- Department of Clinical Pharmacy, School of Pharmacy, Fudan University, Shanghai, China
Background: As an antidiabetic agent, sotagliflozin was recently approved for heart failure (HF). However, its cardiovascular benefits in type 2 diabetic mellitus (T2DM) patients with HF or cardiovascular (CV) risk factors have not been systematically evaluated. The aim of this study is to evaluate the cardiovascular benefits and safety of sotagliflozin in T2DM patients with HF or CV risk factors using Bayesian network meta-analysis.
Methods: Data were retrieved from PubMed, Embase, Web of Science, ClinicalTrials.gov, and Cochrane Library from their inception to 16 August 2023. Randomized controlled trials (RCTs) comparing sotagliflozin with a placebo, dapagliflozin, and empagliflozin in adult T2DM patients with HF or CV risks for at least 12 weeks were included in the study. Data analysis was conducted using R 4.2.3 and Stata 17.0. Cardiovascular efficacy outcomes included HF events (hospitalization or urgent visits for HF), MACE (deaths from CV causes, hospitalizations for HF, nonfatal myocardial infarctions, and strokes), cardiovascular death, the decrease in SBP, and weight loss. Safety outcomes are urinary tract infection, diarrhea, and diabetic ketoacidosis.
Results: Eleven studies with 30,952 patients were included. Compared to dapagliflozin and empagliflozin, 200 mg of sotagliflozin showed the best effect in reducing HF events [OR (95% CI), 0.79 (0.66, 0.94) and 0.90 (0.63, 1.27)]. Compared to dapagliflozin, 200 mg of sotagliflozin [OR (95% CI), 0.76 (0.66, 0.87)] was superior in preventing MACE. Compared to empagliflozin, 200 mg of sotagliflozin [OR (95% CI), 1.46 (1.04, 2.05)] was inferior in preventing CV death. Sotagliflozin showed a poorer SBP decreasing effect than empagliflozin and dapagliflozin [MD (95% CI), 1.30 (0.03, 2.56) and 2.25 (0.35, 4.14), respectively]. There was no significant difference between sotagliflozin and other interventions in weight loss. Sotagliflozin exhibited no increased risk for diabetic ketoacidosis or urinary tract infection among all interventions, however, it showed a mild risk for diarrhea than placebo [OR (95% CI), 1.47 (1.28, 1.69)].
Conclusion: Sotagliflozin displayed moderate CV benefits and acceptable safety. Sotagliflozin can be one of the recommended options for T2DM patients with HF or CV risk factors, which will be important for evidence-based use of sotagliflozin as well as decision-making of T2DM medication.
1 Introduction
As a chronic, irreversible metabolic disease, the prevalence of diabetes has increased sharply (from 4.6% to 9.8%) in the world in 20 years (International Diabetes Federation, 2021). Type 2 diabetes mellitus (T2DM) accounts for about 90% of diabetes (Ahmad et al., 2022). T2DM usually accompanies cardiovascular disease (CVD) such as heart failure (HF), and CVD is the leading cause of morbidity and mortality in T2DM (Joseph et al., 2022). Therefore, one of the main management goals of T2DM is to reduce the risk of cardiovascular (CV) adverse events by using antidiabetic agents (American Diabetes Association, 2021), e.g., sodium-glucose cotransporter 2 inhibitor (SGLT2i).
As a new class of antidiabetic agents, SGLT2i can reduce blood glucose concentration by inhibiting the reabsorption of sodium-glucose in proximal renal tubules and promoting urine sugar excretion. Multiple randomized controlled trials (RCTs) have confirmed that SGLT2i could exert a robust reduction of HF in T2DM adults and additional benefits of weight loss and blood pressure reduction (Leiter et al., 2014; Tikkanen et al., 2015; Wiviott et al., 2019). According to the 2021 American diabetes association (ADA) guidelines (American Diabetes Association, 2021), SGLT2i was recommended to treat T2DM patients with concurrent atherosclerotic cardiovascular disease (ASCVD) or its high-risk factors, HF, or chronic kidney disease. Two widely used SGLT2i, dapagliflozin and empagliflozin, have already been approved to treat HF with or without diabetes.
Sotagliflozin was the first dual SGLT1/2 inhibitor (SGLT1/2i) initially evaluated as an antidiabetic agent in 2019, and its adequate hypoglycemic effect was proved by a meta-analysis (Avgerinos et al., 2022). Subsequently, trials with CV outcomes consistently showed that it could reduce major CV events and hospitalization for HF (Bhatt et al., 2021a; Bhatt et al., 2021b; Cherney et al., 2021; 2023). Sotagliflozin was also approved to treat HF with or without diabetes by the U.S. Food and Drug Administration (FDA) in May 2023, making it a candidate for the recommended regimens for T2DM patients with HF or CV risks. In terms of safety, it was reported that sotagliflozin could increase the risk of ketoacidosis, urinary tract or genital tract infections, diarrhea, and volume depletion events (Musso et al., 2019; Zhou et al., 2022).
It is vital to evaluate the CV efficacy and safety of antidiabetic agents (e.g., sotagliflozin) in T2DM management and clinical drug selection using scientific methods including RCTs and network meta-analysis. However, there was only one RCT that had directly compared the CV benefits and safety of sotagliflozin and empagliflozin at present (Posch et al., 2022). The CV benefits of sotagliflozin in T2DM patients with HF or CV risks have not been systematically evaluated. When lacking direct comparisons, Bayesian network meta-analysis is an effective method to compare multiple treatments by combining direct and indirect evidence (Ahn and Kang, 2021). In this study, cardiovascular benefits and safety of sotagliflozin were evaluated by Bayesian network meta-analysis, which can provide relative rankings of sotagliflozin and other interventions, and will provide important evidence for rational use of sotagliflozin, empagliflozin, and dapagliflozin as well as drug-selection in T2DM patients with HF or CV risks.
2 Materials and methods
This study met the requirement of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension for reporting network meta-analysis (Hutton et al., 2015).
2.1 Search strategy
Five databases (PubMed, Embase, Web of Science, ClinicalTrials.gov, and Cochrane Library were searched for RCTs of sotagliflozin in the treatment of T2DM patients, with a time limit from database creation to 16 August 2023. The databases were searched with the following MeSH (Medical Subject Headings) terms: (1) Sotagliflozin OR LX-4211 [Title] AND (2) Diabetes Mellitus, Type 2 [MeSH] AND (3) Heart Failure [MeSH] OR Cardiovascular Diseases [MeSH] (Supplementary Table S2).
2.2 Eligibility criteria
The eligibility criteria conformed to the PICOS: (1) Population: adult T2DM patients with HF (with or without reduced LVEF) or existing CV risk factors (presence of ASCVD, dyslipidemia, hypertension, obesity, and so on); (2) Intervention: sotagliflozin (orally once-daily of 200 mg and 400 mg); (3) Comparison: SGLT2i (orally once-daily of dapagliflozin 10 mg, orally once-daily of empagliflozin 10 mg and 25 mg); (4) Outcomes: ①HF-events (hospitalizations or urgent visits for HF); ②major adverse cardiovascular events (MACE, including deaths from CV causes, hospitalizations for HF, nonfatal myocardial infarctions, and strokes) ③CV-death; ④decrease in systolic blood pressure (SBP); ⑤body weight (BW) loss; ⑥adverse events, i.e., urinary tract infection (UTI), diarrhea, and diabetic ketoacidosis (DKA); (S) Study type: RCTs. Secondary analyses and phase I studies were excluded. Additionally, hypoglycemic indicators were not evaluated for insufficient data posted by relevant RCTs.
2.3 Data extraction and risk of bias assessment
Data was recorded under the following headings: (1) study profile (title, first author, publication year, study design); (2) baseline characteristics (age, sex, BW, SBP, disease, and so on); (3) predefined outcomes. The risk of bias was assessed with the Cochrane risk of bias tool, including random sequence generation, allocation concealment, performance and detection bias, incomplete outcome data, and selective reporting. Studies were included after a blinded review by two independent reviewers (i.e., Li and Zhu), and referral to a third independent reviewer (i.e., Liang) in case of disagreement.
2.4 Heterogeneity, model fit, and inconsistency assessment
Cochran’s I2 test was used to assess heterogeneity. When I2 <50%, the heterogeneity is not obvious, and fixed-effect models should be applied. Model fit was assessed through the deviance information criterion (DIC), and there is no evidence of inconsistency if the difference between the inconsistency and consistency models is under 5, thus the simpler model with a smaller DIC should be used (Spiegelhalter et al., 2002; Daly et al., 2022). Node-splitting approaches are needed when loops are formed by two or more studies. Markov Chain Monte Carlo algorithm was used for each outcome after 100,000 simulation iterations, 50,000 adaptation iterations, and a thinning interval of 1.
2.5 Data synthesis and analysis
A network meta-analysis was performed with a Bayesian approach using R version 4.2.3 with the GEMTC, JAGS, and MULTINMA packages, and Stata 17.0. Pooled odds ratios (OR) with 95% CIs for dichotomous outcomes and pooled mean differences (MD) with 95% CIs for continuous outcomes were calculated and shown in league tables. The surface under the cumulative ranking curve (SUCRA) was also calculated to rank the effectiveness and safety of each treatment, and a higher SUCRA value indicates a better or safer treatment.
2.6 Publication bias assessment
Publication bias is a common phenomenon in clinical literature in which positive results have a better chance of being published (Dubben and Beck-Bornholdt, 2005), and it is thus caused by unpublished or unnoticed studies. Analysis of publication bias was carried out using Stata 17.0. The comparison-adjusted funnel plots for assessment of publication bias were recommended for network meta-analysis (Higgins et al., 2021). The visually symmetric distribution of scattered dots about the combined effect size line (the central vertical line of the funnel) indicates that the publication bias was not significant.
3 Results
3.1 Study selection and characteristics
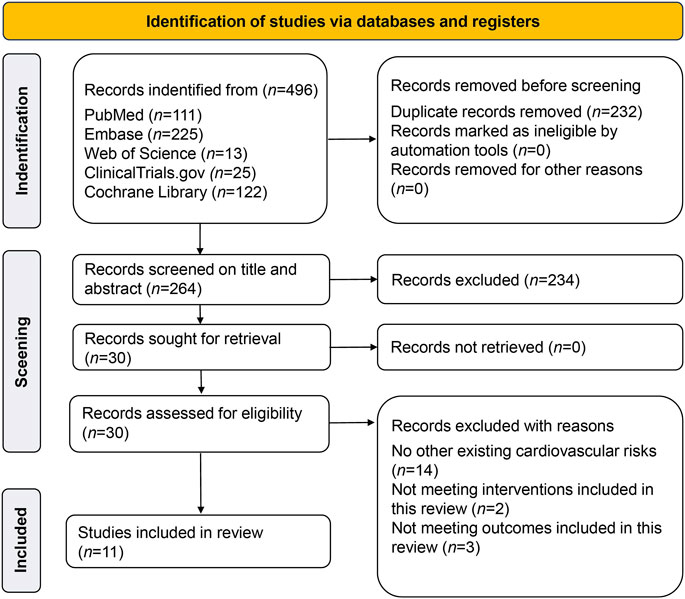
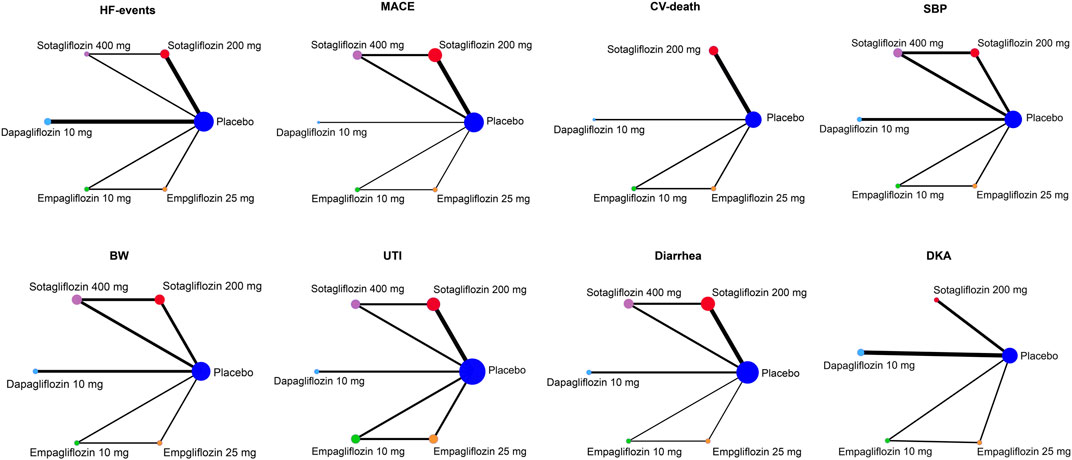
A total of 496 studies were retrieved; 232 duplicates were screened out, 224 studies were excluded after reading the titles and abstracts, and 19 studies were excluded for not meeting inclusion criteria; this left 11 RCTs with 30,952 participants which were finally included (Leiter et al., 2014; Tikkanen et al., 2015; Zinman et al., 2015; McMurray et al., 2019; Phrommintikul et al., 2019; Wiviott et al., 2019; Bhatt et al., 2021a; Bhatt et al., 2021b; Cherney et al., 2021; 2023; Solomon et al., 2022). The PRISMA flow chart is shown in Figure 1. The weighted means of age, BMI, and baseline SBP were 66.2 years, 30.3 kg/m2, 132.1 mmHg, respectively, and the detailed study characteristics were listed in Supplementary Table S1. The network plots of each outcome are illustrated in Figure 2.
3.2 Risk of bias assessment
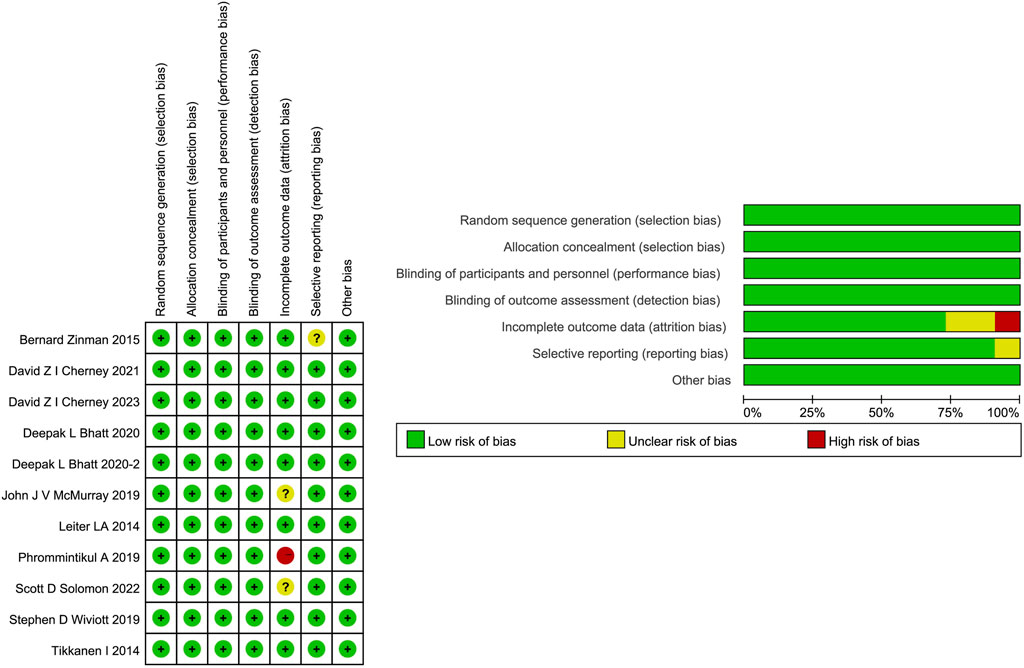
All 11 studies were identified as at low risks of selection and performance bias. However, one study (Phrommintikul et al., 2019) was deemed as having a high risk for incomplete outcome data because no drug safety outcomes were reported. Two studies (McMurray et al., 2019; Solomon et al., 2022) are rated as unclear risks for possibly incomplete safety results. One study (Zinman et al., 2015) was evaluated as at unclear risk for reporting bias due to incomplete baseline information. The detailed risk of bias assessment is shown in Figure 3. Overall, quality was appraised as being of a moderate level.
3.3 Cardiovascular Efficacy outcomes
3.3.1 HF-events
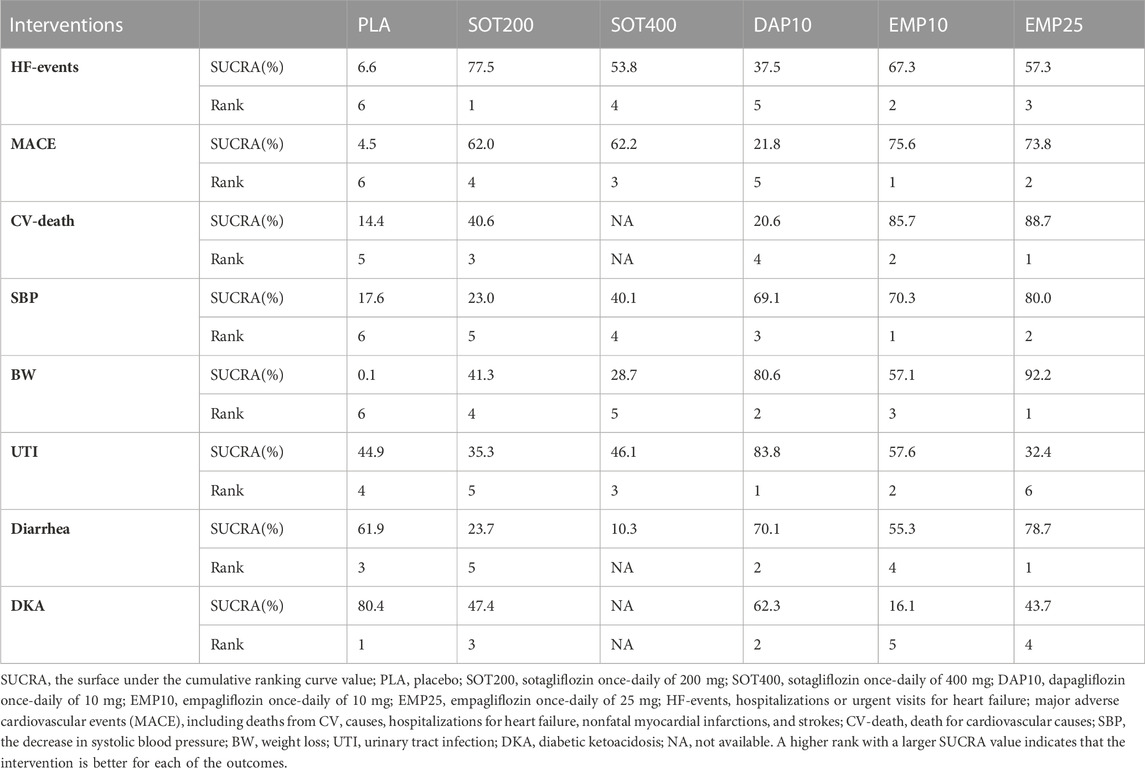
As shown in Figure 4A, 200 mg of sotagliflozin showed superior effect in reducing hospitalization or urgent visits for HF than 10 mg of dapagliflozin (OR, [95% CI], 0.79 [0.66, 0.94]), and showed no significant difference compared with 10 mg and 25 mg of empagliflozin (OR, [95% CI], 0.96 [0.67, 1.37], 0.90 [0.63, 1.27], respectively). There was no significant dose-response relationship between 200 mg and 400 mg of sotagliflozin (OR, [95% CI], 1.01 [0.20, 7.90]). The results of SUCRA (Figure 5A; Table 1) indicated that 200 mg of sotagliflozin exhibited the best effect in reducing hospitalization or urgent visits for HF (77.5%), followed by 10 mg of empagliflozin, 25 mg of empagliflozin, 400 mg of sotagliflozin and 10 mg of dapagliflozin (67.3%, 57.3%, 53.8%, and 37.5%, respectively).
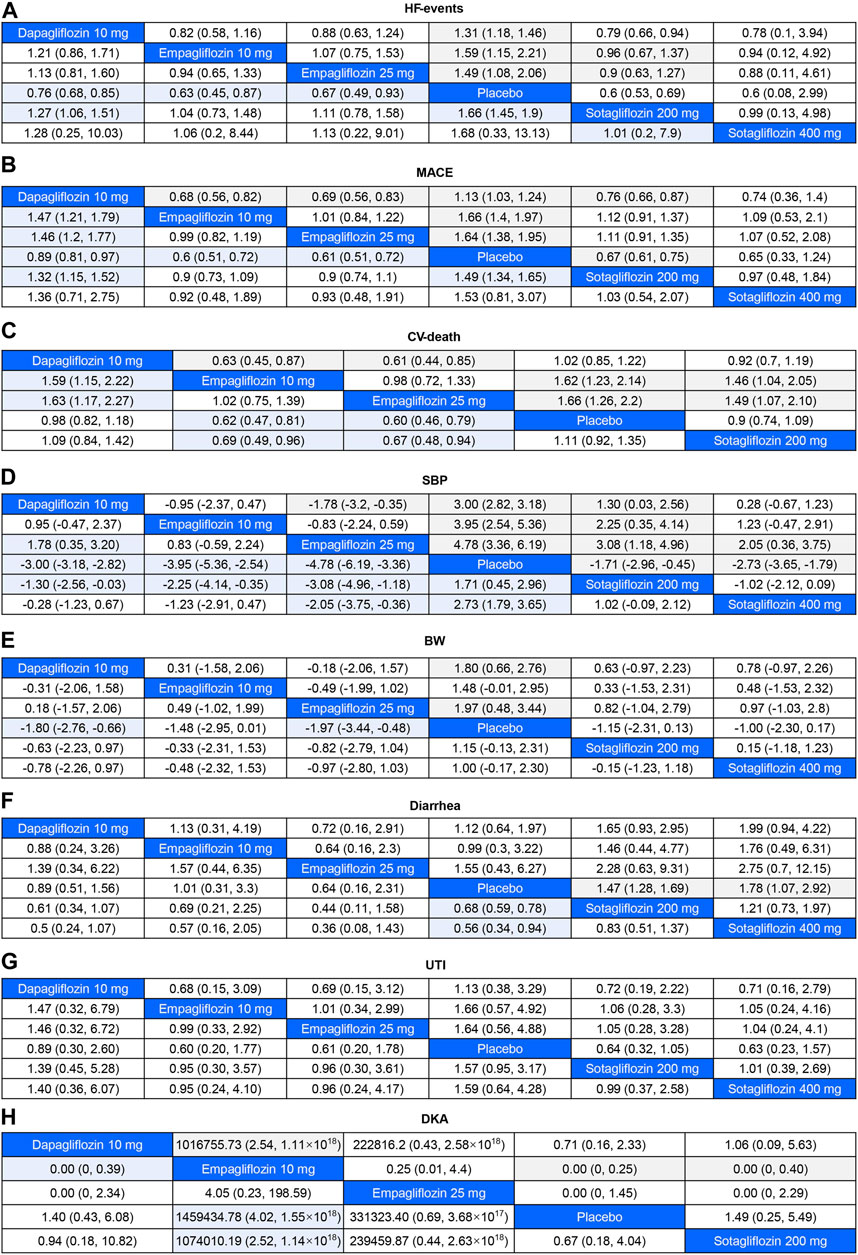
FIGURE 4. League Table for Eight Outcome Measures The colored cells indicated that the results were significant. For odds ratios (OR) in each cell, OR<1 favors column-defining treatment. For mean difference (MD), MD < 0 favors column-defining treatment. HF-events, hospitalizations or urgent visits for heart failure; major adverse cardiovascular events (MACE), including deaths from CV causes, hospitalizations for heart failure, nonfatal myocardial infarctions, and strokes; CV-death, death for cardiovascular causes; SBP, the decrease in systolic blood pressure; BW, body weight loss; UTI, urinary tract infection; DKA, diabetic ketoacidosis. (A), HF-events; (B), MACE; (C), CV-death; (D), SBP; (E), BW; (F), Diarrhea; (G), UTI; (H), DKA.
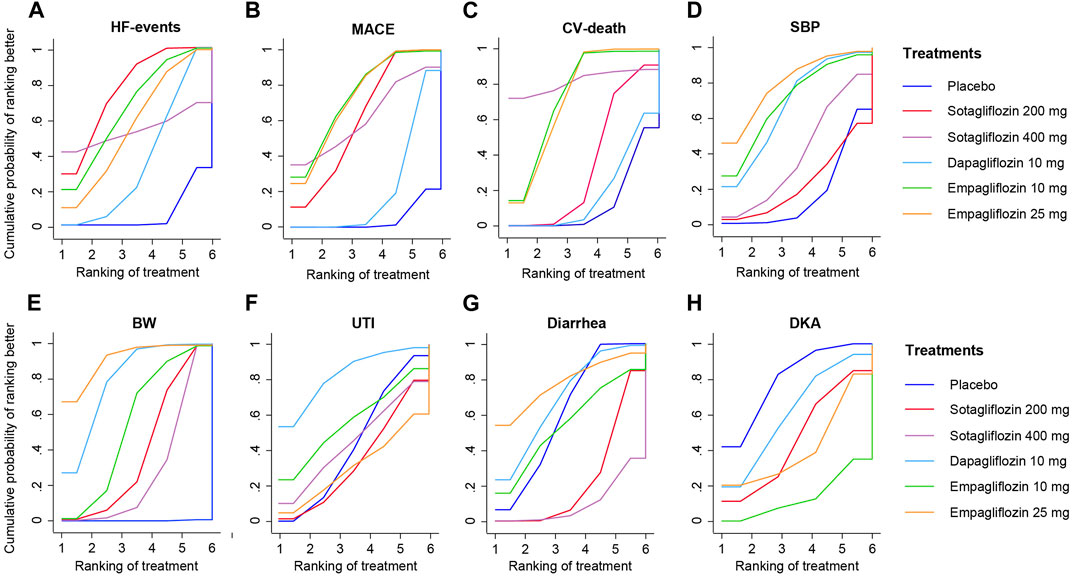
FIGURE 5. SUCRA plot for Eight Outcome Measures. (A), HF-events; (B), MACE; (C), CV-death; (D), SBP; (E), BW; (F), Diarrhea; (G), UTI; (H), DKA.
3.3.2 MACE
As shown in Figure 4B mg of sotagliflozin showed better effect in reducing all MACEs than 10 mg of dapagliflozin (OR, [95% CI], 0.76 [0.66, 0.87], and showed no significantly poorer effect than 10 mg and 25 mg of empagliflozin (OR, [95% CI], 1.12 [0.91, 1.37], 1.11 [0.91, 1.35], respectively). The results of SUCRA (Figure 5B; Table 1) indicated that 10 mg and 25 mg of empagliflozin exhibited superior effects in reducing MACEs (75.6% and 73.8%, respectively), followed by 400 mg, 200 mg of sotagliflozin, and 10 mg of dapagliflozin (62.2%, 62.0%, and 21.8%, respectively).
3.3.3 CV-death
Sotagliflozin showed a poorer effect than 10 mg and 25 mg of empagliflozin (OR, [95% CI], 1.46 [1.04, 2.05], 1.49 [1.07, 2.1], respectively) (Figure 4C) in reducing CV-death. Although the SUCRA (Figure 5C; Table 1) showed that, 200 mg of sotagliflozin (40.6%) ranked higher than 10 mg of dapagliflozin (20.6%) in reducing CV-death, there was no significant difference (OR, [95% CI], 0.92 [0.70, 1.19]). The results of 400 mg of sotagliflozin were not evaluated for heterogeneity.
3.3.4 Blood pressure control
Compared with placebo, 200 mg and 400 mg of sotagliflozin showed significant SBP reduction (OR, [95% CI], −1.71 [-2.96, −0.45], −2.73 [-3.65, −1.79]) (Figure 4D), but they showed the poorest efficacy among all the included interventions in SUCRA (23.0%, 40.1%) (Figure 5D; Table 1). 400 mg of sotagliflozin failed to outperform 10 mg of dapagliflozin and 10 mg of empagliflozin in SBP reduction (OR, [95% CI], 0.28 [-0.67, 1.23], 1.23 [-0.47, 2.91], respectively).
3.3.5 Body weight loss
Compared with other interventions, sotagliflozin did not show a significant body weight loss effect (Figure 4E). The results of SUCRA (Figure 5E; Table 1) indicated that 25 mg of empagliflozin exhibited the best effect in body weight loss (92.2%), followed by 10 mg of dapagliflozin, 10 mg of empagliflozin, 200 mg and 400 mg of sotagliflozin (80.6%, 57.1%, 41.3%, 28.7%, respectively).
3.4 Safety outcomes
As shown in Figure 4F, all the included interventions showed no significantly higher risk for UTI. Compared with placebo, 200 mg and 400 mg of sotagliflozin increased the incidence of diarrhea (OR, [95% CI], 1.47 [1.28, 1.69], 1.78 [1.07, 2.92], respectively), but the other interventions exhibited no significant risk for diarrhea (Figure 4G). Compared with placebo, sotagliflozin was not associated with significant risks of DKA (OR, [95% CI], 1.49 [0.25, 5.49]), and the other interventions showed similar outcomes (Figure 4H). The SUCRA values of safety outcomes (Table 1) indicated that 10 mg of dapagliflozin (83.8%) showed the lowest risks while 25 mg of empagliflozin (32.4%) showed the highest risks in UTI; 10 mg of dapagliflozin (62.3%) showed the lowest risks while 10 mg of empagliflozin (16.1%) showed the highest risks in DKA; and 25 mg of dapagliflozin (78.7%) showed the lowest risks while 400 mg of sotagliflozin (10.3%) showed the highest risks in diarrhea.
3.5 Inconsistency and heterogeneity tests
The difference values of DIC between inconsistency and consistency models in all eight outcomes were less than five (Supplementary Table S3), thus consistency models were applied. For most comparisons, the heterogeneity was not significant. Sensitivity analysis was conducted, and the I2 value of the comparison between placebo and sotagliflozin (200 mg) in CV-death decreased from 86.7% to 0% after eliminating one study (Bhatt et al., 2021a). However, for the following comparisons, heterogeneity could not be decreased because there were only two studies included for each comparison, i.e., placebo versus dapagliflozin in SBP, BW, and UTI; placebo versus 400 mg of sotagliflozin in UTI and DKA, and 200 mg sotagliflozin versus 400 mg of sotagliflozin in SBP (Supplementary Table S4). Therefore, a random-effect model was used.
3.6 Publication bias
The comparison-adjusted funnel plots (Figure 6) suggested that publication bias may exist in CV-death and SBP in the analysis. However, there were fewer than ten studies included in each of the outcomes, so publication bias assessment using funnel plots might not be reliable (Ioannidis and Trikalinos, 2007; Sterne et al., 2011; Dalton et al., 2016).
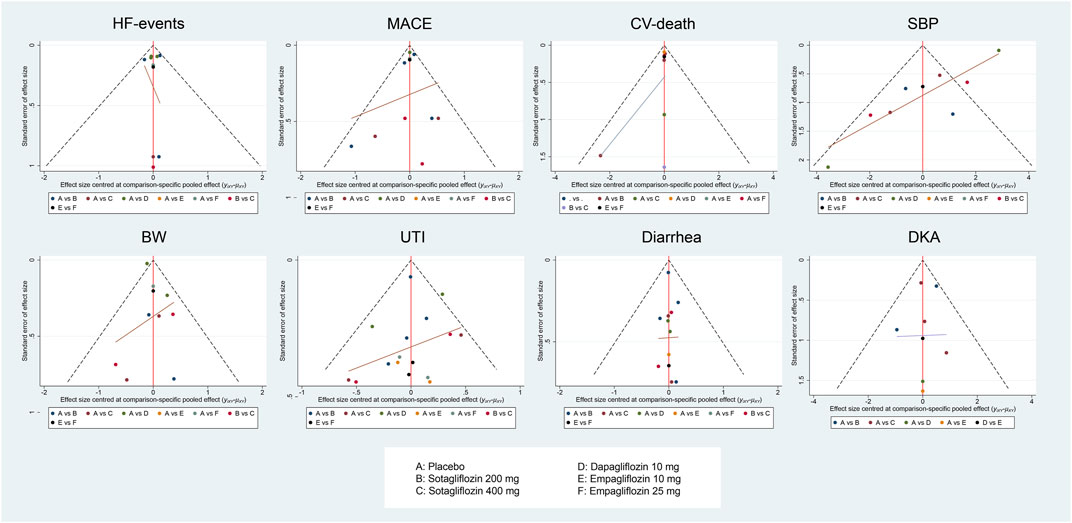
FIGURE 6. Figure 4A, HF-events; Figure 4B, MACE; Figure 4C, CV-death; Figure 4D, SBP; Figure 4E, BW; Figure 4F, Diarrhea; Figure 4G, UTI; Figure 4H, DKA. Comparison-adjusted funnel plot for eight outcomes.
4 Discussion
In the present network meta-analysis, CV benefits and safety of sotagliflozin, as well as dapagliflozin and empagliflozin, were evaluated in T2DM with HF or CV risks. Overall, sotagliflozin, dapagliflozin, and empagliflozin displayed differentiated CV benefits and acceptable safety. Among them, sotagliflozin displayed the best efficacy in avoiding HF hospitalization or urgent visits, moderate effect in reducing MACEs including nonfatal myocardial infarction, strokes, etc., and no superior effect over other interventions in preventing CV-death. A dose-response relationship was not obvious in 200 mg and 400 mg of sotagliflozin, nor as in 10 mg and 25 mg of empagliflozin in terms of CV benefits. Additionally, empagliflozin displayed the best effect in reducing MACE and CV-death, however, only two eligible studies on empagliflozin were included (Tikkanen et al., 2015; Zinman et al., 2015), and further studies are needed to prove its superiority.
Sotagliflozin, dapagliflozin, and empagliflozin were reported to have risks of DKA, UTI, and genital tract infections (Vasilakou et al., 2013; Rosenstock and Ferrannini, 2015; Rieg and Vallon, 2018), however, this study found that there was no significant risk of DKA or UTI among all the included interventions. Generally speaking, dapagliflozin exhibited the best safety, followed by sotagliflozin and empagliflozin according to the SUCRA values. Additionally, only sotagliflozin showed mild risks for diarrhea among all treatments, which was consistent with a previous meta-analysis (Avgerinos et al., 2022). The sotagliflozin-induced diarrhea may be due to its inhibition of SGLT1 (Tsimihodimos et al., 2018), which was associated with the osmotic diarrhea caused by the non-absorbed excess of glucose and galactose in the intestinal lumen (Song et al., 2016).
There was only one published frequentist network meta-analysis of SGLT1/2i versus SGLT2i (Teo et al., 2022). This study conducted a thorough comparison of a variety of SGLT1/2i and SGLT2i with multiple outcomes (i.e., CV, metabolic, and renal indicators) in type 1 and type 2 diabetic patients with or without HF or CV risks. Nevertheless, diabetes without CV risks is not the main indication of SGLT2i or SGLT1/2i according to the ADA guidelines. Consequently, our study refined the inclusion criteria to better meet the guidelines. Furthermore, a frequentist network meta-analysis is generally considered less promising than a Bayesian network meta-analysis in evaluating performance measures in sparse networks (Seide et al., 2020). Therefore, the Bayesian approach was selected in our study.
5 Limitation
In the present study, we evaluated the cardiovascular benefits and safety of sotagliflozin in T2DM patients with HF or CV risk factors using outcome measures, i.e., HF events, MACE, CV death, decrease in SBP and BW, but did not provide any hypoglycemic outcome measures, e.g., glycated hemoglobin (HbA1c). The reason was that only one eligible RCT (Cherney et al., 2021) on sotagliflozin in treating T2DM with HF or CV risk factors had provided any hypoglycemic outcomes. Moreover, the hypoglycemic effect of sotagliflozin has been assessed in previous meta-analyses (Teo et al., 2022) and RCTs (Lexicon Pharmaceuticals, 2021a; Lexicon Pharmaceuticals, 2021b).
HF events are considered as a strong HF and CV prognostic factor, and occurred in 61.3% of HF patients (Chun et al., 2012). Blood pressure control is considered an important factor in HF and CV management (Lee et al., 2022). Thus, both HF events and SBP were included in this study. Nevertheless, HF events do not cover all the prognostic outcome measures of HF, other outcome measures (e.g., B-type natriuretic peptide (BNP), N-terminal pro-BNP (NT-proBNP) level, estimated glomerular filtration rate (eGFR), and ejection fractions) should be included. However, only one study on sotagliflozin (Cherney et al., 2021) reported other HF prognostic outcome measures (i.e., eGFR). Consequently, other prognostic outcomes of HF were not included in the network meta-analysis.
Among all the eligible studies, there were only four publications that reported on sotagliflozin, five studies on dapagliflozin, and two studies on empagliflozin in treating T2DM patients with HF or CV risks, indicating more RCTs on this population need to be conducted so as to reach a solid conclusion. Moreover, a strict search, data selection, and sensitivity analysis were conducted, however, heterogeneity in SBP, BW, UTI, and DKA, and publication bias of CV-death and SBP were not avoided.
Duration of diabetes plays an important role in evaluating CV risks in T2DM patients. An approximately 20% increased risk was associated with every five-year increment in the duration of diabetes (de Jong et al., 2022). Therefore, if the patients without HF or CV risks had been diagnosed with diabetes for long enough, they also met the inclusion criteria. Sub-group analysis on the duration of diabetes could be conducted if only the RCTs had provided more detailed baseline characteristics. More head-to-head RCTs targeting this high-CV risk diabetic population with details on the duration of diabetes need to be conducted so as to provide more reliable evidence on the CV benefits and safety of sotagliflozin. T2DM easily results in diabetic nephropathy, and diabetic nephropathy further increases the risk of HF and ischemic events (Udell et al., 2015). Consequently, outcomes regarding renal functions should also be integrated into RCT designed for T2DM patients with CV risks.
6 Conclusion
Sotagliflozin is optional in treating T2DM patients with HF or CV risk factors and displayed moderate CV benefits and acceptable safety. Overall, empagliflozin and sotagliflozin were superior to dapagliflozin in CV benefits, while dapagliflozin was superior to empagliflozin and sotagliflozin in safety.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
JyL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Visualization, Writing–original draft, Writing–review and editing. CZ: Writing–review and editing, Data curation, Formal Analysis, Investigation. JnL: Data curation, Formal Analysis, Investigation, Writing–review and editing. JH: Data curation, Formal Analysis, Investigation, Writing–review and editing. HL: Writing–review and editing. ZW: Writing–review and editing. RG: Writing–review and editing. JC: Writing–review and editing. SY: Writing–review and editing. LL: Writing–review and editing. FM: Writing–review and editing. GM: Writing–review and editing, Conceptualization, Formal Analysis, Funding acquisition, Project administration, Resources, Supervision, Validation.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (Nos. 82374133, 82074109, 81873078, and 81374051).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1303694/full#supplementary-material
Abbreviations
T2DM, type 2 diabetic mellitus; RCT, randomized controlled trial; SGLT, sodium-glucose co-transporters; SGLT2i, sodium-glucose co-transporters 2 inhibitors; HF, heart failure; CV, cardiovascular; PLA, Placebo; SOT200, once-daily of 200 mg sotagliflozin; SOT400, once-daily of 400 mg sotagliflozin; EMP10, once-daily of 10 mg empagliflozin; EMP25, once-daily of 25 mg empagliflozin; DAP10, once-daily of 10 mg dapagliflozin; HF-events, hospitalization or urgent visit for heart failure; MACE, major adverse cardiovascular events, including deaths from CV causes, hospitalizations for HF, nonfatal myocardial infarctions, and strokes; CV-death, death from cardiovascular causes; SBP, decrease in systolic blood pressure (mm/Hg); BW, decrease in body weight (kg); UTI, urinary tract infection; DKA, incidents of diabetic ketoacidosis; NA, not available.
References
Ahmad, E., Lim, S., Lamptey, R., Webb, D. R., and Davies, M. J. (2022). Type 2 diabetes. Lancet 400 (10365), 1803–1820. doi:10.1016/S0140-6736(22)01655-5
Ahn, E., and Kang, H. (2021). Concepts and emerging issues of network meta-analysis. Korean J. Anesthesiol. 74 (5), 371–382. doi:10.4097/kja.21358
American Diabetes Association (2021). 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2021. Diabetes care 44 (Suppl. ment_1), S111–S124. doi:10.2337/dc21-S009
Avgerinos, I., Karagiannis, T., Kakotrichi, P., Michailidis, T., Liakos, A., Matthews, D. R., et al. (2022). Sotagliflozin for patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes, Obes. Metabolism 24 (1), 106–114. doi:10.1111/dom.14555
Bhatt, D. L., Szarek, M., Pitt, B., Cannon, C. P., Leiter, L. A., McGuire, D. K., et al. (2021a). Sotagliflozin in patients with diabetes and chronic kidney disease. N. Engl. J. Med. 384 (2), 129–139. doi:10.1056/NEJMoa2030186
Bhatt, D. L., Szarek, M., Steg, P. G., Cannon, C. P., Leiter, L. A., McGuire, D. K., et al. (2021b). Sotagliflozin in patients with diabetes and recent worsening heart failure. N. Engl. J. Med. 384 (2), 117–128. doi:10.1056/NEJMoa2030183
Cherney, D. Z., Ferrannini, E., Umpierrez, G. E., Peters, A. L., Rosenstock, J., Carroll, A. K., et al. (2021). Efficacy and safety of sotagliflozin in patients with type 2 diabetes and severe renal impairment. Diabetes, Obes. Metabolism 23 (12), 2632–2642. doi:10.1111/dom.14513
Cherney, D. Z., Ferrannini, E., Umpierrez, G. E., Peters, A. L., Rosenstock, J., Powell, D. R., et al. (2023). Efficacy and safety of sotagliflozin in patients with type 2 diabetes and stage 3 chronic kidney disease. Diabetes, Obes. Metabolism 25, 1646–1657. doi:10.1111/dom.15019
Chun, S., Tu, J. V., Wijeysundera, H. C., Austin, P. C., Wang, X., Levy, D., et al. (2012). Lifetime analysis of hospitalizations and survival of patients newly admitted with heart failure. Circ. Heart Fail. 5 (4), 414–421. doi:10.1161/CIRCHEARTFAILURE.111.964791
Dalton, J. E., Bolen, S. D., and Mascha, E. J. (2016). Publication bias: the elephant in the review. Anesth. analgesia 123 (4), 812–813. doi:10.1213/ANE.0000000000001596
Daly, C., Downing, B. C., and Welton, N. J. (2022). A practical guide to inconsistency checks in Bayesian network meta-analysis.
de Jong, M., Woodward, M., and Peters, S. A. (2022). Duration of diabetes and the risk of major cardiovascular events in women and men: a prospective cohort study of UK Biobank participants. Diabetes Res. Clin. Pract. 188, 109899. doi:10.1016/j.diabres.2022.109899
Dubben, H. H., and Beck-Bornholdt, H. P. (2005). Systematic review of publication bias in studies on publication bias. BMJ 331 (7514), 433–434. doi:10.1136/bmj.38478.497164.F7
Higgins, J. P. T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M. J., et al. (2021). Cochrane handbook for systematic reviews of interventions. version 6.2. Cochrane. (updated February 2021) Available from www.training.cochrane.org/handbook.
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern. Med. 162 (11), 777–784. doi:10.7326/M14-2385
International Diabetes Federation (2021). IDF diabetes atlas. Available from: https://diabetesatlas.org/ (Accesed August 2, 2023).
Ioannidis, J. P., and Trikalinos, T. A. (2007). The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. Cmaj 176 (8), 1091–1096. doi:10.1503/cmaj.060410
Joseph, J. J., Deedwania, P., Acharya, T., Aguilar, D., Bhatt, D. L., Chyun, D. A., et al. (2022). Comprehensive management of cardiovascular risk factors for adults with type 2 diabetes: a scientific statement from the American Heart Association. Circulation 145 (9), e722–e759. doi:10.1161/CIR.0000000000001040
Lee, M. H., Leda, M., Buchan, T., Malik, A., Rigobon, A., Liu, H., et al. (2022). Prognostic value of blood pressure in ambulatory heart failure: a meta-analysis and systematic review. Ambulatory blood pressure predicts heart failure prognosis. Heart Fail. Rev. 27 (2), 455–464. doi:10.1007/s10741-021-10086-w
Leiter, L. A., Cefalu, W. T., de Bruin, T. W., Gause Nilsson, I., Sugg, J., and Parikh, S. J. (2014). Dapagliflozin added to usual care in individuals with type 2 diabetes mellitus with preexisting cardiovascular disease: a 24 week, multicenter, randomized, double-blind, placebo-controlled study with a 28 week extension. J. Am. Geriatrics Soc. 62 (7), 1252–1262. doi:10.1111/jgs.12881
Lexicon Pharmaceuticals (2021a). Efficacy and safety of sotagliflozin versus placebo in patients with type 2 diabetes mellitus not currently treated with antidiabetic therapy, in ClinicalTrials.gov (Bethesda (MD): ational Library of Medicine US). 2016–2019. NLM Identifier: NCT02926937 Available from: https://clinicaltrials.gov/ct2/show/NCT02926937 (Accessed July 15, 2023).
Lexicon Pharmaceuticals (2021b). Efficacy and safety of sotagliflozin versus placebo in patients with type 2 diabetes mellitus on background of metformin, in ClinicalTrials.gov (Bethesda (MD): National Library of Medicine US). 2016–2019. NLM Identifier: NCT02926950. Available from: https://clinicaltrials.gov/ct2/show/NCT02926937 (Accessed July 15, 2023).
McMurray, J. J. V., Solomon, S. D., Inzucchi, S. E., Køber, L., Kosiborod, M. N., Martinez, F. A., et al. (2019). Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 381 (21), 1995–2008. doi:10.1056/NEJMoa1911303
Musso, G., Gambino, R., Cassader, M., and Paschetta, E. (2019). Efficacy and safety of dual SGLT 1/2 inhibitor sotagliflozin in type 1 diabetes: meta-analysis of randomised controlled trials. bmj 365, l1328. doi:10.1136/bmj.l1328
Phrommintikul, A., Wongcharoen, W., Kumfu, S., Jaiwongkam, T., Gunaparn, S., Chattipakorn, S., et al. (2019). Effects of dapagliflozin vs vildagliptin on cardiometabolic parameters in diabetic patients with coronary artery disease: a randomised study. Br. J. Clin. Pharmacol. 85 (6), 1337–1347. doi:10.1111/bcp.13903
Posch, M. G., Walther, N., Ferrannini, E., Powell, D. R., Banks, P., Wason, S., et al. (2022). Metabolic, intestinal, and cardiovascular effects of sotagliflozin compared with empagliflozin in patients with type 2 diabetes: a randomized, double-blind study. Diabetes care 45 (9), 2118–2126. doi:10.2337/dc21-2166
Rieg, T., and Vallon, V. (2018). Development of SGLT1 and SGLT2 inhibitors. Diabetologia 61 (10), 2079–2086. doi:10.1007/s00125-018-4654-7
Rosenstock, J., and Ferrannini, E. (2015). Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes care 38 (9), 1638–1642. doi:10.2337/dc15-1380
Seide, S. E., Jensen, K., and Kieser, M. (2020). A comparison of Bayesian and frequentist methods in random-effects network meta-analysis of binary data. Res. synthesis methods 11 (3), 363–378. doi:10.1002/jrsm.1397
Solomon, S. D., McMurray, J. J., Claggett, B., de Boer, R. A., DeMets, D., Hernandez, A. F., et al. (2022). Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N. Engl. J. Med. 387 (12), 1089–1098. doi:10.1056/NEJMoa2206286
Song, P., Onishi, A., Koepsell, H., and Vallon, V. (2016). Sodium glucose cotransporter SGLT1 as a therapeutic target in diabetes mellitus. Expert Opin. Ther. targets 20 (9), 1109–1125. doi:10.1517/14728222.2016.1168808
Spiegelhalter, D. J., Best, N. G., Carlin, B. P., and Van Der Linde, A. (2002). Bayesian measures of model complexity and fit. J. R. Stat. Soc. Ser. B Stat. Methodol. 64 (4), 583–639. doi:10.1111/1467-9868.00353
Sterne, J. A., Sutton, A. J., Ioannidis, J. P., Terrin, N., Jones, D. R., Lau, J., et al. (2011). Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Bmj 343, d4002. doi:10.1136/bmj.d4002
Teo, Y. N., Ting, A. Z., Teo, Y. H., Chong, E. Y., Tan, J. T. A., Syn, N. L., et al. (2022). Effects of sodium/glucose cotransporter 2 (SGLT2) inhibitors and combined SGLT1/2 inhibitors on cardiovascular, metabolic, renal, and safety outcomes in patients with diabetes: a network meta-analysis of 111 randomized controlled trials. Am. J. Cardiovasc. Drugs 22 (3), 299–323. doi:10.1007/s40256-022-00528-7
Tikkanen, I., Narko, K., Zeller, C., Green, A., Salsali, A., Broedl, U. C., et al. (2015). Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes care 38 (3), 420–428. doi:10.2337/dc14-1096
Tsimihodimos, V., Filippas-Ntekouan, S., and Elisaf, M. (2018). SGLT1 inhibition: pros and cons. Eur. J. Pharmacol. 838, 153–156. doi:10.1016/j.ejphar.2018.09.019
Udell, J. A., Bhatt, D. L., Braunwald, E., Cavender, M. A., Mosenzon, O., Steg, P. G., et al. (2015). Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes and moderate or severe renal impairment: observations from the SAVOR-TIMI 53 Trial. Diabetes care 38 (4), 696–705. doi:10.2337/dc14-1850
Vasilakou, D., Karagiannis, T., Athanasiadou, E., Mainou, M., Liakos, A., Bekiari, E., et al. (2013). Sodium–glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann. Intern. Med. 159 (4), 262–274. doi:10.7326/0003-4819-159-4-201308200-00007
Wiviott, S. D., Raz, I., Bonaca, M. P., Mosenzon, O., Kato, E. T., Cahn, A., et al. (2019). Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 380 (4), 347–357. doi:10.1056/NEJMoa1812389
Zhou, F., Du, N., Zhou, L., Wang, C., Ren, H., and Sun, Q. (2022). The safety of sotagliflozin in the therapy of diabetes mellitus type 1 and type 2: a meta-analysis of randomized trials. Front. Endocrinol. 13, 968478. doi:10.3389/fendo.2022.968478
Keywords: sotagliflozin, sodium-glucose co-transporters 2 inhibitor, type 2 diabetes mellitus, Bayesian network meta-analysis, heart failure, cardiovascular benefits, safety
Citation: Li J, Zhu C, Liang J, Hu J, Liu H, Wang Z, Guan R, Chow J, Yan S, Li L, Ma F and Ma G (2023) Cardiovascular benefits and safety of sotagliflozin in type 2 diabetes mellitus patients with heart failure or cardiovascular risk factors: a bayesian network meta-analysis. Front. Pharmacol. 14:1303694. doi: 10.3389/fphar.2023.1303694
Received: 28 September 2023; Accepted: 06 November 2023;
Published: 17 November 2023.
Edited by:
Linan Zeng, Sichuan University, ChinaCopyright © 2023 Li, Zhu, Liang, Hu, Liu, Wang, Guan, Chow, Yan, Li, Ma and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guo Ma, bWcwMzI4QGZ1ZGFuLmVkdS5jbg==, bWcwMzI4QDEyNi5jb20=
 Jiyifan Li
Jiyifan Li Jingru Liang
Jingru Liang Jiarong Hu
Jiarong Hu Ruifang Guan
Ruifang Guan Guo Ma
Guo Ma