings are consistent with the observed inhibition of TGF-β1 protein expression and Smad2/3 phosphorylation by amygdalin, indicating its potential role in suppressing the TGF-β/smad pathway. Moreover, amygdalin also demonstrates inhibitory effects on the EMT process in BEAS-2B cells stimulated by cigarette smoke in vitro, suggesting its potential use in COPD treatment (Wang et al., 2019). Furthermore, the mechanism by which amygdalin exerts its therapeutic effect may also be related to the inhibition of LPS-induced EMT and TLR4/NF-κB signaling cascade (Si and Zhang, 2021).
Numerous formulas containing ASA have been extensively studied and utilized in the research and treatment of various respiratory diseases such as colds, asthma, COVID-19, and pulmonary fibrosis (Li et al., 2010; Lin et al., 2016; Sun et al., 2018; Bai et al., 2022; Li et al., 2022). This further demonstrates the potential respiratory protection activity of ASA (Table 5).
Limited reports exist on the protective effects of ASA on the digestive system. This section provides a summary of the protective effects of ASA on the digestive tract and digestive glands (Table 5). Studies have shown that 400 mg/kg ASA can enhance the damage caused by gamma-radiation of 5 Gy to the salivary glands of Rattus Norvegicus, specifically affecting the acinar cells. This effect is primarily attributed to the downregulation of EGF protein expression and the upregulation of TGF-β protein expression, indicating that ASA mitigates oxidative damage and inflammatory responses, thereby protecting against salivary gland damage (Abdaulmoneam et al., 2023). In addition, ASA oil has been found to possess gastroprotective effects. In an ethanol-induced rat gastric ulcer model, ASA oil reduces the release of cytokines such as IL-6, increases levels of oxidative stress markers like SOD and CAT, decreases lipid oxidation, and inhibits mucosal cell apoptosis, demonstrating its gastroprotective properties. Recent research also suggests that amygdalin may have potential pancreatic protective effects (Karaboğa et al., 2018). Intravenous injection of 10 mg/kg amygdalin improves pancreatic fibrosis in rats with chronic pancreatitis induced by dibutyldichlorotin, as evidenced by reduced production of profibrotic growth factors and inhibition of pancreatic stellate cell activation. The mechanism may involve improved microcirculation through reduced endothelin-1 expression and upregulated expression of calcitonin gene-related peptide (Zhang et al., 2018). Similarly, ASA ethanol extract can induce apoptosis of pancreatic cancer cells in vitro (Aamazadeh et al., 2020).
In summary, ASA has been found to have a protective effect on parotid glands, pancreas and stomach. Its mechanism of action is believed to involve the inhibition of inflammatory response and oxidative stress, along with the induction of cell apoptosis. However, the specific substances responsible for the therapeutic effects of ASA are still unidentified and the protective effects on other digestive organs and digestive glands have not been defined, thus the protective effects of ASA on the digestive system need to be further investigated.
Diabetes mellitus (DM) is a group of metabolic disorders that poses a significant global health burden, affecting approximately 6% of the population. The majority of diabetic patients (90%–95%) have type II diabetes, while the remaining have type I diabetes. Currently, the options for DM treatment are limited, and long-term use of available drugs may result in severe side effects (Das and Chakrabarti, 2005). ASA has shown specific effects on DM and offers a promising alternative treatment option due to its cost-effectiveness and easy accessibility. Both in vivo and in vitro studies have demonstrated that the antidiabetic activity of ASA is primarily associated with its ability to enhance insulin secretion, leading to reduced blood pressure and mitigation of oxidative stress (Table 5).
In an alloxan-induced rat DM, ASA demonstrated a dose-dependent reduction in blood glucose levels, an increase in body weight, a decrease in lipid peroxidation levels, and an increase in serum CAT levels. ASA significantly increased insulin levels after 8 weeks, and exhibited an inhibitory effect on α-glucosidase, suggesting that its anti-diabetic properties may be attributed to the reduction of oxidative stress caused by glucose, inhibition of α-glucosidase, and significant mediation by elevated insulin (Raafat et al., 2018). Interestingly, ASA also showed a significant reduction in glycosylated hemoglobin levels, indicating its potential to prevent complications associated with DM. Higher levels of Hemoglobin A1C (HbA1c) in diabetic patients are indicative of poorer regulation of blood glucose and an increased risk of diabetes-related complications (Klonoff, 2020). Furthermore, amygdalin was found to alleviate diabetic retinopathy, a complication of DM. In high glucose-stimulated HRECs cells, 40 μM amygdalin demonstrated a significant inhibition on oxidative stress and ferroptosis, evidenced by increased GSH/GSSG ratio, SOD, CAT, GPX4 activity and reduced MDA and ROS levels, as well as significant downregulation of ferroptosis marker proteins including RAS, TFR1, and ACSL4. Notably, the antidiabetic retinopathy effects of amygdalin were found to be associated with the activation of the NRF2/ARE pathway, leading to the activation of NRF2 and HO-1 and an increase in NQO1 protein expression (Li et al., 2023).
Recently, the antihypertensive effects of natural chemical constituents of ASA have attracted great attention from researchers. A polypeptide, Arg-Pro-Pro-Ser-Glu-Asp-Glu-Asp-Gln-Glu, has been identified in ASA albumin lately. This polypeptide acts as a non-competitive inhibitor of angiotensin-converting enzyme (ACE) with an IC50 value of 205.50 μM. Additionally, it has exhibited positive antihypertensive effects on spontaneously hypertensive rats at concentrations of 100 and 150 mg/mL. Although not as effective as 10 mg/kg captopril, this polypeptide has led to a significant decrease in systolic and diastolic blood pressure (Qin et al., 2023). These findings suggest that the polypeptide holds the potential for anti-DM effects and could be utilized in the development of anti-DM drugs. Furthermore, a neutral polysaccharide (AP-1), which has a triple helix structure, has recently been extracted from ASA. AP-1 primarily consists of glucose, arabinose, galactose, and mannose. It has strong inhibition of α-glucosidase enzyme and the ability to scavenge DPPH, ABTS, and Hydroxyl free radicals in vitro (Peng et al., 2023). These findings indicate that AP-1 may serve as a natural anti-oxidant and hypoglycemic agent in the treatment of DM.
Oxidative stress is widely recognized as the underlying cause of both acute and chronic liver diseases (Cui et al., 2021). ASA, a natural source of plant antioxidants, shows promising potential for the treatment of liver diseases. Recent studies have revealed that amygdalin not only alleviates symptoms of Ehrlich ascites cancer but also, helps prevent liver cancer and mitigate associated liver damage when combined with sorafenib. These hepatoprotective effects are attributed to the direct reduction of liver function indicators such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transferase (GGT), as well as the significant antioxidant activity of amygdalin (Attia et al., 2022). In addition, another study also suggests that the key role of ASA in liver protection may be related to oxidative stress (Yurt and Celik, 2011).
ASA has demonstrated hepatoprotective effects at various stages of liver disease development. In the early stages, ASA exhibits anti-inflammatory properties, effectively inhibiting disease progression. The main component of ASA, amygdalin, not only inhibits excessive oxidative stress and reduces the levels of liver injury-related enzymes, but also suppresses the production of TNF-α, IL-6 and IL-1β as well as the expressions of inflammation-related proteins such as iNOS and COX-2, thereby mitigating inflammatory response and providing resistance against acute liver injury (Tang et al., 2019). Hepatic fibrosis, a compensatory pathophysiological process, occurs when the liver is damaged by chronic inflammation, leading to tissue degeneration, inflammatory infiltration, necrosis, and constant repair of liver collagen and extracellular matrix (Tsuchida et al., 2018). Amygdalin, the active ingredient of ASA, has been found to inhibit the activation of hepatic stellate cells induced by transforming growth factors. It also reduces the secretion of cytokines and the levels of ALT and AST, exerting anti-inflammatory effects and protecting the liver from fibrosis (Zhang et al., 2022b). Moreover, amygdalin has a protective effect on advanced liver failure. In the case of acetaminophen-induced acute liver failure, intraperitoneal injection of 2.5 or 5 mg/kg amygdalin has been found to reduce the area of necrosis in liver tissue, lower the levels of liver function-related indicators ALT and AST, and decrease neutrophil and macrophage counts. These effects are associated with the inhibition of oxidative damage, increased protein expression of Nrf2/NQO1/HO1, phospho-AKT, and inhibition of the JNK/RIP3/MLKL signaling pathway (Zhang et al., 2022a).
Overall, ASA and amygdalin have promising liver protection effects both in vivo and in vitro experiments due to their potent anti-oxidant activities (Table 5). However, further research is needed to explore the potential of ASA as a therapeutic drug for different stages of liver disease development (Figure 7).
ASA has therapeutic effects on both renal cell carcinoma and chronic kidney disease, such as renal fibrosis (Table 5). The main component of ASA, amygdalin, inhibits the proliferation and production of transforming growth factors in renal interstitial fibroblasts, which plays a crucial role in the development of renal interstitial fibrosis (Bai et al., 2020). In a rat model of unilateral ureteral obstruction, treatment with amygdalin at concentrations of 3 and 5 mg/kg resulted in reduced renal damage and delayed progression of renal interstitial fibrosis (Guo et al., 2013). However, the accumulation of hydrocyanic acid, a metabolite of amygdalin in ASA, can lead to nervous system depression, limiting its application. Nevertheless, a study found that oral administration of 2 g/kg ASA water extract to rats did not exhibit nephrotoxicity but increased antioxidant activity, manifesting as increased levels of renal function indicators such as urea, creatinine and urea nitrogen as well as increased activities of anti-oxidant enzymes such as SOD and GSH (Zehra and Naz, 2021). In short, although the metabolism of ASA can lead to the accumulation of toxic substances, its rich natural chemical components have shown promising effects in the research of various diseases. Further research is needed to fully understand the impact of ASA on kidney diseases.
In addition to its pharmacological effects described above, ASA oil also exhibits skin protective effects. It can inhibit the growth of human keratinocytes and enhance their programmed cell death, making it a potential treatment option for psoriasis (Li et al., 2016). Furthermore, preliminary clinical studies have shown that massage with ASA oil during early pregnancy can effectively reduce the formation of stretch marks (Timur Taşhan and Kafkasli, 2012). Additionally, ASA extract has demonstrated positive effects in relieving symptoms of dry eye syndrome and dry keratitis (Kim et al., 2016; Hyun et al., 2019). Moreover, ASA has also been found to promote fracture healing (Ying et al., 2020; Trang et al., 2022) and regulate the immune system (Tian et al., 2016) (Table 5).
There is mounting evidence supporting the use of ASA in the treatment of cough, lung, and other respiratory-related diseases. Studies have shown that ASA liquids can reduce the sensitivity of the trachea to ammonia stimulation, thereby relieving cough and promoting intestinal peristalsis (Gao et al., 2012). In a research study investigating the effectiveness of traditional Chinese medicine compounds for treating COVID-19, a total of 166 compounds containing 179 traditional Chinese medicines were collected. Among the candidate prescriptions for COVID-19 treatment selected through complex system entropy and unsupervised hierarchical clustering, ASA ranked third in terms of frequency of use and was included in the first formula (Luo et al., 2020). Furthermore, a data mining analysis examining traditional Chinese medicine prescriptions for respiratory diseases analyzed 562 prescriptions specifically targeting the respiratory system. The results revealed that ASA was utilized in 36.7% of the prescriptions, ranking second after Glycyrrgizae radix et rhizoma—roots and rhizomes of Glycyrrhiza glabra L. (Fabaceae), which was used in 47.2% of the prescriptions (Fu et al., 2013). These findings suggest that ASA holds promise as an effective treatment for respiratory diseases.
The plant kingdom contains many substances that may have the potential to prevent or treat human disease (Zhao et al., 2022b; Sun et al., 2023; Zhao et al., 2023), but these bioactive components (such as vitamins and alkaloids) usually show low bioavailability or biological instability. Recently, various techniques for improving drug delivery have been developed to solve the problems of bioavailability and stability. Nanoparticle is the most promising drug carrier, which can effectively deliver bioactive compounds and improve bioavailability. Currently, a protein belonging to the 11S globulin family was isolated from ASA water extract. This protein is composed of three polypeptides connected by disulfide bonds. Upon heat treatment, these bonds rearrange, resulting in the formation of a spherical-shaped dimer. The unique structure of this protein makes it a potential candidate for use as a nanocarrier. It efficiently encapsulates paclitaxel with a maximum encapsulation efficiency of 92.6% and a maximum release of paclitaxel of 57.4% (Lin et al., 2020). Additionally, a recent study developed liposomes loaded with amygdalin using a molar ratio of Tween 60: cholesterol: dihexadecyl phosphate as 1: 2: 0.1. These liposome-loaded amygdalin formulations demonstrated significant effects in reducing tumor volume, decreasing epidermal hyperplasia, and eliminating edema in a rat tumor model induced by 7,12-dimethylphenanthrene. Surprisingly, the anti-tumor activity of these liposomes surpassed that of tamoxifen, a well-known anti-tumor drug (El-Ela et al., 2022). Moreover, a polypeptide extracted from ASA water extract has displayed the ability to form a complex with zinc ions, exhibiting remarkablely lowering blood pressure effect. This polypeptide shows promise for further development as an antihypertensive drug (Qin et al., 2023).
Numerous studies have demonstrated that formula preparations containing ASA exhibit powerful therapeutic effects in the treatment of lung disease, liver disease, eye disease, and other diseases, especially respiratory diseases. The ASA-containing formulas may significantly relieve symptoms such as fever, cough and runny nose. Table 6 provides a summary of ASA-containing formulations and their clinical applications as outlined in the Chinese Pharmacopoeia 2020 edition.
The main toxic substance in ASA is hydrocyanic acid, which is produced when amygdalin is metabolized. Amygdalin is broken down by β-D-glucosidase into mandelonitrile, which further breaks down into benzaldehyde and hydrocyanic acid. HCN is eventually absorbed into the bloodstream, leading to cyanide poisoning. It is important to note that the toxic doses of amygdalin vary greatly depending on the method of administration. The lethal dose of amygdalin through intravenous injection in humans is 5 g, while oral consumption is 0.5–3.5 mg/kg body weight (Song et al., 2016). When injected intravenously, amygdalin can bypass enzymatic hydrolysis in the gastrointestinal tract, resulting in high blood concentration and detectable amygdalin in the plasma. Additionally, 80% of the injected amygdalin is absorbed by the body within 24 h and eliminated through urine (He et al., 2020). Ingesting 50 ASA consecutively can cause poisoning symptoms in adults, whereas babies can be poisoned by consuming only 5–10 (Chaouali et al., 2013). Cyanide poisoning can lead to rapid hemodynamic and neurological impairment. Studies have shown that hydrocyanic acid can inhibit the activity of cytochrome oxidase in cell mitochondria, causing respiratory inhibition in tissue cells and cell death due to hypoxia. The clinical manifestations of cyanide poisoning depend on the route, duration, dose, and source of exposure. Common symptoms include nausea, vomiting, diarrhea, respiratory failure, hypotension, arrhythmia, cardiac arrest, the odor of bitter almonds, and cherry red skin (Jaszczak-Wilke et al., 2021).
Modern pharmacological research has revealed significant variations in the toxicity of different extracted components of ASA (Table 7). One study found that the median lethal dose (LD50) of lyophilized ASA aqueous extract on Kunming mice was 29.9 g/kg (Song et al., 2016), while another study reported an LD50 of approximately 22.5 g/kg for raw ASA aqueous extract on Kunming mice (Chen and Jia, 2012). However, a separate study administered ASA oil at a dosage of 10 mg/day to Wistar rats for 13 weeks, and no adverse reactions or fatalities were observed (Gandhi et al., 1997). In contrast, when amygdalin was directly administered to Wistar rats, the rats exhibited quadriplegia, muscle-twitching, difficulty in breathing, apnea, and subsequent death, with an LD50 of 880 mg/kg (Adewusi and Oke, 1985). These findings indicate that ASA oil does not exhibit obvious toxicity, whereas ASA water or alcohol extract demonstrates strong toxicity. Furthermore, the toxicity of amygdalin alone is more significant than that of ASA water or alcohol extract.
β-D-glucosidase plays a crucial role in the hydrolysis process of amygdalin. When amygdalin was administered alone, the IC50 of HepG-2 was 458.10 mg/mL. However, co-administration of amygdalin with β-D-glucosidase resulted in a more than 100-fold decrease in IC50 to 3.2 mg/mL, highlighting the critical role of β-D-glucosidase in the pathway of amygdalin poisoning (Zhou et al., 2012). Similarly, there was a notable difference in the IC50 values of PC12 and MDCK cells when amygdalin was administered alone or in combination with β-D-glucosidase. The IC50 of PC12 cells decreased from 35.83 to 5.97 μM, and the IC50 of MDCK cells decreased from 63.97 to 3.93 μM (Song et al., 2016). Although amygdalin itself is stable, it becomes highly toxic after hydrolysis by β-D-glucosidase. Unfortunately, β-D-glucosidase is widely present in humans, animals, plant seeds, and microorganisms. Therefore, it is crucial to explore methods for attenuating amygdalin poisoning and implementing preventive measures.
Traditional Chinese medicine suggests that ASA should undergo processing before use to inhibit the activity of amygdalin and preserve its properties. The 2020 edition of the Chinese Pharmacopoeia states that the main methods for processing and detoxifying ASA include the Clear fried method and the Chan method (Wei et al., 2023). It has been discovered that the combined use of ephedare herba—herbaceous stems of Ephedra sinica Stapf (Ephedraceae) with ASA effectively reduces the toxicity of ASA without impacting the amygdalin content. When mice were orally administered ASA alone, the LD50 was found to be 29.9 g/kg. However, when different ratios of ephedare herba and ASA (MX (4:1), MX (2:1), MX (1:1), MX (1:2), and MX (1:4)) were orally administered, the LD50 of mice was 87.9, 81.6, 81.4, 64.6, and 59.3 g/kg respectively, indicating the detoxification effect of Ephedra sinica Stapf on ASA. Furthermore, the HPLC method was used to measure the difference in amygdalin content among the mentioned groups above. The content of amygdalin in the ASA water extract was found to be 11.77 mg/g. However, co-extraction with ephedra did not result in significant differences in the amygdalin content (Song et al., 2016).
Another detoxification method for ASA has recently been reported. The method involves soaking ASA powder in a 25% sodium chloride solution for 12 h, followed by rinsing with tap water until the liquid becomes clear. This process is repeated once, and then the ASA powder is soaked again in the 25% sodium chloride solution for another 12 h. After rinsing until the liquid is clear, the ASA powder is dried at 45° for 36 h, resulting in the detoxified ASA. This method effectively eliminates the toxic component HCN and significantly reduces the levels of antinutrient factors such as phytates, phytate phosphorus, and oxalate by 71.83%, 23.92%, and 38% respectively compared to raw ASA. The fat content and crude fiber content do not show significant changes. However, there is a reduction in the contents of Vitamin C, β-carotene, minerals, and protein to varying degrees (Tanwar et al., 2018). Overall, this method can be employed in ASA oil and functional food production. Nevertheless, further research is needed to fully explore the medicinal potential of ASA and investigate the effects of different processing methods on ASA.
Studies on the pharmacokinetics of ASA primarily focus on amygdalin and its metabolite prunasin (Table 8). When ASA water extract is administered orally, amygdalin and prunasin can be detected in the plasma of rats, exhibiting significantly different pharmacokinetic parameters, particularly in terms of the maximum concentration (Cmax). After oral administration of ASA water extract, amygdalin is rapidly absorbed with a Tmax at 0.5 h and a Cmax at 223.6 ng/mL. Subsequently, a substantial amount of amygdalin is hydrolyzed to prunasin within a short time, with a Tmax of 0.58 h and a Cmax of 5,212.8 ng/mL (Song et al., 2015). The volume of distribution/bioavailability (Vz/F) of amygdalin is 196.8 L/kg, while the Vz/F of prunasin is 15.9 L/kg, indicating that amygdalin exhibits high tissue distribution specificity and may be concentrated in certain organs compared to prunasin (Helmy et al., 2013). Recent research revealed that the concentration of amygdalin in lung tissue (309.335 ± 13.662 ng/g) was significantly higher than in plasma (44.774 ± 7.397), heart (23.693 ± 6.097), liver (43.391 ± 5.963), spleen (53.745 ± 6.584), and kidney (55.373 ± 4.467) (Yang et al., 2021), suggesting that amygdalin may be concentrated in lung tissue. The elimination half-life (t1/2) of amygdalin and prunasin are 1.15 ± 0.26 h and 2.21 ± 0.52 h, respectively. Similarly, the mean residence time (MRT) for amygdalin and prunasin are 1.33 ± 0.23 h and 1.57 ± 0.22 h, respectively (Song et al., 2015). This observation can be attributed to the hydrolysis of β-D-glucosidase. Additionally, the clearance/bioavailability (CLz/F) of amygdalin is significantly higher at 121.1 ± 31.4 L/kg·h compared to prunasin, which has a CLz/F of only 5.1 ± 0.9 L/kg·h. This difference may be linked to the higher blood concentrations of prunasin. It is worth noting that amygdalin exists in two isomers, D and L, with the latter being stable only at temperatures higher than 40°C (Wahab et al., 2015). After administration of ASA water extract, the plasma concentrations of the two isomers are almost the same, with values of 147.8 ± 34.9 and 138.7 ± 32.4 ng/mL, respectively. However, their metabolites, D-Prunasin and L-Prunasin, exhibit significant differences in concentration, with values of 2,101.4 ± 453.0 and 3,561.2 ± 619.8 ng/mL, respectively. Importantly, the content of L-Prunasin is considerably higher than that of D-Prunasin, indicating stereoselective metabolism of amygdalin. Besides, the bioavailability of amygdalin was found to be only 0.19% ± 0.08% when orally administered to rats, suggesting that amygdalin may have undergone degradation before reaching the intestinal tract. In contrast, prunasin exhibited a higher bioavailability of 64.91% ± 6.30% when administered orally. These findings indicate that amygdalin undergoes deglycosylation metabolism (Zhang et al., 2022b).
Changes in the oral dose of amygdalin lead to variations in its pharmacokinetic parameters. For instance, when rats were orally administered 5 mg/kg of amygdalin, the following parameters were observed: Tmax was 14 min, Cmax was 23.08 ng/mL, area under the plasma concentration curve (0-t) (AUC0–t) was 1,391.77 ng min/mL, area under the plasma concentration curve (0-∞) (AUC0-∞) was 1,569.22 ng min/mL, t1/2 was 28.76 min, and MRT was 53.33 min (Zhang et al., 2022b). However, when the dosage was increased to 100 mg/kg, the following parameters were observed: Tmax was 0.25 h, Cmax was 93.871 ng/mL, AUC0-t was 73.595 ng h/mL (equivalent to 4415.7 ng min/mL), AUC0-∞ was 74.133 ng h/mL (equivalent to 4447.98 ng min/mL), t1/2 was 1.21 h, and MRT was 1.91 h (Yang et al., 2021). Notably, there is little difference in Tmax between the doses of 5 mg/kg and 100 mg/kg, suggesting that the absorption speed of amygdalin may not be affected by dosage. However, as the dose increases, Cmax, AUC, t1/2, and MRT of amygdalin significantly increase. This indicates that higher doses lead to higher peak concentrations of amygdalin and slower elimination, resulting in a longer presence of amygdalin in the body.
Different drug-delivery routes have a significant impact on the absorption, distribution, and elimination of amygdalin. When amygdalin is injected intravenously at a dose of 5 mg/kg, it reaches its Tmax within 2 min, while oral administration takes 14 min. The Cmax after intravenous injection is 34,763.84 ± 18,057.68 ng/mL, compared to only 23.08 ng/mL with oral administration. These indicate that amygdalin is absorbed more rapidly and reaches higher peak plasma concentrations when administered intravenously. Furthermore, the volume of distribution (Vd) for intravenous injection and oral administration is 680.71 ± 257.40 mL/kg and 140,028.28 ± 27,425.92 mL/kg, respectively. This suggests that when amygdalin is administered intravenously, it is primarily distributed in the plasma, whereas after oral administration, it becomes more concentrated. Additionally, the t1/2 of intravenous administration (67.93 ± 24.72 h) is longer than that of oral administration (28.76 ± 7.2 h), and the MRT of intravenous administration (39.42 ± 5.95 min) is shorter than that of oral administration (53.33 ± 10.05 min). These findings indicate that amygdalin remains in the body for a longer duration when administered intravenously (Zhang et al., 2022b).
In addition, the pharmacokinetics of flavonoids in ASA were also investigated. After orally administering 450 mg of ASA skin polyphenols, the plasma was found to contain catechin and naringenin. The Tmax and Cmax values for catechin were 1.4 ± 0.2 h and 44.3 ± 15.6 ng/mL, respectively. For naringenin, the Tmax and Cmax values were 3.3 ± 0.5 h and 19.3 ± 8.2 ng/mL, respectively. Moreover, the Cmax of total flavonoids was 82.3 ± 17.6 ng/mL, which exceeded the levels of catechin and naringenin. This suggests the presence of other unidentified flavonoids in ASA.
Natural medicinal plants have shown significant benefits in treating a range of diseases, including COVID-19 (Setayesh et al., 2022), respiratory diseases (Hajimonfarednejad et al., 2023), mental health disorders such as anxiety and insomnia (Motti and de Falco, 2021), hyperlipidemia (Hashempur et al., 2018), and common fungal infections (Amini et al., 2023). These plants are characterized by their multi-component and multi-target nature, making them vital in the treatment of various illnesses. ASA, a Chinese herbal medicine with a long history of medicinal use, is rich in phytochemical ingredients, active substances, and nutrients. It serves as both a medicinal drug and nutraceutical, with great potential for broad application.
Here, we comprehensively reviewed the phytochemical composition, pharmacological activities, clinical applications, toxicology, and pharmacokinetics studies of ASA. The present study offers a comprehensive summary of the phytochemical composition of ASA, categorizing it into distinct structural types for the first time. It also provides a systematic overview of the pharmacological activities and mechanism of action of ASA. Moreover, the study includes a novel compilation of various detoxification methods before ASA administration, along with an analysis of the alterations in pharmacokinetic parameters after ASA administration. The current research primarily focuses on assessing the anticancer potential of various extracts of ASA and its main component, amygdalin. To date, researchers have successfully isolated and identified 170 chemical components from different ASA extracts. Extensive in vivo and in vitro pharmacological studies have revealed that amygdalin and polyphenols in ASA possess a wide range of pharmacological activities. Furthermore, ASA fatty oil and volatile oil also exhibit specific pharmacological activities in the treatment of certain diseases.
However, there are some aspects worth noting and requiring further research. 1) Amygdalin in ASA exhibits excellent anti-cancer activity in various cell lines. However, most studies conducted so far have been in vitro, with only a few in vivo experiments. Therefore, more preclinical research and translation into clinical studies are needed. 2) It is important to note that ASA is toxic, as amygdalin can be metabolized by β-D-glucosidase, leading to cyanide poisoning. There is limited research on detoxification methods of ASA, and current methods may result in the loss of some active ingredients. Therefore, future research should focus on developing efficient detoxification methods that also preserve the therapeutic properties of ASA. 3) While more than 170 chemical components have been identified in ASA, the pharmacological evaluation has been limited to a few compounds such as amygdalin, its metabolites, total polyphenols, and total volatile oils. Thus, there is an urgent need for in-depth studies on the phytochemistry and pharmacological properties of ASA, particularly the mechanism of action of its bioactive components. 4) ASA and its compounds have shown promising therapeutic effects in the treatment of respiratory diseases in both in vivo and in vitro studies. Some ASA-containing formula preparations have been included in the 2020 edition of the Chinese Pharmacopoeia. Therefore, further investigation into the pharmacological activities and mechanisms of action of these compounds is warranted. 5) Currently, there is a lack of pharmacokinetic data on different ASA extracts and active compounds. Conducting more pharmacokinetic studies on crude ASA extracts and active compounds is crucial for the rational clinical use and development of new drugs.
ASA, a Chinese herbal medicine, is known for its medicinal and food uses. It is rich in phytochemicals and nutrients, making it clinically valuable and potentially useful for food development. Further research is needed to investigate the pharmacological activities of different components of ASA and understand their underlying mechanisms. This study offers a comprehensive analysis of ASA, providing valuable insights for researchers to improve their understanding of ASA and promote the development of ASA as a clinical drug and healthy food.
ST: Conceptualization, Formal Analysis, Data curation, Investigation, Methodology, Writing–original draft. MW: Investigation, Methodology, Writing–review and editing. YP: Investigation, Methodology, Writing–review and editing. YL: Investigation, Methodology, Writing–review and editing. JL: Investigation, Methodology, Writing–review and editing. QT: Writing–review and editing, Data curation, Formal Analysis. TM: Data curation, Formal Analysis, Writing–review and editing. YS: Writing–review and editing, Investigation, Methodology. CZ: Writing–review and editing, Conceptualization. JG: Conceptualization, Writing–review and editing, Funding acquisition, Project administration. HX: Conceptualization, Funding acquisition, Writing–review and editing, Formal Analysis, Supervision.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by the National Natural Science Foundation of China (No. 81573813), the Science and Technology Department of Sichuan Province of China (No. 2023NSFSC0653), the Sichuan Provincial Administration of Traditional Chinese Medicine of China (Nos. 2021XYCZ007), the Health Commission of Sichuan Province of China (No. 21PJ107), and the Excellent Talent Program of Chengdu University of Traditional Chinese Medicine of China (No. GJJJ2021003).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ABTS 2′-Azinobis-(3-ethylbenzthiazoline-6-sulphonate); AchE acetyl cholinesterase; ASA Armeniacae semen amarum; ACE angiotensin-converting enzyme; ALI acute lung injury; ALT alanine aminotransferase; AST aspartate aminotransferase; AUC0–t area under the plasma concentration curve (0-t); AUC0-∞ area under the plasma concentration curve (0-∞); CAT catalase; CL body clearance; CLz/F clearance/bioavailability; Cmax maximum concentration; CNKI China National Knowledge Infrastructure; COPD chronic obstructive pulmonary disease; COX-2 cyclooxygenase-2; DPPH 2,2-diphenyl-1-picrylhydrazyl; DM diabetes mellitus; DMBA 2,2′-Bis (hydroxymethyl)butyric; EMT epithelial-mesenchymal transition; F (%) bioavailability; FRAP ferric reducing anti-oxidant power; GAE gallic acid equivalent; GGT gamma-glutamyl transferase; GSH glutathione; GST glutathione S-transferase; HbA1c hemoglobin A1C; HDL-C high-density lipoprotein cholesterol; IC50 half maximal inhibitory concentration; iNOS inducible nitric oxide synthase; ke elimination rate constant; LD50 median lethal dose; LDL-C low-density lipoprotein cholesterol; LPO lipid peroxide; MDA malondialdehyde; MRT mean residence time; PGE2 prostaglandin E2; ROS reactive oxygen species; SOD superoxide dismutase; t1/2 elimination half-life; TAC total anti-oxidant capacity; Tmax time to peak concentration; Vd volume of distribution; Vz/F distribution/bioavailability.
Aamazadeh, F., Ostadrahimi, A., Rahbar Saadat, Y., and Barar, J. (2020). Bitter apricot ethanolic extract induces apoptosis through increasing expression of Bax/Bcl-2 ratio and caspase-3 in PANC-1 pancreatic cancer cells. Mol. Biol. Rep. 47, 1895–1904. doi:10.1007/s11033-020-05286-w
Abboud, M. M., Al Awaida, W., Alkhateeb, H. H., and Abu-Ayyad, A. N. (2019). Antitumor action of amygdalin on human breast cancer cells by selective sensitization to oxidative stress. Nutr. Cancer 71, 483–490. doi:10.1080/01635581.2018.1508731
Abdaulmoneam, D. M., Bashir, M. H., and El Baz, D. A. H. (2023). Effect of vitamin B17 (amygdalin) found in apricot kernel on the irradiated salivary glands of albino rats. Dent. Med. Probl. 60, 473–481. doi:10.17219/dmp/132387
Adewusi, S. R., and Oke, O. L. (1985). On the metabolism of amygdalin. 1. The LD50 and biochemical changes in rats. Can. J. Physiol. Pharmacol. 63, 1080–1083. doi:10.1139/y85-177
Albogami, S., and Alnefaie, A. (2021). Role of amygdalin in blocking DNA replication in breast cancer in vitro. Curr. Pharm. Biotechnol. 22, 1612–1627. doi:10.2174/1389201022666210203123803
Al-Juhaimi, F. Y., Ghafoor, K., Özcan, M. M., Uslu, N., Babiker, E. E., Ahmed, I. a.M., et al. (2021). Phenolic compounds, antioxidant activity and fatty acid composition of roasted alyanak apricot kernel. J. Oleo Sci. 70, 607–613. doi:10.5650/jos.ess20294
Amini, F., Namjooyan, F., Zomorodian, K., Zareshahrabadi, Z., Shojaei, K., Jaladat, A. M., et al. (2023). The efficacy of complementary treatment with marshmallow (Althaea officinalis L.) on vulvovaginal candidiasis: a randomized double-blinded controlled clinical trial. Explore (NY) 19, 813–819. doi:10.1016/j.explore.2023.04.005
Askar, M. A., El-Sayyad, G. S., Guida, M. S., Khalifa, E., Shabana, E. S., and Abdelrahman, I. Y. (2023). Amygdalin-folic acid-nanoparticles inhibit the proliferation of breast cancer and enhance the effect of radiotherapy through the modulation of tumor-promoting factors/immunosuppressive modulators in vitro. BMC Complement. Med. Ther. 23, 162. doi:10.1186/s12906-023-03986-x
Atriya, A., Majee, C., Mazumder, R., Choudhary, A. N., Salahuddin, , Mazumder, A., et al. (2023). Insight into the various approaches for the enhancement of bioavailability and pharmacological potency of terpenoids: a review. Curr. Pharm. Biotechnol. 24, 1228–1244. doi:10.2174/1389201024666221130163116
Attia, A. A., Salama, A. F., Eldiasty, J. G., Mosallam, S. a.E., El-Naggar, S. A., El-Magd, M. A., et al. (2022). Amygdalin potentiates the anti-cancer effect of Sorafenib on Ehrlich ascites carcinoma and ameliorates the associated liver damage. Sci. Rep. 12, 6494. doi:10.1038/s41598-022-10517-0
Bai, Y., Wang, W., Yin, P., Gao, J., Na, L., Sun, Y., et al. (2020). Ruxolitinib alleviates renal interstitial fibrosis in UUO mice. Int. J. Biol. Sci. 16, 194–203. doi:10.7150/ijbs.39024
Bai, Z., Li, P., Wen, J., Han, Y., Cui, Y., Zhou, Y., et al. (2022). Inhibitory effects and mechanisms of the anti-covid-19 traditional Chinese prescription, Keguan-1, on acute lung injury. J. Ethnopharmacol. 285, 114838. doi:10.1016/j.jep.2021.114838
Bak, E. J., Kim, J., Jang, S., Woo, G. H., Yoon, H. G., Yoo, Y. J., et al. (2013). Gallic acid improves glucose tolerance and triglyceride concentration in diet-induced obesity mice. Scand. J. Clin. Lab. Invest. 73, 607–614. doi:10.3109/00365513.2013.831470
Bakrim, S., Benkhaira, N., Bourais, I., Benali, T., Lee, L. H., El Omari, N., et al. (2022). Health benefits and pharmacological properties of stigmasterol. Antioxidants (Basel) 11, 1912. doi:10.3390/antiox11101912
Blesso, C. N. (2019). Dietary anthocyanins and human health. Nutrients 11, 2107. doi:10.3390/nu11092107
Brown, M. S., and Goldstein, J. L. (2006). Biomedicine. Lowering LDL--not only how low, but how long? Science 311, 1721–1723. doi:10.1126/science.1125884
Cao, X., Xiao, H., Zhang, Y., Zou, L., Chu, Y., and Chu, X. (2010). 1, 5-Dicaffeoylquinic acid-mediated glutathione synthesis through activation of Nrf2 protects against OGD/reperfusion-induced oxidative stress in astrocytes. Brain Res. 1347, 142–148. doi:10.1016/j.brainres.2010.05.072
Cecarini, V., Selmi, S., Cuccioloni, M., Gong, C., Bonfili, L., Zheng, Y., et al. (2022). Targeting proteolysis with cyanogenic glycoside amygdalin induces apoptosis in breast cancer cells. Molecules 27, 7591. doi:10.3390/molecules27217591
Chakraborty, B., Mukerjee, N., Maitra, S., Zehravi, M., Mukherjee, D., Ghosh, A., et al. (2022). Therapeutic potential of different natural products for the treatment of alzheimer's disease. Oxid. Med. Cell Longev. 2022, 6873874. doi:10.1155/2022/6873874
Chang, H. K., Shin, M. S., Yang, H. Y., Lee, J. W., Kim, Y. S., Lee, M. H., et al. (2006). Amygdalin induces apoptosis through regulation of Bax and Bcl-2 expressions in human DU145 and LNCaP prostate cancer cells. Biol. Pharm. Bull. 29, 1597–1602. doi:10.1248/bpb.29.1597
Chang, H. K., Yang, H. Y., Lee, T. H., Shin, M. C., Lee, M. H., Shin, M. S., et al. (2005). Armeniacae semen extract suppresses lipopolysaccharide-induced expressions of cyclooxygenase [correction of cycloosygenase]-2 and inducible nitric oxide synthase in mouse BV2 microglial cells. Biol. Pharm. Bull. 28, 449–454. doi:10.1248/bpb.28.449
Chaouali, N., Gana, I., Dorra, A., Khelifi, F., Nouioui, A., Masri, W., et al. (2013). Potential toxic levels of cyanide in almonds (Prunus amygdalus), apricot kernels (Prunus armeniaca), and almond syrup. ISRN Toxicol. 2013, 610648. doi:10.1155/2013/610648
Chen, C. O., Milbury, P. E., and Blumberg, J. B. (2019). Polyphenols in almond skins after blanching modulate plasma biomarkers of oxidative stress in healthy humans. Antioxidants (Basel) 8, 95. doi:10.3390/antiox8040095
Chen, J., Hu, Y., Mou, X., Wang, H., and Xie, Z. (2021a). Amygdalin alleviates renal injury by suppressing inflammation, oxidative stress and fibrosis in streptozotocin-induced diabetic rats. Life Sci. 265, 118835. doi:10.1016/j.lfs.2020.118835
Chen, J., and Jia, T. (2012). Discuss the significace of processing bitter almond with frying-cooking. Asia-Pacific Tradit. Med. 8, 48–50.
Chen, L., He, M., Zhang, M., Sun, Q., Zeng, S., Zhao, H., et al. (2021b). The Role of non-coding RNAs in colorectal cancer, with a focus on its autophagy. Pharmacol. Ther. 226, 107868. doi:10.1016/j.pharmthera.2021.107868
Chen, W., Wang, J., Yang, H., Sun, Y., Chen, B., Liu, Y., et al. (2022a). Interleukin 22 and its association with neurodegenerative disease activity. Front. Pharmacol. 13, 958022. doi:10.3389/fphar.2022.958022
Chen, Y., Bi, Y., Feng, X., Wang, J., Xu, H., Zhang, T., et al. (2022b). Identification of potential Q-markers of semen armeniacae amarum based on UPLC-MS/MS and metabonomics. Acta Pharm. Sin. 57, 3195–3202. doi:10.16438/j.0513-4870.2022-0581
Chen, Y., Bi, Y., Gao, X., Wang, J., Zhang, C., Sun, Y., et al. (2023). Effect of different storage conditions on the active ingredients of bitter almond. J. Traditional Chin. Med. 64, 167–173. doi:10.13288/j.11-2166/r.2023.02.011
Chen, Y., Ma, J., Wang, F., Hu, J., Cui, A., Wei, C., et al. (2013). Amygdalin induces apoptosis in human cervical cancer cell line HeLa cells. Immunopharmacol. Immunotoxicol. 35, 43–51. doi:10.3109/08923973.2012.738688
Cheng, Y., Yang, C., Zhao, J., Tse, H. F., and Rong, J. (2015). Proteomic identification of calcium-binding chaperone calreticulin as a potential mediator for the neuroprotective and neuritogenic activities of fruit-derived glycoside amygdalin. J. Nutr. Biochem. 26, 146–154. doi:10.1016/j.jnutbio.2014.09.012
Chinese Pharmacopoeia Commission (2020). Chinese pharmacopoeia (Part I). Beijing: China Medical Science Press.
Cui, W., Zhou, H., Liu, Y. Z., Yang, Y., Hu, Y. Z., Han, Z. P., et al. (2023). Amygdalin improves allergic asthma via the thymic stromal lymphopoietin-dendritic cell-OX40 ligand Axis in a mouse model. Iran. J. Allergy Asthma Immunol. 22, 430–439. doi:10.18502/ijaai.v22i5.13993
Cui, Z. Y., Han, X., Jiang, Y. C., Dou, J. Y., Yao, K. C., Hu, Z. H., et al. (2021). Allium victorialis L. Extracts promote activity of FXR to ameliorate alcoholic liver disease: targeting liver lipid deposition and inflammation. Front. Pharmacol. 12, 738689. doi:10.3389/fphar.2021.738689
Czabotar, P. E., Lessene, G., Strasser, A., and Adams, J. M. (2014). Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 15, 49–63. doi:10.1038/nrm3722
Das, S. K., and Chakrabarti, R. (2005). Non-insulin dependent diabetes mellitus: present therapies and new drug targets. Mini Rev. Med. Chem. 5, 1019–1034. doi:10.2174/138955705774575273
De Oliveira, P. A., Kovacs, C., Moreira, P., Magnoni, D., Saleh, M. H., and Faintuch, J. (2017). Unsaturated fatty acids improve atherosclerosis markers in obese and overweight non-diabetic elderly patients. Obes. Surg. 27, 2663–2671. doi:10.1007/s11695-017-2704-8
Desmarchelier, C., Del, V. P. A., Abate-Daga, D., Coussio, J., Gil, R. R., and Silva, G. L. (2005). Antioxidant and free radical scavenging activities of Misodendrum punctulatum, myzodendrone and structurally related phenols. Phytother. Res. 19, 1043–1047. doi:10.1002/ptr.1786
Dikariyanto, V., Berry, S. E., Francis, L., Smith, L., and Hall, W. L. (2021). Whole almond consumption is associated with better diet quality and cardiovascular disease risk factors in the UK adult population: National Diet and Nutrition Survey (NDNS) 2008-2017. Eur. J. Nutr. 60, 643–654. doi:10.1007/s00394-020-02270-9
Do, J. S., Hwang, J. K., Seo, H. J., Woo, W. H., and Nam, S. Y. (2006). Antiasthmatic activity and selective inhibition of type 2 helper T cell response by aqueous extract of semen armeniacae amarum. Immunopharmacol. Immunotoxicol. 28, 213–225. doi:10.1080/08923970600815253
Du, L., and Yu, C. (2023). Textual research on the medicinal properties of bitter almonds. Chin. J. Ethnomedicine Ethnopharmacy 32, 46–49.
Du, X. Q., Shi, L. P., Cao, W. F., Chen, Z. W., Zuo, B., and Hu, J. Y. (2021). Add-on effect of honeysuckle in the treatment of coronavirus disease 2019: a systematic review and meta-analysis. Front. Pharmacol. 12, 708636. doi:10.3389/fphar.2021.708636
Elderdery, A. Y., Alzahrani, B., Hamza, S. M. A., Mostafa-Hedeab, G., Mok, P. L., and Subbiah, S. K. (2022). Synthesis, characterization, and antiproliferative effect of CuO-TiO(2)-chitosan-amygdalin nanocomposites in human leukemic MOLT4 cells. Bioinorg. Chem. Appl. 2022, 1473922. doi:10.1155/2022/1473922
El-Desouky, M. A., Fahmi, A. A., Abdelkader, I. Y., and Nasraldin, K. M. (2020). Anticancer effect of amygdalin (vitamin B-17) on hepatocellular carcinoma cell line (HepG2) in the presence and absence of zinc. Anticancer Agents Med. Chem. 20, 486–494. doi:10.2174/1871520620666200120095525
El-Ela, F. I. A., Gamal, A., Elbanna, H. A., Elbanna, A. H., Salem, H. F., and Tulbah, A. S. (2022). In vitro and in vivo evaluation of the effectiveness and safety of amygdalin as a cancer therapy. Pharm. (Basel) 15, 1306. doi:10.3390/ph15111306
El-Sewedy, T., Salama, A. F., Mohamed, A. E., Elbaioumy, N. M., El-Far, A. H., Albalawi, A. N., et al. (2023). Hepatocellular Carcinoma cells: activity of Amygdalin and Sorafenib in Targeting AMPK/mTOR and BCL-2 for anti-angiogenesis and apoptosis cell death. BMC Complement. Med. Ther. 23, 329. doi:10.1186/s12906-023-04142-1
Feng, L., Luo, H., Xu, Z., Yang, Z., Du, G., Zhang, Y., et al. (2016). Bavachinin, as a novel natural pan-PPAR agonist, exhibits unique synergistic effects with synthetic PPAR-γ and PPAR-α agonists on carbohydrate and lipid metabolism in db/db and diet-induced obese mice. Diabetologia 59, 1276–1286. doi:10.1007/s00125-016-3912-9
Figurová, D., Tokárová, K., Greifová, H., Knížatová, N., Kolesárová, A., and Lukáč, N. (2021). Inflammation, it's regulation and antiphlogistic effect of the cyanogenic glycoside amygdalin. Molecules 26, 5972. doi:10.3390/molecules26195972
Fu, X. J., Song, X. X., Wei, L. B., and Wang, Z. G. (2013). Study of the distribution patterns of the constituent herbs in classical Chinese medicine prescriptions treating respiratory disease by data mining methods. Chin. J. Integr. Med. 19, 621–628. doi:10.1007/s11655-012-1090-2
Gandhi, V. M., Mulky, M. J., Mukerji, B., Iyer, V. J., and Cherian, K. M. (1997). Safety evaluation of wild apricot oil. Food Chem. Toxicol. 35, 583–587. doi:10.1016/s0278-6915(97)00026-4
Gao, L., Wang, J., Li, F., Deng, Y., and Gao, S. (2014). Literature-based analysis on relationship of symptoms, drugs and therapies in treatment of intestinal diseases. J. Tradit. Chin. Med. 34, 106–114. doi:10.1016/s0254-6272(14)60063-7
Gao, L., Wang, J., Li, F., Gao, S., and Deng, Y. (2012). Analysis on clinically drug-used law for lung-intestine related diseases. J. Tradit. Chin. Med. 32, 523–528. doi:10.1016/s0254-6272(13)60064-3
Gao, Z., Li, F. S., and Upur, H. (2011). A study of the law of herbal administration in treating lung-distension by TCM physicians through history using cluster analysis. J. Tradit. Chin. Med. 31, 303–307. doi:10.1016/s0254-6272(12)60008-9
Geng, H., Yu, X., Lu, A., Cao, H., Zhou, B., Zhou, L., et al. (2016). Extraction, chemical composition, and antifungal activity of essential oil of bitter almond. Int. J. Mol. Sci. 17, 1421. doi:10.3390/ijms17091421
Guo, J., Wu, W., Sheng, M., Yang, S., and Tan, J. (2013). Amygdalin inhibits renal fibrosis in chronic kidney disease. Mol. Med. Rep. 7, 1453–1457. doi:10.3892/mmr.2013.1391
Guo, X., Qu, F. X., Zhang, J. D., Zheng, F., Xin, Y., Wang, R., et al. (2023). Amygdalin and exercise training exert a synergistic effect in improving cardiac performance and ameliorating cardiac inflammation and fibrosis in a rat model of myocardial infarction. Appl. Physiol. Nutr. Metab. doi:10.1139/apnm-2023-0135
Hajimonfarednejad, M., Ostovar, M., Hasheminasab, F. S., Shariati, M. A., Thiruvengadam, M., Raee, M. J., et al. (2023). Medicinal plants for viral respiratory diseases: a systematic review on Persian medicine. Evid. Based Complement. Altern. Med. 2023, 1928310. doi:10.1155/2023/1928310
Hashempur, M. H., Mosavat, S. H., Heydari, M., and Shams, M. (2018). Medicinal plants' use among patients with dyslipidemia: an Iranian cross-sectional survey. J. Complement. Integr. Med. 16. doi:10.1515/jcim-2018-0101
He, X. Y., Wu, L. J., Wang, W. X., Xie, P. J., Chen, Y. H., and Wang, F. (2020). Amygdalin - a pharmacological and toxicological review. J. Ethnopharmacol. 254, 112717. doi:10.1016/j.jep.2020.112717
Helmy, S. A., El Bedaiwy, H. M., and Mansour, N. O. (2013). Dose linearity of glimepiride in healthy human Egyptian volunteers. Clin. Pharmacol. Drug Dev. 2, 264–269. doi:10.1002/cpdd.20
Hoshino, A., Costa-Silva, B., Shen, T. L., Rodrigues, G., Hashimoto, A., Tesic Mark, M., et al. (2015). Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335. doi:10.1038/nature15756
Hosny, S., Sahyon, H., Youssef, M., and Negm, A. (2021). Prunus armeniaca L. Seed extract and its amygdalin containing fraction induced mitochondrial-mediated apoptosis and autophagy in liver carcinogenesis. Anticancer Agents Med. Chem. 21, 621–629. doi:10.2174/1871520620666200608124003
Hrichi, S., Rigano, F., Chaabane-Banaoues, R., Oulad El Majdoub, Y., Mangraviti, D., Di Marco, D., et al. (2020). Identification of fatty acid, lipid and polyphenol compounds from Prunus armeniaca L. Kernel extracts. Foods 9, 896. doi:10.3390/foods9070896
Hui, R., Hou, D., Li, T., and Guan, C. (2003). Analysis of volatile components in semen armeniacae amarumby GC-MS with microwave radiation and SDE method. J. Instrum. Analysis 22, 55–57.
Hwang, H. J., Kim, P., Kim, C. J., Lee, H. J., Shim, I., Yin, C. S., et al. (2008). Antinociceptive effect of amygdalin isolated from Prunus armeniaca on formalin-induced pain in rats. Biol. Pharm. Bull. 31, 1559–1564. doi:10.1248/bpb.31.1559
Hyun, S. W., Kim, J., Park, B., Jo, K., Lee, T. G., Kim, J. S., et al. (2019). Apricot kernel extract and amygdalin inhibit urban particulate matter-induced keratoconjunctivitis sicca. Molecules 24, 650. doi:10.3390/molecules24030650
Ibrahim, S. Y., and Abd El-Salam, M. M. (2015). Anti-dermatophyte efficacy and environmental safety of some essential oils commercial and in vitro extracted pure and combined against four keratinophilic pathogenic fungi. Environ. Health Prev. Med. 20, 279–286. doi:10.1007/s12199-015-0462-6
Iwai, K., Kishimoto, N., Kakino, Y., Mochida, K., and Fujita, T. (2004). In vitro antioxidative effects and tyrosinase inhibitory activities of seven hydroxycinnamoyl derivatives in green coffee beans. J. Agric. Food Chem. 52, 4893–4898. doi:10.1021/jf040048m
Jaszczak-Wilke, E., Polkowska, Ż., Koprowski, M., Owsianik, K., Mitchell, A. E., and Bałczewski, P. (2021). Amygdalin: toxicity, anticancer activity and analytical procedures for its determination in plant seeds. Molecules 26, 2253. doi:10.3390/molecules26082253
Jiagang, D., Li, C., Wang, H., Hao, E., Du, Z., Bao, C., et al. (2011). Amygdalin mediates relieved atherosclerosis in apolipoprotein E deficient mice through the induction of regulatory T cells. Biochem. Biophys. Res. Commun. 411, 523–529. doi:10.1016/j.bbrc.2011.06.162
Jiao, H., Li, S., and Tang, Q. (2023). Amygdalin epimers exert discrepant anti-pulmonary fibrosis activity via inhibiting TGF-β1/Smad2/3 pathway. Pulm. Pharmacol. Ther. 81, 102230. doi:10.1016/j.pupt.2023.102230
Jin, F., Wang, J., Regenstein, M. J., and Wang, F. (2018). Effect of roasting temperatures on the properties of bitter apricot (armeniaca sibirica L.) kernel oil. J. Oleo Sci. 67, 813–822. doi:10.5650/jos.ess17212
Jin, H., Cai, C., Li, B., Jin, W., Xia, J., Wang, L., et al. (2019). Modified Si-Jun-Zi-Tang attenuates airway inflammation in a murine model of chronic asthma by inhibiting teff cells via the mTORC1 pathway. Front. Pharmacol. 10, 161. doi:10.3389/fphar.2019.00161
Juengel, E., Afschar, M., Makarević, J., Rutz, J., Tsaur, I., Mani, J., et al. (2016a). Amygdalin blocks the in vitro adhesion and invasion of renal cell carcinoma cells by an integrin-dependent mechanism. Int. J. Mol. Med. 37, 843–850. doi:10.3892/ijmm.2016.2454
Juengel, E., Thomas, A., Rutz, J., Makarevic, J., Tsaur, I., Nelson, K., et al. (2016b). Amygdalin inhibits the growth of renal cell carcinoma cells in vitro. Int. J. Mol. Med. 37, 526–532. doi:10.3892/ijmm.2015.2439
Karaboğa, I., Ovalı, M. A., Yılmaz, A., and Alpaslan, M. (2018). Gastroprotective effect of apricot kernel oil in ethanol-induced gastric mucosal injury in rats. Biotech. Histochem 93, 601–607. doi:10.1080/10520295.2018.1511064
Khosla, P., and Fungwe, T. V. (2001). Conjugated linoleic acid: effects on plasma lipids and cardiovascular function. Curr. Opin. Lipidol. 12, 31–24. doi:10.1097/00041433-200102000-00006
Kim, C. S., Jo, K., Lee, I. S., and Kim, J. (2016). Topical application of apricot kernel extract improves dry eye symptoms in a unilateral exorbital lacrimal gland excision mouse. Nutrients 8, 750. doi:10.3390/nu8110750
Klonoff, D. C. (2020). Hemoglobinopathies and hemoglobin A1c in diabetes mellitus. J. Diabetes Sci. Technol. 14, 3–7. doi:10.1177/1932296819841698
Kopčeková, J., Kolesárová, A., Kováčik, A., Kováčiková, E., Gažarová, M., Chlebo, P., et al. (2018). Influence of long-term consumption of bitter apricot seeds on risk factors for cardiovascular diseases. J. Environ. Sci. Health B 53, 298–303. doi:10.1080/03601234.2017.1421841
Kopčeková, J., Kolesárová, A., Schwarzová, M., Kováčik, A., Mrázová, J., Gažarová, M., et al. (2022). Phytonutrients of bitter apricot seeds modulate human lipid profile and LDL subfractions in adults with elevated cholesterol levels. Int. J. Environ. Res. Public Health 19, 857. doi:10.3390/ijerph19020857
Kopčeková, J., Kováčiková, E., Kováčik, A., Kolesárová, A., Mrázová, J., Chlebo, P., et al. (2021). Consumption of bitter apricot seeds affects lipid and endocrine profile in women. J. Environ. Sci. Health B 56, 378–386. doi:10.1080/03601234.2021.1890513
Kovacikova, E., Kovacik, A., Halenar, M., Tokarova, K., Chrastinova, L., Ondruska, L., et al. (2019). Potential toxicity of cyanogenic glycoside amygdalin and bitter apricot seed in rabbits-Health status evaluation. J. Anim. Physiol. Anim. Nutr. Berl. 103, 695–703. doi:10.1111/jpn.13055
Kung, Y. L., Lu, C. Y., Badrealam, K. F., Kuo, W. W., Shibu, M. A., Day, C. H., et al. (2021). Cardioprotective potential of amygdalin against angiotensin II induced cardiac hypertrophy, oxidative stress and inflammatory responses through modulation of Nrf2 and NF-κB activation. Environ. Toxicol. 36, 926–934. doi:10.1002/tox.23094
Lee, H. H., Ahn, J. H., Kwon, A. R., Lee, E. S., Kwak, J. H., and Min, Y. H. (2014). Chemical composition and antimicrobial activity of the essential oil of apricot seed. Phytother. Res. 28, 1867–1872. doi:10.1002/ptr.5219
Lee, H. M., and Moon, A. (2016). Amygdalin regulates apoptosis and adhesion in Hs578T triple-negative breast cancer cells. Biomol. Ther. Seoul. 24, 62–66. doi:10.4062/biomolther.2015.172
Li, K., Shi, Q., Zhu, H., and Tang, D. (2004). Chemical compositions in bitter almond. J. Northwest For. Univ. 19, 124–126.
Li, K., Yang, W., Li, Z., Jia, W., Li, J., Zhang, P., et al. (2016). Bitter apricot essential oil induces apoptosis of human HaCaT keratinocytes. Int. Immunopharmacol. 34, 189–198. doi:10.1016/j.intimp.2016.02.019
Li, M., Fan, X., Zhou, L., Jiang, M., and Shang, E. (2022). The effect of Ma-Xin-Gan-Shi decoction on asthma exacerbated by respiratory syncytial virus through regulating TRPV1 channel. J. Ethnopharmacol. 291, 115157. doi:10.1016/j.jep.2022.115157
Li, S., Lu, S., Wang, L., Liu, S., Zhang, L., Du, J., et al. (2023). Effects of amygdalin on ferroptosis and oxidative stress in diabetic retinopathy progression via the NRF2/ARE signaling pathway. Exp. Eye Res. 234, 109569. doi:10.1016/j.exer.2023.109569
Li, Y., Chu, F., Li, P., Johnson, N., Li, T., Wang, Y., et al. (2021). Potential effect of Maxing Shigan decoction against coronavirus disease 2019 (COVID-19) revealed by network pharmacology and experimental verification. J. Ethnopharmacol. 271, 113854. doi:10.1016/j.jep.2021.113854
Li, Y., Wang, M. Y., Fan, X. S., Qi, X., Chen, Y., Zhang, H., et al. (2010). Effect of San-ao Decoction, a traditional Chinese prescription, on IL-4 treated normal human bronchial epithelium. J. Ethnopharmacol. 131, 104–109. doi:10.1016/j.jep.2010.06.006
Lin, D., Lin, W., Gao, G., Zhou, J., Chen, T., Ke, L., et al. (2020). Purification and characterization of the major protein isolated from Semen Armeniacae Amarum and the properties of its thermally induced nanoparticles. Int. J. Biol. Macromol. 159, 850–858. doi:10.1016/j.ijbiomac.2020.05.070
Lin, S., Wen, J., Xu, X., Shi, J., Zhang, W., Zheng, T., et al. (2022). Amygdalin induced mitochondria-mediated apoptosis of lung cancer cells via regulating NF[formula: see text]B-1/NF[formula: see text]B signaling cascade in vitro and in vivo. Am. J. Chin. Med. 50, 1361–1386. doi:10.1142/S0192415X22500586
Lin, Y. C., Chang, C. W., and Wu, C. R. (2016). Antitussive, anti-pyretic and toxicological evaluation of Ma-Xing-Gan-Shi-Tang in rodents. BMC Complement. Altern. Med. 16, 456. doi:10.1186/s12906-016-1440-2
Liu, M., Li, S., Wang, X., Zhu, Y., Zhang, J., Liu, H., et al. (2018). Characterization, anti-oxidation and anti-inflammation of polysaccharides by Hypsizygus marmoreus against LPS-induced toxicity on lung. Int. J. Biol. Macromol. 111, 121–128. doi:10.1016/j.ijbiomac.2018.01.010
Liu, P., Feng, Y., Li, H., Chen, X., Wang, G., Xu, S., et al. (2020). Ferrostatin-1 alleviates lipopolysaccharide-induced acute lung injury via inhibiting ferroptosis. Cell Mol. Biol. Lett. 25, 10. doi:10.1186/s11658-020-00205-0
Luo, L., Jiang, J., Wang, C., Fitzgerald, M., Hu, W., Zhou, Y., et al. (2020). Analysis on herbal medicines utilized for treatment of COVID-19. Acta Pharm. Sin. B 10, 1192–1204. doi:10.1016/j.apsb.2020.05.007
Ma, J. X., Xiao, X., Zhou, K. F., Huang, G., Ao, B., Zhang, Y., et al. (2021). Herb pair of Ephedrae Herba-Armeniacae Semen Amarum alleviates airway injury in asthmatic rats. J. Ethnopharmacol. 269, 113745. doi:10.1016/j.jep.2020.113745
Mahboub, H. H., Faggio, C., Hendam, B. M., Algharib, S. A., Alkafafy, M., Abo Hashem, M., et al. (2022). Immune-antioxidant trait, Aeromonas veronii resistance, growth, intestinal architecture, and splenic cytokines expression of Cyprinus carpio fed Prunus armeniaca kernel-enriched diets. Fish. Shellfish Immunol. 124, 182–191. doi:10.1016/j.fsi.2022.03.048
Makarević, J., Rutz, J., Juengel, E., Kaulfuss, S., Reiter, M., Tsaur, I., et al. (2014a). Amygdalin blocks bladder cancer cell growth in vitro by diminishing cyclin A and cdk2. PLoS One 9, e105590. doi:10.1371/journal.pone.0105590
Makarević, J., Rutz, J., Juengel, E., Kaulfuss, S., Tsaur, I., Nelson, K., et al. (2014b). Amygdalin influences bladder cancer cell adhesion and invasion in vitro. PLoS One 9, e110244. doi:10.1371/journal.pone.0110244
Makarević, J., Tsaur, I., Juengel, E., Borgmann, H., Nelson, K., Thomas, C., et al. (2016). Amygdalin delays cell cycle progression and blocks growth of prostate cancer cells in vitro. Life Sci. 147, 137–142. doi:10.1016/j.lfs.2016.01.039
Mamdouh, A. M., Khodeer, D. M., Tantawy, M. A., and Moustafa, Y. M. (2021). In-vitro and in-vivo investigation of amygdalin, metformin, and combination of both against doxorubicin on hepatocellular carcinoma. Life Sci. 285, 119961. doi:10.1016/j.lfs.2021.119961
Mani, J., Neuschäfer, J., Resch, C., Rutz, J., Maxeiner, S., Roos, F., et al. (2020). Amygdalin modulates prostate cancer cell adhesion and migration in vitro. Nutr. Cancer 72, 528–537. doi:10.1080/01635581.2019.1637442
Martínez-Piñeiro, L., Rios, E., Martínez-Gomariz, M., Pastor, T., De Cabo, M., Picazo, M. L., et al. (2003). Molecular staging of prostatic cancer with RT-PCR assay for prostate-specific antigen in peripheral blood and lymph nodes: comparison with standard histological staging and immunohistochemical assessment of occult regional lymph node metastases. Eur. Urol. 43, 342–350. doi:10.1016/s0302-2838(03)00055-1
Mattioli, R., Francioso, A., Mosca, L., and Silva, P. (2020). Anthocyanins: a comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 25, 3809. doi:10.3390/molecules25173809
Messaoudene, M., Pidgeon, R., Richard, C., Ponce, M., Diop, K., Benlaifaoui, M., et al. (2022). A natural polyphenol exerts antitumor activity and circumvents anti-PD-1 resistance through effects on the gut microbiota. Cancer Discov. 12, 1070–1087. doi:10.1158/2159-8290.CD-21-0808
Mikoshiba, S., Takenaka, H., Okumura, T., Someya, K., and Ohdera, M. (2006). The suppressive effect of apricot kernel extract on 5alpha-Androst-16-en-3-one generated by microbial metabolism. Int. J. Cosmet. Sci. 28, 45–52. doi:10.1111/j.1467-2494.2006.00297.x
Ming, T., Tao, Q., Tang, S., Zhao, H., Yang, H., Liu, M., et al. (2022). Curcumin: an epigenetic regulator and its application in cancer. Biomed. Pharmacother. 156, 113956. doi:10.1016/j.biopha.2022.113956
Moola, S., Orchard, A., and Van Vuuren, S. (2022). The antimicrobial and toxicity influence of six carrier oils on essential oil compounds. Molecules 28, 30. doi:10.3390/molecules28010030
Moradipoodeh, B., Jamalan, M., Zeinali, M., Fereidoonnezhad, M., and Mohammadzadeh, G. (2019). In vitro and in silico anticancer activity of amygdalin on the SK-BR-3 human breast cancer cell line. Mol. Biol. Rep. 46, 6361–6370. doi:10.1007/s11033-019-05080-3
Moradipoodeh, B., Jamalan, M., Zeinali, M., Fereidoonnezhad, M., and Mohammadzadeh, G. (2020). Specific targeting of HER2-positive human breast carcinoma SK-BR-3 cells by amygdaline-Z(HER2) affibody conjugate. Mol. Biol. Rep. 47, 7139–7151. doi:10.1007/s11033-020-05782-z
Mosadegh Manshadi, S., Safavi, M., Rostami, S., Nadali, F., and Shams Ardekani, M. R. (2019). Apoptosis induction of armeniacae semen extractin human acute leukemia (NALM-6 and KG-1) cells. Int. J. Hematol. Oncol. Stem Cell Res. 13, 116–121. doi:10.18502/ijhoscr.v13i3.1269
Mosayyebi, B., Mohammadi, L., Kalantary-Charvadeh, A., and Rahmati, M. (2021). Amygdalin decreases adhesion and migration of MDA-MB-231 and MCF-7 breast cancer cell lines. Curr. Mol. Pharmacol. 14, 667–675. doi:10.2174/1874467213666200810141251
Motti, R., and De Falco, B. (2021). Traditional herbal remedies used for managing anxiety and insomnia in Italy: an ethnopharmacological overview. Horticulturae 7, 523. doi:10.3390/horticulturae7120523
Nattagh-Eshtivani, E., Barghchi, H., Pahlavani, N., Barati, M., Amiri, Y., Fadel, A., et al. (2022). Biological and pharmacological effects and nutritional impact of phytosterols: a comprehensive review. Phytother. Res. 36, 299–322. doi:10.1002/ptr.7312
Noureen, S., Noreen, S., Ghumman, S. A., Batool, F., Hameed, H., Hasan, S., et al. (2022). Prunus armeniaca gum-alginate polymeric microspheres to enhance the bioavailability of tramadol hydrochloride: formulation and evaluation. Pharmaceutics 14, 916. doi:10.3390/pharmaceutics14050916
Olasehinde, T. A., and Olaniran, A. O. (2022). Neurotoxicity of anthracene and benz[a]anthracene involves oxidative stress-induced neuronal damage, cholinergic dysfunction and disruption of monoaminergic and purinergic enzymes. Toxicol. Res. 38, 365–377. doi:10.1007/s43188-021-00115-z
Park, H. J., Yoon, S. H., Han, L. S., Zheng, L. T., Jung, K. H., Uhm, Y. K., et al. (2005). Amygdalin inhibits genes related to cell cycle in SNU-C4 human colon cancer cells. World J. Gastroenterol. 11, 5156–5161. doi:10.3748/wjg.v11.i33.5156
Park, J. J., Hwang, S. J., Park, J. H., and Lee, H. J. (2015). Chlorogenic acid inhibits hypoxia-induced angiogenesis via down-regulation of the HIF-1α/AKT pathway. Cell Oncol. (Dordr) 38, 111–118. doi:10.1007/s13402-014-0216-2
Peng, Y., Zhang, Z., Chen, W., Zhao, S., Pi, Y., and Yue, X. (2023). Structural characterization, α-glucosidase inhibitory activity and antioxidant activity of neutral polysaccharide from apricot (Armeniaca Sibirica L. Lam) kernels. Int. J. Biol. Macromol. 238, 124109. doi:10.1016/j.ijbiomac.2023.124109
Perez-Vizcaino, F., and Duarte, J. (2010). Flavonols and cardiovascular disease. Mol. Asp. Med. 31, 478–494. doi:10.1016/j.mam.2010.09.002
Possa, S. S., Leick, E. A., Prado, C. M., Martins, M. A., and Tibério, I. F. (2013). Eosinophilic inflammation in allergic asthma. Front. Pharmacol. 4, 46. doi:10.3389/fphar.2013.00046
Potì, F., Santi, D., Spaggiari, G., Zimetti, F., and Zanotti, I. (2019). Polyphenol health effects on cardiovascular and neurodegenerative disorders: a review and meta-analysis. Int. J. Mol. Sci. 20, 351. doi:10.3390/ijms20020351
Qian, L., Xie, B., Wang, Y., and Qian, J. (2015). Amygdalin-mediated inhibition of non-small cell lung cancer cell invasion in vitro. Int. J. Clin. Exp. Pathol. 8, 5363–5370.
Qiao, Y. N., Zou, Y. L., and Guo, S. D. (2022). Low-density lipoprotein particles in atherosclerosis. Front. Physiol. 13, 931931. doi:10.3389/fphys.2022.931931
Qin, F., Yao, L., Lu, C., Li, C., Zhou, Y., Su, C., et al. (2019). Phenolic composition, antioxidant and antibacterial properties, and in vitro anti-HepG2 cell activities of wild apricot (Armeniaca Sibirica L. Lam) kernel skins. Food Chem. Toxicol. 129, 354–364. doi:10.1016/j.fct.2019.05.007
Qin, N., Chen, C., Zhang, N., Song, L., Li, Y., Guo, L., et al. (2023). Bitter almond albumin ACE-inhibitory peptides: purification, screening, and characterization in silico, action mechanisms, antihypertensive effect in vivo, and stability. Molecules 28, 6002. doi:10.3390/molecules28166002
Qin, Y., Wang, S., Wen, Q., Xia, Q., Wang, S., Chen, G., et al. (2021). Interactions between ephedra sinica and Prunus armeniaca: from stereoselectivity to deamination as a metabolic detoxification mechanism of amygdalin. Front. Pharmacol. 12, 744624. doi:10.3389/fphar.2021.744624
Raafat, K., El-Darra, N., Saleh, F. A., Rajha, H. N., Maroun, R. G., and Louka, N. (2018). Infrared-assisted extraction and HPLC-analysis of Prunus armeniaca L. Pomace and detoxified-kernel and their antidiabetic effects. Phytochem. Anal. 29, 156–167. doi:10.1002/pca.2723
Ramadan, D. T., Ali, M. A. M., Yahya, S. M., and El-Sayed, W. M. (2019). Correlation between antioxidant/antimutagenic and antiproliferative activity of some phytochemicals. Anticancer Agents Med. Chem. 19, 1481–1490. doi:10.2174/1871520619666190528091648
Rodrigues, C. J. C., and De Carvalho, C. (2022). Process development for benzyl alcohol production by whole-cell biocatalysis in stirred and packed bed reactors. Microorganisms 10, 966. doi:10.3390/microorganisms10050966
Rudzińska, M., Górnaś, P., Raczyk, M., and Soliven, A. (2017). Sterols and squalene in apricot (Prunus armeniaca L.) kernel oils: the variety as a key factor. Nat. Prod. Res. 31, 84–88. doi:10.1080/14786419.2015.1135146
Saleem, U., Hussain, L., Shahid, F., Anwar, F., Chauhdary, Z., and Zafar, A. (2022). Pharmacological potential of the standardized methanolic extract of Prunus armeniaca L. In the haloperidol-induced parkinsonism rat model. Evid. Based Complement. Altern. Med. 2022, 3697522. doi:10.1155/2022/3697522
Senica, M., Stampar, F., Veberic, R., and Mikulic-Petkovsek, M. (2017). Fruit seeds of the Rosaceae family: a waste, new life, or a danger to human health? J. Agric. Food Chem. 65, 10621–10629. doi:10.1021/acs.jafc.7b03408
Setayesh, M., Karimi, M., Zargaran, A., Abousaidi, H., Shahesmaeili, A., Amiri, F., et al. (2022). Efficacy of a Persian herbal medicine compound on coronavirus disease 2019 (COVID-19): a randomized clinical trial. Integr. Med. Res. 11, 100869. doi:10.1016/j.imr.2022.100869
Shao, Y., Chen, H., Lin, H., Feng, H., Gong, J., Cao, G., et al. (2022). Exploration on varying patterns of morphological features and quality of armeniacae semen amarum in rancid process based on colorimeter, electronic nose, and GC/MS coupled with human panel. Front. Pharmacol. 13, 599979. doi:10.3389/fphar.2022.599979
Shen, N., Wang, T., Gan, Q., Liu, S., Wang, L., and Jin, B. (2022). Plant flavonoids: classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 383, 132531. doi:10.1016/j.foodchem.2022.132531
Shi, F., Liu, Y., Zhou, X., Shen, P., Xue, R., and Zhang, M. (2022). Disitamab vedotin: a novel antibody-drug conjugates for cancer therapy. Drug Deliv. 29, 1335–1344. doi:10.1080/10717544.2022.2069883
Si, Z., and Zhang, B. (2021). Amygdalin attenuates airway epithelium apoptosis, inflammation, and epithelial-mesenchymal transition through restraining the TLR4/NF-κB signaling pathway on LPS-treated BEAS-2B bronchial epithelial cells. Int. Arch. Allergy Immunol. 182, 997–1007. doi:10.1159/000514209
Simpson, D. S. A., and Oliver, P. L. (2020). ROS generation in microglia: understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants (Basel) 9, 743. doi:10.3390/antiox9080743
Singh, D. (2021). Current updates and future perspectives on the management of renal cell carcinoma. Life Sci. 264, 118632. doi:10.1016/j.lfs.2020.118632
Siri-Tarino, P. W., Sun, Q., Hu, F. B., and Krauss, R. M. (2010). Saturated fat, carbohydrate, and cardiovascular disease. Am. J. Clin. Nutr. 91, 502–509. doi:10.3945/ajcn.2008.26285
Song, S., Chen, F., Xing, X., Ren, M., Ma, Q., Xie, Y., et al. (2015). Concurrent quantification and comparative pharmacokinetic analysis of bioactive compounds in the Herba Ephedrae-Semen Armeniacae Amarum herb pair. J. Pharm. Biomed. Anal. 109, 67–73. doi:10.1016/j.jpba.2015.02.004
Song, S., Ma, Q., Tang, Q., Chen, F., Xing, X., Guo, Y., et al. (2016). Stereoselective metabolism of amygdalin-based study of detoxification of semen armeniacae amarum in the herba ephedrae-semen armeniacae amarum herb pair. J. Ethnopharmacol. 179, 356–366. doi:10.1016/j.jep.2015.12.019
Song, Z., and Xu, X. (2014). Advanced research on anti-tumor effects of amygdalin. J. Cancer Res. Ther. 10 (1), 3–7. doi:10.4103/0973-1482.139743
Spector, A. A., and Kim, H. Y. (2015). Discovery of essential fatty acids. J. Lipid Res. 56, 11–21. doi:10.1194/jlr.R055095
Stevens, J. M., Galyov, E. E., and Stevens, M. P. (2006). Actin-dependent movement of bacterial pathogens. Nat. Rev. Microbiol. 4, 91–101. doi:10.1038/nrmicro1320
Stryjecka, M., Kiełtyka-Dadasiewicz, A., Michalak, M., Rachoń, L., and Głowacka, A. (2019). Chemical composition and antioxidant properties of oils from the seeds of five apricot (Prunus armeniaca L.) cultivars. J. Oleo Sci. 68, 729–738. doi:10.5650/jos.ess19121
Sun, L., Mao, M., Yan, Z., Zuo, C., and Zhang, X. (2018). A Chinese traditional therapy for bleomycin-induced pulmonary fibrosis in mice. Can. Respir. J. 2018, 8491487. doi:10.1155/2018/8491487
Sun, Q., Tao, Q., Ming, T., Tang, S., Zhao, H., Liu, M., et al. (2023). Berberine is a suppressor of Hedgehog signaling cascade in colorectal cancer. Phytomedicine 114, 154792. doi:10.1016/j.phymed.2023.154792
Sun, Z., Ji, N., Ma, Q., Zhu, R., Chen, Z., Wang, Z., et al. (2020). Epithelial-mesenchymal transition in asthma airway remodeling is regulated by the IL-33/cd146 Axis. Front. Immunol. 11, 1598. doi:10.3389/fimmu.2020.01598
Tang, F., Fan, K., Wang, K., and Bian, C. (2019). Amygdalin attenuates acute liver injury induced by D-galactosamine and lipopolysaccharide by regulating the NLRP3, NF-κB and Nrf2/NQO1 signalling pathways. Biomed. Pharmacother. 111, 527–536. doi:10.1016/j.biopha.2018.12.096
Tang, S., Liang, Y., Wang, M., Lei, J., Peng, Y., Tao, Q., et al. (2023). Qinhuo Shanggan oral solution resolves acute lung injury by down-regulating TLR4/NF-κB signaling cascade and inhibiting NLRP3 inflammasome activation. Front. Immunol. 14, 1285550. doi:10.3389/fimmu.2023.1285550
Tanwar, B., Modgil, R., and Goyal, A. (2018). Antinutritional factors and hypocholesterolemic effect of wild apricot kernel (Prunus armeniaca L.) as affected by detoxification. Food Funct. 9, 2121–2135. doi:10.1039/c8fo00044a
Tareen, A. K., Panezai, M. A., Sajjad, A., Achakzai, J. K., Kakar, A. M., and Khan, N. Y. (2021). Comparative analysis of antioxidant activity, toxicity, and mineral composition of kernel and pomace of apricot (Prunus armeniaca L.) grown in Balochistan, Pakistan. Saudi J. Biol. Sci. 28, 2830–2839. doi:10.1016/j.sjbs.2021.02.015
Tavares, L. P., Peh, H. Y., Tan, W. S. D., Pahima, H., Maffia, P., Tiligada, E., et al. (2020). Granulocyte-targeted therapies for airway diseases. Pharmacol. Res. 157, 104881. doi:10.1016/j.phrs.2020.104881
Thakare, V. N., Dhakane, V. D., and Patel, B. M. (2017). Attenuation of acute restraint stress-induced depressive like behavior and hippocampal alterations with protocatechuic acid treatment in mice. Metab. Brain Dis. 32, 401–413. doi:10.1007/s11011-016-9922-y
Tian, H., Yan, H., Tan, S., Zhan, P., Mao, X., Wang, P., et al. (2016). Apricot kernel oil ameliorates cyclophosphamide-associated immunosuppression in rats. Lipids 51, 931–939. doi:10.1007/s11745-016-4166-5
Timur Taşhan, S., and Kafkasli, A. (2012). The effect of bitter almond oil and massaging on striae gravidarum in primiparaous women. J. Clin. Nurs. 21, 1570–1576. doi:10.1111/j.1365-2702.2012.04087.x
Trang, N. M., Kim, E. N., Lee, H. S., and Jeong, G. S. (2022). Effect on osteoclast differentiation and ER stress downregulation by amygdalin and RANKL binding interaction. Biomolecules 12, 256. doi:10.3390/biom12020256
Tsuchida, T., Lee, Y. A., Fujiwara, N., Ybanez, M., Allen, B., Martins, S., et al. (2018). A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer. J. Hepatol. 69, 385–395. doi:10.1016/j.jhep.2018.03.011
Turolo, S., Edefonti, A., Mazzocchi, A., Syren, M. L., Morello, W., Agostoni, C., et al. (2021). Role of arachidonic acid and its metabolites in the biological and clinical manifestations of idiopathic nephrotic syndrome. Int. J. Mol. Sci. 22, 5452. doi:10.3390/ijms22115452
Vahedi-Mazdabadi, Y., Karimpour-Razkenari, E., Akbarzadeh, T., Lotfian, H., Toushih, M., Roshanravan, N., et al. (2020). Anti-cholinesterase and neuroprotective activities of sweet and bitter apricot kernels (Prunus armeniaca L.). Iran. J. Pharm. Res. 19, 216–224. doi:10.22037/ijpr.2019.15514.13139
Vangaveti, V. N., Jansen, H., Kennedy, R. L., and Malabu, U. H. (2016). Hydroxyoctadecadienoic acids: oxidised derivatives of linoleic acid and their role in inflammation associated with metabolic syndrome and cancer. Eur. J. Pharmacol. 785, 70–76. doi:10.1016/j.ejphar.2015.03.096
Wahab, M. F., Breitbach, Z. S., Armstrong, D. W., Strattan, R., and Berthod, A. (2015). Problems and pitfalls in the analysis of amygdalin and its epimer. J. Agric. Food Chem. 63, 8966–8973. doi:10.1021/acs.jafc.5b03120
Wang, B., Sun, T., Sun, L., Li, L., Wan, H., Ding, Z., et al. (2022). Amygdalin attenuates PM2.5-induced human umbilical vein endothelial cell injury via the TLR4/NF-κB and Bcl-2/Bax signaling pathways. Acta Biochim. Biophys. Sin. (Shanghai) 54, 1476–1485. doi:10.3724/abbs.2022136
Wang, B., Zhou, J., He, B., Shi, H., Liang, X., Zhang, Z., et al. (2023). Reveal the patterns of prescriptions for recurrent respiratory tract infections' treatment based on multiple illustrious senior traditional Chinese medicine practitioners. Evid. Based Complement. Altern. Med. 2023, 7982927. doi:10.1155/2023/7982927
Wang, D., Dong, Y., Chen, X., Liu, Y., Wang, J., Wang, X., et al. (2020). Incorporation of apricot (Prunus armeniaca) kernel essential oil into chitosan films displaying antimicrobial effect against Listeria monocytogenes and improving quality indices of spiced beef. Int. J. Biol. Macromol. 162, 838–844. doi:10.1016/j.ijbiomac.2020.06.220
Wang, Y., Gu, W., Kui, F., Gao, F., Niu, Y., Li, W., et al. (2021). The mechanism and active compounds of semen armeniacae amarum treating coronavirus disease 2019 based on network pharmacology and molecular docking. Food Nutr. Res. 65. doi:10.29219/fnr.v65.5623
Wang, Z., Fang, K., Wang, G., Guan, X., Pang, Z., Guo, Y., et al. (2019). Protective effect of amygdalin on epithelial-mesenchymal transformation in experimental chronic obstructive pulmonary disease mice. Phytother. Res. 33, 808–817. doi:10.1002/ptr.6274
Wei, Y., Li, Y., Wang, S., Xiang, Z., Li, X., Wang, Q., et al. (2023). Phytochemistry and pharmacology of Armeniacae semen Amarum: a review. J. Ethnopharmacol. 308, 116265. doi:10.1016/j.jep.2023.116265
Wongjarupong, N., Negron-Ocasio, G. M., Mara, K. C., Prasai, K., Abdallah, M. A., Ahn, K. S., et al. (2021). BALAD and BALAD-2 predict survival of hepatocellular carcinoma patients: a North American cohort study. HPB Oxf. 23, 762–769. doi:10.1016/j.hpb.2020.09.014
Xia, M., Yang, M., Wang, Y., Tian, F., Hu, J., Yang, W., et al. (2020). dl-Mandelic acid exhibits high sperm-immobilizing activity and low vaginal irritation: a potential non-surfactant spermicide for contraception. Biomed. Pharmacother. 126, 110104. doi:10.1016/j.biopha.2020.110104
Xiang, Y., Chen, X., Wang, W., Zhai, L., Sun, X., Feng, J., et al. (2021). Natural product erianin inhibits bladder cancer cell growth by inducing ferroptosis via NRF2 inactivation. Front. Pharmacol. 12, 775506. doi:10.3389/fphar.2021.775506
Xu, S., Xu, X., Yuan, S., Liu, H., Liu, M., Zhang, Y., et al. (2017). Identification and analysis of amygdalin, neoamygdalin and amygdalin amide in different processed bitter almonds by HPLC-ESI-MS/MS and HPLC-DAD. Molecules 22, 1425. doi:10.3390/molecules22091425
Xue, Z., Zhang, D., Guo, L., Zheng, Y., and Zhan, Z. (2022). Herbal textual research on armeniacae semen amarum in famous classical formulas. Chin. J. Exp. Traditional Med. Formulae 28, 207–214. doi:10.13422/j.cnki.syfjx.20211958
Yang, H. Y., Chang, H. K., Lee, J. W., Kim, Y. S., Kim, H., Lee, M. H., et al. (2007). Amygdalin suppresses lipopolysaccharide-induced expressions of cyclooxygenase-2 and inducible nitric oxide synthase in mouse BV2 microglial cells. Neurol. Res. 29 (1), S59–S64. doi:10.1179/016164107X172248
Yang, Y., Xie, B., and Huang, R. (2021). Tissue distribution and plasma pharmacokinetics ofAmygdalin in rats after oral administration. Chin. Manip. Rehabilitation Med. 12, 91–93.
Yiğit, D., Yiğit, N., and Mavi, A. (2009). Antioxidant and antimicrobial activities of bitter and sweet apricot (Prunus armeniaca L.) kernels. Braz J. Med. Biol. Res. 42, 346–352. doi:10.1590/s0100-879x2009000400006
Yin, L., Liu, X., Shao, X., Feng, T., Xu, J., Wang, Q., et al. (2021). The role of exosomes in lung cancer metastasis and clinical applications: an updated review. J. Transl. Med. 19, 312. doi:10.1186/s12967-021-02985-1
Ying, J., Ge, Q., Hu, S., Luo, C., Lu, F., Yu, Y., et al. (2020). Amygdalin promotes fracture healing through TGF-β/smad signaling in mesenchymal stem cells. Stem Cells Int. 2020, 8811963. doi:10.1155/2020/8811963
Yurt, B., and Celik, I. (2011). Hepatoprotective effect and antioxidant role of sun, sulphited-dried apricot (Prunus armeniaca L.) and its kernel against ethanol-induced oxidative stress in rats. Food Chem. Toxicol. 49, 508–513. doi:10.1016/j.fct.2010.11.035
Zannetti, A. (2023). Breast cancer: from pathophysiology to novel therapeutic approaches 2.0. Int. J. Mol. Sci. 24, 2542. doi:10.3390/ijms24032542
Zehra, N., and Naz, L. (2021). Evidence-based hepatic and renal toxicity evaluation of Prunus armeniaca L. seeds in albino Wistar rats. Pak J. Pharm. Sci. 34, 1555–1560. doi:10.19787/j.issn.1008-1879.2021.04.034
Zhang, A. N., Li, N., Chen, Z. C., Guo, Y. L., Tian, C. J., Cheng, D. J., et al. (2023). Amygdalin alleviated TGF-β-induced epithelial-mesenchymal transition in bronchial epithelial cells. Chem. Biol. Interact. 369, 110235. doi:10.1016/j.cbi.2022.110235
Zhang, C., Lin, J., Zhen, C., Wang, F., Sun, X., Kong, X., et al. (2022a). Amygdalin protects against acetaminophen-induced acute liver failure by reducing inflammatory response and inhibiting hepatocyte death. Biochem. Biophys. Res. Commun. 602, 105–112. doi:10.1016/j.bbrc.2022.03.011
Zhang, C., Zhang, D., Wang, Y., Zhang, L., Qi, S., Fang, Q., et al. (2022b). Pharmacokinetics and anti-liver fibrosis characteristics of amygdalin: key role of the deglycosylated metabolite prunasin. Phytomedicine 99, 154018. doi:10.1016/j.phymed.2022.154018
Zhang, J., Gu, H. D., Zhang, L., Tian, Z. J., Zhang, Z. Q., Shi, X. C., et al. (2011). Protective effects of apricot kernel oil on myocardium against ischemia-reperfusion injury in rats. Food Chem. Toxicol. 49, 3136–3141. doi:10.1016/j.fct.2011.08.015
Zhang, L., Yang, S., Sun, L., Shi, D., and Yuan, Z. (2007). GC-MS analysis of fatty acid fractions in the fat oil of bitter almond and its processed products. Chin. Tradit. Pat. Med. 29, 717–719.
Zhang, X., Hu, J., Zhuo, Y., Cui, L., Li, C., Cui, N., et al. (2018). Amygdalin improves microcirculatory disturbance and attenuates pancreatic fibrosis by regulating the expression of endothelin-1 and calcitonin gene-related peptide in rats. J Chin Med Assoc. 81, 437–443. doi:10.1016/j.jcma.2017.09.005
Zhao, H., Ming, T., Tang, S., Ren, S., Yang, H., Liu, M., et al. (2022a). Wnt signaling in colorectal cancer: pathogenic role and therapeutic target. Mol. Cancer 21, 144. doi:10.1186/s12943-022-01616-7
Zhao, H., Ren, S., Yang, H., Tang, S., Guo, C., Liu, M., et al. (2022b). Peppermint essential oil: its phytochemistry, biological activity, pharmacological effect and application. Biomed. Pharmacother. 154, 113559. doi:10.1016/j.biopha.2022.113559
Zhao, H., Tang, S., Tao, Q., Ming, T., Lei, J., Liang, Y., et al. (2023). Ursolic acid suppresses colorectal cancer by down-regulation of wnt/β-catenin signaling pathway activity. J. Agric. Food Chem. 71, 3981–3993. doi:10.1021/acs.jafc.2c06775
Zhao, Y., Zhang, Y., Kong, H., Cheng, G., Qu, H., and Zhao, Y. (2022c). Protective effects of carbon dots derived from armeniacae semen amarum carbonisata against acute lung injury induced by lipopolysaccharides in rats. Int. J. Nanomedicine 17, 1–14. doi:10.2147/IJN.S338886
Zheng, S. Z., Liu, D. J., Sun, P., Yu, G. S., Xu, Y. T., Gong, W., et al. (2014). Feasibility and safety of sorafenib treatment in hepatocellular carcinoma patients with spontaneous rupture. World J. Gastroenterol. 20, 16275–16281. doi:10.3748/wjg.v20.i43.16275
Zhou, C., Qian, L., Ma, H., Yu, X., Zhang, Y., Qu, W., et al. (2012). Enhancement of amygdalin activated with β-D-glucosidase on HepG2 cells proliferation and apoptosis. Carbohydr. Polym. 90, 516–523. doi:10.1016/j.carbpol.2012.05.073
Zhou, X., Bin, X., Huang, R., Luo, H., Huang, F., Xie, M., et al. (2021). Study on chemical constituents differences between armeniacae amarum semen and Persicae semen based on high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry. J. Instrum. Analysis 40, 940–946.
Keywords: Armeniacae semen amarum, Prunus armeniaca L., traditional Chinese medicine, ethnopharmacology, phytochemistry, pharmacology, clinical application, toxicology
Citation: Tang S, Wang M, Peng Y, Liang Y, Lei J, Tao Q, Ming T, Shen Y, Zhang C, Guo J and Xu H (2024) Armeniacae semen amarum: a review on its botany, phytochemistry, pharmacology, clinical application, toxicology and pharmacokinetics. Front. Pharmacol. 15:1290888. doi: 10.3389/fphar.2024.1290888
Received: 08 September 2023; Accepted: 10 January 2024;
Published: 23 January 2024.
Edited by:
Irina Ielciu, University of Medicine and Pharmacy Iuliu Hatieganu, RomaniaReviewed by:
Mohammad Hashem Hashempur, Shiraz University of Medical Sciences, IranCopyright © 2024 Tang, Wang, Peng, Liang, Lei, Tao, Ming, Shen, Zhang, Guo and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinlin Guo, Z3VvNTk2QGNkdXRjbS5lZHUuY24=; Haibo Xu, aGFpYm8ueHVAY2R1dGNtLmVkdS5jbg==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.