- 1Clinical Pharmacy Office, Baoji Central Hospital, Baoji, Shaanxi, China
- 2Department of Pharmacy, Xi’an Jiaotong University Health Science Center, Xi’an, Shaanxi, China
Introduction: Tocilizumab and baricitinib are recommended treatment options for COVID-19 patients with hyperinflammatory response; however, there is a lack of systematic review directly evaluating their efficacy and safety.
Objective: This review was conducted to evaluate the efficacy and safety of tocilizumab and baricitinib in the treatment of hospitalized patients with COVID-19.
Methods: Relevant databases were searched for studies that compared the effect or safety of baricitinib or tocilizumab in hospitalized patients with COVID-19. The mortality was the main outcome. The hospital length of stay or adverse drug reactions were taken into consideration as secondary endpoints. The analyses were performed in Revman 5.3 or Stata 16.0. The protocol and analysis plan were pre-registered in PROSPERO, with the registration number CRD42023408219.
Results: In total, 10 studies with 2,517 patients were included. The overall pooled data demonstrated that, there was no statistically significant difference in the 28-day mortality rate and the hospital length of stay between the tocilizumab and baricitinib (OR = 1.10, 95% CI = 0.80–1.51, p = 0.57; OR = −0.68, 95% CI = −2.24–0.87, p = 0.39). The adverse reactions including secondary infection rate, thrombotic and bleeding events, and acute liver injury of tocilizumab were significantly higher than that of baricitinib. (OR = 1.49, 95% CI = 1.18–1.88, p < 0.001,OR = 1.52, 95% CI = 1.11–2.08, p = 0.009; OR = 1.52, 95% CI = 1.11–2.08, p = 0.009; OR = 2.24, 95% CI = 1.49–3.35, p < 0.001).
Conclusion: In patients hospitalized with COVID-19, no discernible difference in therapeutic efficacy was observed between tocilizumab and baricitinib; however, the group treated with baricitinib demonstrated a significantly lower incidence of adverse effects.
Introduction
As the coronavirus disease 2019 (COVID-19) pandemic has spread across the globe, which has cost millions of lives (Zhu et al., 2020). Severe COVID-19 is always associated with a hyperinflammatory response, which results in the progressive release of proinflammatory cytokines, especially interleukin IL-6, IL-1, and IL-10, interferon, and tumor necrosis factor, provoking a “cytokine storm”. This response can further progress into acute respiratory distress syndrome, multiple organ failure, and even death (Mehta et al., 2020; Rabaan et al., 2021; Jain et al., 2023). The lack of clinically verified universal criteria makes it is difficult to treat the cytokine storm associated with COVID-19. Hence, in addition to antiviral medications (such as nirmatrelvir/ritonavir and remdesivir), immunosuppressive agents (including corticosteroids, tocilizumab, baricitinib, and anakinra) also play a crucial role in the therapeutic management of COVID-19 infection (Peng et al., 2022; Mengato et al., 2023a; Mengato et al., 2023b; Dahms et al., 2023). Corticosteroids are potent cytokine inhibitors, which gained evidence in reducing mortality and disease progression among COVID-19 patients on supplemental oxygen or mechanical ventilation (RECOVERY Collaborative Group et al., 2021). However, despite the administration of corticosteroids in some individuals, the cytokine storm persisted (Fajgenbaum and June 2020; Tang et al., 2021).
One of the major cytokines regulating the inflammatory response in COVID-19, serum IL-6 has been observed to correlate with mortality and has been proposed as a biomarker predictive of disease severity (Liu et al., 2020; McElvaney et al., 2021). Tocilizumab is a recombinant humanized monoclonal antibody against the human IL-6 receptor. Tocilizumab can effectively block cytokine storms by blocking the IL-6 signaling pathway, thereby inhibiting the human immune system and preventing immune cells from attacking human organs and causing damage (Stone et al., 2020; Rosas et al., 2021). Another therapeutic approach for managing COVID-19 is to target the Janus kinase/signal transducer and activator of the transcription (JAK/STAT) pathway, which mediates the signaling of multiple pro-inflammatory cytokines. Baricitinib is a JAK 1/2 inhibitor. Baricitinib interferes with the entry of viruses into host cells and can also block the JAK-STAT pathway to interfere with the antiviral response (Kalil et al., 2021; Marconi et al., 2021).
Numerous studies have demonstrated a lower risk of mortality and mechanical ventilation in severe COVID-19 patients with cytokine storms compared to standard of care (Gupta et al., 2021; Salama et al., 2021; Ely et al., 2022). According to current guidelines, tocilizumab and baricitinib should both be used in severe COVID-19 patients who have signs of systemic inflammation, preferably in conjunction with concurrent corticosteroids (Infectious Diseases Society of America, 2022; COVID-19 Treatment Guidelines Panel, 2019; World Health Organization, 2021). Therefore, which was superior between tocilizumab and baricitinib is still unclear. Then We conducted a meta-analysis to evaluate the efficacy and safety of tocilizumab and baricitinib in treating severe COVID-19 patients.
Methods
This systematic review was performed according to the preferred items for systematic reviews and meta-analyses 2020 guideline (Page et al., 2021). The protocol and analysis plan were preregistered in PROSPERO, with the registration number CRD42023408219 (http://www. crd. york.ac.uk/PROSPERO/). In our report, no ethical approval was needed as this article is based on previously published article.
Study design
The inclusion criteria encompassed randomized controlled trials or cohort studies that assessed the efficacy and safety of baricitinib versus tocilizumab in hospitalized patients with a clinical diagnosis of COVID-19. Trials that incorporated the combination of baricitinib with tocilizumab or had a follow-up duration shorter than 28 days were excluded from the analysis. Only articles with full-text access were considered, while conference proceedings, review articles, commentaries, etc., were excluded.
The primary outcome of this study was the comparison of the in-hospital mortality. The secondary outcomes were the duration of hospitalization, the duration of ICU stay, and the proportion of patients experiencing serious adverse events (SAEs) (including secondary infection rate, venous thromboembolic events, serious bleeding episodes, and acute liver injury).
Search strategy
We conducted a comprehensive search for relevant studies up to April 2023 in PubMed, Embase, Cochrane Library, and the ClinicalTrials.gov website. Our search utilized keywords such as “COVID-19,” “SARS-CoV-2,” “Tocilizumab,” “Baricitinib,” “IL-6 inhibitors,” and “Janus kinase inhibitor.” The detailed search strategy can be found in Supplementary Appendix S1. Additionally, we identified additional references by thoroughly examining the reference lists of included studies and relevant reviews. Two independent reviewers were involved in the selection process based on title and abstract screening. Any disagreements were resolved through discussion with a third reviewer. Titles and abstracts were carefully screened, with full-text review undertaken when necessary due to ambiguity after reading the abstracts.
Data extraction and quality assessment
Using a consistent data extraction spreadsheet, two writers separately extracted the data. The basic features of the articles, population, intervention, comparison group, and outcome of interest were all included in the retrieved data for pooling. No assumptions or oversimplifications were used when extracting the data.
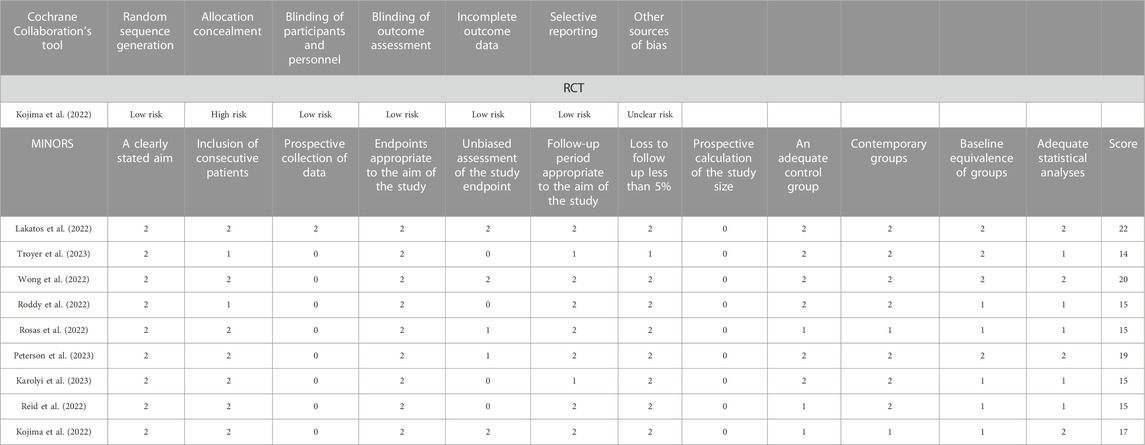
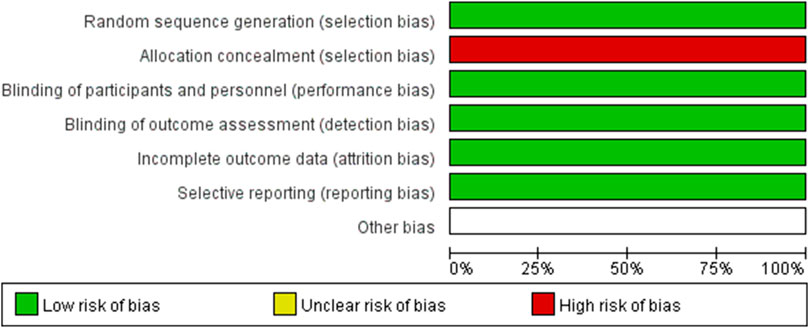
The methodological qualities of all included RCTs were assessed using Cochrane’s tool for bias assessment, which encompassed six specific domains including selection bias, performance bias, detection bias, attrition bias, reporting bias, and other sources of bias. Each domain was classified as low risk of bias, high risk of bias, or unclear risk of bias (Higgins and Green, 2011). The methodological quality of included cohort studies was evaluated using the Methodological Index for Non-Randomized Studies (MINORS) (Slim et al., 2003), which consisted of 12 items with a maximum score of 24.
Statistical analysis
The statistical analyses were conducted using Review Manager 5.3 software and Stata 16.0. The odds ratio (OR) and corresponding 95% confidence intervals (CIs) were utilized as outcome measures. Statistical significance was determined by a p-value less than 0.05, indicating the presence of statistically significant results. Heterogeneity among the studies was estimated using the I2 statistic, with pooled ORs calculated through either a fixed-effect model (in cases where heterogeneity was absent, I2 < 50%) or a random-effect model (when heterogeneity was present, I2 > 50%). Sensitivity analysis was performed employing the one-by-one exclusion method to assess the robustness of findings. Publication bias was evaluated utilizing an Egger funnel plot.
Results
Literature searching
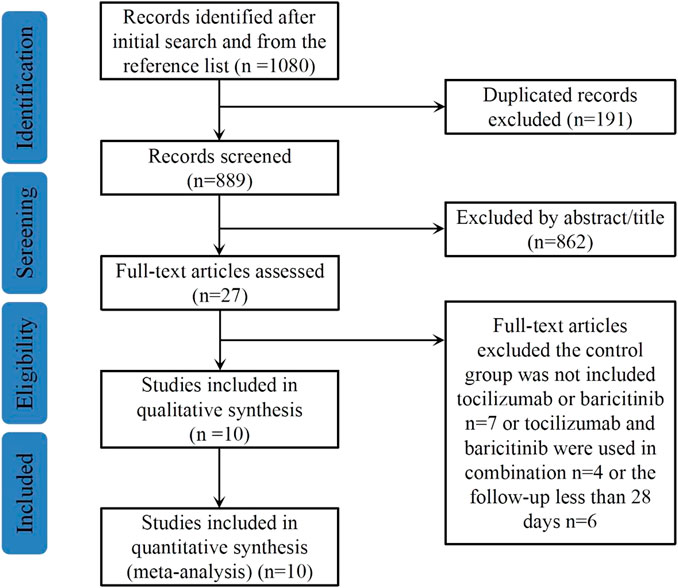
The literature search procedure is shown in Figure 1. A total of 1,080 potentially relevant articles were identified from the above databases. After removing 191 duplicated articles, the titles and abstracts of the remaining 889 articles were screened, 862 articles were then excluded as irrelevant and 27 full-text articles were assessed for eligibility. Finally, a total of 10 articles with 2,517 study participants were included in this meta-analysis (Kojima et al., 2022; Lakatos et al., 2022; Reid et al., 2022; Roddy et al., 2022; Rosas et al., 2022; Wong et al., 2022; Karampitsakos et al., 2023; Peterson et al., 2023; Reid et al., 2023; Troyer et al., 2023).
Study characteristics and quality assessment
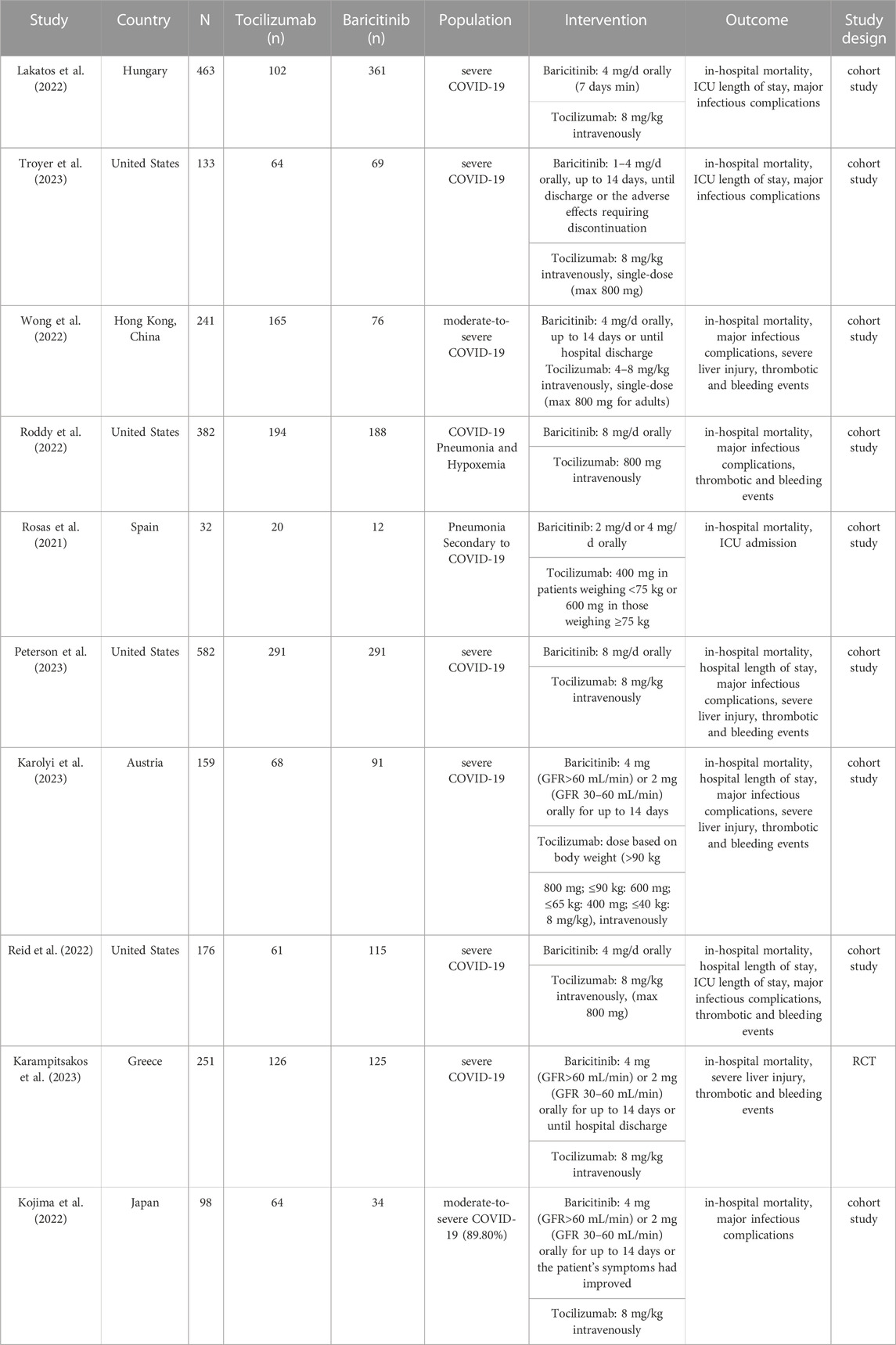
In this meta-analysis with 10 included studies, 1 were RCTs and 9 were cohort studies. The major characteristics of the 10 studies are shown in Table 1. The methodological quality of the RCTs and the comparative cohort studies are shown in Table 2; Figure 2, respectively.
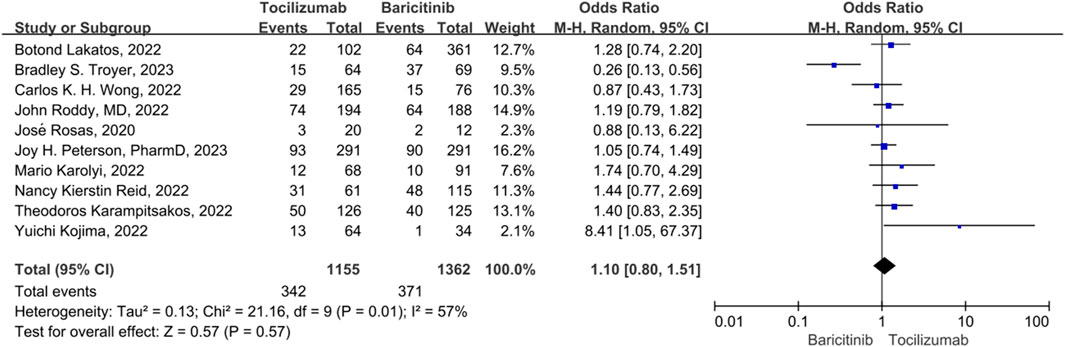
Mortality rate
For mortality, 10 studies were included. The heterogeneity between the 10 studies was statistically different (p = 0.01, I2 = 57%), so a random-effect model was used. There was no statistically significant difference in the 28 days mortality rate between the tocilizumab and baricitinib (OR = 1.10, 95% CI = 0.80–1.51, p = 0.57) (Figure 3).
Hospital length of stay
For length of hospital stay, 3 studies were included. A random model was employed, in that significant heterogeneity was observed among the study (p < 0.01, I2 = 95%). For hospital length of stay, there was no statistically significant difference between the tocilizumab and baricitinib (OR = −0.68, 95% CI = −2.24–0.87, p = 0.39) (Figure 4).
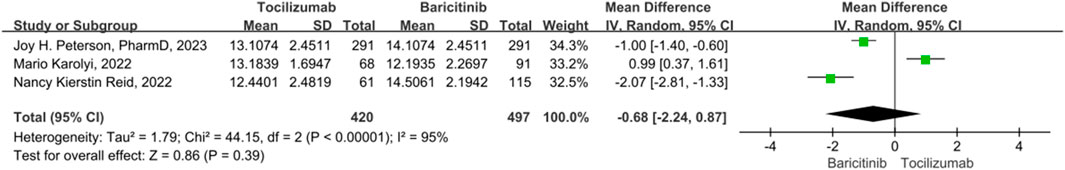
FIGURE 4. The hospital length of stay between tocilizumab and baricitinib in patients with COVID-19.
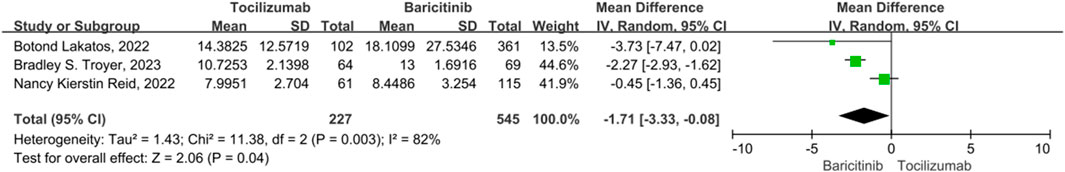
ICU length of stay
There were 3 studies on the ICU length of stay, with significant heterogeneity (p = 0.03, I2 = 82%). The combined results indicate that tocilizumab is associated with a statistically significant reduction in ICU length of stay compared to baricitinib. (OR = −1.71, 95% CI = −3.33–−0.08, p = 0.04) (Figure 5).
Secondary infection rate
Five studies were included in the analysis of secondary infection rates, and no significant heterogeneity was observed among them (p = 0.61, I2 = 0%). Therefore, a fixed-effect model was used to combine the results. The results indicated that tocilizumab had a significantly higher secondary infection rate compared to baricitinib (OR = 1.49, 95% CI = 1.18–1.88, p < 0.001) (Figure 6).
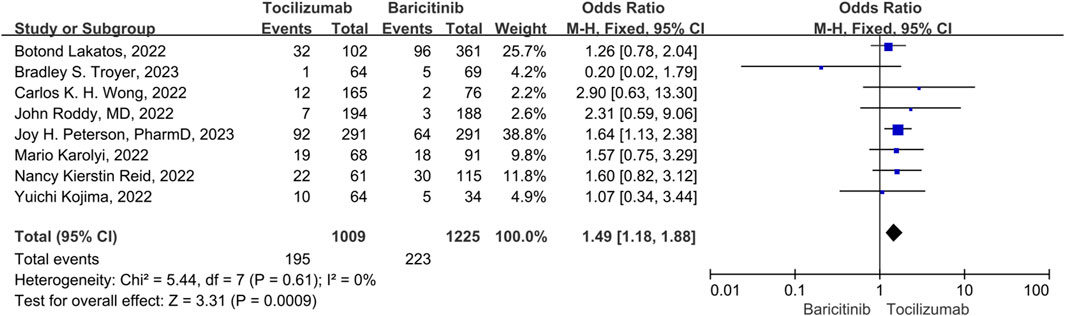
FIGURE 6. The secondary infection rate between tocilizumab and baricitinib in patients with COVID-19.
Thrombotic and bleeding
There were 3 studies included in the thrombotic and bleeding events. No significant heterogeneity was observed among the study (p = 0.31, I2 = 17%), thus a fix-effect model was used to pool the outcomes for studies. The results showed that the thrombotic and bleeding events of tocilizumab significantly was higher than that of the baricitinib (OR = 1.52, 95% CI = 1.11–2.08, p = 0.009) (Figure 7).
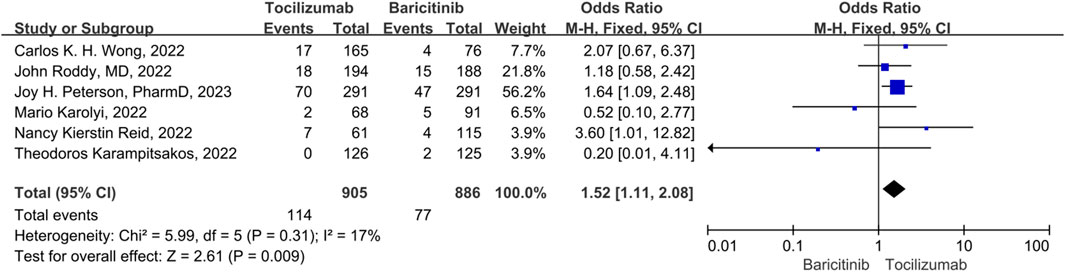
FIGURE 7. Thrombotic and bleeding events between tocilizumab and baricitinib in patients with COVID-19.
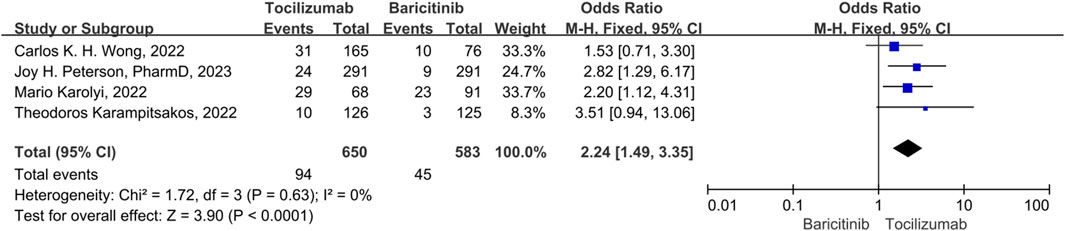
Acute liver injury
There were 4 studies included in the acute liver injury events. A fix-effect model was employed, in that no significant heterogeneity was observed among the studies (p = 0.31, I2 = 17%). The results indicated that the acute liver injury events of tocilizumab was significantly higher than that of the baricitinib (OR = 2.24, 95% CI = 1.49–3.35, p < 0.001) (Figure 8).
Sensitivity analysis
The sensitivity analysis was assessed using a one-by-one elimination method, and the overall findings of the remaining research were statistically unchanged after methodically removing each piece of literature. This indicated that the results of our study were stable (Figure 9).
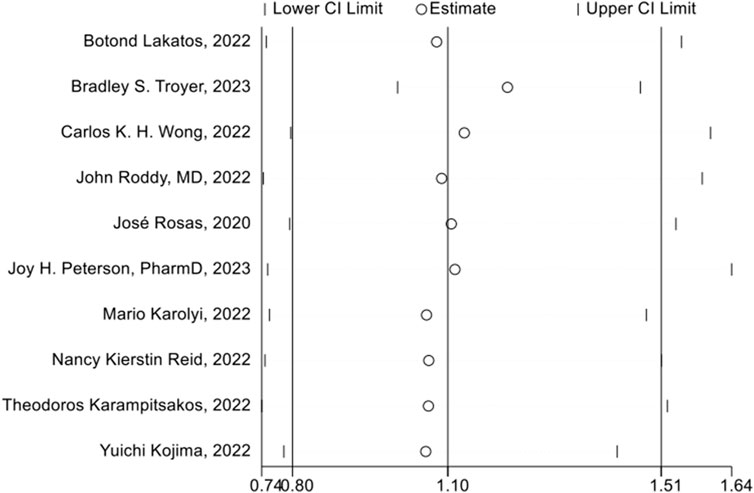
FIGURE 9. Sensitivity analysis of 28 days mortality rate between tocilizumab and baricitinib in patients with COVID-19.
Publication bias
The publication bias of the included studies was evaluated by using funnel plots and Egger’s tests. As no asymmetry of the funnel plot was observed (Figure 10), the plots and the Egger’s test suggested that there was absence of publication in this meta-analysis (t = 0.30, 95% CI = −2.11 to 2.73, p = 0.773) (Figure 11; Table.3).
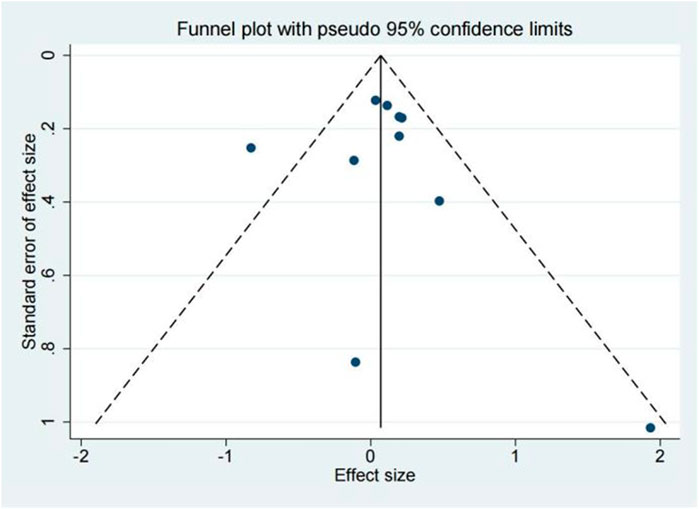
FIGURE 10. Funnel plot of 28 days mortality rate between tocilizumab and baricitinib in patients with COVID-19.
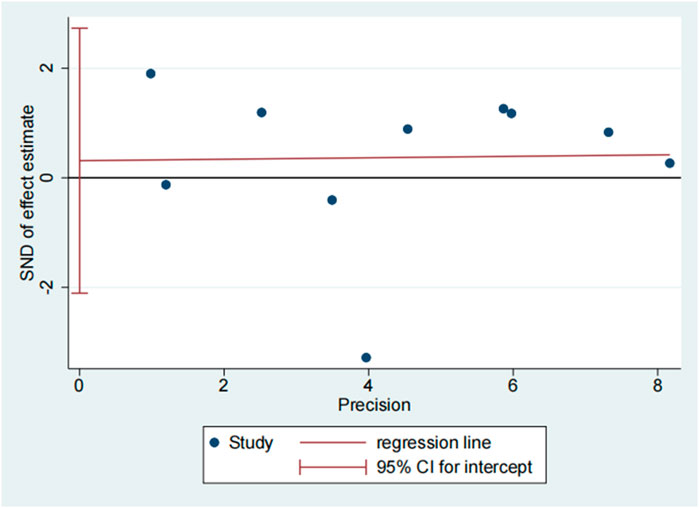
FIGURE 11. Egger’s test of 28 days mortality rate between tocilizumab and baricitinib in patients with COVID-19.
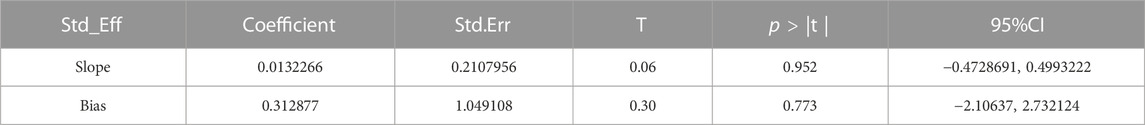
TABLE 3. Egger’s test of 28 days mortality rate between tocilizumab and baricitinib in patients with COVID-19.
Discussion
Summary of results
Although the WHO’s determination that COVID-19 no longer constitutes a PHEIC, it remains an ongoing health issue with a monthly death toll approaching 20,000 worldwide (World Health Organization, 2005). Systemic inflammatory response syndrome is a significant contributor to mortality, and immunomodulatory drugs such as tocilizumab and baricitinib have been widely used in conjunction with dexamethasone (World Health Organization, 2021). Both tocilizumab and baricitinib are thought to be beneficial and are recommended in this patient population, but the relative merits of each have not previously been evaluated. To the best of our knowledge, this is the first meta-analysis to directly evaluate the efficacy and safety of tocilizumab and baricitinib in COVID-19 patients with hyperinflammatory response. In order to compare the therapeutic efficacy of tocilizumab and baricitinib in COVID-19 patients with a hyperinflammatory response, we employed 28 days mortality rate, hospital length of stay, and ICU length of stay as evaluation index. The results of this study showed that there was no statistical difference in 28-day mortality rate (29.61% vs. 27.24%, p = 0.57) and hospital length of stay (p = 0.39) between tocilizumab and baricitinib. However, tocilizumab may result in a shorter ICU stay compared to baricitinib (p = 0.04), possibly due to the slightly higher mortality rate associated with tocilizumab.
Otherwise, we conducted a comparative analysis of the safety profiles of tocilizumab and baricitinib in the treatment of COVID-19 patients in terms of secondary infections, thrombotic and hemorrhagic events, as well as acute liver injury. The results show that the secondary infection rate in tocilizumab were higher than baricitinib among COVID-19 patients (p < 0.001). In addition, compared to baricitinib, tocilizumab may cause greater thrombotic and bleeding events (p = 0.009). Furthermore, tocilizumab caused acute liver damage more frequently than baricitinib (p < 0.001).
Studies from the literature
The previous systematic review demonstrated that both tocilizumab and baricitinib, when compared to standard of care, can reduce the mortality rates and hospital length of stay in patients with predominantly moderate-to-severe COVID-19 (Alkofide et al., 2021; Tharmarajah et al., 2021; Walz et al., 2021; WHO Rapid E et al., 2021). However, there is a lack of head-to-head studies comparing the efficacy and safety of these two treatments. Based on its superior 28-day mortality data, ease of administration, shorter half-life, and lower cost of treatment, baricitinib may be preferred over tocilizumab (Cherian et al., 2022). This finding is consistent with a network meta-analysis that showed no statistically significant difference in mortality rate between the two drugs (Ngamprasertchai et al., 2022).
The JAK-STAT signaling pathway is central to the development of the cytokine storm in COVID-19, which could activate cytokine levels including IL6, IL2, interferon-gamma, etc., (Limen et al., 2022). Baricitinib modulates downstream inflammatory responses via JAK1/JAK2 inhibition and IL-6-induced STAT3 phosphorylation. The anti-cytokine and anti-viral activities of baricitinib are responsible for a rapid reduction in the viral load, inflammatory markers, and IL-6 levels in COVID-19 (Zhang et al., 2020). IL-6 is a pleiotropic cytokine secreted by neutrophils, monocytes, and macrophages and involved in the inflammatory response. This in turn causes impaired oxygen diffusion, and the ensuing inflammation eventually leads to lung fibrosis and multi-organ failure (Gordon et al., 2021). Furthermore, elevated levels of IL-6 have been associated with a hypercoagulable state in patients with COVID-19. Tocilizumab competitively binds to the IL-6 receptor, thereby blocking IL-6-mediated signaling and preventing the assembly of the activated complex with transmembrane proteins. This mitigates immune-mediated damage, lung injury, and reduces oxygen saturation. (Kalil et al., 2021; Rosas et al., 2021; Tharmarajah et al., 2021).
The safety between tocilizumab and baricitinib were still controversial. Numerous prior studies have indicated a lack of substantial disparity in terms of safety between the aforementioned groups; nevertheless, certain recent studies have presented contrasting perspectives. A network meta-analysis was conducted to assess the efficacy and safety of immunomodulators in patients with COVID-19, revealing that tocilizumab may pose a higher risk of infection compared to baricitinib (Ngamprasertchai et al., 2022). Other studies have additionally indicated an elevation in the incidence of thrombosis or acute liver injury among individuals administered tocilizumab as opposed to baricitinib (Karolyi et al., 2023; Peterson et al., 2023; Reid et al., 2023). Our study found that the tocilizumab group had adverse effects more frequently, including secondary infections, thrombotic events, and acute liver damage. The lower rate of adverse effects seen in our study provides valuable insight into the relative safety of short-term use of baricitinib for severe COVID-19. Notably, the pharmacologic half-life of baricitinib is 12 h and that of tocilizumab is up to 13 days (U.S. Food and Drug Administration, 2018; U.S. Food and Drug Administration, 2022)Tocilizumab exhibits a notable capacity to effectively attenuate fever and immune responses triggered by infection for a duration of one or 2 week. Consequently, it is plausible that the incidence of early superinfection might be underestimated (Peterson et al., 2023). Furthermore a single intravenous dose may result in a higher peak blood concentration compared to a daily oral dose, thereby increasing the risk of adverse effects. In addition, although tocilizumab is recommended as a one-time IV infusion for this indication, the full recommended course of baricitinib is 14 days. The duration of baricitinib treatment occasionally fell below a fortnight Thus, a more prolonged drug effect may explain the increased adverse effects seen with tocilizumab.
Strengths and limitations
Our study had several strengths. First, we performed a direct comparison between tocilizumab and baricitinib, which is more compelling than previous studies. Second, our methodology was strictly adhered to the Cochrane Handbook, and the protocol was registered in PROSPERO. Lastly, the number of included articles was the largest, and the evaluation indicators covered efficacy and safety. However, some limitations of our study must be addressed. First, the results were heterogeneous across the included studies. However, Some included studies had inconsistent follow-up time and varying criteria for evaluating efficacy and safety.
In addition, although we did not explore the dominant COVID-19 variants at the time of each study, the effects of variants on the treatment response to immunomodulators were inconclusive. Third, countries or regions might be confounding factors. However, as included studies were multinational and mixed populations were recruited, these will minimize the threat to the validity of this study. Finally, heterogeneity concerning the dosage, timing, and duration of immunomodulator therapy should be further explored, particularly in large-scale clinical trials.
Conclusion
We conducted a meta-analysis to evaluate the efficacy and safety among tocilizumab and baricitinib in treating severe COVID-19 patients. The results showed no difference in the in-hospital mortality rate or hospital length of stay between the two treatments. However, the baricitinib group experienced significantly fewer adverse effects. Although further prospective, randomized trials are needed to further assess this association, our data suggest that baricitinib may be a better choice for treating severe COVID-19 patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Author contributions
JZ: Conceptualization, Data curation, Formal Analysis, Writing–original draft. XF: Investigation, Methodology, Software, Writing–review and editing. XZ: Data curation, Writing–review and editing. FJ: Supervision, Writing–review and editing. YW: Data curation, Writing–review and editing. BY: Software, Writing–review and editing. XL: Data curation, Formal Analysis, Writing–review and editing. DL: Conceptualization, Project administration, Resources, Supervision, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1293331/full#supplementary-material
References
Alkofide, H., Almohaizeie, A., Almuhaini, S., Alotaibi, B., and Alkharfy, K. M. (2021). Tocilizumab and systemic corticosteroids in the management of patients with COVID-19: a systematic review and meta-analysis. Int. J. Infect. Dis. 110, 320–329. doi:10.1016/j.ijid.2021.07.021
Cherian, J. J., Eerike, M., Bagepally, B. S., Das, S., and Panda, S. (2022). Efficacy and safety of baricitinib and tocilizumab in hospitalized patients with COVID-19: a comparison using systematic review and meta-analysis. Front. Pharmacol. 13, 1004308. doi:10.3389/fphar.2022.1004308
COVID-19 Treatment Guidelines Panel (2019). Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health: America. Available at: https://www.covid19treatmentguidelines.nih.gov/ (Accessed on May 8, 2023).
Dahms, K., Mikolajewska, A., Ansems, K., Metzendorf, M. I., Benstoem, C., and Stegemann, M. (2023). Anakinra for the treatment of COVID-19 patients: a systematic review and meta-analysis. Eur. J. Med. Res. 28 (1), 100. doi:10.1186/s40001-023-01072-z
Ely, E. W., Ramanan, A. V., Kartman, C. E., de Bono, S., Liao, R., Piruzeli, M. L. B., et al. (2022). Efficacy and safety of baricitinib plus standard of care for the treatment of critically ill hospitalised adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: an exploratory, randomised, placebo-controlled trial. Lancet Respir. Med. 10 (4), 327–336. doi:10.1016/S2213-2600(22)00006-6
Fajgenbaum, D. C., and June, C. H. (2020). Cytokine storm. N. Engl. J. Med. 383 (23), 2255–2273. doi:10.1056/NEJMra2026131
Gordon, A. C., Angus, D. C., and Derde, L. P. G. (2021). Interleukin-6 receptor antagonists in critically ill patients with covid-19. Reply. N. Engl. J. Med. 385 (12), 1147–1149. doi:10.1056/NEJMc2108482
Gupta, S., Wang, W., Hayek, S. S., Chan, L., Mathews, K. S., Melamed, M. L., et al. (2021). Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 181 (1), 41–51. doi:10.1001/jamainternmed.2020.6252
Higgins, J. P., and Green, S. (2011). Cochrane Handbook for systematic reviews of interventions (version 5.1.0). Available at: http://handbook-5-1.cochrane.org/ (Accessed on April 6, 2023).
Infectious Diseases Society of America (2022). IDSA guidelines on the treatmentand management of patients with COVID-19. Available at: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-andmanagement/ (Accessed on May 8, 2023).
Jain, N. K., Tailang, M., Jain, H. K., Chandrasekaran, B., Sahoo, B. M., Subramanian, A., et al. (2023). Therapeutic implications of current Janus kinase inhibitors as anti-COVID agents: a review. Front. Pharmacol. 14, 1135145. doi:10.3389/fphar.2023.1135145
Kalil, A. C., Patterson, T. F., Mehta, A. K., Tomashek, K. M., Wolfe, C. R., Ghazaryan, V., et al. (2021). Baricitinib plus remdesivir for hospitalized adults with covid-19. N. Engl. J. Med. 384 (9), 795–807. doi:10.1056/NEJMoa2031994
Karampitsakos, T., Papaioannou, O., Tsiri, P., Katsaras, M., Katsimpris, A., Kalogeropoulos, A. P., et al. (2023). Tocilizumab versus baricitinib in hospitalized patients with severe COVID-19: an open label, randomized controlled trial. Clin. Microbiol. Infect. 29 (3), 372–378. doi:10.1016/j.cmi.2022.10.015
Karolyi, M., Gruebl, A., Omid, S., Saak, M., Pawelka, E., Hoepler, W., et al. (2023). Tocilizumab vs. baricitinib in hospitalized severe COVID-19 patients: results from a real-world cohort. Infection 51 (4), 851–858. doi:10.1007/s15010-022-01915-7
Kojima, Y., Nakakubo, S., Takei, N., Kamada, K., Yamashita, Y., Nakamura, J., et al. (2022). Comparative efficacy of tocilizumab and baricitinib administration in COVID-19 treatment: a retrospective cohort study. Med. Kaunas. 58 (4), 513. doi:10.3390/medicina58040513
Lakatos, B., Szabó, B. G., Bobek, I., Kiss-Dala, N., Gáspár, Z., Riczu, A., et al. (2022). Baricitinib vs tocilizumab treatment for hospitalized adult patients with severe COVID-19 and associated cytokine storm: a prospective, investigational, real-world study. Int. J. Infect. Dis. 125, 233–240. doi:10.1016/j.ijid.2022.10.037
Limen, R. Y., Sedono, R., Sugiarto, A., and Hariyanto, T. I. (2022). Janus kinase (JAK)-inhibitors and coronavirus disease 2019 (Covid-19) outcomes: a systematic review and meta-analysis. Expert Rev. Anti Infect. Ther. 20 (3), 425–434. doi:10.1080/14787210.2021.1982695
Liu, B., Li, M., Zhou, Z., Guan, X., and Xiang, Y. (2020). Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 111, 102452. doi:10.1016/j.jaut.2020.102452
Marconi, V. C., Ramanan, A. V., de Bono, S., Kartman, C. E., Krishnan, V., Liao, R., et al. (2021). Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir. Med. 9 (12), 1407–1418. doi:10.1016/S2213-2600(21)00331-3
McElvaney, O. J., Curley, G. F., Rose-John, S., and McElvaney, N. G. (2021). Interleukin-6: obstacles to targeting a complex cytokine in critical illness. Lancet Respir. Med. 9 (6), 643–654. doi:10.1016/S2213-2600(21)00103-X
Mehta, P., McAuley, D. F., Brown, M., Sanchez, E., Tattersall, R. S., and Manson, J. J.HLH Across Speciality Collaboration, UK (2020). COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395 (10229), 1033–1034. doi:10.1016/S0140-6736(20)30628-0
Mengato, D., Mazzitelli, M., Francavilla, A., Bettio, M., Sasset, L., Presa, N., et al. (2023a). Changing patterns and clinical outcomes of hospitalized patients with COVID-19 severe pneumonia treated with remdesivir according to vaccination status: results from a real-world retrospective study. Clin. Exp. Med. 23 (6), 2749–2756. doi:10.1007/s10238-023-01036-x
Mengato, D., Mazzitelli, M., Francavilla, A., Bettio, M., Sasset, L., Presa, N., et al. (2023b). Correction to: changing patterns and clinical outcomes of hospitalized patients with COVID-19 severe pneumonia treated with remdesivir according to vaccination status: results from a real-world retrospective study. Clin. Exp. Med. 23 (6), 2757. doi:10.1007/s10238-023-01088-z
Ngamprasertchai, T., Kajeekul, R., Sivakorn, C., Ruenroegnboon, N., Luvira, V., Siripoon, T., et al. (2022). Efficacy and safety of immunomodulators in patients with COVID-19: a systematic review and network meta-analysis of randomized controlled trials. Infect. Dis. Ther. 11 (1), 231–248. doi:10.1007/s40121-021-00545-0
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Peng, J., Fu, M., Mei, H., Zheng, H., Liang, G., She, X., et al. (2022). Efficacy and secondary infection risk of tocilizumab, sarilumab and anakinra in COVID-19 patients: a systematic review and meta-analysis. Rev. Med. Virol. 32 (3), e2295. doi:10.1002/rmv.2295
Peterson, J. H., Paranjape, N. S., Grundlingh, N., and Priestley, J. L. (2023). Outcomes and adverse effects of baricitinib versus tocilizumab in the management of severe COVID-19. Crit. Care Med. 51 (3), 337–346. doi:10.1097/CCM.0000000000005756
Rabaan, A. A., Al-Ahmed, S. H., Muhammad, J., Khan, A., Sule, A. A., Tirupathi, R., et al. (2021). Role of inflammatory cytokines in COVID-19 patients: a review on molecular mechanisms, immune functions, immunopathology and immunomodulatory drugs to counter cytokine storm. Vaccines (Basel) 9 (5), 436. doi:10.3390/vaccines9050436
RECOVERY Collaborative Group Horby, P., Lim, W. S., Emberson, J. R., Mafham, M., Bell, J. L., and Linsell, L. (2021). Dexamethasone in hospitalized patients with covid-19. N. Engl. J. Med. 384 (8), 693–704. doi:10.1056/NEJMoa2021436
Reid, N. K., Joyner, K. R., and Lewis-Wolfson, T. D. (2022). Baricitinib versus tocilizumab for the treatment of moderate to severe COVID-19. Ann. Pharmacother. 57, 769–775. doi:10.1177/10600280221133376
Reid, N. K., Joyner, K. R., and Lewis-Wolfson, T. D. (2023). Baricitinib versus tocilizumab for the treatment of moderate to severe COVID-19. Ann. Pharmacother. 57 (7), 769–775. doi:10.1177/10600280221133376
Roddy, J., Wells, D., Schenck, K., Santosh, S., and Santosh, S. (2022). Tocilizumab versus baricitinib in patients hospitalized with COVID-19 pneumonia and hypoxemia: a multicenter retrospective cohort study. Crit. Care Explor 4 (5), e0702. doi:10.1097/CCE.0000000000000702
Rosas, I. O., Bräu, N., Waters, M., Go, R. C., Hunter, B. D., Bhagani, S., et al. (2021). Tocilizumab in hospitalized patients with severe covid-19 pneumonia. N. Engl. J. Med. 384 (16), 1503–1516. doi:10.1056/NEJMoa2028700
Rosas, J., Liaño, F. P., Cantó, M. L., Barea, J. M. C., Beser, A. R., Rabasa, J. T. A., et al. (2022). Experience with the use of baricitinib and tocilizumab monotherapy or combined, in patients with interstitial pneumonia secondary to coronavirus COVID19: a real-world study. Reumatol. Clin. Engl. Ed. 18 (3), 150–156. doi:10.1016/j.reumae.2020.10.006
Salama, C., Han, J., Yau, L., Reiss, W. G., Kramer, B., Neidhart, J. D., et al. (2021). Tocilizumab in patients hospitalized with covid-19 pneumonia. N. Engl. J. Med. 384 (1), 20–30. doi:10.1056/NEJMoa2030340
Slim, K., Nini, E., Forestier, D., Kwiatkowski, F., Panis, Y., and Chipponi, J. (2003). Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J. Surg. 73 (9), 712–716. doi:10.1046/j.1445-2197.2003.02748.x
Stone, J. H., Frigault, M. J., Serling-Boyd, N. J., Fernandes, A. D., Harvey, L., Foulkes, A. S., et al. (2020). Efficacy of tocilizumab in patients hospitalized with covid-19. N. Engl. J. Med. 383 (24), 2333–2344. doi:10.1056/NEJMoa2028836
Tang, G., Huang, M., Luo, Y., Liu, W., Lin, Q., Mao, L., et al. (2021). The dynamic immunological parameter landscape in coronavirus disease 2019 patients with different outcomes. Front. Immunol. 12, 697622. doi:10.3389/fimmu.2021.697622
Tharmarajah, E., Buazon, A., Patel, V., Hannah, J. R., Adas, M., Allen, V. B., et al. (2021). IL-6 inhibition in the treatment of COVID-19: a meta-analysis and meta-regression. J. Infect. 82 (5), 178–185. doi:10.1016/j.jinf.2021.03.008
Troyer, B. S., Kovacic Scherrer, N., and Garavaglia, J. (2023). Tocilizumab versus baricitinib for the treatment of COVID-19 in patients with obesity. J. Pharm. Pract. 2023, 089719002311589. doi:10.1177/08971900231158931
U.S. Food and Drug Administration (2018). Olumiant (baricitinib). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/207924s000lbl.pdf (Accessed on June 19, 2023).
U.S. Food and Drug Administration (2022). Actemra (tocilizumab). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/125276s134lbl.pdf (Accessed on June 19, 2023).
Walz, L., Cohen, A. J., Rebaza, A. P., Vanchieri, J., Slade, M. D., Dela Cruz, C. S., et al. (2021). JAK-inhibitor and type I interferon ability to produce favorable clinical outcomes in COVID-19 patients: a systematic review and meta-analysis. BMC Infect. Dis. 21 (1), 47. doi:10.1186/s12879-020-05730-z
WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group Shankar-Hari, M., Vale, C. L., Godolphin, P. J., Fisher, D., Higgins, J. P. T., Spiga, F., et al. (2021). Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA 326 (6), 499–518. doi:10.1001/jama.2021.11330
Wong, C. K. H., Lau, K. T. K., Au, I. C. H., Xiong, X., Chung, M. S. H., Leung, B. Y. C., et al. (2022). Initiation of tocilizumab or baricitinib were associated with comparable clinical outcomes among patients hospitalized with COVID-19 and treated with dexamethasone. Front. Pharmacol. 13, 866441. doi:10.3389/fphar.2022.866441
World Health Organization. (2005) Statement on the fifteenth meeting of the IHR Emergency Committee on the COVID-19 pandemic. Avaliable at: https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemicd (Accessed on June 2, 2023).
World Health Organization (2021). Therapeutics and COVID-19: living guideline. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.3 (Accessed on May 8, 2023).
Zhang, X., Zhang, Y., Qiao, W., Zhang, J., and Qi, Z. (2020). Baricitinib, a drug with potential effect to prevent SARS-COV-2 from entering target cells and control cytokine storm induced by COVID-19. Int. Immunopharmacol. 86, 106749. doi:10.1016/j.intimp.2020.106749
Keywords: COVID-19, immunomodulators, interleukin-6 inhibitors, JAK-STAT inhibitors, tocilizumab, baricitinib, efficacy and safety
Citation: Zhang J, Fan X, Zhang X, Jiang F, Wu Y, Yang B, Li X and Liu D (2023) Efficacy and safety of tocilizumab and baricitinib among patients hospitalized for COVID-19: a systematic review and meta-analysis. Front. Pharmacol. 14:1293331. doi: 10.3389/fphar.2023.1293331
Received: 13 September 2023; Accepted: 03 November 2023;
Published: 22 November 2023.
Edited by:
Pouya Hassandarvish, University of Malaya, MalaysiaReviewed by:
Daniele Mengato, University Hospital of Padua, ItalyRafidah Lani, University of Malaya, Malaysia
Copyright © 2023 Zhang, Fan, Zhang, Jiang, Wu, Yang, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Liu, bGl1ZG9uZzY5MTEyMkAxMjYuY29t
 Jin Zhang
Jin Zhang Xiongxiong Fan1
Xiongxiong Fan1 Dong Liu
Dong Liu