
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 15 November 2023
Sec. Pharmacogenetics and Pharmacogenomics
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1290785
Introduction: Bleeding is one of the most undesirable complications of direct oral anticoagulants (DOACs). While the ryanodine receptor (RYR2) has been related to cardiac diseases, research on bleeding complications is lacking. This study aimed to elucidate the association between RYR2 and bleeding risk to develop the risk scoring system in patients treated with DOACs.
Methods: This study was a retrospective analysis of prospectively collected samples. We selected ten SNPs within the RYR2 gene, and two models were constructed (Model I: demographic factors only, Model II: demographic and genetic factors) in multivariable analysis. Independent risk factors for bleeding were used to develop a risk scoring system.
Results: A total of 447 patients were included, and 49 experienced either major bleeding or clinically relevant non-major bleeding. In Model I, patients using rivaroxaban and experiencing anemia exhibited an increased bleeding risk after adjusting for covariates. Upon incorporating genetic factors into Model I, a significant association with bleeding was also observed in cases of overdosing on DOACs and in patients with a creatinine clearance (CrCl) < 30 mL/min, in addition to rivaroxaban and anemia (Model II). Among genetic factors, RYR2 rs12594 GG, rs17682073 AA, rs3766871 GG, and rs6678625 T alleles were associated with bleeding complications. The area under the receiver operating characteristic curve (AUROC) of Model I was 0.670, whereas that of Model II increased to 0.803, demonstrating better performance with the inclusion of genetic factors. Using the significant variables in Model II, a risk scoring system was constructed. The predicted bleeding risks for scores of 0, 1–2, 3–4, 5–6, 7–8, and 9–10 points were 0%, 1.2%, 4.6%, 15.7%, 41.7%, and 73.3%, respectively.
Conclusion: This study revealed an association between RYR2 and bleeding complications among patients taking DOACs and established a risk scoring system to support individualized DOAC treatment for these patients.
Direct oral anticoagulants (DOACs) are oral anticoagulants that have revolutionized the management of thromboembolic disorders. DOACs have demonstrated comparable efficacy to conventional agents such as coumadin in preventing strokes for patients with atrial fibrillation (AF) and in treating venous thromboembolism (VTE) while exhibiting a reduced risk of bleeding complications (Martin et al., 2016). Unlike warfarin, DOACs exert their effects by directly inhibiting specific clotting factors, including factor Xa or thrombin, without requiring routine monitoring of the international normalized ratio (INR) or dose adjustments. Their rapid onset of action, predictable pharmacokinetics (PKs), and fewer drug-drug interactions collectively position DOACs as an attractive therapeutic alternative to warfarin (Bauer, 2013).
Bleeding is one of the most undesirable complications of anticoagulation treatment (Lip et al., 2018). Although DOACs generally have a safe profile, major bleeding events still occur with a frequency ranging from 3.5% to 5.3% per person-year (Lip et al., 2018). It has also been reported that DOACs may carry risks of gastrointestinal bleeding comparable to or even higher than those associated with warfarin (Feagins and Weideman, 2018). Thus, it is crucial to consider factors that increase the risk of bleeding, such as advanced age, renal impairment, and concomitant use of other medications while on DOAC treatment (Chan et al., 2018). For individuals undergoing anticoagulant treatment, the HAS-BLED score is utilized as a tool to assess the risk of bleeding (Gao et al., 2021). The HAS-BLED encompasses factors such as hypertension, abnormal renal or liver function, stroke, bleeding history or predisposition, an unstable INR, age over 65 years, and the use of drugs or alcohol.
The extent of variability in how individuals respond to DOACs regarding their PKs and pharmacodynamics has not been fully clarified, suggesting the potential involvement of genetic factors. This variability can be understood through gene polymorphisms, such as CES1 and ABCB1, responsible for the activation or transport of DOACs (Raymond et al., 2021; Ferri et al., 2022). In addition, the metabolism of factor Xa inhibitors involves cytochrome P450 (CYP) enzymes, specifically CYP3A4 and CYP3A5 (Raymond et al., 2021). Consequently, prior research has primarily focused on identifying genetic variations within the genes that impact DOAC transport and metabolism (Tseng et al., 2018). Thus, there is a need to identify novel genetic factors associated with DOAC responses.
Ryanodine receptors (RyRs) are Ca2+-induced Ca2+ release channels that play essential roles in neurons, muscle cells, and epithelial cells. Among three isoforms that encode RyR genes (RYR), RYR2 is expressed predominantly in cardiac muscle (Avila et al., 2019), and it has been reported to be associated with cardiac diseases such as arrhythmias and sudden cardiac death (SCD) (Napolitano and Priori, 2007; Steinberg et al., 2023). Abnormalities of perfusion caused by arrhythmias have deleterious effects on hemodynamic instability (Kochiadakis and Kallergis, 2012), which is a significant risk factor for bleeding and mortality (Lee et al., 2013). However, research concerning the impact of RYR2 on bleeding complications is lacking. Therefore, the objective of this study was to elucidate the association between RYR and bleeding risk and to construct the risk scoring system in patients undergoing DOAC treatment.
This retrospective cohort study was conducted at Ewha Womans University Mokdong Hospital and Ewha Womans University Seoul Hospital from June 2018 to December 2021. The study included patients diagnosed with AF who were administered DOACs (namely, apixaban, edoxaban, rivaroxaban, or dabigatran) for a minimum of 3 months. Patients were excluded if they: 1): experienced thromboembolic events during the study period, 2): experienced a bleeding event 1 year after taking DOACs, 3): had minor unverified bleeding events, 4): did not provide a sufficient DNA analysis sample, or 5): withdrew their informed consent. The primary outcome assessed in this study was major bleeding or clinically relevant non-major bleeding (CRNMB), as defined by criteria established by the International Society on Thrombosis and Haemostasis (ISTH) (Schulman et al., 2005; Kaatz et al., 2015). According to the guidelines, cases were considered to have experienced major bleeding if one of the following clinical manifestations was present in the medical chart: 1) fatal bleeding, 2) symptomatic bleeding in a critical organ, or 3) bleeding causing a decrease in hemoglobin level of 20 g/L or more or leading to transfusion of two or more units of white blood or red cells. Patients were recorded as having experienced CRNMB if they had a bleeding history that did not qualify as major bleeding and required any medical or surgical intervention to treat the bleeding.
All procedures were approved by the Ethics Committee of the Institutional Review Board (IRB number: 2019-05-038 and 2018-04-006), and the study adhered to the ethical principles outlined in the 1975 Declaration of Helsinki and its later amendments. Written informed consent was obtained from all participants prior to their enrollment. After obtaining consent, DNA samples were collected prospectively from the blood or saliva of each patient for subsequent DNA analysis. Demographic and clinical information, including sex, age, weight, height, creatinine clearance (CrCl), DOAC prescription data (types, dosage, duration), history of thromboembolic events (stroke, transient ischemic attack, and thromboembolism), bleeding events, comorbidities, smoking and alcohol status, and co-medication, were then retrospectively extracted from the electronic medical records. CYP inhibitors included ritonavir, clarithromycin, quinidine, fluconazole, cyclosporine, amiodarone, dronedarone, diltiazem, verapamil, and imatinib, while CYP inducers included carbamazepine, phenytoin, phenobarbital, and rifampicin. The CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke, vascular disease, age 65–74 years, and sex category; range 0–9) score for stroke risk and the modified HAS-BLED score for bleeding risk were estimated from their components (Lip et al., 2010; Gao et al., 2021). We used a modified HAS-BLED score that excludes the INR from the calculation since the INR is not monitored in patients taking DOACs (Tsu et al., 2015). A score of ≥3 indicates high risks for CHA2DS2-VASc and HAS-BLED scores, so we set the cutoff at 3 in the analysis (Lip et al., 2010; Tsu et al., 2015).
We opted for ten SNPs within the RYR2 gene and included ABCB1 rs3842 as a confounder based on previous research findings (Westaway et al., 2011; Akilzhanova et al., 2014; Francia et al., 2015; Chen et al., 2019; Hopton et al., 2022). Comprehensive information regarding these SNPs, including their chromosomal positions, functional information, and wild-type/variant alleles, was obtained from the National Center for Biotechnology Information (Sayers et al., 2022). To assess the minor allele frequency and linkage disequilibrium in Asian populations, Haploreg 4.1 was employed (Ward and Kellis, 2016).
Genomic DNA from blood samples was collected using the QIAamp DNA Blood Mini Kit (QIAGEN GmbH, Hilden, Germany), while saliva samples were analyzed using OraGene-600 kits (DNA Genotek, Ottawa, ON, Canada) and PrepIT reagents (DNA Genotek, OTT, Canada). We employed the TaqMan SNP genotyping assay (Applied Biosystems, Foster City, CA, United States) to genotype eleven SNPs. All SNPs were checked to ensure they were in Hardy–Weinberg equilibrium.
The chi-squared test was utilized to assess genotyping data for deviations from the Hardy-Weinberg equilibrium. To evaluate the genetic association, we applied both dominant and recessive models, and the most appropriate model was selected based on their effect size and statistical significance.
We employed two analytical approaches: logistic regression and survival analysis. For logistic regression analysis, the characteristics between case and control groups were assessed using Fisher’s exact test or the chi-squared test. A multivariable logistic regression analysis that incorporated variables with p-values <0.05 from the univariate analysis, in addition to age, sex, and ABCB1 rs3842, was performed. Unadjusted and adjusted odds ratios (ORs) with 95% confidence intervals (CIs) were calculated from the univariate and multivariable analyses. The fitness of the prediction model to the data was evaluated using the Hosmer–Lemeshow goodness-of-fit. The discriminative power of the model was assessed using the area under the receiver operating characteristic curve (AUROC). For survival analysis, we set the follow-up time as 1 year, and the time between the initiation of DOACs and the bleeding event was calculated in days. If a patient did not experience any bleeding event within the 1-year study period, he or she was censored. We used whichever came first between the last prescribed date or 365 days as the censored time. We employed Cox proportional hazard regression models and calculated the crude and adjusted hazard ratios (HRs) with 95% CIs.
To develop a risk scoring system, the adjusted OR (AOR) was divided by the minimum AOR observed within the variables and rounded to the closest integer. We subsequently conducted a logistic regression analysis to compare the observed and predicted risks. A p-value <0.05 was considered statistically significant. All the statistical analyses were performed using two-tailed statistics and SPSS version 20.0 (IBM Corp., Armonk, NY, United States).
Of the initial 576 enrolled patients, 447 were included in the final analysis after excluding those not meeting the criteria (Figure 1). Among them, 13 experienced major bleeding, and 36 patients had CRNMB. The demographic and clinical characteristics of the eligible patients are presented in Table 1. The mean age of the participants was 70 years, and 164 patients (36.7%) were female. Patients with severe renal impairment (CrCl less than 30 mL/min) were susceptible to bleeding compared to those with moderate or normal renal function (26.3% vs. 10.3%, p = 0.047). The types of DOACs exhibited a statistically significant difference between the bleeding and non-bleeding groups (p = 0.003). Of the 71 patients on rivaroxaban, 16 (22.5%) experienced a bleeding event, which had the highest incidence, followed by edoxaban (11.6%), apixaban (7.3%), and dabigatran (5.8%). The significant difference in the exposed dosage of DOACs was observed between the bleeding and non-bleeding group (p = 0.018). While 36.4% experienced bleeding in the overdosed group, 9.5% of the standard-dosed patients, and 12.0% of the underdosed patients experienced bleeding, respectively. Moreover, patients with anemia showed higher bleeding incidence than those without anemia (17.8% vs. 8.5%, p = 0.006).
The grouped genotype analysis is shown in Table 2. The genotype distributions were all in Hardy-Weinberg equilibrium, suggesting the representativeness of all samples. Five SNPs of RYR2, specifically rs10925391, rs12594, rs17682073, rs3766871, and rs6678625, and ABCB1 rs3842 showed a significant statistical difference between bleeding and non-bleeding groups. For RYR2 polymorphisms, individuals with the CC genotype for rs10925391 and with the GG genotype for rs12594 experienced more bleeding events than those carrying the A allele (16.5% vs. 9.2%, p = 0.035; 35.3% vs. 9.9%, p = 0.006, respectively). Patients with the wild homozygotes of rs17682073 and rs3766871 had more bleeding than individuals with the variant allele (13.2% vs. 5.6%, p = 0.015; 12.4% vs. 3.0%, p = 0.023, respectively). Furthermore, patients carrying the T allele of rs6678625 tended to experience more bleeding events (27.3% vs. 9.7%, p = 0.006). In addition to RYR2, bleeding events occurred more often in patients with the ABCB1 rs3842 CC genotype (21.7% vs. 9.8%, p = 0.014).
Multivariable regression analyses were conducted in two analytical approaches, incorporating clinical and genetic variables that were significant in the univariate analysis in addition to age and sex. For logistic regression analysis, two models were constructed: Model I included demographic actors only, and Model II included both demographic and genetic factors (Table 3). In Model I, rivaroxaban was associated with a 2.9-fold increased risk of bleeding (95% CI: 1.5–5.9) compared with other DOACs, and patients with anemia showed a 2.5-fold increased bleeding risk (95% CI: 1.3–4.8) compared with those without anemia after adjusting for covariates. When genetic factors were incorporated into Model I, a significant association with bleeding was observed for overdosing of DOACs and CrCl <30 mL/min in addition to the type of DOAC and anemia. Overdosed individuals displayed a 5.2-fold increased risk of bleeding (95% CI: 1.2–23.1), and patients with severely impaired renal function exhibited a 4.0-fold higher risk than those with a CrCl ≥30 mL/min (95% CI: 1.1–14.8). Among the genetic factors, the RYR2 rs3766871 GG genotype had the most significant impact on bleeding risk, increasing the risk for GG genotype carriers by 6.6 times (95% CI: 1.4–32.2) compared with A allele carriers. Individuals with RYR2 rs12594 variant homozygotes (GG) experienced a 5.9-fold increased bleeding risk (95% CI: 1.7–20.7) compared to those carrying the wild-type allele. As for RYR2 rs17682073 and rs6678625, patients with the AA genotype of rs17682073 and the T allele of rs6678625 experienced a 3.5-fold (95% CI: 1.4–8.9) and a 3.7-fold (95% CI: 1.5–9.5) increased risk of bleeding, respectively. The Hosmer–Lemeshow test indicated good model fit for both models (χ2 = 0.734, p = 0.693 for Model I; χ2 = 4.950 and p = 0.550 for Model II). The AUROC of Model I was 0.670 (95% CI: 0.579–0.761), whereas that of Model II showed an increased AUROC of 0.803 (95% CI: 0.735–0.871), demonstrating enhanced performance upon incorporating genetic factors (Figure 2).
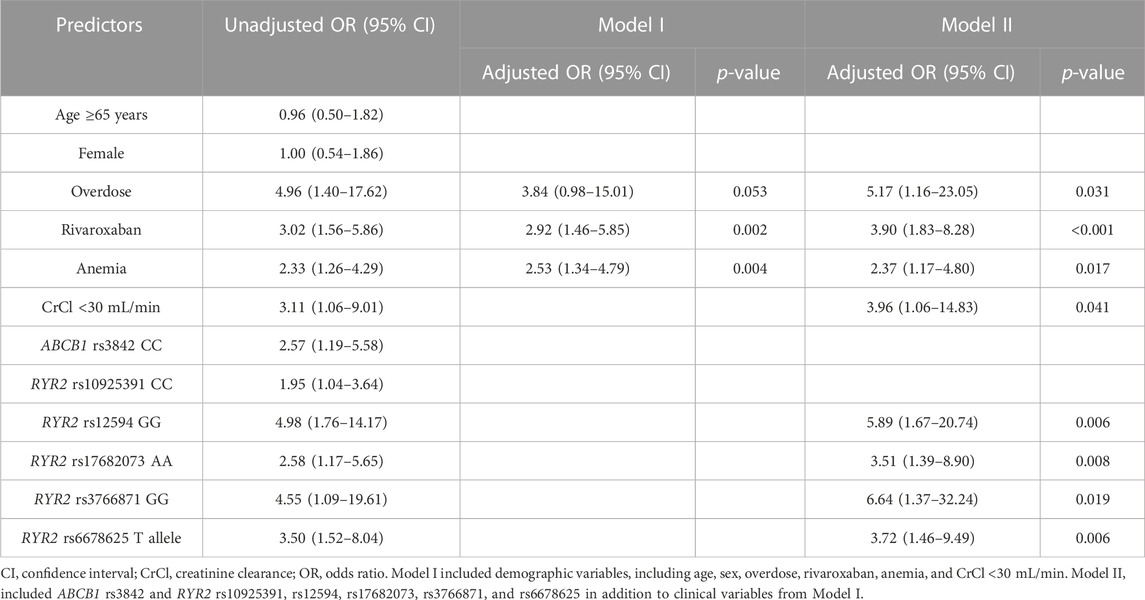
TABLE 3. Univariate and multivariable regression analyses to identify predictors for bleeding in patients treated with direct oral anticoagulants.
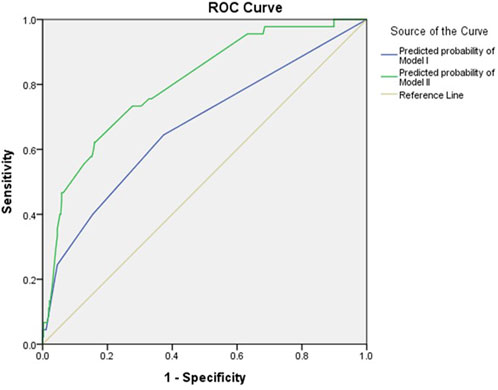
FIGURE 2. AUROC for bleeding using demographic and genetic factors. The blue line represents the predicted probability of Model I, while the green line represents that of Model II. The yellow line is the reference.
The risks of clinical characteristics and gene polymorphisms for bleeding complications were assessed in the survival analysis, as presented in Supplementary Tables S1, S2. Among these variables, those with a significant p-value were incorporated into the multivariable regression analysis (Supplementary Table S3). Rivaroxaban demonstrated a 5.9-fold higher risk of bleeding (95% CI: 1.6–22.1) than dabigatran. Additionally, overdosed individuals showed a 7.5-fold increase in bleeding risk (95% CI: 2.1–27.3) compared to underdosed patients. Among comorbidities, patients with anemia and severe renal impairment exhibited a 3.0-fold (95% CI: 1.6–5.8) and a 3.7-fold (95% CI: 1.3–10.9) higher risk of bleeding than those without these clinical conditions, respectively. For RYR2 polymorphisms, patients carrying the RYR2 rs12594 GG genotype had approximately three times the incidence of bleeding than those with the A allele (95% CI: 1.1–8.3). Individuals with rs17682073 AA genotype and the rs3766871 GG genotype showed a 3.2-fold (95% CI: 1.4–7.5) and a 6.7-fold (95% CI: 1.5–28.8) increased bleeding risks compared to variant allele carriers, respectively. Furthermore, RYR2 rs6678625T allele carriers were more likely to experience bleeding than those with the CC genotype (adjusted HR: 2.9, 95% CI: 1.3–6.3).
Using the significant variables in Model II, a risk scoring system was constructed: the AOR of these variables was divided by the AOR of anemia, which had the smallest value. Consequently, the following points were assigned: overdose (2 points), rivaroxaban (2 points), anemia (1 point), CrCl <30 mL/min (2 points), RYR2 rs12594GG (2 points), rs17682073 AA (1 point), rs6678625 T allele (2 points), and rs3766871GG (3 points). The risk score ranged from 0 to 15, but none of the patients had scores over 11. The risk estimates obtained from the logistic regression curve are depicted in Figure 3. For patients with 0, 1–2, 3–4, 5–6, 7–8, and 9–10 points, the predicted risk of bleeding was 0%, 1.2%, 4.6%, 15.7%, 41.7%, and 73.3%, respectively (Table 4). The AUROC of this logistic regression curve was 0.768 (95% CI: 0.690–0.846, p < 0.001).
Previous studies have demonstrated an association between RYR2 polymorphisms and arrhythmias; however, no study has evaluated bleeding complications. The findings of this study indicated that severe renal impairment (CrCl <30 mL/min), anemia, overdosing of DOACs, and rivaroxaban use significantly increased the bleeding risk. Of genetic factors, RYR2 rs12594, rs17682073, rs3766871, and rs6678625 were associated with bleeding complications. The risk scoring system that was constructed with these variables satisfactorily predicted bleeding risk, achieving an AUROC of 0.768.
RyRs mediate the release of Ca2+ from the endoplasmic and sarcoplasmic reticulum (SR) into the cytosol and convert different extracellular stimuli into intracellular Ca2+ signals, thereby playing a critical role in muscle contraction (Sun and Xu, 2019). Three RYR genes have been identified: RYR1, RYR2, and RYR3. The RYR1 gene (on human chromosome 19q13.1), which is responsible for Ca2+ release in skeletal muscle, has been associated with disorders such as King-Denborough syndrome, rhabdomyolysis-myalgia syndrome, and bleeding abnormalities (Dowling et al., 2011; Lopez et al., 2016; Witting et al., 2018). RYR3 (on 15q13.3-14), which is differentially expressed in the brain, is the least understood isoform of RYRs, and no disease has been conclusively associated with RYR3 mutations. A recent case report has suggested that variants in RYR3 may potentially be linked to nemaline myopathy, but further research is still needed to establish this connection (Nilipour et al., 2018).
RYR2 gene (on 1q42.1-43) encodes the cardiac Ca2+ channel, and mutations have been linked to catecholaminergic polymorphic ventricular tachycardia (CPVT) and SCD (Napolitano and Priori, 2007; Steinberg et al., 2023). In particular, the RYR2 rs3766871, one of the most extensively studied polymorphisms, is a missense variant that leads to the substitution of a serine residue with glycine (Zhang et al., 2003). This substitution causes destabilization of the channel and phosphorylation of protein kinase C, resulting in abnormal spontaneous leaks of Ca2+ from the SR (Milting et al., 2006; Koop et al., 2008). Due to this effect, RYR2 rs3766871 has been investigated in relation to ventricular arrhythmias and SCD in chronic heart failure (Ran et al., 2010; Francia et al., 2015). Francia et al. (2015) demonstrated that the variant allele increased susceptibility to ventricular tachycardia and ventricular fibrillation in patients with heart failure (HR: 3.49; 95% CI: 1.14–10.62). A Chinese cohort study of 1,244 patients with chronic heart failure indicated that the variant allele of rs3766871 in RYR2 was an independent risk factor that increased the risk of cardiac death (HR: 1.92; 95% CI: 1.24–2.94) and ventricular arrhythmias (OR: 1.66; 95% CI: 1.21–2.26) (Ran et al., 2010). Considering that arrhythmia is recognized as a risk factor for stroke, our finding is explicable to some extent.
RYR2 rs12594 is located in the 3′-untranslated region (3′-UTR) of RYR2. Despite being a non-coding part of mRNA, SNPs in the 3′-UTR can influence gene expression through mechanisms such as mRNA degradation, translation, and localization (Mayr, 2017). While its role in bleeding or cardiovascular diseases remains incompletely understood, research has explored its implications in cancer. Chen et al. (2019) observed that the A allele of RYR2 rs12594 was associated with overall survival of patients with astrocytoma, demonstrating the impact of RYR2 on the prognosis of lower-grade brain gliomas. Another case-control study showed a relationship between rs12594 polymorphism and the risk of breast cancer in a Chinese population. The RYR2 rs12594GG genotype was related to decreased breast cancer risk even after being adjusted by age, estrogen receptor status, progesterone receptor status, menopausal status, tumor size, and tumor stage (Wei et al., 2020). Thus, the G allele of rs12594 may contribute to a change in RYR2 expression, thereby increasing susceptibility to bleeding. However, further studies are required due to limited research on cardiovascular diseases.
RYR2 rs6678625 and rs17682073 were associated with an approximately three-fold increased bleeding risk in our study. Despite being intron variants, these SNPs have the potential to influence mRNA splicing, leading to alterations in protein expression or activity (Pagani and Baralle, 2004; Raponi and Baralle, 2010). According to the eQTL analysis by GTEx, the variant allele of rs6678625 had lower RYR2 expression in the aorta and coronary artery (p = 2.6 × 10−6 and 2.8 × 10^−4, respectively) (GTEx Consortium, 2017). Westaway et al. (2011) employed a systematic candidate-gene approach to identify genes linked to SCD risk in a Caucasian population with coronary artery diseases. The findings revealed that RYR2 rs6678625 was one of the SNPs that was significantly associated with SCD, but it lost significance in the replication sample. Based on these findings, it could be speculated that polymorphism of this SNP could potentially alter the expression or activity of RYR2, thereby leading to an elevated risk of bleeding. According to a previous study, RYR2 rs17682073 was identified as one of the SNPs associated with non-autism spectrum disorders in Central European populations (Skafidas et al., 2014); however, the available studies are limited, necessitating additional research.
DOACs are primarily excreted through the kidneys, with excretion rates ranging from 27% to 80% (Steffel et al., 2021). In case of rivaroxaban, the area under the curve increased by 44% in individuals with mild renal impairment (CrCl 50–75 mL/min) and by 64% in those with severe impairment (Kubitza et al., 2010). Therefore, adjusting the dosage of DOACs based on kidney function is imperative. However, there is limited available data on patients with severe chronic kidney disease since individuals with a CrCl <30 mL/min were excluded from all landmark trials of DOACs. Our findings revealed that despite dose adjustments based on kidney function, severe renal impairment remained a significant risk factor for bleeding. A study conducted by Yao et al. (2017) demonstrated a 1.3-fold (95% CI: 1.0–1.6) increased bleeding risk in individuals with moderate to severe renal impairment which is consistent with the findings of our study.
Patients who were prescribed an excessive dose of DOACs showed a five-fold increased bleeding risk according to the findings of our study. This elevated risk of bleeding was associated with increased plasma concentrations resulting of DOACs from reduced excretion and increased dosage. The higher concentrations can lead to more potent pharmacodynamic effects of DOACs, further amplifying the risk of bleeding. Given the increased susceptibility to bleeding risks associated with anticoagulation observed in Asian populations (Gaikwad et al., 2014), it is essential to implement closer monitoring for patients with impaired renal function in order to prevent overdosing of DOACs.
Rivaroxaban was the least safe DOAC with an approximately 4-fold higher risk of bleeding than other DOACs in our study. In a meta-analysis that compared the safety of apixaban and rivaroxaban using real-world data, those treated with apixaban exhibited a reduced risk of major bleeding and CRNMB (relative risk (RR) 0.73, 95% CI: 0.58–0.93 and RR 0.59, 95% CI: 0.50–0.70, respectively) (Aryal et al., 2019). Regarding this result, the safety profile including its PK characteristics of rivaroxaban has been elucidated. As rivaroxaban is given as a once daily dose, the peak concentration is higher than that of other DOACs. For example, the peak-to-trough ratio of rivaroxaban is approximately 10 (at a dose of 10–20 mg once daily), whereas for apixaban, it is approximately 3 (at a dose of 5 mg twice daily) (Aryal et al., 2019). This discrepancy explains the increased incidence of bleeding complications associated with rivaroxaban observed in our study.
Anemia, which refers to low levels of healthy red blood cells or hemoglobin, was associated with increased bleeding in our study. Red blood cells contribute not only to transporting oxygen but also to initiating hemostasis together with platelets and to stabilizing fibrin and clot structure (Lassila and Weisel, 2023). Their role has made anemia an independent risk factor for major bleeding during anticoagulant therapy. In the RE-LY trial with 18,113 randomized patients with AF, anemia was related to major bleeding complications (HR 2.14, 95% CI: 1.87–2.46) and discontinuation of anticoagulants (Westenbrink et al., 2015). In addition, a meta-analysis demonstrated that anemia was associated with a 78% increased risk of major bleeding (HR 1.78, 95% CI: 1.54–2.05) and a 77% increased risk of gastrointestinal bleeding (HR 1.77, 95% CI: 1.23–2.55) (Tu et al., 2021). This explains why many existing bleeding assessment tools incorporate anemia as a constituent factor within their scoring systems (Apostolakis et al., 2012).
Since bleeding is the most undesirable complication in patients taking anticoagulants, many risk scoring systems have been developed; however, most of them are applicable to vitamin K antagonist therapy (Shoeb and Fang, 2013). Among them, the CHA2DS2-VASc score, which was initially designed to predict the risk of stroke, exhibited effective prediction capability for major bleeding in individuals being treated with DOACs, with an AUROC of 0.68 (Yao et al., 2017). However, our risk scoring system that encompasses both clinical and genetic variables showed better prediction ability with an AUROC of 0.80. Considering that there was no significant difference in the CHA2DS2-VASc score or HAS-BLED score between the two groups in their baseline characteristics, genetic factors might considerably influence the onset of bleeding.
This study has a few limitations. While we collected samples prospectively, the risk of bias is associated with the retrospective patient data collection. Furthermore, the inclusion of only Asian participants and the relatively small sample size potentially restrict the generalization of the findings. We did not use multiple test correction methods like Bonferroni correction, often considered excessively conservative for a hypothesis-generating study, to prevent the potential loss of true positives. In addition, drug-gene interactions were not estimated. Given this limitation, careful interpretation is warranted when applying this risk scoring system in clinical settings. Therefore, further extensive cohort studies can validate our findings, thereby contributing to a deeper comprehension of the functional consequences of these polymorphisms. Nevertheless, the implication of a novel risk scoring system that incorporates genetic factors would help to assess susceptibility to bleeding, guide therapeutic interventions, and facilitate personalized medicine approaches.
This study elucidated the association between RYR2 gene polymorphisms and bleeding complications among patients treated with DOACs. After validation of the results, these findings and the constructed risk scoring system may help to predict the bleeding risk for such patients, thereby advocating for individualized DOAC treatment.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the Institutional Review Board of Ewha Womans University Mokdong Hospital and Ewha Womans University Seoul Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
EJ: Conceptualization, Investigation, Writing–original draft. JK: Conceptualization, Investigation, Writing–original draft. SC: Data curation, Writing–original draft. JY: Data curation, Writing–original draft. T-JS: Resources, Writing–original draft. JP: Conceptualization, Resources, Supervision, Writing–review and editing. HG: Conceptualization, Supervision, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Research Foundation (NRF) of Korea grant funded by the Korea government (MSIT) (Grant Number: NRF-2020R1A2C1008120 and NRF-2021R1I1A1A01057412).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1290785/full#supplementary-material
Akilzhanova, A., Guelly, C., Nuralinov, O., Nurkina, Z., Nazhat, D., Smagulov, S., et al. (2014). RYR2 sequencing reveals novel missense mutations in a Kazakh idiopathic ventricular tachycardia study cohort. PloS one 9 (6), e101059. doi:10.1371/journal.pone.0101059
Apostolakis, S., Lane, D. A., Guo, Y., Buller, H., and Lip, G. Y. (2012). Performance of the HEMORR2HAGES, ATRIA, and HAS-BLED bleeding risk–prediction scores in patients with atrial fibrillation undergoing anticoagulation: the AMADEUS (evaluating the use of SR34006 compared to warfarin or acenocoumarol in patients with atrial fibrillation) study. J. Am. Coll. Cardiol. 60 (9), 861–867. doi:10.1016/j.jacc.2012.06.019
Aryal, M. R., Gosain, R., Donato, A., Yu, H., Katel, A., Bhandari, Y., et al. (2019). Systematic review and meta-analysis of the efficacy and safety of apixaban compared to rivaroxaban in acute VTE in the real world. Blood Adv. 3 (15), 2381–2387. doi:10.1182/bloodadvances.2019000572
Avila, G., de la Rosa, J. A., Monsalvo-Villegas, A., and Montiel-Jaen, M. G. (2019). Ca2+ channels mediate bidirectional signaling between sarcolemma and sarcoplasmic reticulum in muscle cells. Cells 9 (1), 55. doi:10.3390/cells9010055
Bauer, K. A. (2013). Pros and cons of new oral anticoagulants. Hematol. Am. Soc. Hematol. Educ. Program 2013 (1), 464–470. doi:10.1182/asheducation-2013.1.464
Chan, N., Sager, P. T., Lawrence, J., Reilly, P., Berkowitz, S., Kubitza, D., et al. (2018). Is there a role for pharmacokinetic/pharmacodynamic-guided dosing for novel oral anticoagulants? Am. Heart J. 199, 59–67. doi:10.1016/j.ahj.2017.10.002
Chen, Q., Sun, Y., Wu, J., Xiong, Z., Niu, F., Jin, T., et al. (2019). LPP and RYR2 gene polymorphisms correlate with the risk and the prognosis of astrocytoma. J. Mol. Neurosci. 69, 628–635. doi:10.1007/s12031-019-01391-z
Dowling, J. J., Lillis, S., Amburgey, K., Zhou, H., Al-Sarraj, S., Buk, S. J., et al. (2011). King–Denborough syndrome with and without mutations in the skeletal muscle ryanodine receptor (RYR1) gene. Neuromuscul. Disord. 21 (6), 420–427. doi:10.1016/j.nmd.2011.03.006
Feagins, L. A., and Weideman, R. A. (2018). GI bleeding risk of DOACs versus warfarin: is newer better? Dig. Dis. Sci. 63 (7), 1675–1677. doi:10.1007/s10620-018-5060-1
Ferri, N., Colombo, E., Tenconi, M., Baldessin, L., and Corsini, A. (2022). Drug-drug interactions of direct oral anticoagulants (DOACs): from pharmacological to clinical practice. Pharmaceutics 14 (6), 1120. doi:10.3390/pharmaceutics14061120
Francia, P., Adduci, C., Semprini, L., Stanzione, R., Serdoz, A., Caprinozzi, M., et al. (2015). RyR2 common gene variant G1886S and the risk of ventricular arrhythmias in ICD patients with heart failure. J. Cardiovasc. Electrophysiol. 26 (6), 656–661. doi:10.1111/jce.12658
Gaikwad, T., Ghosh, K., and Shetty, S. (2014). VKORC1 and CYP2C9 genotype distribution in Asian countries. Thrombosis Res. 134 (3), 537–544. doi:10.1016/j.thromres.2014.05.028
Gao, X., Cai, X., Yang, Y., Zhou, Y., and Zhu, W. (2021). Diagnostic accuracy of the HAS-BLED bleeding score in VKA-or DOAC-treated patients with atrial fibrillation: a systematic review and meta-analysis. Front. Cardiovasc. Med. 8, 757087. doi:10.3389/fcvm.2021.757087
GTEx Consortium (2017). Genetic effects on gene expression across human tissues. Nature 550 (7675), 204–213. Mohammadi Pejman 5 6 Park YoSon 11 Parsana Princy 12 Segrè Ayellet V. 1 Strober Benjamin J. 9 Zappala Zachary 7 8 GCLaAFBAACSEDJRHYJB, P. 19 Volpi Simona 19 NpmAAGPKSLARLNCMHMRASJ, 16 PSLBMEBPA, 137 NCFNCR. doi:10.1038/nature24277
Hopton, C., Tijsen, A. J., Maizels, L., Arbel, G., Gepstein, A., Bates, N., et al. (2022). Characterization of the mechanism by which a nonsense variant in RYR2 leads to disordered calcium handling. Physiol. Rep. 10 (8), e15265. doi:10.14814/phy2.15265
Kaatz, S., Ahmad, D., Spyropoulos, A. C., and Schulman, S.Subcommittee on Control of Anticoagulation (2015). Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J. Thromb. Haemost. 13 (11), 2119–2126. doi:10.1111/jth.13140
Kochiadakis, G. E., and Kallergis, E. M. (2012). Impact of atrial fibrillation on coronary blood flow: a systematic review. J. Atr. Fibrillation 5 (3), 458. doi:10.4022/jafib.458
Koop, A., Goldmann, P., Chen, S. W., Thieleczek, R., and Varsányi, M. (2008). ARVC-related mutations in divergent region 3 alter functional properties of the cardiac ryanodine receptor. Biophysical J. 94 (12), 4668–4677. doi:10.1529/biophysj.107.122382
Kubitza, D., Becka, M., Mueck, W., Halabi, A., Maatouk, H., Klause, N., et al. (2010). Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban, an oral, direct Factor Xa inhibitor. Br. J. Clin. Pharmacol. 70 (5), 703–712. doi:10.1111/j.1365-2125.2010.03753.x
Lassila, R., and Weisel, J. W. (2023). Role of red blood cells in clinically relevant bleeding tendencies and complications. J. Thrombosis Haemostasis 21, 3024–3032. doi:10.1016/j.jtha.2023.05.009
Lee, Y. J., Kim, E. S., Hah, Y. J., Park, K. S., Cho, K. B., Jang, B. K., et al. (2013). Chronic kidney disease, hemodynamic instability, and endoscopic high-risk appearance are associated with 30-day rebleeding in patients with non-variceal upper gastrointestinal bleeding. J. Korean Med. Sci. 28 (10), 1500–1506. doi:10.3346/jkms.2013.28.10.1500
Lip, G. Y., Nieuwlaat, R., Pisters, R., Lane, D. A., and Crijns, H. J. (2010). Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 137 (2), 263–272. doi:10.1378/chest.09-1584
Lip, G. Y., Keshishian, A., Li, X., Hamilton, M., Masseria, C., Gupta, K., et al. (2018). Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients: the ARISTOPHANES study. Stroke 49 (12), 2933–2944. doi:10.1161/STROKEAHA.118.020232
Lopez, R. J., Byrne, S., Vukcevic, M., Sekulic-Jablanovic, M., Xu, L., Brink, M., et al. (2016). An RYR1 mutation associated with malignant hyperthermia is also associated with bleeding abnormalities. Sci. Signal. 9 (435), ra68. doi:10.1126/scisignal.aad9813
Martin, K., Beyer-Westendorf, J., Davidson, B., Huisman, M., Sandset, P., and Moll, S. (2016). Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J. Thrombosis Haemostasis 14 (6), 1308–1313. doi:10.1111/jth.13323
Mayr, C. (2017). Regulation by 3′-untranslated regions. Annu. Rev. Genet. 51, 171–194. doi:10.1146/annurev-genet-120116-024704
Milting, H., Lukas, N., Klauke, B., Körfer, R., Perrot, A., Osterziel, K.-J., et al. (2006). Composite polymorphisms in the ryanodine receptor 2 gene associated with arrhythmogenic right ventricular cardiomyopathy. Cardiovasc. Res. 71 (3), 496–505. doi:10.1016/j.cardiores.2006.04.004
Napolitano, C., and Priori, S. G. (2007). Diagnosis and treatment of catecholaminergic polymorphic ventricular tachycardia. Heart rhythm. 4 (5), 675–678. doi:10.1016/j.hrthm.2006.12.048
Nilipour, Y., Nafissi, S., Tjust, A. E., Ravenscroft, G., Hossein Nejad Nedai, H., Taylor, R. L., et al. (2018). Ryanodine receptor type 3 (RYR3) as a novel gene associated with a myopathy with nemaline bodies. Eur. J. Neurol. 25 (6), 841–847. doi:10.1111/ene.13607
Pagani, F., and Baralle, F. E. (2004). Genomic variants in exons and introns: identifying the splicing spoilers. Nat. Rev. Genet. 5 (5), 389–396. doi:10.1038/nrg1327
Ran, Y., Chen, J., Li, N., Zhang, W., Feng, L., Wang, R., et al. (2010). Common RyR2 variants associate with ventricular arrhythmias and sudden cardiac death in chronic heart failure. Clin. Sci. 119 (5), 215–223. doi:10.1042/CS20090656
Raponi, M., and Baralle, D. (2010). Alternative splicing: good and bad effects of translationally silent substitutions. Febs J. 277 (4), 836–840. doi:10.1111/j.1742-4658.2009.07519.x
Raymond, J., Imbert, L., Cousin, T., Duflot, T., Varin, R., Wils, J., et al. (2021). Pharmacogenetics of direct oral anticoagulants: a systematic review. J. Personalized Med. 11 (1), 37. doi:10.3390/jpm11010037
Sayers, E. W., Bolton, E. E., Brister, J. R., Canese, K., Chan, J., Comeau, D. C., et al. (2022). Database resources of the national center for biotechnology information. Nucleic acids Res. 50 (D1), D20–D26. doi:10.1093/nar/gkab1112
Schulman, S., and Kearon, C.Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis (2005). Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 3 (4), 692–694. doi:10.1111/j.1538-7836.2005.01204.x
Shoeb, M., and Fang, M. C. (2013). Assessing bleeding risk in patients taking anticoagulants. J. thrombosis thrombolysis 35, 312–319. doi:10.1007/s11239-013-0899-7
Skafidas, E., Testa, R., Zantomio, D., Chana, G., Everall, I. P., and Pantelis, C. (2014). Response to Belgard et al. Mol. Psychiatry 19 (4), 407–409. doi:10.1038/mp.2013.186
Steffel, J., Collins, R., Antz, M., Cornu, P., Desteghe, L., Haeusler, K. G., et al. (2021). 2021 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. EP Eur. 23 (10), 1612–1676. doi:10.1093/europace/euab065
Steinberg, C., Roston, T. M., van der Werf, C., Sanatani, S., Chen, S. W., Wilde, A. A., et al. (2023). RYR2-ryanodinopathies: from calcium overload to calcium deficiency. Europace 25 (6), euad156. doi:10.1093/europace/euad156
Sun, Z., and Xu, H. (2019). Ryanodine receptors for drugs and insecticides: an overview. Mini Rev. Med. Chem. 19 (1), 22–33. doi:10.2174/1389557518666180330112908
Tseng, A. S., Patel, R. D., Quist, H. E., Kekic, A., Maddux, J. T., Grilli, C. B., et al. (2018). Clinical review of the pharmacogenomics of direct oral anticoagulants. Cardiovasc. Drugs Ther. 32, 121–126. doi:10.1007/s10557-018-6774-1
Tsu, L. V., Berry, A., Wald, E., and Ehrlich, C. (2015). Modified HAS-BLED score and risk of major bleeding in patients receiving dabigatran and rivaroxaban: a retrospective, case-control study. Consult. Pharmacist®. 30 (7), 395–402. doi:10.4140/TCP.n.2015.395
Tu, S. J., Hanna-Rivero, N., Elliott, A. D., Clarke, N., Huang, S., Pitman, B. M., et al. (2021). Associations of anemia with stroke, bleeding, and mortality in atrial fibrillation: a systematic review and meta-analysis. J. Cardiovasc. Electrophysiol. 32 (3), 686–694. doi:10.1111/jce.14898
Ward, L. D., and Kellis, M. (2016). HaploReg v4: systematic mining of putative causal variants, cell types, regulators and target genes for human complex traits and disease. Nucleic Acids Res. 44 (D1), D877–D881. doi:10.1093/nar/gkv1340
Wei, Y., Wang, X., Zhang, Z., Zhao, C., Chang, Y., Bian, Z., et al. (2020). Impact of NR5A2 and RYR2 3′ UTR polymorphisms on the risk of breast cancer in a Chinese Han population. Breast cancer Res. Treat. 183, 1–8. doi:10.1007/s10549-020-05736-w
Westaway, S. K., Reinier, K., Huertas-Vazquez, A., Evanado, A., Teodorescu, C., Navarro, J., et al. (2011). Common variants in CASQ2, GPD1L, and NOS1AP are significantly associated with risk of sudden death in patients with coronary artery disease. Circ. Cardiovasc Genet. 4 (4), 397–402. doi:10.1161/CIRCGENETICS.111.959916
Westenbrink, B., Alings, M., Connolly, S., Eikelboom, J., Ezekowitz, M., Oldgren, J., et al. (2015). Anemia predicts thromboembolic events, bleeding complications and mortality in patients with atrial fibrillation: insights from the RE-LY trial. J. Thrombosis Haemostasis 13 (5), 699–707. doi:10.1111/jth.12874
Witting, N., Laforet, P., Voermans, N., Roux-Buisson, N., Bompaire, F., Rendu, J., et al. (2018). Phenotype and genotype of muscle ryanodine receptor rhabdomyolysis-myalgia syndrome. Acta Neurol. Scand. 137 (5), 452–461. doi:10.1111/ane.12885
Yao, X., Gersh, B. J., Sangaralingham, L. R., Kent, D. M., Shah, N. D., Abraham, N. S., et al. (2017). Comparison of the CHA2DS2-VASc, CHADS2, HAS-BLED, ORBIT, and ATRIA risk scores in predicting non–vitamin K antagonist oral anticoagulants-associated bleeding in patients with atrial fibrillation. Am. J. Cardiol. 120 (9), 1549–1556. doi:10.1016/j.amjcard.2017.07.051
Keywords: direct oral anticoagulants, bleeding, ryanodine receptors, pharmacogenomics, gene polymorphism
Citation: Jang EJ, Kim JS, Choi SA, Yee J, Song T-J, Park J and Gwak HS (2023) Construction of a risk scoring system using clinical factors and RYR2 polymorphisms for bleeding complications in patients on direct oral anticoagulants. Front. Pharmacol. 14:1290785. doi: 10.3389/fphar.2023.1290785
Received: 08 September 2023; Accepted: 30 October 2023;
Published: 15 November 2023.
Edited by:
Michal Korostynski, Institute of Pharmacology PAS, PolandReviewed by:
Tarik Kivrak, Firat University, TürkiyeCopyright © 2023 Jang, Kim, Choi, Yee, Song, Park and Gwak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hye Sun Gwak, aHNnd2FrQGV3aGEuYWMua3I=; Junbeom Park, cGFya2piQGV3aGEuYWMua3I=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.