
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 19 December 2023
Sec. Pharmacoepidemiology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1301120
This article is part of the Research TopicPharmacovigilance and Pharmacoepidemiology: Public Health and SafetyView all 17 articles
Background: Tenofovir and entecavir demonstrated substantial effectiveness in the reversion of fibrosis and reversed cirrhosis in patients with hepatitis B virus (HBV)-related cirrhosis. However, there has not been a definitive conclusion regarding the association between entecavir and tenofovir on the risk of cirrhosis-related complications. Therefore, this study aimed to investigate the comparative effectiveness between tenofovir and entecavir in HBV-related cirrhosis patients.
Methods: This was a retrospective study using Taiwan’s Health Insurance Research Database. We enrolled newly diagnosed HBV-related cirrhosis patients who initiated entecavir and tenofovir between 2011 and 2019. Treatment groups were determined by the initial HBV antiviral medication prescribed. The primary composite outcome was the development of hepatocellular carcinoma (HCC), death from any causes, and liver transplantation. The secondary outcomes included all the individual components of the primary outcome. The incidence rate was calculated for each outcome for both treatment groups using the Fine–Gray subdistribution hazard models. Propensity score adjustment was used to balance treatment groups.
Results: A total of 7,316 propensity score-matched treatment-naïve patients and 3,524 propensity score-matched treatment-experienced patients were included. Within treatment-naïve patients, those receiving tenofovir showed significantly lower hazards of developing the composite outcome (HR, 0.79; p < 0.0001), hepatocellular carcinoma (HR, 0.86; p = 0.027), mortality (HR, 0.75; p < 0.0001), and liver transplantation (HR, 0.70; p = 0.0189) than those receiving entecavir. As for treatment-experienced patients, tenofovir was associated with a significantly lower risk of the composite outcome (HR, 0.82; p = 0.0033) and hepatocellular carcinoma (HR, 0.60; p < 0.0001), but it did not show a significantly different risk of all-cause mortality (HR, 0.93; p = 0.3374) or liver transplantation (HR, 1.17; p = 0.5112) compared to entecavir.
Conclusion: Tenofovir presented a significantly lower incidence of cirrhosis-related complications than entecavir in patients with hepatitis B virus-related cirrhosis. However, no statistically significant difference in death and liver transplantation was seen in treatment-experienced patients.
Cirrhosis is the leading cause of hepatocellular carcinoma (HCC) and results in approximately 1.16–1.32 million annual deaths globally (GBD, 2017 Cirrhosis Collaborators, 2020). Cirrhosis due to hepatitis B virus infection, namely, hepatitis B virus (HBV)-related cirrhosis, is responsible for over 50% of cirrhosis-related deaths in Asian nations (Sarin et al., 2020). In patients with HBV-related cirrhosis, clinicians would administer HBV antiviral drugs to suppress viral replication, reduce viral load, and thereby prevent cirrhosis progression and even reverse cirrhosis (Marcellin and Asselah, 2013; Calvaruso and Craxì, 2014; Rockey, 2016; Udompap and Kim, 2020).
Among the available nucleos(t)ide analogs (NAs), entecavir (ETV) and tenofovir (TDF/TAF) are recommended as first-line treatments for HBV-related cirrhosis considering their high antiviral efficacy and low rates of resistance (Sarin et al., 2016; European Association for the Study of the Liver, 2017; Terrault et al., 2018). As shown by previous randomized controlled trials, TDF/TAF and ETV demonstrated substantial effectiveness in the reversion of fibrosis and reversed cirrhosis in patients with HBV-related cirrhosis (Schiff et al., 2008; Yokosuka et al., 2010; Schiff et al., 2011; Marcellin et al., 2013).
Previous studies have indicated that TDF/TAF or ETV use may result in different effects on cirrhosis-related outcomes. The reason was that TDF/TAF belongs to the class of acyclic nucleoside phosphonates (ANPs) (De Clercq and Holý, 2005), and its structure differs from that of nucleoside analogs such as ETV. ANPs are characterized by prolonged action (De Clercq and Holý, 2005) and may exhibit better anti-HCC (Sato et al., 2006; Abushahba et al., 2010; Murata and Mizokami, 2023; Yang et al., 2023) and anti-HBV (Murata et al., 2020) effects. However, real-world evidence and experimental research regarding the comparative effectiveness between TDF/TAF and ETV in cirrhosis patients showed conflicting results (Choi et al., 2019; Papatheodoridis et al., 2020; Lee et al., 2021). Therefore, there has not been a definitive conclusion regarding the association between ETV and TDF/TAF on the risk of cirrhosis-related complications. Furthermore, there was a lack of evidence regarding the comparative effectiveness between TDF/TAF and ETV in treatment-experienced cirrhosis patients.
Therefore, this study aimed to investigate the hazards of cirrhosis-related complications, including HCC and liver transplantation, and mortality in patients with HBV-related cirrhosis receiving ETV and TDF/TAF.
This retrospective cohort study was conducted using data from the National Health Insurance Research Database (NHIRD), which covered the healthcare data of approximately 100% of Taiwan’s population (National Health Insurance Administration, 2023a). The healthcare information in the database included that of diagnoses, treatments, operations, and prescription details. The study period was from 1 January 2010 to 31 December 2020. This study was approved by the Institutional Review Board (IRB) of Kaohsiung Medical University Hospital (IRB number: KMUHIRB-E(I)-20230042).
Our study population included newly diagnosed HBV-related cirrhosis patients (adults), who had initiated ETV and TDF/TAF between 2011 and 2019. HBV-related cirrhosis was defined as chronic hepatitis B (CHB) diagnosed with cirrhosis after the initial CHB diagnosis. At least one inpatient visit or three outpatient visits were required to determine the number of CHB patients and for cirrhosis diagnosis. Diagnostic codes from the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) were used to enroll HBV-related cirrhosis patients. The population entry date was defined as the date of the first diagnosis of cirrhosis. The baseline period was the time period within 1 year before the population entry date.
Patients who were below 20 years of age at the population entry date; had incomplete demographic information (including age, gender, or premium insurance); had a history of cirrhosis, liver transplantation, or HCC during the baseline period; or initiated ETV and TDF/TAF together were excluded from the study. Cirrhosis and liver transplantation were identified by the presence of ICD codes, while patients with HCC were defined by the presence of the ICD codes for HCC and inclusion in the Taiwan Cancer Registry long-form database (Kao et al., 2021).
Eligible patients were those with HBV-related cirrhosis who filled their first prescription for either ETV or TDF/TAF after the population entry date. Patients were divided into ETV or TDF/TAF groups based on the initial HBV antiviral medication prescribed after the population entry date. The index date was defined as the first day of receiving ETV or TDF/TAF following the population entry date. Follow-up began on the index date. Patients were stratified into the previously untreated (PUT) cohort and previously treated (PT) cohort (Supplementary eMethods 1) for the analysis.
One primary outcome was evaluated: the composite outcome of HCC, liver transplantation, and all-cause death. Secondary outcomes were individual components of the primary outcome. The detailed definition of each outcome event is shown in Supplementary eMethods 2. Patients who had experienced the outcome event before the index date were excluded from the corresponding outcome analyses. Patients were followed up from the index date to the occurrence of the corresponding outcome, switching antiviral treatment, or the end date of the database (31 December 2020), whichever came first. Patients with discontinuation were censored until they switched treatment or re-initialized treatment. Discontinuation was defined as a gap of more than 30 days between the end of a prescription and the next. In each outcome analysis, patients were not censored if other outcomes (except for the corresponding outcome) had occurred earlier.
Patients’ baseline characteristics and medical information were retrieved from the database. The demographic information including age and gender was obtained from the most recent insurance record prior to the population entry date. Comorbidities were defined as diseases diagnosed at least once in an inpatient or twice in an outpatient setting within 1 year before the population entry date. Detailed information on comorbidities is summarized in Supplementary eTable S1. The Charlson Comorbidity Index (CCI) was used to quantify the comorbidity status of the included patients (Charlson et al., 1987). Co-medications being regarded as confounders were collected (Hayward and Weersink, 2020), and medications prescribed for a minimum of 28 days within the year before the population entry date were co-medications. The disease progression period and treatment gap period were retrieved. The disease progression period was defined as the period between the first CHB diagnosis and the population entry date. The treatment gap period was defined as the period from the population entry date to the index date.
Two propensity score methods, namely, propensity score matching (PSM) and stabilized inverse probability of treatment weighting (IPTW), were used to generate comparable treatment groups before data analyses.
The PSM was performed using the 1:1 nearest-neighbor matching approach, with a caliper width set at 0.2 of the standard deviation of the logit of the propensity score (PS) (Austin, 2011a; Austin, 2011b). Confounders adjusted were age, gender, disease progression time, treatment gap duration, diabetes, hypertension, CCI, HCV/HDV/HEV co-infection, alcoholic cirrhosis, biliary cirrhosis, history of cirrhosis-related complications, and chronic kidney disease (CKD).
HBV-related cirrhosis patients were stratified into PUT patients and PT patients to obtain results. In the baseline analysis, descriptive statistics were stratified by groups. Continuous variables were presented as mean and standard deviation (SD). Categorical variables were represented using the number (N) and percentage (%). To assess the balance in each covariate, standardized mean difference (SMD) was employed, with a value below 0.1 indicating negligible differences between the groups (Austin, 2009a; Austin, 2009b).
Fine–Gray subdistribution hazard models, accounting for the competing risk events of death and liver transplantation, were used to investigate subdistribution HRs with a 95% confidence interval (CI) for each outcome analysis (except for the composite outcome and all-cause death analysis because no competing risk events existed). The proportional hazard assumptions were evaluated before analyses. We conducted sensitivity analyses to evaluate the robustness of our findings. We used the negative control outcome, myocardial infarction, to indirectly evaluate whether potential confounders existed (Lipsitch et al., 2010).
A statistically significant difference was defined as a two-tailed probability value less than 0.05. Data management and statistical analyses were processed with SAS software version 9.4.
The original study population contained 18,351 patients after applying inclusion and exclusion criteria. When PSM was used, 3,658 patients each were included in the ETV and TDF/TAF users in the PUT sub-cohort and 1,762 each were included in the ETV and TDF/TAF users in the PT sub-cohort. After applying stabilized IPTW, a weighted pseudopopulation consisted of 8,204 ETV users and 3,663 TDF/TAF users in the PUT sub-cohort and 4,717 ETV users and 1,764 TDF/TAF users in the PT sub-cohort. The enrollment process for the study population is illustrated in Figure 1. All patients in our study were included in the analysis of death outcome, and the baseline characteristics are presented in Table 1; Supplementary eTable S3. Overall, the mean age ranged from 54 to 57 years, and the majority were men (73%–77%). The mean disease progression period was 2.30–3.69 years. The baseline characteristics of patients for the analysis of the composite outcome, HCC, and liver transplantation are presented in Supplementary eTables S4–S9, respectively.
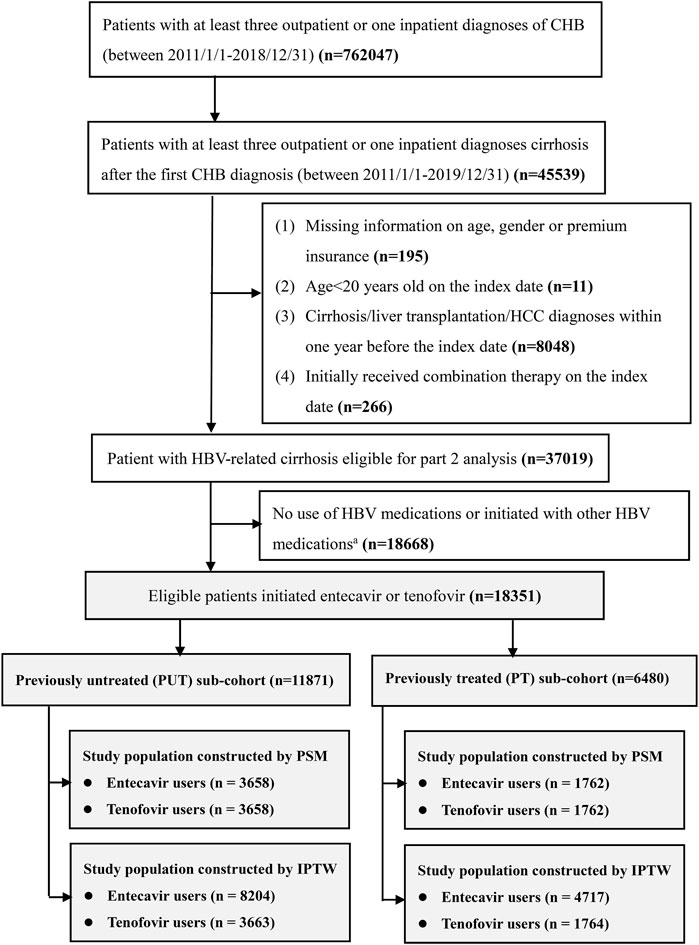
FIGURE 1. Flowchart of patients’ enrollment. CHB, chronic hepatitis B; HCC, hepatocellular carcinoma a; other HBV medications include lamivudine, telbivudine, adefovir, and interferon.
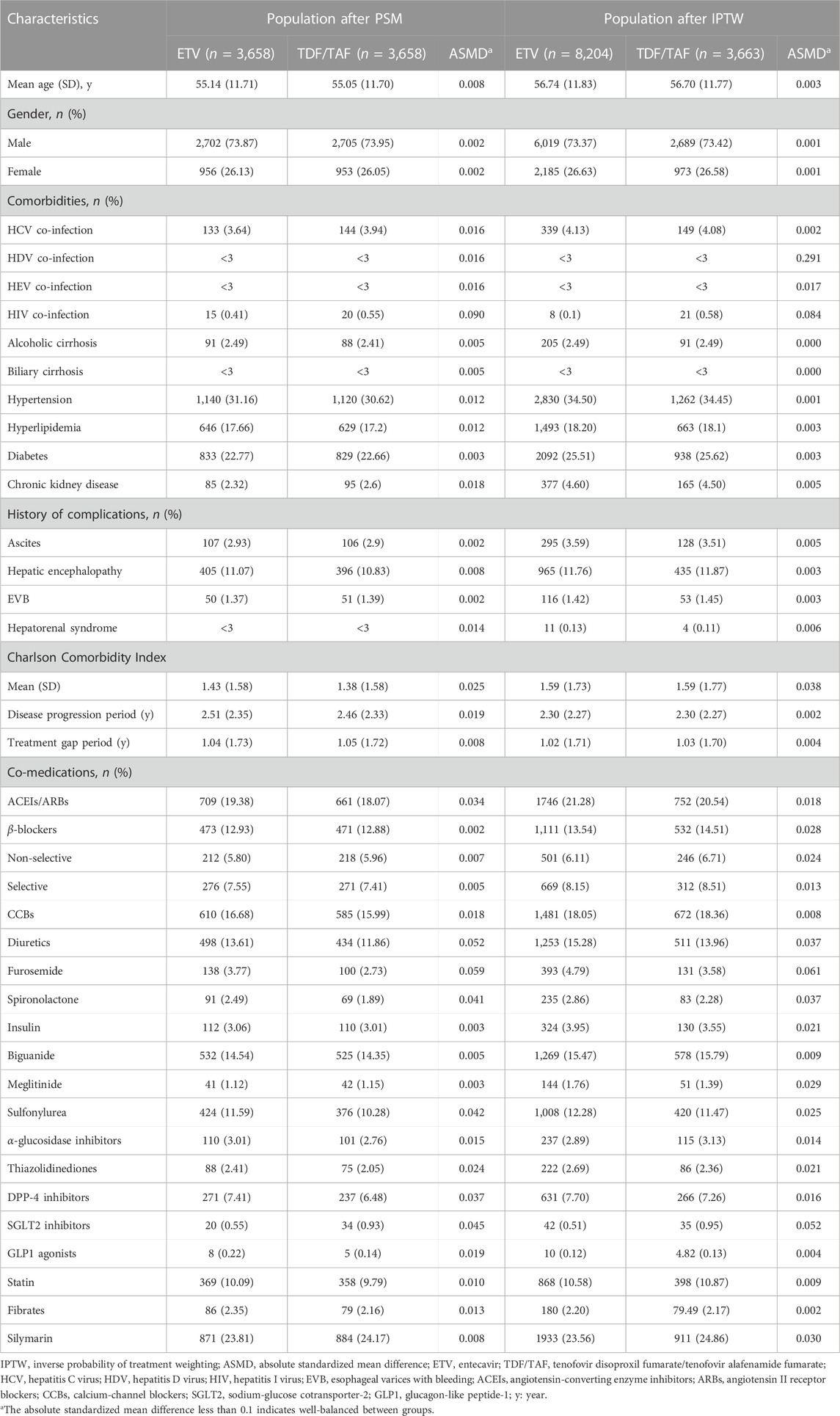
TABLE 1. Baseline characteristics of HBV-related cirrhosis patients within the PUT cohort after applying propensity score methods.
In the analyses with PSM, the incidence rate of the composite outcome, HCC, and mortality was significantly lower in the TDF/TAF users. TDF/TAF showed significantly lower hazards of developing the composite outcome [HR, 0.78 (95% CI, 0.72 to 0.85); p < 0.0001], HCC [HR, 0.87 (95% CI, 0.76 to 0.99); p = 0.0396], mortality [HR, 0.76 (95% CI, 0.68 to 0.83); p < 0.0001], and liver transplantation [HR, 0.72 (95% CI, 0.53 to 0.97); p = 0.0327] in unadjusted analysis accounting for competing risk. After adjusting for baseline confounders, similarly lower hazards of developing the composite outcome, HCC, mortality, and liver transplantation were seen in TDF/TAF users (Table 2 Panel A). The differences in cumulative incidence curves between treatment groups within the PUT cohort for four outcomes are shown in Figure 2. In the analyses with stabilized IPTW, similar hazards of the lower composite outcome, mortality, and liver transplantation were found in TDF/TAF users than in ETV users (Table 2 Panel B; Supplementary eFigure S1).
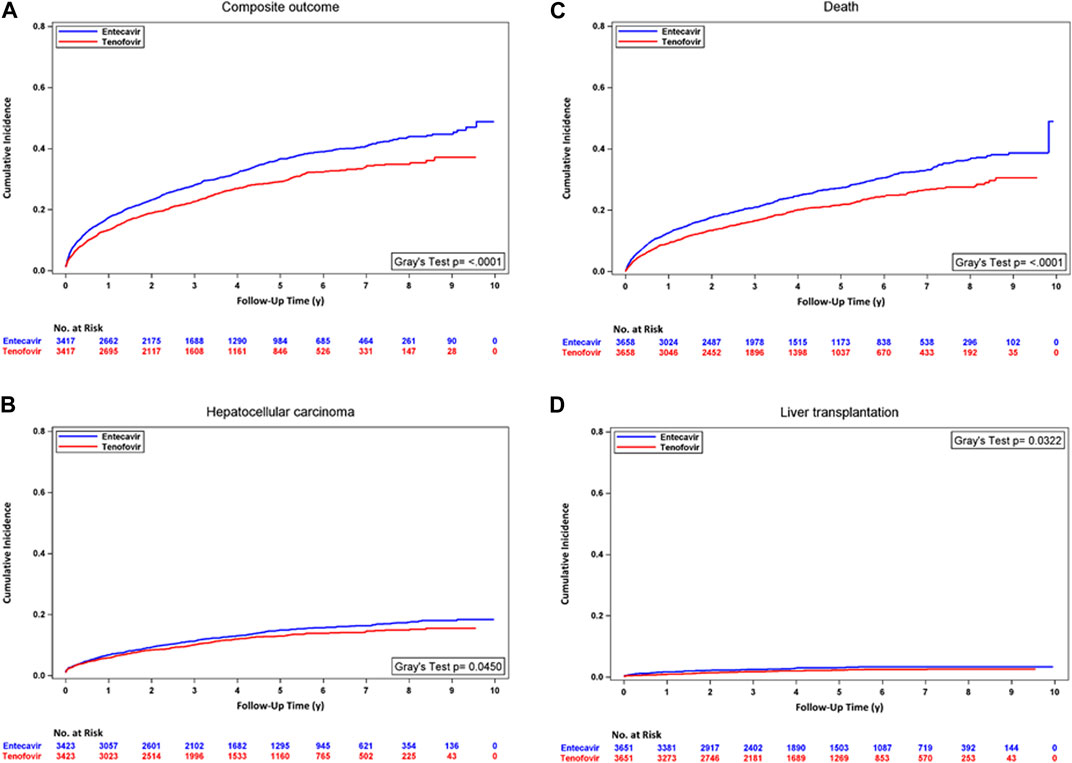
FIGURE 2. Cumulative incidence curves for TDF/TAF users versus ETV users within PUT cohorts after PSM. (A) Composite outcome, (B) hepatocellular carcinoma, (C) death, and (D) liver transplantation.
In the analyses with PSM, the incidence rate of HCC was significantly lower in the TDF/TAF users. In unadjusted analysis accounting for competing risk, TDF/TAF showed significantly lower hazards of developing composite outcomes [HR, 0.81 (95% CI, 0.71 to 0.93); p = 0.0021] and HCC [HR, 0.61 (95% CI, 0.49 to 0.76); p < 0.0001]. TDF/TAF was associated with a lower incidence rate of death, but the result did not achieve statistical significance. After adjusting for baseline confounders, similarly lower hazards of developing composite outcomes and HCC were seen in TDF/TAF users. The risks of death and developing transplantation were not statistically different between the two groups (Supplementary eTable S10 Panel A). The cumulative incidence curves between treatment groups within the PT cohort for four outcomes are shown in Figure 3.
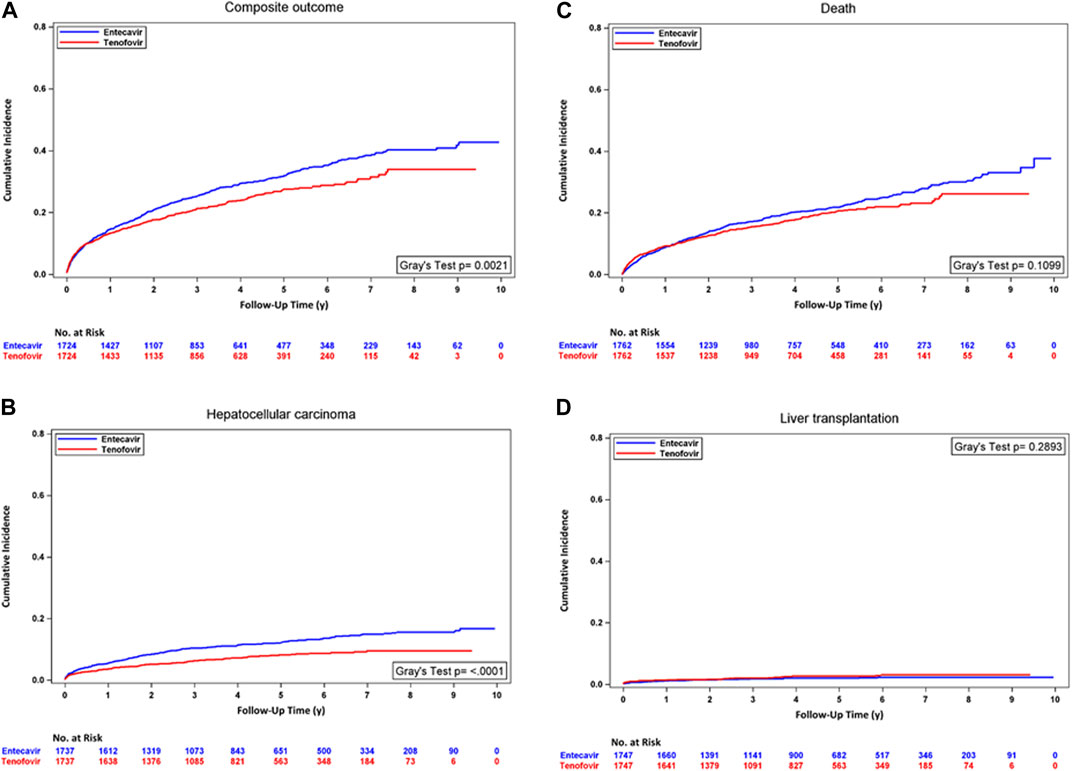
FIGURE 3. Cumulative incidence curves for TDF/TAF users versus ETV users within PT cohorts after PSM. (A) Composite outcome, (B) hepatocellular carcinoma, (C) death, and (D) liver transplantation.
In the analyses with stabilized IPTW, similarly lower incidence rates of the composite outcome and HCC were seen in patients treated with TDF/TAF. TDF/TAF was associated with a lower incidence rate of mortality, but the result did not achieve statistical significance. The univariate and multivariate analyses accounting for competing risk events showed a similar trend of lower composite outcome and HCC hazards in TDF/TAF users than in ETV users. The risks of death and developing transplantation were not statistically different between the two groups (Supplementary eTable S10 Panel B; Supplementary eFigure S2).
Regarding the analysis for the negative control outcome, the outcome did not show a significant association with TDF/TAF treatment (Supplementary eTable S11).
Our study demonstrated a significantly reduced risk of developing cirrhosis-related complications among TDF/TAF users, consistent with previous studies suggesting a lower risk of HCC in individuals with HBV-related cirrhosis who received TDF/TAF than those receiving ETV (Choi et al., 2019). The negative control outcome, namely, MI, supported the conclusion that the lower hazards of cirrhosis-related outcomes and death in TDF/TAF compared to ETV were robust.
Our study could not determine the exact mechanism underlying the better outcomes with TDF/TAF treatment. However, several reasons might explain our findings. First, TDF/TAF might show superior virologic response profiles compared to ETV, as presented in previous studies (Koike et al., 2018; Chen et al., 2019; Choi et al., 2019; Choi et al., 2021). These better virologic outcomes might lead to different levels of effectiveness in preventing cirrhosis-associated complications between TDF/TAF and ETV therapy. Second, the antitumor effects of TDF/TAF have been reported. The reason was that higher interferon-λ3 levels were induced by ANPs (such as TDF/TAF), but not by nucleoside analogs (such as ETV) (Sato et al., 2006; Abushahba et al., 2010; Murata and Mizokami, 2023; Yang et al., 2023). Interferon-λ3 demonstrated potent antitumor effects in murine cancer models, including HCC (Sato et al., 2006; Abushahba et al., 2010; Murata and Mizokami, 2023; Yang et al., 2023). The antitumor activity might explain the differences in risks in developing outcomes between TDF/TAF and ETV. Third, TDF/TAF was anticipated to generate favorable immune responses toward anti-HBV effects. As presented by Murata et al. (2020), TDF/TAF could inhibit interleukin (IL)-10 production and thereby promote the release of IL-12 and tumor necrosis factor (TNF)-α, which was not observed in ETV. Suppressed IL-10 and increased IL-12 would stimulate T cells and NK cells to induce IFN-γ (Henry et al., 2008; Smith et al., 2018). Both IFN-γ and TNF-α promoted anti-HBV effects by inhibiting HBV replication and decreasing HBV covalently closed circular DNA (cccDNA) levels (Cavanaugh et al., 1997; Rehermann and Bertoletti, 2015; Xia et al., 2016).
In the PUT cohort after propensity score matching methods, TDF/TAF showed a significantly lower rate in each outcome. However, TDF/TAF was significantly associated with a lower hazard in the composite outcome and HCC, but not in death or liver transplantation. The inconsistent results among outcomes might be explained as follows: the lack of difference in incidence of death can be attributed to a higher proportion of patients in the ETV groups experiencing deaths unrelated to HCC, compared to the TDF/TAF groups (data not shown). No difference in incidence of liver transplantation represented that most patients received liver transplants because of complications of decompensation rather than HCC (data not shown) (European Association for the Study of the Liver, 2018).
To date, only a few real-world studies have compared cirrhosis-related outcomes between TDF/TAF and ETV in HBV-related cirrhosis patients (Choi et al., 2019; Papatheodoridis et al., 2020). However, real-world evidence investigating the comparative effectiveness between TDF/TAF and ETV in Taiwanese patients with HBV-related cirrhosis was limited. Furthermore, the evidence comparing cirrhosis-related outcomes within treatment-experienced cirrhosis patients was scarce. Our study successfully addresses the current knowledge gap.
The main strengths of our study were as follows: this was a large-scale cohort study using the NHIRD to describe patients’ characteristics and the novel findings that comprehensively evaluated comparative effectiveness between TDF/TAF and ETV in Taiwanese HBV-related cirrhosis patients. Additionally, our findings were consistent with those of a previous cohort (Choi et al., 2019). Moreover, our study addressed the knowledge gap and provided information with comparative effectiveness evidence in patients with prior exposure to NA. Furthermore, our conclusion remained consistent across different propensity score methods and sensitivity analyses.
We acknowledge that some limitations remain in this study. First, HBV-related (e.g., HBV viral load and HBeAg status), liver function-related (e.g., AST and ALT), HCC-related (e.g., α-fetoprotein, family history of HCC, smoking status, alcohol status, and BMI), and cirrhosis-related (platelet count, bilirubin, albumin, prothrombin time, serum creatinine, and fibrosis markers) lab data and Chinese medicine exposure data could not be obtained in our database (Hsu et al., 2014; Chen et al., 2017; Zhang et al., 2021; Kanwal et al., 2023). For HBV-related and liver function-related lab data, the ETV and TDF/TAF could continue to be reimbursed regardless of HBV viral load, HBeAg status, or results of liver function tests in HBV-related cirrhosis patients under the NHI payment guidelines (National Health Insurance Administration, 2023b). Therefore, the absence of information would not substantially affect our findings because the missing information was unlikely to induce treatment selection bias. However, the lack of cirrhosis-related information could impact our ability to assess the severity of liver cirrhosis and hepatic failure. This could misidentify individuals without cirrhosis as having cirrhosis, and vice versa. In addition, the lack of HCC-related data was an unmeasured confounder in our study, which might influence our estimated results. Second, we used ICD codes to identify cirrhosis patients, which hindered our ability to accurately determine cirrhosis status. The generation of misclassification bias resulted from the absence of information concerning diagnostic procedures for cirrhosis in clinical practice (for example, liver biopsy, ultrasound, CT, MRI, and liver stiffness evaluation) (RadiologyInfo, 2022). Third, despite the use of propensity score methods to address confounding variables, unknown or unmeasured confounders might still exist. Fourth, there were potential reasons that would induce selection bias between treatment groups. Given that ETV had been approved 3 years before TDF/TAF, ETV users tended to be older and have more advanced diseases than TDF/TAF users. This “patient warehousing” phenomenon was similarly observed in previous studies (Lok et al., 2016; Hsu et al., 2020; Yip et al., 2020). Moreover, there were a few additional potential explanations for the relatively younger age and milder liver disease of TDF/TAF patients. One reason could be the preference for TDF/TAF among young women of childbearing age due to its safety during pregnancy. Additionally, concerns regarding renal toxicity and osteoporosis might lead to the avoidance of TDF in the elderly population (Sarin et al., 2016; European Association for the Study of the Liver, 2017; Terrault et al., 2018). Nonetheless, because our study was an active comparison design with similar indications, the misclassification population, difference in baseline characteristics, and other unmeasured confounders could be reduced (Yoshida et al., 2015). Fifth, our study used data from the NHIRD; therefore, it is necessary to conduct further studies to validate whether our findings could be extrapolated to other countries or regions.
Our study provided updated information regarding the comparative effectiveness between ETV and TDF/TAF. Further studies could evaluate the comparative cost-effectiveness between two treatments to guide the optimal distribution of healthcare system resources.
TDF/TAF treatment was associated with a significantly lower risk of cirrhosis-related complications, and mortality, in patients with HBV-related cirrhosis compared with ETV treatment. However, no statistically significant difference in death and liver transplantation was seen in treatment-experienced patients. Further studies are necessary to ensure the replicability of our findings.
The data analyzed in this study are subject to the following licenses/restrictions: C-YC had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Data are available from the National Health Insurance Research Database (NHIRD), published by the Bureau of National Health Insurance (BNHI) of the Ministry of Health and Welfare. Owing to the legal restrictions imposed by the Government of Taiwan related to the Personal Information Protection Act, the database cannot be made publicly available. The conclusions presented in this study are those of the authors and do not necessarily reflect the views of the BNHI, the Ministry of Health and Welfare. Requests to access these datasets should be directed to C-YC amsyOTc1NTI1QGhvdG1haWwuY29t.
The studies involving humans were approved by the Institutional Review Board (IRB) of Kaohsiung Medical University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Y-HH: data curation, formal analysis, investigation, methodology, validation, visualization, and writing–original draft. C-WS: conceptualization, investigation, software, and writing–review and editing. C-YC: conceptualization, formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing–original draft, and writing–review and editing. M-JB: conceptualization, funding acquisition, methodology, resources, supervision, and writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by grant (TTMMH-111-05) from Taitung Mackay Memorial Hospital, Taitung, Taiwan.
This study was based in part on data from the NHIRD provided by the Bureau of National Health Insurance (BNHI) of the Ministry of Health and Welfare. The conclusions presented in this study are those of the authors and do not necessarily reflect the views of the BNHI, the Ministry of Health and Welfare. The authors thank the Center for Medical Informatics and Statistics of Kaohsiung Medical University for providing administrative support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1301120/full#supplementary-material
Abushahba, W., Balan, M., Castaneda, I., Yuan, Y., Reuhl, K., Raveche, E., et al. (2010). Antitumor activity of type I and type III interferons in BNL hepatoma model. CII 59 (7), 1059–1071. doi:10.1007/s00262-010-0831-3
Austin, P. C. (2009a). Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun. Statistics - Simul. Comput. 38 (6), 1228–1234. doi:10.1080/03610910902859574
Austin, P. C. (2009b). Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Statistics Med. 28 (25), 3083–3107. doi:10.1002/sim.3697
Austin, P. C. (2011a). An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar. Behav. Res. 46 (3), 399–424. doi:10.1080/00273171.2011.568786
Austin, P. C. (2011b). Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat. 10 (2), 150–161. doi:10.1002/pst.433
Calvaruso, V., and Craxì, A. (2014). Regression of fibrosis after HBV antiviral therapy. Is cirrhosis reversible? Liver international. official J. Int. Assoc. Study Liver 34 (1), 85–90. doi:10.1111/liv.12395
Cavanaugh, V. J., Guidotti, L. G., and Chisari, F. V. (1997). Interleukin-12 inhibits hepatitis B virus replication in transgenic mice. J. virology 71 (4), 3236–3243. doi:10.1128/JVI.71.4.3236-3243.1997
Charlson, M. E., Pompei, P., Ales, K. L., and MacKenzie, C. R. (1987). A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. chronic Dis. 40 (5), 373–383. doi:10.1016/0021-9681(87)90171-8
Chen, C. H., Lee, C. M., Lai, H. C., Hu, T. H., Su, W. P., Lu, S. N., et al. (2017). Prediction model of hepatocellular carcinoma risk in Asian patients with chronic hepatitis B treated with entecavir. Oncotarget 8 (54), 92431–92441. doi:10.18632/oncotarget.21369
Chen, M. B., Wang, H., Zheng, Q. H., Zheng, X. W., Fan, J. N., Ding, Y. L., et al. (2019). Comparative efficacy of tenofovir and entecavir in nucleos(t)ide analogue-naive chronic hepatitis B: a systematic review and meta-analysis. PloS one 14 (11), e0224773. doi:10.1371/journal.pone.0224773
Choi, J., Kim, H. J., Lee, J., Cho, S., Ko, M. J., and Lim, Y. S. (2019). Risk of hepatocellular carcinoma in patients treated with entecavir vs tenofovir for chronic hepatitis B: a Korean nationwide cohort study. JAMA Oncol. 5 (1), 30–36. doi:10.1001/jamaoncol.2018.4070
Choi, W. M., Choi, J., and Lim, Y. S. (2021). Effects of tenofovir vs entecavir on risk of hepatocellular carcinoma in patients with chronic HBV infection: a systematic review and meta-analysis. Clin. gastroenterology hepatology 19 (2), 246–258.e9. doi:10.1016/j.cgh.2020.05.008
De Clercq, E., and Holý, A. (2005). Acyclic nucleoside phosphonates: a key class of antiviral drugs. Nat. Rev. Drug Discov. 4 (11), 928–940. doi:10.1038/nrd1877
European Association for the Study of the Liver (2017). EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 67 (2), 370–398. doi:10.1016/j.jhep.2017.03.021
European Association for the Study of the Liver (2018). EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 69 (2), 406–460. doi:10.1016/j.jhep.2018.03.024
GBD 2017 Cirrhosis Collaborators (2020). The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. lancet Gastroenterology hepatology 5 (3), 245–266. doi:10.1016/S2468-1253(19)30349-8
Hayward, K. L., and Weersink, R. A. (2020). Improving medication-related outcomes in chronic liver disease. Hepatol. Commun. 4 (11), 1562–1577. doi:10.1002/hep4.1612
Henry, C. J., Ornelles, D. A., Mitchell, L. M., Brzoza-Lewis, K. L., and Hiltbold, E. M. (2008). IL-12 produced by dendritic cells augments CD8+ T cell activation through the production of the chemokines CCL1 and CCL17. J. Immunol. Baltim. Md 1950) 181 (12), 8576–8584. doi:10.4049/jimmunol.181.12.8576
Hsu, Y. C., Wong, G. L., Chen, C. H., Peng, C. Y., Yeh, M. L., Cheung, K. S., et al. (2020). Tenofovir versus entecavir for hepatocellular carcinoma prevention in an international consortium of chronic hepatitis B. Am. J. gastroenterology 115 (2), 271–280. doi:10.14309/ajg.0000000000000428
Hsu, Y. C., Wu, C. Y., Lane, H. Y., Chang, C. Y., Tai, C. M., Tseng, C. H., et al. (2014). Determinants of hepatocellular carcinoma in cirrhotic patients treated with nucleos(t)ide analogues for chronic hepatitis B. J. Antimicrob. Chemother. 69 (7), 1920–1927. doi:10.1093/jac/dku041
Kanwal, F., Khaderi, S., Singal, A. G., Marrero, J. A., Loo, N., Asrani, S. K., et al. (2023). Risk factors for HCC in contemporary cohorts of patients with cirrhosis. Hepatology 77 (3), 997–1005. doi:10.1002/hep.32434
Kao, C. W., Chiang, C. J., Lin, L. J., Huang, C. W., Lee, W. C., Lee, M. Y., et al. (2021). Accuracy of long-form data in the Taiwan cancer registry. J. Formos. Med. Assoc. = Taiwan yi zhi 120 (11), 2037–2041. doi:10.1016/j.jfma.2021.04.022
Koike, K., Suyama, K., Ito, H., Itoh, H., and Sugiura, W. (2018). Randomized prospective study showing the non-inferiority of tenofovir to entecavir in treatment-naïve chronic hepatitis B patients. Hepatology Res. 48 (1), 59–68. doi:10.1111/hepr.12902
Lee, S. W., Kim, S. M., Hur, W., Kang, B. Y., Lee, H. L., Nam, H., et al. (2021). Tenofovir disoproxil fumarate directly ameliorates liver fibrosis by inducing hepatic stellate cell apoptosis via downregulation of PI3K/Akt/mTOR signaling pathway. PloS one 16 (12), e0261067. doi:10.1371/journal.pone.0261067
Lipsitch, M., Tchetgen Tchetgen, E., and Cohen, T. (2010). Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiol. Camb. Mass) 21 (3), 383–388. doi:10.1097/EDE.0b013e3181d61eeb
Lok, A. S., McMahon, B. J., Brown, R. S., Wong, J. B., Ahmed, A. T., Farah, W., et al. (2016). Antiviral therapy for chronic hepatitis B viral infection in adults: a systematic review and meta-analysis. Hepatology 63 (1), 284–306. doi:10.1002/hep.28280
Marcellin, P., and Asselah, T. (2013). Long-term therapy for chronic hepatitis B: hepatitis B virus DNA suppression leading to cirrhosis reversal. J. gastroenterology hepatology 28 (6), 912–923. doi:10.1111/jgh.12213
Marcellin, P., Gane, E., Buti, M., Afdhal, N., Sievert, W., Jacobson, I. M., et al. (2013). Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet (London, Engl. 381 (9865), 468–475. doi:10.1016/S0140-6736(12)61425-1
Murata, K., and Mizokami, M. (2023). Possible biological mechanisms of entecavir versus tenofovir disoproxil fumarate on reducing the risk of hepatocellular carcinoma. J. gastroenterology hepatology 38 (5), 683–691. doi:10.1111/jgh.16178
Murata, K., Tsukuda, S., Suizu, F., Kimura, A., Sugiyama, M., Watashi, K., et al. (2020). Immunomodulatory mechanism of acyclic nucleoside phosphates in treatment of hepatitis B virus infection. Hepatology 71 (5), 1533–1545. doi:10.1002/hep.30956
National Health Insurance Administration (2023b). Payment guidelines for antimicrobial agents. Taiwan: Ministry of Health and Welfare. Available at: https://www.nhi.gov.tw/Content_List.aspx?n=E70D4F1BD029DC37&topn=5FE8C9FEAE863B46.
National Health Insurance Administration (2023a). NHI profile. Available at: https://www.nhi.gov.tw/English/Content_List.aspx?n=8FC0974BBFEFA56D&topn=ED4A30E51A609E49.
Papatheodoridis, G. V., Dalekos, G. N., Idilman, R., Sypsa, V., Van Boemmel, F., Buti, M., et al. (2020). Similar risk of hepatocellular carcinoma during long-term entecavir or tenofovir therapy in Caucasian patients with chronic hepatitis B. J. Hepatol. 73 (5), 1037–1045. doi:10.1016/j.jhep.2020.06.011
RadiologyInfo (2022). Cirrhosis of the liver. Available at: https://www.radiologyinfo.org/en/info/cirrhosisliver.
Rehermann, B., and Bertoletti, A. (2015). Immunological aspects of antiviral therapy of chronic hepatitis B virus and hepatitis C virus infections. Hepatology 61 (2), 712–721. doi:10.1002/hep.27323
Rockey, D. C. (2016). Liver fibrosis reversion after suppression of hepatitis B virus. Clin. liver Dis. 20 (4), 667–679. doi:10.1016/j.cld.2016.06.003
Sarin, S. K., Kumar, M., Eslam, M., George, J., Al Mahtab, M., Akbar, S. M. F., et al. (2020). Liver diseases in the asia-pacific region: a lancet gastroenterology & hepatology commission. lancet Gastroenterology hepatology. 5 (2), 167–228. doi:10.1016/S2468-1253(19)30342-5
Sarin, S. K., Kumar, M., Lau, G. K., Abbas, Z., Chan, H. L., Chen, C. J., et al. (2016). Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol. Int. 10 (1), 1–98. doi:10.1007/s12072-015-9675-4
Sato, A., Ohtsuki, M., Hata, M., Kobayashi, E., and Murakami, T. (2006). Antitumor activity of IFN-lambda in murine tumor models. J. Immunol. Baltim. Md 1950) 176 (12), 7686–7694. doi:10.4049/jimmunol.176.12.7686
Schiff, E., Simsek, H., Lee, W. M., Chao, Y. C., Sette, H., Janssen, H. L., et al. (2008). Efficacy and safety of entecavir in patients with chronic hepatitis B and advanced hepatic fibrosis or cirrhosis. Am. J. gastroenterology 103 (11), 2776–2783. doi:10.1111/j.1572-0241.2008.02086.x
Schiff, E. R., Lee, S. S., Chao, Y. C., Kew Yoon, S., Bessone, F., Wu, S. S., et al. (2011). Long-term treatment with entecavir induces reversal of advanced fibrosis or cirrhosis in patients with chronic hepatitis B. Clinical gastroenterology and hepatology: the official clinical practice. J. Am. Gastroenterological Assoc. 9 (3), 274–276. doi:10.1016/j.cgh.2010.11.040
Smith, L. K., Boukhaled, G. M., Condotta, S. A., Mazouz, S., Guthmiller, J. J., Vijay, R., et al. (2018). Interleukin-10 directly inhibits CD8(+) T cell function by enhancing N-glycan branching to decrease antigen sensitivity. Immunity 48 (2), 299–312. doi:10.1016/j.immuni.2018.01.006
Terrault, N. A., Lok, A. S. F., McMahon, B. J., Chang, K. M., Hwang, J. P., Jonas, M. M., et al. (2018). Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 67 (4), 1560–1599. doi:10.1002/hep.29800
Udompap, P., and Kim, W. R. (2020). Development of hepatocellular carcinoma in patients with suppressed viral replication: changes in risk over time. Clin. liver Dis. 15 (2), 85–90. doi:10.1002/cld.904
Xia, Y., Stadler, D., Lucifora, J., Reisinger, F., Webb, D., Hösel, M., et al. (2016). Interferon-γ and tumor necrosis factor-α produced by T cells reduce the HBV persistence form, cccDNA, without cytolysis. Gastroenterology 150 (1), 194–205. doi:10.1053/j.gastro.2015.09.026
Yang, J., Chen, Y., Sun, H., Zhang, X., Wang, J., Liang, Z., et al. (2023). Tenofovir versus entecavir on decreasing risk of HBV-related hepatocellular carcinoma recurrence after liver transplantation. Infect. agents cancer 18 (1), 2. doi:10.1186/s13027-022-00478-4
Yip, T. C., Wong, V. W., Chan, H. L., Tse, Y. K., Lui, G. C., and Wong, G. L. (2020). Tenofovir is associated with lower risk of hepatocellular carcinoma than entecavir in patients with chronic HBV infection in China. Gastroenterology 158 (1), 215–225. doi:10.1053/j.gastro.2019.09.025
Yokosuka, O., Takaguchi, K., Fujioka, S., Shindo, M., Chayama, K., Kobashi, H., et al. (2010). Long-term use of entecavir in nucleoside-naïve Japanese patients with chronic hepatitis B infection. J. Hepatol. 52 (6), 791–799. doi:10.1016/j.jhep.2009.12.036
Yoshida, K., Solomon, D. H., and Kim, S. C. (2015). Active-comparator design and new-user design in observational studies. Nat. Rev. Rheumatol. 11 (7), 437–441. doi:10.1038/nrrheum.2015.30
Keywords: tenofovir, entecavir, effectiveness, hepatitis B virus, cirrhosis
Citation: Huang Y-H, Shen C-W, Chen C-Y and Bair M-J (2023) Comparative effectiveness of tenofovir versus entecavir in patients with hepatitis B virus-related cirrhosis in Taiwan: a retrospective cohort study. Front. Pharmacol. 14:1301120. doi: 10.3389/fphar.2023.1301120
Received: 24 September 2023; Accepted: 30 November 2023;
Published: 19 December 2023.
Edited by:
Eugene Van Puijenbroek, Netherlands Pharmacovigilance Centre Lareb, NetherlandsReviewed by:
Yuzheng Zhuge, Nanjing Drum Tower Hospital, ChinaCopyright © 2023 Huang, Shen, Chen and Bair. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming-Jong Bair, YTU5NjNAbW1oLm9yZy50dw==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.