
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 06 December 2023
Sec. Respiratory Pharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1330518
This article is part of the Research TopicTreatment Strategies for Lung Cancer: A Progress Update and Future PerspectivesView all 6 articles
Lung cancer stands as one of the most prevalent malignancies worldwide, bearing the highest morbidity and mortality rates among all malignant tumors. The treatment of lung cancer primarily encompasses surgical procedures, radiotherapy, and chemotherapy, which are fraught with significant side effects, unfavorable prognoses, and a heightened risk of metastasis and relapse. Although targeted therapy and immunotherapy have gradually gained prominence in lung cancer treatment, diversifying the array of available methods, the overall recovery and survival rates for lung cancer patients remain suboptimal. Presently, with a holistic approach and a focus on syndrome differentiation and treatment, Traditional Chinese Medicine (TCM) has emerged as a pivotal player in the prognosis of cancer patients. TCM possesses characteristics such as targeting multiple aspects, addressing a wide range of concerns, and minimizing toxic side effects. Research demonstrates that Traditional Chinese Medicine can significantly contribute to the treatment or serve as an adjunct to chemotherapy for lung cancer and other lung-related diseases. This is achieved through mechanisms like inhibiting tumor cell proliferation, inducing tumor cell apoptosis, suppressing tumor angiogenesis, influencing the cellular microenvironment, regulating immune system function, impacting signal transduction pathways, and reversing multidrug resistance in tumor cells. In this article, we offer an overview of the advancements in research concerning Traditional Chinese Medicine extracts for the treatment or adjunctive chemotherapy of lung cancer and other lung-related conditions. Furthermore, we delve into the challenges that Traditional Chinese Medicine extracts face in lung cancer treatment, laying the foundation for the development of diagnostic, prognostic, and therapeutic targets.
Primary bronchogenic lung cancer, commonly referred to as lung cancer, represents the most prevalent form of malignant tumor. It encompasses two primary subtypes: small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), with NSCLC constituting a majority, accounting for 85%–90% of cases (Goldstraw et al., 2007). As per data from the 2020 World Cancer Report (GLOBOCAN), the global incidence of lung cancer surged to 2.2 million new cases, contributing to 11.4% of all newly reported cancer cases. Alarmingly, the number of fatalities reached 1.8 million, amounting to 18.0% of total cancer-related deaths. Both the incidence and mortality of this malignancy have consistently held the top position among malignant tumors, displaying a worrisome upward trajectory year after year (Li et al., 2023). Despite the armamentarium of Western medical interventions that encompass comprehensive approaches such as surgery, chemotherapy, radiation therapy, and molecular targeting, a staggering 80% of lung cancer patients already find themselves in the intermediate to advanced stages of the disease. Consequently, their 5-year survival rate remains dishearteningly low, at a mere 14%. Furthermore, these therapies are accompanied by significant side effects. A cascade of common toxic reactions during the chemotherapy process, including anemia, neutropenia, mucositis, or isopermeability, and special toxicity such as ototoxicity, nephrotoxicity, pulmonary toxicity, neurotoxicity, or Raynaud’s syndrome (Zraik and Heß-Busch, 2021). Radiotherapy also bears noticeable side effects, such as lung injury may cause radiation pneumonia, acute respiratory distress syndrome, bronchiolitis obliterans with mechanical pneumonia, and other diseases (Latrèche et al., 2022). While targeted therapy stands out for its precision and reduced side effects compared to other modalities, it is not without its challenges, notably the potential to cause hypertension, anemia, fever, and acute phase reactions (Tímár and Uhlyarik, 2022). With the deepening exploration of traditional Chinese medicine, multiple studies have unveiled its potential to profoundly enhance the quality of life for patients and extend their survival. In clinical applications, traditional Chinese medicine has demonstrated its capacity to inhibit and eliminate tumor cells, while also alleviating the adverse reactions associated with radiotherapy and chemotherapy.
Deregulated cell cycle progression stands as a pivotal catalyst for unbridled cell proliferation, ultimately culminating in the malignant transformation characteristic of cancer. A cornerstone in the quest to combat tumors lies in the inhibition of tumor cell proliferation. Traditional Chinese medicine extracts have emerged as a promising avenue for regulating the cell cycle by modulating the expression of genes integral to tumor proliferation, including cyclin, P53, PCNA, and reactive oxygen species, among others. For instance, a study by Zhu et al. (Zhu et al., 2021) unveiled the potential of licorice extract to curtail the growth of NSCLC. This effect was achieved by downregulating the expression of the CDK4-Cyclin complex, thus obstructing the G0/G1 phase progression in tumor cells. Furthermore, the compound resibufogenin, extracted from Venenum Bufonis, was found to activate GSK-3β in human lung cancer A549 cells, leading to the downregulation of cyclin D1 expression and instigating a G1 phase blockade (Ichikawa et al., 2015). Homoisoflavanone-1, isolated from polygonatum odoratum, exhibited notable prowess in inhibiting NSCLC growth and inducing apoptosis in a dose-dependent manner. This compound primarily arrested the cell cycle in the G2/M phase by activating the p38/p53 signaling pathway. Moreover, it spurred apoptosis in A549 cells through the regulation of mitochondrial/cysteine protease dependence and endoplasmic reticulum stress pathways (Ning et al., 2018). Salvia miltiorrhiza, specifically its methanol extract, tanshinone (CTN), demonstrated the capacity to induce cell cycle arrest in the G2/M phase. This effect was accompanied by an elevation in the expression of p53 and p21, as well as the activation of caspase-3/9 and PARP1. The net result was a pronounced inhibition of cell proliferation and the facilitation of early and late tumor cell apoptosis (Ye et al., 2017). Another extract from Salvia miltiorrhiza, tanshinone IIA, exhibited the ability to induce cell cycle arrest in the G1/S phase, impeding the progression of lung adenocarcinoma. This was achieved through the substantial downregulation of CCNA2, CDK2, AURKA, PLK1, and p-ERK expression (Li et al., 2021). Dioscin, sourced from Rhizoma Dioscoreae Nipponicae, wielded the power to significantly curtail the expression of p-AKT, MMP2, and PCNA, thereby inhibiting the in vitro proliferation, invasion, and migration of lung cancer cells. Additionally, it manifested the capability to reduce lung nodules, mitigate lung injury, and diminish mortality in mouse lung cancer models (Xi et al., 2022). The aqueous extract derived from Hedyotis diffusa, combined with Scutellaria barbata in an equal weight ratio (HDSB11), effectively diminished the expression of NLRP3, procapsase-1, caspase-1, PRAP, Bcl-2, and cyclin D1. This was accompanied by a reduction in the phosphorylation levels of NF-κB, ERK, JNK, and p38 MAPK, resulting in the inhibition of tumor cell proliferation (Lv et al., 2021). Hence, the multitude of traditional Chinese medicine monomers and extracts offer promise in the inhibition of tumor cell proliferation, cell cycle regulation, and the effective treatment of NSCLC.
Apoptosis, known as programmed cell death (PCD), is a meticulously orchestrated process of active cell demise initiated by shifts in the body’s internal and external environment or by death signals orchestrated through gene regulation. Tumors represent a complex spectrum of disorders characterized not only by abnormal cell proliferation and differentiation but also by irregular cell apoptosis. Recent research has unearthed compelling evidence regarding the impact of specific compounds on tumor cells. For instance, imperatorin derived from Angelica dahurica has been shown to modulate gene expression, upregulating p53 and Bax while simultaneously downregulating Mcl-1. This concerted action exerts a robust inhibitory effect on the autonomous growth of lung cancer cells (Choochuay et al., 2013). Ethyl acetate extract from Selaginella doederleinii Hieron intervenes in the cell cycle, effectively blocking the S-phase. This action is achieved by upregulating Bax, downregulating Bcl-2, and activating caspase-9 and caspase-3. The resultant cascade of events leads to the loss of mitochondrial membrane potential and the induction of cell apoptosis (Sui et al., 2016). The combined effects of Ligustrum lucidum and Epimedium are marked by a decrease in α-SMA and Ki-67 protein expression, LC3-II/LC3-I ratio, and Bcl-2/Bax protein ratio. Conversely, they result in an upregulation of caspase-3, p-mTOR, and Bax, all of which inhibit lung tissue cell proliferation and autophagy while promoting apoptosis within the lung tissue and BALF (Ma et al., 2020). Research by Huang et al. (Huang et al., 2022) emphasizes the potential of Hedyotis diffusa extract to regulate VDAC2/3 activity by promoting Bax and inhibiting Bcl-2. This dynamic interaction culminates in iron-induced apoptosis within lung adenocarcinoma cells. Poria cocos extract exerts cytotoxicity on human lung adenocarcinoma cells by elevating Bax expression and Bcl-2 phosphorylation levels. Additionally, it activates caspase-3 and induces apoptosis, an effect associated with mitochondrial perturbation (Lee et al., 2018). Moreover, Tetramethylpyrazine (TMP), as demonstrated by Jiang et al. (Jiang R. et al., 2021), diminishes the expression of NLRP3, curbing the formation of inflammatory complexes, thereby reducing caspase-1 activation, cell apoptosis, and inflammatory responses. Cinnamomum cassia extracts have a dose-dependent inhibition on NSCLC cell proliferation, both in vivo and in vitro. They also induce cell apoptosis (Wu et al., 2017). Polygonatum odoratum extract modulates miR-RNA, Akt-NF-κB, and Akt-mTOR, thereby stimulating apoptosis and autophagy in lung cancer (Wu et al., 2016). Polygonatum odoratum lectin was found to inhibit the Akt-NF-κB pathway to trigger cell apoptosis, simultaneously inducing autophagy by impeding the Akt-mTOR pathway (Li et al., 2014). Acetone extract of Bupleurum scorzonerifolium (AE-BS) exhibits a dose-dependent inhibitory impact on the proliferation of A549 human lung cancer cells. This is achieved through the inhibition of telomerase activity and the activation of cell apoptosis (Cheng et al., 2003). Pure compound dihydroisotanshinone I (DT) extracted from Salvia miltiorrhiza activates caspase-3/9 and PARP1, culminating in cell arrest in the G2/M phase (Wu et al., 2021). The mechanisms underlying traditional Chinese medicine-induced apoptosis in lung cancer cells predominantly involve the regulation of apoptosis-related genes (e.g., p53, C-myc, Bax, Bcl-2), modulation of intracellular Ca2+ concentrations and ACMP, activation of the caspase protease family, inhibition of telomerase activity, and other facets that ultimately induce cell apoptosis and achieve the desired therapeutic effect in NSCLC.
Tumor angiogenesis forms the cornerstone of tumor cell growth and metastasis, and the strategy for anti-tumor angiogenesis revolves around intervening in the regulatory factors of tumor vasculature and their functional connections. Among these factors, Vascular Endothelial Growth Factor (VEGF) and Cyclooxygenase-2 (COX-2) take center stage as pivotal vascular growth factors. Traditional Chinese medicine extracts have demonstrated their capability to hinder tumor growth and metastasis by effectively suppressing key vascular growth factors like VEGF and COX-2. For instance, the extract derived from Hedyotis diffusa exerts control over the Epithelial-Mesenchymal Transition (EMT) by inhibiting COX-2 protein expression. This action effectively curtails the metastatic potential of lung adenocarcinoma cells (Lin et al., 2019). In a separate study, Xie et al. (Xie et al., 2019) showcased the potential of DQA-modified paclitaxel plus ligustrazine micelles in downregulating the expression of VEGF, MMP-2, TGF-β1, and E-cadherin in lung cancer cells, thereby mitigating the metastatic tendencies of these cells. Furthermore, the extract of Ecliptae Herba has been found to significantly lower the levels of COX-2, TGF-β1, MMP-2, and α-SMA, while increasing the MMP-9/TIMP-1 ratio (You et al., 2015). This dual action serves to inhibit the formation of tumor blood vessels. Additionally, studies have revealed that the combination of Astragalus membranaceus and Angelica sinensis can reduce the expression of NF-κB, STAT3, HIF-1α, and VEGF, effectively impeding tumor growth in mice (Wu et al., 2019). Therefore, various traditional Chinese medicine monomers and extracts hold the potential to thwart tumor metastasis by intervening in tumor cell angiogenesis.
Malignant tumors encompass a complex interplay between tumor cells and non-tumor cell components, including fibroblasts, endothelial cells, lymphocytes, macrophages, and extracellular matrix. This ensemble is collectively referred to as the tumor microenvironment. The tumor microenvironment plays a pivotal role in the initiation and progression of tumors, and contemporary research endeavors are dedicated to unraveling the intricate mechanisms through which the tumor microenvironment regulates tumor development. In recent years, investigations have underscored the capacity of traditional Chinese medicine extracts to exert anti-tumor effects by influencing the tumor microenvironment.
Cell adhesion encompasses both homophilic adhesion between cancer cells and heterophilic adhesion between cancer cells and stromal cells. A reduction in the expression of specific adhesion molecules, such as E-cadherin, in tumor cells can lead to diminished homophilic cell adhesion. This, in turn, facilitates the detachment of tumor cells from the extracellular matrix. Epithelial-mesenchymal transition (EMT) denotes the transformation of epithelial cells into mesenchymal cells. It promotes cell metastasis and invasion, imbuing cells with stem cell characteristics, diminishing apoptosis and senescence, fostering immune evasion, and participating in physiological processes like tissue healing, organ fibrosis, and cancer initiation. Notably, during the EMT process, tumor cells develop resistance to chemotherapy and immunotherapy, allowing them to elude immune system surveillance. Metastasis stands as the primary factor contributing to the failure of lung cancer treatment, and inhibiting cell adhesion has emerged as a key focus of research within the realm of traditional Chinese medicine for curbing lung cancer metastasis. Xie et al. (Xie et al., 2019)observed that DQA-modified paclitaxel combined with ligustrazine micelles could downregulate the expression of VEGF, MMP2, TGF-β1, and E-cadherin in lung cancer cells, thus impeding lung cancer cell metastasis. Furthermore, research has revealed that the combination of Trichosanthin (TCS) and TRAIL can regulate the expression of apoptosis-related proteins (caspase 3/8 and PARP), endogenous apoptosis-related proteins (Bcl-2 and Bax), invasion-related proteins (E-cadherin, N-cadherin, Vimentin, ICAM-1, MMP-2, MMP-9), and cell cycle-related proteins (P27, CCNE1, and CDK2) (You et al., 2018). This orchestrated modulation leads to cell apoptosis and S-phase arrest, thereby suppressing cancer cell proliferation and invasion. Cinnamomum cassia extract (CCE) inhibits TGF-β1-induced motility and invasiveness of lung cancer cells by curtailing the adhesion of damaged cells to collagen (Lin et al., 2017). In the context of TGF-β1-induced EMT, dioscin, extracted from Rhizoma Dioscoreae Nipponicae, significantly heightens the expression of epithelial markers (E-cadherin) and reduces interstitial markers (N-cadherin and Snail). This serves to inhibit EMT, effectively restraining the in vitro migration and invasion of lung cancer cells (Lim et al., 2017). Paeonol inhibits TGF-β1-induced EMT while concurrently upregulating the expression of miR-126-5p in lung cancer cells. This dual action results in decreased cell viability, thereby inhibiting the survival and metastasis of human lung cancer cells (Lv et al., 2022).
The successful invasion and metastasis of tumor cells within the body hinge on their ability to breach the formidable barriers presented by the basement membrane (BM) and the extracellular matrix (ECM), thereby gaining entry into the circulatory system. The pivotal steps in the invasion and metastasis process involve tumor cells initially adhering to extracellular matrix components (such as laminin, fibronectin, and collagen) through membrane surface receptors. Subsequently, they execute the degradation of the basement membrane and matrix by secreting an array of proteolytic enzymes. Finally, they undergo directed migration through the compromised basement membrane and matrix, ultimately entering the circulatory system. The expression and activity of matrix metalloproteinases (MMPs) are intricately linked with tumor invasion and metastasis, influencing various phases of tumor development. It is now established that traditional Chinese medicine can modulate the balance between matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) in lung cancer tissue. This modulation leads to the inhibition of urokinase-type plasminogen activator (u-PA) and cathepsin B (CB) secretion, thereby preventing the degradation of the extracellular matrix by lung cancer cells. Dioscin, extracted from Rhizoma Dioscoreae Nipponicae, serves as a potent example of this action. It significantly diminishes the expression of p-AKT, MMP-2, and PCNA, effectively restraining the proliferation, invasion, and migration of A549 and PC-9 cells. This effect extends to a reduction in lung nodules, lung injury, and mortality in mouse lung cancer models (Xi et al., 2022). By inhibiting MMP-2 and u-PA, Cinnamomum cassia extract suppresses the extracellular matrix degradation by lung cancer cells, effectively mitigating TGF-β1-induced invasion and metastasis of lung cancer cells (Lin et al., 2017). Additionally, research by Gao et al. (Gao et al., 2012)underscores the potential of Angelica sinensis extract to reduce MMP-2, MMP-9, TGF-β1, and TIMP-1 expression while elevating TIMP-2 levels. This dual action inhibits the growth and metastasis of lung cancer cells. Sinomenine, extracted from Sinomenium acutum, is another promising compound that reduces the expression of MMP-2, MMP-9, and extracellular matrix metalloproteinase inducer (EMMPRIN/CD147), while concurrently increasing the expression of RECK, TIMP-1, and TIMP-2. This effectively impedes the migration and invasion of lung adenocarcinoma cells (Shen et al., 2020). Shen et al. (Shen et al., 2017) have also identified a similar anti-metastatic mechanism within the extract of Solanum nigrum. Furthermore, solasodine exhibits an ability to reduce the expression of MMP-2, MMP-9, and extracellular matrix metalloproteinase inducer (EMMPRIN) while elevating the expression of RECK, TIMP-1, and TIMP-2, thereby thwarting cell invasion. Through the utilization of gelatin zymography, which measures the activity of matrix metalloproteinases (MMP-2/-9) in NCI-H460 cells, it has been demonstrated that cantharidin inhibits MMP-2/-9 enzyme activity in NCI-H460 cells (Hsia et al., 2016). Hence, an array of traditional Chinese medicines and their extracts wield the potential to influence the trajectory of lung cancer through their impact on the tumor microenvironment.
Presently, research into the anti-tumor immune properties of active compounds within traditional Chinese medicine predominantly encompasses investigations into changes in immune cell activity, the expression of immune molecules, and the presentation of tumor antigens. Licorice extract, for instance, has demonstrated the ability to hinder the growth of NSCLC. It achieves this by elevating the abundance of PD-L1 protein, augmenting antigen presentation, and fostering the infiltration of CD8+ T cells (Zhu et al., 2021). Jiang et al. (Jiang R. et al., 2021)discovered that Tetramethylpyrazine (TMP) can inhibit the polarization of M1-type macrophages in vitro, while to some extent promoting the repolarization of M2-type macrophages. This effect results in the reduction of the transcription and secretion of inflammatory factors (IL-1β, IL-18, TNF-β) induced by lipopolysaccharide (LPS). In the context of lung cancer patients, the plasma activated by phytohemagglutination (PHA) exerts an inhibitory influence on lymphocyte proliferation, CD69 expression, and the production of perforin and granzyme B. This inhibition can be partially or completely reversed by Ganoderma lucidum polysaccharides (Gl-PS) (Sun et al., 2014). Astragalus polysaccharides (PG2) play a pivotal role in significantly augmenting the M1/M2 macrophage polarization rate of NSCLC cells. This action is coupled with a substantial inhibition of cell proliferation, clone formation, and tumor sphere formation. PG2 also promotes the functional maturation of dendritic cells, thus enhancing the T cell-mediated anti-cancer immune response (Bamodu et al., 2019). In an investigation focused on the impact of Trichosanthin (TCS) on the anti-tumor immune response in a 3LL Lewis lung cancer tumor model, the results revealed that TCS elevates the percentage of effector T cells. It fosters robust proliferation of antigen-specific effector T cells and significantly augments the secretion of Th1 cytokines. Moreover, it induces the generation of more memory T cells in tumor-bearing mice. This is accomplished through TCS upregulating the expression of the lung cancer 1 tumor suppressor factor (TSLC1) in 3LL tumor cells and elevating the expression of its ligand class I restrictive T cell-related molecule (CRTAM) in effector T cells. This enhancement, in turn, intensifies the anti-tumor response and induces immune protection (Cai et al., 2011). Furthermore, in their experiment, Zhang et al. (Zhang et al., 2015) noted that Atractylenolide I (AO-1) significantly curbed the production of TNF-α, IL-6, IL-1β, IL-13, and MIF in Bronchoalveolar Lavage Fluid (BALF), along with the production of BALF neutrophils and macrophages. Conversely, AO-1 was found to upregulate the production of IL-10 in BALF. Additionally, AO-I inhibited the expression of Toll-Like Receptor 4 (TLR4) and the activation of Nuclear Factor-Kappa B (NF-κB) induced by lipopolysaccharide (LPS).
The regulation of cellular signaling pathways has emerged as a promising frontier in the battle against lung cancer. Tanshinone IIA, for instance, induces cell cycle arrest in the G1/S phase by orchestrating the CCNA2-CDK2 complex and the AURKA/PLK1 pathway, thereby promoting apoptosis in lung adenocarcinoma cells (Li et al., 2021). In a study by Lin et al. (Lin et al., 2022), Xanthotoxol extracted from Angelica dahurica demonstrated its capacity to trigger cell cycle arrest, foster cell apoptosis, and inhibit Epithelial-Mesenchymal Transition (EMT) processes by downregulating PI3K-AKT signaling. This dual action effectively suppresses the proliferation and metastasis of NSCLC. The administration of Ligustrazine yields significant reductions in the levels of Wnt and β-catenin. Additionally, it downregulates PTEN levels and interrupts the Wnt/β-catenin signaling pathway, resulting in the inhibition of lung cancer cell invasion and proliferation (Dong et al., 2020). Cantharidin exhibits the capability to inhibit the migration and invasion of lung cancer cells, a phenomenon closely associated with the activation of the PI3K-AKT signaling pathway (Kim et al., 2013). Ophiopogon japonicus extract drives an increase in the expression of autophagy-related mediators (including the LC3-II/LC3-I ratio, Atg-3, Atg-7, and Beclin-1) in A549 cells. This activation of autophagy in lung cancer cells is rooted in the inhibition of the PI3K/Akt/mTOR signaling pathway (Chen et al., 2017). Ligustilide, extracted from Angelica sinensis, effectively regulates the proliferation, apoptosis, and aerobic glycolysis of NSCLC cells through the PTEN/AKT signaling pathway (Jiang X. et al., 2021). Astragalus polysaccharides (APS) exert their anti-tumor effects by inhibiting the protein and gene expression of S1PR1, STAT3, and p-STAT3 in the S1PR1/STAT3 signaling pathway (Shen et al., 2023). Glycyrrhizin hampers the migration and invasion of lung cancer cells, an effect intricately linked to its reduction in the activity of the JAK/STAT signaling pathway (Wu et al., 2018). A study by Wang et al. (Wang et al., 2022)unveiled that catalpol, extracted from Radix Rehmanniae, hinders the Nrf2/ARE signaling pathway, leading to a substantial decrease in the proliferation and migration abilities of lung cancer cells. The primary mechanism behind the anti-lung cancer effect of Fritillaria extract revolves around protein phosphorylation. The extract successfully inhibits tumor cell proliferation and AKT phosphorylation, with a predominant role played by the PI3K/AKT signaling pathway (Zhao et al., 2023). Salvia chinensis extract effectively inhibits the transcriptional activity of the Wnt/β-catenin pathway, eliciting significant inhibitory effects on tumor cells in vitro (Wang et al., 2017). Meanwhile, glycyrrhizic acid regulates autophagy related to the PI3K/AKT/mTOR pathway, thereby inhibiting the production of inflammatory factors induced by lipopolysaccharide (LPS) in Acute Lung Injury (ALI) (Qu et al., 2019). A comprehensive summary of the specific extracts and their regulatory mechanisms within traditional Chinese medicine is provided in Table 1.
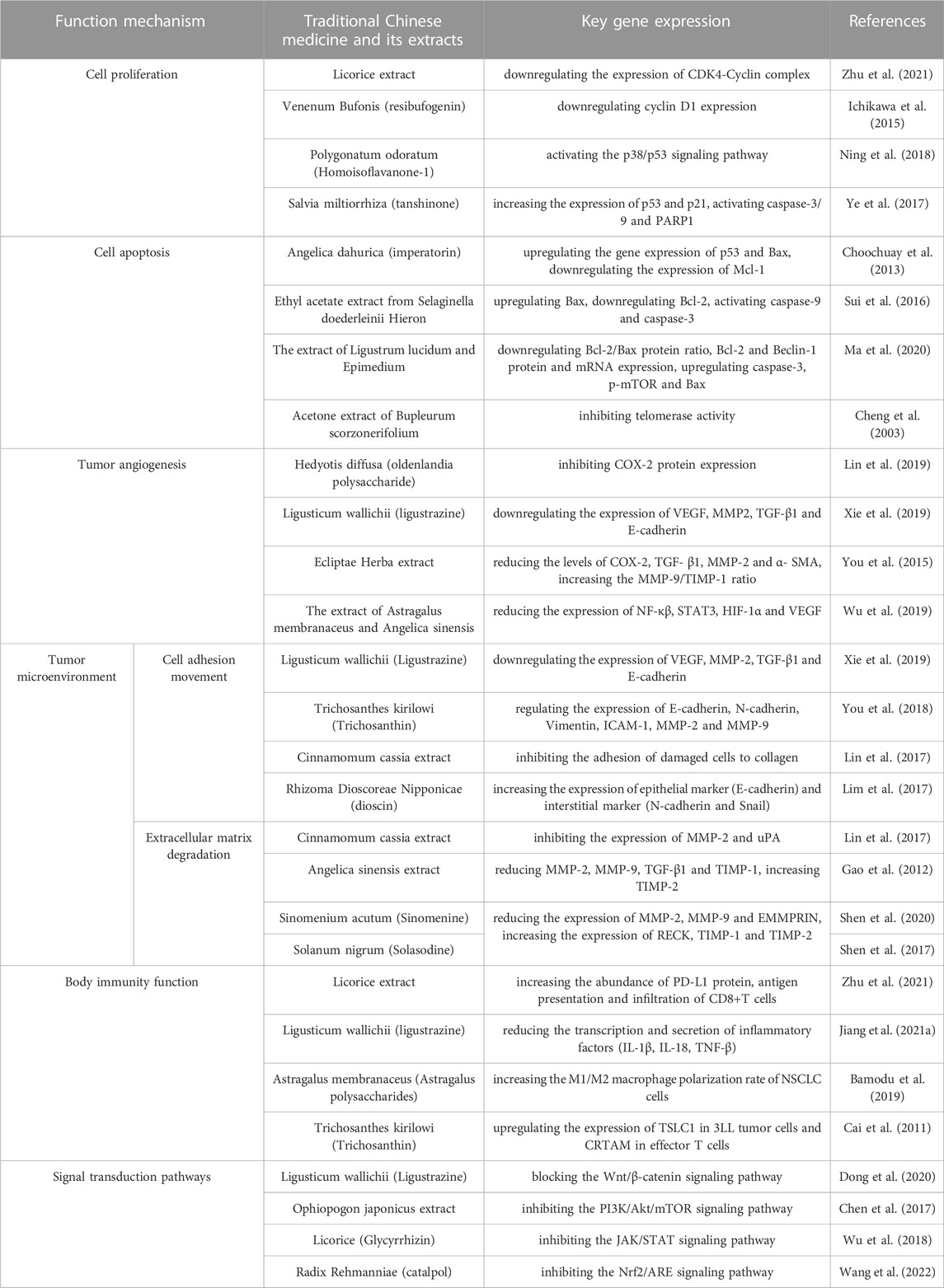
TABLE 1. Mechanism of action of traditional Chinese medicine extract in the treatment of non-small cell lung cancer.
Cisplatin (DDP) is a widely employed chemotherapy drug for lung cancer treatment (Ju et al., 2020; Liu et al., 2022). However, the development of gefitinib resistance (GR) is an inevitable challenge in the course of NSCLC therapy. Emerging research has shown that combining various traditional Chinese medicines with cisplatin can mitigate tumor cell resistance to cisplatin and enhance their anti-tumor potency. Bamodu et al. (Bamodu et al., 2019) reported a significant time-dependent reduction in the M2-associated tumor cell population with astragalus polysaccharides (PG2) treatment. This synergistically improved the anti-cancer efficacy of cisplatin on M2 cells and suppressed the growth of xenograft tumors in a NSCLC mouse model. Furthermore, the presence of PG2 significantly alleviated the side effects linked to cisplatin treatment. Cheng et al. (Cheng Z. et al., 2022)found that the extract of Ophiopogon japonicus exhibited a more pronounced cytotoxic effect on cisplatin-resistant lung cancer cells compared to normal tumor cells. Ophiopogonin B (OP-B) was shown to reduce tumor cell resistance to cisplatin by promoting Caspase-1/GSDMD-dependent pyroptosis. Ganoderma lucidum Polysaccharide (WSG), a glucose-rich polysaccharide derived from Ganoderma lucidum, demonstrated enhanced inhibition of lung cancer both in vitro and in vivo when combined with cisplatin. This combined treatment led to a synergistic reduction in the growth of lung cancer cells, enhancing the apoptosis response mediated by cisplatin. Additionally, WSG reduced the cytotoxic effects of cisplatin on macrophages and normal lung fibroblasts (Qiu et al., 2021). Artemisia argyi extract effectively suppressed both parent and gemcitabine-resistant lung cancer cells by inducing ROS production, mitochondrial membrane depolarization, and apoptosis, while mitigating Epithelial-Mesenchymal Transition (EMT) (Su et al., 2022). The ethanol extract of Centipeda minima intensified cisplatin-induced cell apoptosis in NSCLC xenografts (Gao et al., 2023). The anti-cancer efficacy of cordycepin, both in vitro and in vivo, was found to be comparable to afatinib and even superior to gefitinib. This suggests that cordycepin, either in isolation or combined with existing targeted therapies, might provide additional treatment options for drug-resistant NSCLC (Wei et al., 2019). The combination of Cordyceps sinensis extract Cordycepin and cisplatin exhibited a synergistic effect in inhibiting proliferation and promoting apoptosis in NSCLC cells, both in the presence and absence of cisplatin resistance. This anti-tumor activity was associated with the activation of AMPK and the inhibition of the AKT pathway, thereby enhancing the inhibitory effects of cisplatin on NSCLC (Liao et al., 2021). β-elemene, extracted from Radix curcuma wenyujin, acted synergistically with cisplatin against NSCLC cells by promoting apoptosis and impairing glucose metabolism, combating multidrug resistance, and preserving stemness (Cheng G. et al., 2022). The combination of lithospermum extract shikonin with gefitinib displayed a synergistic anti-tumor effect in vitro and in vivo. The potential molecular mechanisms appeared to be linked to the inhibition of PKM2/STAT3/cyclinD1 (Tang et al., 2018). Experiments have also revealed that gambogenic acid, an extract from garcinia, functioned as an inhibitor of the FGFR signaling pathway, thereby enhancing the efficacy of erlotinib when used in combination (Xu et al., 2018). Aucklandia lappa DC. extract significantly enhanced the effectiveness of gefitinib, suppressing the Muv phenotype of jgIs25. Meanwhile, it also downregulated the mRNA and protein expression of EGFR in jgIs25 (Huang et al., 2017). In a study by Lee et al. (Lee et al., 2011), a low dose of gefitinib, when combined with curcumin, exhibited a significant synergistic effect on apoptosis in a lung cancer cell line. This effect was achieved by reducing the mitochondrial membrane potential and activating caspase-9/caspase-3. Pristimerin inhibited cell proliferation, induced G0/G1 arrest, and promoted cell apoptosis in A549 and NCI-H446 cells. Pristimerin effectively synergized with cisplatin, resulting in the suppression of tumor growth (Zhang et al., 2019). In conclusion, traditional Chinese medicine extracts can synergistically enhance the anti-tumor effects of chemotherapy drugs.
Multidrug resistance (MDR) in lung cancer cells remains a major obstacle to achieving satisfactory chemotherapy outcomes. Consequently, overcoming MDR in tumor cells to enhance chemotherapy effectiveness represents a crucial area of research. Trichosanthin (TCS), by upregulating DR4 and DR5, can trigger cell apoptosis, modulate invasion, influence cell cycle-related proteins, and heighten the sensitivity of NSCLC cells to TRAIL (You et al., 2018). Xu et al. (Xu et al., 2018) reported the successful use of gambogenic acid to overcome erlotinib resistance in NSCLC treatment through the inhibition of the FGFR signaling pathway when administered in combination. Andrographis paniculata addresses cisplatin resistance in tumor cells by acting on the miR-155-5p/SIRT1 axis (Pang et al., 2023). A synergistic inhibitory effect on lung cancer cell proliferation and invasion was observed with the combined use of Scutellaria baicalensis extract baicalin and cisplatin at specific doses and incubation times. The attenuation of cisplatin resistance was associated with the downregulation of MARK2 and p-Akt (Xu et al., 2017). Berberine, derived from Coptis chinensis extract, demonstrated its role as a naturally occurring MET inhibitor. It synergized with osimertinib in overcoming acquired resistance caused by MET amplification (Chen et al., 2022). Ming et al. (Ming et al., 2021) found that fucoxanthin, extracted from Laminaria Japonica, effectively inhibited tumor growth and heightened the sensitivity of lung cancer cells to gefitinib. This effect might be attributed to the inhibition of tumor cell proliferation and the activation of apoptosis. Artemisia annua extract dihydroartemisinin proved effective in overcoming acquired resistance to gefitinib in EGFR-mutant lung adenocarcinoma due to its STAT3 inhibitory activity and low toxicity (Xueting et al., 2017). Triptolide sensitized cancer cells to antitumor drugs both in vitro and in a xenograft tumor model system using lung carcinoma cells. This suggests that triptolide mitigates chemoresistance in cancer cells by targeting the Nrf2 pathway (Zhu et al., 2018). An extraction from Scutellariabarbata D. Don sensitized NSCLC cells to cisplatin treatment by downregulating the SHH signaling pathway (Chen WW. et al., 2021). Tripterygium wilfordii extract celastrol exhibited similar cytotoxic effects on both A549 parental and A549/DDP cisplatin-resistant cells. Celastrol also induced cell apoptosis in a dose- and time-dependent manner in both cell lines, accompanied by ROS accumulation, loss of mitochondrial membrane potentials, and cleavage of PARP and caspases (Shi, 2013). In summary, various traditional Chinese medicines effectively reverse the multidrug resistance observed in response to chemotherapeutic drugs. Their combined use enhances the sensitivity of these enhancers and significantly improves the effectiveness of tumor treatment. The applications of traditional Chinese medicine extracts for NSCLC are detailed in Table 2.
Small cell lung cancer (SCLC) is an aggressive and recalcitrant malady, characterized by rapid growth and early metastatic spread to regional lymph nodes and distant sites. Chemotherapy is the general treatment for SCLC, however, majority of patients who typically exhibit a sensitive response to chemotherapy will swiftly relapse with relatively drug-resistant diseases (Waqar and Morgensztern, 2017; Mi et al., 2020). Despite considerably significant progress in targeted therapy and immunotherapy has been made in treating NSCLC, the therapies have not produced similar effects in SCLC (Byers and Rudin, 2015; Parikh et al., 2016). Although Traditional Chinese Medicine (TCM) is widely applied for patients with SCLC in China, the evidence of TCM in the treatment of SCLC is limited. Compared with chemotherapy alone, those with Chinese herbal medicine and chemotherapy had better therapeutic effects, including better KPS scores, and 5-year survival rate. Moreover, the incidence of gastrointestinal reaction and bone marrow depression was lower (Chen et al., 2020). TCM combined with chemotherapy may improve therapeutic effect, quality of life, and prolong survival time (Chen et al., 2020; Chen Y. et al., 2021; Xu et al., 2021; Qi et al., 2023). Aqueous extract of Artemisia annua played a preventive role against malignancy in the lung cancer models. It also induced apoptosis and decreased intracellular reactive oxygen species (ROS) in the lung cells (Allemailem, 2022). Peng et al. (Peng et al., 2021) revealed that the combination of anlotinib and Brucea Javanica Oil (BJO) significantly inhibited the growth of SCLC liver metastases and angiogenesis more than anlotinib monotherapy. In addition, BJO alleviated some of the side effects associated with anlotinib therapy. The results of the study indicated that the combination of anlotinib with BJO is promisingly active against liver metastases of SCLC, and has clinical potential. Moreover, the combination of gemcitabine (GEM) and BJO resulted in a reduced tumor growth rate and greater apoptosis compared to the vehicle control and GEM monotherapy (Yang et al., 2020). Therefore, traditional Chinese medicine extracts have potential clinical application in SCLC.
In recent years, the aging trend within China’s population has become increasingly prominent, and the incidence of chronic respiratory diseases has been steadily rising. Furthermore, pulmonary fibrosis, as a debilitating interstitial lung condition, presents limited treatment options. Ecliptae Herba extract has shown remarkable efficacy in ameliorating pathological lung tissue changes by reducing the levels of hydroxyproline (HYP), increasing superoxide dismutase (SOD) activity, and decreasing malondialdehyde (MDA) content. This extract, by mitigating oxidative stress, lung tissue inflammation, and epithelial-mesenchymal transition (EMT), exhibits a protective role against pulmonary fibrosis induced by bleomycin (You et al., 2015). Extracts from Radix Pseudostellariae, notably Heterophyllin B, have been found to significantly inhibit EMT and the redifferentiation of lung fibroblasts. Furthermore, it offers protection against bleomycin-induced pulmonary fibrosis by modulating the STING signaling mediated through AMPK and TGF-Smad2/3 pathways (Shi et al., 2022). Catalpol, extracted from Radix Rehmanniae, effectively inhibits NF-κB and MAPK signaling pathways mediated by TLR4, ultimately alleviating bleomycin-induced pulmonary fibrosis (Fu et al., 2014). Meanwhile, Icaritin plays a pivotal role in downregulating the expression of α-SMA and collagen I, effectively inhibiting myofibroblast differentiation, which suggests its potential in treating pulmonary fibrosis (Hua et al., 2021). Furthermore, the research by Li et al. (Li S. et al., 2022) demonstrates the inhibitory effects of pheretima protein on extracellular matrix (ECM), EMT, and anti-inflammatory markers, which contribute to the amelioration of bleomycin-induced pulmonary fibrosis. Alcohol extracts from Sceptridium ternatum (Thunb.) Lyon have exhibited anti-pulmonary fibrosis effects by targeting the SETDB1/STAT3/p-STAT3 signaling pathway (Zou et al., 2023). Qian et al. (Qian et al., 2018) have shown that Astragalus IV significantly inhibits FOXO3a hyperphosphorylation and downregulation induced by TGF-β1/PI3K/Akt, effectively reversing EMT during pulmonary fibrosis progression. Astragalus also exerts antifibrotic effects by inhibiting EMT, ROS, TGF-β1/Smads, apoptosis, and inflammatory pathways (Zhu et al., 2022). Euodia rutaecarpa (Juss.) Benth. extract evodiamine has demonstrated the ability to protect against pulmonary fibrosis-induced inflammatory damage both in vitro and in vivo by inhibiting apoptosis, inflammatory cytokine release, and activating the apelin pathway (Ye et al., 2021). Moreover, Snithin has significantly mitigated bleomycin-induced pulmonary fibrosis by downregulating the mechanism of TGF-β1/NOX4-mediated oxidative stress in lung fibroblasts (Fang et al., 2021). Salvia miltiorrhiza extract Tanshinone IIA has been instrumental in regulating the Keap1/Nrf2 signaling pathway through the activation of Sestrin2, effectively inhibiting pulmonary fibrosis (Li H. et al., 2022). Shenks exhibits its anti-fibrotic properties by blocking the TGF-β1 pathway and regulating oxidative/antioxidant balance (Chu et al., 2017). Peimine has contributed to the amelioration of pulmonary fibrosis by inhibiting M2-type macrophage polarization through the suppression of P38/Akt/STAT6 signals (Cai et al., 2022).
Acute lung injury, one of the most prevalent and severe respiratory conditions, currently lacks effective treatment options. Catalpol has proven to be effective in protecting against lipopolysaccharide-induced acute lung injury (Fu et al., 2014). Similarly, glycyrrhizic acid has shown promise in improving sepsis-induced acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) by inhibiting HMGB1 and ameliorating inflammation in experimental sepsis rat models (Shen et al., 2022). Astragaloside IV, acting through the TLR4/NF-κB signaling pathway, has demonstrated its ability to induce cell autophagy, which, in turn, inhibits the release of inflammatory cytokines and improves the overall inflammatory response (Ying et al., 2021). Indigoin has offered relief in cases of lipopolysaccharide-induced acute lung injury by reducing malondialdehyde (MDA) levels and IL-1β and TNF-α expression in mice (Qi et al., 2017). Spatholobus stem extract has ameliorated acute lung inflammation induced by IAV by inhibiting the MAPK, PI3K-AKT, and oxidative stress pathways (Cai and Zhang, 2022). Furthermore, Euphorbia factor L2 (EFL2), derived from marijuana extract, has exhibited anti-inflammatory effects in a murine acute lung injury model by reducing the levels of inflammatory markers such as interleukin-1 β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), interleukin-8 (IL-8), and myeloperoxidase (MPO) in lung and bronchioalveolar lavage fluid (Zhang et al., 2018). Ren et al. (Ren et al., 2023) found that ergolactone, through its irreversible binding to the NACHT domain of NLRP3, effectively blocked the assembly and activation of NLRP3 inflammasomes. In a lipopolysaccharide-induced acute lung injury model, ergolactone alleviated acute lung injury in wild-type mice. Astragaloside IV targeted PRDX6 and inhibited the activation of the RAC subunit in NADPH oxidase 2, thus suppressing oxidative damage and inflammation in mice with acute lung injury (Cheng et al., 2023). Traditional Chinese medicines, along with their bioactive compounds, such as epigallocatechin-3-gallate (EGCG), kaempferol, isorhamnetin, quercetin, and β-sitosterol, predominantly regulate NF-κB, Nrf2/HO-1, NLRP3, TGF-β/Smad, MAPK, and PI3K/Akt/mTOR pathways, successfully mitigating infection and inflammation across a spectrum of lung-related diseases, including acute lung injury, chronic obstructive pulmonary disease, pulmonary fibrosis, asthma, and lung cancer (Wang et al., 2021). These findings underscore the pivotal role of traditional Chinese medicine extracts in the treatment of lung-related diseases, such as acute lung injury.
The incidence of various lung-related diseases, including cor pulmonale, chronic obstructive pulmonary disease, pulmonary tuberculosis, and asthma, has been steadily on the rise. It is imperative to acknowledge the clinical significance of these diseases. Traditional Chinese medicines have shown promise in addressing these conditions. Astragalus, radix paeoniae rubra, and Psoralea corylifolia have demonstrated their efficacy in reducing pulmonary keratinocytes growth factor (KGF), secretory immunoglobulin A (sIgA), and collagen fiber deposition. These effects lead to improved lung function and provide a valuable treatment approach for cor pulmonale (Jing et al., 2017). Resveratrol, extracted from Polygonum cuspidatum, acts as an activator of SIRT1 and has the potential to alleviate chronic obstructive pulmonary disease by activating the SIRT1 pathway (Ma and Li, 2020). Anemoside B4 has shown significant promise in attenuating the increased levels of inflammatory markers, including IL-1β, TGF-β, IL-8, and TNF-α, in human bronchial epithelial cells exposed to cigarette smoke extract (CSE). This is achieved by inhibiting the MAPK/AP-1/TGF-β signaling pathway, thereby preventing chronic pulmonary obstructive disease induced by cigarette smoke (Ma et al., 2022). Isoliquiritigenin effectively reduces inflammation induced by Mycobacterium tuberculosis (Mtb) through the Notch1/NF-κB and MAPK signaling pathways (Sun et al., 2022). Icariin, characterized by its anti-inflammatory, antioxidant, and immune-regulating properties, presents a multi-target intervention approach to asthma treatment (Yuan et al., 2022). Safranal, an extract from saffron crocus, has been shown to alleviate OVA-induced asthma by inhibiting the MAPK and NF-κB pathways, thus inhibiting mast cell activation and the production of proinflammatory factors (Lertnimitphun et al., 2021). The specific extracts and regulatory mechanisms of traditional Chinese medicine are detailed in Table 3, underscoring the potential of these remedies in addressing a variety of lung-related diseases.
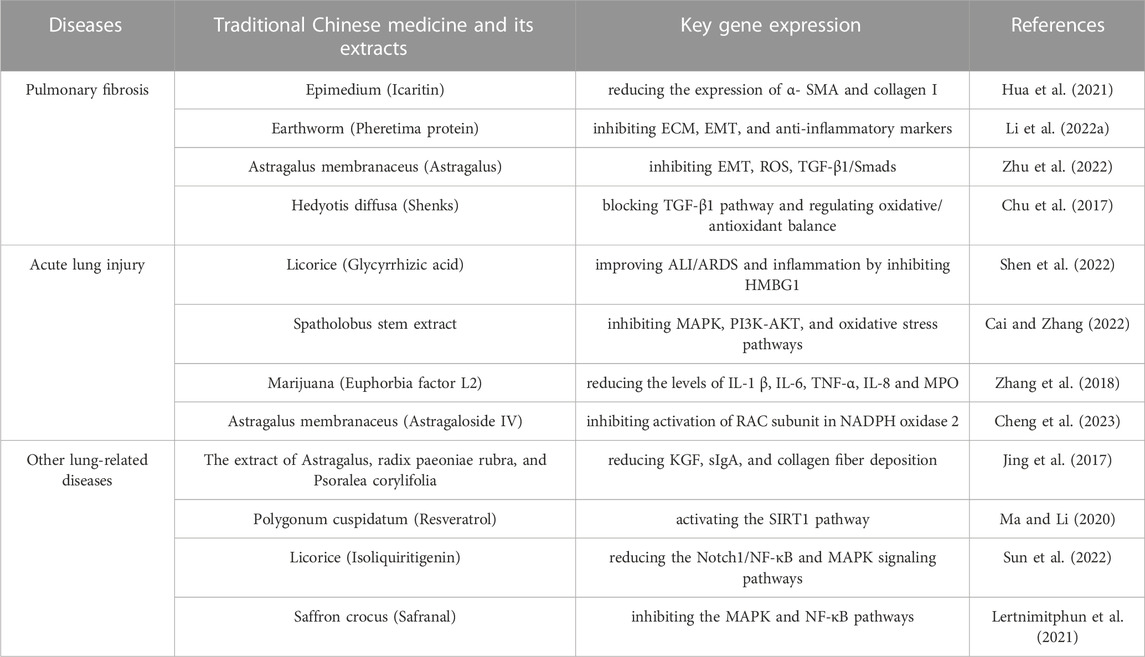
TABLE 3. Mechanism of action of traditional Chinese medicine extract in treating other lung-related diseases.
In conclusion, traditional Chinese medicine offers a multifaceted and layered approach to the prevention and treatment of lung cancer. Experimental research on the anti-lung cancer effects of traditional Chinese medicine has progressed from morphological observations to the microscopic levels of cells, molecules, and genes. Numerous studies have shed light on the mechanisms through which traditional Chinese medicine inhibits lung cancer growth and metastasis. These mechanisms encompass inhibiting tumor cell proliferation, inducing tumor cell apoptosis, and suppressing tumor angiogenesis. Traditional Chinese medicine also affects the cellular microenvironment, regulates immune function, modulates signal transduction pathways, and reverses multidrug resistance in tumor cells. These findings provide a robust scientific foundation for lung cancer treatment with traditional Chinese medicine and contribute to the advancement of the traditional Chinese medicine industry.
However, several challenges persist, including:
1. Overemphasis on traditional Chinese medicine compound formulas and active ingredients at the expense of single Chinese medicine and extracts in anti-lung cancer research.
2. The complex, multifaceted mechanisms of action in traditional Chinese medicine extracts, which pose challenges in pharmacokinetic research, such as determining quantitative standards, sampling times, and concentration standards in plasma or serum.
3. Interferences and uncertainties associated with single drugs and their traditional Chinese medicine extracts, raising doubts about result accuracy.
4. A predominant focus on gene regulation research in the mechanism of action, with less exploration of the interplay between various gene regulations.
5. A predominance of in vitro studies with limited research on in vivo cell apoptosis, hindering the understanding of drug metabolism and induced cell apoptosis in vivo.
To address these issues, we propose that future research and development of anti-lung cancer effects of traditional Chinese medicine single drugs and their extracts should involve interdisciplinary collaboration. This approach, guided by traditional Chinese medicine theory, can delve into the complex biological processes underlying tumor initiation, development, and metastasis. The goal is to develop effective formulations that target lung cancer cells through multiple avenues, including various targets, pathways, and methods. Modern pharmacology, immunology, and molecular biology are steadily unraveling the anti-lung cancer mechanisms of traditional Chinese medicine. Continuous research and exploration in this field aim to harness the full clinical therapeutic potential of traditional Chinese medicine in lung cancer treatment.
ZH: Writing–original draft. YW: Writing–original draft. LH: Writing–review and editing. YH: Writing–original draft, Writing–review and editing. XC: Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Science and Technology Development Project of Jilin Province (Grant No. 20220402071GH).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Allemailem, K. S. (2022). Aqueous extract of Artemisia annua shows in vitro antimicrobial activity and an in vivo chemopreventive effect in a small-cell lung cancer model. Plants (Basel) 11 (23), 3341. doi:10.3390/plants11233341
Bamodu, O. A., Kuo, K. T., Wang, C. H., Huang, W. C., Wu, A. T. H., Tsai, J. T., et al. (2019). Astragalus polysaccharides (PG2) enhances the M1 polarization of macrophages, functional maturation of dendritic cells, and T cell-mediated anticancer immune responses in patients with lung cancer. Nutrients 11 (10), 2264. doi:10.3390/nu11102264
Byers, L. A., and Rudin, C. M. (2015). Small cell lung cancer: where do we go from here? Cancer 121 (5), 664–672. doi:10.1002/cncr.29098
Cai, W., and Zhang, S. L. (2022). Anti-inflammatory mechanisms of total flavonoids from mosla scabra against influenza A virus-induced pneumonia by integrating network pharmacology and experimental verification. Evid. Based Complement. Altern. Med. 2022, 2154485, 2154485. doi:10.1155/2022/2154485
Cai, Y., Xiong, S., Zheng, Y., Luo, F., Jiang, P., and Chu, Y. (2011). Trichosanthin enhances anti-tumor immune response in a murine Lewis lung cancer model by boosting the interaction between TSLC1 and CRTAM. Cell Mol. Immunol. 8 (4), 359–367. doi:10.1038/cmi.2011.12
Cai, Z. H., Tian, Y. G., Li, J. Z., Zhao, P., Li, J. S., Mei, X., et al. (2022). Peimine ameliorates pulmonary fibrosis via the inhibition of M2-type macrophage polarization through the suppression of P38/Akt/STAT6 signals. Biosci. Rep. 42 (10), BSR20220986. doi:10.1042/BSR20220986
Chen, J., Yuan, J., Zhou, L., Zhu, M., Shi, Z., Song, J., et al. (2017). Regulation of different components from Ophiopogon japonicus on autophagy in human lung adenocarcinoma A549Cells through PI3K/Akt/mTOR signaling pathway. Biomed. Pharmacother. 87, 118–126. doi:10.1016/j.biopha.2016.12.093
Chen, S., Bao, Y., Xu, J., Zhang, X., He, S., Zhang, Z., et al. (2020). Efficacy and safety of TCM combined with chemotherapy for SCLC: a systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 146 (11), 2913–2935. doi:10.1007/s00432-020-03353-0
Chen, W. W., Gong, K. K., Yang, L. J., Dai, J. J., Zhang, Q., Wang, F., et al. (2021a). Scutellariabarbata D. Don extraction selectively targets stemness-prone NSCLC cells by attenuating SOX2/SMO/GLI1 network loop. J. Ethnopharmacol. 265, 113295. doi:10.1016/j.jep.2020.113295
Chen, Y., Yu, M., Liu, Z., Zhang, Y., Li, Q., and Yang, G. (2021b). Effects of traditional Chinese medicine combined with chemotherapy for extensive-stage small-cell lung cancer patients on improving oncologic survival: study protocol of a multicenter, randomized, single-blind, placebo-controlled trial. Trials 22 (1), 437. doi:10.1186/s13063-021-05407-1
Chen, Z., Vallega, K. A., Chen, H., Zhou, J., Ramalingam, S. S., and Sun, S. Y. (2022). The natural product berberine synergizes with osimertinib preferentially against MET-amplified osimertinib-resistant lung cancer via direct MET inhibition. Pharmacol. Res. 175, 105998. doi:10.1016/j.phrs.2021.105998
Cheng, C., Liu, K., Shen, F., Zhang, J., Xie, Y., Li, S., et al. (2023). Astragaloside IV targets PRDX6, inhibits the activation of RAC subunit in NADPH oxidase 2 for oxidative damage. Phytomedicine 114, 154795. doi:10.1016/j.phymed.2023.154795
Cheng, G., Li, L., Li, Q., Lian, S., Chu, H., Ding, Y., et al. (2022b). β-elemene suppresses tumor metabolism and stem cell-like properties of non-small cell lung cancer cells by regulating PI3K/AKT/mTOR signaling. Am. J. Cancer Res. 12 (4), 1535–1555.
Cheng, Y. L., Chang, W. L., Lee, S. C., Liu, Y. G., Lin, H. C., Chen, C. J., et al. (2003). Acetone extract of Bupleurum scorzonerifolium inhibits proliferation of A549 human lung cancer cells via inducing apoptosis and suppressing telomerase activity. Life Sci. 73 (18), 2383–2394. doi:10.1016/s0024-3205(03)00648-9
Cheng, Z., Li, Z., Gu, L., Li, L., Gao, Q., Zhang, X., et al. (2022a). Ophiopogonin B alleviates cisplatin resistance of lung cancer cells by inducing Caspase-1/GSDMD dependent pyroptosis. J. Cancer 13 (2), 715–727. doi:10.7150/jca.66432
Choochuay, K., Chunhacha, P., Pongrakhananon, V., Luechapudiporn, R., and Chanvorachote, P. (2013). Imperatorin sensitizes anoikis and inhibits anchorage-independent growth of lung cancer cells. J. Nat. Med. 67 (3), 599–606. doi:10.1007/s11418-012-0719-y
Chu, H., Shi, Y., Jiang, S., Zhong, Q., Zhao, Y., Liu, Q., et al. (2017). Treatment effects of the traditional Chinese medicine Shenks in bleomycin-induced lung fibrosis through regulation of TGF-beta/Smad3 signaling and oxidative stress. Sci. Rep. 7 (1), 2252. doi:10.1038/s41598-017-02293-z
Dong, Y., Yang, Y., Wei, Y., Gao, Y., Jiang, W., and Wang, G. (2020). Ligustrazine eases lung cancer by regulating PTEN and Wnt/β-catenin pathway. Transl. Cancer Res. 9 (3), 1742–1751. doi:10.21037/tcr.2020.03.26
Fang, L., Wang, W., Chen, J., Zuo, A., Gao, H., Yan, T., et al. (2021). Osthole attenuates bleomycin-induced pulmonary fibrosis by modulating NADPH oxidase 4-derived oxidative stress in mice. Oxid. Med. Cell Longev. 2021, 3309944, 3309944. doi:10.1155/2021/3309944
Fu, K., Piao, T., Wang, M., Zhang, J., Jiang, J., Wang, X., et al. (2014). Protective effect of catalpol on lipopolysaccharide-induced acute lung injury in mice. Int. Immunopharmacol. 23 (2), 400–406. doi:10.1016/j.intimp.2014.07.011
Gao, C., Pan, H., Ma, F., Zhang, Z., Zhao, Z., Song, J., et al. (2023). Centipeda minima active components and mechanisms in lung cancer. BMC Complement. Med. Ther. 23 (1), 89. doi:10.1186/s12906-023-03915-y
Gao, M., Zhang, J. H., Zhou, F. X., Xie, C. H., Han, G., Fang, S. Q., et al. (2012). Angelica sinensis suppresses human lung adenocarcinoma A549 cell metastasis by regulating MMPs/TIMPs and TGF-β1. Oncol. Rep. 27 (2), 585–593. doi:10.3892/or.2011.1527
Goldstraw, P., Crowley, J., Chansky, K., Giroux, D. J., Groome, P. A., Rami-Porta, R., et al. (2007). The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J. Thorac. Oncol. 2 (8), 706–714. doi:10.1097/JTO.0b013e31812f3c1a
Hsia, T. C., Yu, C. C., Hsiao, Y. T., Wu, S. H., Bau, D. T., Lu, H. F., et al. (2016). Cantharidin impairs cell migration and invasion of human lung cancer NCI-H460 cells via UPA and MAPK signaling pathways. Anticancer Res. 36 (11), 5989–5997. doi:10.21873/anticanres.11187
Hua, Q., Huang, X., Xie, W., Zhao, F., Cheng, H., Luo, Z., et al. (2021). PPARγ mediates the anti-pulmonary fibrosis effect of icaritin. Toxicol. Lett. 350, 81–90. doi:10.1016/j.toxlet.2021.06.014
Huang, F., Pang, J., Xu, L., Niu, W., Zhang, Y., Li, S., et al. (2022). Hedyotis diffusa injection induces ferroptosis via the Bax/Bcl2/VDAC2/3 axis in lung adenocarcinoma. Phytomedicine 104, 154319. doi:10.1016/j.phymed.2022.154319
Huang, G., Tong, Y., He, Q., Wang, J., and Chen, Z. (2017). Aucklandia lappa DC. extract enhances gefitinib efficacy in gefitinib-resistance secondary epidermal growth factor receptor mutations. J. Ethnopharmacol. 206, 353–362. doi:10.1016/j.jep.2017.06.011
Ichikawa, M., Sowa, Y., Iizumi, Y., Aono, Y., and Sakai, T. (2015). Resibufogenin induces G1-phase arrest through the proteasomal degradation of cyclin D1 in human malignant tumor cells. PLoS One 10 (6), e0129851. doi:10.1371/journal.pone.0129851
Jiang, R., Xu, J., Zhang, Y., Zhu, X., Liu, J., and Tan, Y. (2021a). Ligustrazine alleviate acute lung injury through suppressing pyroptosis and apoptosis of alveolar macrophages. Front. Pharmacol. 12, 680512. doi:10.3389/fphar.2021.680512
Jiang, X., Zhao, W., Zhu, F., Wu, H., Ding, X., Bai, J., et al. (2021b). Ligustilide inhibits the proliferation of non-small cell lung cancer via glycolytic metabolism. Toxicol. Appl. Pharmacol. 410, 115336. doi:10.1016/j.taap.2020.115336
Jing, Y., Zhang, H., Cai, Z., Zhao, Y., Wu, Y., Zheng, X., et al. (2017). Bufei huoxue capsule attenuates pm2.5-induced pulmonary inflammation in mice. Evid. Based Complement. Altern. Med. 2017, 1575793. doi:10.1155/2017/1575793
Ju, Z. S., Sun, B., Bao, D., and Zhang, X. F. (2020). Effect of lncRNA-BLACAT1 on drug resistance of non-small cell lung cancer cells in DDP chemotherapy by regulating cyclin D1 expression. Eur. Rev. Med. Pharmacol. Sci. 24 (18), 9465–9472. doi:10.26355/eurrev_202009_23031
Kim, Y. M., Ku, M. J., Son, Y. J., Yun, J. M., Kim, S. H., and Lee, S. Y. (2013). Anti-metastatic effect of cantharidin in A549 human lung cancer cells. Arch. Pharm. Res. 36 (4), 479–484. doi:10.1007/s12272-013-0044-3
Latrèche, A., Bourbonne, V., and Lucia, F. (2022). Unrecognized thoracic radiotherapy toxicity: a review of literature. Cancer Radiother. 26 (4), 616–621. doi:10.1016/j.canrad.2021.10.008
Lee, J. Y., Lee, Y. M., Chang, G. C., Yu, S. L., Hsieh, W. Y., Chen, J. J., et al. (2011). Curcumin induces EGFR degradation in lung adenocarcinoma and modulates p38 activation in intestine: the versatile adjuvant for gefitinib therapy. PLoS One 6 (8), e23756. doi:10.1371/journal.pone.0023756
Lee, S., Lee, S., Roh, H. S., Song, S. S., Ryoo, R., Pang, C., et al. (2018). Cytotoxic constituents from the sclerotia of poria cocos against human lung adenocarcinoma cells by inducing mitochondrial apoptosis. Cells 7 (9), 116. doi:10.3390/cells7090116
Lertnimitphun, P., Zhang, W., Fu, W., Yang, B., Zheng, C., Yuan, M., et al. (2021). Safranal alleviated OVA-induced asthma model and inhibits mast cell activation. Front. Immunol. 12, 585595. doi:10.3389/fimmu.2021.585595
Li, C., Chen, J., Lu, B., Shi, Z., Wang, H., Zhang, B., et al. (2014). Molecular switch role of Akt in Polygonatum odoratum lectin-induced apoptosis and autophagy in human non-small cell lung cancer A549 cells. PLoS One 9 (7), e101526. doi:10.1371/journal.pone.0101526
Li, C., Lei, S., Ding, L., Xu, Y., Wu, X., Wang, H., et al. (2023). Global burden and trends of lung cancer incidence and mortality. Chin. Med. J. Engl. 136 (13), 1583–1590. doi:10.1097/CM9.0000000000002529
Li, H., Wu, M., Guo, C., Zhai, R., and Chen, J. (2022b). Tanshinone IIA regulates keap1/nrf2 signal pathway by activating Sestrin2 to restrain pulmonary fibrosis. Am. J. Chin. Med. 50 (8), 2125–2151. doi:10.1142/S0192415X22500914
Li, S., Yang, Q., Chen, F., Tian, L., Huo, J., Meng, Y., et al. (2022a). The antifibrotic effect of pheretima protein is mediated by the TGF-β1/Smad2/3 pathway and attenuates inflammation in bleomycin-induced idiopathic pulmonary fibrosis. J. Ethnopharmacol. 286, 114901, 114901. doi:10.1016/j.jep.2021.114901
Li, Z., Zhang, Y., Zhou, Y., Wang, F., Yin, C., Ding, L., et al. (2021). Tanshinone IIA suppresses the progression of lung adenocarcinoma through regulating CCNA2-CDK2 complex and AURKA/PLK1 pathway. Sci. Rep. 11 (1), 23681. doi:10.1038/s41598-021-03166-2
Liao, X. Z., Gao, Y., Zhao, H. W., Zhou, M., Chen, D. L., Tao, L. T., et al. (2021). Cordycepin reverses cisplatin resistance in non-small cell lung cancer by activating AMPK and inhibiting AKT signaling pathway. Front. Cell Dev. Biol. 8, 609285. doi:10.3389/fcell.2020.609285
Lim, W. C., Kim, H., Kim, Y. J., Choi, K. C., Lee, I. H., Lee, K. H., et al. (2017). Dioscin suppresses TGF-β1-induced epithelial-mesenchymal transition and suppresses A549 lung cancer migration and invasion. Bioorg Med. Chem. Lett. 27 (15), 3342–3348. doi:10.1016/j.bmcl.2017.06.014
Lin, C. Y., Hsieh, Y. H., Yang, S. F., Chu, S. C., Chen, P. N., and Hsieh, Y. S. (2017). Cinnamomum cassia extracts reverses TGF-β1-induced epithelial-mesenchymal transition in human lung adenocarcinoma cells and suppresses tumor growth in vivo. Environ. Toxicol. 32 (7), 1878–1887. doi:10.1002/tox.22410
Lin, L., Cheng, K., He, Z., Lin, Q., Huang, Y., Chen, C., et al. (2019). A polysaccharide from Hedyotis diffusa interrupts metastatic potential of lung adenocarcinoma A549 cells by inhibiting EMT via EGFR/Akt/ERK signaling pathways. Int. J. Biol. Macromol. 129, 706–714. doi:10.1016/j.ijbiomac.2019.02.040
Lin, X., Liu, J., Zou, Y., Tao, C., and Chen, J. (2022). Xanthotoxol suppresses non-small cell lung cancer progression and might improve patients' prognosis. Phytomedicine 105, 154364. doi:10.1016/j.phymed.2022.154364
Liu, J. H., Yang, H. L., Deng, S. T., Hu, Z., Chen, W. F., Yan, W. W., et al. (2022). The small molecule chemical compound cinobufotalin attenuates resistance to DDP by inducing ENKUR expression to suppress MYH9-mediated c-Myc deubiquitination in lung adenocarcinoma. Acta Pharmacol. Sin. 43 (10), 2687–2695. doi:10.1038/s41401-022-00890-x
Lv, J., Zhu, S., Chen, H., Xu, Y., Su, Q., Yu, G., et al. (2022). Paeonol inhibits human lung cancer cell viability and metastasis in vitro via miR-126-5p/ZEB2 axis. Drug Dev. Res. 83 (2), 432–446. doi:10.1002/ddr.21873
Lv, Y. X., Pan, H. R., Song, X. Y., Chang, Q. Q., and Zhang, D. D. (2021). Hedyotis diffusa plus Scutellaria barbata suppress the growth of non-small-cell lung cancer via NLRP3/NF-κB/MAPK signaling pathways. Evid. Based Complement. Altern. Med. 2021, 6666499, 6666499. doi:10.1155/2021/6666499
Ma, B. N., and Li, X. J. (2020). Resveratrol extracted from Chinese herbal medicines: a novel therapeutic strategy for lung diseases. Chin. Herb. Med. 12 (4), 349–358. doi:10.1016/j.chmed.2020.07.003
Ma, H., Zhou, Z., Chen, L., Wang, L., and Muge, Q. (2022). Anemoside B4 prevents chronic obstructive pulmonary disease through alleviating cigarette smoke-induced inflammatory response and airway epithelial hyperplasia. Phytomedicine 107, 154431. doi:10.1016/j.phymed.2022.154431
Ma, Z., Tang, X., Gao, Y., Wang, H., Yu, P., and Liu, R. (2020). Combined extracts of epimedii folium and ligustri lucidi fructus with budesonide attenuate airway remodeling in the asthmatic rats by regulating apoptosis and autophagy. Evid. Based Complement. Altern. Med. 2020, 2319409, 2319409. doi:10.1155/2020/2319409
Mi, X., Zhang, X., He, S., Zhang, Z., Qi, R., Jiang, J., et al. (2020). Chinese herbal medicine for small cell lung cancer patients: a protocol for a systematic review and meta-analysis. Med. Baltim. 99 (52), e23746. doi:10.1097/MD.0000000000023746
Ming, J. X., Wang, Z. C., Huang, Y., Ohishi, H., Wu, R. J., Shao, Y., et al. (2021). Fucoxanthin extracted from Laminaria Japonica inhibits metastasis and enhances the sensitivity of lung cancer to Gefitinib. J. Ethnopharmacol. 265, 113302. doi:10.1016/j.jep.2020.113302
Ning, D., Jin, M., Xv, T., Sun, J., and Li, M. (2018). Homoisoflavanone-1 isolated from Polygonatum odoratum arrests the cell cycle and induces apoptosis in A549 cells. Oncol. Lett. 16 (3), 3545–3554. doi:10.3892/ol.2018.9085
Pang, C., Zhang, T., Chen, Y., Yan, B., Chen, C., Zhang, Z., et al. (2023). Andrographis modulates cisplatin resistance in lung cancer via miR-155-5p/SIRT1 axis. Funct. Integr. Genomics 23 (3), 260. doi:10.1007/s10142-023-01186-x
Parikh, M., Riess, J., and Lara, P. N. (2016). New and emerging developments in extensive-stage small cell lung cancer therapeutics. Curr. Opin. Oncol. 28 (2), 97–103. doi:10.1097/CCO.0000000000000264
Peng, S., Dong, W., Chu, Q., Meng, J., Yang, H., DU, Y., et al. (2021). Traditional Chinese medicine Brucea javanica Oil enhances the efficacy of anlotinib in a mouse model of liver-metastasis of small-cell lung cancer. Vivo 35 (3), 1437–1441. doi:10.21873/invivo.12395
Qi, R. Z., He, S. L., Li, Y., Zhao, Y. W., Geng, L., He, J., et al. (2023). Retrospective clinical study on integrated Chinese and western medicine in treatment of limited-stage small cell lung cancer. Chin. J. Integr. Med. 29 (8), 675–682. doi:10.1007/s11655-022-3682-9
Qi, T., Li, H., and Li, S. (2017). Indirubin improves antioxidant and anti-inflammatory functions in lipopolysaccharide-challenged mice. Oncotarget 8 (22), 36658–36663. doi:10.18632/oncotarget.17560
Qian, W., Cai, X., Qian, Q., Zhang, W., and Wang, D. (2018). Astragaloside IV modulates TGF-β1-dependent epithelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. J. Cell Mol. Med. 22 (9), 4354–4365. doi:10.1111/jcmm.13725
Qiu, W. L., Hsu, W. H., Tsao, S. M., Tseng, A. J., Lin, Z. H., Hua, W. J., et al. (2021). WSG, a glucose-rich polysaccharide from Ganoderma lucidum, combined with cisplatin potentiates inhibition of lung cancer in vitro and in vivo. Polym. (Basel) 13 (24), 4353. doi:10.3390/polym13244353
Qu, L., Chen, C., He, W., Chen, Y., Li, Y., Wen, Y., et al. (2019). Glycyrrhizic acid ameliorates LPS-induced acute lung injury by regulating autophagy through the PI3K/AKT/mTOR pathway. Am. J. Transl. Res. 11 (4), 2042–2055.
Ren, M., Chen, J., Xu, H., Li, W., Wang, T., Chi, Z., et al. (2023). Ergolide covalently binds NLRP3 and inhibits NLRP3 inflammasome-mediated pyroptosis. Int. Immunopharmacol. 120, 110292. doi:10.1016/j.intimp.2023.110292
Shen, J., Hua, Z., and Chai, Y. (2022). Glycyrrhizic acid protects experimental sepsis rats against acute lung injury and inflammation. Evid. Based Complement. Altern. Med. 2022, 3571800, 3571800. doi:10.1155/2022/3571800
Shen, K. H., Hung, J. H., Chang, C. W., Weng, Y. T., Wu, M. J., and Chen, P. S. (2017). Solasodine inhibits invasion of human lung cancer cell through downregulation of miR-21 and MMPs expression. Chem. Biol. Interact. 268, 129–135. doi:10.1016/j.cbi.2017.03.005
Shen, K. H., Hung, J. H., Liao, Y. C., Tsai, S. T., Wu, M. J., and Chen, P. S. (2020). Sinomenine inhibits migration and invasion of human lung cancer cell through downregulating expression of miR-21 and MMPs. Int. J. Mol. Sci. 21 (9), 3080. doi:10.3390/ijms21093080
Shen, M., Wang, Y. J., Liu, Z. H., Chen, Y. W., Liang, Q. K., Li, Y., et al. (2023). Inhibitory effect of Astragalus polysaccharide on premetastatic niche of lung cancer through the S1PR1-STAT3 signaling pathway. Evid. Based Complement. Altern. Med. 2023, 4010797, 4010797. doi:10.1155/2023/4010797
Shi, W., Hao, J., Wu, Y., Liu, C., Shimizu, K., Li, R., et al. (2022). Protective effects of heterophyllin B against bleomycin-induced pulmonary fibrosis in mice via AMPK activation. Eur. J. Pharmacol. 921, 174825, 174825. doi:10.1016/j.ejphar.2022.174825
Shi, Z. (2013). Abstract 2245: overcoming cisplatin resistance by celastrol in cancer cells. Cancer Res. 73 (8), 2245. doi:10.1158/1538-7445.AM2013-2245
Su, S. H., Sundhar, N., Kuo, W. W., Lai, S. C., Kuo, C. H., Ho, T. J., et al. (2022). Artemisia argyi extract induces apoptosis in human gemcitabine-resistant lung cancer cells via the PI3K/MAPK signaling pathway. J. Ethnopharmacol. 299, 115658. doi:10.1016/j.jep.2022.115658
Sui, Y., Li, S., Shi, P., Wu, Y., Li, Y., Chen, W., et al. (2016). Ethyl acetate extract from Selaginella doederleinii Hieron inhibits the growth of human lung cancer cells A549 via caspase-dependent apoptosis pathway. J. Ethnopharmacol. 190, 261–271. doi:10.1016/j.jep.2016.06.029
Sun, J., Zhang, Q., Yang, G., Li, Y., Fu, Y., Zheng, Y., et al. (2022). The licorice flavonoid isoliquiritigenin attenuates Mycobacterium tuberculosis-induced inflammation through Notch1/NF-κB and MAPK signaling pathways. J. Ethnopharmacol. 294, 115368. doi:10.1016/j.jep.2022.115368
Sun, L. X., Li, W. D., Lin, Z. B., Duan, X. S., Li, X. F., Yang, N., et al. (2014). Protection against lung cancer patient plasma-induced lymphocyte suppression by Ganoderma lucidum polysaccharides. Cell Physiol. Biochem. 33 (2), 289–299. doi:10.1159/000356669
Tang, J. C., Ren, Y. G., Zhao, J., Long, F., Chen, J. Y., and Jiang, Z. (2018). Shikonin enhances sensitization of gefitinib against wild-type EGFR non-small cell lung cancer via inhibition PKM2/stat3/cyclinD1 signal pathway. Life Sci. 204, 71–77. doi:10.1016/j.lfs.2018.05.012
Tímár, J., and Uhlyarik, A. (2022). On-target side effects of targeted therapeutics of cancer. Pathol. Oncol. Res. 28, 1610694. doi:10.3389/pore.2022.1610694
Wang, H., Wu, J., Fan, H., Ji, Y., Han, C., Li, C., et al. (2022). The impact of catalpol on proliferation, apoptosis, migration, and oxidative stress of lung cancer cells based on Nrf2/ARE signaling. Biomed. Res. Int. 2022, 5621341, 5621341. doi:10.1155/2022/5621341
Wang, J., Wu, Q., Ding, L., Song, S., Li, Y., Shi, L., et al. (2021). Therapeutic effects and molecular mechanisms of bioactive compounds against respiratory diseases: traditional Chinese medicine theory and high-frequency use. Front. Pharmacol. 12, 734450. doi:10.3389/fphar.2021.734450
Wang, N., Tan, H. Y., Chan, Y. T., Guo, W., Li, S., and Feng, Y. (2017). Identification of WT1 as determinant of heptatocellular carcinoma and its inhibition by Chinese herbal medicine Salvia chinensis Benth and its active ingredient protocatechualdehyde. Oncotarget 8 (62), 105848–105859. doi:10.18632/oncotarget.22406
Waqar, S. N., and Morgensztern, D. (2017). Treatment advances in small cell lung cancer (SCLC). Pharmacol. Ther. 180, 16–23. doi:10.1016/j.pharmthera.2017.06.002
Wei, C., Yao, X., Jiang, Z., Wang, Y., Zhang, D., Chen, X., et al. (2019). Cordycepin inhibits drug-resistance non-small cell lung cancer progression by activating AMPK signaling pathway. Pharmacol. Res. 144, 79–89. doi:10.1016/j.phrs.2019.03.011
Wu, C., Zhuang, Y., Jiang, S., Tian, F., Teng, Y., Chen, X., et al. (2017). Cinnamaldehyde induces apoptosis and reverses epithelial-mesenchymal transition through inhibition of Wnt/β-catenin pathway in non-small cell lung cancer. Int. J. Biochem. Cell Biol. 84, 58–74. doi:10.1016/j.biocel.2017.01.005
Wu, C. Y., Yang, Y. H., Lin, Y. S., Chang, G. H., Tsai, M. S., Hsu, C. M., et al. (2021). Dihydroisotanshinone I induced ferroptosis and apoptosis of lung cancer cells. Biomed. Pharmacother. 139, 111585. doi:10.1016/j.biopha.2021.111585
Wu, L., Liu, T., Xiao, Y., Li, X., Zhu, Y., Zhao, Y., et al. (2016). Polygonatum odoratum lectin induces apoptosis and autophagy by regulation of microRNA-1290 and microRNA-15a-3p in human lung adenocarcinoma A549 cells. Int. J. Biol. Macromol. 85, 217–226. doi:10.1016/j.ijbiomac.2015.11.014
Wu, T. H., Yeh, K. Y., Wang, C. H., Wang, H., Li, T. L., Chan, Y. L., et al. (2019). The combination of Astragalus membranaceus and Angelica sinensis inhibits lung cancer and cachexia through its immunomodulatory function. J. Oncol. 2019, 9206951. doi:10.1155/2019/9206951
Wu, X., Wang, W., Chen, Y., Liu, X., Wang, J., Qin, X., et al. (2018). Glycyrrhizin suppresses the growth of human NSCLC cell line HCC827 by downregulating HMGB1 level. Biomed. Res. Int. 2018, 6916797. doi:10.1155/2018/6916797
Xi, P., Niu, Y., Zhang, Y., Li, W., Gao, F., Gu, W., et al. (2022). The mechanism of dioscin preventing lung cancer based on network pharmacology and experimental validation. J. Ethnopharmacol., 292, 115138. doi:10.1016/j.jep.2022.115138
Xie, H. J., Zhao, J., Zhuo-Ma, D., Zhan-Dui, N., Er-Bu, A., and Tsering, T. (2019). Inhibiting tumour metastasis by DQA modified paclitaxel plus ligustrazine micelles in treatment of non-small-cell lung cancer. Artif. Cells Nanomed Biotechnol. 47 (1), 3465–3477. doi:10.1080/21691401.2019.1653900
Xu, L., Meng, X., Xu, N., Fu, W., Tan, H., Zhang, L., et al. (2018). Gambogenic acid inhibits fibroblast growth factor receptor signaling pathway in erlotinib-resistant non-small-cell lung cancer and suppresses patient-derived xenograft growth. Cell Death Dis. 9 (3), 262. doi:10.1038/s41419-018-0314-6
Xu, X. Q., Deng, W. Q., Wang, D. Y., Li, M., Kou, D. L., and Zhang, P. T. (2021). Chinese medicine treatment prolonged survival in small cell lung cancer patients: a clinical observation. Chin. J. Integr. Med. 27 (7), 496–501. doi:10.1007/s11655-020-3197-1
Xu, Z., Mei, J., and Tan, Y. (2017). Baicalin attenuates DDP (cisplatin) resistance in lung cancer by downregulating MARK2 and p-Akt. Int. J. Oncol. 50 (1), 93–100. doi:10.3892/ijo.2016.3768
Xueting, C., Jie, Y., Chunping, H. U., and Peng, C. (2017). Dihydroartemisinin enhances the sensitivity of gefitinib in non-small cell lung cancer cells by inhibiting STAT3. Chin. Sci. Bull. 62 (18), 2013–2019. doi:10.1360/N972017-00204
Yang, H., Tong, Z., Shen, L., Sun, Y. U., Hoffman, R. M., and Huang, J. (2020). Brucea javanica increases survival and enhances gemcitabine efficacy in a patient-derived orthotopic xenograft (PDOX) mouse model of pancreatic cancer. Anticancer Res. 40 (9), 4969–4978. doi:10.21873/anticanres.14500
Ye, C., Zhang, N., Zhao, Q., Xie, X., Li, X., Zhu, H. P., et al. (2021). Evodiamine alleviates lipopolysaccharide-induced pulmonary inflammation and fibrosis by activating apelin pathway. Phytother. Res. 35 (6), 3406–3417. doi:10.1002/ptr.7062
Ye, Y. T., Zhong, W., Sun, P., Wang, D., Wang, C., Hu, L. M., et al. (2017). Apoptosis induced by the methanol extract of Salvia miltiorrhiza Bunge in non-small cell lung cancer through PTEN-mediated inhibition of PI3K/Akt pathway. J. Ethnopharmacol. 200, 107–116. doi:10.1016/j.jep.2016.12.051
Ying, Y., Sun, C. B., Zhang, S. Q., Chen, B. J., Yu, J. Z., Liu, F. Y., et al. (2021). Induction of autophagy via the TLR4/NF-κB signaling pathway by astragaloside Ⅳ contributes to the amelioration of inflammation in RAW264.7 cells. Biomed. Pharmacother. 137, 111271. doi:10.1016/j.biopha.2021.111271
You, C., Sun, Y., Zhang, S., Tang, G., Zhang, N., Li, C., et al. (2018). Trichosanthin enhances sensitivity of non-small cell lung cancer (NSCLC) TRAIL-resistance cells. Int. J. Biol. Sci. 14 (2), 217–227. doi:10.7150/ijbs.22811
You, X. Y., Xue, Q., Fang, Y., Liu, Q., Zhang, C. F., Zhao, C., et al. (2015). Preventive effects of Ecliptae Herba extract and its component, ecliptasaponin A, on bleomycin-induced pulmonary fibrosis in mice. J. Ethnopharmacol. 175, 172–180. doi:10.1016/j.jep.2015.08.034
Yuan, J. Y., Tong, Z. Y., Dong, Y. C., Zhao, J. Y., and Shang, Y. (2022). Research progress on icariin, a traditional Chinese medicine extract, in the treatment of asthma. Allergol. Immunopathol. Madr. 50 (1), 9–16. doi:10.15586/aei.v50i1.490
Zhang, J. L., Huang, W. M., and Zeng, Q. Y. (2015). Atractylenolide I protects mice from lipopolysaccharide-induced acute lung injury. Eur. J. Pharmacol. 765, 94–99. doi:10.1016/j.ejphar.2015.08.022
Zhang, Q., Zhu, S., Cheng, X., Lu, C., Tao, W., Zhang, Y., et al. (2018). Euphorbia factor L2 alleviates lipopolysaccharide-induced acute lung injury and inflammation in mice through the suppression of NF-κB activation. Biochem. Pharmacol. 155, 444–454. doi:10.1016/j.bcp.2018.07.025
Zhang, Y., Wang, J., Hui, B., Sun, W., Li, B., Shi, F., et al. (2019). Pristimerin enhances the effect of cisplatin by inhibiting the miR-23a/Akt/GSK3β signaling pathway and suppressing autophagy in lung cancer cells. Int. J. Mol. Med. 43 (3), 1382–1394. doi:10.3892/ijmm.2019.4057
Zhao, X. Y., Yang, Y. Y., Feng, J. L., and Feng, C. L. (2023). Network pharmacology prediction and experimental validation of trichosanthes-fritillaria thunbergii action mechanism against lung adenocarcinoma. J. Vis. Exp. doi:10.3791/64847
Zhu, J., Huang, R., Yang, R., Xiao, Y., Yan, J., Zheng, C., et al. (2021). Licorice extract inhibits growth of non-small cell lung cancer by down-regulating CDK4-Cyclin D1 complex and increasing CD8+ T cell infiltration. Cancer Cell Int. 21 (1), 529. doi:10.1186/s12935-021-02223-0
Zhu, J., Wang, H., Chen, F., Lv, H., Xu, Z., Fu, J., et al. (2018). Triptolide enhances chemotherapeutic efficacy of antitumor drugs in non-small-cell lung cancer cells by inhibiting Nrf2-ARE activity. Toxicol. Appl. Pharmacol. 358, 1–9. doi:10.1016/j.taap.2018.09.004
Zhu, Y., Chai, Y., Xiao, G., Liu, Y., Xie, X., Xiao, W., et al. (2022). Astragalus and its formulas as a therapeutic option for fibrotic diseases: pharmacology and mechanisms. Front. Pharmacol. 13, 1040350. doi:10.3389/fphar.2022.1040350
Zou, X., Huang, Z., Zhan, Z., Yuan, M., Zhang, Y., Liu, T., et al. (2023). The alcohol extracts of Sceptridium ternatum (Thunb.) Lyon exert anti-pulmonary fibrosis effect through targeting SETDB1/STAT3/p-STAT3 signaling. J. Ethnopharmacol. 313, 116520. doi:10.1016/j.jep.2023.116520
Keywords: Chinese medicine extracts, non-small cell lung cancer, small cell lung cancer, pulmonary fibrosis, acute lung injury, treatment
Citation: He Z, Wang Y, Han L, Hu Y and Cong X (2023) The mechanism and application of traditional Chinese medicine extracts in the treatment of lung cancer and other lung-related diseases. Front. Pharmacol. 14:1330518. doi: 10.3389/fphar.2023.1330518
Received: 31 October 2023; Accepted: 27 November 2023;
Published: 06 December 2023.
Edited by:
Baoming Wang, Chengdu University, ChinaReviewed by:
Guo-Kai Feng, Sun Yat-Sen University Cancer Center, ChinaCopyright © 2023 He, Wang, Han, Hu and Cong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianling Cong, Y29uZ3hsQGpsdS5lZHUuY24=; Yue Hu, eXVlaHVAamx1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.