- 1Personalized Drug Therapy Key Laboratory of Sichuan Province, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 2Department of Pharmacy, People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, China
- 3Department of Pharmacy, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, Chengdu, China
Background: venous thromboembolism (VTE) is one of the most common complications after major orthopaedic surgery. Recent studies have suggested that aspirin may also be effective in preventing VTE, but it is still controversial whether it can be routinely used.
Objectives: To compare the efficacy and safety of aspirin against oral anticoagulants in the prevention of VTE following total hip arthroplasty (THA), total knee arthroplasty (TKA) or hip fracture surgery (HFS).
Methods: Relevant publications have been obtained using electronic search databases such as PubMed, Embase, Web of Science, Cochrane Library, and Clinical Trials. gov. from inception to 20 July 2023. Only RCTs evaluating the efficacy and safety of aspirin compared with oral anticoagulants undergoing major orthopaedic surgery were included in the meta-analysis. The primary outcome reported was any VTE event (including deep vein thrombosis (DVT) and pulmonary embolism (PE)). Secondary outcomes included mortality, major bleeding (including gastrointestinal bleed, cerebrovascular hemorrhage, or any bleeding requiring a return to the theater), minor bleeding (ecchymosis, epistaxis, hematuria), and wound complications. The risk of bias for all included studies was assessed according to the Cochrane Collaboration’s tool.
Results: After screening 974 studies, 12 randomized clinical trials (RCTs) were included, involving 5,088 participants, including 2,540 participants in aspirin, 2,205 participants in rivaroxaban, and 323 participants in warfarin. Aspirin was found to be less effective than oral anticoagulants in thromboprophylaxis after major orthopedic surgery (RR = 1.206, 95% CI 1.053–1.383). After subgroup analysis according to the type of oral anticoagulant, the results showed that aspirin was similar to rivaroxaban and inferior to warfarin. Considering that the studies in the warfarin group were all conducted before 2000, our results need to be further confirmed. In addition, the aspirin group had a higher risk of VTE than the control group in other subgroups, including a follow-up time of ≤3 months, type of procedure as TKA, high-dose aspirin (≥650 mg qd), and no combined use of mechanical prophylaxis. In terms of safety events, aspirin did not show significant differences in major bleeding (RR = 0.952, 95% CI 0.499–1.815), all-cause mortality (RR = 1.208, 95% CI 0.459–3.177), and wound-related events (RR = 0.618, 95% CI 0.333–1.145) compared with oral anticoagulants, and aspirin was associated with a reduction in the risk of minor bleeding (RR = 0.685, 95% CI 0.552–0.850) events and total bleeding (RR = 0.726, 95% CI 0.590–0.892).
Conclusion: Aspirin reduces bleeding risk after major orthopedic surgery compared with oral anticoagulants, but may sacrifice VTE prevention to some extent. Updated evidence is needed to analyze the thromboprophylaxis effects of aspirin in patients undergoing major orthopedic surgery.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=463481, identifier CRD42023463481.
1 Introduction
Patients undergoing major orthopaedic surgery, such as total knee arthroplasty (TKA) and total hip arthroplasty (THA), and hip fracture surgery (HFS), are at elevated risk of venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE) (Piovella et al., 2005; Learmonth et al., 2007; Memtsoudis et al., 2012). Without prevention of thrombosis, post-operative thrombosis of THA and TKA can reach as high as 42%–57% and 40%–80% (Piovella et al., 2005; Geerts et al., 2008; Fisher, 2011), respectively, and up to 2% for fatal PE (Geerts et al., 2004; Howie et al., 2005; Kinov et al., 2014).
Current guidelines recommend prophylaxis should be given for a minimum of 10–14 days in patients undergoing major orthopaedic surgery (Chinese Orthopaedic Association, 2010; Falck-Ytter et al., 2012; Anderson et al., 2019; Gee, 2019). The common pharmacological agents for VTE prophylaxis include direct oral anticoagulants (DOACs) like rivaroxaban, heparin-based agents, vitamin K antagonists, and aspirin. Although there is clinical evidence for the use of these drugs, studies have shown differences in bleeding risk with their use, including major bleeding and increased readmission rates (Zou et al., 2014; Feng et al., 2015; Messerschmidt and Friedman, 2015). According to the Asia-Pacific venous thromboembolism consensus in knee and hip arthroplasty and hip fracture surgery announced in 2021, among all available pharmacological agents for VTE prophylaxis, aspirin is the most appropriate and more cost-effective prophylactic agent than other agents for Asians undergoing elective knee and hip arthroplasty with standard VTE risk, while for patients with elevated VTE risk, DOACs or LMWH are the most appropriate agents (Thiengwittayaporn et al., 2021). However, which pharmacological agent is the most appropriate for Asians undergoing hip fracture surgery still lacks enough supporting evidence. Guideline consensus differs in the prophylaxis of VTE for major orthopaedic surgery.
Randomized controlled trials have been published to evaluate the clinical efficacy and safety of aspirin for the prevention of VTE after THA, TKA, or HFS compared to oral anticoagulants, and the current guideline consensus is still to recommend anticoagulation, but aspirin is also recommended (Jenny et al., 2018; Anderson et al., 2019; Gee, 2019). We conducted a meta-analysis of randomized clinical trials (RCTs) comparing aspirin oral anticoagulants in patients after THA, TKA, or HFS.
2 Materials and methods
We performed a meta-analysis of RCTs according to the guidelines in Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
2.1 Search strategy
A comprehensive search was conducted independently by two individuals using PubMed, Embase, Web of Science, Cochrane Library, and ClinicalTrials.gov databases, covering the period from the inception of the databases until 20 July 2023. The computer-based searches combined terms and combinations of keywords related to the participants (e.g., hip arthroplasty, knee arthroplasty, hip replacement, knee replacement, or hip fractures surgery), drug intervention (e.g., aspirin), comparators (e.g., anticoagulants, warfarin, rivaroxaban, dabigatran, apixaban, or edoxaban), and outcomes (e.g., venous thromboembolism, pulmonary embolism, cerebral hemorrhage, or gastrointestinal hemorrhage). The reference list of all the retrieved articles underwent a manual examination to uncover additional potentially relevant studies. This study was registered in PROSPERO(CRD42023463481).
2.2 Inclusion and exclusion criteria
The search was limited to humans. Case reports of fewer than five neonates and conference abstracts were excluded.
The inclusion criteria encompassed the following: Studies were included if they met the following criteria: 1) Patient underwent major orthopedic surgery including TKA, THA, and HFS; 2) only randomized controlled trials were included; 3) treatment had to involve aspirin and oral anticoagulants, and oral anticoagulants included DOACs and warfarin; 4) the primary study outcome was reported, which was the occurrence of any VTE (including DVT and/or PE) after the procedure, regardless of whether the event was asymptomatic or asymptomatic. Secondary outcomes reported included mortality, major bleeding events (including gastrointestinal bleeding and cerebrovascular bleeding), other bleeding complications, and wound-related problems (e.g., hematomas and infections). There was no limit to the length of follow-up for inclusion in the study.
The exclusion criteria were defined as follows: Those articles that met the following criteria were excluded: 1) articles displaying inconsistent research content; 2) the type of article was review articles, conference abstracts, studies involving animals, case reports or study protocols; 3) articles not in English or Chinese.
2.3 Data extraction and risk of bias assessment
Two reviewers (Z.X.Y and L.N) independently screened the titles and abstracts and reviewed the full text, and articles that met the criteria were included by reviewing the full text, and then study characteristics, baseline characteristics, and prespecified efficacy and safety outcomes were extracted. Selected studies were scored based on The Cochrane Risk of Bias criteria (Cochrane Collaboration, 2011), which includes seven types of risk of bias assessment: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias.
2.4 Statistical methods
We calculated the relative risk (RR) and 95% confidence interval (CI) for each study. Heterogeneity was assessed using the I2 statistic. If I2 ≤ 50% and p ≥ 0.1, indicating that there was no heterogeneity between study outcomes, a fixed-effects model was used. If I2 > 50% and p < 0.1 indicated that there was heterogeneity among the study outcomes, a random effects simulation was used to analyze the source of heterogeneity (Higgins et al., 2003). If the event rate was zero, we corrected for half-integer continuity for all four cells (Friedrich et al., 2007). Publication bias was assessed using the Egger test and Begg’s test. If there was any publication bias, the trim and fill methods were used to adjust for publication bias (Duval and Tweedie, 2000). We performed sensitivity analyses for each outcome by excluding one study at a time to assess the stability of the meta-analysis results. To assess the impact of potential factors on the results of the meta-analysis of the primary outcome, we conducted several subgroup analyses based on study-level characteristics. All statistical analyses were performed using STATA software (version 15.0), and p < 0.05 indicates a statistically significant difference.
3 Result
3.1 Study identification and characteristics
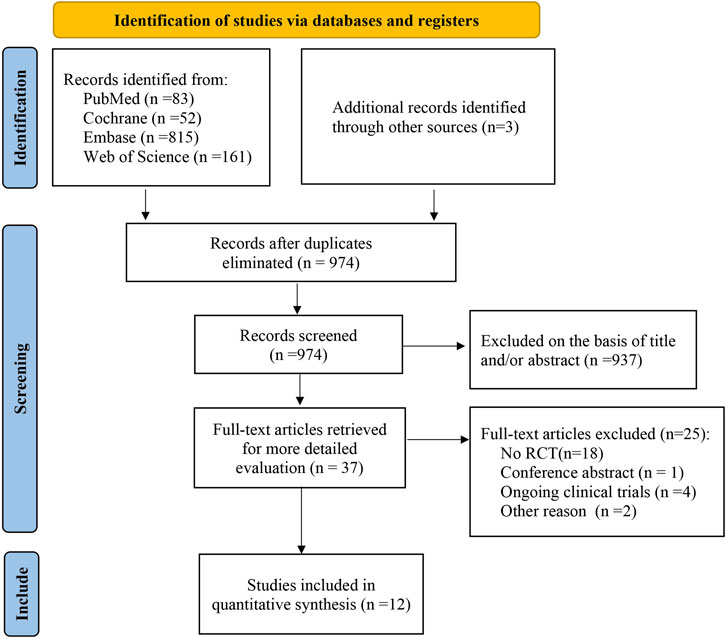
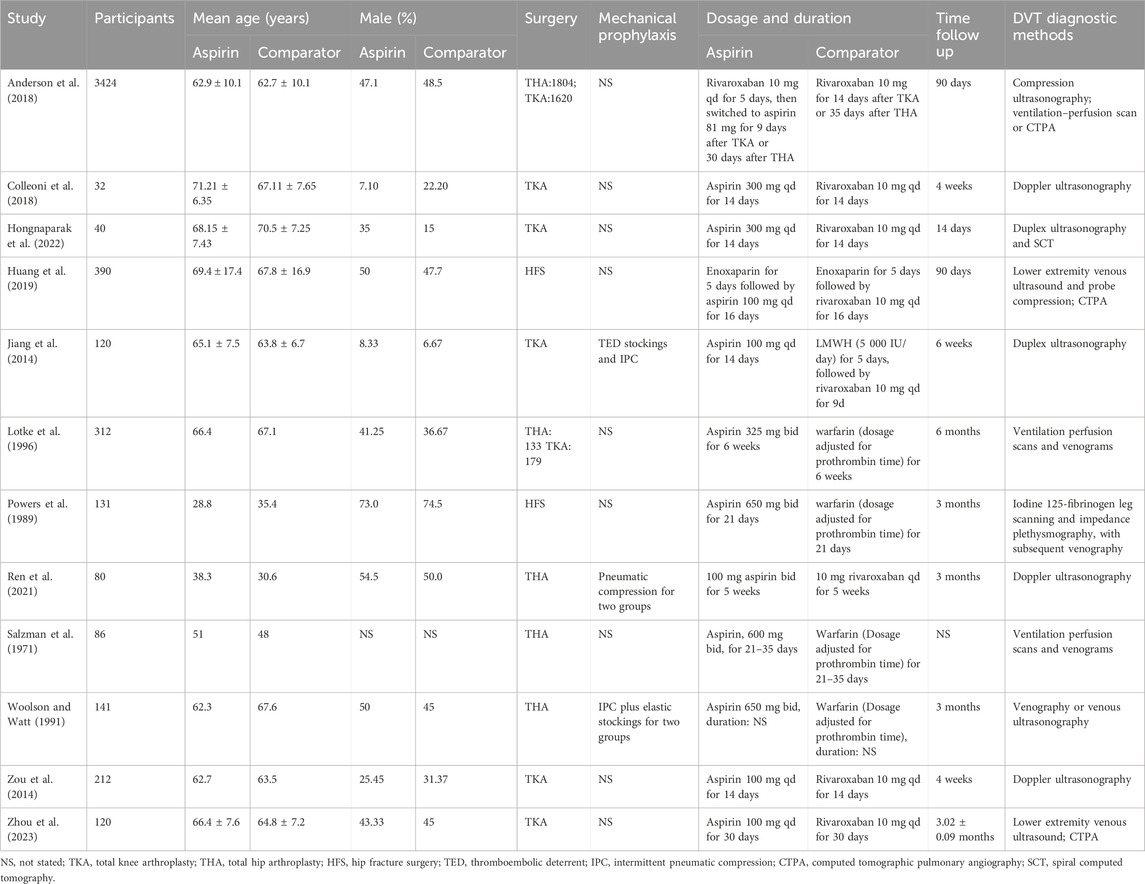
As of July 2020, a total of 974 potentially relevant citations were identified (Figure 1), 937 irrelevant citations were excluded based on title and/or abstract. In addition, one conference abstract, 18 non-RCT studies, four ongoing clinical trials, and three articles were excluded for other reasons. Finally, 12 randomized controlled trials were included. A flowchart for retrieval and inclusion was created based on the PRISMA guidelines (Figure 1). The PRISMA checklist was provided in Supplementary Table S1. The characteristics of the included studies are listed in Table 1. The 12 randomized controlled trials included 5,088 patients, of which 6 studies compared aspirin with rivaroxaban, 4 studies compared aspirin with warfarin, and 2 studies compared aspirin and low-molecular heparin bridged to rivaroxaban. All 12 studies reported VTE outcomes, 11 studies reported fatal events, 10 studies reported major bleeding and minor bleeding, and 8 studies reported wound-related issues.
3.2 Risk of bias
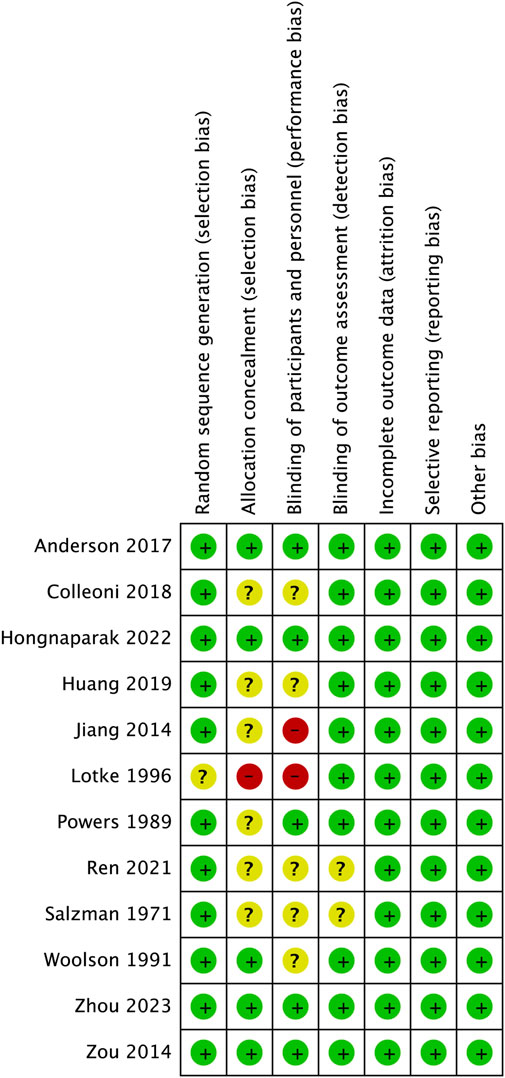
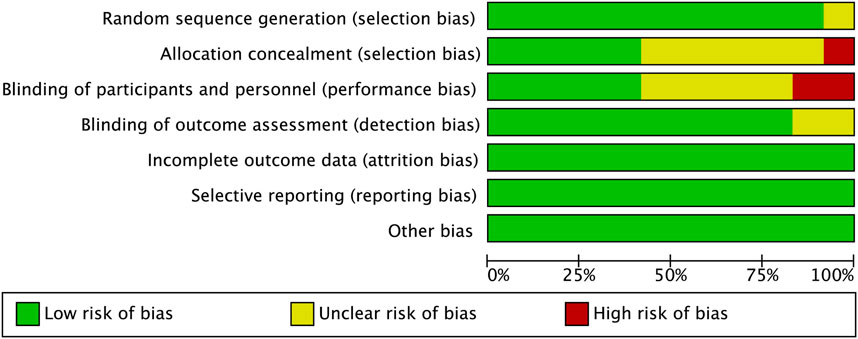
The risk of bias for each study assessed by RevMan 5.3 software is shown in Figures 2, 3. The main bias was allocation concealment and blinding of participants and personnel and personnel and personnel. Allocation blinding was not performed because the control group required subcutaneous injections of LMWH in the study by Jiang et al. (2014). The study of Lotke et al. (1996) was not blinded for the rest of the study except for the diagnostic phase, for which no reason was given. Egger’s test showed no significant publication bias in the results of most studies, except all-cause mortality (p = 0.035). The results of the egger test were listed in Supplementary Table S2.
3.3 Sensitivity analyses
The overall results failed to identify any individual trials that had a significant impact on the results (Supplementary Tables S3–S8).
3.4 Primary outcome
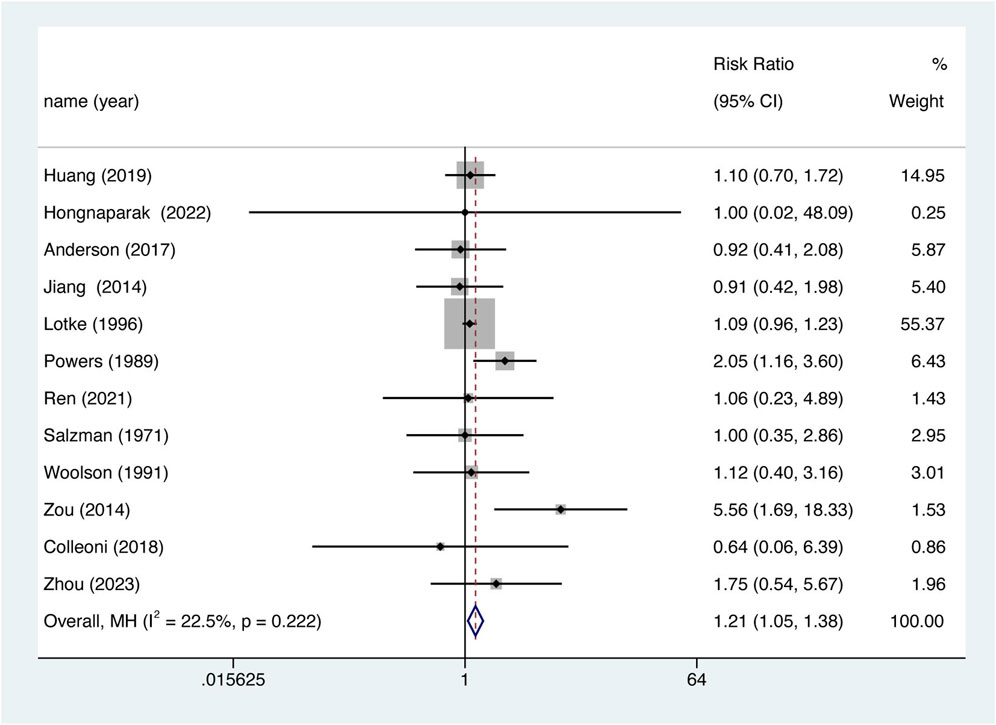
The occurrence of VTE was recorded as an outcome in every study. 12 studies were included in the analysis, Overall, aspirin was associated with lower VTE prevention efficiency. (Figure 4, RR = 1.206, 95% CI 1.053–1.383, p = 0.007), and there was no significant heterogeneity between studies (I2 = 22.5%, df = 11, p = 0.222). The results of the meta-analysis including 95% CI as well as p values for all clinical outcomes are summarized in Supplementary Table S9.
3.5 All-cause mortality
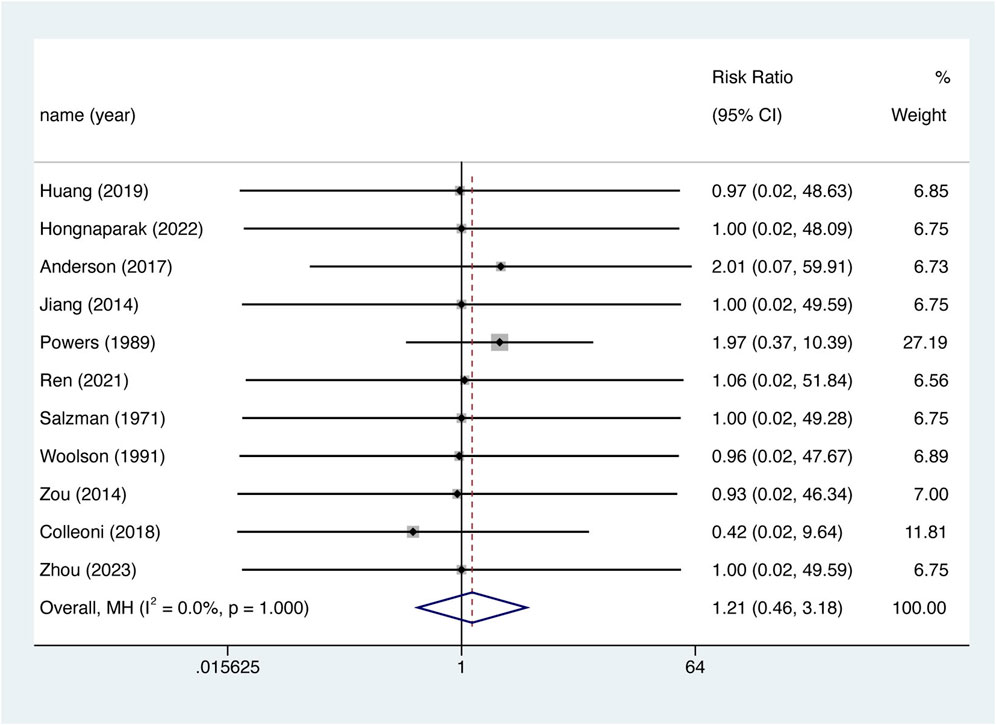
11 studies reported all-cause mortality rates. However, the incidence of fatal events was low, with only three studies reporting such events (Powers et al., 1989; Anderson et al., 2018; Colleoni et al., 2018). Corrections were made using half-integer continuity, and 11 studies were finally included. There was no significant difference in the risk of death between the two groups (Figure 5. RR = 1.208, 95% CI 0.459–3.177, p = 0.702), nor was there significant between-study heterogeneity (I2 = 0%, df = 10, p = 1.000). The Egger test showed publication bias in the results for all-cause mortality (p = 0.035). After the imputation of potentially missing studies using trim-and-fill, the pooled all-cause mortality RR results were similar (RR = 1.217, 95% CI 0.464–3.192, p = 0.689).
3.6 Hemorrhage events
Nine studies reported major bleeding events and minor bleeding events. There was no statistically significant data shown for major bleeding (Figure 6A. RR = 0.952, 95% CI 0.499–1.815, p = 0.882), Instead, aspirin was associated with lower risk of minor bleeding (Figure 6B. RR = 0.685, 95% CI 0.552–0.850, p = 0.001) and total bleeding (Figure 6C. RR = 0.726, 95% CI 0.590–0.892, p = 0.002), and there was no significant heterogeneity between studies (All the I2 were 0.0%).
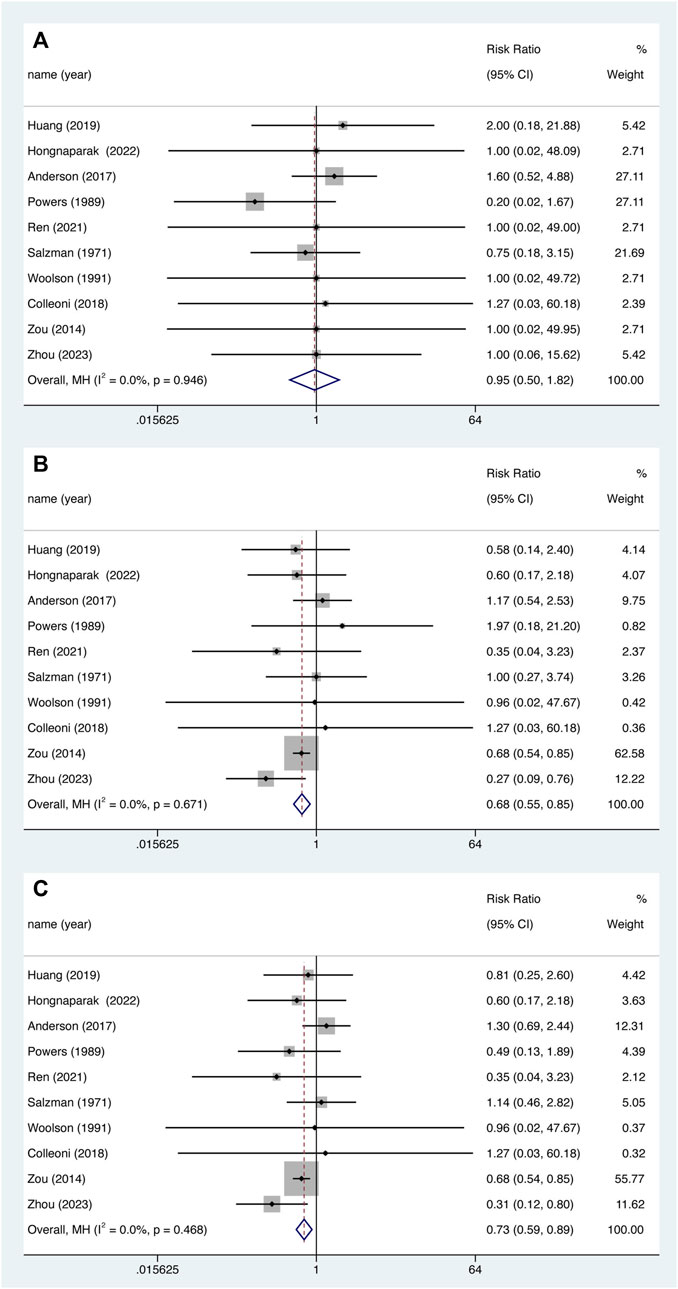
FIGURE 6. Major bleeding (A), minor bleeding (B) and total bleeding events (C) between aspirin and oral anticoagulant.
3.7 Wound-related issues
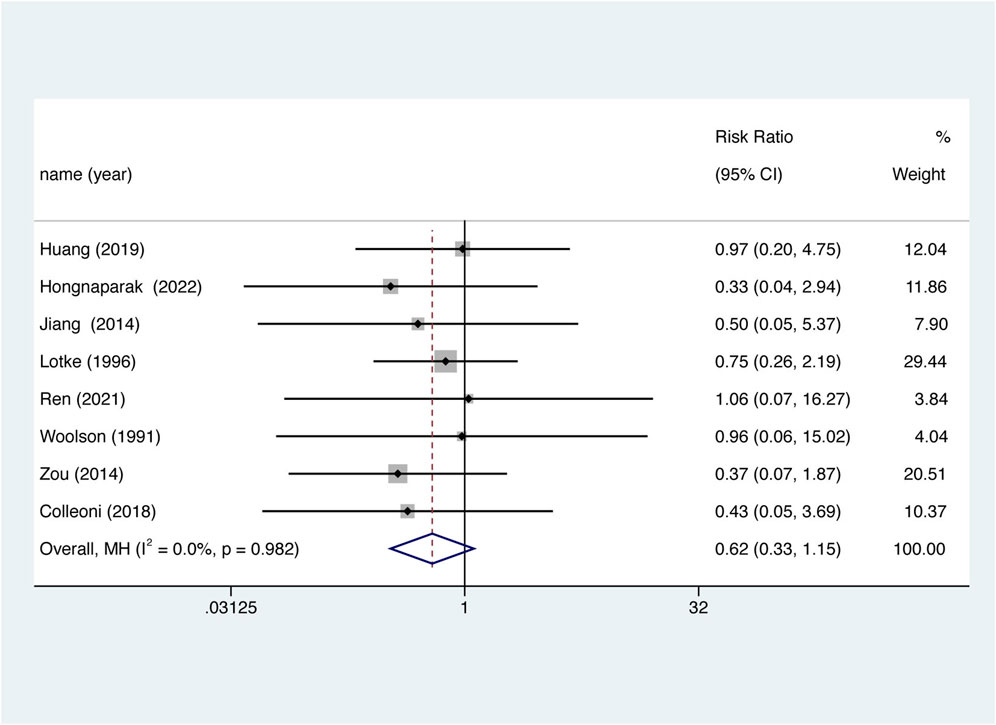
Eight studies reported wound-related issues and were included. There was no significant difference in the risk of wound-related issues between the two groups (Figure 7. RR = 0.618, 95% CI 0.333–1.145, p = 0.126), nor was there significant between-study heterogeneity (I2 = 0%, df = 7, p = 0.982).
3.8 Subgroup
To analyze our primary results more comprehensively, we assessed several potentially confounding variables for subgroup analyses, including the type of comparators (rivaroxaban or warfarin), duration of follow-up (within 3 months or more than 3 months), mechanical prophylaxis of thrombosis (yes or no), country in which the trial was conducted (Asia or non-Asia), type of joint type (TKA, THA or HFS) and aspirin dose (81–162 mg qd, 200–300 mg qd or 650 mg qd). In all pre-2000 studies, the oral anticoagulant (OAC) in the control group was warfarin. The results of the subgroup analysis are shown in Figure 8. There was no significant difference in RR of aspirin compared to OAC in Asia and non-Asia. While in other subgroups, the number of VTE events was higher in the aspirin group than in the control group. These included: follow-up ≤3 months (RR = 1.337, p = 0.027), no combined use of mechanical prophylaxis (RR = 1.230, p = 0.003), type of procedure as TKA (RR = 1.169, p = 0.033), control as warfarin (RR = 1.176, p = 0.021), and a daily dose of aspirin ≥650 mg (RR = 1.183, p = 0.016).
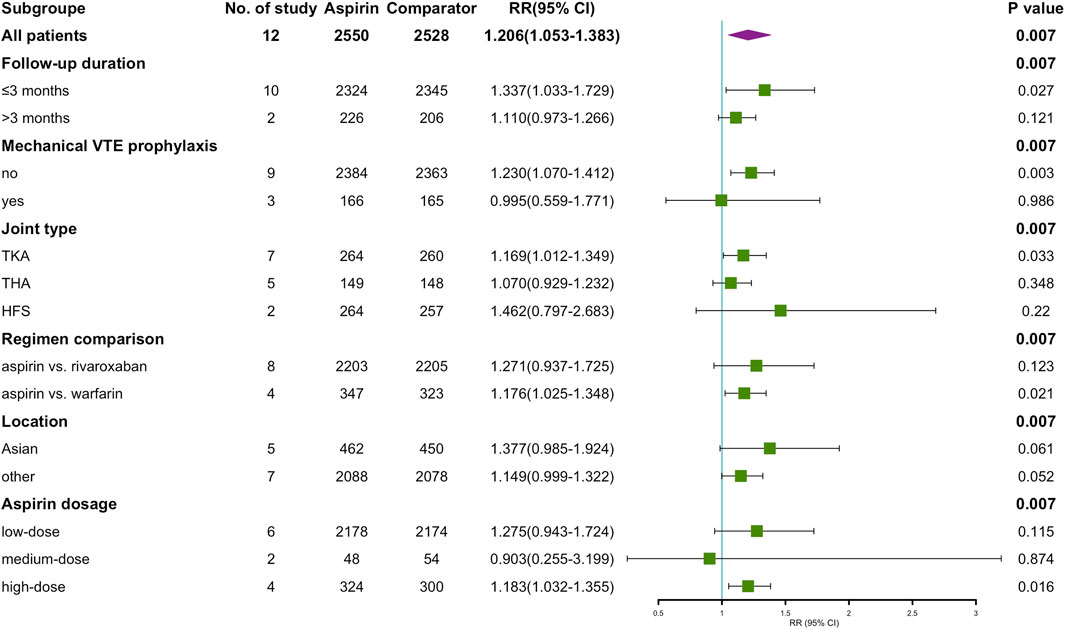
FIGURE 8. Summary of aspirin vs. OAC on VTE occurrence after subgrouping according to study-level characteristics.
4 Discussion
VTE is the most common cause of perioperative hospital deaths, and its complications consume a significant amount of healthcare resources (Reddy et al., 2022). Anticoagulants have been shown to reduce postoperative mortality and VTE-related complications (Hu et al., 2021). Aspirin has the advantage of being widely available, inexpensive, does not require monitoring, and is traditionally considered to have a lower bleeding risk than anticoagulants in the perioperative period (Diep and Garcia, 2020). In the PEP study (O’Brien et al., 2000) 17,000 patients undergoing hip fracture surgery or elective arthroplasty were included were randomly assigned to 160 mg aspirin or placebo per day for 35 days starting preoperatively. Based on this evidence, several clinical guidelines (Falck-Ytter et al., 2012; Anderson et al., 2019; Gee, 2019) consider aspirin as one of the drug choices for thromboprophylaxis. The popularity of aspirin is increasing owing to the guideline recommendations. According to US statistics, oral anticoagulants and aspirin are the main part of anticoagulant prescriptions after hip arthroplasty, and aspirin prescriptions are on the rise year by year (Agarwal et al., 2023).
We included 12 studies evaluating the efficacy and safety of aspirin versus oral anticoagulants for the prevention of VTE after total joint arthroplasty or hip fracture surgery. The analysis of the primary outcome showed that a higher number of VTE occurred with aspirin compared with oral anticoagulants (RR = 1.206, 95% CI 1.053–1.383), this indicated that the clinical efficacy of OAC was superior to that of aspirin. This result is similar to that of a large observational cohort (Richardson et al., 2019). However, previous meta-analyses such as the study conducted by Matharu et al. (2020) and the study conducted by Singjie et al. (2022), comparing the efficacy and safety of aspirin and other anticoagulants, including oral anticoagulants and LMWH, in the prevention of VTE after hip and knee replacement or orthopedic trauma, have shown that there is no statistically significant difference in the prevention of VTE between aspirin and anticoagulants. These results differed from our findings. While it may be related to the different types of anticoagulants in the control group, to explain our findings further, we performed additional subgroup analyses based on the characteristics of the included studies.
First, we defined possible factors that would affect RR, including the medication regimen for thromboprophylaxis, such as aspirin dose, and type of comparator. Follow-up time, trial location, and type of surgery were also considered. To avoid duplicating analyses, the year of publication (pre-2000 vs. post-2000) was not considered. Moreover, current guidelines (Chinese Orthopaedic Association, 2010; Falck-Ytter et al., 2012; Anderson et al., 2019; Gee, 2019) do not standardize the recommended duration of anticoagulation. In addition, the duration of prophylaxis can be influenced by factors such as the type of procedure and individual VTE risk, making it difficult to identify a standard for grouping anticoagulation durations for our analyses (Jones and Al-Horani, 2023).
Given that our control group contained two anticoagulants, warfarin and rivaroxaban, analyzed separately according to the type of anticoagulant, it was found that aspirin and rivaroxaban showed similar effects in preventing venous thrombosis. This is consistent with the results of a large cohort observational study (Bala et al., 2017) and previous meta-analyses (Kapoor et al., 2017; Matharu et al., 2020; Hu et al., 2021). However, in comparison to warfarin, aspirin demonstrated a lower efficacy in preventing VTE. Interestingly, from a pharmacological perspective, aspirin’s antiplatelet effect is immediate, while warfarin’s anticoagulant effect is delayed by 2–3 days, and the response dose-response can be unstable due to patient-related or genetic factors because thrombosis is more likely to occur during the surgical period, the value of delayed anticoagulation may be lower (Della Valle et al., 2006). In a systematic review, warfarin was found to have a higher risk of symptomatic DVT compared to low-dose aspirin (Azboy et al., 2020). We believe that the RCT studies in the warfarin subgroup were all published before 2000, and that both of these indistinguishable factors, i.e., the year of publication and the type of anticoagulant, may have had an impact on the RR for the primary outcome; therefore, we are unable to confirm whether aspirin is not as effective as warfarin in preventing VTE in orthopedic surgery. Of note, the PEPPER trial was a large pragmatic clinical trial (NCT02810704) designed to assess the combined effect of three antithrombotic drugs (aspirin, warfarin, and rivaroxaban) on overall mortality and symptomatic VTE in patients receiving TKA and THA (Pellegrini, Jr et al., 2022). The results from this trial may give us new perspectives.
We also analyzed the results for other subgroups. The subgroups of the trial location did not have statistically significant results for RR (RR = 1.149, p = 0.052). However, aspirin was less effective as an anticoagulant than the control group in the remaining subgroups: follow-up within 3 months, use of high-dose aspirin (325 mg bid), no combination of mechanical thromboprophylaxis, and the type of procedure was TKA. Generally, VTE after major orthopedic surgery occurs more frequently within the first 3 months, the majority of symptomatic VTE (94%) occurs within 2 weeks after joint replacement surgery, with 89% occurring within the first week (Karasavvidis et al., 2022). This difference in risk helps explain the higher number of VTEs in the aspirin group at 3 months of follow-up. In terms of procedure type, the risk of venous thrombosis varies among the three major orthopedic procedures, with TKA having a higher propensity for thrombosis and a slightly higher incidence of VTE(Gionis et al., 2013). Considering that only two studies involving HFS were included in this meta-analysis, with a small sample size, and that in Huang’s study (Huang et al., 2019), the majority of patients were recruited postoperatively, further validation of the effectiveness of aspirin in patients with HFS is needed. Regarding aspirin dosage, recent studies have shown that low-dose aspirin (81 mg, bid) is comparable to high-dose aspirin (325 mg, bid) in preventing VTE after total joint replacement (0.6% vs. 1.3%, p = 0.62) (Shafiei et al., 2023). In the study by Merkow et al. (2021), patients with TKA had a higher incidence of VTE with high-dose aspirin (325 mg, bid) than in the low-dose aspirin group (81 mg, bid) (1.41% vs. 0.23%, p < 0.001). The results of these two studies and ours support the opinion that low-dose aspirin seems to be the more reasonable choice. Moreover, the subgroup analysis results suggest that, in comparison to utilizing aspirin alone, the combined use of mechanical thromboprophylaxis appears to confer clinical benefits, aligning with findings from a systematic review focusing on major orthopedic surgeries (Balk, M.D., M.P.H. et al., 2017). This review demonstrated that in TKA patients, the overall incidence of deep vein thrombosis in the combined mechanical device and antiplatelet group was lower than in the antiplatelet-only group. According to the American Society of Hematology (ASH) guidelines from 2019, combination prophylaxis or mechanical prophylaxis alone is recommended based on individual patient and surgical type VTE and bleeding risk (Anderson et al., 2019).
In terms of safety outcomes, there was no statistical difference between aspirin and OAC in major bleeding, mortality, or wound-related issues (p > 0.05). Also, aspirin was associated with fewer minor bleeding and fewer total bleeding events. In fact, aspirin has been traditionally recognized as having the lowest rate of bleeding complications in surgical patients (Pellegrini et al., 2020). Several large observational cohort studies have demonstrated that the risk of bleeding with postoperative aspirin use in patients with TKA or THA is negligible, making the prospect of aspirin use exciting (Reitman et al., 2003; Lotke and Lonner, 2006; Agaba et al., 2017).
5 Strengths and limitations
There have been many studies on the prevention of VTE with aspirin, including enoxaparin versus aspirin and aspirin versus placebo, and few studies have meta-analyzed aspirin with oral anticoagulants. This study collects research on aspirin versus oral anticoagulants and comprehensively analyzes the efficacy and safety of these two medications in the prevention of VTE after major orthopedic surgery. It provides reference recommendations for the safe use of aspirin for thrombosis prevention in the future. Second, this meta-analysis included 12 RCTs involving a large sample of 5,088 patients, six of which were published in the literature in the last 5 years, with reliable results. In addition, only RCTs were included in this study to avoid unreliable outcomes due to different trial design methods.
Of course, some limitations of this meta-analysis exist. First, although we searched for DOACs including rivaroxaban, dabigatran, and others, limited data prevented us from comparing the efficacy and safety of aspirin with DOACs other than rivaroxaban. Moreover, we performed subgroup analyses of the primary outcomes, but could not exclude all potential confounders in the baseline characteristics because data on individual patient clinical characteristics were not available. Thirdly, any of the factors influencing patient mortality in the included studies were not clearly described and therefore could not be completely avoided. Finally, due to the low incidence of outcomes, the classic half-integer correction was applied, which may have contributed to a slight overestimation of event rates.
6 Conclusion
Available evidence from the RCT suggests that aspirin is statistically inferior to oral anticoagulants regarding the clinical effectiveness of postoperative prophylactic anticoagulation after major orthopedic surgery. Further subgroup analysis revealed that aspirin was similar to rivaroxaban and inferior to warfarin. Considering that the confounding factor of year of publication could not be excluded, our results need to be further validated. In the safety events of all-cause mortality, major bleeding, and wound-related events, there were no statistically significant differences from oral anticoagulants. Aspirin remains promising for use in patients after major orthopedic surgery owing to its advantages in reducing the overall risk of bleeding.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
XZ: Conceptualization, Data curation, Writing–original draft, Writing–review and editing. LN: Data curation, Writing–original draft, Writing–review and editing. YS: Conceptualization, Software, Writing–original draft. LH: Methodology, Software, Writing–original draft. YZ: Software, Supervision, Writing–review and editing. QY: Investigation, Writing–review and editing. YB: Supervision, Visualization, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Key Research and Development Program of China (grant number 2020YFC2005500); the Natural Science Foundation of Sichuan Province (grant number 2022NSFSC0818); and the Special research project on monitoring and evaluation of the use of key clinical drugs by the Specialized Committee on Drug Evaluation of the Chinese Society of Research Hospitals (Y2022FH-YWPJ01-202).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1326224/full#supplementary-material
References
Agaba, P., Kildow, B. J., Dhotar, H., Seyler, T. M., and Bolognesi, M. (2017). Comparison of postoperative complications after total hip arthroplasty among patients receiving aspirin, enoxaparin, warfarin, and factor Xa inhibitors. J. Orthop. 14, 537–543. doi:10.1016/j.jor.2017.08.002
Agarwal, A. R., Das, A., Harris, A., Campbell, J. C., Golladay, G. J., and Thakkar, S. C. (2023). Trends of venous thromboembolism after total hip arthroplasty in the United States: analysis from 2011 to 2019. J. Am. Acad. Orthop. Surg. 31, e376–e384. doi:10.5435/JAAOS-D-22-00708
Anderson, D. R., Dunbar, M., Murnaghan, J., Kahn, S. R., Gross, P., Forsythe, M., et al. (2018). Aspirin or rivaroxaban for VTE prophylaxis after hip or knee arthroplasty. N. Engl. J. Med. 378, 699–707. doi:10.1056/NEJMoa1712746
Anderson, D. R., Morgano, G. P., Bennett, C., Dentali, F., Francis, C. W., Garcia, D. A., et al. (2019). American Society of Hematology 2019 guidelines for management of venous thromboembolism: prevention of venous thromboembolism in surgical hospitalized patients. Blood Adv. 3, 3898–3944. doi:10.1182/bloodadvances.2019000975
Azboy, I., Groff, H., Goswami, K., Vahedian, M., and Parvizi, J. (2020). Low-dose aspirin is adequate for venous thromboembolism prevention following total joint arthroplasty: a systematic review. J. Arthroplasty 35, 886–892. doi:10.1016/j.arth.2019.09.043
Bala, A., Huddleston, J. I., Goodman, S. B., Maloney, W. J., and Amanatullah, D. F. (2017). Venous thromboembolism prophylaxis after TKA: aspirin, warfarin, enoxaparin, or factor xa inhibitors? Clin. Orthop. Relat. Res. 475, 2205–2213. doi:10.1007/s11999-017-5394-6
Balk, M. D., Ellis, M. S. C., Di, M. D., Adam, M. L. I. S., and Trikalinos, M. D. (2017). Venous thromboembolism prophylaxis in major orthopedic surgery: systematic review update. Rockville: Agency for Healthcare Research and Quality AHRQ. doi:10.23970/AHRQEPCCER191
Chinese Orthopaedic Association (2010). Prevention of venous thromboembolism after major orthopaedic surgery: prevention of venous thromboembolism. Orthop. Surg. 2, 81–85. doi:10.1111/j.1757-7861.2010.00068.x
Cochrane Collaboration (2011). Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of. Cochrane handbook for systematic reviews of interventions version 5.
Colleoni, J. L., Ribeiro, F. N., Mos, P. A. C., Reis, J. P., Oliveira, H. R. D., and Miura, B. K. (2018). Venous thromboembolism prophylaxis after total knee arthroplasty (TKA): aspirin vs. rivaroxaban. Rev. Bras. Ortop. (English Ed. 53, 22–27. doi:10.1016/j.rboe.2017.11.007
Della Valle, A. G., Serota, A., Go, G., Sorriaux, G., Sculco, T. P., Sharrock, N. E., et al. (2006). Venous thromboembolism is rare with a multimodal prophylaxis protocol after total hip arthroplasty. Clin. Orthop. Relat. Res. 444, 146–153. doi:10.1097/01.blo.0000201157.29325.f0
Diep, R., and Garcia, D. (2020). Does aspirin prevent venous thromboembolism? Hematology 2020, 634–641. doi:10.1182/hematology.2020000150
Duval, S., and Tweedie, R. (2000). Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56, 455–463. doi:10.1111/j.0006-341X.2000.00455.x
Falck-Ytter, Y., Francis, C. W., Johanson, N. A., Curley, C., Dahl, O. E., Schulman, S., et al. (2012). Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 141, e278S–e325S. doi:10.1378/chest.11-2404
Feng, W., Wu, K., Liu, Z., Kong, G., Deng, Z., Chen, S., et al. (2015). Oral direct factor Xa inhibitor versus enoxaparin for thromboprophylaxis after hip or knee arthroplasty: systemic review, traditional meta-analysis, dose–response meta-analysis and network meta-analysis. Thrombosis Res. 136, 1133–1144. doi:10.1016/j.thromres.2015.10.009
Fisher, W. D. (2011). Impact of venous thromboembolism on clinical management and therapy after hip and knee arthroplasty. Can. J. Surg. 54, 344–351. doi:10.1503/cjs.007310
Friedrich, J. O., Adhikari, N. K., and Beyene, J. (2007). Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med. Res. Methodol. 7, 5. doi:10.1186/1471-2288-7-5
Gee, E. (2019). The National VTE Exemplar Centres Network response to implementation of updated NICE guidance: venous thromboembolism in over 16s: reducing the risk of hospital-acquired deep vein thrombosis or pulmonary embolism (NG89). Br. J. Haematol. 186, 792–793. doi:10.1111/bjh.16010
Geerts, W. H., Bergqvist, D., Pineo, G. F., Heit, J. A., Samama, C. M., Lassen, M. R., et al. (2008). Prevention of venous thromboembolism: American college of chest physicians evidence-based clinical practice guidelines (8th edition). Chest 133, 381S–453S. doi:10.1378/chest.08-0656
Geerts, W. H., Pineo, G. F., Heit, J. A., Bergqvist, D., Lassen, M. R., Colwell, C. W., et al. (2004). Prevention of venous thromboembolism: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 126, 338S–400S. doi:10.1378/chest.126.3_suppl.338S
Gionis, M. N., Ioannou, C. V., Katsamouris, A. N., Katonis, P., Balalis, K., Sfyridaki, K., et al. (2013). The study of the thrombin generation mechanism and the effect of low molecular weight heparin as thromboprophylaxis in patients undergoing total knee and hip replacement. Thrombosis Res. 132, 685–691. doi:10.1016/j.thromres.2013.09.037
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327, 557–560. doi:10.1136/bmj.327.7414.557
Hongnaparak, T., Janejaturanon, J., Iamthanaporn, K., Tanutit, P., and Yuenyongviwat, V. (2022). Aspirin versus rivaroxaban to prevent venous thromboembolism after total knee arthroplasty: a double-blinded, randomized controlled trial. Rev. Bras. Ortop. 57, 741–746. doi:10.1055/s-0041-1735941
Howie, C., Hughes, H., and Watts, A. (2005). Venous thromboembolism associated with hip and knee replacement over a ten-year period: a population-based study. J. Bone & Jt. Surg. Br. 87, 1675–1680. doi:10.1302/0301-620X.87B12.16298
Hu, B., Jiang, L., Tang, H., Hu, M., Yu, J., and Dai, Z. (2021). Rivaroxaban versus aspirin in prevention of venous thromboembolism following total joint arthroplasty or hip fracture surgery: a meta-analysis. J. Orthop. Surg. Res. 16, 135. doi:10.1186/s13018-021-02274-z
Huang, Q., Xing, S., Zeng, Y., Si, H., Zhou, Z., and Shen, B. (2019). Comparison of the efficacy and safety of aspirin and rivaroxaban following enoxaparin treatment for prevention of venous thromboembolism after hip fracture surgery. Orthop. Surg. 11, 886–894. doi:10.1111/os.12542
Jenny, J.-Y., Pabinger, I., Samama, C. M., and Esa Vte Guidelines Task Force, (2018). European guidelines on perioperative venous thromboembolism prophylaxis: aspirin. Eur. J. Anaesthesiology| EJA 35, 123–129. doi:10.1097/EJA.0000000000000728
Jiang, Y., Du, H., Liu, J., and Zhou, Y. (2014). Aspirin combined with mechanical measures to prevent venous thromboembolism after total knee arthroplasty: a randomized controlled trial. Chin. Med. J. 127, 2201–2205. doi:10.3760/cma.j.issn.0366-6999.20132175
Jones, A., and Al-Horani, R. A. (2023). Venous thromboembolism prophylaxis in major orthopedic surgeries and factor XIa inhibitors. Med. Sci. 11, 49. doi:10.3390/medsci11030049
Kapoor, A., Ellis, A., Shaffer, N., Gurwitz, J., Chandramohan, A., Saulino, J., et al. (2017). Comparative effectiveness of venous thromboembolism prophylaxis options for the patient undergoing total hip and knee replacement: a network meta-analysis. J. Thrombosis Haemostasis 15, 284–294. doi:10.1111/jth.13566
Karasavvidis, T., Bouris, V., Xiang, W., Tzavellas, G., Charisis, N., Palaiodimos, L., et al. (2022). Prophylaxis for venous thromboembolic events in elective total hip and TotalKnee arthroplasty. CPD 28, 771–777. doi:10.2174/1381612828666220418090928
Kinov, P., Tanchev, P. P., Ellis, M., and Volpin, G. (2014). Antithrombotic prophylaxis in major orthopaedic surgery: an historical overview and update of current recommendations. Int. Orthop. 38, 169–175. doi:10.1007/s00264-013-2134-8
Learmonth, I. D., Young, C., and Rorabeck, C. (2007). The operation of the century: total hip replacement. Lancet 370, 1508–1519. doi:10.1016/S0140-6736(07)60457-7
Lotke, P. A., and Lonner, J. H. (2006). The benefit of aspirin chemoprophylaxis for thromboembolism after total knee arthroplasty. Clin. Orthop. Relat. Res. 452, 175–180. doi:10.1097/01.blo.0000238822.78895.95
Lotke, P. A., Palevsky, H., Keenan, A. M., Meranze, S., Steinberg, M. E., Ecker, M. L., et al. (1996). Aspirin and warfarin for thromboembolic disease after total joint arthroplasty. Clin. Orthop. Relat. Res. 324, 251–258. doi:10.1097/00003086-199603000-00031
Matharu, G. S., Kunutsor, S. K., Judge, A., Blom, A. W., and Whitehouse, M. R. (2020). Clinical effectiveness and safety of aspirin for venous thromboembolism prophylaxis after total hip and knee replacement: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. 180, 376–384. doi:10.1001/jamainternmed.2019.6108
Memtsoudis, S. G., Pumberger, M., Ma, Y., Chiu, Y., Fritsch, G., Gerner, P., et al. (2012). Epidemiology and risk factors for perioperative mortality after total hip and knee arthroplasty. J. Orthop. Res. 30, 1811–1821. doi:10.1002/jor.22139
Merkow, D. B., Tang, A., Iorio, R., Slover, J. D., Bosco, J. A., and Schwarzkopf, R. (2021). Low dose aspirin is effective in preventing venous thromboembolism in patients undergoing primary total knee arthroplasty. J. Orthop. 24, 26–28. doi:10.1016/j.jor.2021.02.005
Messerschmidt, C., and Friedman, R. J. (2015). Clinical experience with novel oral anticoagulants for thromboprophylaxis after elective hip and knee arthroplasty. Arteriosclerosis, thrombosis, Vasc. Biol. 35, 771–778. doi:10.1161/ATVBAHA.114.303400
O’Brien, J., Duncan, H., Kirsh, G., Allen, V., King, P., Hargraves, R., et al. (2000). Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: pulmonary Embolism Prevention (PEP) trial. Lancet 355, 1295–1302. doi:10.1016/S0140-6736(00)02110-3
Pellegrini, V. D., Eikelboom, J., McCollister Evarts, C., Franklin, P. D., Goldhaber, S. Z., Iorio, R., et al. (2020). Selection bias, orthopaedic style: knowing what we don’t know about aspirin. J. Bone Jt. Surg. 102, 631–633. doi:10.2106/JBJS.19.01135
Pellegrini, V. D., Eikelboom, J. W., Evarts, C. M., Franklin, P. D., Garvin, K. L., Goldhaber, S. Z., et al. (2022). Randomised comparative effectiveness trial of Pulmonary Embolism Prevention after hiP and knee Replacement (PEPPER): the PEPPER trial protocol. BMJ Open 12, e060000. doi:10.1136/bmjopen-2021-060000
Piovella, F., Wang, C., Lu, H., Lee, K., Lee, L., Lee, W., et al. (2005). Deep-vein thrombosis rates after major orthopedic surgery in Asia. An epidemiological study based on postoperative screening with centrally adjudicated bilateral venography. J. thrombosis haemostasis 3, 2664–2670. doi:10.1111/j.1538-7836.2005.01621.x
Powers, P. J., Gent, M., Jay, R. M., Julian, D. H., Turpie, A., Levine, M., et al. (1989). A randomized trial of less intense postoperative warfarin or aspirin therapy in the prevention of venous thromboembolism after surgery for fractured hip. Archives Intern. Med. 149, 771–774. doi:10.1001/archinte.1989.00390040013003
Reddy, G. B., Ovadia, J. E., Yakkanti, R. R., Browne, J. A., and D’Apuzzo, M. R. (2022). Increased morbidity with diagnosis and treatment of pulmonary embolism after total joint arthroplasty: a matched control analysis of 30,000 patients. J. Arthroplasty 37, 948–952. doi:10.1016/j.arth.2022.01.086
Reitman, R. D., Emerson, R. H., Higgins, L. L., and Tarbox, T. R. (2003). A multimodality regimen for deep venous thrombosis prophylaxis in total knee arthroplasty. J. Arthroplasty 18, 161–168. doi:10.1054/arth.2003.50026
Ren, Y., Cao, S.-L., Li, Z., Luo, T., Feng, B., and Weng, X.-S. (2021). Comparable efficacy of 100 mg aspirin twice daily and rivaroxaban for venous thromboembolism prophylaxis following primary total hip arthroplasty: a randomized controlled trial. Chin. Med. J. 134, 164–172. doi:10.1097/CM9.0000000000001305
Richardson, S. S., Schairer, W. W., Sculco, P. K., and Bostrom, M. P. (2019). Comparison of pharmacologic prophylaxis in prevention of venous thromboembolism following total knee arthroplasty. Knee 26, 451–458. doi:10.1016/j.knee.2019.01.004
Salzman, E. W., Harris, W. H., and DeSanctis, R. W. (1971). Reduction in venous thromboembolism by agents affecting platelet function. N. Engl. J. Med. 284, 1287–1292. doi:10.1056/NEJM197106102842303
Shafiei, S. H., Rastegar, M., Mirghaderi, P., Siavashi, B., and Mortazavi, S. M. J. (2023). Comparison of low-dose (162 mg) and high-dose (650 mg) aspirin prophylaxis following total joint arthroplasty: a prospective cohort study. Ann. Med. Surg. Publ. Ahead Print 85, 1461–1467. doi:10.1097/MS9.0000000000000366
Singjie, L. C., Halomoan, R., Saleh, I., Sumargono, E., and Kholinne, E. (2022). Clinical effectiveness and safety of aspirin and other anticoagulants for venous thromboembolism prophylaxis after major orthopedic surgery: a systematic review and meta-analysis of randomized clinical trials. EFORT Open Rev. 7, 792–799. doi:10.1530/EOR-22-0053
Thiengwittayaporn, S., Budhiparama, N., Tanavalee, C., Tantavisut, S., Sorial, R. M., Li, C., et al. (2021). Asia-Pacific venous thromboembolism consensus in knee and hip arthroplasty and hip fracture surgery: Part 3. Pharmacological venous thromboembolism prophylaxis. Knee Surg. Relat. Res. 33, 24–12. doi:10.1186/s43019-021-00100-8
Woolson, S. T., and Watt, J. (1991). Intermittent pneumatic compression to prevent proximal deep venous thrombosis during and after total hip replacement. A prospective, randomized study of compression alone, compression and aspirin, and compression and low-dose warfarin. JBJS 73, 507–512. doi:10.2106/00004623-199173040-00005
Zhou, L.-B., Wang, C.-C., Zhang, L.-T., Wu, T., and Zhang, G.-Q. (2023). Effectiveness of different antithrombotic agents in combination with tranexamic acid for venous thromboembolism prophylaxis and blood management after total knee replacement: a prospective randomized study. BMC Musculoskelet. Disord. 24, 5. doi:10.1186/s12891-022-06117-8
Keywords: major orthopaedic surgery, aspirin, oral anticoagulation, venous thromboembolism prophylaxis, systematic review
Citation: Zheng X, Nong L, Song Y, Han L, Zhang Y, Yin Q and Bian Y (2024) Comparison of efficacy and safety between aspirin and oral anticoagulants for venous thromboembolism prophylaxis after major orthopaedic surgery: a meta-analysis of randomized clinical trials. Front. Pharmacol. 14:1326224. doi: 10.3389/fphar.2023.1326224
Received: 26 October 2023; Accepted: 22 December 2023;
Published: 08 January 2024.
Edited by:
Luigi Vetrugno, University of Studies G. d’Annunzio Chieti and Pescara, ItalyReviewed by:
Adina Turcu-Stiolica, University of Medicine and Pharmacy of Craiova, RomaniaCarmen Fierro, University of Studies G. d’Annunzio Chieti and Pescara, Italy
Copyright © 2024 Zheng, Nong, Song, Han, Zhang, Yin and Bian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan Bian, Ymlhbnl1YW41NjdAMTI2LmNvbQ==; Qinan Yin, eXFuXzA3MTFAMTYzLmNvbQ==
†These authors share first authorship
 Xingyue Zheng
Xingyue Zheng Li Nong
Li Nong Yujie Song1,3†
Yujie Song1,3† Lizhu Han
Lizhu Han Yuan Zhang
Yuan Zhang Qinan Yin
Qinan Yin Yuan Bian
Yuan Bian