
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Pharmacol. , 12 January 2024
Sec. Neuropharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1326815
This article is part of the Research Topic Recent advances in the treatment of epilepsy View all 13 articles
 Ninon Freidel1,2†
Ninon Freidel1,2† Liliane Kreuder1,3†
Liliane Kreuder1,3† Brenden Samuel Rabinovitch1,4,5*
Brenden Samuel Rabinovitch1,4,5* Frank Yizhao Chen1,6
Frank Yizhao Chen1,6 Ryan S. T. Huang1,7
Ryan S. T. Huang1,7 Evan Cole Lewis1,8‡
Evan Cole Lewis1,8‡Psychedelic compounds have been utilized by humans for centuries for medicinal, religious, and tribal purposes. Clinical trial data starting from the early 2000s and continuing today indicates that psychedelics are a clinically efficacious treatment for a variety of neurological and psychiatric disorders. However, all clinical trials examining these substances have excluded any individual with a past or current history of seizures, leaving a large cohort of epilepsy and non-epilepsy chronic seizure disorder patients without anywhere to turn for psychedelic-assisted therapy. These exclusions were made despite any significant evidence that clinically supervised psychedelic use causes or exacerbates seizures in this population. To date, no clinical trial or preclinical seizure model has demonstrated that psychedelics induce seizures. This review highlights several cases of individuals experiencing seizures or seizure remission following psychedelic use, with the overall trend being that psychedelics are safe for use in a controlled, supervised clinical setting. We also suggest future research directions for this field.
Psychedelic-assisted therapy (PAT) has emerged as a clinically efficacious treatment for an array of psychiatric disorders including treatment-resistant depression (TRD) (Carhart-Harris et al., 2016a; Stroud et al., 2018; Palhano-Fontes et al., 2019; Goodwin et al., 2022), major depressive disorder (MDD) (Davis et al., 2021; Sloshower et al., 2023), end-of-life psychiatric distress (Bossis et al., 2016; Agin-Liebes et al., 2020; Rosa et al., 2022), anxiety (Davis et al., 2019), post-traumatic stress disorder (PTSD) (Mithoefer et al., 2018; Mithoefer et al., 2019) and obsessive-compulsive disorder (OCD) (Rodriguez et al., 2013; Kelmendi et al., 2022). PAT has also effectively treated tobacco addiction and alcohol dependence (Krebs and Johansen, 2012; Bogenschutz et al., 2015; Johnson et al., 2017; Sessa et al., 2019). In these clinical trials, both classical psychedelics, such as psilocybin and lysergic acid diethylamide (LSD), and atypical psychedelics, such as ketamine and 3,4-methylenedioxymethamphetamine (MDMA) were integrated into therapy sessions. These forms of PAT are well-tolerated by patients in clinical settings. Recently, psychedelic treatment has been used to treat functional neurological disorder (FND), an umbrella of neurological symptoms including functional movement disorders (FMDs), functional sensory disorders (FSDs), and functional seizures (Butler et al., 2020; Stewart et al., 2020; Argento et al., 2023).
Although psychedelics have shown strong efficacy in treating a diverse array of symptoms, one specific cohort of patients has been excluded from all PAT clinical trials: individuals with a past or current history of seizures.
Individuals with chronic seizure disorders, such as epilepsy and some mitochondrial encephalopathies, have a reduced quality of life and experience disproportionate rates of anxiety and depression compared to the general population (Carhart-Harris and Nutt, 2017; López-Giménez and González-Maeso, 2018). Additionally, ∼10% of individuals with epilepsy experience functional seizures (also referred to as psychogenic, non-epileptic seizures [PNES]), which are not treatable with traditional anti-epileptic pharmacological therapies or surgical interventions due to their psychological underpinnings. These patients require novel, alternative therapies, with conjunctive psychological treatment, such as in PAT.
There is sparse and conflicting published data regarding the safety of psychedelics in the context of chronic and acute seizures. We will demonstrate that most reports are case studies of individuals taking psychedelics recreationally in unsupervised non-clinical settings. The few controlled studies support classical psychedelics as safe and tolerable under clinical supervision, even in patients with a history of epilepsy who currently experience spontaneous, recurrent seizures (SRS).
The safety profile of classical psychedelics in individuals with epilepsy must be characterized to determine if these compounds are safe for use to treat functional seizures and co-morbid neuropsychiatric conditions. Although this review will focus on epilepsy, the data we present is also relevant to individuals with non-epilepsy chronic seizure disorders, such as mitochondrial encephalopathies. This review aims to summarize the complex mosaic of psychedelics in the context of epilepsy and seizures.
All data were extracted from public databases including PubMed, Google Scholar, and ResearchGate. The following search terms were used in different combinations: epilepsy, psychedelics, psilocybin, magic mushrooms, mescaline, LSD, lysergide, lysergic acid diethylamide, MDMA, 3,4-methylenedioxymethamphetamine, methylenedioxymethamphetamine, molly, ecstasy, seizures, chronic seizures, acute seizures, serotonergic psychedelics, and hallucinogen.
Search results were screened by reviewing titles and abstracts. The full text of screened abstracts was reviewed to confirm inclusions.
The inclusion criteria were: 1) any explicit mention of classical and/or atypical psychedelics in the context of acute and/or chronic seizures, and 2) case reports and/or clinical trials involving classical and/or atypical psychedelic use in any seizure disorder or in patients with acute seizures.
A total of 701 papers were collected. 34 papers passed the title and abstract screens. From there, 11 papers passed the full text screen and were included in the analysis (Figure 1).
The classical psychedelics psilocybin, LSD, and mescaline are agonists of the 5-hydroxytryptamine (5-HT) 2A and 2C receptors (López-Giménez and González-Maeso, 2018). The neural circuitry effects of LSD and psilocybin include changes in resting-state functional connectivity (RSFC) between and within distinct brain regions. Most notably, these compounds increase inter-network RSFC, between the default mode network (DMN), executive network (EN), and salience network (SN) structures, while decreasing intra-network RSFC within each set of structures (Carhart-Harris et al., 2014; Carhart-Harris and Nutt, 2017). The precise neurobiological underpinnings of these functional network changes are still not completely understood.
MDMA is an atypical psychedelic, referred to as an empathogen and entactogen (National Academies of Sciences et al., 2022). Although MDMA is serotonergic, it acts via a different mechanism from the classical psychedelics and also possesses some dopaminergic activity (Schenk and Highgate, 2021). MDMA has several mechanisms of action, including increasing presynaptic serotonin release to the synaptic cleft, inhibiting serotonin reuptake at the presynaptic terminal, and some monoamine oxidase inhibition (Kalant, 2001). The subjective effects of MDMA are reported as less hallucinogenic than the classical psychedelics, with increased feelings of empathy and lovingness both outwardly to others and inwardly to oneself (Dumont et al., 2009; Bedi et al., 2010).
Ketamine, an atypical psychedelic and dissociative anesthetic, is an N-methyl-D-aspartate (NMDA) receptor antagonist. The neurobiological mechanisms underlying ketamine’s effects are still being investigated, as ketamine has several mechanisms of action. Ketamine preferentially binds to the NMDA receptors on GABAergic inhibitory interneurons (Gerhard et al., 2020). Ketamine also binds extra-synaptic NMDA receptors on glutamatergic excitatory neurons at lower subanaesthetic doses (Zorumski et al., 2016). At anaesthetic doses, ketamine binds to NMDA receptors on glutamatergic excitatory neurons, inducing overall reduced excitatory transmission, leading to loss of consciousness. Moreover, ketamine may also induce its rapid and persistent anti-depressive effects through its metabolism into hydroxynorketamine (HNK), which is an antagonist of the excitatory ɑ-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor (Zanos et al., 2016), which glutamate is an agonist of.
We identified 10 case reports from 1992 to 2023 in which patients experienced seizures after ingesting a classical or atypical psychedelic substance. Reports in each subsection are ordered by date (oldest to newest). One additional paper, which was not a case report (Serafetinides, 1965) is also described, though this paper is an open-label trial of LSD in individuals with epilepsy who undergo neurosurgery.
Hall et al. (1996) reported a 26-year-old paraplegic male experiencing generalized seizures following the ingestion of an ecstasy (MDMA) tablet (of unknown dosage) alongside concurrent alcohol consumption (Hall et al., 1996). He was treated with 50 mg of intravenous diazepam, dantrolene and anaesthesia (thiopentone, alfentanil and suxamethonium), successfully halting the generalized tonic-clonic seizure. Subsequently, the patient developed hypotension, disseminated intravascular coagulation (DIC), acute renal failure, gross rhabdomyolysis, adult respiratory distress syndrome and hepatic failure. Despite the severity of his condition, he was discharged 17 days later and achieved a complete recovery.
Cooper et al. (1997) reported an accidental ingestion of MDMA (of unknown dosage) resulting in febrile convulsion in a 2-year-old female with a history of speech delay (Cooper and Egleston, 1997). At the time, the patient was undergoing treatment with amoxicillin for an upper respiratory tract infection, but the duration of treatment was not specified. The patient displayed agitation, high fever, rapid heart rate and dilated pupils. She was treated with oxygen, rectal paracetamol, and intravenous diazepam resulting in a full recovery without complications. Initially, the mother did not indicate that the patient ingested any substance, until she was questioned about the patient’s abnormal teeth grinding and oculogyric crisis. Following this, the patient’s urine was analyzed, and the findings showed MDMA and MDA (the metabolite of MDMA) presence. The mother then admitted that the patient had accidently ingested MDMA, which delayed the delivery of treatment. The exact timecourse of the urine analysis and treatment was not described, but it was noted that the patient was admitted to the hospital and underwent the aforementioned treatments prior to the parent’s admission (Cooper and Egleston, 1997).
Magee et al. (1998) reported the case of a 17-year-old female who exhibited generalized seizures 5 and 12 h following the ingestion of an ecstasy (MDMA) tablet (of unknown dosage) with concurrent alcohol consumption (Magee et al., 1998). The patient’s symptoms included drowsiness, incoherent speech, hypotension, and reduced urine sodium levels (115 mmol/L). These symptoms indicated severe dilutional hyponatremia leading to secondary seizure and stupor. The significant salt and water loss resulting from vigorous dancing was effectively treated with intravenous isotonic saline, resulting in a complete recovery.
Huntjens et al. (2022) reported accidental MDMA intoxication in a 14-month-old male toddler with an unremarkable medical history (Huntjens et al., 2022). The patient presented to the emergency department with a generalized tonic-clonic seizure. 2.0 mg (0.2 mg/kg) of intraosseous midazolam was administered, but seizures persisted until the patient received 400 mg (42.5 mg/kg) of intravenous levetiracetam, which successfully terminated the status epilepticus. Blood serum analysis revealed an MDMA concentration of 0.48 mg/L33.
Pauwels et al. (2023), reported two independent incidents where a young child accidently ingested ecstasy (MDMA) and subsequently began experiencing seizures (Pauwels et al., 2013). Patient 1 was a 19-month-old male with an unremarkable medical history who was taken to the hospital due to mowing arm gestures, staring, and eye turning. Urine analysis showed 7.8 mg/L 3,4-methylenedioxyamphetamine (MDA) concentration and 183 mg/L MDMA concentration approximately 1–3 h after initial intoxication. The patient was eventually discharged from the hospital without complications.
Patient 2 was a 20-month-old female with a history of convulsions after a fall on the head. She was taken to the hospital presenting with hyperthermia, tachycardia, and rigidity. Urine analysis revealed a 6 mg/L MDA and 119 mg/L MDMA concentration, along with trace amounts of cocaine (Pauwels et al., 2013). It should be noted, however, that although this report explicitly described trace amounts of cocaine being present in the main text, the table displaying the toxicology results showed a negative result in the cocaine assay, without any urine concentration listed.
Several case studies describe adults experiencing seizures following the recreational use of psychedelics without clinical supervision.
Picker et al. (1992) reported a potential interaction between LSD and fluoxetine, a selective serotonin reuptake inhibitor (SSRI) after a 16-year-old male undergoing 20 mg/day of fluoxetine treatment for approximately 1 year experienced a focal seizure that progressed to a generalized tonic-clonic seizure after ingesting two “blotters” containing LSD at an unknown dosage (Picker et al., 1992). Interestingly, this may have been a case of serotonin syndrome, which is an acute constellation of symptoms caused by excessive serotonin levels. Serotonin syndrome is often reported when different serotonergic drugs are taken concomitantly, such as an SSRI with a large dose of LSD (Boyer and Shannon, 2005; Foong et al., 2018; Francescangeli et al., 2019).
Legriel et al. (2008) reported the case of a 39-year-old male with a history of depression and chronic alcohol abuse (Legriel et al., 2008). The medication history included 75 mg/kg of clomipramine daily to control depression symptoms, which was discontinued after 6 months. The patient reported experiencing a generalized tonic-clonic seizure 3 years prior following ingestion of lysergic acid amine (LSA), which is structurally similar to LSD. The patient was taken to a hospital and experienced mental confusion and mydriasis. Vital signs included a 120 beats/min heart rate, and 185/130 mmHg blood pressure. At the hospital, the patient experienced another seizure that lasted over 10 min, which did cease following intravenous clonazepam administration. The patient was subsequently intubated and mechanically ventilated and was successfully extubated 3 days after admission. The dose of LSA taken was unknown, and the purity was not determined (Legriel et al., 2008).
Aakeroy et al. (2021), reported a male in his late teens with an unremarkable medical history who arrived at the hospital following ingestion of a “blotter” containing LSD and small amounts of N,N-dimethyl tryptamine (DMT), methamphetamine, amphetamine, and MDMA that resulted in the patient experiencing a tonic seizure and other adverse events including vomiting and cyanosis (Aakerøy et al., 2021). Emergency personnel arrived 25 min after the initial onset of symptoms and found the patient in cardiorespiratory arrest. The patient was intubated and received cardiopulmonary resuscitation (CPR) therapy. A comprehensive drug analysis was conducted on a blotter sample identical to the one the patient ingested, which revealed a dosage of 300 μg LSD. Serum and urine samples were collected 3 h after the initial onset of symptoms and LSD was found to have a serum concentration of 4 ng/mL (12.4 nmol/L) and 1.3 ng/mL (4.0 nmol/L) urine concentration (Aakerøy et al., 2021). For comparison, clinical trial LSD dosing is standardized at or around 75 µg, which induces significant mind-altering effects compared to placebo, with observable changes in functional brain activity, detected by functional magnetic resonance imaging (fMRI) (Carhart-Harris et al., 2016b).
Serafetinides (1965) examined the effect of LSD in 20 individuals with a temporal lobectomy to treat their epileptic seizures. 1 μg/kg LSD was given to patients (orally) 2–3 days before, and 1 month after their temporal lobectomy surgeries (Serafetinides, 1965). Scalp electroencephalograph (EEG) recordings were taken during the LSD administration to determine changes in brain waves and epileptic activity. During the pre-operation recordings, 12 patients had no change in epileptic activity, while 5 had a decrease and 1 had increased epileptic EEG activity. During the post-operation recordings, 17 patients had no change in epileptic activity, while 2 had decreased and 1 had increased epileptic activity. Overall, this study indicates LSD may be safe for use in individuals with epilepsy (Serafetinides, 1965).
Lastly, a case study by Blond et al. (2023), reported a significant exacerbation in epileptic seizures following the ingestion of a large dose (3.6 g) of psychedelic mushrooms in a 31-year-old male with a history of refractory frontal epilepsy (Blond and Schindler, 2023). In order to treat the unilateral right temporal epilepsy, the patient had been previously implanted with a responsive neurostimulation system (RNS) that improved morbidity although he continued to experience several focal seizures without awareness per week. According to his RNS data, ingestion of psilocybin at high doses (3.6 g) resulted in 32 long episodes (30 s of prolonged epileptiform activity) while ingestion at low doses (1.5 g) did not change baseline seizure frequency (Blond and Schindler, 2023).
Recently, Argento et al. (2023) reported the case of a 51-year-old female with refractory functional seizures with a daily frequency (Argento et al., 2023). The patient’s medical history included MDD and PTSD. After years of failing behavioural and pharmacological therapies, she enrolled in a ketamine-assisted therapy program. The patient underwent 3 weeks of ketamine-assisted therapy followed by 20 weeks of intermittent therapy and ketamine sessions. During weeks 1, 3, and 4, the patient received 100, 150, and 200 mg of ketamine sublingually, respectively, with an additional 35, 45, and 60 mg intranasal dose, respectively. See Table 1 for the complete KAT treatment regimen. The type of therapy used was psychotherapy by a clinical psychologist specializing in trauma and somatization disorders. Following the treatment, the patient went into remission for the functional seizures, depressive symptoms, and functional movement symptoms (Argento et al., 2023).
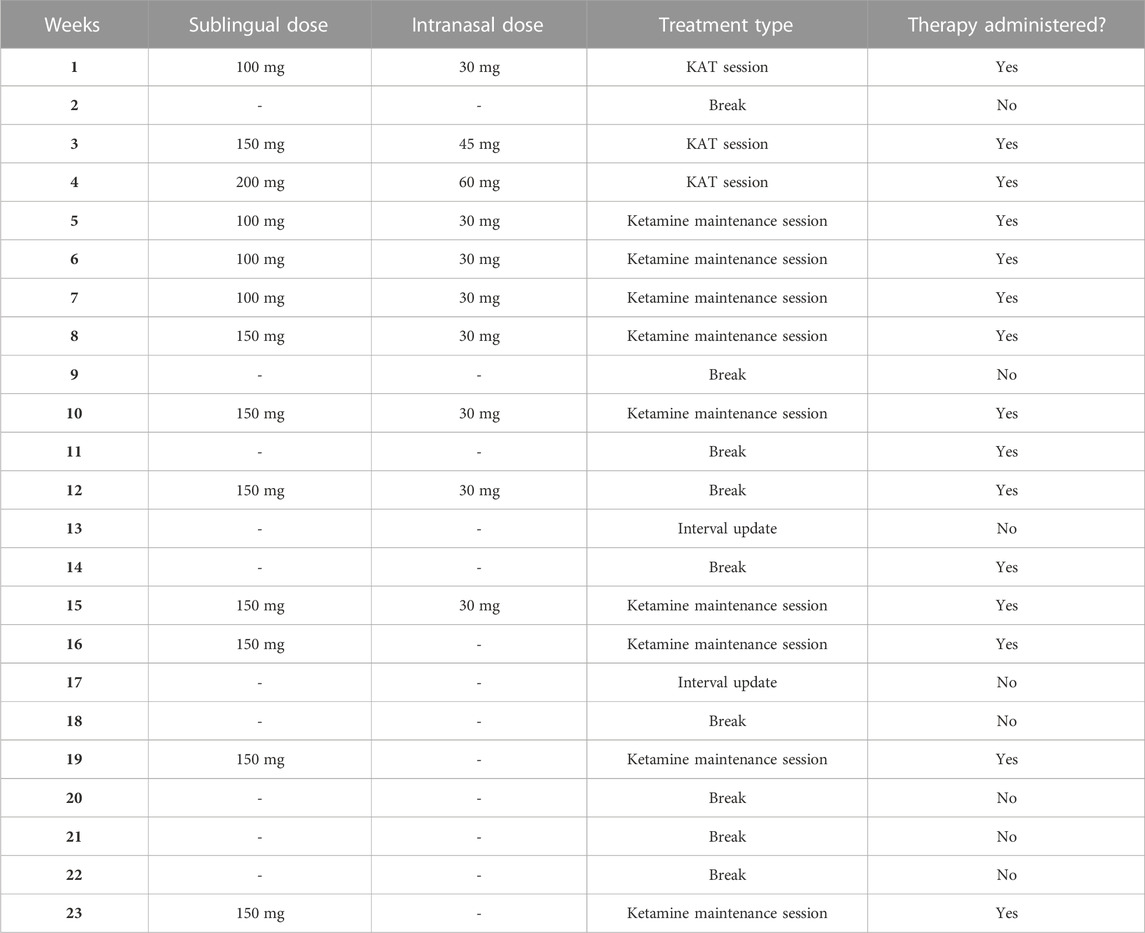
TABLE 1. KAT treatment regimen and timeline from Argento et al., (2023). Adapted from Argento et al., (2023) Figure 2.
In conclusion, there is a deficit of published data on preclinical and clinical uses of psychedelics in the context of epilepsy and non-epilepsy seizure disorders. Most data are case reports of individuals taking unspecified amounts of untested psychedelic drugs in an uncontrolled recreational setting (see Figure 2). Although adverse events were reported, including convulsive seizures, it is unclear whether these events resulted from the psychedelic use, or whether other confounding factors played a role, such as simultaneous alcohol or other drug consumption and concomitant medication, all of which were reported in several cases (see Table 2). Several reports described both accidental and intentional ingestion of other substances, such as cocaine and non-MDMA amphetamines concomitantly with the psychedelic substance (Cooper and Egleston, 1997; Boyer and Shannon, 2005; Huntjens et al., 2022). Additionally, the case reports involving young children ingesting MDMA, though concerning, do not indicate that MDMA induces seizures in individuals with chronic seizure conditions when taken in a controlled clinical setting with appropriate dosing.
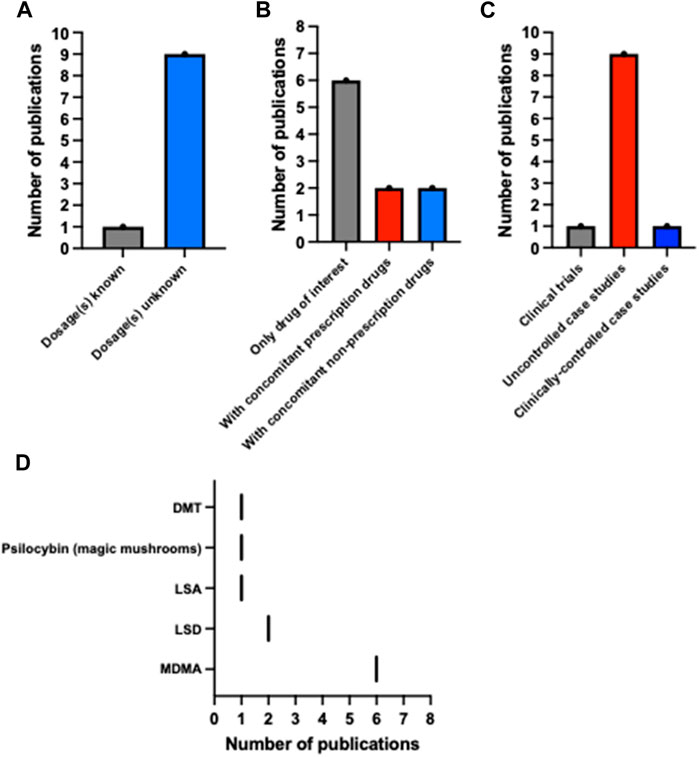
FIGURE 2. Descriptive statistics of the psychedelic and epilepsy literature. Bar plots of the number of publications describing reports where (A) drug dosages were known, (B) multiple drugs (prescription and non-prescription) were ingested, and (C) there was clinical supervision. (D) The number of publications based on the psychedelics ingested in each report. These data highlight variability and sparsity in the literature. Note: Pauwels et al. (2023) counted as n = 2 in this figure due to its reporting of two separate and unrelated cases. (A,B) Do not include Serafetinides (1965) due to this study being a clinical trial. This figure was made using GraphPad Prism 10.
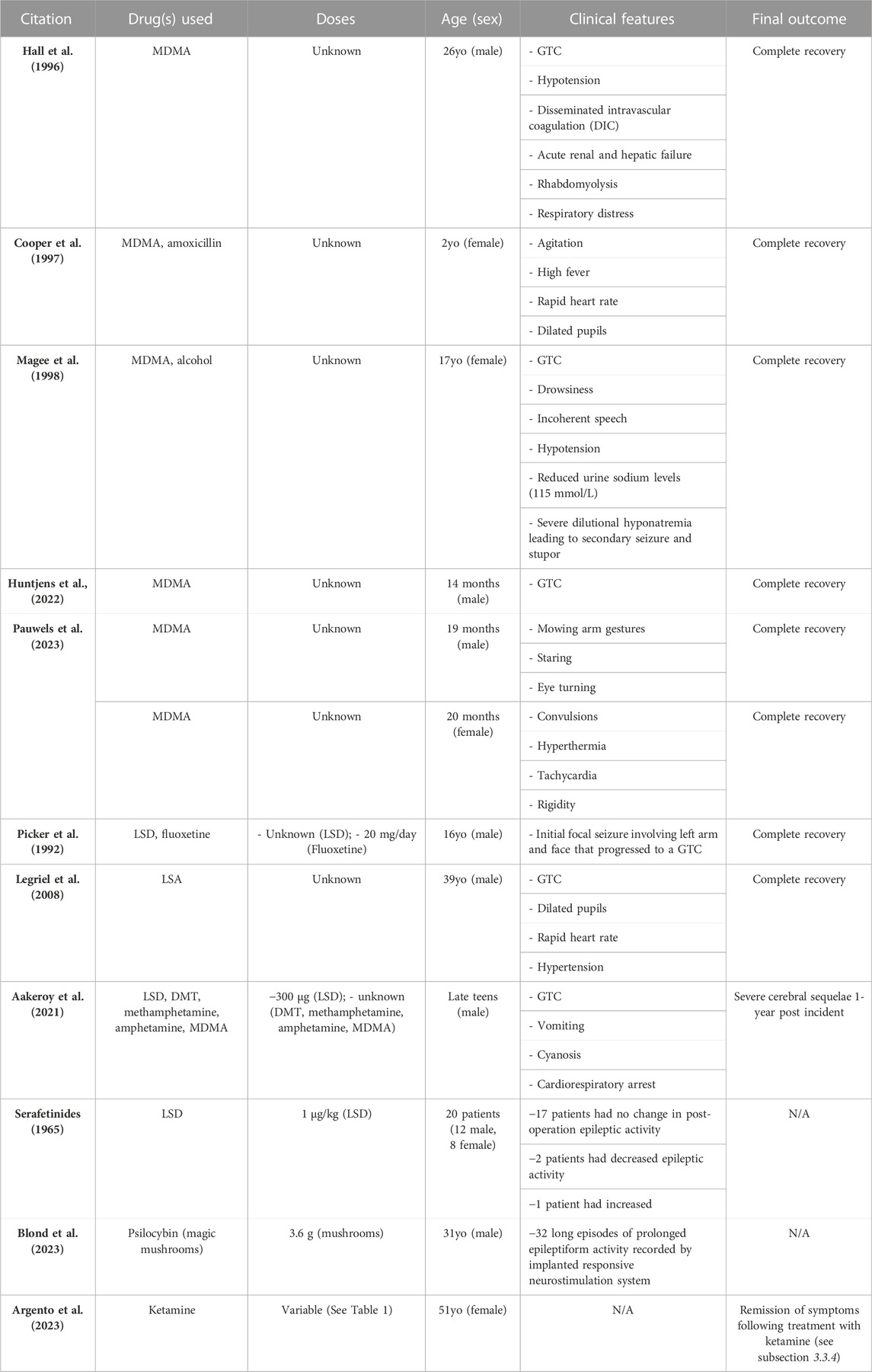
TABLE 2. Summary of all reports described in the results section. Abbreviations used in this table exclusively: years-old (yo); months-old (mo); generalized tonic-clonic seizure (GTC); not applicable (N/A).
It should be noted, however, that in all reports of psychedelic use while under clinical supervision, such as Argento et al. (2023) and Serafetinides (1965), no significant serious adverse events were reported in the individuals who have epilepsy. Although further research is needed, the data we describe in this review indicate that psychedelics may be safe for use in the epilepsy population when taken under clinical supervision in a clinical setting, such as with psychedelic-assisted therapy.
Future research should focus on studying classical psychedelics in preclinical animal and human-derived organoid models of chronic and acute seizures. If it is established that classical psychedelics do not exacerbate seizures in animals, then randomized double-blind placebo-controlled trials should be completed to determine if there is any therapeutic benefit of psychedelics in patients with epilepsy or other chronic seizure disorders to treat their seizures, both epileptic and functional. The field of epilepsy and seizure research is ripe for new data testing classical psychedelic use in acute and chronic seizure disease models. More data is essential to inform clinicians of the potential adverse events or therapeutic benefits of these substances.
NF: Data curation, Writing–original draft, Writing–review and editing. LK: Data curation, Writing–original draft, Writing–review and editing. BR: Writing–review and editing, Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Supervision, Validation, Visualization, Writing–original draft. FC: Writing–review and editing. RH: Writing–review and editing, Visualization. ECL: Supervision, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors thank Maria Paz De Barros Barrento Scavone for her contribution to this work. She assisted in collecting and sorting papers for review. We are grateful to JMCC for their continued support of NCT’s research program.
Author FC was employed by Jamaican Medical Cannabis Corporation. Author ECL is employed by Numinus.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aakerøy, R., Brede, G. I., Stolen, S. B., Krabseth, H. M., Michelsen, L. S., Andreassen, T. N., et al. (2021). Severe neurological sequelae after a recreational dose of LSD. J. Anal. Toxicol. 45, e1–e3. doi:10.1093/jat/bkaa145
Agin-Liebes, G. I., Malone, T., Yalch, M. M., Mennenga, S. E., Ponté, K. L., Guss, J., et al. (2020). Long-term follow-up of psilocybin-assisted psychotherapy for psychiatric and existential distress in patients with life-threatening cancer. J. Psychopharmacol. (Oxf.) 34, 155–166. doi:10.1177/0269881119897615
Argento, E., Omene, E., Jaeger, A. H., Kertes, A., Mitchell, K. A., Necyk, C., et al. (2023). Case report: improvement in refractory functional seizures, depression, and quality of life with ketamine-assisted therapy. Front. Neurosci. 17, 1197409. doi:10.3389/fnins.2023.1197409
Bedi, G., Hyman, D., and de Wit, H. (2010). Is ecstasy an ‘empathogen’? Effects of ±3,4-methylenedioxymethamphetamine on prosocial feelings and identification of emotional states in others. Biol. Psychiatry 68, 1134–1140. doi:10.1016/j.biopsych.2010.08.003
Blond, B. N., and Schindler, E. A. D. (2023). Case report: psychedelic-induced seizures captured by intracranial electrocorticography. Front. Neurol. 14, 1214969. doi:10.3389/fneur.2023.1214969
Bogenschutz, M. P., Forcehimes, A. A., Pommy, J. A., Wilcox, C. E., Barbosa, P. C. R., and Strassman, R. J. (2015). Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J. Psychopharmacol. (Oxf.) 29, 289–299. doi:10.1177/0269881114565144
Bossis, A., Ross, S., Guss, J., Agin-Liebes, G., Malone, T., Cohen, B., et al. (2016). Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J. Psychopharmacol. (Oxf.) 30, 1165–1180. doi:10.1177/0269881116675512
Boyer, E. W., and Shannon, M. (2005). The serotonin syndrome. N. Engl. J. Med. 352, 1112–1120. doi:10.1056/NEJMra041867
Butler, M., Seynaeve, M., Nicholson, T. R., Pick, S., Kanaan, R. A., Lees, A., et al. (2020). Psychedelic treatment of functional neurological disorder: a systematic review. Ther. Adv. Psychopharmacol. 10, 2045125320912125. doi:10.1177/2045125320912125
Carhart-Harris, R., Leech, R., Hellyer, P. J., Shanahan, M., Feilding, A., Tagliazucchi, E., et al. (2014). The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front. Hum. Neurosci. 8, 20. doi:10.3389/fnhum.2014.00020
Carhart-Harris, R. L., Bolstridge, M., Rucker, J., Day, C. M. J., Erritzoe, D., Kaelen, M., et al. (2016a). Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry 3, 619–627. doi:10.1016/S2215-0366(16)30065-7
Carhart-Harris, R. L., Kaelen, M., Bolstridge, M., Williams, T. M., Williams, L. T., Underwood, R., et al. (2016b). The paradoxical psychological effects of lysergic acid diethylamide (LSD). Psychol. Med. 46, 1379–1390. doi:10.1017/S0033291715002901
Carhart-Harris, R. L., and Nutt, D. J. (2017). Serotonin and brain function: a tale of two receptors. J. Psychopharmacol. Oxf. Engl. 31, 1091–1120. doi:10.1177/0269881117725915
Cooper, A. J., and Egleston, C. V. (1997). Accidental ingestion of Ecstasy by a toddler: unusual cause for convulsion in a febrile child. J. Accid. Emerg. Med. 14, 183–184. doi:10.1136/emj.14.3.183
Davis, A. K., Barrett, F. S., May, D. G., Cosimano, M. P., Sepeda, N. D., Johnson, M. W., et al. (2021). Effects of psilocybin-assisted therapy on major depressive disorder: a randomized clinical trial. JAMA Psychiatry 78, 481–489. doi:10.1001/jamapsychiatry.2020.3285
Davis, A. K., So, S., Lancelotta, R., Barsuglia, J. P., and Griffiths, R. R. (2019). 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) used in a naturalistic group setting is associated with unintended improvements in depression and anxiety. Am. J. Drug Alcohol Abuse 45, 161–169. doi:10.1080/00952990.2018.1545024
Dumont, G. J. H., Sweep, F. C. G. J., van der Steen, R., Hermsen, R., Donders, A. R. T., Touw, D. J., et al. (2009). Increased oxytocin concentrations and prosocial feelings in humans after ecstasy (3,4-methylenedioxymethamphetamine) administration. Soc. Neurosci. 4, 359–366. doi:10.1080/17470910802649470
Foong, A.-L., Grindrod, K. A., Patel, T., and Kellar, J. (2018). Demystifying serotonin syndrome (or serotonin toxicity). Can. Fam. Physician 64, 720–727.
Francescangeli, J., Karamchandani, K., Powell, M., and Bonavia, A. (2019). The serotonin syndrome: from molecular mechanisms to clinical practice. Int. J. Mol. Sci. 20, 2288. doi:10.3390/ijms20092288
Gerhard, D. M., Pothula, S., Liu, R. J., Wu, M., Li, X. Y., Girgenti, M. J., et al. (2020). GABA interneurons are the cellular trigger for ketamine’s rapid antidepressant actions. J. Clin. Invest. 130, 1336–1349. doi:10.1172/JCI130808
Goodwin, G. M., Aaronson, S. T., Alvarez, O., Arden, P. C., Baker, A., Bennett, J. C., et al. (2022). Single-dose psilocybin for a treatment-resistant episode of major depression. N. Engl. J. Med. 387, 1637–1648. doi:10.1056/NEJMoa2206443
Hall, A. P., Lyburn, I. D., Spears, F. D., and Riley, B. (1996). An unusual case of Ecstasy poisoning. Intensive Care Med. 22, 670–671. doi:10.1007/BF01709744
Huntjens, D. W., Weersink, E. P. S., Hilarius, D. L., Ran, N. C., and Franssen, E. J. F. (2022). Severe epileptic seizures after accidental MDMA exposure in a 14-month-old child. Clin. Toxicol. Phila. Pa 60, 657. doi:10.1080/15563650.2021.1999464
Johnson, M. W., Garcia-Romeu, A., and Griffiths, R. R. (2017). Long-term follow-up of psilocybin-facilitated smoking cessation. Am. J. Drug Alcohol Abuse 43, 55–60. doi:10.3109/00952990.2016.1170135
Kalant, H. (2001). The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs. CMAJ Can. Med. Assoc. J. 165, 917–928.
Kelmendi, B., Kichuk, S. A., DePalmer, G., Maloney, G., Ching, T. H. W., Belser, A., et al. (2022). Single-dose psilocybin for treatment-resistant obsessive-compulsive disorder: a case report. Heliyon 8, e12135. doi:10.1016/j.heliyon.2022.e12135
Krebs, T. S., and Johansen, P.-Ø. (2012). Lysergic acid diethylamide (LSD) for alcoholism: meta-analysis of randomized controlled trials. J. Psychopharmacol. (Oxf.) 26, 994–1002. doi:10.1177/0269881112439253
Legriel, S., Bruneel, F., Spreux-Varoquaux, O., Birenbaum, A., Chadenat, M. L., Mignon, F., et al. (2008). Lysergic acid amide-induced posterior reversible encephalopathy syndrome with status epilepticus. Neurocrit. Care 9, 247–252. doi:10.1007/s12028-008-9096-5
López-Giménez, J. F., and González-Maeso, J. (2018). Hallucinogens and serotonin 5-ht2a receptor-mediated signaling pathways. Curr. Top. Behav. Neurosci. 36, 45–73. doi:10.1007/7854_2017_478
Magee, C., Staunton, H., Tormey, W., and Walshe, J. J. (1998). Hyponatraemia, seizures and stupor associated with ecstasy ingestion in a female. Ir. Med. J. 91, 178.
Mithoefer, M. C., Feduccia, A. A., Jerome, L., Mithoefer, A., Wagner, M., Walsh, Z., et al. (2019). MDMA-assisted psychotherapy for treatment of PTSD: study design and rationale for phase 3 trials based on pooled analysis of six phase 2 randomized controlled trials. Psychopharmacol. (Berl.) 236, 2735–2745. doi:10.1007/s00213-019-05249-5
Mithoefer, M. C., Mithoefer, A. T., Feduccia, A. A., Jerome, L., Wagner, M., Wymer, J., et al. (2018). 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry 5, 486–497. doi:10.1016/S2215-0366(18)30135-4
National Academies of Scienceset al. (2022). “History and current status of psychedelics and entactogens for the treatment of psychiatric disorders,” in Exploring psychedelics and entactogens as treatments for psychiatric disorders: proceedings of a workshop (Washington, DC: National Academies Press US).
Palhano-Fontes, F., Barreto, D., Onias, H., Andrade, K. C., Novaes, M. M., Pessoa, J. A., et al. (2019). Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychol. Med. 49, 655–663. doi:10.1017/S0033291718001356
Pauwels, S., Lemmens, F., Eerdekens, K., Penders, J., Poesen, K., Desmet, K., et al. (2013). Ecstasy intoxication as an unusual cause of epileptic seizures in young children. Eur. J. Pediatr. 172, 1547–1550. doi:10.1007/s00431-013-2080-x
Picker, W., Lerman, A., and Hajal, F. (1992). Potential interaction of LSD and fluoxetine. Am. J. Psychiatry 149, 843–844. doi:10.1176/ajp.149.6.843b
Rodriguez, C. I., Kegeles, L. S., Levinson, A., Feng, T., Marcus, S. M., Vermes, D., et al. (2013). Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 38, 2475–2483. doi:10.1038/npp.2013.150
Rosa, W. E., Sager, Z., Miller, M., Bernstein, I., Doerner Rinaldi, A., Addicott, K., et al. (2022). Top ten tips palliative care clinicians should know about psychedelic-assisted therapy in the context of serious illness. J. Palliat. Med. 25, 1273–1281. doi:10.1089/jpm.2022.0036
Schenk, S., and Highgate, Q. (2021). Methylenedioxymethamphetamine (MDMA): serotonergic and dopaminergic mechanisms related to its use and misuse. J. Neurochem. 157, 1714–1724. doi:10.1111/jnc.15348
Serafetinides, E. A. (1965). The EEG effects of LSD-25 in epileptic patients before and after temporal lobectomy. Psychopharmacologia 7, 453–460. doi:10.1007/BF00402367
Sessa, B., Sakal, C., O’Brien, S., and Nutt, D. (2019). First study of safety and tolerability of 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy in patients with alcohol use disorder: preliminary data on the first four participants. BMJ Case Rep. CP 12, e230109. doi:10.1136/bcr-2019-230109
Sloshower, J., Skosnik, P. D., Safi-Aghdam, H., Pathania, S., Syed, S., Pittman, B., et al. (2023). Psilocybin-assisted therapy for major depressive disorder: an exploratory placebo-controlled, fixed-order trial. J. Psychopharmacol. (Oxf.) 37, 698–706. doi:10.1177/02698811231154852
Stewart, B., Dean, J. G., Koek, A., Chua, J., Wabl, R., Martin, K., et al. (2020). Psychedelic-assisted therapy for functional neurological disorders: a theoretical framework and review of prior reports. Pharmacol. Res. Perspect. 8, e00688. doi:10.1002/prp2.688
Stroud, J. B., Freeman, T. P., Leech, R., Hindocha, C., Lawn, W., Nutt, D. J., et al. (2018). Psilocybin with psychological support improves emotional face recognition in treatment-resistant depression. Psychopharmacol. (Berl.) 235, 459–466. doi:10.1007/s00213-017-4754-y
Zanos, P., Moaddel, R., Morris, P. J., Georgiou, P., Fischell, J., Elmer, G. I., et al. (2016). NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533, 481–486. doi:10.1038/nature17998
Keywords: psychedelics, seizures, epilepsy, LSD, psilocybin, magic mushrooms, MDMA, ketamine
Citation: Freidel N, Kreuder L, Rabinovitch BS, Chen FY, Huang RST and Lewis EC (2024) Psychedelics, epilepsy, and seizures: a review. Front. Pharmacol. 14:1326815. doi: 10.3389/fphar.2023.1326815
Received: 24 October 2023; Accepted: 07 December 2023;
Published: 12 January 2024.
Edited by:
Mahmoud Rafieian-Kopaei, Shahrekord University of Medical Sciences, IranReviewed by:
Janosch P. Heller, Dublin City University, IrelandCopyright © 2024 Freidel, Kreuder, Rabinovitch, Chen, Huang and Lewis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brenden Samuel Rabinovitch, YnJlbmRlbi5yYWJpbm92aXRjaEB1aG4uY2E=, YnJlbmRlbi5yYWJpbm92aXRjaEBtYWlsLnV0b3JvbnRvLmNh
†These authors share first authorship
‡These authors share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.