
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 22 December 2023
Sec. Experimental Pharmacology and Drug Discovery
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1294810
 Ke-Guang Chen1†
Ke-Guang Chen1† Ye-Hui Zhang1†
Ye-Hui Zhang1† Pan-Pan Ye1†
Pan-Pan Ye1† Xue-Hu Gao2
Xue-Hu Gao2 Lin-Lin Song1
Lin-Lin Song1 Hai-Yan Zhou1
Hai-Yan Zhou1 Qian Li1
Qian Li1 Fu-Rong Zhao1
Fu-Rong Zhao1 Jin-Yi Shi1
Jin-Yi Shi1 Xin-Mei Yang1
Xin-Mei Yang1 Kai Shen2†
Kai Shen2† Sheng Feng2†
Sheng Feng2† Wei Zhao1,3*†
Wei Zhao1,3*†Objectives: INS068 is a novel, soluble, and long-acting insulin analog. In this study, we evaluated the pharmacokinetics and relative bioavailability of two formulations of INS068 in healthy Chinese subjects: a reference formulation packaged in vials and administered via syringe (R), and a test formulation packaged and administered via pen injector (T).
Methods: A randomized, open-label, two-period, two-sequence crossover study was conducted with 24 healthy Chinese subjects. Subjects were randomized and administered subcutaneously in the abdomen at 0.4 U/kg of test or reference INS068 injection according to an open crossover design. INS068 concentrations in the serum were measured using LC-MS/MS, and the pharmacokinetic parameters of maximum concentration (Cmax) and area under the concentration-time curve (AUC0–t and AUC0–∞) were used to evaluate relative bioavailability.
Results: After a single dose at 0.4 U/kg, the median Tmax of INS068 was 12 h for both formulations, and the mean t1/2 for T and R was 13.0 h and 12.6 h, respectively. The geometric means of Cmax and AUC0–∞ were 3.99 nmol/L and 120 h·nmol/L for the T, and 4.05 nmol/L and 117 h·nmol/L for the R, respectively. The geometric mean ratios of Cmax, AUC0–t and AUC0–∞ of T over R were 98.7% (90% CI: 92.7%–105.2%), 102.6% (90% CI: 100.0%–105.3%) and 102.8% (90% CI: 100.1%–105.5%).
Conclusion: The overall PK profile of the two formulations of INS068 injection was comparable in healthy subjects, and the pen injector of INS068 had adequate safety and tolerability, supporting it as a new formulation in a phase III study and bridging PK data from early phase clinical trials.
Clinical Trial Registration: clinicaltrials.gov, identifier: NCT05336071
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by hyperglycemia due to insulin deficiency, insulin resistance, or both. Long-term hyperglycemia is associated with the damage, dysfunction, and failure of various organs, especially the kidneys, eyes, nerves, heart, and blood vessels (American Diabetes Association, 2013). DM is generally classified according to etiological factors, with type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) constituting the majority of cases (UK Prospective Diabetes Study Group, 1998; Holman et al., 2008; Hemmingsen et al., 2013). According to the International Diabetes Federation, there were 382 million people with diabetes in 2013 globally, and the number is expected to rise to 592 million by 2035 (Guariguata et al., 2014). The lifetime risk of diabetes for an average 20-year-old American person in the United States (US) increased from 20.4% for men and 26.7% for women from 1985-1989 to 40.2% for men and 39.3% for women based on 2011 data (Mobasseri et al., 2020). In other words, two out of every five Americans entering adulthood can expect to develop diabetes during their lifetime (Gregg et al., 2014; Lipscombe, 2014).
INS068 is a novel, soluble, long-acting insulin analog developed by Jiangsu Hengrui Pharmaceuticals Co., Ltd., intended to cover basal insulin requirements in patients with T1DM and T2DM. INS068 was designed based on desB30 human insulin, to exert stable and long-acting blood glucose-lowering effects. Nonclinical and clinical trials have demonstrated that INS068 injection has favorable safety profiles and exhibits pharmacodynamics comparable to those of IDeg (US Food and Drug Administration, 2015).
During the clinical development process, the drug substance (DS) and drug product (DP) of INS068 underwent significant changes. INS068 injection, used in Phase 1 and Phase 2 studies so far, was produced with manufacturing process A, packaged in a glass vial, and administered with an insulin syringe, whereas INS068 was planned for use in upcoming Phase 3 clinical trials and for future commercialization is produced with process B and packaged in and administered via a pen injector. Therefore, evaluation of any potential impact of manufacturing process and package changes on the bioavailability and overall PK properties of INS068 is warranted. This study aimed to evaluate the pharmacokinetics and relative bioavailability of INS068 injection between the two formulations in healthy subjects.
This study was conducted from 12 May 2022, to 30 May 2022, at the Clinical Research Center, Shandong Provincial Qianfoshan Hospital, China. Twenty-four healthy male volunteers were recruited based on inclusion and exclusion criteria. Healthy volunteers aged between 18 and 55 years with a body mass index (BMI) between 18 and 27 kg/m2 were eligible to participate. This study was approved by the National Drug Clinical Trial Institution and Research Ethics Committee (REC) of Shandong Provincial Qianfoshan Hospital and Human Genetic Resource Administration of China (HGRAC), and was carried out in accordance with the International Conference on Harmonization (ICH) Good Clinical Practice guidelines (ICH, 2016) and the Declaration of Helsinki (World Medical Association, 2013). Written informed consent was obtained from all the participants prior to the initiation of any study-related activities.
This was a randomized, open-label, two-period, two-sequence crossover study designed to evaluate the PK of two different INS068 injection formulations in healthy subjects. INS068 used in Phase 1 and Phase 2 studies, manufactured with process A, packaged in vial, and administered with a syringe, was designated as the reference (R) product, and INS068 used in Phase 3 studies, manufactured with process B, packaged and administered with pen injector, was designated as the test (T) product.
In the first phase, prior to the commencement of dosing, screening and physical examination were performed within 14 days. The screening procedures included demographic and medical history data collection, general physical examination, and electrocardiography. Laboratory investigations, such as hematology, biochemistry, and urine analysis, were also performed.
The admitted subjects were enrolled and randomized in a 1:1 ratio into two study groups (Figure 1). They were fed a standard light diet in the evening and fasted overnight after dinner for over 10 h. On the morning of study Day 1, the subjects underwent venipuncture (indwelling needles) for infusion of 10% glucose solution and PK sample collection. According to randomization, the subjects received either the reference or test formulation of the INS068 injection at approximately 8 a.m., administered subcutaneously in the abdomen at 0.4 U/kg. A standard breakfast meal was provided approximately 1 h before INS068 administration and water was allowed if needed. Blood samples were collected according to the protocol detailed below to measure INS068 concentrations in the serum. On the morning of study Day 8, the subjects underwent the same procedures as those on Day 1 except that they received a different formulation of INS068 than on Day 1.
Blood samples were collected 1 h before INS068 administration and at 2, 4, 6, 8, 10, 12, 14, 16, 18, 24, 48, 72, and 96 h after administration (14 blood samples in total) to determine the concentration of INS068 in the serum. Immunogenicity samples were collected within 1 h before INS068 administration and then at 16 and 96 h after administration for the analysis of anti-INS068 antibodies on Day 1 and Day 8. Blood samples were centrifuged after standing at room temperature for 30 min to 1 h, and the harvested serum was frozen and maintained at −60°C to −90°C until further analysis. The serum sample was stable for 24 h at room temperature, stable for 144 h at 2°C–8°C, and stable for 748 days at −70°C. Serum INS068 concentrations and anti-INS068 antibodies were measured by Labcorp Pharmaceutical Research & Development (Shanghai) Co., Ltd. (Shanghai, China). Serum INS068 concentrations were measured by LC-MS/MS. The analysis system consists of the HPLC-30AD system (Shimadzu, Japan) and the Sciex Triple Quad 6500 + triple quadrupole tandem mass spectrometry (AB Science, Foster City, California). Appropriate amounts of serum samples, internal standards, and buffer were mixed and transferred to a solid phase extraction (SPE) plate. Water and organic solvent were added as eluent. The collected eluent was blown dry by nitrogen gas, and a complex solution of organic solvent was added. The mixture was taken for injection. The retention time of INS068 was 0.93 min. The detection method has been validated, with accuracy and precision of less than 10%. The lower limit of quantification was 0.04 nmol/L, and the concentration range of the standard curve was 0.04–8 nmol/L.
Assessments were based on the PK parameters of INS068 injected by subcutaneous injection of the two formulations in healthy subjects: AUC0-t, Cmax, AUC0-∞, time to maximum observed concentration (Tmax), terminal elimination half-life (t1/2), apparent volume of distribution (V/F), and apparent clearance (CL/F). The safety assessments included adverse events (AEs), vital signs, physical examination, laboratory tests, 12-lead electrocardiography (ECG), anti-INS068 antibodies, injection site reactions, and hypoglycemic events.
Statistical analyses were performed based on the non-compartmental method using Phoenix WinNonlin (version 8.1) to identify the PK parameters Cmax, AUC0-t, AUC0-∞ (if applicable), t1/2, Tmax, CL/F, and Vz/F of the INS068 injection. SAS software (version 9.4; SAS Institute Inc., Cary, North, USA) was used for statistical analyses. The point estimate with a 90% confidence interval of the least squares mean difference (T over R) was calculated for each PK parameter of Cmax, AUC0-t, and AUC0-∞ after natural logarithmic transformation based on the PK parameter set. The antilogarithmic transformation was also performed to calculate the point estimates of the geometric mean ratios (T over R) and the corresponding 90% confidence intervals.
A total of 56 subjects were screened, of which 24 were enrolled in the study and 32 failed screening. All 24 enrolled subjects completed the trial, including dosing, PK blood collection, safety observation, and follow-up. All subjects were Chinese. Their mean age was 30.3 years (range, 19–48 years), mean height (± standard deviation) was 172.52 ± 5.428 cm, mean weight (± standard deviation) was 68.17 ± 7.432 kg, and mean BMI (± standard deviation) was 22.863 ± 1.848 kg/m2 (Table 1).
The PK population included 24 subjects who were enrolled in the trial. Serum concentrations in the 24 subjects were quantified and analyzed. The mean 96 h serum INS068 concentration-time profiles for the two formulations are shown in Figure 2. The descriptive statistics of the PK parameters of the reference (R) and test (T) formulations of INS068 are shown in Table 2. Following administration of a single dose of 0.4 U/kg, the PK properties of the two INS068 formulations were similar. The median Tmax was 12 h for both T and R, and the mean t1/2 for T and R was 13.0 h and 12.6 h, respectively. The geometric means (GeoMean) of Cmax and AUC0-∞ were 3.99 nmol/L and 120 h·nmol/L for the T, 4.05 nmol/L and 117 h·nmol/L for the R, respectively. The GeoMean ratios (T over R) of Cmax, AUC0-t and AUC0-∞ were 98.7% (90% CI: 92.7%–105.2%), 102.6% (90% CI: 100.0%–105.3%) and 102.8% (90% CI: 100.1%–105.5%), respectively (Table 3).
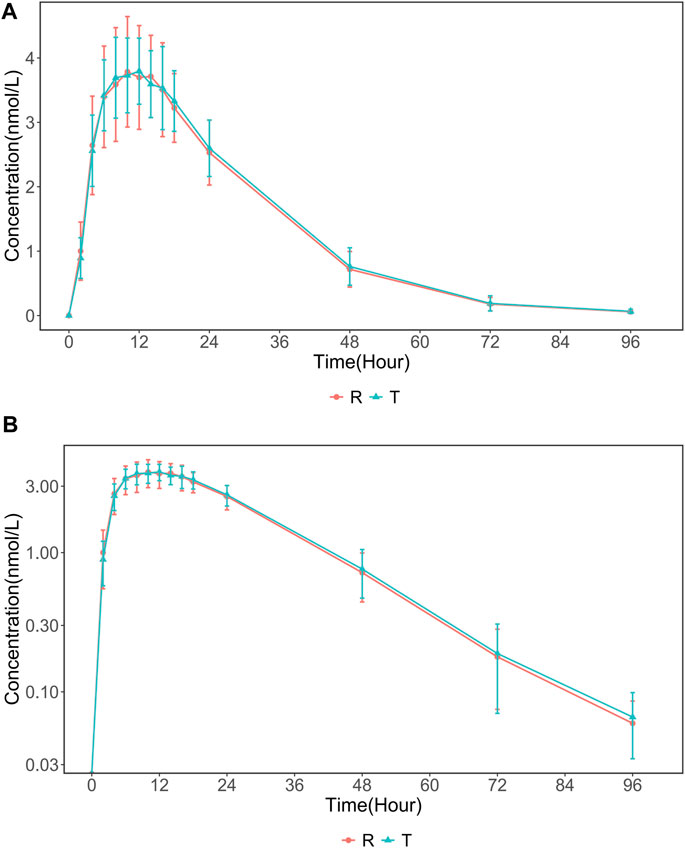
FIGURE 2. The mean (SD) serum INS068 Concentration-Time Profiles after Single Subcutaneous two formulations INS068 in Healthy Male Subjects: (A) non-log transformed data (mean ± SD) and (B) semi-log transformed data.
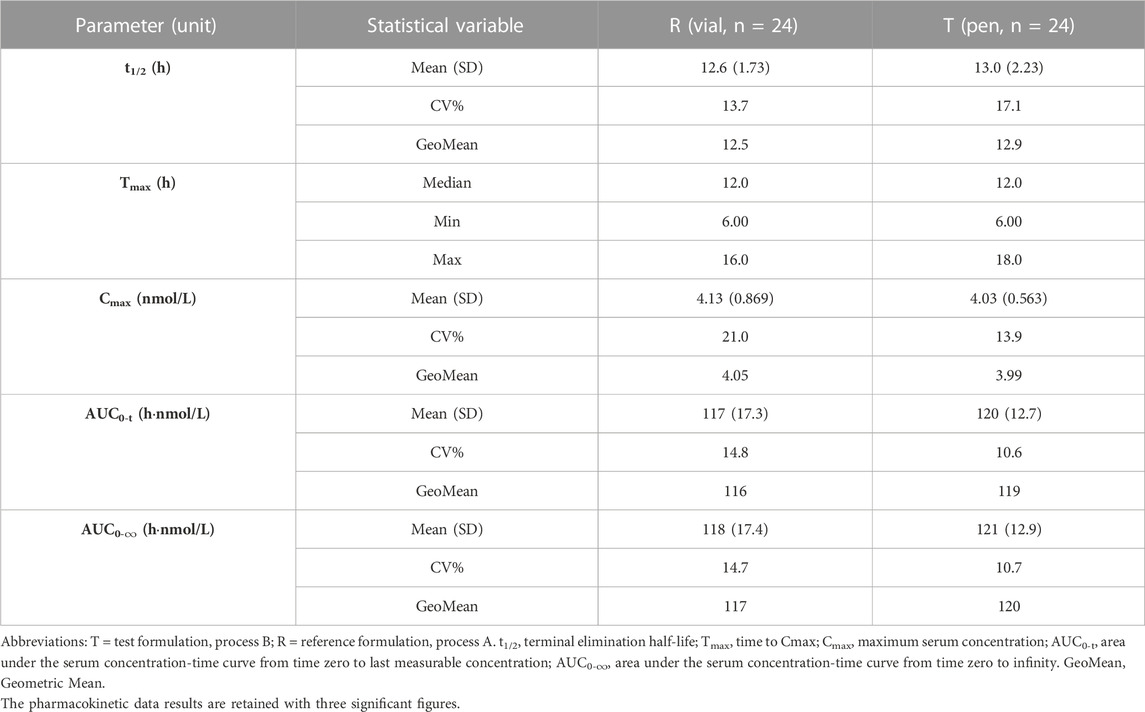
TABLE 2. Summary of the pharmacokinetic parameters of two formulations of INS068 in healthy male subjects.
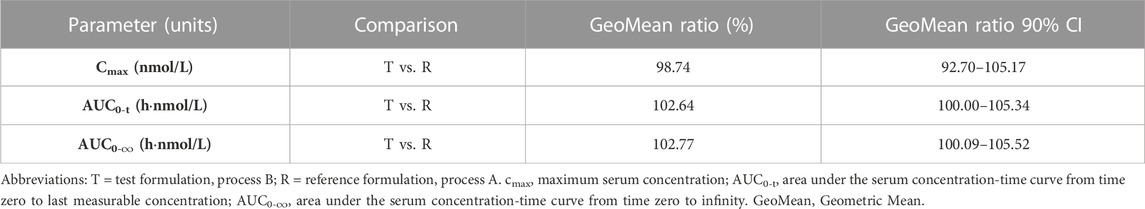
TABLE 3. Summary of the statistical comparisons of the pharmacokinetic parameters of two formulations of INS068 injection.
Both the test and reference formulations of the INS068 injection were well tolerated. All the enrolled subjects completed the study according to the protocol. None of the subjects discontinued or withdrew from the study because of safety issues.
The overall incidence of treatment-emergent adverse events (TEAE) was 29.2% (7/24), of which 16.7% (4/24) were associated with the test formulation, including blood triglyceride levels increased, electrocardiogram ST-T change, and blood creatine phosphokinase increased; 12.5% (3/24) were associated with the reference formulation, including alanine aminotransferase increased, blood pressure increased, dizziness, and diarrhea. The summary of TEAEs is shown in Table 4. All TEAEs were mild in severity (asymptomatic or mild symptoms and no corrective treatment was required). There were no serious adverse events (SAE), deaths, or adverse events of special interest (AESI), nor were there any adverse events that led to dose adjustment or corrective treatment. All TEAEs recovered by the end of the trial, except for one event (increased blood triglycerides) with an unknown outcome.
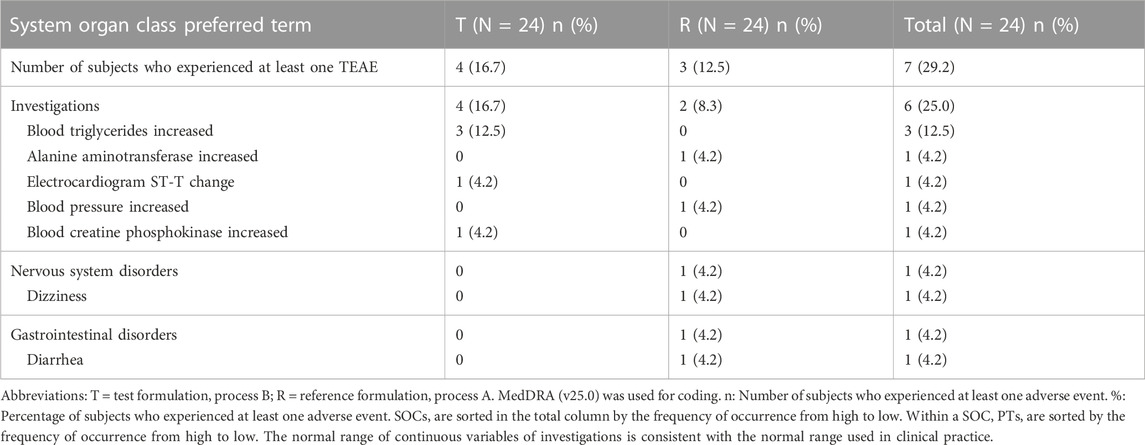
TABLE 4. Summary of treatment-emergent adverse events (TEAEs) by system organ class and preferred terms.
After administration of the test and reference formulations of INS068, there were no significant changes in laboratory results on average, vital signs, ECGs, or physical examination. Nine subjects experienced one hypoglycemic episode after injection of INS068 (including T and R), with an incidence rate of 37.5% (9/24). All hypoglycemic episodes had a level 1 severity according to the 2022 ADA classification (Davies et al., 2022). To date, no injection site reactions have been reported. The Anti-INS068 antibody was detected in 1 of the 24 subjects (4.2%) prior to the administration of INS068. After dosing at 0.4 U/kg, no ADA was detected in the serum of any of the INS068-treated subjects.
INS068 is a novel long-acting human insulin analog. This mode of action is identical to that of human insulin and other insulin analogs, as they all act through the same insulin receptor. INS068 was packaged in a glass vial and administered with an insulin syringe when it was used in Phase 1 and Phase 2 studies. Two Phase 1 studies were conducted in healthy subjects in the US and China respectively. INS068 has slow absorption and metabolism, with Tmax of 12–18 h and t1/2 of 13.3–21.4 h. The AUC0-∞ was directly proportional to the dose, and the mean AUC0-∞ of healthy male subjects in China was 6%–21.7% higher than that in the US. A Phase 1 study conducted in the US type 1 diabetes subjects showed that the PK characteristics of INS068 were similar to IDeg in the dose range of 0.4–0.8 U/kg. An international multicenter Phase 2 study showed that INS068 has good safety, tolerability, and efficacy comparable to IDeg, with glycation levels reduced by 0.98% and 0.97%, respectively. However, INS068 was planned to be packaged and administered via pen injector for upcoming Phase 3 clinical trials and future commercialization. Therefore, the present clinical study compared the PK properties of two INS068 formulations in healthy subjects.
Overall, no statistically significant differences in PK characteristics were observed between the formulations in this study. According to the study results, the PK parameters of the R and T products of INS068 were comparable, and the GeoMean ratios (T over R) of Cmax, AUC0-t and AUC0-∞ and their 90% confidence interval (CI) were between 80% and 125% (Table 3). The GeoMean ratios (T over R) and 90% CI of Cmax, AUC0-t and AUC0-∞ within each group were also calculated as an additional assessment, and all of them fell into the range of 80%–125% (Group A: 92.16%–97.15% for GeoMean ratios and 84.36%–101.23% for 90% CI; Group B: 108.75%–109.24% for GeoMean ratios and 97.82%–121.98% for 90% CI). The CV (%) of the new formulation (T) was lower than that of the old formulation (R). Both the test and reference formulations of the INS068 injection were well tolerated. The results demonstrated that neither the changes in the processes of DS and DP nor the packaging and delivery device affected the bioavailability or overall PK properties of INS068 injection, thereby qualifying the use of the new INS068 product in the upcoming phase 3 studies and for commercialization purposes.
This study has some limitations. First, pharmacodynamics were not investigated in this study, lacking the glucose clamp design, which makes it difficult to reflect the effect of changes in blood glucose in the PK profile of INS068. On the other hand, this study was conducted in healthy male subjects, rather than patients with type 1 diabetes (Korsatko et al., 2013), to include a relatively homogenous cohort of subjects to facilitate the detection of differences between the two formulations, in accordance with regulatory standards (US Food and Drug Administration, 2009; Committee for Medicinal Products for Human Use, 2010). With the inclusion of healthy subjects, a multiple-dose study with a clinically relevant dose would not have been acceptable, due to the risk of hypoglycaemia. We randomized subjects to fixed-dose levels of 0.4 U/kg of INS068 and single dose, which clearly does not reflect the therapeutic use of INS068. In actual clinical practice, the dose of INS068 must be titrated and adjusted to meet individual insulin needs without compromising safety, especially during hypoglycemic events. This is because, in clinical studies, hypoglycemic episodes caused by a lack of personalized basic insulin doses may be considered harmful to subjects.
The PK profiles of the two INS068 formulations were comparable following a single administration to healthy male subjects. Both the test and reference formulations of the INS068 injection were safe and well tolerated. The results support the use of the pen injector in a phase III study and bridge PK data from early phase clinical trials.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Research Ethics Committee (REC) of Shandong Provincial Qianfoshan Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
K-GC: Data curation, Investigation, Methodology, Project administration, Writing–original draft, Writing–review and editing. Y-HZ: Data curation, Investigation, Writing–original draft, Writing–review and editing. P-PY: Data curation, Investigation, Methodology, Validation, Writing–original draft, Writing–review and editing. X-HG: Conceptualization, Data curation, Formal Analysis, Methodology, Writing–original draft. L-LS: Investigation, Project administration, Writing–original draft. H-YZ: Data curation, Investigation, Methodology, Project administration, Writing–original draft. QL: Data curation, Investigation, Project administration, Validation, Writing–original draft. F-RZ: Data curation, Project administration, Writing–original draft. J-YS: Data curation, Resources, Writing–original draft. X-MY: Conceptualization, Data curation, Methodology, Writing–original draft. KS: Data curation, Formal Analysis, Methodology, Writing–original draft, Writing–review and editing. SF: Formal Analysis, Methodology, Validation, Writing–original draft, Writing–review and editing. WZ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Writing–original draft, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. WZ received funding from National Key Research and Development Program of China (2020YFC2008303 and 2020YFC2008306) for his research work. This study was supported by Jiangsu Hengrui Medicine Co., Ltd.
We thank the subjects and their families for their participation in this study as well as the study teams at each study site.
Authors X-HG, KS, and SF were employed by the Jiangsu Hengrui Medicine Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
American Diabetes Association (2013). Diagnosis and classification of diabetes mellitus. Diabetes care 36 (1), S67–S74. doi:10.2337/dc13-S067
Committee for Medicinal Products for Human Use (2010). Guideline on the investigation of bioequivalence. European Medicines Agency. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf (Accessed July 5, 2023).
Davies, M. J., Aroda, V. R., Collins, B. S., Gabbay, R. A., Green, J., Maruthur, N. M., et al. (2022). Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD). Diabetologia 65 (12), 1925–1966. doi:10.1007/s00125-022-05787-2
Gregg, E. W., Zhuo, X., Cheng, Y. J., Albright, A. L., Narayan, K. M., and Thompson, T. J. (2014). Trends in lifetime risk and years of life lost due to diabetes in the USA, 1985-2011: a modelling study. Diabetes & Endocrinol. 2 (11), 867–874. doi:10.1016/S2213-8587(14)70161-5
Guariguata, L., Whiting, D. R., Hambleton, I., Beagley, J., Linnenkamp, U., and Shaw, J. E. (2014). Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 103 (2), 137–149. doi:10.1016/j.diabres.2013.11.002
Hemmingsen, B., Lund, S. S., Gluud, C., Vaag, A., Almdal, T. P., Hemmingsen, C., et al. (2013). Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane database Syst. Rev. (11), CD008143. doi:10.1002/14651858.CD008143.pub3
Holman, R. R., Paul, S. K., Bethel, M. A., Matthews, D. R., and Neil, H. A. (2008). 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 359 (15), 1577–1589. doi:10.1056/NEJMoa0806470
ICH (2016). Integrated addendum to ICH E6(R1): guideline for good clinical practice E6(R2). Available at: https://www.gmp-compliance.org/files/guidemgr/E6_R2__Step_4.pdf (Accessed July 5, 2023).
Korsatko, S., Deller, S., Koehler, G., Mader, J. K., Neubauer, K., Adrian, C. L., et al. (2013). A comparison of the steady-state pharmacokinetic and pharmacodynamic profiles of 100 and 200 U/mL formulations of ultra-long-acting insulin degludec. Clin. drug Investig. 33 (7), 515–521. doi:10.1007/s40261-013-0096-7
Lipscombe, L. L. (2014). The US diabetes epidemic: tip of the iceberg. Diabetes & Endocrinol. 2 (11), 854–855. doi:10.1016/S2213-8587(14)70172-X
Mobasseri, M., Shirmohammadi, M., Amiri, T., Vahed, N., Hosseini Fard, H., and Ghojazadeh, M. (2020). Prevalence and incidence of type 1 diabetes in the world: a systematic review and meta-analysis. Health Promot. Perspect. 10 (2), 98–115. doi:10.34172/hpp.2020.18
UK Prospective Diabetes Study (UKPDS) Group (1998). Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet (London, Engl. 352 (9131), 837–853. doi:10.1016/S0140-6736(98)07019-6
US Food and Drug Administration (2009). Bioavailability and bioequivalence requirements. 21 CFR 21 Part 320. Available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?cfrpart=320 (Accessed July 5, 2023).
US Food and Drug Administration (2015). Pharmacology review(s) for Tresiba. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/203313Orig1s000_203314Orig1s000PharmR.pdf (Accessed July 5, 2023).
Keywords: INS068 injection, pharmacokinetics, relative bioavailability, healthy subjects, insulin analog
Citation: Chen K-G, Zhang Y-H, Ye P-P, Gao X-H, Song L-L, Zhou H-Y, Li Q, Zhao F-R, Shi J-Y, Yang X-M, Shen K, Feng S and Zhao W (2023) Pharmacokinetics and bioavailability of a new long-acting insulin analog in healthy Chinese volunteers: an open, randomized, single-dose, two-period and two-sequence cross-over study. Front. Pharmacol. 14:1294810. doi: 10.3389/fphar.2023.1294810
Received: 15 September 2023; Accepted: 11 December 2023;
Published: 22 December 2023.
Edited by:
Siva Rama Raju Kanumuri, University of Florida, United StatesReviewed by:
Parag Garhyan, Eli Lilly, United StatesCopyright © 2023 Chen, Zhang, Ye, Gao, Song, Zhou, Li, Zhao, Shi, Yang, Shen, Feng and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Zhao, emhhbzR3ZWkyQGhvdG1haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.