
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 27 November 2023
Sec. Pharmacology of Anti-Cancer Drugs
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1294297
This article is part of the Research Topic Repurposing β-blockers for non-cardiovascular diseases View all 5 articles
 Junfeng Guo1,2
Junfeng Guo1,2 Rongxing Liu1
Rongxing Liu1 Fangfang Sheng1
Fangfang Sheng1 Qiuxiang Wu1
Qiuxiang Wu1 Rufu Xu1
Rufu Xu1 Haitao He3
Haitao He3 Gang Zhang3
Gang Zhang3 Junjie Huang3
Junjie Huang3 Zhe Zhang1*
Zhe Zhang1* Rong Zhang1*
Rong Zhang1*Background: Recent reports have suggested that antihypertensive drugs may play an oncogenic role in common cancers, but it is still uncertain whether this could influence the risk of oral cancer. Through two-sample Mendelian randomization (MR), we sought to assess the causal effect of antihypertensive drugs on oral cancer outcomes.
Methods: To proxy the exposure of antihypertensive drugs, we utilized two genetic instruments, including expression quantitative trait loci of drug target genes and genetic variants within or around drug target genes related to blood pressure from genome-wide association studies. Inverse-variance-weighted MR (IVW-MR) and summary-data-based MR (SMR) were employed to compute the instrument effect estimates.
Results: It was observed through IVW-MR analysis that there is a positive relationship between KCNH2 (target of beta-adrenoceptor blockers)–mediated blood pressure and oral cancer (odds ratio [OR] = 1.197, 95% confidence interval [CI] = 1.028–1.394). Similarly, SMR analysis demonstrated that a higher expression of KCNH2 (target of beta-adrenoceptor blockers) was linked to a greater risk of oral cancer (OR = 2.223, 95% CI = 1.094–4.516). Both analyses yielded no consistent evidence of other associations.
Conclusion: This two-sample MR study proposed a latent causal association between KCNH2 (target of beta-adrenoceptor blockers) inhibition and diminished risk of oral cancer.
Data from GLOBOCAN 2020 suggests that oral cancer is the 16th most common type of malignancy worldwide, with an estimated 377,713 new cases annually (Sung et al., 2021; Warnakulasuriya and Kerr, 2021). Oral cancer is becoming a major public health problem, particularly among young men and women, with an increasing prevalence (Sarode et al., 2020). It is a malignancy of epithelial origin, caused by a variety of factors, including genetics, epigenetics, habitual use of tobacco, areca nut, alcohol, microbial agents, and metabolic disorders such as hypertension, hyperglycemia, and dyslipidemia (Verhulst et al., 2019; Seo et al., 2020; Kumari et al., 2022).
Hypertension is a major contributor to cardiovascular complications, accounting for over 10.8 million deaths annually worldwide (GBD, 2019 Risk Factors Collaborators, 2020; Roth et al., 2020). It is a global health problem, affecting 31.1% of adults, and is marked by a high incidence, disability and mortality rate, and low awareness rate (Williams et al., 2018; Wu et al., 2018; Mills et al., 2020). A nationwide population-based study found a significant linear correlation between a 10 mmHg increase in diastolic blood pressure (DBP) and oral cancer in hypertensive patients (Seo et al., 2020). This emphasizes the need to actively control blood pressure to prevent oral cancer. Currently, clinical trials of antihypertensive drugs have been conducted to determine their efficacy and safety; however, there is an increasing focus on the potentially harmful effects these drugs may have on certain types of cancers. A meta-analysis demonstrated that a long-term intake of antihypertensive drugs can increase the risk of kidney cancer (Xie et al., 2020). In addition, antihypertensive drugs may also increase the risk of prostate cancer (Cao et al., 2018). However, there is no evidence of a correlation between oral cancer and antihypertensive drugs. Previous systematic reviews and meta-analyses of antihypertensive drugs have shown that obtaining reliable evidence regarding the risk of neoplasms is complex. Moreover, traditional drug epidemiology research is open to various biases, including immortal time bias, selection bias, and residual or unmeasured confounding, which may influence the precision and trustworthiness of the results.
Mendelian randomization (MR) is a measure for analyzing the causal effects of exposures on disease outcomes, which uses randomly assigned genetic variants inherited from parents as proxies for the exposures (Sanderson et al., 2022). Allocating genetic alleles randomly eliminates any influence of unknown confounding variables and minimizes the occurrence of measurement errors, as is the case with randomization in randomized controlled trials (RCTs) (Walker et al., 2017). Genetic variants of antihypertensive drug targets can be used as proxies to study the impact of their therapeutic inhibition on disease outcomes (Yarmolinsky et al., 2018). Drug target MR can be employed to anticipate drug development and repurposing prospects by utilizing genetic instruments near or within the target genes to simulate the potential effects of drug targets (Ference et al., 2016).
Our study used two-sample MR to analyze the association between antihypertensive drugs and oral cancer to provide useful guidance for the use of antihypertensive drugs. Furthermore, these findings may promote the reutilization of antihypertensive agents as potential oral cancer prevention strategies for future trial designs.
Our two-sample MR study was based on expression quantitative trait loci (eQTLs) studies and summary-level data from genome-wide association studies (GWAS), and this data are publicly available (Figure 1; Supplementary Table S1). All these studies had the necessary approval from the relevant institutional review boards, and the participants had given their informed consents.
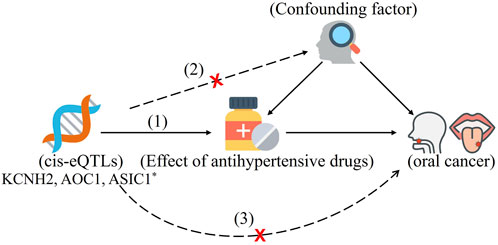
FIGURE 1. The drug-target Mendelian randomization framework in this study. Three assumptions are often required to make causal inference: (1) The chosen instrument is forecastable of the exposure. (2) The instrument is independent of confounding factors. (3) There is no horizontal pleiotropy (the instrument and the outcome are related solely through the exposure). *Common eQTLs SNPs significantly associated with the expression of KCNH2, AOC1, and ASIC1 in blood. They are targets corresponding to antihypertensive drugs.
This study included three common types of antihypertensive drugs: beta-adrenoceptor blockers, potassium-sparing diuretics, and sodium-channel blockers. The publicly accessible eQTL data and DrugBank database (https://www.drugbank.ca/) were used to identify target genes of the active ingredients in each of the antihypertensive drugs (Table 1). We employed available eQTLs for drug target genes as a proxy of exposure to antihypertensive drugs, and obtained the eQTLs summary-level data from the eQTLGen Consortium (https://www.eqtlgen.org/) (Supplementary Table S1). We identified common eQTLs single-nucleotide polymorphisms (SNPs) significantly associated with the expression of KCNH2, AOC1, and ASIC1 in blood (minor allele frequency [MAF] > 1%, p < 5 × 10−8) (Table 2). For this study, only cis-eQTLs, which were eQTLs located within 1 Mb on either side of the encoded gene, were incorporated to generate genetic instruments.
As shown in Table 2, to confirm the detected connection utilizing the eQTLs as an instrument, we proposed an instrument to proxy the exposure of antihypertensive drugs by selecting SNPs associated with DBP at the genome-wide significance level (p < 5 × 10−8) within a 100 kb window of the target gene for each drug. A sample size of 757,601 from the International Consortium of Blood Pressure’s GWAS summary data for DBP was utilized to identify these SNPs, including only common SNPs (MAF >1%) (Evangelou et al., 2018). We permitted the SNPs used as instruments to be in low weak linkage disequilibrium (r2 < 0.3) with each other, to obtain the greatest effectiveness of the instrument for each drug.
The GWAS summary-level data for oral cancer originated from the NHGRI-EBI Catalog of published GWAS (GWAS Catalog), which includes 463,963 Europeans from Croatia, the Czech Republic, France, Germany, Greece, Italy, the Netherlands, Norway, Poland, the Republic of Ireland, Romania, the Russian Federation, Slovakia, Spain, Sweden, Canada, and the United Kingdom. To reduce the risk of collider bias and enable population-level comparisons, we included individuals without oral cancer as controls for all outcomes. All GWAS summary data sources are detailed in Supplementary Table S1.
First, employing eQTL as an instrument, a summary-data-based MR (SMR) method was utilized to generate effect estimates. In addition, this method utilizes summary-level data from GWAS and eQTL studies to analyze the connection between gene expression levels and the outcomes of interest (Zhu et al., 2016). SMR software (version 1.03) was employed for allele harmonization and analysis. Second, employing genetic variants associated with DBP as an instrument, an inverse-variance-weighted MR (IVW-MR) method was utilized to combine effect estimates (Burgess et al., 2013). The TwoSampleMR package (version 0.5.7) in R software was employed for allele harmonization and analysis.
To minimize weak instrument bias, SNPs with an F-statistic >10 were included, and the F-statistic was used to assess the strength of the SNPs employed as the instrument (Burgess et al., 2011). We validated the accuracy of both genetic instruments through positive control analyses. Due to the definite antihypertensive effect of antihypertensive drugs, as a positive control study for the instrument from eQTLs, we examined the relationship between the exposure of interest and DBP. Due to the fact that coronary heart disease (CHD) is one of the main indications for antihypertensive drugs, we examined the relationship between the exposure of interest and CHD as a positive control study for the instruments from the DBP GWAS (Ettehad et al., 2016; Dézsi and Szentes, 2017). The GWAS summary data for CHD from the Coronary ARtery DIsease Genome wide Replication and Meta-analysis plus The Coronary Artery Disease Genetics (CARDIoGRAMplusC4D) Consortium, including 184,305 samples (Nikpay et al., 2015).
In the SMR method, we used the heterogeneity in dependent instruments (HEIDI) test to examine whether the association between gene expression and results was caused by linkage scenarios. A p-value of more than 0.05 in the HEIDI test suggests that the association is not due to linkage. In the IVW-MR method, the Cochran Q test is used to determine the presence or absence of heterogeneity, with p > 0.05 indicating no heterogeneity. We used MR-PRESSO analysis and MR-Egger regression to evaluate the potential horizontal pleiotropy of the SNPs used as instrument variants. MR-PRESSO analysis with a global test of p < 0.05 indicates the existence of horizontal pleiotropic outliers, while MR-Egger regression with p < 0.05 suggests horizontal pleiotropic validity.
Taking multiple testing into account, Bonferroni correction was employed to modify the thresholds of significance level, thus providing a strong evidence for p < 0.017 (3 exposures and 1 outcome) and a suggestive evidence for 0.017 ≤ p < 0.05.
We identified 337, 1430, and 179 cis-eQTLs of drug target gene KCNH2, AOC1, and ASIC1 from the eQTLGen Consortium, and selected the most significant cis-eQTL SNP as a genetic instrument for each drug target gene (Table 2; Supplementary Table S2). In addition, we selected 18, 11, and 6 SNPs within or nearby gene KCNH2, AOC1, and ASIC1 from a GWAS summary data of DBP in the International Consortium of Blood Pressure, respectively (Table 2; Supplementary Table S3). Our F-statistics for all instrument variants were greater than 10, indicating that our study can effectively reduce weak instrument bias (Supplementary Tables S2, S3). Results from the positive control study indicated a significant correlation between exposure to each drug and DBP when eQTLs-proposed instruments were employed (Supplementary Table S4), as well as between exposure to each drug and CHD when DBP GWAS-proposed instruments were employed (Supplementary Table S5). This further confirmed the potency of the chosen genetic instruments.
SMR analysis observed a suggestive evidence that the increased expression of the KCNH2 and ASIC1 genes in blood is linked to an increased risk of oral cancer (KCNH2: OR = 2.223, 95% CI = 1.094–4.516, p = 0.027; ASIC1: OR = 2.742, 95% CI = 1.200–6.265, p = 0.017), while lower expression of the AOC1 gene was associated with a decreased risk of oral cancer (OR = 0.640, 95% CI = 0.429–0.955, p = 0.029) (Figure 2; Supplementary Table S2), indicating that inhibition of KCNH2 and ASIC1 might lower the risk of oral cancer, while upregulation of AOC1 could increase the risk of cancer.
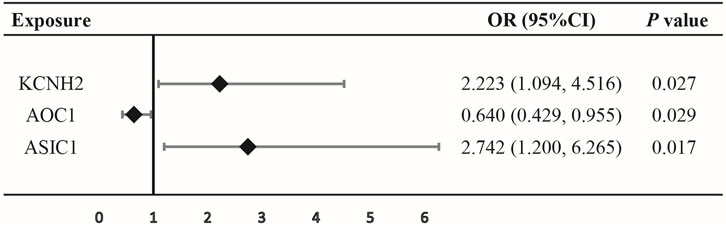
FIGURE 2. Association between expression of gene KCNH2, AOC1, or ASIC1 and oral cancer outcomes by summary-data-based Mendelian randomization (SMR).
IVW-MR analysis also provided suggestive evidence of an association between KCNH2-mediated DBP and risk of oral cancer (OR = 1.197, 95% CI = 1.028–1.394, p = 0.020) (Figure 3; Supplementary Table S6), suggesting that KCNH2 inhibition may be a protective factor against oral cancer. However, no evidence was found, by IVW-MR analysis, to suggest a connection between AOC1, ASIC1-mediated DBP and oral cancer (Figure 3; Supplementary Table S6).
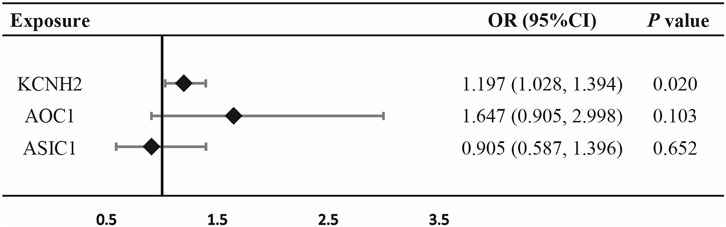
FIGURE 3. Association between diastolic blood pressure (DBP) mediated by gene KCNH2, AOC1, or ASIC1 and oral cancer outcomes by inverse-variance-weighted Mendelian randomization (IVW-MR).
In the SMR method, the HEIDI test indicated that none of the associations observed were caused by a linkage (p > 0.05, Supplementary Table S2).
In the IVW-MR method, Cochran Q test did not reveal any heterogeneity among the reported results (KCNH2, p > 0.05, Supplementary Table S6). MR-PRESSO analysis and MR-Egger regression both indicated that there was no significant overall horizontal pleiotropy in the intercept term (p > 0.05, Supplementary Table S6).
We employed GWAS summary data and cis-eQTL in a two-sample MR analysis to extrapolate the potential impact of antihypertensive drugs on oral cancer. Our study provides suggestive evidence that KCNH2 expression and KCNH2-mediated DBP are positively correlated with oral cancer risk, both of which collectively indicate the potential protective impact of KCNH2 inhibition on oral cancer (OR instrument 1 = 0.450, 95% CI = 0.221–0.914; OR instrument 2 = 0.835, 95% CI = 0.717–0.973). We also observed suggestive evidence of a positive relationship between ASIC1 expression and oral cancer, although this was not validated when using DBP GWAS as an instrument. On the other hand, suggestive evidence that AOC1 expression has a negative association with oral cancer was found; however, this association was not corroborated when using DBP GWAS as an instrument.
Beta-adrenoceptor blockers have been around for years and remain one of the most commonly used medications for cardiovascular diseases. It is recommended as a first-line treatment for hypertension (Chrysant and Chrysant, 2012). Studies have shown that beta-adrenoceptor blockers act on KCNH2, leading to a reduction in blood pressure due to the inhibition of KCNH2 (Bodi et al., 2013). In addition, we also confirmed that KCNH2 is the target gene for beta-adrenoceptor blockers in the DrugBank database. Therefore, this MR study utilized genetic variants linked to KCNH2 expression or KCNH2-mediated DBP as instruments to proxy the exposure of antihypertensive drugs (beta-adrenoceptor blockers). Suggestive evidence from both analyses suggests that KCNH2 inhibition may lower the risk of oral cancer.
The potential association between antihypertensive drugs and cancer risk has been a source of great concern (Tadic et al., 2019; Heisel et al., 2023; Lin et al., 2023; Wang et al., 2023). The results of prior observational studies on the carcinogenic risk of beta-adrenoceptor blockers have been somewhat inconsistent. It has been reported that the use of beta-adrenoceptor blockers could be correlated with a greater risk of breast cancer (Zheng et al., 2021). However, beta-adrenoceptor blockers have also been thought of as potential new treatments for cancer (Engineer et al., 2013). Moreover, a prospective cohort study of 839 individuals monitored over the course of 10 years revealed that beta-adrenoceptor blockers may lead to a decreased risk of cancer (Algazi et al., 2004). Despite the absence of strong evidence, our results provide causal evidence supporting the findings from the above cohort study.
Numerous investigations have assessed the association between antihypertensive drugs and cancer, comprising of randomized controlled studies, basic research, and epidemiological data (Kidoguchi et al., 2022). However, there is currently no relevant study on the relationship between antihypertensive drugs and risk of oral cancer. Our study demonstrated the potential causal relationship between antihypertensive drugs and a decreased risk of oral cancer by using two-sample MR. This is the first and innovative endeavor to investigate the effect of antihypertensive drugs on oral cancer. We employed genetic instruments to proxy drug exposure, aiming to preclude any reverse causal relationships and to minimize confounding bias. In addition, two different genetic instruments were utilized to proxy the studied drugs, which helped to validate each other’s effectiveness estimates. Moreover, sensitivity analyses were conducted to assess the potency of the genetic instruments and the hypothesis of the MR studies.
Despite its novelty, there are some limitations that should be considered when interpreting the results of our study. First, drug-target MR analysis may overestimate the effect of short-term medication use, as they reflect the cumulative, long-term effect of drug target alteration (Zheng et al., 2017). As a result, this study could be more effective in suggesting directions for causal connections of the drugs. Second, there are various classes of antihypertensive drugs, and our study only found a relationship between beta-adrenoceptor blockers and the risk of oral cancer. However, different beta-adrenoceptor blockers have varied pharmacological and pharmacokinetic properties (Khouri et al., 2016). These differing pharmacological properties (e.g., differential absorption rate and plasma half-life) can influence the therapeutic benefit (or experience of adverse effects) of beta-adrenoceptor blockers (Lin et al., 2017). Future evaluation of the potential effects of long-term use of beta-adrenoceptor blockers on cancer risk should therefore include assessment of whether findings are specific to individual agents or classes of beta-adrenoceptor blockers. Third, the efficacy of antihypertensive drugs (beta-adrenoceptor blockers) may differ among subgroups. Nevertheless, since we used data at a summary level, we were not able to carry out subgroup analyses. Consequently, individual level data are needed to gain a more comprehensive understanding in further MR studies. Fourth, despite the multiple sensitivity analyses we conducted to assess the assumptions of the MR study, it was not possible to completely exclude horizontal pleiotropy and/or confounding bias. Fifth, caution should be taken when attempting to apply these findings to other populations, because the study was based primarily on eQTLs and GWAS data from a European population. Sixth, there remains a risk of false-positive findings when examining the protective effect of antihypertensive drugs (beta-adrenoceptor blockers) on oral cancer by applying the Bonferroni correction for multiple tests. However, these findings are preliminary and require confirmatory evidence (from long-term follow-up of clinical trials) in order to guide clinical decision-making.
Our findings present convincing evidence that beta-adrenoceptor blockers are associated with a decreased risk of oral cancer in the European population. In addition, it offers a bright therapeutic prospect for the prevention of oral cancer. Further studies should be conducted to investigate the potential of retargeting or repurposing antihypertensive drugs to expedite the drug development process for oral cancer.
Publicly available datasets were analyzed in this study. This data can be found here: https://www.eqtlgen.org/cis-eqtls.html, http://ftp.ebi.ac.uk/pub/databases/gwas/summary_statistics/, https://www.ukbiobank.ac.uk/, and http://www.cardiogramplusc4d.org/.
The studies involving humans were approved by the relevant institutional review boards. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
JG: Data curation, Investigation, Visualization, Writing–original draft, Writing–review and editing, Formal Analysis. RL: Data curation, Methodology, Software, Writing–original draft, Resources. FS: Formal Analysis, Writing–original draft, Methodology, Visualization. QW: Formal Analysis, Writing–original draft, Methodology, Visualization. RX: Writing–review and editing, Supervision, Validation. HH: Writing–review and editing, Software, Validation. GZ: Writing–review and editing, Software, Validation. JH: Investigation, Writing–original draft. ZZ: Supervision, Writing–review and editing, Conceptualization, Project administration. RZ: Conceptualization, Funding acquisition, Project administration, Writing–review and editing, Supervision.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Chongqing Talent project (CQYC20210303411) and the Chongqing Key Specialty Construction Project of Clinical Pharmacy (Clinical Pharmacy of Cardiovascular Medicine).
We express our appreciation to the participants and investigators who took part in the eQTLGen Consortium, the Global Biodata Coalition, the International Consortium of Blood Pressure, and the CARDIoGRAMplusC4D Consortium.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1294297/full#supplementary-material
CI, confidence interval; CHD, coronary heart disease; DBP, diastolic blood pressure; eQTLs, expression quantitative trait loci; GWAS, genome-wide association studies; HEIDI, heterogeneity in dependent instruments; IVW-MR, Inverse-variance-weighted Mendelian randomization; MAF, minor allele frequency; MR, Mendelian randomization; MR-PRESSO, Mendelian Randomization Pleiotropy RESidual Sum and Outlier; OR, odds ratio; RCTs, randomized controlled trials; SMR, summary-data-based MR; SNPs, single-nucleotide polymorphisms.
Algazi, M., Plu-Bureau, G., Flahault, A., Dondon, M. G., and Lê, M. G. (2004). Could treatments with beta-blockers Be associated with a reduction in cancer risk? Revue d'epidemiologie de sante publique 52 (1), 53–65. doi:10.1016/s0398-7620(04)99022-0
Bodi, I., Franke, G., Pantulu, N. D., Wu, K., Perez-Feliz, S., Bode, C., et al. (2013). Differential effects of the Β-adrenoceptor blockers carvedilol and metoprolol on Sqt1-and sqt2-mutant channels. J. Cardiovasc. Electrophysiol. 24 (10), 1163–1171. doi:10.1111/jce.12178
Burgess, S., Butterworth, A., and Thompson, S. G. (2013). Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37 (7), 658–665. doi:10.1002/gepi.21758
Burgess, S., and Thompson, S. G.CRP CHD Genetics Collaboration (2011). Avoiding bias from weak instruments in mendelian randomization studies. Int. J. Epidemiol. 40 (3), 755–764. doi:10.1093/ije/dyr036
Cao, L., Zhang, S., Jia, C. M., He, W., Wu, L. T., Li, Y. Q., et al. (2018). Antihypertensive drugs use and the risk of prostate cancer: a meta-analysis of 21 observational studies. BMC Urol. 18 (1), 17. doi:10.1186/s12894-018-0318-7
Chrysant, S. G., and Chrysant, G. S. (2012). Current status of Β-blockers for the treatment of hypertension: an update. Drugs today (Barcelona, Spain 1998) 48 (5), 353–366. doi:10.1358/dot.2012.48.5.1782932
Dézsi, C. A., and Szentes, V. (2017). The real role of Β-blockers in daily cardiovascular therapy. Am. J. Cardiovasc. drugs drugs, devices, other interventions 17 (5), 361–373. doi:10.1007/s40256-017-0221-8
Engineer, D. R., Burney, B. O., Hayes, T. G., and Garcia, J. M. (2013). Exposure to acei/arb and Β-blockers is associated with improved survival and decreased tumor progression and hospitalizations in patients with advanced colon cancer. Transl. Oncol. 6 (5), 539–545. doi:10.1593/tlo.13346
Ettehad, D., Emdin, C. A., Kiran, A., Anderson, S. G., Callender, T., Emberson, J., et al. (2016). Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet (London, Engl. 387 (10022), 957–967. doi:10.1016/s0140-6736(15)01225-8
Evangelou, E., Warren, H. R., Mosen-Ansorena, D., Mifsud, B., Pazoki, R., Gao, H., et al. (2018). Genetic analysis of over 1 million People identifies 535 new loci associated with blood pressure traits. Nat. Genet. 50 (10), 1412–1425. doi:10.1038/s41588-018-0205-x
Ference, B. A., Robinson, J. G., Brook, R. D., Catapano, A. L., Chapman, M. J., Neff, D. R., et al. (2016). Variation in Pcsk9 and hmgcr and risk of cardiovascular disease and diabetes. N. Engl. J. Med. 375 (22), 2144–2153. doi:10.1056/NEJMoa1604304
GBD 2019 Risk Factors Collaborators (2020). Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet (London, Engl. 396 (10258), 1223–1249. doi:10.1016/s0140-6736(20)30752-2
Heisel, A. G. U., Vuurboom, M. D., Daams, J. G., de Rie, M. A., Vogt, L., van den Born, B. H., et al. (2023). The use of specific antihypertensive medication and skin cancer risk: a systematic review of the literature and meta-analysis. Vasc. Pharmacol. 150, 107173. doi:10.1016/j.vph.2023.107173
Khouri, C., Jouve, T., Blaise, S., Carpentier, P., Cracowski, J. L., and Roustit, M. (2016). Peripheral vasoconstriction induced by Β-adrenoceptor blockers: a systematic review and a network meta-analysis. Br. J. Clin. Pharmacol. 82 (2), 549–560. doi:10.1111/bcp.12980
Kidoguchi, S., Sugano, N., Yokoo, T., Kaneko, H., Akazawa, H., Mukai, M., et al. (2022). Antihypertensive drugs and cancer risk. Am. J. Hypertens. 35 (9), 767–783. doi:10.1093/ajh/hpac066
Kumari, P., Debta, P., and Dixit, A. (2022). Oral potentially malignant disorders: etiology, pathogenesis, and transformation into oral cancer. Front. Pharmacol. 13, 825266. doi:10.3389/fphar.2022.825266
Lin, S. Y., Huang, H. Y., Chiang, L. T., Huang, L. Y., and Wang, C. C. (2023). Use of calcium channel blockers and risk of breast cancer among women aged 55 Years and older: a nationwide population-based cohort study. Hypertens. Res. 46, 2272–2279. doi:10.1038/s41440-023-01321-y
Lin, T. Y., Chen, C. Y., and Huang, Y. B. (2017). Evaluating the effectiveness of different beta-adrenoceptor blockers in heart failure patients. Int. J. Cardiol. 230, 378–383. doi:10.1016/j.ijcard.2016.12.098
Mills, K. T., Stefanescu, A., and He, J. (2020). The global epidemiology of hypertension. Nat. Rev. Nephrol. 16 (4), 223–237. doi:10.1038/s41581-019-0244-2
Nikpay, M., Goel, A., Won, H. H., Hall, L. M., Willenborg, C., Kanoni, S., et al. (2015). A comprehensive 1,000 genomes-based genome-wide association meta-analysis of coronary Artery disease. Nat. Genet. 47 (10), 1121–1130. doi:10.1038/ng.3396
Roth, G. A., Mensah, G. A., Johnson, C. O., Addolorato, G., Ammirati, E., Baddour, L. M., et al. (2020). Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the gbd 2019 study. J. Am. Coll. Cardiol. 76 (25), 2982–3021. doi:10.1016/j.jacc.2020.11.010
Sanderson, E., Glymour, M. M., Holmes, M. V., Kang, H., Morrison, J., Munafò, M. R., et al. (2022). Mendelian randomization. Nat. Rev. Methods Prim. 2, 6. doi:10.1038/s43586-021-00092-5
Sarode, G., Maniyar, N., Sarode, S. C., Jafer, M., Patil, S., and Awan, K. H. (2020). Epidemiologic aspects of oral cancer. Disease-a-month DM 66 (12), 100988. doi:10.1016/j.disamonth.2020.100988
Seo, J. H., Kim, Y. D., Park, C. S., Han, K. D., and Joo, Y. H. (2020). Hypertension is associated with oral, laryngeal, and esophageal cancer: a nationwide population-based study. Sci. Rep. 10 (1), 10291. doi:10.1038/s41598-020-67329-3
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Tadic, M., Cuspidi, C., Belyavskiy, E., and Grassi, G. (2019). Intriguing relationship between antihypertensive therapy and cancer. Pharmacol. Res. 141, 501–511. doi:10.1016/j.phrs.2019.01.037
Verhulst, M. J. L., Loos, B. G., Gerdes, V. E. A., and Teeuw, W. J. (2019). Evaluating all potential oral complications of diabetes mellitus. Front. Endocrinol. 10, 56. doi:10.3389/fendo.2019.00056
Walker, V. M., Davey Smith, G., Davies, N. M., and Martin, R. M. (2017). Mendelian randomization: a novel approach for the prediction of adverse drug events and drug repurposing opportunities. Int. J. Epidemiol. 46 (6), 2078–2089. doi:10.1093/ije/dyx207
Wang, S., Xie, L., Zhuang, J., Qian, Y., Zhang, G., Quan, X., et al. (2023). Association between use of antihypertensive drugs and the risk of cancer: a population-based cohort study in shanghai. BMC cancer 23 (1), 425. doi:10.1186/s12885-023-10849-8
Warnakulasuriya, S., and Kerr, A. R. (2021). Oral cancer screening: past, present, and future. J. Dent. Res. 100 (12), 1313–1320. doi:10.1177/00220345211014795
Williams, B., Mancia, G., Spiering, W., Agabiti Rosei, E., Azizi, M., Burnier, M., et al. (2018). 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. heart J. 39 (33), 3021–3104. doi:10.1093/eurheartj/ehy339
Wu, Y., Jin, A., Xie, G., Wang, L., Liu, K., Jia, G., et al. (2018). The 20 most important and most preventable health problems of China: a delphi consultation of Chinese experts. Am. J. public health 108 (12), 1592–1598. doi:10.2105/ajph.2018.304684
Xie, Y., Xu, P., Wang, M., Zheng, Y., Tian, T., Yang, S., et al. (2020). Antihypertensive medications are associated with the risk of kidney and bladder cancer: a systematic review and meta-analysis. Aging 12 (2), 1545–1562. doi:10.18632/aging.102699
Yarmolinsky, J., Wade, K. H., Richmond, R. C., Langdon, R. J., Bull, C. J., Tilling, K. M., et al. (2018). Causal inference in cancer epidemiology: what is the role of mendelian randomization? Cancer Epidemiol. Biomarkers Prev. 27 (9), 995–1010. doi:10.1158/1055-9965.Epi-17-1177
Zheng, G., Sundquist, J., Sundquist, K., and Ji, J. (2021). Beta-blockers use and risk of breast cancer in women with hypertension. Cancer Epidemiol. Biomarkers Prev. 30 (5), 965–973. doi:10.1158/1055-9965.Epi-20-1599
Zheng, J., Baird, D., Borges, M. C., Bowden, J., Hemani, G., Haycock, P., et al. (2017). Recent developments in mendelian randomization studies. Curr. Epidemiol. Rep. 4 (4), 330–345. doi:10.1007/s40471-017-0128-6
Keywords: antihypertensive drugs, blood pressure, oral cancer, Mendelian randomization, causal effect
Citation: Guo J, Liu R, Sheng F, Wu Q, Xu R, He H, Zhang G, Huang J, Zhang Z and Zhang R (2023) Association between antihypertensive drugs and oral cancer: a drug target Mendelian randomization study. Front. Pharmacol. 14:1294297. doi: 10.3389/fphar.2023.1294297
Received: 14 September 2023; Accepted: 15 November 2023;
Published: 27 November 2023.
Edited by:
Ayaz Shahid, Western University of Health Sciences, United StatesReviewed by:
Zhang Jiaxing, Guizhou Provincial People’s Hospital, ChinaCopyright © 2023 Guo, Liu, Sheng, Wu, Xu, He, Zhang, Huang, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Zhang, eHFwaGFybWFjeWxhYkAxMjYuY29t; Zhe Zhang, MTUwODY5ODgwMjNAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.