- 1Evidence-Based Medicine Center, School of Basic Medical Sciences, Lanzhou University, Lanzhou, Gansu, China
- 2Department of General Surgery, Taihe Hospital, Hubei University of Medicine, Shiyan, Hubei, China
- 3Center for Evidence-Based Medicine and Clinical Research, Taihe Hospital, Hubei University of Medicine, Shiyan, Hubei, China
- 4Department of Neurosurgery, Taihe Hospital, Hubei University of Medicine, Shiyan, Hubei, China
Objective: A lack of clarity persists regarding the efficacy and risks associated with direct oral anticoagulants (DOACs) in end-stage renal disease (ESRD) patients with atrial fibrillation (AF) undergoing dialysis, primarily due to limited retrospective studies. Therefore, the objective of this study was to evaluate the existing data and propose a practical protocol for the clinical utilization of DOACs in ESRD patients with AF undergoing dialysis.
Methods: PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials were searched for clinical studies evaluating DOACs in ESRD patients with AF on dialysis published up to 2 February 2023. DOACs included warfarin, dabigatran, apixaban, edoxaban, and rivaroxaban. The outcomes were mortality, ischemic stroke, hemorrhagic stroke, any stroke, gastrointestinal bleeding, major bleeding, intracranial bleeding, and minor bleeding.
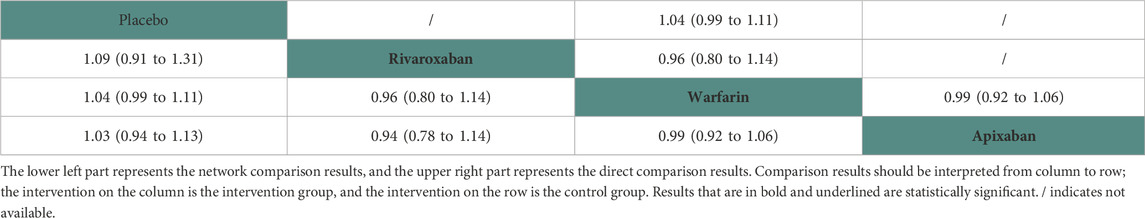
Results: Compared with placebo, apixaban (HR = 0.97, 95% CI: 0.88–1.07), rivaroxaban (HR = 0.91, 95% CI: 0.76–1.10), and warfarin (HR = 0.96, 95% CI: 0.90–1.01) did not reduce mortality. Regarding direct comparisons of mortality, the comparisons of warfarin vs. apixaban (HR = 0.99, 95% CI: 0.92–1.06), placebo vs. warfarin (HR = 1.04, 95% CI: 0.99–1.11), and rivaroxaban vs. warfarin (HR = 0.96, 95% CI: 0.80–1.14) did not significantly reduce mortality. Based on the surface under the cumulative ranking curve, rivaroxaban (75.53%), warfarin (62.14%), and apixaban (45.6%) were the most effective interventions for managing mortality, and placebo (16.74%) was the worst.
Conclusion: In conclusion, rivaroxaban demonstrated efficacy in reducing mortality and the incidence of ischemic stroke, gastrointestinal bleeding, and intracranial hemorrhage. Dabigatran is recommended for the prevention of hemorrhagic stroke. However, caution should be exercised due to the risk of major bleeding. Warfarin can effectively reduce minor bleeding but does not offer significant protection against gastrointestinal or intracranial bleeding. Apixaban was not recommended for mortality reduction or for preventing ischemic or hemorrhagic strokes. Further research will be necessary to establish specific clinical protocols.
1 Introduction
Atrial fibrillation (AF) occurred in more than 10% of end-stage renal disease (ESRD) patients undergoing dialysis (Hijazi et al., 2016). In patients with ESRD receiving dialysis, the coexistence of AF substantially augmented the susceptibility to thrombosis owing to perturbations in atrial contractility; diminished atrial blood perfusion, progression of atrial fibrosis, and impairment and dysfunction of the endothelium; and upregulated the expression of tissue factor, leading to enhanced platelet aggregation and augmented fibrinolysis (Proietti et al., 2018). Anticoagulation was associated with a lower incidence of ischemic stroke, preventing thrombosis and reducing the likelihood of death in patients (Ding et al., 2021). Therefore, the imperative necessity for implementing anticoagulation therapy in patients with AF on dialysis was emphasized (Bonde et al., 2014).
The anticoagulant warfarin impeded the synthesis of coagulation factors and mitigated the risk of thrombosis by inhibiting vitamin K epoxide reductase (Mantha and Ansell, 2012). Multiple studies demonstrated the efficacy of warfarin in preventing ischemic stroke in ESRD patients with AF undergoing dialysis, leading to favorable prognoses (Benz and Eikelboom, 2022; See et al., 2021; Yoon et al., 2017; Ntaios et al., 2017). However, it is crucial to acknowledge that warfarin carries an inherent risk of increasing bleeding tendencies in patients (Baker et al., 2023). Therefore, for individuals with AF on dialysis who were more susceptible to bleeding events, there is a pressing need for the development of safer and more effective anticoagulant medications (Potpara et al., 2012). In recent years, direct oral anticoagulants (DOACs) have been extensively used in anticoagulant therapy. The mechanism of action of DOACs was through direct action on certain clotting factors, mainly the thrombin inhibitor dabigatran and factor Xa inhibitors, including rivaroxaban, apixaban, and edoxaban. DOACs directly acted on coagulation factors, simplified the process of anticoagulation therapy, and had less impact on the clotting pathway, so there was no need for routine monitoring of INR, and the risk of bleeding was relatively low (Franchini et al., 2016). DOACs were currently recommended for the prevention of stroke and systemic thromboembolism in patients with non-renal impaired AF, and these drugs were superior to warfarin in reducing bleeding (Edwina et al., 2023). However, all DOACs were primarily eliminated via renal excretion, with apixaban having a renal clearance of 27% and dabigatran reaching up to 80% (Stamellou and Floege, 2018). Consequently, patients with severe renal impairment (e.g., serum creatinine clearance <25–30 mL/min) or ESRD had been systematically excluded from clinical trials involving DOACs (Pokorney et al., 2020). However, in clinical practice, an increasing number of patients with ESRD and AF were opting for DOACs as an alternative to warfarin therapy after experiencing treatment failure (Lip et al., 2017).
Despite the wealth of evidence supporting anticoagulation used in patients with chronic kidney disease (CKD), there remained a lack of clarity regarding the efficacy and risks associated with DOACs in ESRD patients with AF undergoing dialysis, primarily due to limited retrospective studies lacking network meta-analysis (NMA). Therefore, the objective of this study was to evaluate the existing data and propose a practical protocol for the clinical utilization of DOACs in ESRD patients with AF undergoing dialysis.
2 Methods
2.1 Literature search
Three databases, including PubMed, EMBASE, and the Cochrane Library, were systematically and comprehensively searched to retrieve relevant literature and references published before 02 February 2023. The MeSH terms employed in this study encompassed “renal dialysis,” “hemodialysis,” “chronic kidney disease,” “end-stage renal disease,” “dialysis,” and “atrial fibrillation,” along with the more specific terms of “anticoagulants,” “oral anticoagulants,” “NOACs,” “dabigatran,” “apixaban,” “rivaroxaban,” “edoxaban,” and “warfarin.” The detailed search strategies are deposited in Supplementary Method 1.
2.2 Inclusion and exclusion criteria
The following inclusion criteria were used (Hijazi et al., 2016): participants: adults diagnosed with atrial fibrillation on dialysis (Proietti et al., 2018); interventions: anticoagulant drugs, including DOACs (apixaban, dabigatran, rivaroxaban, and edoxaban) and warfarin (Ding et al., 2021); comparison: none of the anticoagulant drugs (placebo) or other anticoagulant drugs (Bonde et al., 2014); outcomes: mortality, ischemic stroke, hemorrhagic stroke, any stroke, gastrointestinal hemorrhage, major bleeding, intracranial bleeding, and minor bleeding (Mantha and Ansell, 2012); and study design: randomized controlled trials (RCTs) and cohort studies.
The exclusion criteria were as follows (Hijazi et al., 2016): mixed-population study, patients with renal failure but not on dialysis, reported cardiovascular disease (e.g., coronary artery disease, moderate or severe aortic or mitral stenosis, and active endocarditis), traumatic brain injury, and other non-psychological conditions that may have a greater impact on the patient’s mental state (Proietti et al., 2018); no available data in the original research or the data could not be converted (Ding et al., 2021); prevention of relapse trials (Bonde et al., 2014); cross-linking experiments; and duplicate studies (Mantha and Ansell, 2012).
2.3 Data extraction
Data extraction was performed by two independent authors, with the extracted data proofread by a final investigator. If relevant data were not reported in an article, the most recent data were calculated based on the related data reported in the original articles.
2.4 Quality assessment
In the quality appraisal of the RCTs, five aspects, including the randomization process, deviations from the intended intervention, missing outcome data, measurement of the outcome, and selection of the reported result, based on a revised tool for assessing the risk of bias in randomized trials from the Cochrane Handbook (RoB-2) (Sterne et al., 2019), were employed.
For the non-randomized trials, the risk of bias in non-randomized studies of interventions (ROBINS-I) tool (Sterne et al., 2016) was employed. The selected items included confounding bias, subject selection bias, intervention classification bias, bias in deviation from established interventions, missing data bias, endpoint measurement bias, and selective reporting bias. Responses to each question were selected from “Yes,” “Probably Yes,” “No,” “Probably No,” “No Information,” and “Not Applicable.” Any disagreement between the two reviewers was resolved by a third reviewer.
2.5 Statistical analysis
All outcomes were denoted as hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) using the generated network meta-analysis models. The chi-square test was used to assess heterogeneity, with the level of significance set to p < 0.1. I2 values > 40% were interpreted as indicating significant heterogeneity; in such circumstances, a random-effects model was used to conduct meta-analysis. On the other hand, for I2 values ≤ 40%, a fixed-effect model was used instead. The back-calculation method was employed to test the consistency of all outcomes, using separate indirect and direct evidence. The surface under the cumulative ranking curve (SUCRA) for summarizing probabilities was used to provide summary and pooled statistics for the cumulative ranking. Studies with a mean heart failure rate of less than 20% were excluded in the sensitivity analysis. All statistical analyses were performed using the GeMTC package (versions 1.0–2) of R version 4.2.2 (Vienna, Austria).
3 Results
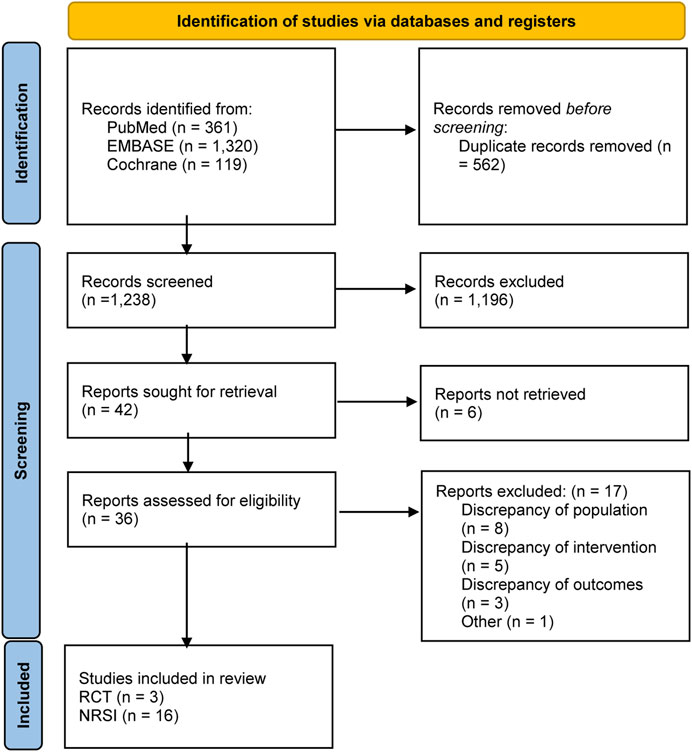
3.1 Study selection
Figure 1 shows the specific screening process. A total of 1,800 articles were retrieved for initial screening, and 562 duplicate articles were first excluded. After the reading of titles and abstracts, 1,238 articles were removed. The articles were comprehensively reviewed, and 17 articles that met the exclusion criteria for this study were removed. A total of 19 studies (Chan et al., 2009; Winkelmayer et al., 2011; Shah et al., 2014; Wakasugi et al., 2014; Chan et al., 2015; Genovesi et al., 2015; Shen et al., 2015; Yodogawa et al., 2016; Kai et al., 2017; Yoon et al., 2017; Siontis et al., 2018; Tan et al., 2019; Mavrakanas et al., 2020; De Vriese et al., 2021; Lin et al., 2021; Pokorney et al., 2022; Sy et al., 2022; Wetmore et al., 2022; Reinecke et al., 2023) were included in this study, comprising three randomized controlled trials (De Vriese et al., 2021; Pokorney et al., 2022; Reinecke et al., 2023) and observational studies.
3.2 Characteristics of included studies
A total of 103,684 subjects were included in the analysis, as detailed in Table 1. Most were over 60 years of age, and most were men. The sample size ranged from 60 (29) to 25,523 (22) participants, and mean follow-up periods were 1 (35) to 4 (25) years. Eight studies (Kai et al., 2017; Siontis et al., 2018; De Vriese et al., 2021; Lin et al., 2021; Pokorney et al., 2022; Sy et al., 2022; Wetmore et al., 2022; Reinecke et al., 2023) used CHA2DS2–VASc scores, and three studies (Chan et al., 2009; Chan et al., 2015; Yodogawa et al., 2016) used the CHADS2 score to score subjects. History of stroke and embolism was present in subjects in eight studies (Shah et al., 2014; Chan et al., 2015; Genovesi et al., 2015; Yodogawa et al., 2016; Kai et al., 2017; Tan et al., 2019; De Vriese et al., 2021). Atrial fibrillation occurred before dialysis in three studies consisting of 11,705 patients (Chan et al., 2009; Wakasugi et al., 2014; Sy et al., 2022) and occurred after dialysis in ten studies comprising 59,204 patients (Winkelmayer et al., 2011; Shah et al., 2014; Chan et al., 2015; Genovesi et al., 2015; Shen et al., 2015; Yodogawa et al., 2016; Kai et al., 2017; Siontis et al., 2018; Tan et al., 2019; Mavrakanas et al., 2020). Heart failure, hypertension, and diabetes were present in approximately 70% of the subjects in most studies. The intervention used in most studies was warfarin (Chan et al., 2009; Winkelmayer et al., 2011; Shah et al., 2014; Wakasugi et al., 2014; Genovesi et al., 2015; Shen et al., 2015; Yodogawa et al., 2016; Kai et al., 2017; Yoon et al., 2017; Tan et al., 2019; Sy et al., 2022), followed by rivaroxaban (Chan et al., 2015; De Vriese et al., 2021; Lin et al., 2021). The doses of rivaroxaban ranged from 10 mg daily (De Vriese et al., 2021) to 20 mg daily (Chan et al., 2015). The apixaban doses ranged from 5 mg daily to 10 mg daily (Siontis et al., 2018; Mavrakanas et al., 2020; Pokorney et al., 2022; Wetmore et al., 2022; Reinecke et al., 2023). Patients on dabigatran were treated with 150 mg daily (84.7%) and with the usual dose of 300 mg daily (15.3%) (Chan et al., 2015). No relevant literature regarding edoxaban treatment in patients with AF on dialysis was included.
3.3 Quality assessment
Information on the quality and bias risk assessment of all the studies is summarized in Supplementary Table S1. Three (De Vriese et al., 2021; Pokorney et al., 2022; Reinecke et al., 2023) of the 19 studies included in this study were RCTs, and the quality evaluation is detailed in Supplementary Table S1A. Three studies (De Vriese et al., 2021; Pokorney et al., 2022; Reinecke et al., 2023) were considered to have concerns regarding bias arising during randomization. Bias due to deviation from the intended intervention and bias due to missing outcome data were considered to pose a low risk for all RCTs performed. The study by De Vriese et al. (2021) was judged to have concerns regarding bias in the selection of reported outcomes, and it was the only study with concerns regarding the overall risk of bias.
The quality evaluation results of the 16 non-randomized studies of interventions (NRSIs) (Chan et al., 2009; Shah et al., 2014; Wakasugi et al., 2014; Chan et al., 2015; Genovesi et al., 2015; Shen et al., 2015; Kai et al., 2017; Siontis et al., 2018; Mavrakanas et al., 2020; Lin et al., 2021) are detailed in Supplementary Table S1B. Among the 16 NRSIs included in this analysis, seven exhibited a moderate risk of bias due to confounding factors (Chan et al., 2009; Wakasugi et al., 2014; Genovesi et al., 2015; Yodogawa et al., 2016; Siontis et al., 2018; Tan et al., 2019; Sy et al., 2022). In terms of participant selection bias, eight studies were deemed to have a moderate risk (Wakasugi et al., 2014; Yodogawa et al., 2016; Kai et al., 2017; Yoon et al., 2017; Tan et al., 2019; Mavrakanas et al., 2020; Lin et al., 2021; Sy et al., 2022). All studies demonstrated a low risk in relation to intervention classification bias. Regarding deviations from intended interventions, four studies (Winkelmayer et al., 2011; Genovesi et al., 2015; Yodogawa et al., 2016; Sy et al., 2022) presented a moderate risk of bias. Three studies (Kai et al., 2017; Mavrakanas et al., 2020; Lin et al., 2021) were found to have a moderate risk of bias due to missing data. With respect to outcome measurement bias, two studies (Tan et al., 2019; Lin et al., 2021) were assessed as having a moderate risk, while one study (Yodogawa et al., 2016) was considered at serious risk. Six studies (Chan et al., 2009; Wakasugi et al., 2014; Chan et al., 2015; Siontis et al., 2018; Mavrakanas et al., 2020; Lin et al., 2021) were considered at low risk concerning the selection of reported results.
3.4 Results of the network and direct-comparison meta-analysis
3.4.1 Mortality
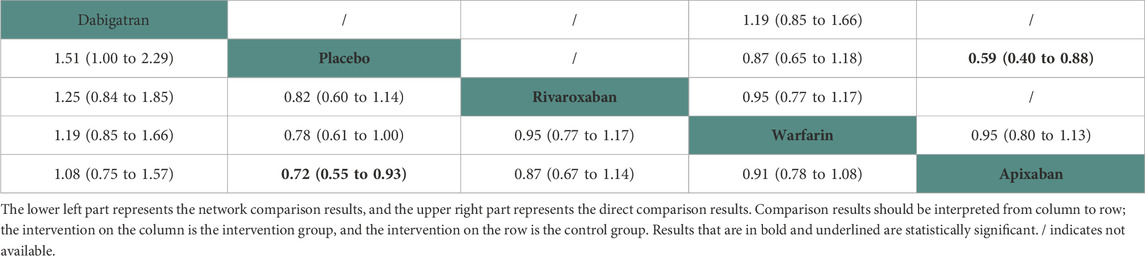
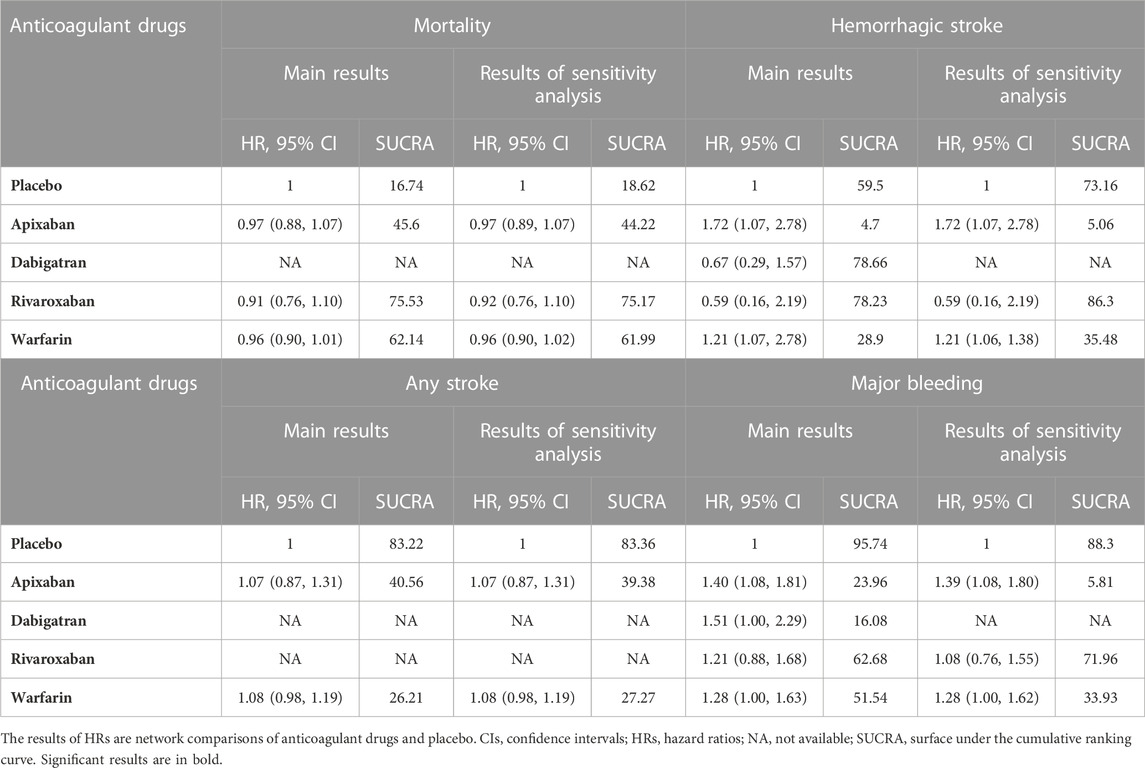
Thirteen studies (Chan et al., 2009; Winkelmayer et al., 2011; Wakasugi et al., 2014; Genovesi et al., 2015; Shen et al., 2015; Yodogawa et al., 2016; Kai et al., 2017; Siontis et al., 2018; Tan et al., 2019; De Vriese et al., 2021; Pokorney et al., 2022; Wetmore et al., 2022; Reinecke et al., 2023) with 62,533 participants using three anticoagulant drugs and a placebo were included in NMA to assess mortality. A network plot of mortality, including three active interventions and a placebo, is depicted in Figure 2A. Compared with placebo based on the NMA results, apixaban (HR = 0.97, 95% CI: 0.88–1.07), rivaroxaban (HR = 0.91, 95% CI: 0.76–1.10), and warfarin (HR = 0.96, 95% CI: 0.90–1.01) did not reduce mortality, as shown in Figure 3A, and comparisons of other anticoagulant drugs for mortality are summarized in Table 2. In the test quantifying overall heterogeneity, significant heterogeneity was found (I2 = 77.6%).
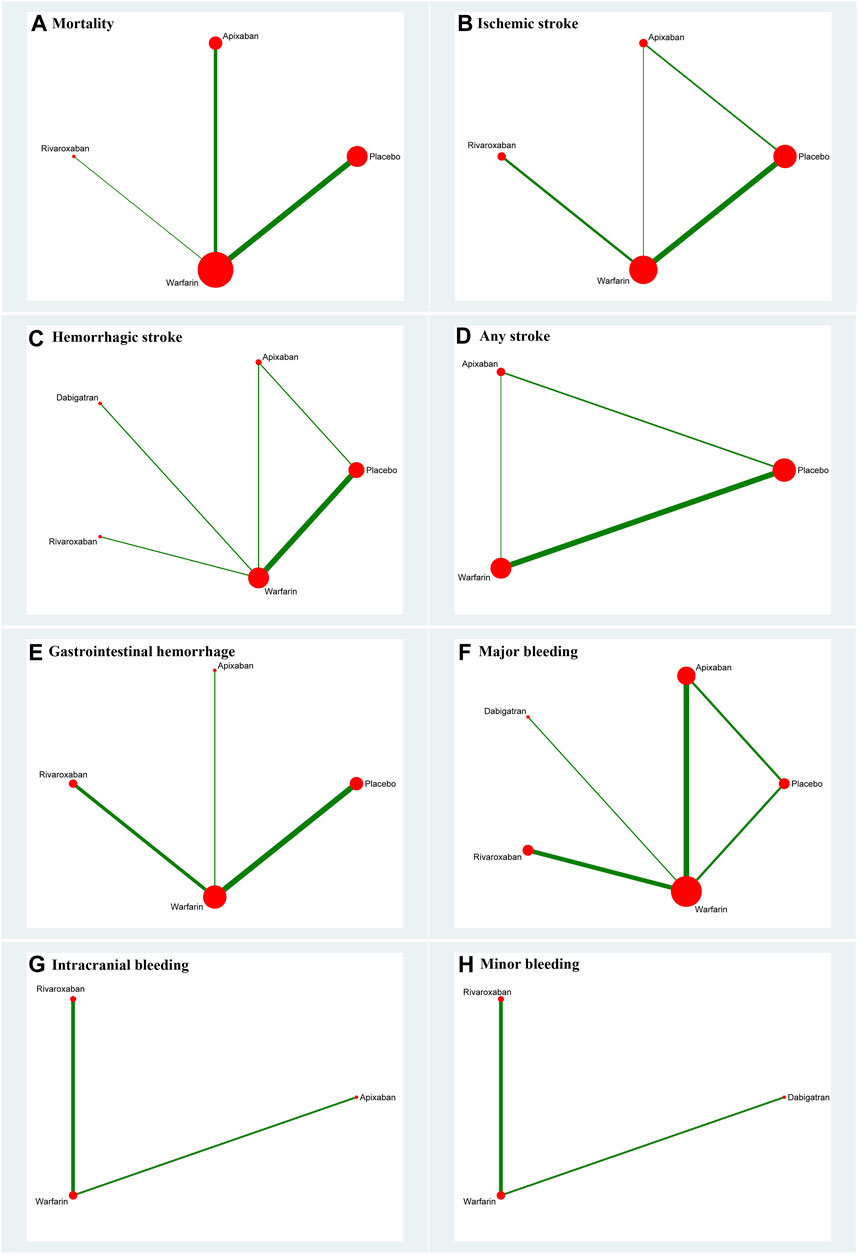
FIGURE 2. Network plot for all outcomes. (A) Mortality; (B) ischemic stroke; (C) hemorrhagic stroke; (D) any stroke; (E) gastrointestinal hemorrhage; (F) major bleeding; (G) intracranial bleeding; and (H) minor bleeding. The size of the nodes corresponds to the number of trials under study. The larger the node, the larger the number of participants in the study. The results of direct comparisons are connected by a line, the thickness of which corresponds to the sum of the sample sizes compared for each pairwise treatment. The thicker the line, the larger the sample size for comparison.
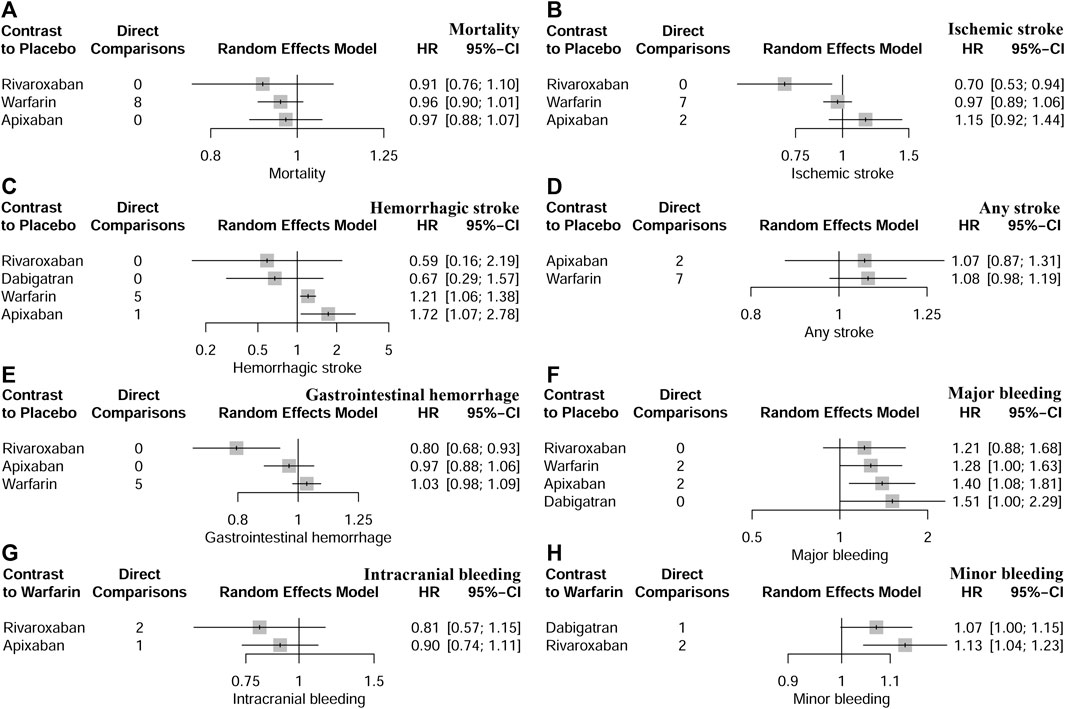
FIGURE 3. Network comparisons of anticoagulant drugs and placebo for all outcomes. (A) indicated mortality, (B) indicated ischaemic stroke, (C) indicated haemorrhagic stroke, (D) indicated any stroke, (E) indicated gastrointestinal haemorrhage, (F) indicated major bleeding, (G) indicated intracranial bleeding, and (H) indicated minor bleeding.
Regarding direct comparisons of mortality, the comparisons of warfarin vs. apixaban (HR = 0.99, 95% CI: 0.92–1.06), placebo vs. warfarin (HR = 1.04, 95% CI: 0.99–1.11), and rivaroxaban vs. warfarin (HR = 0.96, 95% CI: 0.80–1.14) did not significantly reduce mortality (Table 2).
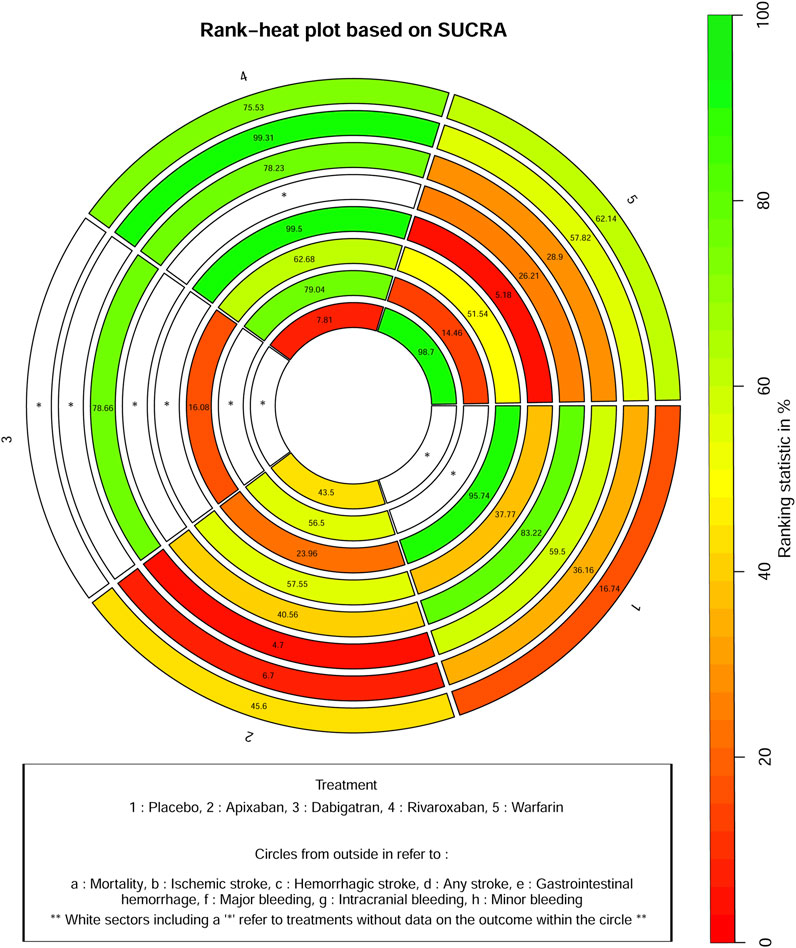
Figure 4 shows that based on SUCRA, rivaroxaban (75.53%), warfarin (62.14%), and apixaban (45.6%) were the most effective interventions for managing mortality, while placebo (16.74%) was least effective.
3.4.2 Ischemic stroke
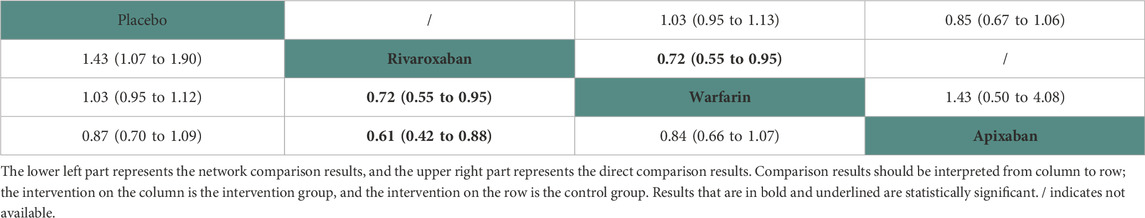
Ten studies (Chan et al., 2009; Winkelmayer et al., 2011; Wakasugi et al., 2014; Shen et al., 2015; Kai et al., 2017; Tan et al., 2019; Mavrakanas et al., 2020; De Vriese et al., 2021; Lin et al., 2021; Pokorney et al., 2022) with 38,750 participants using three anticoagulant drugs and a placebo were included in NMA to assess ischemic stroke. A network plot of ischemic stroke, including three active interventions and a placebo, is depicted in Figure 2B. Compared with placebo based on the NMA results, rivaroxaban (HR = 0.70, 95% CI: 0.53–0.94) reduced the risk of ischemic stroke, while apixaban (HR = 1.15, 95% CI: 0.92–1.44) and warfarin (HR = 0.97, 95% CI: 0.89–1.06) did not reduce the risk, as shown in Figure 3B. The comparisons of other active interventions for ischemic stroke are summarized in Table 3. In the test quantifying overall heterogeneity, obvious heterogeneity was found (I2 = 42.0%).
Regarding direct comparisons of ischemic stroke, the comparisons of rivaroxaban vs. warfarin (HR = 0.72, 95% CI: 0.55–0.95) showed a significantly reduced risk of ischemic stroke, and other comparisons of warfarin vs. apixaban (HR = 1.43, 95% CI: 0.50–4.08), placebo vs. warfarin (HR = 1.03, 95% CI: 0.95–1.13), and placebo vs. apixaban (HR = 0.85, 95% CI: 0.67–1.06) did not reduce the risk (Table 3).
Figure 4 shows that based on SUCRA, rivaroxaban (99.31%), warfarin (57.82%), and placebo (36.16%) were the most effective interventions for managing ischemic stroke, while apixaban (6.70%) was least effective.
3.4.3 Hemorrhagic stroke
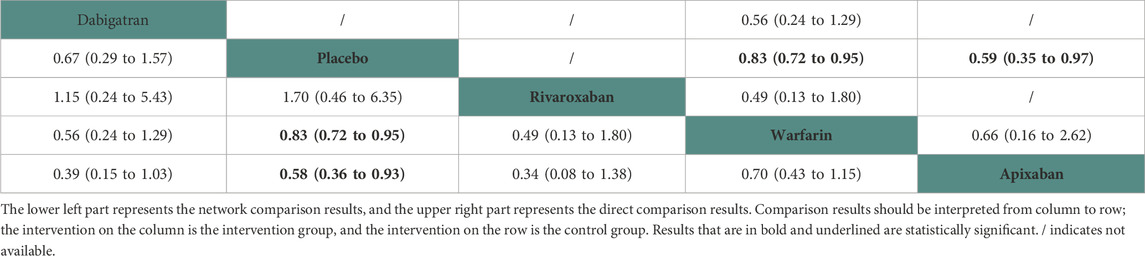
Nine studies (Chan et al., 2009; Winkelmayer et al., 2011; Chan et al., 2015; Shen et al., 2015; Kai et al., 2017; Yoon et al., 2017; Mavrakanas et al., 2020; De Vriese et al., 2021; Pokorney et al., 2022) with 32,821 participants using four anticoagulant drugs and a placebo were included in NMA to assess hemorrhagic stroke. A network plot of hemorrhagic stroke, including three active interventions and a placebo, is depicted in Figure 2C. Compared with placebo based on the NMA results, apixaban (HR = 1.72, 95% CI: 1.72–2.78) and warfarin (HR = 1.21, 95% CI: 1.06–1.38) increased the risk of hemorrhagic stroke, while rivaroxaban (HR = 0.59, 95% CI: 0.16–2.19) and dabigatran (HR = 0.67, 95% CI: 0.29–1.57) did not, as shown in Figure 3C. The comparisons of other active interventions for hemorrhagic stroke are summarized in Table 4. In the test quantifying overall heterogeneity, small heterogeneity was found (I2 = 9.70%).
Regarding direct comparisons of hemorrhagic stroke, the comparisons of placebo vs. warfarin (HR = 0.83, 95% CI: 0.72–0.95) and placebo vs. apixaban (HR = 0.59, 95% CI: 0.35–0.97) revealed a significantly reduced risk of hemorrhagic stroke, but other comparisons of dabigatran vs. warfarin (HR = 0.56, 95% CI: 0.24–1.29) and dabigatran vs. warfarin (HR = 0.49, 95% CI: 0.13–1.80) did not (Table 4).
Figure 4 shows that based on SUCRA, dabigatran (78.66%), rivaroxaban (78.23%), placebo (59.50%), and warfarin (28.90%) were the most effective interventions for managing hemorrhagic stroke, while apixaban (4.70%) was least effective.
3.4.4 Any stroke
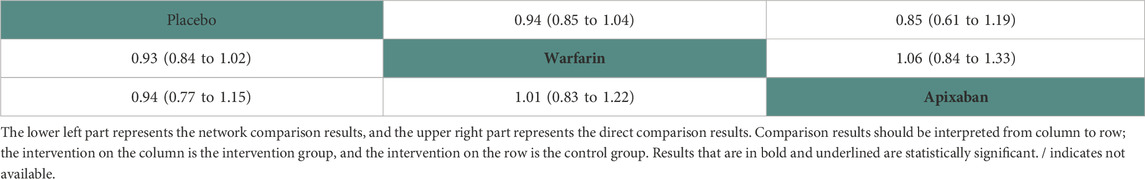
Nine studies (Chan et al., 2009; Winkelmayer et al., 2011; Shah et al., 2014; Shen et al., 2015; Yodogawa et al., 2016; Siontis et al., 2018; Tan et al., 2019; Mavrakanas et al., 2020; Sy et al., 2022) with 47,346 participants using two anticoagulant drugs and a placebo were included in NMA to assess any stroke. A network plot of any stroke, including three active interventions and a placebo, is depicted in Figure 2D. Compared with placebo based on the NMA results, apixaban (HR = 1.07, 95% CI: 0.87–1.31) and warfarin (HR = 1.08, 95% CI: 0.98–1.19) did not increase the risk of any stroke, as shown in Figure 3D. The comparisons of other active interventions for any stroke are summarized in Table 5. In the test quantifying overall heterogeneity, significant heterogeneity was found (I2 = 68.4%).
Regarding direct comparisons of any stroke, the comparisons of placebo vs. warfarin (HR = 0.94, 95% CI: 0.85–1.04), placebo vs. apixaban (HR = 0.85, 95% CI: 0.61–1.19), and warfarin vs. apixaban (HR = 1.06, 95% CI: 0.84–1.33) did not significantly reduce any stroke (Table 5).
Figure 4 shows that, based on SUCRA, placebo (83.22%) and apixaban (40.56%) were the most effective interventions for managing any stroke, while warfarin (26.21%) was least effective.
3.4.5 Gastrointestinal hemorrhage
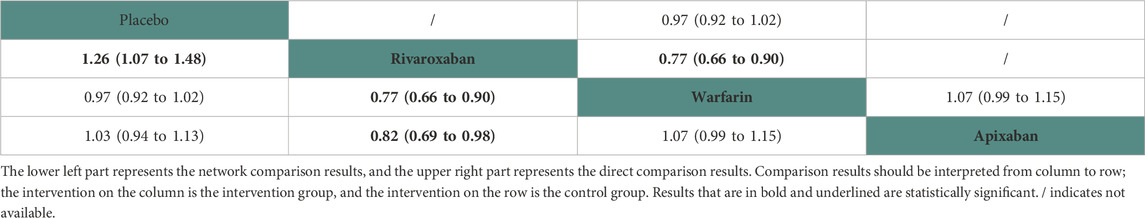
Eight studies (Winkelmayer et al., 2011; Shen et al., 2015; Kai et al., 2017; Yoon et al., 2017; Siontis et al., 2018; Tan et al., 2019; De Vriese et al., 2021; Lin et al., 2021) with 42,683 participants using three anticoagulant drugs and a placebo were included in NMA to assess gastrointestinal hemorrhage. A network plot of gastrointestinal hemorrhage, including three active interventions and a placebo, is depicted in Figure 2E. Compared with placebo based on the NMA results, rivaroxaban (HR = 0.80, 95% CI: 0.68–0.93) reduced the risk of gastrointestinal hemorrhage, while apixaban (HR = 0.97, 95% CI: 0.88–1.06) and warfarin (HR = 1.03, 95% CI: 0.98–1.09) did not (Figure 3E). The comparisons of other active interventions for gastrointestinal hemorrhage are summarized in Table 6. In the test quantifying overall heterogeneity, it did not find heterogeneity (I2 = 0%).
Regarding direct comparisons of gastrointestinal hemorrhage, the comparisons of rivaroxaban vs. warfarin (HR = 0.77, 95% CI: 0.66–0.90) showed significantly reduced gastrointestinal hemorrhage, while other comparisons of placebo vs. warfarin (HR = 0.97, 95% CI: 0.92–1.02) and warfarin vs. apixaban (HR = 1.07, 95% CI: 0.99–1.15) did not (Table 6).
Figure 4 shows that based on SUCRA, rivaroxaban (99.50%), apixaban (57.55%), and placebo (37.77%) were the most effective interventions for managing gastrointestinal hemorrhage, while warfarin (5.18%) was least effective.
3.4.6 Major bleeding
Eleven studies (Chan et al., 2009; Chan et al., 2015; Siontis et al., 2018; Tan et al., 2019; Mavrakanas et al., 2020; De Vriese et al., 2021; Lin et al., 2021; Pokorney et al., 2022; Reinecke et al., 2023) with 72,113 participants using four anticoagulant drugs and a placebo were included in NMA to assess major bleeding. A network plot of major bleeding, including three active interventions and a placebo, is depicted in Figure 2F. Compared with placebo based on the NMA results, apixaban (HR = 1.40, 95% CI: 1.08–1.81) increased the risk of major bleeding, while rivaroxaban (HR = 1.21, 95% CI: 0.88–1.68), warfarin (HR = 1.28, 95% CI: 1.00–1.63), and dabigatran (HR = 1.51, 95% CI: 1.00–2.29) did not, as shown in Figure 3F. The comparisons of other active interventions for major bleeding are summarized in Table 7. In the test quantifying overall heterogeneity, it found significant heterogeneity (I2 = 78.8%).
Regarding direct comparisons of major bleeding, the comparisons of placebo vs. apixaban (HR = 0.59, 95% CI: 0.40–0.88) showed significantly reduced major bleeding, whereas the other comparisons of warfarin vs. apixaban (HR = 0.95, 95% CI: 0.80–1.13), dabigatran vs. warfarin (HR = 1.19, 95% CI: 0.85–1.66), placebo vs. warfarin (HR = 0.87, 95% CI: 0.65–1.18), and rivaroxaban vs. warfarin (HR = 0.95, 95% CI: 0.77–1.17) did not (Table 7).
Figure 4 shows that based on SUCRA, placebo (95.74%), rivaroxaban (62.68%), and warfarin (51.54%) were the most effective interventions for managing major bleeding, while apixaban (23.96%) and dabigatran (16.08%) were least effective.
3.4.7 Intracranial bleeding
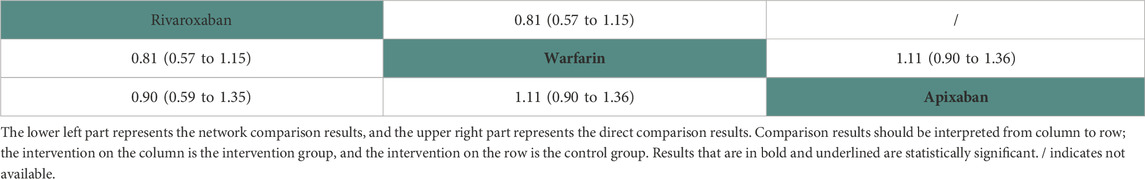
Two studies (Siontis et al., 2018; Lin et al., 2021) with 16,035 participants and three anticoagulant drugs were included in NMA to assess intracranial bleeding. A network plot of intracranial bleeding, including three active interventions and a placebo, is depicted in Figure 2G. Compared with warfarin based on the NMA results, rivaroxaban (HR = 0.81, 95% CI: 0.57–1.15) and apixaban (HR = 0.90, 95% CI: 0.74–1.11) did not increase the risk of intracranial bleeding, as shown in Figure 3G. The comparisons of other active interventions for intracranial bleeding are summarized in Table 8. In the test quantifying overall heterogeneity, it did not find heterogeneity (I2 = 0%).
Regarding direct comparisons of intracranial bleeding, the comparisons of rivaroxaban vs. warfarin (HR = 0.81, 95% CI: 0.57–1.15) and warfarin vs. apixaban (HR = 1.11, 95% CI: 0.90–1.36) did not significantly reduce intracranial bleeding (Table 8).
Figure 4 shows that based on SUCRA, rivaroxaban (79.04%) and apixaban (56.50%) were the most effective interventions for managing intracranial bleeding, while warfarin (14.46%) was least effective.
3.4.8 Minor bleeding
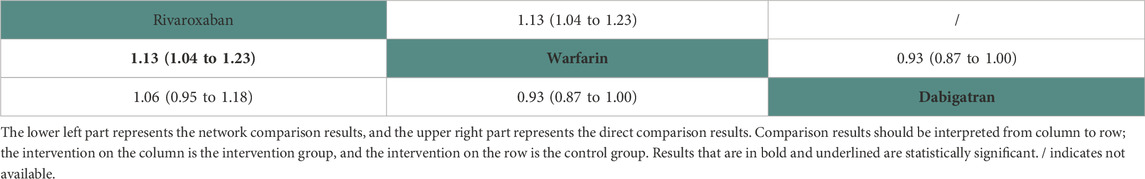
Two studies (Chan et al., 2015; De Vriese et al., 2021) with 23,152 participants and three anticoagulant drugs were included in NMA to assess minor bleeding. A network plot of minor bleeding, including three active interventions and a placebo, is depicted in Figure 2H. Compared with warfarin based on the NMA results, rivaroxaban (HR = 1.13, 95% CI: 1.04–1.23) increased the risk of minor bleeding, while dabigatran (HR = 1.07, 95% CI: 1.00–1.15) did not, as shown in Figure 3H. The comparisons of other active interventions for minor bleeding are summarized in Table 9. In the test quantifying overall heterogeneity, it did not find heterogeneity (I2 = 0%).
Regarding direct comparisons of minor bleeding, the comparisons of rivaroxaban vs. warfarin (HR = 1.13, 95% CI: 1.04–1.23) showed significantly reduced minor bleeding, while those of warfarin vs. dabigatran (HR = 0.93, 95% CI: 0.87–1.00) did not (Table 9).
Figure 4 shows that based on SUCRA, warfarin (98.70%) and apixaban (43.50%) were the most effective interventions for managing minor bleeding, while rivaroxaban (7.81%) was least effective.
3.5 Inconsistency test
Based on separate indirect and direct evidence using the back-calculation method, inconsistencies were not found in any of the outcomes (Supplementary Table S2-9).
3.6 Sensitivity analysis
For sensitivity analysis, studies of Chan et al. (2015) and Yodogawa et al. (2016) with a mean heart failure rate of less than 20% were excluded in outcomes of mortality, hemorrhagic stroke, any stroke, and major bleeding. Table 10 shows that the sensitivity analysis results of reticular versus SUCRA were stable.
3.7 Publication bias
Publication bias was not found in any of the network funnel plots, as shown in Supplementary Table S1-8.
4 Discussion
AF was a prevalent arrhythmia among ESRD patients undergoing dialysis (Chan and Siu, 2015). Both non-valvular AF and ESRD were independent risk factors for stroke and mortality (Bonde et al., 2015). However, the utilization of DOACs in ESRD patients with AF who undergo dialysis remained a controversial subject (Yao et al., 2016). Previous analyses had demonstrated that patients with AF undergoing dialysis were at a significantly elevated risk of stroke and bleeding when compared to those who did not receive anticoagulation therapy (Almutairi et al., 2017). The utilization of DOACs has been consistently increasing in recent years (Caldeira et al., 2015). However, currently, there is a lack of direct head-to-head comparisons between different DOACs. The lack of comprehensive evidence had posed a challenge for clinicians in regard to recommending one oral anticoagulant over another. To gain further insights into this issue, this study conducted a network meta-analysis to evaluate the efficacy of individual oral anticoagulants. This systematic review and meta-analysis of 19 studies revealed no significant differences in mortality, any stroke, or intracranial hemorrhage among rivaroxaban, warfarin, apixaban, and placebo. However, the network meta-analysis demonstrated that rivaroxaban treatment results in a lower incidence of ischemic stroke and gastrointestinal bleeding than did other treatments but causes an increased risk of minor bleeding events. Apixaban and warfarin were associated with an increased risk of hemorrhagic stroke, while apixaban additionally posed a heightened risk of major bleeding. Conventional meta-analysis findings indicate that placebo administration can effectively reduce the incidence of hemorrhagic stroke. Based on SUCRA, rivaroxaban demonstrated superior efficacy in mitigating mortality, ischemic stroke, gastrointestinal bleeding, and intracranial hemorrhage. The most significant reduction in hemorrhagic stroke was observed with dabigatran. Placebo therapy demonstrated the highest efficacy in reducing the incidence of any type of stroke and major bleeding, while warfarin proved to be most effective in reducing minor bleeding.
Patients with ESRD and AF undergoing dialysis had a high mortality rate, but a significant proportion of deaths were not attributable to cardiovascular events. Both the network and traditional meta-analysis indicated that the use of DOACs did not reduce mortality, which was consistent with the literature. In a study (Pan et al., 2017) examining dialysis recipients with AF, DOAC therapy did not lead to a reduction in the risk of all-cause mortality. Furthermore, a meta-analysis (Cohen et al., 2015) encompassing 12 cohort studies involving 17,380 participants revealed that warfarin had no significant impact on mortality among AF patients undergoing hemodialysis. Notably, when considering the comprehensive ranking using SUCRA analysis, rivaroxaban exhibited the greatest reduction in mortality, while apixaban demonstrated the least effect. There was insufficient evidence to support the reduction in mortality with rivaroxaban compared to other DOACs in patients with ESRD and AF undergoing dialysis (Geurts et al., 2022). However, the conclusion appeared plausible based on pharmacokinetic and pharmacodynamic studies demonstrating that a 10-mg dose of rivaroxaban in ESRD patients achieved similar drug levels as a 20-mg dose in healthy individuals, and it was not eliminated through dialysis in ESRD patients (Wetmore et al., 2022). The precise relationship between apixaban and mortality remained uncertain. However, evidence suggested a dose-dependent association, with lower doses of apixaban potentially linked to higher mortality rates. While the standard 5-mg dosage of apixaban was associated with reduced mortality, the administration of a 2.5-mg dose appeared to be correlated with an increase in mortality.
The incidence of AF was significantly elevated in ESRD patients receiving dialysis. A study conducted by Vazquez et al. (2016) revealed that the occurrence of new-onset AF in CKD patients undergoing dialysis was significantly associated with a nine-fold increased risk of stroke. Furthermore, a meta-analysis comprising 21 prospective studies demonstrated an inverse relationship between eGFR <60 mL/min/1.73 m2 and a significantly elevated risk of stroke (Harenberg et al., 2012). An observational study of a randomly selected sample comprising 17,518 patients with ESRD undergoing hemodialysis demonstrated that AF was associated with a 1.8-fold increased risk of stroke (Skjøth et al., 2014). Another investigation revealed the presence of proteinuria thromboembolism by 1.5-fold after adjusting for established stroke risk factors and other potential confounders. The network meta-analysis conducted in this study revealed that rivaroxaban significantly reduced the incidence of ischemic stroke, while apixaban and warfarin were found to be associated with an increased incidence of hemorrhagic stroke. The efficacy of apixaban in reducing thromboembolism had been demonstrated, with a dosage of 5 mg administered twice daily showing superior results compared to warfarin. In accordance with the KDIGO 2012 guidelines (Gordon et al., 2023), utilizing a lower dosage of 2.5 mg apixaban for stroke prevention in patients with ESKD and AF was tentatively considered. This observed discrepancy arose from previous studies that combined ischemic stroke and hemorrhagic stroke as a single outcome. Rates of stroke did not show any significant association with the use of DOACs. Given that vascular factors were the primary cause of stroke in patients with ESRD, the potential benefits of anticoagulant therapy in reducing stroke risk were limited. Conventional meta-analyses had suggested that the non-use of anticoagulant therapy could potentially decrease the incidence of hemorrhagic strokes. Compared to the non-use of anticoagulant therapy, the use of warfarin-based DOAC therapy had been suggested to confer significant benefits in the prevention of thromboembolic events and all-cause mortality, without an increased risk of bleeding events.
According to the SUCRA comprehensive ranking, rivaroxaban demonstrated the greatest reduction in ischemic stroke, while apixaban showed the least. Dabigatran was found to be most effective in reducing hemorrhagic stroke incidence, whereas apixaban was the least effective. Placebo exhibited the highest efficacy in reducing any type of stroke, with warfarin being the most effective in managing minor bleeding. According to the AHA/ACC/HRS guidelines (January et al., 2019), for patients with non-valvular AF and CHA2DS2-VASc scores of 2 or higher and end-stage CKD (creatinine clearance <15 mL/min) or who were on dialysis, warfarin (INR 2.0–3.0) or apixaban was recommended. However, Chan and Siu (2015) found that, in patients with AF, warfarin use was associated with a significantly increased risk of new stroke compared to non-use of warfarin. The more detailed conclusion was that there was no observed association between warfarin and ischemic stroke in patients with ESRD, but an association existed between warfarin and an increased risk of hemorrhagic stroke in patients with AF. In summary, the findings of this study supported the notion that warfarin did not confer any benefit in reducing the incidence of stroke among ESRD patients receiving dialysis, aligning with the overall conclusion drawn from this investigation.
In our network meta-analysis, rivaroxaban demonstrated a reduction in the risk of gastrointestinal bleeding. However, it was associated with an increased risk of minor bleeding. Conversely, apixaban was found to increase the risk of major bleeding. Neither network nor traditional meta-analysis indicated that DOACs were ineffective in reducing intracranial hemorrhage. Consistent with the present study (Buckley et al., 2022), the use of DOACs did not yield beneficial outcomes in terms of reducing bleeding rates among ESRD patients with AF undergoing dialysis. Among patients receiving dialysis and diagnosed with AF, treatment with DOACs was associated with a 28% increased risk of bleeding events. In a meta-analysis comprising 12 cohort studies involving 17,380 participants, the use of warfarin in AF patients undergoing hemodialysis was associated with a 21% higher risk of bleeding (Briere et al., 2019). According to the SUCRA comprehensive ranking, the administration of rivaroxaban significantly decreased the incidence of gastrointestinal and intracranial bleeding to the greatest extent, while warfarin exhibited the least favorable outcomes. Conversely, minor bleeding showed the opposite pattern. The rate of major bleeding was most effectively reduced by placebo and least effectively reduced by dabigatran. A study utilizing a US insurance claims database demonstrated that in AF patients with ESRD, the risk of ischemic stroke/systemic embolism was associated with a lower risk of major bleeding when the patients were treated with rivaroxaban compared to warfarin (Lip et al., 2016). Siontis et al. (2018) investigated 2,351 AF patients with ESRD receiving apixaban and 23,172 receiving warfarin and concluded that apixaban significantly decreased the risk of major bleeding. Notably, the annual bleeding rate, particularly intracranial bleeding, was found to be high for both apixaban and warfarin in this study, with more than two-thirds of patients discontinuing DOACs within 1 year.
Furthermore, it was worth mentioning that DOACs exhibit reduced efficacy in mitigating bleeding events among ESRD patients with AF undergoing dialysis, which may be attributed to the association between CKD and an increased risk of bleeding. Platelet dysfunction in individuals with ESRD, impaired renal clearance, and concurrent heparin use during hemodialysis collectively heightened the susceptibility to bleeding among dialysis recipients utilizing DOACs. Considering that hemodialysis is usually conducted three times per week, administering DOACs once or twice daily may lead to drug accumulation over the extended intervals between treatment sessions (Kumar et al., 2019). Later, as drug accumulation occurs, the efficacy of DOACs diminishes, exacerbating bleeding in parallel with deteriorating renal function. CKD patients undergoing oral anticoagulant therapy were susceptible to glomerular hemorrhage and renal tubular obstruction due to excessive anticoagulation, as well as anticoagulant-related nephropathy that further compromised renal function. Moreover, when considering the utilization of DOACs in ESRD patients, it was crucial to account for the degree of decline in renal function since studies had demonstrated heterogeneity across CKD stages concerning all-cause mortality, thromboembolic events, and bleeding incidents.
A nationwide cohort study conducted in 2016 identified DOACs as safe and effective alternatives to warfarin therapy, demonstrating their potential for clinical use (Larsen et al., 2016). Regarding the prevention of ischemic stroke alone, no significant disparities were observed between DOACs and warfarin. However, when considering the combined endpoint of ischemic stroke and systemic embolism, rivaroxaban posed a risk lower than that posed by warfarin. In contrast, the effects of dabigatran and apixaban were not statistically significant. Apixaban and dabigatran were associated with a decreased risk of mortality compared to rivaroxaban or warfarin. Meanwhile, a 2021 meta-analysis concluded that apixaban and rivaroxaban may serve as potential alternatives to warfarin anticoagulants because they did not increase the risk of major bleeding or stroke (Abdullah et al., 2021). In contrast, See et al. (2021) concluded that DOACs were not more favorable than warfarin in terms of efficacy and safety in patients with AF on dialysis. The use of DOACs was not associated with a reduced risk of ischemic stroke/systemic embolism in patients with ESRD combined with AF. A network meta-analysis from 2021 also suggested that DOACs were superior to warfarin in preventing thromboembolic events and reducing bleeding risk in AF patients with glomerular filtration rates of 15–60 mL/min (Su et al., 2021). However, further high-quality evidence was still required to establish tailored treatment regimens for DOACs in diverse populations.
Clinical implication
This NMA examined the previous three RCTs and sixteen observational cohorts of anticoagulant drugs and compared the effects of four anticoagulant drugs used for patients with atrial fibrillation on dialysis. Our study demonstrated that the effectiveness and safety of these four anticoagulant drugs had different disadvantages and advantages. Among them, rivaroxaban showed relatively better efficacy and safety than the remaining anticoagulant drugs. This contrasts with the latest AHA/ACC/HRS guidelines (January et al., 2019), which recommend warfarin and apixaban for patients with non-valvular AF on dialysis. Therefore, the decision evaluation based on NMA in this study provided new evidence for guidelines and clinicians, offering new insights into the use of anticoagulant therapy in therapeutic regimens for AF patients on dialysis.
Advantages and limitations
This meta-analysis had several advantages. First, NMA and conventional meta-analysis were employed in this study; they confirmed each other and enhanced the strength of the evidence. Previous studies, including RCTs and meta-analyses, provided conflicting conclusions, and our study confirmed their points of contradiction. Second, studies with a heart failure rate of less than 20% were excluded; sensitivity analysis showed that the network results were stable and confirmed the reliability and credibility of our evidence. Third, in the absence of direct head-to-head studies between various DOACs, warfarin, and placebo, this study compared and ranked the effectiveness of anticoagulant drugs used for patients with AF on dialysis using NMA, which provided a theoretical basis for clinical staff to select the anticoagulant drugs.
This study had several limitations. Regarding the data analysis, the presence of statistical heterogeneity in the outcome analyses and the inherent clinical and methodological heterogeneity may have exerted an influence on our findings. Our study employed an intention-to-treat design and did not account for changes or discontinuation of DOACs, leading to variations in patient categorization. Furthermore, both adjusted and unadjusted outcomes were amalgamated in observational studies, which could have impacted our results. In most studies, the incidence rate of events was low, and the 95% CI of the effect measure was wide. The network structure was highly sparse, resulting in limited power for consistency testing and minimal opportunity for cycle testing. Consequently, it was not feasible to estimate differences between models in a network meta-analysis. Regarding the study design, various DOACs exhibited varying degrees of renal excretion. Therefore, decisions regarding DOAC selection or dosage may have been influenced by individual patient characteristics such as renal function and weight, which could have introduced potential bias. The inclusion of studies did not explicitly mention the patients’ prior use of oral anticoagulants, which also introduced lead time bias into the study. Furthermore, it was not feasible to conduct stratified subgroup analyses based on varying follow-up durations and different selection criteria.
5 Conclusion
In conclusion, rivaroxaban demonstrated efficacy in reducing mortality and the incidence of ischemic stroke, gastrointestinal bleeding, and intracranial hemorrhage. Dabigatran was recommended for the prevention of hemorrhagic strokes. However, caution should be exercised due to the risk of major bleeding. Warfarin could effectively reduce minor bleeding but did not offer significant protection against gastrointestinal or intracranial bleeding. Apixaban was not recommended for mortality reduction or for preventing ischemic or hemorrhagic strokes. Further RCTs are warranted to establish specific clinical protocols.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
X-FS: conceptualization, data curation, formal analysis, methodology, project administration, software, writing–original draft, and writing–review and editing. CZ: data curation, formal analysis, investigation, methodology, software, visualization, and writing–original draft. JH: data curation, formal analysis, methodology, resources, and writing–original draft. TZ: data curation, formal analysis, methodology, project administration, and writing–original draft. BM: conceptualization, formal analysis, investigation, methodology, resources, supervision, writing–original draft, and writing–review and editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1320939/full#supplementary-material
References
Abdullah, H. M., Ullah, W., Jafar, M. S., van Zyl, M., Saeed, R., Alam, M., et al. (2021). Safety and efficacy of apixaban, rivaroxaban, and warfarin in end-stage renal disease with atrial fibrillation: a systematic review and meta-analysis. Cardiovasc. revascularization Med. Incl. Mol. Interv. 30, 26–32. doi:10.1016/j.carrev.2020.09.041
Almutairi, A. R., Zhou, L., Gellad, W. F., Lee, J. K., Slack, M. K., Martin, J. R., et al. (2017). Effectiveness and safety of non-vitamin K antagonist oral anticoagulants for atrial fibrillation and venous thromboembolism: a systematic review and meta-analyses. Clin. Ther. 39 (7), 1456–1478. doi:10.1016/j.clinthera.2017.05.358
Baker, R. I., Gilmore, G., Chen, V., Young, L., Merriman, E., Curnow, J., et al. (2023). Direct oral anticoagulants or vitamin K antagonists in emergencies: comparison of management in an observational study. Res. Pract. thrombosis haemostasis 7 (5), 100196. doi:10.1016/j.rpth.2023.100196
Benz, A. P., and Eikelboom, J. W. (2022). Apixaban compared with warfarin in patients with atrial fibrillation and end-stage renal disease: lessons learned. Circulation 146 (23), 1746–1748. doi:10.1161/CIRCULATIONAHA.122.061647
Bonde, A. N., Kamper, A. L., and Olesen, J. B. (2015). Reply: clinical benefit of warfarin in dialysis patients with atrial fibrillation. J. Am. Coll. Cardiol. 66 (11), 1311. doi:10.1016/j.jacc.2015.05.081
Bonde, A. N., Lip, G. Y., Kamper, A. L., Hansen, P. R., Lamberts, M., Hommel, K., et al. (2014). Net clinical benefit of antithrombotic therapy in patients with atrial fibrillation and chronic kidney disease: a nationwide observational cohort study. J. Am. Coll. Cardiol. 64 (23), 2471–2482. doi:10.1016/j.jacc.2014.09.051
Briere, J. B., Bowrin, K., Millier, A., Toumi, M., Wojciechowski, P., and Taieb, V. (2019). Number needed to treat based on real-world evidence for non-vitamin K antagonist oral anticoagulants versus vitamin K antagonist oral anticoagulants in stroke prevention in patients with non-valvular atrial fibrillation. J. Med. Econ. 22 (8), 760–765. doi:10.1080/13696998.2019.1606001
Buckley, B. J. R., Lane, D. A., Calvert, P., Zhang, J., Gent, D., Mullins, C. D., et al. (2022). Effectiveness and safety of apixaban in over 3.9 million people with atrial fibrillation: a systematic review and meta-analysis. J. Clin. Med. 11 (13), 3788. doi:10.3390/jcm11133788
Caldeira, D., Rodrigues, F. B., Barra, M., Santos, A. T., de Abreu, D., Gonçalves, N., et al. (2015). Non-vitamin K antagonist oral anticoagulants and major bleeding-related fatality in patients with atrial fibrillation and venous thromboembolism: a systematic review and meta-analysis. Heart (British Card. Soc. 101 (15), 1204–1211. doi:10.1136/heartjnl-2015-307489
Chan, K. E., Edelman, E. R., Wenger, J. B., Thadhani, R. I., and Maddux, F. W. (2015). Dabigatran and rivaroxaban use in atrial fibrillation patients on hemodialysis. Circulation 131 (11), 972–979. doi:10.1161/CIRCULATIONAHA.114.014113
Chan, K. E., Lazarus, J. M., Thadhani, R., and Hakim, R. M. (2009). Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J. Am. Soc. Nephrol. JASN. 20 (10), 2223–2233. doi:10.1681/ASN.2009030319
Chan, P. H., and Siu, C. W. (2015). Clinical benefit of warfarin in dialysis patients with atrial fibrillation. J. Am. Coll. Cardiol. 66 (11), 1310–1311. doi:10.1016/j.jacc.2015.03.601
Cohen, A. T., Hamilton, M., Mitchell, S. A., Phatak, H., Liu, X., Bird, A., et al. (2015). Comparison of the novel oral anticoagulants apixaban, dabigatran, edoxaban, and rivaroxaban in the initial and long-term treatment and prevention of venous thromboembolism: systematic review and network meta-analysis. PloS one 10 (12), e0144856. doi:10.1371/journal.pone.0144856
De Vriese, A. S., Caluwé, R., Van Der Meersch, H., De Boeck, K., and De Bacquer, D. (2021). Safety and efficacy of vitamin K antagonists versus rivaroxaban in hemodialysis patients with atrial fibrillation: a multicenter randomized controlled trial. J. Am. Soc. Nephrol. JASN 32 (6), 1474–1483. doi:10.1681/ASN.2020111566
Ding, W. Y., Gupta, D., Wong, C. F., and Lip, G. Y. H. (2021). Pathophysiology of atrial fibrillation and chronic kidney disease. Cardiovasc. Res. 117 (4), 1046–1059. doi:10.1093/cvr/cvaa258
Edwina, A. E., Dia, N., Dreesen, E., Vanassche, T., Verhamme, P., Spriet, I., et al. (2023). Insights into the pharmacokinetics and pharmacodynamics of direct oral anticoagulants in older adults with atrial fibrillation: a structured narrative review. Clin. Pharmacokinet. 62 (3), 351–373. doi:10.1007/s40262-023-01222-w
Franchini, M., Liumbruno, G. M., Bonfanti, C., and Lippi, G. (2016). The evolution of anticoagulant therapy. Blood Transfus. 14 (2), 175–184. doi:10.2450/2015.0096-15
Genovesi, S., Rossi, E., Gallieni, M., Stella, A., Badiali, F., Conte, F., et al. (2015). Warfarin use, mortality, bleeding and stroke in haemodialysis patients with atrial fibrillation. Nephrol. Dial. Transplant. 30 (3), 491–498. doi:10.1093/ndt/gfu334
Geurts, S., van der Burgh, A. C., Bos, M. M., Ikram, M. A., Stricker, B. H. C., Deckers, J. W., et al. (2022). Disentangling the association between kidney function and atrial fibrillation: a bidirectional Mendelian randomization study. Int. J. Cardiol. 355, 15–22. doi:10.1016/j.ijcard.2022.03.004
Gordon, C. E., Adam, G. P., Jadoul, M., Martin, P., and Balk, E. M. (2023). Kidney transplantation from hepatitis C virus-infected donors to uninfected recipients: a systematic review for the KDIGO 2022 hepatitis C clinical practice guideline update. Am. J. kidney Dis. 82 (4), 410–418. doi:10.1053/j.ajkd.2022.12.019
Harenberg, J., Marx, S., Diener, H. C., Lip, G. Y., Marder, V. J., Wehling, M., et al. (2012). Comparison of efficacy and safety of dabigatran, rivaroxaban and apixaban in patients with atrial fibrillation using network meta-analysis. Int. angiology a J. Int. Union Angiology 31 (4), 330–339.
Hijazi, Z., Hohnloser, S. H., Andersson, U., Alexander, J. H., Hanna, M., Keltai, M., et al. (2016). Efficacy and safety of apixaban compared with warfarin in patients with atrial fibrillation in relation to renal function over time: insights from the ARISTOTLE randomized clinical trial. JAMA Cardiol. 1 (4), 451–460. doi:10.1001/jamacardio.2016.1170
January, C. T., Wann, L. S., Calkins, H., Chen, L. Y., Cigarroa, J. E., Cleveland, J. C., et al. (2019). 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons. Circulation 140 (2), e125–e151. doi:10.1161/CIR.0000000000000665
Kai, B., Bogorad, Y., Nguyen, L. N., Yang, S. J., Chen, W., Spencer, H. T., et al. (2017). Warfarin use and the risk of mortality, stroke, and bleeding in hemodialysis patients with atrial fibrillation. Heart rhythm. 14 (5), 645–651. doi:10.1016/j.hrthm.2017.01.047
Kumar, S., Lim, E., Covic, A., Verhamme, P., Gale, C. P., Camm, A. J., et al. (2019). Anticoagulation in concomitant chronic kidney disease and atrial fibrillation: JACC review topic of the week. J. Am. Coll. Cardiol. 74 (17), 2204–2215. doi:10.1016/j.jacc.2019.08.1031
Larsen, T. B., Skjøth, F., Nielsen, P. B., Kjældgaard, J. N., and Lip, G. Y. (2016). Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ Clin. Res. ed) 353, i3189. doi:10.1136/bmj.i3189
Lin, Y. C., Chen, B. L., Shih, C. M., Lin, F. Y., Chen, C. W., Hsu, C. Y., et al. (2021). Effectiveness and safety of rivaroxaban versus warfarin in Taiwanese patients with end-stage renal disease and nonvalvular atrial fibrillation: a real-world nationwide cohort study. PloS one 16 (4), e0249940. doi:10.1371/journal.pone.0249940
Lip, G. Y., Mitchell, S. A., Liu, X., Liu, L. Z., Phatak, H., Kachroo, S., et al. (2016). Relative efficacy and safety of non-Vitamin K oral anticoagulants for non-valvular atrial fibrillation: network meta-analysis comparing apixaban, dabigatran, rivaroxaban and edoxaban in three patient subgroups. Int. J. Cardiol. 204, 88–94. doi:10.1016/j.ijcard.2015.11.084
Lip, G. Y. H., Skjøth, F., Nielsen, P. B., Kjældgaard, J. N., and Larsen, T. B. (2017). Effectiveness and safety of standard-dose nonvitamin K antagonist oral anticoagulants and warfarin among patients with atrial fibrillation with a single stroke risk factor: a nationwide cohort study. JAMA Cardiol. 2 (8), 872–881. doi:10.1001/jamacardio.2017.1883
Mantha, S., and Ansell, J. (2012). An indirect comparison of dabigatran, rivaroxaban and apixaban for atrial fibrillation. Thrombosis haemostasis 108 (3), 476–484. doi:10.1160/TH12-02-0093
Mavrakanas, T. A., Garlo, K., and Charytan, D. M. (2020). Apixaban versus No anticoagulation in patients undergoing long-term dialysis with incident atrial fibrillation. Clin. J. Am. Soc. Nephrol. CJASN. 15 (8), 1146–1154. doi:10.2215/CJN.11650919
Ntaios, G., Papavasileiou, V., Makaritsis, K., Vemmos, K., Michel, P., and Lip, G. Y. H. (2017). Real-World setting comparison of nonvitamin-K antagonist oral anticoagulants versus vitamin-K antagonists for stroke prevention in atrial fibrillation: a systematic review and meta-analysis. Stroke 48 (9), 2494–2503. doi:10.1161/STROKEAHA.117.017549
Pan, K. L., Singer, D. E., Ovbiagele, B., Wu, Y. L., Ahmed, M. A., and Lee, M. (2017). Effects of non-vitamin K antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and valvular heart disease: a systematic review and meta-analysis. J. Am. Heart Assoc. 6 (7), e005835. doi:10.1161/JAHA.117.005835
Pokorney, S. D., Black-Maier, E., Hellkamp, A. S., Friedman, D. J., Vemulapalli, S., Granger, C. B., et al. (2020). Oral anticoagulation and cardiovascular outcomes in patients with atrial fibrillation and end-stage renal disease. J. Am. Coll. Cardiol. 75 (11), 1299–1308. doi:10.1016/j.jacc.2020.01.019
Pokorney, S. D., Chertow, G. M., Al-Khalidi, H. R., Gallup, D., Dignacco, P., Mussina, K., et al. (2022). Apixaban for patients with atrial fibrillation on hemodialysis: a multicenter randomized controlled trial. Circulation 146 (23), 1735–1745. doi:10.1161/CIRCULATIONAHA.121.054990
Potpara, T. S., Polovina, M. M., Licina, M. M., Stojanovic, R. M., Prostran, M. S., and Lip, G. Y. (2012). Novel oral anticoagulants for stroke prevention in atrial fibrillation: focus on apixaban. Adv. Ther. 29 (6), 491–507. doi:10.1007/s12325-012-0026-8
Proietti, M., Romanazzi, I., Romiti, G. F., Farcomeni, A., and Lip, G. Y. H. (2018). Real-World use of apixaban for stroke prevention in atrial fibrillation: a systematic review and meta-analysis. Stroke 49 (1), 98–106. doi:10.1161/STROKEAHA.117.018395
Reinecke, H., Engelbertz, C., Bauersachs, R., Breithardt, G., Echterhoff, H. H., Gerß, J., et al. (2023). A randomized controlled trial comparing apixaban with the vitamin K antagonist phenprocoumon in patients on chronic hemodialysis: the AXADIA-AFNET 8 study. Circulation 147 (4), 296–309. doi:10.1161/CIRCULATIONAHA.122.062779
See, L. C., Lee, H. F., Chao, T. F., Li, P. R., Liu, J. R., Wu, L. S., et al. (2021). Effectiveness and safety of direct oral anticoagulants in an asian population with atrial fibrillation undergoing dialysis: a population-based cohort study and meta-analysis. Cardiovasc. drugs Ther. 35 (5), 975–986. doi:10.1007/s10557-020-07108-4
Shah, M., Avgil Tsadok, M., Jackevicius, C. A., Essebag, V., Eisenberg, M. J., Rahme, E., et al. (2014). Warfarin use and the risk for stroke and bleeding in patients with atrial fibrillation undergoing dialysis. Circulation 129 (11), 1196–1203. doi:10.1161/CIRCULATIONAHA.113.004777
Shen, J. I., Montez-Rath, M. E., Lenihan, C. R., Turakhia, M. P., Chang, T. I., and Winkelmayer, W. C. (2015). Outcomes after warfarin initiation in a cohort of hemodialysis patients with newly diagnosed atrial fibrillation. Am. J. kidney Dis. 66 (4), 677–688. doi:10.1053/j.ajkd.2015.05.019
Siontis, K. C., Zhang, X., Eckard, A., Bhave, N., Schaubel, D. E., He, K., et al. (2018). Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation 138 (15), 1519–1529. doi:10.1161/CIRCULATIONAHA.118.035418
Skjøth, F., Larsen, T. B., Rasmussen, L. H., and Lip, G. Y. (2014). Efficacy and safety of edoxaban in comparison with dabigatran, rivaroxaban and apixaban for stroke prevention in atrial fibrillation. An indirect comparison analysis. Thrombosis haemostasis 111 (5), 981–988. doi:10.1160/TH14-02-0118
Stamellou, E., and Floege, J. (2018). Novel oral anticoagulants in patients with chronic kidney disease and atrial fibrillation. Nephrol. Dial. Transplant. 33 (10), 1683–1689. doi:10.1093/ndt/gfx322
Sterne, J. A., Hernán, M. A., Reeves, B. C., Savović, J., Berkman, N. D., Viswanathan, M., et al. (2016). ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ Clin. Res. ed) 355, i4919. doi:10.1136/bmj.i4919
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ Clin. Res. ed) 366, l4898. doi:10.1136/bmj.l4898
Su, X., Yan, B., Wang, L., Lv, J., Cheng, H., and Chen, Y. (2021). Oral anticoagulant agents in patients with atrial fibrillation and CKD: a systematic review and pairwise network meta-analysis. Am. J. kidney Dis. 78 (5), 678–689.e1. doi:10.1053/j.ajkd.2021.02.328
Sy, J., Wenziger, C., Marroquin, M., Kalantar-Zadeh, K., Kovesdy, C., and Streja, E. (2022). Warfarin use, stroke, and bleeding risk among pre-existing atrial fibrillation US veterans transitioning to dialysis. Nephron 146 (4), 360–368. doi:10.1159/000521494
Tan, J., Bae, S., Segal, J. B., Zhu, J., Alexander, G. C., Segev, D. L., et al. (2019). Warfarin use and the risk of stroke, bleeding, and mortality in older adults on dialysis with incident atrial fibrillation. Nephrol. Carlt. Vic. 24 (2), 234–244. doi:10.1111/nep.13207
Vazquez, F. J., Gonzalez, J. P., LeGal, G., Carrier, M., and Gándara, E. (2016). Risk of major bleeding in patients receiving vitamin K antagonists or low doses of aspirin. A systematic review and meta-analysis. Thrombosis Res. 138, 1–6. doi:10.1016/j.thromres.2015.12.013
Wakasugi, M., Kazama, J. J., Tokumoto, A., Suzuki, K., Kageyama, S., Ohya, K., et al. (2014). Association between warfarin use and incidence of ischemic stroke in Japanese hemodialysis patients with chronic sustained atrial fibrillation: a prospective cohort study. Clin. Exp. Nephrol. 18 (4), 662–669. doi:10.1007/s10157-013-0885-6
Wetmore, J. B., Weinhandl, E. D., Yan, H., Reyes, J. L., Herzog, C. A., and Roetker, N. S. (2022). Apixaban dosing patterns versus warfarin in patients with nonvalvular atrial fibrillation receiving dialysis: a retrospective cohort study. Am. J. kidney Dis. 80 (5), 569–579.e1. doi:10.1053/j.ajkd.2022.03.007
Winkelmayer, W. C., Liu, J., Setoguchi, S., and Choudhry, N. K. (2011). Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin. J. Am. Soc. Nephrol. CJASN 6 (11), 2662–2668. doi:10.2215/CJN.04550511
Yao, X., Abraham, N. S., Sangaralingham, L. R., Bellolio, M. F., McBane, R. D., Shah, N. D., et al. (2016). Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation. J. Am. Heart Assoc. 5 (6), e003725. doi:10.1161/JAHA.116.003725
Yodogawa, K., Mii, A., Fukui, M., Iwasaki, Y. K., Hayashi, M., Kaneko, T., et al. (2016). Warfarin use and incidence of stroke in Japanese hemodialysis patients with atrial fibrillation. Heart vessels 31 (10), 1676–1680. doi:10.1007/s00380-015-0777-7
Keywords: end-stage renal disease, dialysis, atrial fibrillation, direct oral anticoagulants, warfarin, network meta-analysis
Citation: Shen X-F, Zhang C, Hu J, Zhang T and Ma B (2023) Anticoagulant drugs for patients with atrial fibrillation on dialysis: a systematic analysis and network meta-analysis. Front. Pharmacol. 14:1320939. doi: 10.3389/fphar.2023.1320939
Received: 13 October 2023; Accepted: 27 November 2023;
Published: 15 December 2023.
Edited by:
Danilo Menichelli, Sapienza University of Rome, ItalyReviewed by:
Arianna Pannunzio, Sapienza University of Rome, ItalyZhiyan Liu, First Hospital, Peking University, China
Copyright © 2023 Shen, Zhang, Hu, Zhang and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Ma, a2l0dHltYjIwMTdAMTYzLmNvbQ==
 Xian-Feng Shen
Xian-Feng Shen Chao Zhang
Chao Zhang Jun Hu2
Jun Hu2 Bin Ma
Bin Ma