
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Pharmacol. , 09 January 2024
Sec. Obstetric and Pediatric Pharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1340452
Background: The infusion of phenylephrine to prevent spinal-induced hypotension (SIH) in cesarean delivery may decrease the rostral spread of a spinal local anesthetic. We hypothesized that infusion of norepinephrine may decrease the rostral spread of spinal anesthesia, similar to that caused by phenylephrine. The aim of this study was to compare the block height of spinal anesthesia in the presence or absence of norepinephrine infusion administered to prevent SIH during cesarean delivery.
Methods: Eighty patients were enrolled and allocated into groups receiving a norepinephrine infusion (group N) or saline infusion (group C). After intrathecal injection of hyperbaric bupivacaine 10 mg, the block height for cold and pinprick sensation was checked 10 and 20 min after the injection. The demographic characteristics, spinal anesthesia, side effects, and neonatal outcomes were also recorded.
Results: The block height for cold and pinprick sensation was similar between the two groups, although the incidence of hypotension was significantly lower (p < 0.00) in group N than in group C. Systolic blood pressure was also more stable in group N than in group C, with the incidence of interventions being significantly lower in group N. There was no significant difference in patient satisfaction between the two groups.
Conclusion: Evidence from this study suggested that prophylactic norepinephrine infusion does not reduce the rostral spread of spinal anesthesia in pregnancy. We suggest that it is not necessary to increase the dose of an intrathecal local anesthetic for cesarean delivery when prophylactic norepinephrine is administered.
Clinical Trial Registration: https://www.chictr.org.cn/bin/project/edit?pid=152899, identifier [ChiCTR2200057439].
Hypotension is a common side effect of spinal anesthesia in pregnant women undergoing cesarean delivery Chooi et al. (2020); Massoth et al. (2020). To prevent spinal-induced hypotension (SIH) and its associated intraoperative nausea and vomiting (INOV), prophylactic infusion of either phenylephrine or norepinephrine is an ideal strategy and well-accepted technique, used often during cesarean delivery Chooi et al. (2020); Fu et al. (2020); Massoth et al. (2020); Ngan Kee et al. (2020); Wei et al. (2020); Xiao et al. (2020). However, infusion of phenylephrine to prevent SIH is associated with a decrease in the rostral spread of spinal anesthesia in pregnant women undergoing cesarean delivery Cooper and Mowbray (2003); Cooper et al. (2004). This decrease is possibly due to the alpha agonist effects of phenylephrine on epidural venous tone, although the exact mechanism is still unknown Cooper and Mowbray (2003); Cooper et al. (2004). Currently, there is also no evidence on the effects of norepinephrine on epidural venous tone. Therefore, we hypothesized, preventive norepinephrine infusion may also decrease the rostral spread of spinal anesthesia, similar to that observed with phenylephrine. Accordingly, we designed a study to determine the effect of preventive norepinephrine infusion on the spread of spinal anesthesia. The null hypothesis of this study was that norepinephrine infusion does not affect the spread of spinal anesthesia.
The study was approved by the Jiaxing University Affiliated Women and Children Hospital’s Institutional Review Board (IRB KY-2022-05, date of approval: 19 January 2022). All parturients recruited in this study signed written, informed consent. We registered the clinical trial in the Chinese Clinical Trials Registry at https://www.chictr.org.cn/(ChiCTR2200057439, principal investigator: Yu-Fang Dong, date of registration: 12 March 2022) before enrollment of the parturients, which was initiated on 15 March 2022, and concluded on 21 June 2022.
The study was a prospective, randomized, double-blind design.
Consecutive parturients who met the following inclusion criteria and were scheduled for elective cesarean delivery were enrolled in the study. The inclusion criteria were as Society of Anesthesiologists (ASA) physical status < Ⅲ, singleton pregnancy at term (37 weeks ≤ gestation age ≤41 weeks), age 20–40 years, height 158–170 cm, and body mass index (BMI) ≤ 35 kg/m2. The exclusion criteria were preeclampsia or preexisting hypertension, preexisting or gestational diabetes, intrauterine growth retardation or preterm delivery, allergic to norepinephrine and local anesthetics, and any contraindications to spinal or epidural anesthesia, including a bleeding disorder, local infection, or intracranial hypertension.
Randomization of the parturients was carried out by a research assistant who used a numbered sheet generated by an online randomization generator (https://www.random.org/sequences/) to allocate them to receive an infusion of either norepinephrine (group N) or saline (group C). The randomized scheme was then concealed in sequentially numbered, opaque envelopes, with one opened for each patient enrolled.
All parturients enrolled in the study fasted without solid food for 8 h and water for 2 h. No premedication was administered. Upon arrival in the operating room, standard monitoring for vital signs was applied for continuous measurement, including non-invasive blood pressure, electrocardiogram, and pulse oximetry. The baseline values for systolic blood pressure (SBP) and heart rate (HR) were calculated as the mean of three continuous measurements of SBP and HR at 3-min intervals, 5 min after the patient had become calm. Peripheral intravenous access in the left upper limb was then created through an 18-G trocar. No prehydration was given.
Combined spinal-epidural anesthesia was achieved via a needle-through-needle technique at the ascertained L3-4 vertebral interspace, which was located by ultrasound assessment, with the patient in the left lateral position under regional anesthesia. The epidural space was assessed by the loss-of-resistance of saline (<2 mL) using an 18-gauge Tuohy. A 25-gauge Whitacre needle was then passed through the Tuohy needle to reach the subarachnoid space. After the emergence of clear cerebrospinal fluid (CSF), 10 mg of hyperbaric bupivacaine (2 mL) was injected into the subarachnoid space over 15 s, with the bevel rostral. Before removal of the Whitacre needle, gentle aspiration was applied by the syringe to ensure that CSF could be withdrawn, which indicated successful delivery of the intrathecal medications had occurred. An epidural nylon catheter with multiple holes (three holes) was then inserted 3–4 cm into the epidural space.
Immediately after the intrathecal injection, an infusion of 0.1 ㎍/kg/min norepinephrine, or saline, was prepared by a fixed anesthesia assistant who knew the patients’ grouping but did not participate in patient care and data collection. The infusions were administered using a syringe pump. Meanwhile, a coload of 10 mL/kg/h of warmed Lactate Ringer solution was infused. Hypotension was defined as an absolute SBP value <90 mmHg or a decrease ≥20% in the baseline value, hypertension as a SBP ≥120% of baseline value, and bradycardia as an HR < 50 bpm. According to the study protocol, we treated hypotension accompanied by an increase in HR with 50 ㎍ of phenylephrine, hypotension occurring with bradycardia with 0.5 mg atropine and/or 6 mg ephedrine, and hypertension by stopping the infusion of norepinephrine and restarting when SBP was <120% of the baseline value.
The primary outcome in this study was block height measured 10 and 20 min after the intrathecal injection. Alcohol wipes were used to assess cold sensation, with the patients asked to report changes in temperature perception. A blunted epidural needle was used to assess pinprick sensation and the patients were required to report if the blunt needle caused pain, felt sharp, or both. The secondary outcomes of this study were: side effects such as hypotension; hypertension; bradycardia; shivering; nausea and vomiting; physician interventions including treatment of hypotension, hypertension, or bradycardia; surgical data including duration of surgery and duration from intrathecal injection to infant delivery; patient satisfaction was evaluated immediately following the completion of surgery using a 0–5 scale, where 0 signifies dissatisfaction and five represents the highest level of satisfaction; and neonatal outcomes including 1, 5 min Apgar score and umbilical arterial pH value; and patient satisfaction (0–5, where 0 presents most unsatisfied, 5 presents most satisfied). Demographic characteristics including maternal age, height, weight, and gestational age were also recorded.
Group sample sizes of 24 and 24 achieve 90% power to show a difference in means when there is a difference of 2.0 (significance for clinical practice) between the null hypothesis mean difference of 0.0 and the actual mean difference of 2.0 at the 0.050 significance level (alpha) using a two-sided Mann-Whitney-Wilcoxon Test. These results are based on 2000 Monte Carlo samples from the null distributions: Normal (M0 S) and Normal (M0 S), and the alternative distributions: Normal (M0 S) and Normal (M1 S). To account for possible dropouts, we enrolled 40 parturients for each group in this study.
The Kolmogorov-Smirnov test was used to assess the distribution of the univariable data. Normally distributed data, including the demographic characteristics, surgery time, and umbilical arterial pH value were expressed as mean ± standard deviation (SD), with differences analyzed using Student’s t-test. Non-normally distributed data, such as block height, were expressed as median (range) and were analyzed using the Mann-Whitney U test. The incidence of side effects was expressed as a number (incidence) and tested using Fisher’s exact test. A p-value <0.05 was regarded as statistically significant (two-sided). The analyses were performed using GraphPad Prism version 5.0 (GraphPad Software Inc., San Diego, CA, United States).
A total of 91 patients were enrolled for the assessment of eligibility. Of these 91 patients, 7 did not meet the inclusion criteria and 4 declined to participate, leaving 80 patients in the final analysis. The patients were then randomized into one of the two study groups. The CONSORT flow diagram is shown in Figure 1. As shown in Table 1, there was no significant difference in the demographic characteristics and surgical data between the two groups.
Block heights were similar between the two groups for cold and pinprick sensation 10 and 20 min after the intrathecal injection (Figure 2). With the exception of seven patients in group N and nine patients in the group C whose block height for cold sensation reached T5 20 min after the injection, all the remaining patients recorded a response ≥ T4. All 12 patients did not require an epidural top-up. One patient in group N whose block height for pinprick sensation only reached T7 received an epidural injection of 5 mL of 2% lidocaine 20 min after the intrathecal injection. No patient in group C required this extra treatment. Only one patient in group C complained of being uncomfortable and felt pain during surgery, and was therefore administered 10 mL of 2% lidocaine epidurally. There was no significant difference (p = 0.26) in patient satisfaction between group N (5, (4-5)) and group C (5, (2-5)), with no patient requiring general anesthesia.
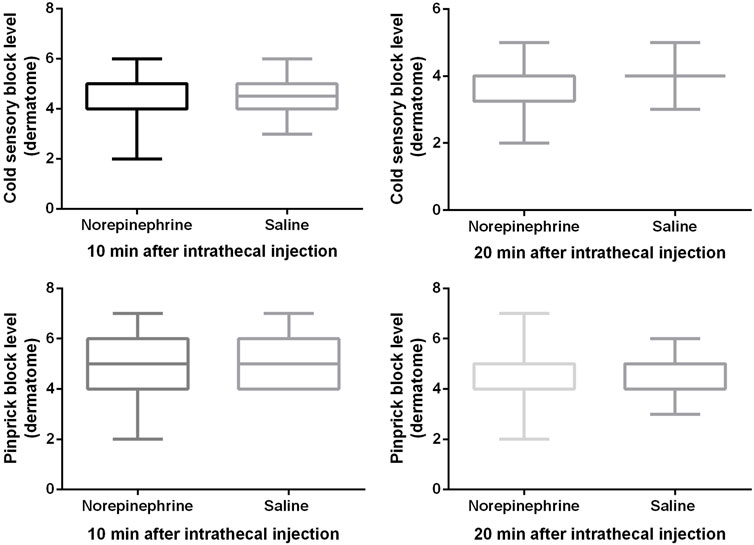
FIGURE 2. Block height for cold and pinprick sensation. The cold sensory block level was 4 (4, 5) vs. 4.5 (4, 5) at 10 min, and 4 (3, 4) vs. 4 (4, 4) at 20 min after intrathecal injection. The pinprick sensory block level was 5 (4, 6) vs. 5 (4, 6) at 10 min, and 4 (4, 5) vs. 4 (4, 5) at 20 min after intrathecal injection. Boxplots show median, 10th, 25th, 75th, and 90th percentiles. There was no significant difference between the two groups (Mann-Whitney U test, all p values > 0.05).
Subgroup (patients who used phenylephrine to treat hypotension were excluded) analysis showed that there was no difference in the cold or pinprick sensation for 10, and 20 min after intrathecal injection between the two groups, which are shown in Table 2.
The incidence of side effects is shown in Table 3. The incidence of hypotension was significantly lower (p < 0.001) in group N (10%) than in group C (57.5%). The incidence of nausea and vomiting was lower (p = 0.048) in group N than in group C. As shown in Figure 3, SBP measured at 1-min intervals in the first 20 min after the intrathecal injection, was closer to the baseline value in group N than in group C, with no significant difference in the area under the curve between the two groups (p > 0.05). Two patients in group N experienced reactive hypertension, although there was no significant difference in incidence between the two groups (p = 0.494). There was also no significant difference in the incidence of shivering between the two groups. The incidence of physician interventions was significantly lower in group N than in group C, while patient satisfaction showed no significant difference between the two groups.
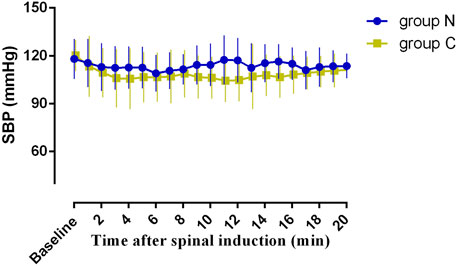
FIGURE 3. Systolic blood pressure 20 min after the intrathecal injection. The area under the curve (mean ± SD) was not significantly different between the two groups (2273 ± 40, and 2161 ± 46 min·mmHg in group N and group C, p < 0.05).
The neonatal outcomes are summarized in Table 3 that shows there were no significant differences in the 1 and 5-min Apgar scores and umbilical arterial pH value between the two groups.
The results of this study suggested that norepinephrine infusion to prevent spinal-induced hypotension in cesarean delivery does not affect the rostral spread of spinal anesthesia. This result underlies that it is not necessary to increase the dose of intrathecal bupivacaine in order to reach a satisfactory level of anesthesia when prophylactic norepinephrine infusion is used to manage post-spinal blood pressure. Previous studies have suggested that when phenylephrine is used as a preventive infusion in cesarean delivery this may lead to a decrease in the rostral spread of spinal anesthesia. Cooper et al. (2004) Recently, it has been reported that norepinephrine has sufficient potency to act as an alternate to phenylephrine in obstetric anesthesia. Ngan Kee et al. (2015); Mercier et al. (2019); Theodoraki et al. (2020) However, there is only limited data on the effect of norepinephrine on intrathecal anesthetic spread. The strength of the current study was that it provided evidence on the effect of norepinephrine in this context.
The requirement for an intrathecal local anesthetic for pregnant women undergoing cesarean delivery is lower than that for non-pregnant women undergoing surgery under spinal anesthesia. This is due to dilation of the epidural veins that leads to a reduction in lumbosacral CSF volume. Cooper et al. (2004) Similarly, evidence has shown that an epidural injection of 10 mL of saline slightly increases the volume of the epidural space and subsequently decreases the volume of lumbosacral CSF, thereby increasing the rostral spread of spinal anesthesia. Stienstra et al. (1999)Therefore, we assumed that both norepinephrine and phenylephrine may reduce the spread of spinal anesthesia by contracting the epidural veins and offsetting the physiological expansion associated with pregnancy.
However, data from the current study showed no decremental spread in spinal anesthesia, a result which is contrary to our hypothesis and different from those of a previous study Cooper et al. (2004). After carefully reviewing the protocols of Cooper (Cooper et al., 2004) and our studies, we found the specific gravity of the local anesthetics used in the two studies were different (10 mg of plain levobupivacaine in Cooper’s study compared to 10 mg of hyperbaric bupivacaine in the current study). We concluded that this was the most likely reason for the different findings between the two studies. In the supine position, a hyperbaric local anesthetic solution spreads rostrally under the action of gravity, until the thoracic curve of the vertebral canal is reached and limits further spread Hocking and Wildsmith (2004); Loubert et al. (2011). In contrast, the spread of a plain anesthetic solution is dependent mainly on the flow of CSF rather than restricted by a physiological structure. Clinical trials also provided evidence that intrathecal plain bupivacaine was more effective for rostral spread than that of hyperbaric bupivacaine following epidural volume extension Tyagi et al. (2008). We therefore consider that the difference in CSF flow caused by vasopressors has less effect on the rostral spread of hyperbaric bupivacaine than that caused by plain levobupivacaine, due to the spread of hyperbaric bupivacaine being more dependent on the effect of gravity.
Another mechanism causing this phenomenon may be the different pressures in the epidural space caused by phenylephrine and norepinephrine. In their study, Cooper (Cooper et al., 2004) used 67 ㎍/min of phenylephrine, whereas we used 0.1 ㎍/kg/min of norepinephrine, equivalent to 7.1 ㎍/min based on the mean body weight of group N (71 kg). Evidence shows that the efficacy ratio for continuous infusion of norepinephrine to phenylephrine is approximately 1:6 Qian et al. (2022). Therefore, 67 ㎍/min of phenylephrine has a greater vasoconstrictive effect than 7.1 ㎍/min of norepinephrine (equivalent to 42.6 ㎍/min of phenylephrine). This results in lower epidural pressure which may reduce the rostral spread of the intrathecal local anesthetic. Further clinical investigations are therefore warranted to confirm the equivalent vasopressor effect of spinal spread.
It could be argued that our use of phenylephrine to treat hypotension could possibly confound our results, although there was no evidence to show that an intravenous bolus of rescued phenylephrine affected the rostral spread of spinal anesthesia. Taking this into account, we conducted a subgroup analysis, in which patients who suffered hypotension and received phenylephrine were excluded. The analysis showed no difference in cold or pinprick sensation at 10, and 20 min after the spinal injection between the two groups. Despite this, further studies with large simple size are warranted.
That we chose 0.1 ㎍/kg/min of norepinephrine as the infusions rate was based on the results of our previous studies, in which we found 0.1 ㎍/kg/min of norepinephrine would be an ideal initial and safe dose for preventing SIH during cesarean delivery for maintaining the haemodynamics and without any peripheral venous complications Xu et al. (2021). Although of that, there remain concerns regarding the safety of peripheral administration of a norepinephrine infusion using such a large dose. However, prior data have shown that peripheral venous delivery of norepinephrine is safe and feasible. A recent large multicenter study of non-obstetric patients showed no significant correlation between the use of peripheral norepinephrine infusions and adverse events Pancaro et al. (2020). Similarly, the absence of peripheral venous complications in our study suggests that the administration of norepinephrine via the peripheral venous route is both safe and feasible. Nevertheless, we recommend peripheral administration of norepinephrine via a large vein with concurrent intravenous fluid infusion through the same vein.
Similar to previous studies (Ngan Kee et al., 2004; Siddik-Sayyid et al., 2014; George et al., 2018),our results showed that prophylactic vasopressor infusion to prevent SIH was superior to an intravenous bolus administration of vasopressor to treat hypotension, because of more stable hemodynamics state (Figure 3) and a lower incidence of hypotension. Our study also showed a low incidence of reactive hypertension with an infusion of 0.1 ㎍/kg/min norepinephrine, a result which was in accordance with that of our previous study Xu et al. (2021). The higher incidence and frequency of physician intervention required in the saline group suggested that without a preventive strategy to manage post-spinal hemodynamics, this would inevitably result in a heavier workload for obstetric anesthesia care providers. Although patients without prophylactic norepinephrine experienced a high incidence of hypotension, but there was no significant difference in patient satisfaction between the two groups due to the comparable incidence of nausea and vomiting, resulting from prompt observation and management.
We acknowledge there are several limitations in the current study. First, phenylephrine was used to treat hypotension in the control group, which would have influenced the results of the rostral spread observed with intrathecal hyperbaric bupivacaine. However, there was no evidence to show that a bolus of phenylephrine could influence spinal spread. We choose phenylephrine rather than ephedrine to treat hypotension because it is superior for maintaining the pH value of the umbilical artery blood. Further studies are therefore needed to compare the direct effects of infusion of phenylephrine or norepinephrine on the rostral spread of spinal medications. Second, the sample size calculated for the current study was for primary outcomes, but not for secondary outcomes. There are inevitable statistical errors that may occur and therefore the conclusions derived from these results can only be regarded as explorative for clinical practice. Third, no objective indicators were used to assess block height in the current study. Therefore, the assessment of block height was subjective, with different people having different standards for their post-spinal perception of cold and pinprick sensation. This will have inevitably affected the results. Finally, the observed side effects in this study may not accurately reflect the impact on neonatal outcomes. Nonetheless, studies conducted in this context have demonstrated that similar to phenylephrine, norepinephrine is both safe and effective for neonates, even in women with fetal compromise Mohta et al. (2022).
In summary, this study suggested that prophylactic infusion of norepinephrine does not reduce the rostral spread of intrathecal hyperbaric bupivacaine in pregnancy, and that it is not necessary to increase the dose of the intrathecal local anesthetic. However, further studies are warranted to compare the direct effects of phenylephrine and norepinephrine infusions on the rostral spread of spinal medications and intrathecal dose requirement of local anesthetic.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by Jiaxing University Affiliated Women and Children Hospital’s Institutional Review Board (IRB KY-2022-05, date of approval: 19 January 2022). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Y-FD: Investigation, Methodology, Writing–original draft. JiQ: Investigation, Methodology, Writing–original draft. JiW: Investigation, Writing–original draft. L-ZW: Investigation, Methodology, Writing–original draft. X-HQ: Formal Analysis, Methodology, Writing–review and editing. FX: Formal Analysis, Methodology, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We gave our sincerest thanks to all the staff in the operating theater and all obstetric staff involved for their kind help in study delivery.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Chooi, C., Cox, J. J., Lumb, R. S., Middleton, P., Chemali, M., Emmett, R. S., et al. (2020). Techniques for preventing hypotension during spinal anaesthesia for caesarean section. Cochrane Database Syst. Rev. 7 (7), Cd002251. doi:10.1002/14651858.CD002251.pub4
Cooper, D. W., Jeyaraj, L., Hynd, R., Thompson, R., Meek, T., Ryall, D. M., et al. (2004). Evidence that intravenous vasopressors can affect rostral spread of spinal anesthesia in pregnancy. Anesthesiology 101 (1), 28–33. doi:10.1097/00000542-200407000-00007
Cooper, D. W., and Mowbray, P. (2003). Can choice of vasopressor therapy affect rostral spread of spinal anaesthetic? Anesthesiology 98 (6), 1524. doi:10.1097/00000542-200306000-00051
Fu, F., Xiao, F., Chen, W., Yang, M., Zhou, Y., Ngan Kee, W. D., et al. (2020). A randomised double-blind dose-response study of weight-adjusted infusions of norepinephrine for preventing hypotension during combined spinal-epidural anaesthesia for Caesarean delivery. Br. J. Anaesth. 124 (3), e108–e114. doi:10.1016/j.bja.2019.12.019
George, R. B., McKeen, D. M., Dominguez, J. E., Allen, T. K., Doyle, P. A., and Habib, A. S. (2018). A randomized trial of phenylephrine infusion versus bolus dosing for nausea and vomiting during Cesarean delivery in obese women. Can. J. Anaesth. 65 (3), 254–262. doi:10.1007/s12630-017-1034-6
Hocking, G., and Wildsmith, J. A. (2004). Intrathecal drug spread. Br. J. Anaesth. 93 (4), 568–578. doi:10.1093/bja/aeh204
Loubert, C., Hallworth, S., Fernando, R., Columb, M., Patel, N., Sarang, K., et al. (2011). Does the baricity of bupivacaine influence intrathecal spread in the prolonged sitting position before elective cesarean delivery? A prospective randomized controlled study. Anesth. Analg. 113 (4), 811–817. doi:10.1213/ANE.0b013e3182288bf2
Massoth, C., Töpel, L., and Wenk, M. (2020). Hypotension after spinal anesthesia for cesarean section: how to approach the iatrogenic sympathectomy. Curr. Opin. Anaesthesiol. 33 (3), 291–298. doi:10.1097/ACO.0000000000000848
Mercier, F. J., Soued, M., Morau, E., and Ngan Kee, W. D. (2019). Noradrenaline for haemodynamic control in obstetric anaesthesia: is it now a suitable alternative to phenylephrine? Anaesth. Crit. Care Pain Med. 38 (6), 591–593. doi:10.1016/j.accpm.2019.10.006
Mohta, M., Bambode, N., Chilkoti, G. T., Agarwal, R., Malhotra, R. K., and Batra, P. (2022). Neonatal outcomes following phenylephrine or norepinephrine for treatment of spinal anaesthesia-induced hypotension at emergency caesarean section in women with fetal compromise: a randomised controlled study. Int. J. Obstet. Anesth. 49, 103247–103317. doi:10.1016/j.ijoa.2021.103247
Ngan Kee, W. D., Khaw, K. S., Ng, F. F., and Lee, B. B. (2004). Prophylactic phenylephrine infusion for preventing hypotension during spinal anesthesia for cesarean delivery. Anesth. Analg. 98 (3), 815–821. table of contents. doi:10.1213/01.ane.0000099782.78002.30
Ngan Kee, W. D., Lee, S. W. Y., Ng, F. F., and Lee, A. (2020). Norepinephrine or phenylephrine during spinal anaesthesia for Caesarean delivery: a randomised double-blind pragmatic non-inferiority study of neonatal outcome. Br. J. Anaesth. 125 (4), 588–595. doi:10.1016/j.bja.2020.05.057
Ngan Kee, W. D., Lee, S. W. Y., Ng, F. F., Tan, P. E., and Khaw, K. S. (2015). Randomized double-blinded comparison of norepinephrine and phenylephrine for maintenance of blood pressure during spinal anesthesia for cesarean delivery. Anesthesiology 122 (4), 736–745. doi:10.1097/ALN.0000000000000601
Pancaro, C., Shah, N., Pasma, W., Saager, L., Cassidy, R., van Klei, W., et al. (2020). Risk of major complications after perioperative norepinephrine infusion through peripheral intravenous lines in a multicenter study. Anesth. Analg. 131 (4), 1060–1065. doi:10.1213/ANE.0000000000004445
Qian, J., Zhao, Y. P., Deng, J. L., Wang, L. Z., Xiao, F., Shen, B., et al. (2022). Determination of the relative potency of norepinephrine and phenylephrine given as infusions for preventing hypotension during combined spinal-epidural anesthesia for cesarean delivery: a randomized up-and-down sequential allocation study. Front. Pharmacol. 13, 942005. doi:10.3389/fphar.2022.942005
Siddik-Sayyid, S. M., Taha, S. K., Kanazi, G. E., and Aouad, M. T. (2014). A randomized controlled trial of variable rate phenylephrine infusion with rescue phenylephrine boluses versus rescue boluses alone on physician interventions during spinal anesthesia for elective cesarean delivery. Anesth. Analg. 118 (3), 611–618. doi:10.1213/01.ane.0000437731.60260.ce
Stienstra, R., Dilrosun-Alhadi, B. Z., Dahan, A., van Kleef, J. W., Veering, B. T., and Burm, A. G. (1999). The epidural "top-up" in combined spinal-epidural anesthesia: the effect of volume versus dose. Anesth. Analg. 88 (4), 810–814. doi:10.1097/00000539-199904000-00024
Theodoraki, K., Hadzilia, S., Valsamidis, D., and Stamatakis, E. (2020). Prevention of hypotension during elective cesarean section with a fixed-rate norepinephrine infusion versus a fixed-rate phenylephrine infusion. Α double-blinded randomized controlled trial. Int. J. Surg. 84, 41–49. doi:10.1016/j.ijsu.2020.10.006
Tyagi, A., Kumar, A., Sethi, A. K., and Mohta, M. (2008). Epidural volume extension and intrathecal dose requirement: plain versus hyperbaric bupivacaine. Anesth. Analg. 107 (1), 333–338. doi:10.1213/ane.0b013e3181734436
Wei, C., Qian, J., Zhang, Y., Chang, X., Hu, H., and Xiao, F. (2020). Norepinephrine for the prevention of spinal-induced hypotension during caesarean delivery under combined spinal-epidural anaesthesia: randomised, double-blind, dose-finding study. Eur. J. Anaesthesiol. 37 (4), 309–315. doi:10.1097/EJA.0000000000001152
Xiao, F., Shen, B., Xu, W. P., Feng, Y., Ngan Kee, W. D., and Chen, X. Z. (2020). Dose-response study of 4 weight-based phenylephrine infusion regimens for preventing hypotension during cesarean delivery under combined spinal-epidural anesthesia. Anesth. Analg. 130 (1), 187–193. doi:10.1213/ANE.0000000000004092
Xu, W., Drzymalski, D. M., Ai, L., Yao, H., Liu, L., and Xiao, F. (2021). The ED(50) and ED(95) of prophylactic norepinephrine for preventing post-spinal hypotension during cesarean delivery under combined spinal-epidural anesthesia: a prospective dose-finding study. Front. Pharmacol. 12, 691809. doi:10.3389/fphar.2021.691809
Keywords: norepinephrine, spinal anesthesia, hypotension, pregnancy, bupivacaine
Citation: Dong Y-F, Qian J, Wang J, Wang L-Z, Qian X-H and Xiao F (2024) Prophylactic infusion of norepinephrine does not affect the rostral spread of spinal anesthesia in pregnancy: a prospective, randomized, double-blinded study. Front. Pharmacol. 14:1340452. doi: 10.3389/fphar.2023.1340452
Received: 18 November 2023; Accepted: 28 December 2023;
Published: 09 January 2024.
Edited by:
Kassiani Theodoraki, National and Kapodistrian University of Athens, GreeceReviewed by:
Carlos Alan Dias-Junior, São Paulo State University, BrazilCopyright © 2024 Dong, Qian, Wang, Wang, Qian and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xing-Hua Qian, cXhoMDkzMEBzaW5hLmNvbQ==; Fei Xiao, MTM3MDY1OTc1MDFAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.