
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pharmacol. , 09 November 2023
Sec. Pharmacogenetics and Pharmacogenomics
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1288068
Drug-induced liver injury (DILI) is one of the serious adverse drug reactions (ADRs), which belongs to immune-mediated adverse drug reactions (IM-ADRs). As an essential health drug, albendazole has rarely been reported to cause serious liver damage. A young man in his 30 s developed severe jaundice, abnormal transaminases, and poor blood coagulation mechanism after taking albendazole, and eventually developed into severe liver failure. The patient was found heterozygous of HLA-B*15:02 and HLA-B*13:01 through HLA-targeted sequencing, which may have a pathogenic role in the disease. This case report summarizes his presentation, treatment, and prognosis. A useful summary of the diagnosis and associated genetic variant information is provided.
Adverse drug reactions (ADRs) are a major problem affecting patient drug use safety (Ribeiro-Vaz et al., 2016; Alfirevic and Pirmohamed, 2017). According to the latest statistics from the World Health Organization, drug-induced damage has risen to the fifth cause of death in the world. The proportion of patients hospitalized due to irrational drug use is 10%–20%, and 5% of the patients died from serious ADRs (Fossouo Tagne et al., 2023). ADRs are divided into predictable type A adverse reactions and unpredictable type B adverse reactions (Bohm et al., 2018). Type B adverse reactions often have more severe consequences, since type B is an immune-mediated adverse drug reactions (IM-ADRs) independent of drug activity (Karnes et al., 2019).
According to the type of immune cells, IM-ADRs can be divided into B cell-mediated IM-ADRs and T cell-mediated IM-ADRs (Yip et al., 2015). The former clinical phenotype develops rapidly and manifests as severe anaphylaxis and mast cell reactions. The latter clinical phenotype is slightly delayed and usually involves vital organs such as the skin, liver, kidney, and pancreas (White et al., 2015), which can lead to the production of many phenotypes, such as Steven-Johnson Syndrome (SJS)/Toxic Epidermal Necrosis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and drug-induced liver injury (DILI). In this study, we focus on DILI, which is an autoimmune disease in the broad sense. DILI can lead to life-threatening liver failure and accounts for 7%–15% of acute liver failure cases in Europe and the United States (Leise et al., 2014). In recent years, many studies have found that DILI shows strong human leukocyte antigen (HLA) associations (such as HLA-B*57:01 and flucloxacillin-induced liver injury (Daly et al., 2009); HLA-DQA1*02:01 and lapatinib-induced liver injury (Spraggs et al., 2011)). HLA is a product encoded by the HLA gene complex. HLA participates in the regulation of the immune system by presenting antigenic peptides to T lymphocytes (Redwood et al., 2018). Here we report a young man with albendazole-induced acute liver failure who carries pathogenic HLA alleles. The role of HLA testing in the diagnosis and prevention of IM-ADRs is highlighted and a review of published similar cases is provided.
A young man in his thirties presented to the department of hepatology with a 20-day history of jaundice and abnormal liver function. His mental status was average, with transient appetite loss and poor sleep quality. The disease was exacerbated after admission to the hospital, as the iris and skin of the patient severely yellowed. Additionally, the blood coagulation mechanism deteriorated, and acute liver failure occurred. Before the onset of liver injury, the patient took albendazole tablets orally according to the recommended dose. The patient has a fever after taking the medicine, which was followed by yellow urine and fatigue. He had no preexisting medical conditions. The patient described that he had injured his Achilles tendon playing basketball in 2012 and recovered after surgery. He had two abnormal liver functions due to taking antiparasitic agents in the past. The first time was in childhood, and the drugs used were unknown. The second time was in 2018, and the drug taken was albendazole. Since the condition improved after treatment, the patient did not pay attention to this phenomenon. He had quit smoking for many years but drank alcohol intermittently.
The differential diagnosis of unexplained liver injury includes drug-induced liver injury, viral hepatitis, autoimmune liver disease, genetically related liver injury, alcoholic liver disease, parasitic infection, and hepatolithiasis with biliary tract infection. In this case, the patient had no family history of liver disease, and his urine copper and serum ceruloplasmin levels were normal. The patient did not drink alcohol for 1 week before taking albendazole, and also did not have the typical pathological manifestations of alcoholic liver disease. Hereditary liver disease and alcoholic liver disease could be ruled out in advance. Routine blood tests, abdominal B-ultrasound, magnetic resonance imaging (MRI) and magnetic resonance cholangiopancreatography (MRCP) results were normal. Hepatolithiasis combined with biliary tract infection was not considered. A full set of parasite tests was negative, and hepatitis A, B, C, D, E virus, Epstein‒Barr virus, and cytomegalovirus were all negative. A full set of systemic lupus erythematosus, immunity checks, and IgG4 immunoglobulin levels were normal. MRI did not show typical primary sclerosing cholangitis (PSC) bile duct withered branch changes. The patient had a clear medication history and a similar experience after taking albendazole. To make an exclusive diagnosis, a liver biopsy was performed, and the biopsy results supported the diagnosis (Figure 1).
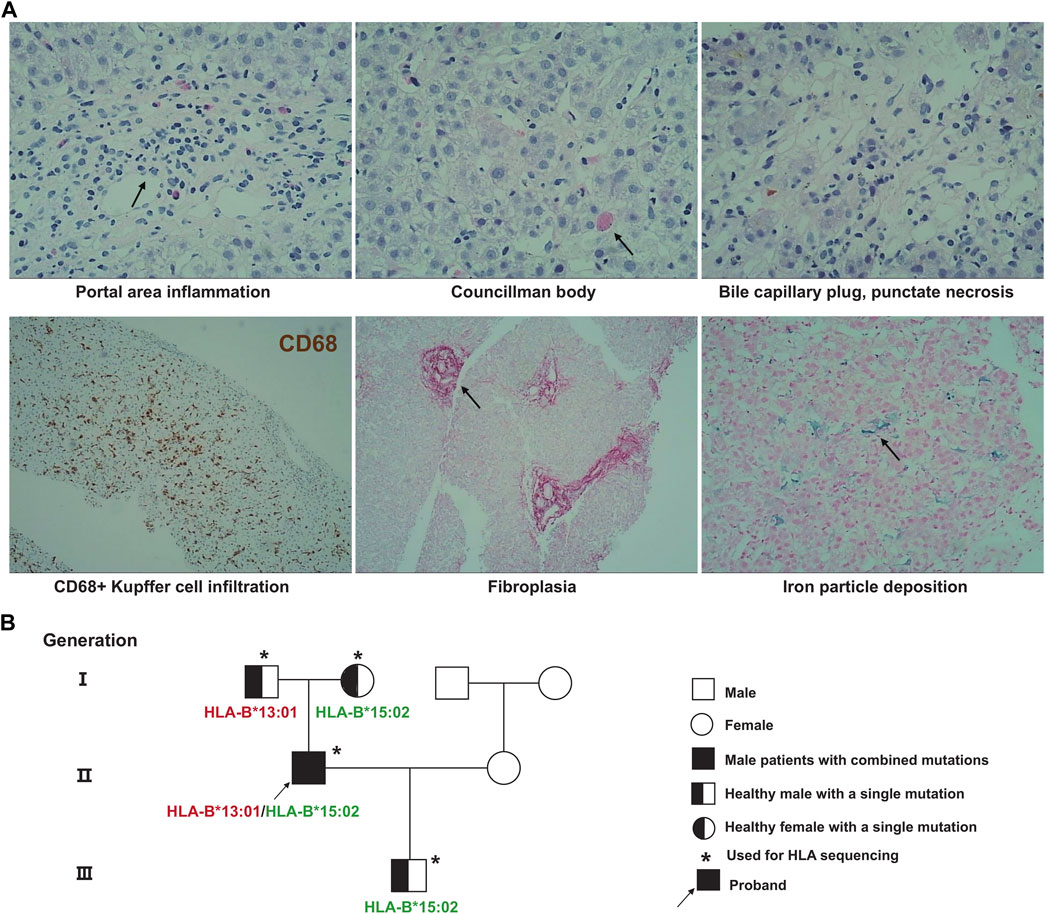
FIGURE 1. Patient pathological results of liver biopsy and genetic pedigree of HLA polymorphisms. (A) The pathological results of liver puncture showed obvious portal area inflammation, eosinophil infiltration, and CD68+ Kupffer cell infiltration; (B) HLA targeted sequencing results of patients and their families.
Each time the patient took an antiparasitic drug, he developed liver damage of increasing severity. To explore the cause of the disease, we used whole-exome sequencing to detect genetic variants carried by the patient that were consistent with the main clinical phenotype and had clear clinical significance, however, genetic variants that could explain the association with albendazole-induced DILI were not identified. At the same time, we collected the patient’s stool samples for 16s RNA sequencing. The results showed that the gut bacteria levels of patient were within the normal range. The detection values of the nine functional core bacteria, including Bacteroides, Blautia, Coprococcus, Clostridium, Faecalibacterium, RoseburiaP, Hascolarctobacterium, Ruminococcus, and Subdoligranulum, were not deviate from the reference range. We took blood samples from the patient and his family for HLA-targeted sequencing; this technique is a next-generation sequencing (NGS) strategy developed using Illumina sequencing by synthesis (SBS) technology. The sequencing range is the full length of the gene. Genomic DNA for sequencing was extracted from peripheral blood samples, which were performed using the MagPure Fast Blood DNA Kit (Magen Biotech) and quantified using agarose gel electrophoresis. The library construction is completed by NimbleGen kit (Roche Biotech), which can efficiently enrich the human HLA region and its flanks with a total of 4.97 Mb (chr6:28477797–33448354). Fragments between 180 and 280 bp in length were extracted and sequenced using the Illumina NovaSeq6000 system. The sequencing depth was 100x and the coverage was 99%. We found that patient was heterozygous for HLA-B*15:02:01:01 and HLA-B*13:01:01:01. Interestingly, this phenomenon was not observed in any of the patient’s family members, who also took albendazole without developing any adverse effects. Both HLA-B*15:02 and HLA-B*13:01 are alleles of the HLA-B gene. These genotypes consist of serial single-base substitutions. Compared with the HLA-B reference sequence, the HLA-B*15:02 allele has 41 single nucleotide polymorphisms (SNPs) and the HLA-B*13:01 allele has 54 SNPs. Detailed genetic information is shown in Figure 2. These base substitutions cause changes in the amino acid sequence, making the final encoded protein a mutant protein. Studies have shown that mutated HLA proteins can activate the immune system by binding and presenting drugs to T cell receptors, causing adverse drug reactions (Deshpande et al., 2021). Thus, we speculated that these alleles may increase the susceptibility to this disease. The patient not only carries HLA-B*15:02 and HLA-B*13:01, but also has HLA-A*02:03, HLA-A*24:02, HLA-C*03:04, HLA-C*08:01, HLA-DRB1*12:02, HLA-DRB1*16:02, HLA-DQA1*06:01, HLA-DQA1*01:02, HLA-DQB1*05:02, HLA-DQB1*03:01, HLA-DPB1*21:01, HLA-DPB1*05:01, and HLA-DRB3*03:01. Previous studies have found that HLA-DRB1*03:01, HLA-DRB1*04:01 and HLA-DRB1*07:01 are strongly associated with susceptibility to autoimmune hepatitis (de Boer et al., 2014; Terziroli Beretta-Piccoli et al., 2022), and patients with HLA-B8 will accelerate the development of alcoholic cirrhosis (Saunders et al., 1982). However, HLA alleles for AIH susceptibility-associated or accelerated alcoholic hepatitis were not detected in the patient.
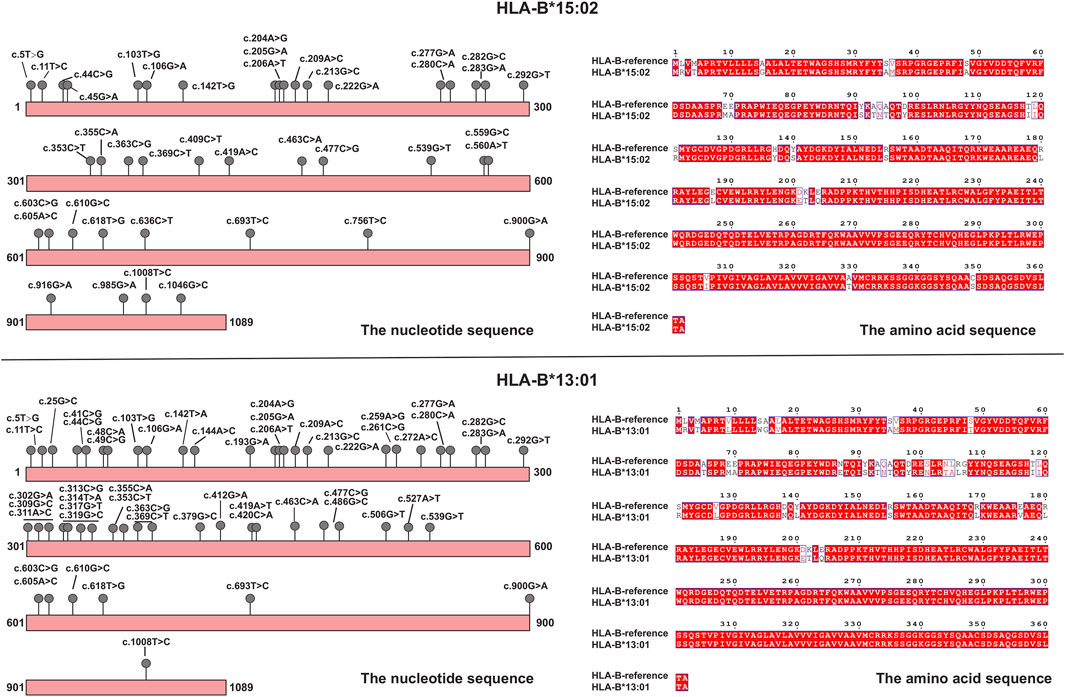
FIGURE 2. Schematic diagrams of mutations in HLA alleles (HLA-B*15:02 and HLA-B*13:01), and amino acid changes compared to the reference.
After hospitalization, the patient was advised to rest in bed, reduce activities, and eat a high-quality protein diet. Additionally, his vital signs were monitored, and routine blood tests, including liver function, coagulation function, blood ammonia, C-reactive protein (CRP), procalcitonin (PCT), and other indicators, to protect against the development and aggravation of liver failure and coinfection. In terms of drugs, reduced glutathione, magnesium isoglycyrrhizinate injection, and ursodeoxycholic acid capsules were administered. Plasma and cryoprecipitate were intermittently infused to improve blood coagulation function, and energy support therapy was performed. After the patient was discharged from the hospital, liver function was monitored weekly until normalization. During this period, glutathione tablets should be continued for liver protection treatment. Any known drugs to produce ADR related to HLA-B*15:02 and HLA-B*13:01 were excluded from the patient’s medication list, such as carbamazepine and chlorphenylsulfone, to reduce the risk of secondary damage.
The patient gradually recovered liver function through drug treatment, energy support, intermittent infusion of plasma, and coagulation factors. This was accompanied by a decrease in jaundice and an improvement in the coagulation mechanism. After 6 days of symptomatic treatment for jaundice reduction, the patient’s condition improved, all indicators were close to normal, and the skin and sclera had no yellow coloring (Table 1). After being discharged from the hospital, the patient was taking glutathione tablets to protect the liver and rechecked once a week. The half-year follow-up showed that the patient had no symptoms, such as increased jaundice, yellow urine, and fatigue. Enzyme indicators and bilirubin fully recovered 2 months after discharge. In addition, the list of drugs given for the carried HLA alleles also provide clinical references for the patient, and the patient has not experienced liver damage caused by drugs thus far.
As a broad-spectrum antiparasitic drug, albendazole can selectively and irreversibly inhibit the aggregation of intestinal parasites and intestinal parietal cell cytoplasmic microtubule system, thereby blocking the uptake of various nutrients and glucose and absorption (Eid et al., 2020). Albendazole has been included in the list of essential medicines by the World Health Organization, and has low toxicity and high efficiency. Reported side effects of the drug include diarrhea, abdominal pain, dizziness, fever, and rash (Bagheri et al., 2004). However, liver injury or acute liver failure rarely occurs, and the pathological mechanism of liver injury is unclear. In vitro experiments have demonstrated rapid conversion of albendazole to a sulphoxide (ABS) and subsequently a sulphone (ABSO). ABS is considered an active substance that performs pharmacological effects, while ABSO is an inert compound (Gottschall et al., 1990). The production of ABS in human liver is mediated through flavin monoxygenases (FMO) and cytochrome P450 reductase (CYP), mainly CYP3A4 (Rawden et al., 2000). Therefore, functional alleles on the gene encoding CYP3A4, such as CYP3A4*4, CYP3A4*5, CYP3A4*6, CYP3A4*21 and CYP3A4*22, will affect the efficacy and toxicity of albendazole. ADRs are divided into dose-dependent type A and dose-independent type B. CYP alleles may induce other adverse effects of albendazole (dose-dependent). However, DILI is a dose-independent and unpredictable ADR, it may be less affected by CYP alleles.
To date, a literature search reveals only 10 cases of albendazole-induced liver injury (Table 2), one in which drug-induced liver failure requiring liver transplantation developed (Aasen et al., 2018; Piloiu and Dumitrascu, 2021). According to Table 2, personal history of hepatic parasites is a recurrent indication. Albendazole plays an important role in the treatment of parasitic infections, either as monotherapy or as an adjunct to percutaneous drainage or surgery. Therefore, when using albendazole in the treatment of parasitic infections, clinicians should be aware of the potential for hepatic injury and routinely monitor liver function tests throughout the course of treatment. In addition to a history of parasitic disease, a history of alcohol consumption is an important factor interfering with the diagnosis of drug-induced liver injury. Alcohol is mainly metabolized by the liver, and alcohol consumption increases the burden on the liver and may lead to worsening liver injury. At this time, if the patient carries disease susceptibility-related HLA alleles, the risk of severe liver injury increases exponentially. The description of our patient emphasizes the safe use of albendazole within this context. The patient’s alertness to albendazole is not strong. Safety, high efficiency, and low toxicity have become the stereotype of albendazole, and it is difficult for patients to realize that the onset of symptoms may represent an ADR. Due to the immune memory produced by the body, when exposed to the same stimulus, a stronger immune response will appear, causing more severe liver damage.
Recent studies have shown that there is a strong genetic susceptibility to IM-ADRs. And these variants point to the region where the human leukocyte antigen is located (Hetherington et al., 2002; Tassaneeyakul et al., 2009; Kang et al., 2011; Garon et al., 2017). HLA is divided into class I and class II, both of which are distributed on the cell surface and play the role of presenting antigens (Gibson et al., 2023). However, it should be noted that class I HLA molecules present endogenous antigens, while class II responsible for exogenous antigens. The immune response is initiated through the formation of MHC-antigen peptide-TCR complexes (White et al., 2015). Correlative clinical phenotypes include severe cutaneous adverse reactions, DILI, and hypersensitivity syndromes, which affect multiple organs simultaneously. In our study, HLA-targeted sequencing revealed this patient to be heterozygous for HLA-B*15:02 and HLA-B*13:01. HLA-B*15:02 is associated with severe cutaneous adverse reactions of aromatic antiepileptic drugs (AEDs), including phenytoin (Man et al., 2007; Lochar et al., 2008), lamotrigine (Shi et al., 2011), carbamazepine (Chen et al., 2011), and oxcarbazepine (Che et al., 2009). In addition, adverse reactions of some antibiotics (sulfamethoxazole/trimethoprim (Kongpan et al., 2015), and dapsone (Tempark et al., 2017)) were related to HLA-B*15:02. A large clinical trial in Asians revealed a strong association of carbamazepine adverse reactions with HLA-B*15:02 (OR = 1,357, 95% CI = 193–8838, and p-value as p = 1.6 × 10−41) (Chen et al., 2011). The phenotypes involved are Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug reactions with eosinophilia and systemic symptoms (DRESS). HLA-B*13:01 was strongly associated with trichloroethylene-induced hypersensitivity dermatitis (OR = 27.5, 95%CI = 3.5–55.7, and p-value as p = 1.48 × 10−21) (Li et al., 2007) and dapsone-induced hypersensitivity reactions (OR = 20.53, 95%CI = 11.55–36.48, and p-value as p = 6.84 × 10−25) (Zhang et al., 2013). The frequencies of HLA-B*15:02 and HLA-B*13:01 in Chinese population are 0.1287 and 0.0680 respectively (Trachtenberg et al., 2007; Yao et al., 2009). However, the patient’s family members carried only one of the two HLA polymorphisms and did not develop DILI after taking the drug. This is an interesting clinical phenomenon, and we highly suspect that HLA alleles play an important role in disease susceptibility.
From the patient’s perspective, the medication recommendations based on their HLA genotype will help to prevent future ADRs.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NCBI BioProject (https://www.ncbi.nlm.nih.gov/bioproject/), PRJNA992780.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
J-ML: Data curation, Funding acquisition, Investigation, Resources, Validation, Visualization, Writing–original draft. YZ: Conceptualization, Data curation, Formal Analysis, Investigation, Validation, Visualization, Writing–original draft. ZZ: Data curation, Investigation, Validation, Writing–review and editing. J-JC: Funding acquisition, Investigation, Validation, Visualization, Writing–original draft. J-YY: Conceptualization, Data curation, Funding acquisition, Investigation, Supervision, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (82373962, 82073943, and 82204533), Scientific research project of Furong laboratory of Central South University (No. 2023SK2083) and Hunan Province Clinical Medical Technology Innovation Guidance Project (2021SK50910).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aasen, T. D., Nasrollah, L., and Seetharam, A. (2018). Drug-induced liver failure requiring liver transplant: report and review of the role of albendazole in managing echinococcal infection. Exp. Clin. Transpl. 16 (3), 344–347. doi:10.6002/ect.2015.0313
Alfirevic, A., and Pirmohamed, M. (2017). Genomics of adverse drug reactions. Trends Pharmacol. Sci. 38 (1), 100–109. doi:10.1016/j.tips.2016.11.003
Bagheri, H., Simiand, E., Montastruc, J. L., and Magnaval, J. F. (2004). Adverse drug reactions to anthelmintics. Ann. Pharmacother. 38 (3), 383–388. doi:10.1345/aph.1D325
Ben Fredj, N., Chaabane, A., Chadly, Z., Ben Fadhel, N., Boughattas, N. A., and Aouam, K. (2014). Albendazole-induced associated acute hepatitis and bicytopenia. Scand. J. Infect. Dis. 46 (2), 149–151. doi:10.3109/00365548.2013.835068
Bilgic, Y., Yilmaz, C., Cagin, Y. F., Atayan, Y., Karadag, N., and Harputluoglu, M. M. M. (2017). Albendazole induced recurrent acute toxic hepatitis: a case report. Acta Gastroenterol. Belg 80 (2), 309–311.
Bohm, R., Proksch, E., Schwarz, T., and Cascorbi, I. (2018). Drug hypersensitivity. Dtsch. Arztebl Int. 115 (29-30), 501–512. doi:10.3238/arztebl.2018.0501
Chen, P., Lin, J. J., Lu, C. S., Ong, C. T., Hsieh, P. F., Yang, C. C., et al. (2011). Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N. Engl. J. Med. 364 (12), 1126–1133. doi:10.1056/NEJMoa1009717
Chen, Y. C., Chu, C. Y., and Hsiao, C. H. (2009). Oxcarbazepine-induced Stevens-Johnson syndrome in a patient with HLA-B*1502 genotype. J. Eur. Acad. Dermatol Venereol. 23 (6), 702–703. doi:10.1111/j.1468-3083.2008.02988.x
Choi, G. Y., Yang, H. W., Cho, S. H., Kang, D. W., Go, H., Lee, W. C., et al. (2008). Acute drug-induced hepatitis caused by albendazole. J. Korean Med. Sci. 23 (5), 903–905. doi:10.3346/jkms.2008.23.5.903
Daly, A. K., Donaldson, P. T., Bhatnagar, P., Shen, Y., Pe'er, I., Floratos, A., et al. (2009). HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat. Genet. 41 (7), 816–819. doi:10.1038/ng.379
de Boer, Y. S., van Gerven, N. M., Zwiers, A., Verwer, B. J., van Hoek, B., van Erpecum, K. J., et al. (2014). Genome-wide association study identifies variants associated with autoimmune hepatitis type 1. Gastroenterology 147 (2), 443–452.e5. doi:10.1053/j.gastro.2014.04.022
Deshpande, P., Hertzman, R. J., Palubinsky, A. M., Giles, J. B., Karnes, J. H., Gibson, A., et al. (2021). Immunopharmacogenomics: mechanisms of HLA-associated drug reactions. Clin. Pharmacol. Ther. 110 (3), 607–615. doi:10.1002/cpt.2343
Eid, R. K., Ashour, D. S., Essa, E. A., El Maghraby, G. M., and Arafa, M. F. (2020). Chitosan coated nanostructured lipid carriers for enhanced in vivo efficacy of albendazole against Trichinella spiralis. Carbohydr. Polym. 232, 115826. doi:10.1016/j.carbpol.2019.115826
Fossouo Tagne, J., Yakob, R. A., Dang, T. H., McDonald, R., and Wickramasinghe, N. (2023). Reporting, monitoring, and handling of adverse drug reactions in Australia: scoping review. JMIR Public Health Surveill. 9, e40080. doi:10.2196/40080
Garon, S. L., Pavlos, R. K., White, K. D., Brown, N. J., Stone, C. A., and Phillips, E. J. (2017). Pharmacogenomics of off-target adverse drug reactions. Br. J. Clin. Pharmacol. 83 (9), 1896–1911. doi:10.1111/bcp.13294
Gibson, A., Deshpande, P., Campbell, C. N., Krantz, M. S., Mukherjee, E., Mockenhaupt, M., et al. (2023). Updates on the immunopathology and genomics of severe cutaneous adverse drug reactions. J. Allergy Clin. Immunol. 151 (2), 289–300 e4. doi:10.1016/j.jaci.2022.12.005
Gottschall, D. W., Theodorides, V. J., and Wang, R. (1990). The metabolism of benzimidazole anthelmintics. Parasitol. Today 6 (4), 115–124. doi:10.1016/0169-4758(90)90228-v
Gozukucuk, R., Abci, I., and Guclu, M. (2013). Albendazole-induced toxic hepatitis: a case report. Turk J. Gastroenterol. 24 (1), 82–84. doi:10.4318/tjg.2013.0426
Hetherington, S., Hughes, A. R., Mosteller, M., Shortino, D., Baker, K. L., Spreen, W., et al. (2002). Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet 359 (9312), 1121–1122. doi:10.1016/S0140-6736(02)08158-8
Kang, H.-R., Jee, Y. K., Kim, Y.-S., Lee, C. H., Jung, J.-W., Kim, S. H., et al. (2011). Positive and negative associations of HLA class I alleles with allopurinol-induced SCARs in Koreans. Pharmacogenet Genomics 21 (5), 303–307. doi:10.1097/FPC.0b013e32834282b8
Karnes, J. H., Miller, M. A., White, K. D., Konvinse, K. C., Pavlos, R. K., Redwood, A. J., et al. (2019). Applications of immunopharmacogenomics: predicting, preventing, and understanding immune-mediated adverse drug reactions. Annu. Rev. Pharmacol. Toxicol. 59, 463–486. doi:10.1146/annurev-pharmtox-010818-021818
Kongpan, T., Mahasirimongkol, S., Konyoung, P., Kanjanawart, S., Chumworathayi, P., Wichukchinda, N., et al. (2015). Candidate HLA genes for prediction of co-trimoxazole-induced severe cutaneous reactions. Pharmacogenet Genomics 25 (8), 402–411. doi:10.1097/FPC.0000000000000153
Leise, M. D., Poterucha, J. J., and Talwalkar, J. A. (2014). Drug-induced liver injury. Mayo Clin. Proc. 89 (1), 95–106. doi:10.1016/j.mayocp.2013.09.016
Li, H., Dai, Y., Huang, H., Li, L., Leng, S., Cheng, J., et al. (2007). HLA-B*1301 as a biomarker for genetic susceptibility to hypersensitivity dermatitis induced by trichloroethylene among workers in China. Environ. Health Perspect. 115 (11), 1553–1556. doi:10.1289/ehp.10325
Locharernkul, C., Loplumlert, J., Limotai, C., Korkij, W., Desudchit, T., Tongkobpetch, S., et al. (2008). Carbamazepine and phenytoin induced Stevens-Johnson syndrome is associated with HLA-B*1502 allele in Thai population. Epilepsia 49 (12), 2087–2091. doi:10.1111/j.1528-1167.2008.01719.x
Man, C. B., Kwan, P., Baum, L., Yu, E., Lau, K. M., Cheng, A. S., et al. (2007). Association between HLA-B*1502 allele and antiepileptic drug-induced cutaneous reactions in Han Chinese. Epilepsia 48 (5), 1015–1018. doi:10.1111/j.1528-1167.2007.01022.x
Marin Zuluaga, J. I., Marin Castro, A. E., Perez Cadavid, J. C., and Restrepo Gutierrez, J. C. (2013). Albendazole-induced granulomatous hepatitis: a case report. J. Med. Case Rep. 7, 201. doi:10.1186/1752-1947-7-201
Nandi, M., Sarkar, S., Karaagaç, A. T., Yildirim, A. I., Sharma, J., Mantan, M., et al. (2013). Correspondence. Indian Pediatr. 50 (11), 1064–1068. doi:10.1007/s13312-013-0285-8
Piloiu, C., and Dumitrascu, D. L. (2021). Albendazole-induced liver injury. Am. J. Ther. 28 (3), e335–e340. doi:10.1097/MJT.0000000000001341
Rawden, H. C., Kokwaro, G. O., Ward, S. A., and Edwards, G. (2000). Relative contribution of cytochromes P-450 and flavin-containing monoxygenases to the metabolism of albendazole by human liver microsomes. Br. J. Clin. Pharmacol. 49 (4), 313–322. doi:10.1046/j.1365-2125.2000.00170.x
Redwood, A. J., Pavlos, R. K., White, K. D., and Phillips, E. J. (2018). HLAs: key regulators of T-cell-mediated drug hypersensitivity. HLA 91 (1), 3–16. doi:10.1111/tan.13183
Ribeiro-Vaz, I., Silva, A. M., Costa Santos, C., and Cruz-Correia, R. (2016). How to promote adverse drug reaction reports using information systems - a systematic review and meta-analysis. BMC Med. Inf. Decis. Mak. 16, 27. doi:10.1186/s12911-016-0265-8
Rios, D., and Restrepo, J. C. (2013). Albendazole-induced liver injury: a case report. Colomb. Med. (Cali). 44 (2), 118–120. doi:10.25100/cm.v44i2.1021
Saunders, J. B., Wodak, A. D., Haines, A., Powell-Jackson, P. R., Portmann, B., Davis, M., et al. (1982). Accelerated development of alcoholic cirrhosis in patients with HLA-B8. Lancet 1 (8286), 1381–1384. doi:10.1016/s0140-6736(82)92500-4
Shah, C., Mahapatra, A., Shukla, A., and Bhatia, S. (2013). Recurrent acute hepatitis caused by albendazole. Trop. Gastroenterol. 34 (1), 38–39. doi:10.7869/tg.2012.90
Shi, Y. W., Min, F. L., Liu, X. R., Zan, L. X., Gao, M. M., Yu, M. J., et al. (2011). Hla-B alleles and lamotrigine-induced cutaneous adverse drug reactions in the Han Chinese population. Basic Clin. Pharmacol. Toxicol. 109 (1), 42–46. doi:10.1111/j.1742-7843.2011.00681.x
Spraggs, C. F., Budde, L. R., Briley, L. P., Bing, N., Cox, C. J., King, K. S., et al. (2011). HLA-DQA1*02:01 is a major risk factor for lapatinib-induced hepatotoxicity in women with advanced breast cancer. J. Clin. Oncol. 29 (6), 667–673. doi:10.1200/JCO.2010.31.3197
Tassaneeyakul, W., Jantararoungtong, T., Chen, P., Lin, P.-Y., Tiamkao, S., Khunarkornsiri, U., et al. (2009). Strong association between HLA-B*5801 and allopurinol-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharmacogenet Genomics 19 (9), 704–709. doi:10.1097/FPC.0b013e328330a3b8
Tempark, T., Satapornpong, P., Rerknimitr, P., Nakkam, N., Saksit, N., Wattanakrai, P., et al. (2017). Dapsone-induced severe cutaneous adverse drug reactions are strongly linked with HLA-B*13: 01 allele in the Thai population. Pharmacogenet Genomics 27 (12), 429–437. doi:10.1097/FPC.0000000000000306
Terziroli Beretta-Piccoli, B., Mieli-Vergani, G., and Vergani, D. (2022). Autoimmmune hepatitis. Cell Mol. Immunol. 19 (2), 158–176. doi:10.1038/s41423-021-00768-8
Trachtenberg, E., Vinson, M., Hayes, E., Hsu, Y. M., Houtchens, K., Erlich, H., et al. (2007). HLA class I (A, B, C) and class II (DRB1, DQA1, DQB1, DPB1) alleles and haplotypes in the Han from southern China. Tissue Antigens 70 (6), 455–463. doi:10.1111/j.1399-0039.2007.00932.x
White, K. D., Chung, W. H., Hung, S. I., Mallal, S., and Phillips, E. J. (2015). Evolving models of the immunopathogenesis of T cell-mediated drug allergy: the role of host, pathogens, and drug response. J. Allergy Clin. Immunol. 136 (2), 219–234. quiz 35. doi:10.1016/j.jaci.2015.05.050
Yao, Y., Shi, L., Shi, L., Matsushita, M., Yu, L., Lin, K., et al. (2009). Distribution of HLA-A, -B, -Cw, and -DRB1 alleles and haplotypes in an isolated Han population in Southwest China. Tissue Antigens 73 (6), 561–568. doi:10.1111/j.1399-0039.2009.01237.x
Yip, V. L., Alfirevic, A., and Pirmohamed, M. (2015). Genetics of immune-mediated adverse drug reactions: a comprehensive and clinical review. Clin. Rev. Allergy Immunol. 48 (2-3), 165–175. doi:10.1007/s12016-014-8418-y
Keywords: IM-ADRs, DILI, HLA polymorphism, genetic susceptibility, liver injury
Citation: Liao J-M, Zhan Y, Zhang Z, Cui J-J and Yin J-Y (2023) HLA-targeted sequencing reveals the pathogenic role of HLA-B*15:02/HLA-B*13:01 in albendazole-induced liver failure: a case report and a review of the literature. Front. Pharmacol. 14:1288068. doi: 10.3389/fphar.2023.1288068
Received: 03 September 2023; Accepted: 27 October 2023;
Published: 09 November 2023.
Edited by:
Charity Nofziger, Pharmgenetix GmbH, AustriaReviewed by:
Ramcés Falfán-Valencia, National Institute of Respiratory Diseases-Mexico (INER), MexicoCopyright © 2023 Liao, Zhan, Zhang, Cui and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ji-Ye Yin, eWluaml5ZUBjc3UuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.