
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Pharmacol. , 22 November 2023
Sec. Obstetric and Pediatric Pharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1281382
This article is part of the Research Topic Women in Obstetric and Pediatric Pharmacology: 2023 View all 6 articles
 Marcelo R. Luizon1,2*†‡
Marcelo R. Luizon1,2*†‡ Daniela A. Pereira1†
Daniela A. Pereira1† Izabela Mamede3
Izabela Mamede3 Carla S. Ceron4
Carla S. Ceron4 Ricardo C. Cavalli5
Ricardo C. Cavalli5 Ana C. Palei6*‡
Ana C. Palei6*‡ Valeria C. Sandrim2*‡
Valeria C. Sandrim2*‡Preeclampsia (PE) is a multisystem disorder that affects 2%–8% of pregnancies worldwide and is a leading cause of maternal and fetal morbidity and mortality (Duley, 2009). PE is defined as new-onset maternal blood pressure greater than 140/90 mmHg after the 20th week of pregnancy that occurs along with proteinuria or other indications of renal insufficiency, thrombocytopenia, liver dysfunction, pulmonary edema, and cerebral disturbances (American College of Obstetricians and Gynecologists, 2013). The pathogenesis of PE is multifactorial with recognized placental, vascular, renal, and immunological contributions (Turbeville and Sasser, 2020).
PE is characterized by defective placentation, abnormal spiral artery remodeling, placental ischemia, oxidative stress at the maternal-fetal interface, and angiogenic imbalance in the maternal circulation, thereby resulting in endothelial dysfunction and end-organ damage (Phipps et al., 2019). Noteworthy, several studies ratify the relationship of PE with future risk for cardiovascular disease, and accumulating evidence suggests an association of PE with long-term renal disease, although further studies on the mechanisms underlying this increased risk are needed (Turbeville and Sasser, 2020). However, significant knowledge gaps still exist in identifying the mechanisms that link placental ischemia to maternal systemic vascular and renal dysfunction (Wang et al., 2023).
Current treatment strategies for PE focus on stabilizing the maternal symptoms in order to prolong pregnancy and allow additional fetal development, and managing maternal hypertension is a priority (Townsend et al., 2016; Wang et al., 2023). Antihypertensive drugs used to control high blood pressure, such as methyldopa, nifedipine, hydralazine, and labetalol have the potential to prolong gestation, decreasing obstetric and perinatal complications in PE (Berzan et al., 2014; Peracoli et al., 2019; American College of Obstetricians and Gynecologists, 2020). However, a large subgroup of patients with PE is nonresponsive to antihypertensive therapy, being more susceptible to develop adverse maternal and fetal outcomes (Luizon et al., 2017a).
In this opinion article, we contribute with viewpoints on the definition of phenotypes for the subgroup of patients with PE classified as nonresponsive to antihypertensive therapy, and how studies focused on this nonresponsive subgroup of patients with PE can yield insights into molecular mechanisms underlying the endothelial dysfunction as well as the associated cardiovascular and renal complications in PE.
Our group has previously used a criteria to define antihypertensive therapy responsiveness based on the evaluation of clinical and laboratory parameters following the administration of methyldopa, nifedipine and hydralazine (Sandrim et al., 2010a; Palei et al., 2012b; Luizon et al., 2014; Sandrim et al., 2015; Luizon et al., 2017a; Luizon et al., 2017b; Pereira et al., 2021). Notably, patients with PE classified as nonresponsive to antihypertensive therapy were markedly associated with the worst clinical parameters. However, many of the clinical findings we used on the definition of antihypertensive therapy responsiveness are shared with the definition of severe features in PE, including thrombocytopenia, abnormally elevated levels of liver enzymes and/or creatinine in the blood, persistent right upper quadrant or epigastric pain, and new-onset cerebral and visual disturbances (American College of Obstetricians and Gynecologists, 2013). Thus, it is possible that our criteria of responsiveness denote disease severity instead (Luizon et al., 2017a).
Noteworthy, we have recently better characterized the clinical phenotype of patients with PE classified as nonresponsive to antihypertensive therapy (Pereira et al., 2021). The nonresponsive subgroup showed higher blood pressure, fasting glucose, creatinine, proteinuria, alanine aminotransferase, and soluble fms-like tyrosine kinase-1 (sFlt-1) levels, in opposition to lower gestational age at delivery and newborn weight (Figure 1). Other symptoms related to impairment of the central nervous system, blood cells (hemolysis and thrombocytopenia), and fetal development were also recorded (Pereira et al., 2021). Furthermore, PE can be classified according to gestational age of onset of symptoms into early-onset PE (<34 weeks) and late-onset PE (≥34 weeks). Remarkably, the subgroup nonresponsive to antihypertensive therapy showed higher percentage of early-onset PE (47.3% versus 6.3%), preterm birth (61.5% versus 11.6%), and intrauterine growth restriction (50.5% versus 14.3%) than the responsive subgroup (Pereira et al., 2021).
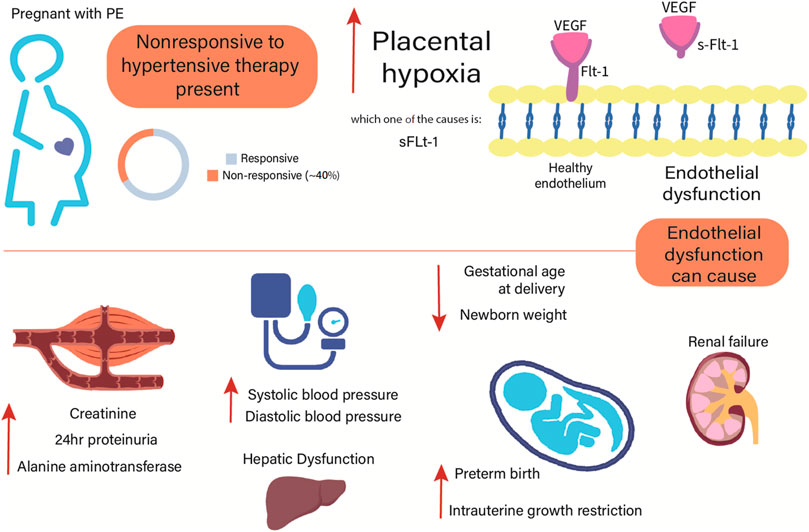
FIGURE 1. Clinical characteristics of patients with preeclampsia classified as nonresponsive to current antihypertensive therapy. Compared to the responsive group, the nonresponsive group showed higher systolic and diastolic blood pressure, creatinine, proteinuria, alanine aminotransferase, and sFlt-1 levels (Pereira et al., 2021). Adverse outcomes in the mother and fetal development may be implicated to the endothelial dysfunction observed in PE. Conversely, the nonresponsive group showed lower gestational age at delivery and newborn weight than the responsive group (Pereira et al., 2021). sFlt-1, soluble fms-like tyrosine kinase-1; VEGF, vascular endothelial growth factor.
Although a large subgroup (∼46%) of patients with PE are nonresponsive to currently approved antihypertensive therapy during pregnancy (Luizon et al., 2017a), the underlying molecular mechanisms are unclear. In the next sessions, we contribute with viewpoints on the interpretation of findings from previous studies focused on this nonresponsive subgroup of patients with PE and how they can yield insights into the underlying mechanisms of increased cardiovascular and renal risk in PE.
Antiangiogenic factors released into the maternal circulation, including sFlt-1 as result of placental ischemia/hypoxia contribute to the widespread endothelial dysfunction and proteinuria found in PE (Maynard et al., 2003; Powe et al., 2011). Circulating nitrite concentrations (a marker of endogenous NO formation) is a suitable approach to understand the underlying molecular mechanisms of endothelial dysfunction in healthy subjects (Metzger et al., 2013), healthy pregnancy and in PE (Sandrim et al., 2010b), as reviewed elsewhere (Luizon et al., 2018). Notably, PE is characterized by reduced bioavailability of NO, which is inversely related to sFlt-1 (Sandrim et al., 2008).
Endothelial dysfunction in PE has also been associated with dysregulation of adipocytokines (Mori et al., 2010). Specifically, visfatin/eNAMPT is an adipocytokine that has been proposed as a marker of endothelial dysfunction and vascular damage (Romacho et al., 2013), and visfatin/eNAMPT was shown to produce in vivo endothelial dysfunction in mice via toll-like receptor-4 (TLR4)-mediated pathway (Romacho et al., 2020). Potential interactions among visfatin/eNAMPT, TLR4, and inflammatory cytokines in PE should be further considered (Nunes et al., 2022). Briefly, possible visfatin/eNAMPT mechanisms of action by binding to TLR4 activates NF-kB phosphorylation and its translocation to the nucleus, where it acts by activating the NLRP3 inflammasome, responsible for the release of inflammatory cytokines. Activation of NLRP3 also releases reactive oxygen species, increasing oxidative stress, and therefore inhibiting NO formation, as reviewed elsewhere (Nunes et al., 2022).
Most studies provided evidence for increased visfatin levels in pregnant women with PE, despite discordant findings (Amiri-Dashatan et al., 2022). Although we have found no differences in the circulating levels of visfatin/eNAMPT between health pregnancy and PE (Luizon et al., 2015), visfatin/eNAMPT levels were inversely related to circulating NO and positively related to sFlt-1 levels in PE (Pereira et al., 2019). Notably, the same correlations were significantly only in the subgroup of patients with PE classified as nonresponsive to antihypertensive therapy, who showed higher proteinuria and plasma sFlt-1 levels (Pereira et al., 2021).
Visfatin impairs endothelium-dependent relaxation through nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) oxidase activity, which leads to release of superoxide anions (Vallejo et al., 2011). These anions scavenge NO to generate peroxynitrite, which stimulates the expression of inducible NO synthase enzyme and intercellular cell adhesion molecule-1, a well-known marker of endothelial dysfunction (Goulopoulou and Davidge, 2015). Superoxide anions also induce the uncoupling of endothelial NO synthase (Brennan et al., 2014), further decreasing NO bioavailability. During pregnancy, an increase in oxidative stress is observed as a result of the normal systemic inflammatory response, reflected as higher levels of circulating reactive oxygen species. This increased oxidative stress does not cause tissue damage because it is counter-balanced by the synthesis of antioxidants (Chiarello et al., 2020). However, in PE, placental ischemia/hypoxia exacerbates increased oxidative stress to a level that is harmful for the mother and fetal health by decreasing NO bioavailability as well as via several other mechanisms (Chiarello et al., 2020; Guerby et al., 2021).
Taken together, the evidence provided in the preceding paragraphs suggests that visfatin/eNAMPT may inhibit NO formation and upregulate sFlt-1, which may be due to the increased oxidative stress observed in PE. In view of the complex deleterious impact that adipocytokines have on the endothelium and vascular homeostasis (McElwain et al., 2020), these potential mechanisms may underly the role of visfatin/eNAMPT in vascular dysfunction in PE, as reviewed elsewhere (Ceron et al., 2022; Ceron et al., 2023), and perhaps serve as a link between PE and the occurrence of cardiovascular and renal diseases later in life.
MMP-2 is expressed by a variety of cells including trophoblasts, endothelial cells, and fibroblasts, playing a major role in embryogenesis, placental morphogenesis, and cardiovascular and renal function (Palei et al., 2013). We have found higher circulating MMP-2 levels in patients with PE classified as nonresponsive to antihypertensive therapy (Palei et al., 2012a).
In addition, we examined the differential gene expression in human umbilical vein endothelial cells (HUVECs) incubated with plasma from patients with PE classified according to antihypertensive therapy (nonresponsive relative to responsive patients), and we identified interactions among genes and antihypertensive drugs used in PE (Luizon et al., 2016). Notably, genes that were downregulated or upregulated in HUVECs incubated with plasma from nonresponsive PE patients were reported as upregulated or downregulated by nifedipine and hydralazine, respectively. Notably, while MMP-2 was found to be upregulated in these HUVECs (Luizon et al., 2016), hydralazine treatment was shown to decrease MMP-2 expression in spontaneously hypertensive rats (Kodavanti et al., 2013).
Hypoxia can stimulate MMP-2 expression, and MMP-2 is capable of cleaving big endothelin (ET)-1 and induce hypertension through the generation of ET-1, a potent vasoconstrictor (Fernandez-Patron et al., 1999). Interestingly, we found that plasma from patients with PE stimulated microRNA expression in HUVECs that were negatively related to ET-1 levels (Caldeira-Dias et al., 2018). Considering that agents that improve endothelial function in PE hold promise to alleviate clinical symptoms (Sasser et al., 2015), microRNAs may be useful candidates for therapeutic intervention in the management of hypertension in PE (Caldeira-Dias et al., 2018).
As aforementioned, patients with PE classified as nonresponsive to antihypertensive therapy showed higher plasma creatinine and proteinuria (Figure 1) than responsive patients (Pereira et al., 2021). Creatinine is considered a fairly reliable indicator of kidney function. Proteinuria is also associated with impaired renal function in PE, being the amount of protein loss related to disease severity (Fishel Bartal et al., 2022). Patients with PE have angiogenic imbalance represented by increased circulating sFlt-1 and soluble endoglin, but reduced vascular endothelial growth factor (VEGF) and placental growth factor levels, which together damage the endotelium of the glomerular filtration barrier (Fishel Bartal et al., 2022). In this regard, clinical and experimental studies reviewed elsewhere (Cruz et al., 2021) have shown a compelling role for the full-length MMP-2 in ischemic renal injury, progressive renal fibrosis, and diabetic nephropathy. Interestingly, evidence suggests that MMP-2 is able to cleave the extracellular domain of the VEGF receptor-2, thereby leading to endothelial apoptosis and vascular rarefaction (Tran et al., 2010; Wang et al., 2014).
While the extracellular activity of MMP-2 is mainly regulated via inhibition by TIMPs, a novel intracellular N-terminal truncated isoform of MMP-2 has been discovered, which is induced by hypoxia and oxidative stress by activation of a latent promoter located in the first intron of MMP-2 (Lovett et al., 2012). We have previously proposed that the latent promoter of this MMP-2 isoform undergoes epigenetic regulation via its overlap with histone modifications, a putative active enhancer element, and binding sites for transcription factors that are known to cooperate in hypoxia-induced gene transcription (Cruz et al., 2021).
The balance between MMPs and TIMPs is key to the stability of the extracellular matrix and normal function of these proteases during pregnancy. Although TIMP-3 is able to bind and inhibit MMP-2, it has also been reported that activation of the pro-form of MMP-2 by Matrix type 3-MMP may be enhanced by TIMP-3 in a dose-dependent manner (Zhao et al., 2004). Furthermore, TIMP-3 has MMP-independent functions, such as the inhibition of angiogenesis by blocking the binding of VEGF to VEGF receptor-2 and hindering downstream signaling (Qi et al., 2003). Regarding the role of TIMP-3 in PE, the promoter polymorphism rs9619311 was not associated with response to antihypertensive therapy (Luizon et al., 2014). However, we identified a higher number of significant differentially methylated probes located on the TIMP-3 promoter, as well as an increased TIMP-3 expression in corresponding placental samples from early-onset PE compared to controls, which denotes DNA methylation of TIMP-3 promoter as an epigenetic mechanism in PE (Cruz et al., 2022). Recently, we found that circulating TIMP-3 is increased in patients with PE compared with healthy pregnancy, and these TIMP-3 levels were positively correlated with MMP-2 and TIMP-1 concentrations in PE (Palei et al., 2022). These findings may contribute to understand the relevance of TIMP-3 in the pathophysiology of PE.
Here, we discussed potential mechanisms underlying endothelial dysfunction and associated long-term cardiovascular and renal risk in PE based on findings from studies focused on patients with PE classified as nonresponsive to antihypertensive therapy. Effects on NO bioavailability highlight the potential role of visfatin/NAMPT on mediating endothelial dysfunction, which might contribute to increased cardiovascular events in PE. Moreover, we provided insights into the epigenetic control of MMP-2 and TIMP-3 expression that may contribute to cardiovascular and renal complications in PE. Finally, epigenetic regulation of the vascular endothelium should be further considered as potential drug targets to improve antihypertensive responsiveness in PE.
ML: Conceptualization, Funding acquisition, Project administration, Supervision, Writing–original draft, Writing–review and editing. DP: Conceptualization, Writing–original draft, Writing–review and editing. IM: Conceptualization, Writing–original draft, Writing–review and editing. CC: Conceptualization, Writing–original draft, Writing–review and editing. RC: Conceptualization, Project administration, Writing–review and editing. AP: Conceptualization, Funding acquisition, Project administration, Writing–original draft, Writing–review and editing. VS: Conceptualization, Funding acquisition, Project administration, Supervision, Writing–original draft, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Council for Scientific and Technological Development (CNPq/Brazil; grants #312599/2019-6, #308504/2021-6, and #302076/2022-0), Minas Gerais Research Foundation (FAPEMIG/Brazil; grant APQ-01960-18), São Paulo Research Foundation (FAPESP/Brazil; grants #2019/07230-8 and #2021/12010-7), Coordination for the Improvement of Higher Education Personnel (CAPES/Brazil) (Finance Code 001), and The National Institutes of Health (NIH/United States; grants K01HL159032, R01HL148191, and U54GM115428).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
American College of Obstetricians and Gynecologists (2013). Hypertension in pregnancy. Report of the American college of obstetricians and gynecologists' task force on hypertension in pregnancy. Obstet. Gynecol. 122, 1122–1131. doi:10.1097/01.AOG.0000437382.03963.88
American College of Obstetricians and Gynecologists (2020). Gestational hypertension and preeclampsia: ACOG practice bulletin, number 222. Obstet. Gynecol. 135, e237–e260. doi:10.1097/AOG.0000000000003891
Amiri-Dashatan, N., Koushki, M., Hosseini, H., Khodabandehloo, H., Fathi, M., Doustimotlagh, A. H., et al. (2022). Association between circulating visfatin and pre-eclampsia: a systematic review and meta-analysis. J. Matern. Fetal Neonatal Med. 35, 2606–2618. doi:10.1080/14767058.2020.1789581
Berzan, E., Doyle, R., and Brown, C. M. (2014). Treatment of preeclampsia: current approach and future perspectives. Curr. Hypertens. Rep. 16, 473. doi:10.1007/s11906-014-0473-5
Brennan, L. J., Morton, J. S., and Davidge, S. T. (2014). Vascular dysfunction in preeclampsia. Microcirculation 21, 4–14. doi:10.1111/micc.12079
Caldeira-Dias, M., Luizon, M. R., Deffune, E., Tanus-Santos, J. E., Freire, P. P., Carvalho, R. F., et al. (2018). Preeclamptic plasma stimulates the expression of miRNAs, leading to a decrease in endothelin-1 production in endothelial cells. Pregnancy Hypertens. 12, 75–81. doi:10.1016/j.preghy.2018.03.001
Ceron, C. S., Luizon, M. R., and Palei, A. C. (2023). The potential role of visfatin in mediating vascular dysfunction and hypertension. J. Cardiovasc Pharmacol. 82, 347–349. doi:10.1097/FJC.0000000000001457
Ceron, C. S., Pereira, D. A., Sandrim, V. C., and Luizon, M. R. (2022). Potential roles of visfatin/NAMPT on endothelial dysfunction in preeclampsia and pathways underlying cardiac and vascular remodeling. J. Cell Physiol. 237, 10–12. doi:10.1002/jcp.30572
Chiarello, D. I., Abad, C., Rojas, D., Toledo, F., Vazquez, C. M., Mate, A., et al. (2020). Oxidative stress: normal pregnancy versus preeclampsia. Biochim. Biophys. Acta Mol. Basis Dis. 1866, 165354. doi:10.1016/j.bbadis.2018.12.005
Cruz, J. O., Conceicao, I., Sandrim, V. C., and Luizon, M. R. (2022). Comprehensive analyses of DNA methylation of the TIMP3 promoter in placentas from early-onset and late-onset preeclampsia. Placenta 117, 118–121. doi:10.1016/j.placenta.2021.12.003
Cruz, J. O., Silva, A. O., Ribeiro, J. M., Luizon, M. R., and Ceron, C. S. (2021). Epigenetic regulation of the N-terminal truncated isoform of matrix metalloproteinase-2 (NTT-MMP-2) and its presence in renal and cardiac diseases. Front. Genet. 12, 637148. doi:10.3389/fgene.2021.637148
Duley, L. (2009). The global impact of pre-eclampsia and eclampsia. Semin. Perinatol. 33, 130–137. doi:10.1053/j.semperi.2009.02.010
Fernandez-Patron, C., Radomski, M. W., and Davidge, S. T. (1999). Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circ. Res. 85, 906–911. doi:10.1161/01.res.85.10.906
Fishel Bartal, M., Lindheimer, M. D., and Sibai, B. M. (2022). Proteinuria during pregnancy: definition, pathophysiology, methodology, and clinical significance. Am. J. Obstet. Gynecol. 226, S819–S834. doi:10.1016/j.ajog.2020.08.108
Goulopoulou, S., and Davidge, S. T. (2015). Molecular mechanisms of maternal vascular dysfunction in preeclampsia. Trends Mol. Med. 21, 88–97. doi:10.1016/j.molmed.2014.11.009
Guerby, P., Tasta, O., Swiader, A., Pont, F., Bujold, E., Parant, O., et al. (2021). Role of oxidative stress in the dysfunction of the placental endothelial nitric oxide synthase in preeclampsia. Redox Biol. 40, 101861. doi:10.1016/j.redox.2021.101861
Kodavanti, U. P., Thomas, R. F., Ledbetter, A. D., Schladweiler, M. C., Bass, V., Krantz, Q. T., et al. (2013). Diesel exhaust induced pulmonary and cardiovascular impairment: the role of hypertension intervention. Toxicol. Appl. Pharmacol. 268, 232–240. doi:10.1016/j.taap.2013.02.002
Lovett, D. H., Mahimkar, R., Raffai, R. L., Cape, L., Maklashina, E., Cecchini, G., et al. (2012). A novel intracellular isoform of matrix metalloproteinase-2 induced by oxidative stress activates innate immunity. PLoS One 7, e34177. doi:10.1371/journal.pone.0034177
Luizon, M. R., Belo, V. A., Palei, A. C., Amaral, L. M., Lacchini, R., Sandrim, V. C., et al. (2015). Effects of NAMPT polymorphisms and haplotypes on circulating visfatin/NAMPT levels in hypertensive disorders of pregnancy. Hypertens. Res. 38, 361–366. doi:10.1038/hr.2015.15
Luizon, M. R., Caldeira-Dias, M., Deffune, E., Fernandes, K. S., Cavalli, R. C., Tanus-Santos, J. E., et al. (2016). Antihypertensive therapy in pre-eclampsia: effects of plasma from nonresponsive patients on endothelial gene expression. Pharmacogenomics 17, 1121–1127. doi:10.2217/pgs-2016-0033
Luizon, M. R., Palei, A. C., Cavalli, R. C., and Sandrim, V. C. (2017a). Pharmacogenetics in the treatment of pre-eclampsia: current findings, challenges and perspectives. Pharmacogenomics 18, 571–583. doi:10.2217/pgs-2016-0198
Luizon, M. R., Palei, A. C., Sandrim, V. C., Amaral, L. M., Machado, J. S., Lacchini, R., et al. (2014). Tissue inhibitor of matrix metalloproteinase-1 polymorphism, plasma TIMP-1 levels, and antihypertensive therapy responsiveness in hypertensive disorders of pregnancy. Pharmacogenomics J. 14, 535–541. doi:10.1038/tpj.2014.26
Luizon, M. R., Palei, A. C. T., Belo, V. A., Amaral, L. M., Lacchini, R., Duarte, G., et al. (2017b). Gene-gene interactions in the NAMPT pathway, plasma visfatin/NAMPT levels, and antihypertensive therapy responsiveness in hypertensive disorders of pregnancy. Pharmacogenomics J. 17, 427–434. doi:10.1038/tpj.2016.35
Luizon, M. R., Pereira, D. A., and Sandrim, V. C. (2018). Pharmacogenomics of hypertension and preeclampsia: focus on gene-gene interactions. Front. Pharmacol. 9, 168. doi:10.3389/fphar.2018.00168
Maynard, S. E., Min, J. Y., Merchan, J., Lim, K. H., Li, J., Mondal, S., et al. (2003). Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Invest. 111, 649–658. doi:10.1172/JCI17189
Mcelwain, C. J., Tuboly, E., Mccarthy, F. P., and Mccarthy, C. M. (2020). Mechanisms of endothelial dysfunction in pre-eclampsia and gestational diabetes mellitus: windows into future cardiometabolic health? Front. Endocrinol. (Lausanne) 11, 655. doi:10.3389/fendo.2020.00655
Metzger, I. F., Luizon, M. R., Lacchini, R., Ishizawa, M. H., and Tanus-Santos, J. E. (2013). Effects of endothelial nitric oxide synthase tagSNPs haplotypes on nitrite levels in black subjects. Nitric Oxide 28, 33–38. doi:10.1016/j.niox.2012.10.002
Mori, T., Shinohara, K., Wakatsuki, A., Watanabe, K., and Fujimaki, A. (2010). Adipocytokines and endothelial function in preeclamptic women. Hypertens. Res. 33, 250–254. doi:10.1038/hr.2009.222
Nunes, P. R., Ceron, C. S., Luizon, M. R., and Sandrim, V. C. (2022). Interaction among extracellular nicotinamide phosphoribosyltransferase, toll-like receptor-4, and inflammatory cytokines in pre-eclampsia. Am. J. Reprod. Immunol. 87, e13514. doi:10.1111/aji.13514
Palei, A. C., Cruz, J. O., Chaguri, J. L., Peracoli, J. C., Romao-Veiga, M., Ribeiro-Vasques, V. R., et al. (2022). Circulating levels of tissue inhibitor of metalloproteinase 3, a protein with inhibitory effects on angiogenesis, are increased in pre-eclampsia. Int. J. Gynaecol. Obstet. 161, 544–551. doi:10.1002/ijgo.14552
Palei, A. C., Granger, J. P., and Tanus-Santos, J. E. (2013). Matrix metalloproteinases as drug targets in preeclampsia. Curr. Drug Targets 14, 325–334. doi:10.2174/1389450111314030004
Palei, A. C., Sandrim, V. C., Amaral, L. M., Machado, J. S., Cavalli, R. C., Lacchini, R., et al. (2012a). Effects of matrix metalloproteinase (MMP)-2 polymorphisms on responsiveness to antihypertensive therapy of women with hypertensive disorders of pregnancy. Basic Clin. Pharmacol. Toxicol. 111, 262–267. doi:10.1111/j.1742-7843.2012.00905.x
Palei, A. C., Sandrim, V. C., Amaral, L. M., Machado, J. S., Cavalli, R. C., Lacchini, R., et al. (2012b). Matrix metalloproteinase-9 polymorphisms affect plasma MMP-9 levels and antihypertensive therapy responsiveness in hypertensive disorders of pregnancy. Pharmacogenomics J. 12, 489–498. doi:10.1038/tpj.2011.31
Peracoli, J. C., Borges, V. T. M., Ramos, J. G. L., Cavalli, R. C., Costa, S., Oliveira, L. G., et al. (2019). Pre-eclampsia/Eclampsia. Rev. Bras. Ginecol. Obstet. 41, e1–e2. doi:10.1055/s-0040-1702167
Pereira, D. A., Sandrim, V. C., Palei, A. C., Amaral, L. M., Belo, V. A., Lacchini, R., et al. (2021). NAMPT single-nucleotide polymorphism rs1319501 and visfatin/NAMPT affect nitric oxide formation, sFlt-1 and antihypertensive therapy response in preeclampsia. Pharmacogenomics 22, 451–464. doi:10.2217/pgs-2021-0006
Pereira, D. A., Sandrim, V. C., Palei, A. C. T., Tanus-Santos, J. E., Belo, V. A., Cavalli, R. C., et al. (2019). NAMPT levels are inversely related to nitric oxide formation and positively related to soluble fms-like tyrosine kinase-1 levels in preeclampsia. Pregnancy Hypertens. 18, 137–140. doi:10.1016/j.preghy.2019.09.022
Phipps, E. A., Thadhani, R., Benzing, T., and Karumanchi, S. A. (2019). Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 15, 275–289. doi:10.1038/s41581-019-0119-6
Powe, C. E., Levine, R. J., and Karumanchi, S. A. (2011). Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 123, 2856–2869. doi:10.1161/CIRCULATIONAHA.109.853127
Qi, J. H., Ebrahem, Q., Moore, N., Murphy, G., Claesson-Welsh, L., Bond, M., et al. (2003). A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat. Med. 9, 407–415. doi:10.1038/nm846
Romacho, T., Sanchez-Ferrer, C. F., and Peiro, C. (2013). Visfatin/Nampt: an adipokine with cardiovascular impact. Mediat. Inflamm. 2013, 946427. doi:10.1155/2013/946427
Romacho, T., Valencia, I., Ramos-Gonzalez, M., Vallejo, S., Lopez-Esteban, M., Lorenzo, O., et al. (2020). Visfatin/eNampt induces endothelial dysfunction in vivo: a role for Toll-Like Receptor 4 and NLRP3 inflammasome. Sci. Rep. 10, 5386. doi:10.1038/s41598-020-62190-w
Sandrim, V. C., Palei, A. C., Eleuterio, N., Tanus-Santos, J. E., and Cavalli, R. C. (2015). Antihypertensive therapy in preeclampsia is not modulated by VEGF polymorphisms. Arch. Gynecol. Obstet. 291, 799–803. doi:10.1007/s00404-014-3475-2
Sandrim, V. C., Palei, A. C., Luizon, M. R., Izidoro-Toledo, T. C., Cavalli, R. C., and Tanus-Santos, J. E. (2010a). eNOS haplotypes affect the responsiveness to antihypertensive therapy in preeclampsia but not in gestational hypertension. Pharmacogenomics J. 10, 40–45. doi:10.1038/tpj.2009.38
Sandrim, V. C., Palei, A. C., Metzger, I. F., Gomes, V. A., Cavalli, R. C., and Tanus-Santos, J. E. (2008). Nitric oxide formation is inversely related to serum levels of antiangiogenic factors soluble fms-like tyrosine kinase-1 and soluble endogline in preeclampsia. Hypertension 52, 402–407. doi:10.1161/HYPERTENSIONAHA.108.115006
Sandrim, V. C., Palei, A. C., Sertorio, J. T., Cavalli, R. C., Duarte, G., and Tanus-Santos, J. E. (2010b). Effects of eNOS polymorphisms on nitric oxide formation in healthy pregnancy and in pre-eclampsia. Mol. Hum. Reprod. 16, 506–510. doi:10.1093/molehr/gaq030
Sasser, J. M., Murphy, S. R., and Granger, J. P. (2015). Emerging drugs for preeclampsia--the endothelium as a target. Expert Opin. Emerg. Drugs 20, 527–530. doi:10.1517/14728214.2015.1062875
Townsend, R., O'brien, P., and Khalil, A. (2016). Current best practice in the management of hypertensive disorders in pregnancy. Integr. Blood Press Control 9, 79–94. doi:10.2147/IBPC.S77344
Tran, E. D., Delano, F. A., and Schmid-Schonbein, G. W. (2010). Enhanced matrix metalloproteinase activity in the spontaneously hypertensive rat: VEGFR-2 cleavage, endothelial apoptosis, and capillary rarefaction. J. Vasc. Res. 47, 423–431. doi:10.1159/000281582
Turbeville, H. R., and Sasser, J. M. (2020). Preeclampsia beyond pregnancy: long-term consequences for mother and child. Am. J. Physiol. Ren. Physiol. 318, F1315-F1326–F1326. doi:10.1152/ajprenal.00071.2020
Vallejo, S., Romacho, T., Angulo, J., Villalobos, L. A., Cercas, E., Leivas, A., et al. (2011). Visfatin impairs endothelium-dependent relaxation in rat and human mesenteric microvessels through nicotinamide phosphoribosyltransferase activity. PLoS One 6, e27299. doi:10.1371/journal.pone.0027299
Wang, B., Li, B. W., Li, H. W., Li, A. L., Yuan, X. C., Wang, Q., et al. (2014). Enhanced matrix metalloproteinases-2 activates aortic endothelial hypermeability, apoptosis and vascular rarefaction in spontaneously hypertensive rat. Clin. Hemorheol. Microcirc. 57, 325–338. doi:10.3233/CH-131713
Wang, X., Shields, C. A., Ekperikpe, U., Amaral, L. M., Williams, J. M., and Cornelius, D. C. (2023). Vascular and renal mechanisms of preeclampsia. Curr. Opin. Physiology 33, 100655. doi:10.1016/j.cophys.2023.100655
Zhao, H., Bernardo, M. M., Osenkowski, P., Sohail, A., Pei, D., Nagase, H., et al. (2004). Differential inhibition of membrane type 3 (MT3)-matrix metalloproteinase (MMP) and MT1-MMP by tissue inhibitor of metalloproteinase (TIMP)-2 and TIMP-3 rgulates pro-MMP-2 activation. J. Biol. Chem. 279, 8592–8601. doi:10.1074/jbc.M308708200
Keywords: antihypertensive agents, endothelial dysfunction, genetic polymorphisms, matrix metalloproteinase (MMP)-2, nicotinamide phosphoribosyltransferase (NAMPT), nitric oxide, preeclampsia, tissue inhibitor of metalloproteinase (TIMP)-3
Citation: Luizon MR, Pereira DA, Mamede I, Ceron CS, Cavalli RC, Palei AC and Sandrim VC (2023) Antihypertensive therapy responsiveness and adverse outcomes in preeclampsia: insights into molecular mechanisms underlying cardiovascular and renal complications. Front. Pharmacol. 14:1281382. doi: 10.3389/fphar.2023.1281382
Received: 22 August 2023; Accepted: 13 November 2023;
Published: 22 November 2023.
Edited by:
Ariela Hoxha, University Hospital of Padua, ItalyReviewed by:
Fernanda Regina Giachini, Federal University of Mato Grosso, BrazilCopyright © 2023 Luizon, Pereira, Mamede, Ceron, Cavalli, Palei and Sandrim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marcelo R. Luizon, bXJsdWl6b25AdWZtZy5icg==; Ana C. Palei, YXBhbGVpQHVtYy5lZHU=; Valeria C. Sandrim, dmFsZXJpYS5zYW5kcmltQHVuZXNwLmJy
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.