- 1Central Asian Center for Development Studies, New Uzbekistan University, Tashkent, Uzbekistan
- 2School of Engineering, Central Asian University, Tashkent, Uzbekistan
- 3Institute of Botany, Academy of Sciences of Republic of Uzbekistan, Tashkent, Uzbekistan
- 4Department of Chemistry, Biochemistry and Environmental Protection, Faculty of Sciences, University of Novi Sad, Novi Sad, Serbia
- 5Department of Plant Sciences, Quaid-i-Azam University, Islamabad, Pakistan
- 6Department of Forestry and Landscape Design, Tashkent State Agrarian University, Tashkent, Uzbekistan
- 7Department of Education and Training Management, Tashkent International University of Education, Tashkent, Uzbekistan
- 8College of Landscape Architecture, Jiangsu Vocational College of Agriculture and Forestry, Zhenjiang, China
- 9Faculty of Biology and Biotechnology, Al-Farabi Kazakh National University, Almaty, Kazakhstan
- 10Higher School of Natural Sciences, Astana International University, Astana, Kazakhstan
- 11State Key Laboratory of Rice Biology and Breeding, Ministry of Agricultural and Rural Affairs Laboratory of Molecular Biology of Crop Pathogens and Insects, Zhejiang University, Hangzhou, China
- 12Department of Genetics, Faculty of Natural and Agricultural Sciences, University of the Free State, Bloemfontein, South Africa
- 13Department of Environmental Sciences, COMSATS University Islamabad, Abbottabad Campus, Abbottabad, Pakistan
- 14Department of Ecology and Botany, Andijan State University, Andijan, Uzbekistan
- 15Department of Ecology Monitoring, National University of Uzbekistan, Tashkent, Uzbekistan
- 16Biology Department, College of Science, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
- 17Department Faculty and Hospital Therapy -1, Occupational Pathology, Tashkent Medical Academy, Tashkent, Uzbekistan
- 18Centre d’Ecologie Fonctionnelle et Evolutive, Centre National de Recherche Scientifique, Ecole Pratique des Hautes Etudes, Institut pour la Recherche et le Développement, University of Montpellier, Montpellier, France
- 19Laboratory of Botany, Phytochemistry and Mycology, Faculty of Pharmacy, University of Montpellier, Montpellier, France
Despite its millennial existence and empirical documentation, the ethnological knowledge of herbs is a more recent phenomenon. The knowledge of their historical uses as food, medicine, source of income and small-scale businesses, and the sociological impacts are threatened due to the slow ethnobotanical research drive. Species of the genus Solanum have long been extensively used in folk medicine to treat various illnesses of humans since the dawn of civilization. All data were systematically obtained from papers, monographs, and books written in Uzbek, Russian, and English through various scientific online databases, including Google, Google Scholar, PubMed, Scopus, Semantic Scholar, Science Direct, and Web of Science using specific keywords focused on eight Solanum species. Eight native and non-native Solanum species as S. dulcamara L., S. lycopersicum L., S. melongena L., S. nigrum L., S. rostratum Dunal., S. sisymbriifolium Lam., S. tuberosum L., and S. villosum Mill. have been recorded in Uzbekistan of Central Asia. In this article we presented recently obtained data on the diversity, morphological characteristics, global distribution, habitat, population status, phenology, reproduction, pharmacology and phytochemistry of these Solanum species in Uzbekistan. Furthermore, relying on a combination of literature reviews and analyses from various scientific papers, we focus on food consumption coupled with global ethnobotanical and ethnopharmacological uses in human diseases of the Solanum species growing in Uzbekistan. Since the dawn of civilization, these eight cultivated and non-cultivated species of Solanum have provided sustainable resources of medicinal plants in Uzbekistan to prevent and treat various human diseases. Based on the collected data, it was shown that Solanum species have not been studied ethnobotanically and ethnomedicinally in Uzbekistan and it is necessary to conduct phytochemical and biotechnological research on them in the future. Traditional uses and scientific evaluation of Solanum indicate that S. nigrum, S. sisymbriifolium and S. tuberosum are one of the most widely used species in some parts of the world. Although considerable progress has been made to comprehend the chemical and biological properties of S. nigrum and S. tuberosum species, more research on the pharmacology and toxicology of these species is needed to ensure the safety, efficacy, and quality of their biologically active extracts and isolated bioactive compounds. Additionally, conducting additional research on the structure-activity relationship of certain isolated phytochemicals has the potential to enhance their biological efficacy and advance the scientific utilization of traditional applications of Solanum taxa.
Introduction
Despite its millennial existence and empirical documentation, the ethnological knowledge of herbs is a more recent phenomenon. Knowledge of their historical uses as food, medicine, source of income and small-scale businesses, and the sociological impacts are threatened due to the slow ethnobotanical research drive. The poor documentation and lack of study of medicinal plants in many developing countries has created inconsistencies in their uses in relation to the practice of traditional medicine, food and mythological beliefs. Their relevance in modern-day pharmaceutics and nutraceuticals is a product of human experimentations over time. Factors that may be anthropogenic, ethnographic, and environmental have been implicated in herb underutilization and under-exploration of plants, algae, and fungi, including animals in Central Asia. Ethnobiological literature on Central Asia is scant, random, limited in scope and fraught with taxonomic inconsistencies (Khojimatov et al., 2023a; Gafforov et al., 2023). Hence, this study is based on an extant ethnobotanical treatise and aims to represent an integrative knowledge of the beneficial Solanum species of Uzbekistan, their uses in indigent cultures, encompassing a brief phytochemical overview.
With 102 genera and roughly 2,500 species, the flowering plants of Solanaceae (order Solanales), also known as the nightshade or potato family, is very important economically as a source of food and medicine (Särkinen et al., 2018; Kaunda and Zhang, 2019; Chidambaram et al., 2022). Solanum with ca. 1,250 species, is the largest genus in the Solanaceae and one of most species-rich genera of flowering plants with contains members spread all over the world, and in temperate zones, there are very few species, while the entire United States and Canada only have roughly 50 species from various genera of Solanaceae (Morris and Taylor, 2017; Gagnon et al., 2022). Its dark colloquial name of “nightshade” comes from the deadly alkaloids found in some family species. South America, where most nightshade species are thought to originate, is home to many of these species. The richest in terms of species diversity are the continents of Africa and Australia. The Solanaceae family is primarily found in tropical and temperate regions, from desert areas to tropical woods. The Solanaceae members have been found on various continents due to their Neotropical origin (Dupin et al., 2016; Tovar et al., 2021). Solanaceae species are used in folk medicine, traditional culture, pharmacology and ornamental gardening. Like the whole world, Uzbekistan also depends heavily on some members of this family as food crops. For instance, food crops produced 540 million tons worldwide in 2010 on 28 million hectares of land. However, this only applies to the four principal crops: potatoes, tomatoes, eggplants, and peppers. It does not apply to many other cultivated species or numerous semi-cultivated, wild-collected species. The main problem of tomato yield in Uzbekistan is the post-harvest activities and due to which a lot of crop production can be wasted (Padalia, 2014). The members are often herbs and can be annuals, biennials, or perennials, while certain species can also be shrubs or small trees.
More than 1,200 wild medicinal plants in Uzbekistan have been studied and described (Khojimatov, 2021). However, many medicinal plants found in Uzbekistan have not been thoroughly scientifically evaluated for their potential value in ethnobotany and ethnomedicine, such as a member of the Solanaceae family. This review aims to investigate the diversity, ethnobotanical uses, and brief details of the phytochemistry and pharmacology of eight Solanum species that are cultivated in Uzbekistan, both native and non-native.
Materials and methods
The geographic location, vegetation biomes and climate of the study area
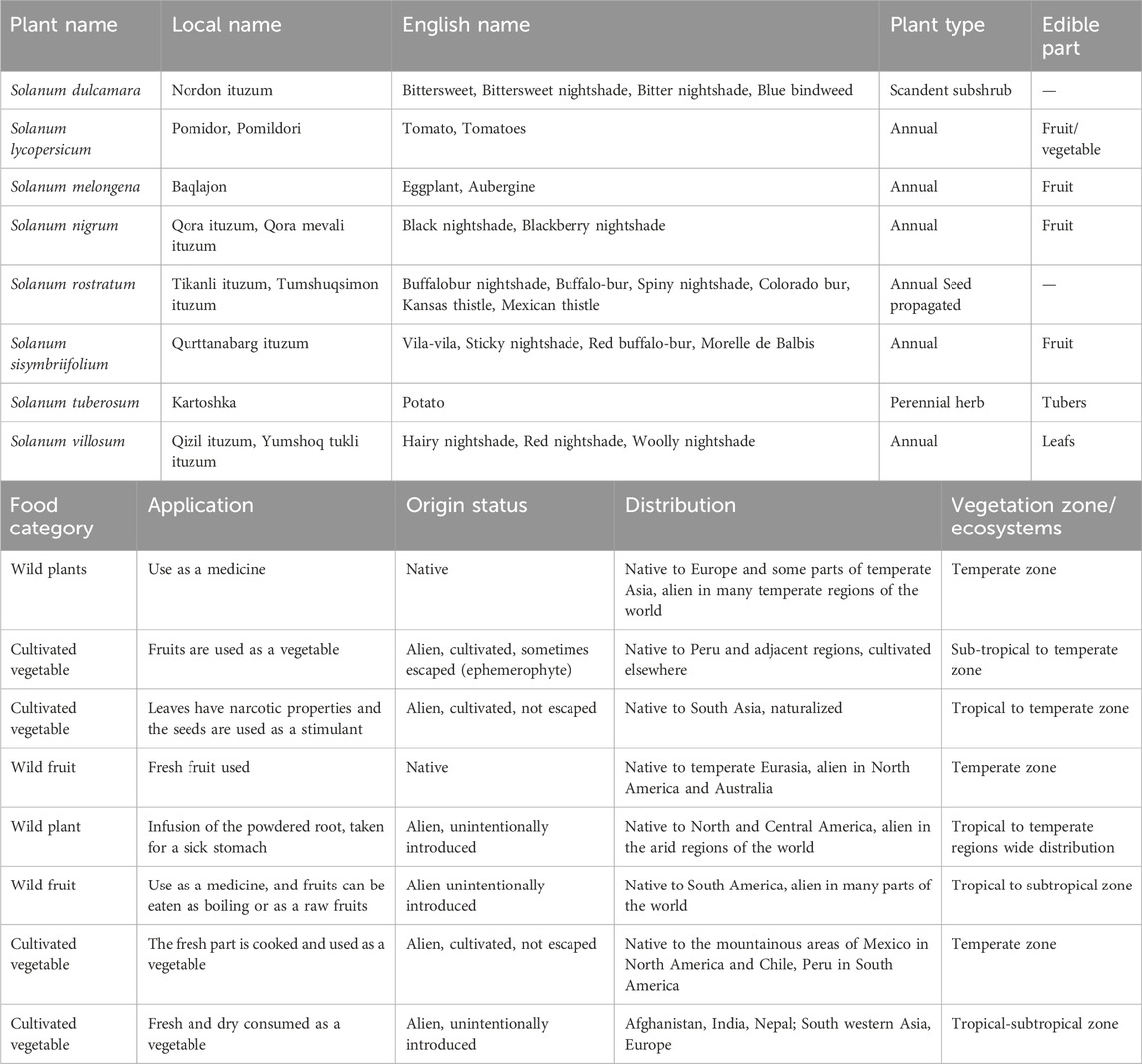
Uzbekistan, located in Central Asia, which covers a total area of 447,400 km2 (Figure 1), has a diversity of habitats of global and regional importance for ecological functions.
FIGURE 1. Map of Uzbekistan (Gafforov et al., 2017).
Uzbekistan’s varied landscapes, consisting of high mountain ranges, vast steppes, deserts and riparian wetlands result in a high diversity of habitats. The mountains of the Central Asia biodiversity region within the largest floral geographic region of temperate Asia consist of two major mountain systems, the Pamir and the Tien Shan. The mountainous areas occupy 15% of the territory of Uzbekistan. The highest point in Uzbekistan is the peak of Hazrati Sultan in the Hissar mountain range (4,643 m, 15,233 ft.) in the Surkhandarya region in Southern Uzbekistan (Gafforov, 2017).
The largest biomes in Uzbekistan are temperate grasslands, savannas, and shrublands. Uzbekistan also contains mountain grasslands and shrublands, deserts, and xeric shrublands, as well as temperate coniferous forests biomes. Despite the mountainous nature of Central Asia, forests cover a relatively small proportion of each country. Much of the forest area is dominated by small trees of the genus Haloxylon Bunge ex E. Fenzl (Amaranthaceae) and other shrubs, particularly in desert and semi-desert areas of Uzbekistan. In moist, mountainous areas the main species are Juniperus spp., Populus spp., Salix spp., Juglans regia L., Pistacia vera L., Malus sieversii (Ledeb.) M. Roem., and M. niedzwetzkyana Dieck ex Koehne, Prunus communis L., P. sogdiana Vassilcz., P. ferganica Lincz, Pyrus bucharica Litv., and P. korschinskyi Litv., Sorbus persica Hedl., and other deciduous forest trees, fruit-bearing trees, and shrubs (Botman, 2009; Gafforov et al., 2017). The flora of Uzbekistan includes 4,500 species of vascular plants, of which about 400 species are endemic, rare, and relict and about 200 species are used in foods, and 1,200 species are used as medicinal plants (Khojimatov et al., 2023a,b). According to the World Geographical Scheme for Recording Plant Distribution system, the region of Uzbekistan belongs to the Central Asian botanical flora (Brummit, 2001). The main ecological forest types in Uzbekistan are mountain, desert, and flood-plain forests (Figure 2).

FIGURE 2. Forest types in the study area. (A) Mountain juniper forests; (B) Wild fruit tree forests in the mountain; (C) Desert saxaul (Haloxylon spp.) forests; (D) Tugai forests (Gafforov et al., 2020).
Uzbekistan is one of the major producers of fruits and vegetables among the Commonwealth of Independent States (CIS) nations due to fertile land. Many farms focus on growing specific crop species, such as potatoes, tomatoes, peppers, melons, and watermelons, in order to maximize productivity and profitability. In both open fields and greenhouses, tomatoes are the most popular vegetable crop in Uzbekistan. Fresh tomatoes are a profitable crop that can boost the profitability of greenhouse farmers because 20% of their production in open fields and roughly 60% in protected regions are exported.
Population of Uzbekistan
Nowadays, the population of Uzbekistan is more than 35,163,944 people (Macrotrends, 2023). Many nationalities and ethnic groups, such as Uzbeks, make up more than four-fifths of the population, followed by Tajiks, Kazakhs, Tatars, Russians, Karakalpaks and other Germans, Greeks, Kyrgyz, Meskhetian Turks, Slavs Turkmens, Uighurs, and Ukrainians. In addition, numerous Diasporas in Uzbekistan are Armenians, Azerbaijanis, Georgians, Iranians, Koreans, and many other nationalities (Lubin, 1984).
Data collection
We have obtained all data from papers, monographs, and books written in Uzbek, Russian, and English in indexed and non-indexed journals by using online bibliographic databases: Google, Google Scholar, PubMed, Scopus, Semantic Scholar, Web of Science, and ScienceDirect Navigator, as well as some local library sources, and other available scientific materials, focused on eight Solanum species. As a result, approximately 270 published articles were found in which some studies were selected for the diversity, geographical distribution, habitat, taxonomy, morphological characteristics, ethnobotany, and uses in ethnomedicinal of the selected plants of the genus Solanum. Moreover, we investigated the reference lists of 190 selected literature sources from the year range 1930–2023 to acquire a more comprehensive and precise dataset of information. In addition, the scientific names of the plants were checked for potential synonyms in Plants of the World Online (POWO) (Powo, 2023), and a current list of Solanum species was compiled as well.
Results and discussion
Diversity of Solanum species in Uzbekistan
The largest genus of Solanaceae, Solanum L., has over 1,250 species, making it economically and culturally significant for its food crops (Kaunda and Zhang, 2019; Gagnon et al., 2022). This perennial, frost-sensitive shrub needs bright, humid weather. This genus has spread throughout the Old World, including Australia, Africa, as well as North and South America, Europe, and Asia. Its main producers are India, Pakistan, Sri Lanka, Bangladesh, China, Japan, Uzbekistan, and Syria (Devaux et al., 2021). Uzbekistan grows along agricultural lands, built-up regions, roadside ditches, lowland river basins, and disturbed places. Based on the Plants of the World Online database and recently published articles in Uzbekistan, the scientific names of eight species of the genus Solanum are listed: Solanum dulcamara L., S. lycopersicum L., S. melongena L., S. nigrum L., S. rostratum Dunal., S. sisymbriifolium Lam., S. tuberosum L., and Solanum villosum Mill. Solanum encompasses a limited number of species (Table 1).
Several species, particularly Solanum nigrum and S. dulcamara, are considered nightshades and highly poisonous. The potato (S. tuberosum), tomato (S. lycopersicum), and eggplant (S. melongena) are three food crops of significant economic importance that belong to the wide and diversified genus Solanum of flowering plants (aborigine, brinjal). It also includes various plants grown for their decorative blooms and fruits and the so-called horse nettles, which are unrelated to the Urtica genus of real nettles. Solanum species have many different growth habits, including annuals, perennials, vines, subshrubs, shrubs, and tiny trees. Many once separate genera, like Lycopersicon (the tomato group) and Cyphomandra, are now subgenera or sections of Solanum. Of the eight selected species, only two are native: a sub shrubby climbing S. dulcamara and an annual S. nigrum. All others are invasive: S. lycopersicum, S. melongena and S. tuberosum are widely cultivated, and three other species, i.e., S. villosum from the Mediterranean area, while S. rostratum and S. sisymbriifolium from North and South America, respectively are unintentionally introduced species that can be classified as the least naturalized species in the lowland dump places of the country.
Fresh fruit yields of 45–65 tons/ha under irrigation, of which 85 to 95 percent is moisture, are considered to be good commercial yields. For the purposes of this investigation, a 15% dry matter content was assumed. FAOSTAT estimates that Uzbekistan’s yields are higher than the global average but lower than those of the major Mediterranean Sea producing nations (Spain, Italy, etc.). Fresh yields in Uzbekistan typically range between 25 and 35 tons per hectare, according to both FAOSTAT and municipal figures.
Economic importance of the Solanum in Uzbekistan
Uzbekistan has reforming its economic and agricultural policies and given priority to the development of the horticultural subsector. Uzbekistan is well known for its delicious fruits and vegetables; with its entrepreneurial dynamics, it has enormous potential to become a key player in the production and export of horticultural products as well as value-added food products. Among them economically important potato and tomato crops are planted in Uzbekistan and a high yield is obtained from them every year. According Ministry of Agriculture of Uzbekistan in January-December 2022, 301,000 tons of agricultural products were grown in greenhouses (East-Fruit, 2022; Future Food Production, 2022). Of these, 211 thousand tons of tomatoes were harvested. Potatoes for the 2022 harvest in all categories of farms in Uzbekistan are planted on 243,000 ha, which is 55%, 86,000 ha more than last year. Accordingly, the forecasted crop volume in 2022 was 4.2 million tons, which is 26% as 850,000 tons more than in 2021. However, many Solanum cultivars have lost their yield due to damage caused by various pathogens. As a result, it causes great loses to the economy of the state (Gafforov et al., 2022).
Botanical traits and distribution, taxonomy, habitat, ecology, phytochemistry and pharmacology
Solanum is one of the largest genus of flowering plants (Frodin, 2004), well known for the Black Nightshade, Solanum nigrum (Solanaceae). The botanical traits of S. dulcamara, S. lycopersicum, S. melongena, S. nigrum, S. rostratum, S. sisymbriifolium, S. tuberosum, and S. villosum are as follows.
Taxonomic treatment
Solanum dulcamara L. Sp. Pl.: 185 (1753) (Table 1; Figure 3)

FIGURE 3. Solanum dulcamara. (A,B) Habit and leaves; (C) Inflorescence; (D) Fully mature fruits (Tashkent Botanical Garden: 17.11.2022, Photo credit by Yusufjon Gafforov and Trobjon Makhkamov).
Synonyms: Dulcamara flexuosa Moench, Lycopersicon dulcamara (L.) Medik, Solanum ruderale Salisb., nom. superfl., S. scandens Neck., nom. superfl.
Description: Unarmed or pubescent semi-shrub with a few upright, ascending, or occasionally climbing shoots coming from the base. (5)7–10 cm long, (2.5)4–5 cm wide, broadly ovate, with a distinct unequal or slightly kidney-shaped, less frequently wedge-shaped base, noticeably attenuated towards the apex, decreasing up the stem, and 2–3 times shorter than the blade on the petioles. Leaves can be whole or have upper leaves incised into two obtuse, ovate, horizontally or upwardly directed lobes. Inflorescence flat corymbose panicle with 10–25 flowers and 1–2 branches at the base. Peduncles that are 3–4.5 cm long and pedicels that are heavily pubescent or nearly glabrous. Calyx (1.5–), 2 (–2.5) mm long, three lobes, and pubescent or hairless. Corolla purple, 15–17 mm in diameter, with 3–4 mm broad, especially in the bud, oblong-ovate lobes, and fluffy at the top. Embryos merged. Red, round, (6) 7–8 mm in diameter berries are present (Solanum dulcamara, 2023).
Phenology: Flowers in June-September and fruits in July-September.
Reproduction: By seeds.
Population status: Common, forming dense groups.
Global Distribution: Solanum dulcamara is a native plant of several African countries (Algeria, Morocco, and Tunisia). Asia (Afghanistan, China, Inner Mongolia, Iran, Iraq, Japan, Kazakhstan, Khabarovsk, Kirgizstan, Mongolia, Myanmar, Pakistan, Palestine, Tajikistan, Turkmenistan, Uzbekistan, Vietnam). Europe (Albania, Austria, Belarus, Belgium, Bulgaria, Denmark, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Netherlands, Norway, Poland, Portugal, Romania, Russia, Serbia, Slovakia, Spain, Sweden, Switzerland, Turkey, Ukraine, United Kingdom). It is introduced to North America (Canada, United States) and South America (Brazil).
Habitat: Solanum dulcamara grows in roadsides, disturbed grounds, mountain river valleys and lakesides, lowland river valleys and sides of irrigation canals, built-up areas, and agricultural lands.
Phytochemistry and Pharmacology: The chemical composition of different parts of S. dulcamara was discussed especially in terms of alkaloid identification, bioactivity and isolation. Cansever and Turker (2007) found in their research that methanolic extracts derived from leaves and stems of S. dulcamara grown in natural field conditions demonstrated effective antibacterial properties against Staphylococcus epidermidis, S. aureus, Klebsiella pneumonia, Salmonella typhimurium, and Serratia marcescens. Notably, the antibacterial efficacy was higher in field-grown plant material compared to in vitro-grown material. Furthermore, the methanolic extracts exhibited superior antitumor activity compared to water extracts, with field-grown leaves and stems displaying greater efficacy than their in vitro-grown counterparts (Cansever and Turker, 2007). Two years later, Kumar et al. (2009), reported that the plant produces a high content of a specific alkaloid: β-solamarin (roots), solanine (unripe fruits) and solasodine (flowers). All parts of the plant contain various glycoalkaloids of a wide structural variety (solamarin, solamargine, solanine, solasodine, tomatidine), phenolic compounds (biflavonoids) and steroids (β-sitosterol and stigmasterol) (Rosa-Martínez et al., 2015; Popova et al., 2021). Additionally, Rosa-Martínez et al. (2015) isolated and examined flavonoids from S. dulcamara to investigate their potential anti-hyperglycemic properties.
Solanum lycopersicum L. Sp. Pl.: 185 (1753) (Table 1; Figure 4)

FIGURE 4. Solanum lycopersicum. (A,B) Habit; (C,D) Inflorescence in hairy individual; (E) Immature infructescence; (F) Fully mature fruits (Tashkent province, Qibray district, TashGres, Greenhouse, 28.12.2022, Photo credit by Yusufjon Gafforov and Trobjon Makhkamov).
Synonyms: Lycopersicon esculentum Mill., L. esculentum subsp. typicum Luckwill, not validly publ., L. lycopersicum (L.) H. Karst., nom. rej., L. pomum-amoris Moench, nom. superfl., L. solanum-lycopersicum Hill, nom. superfl., Solanum lycopersicum var. esculentum (Mill.) Voss.
Description: The two distinguishing features of Solanum lycopersicum are its little, soapy-smelling, green fruits with a disagreeable flavor and its small compound leaves with thick, rounded leaflets. Leaflets are disseminated at the edge blastozone and go through developmental stages like leaves. The tomato is a perennial plant in the Solanaceae, frequently grown as an annual herb. It usually reaches a height of 1–3 m and has a flimsy woody stem that scrambles over neighboring plants. Tomatoes can be oblong, round, flat on top and bottom, or pear-shaped. The fruit is a tasty, brightly colored, frequently red berry that is typically much larger in cultivated varieties than it is in wild plants. This is because of the pigment lycopene (Solanum lycopersicum, 2023).
Phenology: The time of flowering and fruiting depends on early or late varieties of tomato and it depends on which region of Uzbekistan it is planted.
Reproduction: By seeds.
Population status: Common/Cultivated.
Global distribution: S. lycopersicum is native to South America but was introduced into countries of Asia, Europe and North America, where they soon became popular and were exported around the world. There are no naturally growing wild species of tomato in Uzbekistan.
Habitat: Not known in a truly wild situation. Wild tomatoes are native to western South America and distributed from Ecuador to northern Chile (Darwin et al., 2003; Peralta and Spooner, 2005). They flourish in a range of environments, including those with arid coastal lowlands and surrounding regions where the pacific winds are scarcer in the fall and wet climates, isolated valleys in the high Andes, and harsh deserts as the Atacama Desert in northern Chile.
Phytochemistry and Pharmacology: The chemical composition of S. lycopersicum covers a broad spectrum of bioactive coumpouds and is very well documented, based mainly on phenolic compounds, carotenoids and alkaloids as the most present (Helmja et al., 2007; Iijima et al., 2009; Choi et al., 2011; Hövelmann et al., 2019). Choi et al. (2011) determined phenolic compounds viz. caffeic acid-hexose isomer I, caffeic acid-hexose isomer II, caffeoyl-quinic acid, 5-caffeoylquinic acid, caffeoyl-quinic acid isomer, quercetin-3-apiosylrutinoside, quercetin-3-rutinoside, dicaffeoylquinic acid, tricaffeoylquinic acid, naringenin chalcone, and naringenin in different varieties of S. lycopersicum. According to Elizalde-Romero et al. (2021), S. lycopersicum is an important source of lycopene, whereas different preparation of the plant (tangerine tomato juice, red tomato juice, tomato paste, fresh tomato, and tangerine sauce) contained lycopene in both forms, as cis-, and trans-lycopene. All parts of this plant, including fruits, leaves, and stems, contain steroidal glycoalkaloids as α-tomatine and dehydrotomatine (Ostreikova et al., 2022).
Free amino acid and phenolic derivatives were investigated for antioxidative and cytotoxic properties (Choi et al., 2011). Rosa-Martínez et al. (2015) reported that S. lycopersicum is the primary food source of lycopene, a significant source of vitamin C and vitamin E as well as of both flavonoids naringenin and rutin with antioxidant properties. Recent findings also identify a clear connection between tomato and positive effects on metabolic syndrome as hypertension and cardiovascular disease (Alam et al., 2019; Ani et al., 2022). Indeed, Ani et al. (2022) showed a possible mechanism of antihypertensive property of lycopene-rich extract of Solanum lycopersicon in Wistar rats.
Solanum melongena L. Sp. Pl.: 186 (1753) (Table 1; Figure 5)

FIGURE 5. Solanum melongena. (A) Habit; (B) Inflorescence; (C) immature infructescence; (D) Fully mature fruits (Tashkent province, Qibray district, eggplant farmer area). Photo credit by Yusufjon Gafforov and Trobjon Makhkamov).
Synonyms: Melongena esculenta (Dunal) Grecescu, M. incurva Mill., M. ovata Mill.,
M. spinosa Mill., M. teres Mill., Solanum aethiopicum var. violaceum Dunal, S. album Lour., S. album Noronha, not validly publ., S. album var. richardii Dunal, S. album var. rumphii Dunal, S. edule Schumach. & Thonn., S. edule var. multifidum Dunal, S. esculentum Dunal, S. esculentum var. aculeatum Dunal, Solanum esculentum var. subinerme Dunal, S. heteracanthum Dunal, S. indicum Roxb., nom. illeg., S. lagenarium Dunal, S. melongena subsp. agreste Dikii, S. melongena var. angustum Dikii, S. melongena var. cylindricum Dikii, S. melongena var. esculentum (Dunal) Walp., S. melongena var. giganteum (Alef.) Dikii, S. melongena var. globosi Dikii, S. melongena var. leucoum (Alef.) Dikii, S. melongena var. ovigera Pers., S. melongena var. racemiflorum Dikii, S. melongena var. racemosum Dikii, S. melongena var. serpentinum L.H. Bailey, S. melongena var. stenoleucum (Alef.) Dikii, S. melongena var. variegatum (Alef.) Dikii, S. melongena var. violaceum (Alef.) Dikii, S. melongena var. viride Dikii, S. melongenum St.-Lag., S. oviferum Salisb., S. ovigerum Dunal, S. ovigerum var. album Sweet, S. ovigerum var. insanum Blume, S. ovigerum var. luteum Sweet, S. ovigerum var. oblongocylindricum Dunal, nom. superfl., S. ovigerum var. ovum-album Dunal, S. ovigerum var. ovum-luteum Dunal, S. ovigerum var. ovum-rubens Dunal, S. ovigerum var. ruber Sweet, S. ovigerum subsp. sinuatorepandum Dunal, S. ovigerum subsp. subrepandum Dunal, S. ovigerum var. violaceum Sweet, S. plumieri Dunal, S. pressum Dunal, S. pseudoundatum Blume, S. pseudoundatum var. albiflorum Blume, S. pseudoundatum var. atropurpurascens Blume, S. pseudoundatum var. leucocarpon Blume, S. requienii Dunal, S. sativum Dunal, S. sativum var. albiflorum (Blume) Dunal, S. sativum var. atropurpurascens (Blume) Dunal, S. sativum var. leucocarpon (Blume) Dunal, S. serpentinum Noronha, S. tomentosum Hasselt ex Miq., not validly publ., S. trilobatum Noronha, not validly publ., S. trongum Poir., S. trongum var. divaricatum Dunal, S. trongum var. rumphii Dunal, S. violaceum DC. ex Dunal, not validly publ., S. zeylanicum Scop.
Description: Annual succulent up to 90 cm tall. Few prickles and stellate hairs on the stem and branches. Oval to rhomboid-ovate, sinuate to lobed leaves, 5–20 × 4–15 cm. Purple to pale violet, solitary or in clusters of up to five, recurved pedicel, up to 5 cm long. Campanulate, sparsely prickled, 15–18 mm long calyx that enlarges in fruit. The lobes of the corolla’s limb are triangular-ovate and stellate-tomentose on the outside. The thread of stamen supporting the filament is 3–4 mm long filaments. Berry, 8–15 cm long, ovoid to subglobose to elongated in shape, typically dark purple or different color variants. Three mm long, highly rugose subreniform seeds (Solanum melongena, 2023).
Phenology: The time of flowering and fruiting depends on early or late varieties of eggplants and it varies on which region of Uzbekistan it is planted.
Reproduction: By seeds, propagation by rooting healthy shoots is also possible.
Population status: Common/Cultivated.
Global distribution: S. melongena is native to Southeast Asia (China South-Central, Laos, Malaya, Myanmar, Thailand, and Vietnam) and has been cultivated in southern and eastern Asia regions since prehistory for food purposes.
Habitat: Cultivated S. melongena cultivars are planted on agricultural lands.
Phytochemistry and Pharmacology: The phytochemical constituents of S. melongena are different alkaloids as amides and glycoalkaloids (pyrrolidine, quinazolizidine and tropane), phenolic acids, phenylpropanoids, polyphenols (anthocyanins, flavonoids), steroidal saponins, sterols and tetracyclic triterpenes (Rosa-Martínez et al., 2015; Sun et al., 2015; Lelario et al., 2019; Chen et al., 2021; Ralte et al., 2021; Jit et al., 2022). Kacjan Maršić et al. (2014) reported that the main phenolic compounds of S. melongena were chlorogenic acid, delphinidin-3-rutinoside, quercetin-3-glucoside and quercetin-3-galactoside. Sun et al. (2014) isolated ten polycyclic aromatic lignanamides from S. melongena roots, including four new melongenamides. Then Sun et al. (2015) characterized 16 phenylpropanoid amides, and among them four compounds (N-cis-feruloylnoradrenline, N-trans-sinapoyloctopamine, N-trans-caffeoyloctopamine, and N-trans-feruloylnoradrenline) were isolated from the genus Solanum for the first time. Yang et al. (2018) isolated and characterized six steroidal saponins, including five new cholestane saponins (abutilosides P-T), one new steroidal alkaloid (abutiloside U), along with one new natural product named as (25R)-3β,16α,26-trihydroxy-5-en-cholestan-22-one-3-O-α-L-rhamnopyranosyl-(1 → 4)-β-D-glucopyranoside, abutiloside P, and three know steroids (abutiloside G, solaviaside B, and tumacone).
Antiinflammatory lignanamides exhibited inhibition of nitric oxide production in lipopolysaccharide-induced RAW 264.7 macrophages (Sun et al., 2014). Anticholinesterase and antioxidant activities of glycoalkaloids were discussed by Lelario et al. (2019). Jit et al. (2022) demonstrated that S. melongena contains two phenolic compounds (chlorogenic acid, ferulic acid) with radioprotective activity.
Solanum nigrum L. Sp. Pl.: 186 (1753) (Table 1; Figure 6)

FIGURE 6. Solanum nigrum. (A,B) Habit; (C,D) Inflorescence and immature infructescence; (E,F) Fully mature fruits. (Tashkent province, Pskent district, 29.09.2022, Photo credit by Yusufjon Gafforov and Trobjon Makhkamov).
Synonyms: Solanum humile Salisb., nom. superfl., S. morella Desf, nom. superfl., S. morella subsp. nigrum (L.) Rouy, nom. superfl., S. nigrum var. genuinum Hassl., not validly publ., S. nigrum var. humile Macloskie, not validly publ., S. nigrum var. legitimum Neilr., not validly publ., S. vulgatum Baumg., nom. superfl., S. vulgatum var. nigrum (L.) Spenn., nom. superfl.
Description: It is an annual plant having a stem that is upright, decumbent, splayed-branched from the base, cylindrical below, flattened-cylindrical, with slightly projecting non-serrated ribs, and 25–50 (75) cm high. The leaves are 3–5–7 cm long, 2–4 cm wide, shorter than blades on petioles, dark green, juicy, glabrous or, especially young, with sparse short hairs, denser along the veins, oblong-ovate or rhombic-ovate, gradually narrowing from the middle into an acute apex, with an unequal wedge-shaped base widely descending onto the petiole, at the bottom notched-toothed. The extra-axillary inflorescences are umbellate, or racemose corymbose with 3–8 flowers each. Pedicels with the same, but more thick hairs, drooping, spaced at fruits; peduncles glabrous or, more frequently, with upward-directed adpressed hairs, and 2–2.5 mm long, cylindrically campanulate, glabrous or more frequently hairy, and one-third incised into blunt teeth. Corolla is white, 6–7 mm in diameter, and has lobes on the outer that are ovate-lanceolate and quickly pubescent. The berry is 6–10 mm long, black, and round. Yellow, almost reniform, somewhat elongated at one end, fine-meshed seeds measure 2 mm in length (Solanum nigrum, 2023).
Phenology: Flowers in June-October, fruits in July-August.
Reproduction: By seeds.
Population status: Common, sometimes forming dense groups in croplands.
Global Distribution: This species occurs on all continents except Antarctica; it is species native to Eurasia (Western Europe to Japan), northern Africa, and Australia, sporadically introduced in South Africa and naturalized locally in temperate North America (Särkinen et al., 2018).
Habitat: Disturbed grounds, mountain river valleys and lakesides, lowland river valleys and sides of irrigation canals, dry riverbeds, alluvial fans and gravel deposits, built-up areas, and agricultural lands.
Phytochemistry and Pharmacology: Jain et al. (2011) reported major bioactive compounds in S. nigrum that include glycoalkaloids, glycoproteins, and polysaccharides. The glycoalkaloids include solamargine, solanine, solasonine (Jain et al., 2011; Sivakumar et al., 2020). In 2022, Chen et al. (2022) claimed that about 188 phytochemicals were separated and identified from S. nigrum, containing alkaloids, flavonoids, organic acids, phenylpropanoids and their glycosides, polysaccharides and steroids.
Using streptozotocin-induced diabetic mice model, Nyaga et al. (2019) showed significant antidiabetic effect of S. nigrum var. sarrachoides and hence their use in folklore medicine. Chen et al. (2022) also mentioned that S. nigrum has nutrients essential for humans and of great importance to human eye and skin health (α-carotene, β-carotene, ferulic acid ester). Steroidal saponins and steroidal alkaloids have been considered as the main bioactive components of S. nigrum, exhibiting various biological activities (Li et al., 2023). Then Wang et al. (2023) proved that the steroidal saponins from S. nigrum had broad-spectrum cytotoxic activity against various human leukemia cancer cell lines.
Solanum rostratum Dunal. Hist. Nat. Solanum: 234 (1813) (Table 1; Figure 7)

FIGURE 7. Solanum rostratum. (A) Habit and leaves [Photo credit by Frankie Coburn (Swbiodiversity, 2023a)]; (B) Inflorescence and immature infructescence [Photo credit by Patrick Alexander (Swbiodiversity, 2023b)]; (C) Inflorescence [Photo credit by Frankie Coburn (Swbiodiversity, 2023c)]; (D) Mature fruits [Photo credit by Patrick Alexander (Swbiodiversity, 2023d)].
Synonyms: Androcera rostrata (Dunal) Rydb., Nycterium rostratum (Dunal) Link.
Description: Annual herbs that are seated with thorns that resemble needles and pubescent with stellate hairs. Up to 60 cm high, tall, splayed-branched stem. The petioles are 1–2 times shorter than the plate; the leaves have stellate hairs on both sides and are oblong-ovate to ovate in shape. They are pinnately divided into obovate segments, which are then divided into rounded-oblong lobes 7–10 cm long and 4–7 cm wide. Racemes with three to eight flowers each are made up of flowers on short stalks. Campanulate, 1.5–2.5 mm long, stellate-haired, and two-thirds of its length divided into lanceolate lobes, the calyx is maintained in fruit. Yellow, 3–4 mm in diameter, slightly zygomorphic corolla with ovate-lanceolate lobes. Nearly equal in length, the fifth anther is much longer and more strongly bent. Berry drying out and cracking inside a growing cup. Seeds are brown, unevenly angular, and fine-meshed (BSBI Species Accounts Archive, 2010).
Phenology: Flowers in August, fruits in September.
Reproduction: By seeds.
Population status: Common, found in dense groups.
Global distribution: Africa (Libya, Morocco, Tunisia, South Africa). Asia (Azerbaijan, Bangladesh, China, India, Japan, Kazakhstan, Palestine, South Korea, Uzbekistan). Europe (Albania, Austria, Belgium, Bulgaria, Denmark, France, Germany, Hungary, Ireland, Latvia, Lithuania, Moldova, Norway, Russia, Slovakia, Ukraine, United Kingdom). North America (Canada, Mexico, United States). Oceania (Australia, New Zealand).
Habitat: S. rostratum occurring in roadsides, disturbed grounds, lowland river valleys and sides of irrigation canals, built-up areas, and agricultural lands.
Invasiveness: S. rostratum is a fast-growing, vigorous weed native to North-Central America, South America, and it includes the countries Panama, Costa Rica, Nicaragua, Honduras, El Salvador, Guatemala, and Belize. Now widely introduced or migrated into several countries of Europe, Asia, South Africa, and Australia. The species invade ecosystems by forming dense colonies, and a single plant can produce hundreds of seeds (Vallejo-Marin, 2010) which are dispersed by both biotic and abiotic vectors and self-propelled by its dehiscent fruit. The species is a declared noxious weed in Central Asia and is listed as invasive alien plant species in Uzbekistan.
Phytochemistry and Pharmacology: Various studies have shown that S. rostratum contains alkaloids, flavonoids and steroids (Chang et al., 2017; Huang et al., 2017; Omar et al., 2018). Omar et al. (2018) reported the isolation and structure elucidation of linalyl-β-glucopyranoside and apigenin-7-O-glucoside. Liu et al. (2021b) isolated pyrrole alkaloids from the leaves of S. rostratum and identified three pairs of novel enantiomeric pyrrole alkaloids: (2′R)-caffeicpyrrole A, (2′S)-caffeicpyrrole A, (2′R)-caffeicpyrrole B, (2′S)-caffeicpyrrole B, (2′R)-caffeicpyrrole C, and (2′S)-caffeicpyrrole C. Authors such as Omar et al. (2018) and Valadez Vega et al. (2019) demonstrated the antioxidant and anti-carcinogenic effects of S. rostratum.
Solanum sisymbriifolium Lam. Tabl. Encycl. 2: 25 (1794) (Table 1; Figure 8)
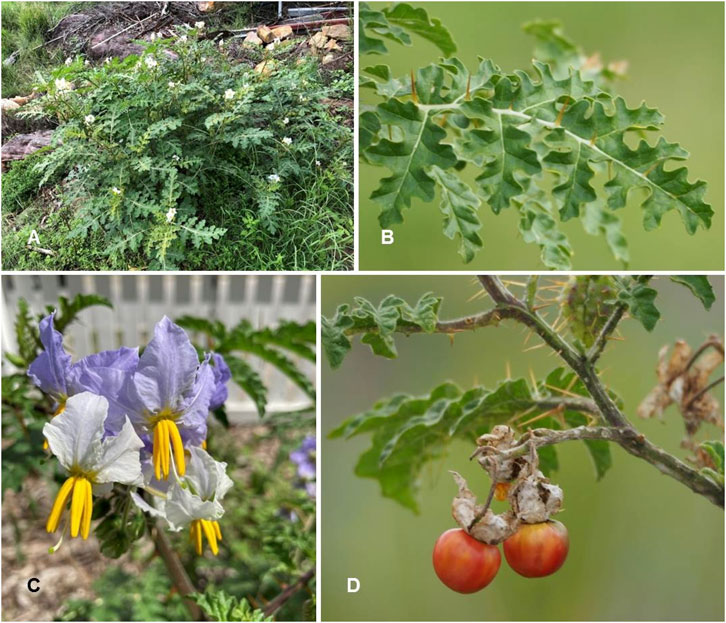
FIGURE 8. Solanum sisymbriifolium. (A) Habit [Photo credit by Inaturalist (2023d)]; (B) Leaves [Photo credit by Inaturalist (2023c)]; (C) Inflorescence [Photo credit by Phillip Mayhair (Inaturalist, 2023a)]; (D) Fully mature fruits [Photo credit by Inaturalist (2023b)].
Synonyms: Solanum balbisii Dunal, S. balbisii var. bipinnata Hook, S. balbisii var. oligospermum Sendtn, S. balbisii var. purpureum Hook, S. bipinnatifidum Larrañaga, S. brancaefolium J. Jacq, S. decurrens Balb, S. edule Vell. nom. illeg., S. formosum Weinm., S. inflatum Hornem., S. mauritianum Willd. ex Roth, S. opuliflorum Port. ex Dunal, S. pilosum Raf., S. rogersii S. Moore, S. sabeanum Buckley, S. sisymbriifolium f. albiflorum Kuntze, S. sisymbriifolium var. bipinnatipartitum Dunal, S. sisymbriifolium var. brevilobum Dunal, S. sisymbriifolium var. gracile Mattos, S. sisymbriifolium var. heracleifolium Sendtn, S. sisymbriifolium f. lilacinum Kuntze, S. sisymbriifolium var. macrocarpum Kuntze, S. sisymbriifolium var. oligospermum (Sendtn.) Dunal, S. sisymbriifolium purpureiflorum Dunal, S. subviscidum Schrank, S. thouinii C. C. Gmel., S. viscidum Schweigg, S. viscosum Lag, S. xanthacanthum Willd.
Description: Annual herbs with enormous needle-like spines and pubescent stellate with sticky glandular hairs. 50–150 cm tall erect, branching stem. On petioles studded with spines and 2–3 times shorter than the blade, the leaves are elliptical, whole or pinnately divided into oblong serrated segments, 5–10 cm long, and 3–5 cm wide. The leaves also have stellate hairs on both sides. Racemose inflorescences of flowers on long stalks. Calyx campanulate, 1 cm long, roughly half-carved into linear-triangular lobes, preserved throughout fruiting, significantly enlarged and ripped into five turning sections. White or bluish corolla with a limb divided into ovoid lobes, 3–4 mm in diameter, regular, glabrous on the outside with stellate hairs. Five anthers, each equal. The berry is 1–2 cm in diameter and bright crimson inside a growing calyx: Brown, kidney-shaped seeds.
Phenology: Flowers in September, fruits in October.
Reproduction: By seeds.
Population status: By seeds and rhizomes.
Global distribution: Africa (Benin, Kenya, Morocco, South Africa). Asia (China, India, Republic of Korea, Uzbekistan). Europe: (Austria, Belgium, Czech Republic, Denmark, Germany, Ireland, Estonia, Finland, France, Hungary, Italy, Latvia, Lithuania, Netherlands, Norway, Portugal, Spain, Sweden, Turkey, United Kingdom, Ukraine). North America (Canada, United States). South America (Argentina, Bolivia, Brazil, Chile, Colombia, Ecuador, Paraguay, Peru, Uruguay, Venezuela). Oceania and Western Australia.
Habitat: This species occurs in agricultural lands and includes irrigated crops and pastures. The species grows in ruderal and disturbed habitats in urban and semi-urban areas. The species also grows in coastal areas, roadsides, disturbed grounds, lowland river valleys, and built-up areas.
Invasiveness: Solanum sisymbriifolium is native to South America and has been introduced into other regions for ornamental purposes. However, as S. sisymbriifolium tends to be invasive, its introduction as a trap crop or cultivated plant into a new region should be considered thoroughly before implementation.
Phytochemistry and Pharmacology: S. sisymbriifolium is an important source of alkaloids (cuscohygrine, solacaproine, solamine, solasodiene and solasodine), phenolics (caffeic acid, chlorogenic acid, dihydrocaffeic acid, ferulic acid), flavonoids (kaempferol-3-rutinose, rutin), and saponins as isonuatigenin-3-O-β-solatriose (Ibarrola et al., 1996; Ferro et al., 2005; Gupta et al., 2014; More, 2019). Figueiredo et al. (2021) identified steroidal saponins as nuatigenin-3-O-β-chacotriose (nuatigenoside) from the roots of S. sisymbriifolium.
Biological activities of different parts of S. sisymbriifolium were reported including anticancer, anti-diabetic, antimicrobial, antioxidant, hepatoprotective, and hypotensive properties (Ibarrola et al., 1996; Ferro et al., 2005; Gupta et al., 2014; More, 2019; Gebrewbet et al., 2023).
Solanum tuberosum L. Sp. Pl.: 185 (1753) (Table 1; Figure 9)

FIGURE 9. Solanum tuberosum. (A,B) Habit and leaves; (C) Inflorescence; (D) Fully mature fruits (Tashkent province, Qibray district, early potato farmer area). Photo credit by Yusufjon Gafforov and Trobjon Makhkamov.
Synonyms: Lycopersicon tuberosum (L.) Mill., Solanum tuberosum var. cultum, nom. superfl., S. tuberosum var. vulgare Hook. f., nom. inval.
Description: Herbs that are 30–80 cm tall, erect or spreading, glabrous or sparingly pubescent, and have simple and glandular hairs. Stolons harbouring underground tubers that can be white, scarlet, or purplish and are fleshy, globose, oblate, or elliptic in shape. Petiole 2.5–5 cm; leaflet blade oval or oblong, typically sparingly pilose; interrupted odd-pinnate leaves with 6–8 pairs of leaflets and smaller, uneven interstitial leaflets; Panicles that resemble terminal, leaf-opposing, or axillary inflorescences have several flowers and few stems. From mid, the pedicel articulates by 1–2 cm. The lanceolate, sparsely pubescent calyx lobes. Corolla spins measure 2.5–3 cm in diameter and have deltate lobes (5 mm long). It could be white, blue-purple, pink, or purple. The anthers are 5–6 mm in length; the evident ovaries are about 8 mm in style, and the filaments are around 1 mm. Berry green or yellowish green, smooth, globose, frequently striped and about 1.5 cm in diameter (Stern et al., 2013; Solanum tuberosum, 2023).
Phenology: The time of flowering and fruiting depends on early or late varieties of potatoes and it depends on which region of Uzbekistan it is planted. Most potatoes were planted in the Samarkand area on almost 17,000 ha of land in 2017, followed by the Tashkent region (almost 15,000 ha in 2017) (Netherlands Enterprise Agency, 2020). Two harvests per year are carried out in Uzbekistan, namely, first the spring harvest from February to June and the shorter autumn harvest from the end of July to the end of October.
Reproduction: By tuber, rhizomes and seeds.
Population status: Common.
Global distribution: S. tuberosum is native to South America (Argentina, Bolivia, Chile, Colombia, Ecuador, Peru, and Venezuela). It is cultivated worldwide in over one hundred countries throughout Africa, Asia, Australia, Europe, and North America.
Habitat: Cultivated S. tuberosum cultivars are planted on agricultural lands.
Phytochemistry and Pharmacology: Secondary metabolites in S. tuberosum tubers include both phytonutrients and various secondary metabolites. Camire et al. (2009) reported that S. tuberosum is an important source of vitamins (vitamin C, B1, B2, B3, B5, B6, E, folic acid, β-carotene, etc.), and minerals as Fe, Mg, P, and Zn. Furthermore, Drewnowski and Rehm (2013) reported that S. tuberosum is the most affordable source of vitamin C, potassium and fibre providing about 10% of its daily value. Anjum Sahair et al. (2018) reported phenolic derivatives as chlorogenic acid, caffeic acid, gallic acid and protocatechuic acid. The protein content of S. tuberosum is comparable to that of cereals, and nutritionally, potato protein is similar to that of whole eggs (Franková et al., 2022). Among the secondary metabolites, glycoalkaloids are considered as the most common. Based on the literature data (Shakya and Navarre, 2008), various cultivars of S. tuberosum contain less than 20 mg/100 g f.w. of total glycoalkaloids, whereas chaconine and solanine are thought to comprise up to 90% of the total glycoalkaloid content of domesticated ones, with chaconine frequently being more prevalent than solanine. According to Fogelman et al. (2019), the tuber of S. tuberosum contains various secondary metabolites, including anthocyanidins such as peonidin and pelargonidin, carotenoids primarily composed of xanthophylls, phenolic acids: caffeic acid and coumaric acid, along with a few unidentified acids structurally similar to chlorogenic, hydroxycinnamic, and coumaric acid. Additionally, the tuber harbors flavonol, specifically kaempferol, as well as toxic steroidal glycoalkaloids (SGAs). Two years afterward, in 2021, Sampaio et al. (2021) conducted an analysis of ten varieties of colored potato peels, revealing the presence of both non-anthocyanin and anthocyanin phenolic compounds in the examined samples. Among the non-anthocyanin phenolics, caffeic and caffeoylquinic acid were present in the highest concentrations across all samples. Additionally, O-glycosylated flavonoid derivatives and polyamine derivatives were detected. In terms of anthocyanins, all tentatively identified compounds were acylated with a hydroxycinnamic acid. In the same study, the researchers observed that all examined samples exhibited both antioxidant and antitumor activities, demonstrating no adverse effects. The Rosemary variety extract of S. tubersoum displayed the most favorable results in terms of antioxidant and antitumor effects and was the sole sample to exhibit anti-inflammatory activity (Sampaio et al., 2021).
Recently, Baur et al. (2022) showed the light impact on quantitation of toxic steroidal glycoalkaloids and identification of newly identified saponins from S. tuberosum tubers.
The wide variety of phytonutrients found in potatoes, including anthocyanins, carotenoids, minerals, polyphenols and vitamins, have the potential to improve human health and diet (Mishra et al., 2020; Franková et al., 2022; Kowalczewski et al., 2022). Nutritional value of S. tuberosum was highlighted by authors (Fogelman et al., 2019; Dereje et al., 2021). Being rich in carbohydrates, vitamins and antioxidants, the potato is a staple food and potato starch has unique properties compared to cereal starches (Xu et al., 2023). In addition, Rosas-Cruz et al. (2020) postulated that the wound healing mechanism of S. tuberosum-based ointment is related to phytoconstituents as phenolic compounds that exert antioxidant, antimicrobial, and anti-inflammatory effects, which contributes to the optimal healing process.
Solanum villosum Mill. Gard. Dict. ed. 8.: n. 2 (1768) (Table 1; Figure 10)

FIGURE 10. Solanum villosum. (A) Habit and leaves (Photo credit by Julien Renoult); (B) Inflorescence [Photo credit by Julien Renoult (Inaturalist, 2023f)]; (C) Inflorescence and immature infructescence (Photo credit by Corentin Desseux); (D) Fully mature fruits [Photo credit by Corentin Desseux (Inaturalist, 2023e)].
Synonyms: Solanum luteum subsp. villosum (Mill.) Dostál
Description: Grayish annual herbs having stem 15–70 cm high, erect or, less frequently, decumbent, splayed-branched from the base up, cylindrical in the bottom half, higher like the branches, not usually tetrahedral, indistinctly ribbed and especially densely covered in short, upward-facing hairs when young. The petiole’s blades are shorter than the leaves because the leaves are bluish-greenin young stage, densely pubescent on both sides with short, semi-appressed hairs, later becoming bare, ovate, oblong-ovate or rhombic-ovate, obtuse at the apex or gradually narrowed from the middle, with almost rounded or more frequently wedge-shaped leaves, descending to the petioles. Corymbose, extra-axillary, and 4–8 flowered inflorescences. Campanulate, subappressedly pubescent, ranging in length from 2 to 2.5 mm, with a third bluntly toothed. Corolla is white, 4.5–5 mm in diameter triangular ovate limb lobes, and is externally somewhat pubescent. Berry is spherical, orange-red or brownish-red, and 7–10 mm in diameter. Berry is spherical, orange-red or brownish-red, and 7–10 mm in diameter. Yellow, reniform, 2 mm long, and fine-meshed seeds are produced (Solanaceaesource, 2023).
Phenology: Flowers in June - October, fruits in July–September.
Reproduction: By seeds.
Population status: Common, found in small groups.
Global distribution: Africa (Algeria, Angola, Burundi, Egypt, Eritrea, Ethiopia, Kenya, Libya, Madeira, Malawi, Morocco, Mozambique, Nigeria, Somalia, Sudan, Tanzania, Tunisia, Uganda), Asia (Afghanistan, Bangladesh, China, India, Iran, Iraq, Italy, Kazakhstan, Kirgizstan, Korea, Myanmar, Nepal, Oman, Pakistan, Palestine, Saudi Arabia, Tajikistan, Taiwan, Turkmenistan, Uzbekistan, Vietnam, Yemen, Zambia, Zimbabwe), Europe (Albania, Austria, Belgium, Belarus, Bulgaria, Denmark, France, Germany, Greece, Ireland, Hungary, Netherlands, Poland, Portugal, Romania, Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland, Turkey, United Kingdom, Ukraine), North America (United States), Oceania (Australia, New Zealand).
Habitat: Solanum villosum is a prevalent species found on roadsides, disturbed grounds, mountain river valleys and lakesides, lowland river valleys and sides of irrigation canals, built-up areas, and agricultural lands.
Food: S. villosum is more commonly cultivated in eastern Africa, and many specimen labels note that the fruits of S. villosum are particularly prized by children (Keding et al., 2007).
Phytochemistry and Pharmacology: Numerous phytochemicals as alkaloids, amino acids, carbohydrates, fatty acids, flavonoids, glycosides, phenols, proteins, saponins, steroids, tannins, and terpenoids were identified from S. villosum (Venkatesh et al., 2014a; Chowdhury et al., 2015; Ben-Abdullah et al., 2018; Zahara et al., 2019). Ahmad et al. (2019) summarized that leaves of S. villosum contain high levels of nutrients such as carbohydrates and proteins, minerals (Ca, Fe, and P), and vitamins (especially vitamins A, B and C). According to Wojdyło et al. (2019), recommended daily intake for Ca is 1,200 mg for adults, and Ca content in S. villosum is 442 mg/100 g d.w. which was higher than reported in other Solanum species, i.e., S. retroflexum (199 mg/100 g d.w.). Furthermore, Ben-Abdullah et al. (2018) reported for the first time the impact of salt stress induced by NaCl on the production of carotenoids (β-carotene, lutein), glycoalkaloids (GAs) (β-solamagine, α-solasonine), and phenolic compounds (caffeic acid, quercetin, quercetin 3-β-D-glcoside) in S. villosum.
Based on traditional medicine in Southern India, free radical scavenging activity was reported for S. villosum (Venkatesh et al., 2014b). Furthermore, according to Zahara et al. (2019), S. villosum has high nutritional value and used as an Ayurvedic herb with multiple medicinal properties (antifibrotic, antimicrobial and hepatoprotective activity), it is a good source of pharmaceutical agents such as steroidal alkaloids, phenolic compounds, saponins, etc. Indeed, the hydromethanol immature fruit extract possess a high molluscicidal activity against Galba truncatula intermediate host of trematode Fasciola hepatica, a causal agent of fascioliasis in humans (Chowdhury et al., 2008). Chloroform and methanol extracts of S. villosum green berries showed a potential larvicidal biocontrol activity against Aedes aegypti (= Stegomyia aegypti), mosquito that can mainly spread dengue fever, chikungunya, Zika fever and yellow fever (Chowdhury et al., 2008). Two years later, Abdel-Sattar et al. (2010) reported that methanolic, petroleum ether, chloroform, ethyl acetate and aqueous extracts of S. villosum showed significant antiprotozoal activity against Plasmodium falciparum, Trypanosoma brucei, T. cruzi and Leishmania infantum. On the other hand, glycoalkaloids (solanine and solasodine) from butanol extract of S. villosum fruit possessed anticancer potential on LIM-1863 human colon carcinoma cell line (Venkatesh et al., 2014a). Five years later, Nyaga et al. (2019) reported the antidiabetic property of this plant in a streptozotocin-induced diabetic mice model. The authors proposed that the presence of flavonoids, alkaloids, tannins, saponins, phenols, and glycosides in the plant makes it a potential candidate for novel diabetes therapies, especially considering its demonstrated lack of toxicity. A crude extract comparison showed in vitro antimicrobial activities of Solanum villosum (AbdelGawwad et al., 2020). Recently, Staveckiene et al. (2023) demonstrated the effect of ripening stages on the accumulation of polyphenols and antioxidant activity of the fruit extracts of S. villosum.
Ethnobotanical study and uses of the eight Solanum species from Uzbekistan
In the 21st century’s third decade, scientists are still making remarkable strides in the domain of ethnopharmacology and research related to different kinds of medicinal plants (Kim et al., 2023). Regarding this, the World Health Organization reported that indigenous or native populations worldwide still practice traditional medicine using plants as their primary source of treatment and have built their medicinal systems based on their theories, beliefs, and experiences (Mir et al., 2021; Nanjala et al., 2022). Before the modern scientific practice, the traditional herbal medicine was used in healthcare, and most people worldwide still rely on herbal health practices today (Das and Barua, 2013). Recently, more than 80% of the people in Asian and African countries depend on it for primary healthcare (Nanjala et al., 2022). However, despite herbs being used as effective medicines for centuries, the majority lack scientific support and are unexplored. A major approach in ethnopharmacology—ethnobotany, ethnomycology and ethnozoology in particular—is how to transmit knowledge in these fields over a long period, over a large geographical area, or between geographically, culturally or scientifically different regions of the world (Cunningham and Yang, 2011; Fuller, 2013; Guerrero-Gatica et al., 2020). Therefore, ethnobotanists can help rescue this disappearing knowledge and return it to local communities (Ramachandran et al., 2009; Ford, 2015; Belichenko et al., 2022). In general, ethnobotany is the study of the direct interrelationship between human beings and plants, which examines how human communities have used plants to meet their spiritual and practical requirements (Galvis-Tarazona et al., 2022). To ensure the sustainability of plants in the future, it is also crucial to understand the socio-ecological dynamics surrounding their current distribution, trade, and protection (Kunwar et al., 2020).
Solanum is a globally distributed genus; it is rich in various classes of bioactive metabolites and has been used by different tribes all over the world for centuries in traditional medicine and human nutrition (Anjum Sahair et al., 2018; Chidambaram et al., 2022). Central Asia includes five countries: Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan, and Uzbekistan, with about 9,800 vascular plant species and among them, Uzbekistan with over 4,500 species, has a central position in the region (Khojimatov et al., 2020). About 600 plant species have been used in traditional medicine, but only about 200 species have been phytochemically characterized, and some of them (about 150 species) were included in the original Pharmacopoeia of Uzbekistan (Khojimatov et al., 2020).
Although traditional medicine in Central Asia has a vast history that dates back many centuries, its most notable era was between the 10th and 11th centuries (Tayjanov et al., 2021; Gafforov et al., 2023). Abū-ʿAlī al-Ḥusayn Ibn-ʿAbdallāh Ibn-Sīnā (Avicenna, 1930) (arab. ابن سینا, Ibn Sina) was born in 980 in the village of Afshana, present day Bukhara region in Uzbekistan and died in 1037 in Hamadan (Tayjanov et al., 2021). He was one of the early scientists exploring folk medicines’ secrets in medieval East. The “Book of Healing” (Kitab al-Shifa), followed by “The Canon of Medicine” were both written by Avicenna. “The Canon of Medicine” (arab. القانون في الطب, Al-Qānūn fī al-ṭibb) the main medical work of Avicenna is a genuine medical encyclopedia. For many centuries, the work of Avicenna served as the main medical guide of many countries. The Canon was divided into five books. First book of Canon covered general medical and physiological principles and general therapeutic procedures as well. The second book of the Canon is an encyclopedia of medicines (Materia Medica). Descriptions of about 800 therapeutic compounds derived from plants, minerals, and animals may be found. These descriptions were developed on the basis of the identification and management of diseases that affect the entire body. Disorders of various organs were covered in the chapter of the third book on specific pathology. Fifth book is kind of pharmacopoeia and contains lists of about 650 medicinal compounds including their uses and medicinal effects. In general, it combines the experiments of medicine of ancient. Of the 810 drugs listed in the book of Canon, 515 are medicinal plants (and their agents), 125 products of animal origin, and 85 minerals (Tayjanov et al., 2021). Also, of the selected Solanum species, Avicenna documented the traditional use of S. nigrum in the form of juice as the eye remedy in the conditions where children’s eyes swelled after crying, and hepatoprotective agent (Table 2).
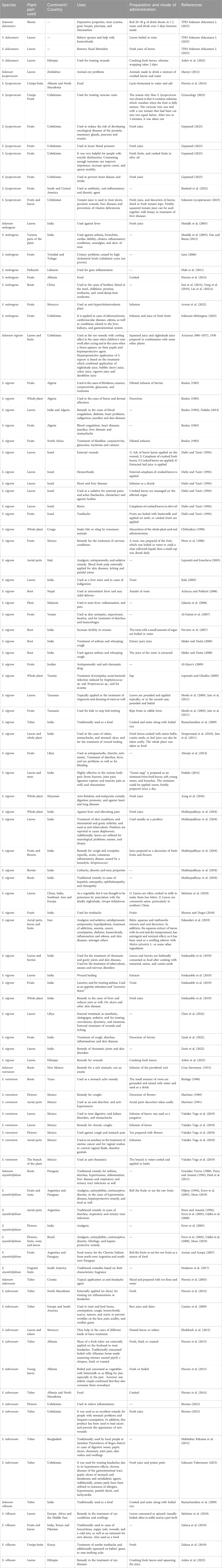
TABLE 2. Ethnobotanical, ethnopharmacological and food uses of the eight Solanum species from Uzbekistan (species name, plant part, country, uses, mode of administration, and used references).
In addition, Avicenna also described usage of nightshade in the treatment of various diseases, for example, an ointment from nightshade has been used for the treatment of headaches and different types of tumors (earlobes and meninges); also plant fruits juice has been used for eyes and throat diseases, and as a sleeping pill as well. Moreover, nightshade fruits were used as hemostatic and diuretic agents for excessive menstrual bleeding, and also for diseases of the kidneys and bladder as well. According to Avicenna “The Canon of Medicine,” nightshade also relieves pain (Khojimatov, 2021; Boboev et al., 2023).
Historically Central Asian botanists, especially in the 20th century, made significant contributions to developing pharmacognosy, pharmacology and phytotherapy (Khojimatov et al., 2020; 2023a). Sezik et al. (2004) worked on examining folk medicine in Uzbekistan, which resulted in 177 folk remedies in the surveyed area of Jizzakh, Samarqand, and Tashkent provinces. Among these folk remedies, 162 were obtained from 79 different plant species belonging to 31 families, including 15 animal-originated remedies belonging to eight animals (Khojimatov et al., 2020; 2023a). Nonetheless, almost no research has been done on the application of members of the Solanaceae family, particularly the species of the genus Solanum, in folk medicine in Uzbekistan. Recently, there is limited information available concerning the ethnobotanical use of three species, S. lycopersicum, S. melongena and S. tuberosum within the region of Uzbekistan (Solanum-Lycopersicum, 2023; Solanum-Melongena, 2023; Solanum-Tuberosum, 2023).
Solanum species have a long history of uses both as edible and as ethnomedicinal plants in different traditional practices around the world. The species have long been used in folk medicine to treat various illnesses, including constipation, eczema, hemorrhoids, heart diseases, herpes, inflammations, rheumatism, wounds, etc. (Kaunda and Zhang, 2019). Various parts of many species belonging to the Solanum section are widely used in medicine all over the world. Their use as such is recorded from the earliest times, and various species, especially S. nigrum, are mentioned and often illustrated in all of the ancient herbals, with Dioscorides being one of the first to record their medicinal properties. Since then, S. nigrum has continued to be widely acclaimed for its medicinal effects in every country where the taxon is found. The previous study demonstrates that the Solanum species, as the most famous member of the Solanaceae family, has noticeable traditional applications that mainly originate from South America, Asia, Africa and Europe. In American and Asian countries, particularly India, Brazil, Mexico, and China, there are special reports on the traditional applications of Solanum species. Besides Solanum in Solanaceae in Uzbekistan, Khojimatov et al. (2020) reported traditional uses of the two species from the Solanaceae family (Datura stramonium L. and Hyoscyamus niger L.).
Therefore, this study aimed to review the ethnobotanical knowledge of Solanum species (Table 2). Based on the available literature data, ethnographic, ethnopharmacological and food uses of the eight Solanum species from Uzbekistan are reported. Among them, S. nigrum is one of the largest, most variable/widespread species of the genus Solanum (Eskandari et al., 2019) and the most used in traditional medicine worldwide, while on the other hand, S. dulcamara and S. melongena are practically not documented. Among the selected Solanum species, the most literature data were based on S. tuberosum followed by S. lycopersicum, S. nigrum, S. melongena, S. villosum, S. rostratum, while S. dulcamara and S. sisymbriifolium were least examined and explored.
Chen et al. (2022) reviewed that the first known record describing the medicinal use of S. nigrum was found in Yao Xing Lun (药性论, Tang Dynasty) (Editorial Committee of State Administration of Traditional Chinese Medicine, 1999). Since then, its medicinal use was increasingly reported in many other well-known classical, traditional Chinese medicine (TCM) monographs, including Ben Cao Gang Mu (本草纲目, Ming Dynasty), Ben Cao Gang Mu Shi Yi (本草纲目拾遗, Qing Dynasty), Ben Cao Tu Jing (本草图经, Song Dynasty), Dian Nan Ben Cao (滇南本草, Ming Dynasty), Dian Nan Ben Cao Tu Shuo (滇南本草图说, Ming Dynasty), Jiu Huang Ben Cao (救荒本 草, Ming Dynasty), Shi Liao Ben Cao (食疗本草, Tang Dynasty), and Xin Xiu Ben Cao (新修本草, Tang Dynasty). In all of these major TCM monographs, it was recorded that S. nigrum has different medicinal properties and TCM herbs or classical prescriptions containing S. nigrum have been used as decoction, granules, pills, powders, and tablets (Chen et al., 2022). The traditional usages of S. nigrum, commonly used worldwide, are presented in Table 2. Melongianum (S. melongena) was domesticated in Vavilov’s Chinese center (Vavilov, 1992) or Indo-Chinese center (Vavilov, 1992) and was known in the Middle Ages as well (Carnevale Schianga, 2011). It is included in the Tractatus de herbis and other similar manuscripts (Touwaide and Appetiti, 2013).
Conclusion
Species of the genus Solanum are considered either valuable foodstuffs and/or important medicinal plants due to their wide range of applications in the field of ethnobotany. The diversity of Solanum as food brings undeniable health benefits to the population through the presence of starch (source of sugar), lycopene and phenolics (antioxidant), anthocyanins possess antidiabetic, anticancer, anti-inflammatory, antimicrobial, and anti-obesity effects, as well as prevent cardiovascular diseases.
Generally, the diverse phytochemical profiles of these Solanum species, encompassing alkaloids, flavonoids, sterols, saponins, and various other bioactive compounds, underscore their potential contributions to medicine and nutrition.
In conclusion, the review paper highlights the ethnobotanical significance of eight Solanum species in Uzbekistan, revealing their economic, nutritional, and medicinal values. These species demonstrate a diverse range of traditional uses, exhibiting potential as antibacterial, antifungal, anti-inflammatory, anticancer, and antioxidant agents. Nevertheless, the research also highlights a decline in the transmission of traditional knowledge. This underscores the importance of ongoing phytochemical investigations to fully leverage the medicinal capabilities of Solanum species. The discoveries presented in this study offer valuable insights for future research and the development of innovative pharmaceutical solutions. They encourage the exploration of new plant sources and the utilization of advanced pharmaceutical methodologies in the pursuit of potential drug development.
Author contributions
YG: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. MR: Formal Analysis, Investigation, Methodology, Resources, Validation, Writing–original draft, Writing–review and editing. MuZ: Investigation, Methodology, Writing–original draft, Writing–review and editing. TM: Investigation, Resources, Writing–review and editing. MY: Investigation, Resources, Software, Writing–review and editing. J-JC: Funding acquisition, Project administration, Resources, Validation, Writing–review and editing. MoZ: Investigation, Writing–review and editing. MW: Investigation, Resources, Writing–review and editing. SG: Formal Analysis, Investigation, Writing–review and editing. AY: Investigation, Resources, Writing–review and editing. OM: Investigation, Writing–review and editing. AAA: Investigation, Writing–review and editing. SR: Supervision, Writing–review and editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by State Scientific and Technical Program of Institute of Botany of Uzbekistan Academy of Sciences, (2021–2024), Agency for Innovative Development of the Republic of Uzbekistan (Project no. AL 2021090820) and Jiangsu Qinglan Project is mine talent project of Jiangsu Province (2022) and the Science Fund of the Jiangsu Vocational College of Agriculture and Forestry (2020kj003 and 2021kj91).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1287793/full#supplementary-material
References
Abdelgawwad, M. R., Mahmood, A., Farraj, D. A., El-Abedein, A. I., Mahmoud, A. H., and Bukhari, S. M. (2020). In-vitro antimicrobial activities of Solanum villosum (L.) lam; crude extract solvent comparison. J. King Saud. Univ. Sci. 32, 2129–2133. doi:10.1016/j.jksus.2020.01.035
Abdel-Sattar, E., Maes, L., and Salama, M. M. (2010). In vitro activities of plant extracts from Saudi Arabia against malaria, leishmaniasis, sleeping sickness and Chagas disease. Phytother. Res. 24 (9), 1322–1328. doi:10.1002/ptr.3108
Aburjai, T. A., Oun, I. M., Auzi, A. A., and Hudaib, M. M. (2014). Volatile oil constituents off and leaves of Solanum nigrum L. growing in Libya. J. Essent. Oil-Bear Plants 17 (3), 397–404. doi:10.1080/0972060x.2014.895194
Acharya, E. S., and Pokhrel, B. M. (2006). Ethno–medicinal plants used by bantar of bhaudaha, morang, Nepal. Our Nat. 4 (1), 96–103. doi:10.3126/on.v4i1.508
Ahmad, N., Bibi, Y., Zahara, K., Bibi, F., Sadaf, H. M., and Sardar, N. (2019). An insight to therapeutic potential and phytochemical profile of Solanum villosum (L). Med. Drug Discov. 2, 100007. doi:10.1016/j.medidd.2019.100007
Alam, P., Raka, M. A., Khan, S., Sarker, J., Ahmed, N., Nath, P., et al. (2019). A clinical review of the effectiveness of tomato (Solanum lycopersicum) against cardiovascular dysfunction and related metabolic syndrome. J. Herb. Med. 16, 100235. doi:10.1016/j.hermed.2018.09.006
Al-Fatimi, M., Wurster, M., Schröder, G., and Lindequist, U. (2007). Antioxidant, antimicrobial and cytotoxic activities of selected medicinal plants from Yemen. J. Ethnopharmacol. 111, 657–666. doi:10.1016/j.jep.2007.01.018
Al-Qura’n, S. (2009). Ethnopharmacological survey of wild medicinal plants in Showbak, Jordan. J. Ethnopharmacol. 123 (4), 45–50. doi:10.1016/j.jep.2009.02.031
Ani, C. O., Nweke, M. L., Okeke, O. P., Okolo, K. O., Ndubuisi, R. N., Okorie, P. O., et al. (2022). Investigation of the effect and possible mechanism of antihypertensive activity of lycopene-rich extract of Solanum lycopersicon in Wistar rats. Int. J. Clin. Exp. Physiol. 9 (2), 80–88. doi:10.5530/ijcep.2022.9.2.17
Anjum Sahair, R., Sneha, S., Raghu, N., Ts, G., Karthikeyan, M., Gnanasekaran, A., et al. (2018). Solanum tuberosum L: botanical, phytochemical, pharmacological and nutritional significance. Int. J. Phytomed. 10 (3), 115–124. doi:10.5138/09750185.2256
Arenas, P., and Scarpa, G. (2007). Edible wild plants of the chorote Indians, gran chaco, Argentina. Bot. J. Linn. Soc. Lond. 153 (1), 73–85. doi:10.1111/j.1095-8339.2007.00576.x
Arrout, A., El Ghallab, Y., El Otmani, I. S., and Said, A. A. (2022). Ethnopharmacological survey of plants prescribed by herbalists for traditional treatment of hypercholesterolemia in Casablanca, Morocco. J. Herb. Med. 36, 100607. doi:10.1016/j.hermed.2022.100607
Asfaw, A., Lulekal, E., Bekele, T., Debella, A., Abebe, A., and Degu, S. (2023). Documentation of traditional medicinal plants use in Ensaro District, Ethiopia: implications for plant biodiversity and indigenous knowledge conservation. J. Herb. Med. 38, 100641. doi:10.1016/j.hermed.2023.100641
Aung, H. T., Sein, M. M., Aye, M. M., and Thu, Z. M. (2016). A review of traditional medicinal plants from Kachin State, Northern Myanmar. Nat. Prod. Commun. 11 (3)–64. 1934578X1601100. doi:10.1177/1934578x1601100310
Avicenna (980–1037) (1930). A treatise on the Canon of medicine of Avicenna, incorporating a translation of the first book. Gruner, O.C.; London: Luzac & Co, UK.
Baur, S., Bellé, N., Hausladen, H., Wurzer, S., Brehm, L., Stark, T. D., et al. (2022). Quantitation of toxic steroidal glycoalkaloids and newly identified saponins in post-harvest light-stressed potato (Solanum tuberosum L.) Varieties. J. Agric. Food Chem. 70, 8300–8308. doi:10.1021/acs.jafc.2c02578
Belichenko, O., Kolosova, V. B., Kalle, R., and Sõukand, R. (2022). Green pharmacy at the tips of your toes: medicinal plants used by Setos and Russians of Pechorsky District, Pskov Oblast (NW Russia). J. Ethnobiol. Ethnomed. 18 (1), 46. doi:10.1186/s13002-022-00540-w
Ben-Abdallah, S., Zorrig, W., Amyot, L. M., Renaud, J. B., Hannoufa, A., Lachâal, M., et al. (2018). Potential production of polyphenols, carotenoids and glycoalkaloids in Solanum villosum Mill. under salt stress. Biologia 74, 309–324. doi:10.2478/s11756-018-00166-y
Birmiss (2023). Xalq tabobatida gullar kartoshka xalq dorilar, retseptlar. Available online: https://uz.birmiss.com/xalq-tabobatida-gullar-kartoshka-xalq-dorilar-retseptlar/ (accessed on April 3, 2023).
Boboev, S., Makhkamov, T., Bussmann, R. W., Zafar, M., and Yuldashev, A. (2023). Anatomical and phytochemical studies and ethnomedicinal uses of Colchicum autumnale L. Ethnobot. Res. Appl. 25, 1–9. doi:10.32859/era.25.6.1-9
Botman, E. (2009). Forest rehabilitation in the republic of Uzbekistan. Bull. IUFRO World Ser. 20 (4), 253–299.
Boulos, L. (1983). Medicinal plants of North Africa. Algonac, Michigan, USA: Reference Publications, Inc., 286.
Brummitt, R. K. (2001). World geographical Scheme for recording plant distributions: edition 2. International working group on taxonomic data bases for plant Sciences (TDWG). Pittsburgh: Carnegie Mellon University.
BSBI Species Accounts Archive (2010). Solanum rostratum. Available online: https://sppaccounts.bsbi.org/content/solanum-rostratum-2.html ((accessed on March 10, 2023).
Buabeid, M. A., Arafa, E. S. A., Rani, T., Ahmad, F. U., Ahmed, H., Hassan, W., et al. (2022). Effects of Solanum lycopersicum L. (tomato) against isoniazid and rifampicin induced hepatotoxicity in wistar albino rats. Braz. J. Biol. 84, e254552. doi:10.1590/1519-6984.254552
Burlage, H. M. (1968). Index of plants of Texas with reputed medicinal and poisonous properties. Texas: Austin.
Camire, M. E., Kubow, S., and Donnelly, D. J. (2009). Potatoes and human health. Crit. Rev. Food Sci. Nutr. 49 (10), 823–840. doi:10.1080/10408390903041996
Cansever, E., and Turker, A. U. (2007). In vitro culture and biological activity of Solanum dulcamara, a medicinal plant. Planta Med. 73, 182. doi:10.1055/s-2007-986963
Carnevale Schianca, E. (2011). La cucina medievale: lessico, storia, preparazioni; Leo S. Olschki, Florence. Biblioteca dell' “Archivum Romanicum”. Ser. I Storia, Lett. Paleogr. 386:1–799. Available at: https://www.olschki.it/libro/9788822260734.
Chang, L., Shao, Q., Xi, X., Chu, Q., and Wei, Y. (2017). Separation of four flavonol glycosides from Solanum rostratum Dunal using aqueous two-phase flotation followed by preparative high-performance liquid chromatography. J. Sep. Sci. 40 (3), 804–812. doi:10.1002/jssc.201600922
Chen, F., Zhou, J., Zhang, Y., Chen, Y., Wang, Y., Zhao, Y., et al. (2021). Five new steroidal saponins from the seeds of Solanum melongena L. Phytochem. Lett. 41, 21–26. doi:10.1016/j.phytol.2020.10.008
Chen, X., Dai, X., Liu, Y., Yang, Y., Yuan, Y., He, X., et al. (2022). Solanum nigrum Linn.: an insight into current research on traditional uses, phytochemistry, and pharmacology. Front. Pharmacol. 13, 918071. doi:10.3389/fphar.2022.918071
Chidambaram, K., Alqahtani, T., Alghazwani, Y., Aldahish, A. A., Annadurai, S., Venkatesan, K., et al. (2022). Medicinal plants of Solanum species: the promising sources of phyto-insecticidal compounds. J. Trop. Med., 2022, 4952221. doi:10.1155/2022/4952221
Chifundera, K. (1998). Livestock diseases and the traditional medicine in the bushi area, kivu province, democratic republic of Congo. Afr. Study Monogr. 19, 13–34. doi:10.14989/68167
Choi, S., Kim, H., Kim, H., Lee, I., Kozukue, N., Levin, C. E., et al. (2011). Free amino acid and phenolic contents and antioxidative and cancer cell-inhibiting activities of extracts of 11 greenhouse-grown tomato varieties and 13 tomato-based foods. J. Agric. Food Chem. 59 (24), 12801–12814. doi:10.1021/jf202791j
Chowdhury, N., Ghosh, A., and Chandra, G. (2008). Mosquito larvicidal activities of Solanum villosum berry extract against the dengue vector Stegomyia aegypti. BMC Complement. Altern. Med. 8, 10–18. doi:10.1186/1472-6882-8-10
Chowdhury, N., Paramanik, M., Sarkar, N., Laskar, S., and Chandra, G. (2015). Fatty acid analysis of leaf and berry of Solanum villosum Mill. and its prospect as diet and bactericide. Anal. Chem. Lett. 5 (5), 260–266. doi:10.1080/22297928.2015.1137225
Coxe Stevenson, M. (1915). “Ethnobotany of the zuni Indians,” in 13th annual report of the Bureau of American Ethnology, 31–102. 1908–1909.
Cunningham, A. B., and Yang, X. (2011). “Mushrooms in forests and woodlands: resource management, values and local livelihoods,” in Earthscan, routledge. 1, 240.
Dafni, A., and Yaniv, Z. (1994). Solanaceae as medicinal plants in Israel. J. Ethnopharmacol. 44 (1), 11–18. doi:10.1016/0378-8741(94)90093-0
Darwin, S. C., Knapp, S., and Peralta, I. E. (2003). Taxonomy of tomatoes in the galápagos islands: native and introduced species of Solanum section lycopersicon (Solanaceae). Syst. Biodivers. 1 (1), 29–53. doi:10.1017/s1477200003001026
Das, M., and Barua, N. (2013). Pharmacological activities of Solanum melongena Linn. (Brinjal plant). Int. J. Green Pharm. 7 (4), 274–277. doi:10.4103/0973-8258.122049
Dereje, B., and Chibuzo, N. (2021). Nutritional composition and biochemical properties of Solanum tuberosum. IntechOpen. doi:10.5772/intechopen.98179
Devaux, A., Goffart, J. P., Kromann, P., Andrade-Piedra, J. L., Polar, V., and Hareau, G. (2021). The potato of the future: opportunities and challenges in sustainable agri-food systems. Potato Res. 64, 681–720. doi:10.1007/s11540-021-09501-4
Diab, R., Mounayar, A., Maalouf, É. I., and Chahine, R. (2011). Beneficial effects of Solanum melongena (Solanaceae) peduncles extracts, in periodontal diseases. J. Med. Plant Res. 5 (11), 2309–2315.
Drewnowski, A., and Rehm, C. D. (2013). Vegetable cost metrics show that potatoes and beans provide most nutrients per penny. PLoS ONE 8, e63277. doi:10.1371/journal.pone.0063277
Dupin, J., Matzke, N. J., Särkinen, T., Knapp, S., Olmstead, R. G., Bohs, L., et al. (2016). Bayesian estimation of the global biogeographical history of the Solanaceae. J. Biogeogr. 44 (4), 887–899. doi:10.1111/jbi.12898
East-Fruit (2022). Greenhouse-Tomato-Production-In-Uzbekistan-Increased. Available online: https://east-fruit.com/en/news/greenhouse-tomato-production-in-uzbekistan-increased-by-26-in-2022/ (accessed on July 9, 2023).
Elizalde-Romero, C. A., Montoya-Inzunza, L. A., Contreras-Angulo, L. A., Heredia, J. B., and Gutiérrez-Grijalva, E. P. (2021). Solanum fruits: phytochemicals, bioaccessibility and bioavailability, and their relationship with their health-promoting effects. Front. Nutr. 8, 790582. doi:10.3389/fnut.2021.790582
Eskandari, M., Assadi, M., Shirzadian, S., and Mehregan, I. (2019). Ethnobotanical study and distribution of the Solanum section Solanum species (Solanaceae) in Iran. J. Med. Plants 18 (71), 85–98. doi:10.29252/jmp.3.71.85
Ferro, E. A., Alvarenga, N. L., Ibarrola, D. A., Hellion-Ibarrola, M. C., and Ravelo, A. G. (2005). A new steroidal saponin from Solanum sisymbriifolium roots. Fitoterapia 76, 577–579. doi:10.1016/j.fitote.2005.04.008
Figueiredo, G. G., Coronel, O. A., Trabuco, A. C., Bazán, D. E., Russo, R. R., Alvarenga, N., et al. (2021). Steroidal saponins from the roots of Solanum sisymbriifolium Lam. (Solanaceae) have inhibitory activity against dengue virus and yellow fever virus. Braz. J. Med. Biol. Res. 54 (7), e10240. doi:10.1590/1414-431X2020e10240
Filipoy, A. J. (1994). Medicinal plants of the pilaga of central chaco. J. Ethnopharmacol. 44 (3), 181–193. doi:10.1016/0378-8741(94)01185-0
Fogelman, E., Oren-Shamir, M., Hirschberg, J., Mandolino, G., Parisi, B., Ovadia, R., et al. (2019). Nutritional value of potato (Solanum tuberosum) in hot climates: anthocyanins, carotenoids, and steroidal glycoalkaloids. Planta 249, 1143–1155. doi:10.1007/s00425-018-03078-y
Ford, C. J. (2015). Weed women, all night vigils, and the secret life of plants: negotiated epistemologies of ethnogynecological plant knowledge in American history; Dissertation. New England, United States: Antioch University New England, 296.
Franková, H., Musilová, J., Árvay, J., Harangozo, Ľ., Šnirc, M., Vollmannová, A., et al. (2022). Variability of bioactive substances in potatoes (Solanum tuberosum L.) depending on variety and maturity. Agronomy 12, 1454. doi:10.3390/agronomy12061454
Frodin, D. G. (2004). History and concepts of big plant genera. Taxon 53 (3), 753–776. doi:10.2307/4135449
Fuller, R. (2013). Ethnobotany: major developments of a discipline abroad, reflected in New Zealand. N. Z. J. Bot. 51 (2), 116–138. doi:10.1080/0028825x.2013.778298
Future Food Production (2022). Greenhouse-Tomato-Production-In-Uzbekistan-Increased-By-26-In-2022. Available online: https://www.futurefoodproduction.com/post/greenhouse-tomato-production-in-uzbekistan-increased-by-26-in-2022/ (accessed on July 9, 2023).
Gafforov, Y., Ordynets, A., Langer, E., Yarasheva, M., Gugliotta, A. M., Schigel, D., et al. (2020). Species diversity with comprehensive annotations of wood-inhabiting poroid and corticioid fungi in Uzbekistan. Front. Microbiol. 11, 598321. doi:10.3389/fmicb.2020.598321
Gafforov, Y., Rašeta, M., Rapior, S., Yarasheva, M., Wang, X., Zhou, L., et al. (2023). Macrofungi as medicinal resources in Uzbekistan: biodiversity, ethnomycology, and ethnomedicinal practices. J. Fungi 9 (9), 922. doi:10.3390/jof9090922
Gafforov, Y., Riebesehl, J., Ordynets, A., Langer, E., Yarasheva, M., Ghobad-Nejhad, M., et al. (2017). Hyphodontia (hymenochaetales, basidiomycota) and similar taxa from central Asia. Botany 95, 1041–1056. doi:10.1139/cjb-2017-0115
Gafforov, Y., Teshaboeva, S., Kholmuradova, T., Makhkamov, T., Abduboyeva, N., Abdurazakov, A., et al. (2022). “Biodiversıty of fungi and fungus like organism on Solanum species in Uzbekistan,” in 3rd International Eurasian Mycology Congress, Van, Turkey, September 2022.
Gafforov, Y. S. (2017). A preliminary checklist of ascomycetous microfungi from Southern Uzbekistan. Mycosphere 8 (4), 660–696. doi:10.5943/mycosphere/8/4/12
Gagnon, E., Hilgenhof, R., Orejuela, A., McDonnell, A., Sablok, G., Aubriot, X., et al. (2022). Phylogenomic discordance suggests polytomies along the backbone of the large genus Solanum (Solanaceae). Am. J. Bot. 109, 580–601. doi:10.1002/ajb2.1827
Galvis-Tarazona, D. Y., Ojeda-Pérez, Z. Z., and Arias-Moreno, D. M. (2022). Cultural and ethnobotanical legacy of native potatoes in Colombia. J. Ethnobiol. Ethnomed. 18, 59. doi:10.1186/s13002-022-00557-1
Gebrewbet, G. H., and Hndeya, A. G. (2023). Phytochemical screening and antibacterial activity studies on the crude leaf extract of Solanum sisymbriifolium: traditional Ethiopian medicinal plant. Adv. Gut Microbiome Res., 2023, 5525606. doi:10.1155/2023/5525606
Gepamed (2023). Gepamed. Available online: https://gepamed.uz/news/article_detail.php?action=detail&code=679 (accessed on April 3, 2023).
González Torres, D. M. (1980). Catálogo de plantas medicinales (y alimenticias y útiles) usadas en Paraguay. Asuncion, Paraguay: Editorial Comuneros, 456.
Guerrero-Gatica, M., Mujica, M. I., Barceló, M. I., Vio-Garay, M. F., Gelcich, S., and Armesto, J. J. (2020). Traditional and local knowledge in Chile: review of experiences and insights for management and sustainability. Sustainability 12 (5), 1767. doi:10.3390/su12051767
Gupta, V., Simlai, A., Tiwari, M., Bhattacharya, K., and Roy, A. (2014). Phytochemical contents, antimicrobial and antioxidative activities of Solanum sisymbriifolium. J. Appl. Pharm. Sci. 4 (03), 075–080. doi:10.7324/japs.2014.40315
Gynecology (2023). Yashil-pomidor-varikozga-davo. Available online: https://dawo.uz/gynecology/227-yashil-pomidor-varikozga-davo.html (accessed on April 3, 2023).
Heinrich, M., Mah, J., and Amirkia, V. (2021). Alkaloids used as medicines: structural phytochemistry meets biodiversity-an update and forward look. Molecules 26, 1836. doi:10.3390/molecules26071836
Helmja, K., Vaher, M., and Kaljurand, M. (2007). Characterization of bioactive compounds contained in vegetables of the Solanaceae family by capillary electrophoresis. Proc. Est. Acad. Sci. Chem. 56 (4), 172–186. doi:10.3176/chem.2007.4.02
Hövelmann, Y., Hahn, M., Hübner, F., and Humpf, H. U. (2019). Detection of novel cytotoxic imidazole alkaloids in tomato products by LC-MS/MS. J. Agric. Food Chem. 67 (13), 3670–3678. doi:10.1021/acs.jafc.9b00461
Huang, H. J., Ling, T. J., Wang, H. M., Cao, A. C., Zhang, C. X., and Wei, S. H. (2017). One new flavonoid from Solanum rostratum. Nat. Prod. Res. 31, 1831–1835. doi:10.1080/14786419.2017.1290621
Ibarrola, D. A., Ibarrola, M., Vera, C., Montalbetti, Y., and Ferro, E. A. (1996). Hypotensive effect of crude root extract of Solanum sisymbriifolium (Solanaceae) in normo- and hypertensive rats. J. Ethnopharmacol. 54 (1), 7–12. doi:10.1016/0378-8741(96)01442-0
Iijima, Y., Fujiwara, Y., Tokita, T., Ikeda, T., Nohara, T., Aoki, K., et al. (2009). Involvement of ethylene in the accumulation of esculeoside A during fruit ripening of tomato (Solanum lycopersicum). J. Agric. Food Chem. 57 (8), 3247–3252. doi:10.1021/jf8037902
Inaturalist (2023a). Photo 138991911, (c) Phillip Mayhair, all rights reserved, uploaded by Phillip Mayhair. Available online: https://www.inaturalist.org/photos/138991911 (accessed on March 8, 2023).
Inaturalist (2023b). Photo 27490948, no rights reserved, uploaded by 葉子. Available online: https://www.inaturalist.org/photos/27490948 (accessed on March 8, 2023).
Inaturalist (2023c). Photo 27490953, no rights reserved, uploaded by 葉子. Available online: https://www.inaturalist.org/photos/27490953 (accessed on March 8, 2023).
Inaturalist (2023d). Photo 67875298, (c) polyscias099, some rights reserved (CC BY-NC). Available online: https://www.inaturalist.org/photos/67875298 (accessed on March 8, 2023).
Inaturalist (2023e). Red nightshade solanum villosum. Available online: https://www.inaturalist.org/observations/141266299 (accessed on March 8, 2023).
Inaturalist (2023f). Red nightshade solanum villosum. Available online: https://www.inaturalist.org/observations/141644561 (accessed on March 8, 2023).
Jain, R., Sharma, A., Gupta, S., Sarethy, I. P., and Gabrani, R. (2011). Solanum nigrum: current perspectives on therapeutic properties. Altern. Med. Rev. 16 (1), 78–85.
Jit, B. P., Pattnaik, S., Arya, R., Dash, R., Sahoo, S. S., Pradhan, B., et al. (2022). Phytochemicals: a potential next generation agent for radioprotection. Phytomedicine 106, 154188. doi:10.1016/j.phymed.2022.154188
Kacjan Maršić, N., Mikulič-Petkovšek, M., and Štampar, F. (2014). Grafting influences phenolic profile and carpometric traits of fruits of greenhouse-grown eggplant (Solanum melongena L.). J. Agric. Food Chem. 62, 10504–10514. doi:10.1021/jf503338m
Kala, C. P. (2005). Ethnomedicinal botany of the Apatani in the Eeastern Himalayan region of India. J. Ethnobiol. Ethnomed. 1, 11. doi:10.1186/1746-4269-1-11
Karimov, U. I., and Khurshit, E. U. (1993). Abu Ali ibn Sina. Kanon vrachebnoy nauki. Tashkent, Uzbekistan: Fan Publishing House of Academy of Sciences Republic of Uzbekistan. (In Russian).
Kaunda, J. S., and Zhang, Y. (2019). The genus Solanum: an ethnopharmacological, phytochemical and biological properties review. Nat. Prod. Bioprospect. 9, 77–137. doi:10.1007/s13659-019-0201-6
Keding, G., Weinberger, K., Swai, I., and Mndiga, H. (2007). Diversity, traits and use of traditional vegetables in Tanzania. Technical Bulletin No. 40. Shanhua, Taiwan: AVRDC-The World Vegetable Center, 53.
Khabbach, A., Libiad, M., Ennabili, A., and Bousta, D. (2012). Medicinal and cosmetic use of plants from the province of Taza, Northern Morocco. Bol. Latinoam. Caribe. Plantas Med. Aromat. 11 (1), 46–60.
Khojimatov, O. K. (2021). Medicinal plants of Uzbekistan (properties, application and rational use). Tashkent: Manawiyat Publishing House, 328. (In Russian).
Khojimatov, O. K., Bussmann, R. W., and Gafforov, Y. (2023b). “Uzbekistan – ecosystems, biodiversity, history and culture,” in Ethnobiology of Uzbekistan. Ethnobiology. Editors O. K. Khojimatov, Y. Gafforov, and R. W. Bussmann (Cham: Springer). doi:10.1007/978-3-031-23031-8_1
Khojimatov, O. K., Gafforov, Y., and Bussmann, R. W. (2023a). Ethnobiology of Uzbekistan ethnomedicinal knowledge of mountain communities. Switzerland: Springer Cham, 1513. doi:10.1007/978-3-031-23031-8
Khojimatov, O. K., Khamaraeva, D. T., Khujanov, A. N., and Bussmann, R. W. (2020). An overview of ethnomedicinal plants of Uzbekistan. Ethnobot. Res. Appl. 20, 1–19. doi:10.32859/era.20.08.1-19
Kim, C.-H., Heinrich, M., Yen, H.-R., Echeverria, J., and Lu, A. (2023). Editorial: insights in ethnopharmacology: 2022. Front. Pharmacol. 14:1264063. doi:10.3389/fphar.2023.1264063
Kowalczewski, P. Ł., Olejnik, A., Świtek, S., Bzducha-Wróbel, A., Kubiak, P., Kujawska, M., et al. (2022). Bioactive compounds of potato (Solanum tuberosum L.) juice: from industry waste to food and medical applications. Crit. Rev. Plant Sci. 41, 52–89. doi:10.1080/07352689.2022.2057749
Kumar, P., Sharma, B., and Bakshi, N. (2009). Biological activity of alkaloids from Solanum dulcamara L. Nat. Prod. Res. 23 (8), 719–723. doi:10.1080/14786410802267692
Kunwar, R. M., Adhikari, Y. P., Sharma, H. P., Rimal, B., Devkota, H. P., Charmakar, S., et al. (2020). Distribution, use, trade and conservation of Paris polyphylla Sm. in Nepal. Glob. Ecol. Conserv. 23, e01081. doi:10.1016/j.gecco.2020.e01081
Lans, C. A. (2006). Ethnomedicines used in Trinidad and Tobago for urinary problems and diabetes mellitus. J. Ethnobiol. Ethnomed. 2, 45. doi:10.1186/1746-4269-2-45
Lelario, F., De Maria, S., Rivelli, A. R., Russo, D., Milella, L., Bufo, S. A., et al. (2019). A complete survey of glycoalkaloids using LC-FTICR-MS and IRMPD in a commercial variety and a local landrace of eggplant (Solanum melongena L.) and their anticholinesterase and antioxidant activities. Toxins 11 (4), 230. doi:10.3390/toxins11040230
Leporatti, M. L., and Ghedira, K. (2009). Comparative analysis of medicinal plants used in traditional medicine in Italy and Tunisia. J. Ethnobiol. Ethnomed. 5, 31. doi:10.1186/1746-4269-5-31
Leporatti, M. L., and Ivancheva, S. (2003). Preliminary comparative analysis of medicinal plants used in the traditional medicine of Bulgaria and Italy. J. Ethnopharmacol. 87, 123–142. doi:10.1016/s0378-8741(03)00047-3
Li, S., Zhao, Y., Gao, W., Zhang, L., Yu, H., and Wu, H. (2023). Steroidal constituents from Solanum nigrum. Fitoterapia 169, 105603. doi:10.1016/j.fitote.2023.105603
Liu, Y., Yin, X., Sun, Y., Liu, Y., Lu, D., Zhou, Y., et al. (2021a). A new phenylpropanoid from the roots of Solanum melongena L. and evaluation of anti-inflammatory activity. Rec. Nat. Prod. 15, 261–266. doi:10.25135/rnp.211.20.10.1837
Liu, Z., Wang, M., Tian, M., Yuan, L., Yu, B., Qu, B., et al. (2021b). Pyrrole alkaloids from Solanum rostratum and their chemical defense function against Henosepilachna vigintioctomaculata. Fitoterapia 155, 105031. doi:10.1016/j.fitote.2021.105031
Lubin, N. (1984). Labour and nationality in soviet central Asia: an uneasy compromise. Publishing House Palgrave Macmillan, 305. Available online: https://www.amazon.fr/Labour-Nationality-Soviet-Central-Asia/dp/069107674X ((accessed on March 5, 2023).
Macrotrends (2023). Uzbekistan population. Available online: https://www.macrotrends.net/countries/UZB/uzbekistan/population (accessed on February 4, 2023).
Mahbubur Rahman, A. H. M., Akter, S., Rani, R., and Rafiul Islam, A. K. M. (2015). Taxonomic study of leafy vegetables at Santahar Pouroshova of District Bogra, Bangladesh with emphasis on medicinal plants. Int. J. Adv. Res. Publ. 3 (5), 1019–1036.
Maroyi, A. (2012). Use of traditional veterinary medicine in Nhema communal area of the Midlands province, Zimbabwe. Afr. J. Tradit. Complement. Altern. Med. 9 (3), 315–322. doi:10.4314/ajtcam.v9i3.3
Martinez, M. (1969). Las plantas medicinales de Mexico. 5. Mexico City: Ediciones Botas-Mexico, 627.
Martinez, M. (1991). Las plantas medicinales de Mexico. 5. Mexico City: Ediciones Botas-Mexico, 656.
Mir, A. Y., Yaqoob, U., Hassan, M., Bashir, F. M., Zanit, S. B., Haq, S. M., et al. (2021). Ethnopharmacology and phenology of high-altitude medicinal plants in Kashmir, Northern Himalaya. Ethnob. Res. Appl. 22, 17. doi:10.32859/era.22.17.1-15
Mishra, T., Raigond, P., Thakur, N., Dutt, S., and Singh, B. (2020). Recent updates on healthy phytoconstituents in potato: a nutritional depository. Potato Res. 63, 323–343. doi:10.1007/s11540-019-09442-z
More, G. K. (2019). A review of the ethnopharmacology, phytochemistry and pharmacological relevance of the South African weed Solanum sisymbriifolium Lam. (Solanaceae). Environ. Dev. Sustain. 21, 37–50. doi:10.1007/s10668-017-0042-6
Morris, W. L., and Taylor, M. A. (2017). The solanaceous vegetable crops: potato, tomato, pepper, and eggplant. Encyclopedia of Applied Plant Sciences, 3, 55–58.
Moshi, M. J., Otieno, D. F., Mbabazi, P. K., and Weisheit, A. (2009). The ethnomedicine of the Haya people of Bugabo ward, Kagera Region, north western Tanzania. J. Ethnobiol. Ethnomed. 5, 24. doi:10.1186/1746-4269-5-24
Mukhopadhyay, G., Sarkar, S., Kundu, S., Kundu, S., Sarkar, P., Sarkar, S., et al. (2018). Ethno-pharmacological activity of Solanum nigrum. J. Pharm. Innov. 7 (10), 692–698.
Mutalik, S., Paridhavi, K., Rao, C. M., and Udupa, N. (2003). Antipyretic and analgesic effect of leaves of Solanum melongena Linn. in rodents. Indian J. Pharmacol. 35, 312315.
Nanjala, C., Odago, W. O., Rono, P. C., Waswa, E. N., Mutinda, E. S., Oulo, M. A., et al. (2022). A review on ethnobotany, phytochemistry, and pharmacology of the genus Didymocarpus Wall. (Gesneriaceae). J. Ethnopharmacol. 295, 115404. doi:10.1016/j.jep.2022.115404
Netherlands Enterprise Agency (2020). Market study on plant propagation. Available online: https://www.rvo.nl/sites/default/files/2020/04/Market-study-on-plant-propagation-material-Uzbekistan.pdf (accessed on July 9 2023).
Nyaga, S. N., Mathiu, P. M., Onyango, C. M., and Areba, G. O. (2019). Antidiabetic properties of Solanum villosum and Solanum nigrum var. sarrachoides in a streptozotocin-induced diabetic mice model. Int. J. Basic Clin. Pharmacol. 8 (11), 2396–2402. doi:10.18203/2319-2003.ijbcp20194774
Omar, T., Noman, L., Mohamed, B., Altuntas, F. O., and Demirtaş, I. (2018). Phytochemical constituents and antioxidant effect of Solanum rostratum species from Algeria. Asian J. Pharm. Clin. Res. 11 (6), 219–223. doi:10.22159/ajpcr.2018.v11i6.24951
Ostreikova, T. O., Kalinkina, O. V., Bogomolov, N. G., and Chernykh, I. V. (2022). Glycoalkaloids of plants in the family Solanaceae (Nightshade) as potential drugs. Pharm. Chem. J. 56 (7), 948–957. doi:10.1007/s11094-022-02731-x
Padalia, K. (2014). Gewai saag: a folk medicine used by the tribal people of Central Himalayan region. Indian J. Tradit. Knowl. 1 (1), 144–146.
Parveen, U. B., Roy, S. M., and Kumar, A. (2007). Traditional uses of medicinal plants among the rural communities of Churu district in the Thar Desert, India. J. Ethnopharmacol. 113 (3), 387–399. doi:10.1016/j.jep.2007.06.010
Pasdaran, A., Pasdaran, A., and Mamedov, N. A. (2017). Antibacterial and antioxidant activities of the volatile composition of the flower and fruit of Solanum sisymbriifolium (Litchi Tomato). Pharm. Sci. 23, 66–71. doi:10.15171/ps.2017.10
Patel, K., Singh, R. B., and Patel, D. K. (2013). Medicinal significance, pharmacological activities, and analytical aspects of solasodine: a concise report of current scientific literature. J. Acute Dis. 2 (2), 92–98. doi:10.1016/s2221-6189(13)60106-7
Peralta, I. E., and Spooner, D. M. (2005). Morphological characterization and relationships of wild tomatoes (Solanum L. Section Lycopersicon). Monogr. Syst. Bot. Mo. Bot. Gard. 104, 227–257.
Perez, C., and Anesini, C. (1994). Inhibition of Pseudomonas aeruginosa by Argentinean medicinal plants. Fitoterapia 65 (2), 169–172.
Pérez, G. R. M., Perez, L. J. A., Garcia, D. L. M., and Sossa, M. H. (1988). Neuropharmacological activity of Solanum nigrum fruit. J. Ethnopharmacol. 62, 43–48. doi:10.1016/s0378-8741(98)00059-2
Pieroni, A., Cianfaglione, K. M., Nedelcheva, A., Hajdari, A., Mustafa, B., and Quave, C. L. (2014). Resilience at the border: traditional botanical knowledge among Macedonians and Albanians living in Gollobordo, Eastern Albania. J. Ethnobiol. Ethnomed. 10, 31. doi:10.1186/1746-4269-10-31
Pieroni, A., Giusti, M. E., Münz, H., Lenzarini, C., Turković, G., and Turković, A. (2003). Ethnobotanical knowledge of the istro-Romanians of žejane in Croatia. Fitoterapia 74 (7–8), 710–719. doi:10.1016/j.fitote.2003.06.002
Pieroni, A., Rexhepi, B., Nedelcheva, A., Hajdari, A., Mustafa, B., Kolosova, V. B., et al. (2013). One century later: the folk botanical knowledge of the last remaining Albanians of the upper Reka Valley, Mount Korab, Western Macedonia. J. Ethnobiol. Ethnomed. 9, 22. doi:10.1186/1746-4269-9-22
Popova, V. T., Stoyanova, M. A., Ivanova, T. A., Stoyanova, A. S., and Dimitrova-Dyulgerova, I. Z. (2021). Phytochemical composition of leaves and stems of Solanum nigrum L. and Solanum dulcamara L. (Solanaceae) from Bulgaria. IOP Conf. Ser. Mater. Sci. Eng. 1031, 012091. doi:10.1088/1757-899x/1031/1/012091
Powo (2023). Powo science kew. Available online: https://powo.science.kew.org/ (accessed on February 3, 2023).
Ralte, L., Bhardwaj, U., and Singh, Y. T. (2021). Traditionally used edible Solanaceae plants of Mizoram, India have high antioxidant and antimicrobial potential for effective phytopharmaceutical and nutraceutical formulations. Heliyon 7, e07907. doi:10.1016/j.heliyon.2021.e07907
Ramachandran, V. S., Joseph, S., and Aruna, R. (2009). Ethnobotanical studies from Amaravathy range of Indira Gandhi Wildlife Sanctuary, western ghats, Coimbatore district, southern India. Ethnobot. Leafl. 13, 1069–1087.
Rosa-Martínez, E., García-Martínez, M. D., Adalid-Martínez, A. M., Pereira-Dias, L., Casanova, C., Soler, E., et al. (2015). A new biflavonoid from Solanum dulcamara L. and investigation of anti-hyperglycaemic activity of its fruit extract. Nat. Prod. Res. 29 (4), 308–314. doi:10.1080/14786419.2014.928878
Rosas-Cruz, G. P., Silva-Correa, C. R., Calderón-Peña, A. A., Torre, V. E., Aspajo-Villalaz, C. L., Cruzado-Razco, J. L., et al. (2020). Wound healing activity of an ointment from Solanum tuberosum L. "Tumbay yellow potato" on Mus musculus balb/c. Polym. J. 12 (6), 1268–1275. doi:10.5530/pj.2020.12.175
Sampaio, S. L., Petropoulos, S. A., Días, M. I., Pereira, C., Calhelha, R. C., Fernandes, Â., et al. (2021). Phenolic composition and cell-based biological activities of ten coloured potato peels (Solanum tuberosum L.). Food Chem. 363, 130360. doi:10.1016/j.foodchem.2021.130360
Särkinen, T. E., Poczai, P., Barboza, G. E., van der Weerden, G. M., Baden, M., and Knapp, S. (2018). A revision of the Old world black nightshades (morelloid clade of solanum L., Solanaceae). PhytoKeys 106, 1–223. doi:10.3897/phytokeys.106.21991
Semanticscholar (2023). A free, AI-powered research tool for scientific literature. Available online: https://www.semanticscholar.org/ (accessed on February 22, 2023).
Sezik, E., Yeşilada, E., Shadidoyatov, H., Kulivey, Z., Nigmatullaev, A. M., Aripov, H. N., et al. (2004). Folk medicine in Uzbekistan. I. Toshkent, djizzax, and Samarqand provinces. J. Ethnopharmacol. 92 (2–3), 197–207. doi:10.1016/j.jep.2004.02.016
Shakya, R., and Navarre, D. A. (2008). LC-MS analysis of solanidane glycoalkaloid diversity among tubers of four wild potato species and three cultivars (Solanum tuberosum). J. Agric. Food Chem. 56 (16), 6949–6958. doi:10.1021/jf8006618
Sharma, S. B., and Dogra, K. S. (2018). Utilization of Solanaceae for dental care management in India. Pharma Innov. 7 (5), 71–74.
Sikdar, M., and Dutta, U. (2008). Traditional phytotherapy among the Nath people of Assam. Stud. Ethno-Med. 2 (1), 39–45. doi:10.1080/09735070.2008.11886313
Sivakumar, D., Phan, A. D. T., Slabbert, R. M., Sultanbawa, Y., and Remize, F. (2020). Phytochemical and nutritional quality changes during irrigation and postharvest processing of the underutilized vegetable African Nightshade. Front. Nutr. 7, 576532. doi:10.3389/fnut.2020.576532
Sivaperumal, R., Ramya, S., Ravi, A. V., Rajasekaran, C., and Jayakumararaj, R. (2010). Ethnopharmacological studies on the medicinal plants used by tribal inhabitants of Kottur Hills, Dharmapuri, Tamilnadu, India. Int. Sci. Technol. 5, 57–64.
Solanaceaesource (2023). Solanum villosum Mill. Available online: https://solanaceaesource.myspecies.info/taxonomy/term/110367/descriptions (accessed on March 8, 2023).
Solanum dulcamara (2023). Plants. Available online: https://plants.ces.ncsu.edu/plants/solanum-dulcamara/ (accessed on March 8, 2023).
Solanum-Lycopersicum (2023). Plant. Available online: https://www.k12.uz/plant/solanum-lycopersicum-l/ (accessed on November 7 2023)
Solanum lycopersicum (2023). Plants. Available online: https://plants.ces.ncsu.edu/plants/solanum-lycopersicum/ (accessed on March 8, 2023).
Solanum-Melongena (2023). Plant. Available online: https://www.k12.uz/plant/solanum-melongena-l/ (accessed on November 7, 2023).
Solanum melongena (2023). Plants. Available online: https://plants.ces.ncsu.edu/plants/solanum-melongena/ (accessed on March 8, 2023).
Solanum nigrum (2023). Plants. Available online: https://plants.ces.ncsu.edu/plants/solanum-nigrum/ (accessed on March 8, 2023).
Solanum-Tuberosum (2023). Plant. Available online: https://www.k12.uz/plant/solanum-tuberosum-l/ (accessed on November 7, 2023).
Solanum tuberosum (2023). Plants. Available online: https://plants.ces.ncsu.edu/plants/solanum-tuberosum/ (accessed on March 8, 2023).
Song, Y., Mei, T., Liu, Y., Kong, S., Zhang, J., Xie, M., et al. (2021). Metabolites identification of chemical constituents from the eggplant (Solanum melongena L.) calyx in rats by UPLC/ESI/qTOF-MS analysis and their cytotoxic activities. Front. Pharmacol. 12, 655008. doi:10.3389/fphar.2021.655008
Sonkamble, P. S., Nagoba, S. N., Vikram, S., Sarukh, V. S., and Shimge, K. R. (2019). Systematic review on Solanum nigrum. World J. Pharm. Life Sci. 5 (2), 109–112.
Staveckienė, J., Kulaitienė, J., Levickienė, D., Vaitkevičienė, N., and Vaštakaitė-Kairienė, V. (2023). The effect of ripening stages on the accumulation of polyphenols and antioxidant activity of the fruit extracts of Solanum species. Plants 12, 2672. doi:10.3390/plants12142672
Stern, S., Bohs, L., Giacomin, L. L., Stehmann, J. R., and Knapp, S. (2013). A revision of Solanum section Gonatotrichum bitter (Solanaceae). Syst. Bot. 38 (2), 471–496. doi:10.1600/036364413x666624
Sun, J., Gu, Y., Su, X., Li, M., Huo, H., Zhang, J., et al. (2014). Anti-inflammatory lignanamides from the roots of Solanum melongena L. Fitoterapia 98, 110–116. doi:10.1016/j.fitote.2014.07.012
Sun, J., Huo, H., Zhang, J., Huang, Z., Zheng, J., Zhang, Q., et al. (2015). Phenylpropanoid amides from the roots of Solanum melongena L. (Solanaceae). Biochem. Syst. Ecol. 58, 265–269. doi:10.1016/j.bse.2014.12.018
Swbiodiversity (2023a). Swbiodiversity seinet. Available online: https://swbiodiversity.org/seinet/imagelib/imgdetails.php?imgid=1368429 (accessed on March 8, 2023).
Swbiodiversity (2023b). Swbiodiversity seinet. Available online: https://swbiodiversity.org/seinet/imagelib/imgdetails.php?imgid=254683 (accessed on March 8, 2023).
Swbiodiversity. (2023c). Swbiodiversity seinet. Available online: https://swbiodiversity.org/seinet/imagelib/imgdetails.php?imgid=1368428 (accessed on March 8 2023).
Swbiodiversity (2023d). Swbiodiversity seinet. Available online: https://swbiodiversity.org/seinet/imagelib/imgdetails.php?imgid=254680 (accessed on March 8, 2023).
Tayjanov, K., Khojimatov, O. K., Gafforov, Y., Makhkamov, T., Normakhamatov, N., and Bussmann, R. W. (2021). Plants and fungi in the ethnomedicine of the medieval East - a review. Ethnobot. Res. Appl. 22, 46. doi:10.32859/era.22.46.1-20
TF03 Solanum dulcamara L (2023). kent-ethnobotanical-herbarium. Available online: https://research.kent.ac.uk/kent-ethnobotanical-herbarium/record/tf03-solanum-dulcamara-l (accessed on March 8, 2023).
Touwaide, A., and Appetiti, E. (2013). Knowledge of Eastern materia medica (Indian and Chinese) in pre-modern Mediterranean medical traditions: a study in comparative historical ethnopharmacology. J. Ethnopharmacol. 148 (2), 361–378. doi:10.1016/j.jep.2013.03.068
Tovar, J. D., André, T., Wahlert, G. A., Bohs, L., and Giacomin, L. L. (2021). Phylogenetics and historical biogeography of Solanum section Brevantherum (Solanaceae). Mol. Phylogenet. Evol. 162, 107195. doi:10.1016/j.ympev.2021.107195
Uddin, S. J., Rouf, R., Shilpi, J. A., Alamgir, M., Nahar, L., and Sarker, S. D. (2008). Screening of some Bangladeshi plants for in vitro antibacterial activity. Orient. Pharm. Exp. Med. 8 (3), 316–321. doi:10.3742/opem.2008.8.3.316
Valadez Vega, M. D., Izquierdo Vega, J. A., Villagómez Ibarra, J. R., Sánchez Gutiérrez, M., Madrigal Santillán, E. O., Morales Gonzalez, J. A., et al. (2019). Assessments of antioxidant content and the anti-carcinogenic effect of extracts of Solanum rostratum Dunal in human cancer cells. Acta Pol. Pharm. 76 (3), 493–502. doi:10.32383/appdr/100501
Vallejo-Marin, M. (2010). BSBI Species Accounts Archive: Solanum rostratum. Botanical Society of the British Isles website. Available at: https://sppaccounts.bsbi.org/content/solanum-rostratum-2.html
Vavilov, N. I. (1992). Origin and geography of cultivated plants. Cambridge: Cambridge University Press, 498.
Venkatesh, R., Kalaivani, K., and Vidya, R. (2014a). Analysis of phytochemicals and free radical scavenging activity of Solanum villosum (Mill). - a traditional medicinal plant in Southern India. World J. Pharm. Pharm. Sci. 3, 741–755.
Venkatesh, R., Kalaivani, K., and Vidya, R. (2014b). Toxicity assessment of ethanol extract of Solanum villosum (mill) on wistar albino rats. Int. J. Pharma Sci. Res. 5, 406–412.
Wang, Y., Wang, S., Xu, J., Wang, Y., Xiang, L., and He, X. (2023). Total steroidal saponins from black nightshade (Solanum nigrum L.) overcome tumor multidrug resistance by inducing autophagy-mediated cell death in vivo and in vitro. Phytother. Res. 37, 3009–3024. doi:10.1002/ptr.7796
Wojdyło, A., Lech, K., Nowicka, P., Hernandez, F., Figiel, A., and Carbonell-Barrachina, A. A. (2019). Influence of different drying techniques on phenolic compounds, antioxidant capacity and colour of Ziziphus jujube Mill fruits. Molecules 24, 2361. doi:10.3390/molecules24132361
Xu, J., Li, Y., Kaur, L., Singh, J., and Zeng, F. (2023). Functional food based on Potato. Foods 12, 2145. doi:10.3390/foods12112145
Yang, B., Yin, X., Liu, Y., Ye, H., Zhang, M., Guan, W., et al. (2019). Bioassay-guided isolation of lignanamides with potential anti-inflammatory effect from the roots of Solanum melongena L. Phytochem. Lett. 30, 160–164. doi:10.1016/j.phytol.2019.01.020
Yang, B., Yin, X., Liu, Y., Zhao, D., and Kuang, H. (2018). New steroidal saponins from the roots of Solanum melongena L. Fitoterapia 128, 12–19. doi:10.1016/j.fitote.2018.04.021
Zahara, K., Ahmad, N., Bibi, Y., Bibi, F., Sadaf, H. M., and Sardar, N. (2019). An insight to therapeutic potential and phytochemical profile of Solanum villosum (L). Med. Drug Discov. 2, 100007. doi:10.1016/j.medidd.2019.100007
Zakaria, Z. A., Gopalan, H. K., Zainal, H., Mohd Pojan, N. H., Morsid, N. A., Aris, A., et al. (2006). Antinociceptive, anti-inflammatory and antipyretic effects of Solanum nigrum chloroform extract in animal models. Yakugaku Zasshi J. Pharm. Soc. Jpn. 126 (11), 1171–1178. doi:10.1248/yakushi.126.1171
Keywords: Avicenna, biodiversity, Central Asia, ethnomedicine, flowering plant, folk medicine, Solanaceae
Citation: Gafforov Y, Rašeta M, Zafar M, Makhkamov T, Yarasheva M, Chen J-J, Zhumagul M, Wang M, Ghosh S, Abbasi AM, Yuldashev A, Mamarakhimov O, Alosaimi AA, Berdieva D and Rapior S (2024) Exploring biodiversity and ethnobotanical significance of Solanum species in Uzbekistan: unveiling the cultural wealth and ethnopharmacological uses. Front. Pharmacol. 14:1287793. doi: 10.3389/fphar.2023.1287793
Received: 02 September 2023; Accepted: 21 December 2023;
Published: 24 January 2024.
Edited by:
Ana Hortência Fonsêca Castro, Universidade Federal de São João del-Rei, BrazilReviewed by:
Mairon Coimbra, Universidade Federal de São João del-Rei, BrazilJuliana Cristina Dos Santos Almeida Bastos, Universidade Federal de São João del-Rei, Brazil
Olha Mykhailenko, University College London, United Kingdom
Copyright © 2024 Gafforov, Rašeta, Zafar, Makhkamov, Yarasheva, Chen, Zhumagul, Wang, Ghosh, Abbasi, Yuldashev, Mamarakhimov, Alosaimi, Berdieva and Rapior. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yusufjon Gafforov, eS5nYWZmb3JvdkBuZXd1dS51eg==, eXVnYWZmb3JvdkB5YWhvby5jb20=; Jia-Jia Chen, amlhamlhY2hlbkBqc2FmYy5lZHUuY24=
 Yusufjon Gafforov
Yusufjon Gafforov Milena Rašeta
Milena Rašeta Muhammad Zafar
Muhammad Zafar Trobjon Makhkamov
Trobjon Makhkamov Manzura Yarasheva
Manzura Yarasheva Jia-Jia Chen
Jia-Jia Chen Moldir Zhumagul9,10
Moldir Zhumagul9,10 Mengcen Wang
Mengcen Wang Soumya Ghosh
Soumya Ghosh Akramjon Yuldashev
Akramjon Yuldashev Sylvie Rapior
Sylvie Rapior