- 1West China School of Pharmacy, Sichuan University, Chengdu, China
- 2Department of Pharmacy, Evidence-Based Pharmacy Center, West China Second University Hospital, Sichuan University, Chengdu, China
- 3Key Laboratory of Birth Defects and Related Diseases of Women and Children, Sichuan University, Ministry of Education, Chengdu, China
A Corrigendum on
Advances in the study of acetaminophen-induced liver injury
by Li X, Ni J and Chen L (2023). Front. Pharmacol. 14:1239395. doi: 10.3389/fphar.2023.1239395
In the published article, there was an error in the caption for Figures 2, 3, 4, 6 as published. The previous captions for these did not attribute the figures to their citing source. The corrected figure captions appear below.
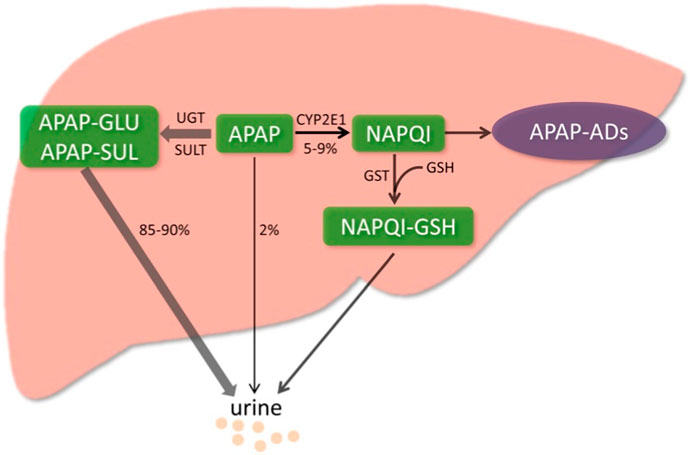
FIGURE 2. Metabolic activation pathway of acetaminophen. Generally, NAPQI is detoxified by conjugating with GSH. However, excessive NAPQI depletes GSH following APAP overdose, leading to the formation of APAP protein adducts (APAP-Ads) through the covalent binding of sulfhydryl groups in cellular proteins. (Yan et al., 2018; reprinted from Redox Biology with permission).
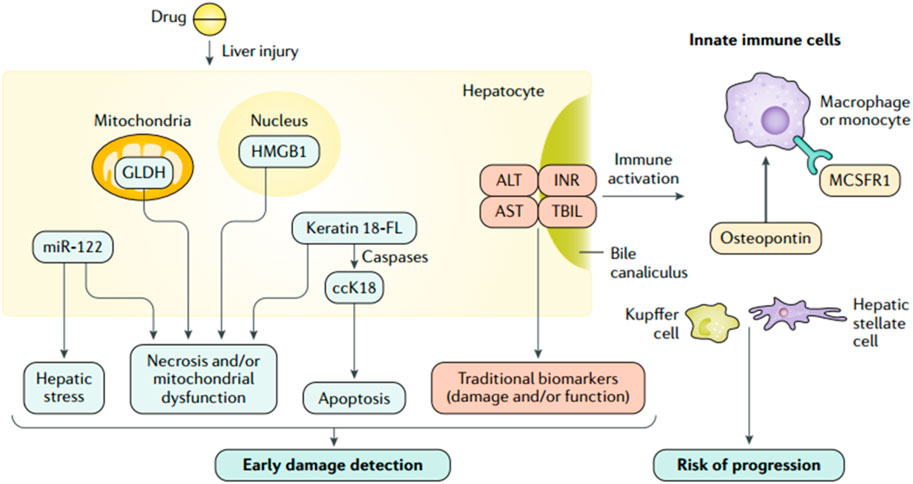
FIGURE 3. Traditional and investigational DILI biomarkers (Andrade et al., 2019a; reprinted from Nature Reviews Disease Primers with permission).
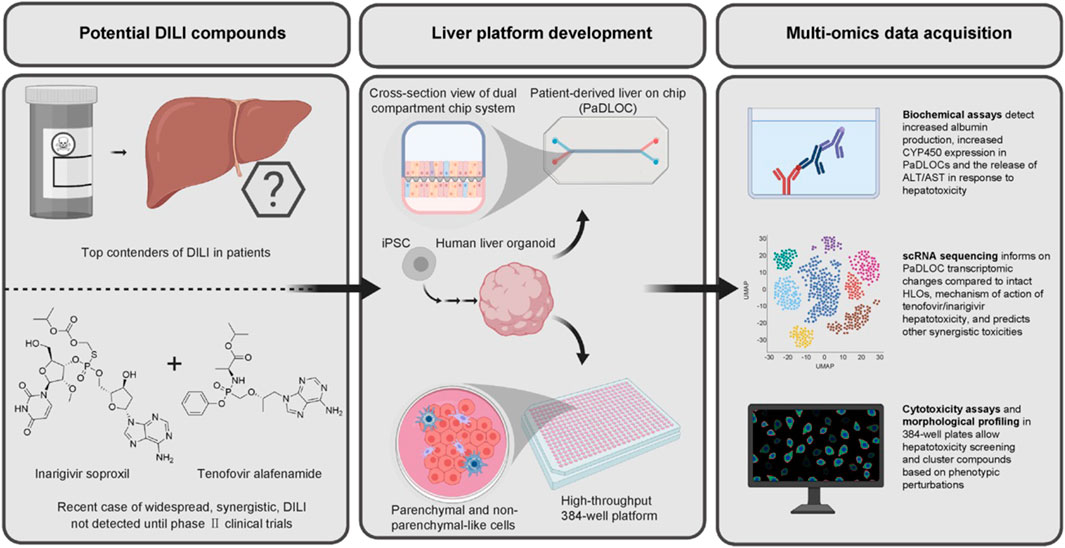
FIGURE 4. HLO-based screening platform for DILI risk prediction (Zhang et al., 2023; reprinted from Journal of Hepatology with permission).
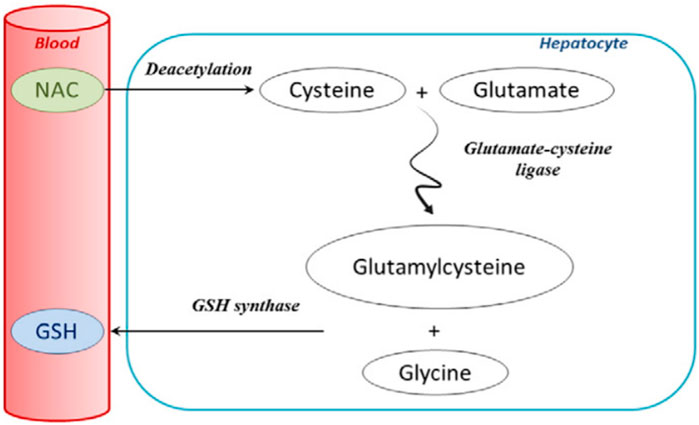
FIGURE 6. Extensive first-pass metabolism of NAC in the liver after oral administration (Lasram et al., 2015; reprinted from Clinical Biochemistry with permission).
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: drug-induced liver injury, acetaminophen-induced liver injury, diagnosis, screening, prevention and management
Citation: Li X, Ni J and Chen L (2023) Corrigendum: Advances in the study of acetaminophen-induced liver injury. Front. Pharmacol. 14:1283596. doi: 10.3389/fphar.2023.1283596
Received: 26 August 2023; Accepted: 13 September 2023;
Published: 21 September 2023.
Approved by:
Frontiers Editorial Office, Frontiers Media SA, SwitzerlandCopyright © 2023 Li, Ni and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiaqi Ni, amlhcWluaTAwN0AxNjMuY29t; Li Chen, Y2hlbmxfaHhleUBzY3UuZWR1LmNu
 Xinghui Li
Xinghui Li Jiaqi Ni
Jiaqi Ni Li Chen
Li Chen