- 1Division of Endocrinology and Nutrition, Cliniques Universitaires St-Luc and Institut de Recherche Expérimentale et Clinique (IREC), Université Catholique de Louvain, Brussels, Belgium
- 2Laboratoire Tilman, Baillonville, Belgium
- 3B-STAT, Centre Hospitalier Universitaire, Liège, Belgium
- 4Division of Cardiology, Cliniques Universitaires St-Luc and Pôle de Recherche Cardiovasculaire, Institut de Recherche Expérimentale et Clinique (IREC), Université Catholique de Louvain, Brussels, Belgium
- 5Nuffield Division of Clinical Laboratory Sciences, Radcliffe Department of Medicine, University of Oxford, Oxford, United Kingdom
- 6Department of Biochemistry, Liaquat University of Medical and Health Sciences, Jamshoro, Pakistan
Background: Hyperlipidemia is associated with a higher rate of cardiovascular, cerebrovascular, and peripheral vascular disease. Conventional drugs such as statins are effective in controlling hyperlipidemia; however, they are associated with various side effects, especially myalgia. Nutraceutical lipid-lowering interventions are becoming increasingly popular, particularly among patients who are intolerant or refractory to statins. Substantial preclinical and clinical evidence suggests that extracts of amla, walnut, and olive, and red yeast rice (RYR) powder possess significant antihyperlipidemic effects.
Aims: This study aimed to evaluate the efficacy, safety, and patient satisfaction of a combined supplementation of standardized dry extracts of amla fruit (500 mg), walnut leaves (50 mg), olive fruit (25 mg), and RYR powder (33.6 mg) (Cholesfytol NG®) in hypercholesterolemic patients.
Methods: This was a real-life setting, retrospective, observational, single-arm, non-randomized study in hypercholesterolemic patients (total cholesterol (TC) ≥ 200 mg/dL or low-density lipoprotein-cholesterol (LDL-C) ≥ 130 mg/dL), enrolled at 57 general practitioner (GP) surgeries in Belgium from March 2020 to January 2022. These patients received a GP-prescribed daily single dosage of two oral tablets of Cholesfytol NG® supplementation for 2 months to overcome their hypercholesterolemia in the absence of a conventional lipid-lowering drug (n = 208) or with a lipid-lowering drug (n = 13). At 2-month follow-up, the lipid profile was re-evaluated, alongside a patient’s questionnaire on treatment general satisfaction and willingness to pursue supplementation.
Results: After supplementation, TC decreased by 15%, LDL-C by 19%, non-high-density lipoprotein-cholesterol (non-HDL-C) by 20% (all p < 0.0001), triglycerides (TG) by 9% (p = 0.0028) (−18.4%, p = 0.0042, in patients with baseline TG > 180 mg/dL, n = 58), and remnant cholesterol (RC) by 12% (p = 0.0001). These changes were unaffected by statin intolerance status in patients who received Cholesfytol NG® alongside statin. The supplement was well tolerated by all patients, and no serious adverse events or supplement-emergent effects were reported. Most patients were satisfied with the supplementation and wanted to pursue the nutraceutical.
Conclusion: According to the results of this study, a combined supplementation of amla, walnut, and olive extracts, and RYR powder exerts a significant antihyperlipidemic effect, leading to a decrease in circulatory LDL-C and RC levels in patients with hypercholesterolemia. The supplementation bears excellent safety and tolerability, and is rated as satisfactory and pursuable, even among patients with statin intolerance.
Clinical Trial Registration: clinicaltrials.gov; identifier number: NCT06002893
1 Introduction
Hypercholesterolemia is defined as elevated blood levels of total cholesterol (TC) and/or low-density lipoprotein-cholesterol (LDL-C, also known as ‘bad’ cholesterol) or non-high-density lipoprotein-cholesterol (non-HDL-C, all the ‘bad’ cholesterol or TC burden) and is diagnosed by a blood lipid profile test. Hypercholesterolemia is among the leading modifiable risk factors of atherosclerotic cardiovascular disease (ASCVD) including cerebrovascular disease, coronary heart disease, peripheral arterial disease, and aortic disease (Keys, 1980; Castelli et al., 1986; Lacoste et al., 1995; Stamler, 1996), especially in at-risk patients, such as those with pre-existing atherosclerotic disease, hypertension, impaired plasma glucose, and low high-density lipoprotein-cholesterol (HDL-C, also known as ‘good’ cholesterol) levels, diabetes, older age, and cigarette smoking. In addition, high blood plasma triglyceride (TG) levels add another independent risk factor for the development of coronary artery disease (CAD) and cardiovascular events (Collaboration et al., 2004; Sarwar et al., 2007; Nordestgaard and Varbo, 2014). Hypercholesterolemia is generally asymptomatic until substantial atherosclerosis has developed. Complications of hypercholesterolemia and atherosclerosis are diverse and include sudden cardiac arrest, myocardial infarction, ischemic stroke, ischemic cardiomyopathy, acute limb ischemia, claudication, and erectile dysfunction. Among the key risk factors of secondary hypercholesterolemia are excessive consumption of saturated fats, trans-fatty acids, cholesterol-rich diets, and a sedentary lifestyle. Other associations include obesity, diabetes, hypothyroidism, nephrotic syndrome, and cholestasis.
Treatment of hypercholesterolemia typically involves lifestyle modifications such as nutritional changes (adopting low-cholesterol and low-saturated fat food diet), regular physical activity/exercise, smoking cessation, weight loss, and conventional drug treatment including 3-hydroxy-3-methyl glutaryl-CoA (HMG-CoA) reductase inhibitors (statins), selective cholesterol absorption inhibitors (SCAIs), bile acid sequestrants, proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, adenosine triphosphate-citrate lyase (ACL) inhibitors, nicotinic acid, fibric acid derivatives, and omega-3 fatty acids and fatty acid esters (Authors/Task Force Members ESC Committee for Practice Guidelines (CPG) ESC National Cardiac Societies, 2019). Among these medications, statins have proven to be most effective in reducing LDL-C levels and decreasing ASCVD risk by 15%–37%, and they are recommended as a first-line therapy for the primary and secondary prevention of ASCVD. In patients with high CV risk, to achieve additional LDL-C reduction and reduce ASCVD risk, the current guidelines for cholesterol management recommend two drugs, Ezetimibe (a SCAI) or a PCSK9 inhibitor (Alirocumab and Evolocumab), alongside statin (Choi and Na, 2019). However, besides LDL-C, there is also a substantial residual CV risk driven by elevated levels of remnant cholesterol (RC). Remnant cholesterol, defined as the cholesterol content of all non-LDL and non-HDL particles, has been recognized as an individual cardiovascular risk factor, independent of LDL-C, particularly in patients with metabolic disorders (Chait et al., 2020; Ginsberg et al., 2021). Extensive studies have revealed that RC levels are substantially linked with a greater risk of CAD as well as major cardiovascular adverse incidences (Castañer et al., 2020; Zhao et al., 2020; Shao et al., 2022), even when LDL-C is at goal on effective statin (±Ezetimibe) treatment (Varbo et al., 2014; Sandesara et al., 2019; Castañer et al., 2020; Ginsberg et al., 2021; Quispe et al., 2021; Doi et al., 2023).
Although conventional lipid-lowering drugs (LLDs) can significantly decrease LDL-C levels, these medications have diverse side effects (Feingold, 2021); for instance, statins can cause muscle aches, tenderness or weakness (myalgia), joint pain, liver injury, increase in blood sugar, nausea, indigestion, and upset stomach. Statin-associated muscle symptoms (SAMS) are clinically the adverse side effects experienced by over 70% of patients, resulting in discontinuation of statin therapy. Statin intolerance has been implicated with an increased risk of recurrent cardiac incidents. Other LLDs such as Ezetimibe can cause dizziness, upset stomach, and diarrhea; side effects of fibrates include indigestion, abdominal pain, and fatigue; bile acid resins can cause constipation, bloating, stomach pain, and nausea. Niacin consumption can cause flushing, itching, increased heart rate, and heartburn, while omega-3 fatty acids can lead to taste disturbances, “fish burps,” indigestion, and upset stomach.
Indeed, as a first-line approach, adopting lifestyle changes such as optimized nutrition aimed to reduce modifiable risk factors at the individual level, is an effective strategy in the management of lifestyle-related health conditions. In recent years, there has been a growing scientific and clinical interest in the therapeutic properties of functional nutritional supplements, both in the prevention and management of lifestyle-related health conditions such as hyperlipidemia, low HDL-C level, abdominal obesity, hyperglycemia, and hypertension (Vanhoutte, 2009; Suganya et al., 2016). Specifically, for the management of hyperlipidemia, among the several botanicals with demonstrated biological activities, the extracts of Emblica officinalis Gaertn. or Phyllanthus emblica L. fruit, commonly known as Indian gooseberry or amla, Juglans regia L. (walnut) leaves, Olea europaea L. (olive) fruit, and Monascus purpureus (red yeast rice or RYR) powder have emerged as an effective antihyperlipidemic agents with an excellent safety and tolerability profile (Figure 1).

FIGURE 1. New-generation Cholesfytol NG® is a dietary supplement consisting of a combination of (L to R) standardised dry extracts of amla fruit (500 mg), walnut leaves (50 mg), olive fruit (25 mg, equivalent to 5 mg hydroxytyrosol), and RYR (33.6 mg, equivalent to 1.45 mg of monacolines) powder, which produces a synergistic antihypercholesterolemic effect arising from the biologically active chemical constituents of the extracts.
1.1 Amla extract as natural medicine
For centuries, in Ayurveda, amla has been utilised for diverse health conditions including CAD and metabolic disorder conditions such as hypertension, type 2 diabetes, and obesity. Modern pharmacological studies have demonstrated antioxidant, antihyperglycemic, antihyperlipidemic, and anti-inflammatory therapeutic effects of amla, both in human and animal model studies (Yokozawa et al., 2007; Antony et al., 2008a; Akhtar et al., 2011; Gul et al., 2022). Amla reportedly contains a wide range of compounds, including quercetin, corilagin, phyllanemblinins, ellagic acid, chebulic acid, emblicanins, phyllaemblic acid, pedunculagin, methyl gallate, punigluconin, chlorogenic acid, gallic acid, and other compounds (Gul et al., 2022).
1.2 Walnut extract as natural medicine
Walnut extract has been traditionally utilized for various medicinal purposes including hyperlipidemia, diabetes, and heart diseases (Delaviz et al., 2017; Gupta et al., 2019; Sharma et al., 2022). Current evidence suggests that walnut extract is antioxidative, anti-inflammatory, antidiabetic, antihyperlipidemic, hypolipidemic, antihypertensive, and hepato- and nephro-protective (Delaviz et al., 2017; Gupta et al., 2019; Sharma et al., 2022). Walnut leaves are rich in antioxidants including phenolic acids (largely caffeoylquinic acids) and flavonoids, consisting mainly of juglone (5-hydroxy-1,4-naphthoquinone), quercetin, and its derivatives (Delaviz et al., 2017; Gupta et al., 2019; Sharma et al., 2022).
1.3 Olive extract as natural medicine
Olive has been used for centuries as a traditional medicine for multiple health conditions such as hyperglycemia, hypercholesterolemia, hyperuricemia, hypertension, and inflammation (Hashmi et al., 2015; Visioli et al., 2020). Olive extract exhibits a wide array of therapeutic effects including antidyslipidemic, antidiabetic, antioxidant, antihypertensive, cardioprotective, and anti-inflammatory (Hashmi et al., 2015; Visioli et al., 2020).
1.4 RYR powder as natural medicine
RYR exhibits diverse biological activities including hypolipidemic, antiatherosclerotic, antidiabetic, antiobesity, immunomodulatory, anti-inflammatory, and antihypertensive (Zhu et al., 2019). RYR is a proven effective agent against hypercholesterolemia (Cicero et al., 2019). In terms of its chemical composition, over 100 compounds have been isolated from RYR including monacolins, organic acids, flavonoids, polysaccharides, sterols, pigments, decalin derivatives, and others (Zhu et al., 2019).
In an effort to treat hypercholesterolemia and higher TG levels without side effects in a natural way, an optimized oral dietary supplement consisting of a combination of standardized dry extracts of amla fruit, walnut leaves, olive fruit, and RYR powder has been developed (new-generation Cholesfytol NG®; Tilman s. a., Baillonville, Belgium). The different extract components of this product have documented complementary effects on dyslipidemia and hypercholesterolemia (Bulotta et al., 2014; Hu et al., 2014; Barbagallo et al., 2015; Efentakis et al., 2015; Verhoeven et al., 2015; Sahebkar et al., 2016; Cicero et al., 2017; Tshongo Muhindo et al., 2017; Hermans et al., 2020). According to the reported literature, LDL-C lowering is achieved with RYR monacolin K (MK) (Endo, 1980; Lin et al., 2008; Pérez-Jiménez et al., 2018) and amla PCSK9 inhibition (Variya et al., 2016; Variya et al., 2018). A TG-lowering effect of this nutraceutical is also present, as amla is a natural nuclear receptor agonist of peroxisome proliferator-activated receptor alpha (PPAR-α) (Variya et al., 2016). The walnut leaves extract also lowers TG due to ellagitannins’ hypotriglyceridemic activity via an increase in the peroxisomal β-oxidation of fatty acids in the liver (Shimoda et al., 2009). Furthermore, amla and olive extracts are strong antioxidants, and both improve endothelial function (Shimoda et al., 2009; Bulotta et al., 2014; Hu et al., 2014; Efentakis et al., 2015).
To date, there have been no prospective real-life studies on the effect of an association of extracts of amla, walnut, olive, and RYR powder on different aspects of the hyperlipidemia including RC in patients with or without a history of statin intolerance. The present real-life setting, pragmatic, and observational exploratory study aimed to evaluate the safety and clinical efficacy of general practitioner (GP)-prescribed Cholesfytol NG® supplementation (single dose of two tablets a day for 2 months) on LDL-C, HDL-C, non-HDL-C, and RC in patients with hypercholesterolemia in a Belgian population.
2 Materials and methods
2.1 Study design and patients
This was a real-life setting, retrospective, single-arm non-controlled, non-randomized, and open-label observational clinical study of a random population of hypercholesterolemic patients, enrolled at 57 GP surgeries in Belgium from 19 March 2020 to 31 January 2022. These patients received a GP prescription of Cholesfytol NG® supplement, taken as a single dose of two tablets a day in the evening for 2 months in the absence of an LLD (94.1%) or with an LLD (5.9%). Patients were assessed at the study inclusion visit (T0) and the 2-months follow-up visit (T1) at the GP. As this was an observational study of data collection of patients’ medical records from GPs, ethics approval was not required as per the Belgian law (7 May 2004 Art. 3 §2) regarding research involving human subjects. The study was registered in the clinicaltrials.gov registry (identifier number NCT06002893).
Patients were required to meet the following inclusion criteria: (1) age ≥ 21 years; (2) both male or female; (3) TC ≥ 200 mg/dL; (4) LDL-C ≥ 130 mg/dL; (5) with or without a history of myalgia complaints and/or diabetes; (6) No prior treatment of cholesterol lowering agents and/or patients whose cholesterol-lowering treatment did not allow them to reach LDL-C target; (7) patients who had stopped their cholesterol-lowering treatment because of side effects including myalgia. (8) Cholesfytol NG® 240 supplement prescription as a single dose of 2 tablets a day for 2-months. Exclusion criteria included pregnant or breastfeeding patients.
2.2 Cholesfytol NG® supplement
Cholesfytol NG® supplement is a new-generation optimized oral dietary supplement manufactured by Tilman s. a., Baillonville, Belgium, and contains 500 mg of dry extract of amla fruit, standardized to ≥60.0% hydrolyzable tannins, which includes compounds like emblicanin-A, emblicanin-B, punigluconin, and pedunculagin; 50 mg walnut leaves dry extract standardized to ≥0.15% 3-O-caffeoylquinic acid (3-CQA); 25 mg olive fruit dry extract standardized to contain 20% hydroxytyrosol (5 mg); and 33.6 mg of RYR powder (equivalent to 1.45 mg of monacolines).
2.3 Variables
The data collected for each patient includes demographics (age and sex), baseline, and 2-months follow-up clinical data: lipids [measured by CardioChek Plus; TC (mg/dL); LDL-C (mg/dL; Friedewald’s formula); HDL-C (mg/dL); TG (mg/dL)]; non-HDL-C (mg/dL) was calculated as [TC minus HDL-C]; RC (mg/dL) was calculated as [TC minus LDL-C minus HDL-C] (Varbo et al., 2014); plasma glucose was measured with CardioChek Plus (mg/dL); systolic and diastolic blood pressure (SBP and DBP); and waist circumference (WC, cm). Patients were asked about their current treatment (or already tried in the case of LLDs), about any intolerance to statins, and the type of LLD they are using (statins, fibrates, Ezetimibe, statin + Ezetimibe, other LLDs including RYR and omega-3 supplements, or other dietary supplements). The use of BP-lowering and glucose-lowering drugs was also recorded. During treatment, the occurrence of SAMS side effects were also documented (Stroes et al., 2015). A general patient questionnaire was also filled in, to assess patient’s general satisfaction and desire to continue Cholesfytol NG® supplementation beyond the study period. All information was recorded in a case report form (CRF).
2.4 Statistical analyses
The results are presented as mean and standard deviation (SD) and median and interquartile range (IQR) for quantitative variables and as frequency tables (numbers and percentages) for categorical variables. The association between two quantitative variables was measured by the correlation coefficient. The comparison of the means of the same quantitative variable before and after treatment was performed by a paired-sample t-test as well as by the Wilcoxon signed-rank test. To compare the means of the two groups, the t-test for unpaired samples or the Kruskal–Wallis test was used. To compare proportions, the chi-squared test or Fisher exact test was used. The mean changes (in pure values and percentages compared to the baseline value) of the different parameters after treatment were also presented with their 95% confidence intervals (95% CI). The analysis of patient satisfaction according to different covariates was studied by ordinal logistic regression. The association between satisfaction and the covariate was quantified by the odds ratio (OR) and its 95% CI. The results were considered significant at the 5% uncertainty level (p < 0.05). All statistical calculations were performed on the maximum amount of data present. Missing data were not replaced or imputed. Analyses were performed using SAS® version 9.4 and R version 4.1.1 statistical software.
3 Results
3.1 Patients’ enrollment
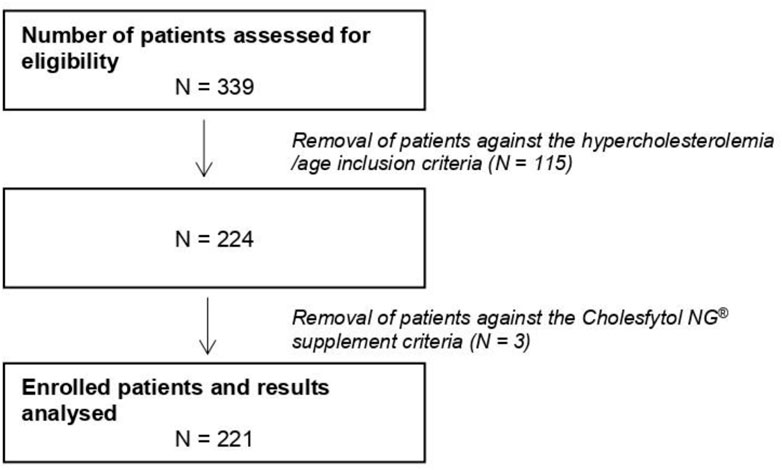
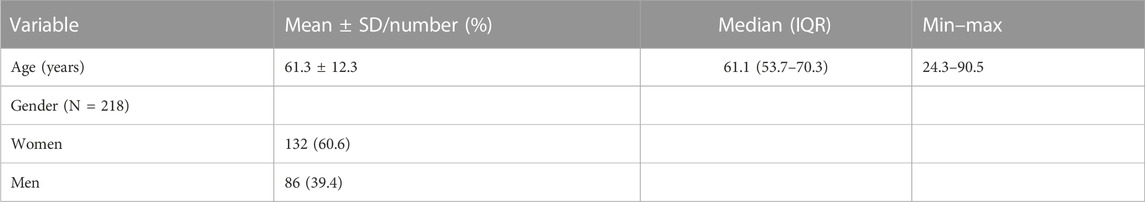
Figure 2 shows the study consort flow diagram. In total, 339 patients were assessed for eligibility, out of which 115 patients were excluded due to not fulfilling some of the inclusion criteria (i.e., TC < 200 mg/dL, LDL-C < 130 mg/dL, or age <21 years). Furthermore, three patients were excluded: for two of them, Cholesfytol NG® supplementation was no longer deemed necessary by the GP, while for one patient, the prescription of supplementation was not two tablets per day, leaving the study population to a total of 221 patients. Of these 221 patients, 119 had a minor violation to compliance with the study supplement period, i.e., 8 ± 2 weeks, while the remaining 102 patients complied with the supplement period of 2 months. Table 1 shows the demographics of patients. The mean age of patients was 61 ± 12 years, of whom 39.4% were men.
3.2 Patients’ history and treatment for hypercholesterolemia
At the time of supplement prescription/enrollment, among the total 221 patients, 143 (64.7%) had never received or tried an LLD before, 49 (22.1%) had stopped using LDDs, and 29 (13.1%) were still using LLDs. Of these 78 (35.3%) patients, 54 (69.2%) had received one LLD, 19 (24.3%) two LLDs, and five (6.4%) three LLDs. Statins were by far the most predominant LLDs (23%), followed by RYR supplement (12%), while fibrates, Ezetimibe, a combination of statin and Ezetimibe, omega-3 fatty acids, other dietary supplements, and other LLDs barely reached a few percents. There was a history of statin intolerance in 23% of patients, 33% of patients were taking BP-lowering medications, and 5% of patients were taking glucose-lowering drugs. Among the 29 patients who were on LLDs at enrollment, 13 (5.9%) kept their LLDs or were prescribed another LLD (mainly prescription statins and dietary supplements other than RYR and omega-3 fatty acids) together with Cholesfytol NG® supplement, while the remaining 16 (7.2%) patients were switched to Cholesfytol NG® supplement (alone). Overall, a total of 208 (94.1%) patients received Cholesfytol NG® supplement prescription as a single dose of two tablets a day for 2 months, in the absence of an LLD/other supplement for the management of their hypercholesterolemia. The majority of patients were compliant (89.1%) with the supplementation.
3.3 Lipid changes between T0 and T1
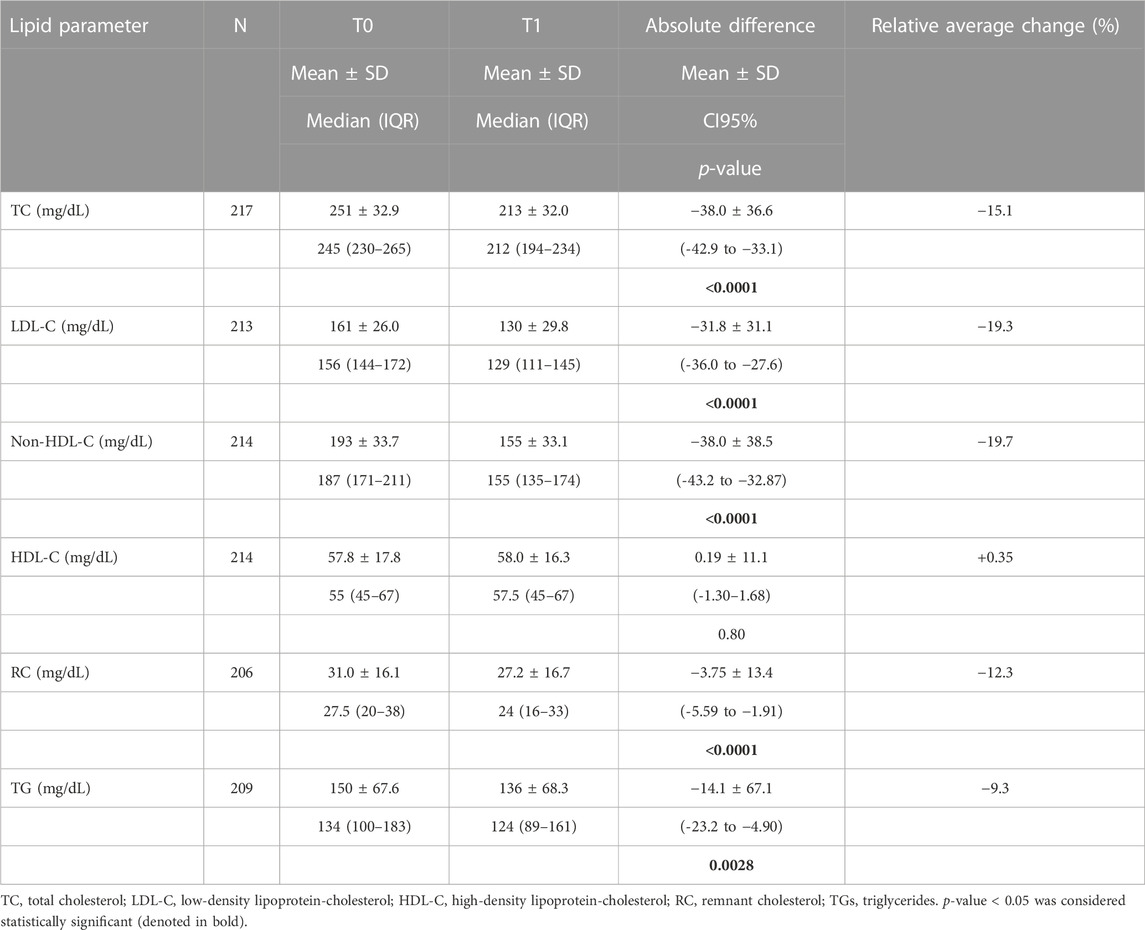
Table 2 shows the effect of Cholesfytol NG® supplementation on patients’ lipid profile parameters. At T0, the mean lipid values were as follows: TC: 251 ± 33 mg/dL, LDL-C: 163 ± 29 mg/dL, non-HDL-C: 194 ± 34 mg/dL, HDL-C: 58 ± 18 mg/dL, RC: 31 ± 16 mg/dL, and TG: 152 ± 69 mg/dL. At T1, there was a significant decrease in TC [-38 ± 37 mg (relative drop: −15.1%), p < 0.0001]. The same was true for LDL-C [-32 ± 31 mg/dL (−19.3%), p < 0.0001], non-HDL-C [-38 ± 39 mg/dL (−19.7%), p < 0.0001], RC [-4 ± 13 mg/dL (−12.3%), p < 0.0001], and TG [-14 ± 67 mg/dL (−9.3%), p = 0.0028]. HDL-C remained stable (0.19 ± 11.1 mg/dL, p = 0.80). Among the 58 patients with baseline TG > 180 mg/dL, the latter decreased from 234 ± 60 mg/dL to 191 ± 89 mg/dL (−18.4%), p = 0.0042). This decrease differed significantly (p = 0.0089) from patients with TG ≤ 180 mg/dL at baseline, among whom the relative decrease was 2.5% (NS), non-significant.
3.4 Non-lipid changes between T0 and T1
Table 3 shows the effect of Cholesfytol NG® supplementation on patients’ fasting plasma glycemia, tensor, and biometric parameters. The mean plasma glucose at T0 was 96 ± 18 mg/dL, SBP was 132 ± 14 mmHg, DBP was 79 ± 8 mmHg, and WC was 97 ± 15 cm. At T1, the mean plasma glucose was 94 ± 14 mg/dL, SBP was 128 ± 11 mmHg, DBP was 79 ± 8 mmHg, and WC was 96 ± 15 cm. The mean decrease in glucose was −2.35 ± 14.5 mg/dL (−2.5%), p = 0.026. SBP decreased by 4 ± 12 mmHg (−3%), p < 0.0001. DBP remained stable (p = 0.21). WC decreased by −0.85 ± 3 cm (−1%), p = 0.020.
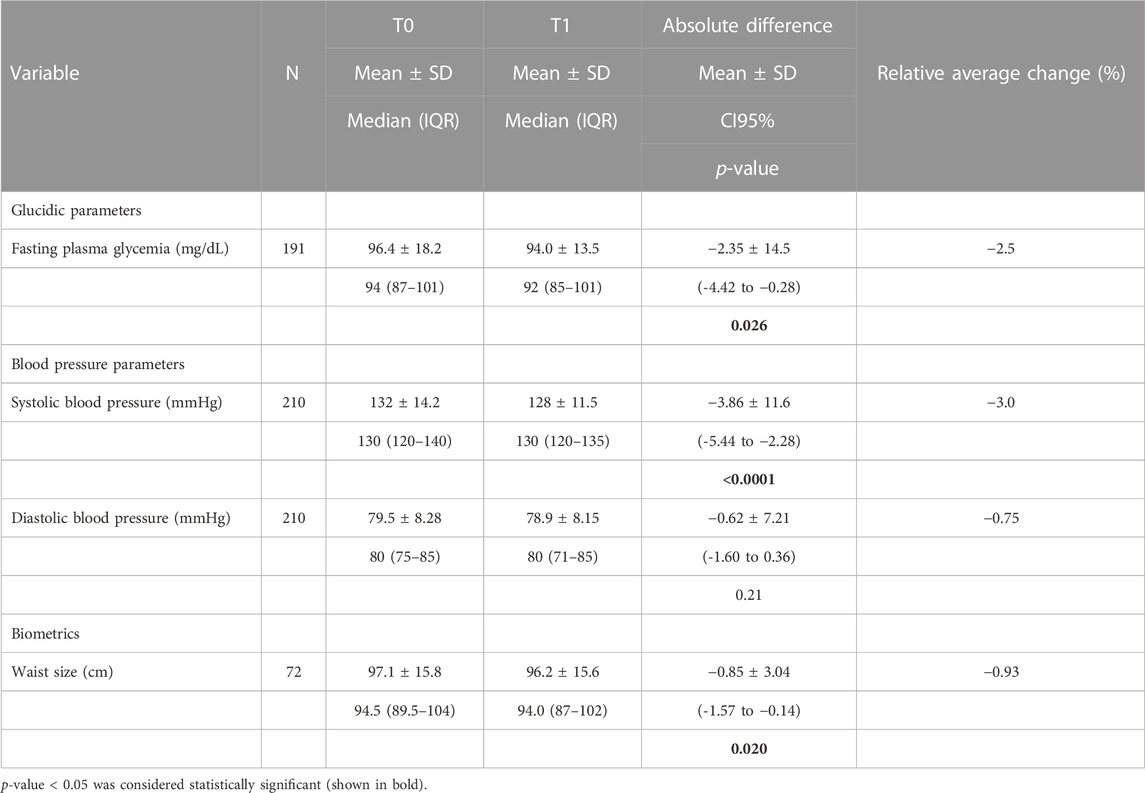
TABLE 3. Effect of Cholesfytol NG® supplementation on patients’ fasting plasma glycemia, tensor, and biometric parameters (N = 221).
3.5 Safety and tolerability of supplementation
The supplement was well tolerated, and no adverse events or supplement-associated effects were reported by any of the study participants. During the study period of a median of 71 days (IQR 62–91) of taking the nutraceutical, a small number of patients (6%) reported mild gastrointestinal effects.
3.6 Cholesfytol NG® supplement satisfaction
Overall, most patients (79%) were (very) satisfied with the treatment at the end of follow-up (T1); 86% wanted to continue, and 82% felt an improvement in their condition. The level of patient satisfaction was studied according to demographic characteristics and changes (%) in each metabolic parameter by ordinal logistic regression, with very dissatisfied and dissatisfied patients combined (Table 4). The results showed that the greater the drop in TC (OR = 1.07, p < 0.0001), LDL-C (OR = 1.05, p < 0.0001), and non-HDL-C (OR = 1.05, p < 0.0001), the greater the satisfaction of patients.
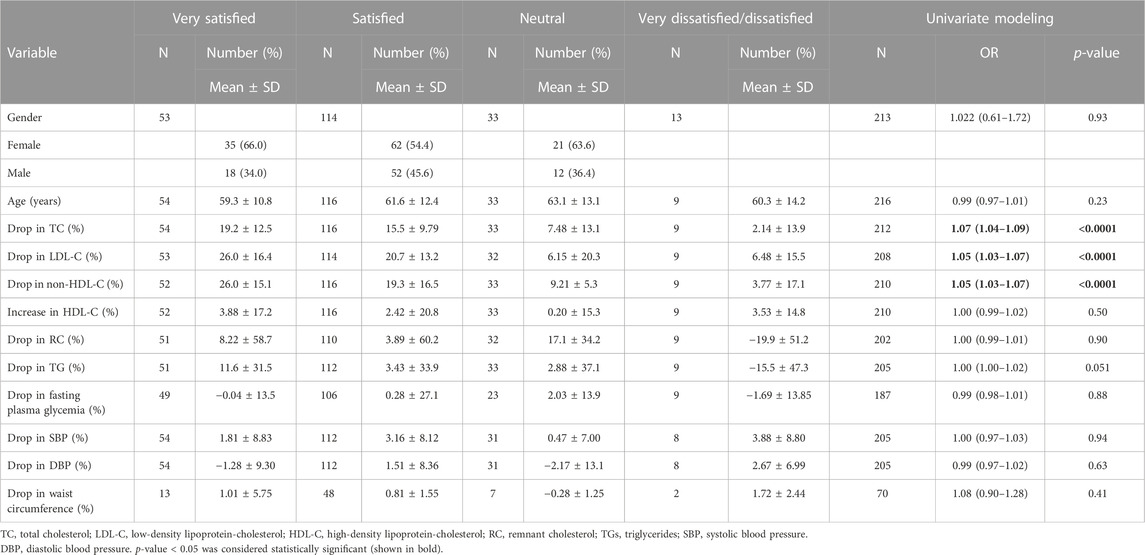
TABLE 4. Patient’s demographic characteristics and changes (%) in metabolic parameters and supplement satisfaction by ordinal logistic regression at univariate level (N = 221).
4 Discussion
The main objective of the present exploratory study conducted by GPs in patients (N = 221) suffering from hypercholesterolemia was to evaluate the efficacy and safety of a combined supplement of standardized dry extracts of amla fruit, walnut leaves, olive fruit, and RYR powder, taken as two tablets/day for a period of 2 months, in the absence of conventional LLD treatment (94.1%), and in a small number of patients (5.9%) with statins/another dietary supplement. Figure 3 summarizes the treatment outcomes for these patients. As regards lipid parameters, TC decreased by 15%, LDL-C by 20%, and non-HDL-C by 16% under real-life conditions. It was not accompanied by side effects usually associated with prescription statins (Gopa et al., 2012; Stroes et al., 2015; Dugani et al., 2016). Reductions of such magnitudes are clinically relevant, especially as they are achieved using a nutraceutical with a most satisfactory safety profile (Baigent et al., 2005). Next to the basic lipid profile, the study evaluated the effect of a nutraceutical on RC. At baseline, patients’ mean RC levels exceeded the upper limit of 30 mg/dL, which corresponds to an excess of atherogenic cholesterol unrelated to LDL-C (Varbo et al., 2014; Sandesara et al., 2019; Castañer et al., 2020; Ginsberg et al., 2021; Quispe et al., 2021; Doi et al., 2023). On treatment, the mean RC level decreased by 12.3%, putting it below the pathological threshold of 30 mg/dL. These results show that in addition to lowering the LDL-C cardiovascular risk, this nutraceutical also has a beneficial impact on lowering the residual risk associated with RC.
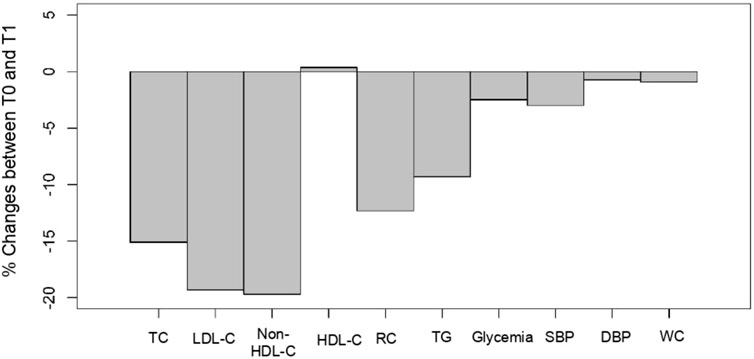
FIGURE 3. Cholesfytol NG® supplement effect. Mean change (%) in patients’ metabolic parameters between T0 and T1 (N = 221). TC, total cholesterol; LDL-C, low-density lipoprotein-cholesterol; HDL-C, high-density lipoprotein-cholesterol; RC, remnant cholesterol; TGs, triglycerides; SBP, systolic blood pressure; DBP, diastolic blood pressure; and WC, waist circumference.
The supplementation also resulted in a significant decrease of 3% in SBP and 1% in WC (Figure 3). There was also an improvement of 2.5% in patients’ plasma glycemia. In those patients who fully complied with the 2-months supplementation, a drop of 4% and 2% in SBP and DBP, respectively, was observed.
This supplementation may be envisaged to lower LDL-C and RC levels, while also avoiding the risk of side effects, on a long-term basis as an addition in primary prevention settings while also avoiding the risk of side effects, when the distance to LDL-C or distance to RC can be bridged and/or in patients who do not wish to take prescription statins over the long term when baseline cardiovascular risk is low (P et al., 2013). This nutraceutical could also be considered an add-on option for patients in primary or secondary cardiovascular prevention when they do not reach their LDL-C target despite receiving the best standards of care (including statins) and/or when experiencing SAMS on high-intensity statin therapy (Stroes et al., 2015).
The clinically significant reductions in patients’ lipid profile achieved with Cholesfytol NG® supplementation is the result of synergistic antihypercholesterolemic effects arising from the supplement component extracts (active chemical constituents), as reported in previous randomized clinical trials (RCTs). The treatment benefits of amla in hypercholesterolemia and atherosclerosis are well established in animal experiments (Rajak et al., 2004; Yokozawa et al., 2007; Variya et al., 2018) and human studies (Antony et al., 2008a; Antony et al., 2008b; Gopa et al., 2012; Usharani et al., 2013; Sinha et al., 2014; Upadya et al., 2019; Usharani et al., 2019) with excellent safety and tolerability profile. In a recent double-blind, placebo-controlled RCT involving patients with metabolic syndrome, a daily 1,000 mg amla fruit extract supplementation for 12 weeks led to a significant mean % change in LDL-C (−21.8%), TC (−11.11%), TG (−19.22%), and HDL-C (+22.16%) as compared to the placebo group (Usharani et al., 2019). In another study in dyslipidemic patients, the same dosage and duration of amla fruit extract supplementation resulted in a significant reduction in LDL-C, VLDL-C, TC, and TG levels in comparison to the placebo group (Upadya et al., 2019). In a study by Gopa et al., a 500 mg amla extract capsule/day intake for 42 days was non-inferior to 20 mg simvastatin in lowering LDL-C (Gopa et al., 2012). The possible mechanism of action of amla in regulating dyslipidemia is via PCSK9-inhibition, PPAR-α agonism (Variya et al., 2016; Variya et al., 2018), as well as its strong antioxidant effects (Antony et al., 2008a; Gul et al., 2022). As compared to the native LDL-C, oxidized LDL-C are considered more harmful to the arterial wall. Oxidized LDL-C are pro-oxidants and can lead to tissue damage and stimulate the development of atherosclerotic lesions (Navab et al., 1996). Overall, there is substantial preclinical and clinical evidence to suggest the antidyslipidemic pharmacological effect of the amla fruit extract.
Red yeast rice is also well known for its hypolipidemic effect, demonstrated by many RCTs (Journoud and Jones, 2004; Becker et al., 2009; Cicero et al., 2019; Wang et al., 2019; Cicero et al., 2021; Li et al., 2022). Currently, RYR is among the most widely utilized dietary supplements for the management of high cholesterol levels in Asian and European countries (Sahebkar et al., 2016). Many clinicians use RYR as a substitute treatment for their hyperlipidemic patients who are intolerant to statin, due to its excellent safety and tolerability profile (Fogacci et al., 2019). RYR has a comparable lipid-lowering activity to statins and lowers the cardiovascular disease risk in patients with statin intolerance or those who do not meet their goals after a single course of treatment with statins or ezetimibe. RYR has been suggested as a treatment option for lowering LDL-C levels and ASCVD risk for people with mild-to-moderate hypercholesterolemia who are ineligible for statin therapy, particularly those who are unable to implement lifestyle modifications, and also for those who are otherwise eligible for statin therapy but unwilling to take a pharmacologic therapy (Cicero et al., 2023). The antihyperlipidemic therapeutic effect of RYR is believed to be due to its principal active constituent monacolins, more specifically monacolin K (2.9 mg from Cholesfytol NG® supplementation). Monacolin K has the same chemical structure as Lovastatin (a type of statin) and is a competitive inhibitor of the cholesterol production pathway’s rate-limiting enzyme HMG-CoA reductase (HMGR) (Endo, 1980; Istvan and Deisenhofer, 2001; Pérez-Jiménez et al., 2018). In a recent meta-analysis study of 20 RCTs consisting of an overall patient population of 6,663, RYR supplementation for a period of 2 months to 2 years was associated with reduction in serum LDL-C levels by 1.02 mmol/L (∼39.4 mg/dL) (with 95% confidence) as compared to the placebo group, exhibiting substantial lipid-lowering efficacy comparable to the treatment effect of low-dose statins (e.g., 10 mg Simvastatin, 20 mg Lovastatin, and 40 mg Pravastatin) (Gerards et al., 2015). The study also reported a decrease in levels of TG, as well as an improvement in HDL-C levels. In another double-blind, placebo-controlled RCT, an RYR supplementation for 16 weeks led to a significant reduction in patients’ serum LDL-C (23.0%) and TC (15.5%) levels as compared to the placebo group (Bogsrud et al., 2010).
Walnut extracts have also been extensively studied for their antihyperlipidemic effect. A recent meta-analysis study of 26 RCTs has shown that supplementation of the walnut diet was associated with improvement in the blood lipid profile without having a negative impact on body weight or blood pressure (Guasch-Ferré et al., 2018). In another meta-analysis study of 13 RCTs by Banel et al., high-walnut-enriched diets significantly decreased LDL-C and TC (Banel and Hu, 2009). A large body of animal model studies also supports the antihyperlipidemic effect of walnut leaves extract supplementation, leading to a significant reduction in TC, LDL-C, VLDL-C very-low-density lipoprotein (VLDL) cholesterol, and TG levels and improvement in HDL-C levels (Mahmoodi et al., 2011; Jelodar et al., 2020; Azh et al., 2021).
The antidyslipidemic and/or cardioprotective properties of olive fruit extract have been extensively investigated in both animal and human studies and attributed to the strong antioxidative effects of its polyphenolic constituents, predominantly hydroxytyrosol or HXT (received as 10 mg from Cholesfytol NG® supplementation) and its derivatives (Raederstorff, 2009). Several RCTs have reported an improvement in the lipid profile and a decrease in oxidized LDL-C levels after an intake of olive diets rich in HXT (Marrugat et al., 2004; Covas et al., 2006; de la Torre-Carbot et al., 2010; Mateos et al., 2016; Fonollá et al., 2021; Cicero et al., 2022). As a matter of fact, the European Food and Safety Agency (EFSA) published a report in 2011 asserting that consuming 5 mg of HXT (or its derivatives) from olives on a daily basis protects LDL particles from oxidative damage (EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA), 2011). Hydroxytyrosol is listed on the US Food and Drug Administration (FDA) GRAS (Generally Recognized as Safe) status list (Nova, 2019), supporting its excellent safety profile.
Besides demonstrating the antihypercholesterolemic efficacy, this study also affirmed the excellent safety and tolerability of Cholesfytol NG® supplementation, thus avoiding the risk of side effects. Although statins are the most commonly used LLDs in clinical practice to treat hypercholesterolemia/LDL-C both in primary and secondary prevention of cardiovascular events, they can sometimes harm muscles, resulting in inflammation and discomfort. Impairment in memory, hyperglycemia, and liver injury are other negative consequences associated with statin therapy (Joseph et al., 2015). These complications pose additional health risks, especially for older patients like those in this study. In 2012, the FDA amended the safety profile of statins to include a warning about the possibility of elevated fasting plasma glucose and HbA1c levels (Feingold, 2021).
We acknowledge that our study has the inherent limitations of an observational, uncontrolled, open-label, and short-term design with a limited patient cohort. Since this was a clinical evaluation in a real-life setting, the GPs had complete discretion over which patients the supplementation was prescribed, and the Cholesfytol NG® supplement prescription was not based on a set of predetermined criteria. The absence of a control group makes it impossible to assess possible confounding effects, including lifestyle, over the observation period. Since patients remained in usual care over a relatively short study period and were not given instructions to reinforce lifestyle or change diet, such confounders are unlikely. The sample of mostly Caucasian patients who took the nutraceutical in a compliant manner may not necessarily be superimposable to some hypercholesterolemic patients followed by GPs or specialists. On the other hand, the study has the merits of having been carried out in real-life settings in hypercholesterolemic subjects with broad inclusion criteria, which also included a substantial number of patients intolerant to prescription statins, with an overall patient satisfactory compliance.
In conclusion, a nutraceutical combining dry extract of amla fruit, walnut leaves, olive fruit, and RYR powder significantly decreases LDL-C and RC levels in hypercholesterolemic patients in real-life setting. The supplementation bears excellent safety and tolerance and is rated as satisfactory, even among patients with statin intolerance.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
This study did not require ethics approval according to the Belgian law (7 May 2004 Art. 3 §2) concerning experiments on humans, as this was a retrospective observational study of patients’ history of treatment.
Author contributions
MH: methodology, writing–original draft, and writing–review and editing. YD: conceptualization, supervision, and writing–review and editing. IJ: conceptualization, data curation, and writing–review and editing. LS: formal analysis and writing–review and editing. AA: data curation and writing–review and editing. SA: writing–review and editing. MR: writing–review and editing. AK: writing–original draft and writing–review and editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article. This study was carried out in the absence of any external funding.
Acknowledgments
The authors thank all the GP surgeries who participated in this study and helped in the collection of the data.
Conflict of interest
Authors YD and IJ were employed by Laboratoire Tilman, Belgium.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akhtar, M. S., Ramzan, A., Ali, A., and Ahmad, M. (2011). Effect of Amla fruit (Emblica officinalis Gaertn.) on blood glucose and lipid profile of normal subjects and type 2 diabetic patients. Int. J. Food Sci. Nutr. 62 (6), 609–616. doi:10.3109/09637486.2011.560565
Antony, B., Benny, M., and Kaimal, T. N. B. (2008a). A Pilot clinical study to evaluate the effect of Emblica officinalis extract (Amlamax™) on markers of systemic inflammation and dyslipidemia. Indian J. Clin. Biochem. 23 (4), 378–381. doi:10.1007/s12291-008-0083-6
Antony, B., Merina, B., and Sheeba, V. (2008b). Amlamax in the management of dyslipidemia in humans. Indian J. Pharm. Sci. 70 (4), 504–507. doi:10.4103/0250-474x.44604
Authors/Task Force Members ESC Committee for Practice Guidelines (CPG) ESC National Cardiac Societies (2019). 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis 290, 140–205. doi:10.1016/j.atherosclerosis.2019.08.014
Azhar, R., Ali, S., Khan, H., Farooq, F., Noureen, F., and Anjum, A. F. (2021). Effect of ethanolic extract of walnut leaves on lipid profile and atherogenic index in hypercholesterolemic rats. Pak. J. Med. Health Sci. 15, 3955–3958. doi:10.53350/pjmhs2115123955
Baigent, C., Keech, A., Kearney, P. M., Blackwell, L., Buck, G., Pollicino, C., et al. (2005). Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 366 (9493), 1267–1278. doi:10.1016/s0140-6736(05)67394-1
Banel, D. K., and Hu, F. B. (2009). Effects of walnut consumption on blood lipids and other cardiovascular risk factors: a meta-analysis and systematic review. Am. J. Clin. Nutr. 90 (1), 56–63. doi:10.3945/ajcn.2009.27457
Barbagallo, C. M., Cefalù, A. B., Noto, D., and Averna, M. R. (2015). Role of nutraceuticals in hypolipidemic therapy. Front. Cardiovasc Med. 2, 22. doi:10.3389/fcvm.2015.00022
Becker, D. J., Gordon, R. Y., Halbert, S. C., French, B., Morris, P. B., and Rader, D. J. (2009). Red yeast rice for dyslipidemia in statin-intolerant patients: a randomized trial. Ann. Intern Med. 150 (12), 830–839. doi:10.7326/0003-4819-150-12-200906160-00006
Bogsrud, M. P., Ose, L., Langslet, G., Ottestad, I., Strøm, E. C., Hagve, T. A., et al. (2010). HypoCol (red yeast rice) lowers plasma cholesterol - a randomized placebo controlled study. Scand. Cardiovasc J. 44 (4), 197–200. doi:10.3109/14017431003624123
Bulotta, S., Celano, M., Lepore, S. M., Montalcini, T., Pujia, A., and Russo, D. (2014). Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 12, 219. doi:10.1186/s12967-014-0219-9
Castañer, O., Pintó, X., Subirana, I., Amor, A. J., Ros, E., Hernáez, Á., et al. (2020). Remnant cholesterol, not LDL cholesterol, is associated with incident cardiovascular disease. J. Am. Coll. Cardiol. 76 (23), 2712–2724. doi:10.1016/j.jacc.2020.10.008
Castelli, W. P., Garrison, R. J., Wilson, P. W. F., Abbott, R. D., Kalousdian, S., and Kannel, W. B. (1986). Incidence of coronary heart disease and lipoprotein cholesterol levels: the framingham study. JAMA 256 (20), 2835–2838. doi:10.1001/jama.1986.03380200073024
Chait, A., Ginsberg, H. N., Vaisar, T., Heinecke, J. W., Goldberg, I. J., and Bornfeldt, K. E. (2020). Remnants of the triglyceride-rich lipoproteins, diabetes, and cardiovascular disease. Diabetes 69 (4), 508–516. doi:10.2337/dbi19-0007
Choi, J. Y., and Na, J. O. (2019). Pharmacological strategies beyond statins: ezetimibe and PCSK9 inhibitors. J. Lipid Atheroscler. 8 (2), 183–191. doi:10.12997/jla.2019.8.2.183
Cicero, A. F. G., Colletti, A., Bajraktari, G., Descamps, O., Djuric, D. M., Ezhov, M., et al. (2017). Lipid lowering nutraceuticals in clinical practice: position paper from an International Lipid Expert Panel. Arch. Med. Sci. 13 (5), 965–1005. doi:10.5114/aoms.2017.69326
Cicero, A. F. G., Fogacci, F., and Banach, M. (2019). Red yeast rice for hypercholesterolemia. Methodist Debakey Cardiovasc J. 15 (3), 192–199. doi:10.14797/mdcj-15-3-192
Cicero, A. F. G., Fogacci, F., Di Micoli, A., Veronesi, M., Grandi, E., and Borghi, C. (2022). Hydroxytyrosol-rich olive extract for plasma cholesterol control. Appl. Sci. 12 (19), 10086. doi:10.3390/app121910086
Cicero, A. F. G., Fogacci, F., Stoian, A. P., and Toth, P. P. (2023). Red yeast rice for the improvement of lipid profiles in mild-to-moderate hypercholesterolemia: a narrative review. Nutrients 15 (10), 2288. doi:10.3390/nu15102288
Cicero, A. F. G., Fogacci, F., and Zambon, A. (2021). Red yeast rice for hypercholesterolemia: JACC focus seminar. J. Am. Coll. Cardiol. 77 (5), 620–628. doi:10.1016/j.jacc.2020.11.056
Collaboration, APCS, Barzi, F., Jamrozik, K., Lam, T. H., Ueshima, H., Whitlock, G., et al. (2004). Serum triglycerides as a risk factor for cardiovascular diseases in the Asia-Pacific region. Circulation 110 (17), 2678–2686. doi:10.1161/01.CIR.0000145615.33955.83
Covas, M.-I., Nyyssönen, K., Poulsen, H. E., Kaikkonen, J., Zunft, H.-J. F., Kiesewetter, H., et al. (2006). The effect of polyphenols in olive oil on heart disease risk factors: a randomized trial. Ann. Intern Med. 145 (5), 333–341. doi:10.7326/0003-4819-145-5-200609050-00006
de la Torre-Carbot, K., Chávez-Servín, J. L., Jaúregui, O., Castellote, A. I., Lamuela-Raventós, R. M., Nurmi, T., et al. (2010). Elevated circulating LDL phenol levels in men who consumed virgin rather than refined olive oil are associated with less oxidation of plasma LDL. J. Nutr. 140 (3), 501–508. doi:10.3945/jn.109.112912
Delaviz, H., Mohammadi, J., Ghalamfarsa, G., Mohammadi, B., and Farhadi, N. (2017). A review study on phytochemistry and pharmacology applications of Juglans regia plant. Pharmacogn. Rev. 11 (22), 145–152. doi:10.4103/phrev.phrev_10_17
Doi, T., Nordestgaard, B. G., and Langsted, A. (2023). Can remnant cholesterol (triglyceride-rich lipoproteins) reclassify estimated risk of atherosclerotic cardiovascular disease? Curr. Opin. Endocrinol. Diabetes Obes. 30 (2), 128–135. doi:10.1097/med.0000000000000799
Dugani, S. B., Akinkuolie, A. O., Paynter, N., Glynn, R. J., Ridker, P. M., and Mora, S. (2016). Association of lipoproteins, insulin resistance, and rosuvastatin with incident type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol. 1 (2), 136–145. doi:10.1001/jamacardio.2016.0096
Efentakis, P., Iliodromitis, E. K., Mikros, E., Papachristodoulou, A., Dagres, N., Skaltsounis, A. L., et al. (2015). Effects of the olive tree leaf constituents on myocardial oxidative damage and atherosclerosis. Planta Med. 81 (8), 648–654. doi:10.1055/s-0035-1546017
EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) (2011). Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), mainte: polyphenols in olive related health claims. EFSA J. 9 (4), 2033. doi:10.2903/j.efsa.2011.2033
Endo, A. (1980). Monacolin K, a new hypocholesterolemic agent that specifically inhibits 3-hydroxy-3-methylglutaryl coenzyme A reductase. J. Antibiotics 33 (3), 334–336. doi:10.7164/antibiotics.33.334
Feingold, K. R. (2021). Cholesterol lowering drugs. https://www.ncbi.nlm.nih.gov/books/NBK395573/(Accessed October 11, 2023).
Fogacci, F., Banach, M., Mikhailidis, D. P., Bruckert, E., Toth, P. P., Watts, G. F., et al. (2019). Safety of red yeast rice supplementation: a systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 143, 1–16. doi:10.1016/j.phrs.2019.02.028
Fonollá, J., Maldonado-Lobón, J. A., Luque, R., Rodríguez, C., Bañuelos, Ó., López-Larramendi, J. L., et al. (2021). Effects of a combination of extracts from olive fruit and almonds skin on oxidative and inflammation markers in hypercholesterolemic subjects: a randomized controlled trial. J. Med. Food 24 (5), 479–486. doi:10.1089/jmf.2020.0088
Gerards, M. C., Terlou, R. J., Yu, H., Koks, C. H., and Gerdes, V. E. (2015). Traditional Chinese lipid-lowering agent red yeast rice results in significant LDL reduction but safety is uncertain - a systematic review and meta-analysis. Atherosclerosis 240 (2), 415–423. doi:10.1016/j.atherosclerosis.2015.04.004
Ginsberg, H. N., Packard, C. J., Chapman, M. J., Borén, J., Aguilar-Salinas, C. A., Averna, M., et al. (2021). Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur. Heart J. 42 (47), 4791–4806. doi:10.1093/eurheartj/ehab551
Gopa, B., Bhatt, J., and Hemavathi, K. G. (2012). A comparative clinical study of hypolipidemic efficacy of Amla (Emblica officinalis) with 3-hydroxy-3-methylglutaryl-coenzyme-A reductase inhibitor simvastatin. Indian J. Pharmacol. 44 (2), 238–242. doi:10.4103/0253-7613.93857
Guasch-Ferré, M., Li, J., Hu, F. B., Salas-Salvadó, J., and Tobias, D. K. (2018). Effects of walnut consumption on blood lipids and other cardiovascular risk factors: an updated meta-analysis and systematic review of controlled trials. Am. J. Clin. Nutr. 108 (1), 174–187. doi:10.1093/ajcn/nqy091
Gul, M., Liu, Z.-W., Haq, I.-U., Rabail, R., Faheem, F., Walayat, N., et al. (2022). Functional and nutraceutical significance of amla (Phyllanthus emblica L.): a review. Antioxidants 11 (5), 816. doi:10.3390/antiox11050816
Gupta, A., Behl, T., and Panichayupakaranan, P. (2019). A review of phytochemistry and pharmacology profile of Juglans regia. Obes. Med. 16, 100142. doi:10.1016/j.obmed.2019.100142
Hashmi, M. A., Khan, A., Hanif, M., Farooq, U., and Perveen, S. (2015). Traditional uses, phytochemistry, and pharmacology of Olea europaea (olive). Evid. Based Complement. Altern. Med. 2015, 541591. doi:10.1155/2015/541591
Hermans, M. P., Lempereur, P., Salembier, J. P., Maes, N., Albert, A., Jansen, O., et al. (2020). Supplementation effect of a combination of olive (Olea europea L.) leaf and fruit extracts in the clinical management of hypertension and metabolic syndrome. Antioxidants (Basel) 9 (9), 872. doi:10.3390/antiox9090872
Hu, T., He, X. W., Jiang, J. G., and Xu, X. L. (2014). Hydroxytyrosol and its potential therapeutic effects. J. Agric. Food Chem. 62 (7), 1449–1455. doi:10.1021/jf405820v
Istvan, E. S., and Deisenhofer, J. (2001). Structural mechanism for statin inhibition of HMG-CoA reductase. Science 292 (5519), 1160–1164. doi:10.1126/science.1059344
Jelodar, G., Mohammadi, M., Akbari, A., and Nazifi, S. (2020). Cyclohexane extract of walnut leaves improves indices of oxidative stress, total homocysteine and lipids profiles in streptozotocin-induced diabetic rats. Physiol. Rep. 8 (1), e14348. doi:10.14814/phy2.14348
Joseph, J. P., Afonso, M., Berdaï, D., Salles, N., Bénard, A., Gay, B., et al. (2015). Benefits and risks for primary prevention with statins in the elderly. Presse Med. 44 (12), 1219–1225. doi:10.1016/j.lpm.2015.09.015
Journoud, M., and Jones, P. J. H. (2004). Red yeast rice: a new hypolipidemic drug. Life Sci. 74 (22), 2675–2683. doi:10.1016/j.lfs.2003.10.018
Keys, A. (1980). Seven countries: a multivariate analysis of death and coronary heart disease. Harvard University Press.
Lacoste, L., Lam, J. Y., Hung, J., Letchacovski, G., Solymoss, C. B., and Waters, D. (1995). Hyperlipidemia and coronary disease: correction of the increased thrombogenic potential with cholesterol reduction. Circulation 92 (11), 3172–3177. doi:10.1161/01.cir.92.11.3172
Li, P., Wang, Q., Chen, K., Zou, S., Shu, S., Lu, C., et al. (2022). Red yeast rice for hyperlipidemia: a meta-analysis of 15 high-quality randomized controlled trials. Front. Pharmacol. 12, 819482. doi:10.3389/fphar.2021.819482
Lin, Y.-L., Wang, T.-H., Lee, M.-H., and Su, N.-W. (2008). Biologically active components and nutraceuticals in the Monascus-fermented rice: a review. Appl. Microbiol. Biotechnol. 77, 965–973. doi:10.1007/s00253-007-1256-6
Mahmoodi, M., Eghbali, H., zijoud, S., Pour-Rashidi, A., Mohamadi, A., Borhani, M., et al. (2011). Study of the effects of walnut leaf on some blood biochemical parameters in hypercholesterolemic rats. Biochem. Anal. Biochem., 1. doi:10.4172/2161-1009.1000103
Marrugat, J., Covas, M.-I., Fitó, M., Schröder, H., Miró-Casas, E., Gimeno, E., et al. (2004). Effects of differing phenolic content in dietary olive oils on lipids and LDL oxidation-a randomized controlled trial. Eur. J. Nutr. 43 (3), 140–147. doi:10.1007/s00394-004-0452-8
Mateos, R., Martínez-López, S., Arévalo, G. B., Amigo-Benavent, M., Sarriá, B., and Bravo-Clemente, L. (2016). Hydroxytyrosol in functional hydroxytyrosol-enriched biscuits is highly bioavailable and decreases oxidised low density lipoprotein levels in humans. Food Chem. 205, 248–256. doi:10.1016/j.foodchem.2016.03.011
Navab, M., Berliner, J. A., Watson, A. D., Hama, S. Y., Territo, M. C., Lusis, A. J., et al. (1996). The yin and yang of oxidation in the development of the fatty streak. A review based on the 1994 george lyman duff memorial lecture. Arterioscler. Thromb. Vasc. Biol. 16 (7), 831–842. doi:10.1161/01.atv.16.7.831
Nordestgaard, B. G., and Varbo, A. (2014). Triglycerides and cardiovascular disease. Lancet 384 (9943), 626–635. doi:10.1016/s0140-6736(14)61177-6
Nova, M. (2019). GRAS notice (GRN) No. 876; office of food additive safety. https://www.fda.gov/media/134474/download (Accessed September 14, 2022).
Palmer, M. K., Nicholls, S. J., Lundman, P., Barter, P. J., and Karlson, B. W. (2013). Achievement of LDL-C goals depends on baseline LDL-C and choice and dose of statin: an analysis from the VOYAGER database. Eur. J. Prev. Cardiol. 20 (6), 1080–1087. doi:10.1177/2047487313489875
Pérez-Jiménez, F., Pascual, V., Meco, J. F., Pérez Martínez, P., Delgado Lista, J., Domenech, M., et al. (2018). Document of recommendations of the SEA 2018. Lifestyle in cardiovascular prevention. Clinica Investig. Arterioscler. 30 (6), 280–310. doi:10.1016/j.arteri.2018.06.005
Quispe, R., Martin, S. S., Michos, E. D., Lamba, I., Blumenthal, R. S., Saeed, A., et al. (2021). Remnant cholesterol predicts cardiovascular disease beyond LDL and ApoB: a primary prevention study. Eur. Heart J. 42 (42), 4324–4332. doi:10.1093/eurheartj/ehab432
Raederstorff, D. (2009). Antioxidant activity of olive polyphenols in humans: a review. Int. J. Vitam. Nutr. Res. 79 (3), 152–165. doi:10.1024/0300-9831.79.3.152
Rajak, S., Banerjee, S. K., Sood, S., Dinda, A. K., Gupta, Y. K., Gupta, S. K., et al. (2004). Emblica officinalis causes myocardial adaptation and protects against oxidative stress in ischemic-reperfusion injury in rats. Phytother. Res. 18 (1), 54–60. doi:10.1002/ptr.1367
Sahebkar, A., Serban, M. C., Gluba-Brzózka, A., Mikhailidis, D. P., Cicero, A. F., Rysz, J., et al. (2016). Lipid-modifying effects of nutraceuticals: an evidence-based approach. Nutrition 32 (11-12), 1179–1192. doi:10.1016/j.nut.2016.04.007
Sandesara, P. B., Virani, S. S., Fazio, S., and Shapiro, M. D. (2019). The forgotten lipids: triglycerides, remnant cholesterol, and atherosclerotic cardiovascular disease risk. Endocr. Rev. 40 (2), 537–557. doi:10.1210/er.2018-00184
Sarwar, N., Danesh, J., Eiriksdottir, G., Sigurdsson, G., Wareham, N., Bingham, S., et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. (2007) 115(4):450–458. doi:10.1161/CIRCULATIONAHA.106.637793
Shao, Q., Yang, Z., Wang, Y., Li, Q., Han, K., Liang, J., et al. (2022). Elevated remnant cholesterol is associated with adverse cardiovascular outcomes in patients with acute coronary syndrome. J. Atheroscler. Thromb. 29 (12), 1808–1822. doi:10.5551/jat.63397
Sharma, M., Sharma, M., and Sharma, M. (2022). A comprehensive review on ethnobotanical, medicinal and nutritional potential of walnut (Juglans regia L.). Proc. Indian Natl. Sci. Acad. 88 (4), 601–616. doi:10.1007/s43538-022-00119-9
Shimoda, H., Tanaka, J., Kikuchi, M., Fukuda, T., Ito, H., Hatano, T., et al. (2009). Effect of polyphenol-rich extract from walnut on diet-induced hypertriglyceridemia in mice via enhancement of fatty acid oxidation in the liver. J. Agric. Food Chem. 57 (5), 1786–1792. doi:10.1021/jf803441c
Sinha, R., Sharma, N., Advani, U., Dadheech, G., Kulshreshtha, S., and Parakh, R. (2014). Comparitive study of hypolipidemic effects of atorvastatin with emblica officinalis (amla) in patients of type II hyperlipidemia, 2799–2810.
Stamler, J. (1996). Is the relationship between serom cholesterol and risk of premature death from coronary heart disease continuous and graded? JAMA 276, 882–888.
Stroes, E. S., Thompson, P. D., Corsini, A., Vladutiu, G. D., Raal, F. J., Ray, K. K., et al. (2015). Statin-associated muscle symptoms: impact on statin therapy-European atherosclerosis society consensus Panel statement on assessment, aetiology and management. Eur. Heart J. 36 (17), 1012–1022. doi:10.1093/eurheartj/ehv043
Suganya, N., Bhakkiyalakshmi, E., Sarada, D. V. L., and Ramkumar, K. M. (2016). Reversibility of endothelial dysfunction in diabetes: role of polyphenols. Br. J. Nutr. 116 (2), 223–246. doi:10.1017/S0007114516001884
Tshongo Muhindo, C., Ahn, S. A., Rousseau, M. F., Dierckxsens, Y., and Hermans, M. P. (2017). Efficacy and safety of a combination of red yeast rice and olive extract in hypercholesterolemic patients with and without statin-associated myalgia. Complement. Ther. Med. 35, 140–144. doi:10.1016/j.ctim.2017.10.014
Upadya, H., Prabhu, S., Prasad, A., Subramanian, D., Gupta, S., and Goel, A. (2019). A randomized, double blind, placebo controlled, multicenter clinical trial to assess the efficacy and safety of Emblica officinalis extract in patients with dyslipidemia. BMC Complement. Altern. Med. 19 (1), 27. doi:10.1186/s12906-019-2430-y
Usharani, P., Fatima, N., and Muralidhar, N. (2013). Effects of Phyllanthus emblica extract on endothelial dysfunction and biomarkers of oxidative stress in patients with type 2 diabetes mellitus: a randomized, double-blind, controlled study. Diabetes Metab. Syndr. Obes. 6, 275–284. doi:10.2147/dmso.S46341
Usharani, P., Merugu, P. L., and Nutalapati, C. (2019). Evaluation of the effects of a standardized aqueous extract of Phyllanthus emblica fruits on endothelial dysfunction, oxidative stress, systemic inflammation and lipid profile in subjects with metabolic syndrome: a randomised, double blind, placebo controlled clinical study. BMC Complement. Altern. Med. 19 (1), 97. doi:10.1186/s12906-019-2509-5
Vanhoutte, P. M. (2009). Endothelial dysfunction - the first step toward coronary arteriosclerosis. Circ. J. 73 (4), 595–601. doi:10.1253/circj.CJ-08-1169
Varbo, A., Benn, M., and Nordestgaard, B. G. (2014). Remnant cholesterol as a cause of ischemic heart disease: evidence, definition, measurement, atherogenicity, high risk patients, and present and future treatment. Pharmacol. Ther. 141 (3), 358–367. doi:10.1016/j.pharmthera.2013.11.008
Variya, B. C., Bakrania, A. K., Chen, Y., Han, J., and Patel, S. S. (2018). Suppression of abdominal fat and anti-hyperlipidemic potential of Emblica officinalis: upregulation of PPARs and identification of active moiety. Biomed. Pharmacother. 108, 1274–1281. doi:10.1016/j.biopha.2018.09.158
Variya, B. C., Bakrania, A. K., and Patel, S. S. (2016). Emblica officinalis (Amla): a review for its phytochemistry, ethnomedicinal uses and medicinal potentials with respect to molecular mechanisms. Pharmacol. Res. 111, 180–200. doi:10.1016/j.phrs.2016.06.013
Verhoeven, V., Van der Auwera, A., Van Gaal, L., Remmen, R., Apers, S., Stalpaert, M., et al. (2015). Can red yeast rice and olive extract improve lipid profile and cardiovascular risk in metabolic syndrome? a double blind, placebo controlled randomized trial. BMC Complement. Altern. Med. 15, 52. doi:10.1186/s12906-015-0576-9
Visioli, F., Davalos, A., López de Las Hazas, M. C., Crespo, M. C., and Tomé-Carneiro, J. (2020). An overview of the pharmacology of olive oil and its active ingredients. Br. J. Pharmacol. 177 (6), 1316–1330. doi:10.1111/bph.14782
Wang, T. J., Lien, A. S., Chen, J. L., Lin, C. H., Yang, Y. S., and Yang, S. H. (2019). A randomized clinical efficacy trial of red yeast rice (Monascus pilosus) against hyperlipidemia. Am. J. Chin. Med. 47 (2), 323–335. doi:10.1142/s0192415x19500150
Yokozawa, T., Kim, H. Y., Kim, H. J., Okubo, T., Chu, D. C., and Juneja, L. R. (2007). Amla (Emblica officinalis Gaertn.)prevents dyslipidaemia and oxidative stress in the ageing process. Br. J. Nutr. 97 (6), 1187–1195. doi:10.1017/S0007114507691971
Zhao, Q., Zhang, T. Y., Cheng, Y. J., Ma, Y., Xu, Y. K., Yang, J. Q., et al. (2020). Prognostic impact of estimated remnant-like particle cholesterol in patients with differing glycometabolic status: an observational cohort study from China. Lipids Health Dis. 19 (1), 179. doi:10.1186/s12944-020-01355-y
Keywords: Cholesfytol NG®, hyperlipidemia, LDL-C, remnant cholesterol, amla, red yeast rice, olive, walnut
Citation: Hermans MP, Dierckxsens Y, Janssens I, Seidel L, Albert A, Ahn SA, Rousseau MF and Khan A (2023) The antihyperlipidemic effect of a combined supplement of standardized dry extracts of amla (Emblica officinalis), walnut (Juglans regia), olive (Olea europaea) and red yeast rice (Monascus purpureus) powder: Reduction in circulatory low-density lipoprotein-cholesterol (LDL-C) and remnant cholesterol (RC) levels in patients with hypercholesterolemia. Front. Pharmacol. 14:1280234. doi: 10.3389/fphar.2023.1280234
Received: 19 August 2023; Accepted: 18 October 2023;
Published: 27 November 2023.
Edited by:
Anthony Booker, University of Westminster, United KingdomReviewed by:
Federica Fogacci, University of Bologna, ItalyDiana Simona Antal, Victor Babes University of Medicine and Pharmacy, Romania
Copyright © 2023 Hermans, Dierckxsens, Janssens, Seidel, Albert, Ahn, Rousseau and Khan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amjad Khan, QW1qYWQua2hhbkByZG0ub3guYWMudWs=, QW1qYWRraGFuQGx1bWhzLmVkdS5waw==
 Michel P. Hermans1
Michel P. Hermans1 Laurence Seidel
Laurence Seidel Adelin Albert
Adelin Albert Amjad Khan
Amjad Khan