
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Pharmacol. , 12 January 2024
Sec. Ethnopharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1334160
This article is part of the Research Topic Preventing and Treating Liver Diseases: Medicinal and Food Plants, their metabolites as potential options View all 17 articles
 Allah Nawaz1,2*†
Allah Nawaz1,2*† Azhar Manzoor3
Azhar Manzoor3 Saeed Ahmed4
Saeed Ahmed4 Naveed Ahmed5
Naveed Ahmed5 Waseem Abbas2
Waseem Abbas2 Mushtaq Ahmad Mir6
Mushtaq Ahmad Mir6 Muhammad Bilal7
Muhammad Bilal7 Alisha Sheikh8
Alisha Sheikh8 Saleem Ahmad9
Saleem Ahmad9 Ishtiaq Jeelani2*†
Ishtiaq Jeelani2*† Takashi Nakagawa2
Takashi Nakagawa2Hepatitis C virus (HCV) infection is a significant global health concern, prompting the need for effective treatment strategies. This in-depth review critically assesses the landscape of HCV treatment, drawing parallels between traditional interferon/ribavirin therapy historically pivotal in HCV management and herbal approaches rooted in traditional and complementary medicine. Advancements in therapeutic development and enhanced clinical outcomes axis on a comprehensive understanding of the diverse HCV genome, its natural variations, pathogenesis, and the impact of dietary, social, environmental, and economic factors. A thorough analysis was conducted through reputable sources such as Science Direct, PubMed, Scopus, Web of Science, books, and dissertations. This review primarily focuses on the intricate nature of HCV genomes and explores the potential of botanical drugs in both preventing and treating HCV infections.
Hepatitis C virus (HCV) infection is a significant global health issue and is regarded as one of the primary causes of mortality worldwide. According to the World Health Organization (WHO), the worldwide prevalence of HCV infections is currently reported to be 58 million, with an estimated 1.5 million new HCV infections occurring each year. This high incidence results in approximately 290,000 deaths annually attributed to HCV infection (WHO, 2022). While most cases of HCV infection remain asymptomatic, the disease can advance to chronic conditions like liver fibrosis, liver cirrhosis, hepatocellular carcinoma or liver failure. Notably, liver cirrhosis emerges in around 20% of individuals with chronic hepatitis C. Chronic liver disease is mainly attributed to excessive alcohol consumption and persistent infections with hepatitis B and C viruses (Singh and Hoyert, 2000). Several factors contribute to an elevated risk of HCV infection, including alcohol consumption, immunosuppression, and acquisition of HCV after the age of 40.
Recent research has elucidated that the impact of HCV extends beyond hepatocytes, leading to extrahepatic manifestations such as lymphoproliferative disorders, insulin resistance, type 2 diabetes, renal disease, and neurological disorders (Jacobson et al., 2010; Ito et al., 2011; Stanaway et al., 2016; Vanni et al., 2016; Drazilova et al., 2018; Moustafa et al., 2020). Consequently, there is an urgent need to identify new, and intensive treatment approaches capable of addressing both intra- and extrahepatic manifestations of HCV. The therapeutic approach for HCV is undergoing dynamic advancements aimed at attaining optimal responses and sustained viral eradication over the long term. The introduction of Interferon alpha-2b in 1986 marked an initial milestone in the pursuit of a curative approach for HCV (Tong et al., 1997; Hoofnagle et al., 1986). However, the sustained virologic response (SVR) rate with interferon monotherapy was limited to 10%–20% (Farrell et al., 1998; McHutchison et al., 1998). Subsequent research revealed that Ribavirin, an orally active synthetic guanosine analogue with antiviral and immunomodulatory properties, could enhance treatment outcomes when combined with interferon therapy (Bodenheimer et al., 1997; Davis et al., 1998). The administration of interferon and ribavirin therapy for the treatment of HCV can induce several adverse effects, including flu-like symptoms, nausea, vomiting, depression, insomnia, weight loss, anemia, and skin reactions. A significant advancement in HCV treatment has emerged with the introduction of various oral regimens that incorporate Direct-Acting Antivirals (DAAs), each characterized by distinct mechanisms of action (Asselah et al., 2018; Christensen et al., 2018). These treatments boast a favorable safety profile and are generally well-tolerated, resulting in a remarkable increase in SVR rates, often nearing 100%.
DAAs offer several advantages, including minimal side effects, short treatment durations (typically 8–12 weeks), and a low likelihood of viral resistance development. Targeting specific steps in the HCV life cycle, DAAs provide highly effective, well-tolerated, and curative treatment options. With cure rates often exceeding 95% across diverse patient populations and HCV genotypes, DAAs have a high barrier to the development of drug resistance. This approach has revolutionized HCV management, allowing healthcare providers to offer curative therapies to a broad range of patients, including those with co-infections (e.g., HIV) and special populations such as individuals with cirrhosis, transplant recipients, and people who inject drugs. While DAAs offer significant advantages, their high cost has raised concerns about access to treatment in some regions. Efforts are ongoing to make these medications more accessible globally.
In contrast, herbal treatments for HCV represent a traditional and alternative approach rooted in centuries-old natural remedies. Although some herbal products have been investigated for their hepatoprotective and potential antiviral properties, scientific evidence supporting their efficacy and safety in HCV management is limited and inconsistent. Herbs such as milk thistle, licorice root, and curcumin have been explored for potential benefits, but clinical study results are inconclusive. Moreover, the lack of standardization and quality control in herbal products raises concerns about consistency and safety.
On a global scale, chronic liver disease and cirrhosis rank as the 10th leading cause of death. This condition affects both males and females disproportionately between the ages of 35 and 64, making it the 5th leading cause of mortality among individuals aged 45 to 64. Approximately 70% of individuals chronically infected with HCV develop progressive liver disease, and HCV infection accounts for the chronic liver disease in 40% of all affected patients (Nainan et al., 2006). According to the World Health Organization (WHO), approximately 200 million people are currently living with HCV, with an annual incidence of 3.5–4.0 million new infections. The prevalence of HCV varies globally, with notable regional distinctions. These insights into the global epidemiology of HCV underscore the importance of comprehensive strategies for prevention, diagnosis, and management on a worldwide scale.
The liver, the largest solid organ in the body, plays a pivotal role in diverse physiological functions. Comprising various cell types, the liver includes hepatocytes as the predominant cell type, along with non-parenchymal cells, hepatic stellate cells, endothelial cells, and Kupffer cells. The latter are resident macrophages that mainly regulate liver homeostasis during liver inflammation (Kazankov et al., 2019; Sakai et al., 2019; Alharthi et al., 2020). Immune cells are key players in various metabolic diseases, including insulin resistance, fatty liver, and atherosclerosis. HCV, upon binding to extrahepatic peripheral B cells (CD81), triggers dysregulation within the immune system. This infection results in the chronic stimulation of lymphocytes, ultimately leading to the expansion of B-cell clones. This process then activates the complement system and antibody production to form immune complexes, resulting in tissue damage (Jacobson et al., 2010; Ito et al., 2011; Moustafa et al., 2020) (Figure 1A).
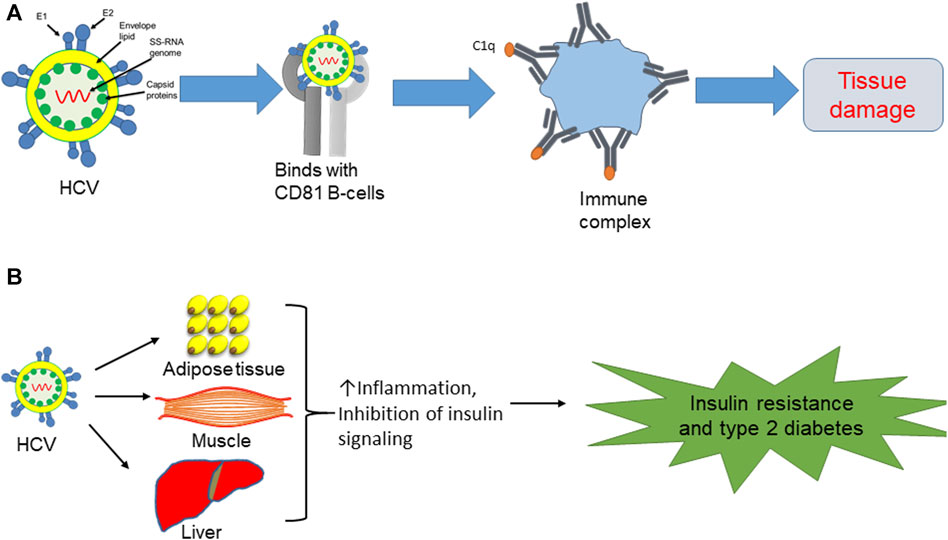
FIGURE 1. (A) HCV binds with CD81 B cells, leading to antibody production and formation of immune complexes, which then initiate tissue damage. (B) Direct effect of HCV on insulin resistance and type 2 diabetes via induction of proinflammatory cytokines in adipose tissue and liver, while also blocking insulin signaling in muscle.
HCV is involved in extrahepatic manifestations of metabolic disorders, particularly insulin resistance and type 2 diabetes, due to the aberrant activation of inflammatory cytokines. In the context of chronic HCV infections, there is a notable production of inflammatory cytokines, including interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), within the liver. These cytokines, in turn, disrupt insulin signaling pathways, leading to the development of insulin resistance. Beyond the heightened risk of type 2 diabetes associated with HCV infections, individuals with chronic HCV infections also exhibit an elevated prevalence of cardiac-cerebrovascular disorders. This intricate relationship underscores the systemic impact of HCV beyond the liver and emphasizes the potential role of viral-induced inflammatory responses in contributing to broader metabolic and cardiovascular complications (Drazilova et al., 2018; Moustafa et al., 2020). HCV infection seems to accelerate the inflammatory response and hinder insulin signaling in metabolically responsive tissues, including the liver, adipose tissue, and skeletal muscle (Figure 1B). The dysregulation of inflammatory pathways and interference with insulin signaling in these tissues contribute to the development of metabolic disturbances, including insulin resistance, highlighting the systemic effects of HCV infection beyond the liver (Fujisaka et al., 2009; Fujisaka et al., 2013; Fujisaka et al., 2016; Nawaz et al., 2017a; Nawaz et al., 2017b; Takikawa et al., 2019). Indeed, aging-induced inflammation plays a substantial role in the onset of diverse disorders, including diabetes, atherosclerosis, and mitochondrial dysfunction. The inflammatory milieu associated with aging can amplify the overall impact of HCV infection on the body’s health, exacerbating its effects on metabolic and physiological processes (Zhang et al., 2016; Chia et al., 2018; Kazankov et al., 2019; Luo et al., 2019; Reidy et al., 2019). Consequently, HCV infection is correlated with metabolic complications due to the abnormal activation of inflammatory cytokines.
The use of various medicinal plants for managing chronic hepatitis C infections has been a part of traditional medicine in different cultures for centuries. Many societies have relied on natural remedies derived from plants to address various health issues, including liver ailments. It is important to note that while traditional practices often involve the use of medicinal plants, the effectiveness and safety of these remedies can vary, and scientific research is essential to validate their therapeutic potential. Botanical drug(s), individually or in combination, are effective against HCV infection, blocking the virus’s entry, translation, replication, and assembly (summarized in Table 1). Molecular studies have also demonstrated that medicinal plants can be used to develop anti-HCV drugs. Silymarin, derived from the seeds of the milk thistle plant (Silybum marianum: family Asteraceae), is known to inhibit inflammatory cytokines and other transcriptional factors, viral entry into hepatocytes, and viral replication (Polyak et al., 2007). Quercetin is reported to block HCV replication via a direct inhibitory effect on NS3 polymerase and IRES activity (Gonzalez et al., 2009; Bachmetov et al., 2012). Ladanein and Limonium sinense block viral entry through effects on post-attachment entry steps, including uncoating, fusion, and endocytosis (Haid et al., 2012; Hsu et al., 2015; Jardim et al., 2018). Magnolia officinalis, a member of Magnoliaceae family, blocks HCV translation (Lam et al., 2016). Furthermore, glycyrrhizin, an inherent compound present in the roots of the Glycyrrhiza glabra plant from the Fabaceae family, demonstrates anti-HCV activity. It acts specifically by impeding viral translation and replication, leading to a reduction in viral titer (Ashfaq et al., 2011; Matsumoto et al., 2013; Ashfaq and Idrees, 2014; Li et al., 2014; Li et al., 2019).
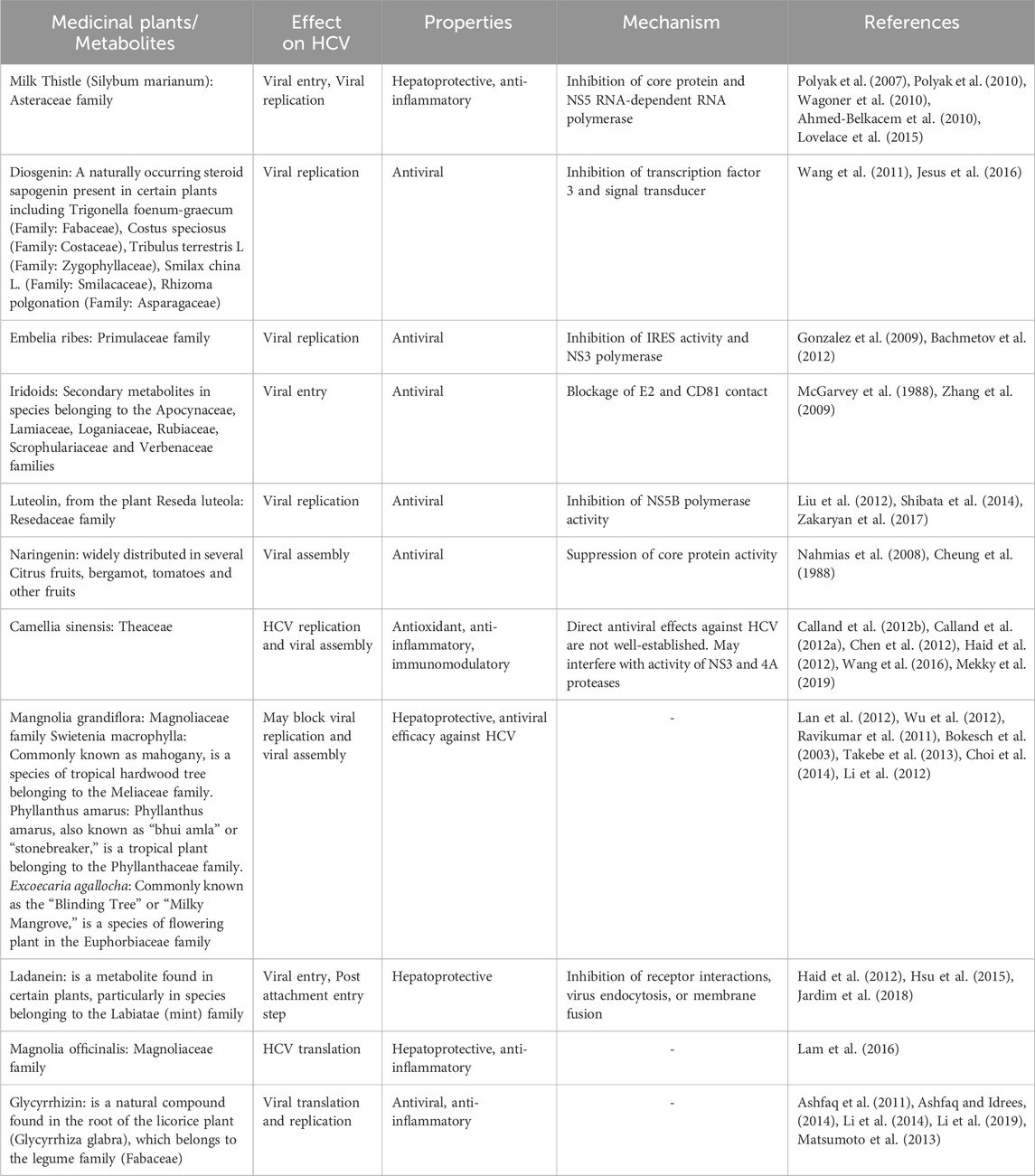
TABLE 1. Compilation of medicinal plants and their metabolites, along with potential mechanisms of action against HCV.
We previously found that a polyherbal formulation comprising five medicinal plants, including Silymarin, showed comparable results to interferon and ribavirin therapy in reducing viral loads in patients with HCV (Nawaz et al., 2015; Nawaz et al., 2016). In addition, polyherbal formulation has been found to have minimal or no side effects and was well-tolerated by patients, while also contributing to an improvement in quality of life. A recent cross-sectional study conducted on patients with HCV infection showed that herbal medicines are safe and effective against HCV (Nsibirwa et al., 2020). Complementary and alternative medicine therapies (CAM) may offer potential benefits in alleviating the chronic liver disease associated with HCV, even though they may not directly inhibit or eliminate the viral infection itself. Some CAM therapies have shown biological effects, including antioxidant, anti-fibrotic, or immune-modulating activities, which could contribute to the amelioration of the disease (Ferenci et al., 1989). Here are some host factors targeted by CAM therapies that have been studied in the context of viral infections: 1) Immune system modulation: CAM therapies such as herbal supplements, vitamins, and minerals are often used with the aim of modulating the immune system. For example, certain herbs like echinacea (Asteraceae family) and astragalus (Fabaceae family) are believed to have immune-enhancing properties. 2) Some CAM approaches involve the use of herbs and supplements with purported antiviral effects. Examples include elderberry, garlic, and licorice root, which have been studied for their potential to inhibit viral replication. 3) Stress can negatively impact the immune system, making the body more susceptible to infections. Mind-body practices such as meditation, yoga, and acupuncture, which fall under the CAM umbrella, aim to reduce stress and promote overall wellbeing. 4) CAM often emphasizes the importance of a balanced and nutrient-rich diet to support overall health. Nutritional interventions, including dietary supplements and specific diets, may be recommended to enhance the host’s nutritional status, potentially supporting the immune response. 5) Probiotics, considered as CAM intervention, focus on supporting a healthy balance of gut bacteria. Since the gut microbiome plays a role in immune function, some CAM practitioners recommend probiotics to enhance the body’s defense mechanisms. It is important to note that the scientific evidence supporting these interventions can vary, and further research is often needed for validation.
In China, a combination of seven botanical drugs known as Sho-Sai-Ko or xiao-chai-hu-lang has been used to treat hepatitis C, leading to improvements in liver pathogenicity among selected hepatitis C patients who were not suitable candidates for interferon-based treatments (Deng et al., 2011). Hence, alternative herbal therapy presents a promising option for the treatment of hepatitis C (Nawaz et al., 2015). In Egypt, a year-long randomized double-blind trial was conducted with Silymarin on patients diagnosed with HCV. Despite the safe administration of Silymarin throughout the trial period, the study yielded discouraging outcomes with no significant improvements observed in terms of HCV viral load and serum ALT levels (Tanamly et al., 2004). On the contrary, Hepcinal, a formulation comprising Silymarin alongside four other drugs, exhibited enhanced clinical, biochemical, and serological responses (Nawaz et al., 2016). Shifting our focus to herbal treatments, we delve into their historical roots and significance in HCV management. Prominent herbs such as licorice root (Glycyrrhiza glabra, Fabaceae family), and curcumin (Curcuma longa L. (turmeric) of ginger family (Zingiberaceae)) have been subject to scientific scrutiny for their potential hepatoprotective and antiviral properties. Metabolites including flavonoids, alkaloids, and polyphenols present in Phyllanthus niruri L. (Phyllanthaceae family), Glycyrrhiza glabra (licorice), and Silybum marianum L.) Gaertn (milk thistle) (Asteraceae family) have shown potential in inhibiting viral replication. Many medicinal plants including Schisandra chinensis (Schisandraceae family) and Picrorhiza kurroa (Plantaginaceae family), possess hepatoprotective properties, protecting liver from further damage. Some herbs, such as Curcuma longa (turmeric) (Zingiberaceae family), exhibit anti-inflammatory property that may help to mitigate the immune response and reduce liver inflammation. Overall, botanical drug(s), individually or in combination, are effective against HCV infection, blocking viral entry, translation, replication, and assembly. Furthermore, we acknowledge the challenges posed by the lack of standardization and quality control in herbal products. However, it is crucial to emphasize the need for further pharmacological investigations and large-scale clinical trials to validate the clinical safety and efficacy of these botanical drugs. We have summarized the effectiveness of selected botanical drugs and their ability to inhibit HCV activity in Figures 2A,B. In summary, while some botanical drugs may offer potential benefits for liver health, their use for HCV treatment should be approached with caution. Consultation with a healthcare provider and adherence to conventional medical treatments are essential for managing HCV effectively. As far as our current knowledge extends, there are no large-scale cross-sectional studies available that comprehensively demonstrate the clinical and serological outcomes of botanical drugs in treating HCV. However, in smaller-scale studies, researchers have documented the potential benefits of medicinal plants in combating HCV infection and have elucidated some of the molecular mechanisms involved. Additional research is needed to further understand the mechanisms underlying these improvements resulting from herbal treatments, as well as to provide additional evidence regarding their effectiveness and safety.
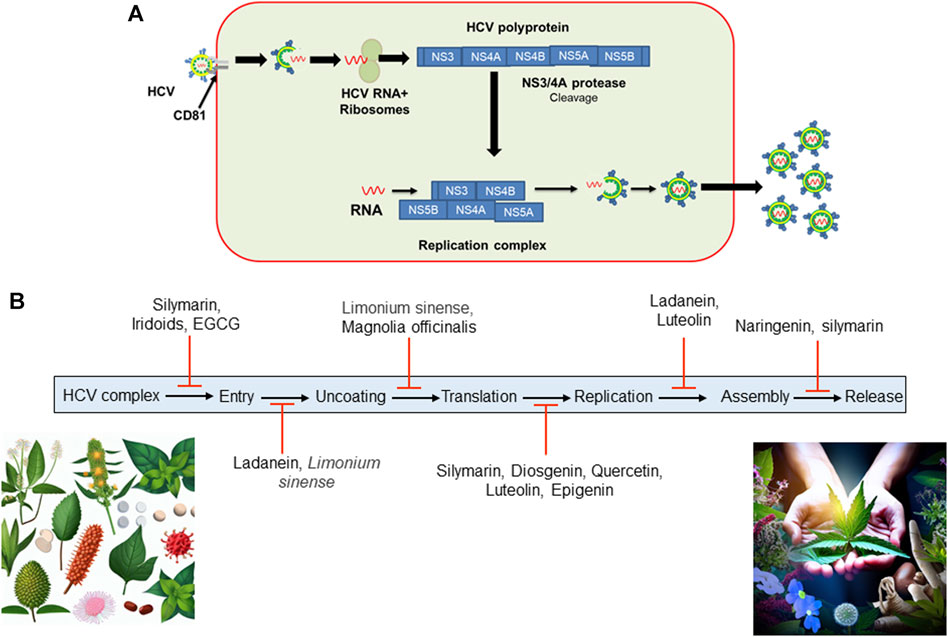
FIGURE 2. (A) HCV entry into hepatocytes. HCV complex enters into hepatocytes and uses a CD81 B-cell as a reservoir. After entry and fusion, the viral genome is released into the cytosol, accompanied by translation and replication. Once replication is complete, HCV assembles a new viral coat and is released from the host cell to infect other cells. (B) Certain botanical drugs have been documented to impede HCV activity by disrupting its replication cycle at various stages. (EGCG, Epigallocatechin-3-gallate found in green tea extract; HCV, Hepatitis C Virus).
The emergence of new therapeutic approaches holds promise for curing a greater number of HCV patients. The availability of potent natural or botanical drugs for HCV infection is a positive development. Consequently, there should be a heightened focus on the screening and identification of potent medicinal plants for the management and treatment of both acute and chronic HCV infections. This exploration of botanical drugs may lead to more effective and accessible treatments for individuals affected by HCV.
AN: Conceptualization, Supervision, Writing–original draft, Writing–review and editing. AM: Writing–review and editing. SeA: Writing–original draft. NA: Writing–original draft. WA: Writing–review and editing. MAM: Writing–review and editing. MB: Writing–review and editing. AS: Writing–review and editing. SlA: Writing–review and editing. IJ: Writing–original draft. TN: Reviewing final draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article from Japan Foundation for Applied Enzymology (a grant for Front Runner of Future Diabetes Research (to AN). This work was supported by the Young Research Grant from the Japan Diabetes Society (to AN) and Cell Science Foundation (to AN).
We would like to thank Editage (www.editage.com) for English language editing. The authors extend their appreciation to the all members of First Department of Internal Medicine and Department of Molecular and Medical Pharmacology, University of Toyama, Toyama, Japan.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahmed-Belkacem, A., Ahnou, N., Barbotte, L., Wychowski, C., Pallier, C., Brillet, R., et al. (2010). Silibinin and related compounds are direct inhibitors of hepatitis C virus RNA-dependent RNA polymerase. Gastroenterology 138, 1112–1122. doi:10.1053/j.gastro.2009.11.053
Alharthi, J., Latchoumanin, O., George, J., and Eslam, M. (2020). Macrophages in metabolic associated fatty liver disease. World J. Gastroenterol. 26, 1861–1878. doi:10.3748/wjg.v26.i16.1861
Ashfaq, U. A., and Idrees, S. (2014). Medicinal plants against hepatitis C virus. World J. Gastroenterol. 20, 2941–2947. doi:10.3748/wjg.v20.i11.2941
Ashfaq, U. A., Masoud, M. S., Nawaz, Z., and Riazuddin, S. (2011). Glycyrrhizin as antiviral agent against hepatitis C virus. J. Transl. Med. 9, 112. doi:10.1186/1479-5876-9-112
Asselah, T., Marcellin, P., and Schinazi, R. F. (2018). Treatment of hepatitis C virus infection with direct-acting antiviral agents: 100% cure?. Liver Int. 38 (1), 7–13. doi:10.1111/liv.13673
Bachmetov, L., Gal-Tanamy, M., Shapira, A., Vorobeychik, M., Giterman-Galam, T., Sathiyamoorthy, P., et al. (2012). Suppression of hepatitis C virus by the flavonoid quercetin is mediated by inhibition of NS3 protease activity. J. Viral Hepat. 19, e81–e88. doi:10.1111/j.1365-2893.2011.01507.x
Bodenheimer, H. C., Lindsay, K. L., Davis, G. L., Lewis, J. H., Thung, S. N., and Seeff, L. B. (1997). Tolerance and efficacy of oral ribavirin treatment of chronic hepatitis C: a multicenter trial. Hepatology 26, 473–477. doi:10.1002/hep.510260231
Bokesch, H. R., O'Keefe, B. R., Mckee, T. C., Pannell, L. K., Patterson, G. M., Gardella, R. S., et al. (2003). A potent novel anti-HIV protein from the cultured cyanobacterium Scytonema varium. Biochemistry 42, 2578–2584. doi:10.1021/bi0205698
Calland, N., Albecka, A., Belouzard, S., Wychowski, C., Duverlie, G., Descamps, V., et al. (2012a). (-)-Epigallocatechin-3-gallate is a new inhibitor of hepatitis C virus entry. Hepatology 55, 720–729. doi:10.1002/hep.24803
Calland, N., Dubuisson, J., Rouillé, Y., and Séron, K. (2012b). Hepatitis C virus and natural compounds: a new antiviral approach? Viruses 4, 2197–2217. doi:10.3390/v4102197
Chen, C., Qiu, H., Gong, J., Liu, Q., Xiao, H., Chen, X. W., et al. (2012). (-)-Epigallocatechin-3-gallate inhibits the replication cycle of hepatitis C virus. Arch. Virol. 157, 1301–1312. doi:10.1007/s00705-012-1304-0
Cheung, R., Dickins, J., Nicholson, P. W., Thomas, A. S., Smith, H. H., Larson, H. E., et al. (1988). Compliance with anti-tuberculous therapy: a field trial of a pill-box with a concealed electronic recording device. Eur. J. Clin. Pharmacol. 35, 401–407. doi:10.1007/BF00561372
Chia, C. W., Egan, J. M., and Ferrucci, L. (2018). Age-related changes in glucose metabolism, hyperglycemia, and cardiovascular risk. Circulation Res. 123, 886–904. doi:10.1161/CIRCRESAHA.118.312806
Choi, M., Kim, Y. M., Lee, S., Chin, Y. W., and Lee, C. (2014). Mangosteen xanthones suppress hepatitis C virus genome replication. Virus Genes 49, 208–222. doi:10.1007/s11262-014-1098-0
Christensen, S., Buggisch, P., Mauss, S., Böker, K. H. W., Schott, E., Klinker, H., et al. (2018). Direct-acting antiviral treatment of chronic HCV-infected patients on opioid substitution therapy: still a concern in clinical practice? Addiction 113, 868–882. doi:10.1111/add.14128
Davis, G. L., Esteban-Mur, R., Rustgi, V., Hoefs, J., Gordon, S. C., Trepo, C., et al. (1998). Interferon alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. International Hepatitis Interventional Therapy Group. N. Engl. J. Med. 339, 1493–1499. doi:10.1056/NEJM199811193392102
Deng, G., Kurtz, R. C., Vickers, A., Lau, N., Yeung, K. S., Shia, J., et al. (2011). A single arm phase II study of a Far-Eastern traditional herbal formulation (sho-sai-ko-to or xiao-chai-hu-tang) in chronic hepatitis C patients. J. Ethnopharmacol. 136, 83–87. doi:10.1016/j.jep.2011.04.008
Drazilova, S., Gazda, J., Janicko, M., and Jarcuska, P. (2018). Chronic hepatitis C association with diabetes mellitus and cardiovascular risk in the era of DAA therapy. Can. J. Gastroenterology Hepatology 2018, 6150861. doi:10.1155/2018/6150861
Farrell, G. C., Bacon, B. R., and Goldin, R. D. (1998). Lymphoblastoid interferon alfa-n1 improves the long-term response to a 6-month course of treatment in chronic hepatitis C compared with recombinant interferon alfa-2b: results of an international randomized controlled trial. Clinical Advisory Group for the Hepatitis C Comparative Study. Hepatology 27, 1121–1127. doi:10.1002/hep.510270429
Ferenci, P., Dragosics, B., Dittrich, H., Frank, H., Benda, L., Lochs, H., et al. (1989). Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J. Hepatol. 9, 105–113. doi:10.1016/0168-8278(89)90083-4
Fujisaka, S., Usui, I., Bukhari, A., Ikutani, M., Oya, T., Kanatani, Y., et al. (2009). Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes 58, 2574–2582. doi:10.2337/db08-1475
Fujisaka, S., Usui, I., Ikutani, M., Aminuddin, A., Takikawa, A., Tsuneyama, K., et al. (2013). Adipose tissue hypoxia induces inflammatory M1 polarity of macrophages in an HIF-1α-dependent and HIF-1α-independent manner in obese mice. Diabetologia 56, 1403–1412. doi:10.1007/s00125-013-2885-1
Fujisaka, S., Usui, I., Nawaz, A., Takikawa, A., Kado, T., Igarashi, Y., et al. (2016). M2 macrophages in metabolism. Diabetol. Int. 7, 342–351. doi:10.1007/s13340-016-0290-y
Gonzalez, O., Fontanes, V., Raychaudhuri, S., Loo, R., Loo, J., Arumugaswami, V., et al. (2009). The heat shock protein inhibitor Quercetin attenuates hepatitis C virus production. Hepatology 50, 1756–1764. doi:10.1002/hep.23232
Haid, S., Novodomská, A., Gentzsch, J., Grethe, C., Geuenich, S., Bankwitz, D., et al. (2012). A plant-derived flavonoid inhibits entry of all HCV genotypes into human hepatocytes. Gastroenterology 143, 213–222. doi:10.1053/j.gastro.2012.03.036
Hoofnagle, J. H., Mullen, K. D., Jones, D. B., Rustgi, V., Di Bisceglie, A., Peters, M., et al. (1986). Treatment of chronic non-A,non-B hepatitis with recombinant human alpha interferon. A preliminary report. N. Engl. J. Med. 315, 1575–1578. doi:10.1056/NEJM198612183152503
Hsu, W.-C., Chang, S.-P., Lin, L.-C., Li, C.-L., Richardson, C. D., Lin, C.-C., et al. (2015). Limonium sinense and gallic acid suppress hepatitis C virus infection by blocking early viral entry. Antivir. Res. 118, 139–147. doi:10.1016/j.antiviral.2015.04.003
Ito, M., Kusunoki, H., and Mizuochi, T. (2011). Peripheral B cells as reservoirs for persistent HCV infection. Front. Microbiol. 2, 177. doi:10.3389/fmicb.2011.00177
Jacobson, I. M., Cacoub, P., Dal Maso, L., Harrison, S. A., and Younossi, Z. M. (2010). Manifestations of chronic hepatitis C virus infection beyond the liver. Clin. Gastroenterol. Hepatol. 8, 1017–1029. doi:10.1016/j.cgh.2010.08.026
Jardim, A. C. G., Shimizu, J. F., Rahal, P., and Harris, M. (2018). Plant-derived antivirals against hepatitis c virus infection. Virology J. 15, 34. doi:10.1186/s12985-018-0945-3
Jesus, M., Martins, A. P., Gallardo, E., and Silvestre, S. (2016). Diosgenin: recent highlights on Pharmacology and analytical methodology. J. Anal. Methods Chem. 2016, 4156293. doi:10.1155/2016/4156293
Kazankov, K., Jørgensen, S. M. D., Thomsen, K. L., Møller, H. J., Vilstrup, H., George, J., et al. (2019). The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat. Rev. Gastroenterology Hepatology 16, 145–159. doi:10.1038/s41575-018-0082-x
Lam, P., Cheung, F., Tan, H. Y., Wang, N., Yuen, M. F., and Feng, Y. (2016). Hepatoprotective effects of Chinese medicinal herbs: a focus on anti-inflammatory and anti-oxidative activities. Int. J. Mol. Sci. 17, 465. doi:10.3390/ijms17040465
Lan, K. H., Wang, Y. W., Lee, W. P., Lan, K. L., Tseng, S. H., Hung, L. R., et al. (2012). Multiple effects of Honokiol on the life cycle of hepatitis C virus. Liver Int. 32, 989–997. doi:10.1111/j.1478-3231.2011.02621.x
Li, J. Y., Cao, H. Y., Liu, P., Cheng, G. H., and Sun, M. Y. (2014). Glycyrrhizic acid in the treatment of liver diseases: literature review. Biomed. Res. Int. 2014, 872139. doi:10.1155/2014/872139
Liu, M. M., Zhou, L., He, P. L., Zhang, Y. N., Zhou, J. Y., Shen, Q., et al. (2012). Discovery of flavonoid derivatives as anti-HCV agents via pharmacophore search combining molecular docking strategy. Eur. J. Med. Chem. 52, 33–43. doi:10.1016/j.ejmech.2012.03.002
Li, X., Sun, R., and Liu, R. (2019). Natural products in licorice for the therapy of liver diseases: progress and future opportunities. Pharmacol. Res. 144, 210–226. doi:10.1016/j.phrs.2019.04.025
Li, Y., Yu, S., Liu, D., Proksch, P., and Lin, W. (2012). Inhibitory effects of polyphenols toward HCV from the mangrove plant Excoecaria agallocha L. Bioorg Med. Chem. Lett. 22, 1099–1102. doi:10.1016/j.bmcl.2011.11.109
Lovelace, E. S., Wagoner, J., Macdonald, J., Bammler, T., Bruckner, J., Brownell, J., et al. (2015). Silymarin suppresses cellular inflammation by inducing reparative stress signaling. J. Nat. Prod. 78, 1990–2000. doi:10.1021/acs.jnatprod.5b00288
Luo, H., Mu, W. C., Karki, R., Chiang, H. H., Mohrin, M., Shin, J. J., et al. (2019). Mitochondrial stress-initiated aberrant activation of the NLRP3 inflammasome regulates the functional deterioration of hematopoietic stem cell aging. Cell Rep. 26, 945–954. doi:10.1016/j.celrep.2018.12.101
Matsumoto, Y., Matsuura, T., Aoyagi, H., Matsuda, M., Hmwe, S. S., Date, T., et al. (2013). Antiviral activity of glycyrrhizin against hepatitis C virus in vitro. PLoS One 8, e68992. doi:10.1371/journal.pone.0068992
Mcgarvey, P., Helling, R. B., Lee, J. Y., Engelke, D. R., and El-Gewely, M. R. (1988). Initiation of rrn transcription in chloroplasts of Euglena gracilis bacillaris. Curr. Genet. 14, 493–500. doi:10.1007/BF00521275
Mchutchison, J. G., Gordon, S. C., Schiff, E. R., Shiffman, M. L., Lee, W. M., Rustgi, V. K., et al. (1998). Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N. Engl. J. Med. 339, 1485–1492. doi:10.1056/NEJM199811193392101
Mekky, R. Y., El-Ekiaby, N., El Sobky, S. A., Elemam, N. M., Youness, R. A., El-Sayed, M., et al. (2019). Epigallocatechin gallate (EGCG) and miR-548m reduce HCV entry through repression of CD81 receptor in HCV cell models. Arch. Virol. 164, 1587–1595. doi:10.1007/s00705-019-04232-x
Moustafa, H., Madkour, M., Hamed, F., Abouelnazar, S., Abo Elwafa, R., and Moaaz, M. (2020). Modulation of memory B cell phenotypes and toll-like receptor-7 in chronic hepatitis C virus infection during direct-acting antiviral interferon-free therapy: correlation with interleukin-7. Viral Immunol. 34, 227–240. doi:10.1089/vim.2020.0110
Nahmias, Y., Goldwasser, J., Casali, M., Van Poll, D., Wakita, T., Chung, R. T., et al. (2008). Apolipoprotein B-dependent hepatitis C virus secretion is inhibited by the grapefruit flavonoid naringenin. Hepatology 47, 1437–1445. doi:10.1002/hep.22197
Nainan, O. V., Alter, M. J., Kruszon-Moran, D., Gao, F.-X., Xia, G., Mcquillan, G., et al. (2006). Hepatitis C virus genotypes and viral concentrations in participants of a general population survey in the United States. Gastroenterology 131, 478–484. doi:10.1053/j.gastro.2006.06.007
Nawaz, A., Aminuddin, A., Kado, T., Takikawa, A., Yamamoto, S., Tsuneyama, K., et al. (2017a). CD206+ M2-like macrophages regulate systemic glucose metabolism by inhibiting proliferation of adipocyte progenitors. Nat. Commun. 8, 286. doi:10.1038/s41467-017-00231-1
Nawaz, A., Kado, T., Igarashi, Y., Kunimasa, Y., Usui, I., Fujisaka, S., et al. (2017b). Adipose tissue-resident macrophages and obesity. RADS J. Pharm. Pharm. Sci. 5, 3. (2017): Contents of Volume 5(3) July-September 2017.
Nawaz, A., Nazar, H., Usmanghani, K., Sheikh, Z. A., Chishti, M. A., and Ahmad, I. (2016). Ways to manage hepatitis C without cirrhosis: treatment by comparison of coded eastern medicine hepcinal with interferon alpha 2b and ribavirin. Pak J. Pharm. Sci. 29, 919–927.
Nawaz, A., Zaidi, S. F., Usmanghani, K., and Ahmad, I. (2015). Concise review on the insight of hepatitis C. J. Taibah Univ. Med. Sci. 10, 132–139. doi:10.1016/j.jtumed.2014.08.004
Nsibirwa, S., Anguzu, G., Kamukama, S., Ocama, P., and Nankya-Mutyoba, J. (2020). Herbal medicine use among patients with viral and non-viral Hepatitis in Uganda: prevalence, patterns and related factors. BMC Complementary Med. Ther. 20, 169. doi:10.1186/s12906-020-02959-8
Polyak, S. J., Morishima, C., Lohmann, V., Pal, S., Lee, D. Y., Liu, Y., et al. (2010). Identification of hepatoprotective flavonolignans from silymarin. Proc. Natl. Acad. Sci. U. S. A. 107, 5995–5999. doi:10.1073/pnas.0914009107
Polyak, S. J., Morishima, C., Shuhart, M. C., Wang, C. C., Liu, Y., and Lee, D. Y. (2007). Inhibition of T-cell inflammatory cytokines, hepatocyte NF-kappaB signaling, and HCV infection by standardized Silymarin. Gastroenterology 132, 1925–1936. doi:10.1053/j.gastro.2007.02.038
Ravikumar, Y. S., Ray, U., Nandhitha, M., Perween, A., Raja Naika, H., Khanna, N., et al. (2011). Inhibition of hepatitis C virus replication by herbal extract: Phyllanthus amarus as potent natural source. Virus Res. 158, 89–97. doi:10.1016/j.virusres.2011.03.014
Reidy, P. T., Mckenzie, A. I., Mahmassani, Z. S., Petrocelli, J. J., Nelson, D. B., Lindsay, C. C., et al. (2019). Aging impairs mouse skeletal muscle macrophage polarization and muscle-specific abundance during recovery from disuse. Am. J. Physiology-Endocrinology Metabolism 317, E85–E98. doi:10.1152/ajpendo.00422.2018
Sakai, M., Troutman, T. D., Seidman, J. S., Ouyang, Z., Spann, N. J., Abe, Y., et al. (2019). Liver-derived signals sequentially reprogram myeloid enhancers to initiate and maintain kupffer cell identity. Immunity 51, 655–670. doi:10.1016/j.immuni.2019.09.002
Shibata, C., Ohno, M., Otsuka, M., Kishikawa, T., Goto, K., Muroyama, R., et al. (2014). The flavonoid apigenin inhibits hepatitis C virus replication by decreasing mature microRNA122 levels. Virology 462-463, 42–48. doi:10.1016/j.virol.2014.05.024
Singh, G. K., and Hoyert, D. L. (2000). Social epidemiology of chronic liver disease and cirrhosis mortality in the United States, 1935-1997: trends and differentials by ethnicity, socioeconomic status, and alcohol consumption. Hum. Biol. 72, 801–820.
Stanaway, J. D., Flaxman, A. D., Naghavi, M., Fitzmaurice, C., Vos, T., Abubakar, I., et al. (2016). The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet 388, 1081–1088. doi:10.1016/S0140-6736(16)30579-7
Takebe, Y., Saucedo, C. J., Lund, G., Uenishi, R., Hase, S., Tsuchiura, T., et al. (2013). Antiviral lectins from red and blue-green algae show potent in vitro and in vivo activity against hepatitis C virus. PLoS One 8, e64449. doi:10.1371/journal.pone.0064449
Takikawa, A., Usui, I., Fujisaka, S., Tsuneyama, K., Okabe, K., Nakagawa, T., et al. (2019). Macrophage-specific hypoxia-inducible factor-1α deletion suppresses the development of liver tumors in high-fat diet-fed obese and diabetic mice. J. Diabetes Investig. 10, 1411–1418. doi:10.1111/jdi.13047
Tanamly, M. D., Tadros, F., Labeeb, S., Makld, H., Shehata, M., Mikhail, N., et al. (2004). Randomised double-blinded trial evaluating silymarin for chronic hepatitis C in an Egyptian village: study description and 12-month results. Dig. Liver Dis. 36, 752–759. doi:10.1016/j.dld.2004.06.015
Tong, M. J., Blatt, L. M., Mchutchison, J. G., Co, R. L., and Conrad, A. (1997). Prediction of response during interferon alfa 2b therapy in chronic hepatitis C patients using viral and biochemical characteristics: a comparison. Hepatology 26, 1640–1645. doi:10.1002/hep.510260637
Vanni, E., Bugianesi, E., and Saracco, G. (2016). Treatment of type 2 diabetes mellitus by viral eradication in chronic hepatitis C: myth or reality? Dig. Liver Dis. 48, 105–111. doi:10.1016/j.dld.2015.10.016
Wagoner, J., Negash, A., Kane, O. J., Martinez, L. E., Nahmias, Y., Bourne, N., et al. (2010). Multiple effects of silymarin on the hepatitis C virus lifecycle. Hepatology 51, 1912–1921. doi:10.1002/hep.23587
Wang, Y. J., Pan, K. L., Hsieh, T. C., Chang, T. Y., Lin, W. H., and Hsu, J. T. (2011). Diosgenin, a plant-derived sapogenin, exhibits antiviral activity in vitro against hepatitis C virus. J. Nat. Prod. 74, 580–584. doi:10.1021/np100578u
Wang, Y., Li, J., Wang, X., Peña, J. C., Li, K., Zhang, T., et al. (2016). (-)-Epigallocatechin-3-Gallate enhances hepatitis C virus double-stranded RNA intermediates-triggered innate immune responses in hepatocytes. Sci. Rep. 6, 21595. doi:10.1038/srep21595
WHO (2022). Hepatitis C (2021). Available online at: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (Accessed May 7, 2022).
Wu, S. F., Lin, C. K., Chuang, Y. S., Chang, F. R., Tseng, C. K., Wu, Y. C., et al. (2012). Anti-hepatitis C virus activity of 3-hydroxy caruilignan C from Swietenia macrophylla stems. J. Viral Hepat. 19, 364–370. doi:10.1111/j.1365-2893.2011.01558.x
Zakaryan, H., Arabyan, E., Oo, A., and Zandi, K. (2017). Flavonoids: promising natural compounds against viral infections. Archives Virology 162, 2539–2551. doi:10.1007/s00705-017-3417-y
Zhang, H., Rothwangl, K., Mesecar, A. D., Sabahi, A., Rong, L., and Fong, H. H. (2009). Lamiridosins, hepatitis C virus entry inhibitors from Lamium album. J. Nat. Prod. 72, 2158–2162. doi:10.1021/np900549e
Keywords: hepatitis C virus, direct-acting antiviral agents (DAAs), botanical drugs, hepatitis C, hepatoprotective and antiviral properties of medicinal plants
Citation: Nawaz A, Manzoor A, Ahmed S, Ahmed N, Abbas W, Mir MA, Bilal M, Sheikh A, Ahmad S, Jeelani I and Nakagawa T (2024) Therapeutic approaches for chronic hepatitis C: a concise review. Front. Pharmacol. 14:1334160. doi: 10.3389/fphar.2023.1334160
Received: 06 November 2023; Accepted: 18 December 2023;
Published: 12 January 2024.
Edited by:
Rongrui Wei, Jiangxi University of Traditional Chinese Medicine, ChinaReviewed by:
Sidra Rehman, COMSATS University, Islamabad Campus, PakistanCopyright © 2024 Nawaz, Manzoor, Ahmed, Ahmed, Abbas, Mir, Bilal, Sheikh, Ahmad, Jeelani and Nakagawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Allah Nawaz, YWxsYWgubmF3YXpAam9zbGluLmhhcnZhcmQuZWR1; Ishtiaq Jeelani, aWplZWxhbmlAdWNzZC5lZHU=
†Present address: Allah Nawaz, Joslin Diabetes Center, Harvard Medical School, Harvard University, Boston, MA, United States
Ishtiaq Jeelani, Department of Medicine, Division of Endocrinology and Metabolism, University of California, San Diego, CA, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.