
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 19 January 2024
Sec. Ethnopharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1328903
Background: Throughout the history, nature has provided mankind with most of their basic needs, which include food, shelter, medicine, clothes, flavours, scents as well as raw materials. Given that they are an integral part of cultural heritage, medicinal plants have played a significant role in human healthcare systems around the world. Investigating various biological resources for use as medicines requires ethnomedicinal studies.
Methods: Data on utilization of ethnomedicinal plants from local healers in Kenya’s Mosop Sub-County in Nandi County was documented through open-ended, semi-structured questionnaires. A number of quantitative indices, such as the Use Citation (UC), Informant Consensus Factor (ICF), Use Value (UV), Frequency of Citation (FoC) and Relative Frequency of Citation (RFC) were used to convey the potential medical benefits, vitality and variety of the ethnomedicine.
Results: 102 informants provided information on 253 ethnomedicinal plant species, classified into 74 families. There were 249 native plant species identified, along with few exotic species: Senegalia senegal (L.) Britton, Persea americana Mill, Carica papaya L. and Solanum betaceum Cav. Of all recorded species, 32% and 27% were herbs and trees, respectively. Among plant parts, leaves were most frequently utilized (27%) and roots (26%), while decoctions (21%) were the most widely used formulations. The dominant family was Asteraceae, with 28 species, followed by Lamiaceae, with 19 species. The highest ICF value was 0.778 for a number of parasitic and infectious illnesses, including ringworms, athlete’s foot rot, tetanus, typhoid, intestinal parasites, abscesses, malaria, and amoebiasis. The study’s data validates the region’s widespread use of traditional medicinal plant remedies.
Conclusion: The current study will lay a foundation of knowledge for future research investigations. The abundance of knowledge regarding ethnomedicinal species and their medicinal applications will stimulate further phytochemical and pharmacological research, which could lead to the discovery of potentially significant pharmaceuticals.
Plants have been employed for generations in traditional medicinal therapies by different communities around the world (Vitalini et al., 2013; Nguyen et al., 2019). Herbal medicine is strongly connected to African traditional medicine and is occasionally used in connection with various African societies. It is the most established and extensively utilized primary health self-care system in rural areas, with utilization across all countries and cultures (Idu et al., 2010; Mutie et al., 2020). According to estimates, almost 70% of Kenyans have at some point in their lives used medicinal plants for medical purposes despite the availability of conventional pharmaceuticals (Awiti, 2014; Nankaya et al., 2020). The utilization of plant based medicinal products for the treatment and management of various diseases continues to flourish globally. This is due to a myriad of challenges including decreased efficacy or ineffectiveness of available drugs in treatment of diseases such as malaria, HIV/AIDS, asthma, cancer and diabetes, drug and multidrug resistance as well as the slow pace of discovery of pharmaceuticals with novel modes of action (Mahomoodally, 2013; Mutie et al., 2020). Furthermore, the use of traditional medicinal preparations is always cheap, readily available and easy to prepare and use compared to the contemporary medication which has an associated expense (Antwi-Baffour et al., 2014; Kimutai et al., 2019; Wanjohi et al., 2020a).
Kenya is home to 6,293 native species of vascular plants, of which over 1,200 are valuable medicinally. This diversity makes Kenya a hotspot for ethnomedicinal biodiversity (Melly et al., 2020; Mutie et al., 2020; Mutungi, 2023). Various ethnobotanical researches involving different ethnic groups in Kenya have been done and local communities have accumulated vital traditional knowledge regarding their usage (Jeruto et al., 2008a; Omwenga et al., 2015; Kimutai et al., 2019; Wanjohi et al., 2020b; Mutie et al., 2020; Nankaya et al., 2020). Community members who identify themselves as belonging to a certain culture share experiential knowledge, which serves as the primary representation of ethnobotanical knowledge within a given microsystem. The core body of knowledge is usually associated with the resources that are most easily accessible to the local population; unfortunately this is altered by the entrance of foreign ideas and practices (Ashebo, 2019; Turner et al., 2022). Communities’ cultural identities are defined in part by the indigenous knowledge of how to use and prepare herbs used in traditional medicine. This information also serves as proof of the communities’ historical linkages. In spite of this, the majority of ethnobotanical knowledge about herbal remedies and therapeutic approaches is still not documented (Leonti and Casu, 2013; Zubaidah et al., 2020; Seile et al., 2022). This is exacerbated by the fact that ethnomedicinal knowledge is primarily transmitted orally in Kenyan communities, to limited family members who might not even be eager to acquire the skill (Kipkore et al., 2014; Omwenga et al., 2015).
Ethnomedicine as a science enables the conversion of traditional knowledge from African traditional medicine into knowledge-based research field. This includes understanding the traditional healthcare system and identifying plant-derived substances for therapeutic purposes (Jadid et al., 2020; Tefera and Yihune, 2019; Turner, 2014). Thus, ethnobotanical research that records the uses of plants for medicinal purposes serves as the basis for future phytochemical and pharmacological studies that may form the process of development of innovative treatments and products as well as for the sustainable management of plants (Heinrich, 2000; Jadid et al., 2020). Furthermore, there is a shift in lifestyle globally where there is growing demand for natural organic products. The demand for natural therapeutic products on both domestic and globally has in the recent times increased and sales of products made from medicinal plants such as herbal nutritional supplements, herbal-based cosmetics and herbal healthcare formulations has resulted in considerable economic gains (Ved and Goraya, 2007; Dzoyem et al., 2013; Biagi et al., 2016; Samet and Cikili, 2016). Secondary metabolites from plants can also be valorized for use in the agriculture sector as green and healthy alternatives to chemical pesticides and insecticides because they are generally less toxic, biodegradable, harmless to unintended creatures, and do not affect hosts. Hence the reason plants become the better option as opposed to the synthetics (Naboulsi et al., 2018; Fortunati et al., 2019; Khursheed et al., 2022; Ngegba et al., 2022; Zhao et al., 2022).
While a number of ethnobotanical investigations have been conducted in various locations around Nandi County, including the Aldai sub county (Jeruto et al., 2008b), Tindiret sub county (Kigen et al., 2016), none has been conducted in Mosop Sub-county to gather information about the ethnomedical plants and their applications. Thus, the current study set out to document ethnobotanical knowledge amongst the residents of Mosop Sub County traditionally use. The collected data was qualitatively and quantitatively analyzed using several statistical indices to ascertain the medicinal plant diversity, ethnomedicinal richness as well validate the importance of the cited medicinal species. To get the most out of herbal remedies, researchers should investigate appropriate preparation and dosage formulation techniques as well as traditional medicinal practitioner must work with scientific institutions to aid in the discovery of pharmaceutically active products based on indigenous knowledge.
Mosop Sub County, which covers 602 km2, is situated in Kenya’s North Rift Valley to the north of Nandi County. The county is bordered by Uasin Gishu County to the North and East, Kericho County to the South East, Kisumu County to the South, Vihiga County to the South West and Kakamega County to the West (Figure 1). The Equator defines Nandi County from the south, and it stretches north to latitude 0034′N. Both the eastern and western boundaries extend to longitudes of 35025′E and 34045′E, respectively. Rich volcanic soils, chilly rainy environment with temperatures between 15°C and 26°C, and an annual rainfall of 1,200 to 2,000 mm characterize it (http://nandi.co.ke/). This results in a variety of ecological zones, including woods, shrubs, and savannah grassland with swamps along the escarpment, and large areas of the Nandi forest at the top. This region offers a very large topography with a rich biodiversity of various plant species, as well as, in some cases, largely intact native forests along the Teressia, Kaptaroi, and Nandi North forests, which are close to the rich biodiversity of Kakamega forests. The region’s plant biodiversity is exceptionally rich and diverse, and it is frequently said to be a transitional zone between the afro-montane forests of Kenya’s highlands and the lowland forests that traverse Africa from the Zaire basin to western Kenya (Blackett et al., 1994; Girma et al., 2015). Trees, small trees, bushes, shrubs, pteridophytes, creepers, lianas, climbers and succulents are just a few of the many plant species that make up the natural ecosystem of the study site. The traditional medicinal practitioners treat and manage a range of illnesses and diseases affecting the locals by using the many medicinal plants species present in the beautiful distinct habitats in the study site. The study site was chosen because it was the first of its kind in the area and the ease of being able to interact with the respondents in the local dialect.
The National Commission for Science and Innovation (NACOSTI), Kenya, provided the research permit (NACOSTI/P/21/12175). Before the interview, all respondents provided oral informed consent. The respondents were informed that their information would only be used for scientific research purposes and would not be used for commercial gain. They were also assured that their identities would remain anonymous.
Guided field trips and an exploratory semi-structured questionnaire approach were used in the study. One or more informants observed the particular plants in their natural habitat during field trips, gathering samples for subsequent botanical identification. Using this technique for gathering data, we collected medicinal plants for identification and preservation, gathered botanical knowledge, and obtained information on their therapeutic applications.
The kind help of local administration personnel allowed for the identification of herbal practitioners prior to the field survey. The traditional medicinal practitioners were chosen based on their willingness to participate in consultative meetings with researchers. This was important since the practitioner needed to know exactly why we were asking for information before they could participate. The interviewees were either active practitioners at the time of the study or former practitioners who had to cut back from the practice owing to aging, commitments or any other personal reasons. Semi-structured open-ended questionnaires were used to record the interviews conducted both at the practitioners’ homesteads and during field sampling with practitioners. All interviews were conducted in the native Nandi dialect and subsequently translated into English. Data gathered included the informant’s name, age, sex, occupation, and educational attainment as well as botanical details including the plant’s local name, source, parts used, and therapeutic uses, as well as preparation and administration techniques. A voucher specimen for the herbarium was collected for every plant species mentioned using standard botanical procedures for further identification and confirmation using the relevant taxonomic keys at Egerton University. Images of all of the mentioned medicinal plants were also taken in order to support the identification processes. The field work was performed between August 2019 and June 2021 where all the traditional practitioners’ responses to structured questionnaires and interviews were used to gather pertinent information.
The data analysis process comprised both qualitative and quantitative approaches. For the purpose of investigating the sociocultural influence on ethnomedicinal data, gender and age of the practitioner were analyzed. Between the quantity of reported medicinal plants species used and gender of the practitioner, a one-way ANOVA analysis was run on Version 25.00 of SPSS. The data was expressed as the mean and standard error of the mean on the quantity of plants species reported by each gender. To further examine any potential relationships between ethnobotanical data and demographics of the practitioners, regression analysis was done to clearly indicate the underlying relationship between the age of the practitioners’ and the quantity of medicinal plant species reported. Moreover, the study tested the validity of the data quantitatively using UC, UV, RFC, FL and ICF.
The formula for calculating the informant consensus factor (ICF) is ICF = Nur-Nt/Nur-1, where Nur denotes the number of use reports from informants for a particular plant-use category and Nt denotes the total number of species that are used for that plant use category for all informants (Canales et al., 2005). ICF values fall between 0.00 and 1.00. UV was used to determine the relative importance on uses of the plant species and was calculated from the sum of the informant species use citation for a particular medicinal plant species divided by the total number of informants (Ni)who reported that species. The UV was calculated according to Hoffman and Gallaher as follows: UV = [∑UVis/(Ni)] (Hoffman and Gallaher, 2007). RFC is an index determined by dividing the number of informants citing the use of a medicinal plant species also known as frequency of citation (FC) by total number of informants in the survey (N) was also calculated using the formula RFC = FC/N (0 < RFC < 1). Where FC = Number of times a particular species was mentioned/total number of times that all species were mentioned × 100 (Tardío and Pardo-de-Santayana, 2008). Fidelity level determines the specific uses of each plant species and preference over other species. It expresses the specificity of disease treated by a reported medicinal plant species. It is calculated by using a formula adopted by Khan et al. (2015). FL= (Ip/Iu) × 100. Where “Ip” is the number of informants who share their knowledge about a given species for the treatment of a specific disease and “Iu” is the total number of all informants who reported all uses about a given plant species (Al-Qura’n, 2009).
The study included 102 practitioners as participants. They were engaged in various occupations to support themselves, such as: Carpentry (2), Casual labours (7), Catering (1), Cook in local school (1), Electrical job (1), Farming (41), Hawking (1), Herbalist (11), Herdsman (2), House wives/Home makers (11), Masonry (1), Milk vending (3), Plumbing (1), Retired civil servants (2), Security (2), Shopkeepers (2), Tailoring (3), Teaching (2), Trading (2), traditional midwifery (1), boutique (1) and businesses (4) as presented in Figure 2. The age of the practitioners’ ranged from 35 to 97 years with highest number of practitioners’ being between 45 and 49 years of age. Only seventeen practitioners’ were over 70 years of age. Forty five practitioners’ were between 35 and 49 years old, while forty practitioners’ were between 40 and 69 years old. Among the age groups male practitioners’ were more than the female (Table 1). There were 74 male and 28 female practitioners’ that gave 72.5% and 27.5%, respectively. Most of the practitioners’ had an impressive experience of practicing traditional medicinal therapy with the highest numbers having 10–20 years of experience (37.3%) The numbers declined progressively with the age of the practitioners’ with only 3% of the practitioners’ having the longevity of experience of above 60 years (Table 1). The study observed that older practitioners’ offered rich information that was more profound and detailed since they possessed a greater amount of significant oral tradition knowledge acquired through experience obtained through a lengthy accumulation of generational old traditional medical procedures and therapies as demonstrated by regression analysis done on informant’s age versus the number of medicinal plants species reported by them.
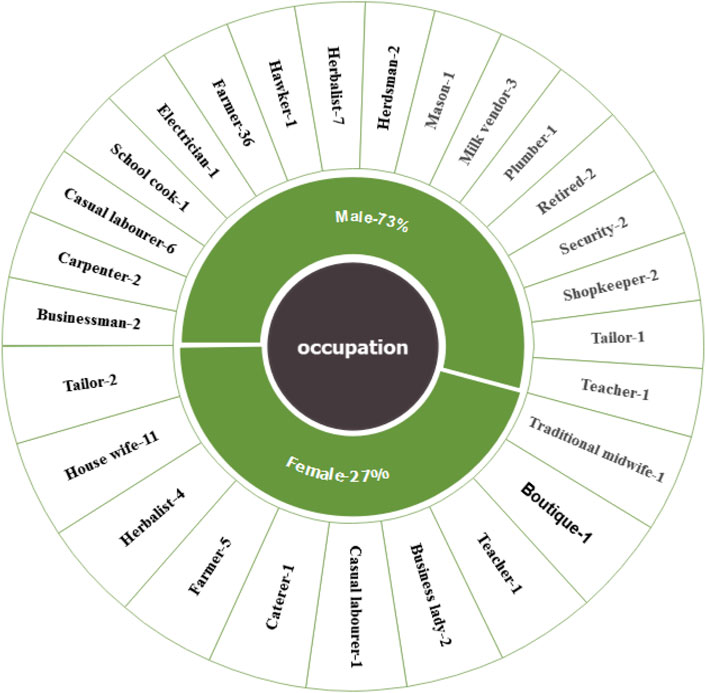
FIGURE 2. Occupation of the respondents from the study area presented per gender. The inner part of the chart in green shows the gender and the outer part giving the specific occupation per gender.
Knowledge transfer was mainly done through apprenticeship and with a family member (65%), apprenticeship and a practitioner (16%), whereas knowledge acquisition via apprenticeship and community elders’ as well as community elders’ was at 8% each. The least mode of acquisition was at 3% through a practitioner. It is apparent from Table 1 that the fundamental sources of knowledge held by the elderly can be attributed to the experiences gathered every day of their lifetime on the traditional usage of medicinal plants and its related skill set. In terms of education, we observed that the majority of the practitioners’ had only basic schooling (24.5% were illiterate), 18.6% had completed both primary and secondary school (sixteen percent each), and only 3.9% had completed their tertiary education (Table 1). The respondents were actively engaged in training for knowledge transfer through a combination of channels at 53.9% as depicted (Figure 3). This comparatively fair rate of knowledge transfer may be due to the fact that most conventional practitioners frequently “ring-fence” their specific area of expertise or pass away without passing on their knowledge and skills to younger generations. Indeed, many practitioners preserved their therapeutic healing secrets; when they pass away without passing on their expertise, that information also disappears with them. It is important to highlight the fact that the younger generation has considerably less knowledge about forests, medicinal plants and their traditional application owing to the fact that they have been cut off from their communities by the current contemporary educational system; and graduates and the learning processes distances them from their villages and their traditions. These result findings further indicate that 70% of the respondents treated various ailments in collaboration with contemporary medicine as well as 71% of the practitioners were making referrals to seek further modern medical attention in cases where the prescribed traditional therapy was not well effective (Figure 3). However, all the practitioners’ had a strong perception that their therapies and remedies were very effective.
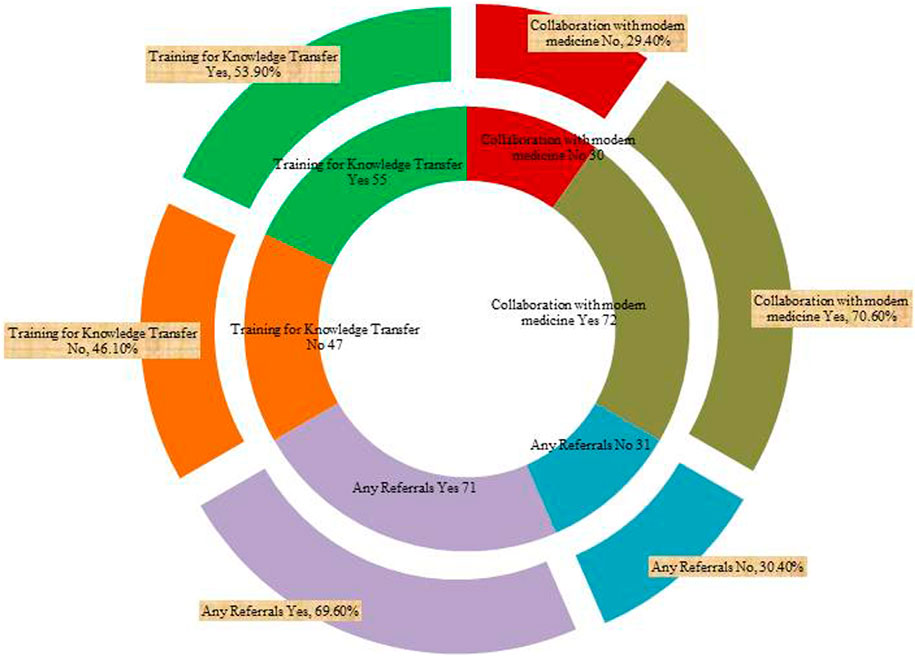
FIGURE 3. Count of respondents on referrals, training and collaboration with modern medicine. The inner part of the chart gives the numbers on referrals, training and collaboration whereas the outer part of the chart gives their corresponding percentages.
To find out if there were differences in diversity of knowledge in medicinal plant species used to treat and manage different ailments and diseases in Mosop amongst both genders of the practitioners’, one-way ANOVA demonstrated that the number of medicinal plants species cited by women and men practitioners’ were not significantly different statistically (p = 0.844). Despite this, male practitioners reported, on average, a higher medicinal plant species count (152.0401 ± 7.253) than their female counter parts (149.250 ± 12.700) (Mean ± S.E.). This corresponded with the study’s greater participation rate of male practitioners. However, on considering the age of the respondents and their knowledge on medicinal plants, there was a positive correlation (R2 = 0. 277, p < 0.05) as in Figure 4. As a result, older individuals typically had greater knowledge of medicinal plants than younger practitioners’.
The most dominant families in terms of the highest reported medicinal plant species were Asteraceae (28 species), followed by Lamiaceae (19 species), Fabaceae (19 species) and Acanthaceae (12 species) as in Figure 5. 34 families were represented by only a single medicinal plant species. We identified 253 species divided into 74 families of medicinal plants used in the treatment and management of different ailments and diseases in Mosop Sub-County (Supplementary Table S1). The degree of ethnobotanical richness in the study area is directly accredited to its diverse flora. In this study most of the medicinal plant species were herbs (32%), followed by trees (26%), shrub/small trees (12%), shrub/tree at 12%, climbers (8%) and others (2%) as in Figure 6. The only epiphyte was Phragmanthera usuiensis (Oliver) M.G.Gilbert. Medicinal plants status was assessed using the criteria from the International Union for Conservation of Nature (IUCN) (https://www.iucnredlist.org). The study showed that Warburgia ugandensis Sprague as critically endangered (CE), Tiliacora kenyensis Troupin as endangered (EN); Polyscias kikuyuensis Summerh. as near threatened (NT); Solanum betaceum Cav. and Carica papaya L. as data deficient (DD); Zanthoxylum chevalieri Engl., Justicia flava Vahl and Prunus africana (Hook.f.) Kalkman were vulnerable (VU). The remaining medicinal plant species assessed as of least concern (LC) and not evaluated (NE) were 101 and 144, respectively. Juniperus procera Hochst. ex Endl., Justicia flava Vahl, Microglossa pyrifolia (Lam.) Kuntze, Carica papaya L., Ehretia cymosa Thonn., Olea welwitschii Gilg & G.Schellenb and Protea gaguedi J.F.Gmel. were assessed as LC with an alert that their numbers were decreasing.
The most commonly used plant parts were leaves (27%), followed by the roots (26%), stem bark (11%), whole plant (6%), stems, shoots, seeds and root bark 3% each; aerial part and leafy twigs (2%) and 1% were the gum, tuber inflorescence, tender stalks, sap, resin, pods, panicles, oil, latex, flower buds and berries as in Figure 7. The leaves were the portion that was most frequently used. The roots and the stem bark were also the most preferred parts of the medicinal plant parts to be used in preparing the herbal remedies. Tender stalks, flowers and the fruits with 48, 38 and 43 entries were also amongst the most popular parts of the medicinal plant sort by the respondents. The most used preparation method was the decoction (21%), followed by extract (14%), Infusion (13%) and the least medicinal preparation were in form of suppositories, lotions, sprays and syrups (Figure 8). Due to its ease of use and preparation, the decoction method was more widely used. While using the decoctions, some additives, such as honey, goat soup, ghee or milk were added to change their flavour. Extracts preparations were obtained adding little water to macerated/pounded/pulverized medicinal plant material with or without filtration. Macerated/pounded/pulverized/powdered medicinal plant material were soaked in hot or cold water and filtered to prepare infusions. The choice of infusion method depended on the desired end product and the properties of the plant material being infused and more so the ailment it is supposed to treat or manage. Closely related to the extract and the infusions were the juice preparations where by the liquid was extracted from medicinal plant material without the addition of any solvent or solution to aid the process. Juices were made exclusively from the raw natural phytochemical constituents of the specific medicinal plant species; no additional ingredients were added.
The present work was the first ever study to record quantitative data of the medicinal plants of Mosop Sub County in Nandi County, including relative frequency of citation, use value, use citation, informant consensus factor and relative frequency of citation. The UV of each medicinal plant was determined to assess the commonness in use of each plant in the study area of Mosop. The UV of the encountered medicinal plant species ranged from 0.53 to 36.54 (Supplementary Table S2). The important medicinal plant species with high use value were Vachellia nilotica (L.) P. J. H. Hurter & Mabb., Vangueria infausta Burch., Zanthoxylum asiaticum (L.) Appelhans, Groppo & J.Wen, Clutia abysinicca Jaub.& Spach; and Croton macrostachyus Del. While a low value may indicate that a plant is infrequently used, it does not always indicate inefficiency. The lower UV was caused by respondents’ lack of knowledge about the plant species. Cyathula cylindrica Moq (1.89), Scepocarpus hypselodendron (Hochst. ex A.Rich.) T.Wells & A.K.Monro (1.57) and Tiliacora triandra Troupin (0.53) had the low use values.
The range of RFC was from 0.53 to 1.00. The highest value of RFC (1.00) was found in Lantana trifolia L, Ziziphus mucronata Wild, Tragia brevipes Pax, Zanthoxylum chalybeum Engl., Erythrococca Bongensis Pax, Grewia similis K. Schum, Entada abyssinica Steudel ex A.Rich. and Warburgia ugandensis Sprague (Supplementary Table S2). These result findings shows how frequently these particular plant species are used in the study area to prepare remedies for different ailments. The International Classification of Diseases (ICD-10) version of 2016 aided the clustering of various diseases and disorders into several categories. The entire spectrum of illnesses, ailments, traumas, and other connected medical problems is represented by ICD-10. Based on the documented use of medicinal plant species in the study, 18 categories were identified. ICF values range from 0.00 to 1.00. Higher ICF values are indicative of the fact that only a handful of medicinal plant species are recognized to be given by a higher number of practitioners, whereas lower values show that practitioners are at odds over which species to use in treating or managing a specific ailment or disease. The 18 diseases categories demonstrate the wide range of applications of medicinal plant species from the study area. The ICF values for each ailment category ranged from 0.13 to 0.78 (Table 2).
A relative informant consensus regarding the usage of medicinal plants species to treat or manage an illness or diseases is indicated by an ICF score greater than 0.5. On the other hand, ICF values less than 0.5 suggested that practitioners’ exchange little information about medicinal plant species therapies. The highest ICF (0.778) was reported for the category of certain infectious and parasitic diseases with 225 medicinal plant species and 1,010 UCs, followed secondly by the diseases of the respiratory system category (0.777) with 189 plant species and 884 UCs. In third place was digestive system diseases category (0.770), with 217 medicinal plant species and 942 UCs. Our ICFs values shows that the practitioners’ have a strong consistent know-how when it comes to choosing and applying medicinal plants species to treat and manage illnesses and diseases that gave high ICF values. The highest ICF (0.778) reported for the category of certain infectious and parasitic diseases was dominantly due to a high citation of respiratory associated conditions. This could be attributed to the climate in the research area, as well as the fact that relative clinical indications are more prevalent for locals seeking the services of the practitioners’ to recognize easily.
Our results showed that FL values ranged from 10% to 93% (Table 3). Senegalia senegal (L.) Britton demonstrated the highest FL for throat infection (93%), followed by Gynandropsis gynandra (L.) Briq. (92.86%) and Baccharoides lasiopus (O.Hoffm.) H. Rob. (91.67%) for relieving body pain. Low percentage of FL belonged to Hibiscus diversifolius Jacq used in nerve diseases (10.10%) and heart diseases and fluid retention at 11.11%. All low FLs in this study were conditions managed using Hibiscus diversifolius Jacq and Triumfetta macrophylla K. Schum (10.10%–21.21%) shared the same local name “Meswot” belonging to the family Malvaceae. The low fidelity levels observed could be the due to the limited number of species from this family used to treat and manage different ailments and diseases. Additionally, it may suggest that Mosop residents in Nandi County are not well-informed about the utilization of this medicinal shrub. Medicinal plant species with high FL values can be utilized as a basis of further phytochemical and pharmacological evaluation to identify significant bioactive chemicals compounds. This emphasizes how vital it is to use an ethnomedicinal focused method of bio prospecting in order to identify and find new phytocompounds or plant-based products that may be used in a variety of disciplines.
The study found a precise correlation between the relative relevance of using medicinal plants and the local importance of each species (Figure 9). The Pearson correlation coefficient between RFCs and UVs was 1.00 (p-value <0.05). Despite this finding, some medicinal plant species exhibited high RFC and UV values, whereas others had low UV values but were still highly significant to the community. For instance, Tiliacora triandra (Colebr.) Diels with the least UV of 0.53 had an RFC of 0.53; Tabernaemontana stapfiana Britten had a UV of 0.55 and RFC value of 0.55; Sida cordifolia L had a UV of 1.57 and an RFC of 0.73 and Leucas calostachys Oliv. Had a UV of 1.89 and RFC of 0.8 (Supplementary Table S2). Their RFC values are above the half mark (0.5) suggesting that these specific species are commonly used in the study area to prepare remedies for various illnesses and diseases. It simply indicates that the subjective worth these botanical indexes hold for the local population is not always reflected in them. Therefore future research could look into such medicinal plant species with the highest RFCs would be the choice of medicinal plant species for future research on bioactive phytochemicals, drug discovery and development. These medicinal plant species were very important from a community’s medicinal perspective.
The importance of medicinal plants for healthcare is one reason why research on them continues to receive a lot of attention both nationally and globally. It is imperative to have a thorough knowledge both scientifically and culturally through acquired traditional knowledge to promote the use traditional medicinal therapies as an important complement to conventional medicine. Therefore, more research must be done to ascertain the validity and efficacy of medicinal plants before making them available to humans as an alternative medicine. The current study found that the residents hold a very rich cultural tradition of medicinal plant use in treatment and management of different ailments and diseases. In this study, male practitioners’ were more than their female counter parts. However, the range of plant species used and their mode of preparation were quite comparable in both male and female healers. Age of the respondents on the other hand showed a positive correlation to the knowledge on the diversity of medicinal plant species used by the community. The older responders provided more information since they have access to a greater amount of the oral tradition knowledge passed down from generations before them (Miguéis et al., 2019; Dapar et al., 2020; Weldearegay and Awas, 2021). This outcome is based on a great deal of experience, indicating that knowledge on medicinal plants develops over a long period of time (Dapar et al., 2020; Khakurel et al., 2022).
Torres-Avilez et al. (2016) also did not find a significant difference between men and women in terms of their knowledge on traditional herbal medicine because these differences are not unidirectional and can only be detected on small scales of studies. A greater knowledge gap between both genders is not likely or improbable (Torres-Avilez et al., 2016; Khakurel et al., 2022). There have also been various reports of studies on ethnomedicinal knowledge and applications among Kenya’s various communities consistent with the current study reporting a higher proportion of male participants than the females (Kimondo et al., 2015; Ochwang’i et al., 2014; Wanjohi et al., 2020b) and indeed other regional and international studies (Amjad et al., 2017; Faruque et al., 2018; Okori et al., 2022). However, these findings does not support the notion that women are considered repositories traditional medicinal knowledge owing to their critical role as care givers in every community setting (Imperato, 1981; Rasmussen, 1998; Tugume and Nyakoojo, 2019).
The most noticeable families in terms of the greatest number of species of medicinal plants documented were Asteraceae, Lamiaceae, Fabaceae and Acanthaceae. This study findings supports evidence from previous observations where Asteraceae was among the most reported families with species used dominantly in traditional therapeutic preparations due to the large number of its bioactive phytocompounds from Asteraceae (Jeruto et al., 2008b; Gakuya et al., 2020; Mokua et al., 2021). The fact that the same plant species are used to cure the same illness in several locations demonstrates their vast spread and the fact that these plant species are successful in treating the precise illnesses (Bekalo et al., 2009; Amjad et al., 2017; Faruque et al., 2018; Tugume and Nyakoojo, 2019). Fabaceae is the largest plant family in Kenya with 576 species, followed by Asteraceae (403), Malvaceae (219), Lamiaceae (206) and Euphorbiaceae (219) (Zhou et al., 2017; Nankaya et al., 2020). Asteraceae and Lamiaceae species are herbaceous weeds that grow in disturbed regions, making them easily accessible (Nankaya et al., 2020).
The widespread use of herbaceous medicinal plants may be due to their abundance in the research area. Every part of the plant was employed by the local herbalists, but this study indicated that the leaves were the most often used component. This is probably because they are the easiest to gather and are likely to contain the most bioactive compounds. Our results corroborate those of (Tugume and Nyakoojo, 2019; Abdullah et al., 2021; Usman et al., 2021) who found that leaves were the most often used component of the plants. We realized that the most preferred preparation method was decoction. This is consistent with other research that found decoction to be the most popular preparation technique due to its simplicity in preparation (Salinitro et al., 2017; Nankaya et al., 2019; Junsongduang et al., 2020).
Medicinal plant species with high use may have a great potential for healing illnesses, however medicinal plant species with low RFC or UV likewise ought not to be disregarded as there is a possibility that a gradual loss of knowledge could result for not providing information about such medicinal plant species to the next-generation (Zenderland et al., 2019; Cordero et al., 2022; Okori et al., 2022). Identification of the most significant medicinal plant species depends on profound quantitative analysis of data and subjective interpretation of ethnobotanical data acquired in the field to ascertain their authenticity for development of marketable products. As their preferred usage may put their natural populations at risk from overharvesting, these species should also be given priority for conservation (Amjad et al., 2017; Wali et al., 2022; Ullah et al., 2023).
High ICF values are obtained when a considerable number of informants report to have used one or a small number of medicinal plant species to treat or manage a certain illness or diseases. Low ICF values on the other hand suggest that practitioners’ have divergent opinions on the best medicinal plant species to utilize. Additionally, a low ICF score indicates that less traditional treatments are being used due to the accessibility of conventional medications that offer contemporary substitutes for traditional medicinal therapies (Friedman et al., 1986; Faruque et al., 2018). The ICF values may vary from culture to culture due to the variations in medicinal plants species that are identified and used in various regions as well as the illnesses and diseases that these medicinal plants species are used to treat and manage. High ICF values can be utilized to pinpoint very significant medicinal plant species while looking for biologically active compounds.
The study’s findings showed that there is a significant diversity of medicinal plants in Mosop of Nandi County of Kenya. This being the first ethnobotanical undertaking in the study site it forms a basis of knowledge for future research. The wealth of information on ethnomedicinal plant species and their therapeutic uses could inspire further phytochemical and pharmacological investigations that may result in the development of potentially significant pharmaceuticals. To use traditional medicinal preparations as valuable complement to conventional medicine, more research must be done to ascertain the validity and efficacy of the plants before extending their use to other communities. Elderly practitioners were the main sources of indigenous knowledge and are frequently knowledgeable about the local flora and fauna. Respect for their knowledge must be shown and it must be further investigated, documented, validated, and applied to the advantage of both the community and the general public. Medicinal plant species with the highest use reports were used to treat certain infectious and parasitic category of diseases. The present study’s findings can be used to create or enhance initiatives such as in situ establishment of medicinal plant gardens at particular sites in collaboration with the local population.
The study documented the diversity of medicinal plants used by the community of Mosop in Nandi-Kenya and gathered data from traditional medicine practitioners about the different parts used and the most effective way to administer them. Utilizing statistical methods, this study shows the relationship between the identified disease categories and the medicinal plant species used by determining the quantitative relevance of the data collected using a variety of indicators. The results show the diversity and richness of 253 species of medicinal plants spanning 74 families. The medicinal plant species that have been used to treat specific infectious and parasitic disease categories have the most use reports. The wealth of information from the study will encourage further phytochemical and pharmacological investigations that may result in the development of potentially significant pharmaceuticals. Global warming is having an increasing impact on biological richness, thus the world will have to deal with its effects as they become more obvious. The results, in our opinion, will be of interest to environmental conservationists who are trying to support biodiversity through initiatives such as the in situ establishment of medicinal plant gardens at particular sites in collaboration with the local population.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
ZM: Data curation, Resources, Visualization, Writing–original draft, Writing–review and editing. SN: Conceptualization, Formal Analysis, Methodology, Validation, Writing–review and editing. FT: Data curation, Investigation, Resources, Writing–original draft, Writing–review and editing. SI: Conceptualization, Methodology, Resources, Writing–original draft, Writing–review and editing. MO: Conceptualization, Data curation, Resources, Supervision, Writing–review and editing.
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
This research is a component of the doctoral dissertation (Ph.D.) of ZM. We sincerely thank the survey respondents for providing their information and field assistance for research logistical support. Thank you also to the authors for the valued contributions. We are also grateful to the local administration and forest service officer of Nandi County, Kenya for facilitating the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1328903/full#supplementary-material
Abdullah, W. O., Omar, B., Abd Ghani, M. K., Unyah, N. Z., Hamat, R. A., Rayani, M., et al. (2011). In vitro antiplasmodial activity and cytotoxicity of ten plants used as traditional medicine in Malaysia. J. Sains Kesihat. Malays. 9 (2), 5–8.
Abdullah, , Khan, S. M., Kashif, R. A., Haq, Z. U., Ahmad, Z., Haq, A. U., et al. (2021). “Ethnobotanical appraisal of medicinal plants from Bajaur; A remote area of the Khyber Pakhtunkhwa province of Pakistan,” in Ethnobiology of mountain communities in asia (Cham: Springer), 277–293.
Abioye, A. V., Mohammed, Z., Ahmed, A., and Ayeni, A. (2019). Evaluation of the analgesic potential of Basella alba (L.) leaves (Basellaceae). Trop. J. Nat. Prod. Res. (TJNPR) 3 (1), 22–25. doi:10.26538/tjnpr/v3i1.5
Aboyade, O., Yakubu, M., Grierson, D., and Afolayan, A. (2009). Studies on the toxicological effect of the aqueous extract of the fresh, dried and boiled berries of Solanum aculeastrum Dunal in male Wistar rats. Hum. Exp. Toxicol. 28 (12), 765–775. doi:10.1177/0960327109354545
Addo-Mensah, A., and Holland, D. P. (2022). Evaluation of the antimicrobial activity of vangueria volkensii bark, fruit, leaf, and stem extracts. J. Med. Plants 10 (2), 208–214. doi:10.22271/plants.2022.v10.i1c.1383
Adebayo, E. A., and Ishola, R. O. (2009). Phytochemical and antimicrobial screening of crude extracts from the root, stem bark, and leaves of Terminalia glaucescens. Afr. J. Pharm. Pharmacol. 3 (5), 217–221.
Adedapo, A. A., Jimoh, F. O., Koduru, S., Afolayan, A. J., and Masika, P. J. (2008). Antibacterial and antioxidant properties of the methanol extracts of the leaves and stems of Calpurnia aurea. BMC Complement. Altern. Med. 8, 53–58. doi:10.1186/1472-6882-8-53
Adeleye, O., Bamiro, O., Bakre, L., Badru, A., Balogun-Agbaje, O., Olusola, A., et al. (2021). In vivo anti-inflammatory assessment of a topical formulation containing Ehretia cymosa extract mediated-silver nanoparticles. Niger. J. Pharm. Res. 17 (2), 179–188. doi:10.4314/njpr.v17i2.4
Adewole, E., Yusuf, B., Ebitimitula, O. E., Ojo, A., Adewumi, D. F., Oludoro, O., et al. (2022). Phytochemicals profile and in-vitro antidiabetic potentials of fractionated extracts of Entada africana and Leptadenia hastata. Sci. Pharm. Sci. 3 (37), 65–73. doi:10.15587/2519-4852.2022.255744
Adongo, J. O., Josiah, O. O., Alice, W. N., and Joseph, W. M. (2012). Antimicrobial activity of the root extracts of tylosema fassoglensis schweinf. Torre & hillc (caesalpiniaceae). Egerton: Egerton University.
Agunu, A., Yusuf, S., Andrew, G. O., Zezi, A. U., and Abdurahman, E. M. (2005). Evaluation of five medicinal plants used in diarrhoea treatment in Nigeria. J. Ethnopharmacol. 101 (1-3), 27–30. doi:10.1016/j.jep.2005.03.025
Akhtar, M. A., Raju, R., Beattie, K. D., Bodkin, F., and Münch, G. (2016). Medicinal plants of the Australian aboriginal Dharawal people exhibiting anti-inflammatory activity. Evidence-Based Complement. Altern. Med. 2016, 2935403. doi:10.1155/2016/2935403
Akindele, A. J., Salako, O. A., Sofidiya, M. O., Ajibulu, A. J., Osiagwu, D. D., and Adeyemi, O. O. (2016). Gastroprotective effects of the aqueous seed extract of Entada gigas (Linn.) Fawc. and Rendle (Fabaceae) in ulcer models in rats. Afr. J. Pharmacol. Ther. 5 (3).
Alabi, A. O., Ajayi, A. M., Omorogbe, O., and Umukoro, S. (2019). Anti-nociceptive and anti-inflammatory effects of an aqueous extract of blended leaves of Ocimum gratissimum and Psidium guajava. Clin. Phytoscience 5 (1), 34–39. doi:10.1186/s40816-019-0130-2
Aldhaher, A., Langat, M., Schwikkard, S., Carew, M., and Mulholland, D. (2016). New terpenoids from Croton dichogamus Pax. Planta Medica 82 (S 01), S1–S381. doi:10.1055/s-0036-1596327
Ali, A., Akhtar, N., Khan, B. A., Khan, M. S., and Rasul, A. (2012). Acacia nilotica: a plant of multipurpose medicinal uses. J. Med. plants Res. 6 (9), 1492–1496. doi:10.5897/jmpr11.1275
Ali, N. A. A., Al-Fatimi, M. A., Crouch, R. A., Denkert, A., Setzer, W. N., and Wessjohann, L. (2013). Antimicrobial, antioxidant, and cytotoxic activities of the essential oil of Tarchonanthus camphoratus. Nat. Product. Commun. 8 (5), 1934578X1300800. doi:10.1177/1934578x1300800534
Aljohani, A. S., Alhumaydhi, F. A., Rauf, A., Hamad, E. M., and Rashid, U. (2022). In vivo and in vitro biological evaluation and molecular docking studies of compounds isolated from Micromeria biflora (buch. Ham. Ex D. Don) benth. Molecules 27 (11), 3377. doi:10.3390/molecules27113377
Al-Qura’n, S. (2009). Ethnopharmacological survey of wild medicinal plants in Showbak, Jordan. J. Ethnopharmacol. 123 (1), 45–50. doi:10.1016/j.jep.2009.02.031
Amadi, B., Bello, R., and Ohiri, R. (2022). Ameliorative potentials of dichloromethane extract of Musa acuminata latundan bract in indomethacin administered wistar rats. Asian J. Plant Soil Sci. 7 (1), 171–184.
Amjad, M. S., Qaeem, M. f., Ahmad, I., Khan, S. U., Chaudhari, S. K., Zahid Malik, N., et al. (2017). Descriptive study of plant resources in the context of the ethnomedicinal relevance of indigenous flora: a case study from Toli Peer National Park, Azad Jammu and Kashmir, Pakistan. PLoS ONE 12 (2), e0171896. doi:10.1371/journal.pone.0171896
Annan, K., and Dickson, R. (2008). Evaluation of wound healing actions of Hoslundia opposita vahl, Anthocleista nobilis G. Don. and Balanites aegyptiaca L. J. Sci. Technol. (Ghana) 28 (2), 26–35. doi:10.4314/just.v28i2.33091
Anthoney, S. T., Jackie, O., Edwin, M., and Erick, T. (2015). In vitro antibacterial activity of the aqua extract of Phytolacca dodecandra roots against laboratory strains of selected human pathogenic organisms. Int. J. Bioassays 4 (05), 3903–3909.
Antwi-Baffour, S. S., Bello, A. I., Adjei, D. N., Mahmood, S. A., and Ayeh-Kumi, P. F. (2014). The place of traditional medicine in the African society: the science, acceptance and support. Am. J. Health Res. 2 (2), 49–54. doi:10.11648/j.ajhr.20140202.13
Arooj, B., Asghar, S., Saleem, M., Khalid, S. H., Asif, M., Chohan, T., et al. (2023). Anti-inflammatory mechanisms of eucalyptol rich Eucalyptus globulus essential oil alone and in combination with flurbiprofen. Inflammopharmacology 31, 1849–1862. doi:10.1007/s10787-023-01237-6
Ashebo, T. (2019). A review on the qualitative method of the study of people-plants relationship in their environment. Int. J. Environ. Sci. Nat. Resour. 22 (1), 21–29. doi:10.19080/IJESNR.2019.22.556078
Asumang, P., Boakye, Y. D., Agana, T. A., Yakubu, J., Entsie, P., Akanwariwiak, W. G., et al. (2021). Antimicrobial, antioxidant and wound healing activities of methanol leaf extract of Bridelia micrantha (Hochst.) Baill. Sci. Afr. 14, e00980. doi:10.1016/j.sciaf.2021.e00980
Asuzu, P. C., Aryee, A. N. A., Trompeter, N., Mann, Y., Besong, S. A., Duncan, R. L., et al. (2021). In vitro assessment of efficacy and cytotoxicity of Prunus africana extracts on prostate cancer C4-2 cells. bioRxiv, Available at: https://doi.org/10.1101/2021.03.14.435338.
Awiti, J. O. (2014). Poverty and health care demand in Kenya. BMC health Serv. Res. 14 (1), 560. doi:10.1186/s12913-014-0560-y
Bandyopadhyay, U., Biswas, K., Sengupta, A., Moitra, P., Dutta, P., Sarkar, D., et al. (2004). Clinical studies on the effect of Neem (Azadirachta indica) bark extract on gastric secretion and gastroduodenal ulcer. Life Sci. 75 (24), 2867–2878. doi:10.1016/j.lfs.2004.04.050
Bbosa, G. S. (2014). Antiplasmodial activity of leaf extracts of Zanthoxylum chalybeum engl. Br. J. Pharm. Res. 4 (6), 705–713. doi:10.9734/bjpr/2014/6528
Bbosa, G. S., Kyegombe, D. B., Lubega, A., Musisi, N., Ogwal-Okeng, J., and Odyek, O. (2013). Anti-Plasmodium falciparum activity of Aloe dawei and Justicia betonica. Afr. J. Pharm. Pharmacol. 7 (31), 2258–2263. doi:10.5897/AJPP12.479
Bekalo, T. H., Woodmatas, S. D., and Woldemariam, Z. A. (2009). An ethnobotanical study of medicinal plants used by local people in the lowlands of Konta Special Woreda, southern nations, nationalities and peoples regional state, Ethiopia. J. Ethnobiol. Ethnomedicine 5, 26–15. doi:10.1186/1746-4269-5-26
Bhajoni, P. S., Meshram, G. G., and Lahkar, M. (2016). Evaluation of the Antiulcer Activity of the Leaves of Azadirachta indica an Experimental Study. Integr. Med. Int. 3 (1-2), 10–16. doi:10.1159/000442750
Biagi, M., Pecorari, R., Appendino, G., Miraldi, E., Magnano, A. R., Governa, P., et al. (2016). Herbal products in Italy: the thin line between phytotherapy, nutrition and parapharmaceuticals; a normative overview of the fastest growing market in Europe. Pharmaceuticals 9 (4), 65. doi:10.3390/ph9040065
Blackett, H., Lukandu, G., and Obara, A. (1994). Forest inventory report No. 10: north Nandi, Kaptaroi and Teressia.
Bouriah, N., Bendif, H., Peron, G., Miara, M. D., Dall'Acqua, S., Flamini, G., et al. (2021). Composition and profiling of essential oil, volatile and crude extract constituents of Micromeria inodora growing in western Algeria. J. Pharm. Biomed. Anal. 195, 113856. doi:10.1016/j.jpba.2020.113856
Bum, E. N., Ngah, E., Ngo Mune, R. M., Ze Minkoulou, D. M., Talla, E., Moto, F. C. O., et al. (2012). Decoctions of Bridelia micrantha and Croton macrostachyus may have anticonvulsant and sedative effects. Epilepsy & Behav. 24 (3), 319–323. doi:10.1016/j.yebeh.2012.03.028
Burkill, H. M. (1995). The useful plants of west tropical Africa, Vols. 1-3. Kew: Royal Botanic Gardens.
Canales, M., Hernández, T., Caballero, J., De Vivar, A. R., Avila, G., Duran, A., et al. (2005). Informant consensus factor and antibacterial activity of the medicinal plants used by the people of San Rafael Coxcatlán, Puebla, México. J. Ethnopharmacol. 97 (3), 429–439. doi:10.1016/j.jep.2004.11.013
Chepng’etich, J., Ngule, C., Jepkorir, M., Mwangangi, R., Njuguna, D., Ndung’u, J., et al. (2018). Total phenolic content and in vitro antiproliferative activity of Tragia brevipes (Pax) and Tetradenia riparia (Hochst) leaves extract. Eur. J. Med. Plants 22 (4), 1–10. doi:10.9734/EJMP/2018/40058
Cho, Y.-C., Kim, Y. R., Kim, B. R., Bach, T. T., and Cho, S. (2016). Thunbergia alata inhibits inflammatory responses through the inactivation of ERK and STAT3 in macrophages. Int. J. Mol. Med. 38 (5), 1596–1604. doi:10.3892/ijmm.2016.2746
Chun, K., and Kundu, J. (2013). Analgesic, anti-inflammatory and diuretic activity of methanol extract of Flacourtia indica. Arch. Basic Appl. Med. 1 (1), 39–44.
Cordero, C. S., Meve, U., and Alejandro, G. J. D. (2022). Ethnobotanical documentation of medicinal plants used by the indigenous panay bukidnon in lambunao, iloilo, Philippines. Front. Pharmacol. 12, 790567. doi:10.3389/fphar.2021.790567
Danyaal, M. (2020). The isolation and purification of chemical constituents of'Croton megalocarpus' Hutch husks. Kingston: Kingston University.
Dapar, M. L. G., Alejandro, G. J. D., Meve, U., and Liede-Schumann, S. (2020). Quantitative ethnopharmacological documentation and molecular confirmation of medicinal plants used by the Manobo tribe of Agusan del Sur, Philippines. J. Ethnobiol. Ethnomedicine 16 (1), 14–60. doi:10.1186/s13002-020-00363-7
Da Silva, G., Taniça, M., Rocha, J., Serrano, R., Gomes, E. T., Sepodes, B., et al. (2011). In vivo anti-inflammatory effect and toxicological screening of Maytenus heterophylla and Maytenus senegalensis extracts. Hum. Exp. Toxicol. 30 (7), 693–700. doi:10.1177/0960327110379242
Dawa, I., Yakubu, J., Mamza, U., Balami, V., Sodipo, O., Abdulrahman, F., et al. (2021). Antimicrobial activities of methanol leaf extract of Carissa edulis Vahl (Apocynaceae). Bull. Pure Appl. Sciences-Chemistry 40 (2), 96–103. doi:10.5958/2320-320x.2021.00016.9
de Boer, H. J., Kool, A., Broberg, A., Mziray, W. R., Hedberg, I., and Levenfors, J. J. (2005). Anti-fungal and anti-bacterial activity of some herbal remedies from Tanzania. J. Ethnopharmacol. 96 (3), 461–469. doi:10.1016/j.jep.2004.09.035
Degu, A., Engidawork, E., and Shibeshi, W. (2016). Evaluation of the anti-diarrheal activity of the leaf extract of Croton macrostachyus Hocsht. ex Del.(Euphorbiaceae) in mice model. BMC Complement. Altern. Med. 16, 379–411. doi:10.1186/s12906-016-1357-9
del Carmen Juárez-Vázquez, M., and Jiménez-Arellanes, M. A. (2019). Phytochemical investigation, anti-inflammatory and antinociceptive activities from some species of Cleomaceae family: a systematic review. Adv. Med. Plans Res. 7 (4), 107–128. doi:10.30918/ampr.74.19.039
Demgne, O. M. F., Tchinda, C. F., Mbaveng, A. T., Beng, V. P., and Kuete, V. (2022). Antibacterial and antibiotic-potentiating activities of nine Cameroonian medicinal plants against multidrug-resistant bacteria expressing active efflux pumps. Invest. Med. Chem. Pharmacol. 5 (1), 1–11. doi:10.31183/imcp.2022.00058
Derso, S. (2020). Phytochemical investigation and determination of antibacterial activity of calpurnia aurea (digita) seed and leaf extracts. Ethiopia: Selam Derso. Available at: http://hdl.handle.net/123456789/3096.
Dhone, P. G., Ekka, A., Sinha, P. K., and Sahoo, A. (2022). In vivo antipyretic activity of Scutia myrtina acute oral toxicity study. Int. J. Health Sci. 6 (3), 9706–9711. doi:10.53730/ijhs.v6nS3.8548
Diwane, C., Patil, R., Vyavahare, P., and Bhambar, R. (2015). Protective effect of Rubia cordifolia in paclitaxel-induced neuropathic pain in experimental animals. Indian J. Pain 29, 150–154. doi:10.4103/0970-5333.165833
Dorababu, M., Prabha, T., Priyambada, S., Agrawal, V. K., Aryya, N. C., and Goel, R. K. (2004). Effect of Bacopa monniera and Azadirachta indica on gastric ulceration and healing in experimental NIDDM rats. Indian J. Exp. Biol. 42 (4), 389–397.
Dula, D., and Zelalem, A. (2018). Antioxidant activity assessment of Calpurnia aurea root extract. Nat. Prod. Chem. Res. 6 (307), 2. doi:10.4172/2329-6836.1000307
Dutta, S., and Das, S. (2010). A study of the anti-inflammatory effect of the leaves of Psidium guajava Linn. on experimental animal models. Pharmacogn. Res. 2 (5), 313–317. doi:10.4103/0974-8490.72331
Dzoyem, J. P., Tshikalange, E., and Kuete, V. (2013). Medicinal plants market and industry in Africa. Med. Plant Res. Afr. 2013, 859–890. doi:10.1016/b978-0-12-405927-6.00024-2
El-Ahmady, S. H., Ashour, M. L., and Wink, M. (2013). Chemical composition and anti-inflammatory activity of the essential oils of Psidium guajava fruits and leaves. J. Essent. Oil Res. 25 (6), 475–481. doi:10.1080/10412905.2013.796498
Emam, J. A., Yaya, E. E., Choudhary, M. I., Yousuf, S., and Gebremedhin, T. M. (2021). In vitro anticancer activities of selected Ethiopian plant extracts on HeLa and PC3 cell lines. Ethiop. Pharm. J. 37 (1), 77–82. doi:10.4314/epj.v37i1.6
Erasto, P., Mbwambo, Z., Nondo, R., and Lall, N. (2011). Antimycobacterial, antioxidant activity and toxicity of extracts from the roots of <i&gt;Rauvolfia vomitoria&lt;/i&gt; and <i&gt;R. caffra&lt;/i&gt;. Spatula DD 1 (73), 73. doi:10.5455/spatula.20110514043359
Ezeonwumelu, J. O., Kawooya, G. N., Okoruwa, A. G., Dare, S. S., Ebosie, J. C., Akunne, A. A., et al. (2019). Phytochemical screening, toxicity, analgesic and anti-pyretic studies of aqueous leaf extract of Plectranthus barbatus [Andrews. Engl.] in rats. Pharmacol. Pharm. 10 (04), 205–221. doi:10.4236/pp.2019.104018
Falé, P. L., Madeira, P. J. A., Florêncio, M. H., Ascensão, L., and Serralheiro, M. L. M. (2011). Function of Plectranthus barbatus herbal tea as neuronal acetylcholinesterase inhibitor. Food & Funct. 2 (2), 130–136. doi:10.1039/c0fo00070a
Fanta Yadang, S. A., Taiwe Sotoing, G., Ngatcha Zouakeu, K. S., Khan, M. A., Agbor, G. A., Ur-Rahman, N., et al. (2019). Quantification of bioactive compounds and evaluation of the antioxidant activity of Carissa edulis Valh (Apocynaceae) leaves. Sci. World J. 2019, 7549620. doi:10.1155/2019/7549620
Faruque, M. O., Uddin, S. B., Barlow, J. W., Hu, S., Dong, S., Cai, Q., et al. (2018). Quantitative ethnobotany of medicinal plants used by indigenous communities in the Bandarban District of Bangladesh. Front. Pharmacol. 9, 40. doi:10.3389/fphar.2018.00040
Farzana, M., and Al Tharique, I. (2014). A review of ethnomedicine, phytochemical and pharmacological activities of Acacia nilotica (Linn) willd. J. Pharmacogn. Phytochem. 3 (1), 84–90.
Farzana, S., Saha, S. P., Sultana, N., and Khan, M. I. (2019). Gastroprotective effect of Azadirachta indica leaves (neem) extract on aspirin induced gastric ulcer in rats. Delta Med. Coll. J. 7 (2), 61–65. doi:10.3329/dmcj.v7i2.45542
Feyisa, K., Feyisa, W., Girma, T., and Kemal, T. (2022). Traditional medicinal plants used for the treatment of urological and urogenital diseases in Ethiopia: a review. Pharmacogn. J. 14 (3), 722–733. doi:10.5530/pj.2022.14.92
Fortunati, E., Mazzaglia, A., and Balestra, G. M. (2019). Sustainable control strategies for plant protection and food packaging sectors by natural substances and novel nanotechnological approaches. J. Sci. Food Agric. 99 (3), 986–1000. doi:10.1002/jsfa.9341
Friedman, J., Yaniv, Z., Dafni, A., and Palewitch, D. (1986). A preliminary classification of the healing potential of medicinal plants, based on a rational analysis of an ethnopharmacological field survey among Bedouins in the Negev Desert, Israel. J. Ethnopharmacol. 16 (2-3), 275–287. doi:10.1016/0378-8741(86)90094-2
Fyhrquist, P., Laakso, I., Garcia Marco, S., Julkunen-Tiitto, R., and Hiltunen, R. (2014). Antimycobacterial activity of ellagitannin and ellagic acid derivate rich crude extracts and fractions of five selected species of Terminalia used for treatment of infectious diseases in African traditional medicine. South Afr. J. Bot. 90, 1–16. doi:10.1016/j.sajb.2013.08.018
Gakuya, D. W., Okumu, M. O., Kiama, S. G., Mbaria, J. M., Gathumbi, P. K., Mathiu, P. M., et al. (2020). Traditional medicine in Kenya: past and current status, challenges, and the way forward. Sci. Afr. 8, e00360. doi:10.1016/j.sciaf.2020.e00360
Giang, N. T. H., Van Ngot, P., and Thanh, D. T. N. (2021). Morphological, anatomical and antibacterial characteristics of Leonotis nepetifolia plants growing in Binh Thuan Province, Vietnam. GSC Biol. Pharm. Sci. 14 (2), 053–063. doi:10.30574/gscbps.2021.14.2.0041
Gichui, W. G. (2016). Antinociceptive activities of extracts of Croton megalocarpus hutch (Eurphobiaceae) using animal models. Nairobi: University of Nairobi.
Girma, A., Fischer, E., and Dumbo, B. (2015). Vascular plant diversity and community structure of Nandi forests, western Kenya. J. East Afr. Nat. Hist. 103 (2), 125–152. doi:10.2982/028.103.0202
Göger, G., Karaca, N., Altınbaşak, B. B., Demirci, B., and Demirci, F. (2020). In vitro antimicrobial, antioxidant and anti-inflammatory evaluation of Eucalyptus globulus essential oil. Nat. Volatiles Essent. Oils 7 (3), 1–11. doi:10.37929/nveo.759607
Guchu, B. M., Machocho, A. K., Mwihia, S. K., and Ngugi, M. P. (2020). In vitro antioxidant activities of methanolic extracts of Caesalpinia volkensii Harms., Vernonia lasiopus O. Hoffm., and Acacia hockii De Wild. Evidence-based Complement. Altern. Med. 2020, 3586268. doi:10.1155/2020/3586268
Gudu, G. J., Naka Keta, J., Bala Gudu, N., Abubakar, M., and Zinatu, K. (2023). Phytochemical analysis of some plants used for treatments of respiratory tract disease in zuru metropolis. World 8 (3), 53–56. doi:10.11648/j.wjac.20230803.11
Gwatidzo, L., Leo, C., and Cexton, M. N. M. M. (2018). In vitro anti-inflammatory activity of Vangueria infausta: an edible wild fruit from Zimbabwe. Afr. J. Pharm. Pharmacol. 12 (13), 168–175. doi:10.5897/ajpp2018.4894
Hamza, R. Z., Al-Motaani, S. E., and Al-Talhi, T. (2021). Therapeutic and ameliorative effects of active compounds of Combretum molle in the treatment and relief from wounds in a diabetes mellitus experimental model. Coatings 11 (3), 324. doi:10.3390/coatings11030324
Hassan, H. S., Sule, M., Musa, M., Emmanuel, A., Ibrahim, H., Hassan, A., et al. (2010). Analgesic and anti-inflammatory activities of the saponins extract of Carissa edulis root in rodents. Int. J. Biol. Chem. Sci. 4 (4). doi:10.4314/ijbcs.v4i4.63065
Hassan, L., Mshelia, H. E., Umar, K. J., Kangiwa, S. M., Ogbiko, C., and Yusuf, A. J. (2018). Phytochemical Screening, Isolation and Characterization of Beta-Sitosterol from ethyl acetate Extract of Stem Bark of Entada africana (Fabaceae) Guill. et Perr. J. Chem. Soc. Niger. 43 (3).
Heinrich, M. (2000). Ethnobotany and its role in drug development. Phytotherapy Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 14 (7), 479–488. doi:10.1002/1099-1573(200011)14:7<479::aid-ptr958>3.0.co;2-2
Hikaambo, C. N., Chilala, P., Ndubi, F., Mayoka, G., Kampamba, M., Kabuka, R., et al. (2023). Antimicrobial activities of Solanum aculeastrum fruit extract against Escherichia coli, Staphylococcus aureus and Candida albicans: significance of african traditional medicine in combating infections and attaining universal health coverage. Pharmacol. Pharm. 14 (5), 176–188. doi:10.4236/pp.2023.145013
Hirudkar, J. R., Parmar, K. M., Prasad, R. S., Sinha, S. K., Lomte, A. D., Itankar, P. R., et al. (2020). The antidiarrhoeal evaluation of Psidium guajava L. against enteropathogenic Escherichia coli induced infectious diarrhoea. J. Ethnopharmacol. 251, 112561. doi:10.1016/j.jep.2020.112561
Hoffman, B., and Gallaher, T. (2007). Importance indices in ethnobotany. Ethnobot. Res. Appl. 5, 201–218. doi:10.17348/era.5.0.201-218
Hou, Y., Cao, S., Brodie, P. J., Callmander, M. W., Ratovoson, F., Rakotobe, E. A., et al. (2009). Antiproliferative and antimalarial anthraquinones of Scutia myrtina from the Madagascar forest. Bioorg. Med. Chem. 17 (7), 2871–2876. doi:10.1016/j.bmc.2009.02.022
Hussain, F., Poddar, S. K., Ganguly, A., and Rahman, S. A. (2016). Investigation of CNS depressant, anti-diarrheal and cytotoxic activities of crude methanolic extracts of Acacia nilotica and Justicia adhatoda root. Indo Am. J. Pharm. Res. 6 (1), 3954–3961.
Idu, M., Erhabor, J. O., and Efijuemue, H. M. (2010). Documentation on medicinal plants sold in markets in Abeokuta, Nigeria. Trop. J. Pharm. Res. 9 (2). doi:10.4314/tjpr.v9i2.53696
Ilodigwe, E. E., and Akah, P. A. (2009). Spathodea campanulata: an experimental evaluation of the analgesic and anti-inflammatory properties of a traditional remedy. Asian J. Med. Sci. 1 (2), 35–38.
Imperato, P. J. (1981). The role of women in traditional healing among the Bambara of Mali. Trans. R. Soc. Trop. Med. Hyg. 75 (6), 766–770. doi:10.1016/0035-9203(81)90406-5
Ior, I., Otimenyin, I., and Umar, M. (2012). Anti-inflammatory and analgesic activities of the ethanolic extract of the leaf of Syzygium guineense in rats and mice. IOSR J. Pharm. 2 (4), 33–36. doi:10.9790/3013-24303336
Islam, M. A. F., Masuma, R., Rakibur Rahman, A. M., Shohel Hossain, M., Nahid Hasan, M., and Jubayer, A. (2023). Evaluation of analgesic, anti-inflammatory and antipyretic properties of the Flacourtia indica extract in laboratory animal. J. Phytomolecules Pharmacol. 2023. doi:10.56717/jpp.2022.v01i02.009
Islam, M. H., Rahman, K. H., Rahman, S., and Rahmatullah, M. (2015). Preliminary antihyperglycemic, antinociceptive activity, phytochemical analysis and toxicity studies on leaves of Urena lobata L. J. Chem. Pharm. Res. 7 (4), 559–563.
Jadid, N., Kurniawan, E., Himayani, C. E. S., Prasetyowati, I., Purwani, K. I., Muslihatin, W., et al. (2020). An ethnobotanical study of medicinal plants used by the Tengger tribe in Ngadisari village, Indonesia. PLoS ONE 15 (7), e0235886. doi:10.1371/journal.pone.0235886
Jaiswal, A., Verma, M., Kohli, S., Chauhan, D. N., Shah, K., and Chauhan, N. S. (2023). Babool (Acacia nilotica) and oral health. Pharmacol. Stud. Nat. Oral Care 2023, 597–606. doi:10.1002/9781394167197.ch31
Jang, M., Jeong, S. W., Cho, S. K., Ahn, K. S., Lee, J. H., Yang, D. C., et al. (2014). Anti-inflammatory effects of an ethanolic extract of guava (Psidium guajava L.) leaves in vitro and in vivo. J. Med. food 17 (6), 678–685. doi:10.1089/jmf.2013.2936
Jepkorir, M., Nyanjom, S. G., Kamau, S., Chepng'etich, J., Kipkoech, G., and Mwitari, P. G. (2023). In vivo anti-inflammatory activity, safety and gene expression profiles of Carissa edulis, Withania somnifera, Prunus africana and Rhamnus prinoides for potential management of rheumatoid arthritis. Sci. Afr. 22, e01933. doi:10.1016/j.sciaf.2023.e01933
Jeruto, P., Lukhoba, C., Ouma, G., Otieno, D., and Mutai, C. (2008). An ethnobotanical study of medicinal plants used by the Nandi people in Kenya. J. Ethnopharmacol. 116 (2), 370–376. doi:10.1016/j.jep.2007.11.041
Jeruto, P., Lukhoba, C., Ouma, G., Otieno, D., and Mutai, C. (2008a). An ethnobotanical study of medicinal plants used by the Nandi people in Kenya. J. Ethnopharmacol. 116 (2), 370–376. doi:10.1016/j.jep.2007.11.041
Jeruto, P., Lukhoba, C. L., Ouma, G., Otieno, D., and Mutai, C. (2008b). Herbal treatments in Aldai and Kaptumo divisions in Nandi district, Rift valley province, Kenya. Afr. J. Traditional, Complementary Altern. Med. 5 (1), 103–105.
Jeruto, P., Nyangacha, R., and Mutai, C. (2015). In vitro and in vivo antiplasmodial activity of extracts of selected Kenyan medicinal plants. Afr. J. Pharm. Pharmacol. 9 (16), 505. doi:10.5897/ajpp2013.3886
Junsongduang, A., Kasemwan, W., Lumjoomjung, S., Sabprachai, W., Tanming, W., and Balslev, H. (2020). Ethnomedicinal knowledge of traditional healers in Roi Et, Thailand. Plants 9 (9), 1177. doi:10.3390/plants9091177
Jurbe, G., Ntim, P. S., Ajayi, T. A., Chuwkuka, J. U., Dawurung, C. J., Makoshi, M. S., et al. (2015). Phytochemical Screening and Antidiarrheal Evaluation of Acetone Extract of Acacia sieberiana var woodii (Fabaceae) stem bark in wistar rats. Acad. J. Pham. Pharmacol. 3 (1), 1–6.
Kalusalingam, A., Samuel, A. J. S. J., Khan, A., and Vimala, A. G. K. A. (2018). Preliminary phytochemical screening and evaluation of analgesic activity of Basella alba linn. KPJ Med. 7 (1), 38.
Kamau, J., and Pm, N. (2016). Anti-inflammatory activity of methanolic leaf extract of Kigelia africana (Lam.) Benth and stem bark extract of Acacia hockii De Wild in Mice. J. Dev. Drugs 5 (2), 1–8. doi:10.4172/2329-6631.1000156
Kamau, K. (2016). Antipyretic and anti-inflammatory properties of methanolic extracts of kigelia africana (lam.) benth and Acacia hockii de Wild in animal models. Kenya: School of Pure and Applied Sciences, Kenyatta University Nairobi.
Kamau, P. K., Zipporah, N., Francis, M. N., and John, T. (2020). In vitro antiplasmodial, cytotoxicity assay and partial chemical characterization of Kenyan Physalis peruviana L.(Solanaceae family) extracts. J. Med. Plants Res. 14 (2), 73–80. doi:10.5897/jmpr2019.6882
Kareru, P., Gachanja, A. N., Keriko, J. M., and Kenji, G. M. (2008). Antimicrobial activity of some medicinal plants used by herbalists in eastern province, Kenya. Afr. J. Tradit. Complement. Altern. Med. 5 (1), 51–55. doi:10.4314/ajtcam.v5i1.31256
Kariuki, S. (2015). Study of crude extracts of Ajuga remota benth (labiatae) as potential anti-malarial drug. J. Environ. Sustain. Adv. Res. 1.
Kasture, S., Kasture, V., and Chopde, C. (2001). Anti-inflammatory activity of Rubia cordifolia roots. J. Nat. Remedies 1 (2), 111–115.
Kathare, J., Mbaria, J. M., Nguta, J. M., and Moriasi, G. A. (2021b). Antimicrobial, cytotoxicity, acute oral toxicity and qualitative phytochemical screening of the aqueous and methanolic extracts of Physalis peruviana L (Solanaceae). Appl. Microbiol. Open Access 7, 189.
Kathare, J. M., Mbaria, J. M., Nguta, J. M., Moriasi, G. A., and Mainga, A. O. (2021a). Antimicrobial efficacy, cytotoxicity, acute oral toxicity, and phytochemical investigation of the aqueous and methanolic stem bark extracts of Bridellia micrantha (hochst.) baill. Pharmacogn. J. 13 (5), 1248–1256. doi:10.5530/pj.2021.13.158
Khakurel, D., Uprety, Y., Ahn, G., Cha, J.-Y., Kim, W.-Y., Lee, S.-H., et al. (2022). Diversity, distribution, and sustainability of traditional medicinal plants in Kaski district, western Nepal. Front. Pharmacol. 13, 1076351. doi:10.3389/fphar.2022.1076351
Khan, M. P. Z., Ahmad, M., Zafar, M., Sultana, S., Ali, M. I., and Sun, H. (2015). Ethnomedicinal uses of edible wild fruits (EWFs) in Swat Valley, Northern Pakistan. J. Ethnopharmacol. 173, 191–203. doi:10.1016/j.jep.2015.07.029
Khursheed, A., Rather, M. A., Jain, V., Rasool, S., Nazir, R., Malik, N. A., et al. (2022). Plant based natural products as potential ecofriendly and safer biopesticides: a comprehensive overview of their advantages over conventional pesticides, limitations and regulatory aspects. Microb. Pathog. 173, 105854. doi:10.1016/j.micpath.2022.105854
Kigen, C. K. (2019). In silico prediction of anti-malarial activity and pharmacokinetic properties of herbal derivatives of Ajuga remota and Azadirachta indica. Juja, Kenya: Bachelor of Science degree in Medical Biochemistry in the Jomo Kenyatta University of Agriculture and Technology.
Kigen, G., Maritim, A., Some, F., Kibosia, J., Rono, H., Chepkwony, S., et al. (2016). Ethnopharmacological survey of the medicinal plants used in Tindiret, Nandi County, Kenya. Afr. J. Traditional, Complementary Altern. Med. 13 (3), 156–168. doi:10.21010/ajtcam.v13i3.19
Kimondo, J., Miaron, J., Mutai, P., and Njogu, P. (2015). Ethnobotanical survey of food and medicinal plants of the Ilkisonko Maasai community in Kenya. J. Ethnopharmacol. 175, 463–469. doi:10.1016/j.jep.2015.10.013
Kimutai, N., Ogutu, P. A., and Jeruto, C. M. P. (2019). Ethnobotanical study of selected medicinal plants used against bacterial infections in Nandi county, Kenya. J. Med. Plants 7 (4), 103–108.
Kipkore, W., Wanjohi, B., Rono, H., and Kigen, G. (2014). A study of the medicinal plants used by the Marakwet Community in Kenya. J. Ethnobiol. Ethnomedicine 10 (1), 24–22. doi:10.1186/1746-4269-10-24
Kipngeno, C. D. (2019). Screening and characterization of some anticancer compounds from Salicaceae, Myrtaceae, Euphorbiaceae and Solanaceae families. Egerton: Egerton University.
Koduru, S., Grierson, D. G., van de Venter, M. V., and Afolayan, A. A. (2006). In vitro antitumour activity of Solanum aculeastrum berries on three carcinoma cells. Int. J. Cancer Res. 2 (4), 397–402. doi:10.3923/ijcr.2006.397.402
Komakech, R., Yim, N. H., Shim, K. S., Jung, H., Byun, J. E., Lee, J., et al. (2022). Root extract of a micropropagated Prunus africana medicinal plant induced apoptosis in human prostate cancer cells (PC-3) via caspase-3 activation. Evidence-Based Complement. Altern. Med. 2022, 8232851. doi:10.1155/2022/8232851
Koriem, K. M., Arbid, M. S., and Saleh, H. N. (2019). Antidiarrheal and protein conservative activities of Psidium guajava in diarrheal rats. J. Integr. Med. 17 (1), 57–65. doi:10.1016/j.joim.2018.12.001
Kubera Sampath Kumar, S., Prakash, C., Ramesh, P., Sukumar, N., Balaji, J., and Palaniswamy, N. (2021). Study of wound dressing material coated with natural extracts of Calotropis Gigantean, Eucalyptus Globulus and buds of Syzygium Aromaticum solution enhanced with rhEGF (REGEN-DTM 60). J. Nat. Fibers 18 (12), 2270–2283. doi:10.1080/15440478.2020.1726239
Kumar, V., Bhat, Z. A., Kumar, D., Bohra, P., and Sheela, S. (2011). In-vitro anti-inflammatory activity of leaf extracts of Basella alba linn. Var. alba. Int. J. Drug Dev. Res. 3 (2), 176–179.
Kuria, J. M. (2014). Efficacy of aspilia pluriseta schweinf in cutaneous wound healing in a mouse model. Nairobi: University of Nairobi.
Kuria, J. M., Mbaria, J. M., Gathumbi, P. K., and Kiama, S. G. (2015). Influence of Aspilia pluriseta Schweinf (Asteraceae) on the healing of dermal excision wounds (mouse model) and skin sensitization activity (Guinea pig model). Afr. J. Pharmacol. Ther. 4 (3).
Kuria, K., De Coster, S., Muriuki, G., Masengo, W., Kibwage, I., Hoogmartens, J., et al. (2001). Antimalarial activity of Ajuga remota Benth (Labiatae) and Caesalpinia volkensii Harms (Caesalpiniaceae): in vitro confirmation of ethnopharmacological use. J. Ethnopharmacol. 74 (2), 141–148. doi:10.1016/s0378-8741(00)00367-6
Lacroix, D., Prado, S., Kamoga, D., Kasenene, J., Namukobe, J., Krief, S., et al. (2011). Antiplasmodial and cytotoxic activities of medicinal plants traditionally used in the village of Kiohima, Uganda. J. Ethnopharmacol. 133 (2), 850–855. doi:10.1016/j.jep.2010.11.013
Legesse, B. A., Tamir, A., and Bezabeh, B. (2019). Phytochemical screening and antibacterial activity of leaf extracts of Dovyalis abyssinica. J. Emerg. Technol. Innov. Res. 6 (6), 453–465.
Leonti, M., and Casu, L. (2013). Traditional medicines and globalization: current and future perspectives in ethnopharmacology. Front. Pharmacol. 4, 92. doi:10.3389/fphar.2013.00092
Lobo, R., and Ballal, M. (2011). Screening for antidiarrheal activity of Psidium guajava: a possible alternative in the treatment against diarrhea causing enteric pathogens. J. Chem. 3, 961–967.
Lozoya, X., Reyes-Morales, H., Chávez-Soto, M. A., Martínez-García, M. d. C., Soto-González, Y., and Doubova, S. V. (2002). Intestinal anti-spasmodic effect of a phytodrug of Psidium guajava folia in the treatment of acute diarrheic disease. J. Ethnopharmacol. 83 (1-2), 19–24. doi:10.1016/s0378-8741(02)00185-x
Lu, J., Mao, D., Li, X., Ma, Y., Luan, Y., Cao, Y., et al. (2020). Changes of intestinal microflora diversity in diarrhea model of KM mice and effects of Psidium guajava L. as the treatment agent for diarrhea. J. Infect. Public Health 13 (1), 16–26. doi:10.1016/j.jiph.2019.04.015
Mahishi, P., Srinivasa, B., and Shivanna, M. (2005). Medicinal plant wealth of local communities in some villages in Shimoga District of Karnataka, India. J. Ethnopharmacol. 98 (3), 307–312. doi:10.1016/j.jep.2005.01.035
Mahomoodally, M. F. (2013). Traditional medicines in Africa: an appraisal of ten potent African medicinal plants. Evidence-Based Complementary Altern. Med. 2013, 617459. doi:10.1155/2013/617459
Maina, G. S. (2015). Antipyretic properties of dichloromethane: methanolic leaf and root bark extracts of Carissa edulis in rats. Asian J. Biomed. Pharm. Sci. 5 (43), 12–20. doi:10.15272/ajbps.v5i43.681
Mangussad, D., Sucgang, A. T., Clemencia, M. C. M., Manalo, M., Uy, L. Y., and Torio, M. A. (2021). Isolation and characterization of the total protein in’Lakatan’Banana (Musa acuminata colla) with bioactive peptides exhibiting antioxidative and antihypertensive activities. Philipp. Agric. Sci. Philipp. 104 (3).
Manikandan, P. A. (2005). Folk herbal medicine: a survey on the paniya tribes of Mundakunnu village of the Nilgiri hills, South India. Anc. Sci. life 25 (1), 21–27.
Mans, D. R., Friperson, P., Pawirodihardjo, J., and Djotaroeno, M. (2022). Phenolic compounds and antioxidant activities of eight species of fabaceae that are commonly used in traditional medical practices in the republic of Suriname. Med. Plants 2022, 106076. IntechOpen. doi:10.5772/intechopen.106076
Manzo, L. M., Moussa, I., and Ikhiri, K. (2017). Phytochemical screening of selected medicinal plants used against diarrhea in Niger, West Africa. Int. J. Herb. Med. 5 (4), 32–38.
Maobe, M. A., Gatebe, E., Gitu, L., and Rotich, H. (2013). Preliminary phytochemical screening of eight selected medicinal herbs used for the treatment of diabetes, malaria and pneumonia in Kisii region, southwest Kenya. Eur. J. Appl. Sci. 5 (10), 01–06. doi:10.5829/idosi.ejas.2013.5.1.65127
Mariita, R. M., Orodho, J. A., Okemo, P. O., and Mbugua, P. K. (2010). Antifungal, antibacterial and antimycobacterial activity of Entada abysinnica Steudel ex A. Rich (Fabaceae) methanol extract. Pharmacogn. Res. 2 (3), 163–168. doi:10.4103/0974-8490.65511
Marquardt, P., Seide, R., Vissiennon, C., Schubert, A., Birkemeyer, C., Ahyi, V., et al. (2020). Phytochemical characterization and in vitro anti-inflammatory, antioxidant and antimicrobial activity of Combretum collinum Fresen leaves extracts from Benin. Molecules 25 (2), 288. doi:10.3390/molecules25020288
Masoko, P., and Makgapeetja, D. M. (2015). Antibacterial, antifungal and antioxidant activity of Olea africana against pathogenic yeast and nosocomial pathogens. BMC Complement. Altern. Med. 15 (1), 409–9. doi:10.1186/s12906-015-0941-8
Matara, D. N., Nguta, J. M., Musila, F. M., and Mapenay, I. (2021). Phytochemical analysis and investigation of the antimicrobial and cytotoxic activities of Croton dichogamus pax crude root extracts. Evidence-Based Complement. Altern. Med. 2021, 2699269. doi:10.1155/2021/2699269
Matasyoh, J. C., Kiplimo, J. J., Karubiu, N. M., and Hailstorks, T. P. (2007). Chemical composition and antimicrobial activity of the essential oil of Satureja biflora (Lamiaceae). Bull. Chem. Soc. Ethiop. 21 (2), 249–254. doi:10.4314/bcse.v21i2.21204
Matebie, W. A., Zhang, W., and Xie, G. (2019). Chemical composition and antimicrobial activity of essential oil from Phytolacca dodecandra collected in Ethiopia. Molecules 24 (2), 342. doi:10.3390/molecules24020342
Meharie, B. G., Amare, G. G., and Belayneh, Y. M. (2020). Evaluation of hepatoprotective activity of the crude extract and solvent fractions of Clutia abyssinica (euphorbiaceae) leaf against CCl4-induced hepatotoxicity in mice. J. Exp. Pharmacol. 12, 137–150. doi:10.2147/JEP.S248677
Mehboob, M., Naureen, I., Saleem, A., and Amanat, A. (2022). Medicinal and nutritional importance of Lagenaria siceraria (Lauki). Saudi J. Biomed. Res. 7 (2), 67–73. doi:10.36348/sjbr.2022.v07i02.001
Melese, A., Dobo, B., and Mikru, A. (2019). Antibacterial activities of Calpurnia aurea and Ocimum lamiifolium extracts against selected gram positive and gram-negative bacteria. Ethiop. J. Sci. Technol. 12 (3), 203–220. doi:10.4314/ejst.v12i3.2
Melly, D. K., Kipkoech, S., Muema, B. W., Kamau, P., Malombe, I., Hu, G., et al. (2020). An annotated checklist of the vascular flora of South and North Nandi Forests, Kenya. PhytoKeys 155, 87–139. doi:10.3897/phytokeys.155.51966
Miaffo, D., Wansi, S. L., Mbiantcha, M., and Poualeu, S. L. K. (2015). Toxicological evaluation of aqueous and acetone extracts of Combretum molle twigs in Wistar Rats. eJBio 11, 33–45.
Migabo, H., Munyeshyaka, E., Izere, C., Yadufashije, C., Niyonzima, F., and Habyarimana, T. (2023). Evaluation of phytochemical profile and antimicrobial activity of Tragia brevipes extracts against pathogenic bacteria. J. Adv. Biotechnol. Exp. Ther. 6 (1), 140–148. doi:10.5455/jabet.2023.d113
Miguéis, G. d. S., da Silva, R. H., Damasceno Junior, G. A., and Guarim-Neto, G. (2019). Plants used by the rural community of Bananal, Mato Grosso, Brazil: aspects of popular knowledge. PLoS ONE 14 (1), e0210488. doi:10.1371/journal.pone.0210488
Milugo, T. K., Omosa, L. K., Ochanda, J. O., Owuor, B. O., Wamunyokoli, F. A., Oyugi, J. O., et al. (2013). Antagonistic effect of alkaloids and saponins on bioactivity in the quinine tree (Rauvolfia caffra sond.): further evidence to support biotechnology in traditional medicinal plants. BMC Complement. Altern. Med. 13, 285–286. doi:10.1186/1472-6882-13-285
Misar, A., Bhagat, R., and Mujumdar, A. (2007). Antidiarrhoeal activity of Acacia nilotica Willd. bark methanol extract. Hindustan Antibiot. Bull. 49 (1-4), 14–20.
Mohapatra, B. B., Das, M. C., Dinda, S. C., and Nagoji, K. E. V. (2012). Anti-ulcer activity of aqueous and ethanolic leaf extract of neem (Azadirachta indica) in albino rats. J. Pharm. Res. 5 (3), 1571.
Mokoka, T. A., McGaw, L. J., Mdee, L. K., Bagla, V. P., Iwalewa, E. O., and Eloff, J. N. (2013). Antimicrobial activity and cytotoxicity of triterpenes isolated from leaves of Maytenus undata (Celastraceae). BMC Complement. Altern. Med. 13, 111–119. doi:10.1186/1472-6882-13-111
Mokua, S. K., Mbaria, J. M., Maitho, T. E., and Moriasi, G. A. (2021). Ethnobotanical documentation, phytochemical screening, and cytotoxicity evaluation of medicinal plants used to manage snakebite envenomation in Mwingi West subcounty, Kenya. Evidence-Based Complementary Altern. Med. 2021, 4167296–4167312. doi:10.1155/2021/4167296
Molander, M., Nielsen, L., Søgaard, S., Staerk, D., Rønsted, N., Diallo, D., et al. (2014). Hyaluronidase, phospholipase A2 and protease inhibitory activity of plants used in traditional treatment of snakebite-induced tissue necrosis in Mali, DR Congo and South Africa. J. Ethnopharmacol. 157, 171–180. doi:10.1016/j.jep.2014.09.027
Mollika, S., Islam, N., Parvin, N., Kabir, A., Sayem, M. W., Luthfunnesa, S. R., et al. (2014). Evaluation of analgesic, anti-inflammatory and CNS activities of the methanolic extract of Syzygium samarangense leave. Glob. J. Pharmacol. 8 (1), 39–46. doi:10.5829/idosi.gjp.2014.8.1.81223
Mota, V. S., Turrini, R. N. T., and Poveda, V. B. (2015). Antimicrobial activity of Eucalyptus globulus oil, xylitol and papain: a pilot study. Rev. Esc. Enferm. Usp. 49, 0216–0220. doi:10.1590/S0080-623420150000200005
Mugaba, M. (2023). Phytochemical constitunents analysis and formulation of herbal syrup from thunbergia alata extracts for the management of diarrhoea. Busia, Uganda: Busitema University.
Muganga, R., Angenot, L., Tits, M., and Frédérich, M. (2010). Antiplasmodial and cytotoxic activities of Rwandan medicinal plants used in the treatment of malaria. J. Ethnopharmacol. 128 (1), 52–57. doi:10.1016/j.jep.2009.12.023
Muithya, J. N. (2010). Phytochemical and in vitro anti-microbial screening of echinops hispidus fresen. And grewia similis K. Schum. Nairobi, Kenya: School of Pure and Applied Science Kenyatta University.
Mukungu, N., Abuga, K., Okalebo, F., Ingwela, R., and Mwangi, J. (2016). Medicinal plants used for management of malaria among the Luhya community of Kakamega East sub-County, Kenya. J. Ethnopharmacol. 194, 98–107. doi:10.1016/j.jep.2016.08.050
Mulaudzi, R., Ndhlala, A. R., Kulkarni, M. G., Finnie, J. F., and Van Staden, J. (2013). Anti-inflammatory and mutagenic evaluation of medicinal plants used by Venda people against venereal and related diseases. J. Ethnopharmacol. 146 (1), 173–179. doi:10.1016/j.jep.2012.12.026
Müller-Jakic, B., Müller, M., Pröbstle, A., Johns, T., and Bauer, R. (1993). Anti-inflammatory activity of Zanthoxylum chalybeum extracts and identification of protoberberine and benzophenanthridine alkaloids by GC-MS and HPLC. Planta Medica 59 (1), A664. doi:10.1055/s-2006-959934
Muriithi, N. J., Maina, G. S., Maina, M. B., Mworia, J. K., Juma, K. K., Umar, A., et al. (2015). Determination of hematological effects of methanolic leaf extract of Vernonia lasiopus in normal mice. J. Blood Lymph. 05 (03). doi:10.4172/2165-7831.1000139
Musila, M., Dossaji, S. F., Nguta, J. M., Lukhoba, C. W., and Munyao, J. M. (2013). In vivo antimalarial activity, toxicity and phytochemical screening of selected antimalarial plants. J. Ethnopharmacol. 146 (2), 557–561. doi:10.1016/j.jep.2013.01.023
Mutie, F. M., Gao, L.-L., Kathambi, V., Rono, P. C., Musili, P. M., Ngugi, G., et al. (2020). An ethnobotanical survey of a dryland botanical garden and its environs in Kenya: the mutomo hill plant sanctuary. Evidence-Based Complementary Altern. Med. 2020, 1543831. doi:10.1155/2020/1543831
Mutungi, M. (2023). Evidence-based conservation of threatened medicinal plants in dry lands of Kenya: community awareness, and mass propagation.
Naboulsi, I., Aboulmouhajir, A., Kouisni, L., Bekkaoui, F., and Yasri, A. (2018). Plants extracts and secondary metabolites, their extraction methods and use in agriculture for controlling crop stresses and improving productivity: a review. Acad. J. Med. Plants 6, 223–240.
Nambooze, J., Erukainure, O. L., and Chukwuma, C. I. (2022). Phytochemistry of Prunus africana and its therapeutic effect against prostate cancer. Comp. Clin. Pathol. 31 (5), 875–893. doi:10.1007/s00580-022-03382-w
Namuga, C., Muwonge, H., Lubwama, M., Janet, N., Sekulima, T., and Kirabira, J. B. (2022). Antibacterial activities of Bidens pilosa L, Hoslundia opposita Vahl, and Ageratum conyzoides L against some common wound pathogens. Afr. J. Pharm. Pharmacol. 16 (5), 64–78. doi:10.5897/AJPP2022.5296
Nankaya, J., Gichuki, N., Lukhoba, C., and Balslev, H. (2019). Medicinal plants of the Maasai of Kenya: a review. Plants 9 (1), 44. doi:10.3390/plants9010044
Nankaya, J., Gichuki, N., Lukhoba, C., and Balslev, H. (2020). Medicinal plants of the Maasai of Kenya: a review. Plants 9 (1), 44. doi:10.3390/plants9010044
Narapusetty, N., Sivaiah, O., Balanasaraiah, B., Haranadhbabu, M., Prasad, B., Crown, B. H., et al. (2017). Anti-Inflammatory activity of ethanolic extract of Basella alba in acute and sub-acute model. Asian J. Pharm. Res. 7 (2), 88–93. doi:10.5958/2231-5691.2017.00015.6
Nardos, A., and Makonnen, E. (2017). In vivo antiplasmodial activity and toxicological assessment of hydroethanolic crude extract of Ajuga remota. Malar. J. 16, 25–28. doi:10.1186/s12936-017-1677-3
Narendhirakannan, R., Subramanian, S., and Kandaswamy, M. (2007). Anti-inflammatory and lysosomal stability actions of Cleome gynandra L. studied in adjuvant induced arthritic rats. Food Chem. Toxicol. 45 (6), 1001–1012. doi:10.1016/j.fct.2006.12.009
Ngaffo, C. M., Kamga, J., Guefack, M. G. F., and Kuete, V. (2022). The antiproliferative extract from the leaves of Acacia sieberiana var. woodii (Fabaceae) is harmless as evidenced by acute and subacute toxicity studies in rats. South Afr. J. Bot. 150, 217–224. doi:10.1016/j.sajb.2022.07.033
Ngegba, P. M., Cui, G., Khalid, M. Z., and Zhong, G. (2022). Use of botanical pesticides in agriculture as an alternative to synthetic pesticides. Agriculture 12 (5), 600. doi:10.3390/agriculture12050600
Ngoufack Azanze, E., Mbiantcha, M., Madjo, K. Y. K., Yousseu, N. W., Fagni Njoya, Z. L., Adjouzem, C. F., et al. (2023). Markhamia lutea leaves aqueous and ethanolic extract with curative anti-inflammatory activity attenuates paclitaxel toxicity in rat’s intestine. J. Complement. Integr. Med. 2023 (0). doi:10.1515/jcim-2023-0017
Ngugi, D. N. (2014). Study of antiplasmodial activity, cytotoxicity and acute toxicity of Zanthoxylum chalybeum ENGL, and Vernonia lasiopus o. Hoffman. Nairobi: University of Nairobi.
Nguyen, T. S., Xia, N. H., Van Chu, T., and Van Sam, H. (2019). Ethnobotanical study on medicinal plants in traditional markets of Son La province, Vietnam. For. Soc. 3 (2), 171–192. doi:10.24259/fs.v3i2.6005
Nicholas, K. (2017). In-Vitro cytotoxicity of three selected medicinal plant extracts from Kenya. World J. Pharm. Pharm. Sci. 6 (7), 144–152. doi:10.20959/wjpps20176-9263
Nile, S. H., and Keum, Y. S. (2018). Chemical composition, antioxidant, anti-inflammatory and antitumor activities of Eucalyptus globulus Labill. India: NISCAIR-CSIR.
Njau, E.-F., Alcorn, J., Ndakidemi, P., Chirino-Trejo, M., and Buza, J. (2014). Antimicrobial and antioxidant activity of crude extracts of Rauvolfia caffra var. caffra (Apocynaceae) from Tanzania. Int. J. Biol. 6 (4). doi:10.5539/ijb.v6n4p156
Njenga, D., Irungu, B., Mbaria, J., Mutai, C., and Nguta, J. (2016). Antiplasmodial activity, cytotoxicity and acute toxicity of Zanthoxylum chalybeum engl. World J. Pharm. Pharm. Sci. 5, 208–217.
Njeru, S. N., and Muema, J. M. (2020). Antimicrobial activity, phytochemical characterization and gas chromatography-mass spectrometry analysis of Aspilia pluriseta Schweinf. extracts. Heliyon 6 (10), e05195. doi:10.1016/j.heliyon.2020.e05195
Njeru, S. N., and Muema, J. M. (2021). In vitro cytotoxicity of Aspilia pluriseta Schweinf. extract fractions. BMC Res. Notes 14 (1), 57–64. doi:10.1186/s13104-021-05472-4
Njume, C., Afolayan, A. J., Samie, A., and Ndip, R. N. (2011). In-vitro anti-Helicobacter pylori activity of acetone, ethanol and methanol extracts of the stem bark of Combretum molle (Combretaceae). J. Med. Plants Res. 5 (14), 3210–3216.
Nkomo, L., Green, E., and Ndip, R. (2011). Preliminary phytochemical screening and in vitro anti-Helicobacter pylori activity of extracts of the leaves of Lippia javanica. Afr. J. Pharm. Pharmacol. 5 (20), 2184–2192. doi:10.5897/ajpp10.168
Nkomo, L. P. (2010). In vitro bioactivity of crude extracts of Lippia javanica on clinical isolates of Helicobacter pylori: preliminary phytochemical screening. Eastern Cape, South Africa: University of Fort Hare. Available at: http://hdl.handle.net/10353/508.
Ochwang’i, D. O., Kimwele, C. N., Oduma, J. A., Gathumbi, P. K., Mbaria, J. M., and Kiama, S. G. (2014). Medicinal plants used in treatment and management of cancer in Kakamega County, Kenya. J. Ethnopharmacol. 151 (3), 1040–1055. doi:10.1016/j.jep.2013.11.051
Odo, C. E. (2016). Effect of the methanol extract of the leaves of Azadirachta indica on ethanol-induced gastric ulcer in rats. J. Pharm. Res. 10 (1), 41–45.
Ofusori, D. A., Falana, B. A., Ofusori, A. E., Abayomi, T. A., Ajayi, S. A., and Ojo, G. B. (2010). Gastroprotective effect of aqueous extract of neem Azadirachta indica on induced gastric lesion in rats. Int. J. Biol. Med. Res. 1 (4), 219–222.
Okach, D., Nyunja, A., and Opande, G. (2013). Phytochemical screening of some wild plants from Lamiaceae and their role in traditional medicine in Uriri District-Kenya. Int. J. Herb. Med. 1 (5), 135–143.
Okello, D., Gathirwa, J., Wanyoko, A., Komakech, R., Chung, Y., Gang, R., et al. (2023). Comparative antiplasmodial activity, cytotoxicity, and phytochemical contents of Warburgia ugandensis stem bark against Aspilia africana wild and in vitro regenerated tissues. J. Plant Biotechnol. 50 (1), 13–107. doi:10.5010/jpb.2023.50.013.097
Okori, B. C., Oryema, C., Opiro, R., Amos, A., Obici, G. I., Rutaro, K., et al. (2022). Ethnobotanical survey of plants locally used in the control of termite pests among rural communities in northern Uganda. CABI Agric. Biosci. 3 (1), 37–10. doi:10.1186/s43170-022-00109-3
Olajide, O. A., Akinola Alada, A., and Kolawole, O. T. (2005). Anti-inflammatory properties of entada abyssinica. Leaves. Pharm. Biol. 43 (7), 583–585. doi:10.1080/13880200500301654
Oloro, J., Tanayen, J. K., Barbra, K., Lawrence, I., Paul, W., Francis, B., et al. (2016). Phytochemical and efficacy study on four herbs used in erectile dysfunction: Mondia whiteii, Cola acuminata, Urtica massaica, and Tarenna graveolens. Afr. J. Pharm. Pharmacol. 10, 785–790. doi:10.5897/ajpp2015.4405
Omara, T. (2020). Antimalarial plants used across Kenyan communities. Evidence-Based Complement. Altern. Med. 2020. doi:10.1155/2020/4538602
Omeh, Y. N., Onoja, S. O., Ezeja, M. I., and Okwor, P. O. (2014). Subacute antidiabetic and in vivo antioxidant effects of methanolic extract of Bridelia micrantha (Hochst Baill) leaf on alloxan-induced hyperglycaemic rats. J. Complement. Integr. Med. 11 (2), 99–105. doi:10.1515/jcim-2013-0067
Omodamiro, O. D., Ajah, O., Jimoh, M. A., and Ewa-Ibe, C. (2021). Evaluation of sub-chronic toxicity, anti-inflammatory and diuretic effect of ethanol leaves extract Ficus capensis in albino rat. Anim. Res. Int. 18 (2), 4073–4082.
Omwenga, E., Hensel, A., Shitandi, A., and Goycoolea, F. (2015). Ethnobotanical survey of traditionally used medicinal plants for infections of skin, gastrointestinal tract, urinary tract and the oral cavity in Borabu sub-county, Nyamira county, Kenya. J. Ethnopharmacol. 176, 508–514. doi:10.1016/j.jep.2015.11.032
Onwuka, N. A., Ezike, A. C., Ettebong, E. D., Tologbonse, A. A., and Onyeukwu, N. J. (2016). Evaluation of the immunomodulatory activity of Hoslundia opposita Vahl (Lamiaceae) leaf extract. Int. J. Pharmacogn. Phytochem. Res. 8, 1–7.
Owolabi, A. O., Ndako, J. A., Owa, S. O., Oluyori, A. P., Oludipe, E. O., Akinsanola, B. A., et al. (2022). In vitro antimicrobial appraisal of the potentials of Morinda lucida against someselected bacteria. Trop. J. Nat. Prod. Res. 6 (3), 380–387. doi:10.26656/fr.2017.6(4).424
Oyedeji-Amusa, M., Van Vuuren, S., and Van Wyk, B. E. (2020). Antimicrobial activity and toxicity of extracts from the bark and leaves of South African indigenous Meliaceae against selected pathogens. South Afr. J. Bot. 133, 83–90. doi:10.1016/j.sajb.2020.06.032
Pacifica, B., Nyanchongi, B., and Masai, R. (2018). Ethnobotanical survey of medicinal plants used for treatment of malaria by Kipsigis people in Kericho County, Kenya. IOSR J. Pharm. Biol. Sci. 13, 24–30. doi:10.9790/3008-1304062430
Parra-Delgado, H., García Ruiz, G., Nieto Camacho, A., and Martínez-Vázquez, M. (2004). Anti-inflammatory activity of some extracts and isolates from Leonotis nepetaefolia on TPA-induced edema model. Rev. Soc. Quím. México 48 (4), 293–295.
Patel, A., Patel, T., Macwan, C., Patel, M., Chauhan, K., and Patel, J. (2010). Evaluation of anti inflammatory and analgesic activity of roots of Rubia cordifolia in rats. J. Pharm. Sci. Res. 2 (12), 809.
Patterson, S., Waters, M. E., Braman, N., Willson, R., Hill, R. A., Magolan, J., et al. (2023). Garcinia buchananii stem bark extract and its bioactive constituents manniflavanone, GB-2 and buchananiflavanone attenuate intestinal inhibitory neuromuscular transmission. J. Smooth Muscle Res. 59, 34–57. doi:10.1540/jsmr.59.34
Prathibha, M., and Jayaramu, S. (2019). Phytochemical screening and in vitro antihelmintic properties of Justicia betonica L. Int. J. Pharm. Biol. Sci. 9 (1), 1426–1430. doi:10.21276/ijpbs.2019.9.1.188
Pushpan, R., Karra, N., Nariya, M. B., and K., A. V. (2017). Evaluation of anti-arthritic potential of Leonotis nepetifolia (L.) R. Br. against Freund’s adjuvant induced arthritis. J. Ayurveda Integr. Med. Sci. 2 (05), 59–66. doi:10.21760/jaims.v2i05.10255
Raja, N. L., and Sundar, K. (2016). Psidium guajava linn confers analgesic effects on mice. J. Pharm. Sci. Res. 8 (6), 412.
Raji, Y., Ogunwande, I. A., Osadebe, C. A., and John, G. (2004). Effects of Azadirachta indica extract on gastric ulceration and acid secretion in rats. J. Ethnopharmacol. 90 (1), 167–170. doi:10.1016/j.jep.2003.09.020
Rao, D. M., Rao, U., and Sudharshanam, G. (2006). Ethno-medico-botanical studies from rayalaseema region of southern eastern ghats, Andhra Pradesh, India. Ethnobot. Leafl. 2006 (1), 21.
Rasmussen, S. J. (1998). Only women know trees: medicine women and the role of herbal healing in Tuareg culture. J. Anthropol. Res. 54 (2), 147–171. doi:10.1086/jar.54.2.3631728
Rattan, R. (2023). Bioactive flavonoids from Acanthaceae species-A review. Int. J. Emerg. Technol. Innov. Res. 10 (6), h16–h21.
Rauf, A., Bawazeer, S., Rashid, U., El-Esawi, M. A., Khan, M. H., Shah, S. U. A., et al. (2021). Antiglycation and enzyme inhibitory potential of salicylalazine isolated from Micromeria biflora (Buch.-Ham. ex D. Don) Benth. South Afr. J. Bot. 143, 344–349. doi:10.1016/j.sajb.2020.12.021
Ray, J., Goyal, P., and Aggarwal, B. K. (2015). Approach of Eucalyptus globulus plant parts for human health safety and toxicological aspects. Brit Open J. Plant Sci. 1 (1), 1–10.
Roger, T., Pierre-Marie, M., Igor, V. K., and Patrick, V. (2015). Phytochemical screening and antibacterial activity of medicinal plants used to treat typhoid fever in Bamboutos division, West Cameroon. J. Appl. Pharm. Sci. 5 (6), 034–049. doi:10.7324/japs.2015.50606
Salinitro, M., Vicentini, R., Bonomi, C., and Tassoni, A. (2017). Traditional knowledge on wild and cultivated plants in the kilombero valley (morogoro region, Tanzania). J. Ethnobiol. Ethnomedicine 13, 17–14. doi:10.1186/s13002-017-0146-y
Samet, H., and Cikili, Y. (2016). Importance of medicinal and aromatic plants as an alternative crop in the rural development of Turkey. J. Rural Community Dev. 10 (4).
Sanni, S., Thilza, I. B., Talle, M., Mohammed, S. A., Sanni, F. S., Okpoli, L. A., et al. (2010). The effect of Acacia nilotica pob ethyl acetate fraction on induced diarrhea in albino rats. N. Y. Sci. J. 3, 16–20.
Seile, B. P., Bareetseng, S., Koitsiwe, M. T., and Aremu, A. O. (2022). Indigenous knowledge on the uses, sustainability and conservation of African ginger (Siphonochilus aethiopicus) among two communities in Mpumalanga province, South Africa. Diversity 14 (3), 192. doi:10.3390/d14030192
Sekhar, N. C., Jayasree, T., Ubedulla, S., Dixit, R., V S, M., and J, S. (2014). Evaluation of antinociceptive activity of aqueous extract of bark of Psidium guajava in albino rats and albino mice. J. Clin. Diagn. Res. JCDR 8 (9), HF01–HF04. doi:10.7860/JCDR/2014/8288.4811
Shen, C.-H., Liu, C. T., Song, X. J., Zeng, W. Y., Lu, X. Y., Zheng, Z. L., et al. (2018). Evaluation of analgesic and anti-inflammatory activities of Rubia cordifolia L. by spectrum-effect relationships. J. Chromatogr. B 1090, 73–80. doi:10.1016/j.jchromb.2018.05.021
Siani, A. C., Souza, M. C., Henriques, M. G. M. O., and Ramos, M. F. S. (2013). Anti-inflammatory activity of essential oils from Syzygium cumini and Psidium guajava. Pharm. Biol. 51 (7), 881–887. doi:10.3109/13880209.2013.768675
Silva, J., Abebe, W., Sousa, S. M., Duarte, V. G., Machado, M. I. L., and Matos, F. J. A. (2003). Analgesic and anti-inflammatory effects of essential oils of Eucalyptus. J. Ethnopharmacol. 89 (2-3), 277–283. doi:10.1016/j.jep.2003.09.007
Singh, R., Singh, B., Singh, S., Kumar, N., Kumar, S., and Arora, S. (2010). Umbelliferone–An antioxidant isolated from Acacia nilotica (L.) Willd. ex. Del. Food Chem. 120 (3), 825–830. doi:10.1016/j.foodchem.2009.11.022
Sivakumar, K., Rajasekaran, A., Ponnilavarasan, I., Somasundaram, A., and Sivasakthi, R. (2010). Antiulcer and analgesic activity of the ethanol extract of Cleome gynandra linn leaves. Res. J. Pharm. Technol. 3 (3), 766–769.
Stark, T. D., Salger, M., Frank, O., Balemba, O. B., Wakamatsu, J., and Hofmann, T. (2015). Antioxidative compounds from Garcinia buchananii stem bark. J. Nat. Prod. 78 (2), 234–240. doi:10.1021/np5007873
Tanko, Y., Mohammed, A., Okasha, M. A., Umar, A. H., and Magaji, R. A. (2008). Anti-nociceptive and anti-inflammatory activities of ethanol extract of Syzygium aromaticum flower bud in wistar rats and mice. Afr. J. Tradit. Complement. Altern. Med. 5 (2), 209–212. doi:10.4314/ajtcam.v5i2.31275
Tardío, J., and Pardo-de-Santayana, M. (2008). Cultural importance indices: a comparative analysis based on the useful wild plants of Southern Cantabria (Northern Spain). Econ. Bot. 62 (1), 24–39. doi:10.1007/s12231-007-9004-5
Tefera, T., and Yihune, M. (2019). Ethnobotanical study on medicinal plants used by indigenous people in Tenta District, South Wollo, Ethiopia. J. Med. Plants Res. 13 (2), 47–54. doi:10.5897/jmpr2018.6599
Tegegne, H., and Woldegiorgis, D. (2023). Testing the skin with Histofarcin and examining the in-vitro antifungal properties of Croton Macrostachyus against the mycelial form of Histoplasma capsulatum Variety Farciminosum, isolated from a horse in Adama City, Ethiopia. Available at: https://doi.org/10.21203/rs.3.rs-3583121/v1.
Teke, G. N., Lunga, P. K., Wabo, H. K., Kuiate, J. R., Vilarem, G., Giacinti, G., et al. (2011). Antimicrobial and antioxidant properties of methanol extract, fractions and compounds from the stem bark of Entada abyssinica Stend ex A. Satabie. BMC Complement. Altern. Med. 11 (1), 57–58. doi:10.1186/1472-6882-11-57
Tomen, I., Guragac, F., Keleş, H., Reunanen, M., and Kupeli-Akkol, E. (2017). Characterization and wound repair potential of essential oil Eucalyptus globulus Labill. Fresenius Environ. Bull. 26 (11).
Torres-Avilez, W., Medeiros, P. M. d., and Albuquerque, U. P. (2016). Effect of gender on the knowledge of medicinal plants: systematic review and meta-analysis. Evidence-Based Complementary Altern. Med. 2016, 6592363. doi:10.1155/2016/6592363
Tugume, P., and Nyakoojo, C. (2019). Ethno-pharmacological survey of herbal remedies used in the treatment of paediatric diseases in Buhunga parish, Rukungiri District, Uganda. BMC Complementary Altern. Med. 19 (1), 353–410. doi:10.1186/s12906-019-2763-6
Tumen, I., Süntar, I., Eller, F. J., Keleş, H., and Akkol, E. K. (2013). Topical wound-healing effects and phytochemical composition of heartwood essential oils of Juniperus virginiana L., Juniperus occidentalis Hook., and Juniperus ashei J. Buchholz. J. Med. food 16 (1), 48–55. doi:10.1089/jmf.2012.2472
Turner, N. (2014). Ancient pathways, ancestral knowledge: ethnobotany and ecological wisdom of indigenous peoples of northwestern North America (Vol. 74). Canada: McGill-Queen’s University Press. Available at: https://hdl.handle.net/2027/heb33492.0001.001.
Turner, N. J., Cuerrier, A., and Joseph, L. (2022). Well grounded: indigenous Peoples' knowledge, ethnobiology and sustainability. People Nat. 4 (3), 627–651. doi:10.1002/pan3.10321
Tyagi, S., Singh, M., Singh, D., Yadav, I., Singh, S., and Mansoori, M. H. (2011). Anti-asthmatic potential of Flacourtia indica merr. Afr. J. Basic Appl. Sci. 3 (5), 201–204.
Tyavambiza, C., Dube, P., Goboza, M., Meyer, S., Madiehe, A. M., and Meyer, M. (2021). Wound healing activities and potential of selected african medicinal plants and their synthesized biogenic nanoparticles. Plants 10 (12), 2635. doi:10.3390/plants10122635
Ullah, H., Qureshi, R., Munazir, M., Bibi, Y., Saboor, A., Imran, M., et al. (2023). Quantitative ethnobotanical appraisal of shawal valley, south waziristan, khyber pakhtunkhwa, Pakistan. Ethnobot. Res. Appl. 25, 1–17. doi:10.32859/era.25.527.1-17
Umar, S. I., Lawal, B., Mohammed, B. A., Obiekezie, C. I., Adewuyi, A. H., Babalola, S. B., et al. (2019). Antioxidant and antimicrobial activities of naturally occurring flavonoids from M. heterophylla and the safety evaluation in Wistar rats. Iran. J. Toxicol. 13 (4), 39–44. doi:10.32598/ijt.13.4.516.2
Usman, M., Ditta, A., Ibrahim, F. H., Murtaza, G., Rajpar, M. N., Mehmood, S., et al. (2021). Quantitative ethnobotanical analysis of medicinal plants of high-temperature areas of Southern Punjab, Pakistan. Plants 10 (10), 1974. doi:10.3390/plants10101974
Ved, D., and Goraya, G. (2007). Demand and supply of medicinal plants in India. Bangalore, India: NMPB, New Delhi & FRLHT, 18.
Vigo, E., Cepeda, A., Gualillo, O., and Perez-Fernandez, R. (2004). In-vitro anti-inflammatory effect of Eucalyptus globulus and Thymus vulgaris: nitric oxide inhibition in J774A. 1 murine macrophages. J. Pharm. Pharmacol. 56 (2), 257–263. doi:10.1211/0022357022665
Vijayasanthi, M., Doss, A., and Kannan, K. (2015). Anti-inflammatory activity of Spathodea campanulata P. Beauv. leaves against carrageenan induced paw edema. Bio Technology-Elixir Int. J. 78, 29450–29452.
Vitalini, S., Iriti, M., Puricelli, C., Ciuchi, D., Segale, A., and Fico, G. (2013). Traditional knowledge on medicinal and food plants used in Val San Giacomo (Sondrio, Italy)—an alpine ethnobotanical study. J. Ethnopharmacol. 145 (2), 517–529. doi:10.1016/j.jep.2012.11.024
Waiganjo, N., Ochanda, H., and Yole, D. (2016). Phytochemical analysis of the selected five plant extracts.
Wakeel, O. K., Abe, A. I., Awosan, O. B., Olapade, M. K., Olatoyan-Layonu, T. J., Olowe, O. A., et al. (2021). Anti-Nociceptive and anti-inflammatory effects of stem bark extract of Ficus capensis thunb (moraceae) by bioactivity fractionation. Anti-Inflammatory Anti-Allergy Agents Med. Chem. Former. Curr. Med. Chemistry-Anti-Inflammatory Anti-Allergy Agents) 20 (2), 206–218. doi:10.2174/1871523019666200825194616
Wali, R., Khan, M. F., Mahmood, A., Mahmood, M., Qureshi, R., Ahmad, K. S., et al. (2022). Ethnomedicinal appraisal of plants used for the treatment of gastrointestinal complaints by tribal communities living in Diamir district, Western Himalayas, Pakistan. PLoS ONE 17 (6), e0269445. doi:10.1371/journal.pone.0269445
Wanjohi, B. K., Njenga, E. W., Sudoi, V., Kipkore, W. K., Moore, H. L., and Davies, M. I. (2020a). Ecological knowledge of indigenous plants among the marakwet community (embobut basin), elgeyo marakwet county (Kenya). Ethnobot. Res. Appl. 20, 1–16. doi:10.32859/era.20.1.1-16
Wanjohi, B. K., Sudoi, V., Njenga, E. W., and Kipkore, W. K. (2020b). An ethnobotanical study of traditional knowledge and uses of medicinal wild plants among the marakwet community in Kenya. Evidence-Based Complementary Altern. Med. 2020, 3208634. doi:10.1155/2020/3208634
Wekesa, D., Mambo, F., Barasa, E., Soita, K., Gosar, A. A., Kitungulu, N., et al. (2023). Antiplasmodial and cytotoxic activities of selected medicinal plants in Western Kenya. J. Phytopharm. 12 (4), 218–229. doi:10.31254/phyto.2023.12402
Weldearegay, E. M., and Awas, T. (2021). Ethnobotanical study in and around sirso natural forest of melokoza district, gamo goffa zone, southern Ethiopia. Ethnobot. Res. Appl. 22, 1–24. doi:10.32859/era.22.27.1-24
Were, P. S., Kutima, H. L., Suba, H. O., and Walyambillah, W. (2020). Warburgia ugandensis: a potent in vivo phytomedicine against Plasmodium knowlesi. J. Pharmacogn. Phytochem. 9 (5), 01–05.
Wetungu Martin, W., Matasyoh, J., and Kinyanjui, T. (2014). Antimicrobial activity of solvent extracts from the leaves of Tarchonanthus camphoratus (Asteraceae). J. Pharmacogn. Phytochem. 3 (1), 123–127.
Woode, E., Ansah, C., Ainooson, G. K., Abotsi, W. M., Mensah, A. Y., and Duweijua, M. (2007). Anti-inflammatory and antioxidant properties of the root extract of Carissa edulis (Forsk.) Vahl (Apocynaceae). J. Sci. Technol. (Ghana) 27 (3), 5–15. doi:10.4314/just.v27i3.33054
Wuerger, G., McGaw, L. J., Franz, C., and Eloff, J. N. (2009). Evaluation of the activity of leaf extracts of trees that have been used to treat diarrhoea in humans and animals. Afr. J. Tradit. Complement. Altern. Med. 2009, 475–476.
Würger, G. (2010). A rational in vitro evaluation of 53 medicinal plants used in the treatment of diarrhoea and the potential use of Deinbollia oblongifolia (Sapindaceae) extracts. Pretoria: University of Pretoria.
Yacob, T., Shibeshi, W., and Nedi, T. (2016). Antidiarrheal activity of 80% methanol extract of the aerial part of Ajuga remota Benth (Lamiaceae) in mice. BMC Complement. Altern. Med. 16 (1), 303–308. doi:10.1186/s12906-016-1277-8
Yusuf, A., and Abdullahi, M. (2019). The phytochemical and pharmacological actions of Entada africana Guill. & Perr. Heliyon 5 (9), e02332. doi:10.1016/j.heliyon.2019.e02332
Zayede, D., Mulaw, T., and Kahaliw, W. (2020). Antidiarrheal activity of hydromethanolic root extract and solvent fractions of Clutia abyssinica Jaub. & Spach. (Euphorbiaceae) in mice. Evidence-Based Complement. Altern. Med. 2020, 5416749. doi:10.1155/2020/5416749
Zenderland, J., Hart, R., Bussmann, R. W., Paniagua Zambrana, N. Y., Sikharulidze, S., Kikvidze, Z., et al. (2019). The use of “Use Value”: quantifying importance in ethnobotany. Econ. Bot. 73, 293–303. doi:10.1007/s12231-019-09480-1
Zhao, R., Wang, H.-H., Gao, J., Zhang, Y.-J., Li, X., Zhou, J.-J., et al. (2022). Plant volatile compound methyl benzoate is highly effective against Spodoptera frugiperda and safe to non-target organisms as an eco-friendly botanical-insecticide. Ecotoxicol. Environ. Saf. 245, 114101. doi:10.1016/j.ecoenv.2022.114101
Zhou, Y., Liu, B., Mbuni, Y., Yan, X., Mwachala, G., Hu, G., et al. (2017). Vascular flora of Kenya, based on the flora of tropical East Africa. PhytoKeys 90, 113–126. doi:10.3897/phytokeys.90.20531
Keywords: ethnomedicinal plants, traditional medicine, Kenyan medicinal plants, herbal medicine, quantitative indices
Citation: Maiyo ZC, Njeru SN, Toroitich FJ, Indieka SA and Obonyo MA (2024) Ethnobotanical study of medicinal plants used by the people of Mosop, Nandi County in Kenya. Front. Pharmacol. 14:1328903. doi: 10.3389/fphar.2023.1328903
Received: 27 October 2023; Accepted: 27 December 2023;
Published: 19 January 2024.
Edited by:
François Chassagne, IRD UMR152 Pharmacochimie et Biologie Pour le Développement (PHARMADEV), FranceReviewed by:
Jean-Marc Dubost, National Museum of Natural History, FranceCopyright © 2024 Maiyo, Njeru, Toroitich, Indieka and Obonyo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Z. C. Maiyo, Y21haXlAZ21haWwuY29t, emlwcG9yYWgubWFpeW9AQGVnZXJ0b24uYWMua2U=; M. A. Obonyo, b2JvbnlvQGdtYWlsLmNvbQ==, bWVzaGFjay5vYm9ueW9AZWdlcnRvbi5hYy5rZQ==
†ORCID: Z. C. Maiyo, orcid.org/0000-0001-9674-4036; S. N. Njeru, orcid.org/0000-0003-1154-0777; F. J. Toroitich, orcid.org/0000-0001-8930-3128; S. A. Indieka, orcid.org/0000-0002-1630-9075; M. A. Obonyo, orcid.org/0000-0002-5826-7109
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.