- 1Department of Anesthesia, Shaoxing People’s Hospital, Shaoxing, China
- 2Department of Anesthesiology, Jiaxing Women and Children’s Hospital, Jiaxing, China
Background: Supine hypotensive syndrome is a common complication in late pregnancy. This study aims to explore the effects of ondansetron on the prevention of supine hypotensive syndrome during spinal anesthesia for cesarean section.
Methods: A total of 80 women undergoing elective cesarean delivery were randomly assigned to two groups (the ondansetron group and the control group), with 40 cases in each group. The ondansetron group received 0.075 mg/kg of ondansetron intravenously 5 min before the induction of spinal anesthesia; the control group was given the same volume of saline solution. The blood pressure and heart rate were measured. Umbilical artery pH was analyzed, and the incidence of nausea and vomiting and vasoconstrictor drug usage were noted.
Results: The incidence of supine hypotensive syndrome, nausea and vomiting, and vasoconstrictor drug use were significantly lower in the ondansetron group than the control group (2.5% vs. 20%, p = 0.029; 2.5% vs. 22.5%, p = 0.007; and 5% vs. 22.5%, p = 0.012, respectively). Umbilical artery pH was higher in the ondansetron group than the control group, and statistical significance was observed (7.31 ± 0.03 vs. 7.28 ± 0.04, p = 0.002). The maternal hemodynamic parameters and the neonatal Apgar score were similar between the two groups.
Conclusion: Ondansetron can effectively prevent supine hypotensive syndrome, reduce the incidence of nausea, vomiting, and vasoconstrictor drug use, and improve neonatal umbilical arterial pH during spinal anesthesia for cesarean section.
Clinical Trial Registration: https://www.chictr.org.cn/, identifier ChiCTR180018756.
1 Introduction
Supine hypotensive syndrome is a common complication during the perioperative period. It is characterized by severe hypotension, accompanied by dizziness, chest tightness, and paleness, which is caused when the gravid uterus compresses the inferior vena cava during pregnancy (De-Giorgio et al., 2012; Humphries et al., 2020). Supine hypotensive syndrome can lead to fetal hypoperfusion and affect the health of the mother and infant, so it is very important to take effective measures to prevent and treat supine hypotensive syndrome during cesarean section. Postural intervention is an effective measure for preventing and treating supine hypotensive syndrome during caesarean section, which is done by turning the woman on her side (Kinsella and Lohmann, 1994) In addition, fluid preload and vasoconstrictors can effectively prevent the episode of hypotension after spinal anesthesia (Massoth et al., 2022).
Ondansetron is a highly selective serotonergic antagonist. It is often used to prevent and treat nausea and vomiting caused by cancer chemotherapy and surgery. Literature reports that ondansetron could suppress serotonin-induced vasodilation by reducing the Bezold–Jarisch reflex (Ortiz-Gómez et al., 2014; Ortiz-Gómez et al., 2017). This study aimed at investigating whether ondansetron could prevent supine hypotensive syndrome in cesarean sections under spinal anesthesia.
2 Materials and methods
This study was conducted in accordance with the Declaration of Helsinki and approved by the hospital’s Ethics Committee on 6 November 2019. This trial was registered in the Chinese Clinical Trial Registry (registration number: ChiCTR180018756). All parturients signed written informed consent.
From November 2019 to March 2021, a total of 80 term parturient women undergoing elective cesarean section without symptoms of supine hypotension were included in this trial. The inclusion criteria were as follows: singleton, American Society of Anesthesiologists (ASA) physical status I–II, gestational age >37 weeks, height ranged from 153 to 175 cm, and weight ranged between 55 and 90 kg. The exclusion criteria included pregnancy-related hypertensive disease, cerebrovascular disease, and any spinal anesthesia contraindications. The parturients were randomly divided into the ondansetron and control groups using a computer-generated random number code. The surgeons, anesthetists, and investigators were blinded to the group allocation.
No premedication was given to the parturients. After entering the operating center, the vital signs, including the electrocardiogram (ECG), non-invasive blood pressure (BP), pulse oxygen saturation (SpO2), and heart rate (HR), were monitored using an anesthesia monitor. The hemodynamic parameters were measured at 2-min intervals. The baseline values of BP and HR were calculated as the means of three successive measurements. Subsequently, an 18-gauge intravenous cannula was inserted into the vein in the forearm. Lactated Ringer’s solution was infused at a rate of 10 mL kg−1·h−1 until delivery. Thereafter, it was infused at a rate of 5 mL·kg−1 h−1.
The ondansetron group received 0.075 mg/kg ondansetron intravenously 5 min before the induction of spinal anesthesia, and the control group received an equal volume of normal saline solution. These solutions were prepared by nurses who were not involved in this study. Anesthesia puncture was carried out in the left lateral decubitus position. At the estimated L3 to L4 vertebral interspace, an 18-gauge Tuohy needle was inserted into the epidural space, and a 25-gauge spinal needle was inserted into the subarachnoid space via the Tuohy needle. After free cerebrospinal fluid flow was observed, a mixture of 0.5% hyperbaric bupivacaine 10 mg and sufentanil 2.5 μg (2 mL in total) was administered intrathecally over 10 s with the needle orifice facing cephalad, and an epidural catheter was inserted 3–4 cm cephalad into the epidural space. The parturients were immediately returned to the supine position with a 10-degree tilt to the left side after spinal anesthesia. The systolic blood pressure (SBP), diastolic blood pressure (DBP), and HR were noted at the following time points: 5 min before spinal anesthesia induction (T0), 5 min after spinal anesthesia induction (T1), at skin incision (T2), and delivery (T3). Vasoconstrictors were administered for the following indications: a. mean arterial pressure <60 mmHg; b. nausea and vomiting; c. stuffiness and palpitation or dizziness; and d. patients complained of discomfort as hypotension or hypotension-related discomfort. A rescue bolus dose of phenylephrine 50 µg was given when any of the above conditions occurred. Atropine 0.3–0.5 mg was administered intravenously when HR was <50 beats/min. After a bilateral T6 sensory block was achieved, a surgical incision was allowed. After delivery, umbilical artery blood was drawn for analysis, 5 U of oxytocin was administered muscularly in utero, and 3 U of oxytocin was administered by intravenous bolus.
The Apgar scores of the newborns at 1 and 5 min and the pH of the umbilical artery were evaluated. The incidence of maternal hypotension, bradycardia, nausea and vomiting, and vasoconstrictor drug use were noted during spinal anesthesia. Maternal hypotension was defined as an SBP <80% of baseline. Bradycardia was defined as HR < 60 beats/min. Supine hypotensive syndrome was defined as severe hypotension (SBP drop by more than 30% of baseline), accompanied by dizziness, chest tightness, and paleness. Vasoconstrictor drugs were used when supine hypotensive syndrome occurred or patients complained of nausea and vomiting, had an SBP below 70% of baseline, or showed other hypotension-related complications. The maximum sensory block level was measured within the first 10 min after intrathecal administration using a pinprick at 2-min intervals, the induction-to-incision interval, and the induction-to-delivery interval, and the minimal mean arterial pressure was also recorded.
2.1 Statistical analysis
The incidence of supine hypotensive syndrome was the primary outcome of this trial. The size of the sample was calculated in accordance with our pilot study. It was estimated that a sample size of 32 women in each group could detect a difference of 60% in the incidence of supine hypotensive syndrome between the two groups before delivery, with an alpha error of 0.05 and a power of 0.8. To account for dropouts, the sample size in each group was increased to 40. The quantitative data with a normal distribution were analyzed using the t-test and analysis of variance (ANOVA). Categorical variables were compared using the chi-squared test or Fisher’s exact test. A p-value of <0.05 was considered statistically significant.
3 Results
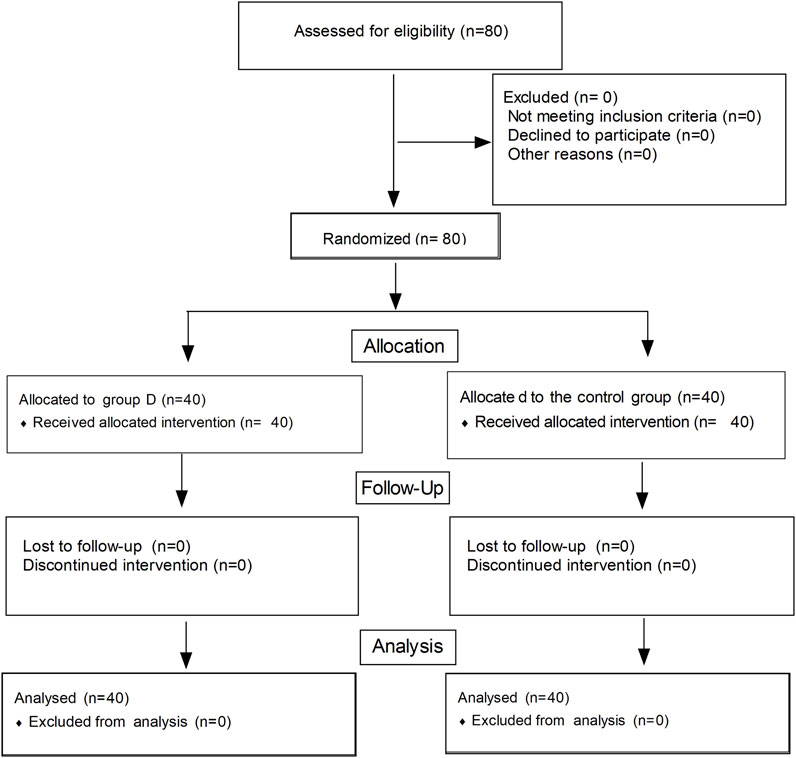
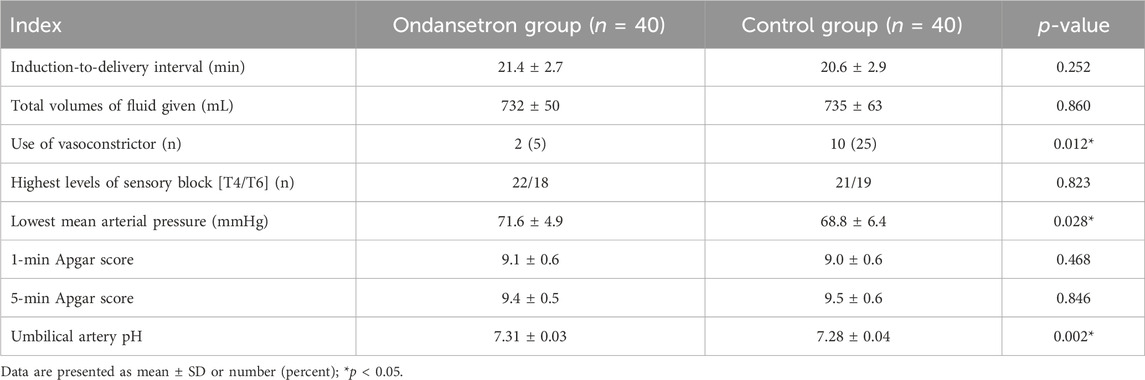
The flow diagram of this study is shown in Figure 1. A total of 80 women were enrolled in this study and completed the trial. There were no significant differences in terms of maternal age, height, body mass index, and gestational week between the two groups (Table 1). No significant differences were observed in terms of the induction-to-delivery interval, highest levels of sensory block, and total volume of fluid given; there was no statistical significance (p > 0.05). The rate of vasoconstrictor usage was significantly lower in the ondansetron group than the control group (5% vs. 22.5%, p = 0.012). The minimal mean arterial pressure was higher in the ondansetron group than the control group (71.4 ± 4.9 vs. 68.8 ± 6.4 mmHg, p = 0.028). Umbilical artery pH was higher in the ondansetron group than the control group; there was a statistical significance (7.31 ± 0.03 vs. 7.28 ± 0.04, p = 0.002), but there were no statistical significances in terms of the Apgar score at 1 min and 5 min between the two groups (p > 0.05) (Table 2).
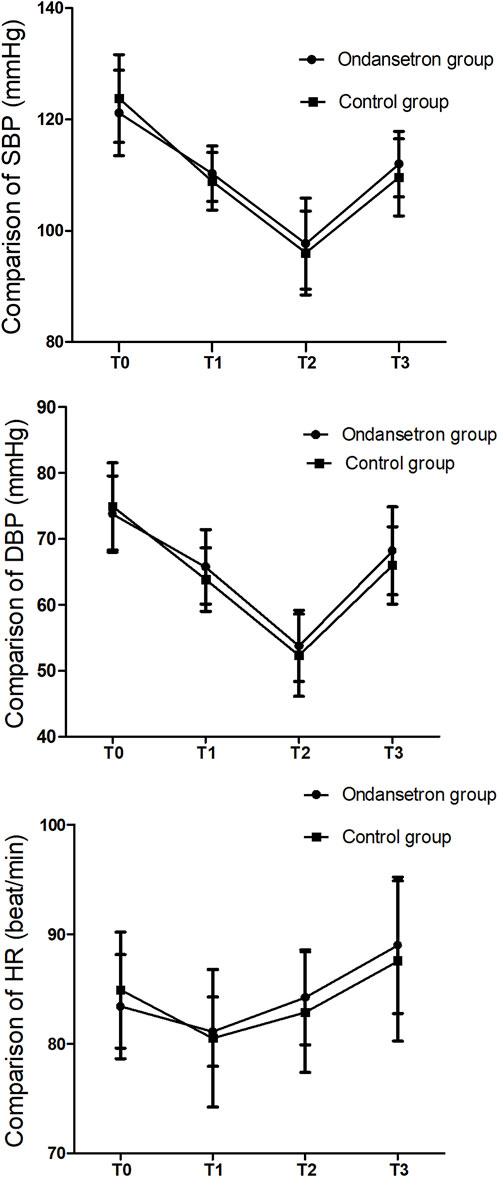
The blood pressure and HR did not change significantly before and after drug administration. The SBP, DBP, and HR were similar at each time point after spinal anesthesia; there were no significant differences between the two groups (Figure 2).
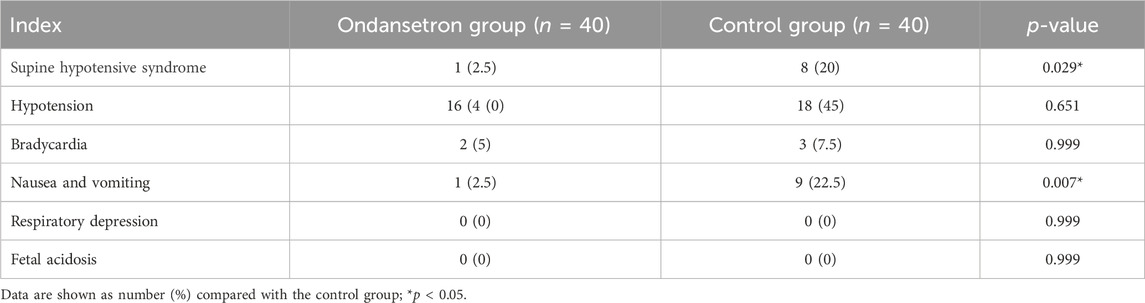
The incidence of supine hypotensive syndrome, nausea, and vomiting was significantly lower in the ondansetron group than the control group (2.5% vs. 20%, p = 0.029; 2.5% vs. 22.5%, p = 0.007, respectively), but there were no statistical significances in terms of hypotension, bradycardia, respiratory depression, and fatal acidosis between the two groups (p > 0.05) (Table 3).
4 Discussion
In this study, we found that ondansetron can effectively prevent supine hypotensive syndrome, reduce the incidence of nausea, vomiting, and vasoconstrictor drug use, and improve neonatal umbilical artery pH during spinal anesthesia for cesarean section.
In the present study, the blood pressure and heart rate 5 min after ondansetron administration had no obvious changes, which indicated that ondansetron had no effects on maternal hemodynamics before and after spinal anesthesia. In our study, only one case of supine hypotensive syndrome was observed; almost all parturients who were administered ondansetron were able to tolerate lying supine during spinal anesthesia without experiencing any symptoms except hypotension. The finding demonstrated that ondansetron can effectively prevent supine hypotensive syndrome during spinal anesthesia for cesarean section. The relevant reasons are as follows: first, ondansetron is a serotonin receptor antagonist; it can inhibit the Bezold–Jarisch reflex and reduce spinal anesthesia-induced vasodilation. Second, ondansetron may cause a dose-dependent increase in blood pressure by acting on serotonin receptors located at the medial septum/vertical limb of the diagonal band complex (Urzedo-Rodrigues et al., 2011). Finally, animal studies indicate that serotonin pathways in the rat brain are a complicated and multifactorial system that regulates blood pressure (Fregoneze et al., 2011); thus, the peripheral and central mechanisms can be involved. Our study indicated that the minimal mean arterial pressure was higher in the ondansetron group than the control group (71.4 ± 4.9 vs. 68.8 ± 6.4 mmHg, p = 0.028). Ondansetron can inhibit the Bezold–Jarisch reflex and mitigate hypotension after spinal anesthesia in the event of a further drop in blood pressure. Ondansetron can attenuate serotonin-induced vasodilation and lead to a slight increase in blood pressure, so ondansetron could not decrease the incidence of maternal hypotension during spinal anesthesia for cesarean section. The present study also showed that ondansetron could significantly reduce the incidence of maternal nausea and vomiting. There is a controversial issue about ondansetron reducing the incidence of hypotension in a cesarean section. Wang et al. (2014) reported that intravenous ondansetron (4 mg) prior to spinal anesthesia can reduce the incidence of hypotension in cesarean sections by 30% vs. 60%, which is contrary to our findings. The reason may be related to the small sample size. However, some studies indicated that ondansetron could not reduce the incidence of hypotension in parturient women (Owczuk et al., 2008; Marciniak et al., 2015), which is in agreement with our findings. Ondansetron could attenuate the fall of systolic and mean blood pressure but did not decrease the incidence of hypotension induced by spinal anesthesia. During cesarean sections, the occurrence of nausea and vomiting was mainly associated with hypotension. Hypotension stimulates the central nervous system, which leads to the occurrence of nausea and vomiting. Moreover, ondansetron reduces the occurrence of nausea and vomiting by inhibiting the emetic center in the brain. In this study, we found a strange phenomenon: the parturient women who were administered ondansetron were able to tolerate hypotension better and did not feel any discomfort. Because of ethical issues, the vasoconstrictor drugs were given when mean arterial pressure was <60 mmHg (the lowest blood pressure that an important organ can tolerate) in this study, thus failing to investigate the minimum mean arterial pressure that can be tolerated.
In this study, the umbilical artery pH was higher in the ondansetron group than the control group, but no fetal acidosis (umbilical artery pH less than 7.2) was observed. Umbilical artery pH is a sensitive index that reflects fetal asphyxia. Umbilical artery pH is mainly associated with fetal perfusion. Ondansetron plays an important role in increasing fetal perfusion by inhibiting the Bezold–Jarisch reflex and reducing serotonin-induced vasodilation after spinal anesthesia. The 5-min Apgar scores of the neonates were all greater than 9 in both groups, which showed that ondansetron had no adverse effect on the neonates. Pasternak et al. (2013) reported that more than 600,000 pregnant women treated with ondansetron had no adverse effects on the neonates. In addition, Einarson et al. (2004) reported that ondansetron administered during pregnancy was not associated with an increased risk for major birth defects above the baseline level. These findings were consistent with the results of our study.
4.1 Limitations
Genetic polymorphism, baseline sympathovagal balance, and environmental and individual factors may influence spinal anesthesia-induced hypotension during elective cesarean section, which increases outcome variability (Yu et al., 2021). Moreover, research with a large sample size is needed to explore the safety of the serotonin receptor antagonist in fetuses.
5 Conclusion
Ondansetron can effectively prevent supine hypotensive syndrome, reduce the incidence of nausea, vomiting, and vasoconstrictor drug usage, and improve neonatal umbilical arterial pH during spinal anesthesia for cesarean section.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Jiaxing Women and Children’s Hospital’s Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Study design and data analysis: YZ and FX. Patient recruitment and data collection: WZ. Writing of the paper: FX and WZ. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors would like to thank their colleagues for their valuable contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
De-Giorgio, F., Grassi, V. M., Vetrugno, G., d'Aloja, E., Pascali, V. L., and Arena, V. (2012). Supine hypotensive syndrome as the probable cause of both maternal and fetal death. J. Forensic Sci. 57, 1646–1649. doi:10.1111/j.1556-4029.2012.02165.x
Einarson, A., Maltepe, C., Navioz, Y., Kennedy, D., Tan, M. P., and Koren, G. (2004). The safety of ondansetron for nausea and vomiting of pregnancy: a prospective comparative study. BJOG 111, 940–943. doi:10.1111/j.1471-0528.2004.00236.x
Fregoneze, J. B., Oliveira, E. F., Ribeiro, V. F., Ferreira, H. S., and De Castro E Silva, E. (2011). Multiple opioid receptors mediate the hypotensive response induced by central 5-HT (3) receptor stimulation. Neuropeptides 45, 219–227. doi:10.1016/j.npep.2011.03.004
Humphries, A., Mirjalili, S. A., Tarr, G. P., Thompson, J. M. D., and Stone, P. (2020). Hemodynamic changes in women with symptoms of supine hypotensive syndrome. Acta Obstet. Gynecol. Scand. 99 (5), 631–636. doi:10.1111/aogs.13789
Kinsella, S. M., and Lohmann, G. (1994). Supine hypotensive syndrome. Obstet. Gynecol. 83 (5), 774–788.
Marciniak, A., Owczuk, R., Wujtewicz, M., Preis, K., and Majdyło, K. (2015). The influence of intravenous ondansetron on maternal blood haemodynamics after spinal anaesthesia for caesarean section: a double-blind, placebo-controlled study. Ginekol. Pol. 86, 461–467. doi:10.17772/gp/2405
Massoth, C., Chappell, D., Kranke, P., and Wenk, M. (2022). Supine hypotensive syndrome of pregnancy: a review of current knowledge. Eur. J. Anaesthesiol. 39 (3), 236–243. doi:10.1097/EJA.0000000000001554
Ortiz-Gómez, J. R., Palacio-Abizanda, F. J., Morillas-Ramirez, F., Fornet-Ruiz, I., Lorenzo-Jiménez, A., and Bermejo-Albares, M. L. (2014). The effect of intravenous ondansetron on maternal haemodynamics during elective caesarean delivery under spinal anaesthesia: a double-blind, randomised, placebo-controlled trial. Int. J. Obstet. Anesth. 23, 138–143. doi:10.1016/j.ijoa.2014.01.005
Ortiz-Gómez, J. R., Palacio-Abizanda, F. J., Morillas-Ramirez, F., Fornet-Ruiz, I., Lorenzo-Jiménez, A., and Bermejo-Albares, M. L. (2017). Reducing by 50% the incidence of maternal hypotension during elective caesarean delivery under spinal anesthesia: effect of prophylactic ondansetron and/or continuous infusion of phenylephrine - a double-blind, randomized, placebo-controlled trial. Saudi J. Anaesth. 11 (4), 408–414. doi:10.4103/sja.SJA_237_17
Owczuk, R., Wenski, W., Polak-Krzeminska, A., Twardowski, P., Arszułowicz, R., Dylczyk-Sommer, A., et al. (2008). Ondansetron given intravenously attenuates arterial blood pressure drop due to spinal anesthesia: a double-blind, placebo-controlled study. Reg. Anesth. Pain Med. 33, 332–339. doi:10.1016/j.rapm.2008.01.010
Pasternak, B., Svanstrom, H., and Hviid, A. (2013). Ondansetron in pregnancy and risk of adverse fetal outcomes. N. Engl. J. Med. 368, 814–823. doi:10.1056/NEJMoa1211035
Urzedo-Rodrigues, L. S., Ferreira, H. S., Almeida, D. O., Medeiros, J. P., Batista, A., de Castro e Silva, E., et al. (2011). Blockade of 5-HT3 receptors at septal area increase blood pressure in unanaesthetized rats. Auton. Neurosci. 159, 51–61. doi:10.1016/j.autneu.2010.07.028
Wang, M., Zhuo, L., Wang, Q., Shen, M. K., Yu, Y. Y., Yu, J. J., et al. (2014). Efficacy of prophylactic intravenous ondansetron on the prevention of hypotension during cesarean delivery: a dose-dependent study. Int. J. Clin. Exp. Med. 7, 5210–5216.
Keywords: ondansetron, supine hypotensive, syndrome, spinal anesthesia, cesarean section
Citation: Zhang Y, Xiao F and Zhang W (2024) Intravenous ondansetron for the prevention of supine hypotensive syndrome during spinal anesthesia for cesarean section: a randomized controlled trial. Front. Pharmacol. 15:1194196. doi: 10.3389/fphar.2024.1194196
Received: 26 March 2023; Accepted: 03 January 2024;
Published: 18 January 2024.
Edited by:
Kassiani Theodoraki, National and Kapodistrian University of Athens, GreeceReviewed by:
Mahmood Moosazadeh, Mazandaran University of Medical Sciences, IranDavid R. Drover, Stanford University, United States
Copyright © 2024 Zhang, Xiao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wangping Zhang, emhhbmc2NTA2NzlAMTYzLmNvbQ==
†These authors share first authorship
 Yuan Zhang1†
Yuan Zhang1† Wangping Zhang
Wangping Zhang