- 1General Practice, Shenzhen Hospital of Integrated Traditional Chinese and Western Medicine, Shenzhen, Guangdong, China
- 2Traditional Chinese Medicine, Nanfang Hospital, Southern Medical University, Guangdong, China
Background: Non-small cell lung cancer (NSCLC) poses a serious threat to human health. Several clinical studies have reported the benefits of Chinese herbal injections (CHIs) in combination with docetaxel and cisplatin (DP). This multidimensional network meta-analysis aimed to investigate the preferred regimen of CHIs in combination with DP for the treatment of NSCLC.
Methods: Multiple databases were searched to identify randomized controlled trials (RCTs) of CHIs for NSCLC from the database inception to 30 April 2023. Studies that met the inclusion criteria and exhibited good methodological quality were included. Data analysis was conducted using Stata 15.0 and R 4.2.1 software. An odds ratio (OR) was used as the effect size, and the surface under the cumulative ranking curve (SCURA) was employed to rank the evaluated treatments.
Results: The network meta-analysis included 85 eligible RCTs, encompassing 6,580 patients and 11 CHIs. Astragalus Injection combined with DP was identified as the most effective regimen for improving the response rate (SUCRAs: 90.25%). Brucea Javanica Oil Milk Injection combined with DP proved most effective in ameliorating the quality of life (SUCRAs: 76.89%). Shenfu Injection combined with DP emerged as the most effective for enhancing CD3+ and CD4+ (SUCRAs: 93.75%, 88.50%). Kanglaite Injection combined with DP exhibited the best efficacy in improving CD8+ (SUCRAs: 88.96%). Brucea Javanica Oil Milk Injection combined with DP was the most potent regimen for enhancing CD4+/CD8+ (SUCRAs: 93.13%).
Conclusion: CHIs in combination with DP outperformed DP alone in NSCLC patients. Astragalus Injection plus DP, Brucea Javanica Oil Milk Injection plus DP, Shenfu Injection plus DP, Kanglaite Injection plus DP, and Brucea Javanica Oil Milk Injection plus DP were significantly effective. However, further multicenter and well-designed RCTs are required to validate our findings.
1 Background
According to the most recent data from the World Health Organization’s International Agency for Research on Cancer (IARC), there were 19.29 million new cancer cases and 9.96 million cancer deaths in 2020. Among these, lung cancer is the predominant type, accounting for 11.6% of all cases, and it is the leading cause of cancer-related deaths, responsible for 18.4% of the total (Ettinger et al., 2013; Ferlay et al., 2015; Bray et al., 2018). Lung cancer has a poor prognosis, with a 5-year survival rate of only 16.8% (Ettinger et al., 2013; Ferlay et al., 2015; Bray et al., 2018). It can be categorized based on histology into non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) (Miller et al., 2018). NSCLC comprises roughly 85% of all lung cancer cases, and unfortunately, approximately 50% of NSCLC patients are diagnosed at an advanced stage (La Fleur et al., 2019). Early-stage NSCLC is typically treated with surgery-based comprehensive therapy, while intermediate- and late-stage NSCLC is commonly managed with a combination of radiotherapy, chemotherapy, targeted therapy, and immunotherapy. Although these treatments can be effective for some patients in the short term, they often come with a highly toxic physiological environment and a risk of adverse events (Pfister et al., 2004).
In China, the combination of Traditional Chinese Medicine (TCM) and chemotherapy has been widely employed in the treatment of cancer (Qi et al., 2010). Studies have reported the benefits of TCM as an adjuvant therapy for cancer, particularly in terms of slowing down disease progression and alleviating chemotherapy-induced complications and adverse events (Hofseth and Wargovich, 2007; Konkimalla and Efferth, 2008). Chinese Herbal Injections (CHIs), a significant component of Traditional Chinese Medicine (TCM), have seen increased utilization in the management of cancer, particularly in cases of non-small cell lung cancer (NSCLC). Commonly practiced combinations of CHIs with chemotherapy include CHIs combined with vinorelbine plus cisplatin (NP), CHIs combined with gemcitabine plus cisplatin (GP), CHIs combined with paclitaxel plus cisplatin (TP), CHIs combined with paclitaxel plus carboplatin (TC), CHIs combined with pemetrexed plus cisplatin (PP), and CHIs combined with docetaxel plus cisplatin (DP). Several clinical studies have demonstrated that the combination of CHIs with the above-mentioned chemotherapeutic regimens has yielded positive outcomes in the treatment of NSCLC. This approach can effectively regulate the body’s immune function, reduce the toxic side effects of chemotherapeutic drugs, enhance treatment efficacy, improve the prognosis of advanced NSCLC patients, enhance their quality of life (QoL), and extend their survival period (Zhihao, 2016; Yanlin et al., 2019; Guofu et al., 2020; Shaoguang, 2021; Shuping and Xiaobin, 2022; Xing et al., 2022).
Several network meta-analyses of CHIs with NP, GP, or TP have been conducted (Ni et al., 2020a; Ni et al., 2020b; Wang et al., 2021). Nevertheless, the optimal regimen for combining CHIs with DP in the treatment of NSCLC remains uncertain, which could pose challenges for clinicians in clinical practice. Direct comparisons among herbal injections are lacking. In contrast, network meta-analysis (NMA) enables the integration of comparisons made in clinical trials and concurrent interventions to assess their relative efficacy (Salanti et al., 2014). Therefore, this study systematically evaluates the effectiveness and safety of different CHIs combined with DP regimens for NSCLC through NMA. The aim is to provide evidence-based guidance for clinicians in selecting the most appropriate treatment strategy.
2 Methodology
The meta-analysis was conducted following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) and their specific requirements for Network Meta-Analysis (NMA). The study protocol was registered in the International Prospective Register of Systematic Reviews under the registration number CRD42023445523.
2.1 Search strategy
In this network meta-analysis (NMA), a comprehensive literature search was carried out in electronic databases, including Web of Science, Embase, PubMed, Cochrane Library, CNKI, Wanfang, CQVIP, and CBMDisc. There were no restrictions on publication year, language, or blinding method. The search period extended from the inception of each database up to 30 April 2023. To identify relevant publications, a combination of MeSH terms and free text search terms was applied in the search, with a focus on three main topics: 1) NSCLC, 2) injection, and 3) randomized controlled trials (RCTs). The specific search strategy used is detailed in Attachment 1. Additionally, the reference lists of published systematic reviews were manually examined to supplement the search and ensure its comprehensiveness.
2.2 Inclusion and exclusion criteria
The literature meeting the following criteria was included in this study: Study population: The study encompassed patients diagnosed with NSCLC through cytology or pathology, regardless of gender, age, race, region, or nationality. Patients with NSCLC who had other concurrent tumors were excluded. Interventions: The control group received DP chemotherapy alone, while the study group received a type of CHI combined with DP. Type of study: Only randomized controlled trials (RCTs) were considered. Outcome indicators: The study assessed the following outcome measures: a) Response rate: Response rate was evaluated according to the WHO criteria for assessing solid tumor efficacy. It was categorized into four tiers: Complete response (CR): The complete disappearance of the patient’s visible lesion within 1 month after treatment. Partial response (PR): A reduction of ≥50% in the tumor size of a single lesion or >50% reduction in the vertical diameter product of the two largest tumors in a multiloculated lesion. Stable disease (SD): Refers to no significant change in the patient’s disease for at least 4 weeks and <25% increase or <50% decrease in estimated tumor size. Progressive disease (PD): Involves ≥25% increase in the estimated size of a new or original lesion. In this study, the response rate was calculated as (CR + PR)/total number of patients × 100%. b) Quality of Life (QoL): QoL was assessed using the Karnofsky (Kahn) scale. An increase of ≥10 points in the Karnofsky score after the course of treatment compared to before treatment was considered an improvement, a decrease of ≥10 points indicated a decrease, and an increase or decrease of <10 points was classified as a stable condition. In this study, the improvement rate was calculated as the number of patients with improved QoL/total number of patients × 100%. c) Adverse effects: Adverse effects included leukopenia, hemoglobinopenia, and thrombocytopenia. These were classified into five levels according to the Acute and Subacute Toxicity Criteria for chemotherapeutic drugs developed by WHO: 0, I, II, III, and IV. The incidence of adverse effects was calculated as the number of patients with adverse effects/sample size × 100%. d) Immune cell indicators: Immune cell indicators included CD3+, CD4+, CD8+, and CD4+/CD8+.
The following types of literature were excluded from this study: 1) Animal or cellular experiments, case reports, research proposals, review articles, letters, editorials, and conference summaries; 2) Literature with missing research data or significant errors; 3) Duplicate publications; 4) Literature for which the full text was not available.
2.3 Data extraction
The search results were imported into EndNote, and a two-step screening process was employed. Two investigators independently screened the papers by referring to the inclusion and exclusion criteria based on the title and abstract. Subsequently, full texts were reviewed for a second screening. Any disagreements that arose were resolved through discussion or by seeking advice from a third investigator. Two investigators utilized Excel 2016 to independently extract data from the included literature. This information encompassed the first author, year of publication, country of origin, details on randomization and blinding, descriptions of the interventions and control groups, duration of the treatment, characteristics of the study population, and the outcome indicators. These details were organized in a table of baseline characteristics.
2.4 Quality assessment
The Cochrane Risk of Bias Assessment Tool (RoB2.0) (Higgins and Green, 2022) was utilized to evaluate the included studies in six domains: bias due to the randomization process, bias due to deviation from the defined interventions, bias due to missing outcome data, bias due to outcome measurement, bias due to selective reporting of outcomes, and other sources of bias. Two investigators conducted independent assessments for each of these six domains in every study, categorizing them as “low risk,” “high risk,” or “possible risk.” In cases where discrepancies arose, they were resolved through discussion or by seeking input from a third investigator. The results of the evaluations were presented in a risk-of-bias plot.
2.5 Statistical analysis
In the analysis, the response rate and Quality of Life (QoL) were presented as risk ratios (RR) with 95% confidence intervals (CI). The immune cell indicators (CD3+, CD4+, CD8+, CD4+/CD8+) were displayed as weighted mean differences (MD) with 95% CI. To account for heterogeneity between trials, a Bayesian hierarchical random-effects model was initially employed for comparing various treatment options for NSCLC (Dias et al., 2013; Mills et al., 2013). All calculations and graphical representations were generated using R 4.2.1 software and Stata 15.1 software. The Markov chain Monte Carlo (MCMC) simulation was carried out based on the likelihood function and prior assumptions using Bayesian inference with R 4.2.1 software. It involved 500,000 iterations and 20,000 annealing settings to explore the posterior distributions of the examined nodes (Dias et al., 2012; Bois, 2013; Hamra et al., 2013). The node splitting method was used to assess local inconsistency in outcomes with closed loops. A network graph was created to illustrate the relationships among the different treatments. Additionally, a comparison-adjusted funnel plot was utilized to assess potential publication bias (Chaimani et al., 2013; Whegang Youdom et al., 2017). Furthermore, surface under the cumulative ranking probability (SUCRA) values were employed to rank the evaluated treatments. SUCRA values range from 0 to 1, where a higher SUCRA value corresponds to a higher ranking for NSCLC compared to other treatments (Rücker and Schwarzer, 2015; Trinquart et al., 2016). A league table was generated to present the comparisons between each pair of interventions within each outcome.
3 Results
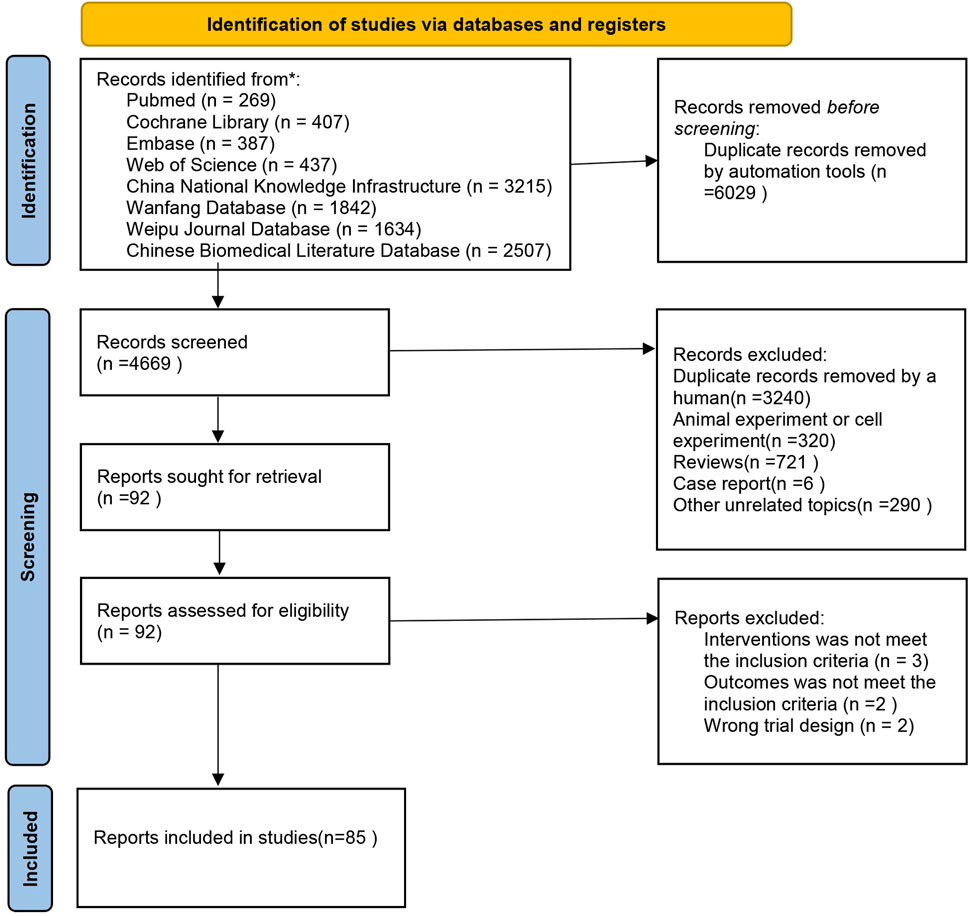
The initial search produced a total of 10,698 articles. After removing 6,029 duplicates, a further 4,669 articles were excluded following an initial review of titles and abstracts. The remaining literature underwent a comprehensive assessment based on the inclusion and exclusion criteria. Ultimately, 85 articles met the criteria for inclusion. You can refer to Figure 1 for a detailed overview of the screening process.
3.1 Inclusion studies and characteristics
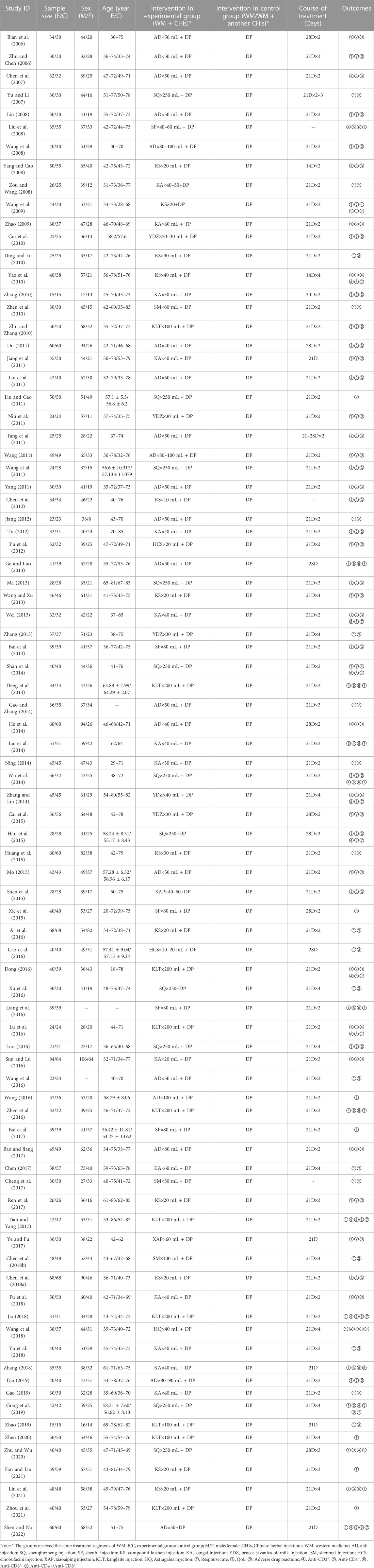
The 85 included studies were all from China and involved a total of 6,580 patients. Among these, 3,307 were in the experimental group, and 3,273 were in the control group. There were 11 CHIs involved, which included Aidi Injection plus DP (20 articles), Shenqi Fuzheng Injection plus DP (11 articles), Shenfu Injection plus DP (5 articles), Compound Kushen Injection plus DP (12 articles), Kangai Injection plus DP (14 articles), Brucea Javanica Oil Milk Injection plus DP (5 articles), Shenmai Injection plus DP (3 articles), Cinobufacini Injection plus DP (2 articles), Xiaoaiping Injection plus DP (2 articles), Kanglaite Injection plus DP (10 articles), and Astragalus Injection combined with DP (1 article). The basic characteristics of the included literature are presented in Table 1.
3.2 Methodological quality
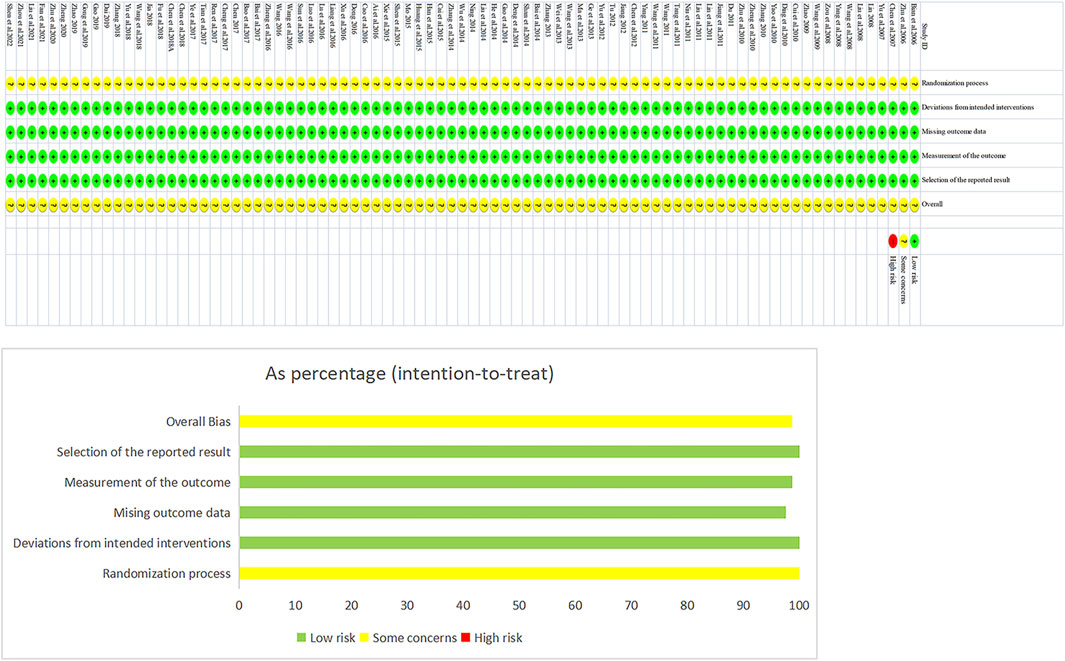
The risk-of-bias assessment results for the 85 included studies are shown in Figure 2. In terms of bias in randomization, all 85 studies were assessed as having a potential risk due to the lack of a description of randomization and allocation concealment. All studies were assessed as having a low risk of bias in terms of deviations from established interventions, missing data on outcomes, measurements, and selective reporting. No other sources of bias were found in any of the included studies, which were assessed to be at low risk. Taken together, the risk of bias in the included literature was low.
3.3 Network analysis results
3.3.1 Network diagram
The 85 included studies (Bian et al., 2006; Zhu and Chen, 2006; Chen et al., 2007; Yu and Li, 2007; Lin, 2008; Liu et al., 2008; Wang et al., 2008; Yang and Cao, 2008; Zou and Wang, 2008; Wang et al., 2009; Zhao, 2009; Cui et al., 2010; Ding and Lu, 2010; Yao et al., 2010; Zhang, 2010; Zhen et al., 2010; Zhu and Zhang, 2010; Du, 2011; Jiang et al., 2011; Lin et al., 2011; Liu and Gao, 2011; Niu et al., 2011; Tang et al., 2011; Wang, 2011; Wang et al., 2011; Yang, 2011; Chen et al., 2012; Jiang, 2012; Tu, 2012; Yu et al., 2012; Ge and Luo, 2013; Ma, 2013; Wang and Xu, 2013; Wei, 2013; Zhang, 2013; Bai et al., 2014; Shan et al., 2014; Deng et al., 2014; Gao and Zhang, 2014; He et al., 2014; Liu et al., 2014; Ning, 2014; Wu et al., 2014; Zhang and Liu, 2014; Cai et al., 2015; Han et al., 2015; Huang et al., 2015; Mo, 2015; Shen et al., 2015; Xie et al., 2015; Ai et al., 2016; Cao et al., 2016; Dong, 2016; Xu et al., 2016; Liang et al., 2016; Lu et al., 2016; Luo, 2016; Sun and Lu, 2016; Wang et al., 2016; Wang, 2016; Zhen et al., 2016; Bai et al., 2017; Bao and Jiang, 2017; Chen, 2017; Cheng et al., 2017; Ren et al., 2017; Tian and Yang, 2017; Ye and Fu, 2017; Chen et al., 2018b; Chen et al., 2018a; Fu et al., 2018; Jia, 2018; Wang et al., 2018; Yu et al., 2018; Zhang, 2018; Dai, 2019; Gao, 2019; Gong et al., 2019; Zhao, 2019; Zhen, 2020; Zhu and Wu, 2020; Fan and Liu, 2021; Liu et al., 2021; Zhou et al., 2021; Shen and Na, 2022) covered 11 different CHI interventions: Aidi Injection (20 RCTs), Astragalus Injection (1 RCT), Compound Kushen Injection (12 RCTs), Kangai Injection (14 RCTs), Kanglaite Injection (10 RCTs), Shenfu Injection (5 RCTs), Shenmai Injection (3 RCTs), Shenqi Fuzheng Injection (11 RCTs), Xiaoaiping Injection (2 RCTs), Brucea Javanica Oil Milk Injection (5 RCTs), and Cinobufacini Injection (2 RCTs). The network structure between these interventions is depicted in Figure 3. In the figure, the thickness of the lines corresponds to the volume of literature involved in the pairwise comparisons, and the size of the circles’ diameter is proportional to the number of participants included in each intervention.
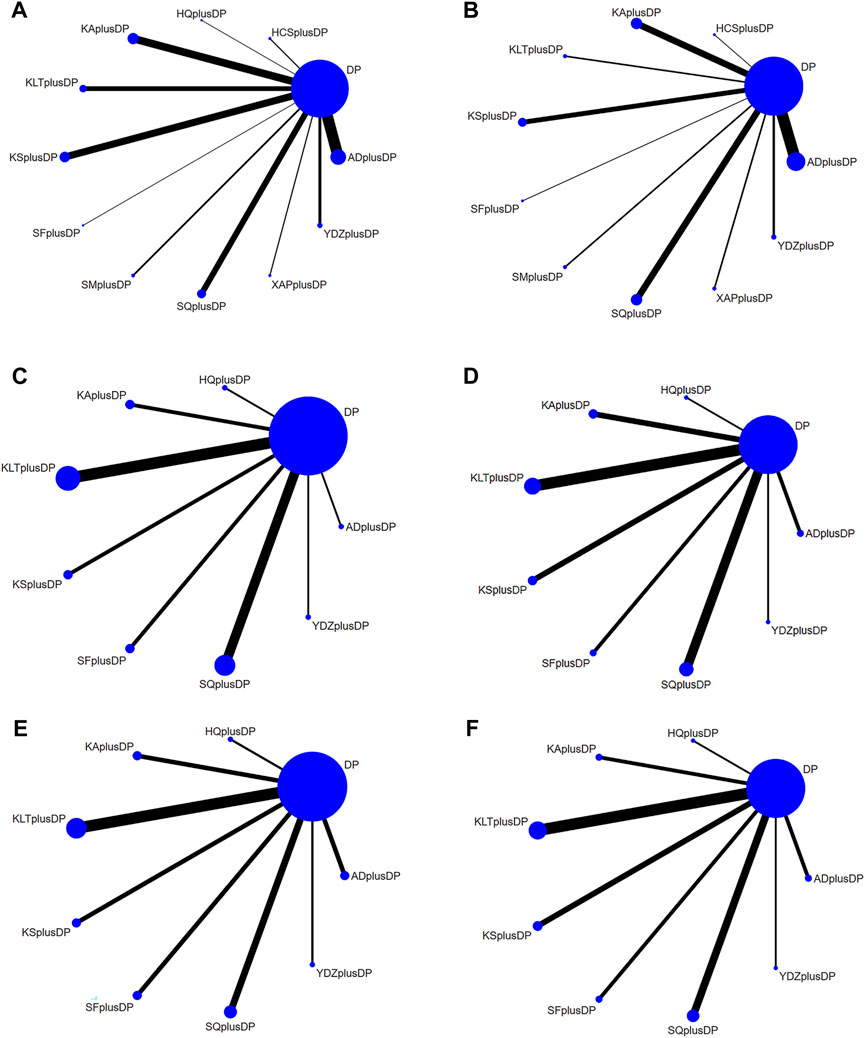
FIGURE 3. Network Diagram. (A) Response rate network structure; (B) QoL network structure; (C) CD3+ network structure; (D) CD4+ network structure; (E) CD8+ network structure; (F) CD4+/CD8+ network structure.
3.3.2 Response rate
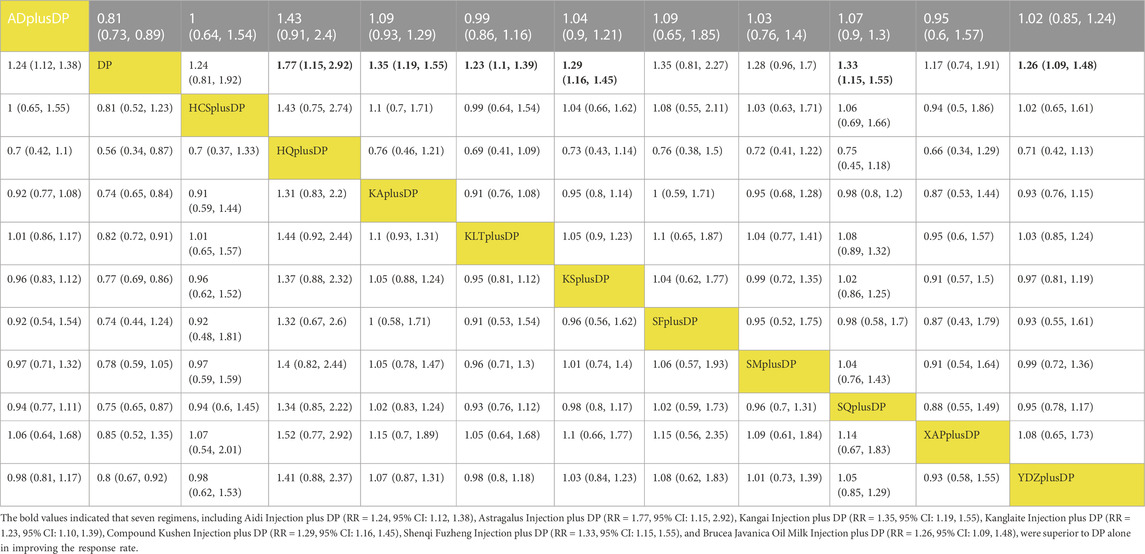
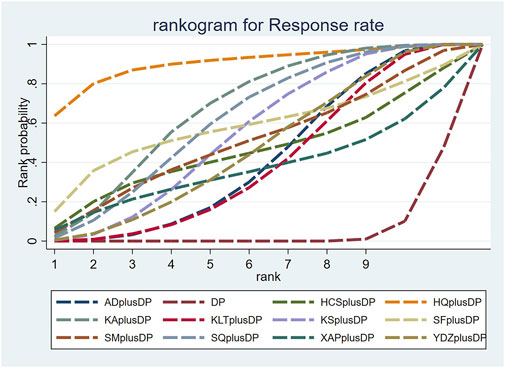
Seventy-six studies reported information on the response rate. The results indicated that seven regimens, including Aidi Injection plus DP (RR = 1.24, 95% CI: 1.12, 1.38), Astragalus Injection plus DP (RR = 1.77, 95% CI: 1.15, 2.92), Kangai Injection plus DP (RR = 1.35, 95% CI: 1.19, 1.55), Kanglaite Injection plus DP (RR = 1.23, 95% CI: 1.10, 1.39), Compound Kushen Injection plus DP (RR = 1.29, 95% CI: 1.16, 1.45), Shenqi Fuzheng Injection plus DP (RR = 1.33, 95% CI: 1.15, 1.55), and Brucea Javanica Oil Milk Injection plus DP (RR = 1.26, 95% CI: 1.09, 1.48), were superior to DP alone in improving the response rate, and the differences were statistically significant. Other interventions did not show significant differences in pairwise comparisons (see Table 2). According to the cumulative probability results, Astragalus Injection plus DP (SUCRAS: 90.25%), Kangai Injection plus DP (SUCRAS: 67.26%), and Shenqi Fuzheng Injection plus DP (SUCRAS: 61.90%) were likely to be the top three effective regimens in improving the response rate (see Figure 4).
3.3.3 QoL
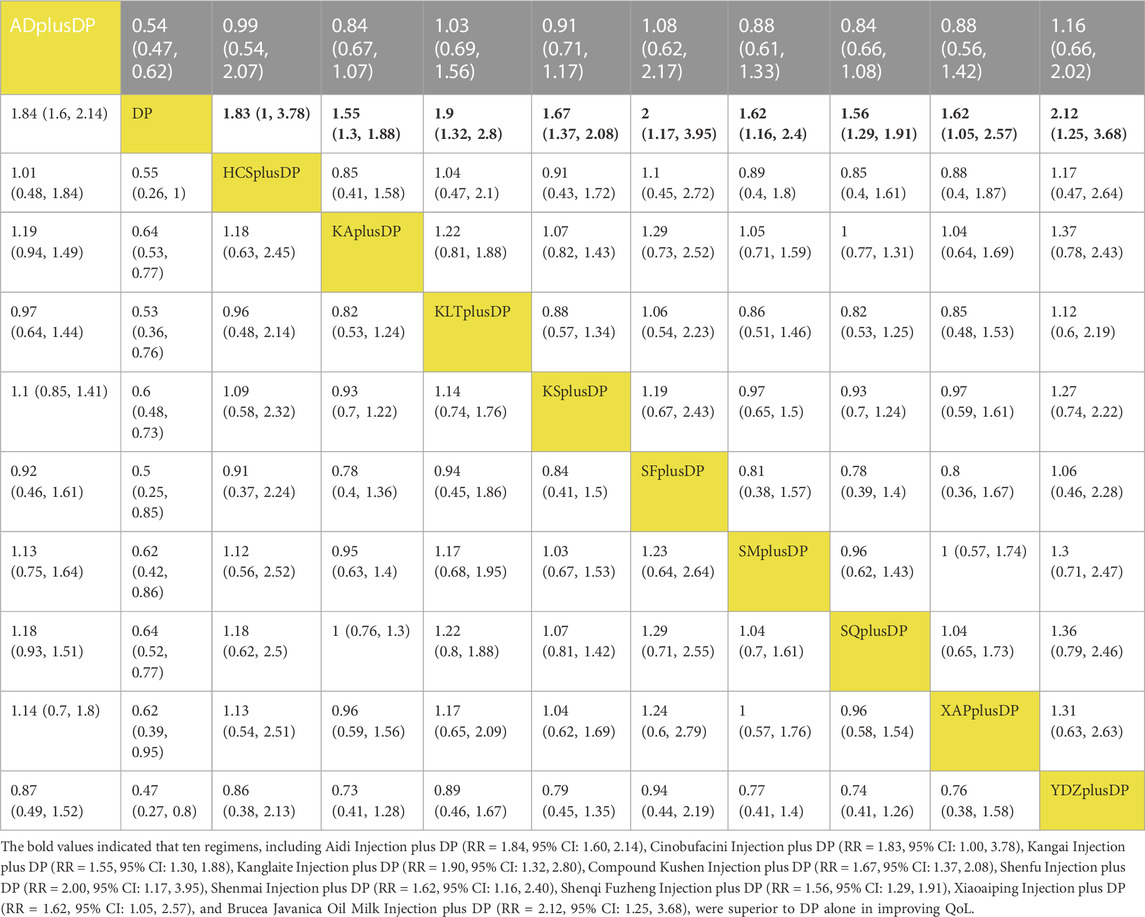
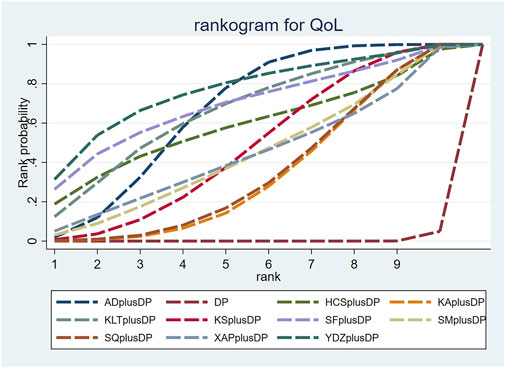
Forty-seven studies reported information on Quality of Life (QoL). The results indicated that ten regimens, including Aidi Injection plus DP (RR = 1.84, 95% CI: 1.60, 2.14), Cinobufacini Injection plus DP (RR = 1.83, 95% CI: 1.00, 3.78), Kangai Injection plus DP (RR = 1.55, 95% CI: 1.30, 1.88), Kanglaite Injection plus DP (RR = 1.90, 95% CI: 1.32, 2.80), Compound Kushen Injection plus DP (RR = 1.67, 95% CI: 1.37, 2.08), Shenfu Injection plus DP (RR = 2.00, 95% CI: 1.17, 3.95), Shenmai Injection plus DP (RR = 1.62, 95% CI: 1.16, 2.40), Shenqi Fuzheng Injection plus DP (RR = 1.56, 95% CI: 1.29, 1.91), Xiaoaiping Injection plus DP (RR = 1.62, 95% CI: 1.05, 2.57), and Brucea Javanica Oil Milk Injection plus DP (RR = 2.12, 95% CI: 1.25, 3.68), were superior to DP alone in improving QoL, and the differences were statistically significant. Other interventions did not show significant differences in pairwise comparisons (see Table 3). According to the cumulative probability results, Brucea Javanica Oil Milk Injection plus DP (SUCRAs: 76.89%), Shenfu Injection plus DP (SUCRAs: 69.49%), and Aidi Injection plus DP (SUCRAs: 66.94%) were likely to be the top three effective regimens in improving QoL (see Figure 5).
3.3.4 CD3+
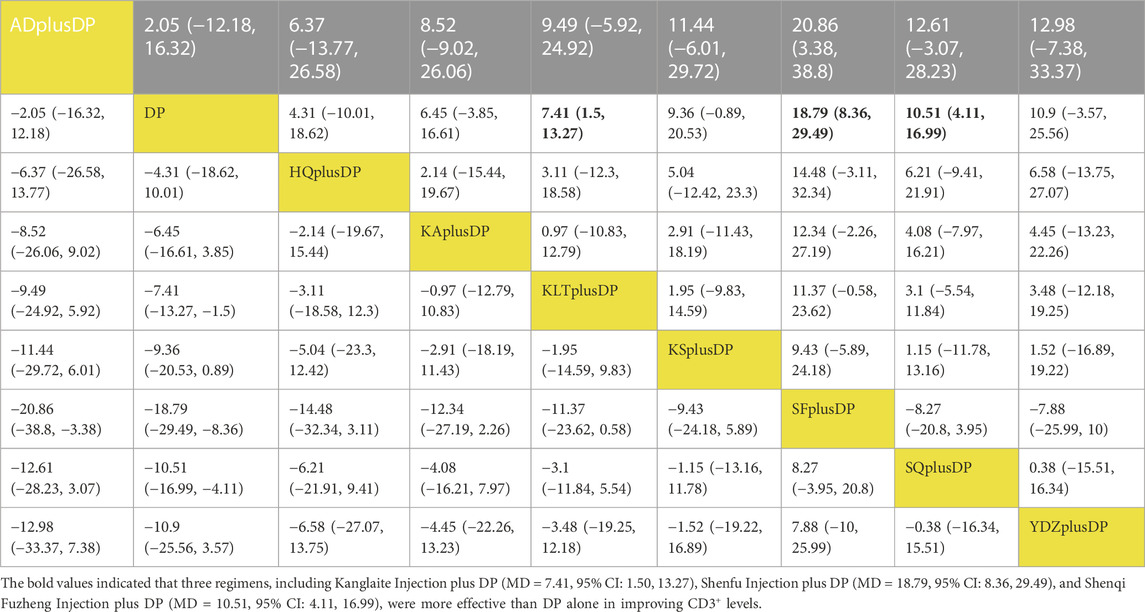
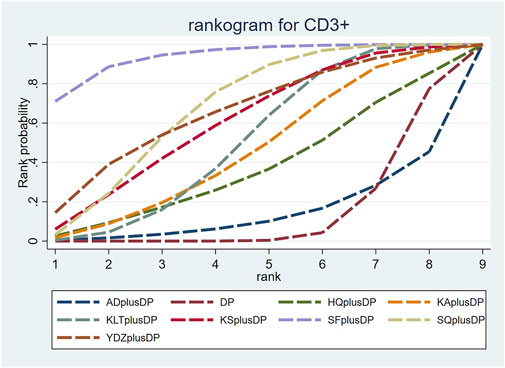
Twenty studies reported information on CD3+ levels. The results indicated that three regimens, including Kanglaite Injection plus DP (MD = 7.41, 95% CI: 1.50, 13.27), Shenfu Injection plus DP (MD = 18.79, 95% CI: 8.36, 29.49), and Shenqi Fuzheng Injection plus DP (MD = 10.51, 95% CI: 4.11, 16.99), were more effective than DP alone in improving CD3+ levels, and the differences were statistically significant. Shenfu Injection plus DP (MD = 20.86, 95% CI: 3.38, 38.80) was found to be more effective in improving CD3+ levels compared to Aidi Injection plus DP. Other interventions did not show significant differences in pairwise comparisons (see Table 4). According to the cumulative probability results, Shenfu Injection plus DP (SUCRAs: 93.75%), Shenqi Fuzheng Injection plus DP (SUCRAs: 67.81%), and Brucea Javanica Oil Milk Injection plus DP (SUCRAs: 65.69%) were likely to be the top three approaches for improving CD3+ levels (see Figure 6).
3.4.5 CD4+
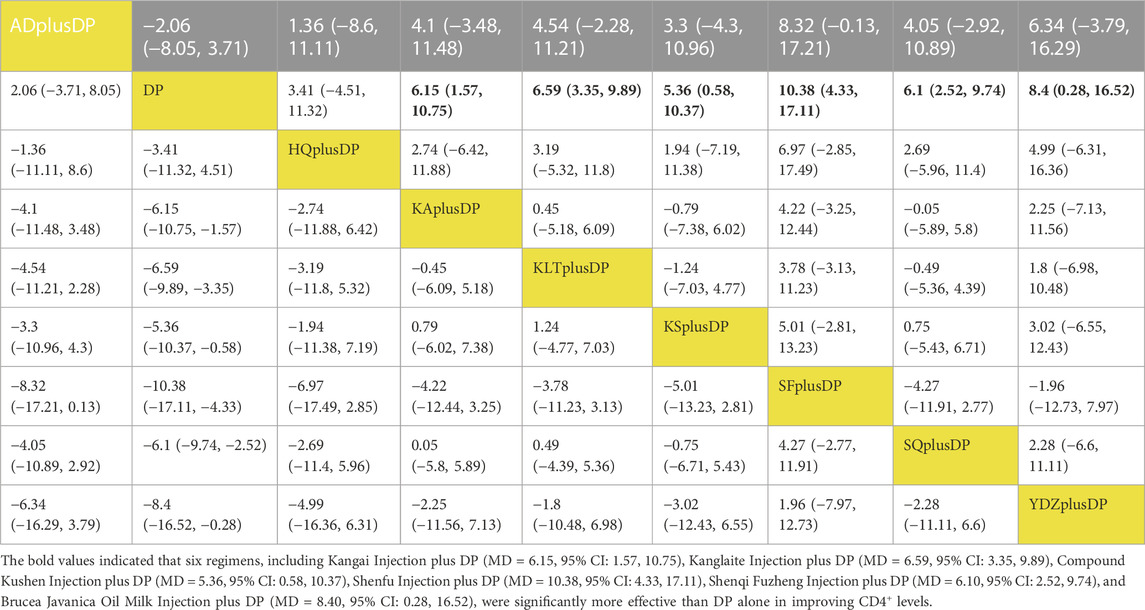
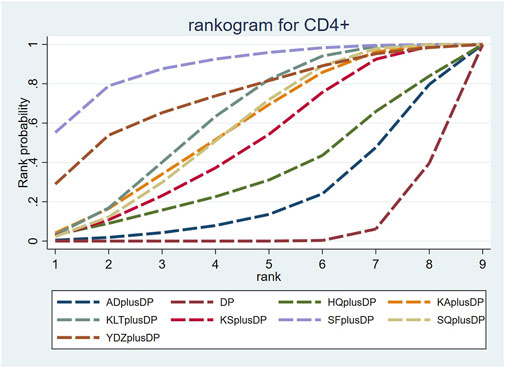
Twenty-three studies reported information on CD4+ levels. The results indicated that six regimens, including Kangai Injection plus DP (MD = 6.15, 95% CI: 1.57, 10.75), Kanglaite Injection plus DP (MD = 6.59, 95% CI: 3.35, 9.89), Compound Kushen Injection plus DP (MD = 5.36, 95% CI: 0.58, 10.37), Shenfu Injection plus DP (MD = 10.38, 95% CI: 4.33, 17.11), Shenqi Fuzheng Injection plus DP (MD = 6.10, 95% CI: 2.52, 9.74), and Brucea Javanica Oil Milk Injection plus DP (MD = 8.40, 95% CI: 0.28, 16.52), were significantly more effective than DP alone in improving CD4+ levels, and the differences were statistically significant. Other interventions did not show significant differences in pairwise comparisons (see Table 5). According to the cumulative probability results, Shenfu Injection plus DP (SUCRAs: 88.50%), Brucea Javanica Oil Milk Injection plus DP (SUCRAs: 73.28%), and Kanglaite Injection plus DP (SUCRAs: 62.35%) were likely to be the top three regimens in improving CD4+ levels (see Figure 7).
3.4.6 CD8+
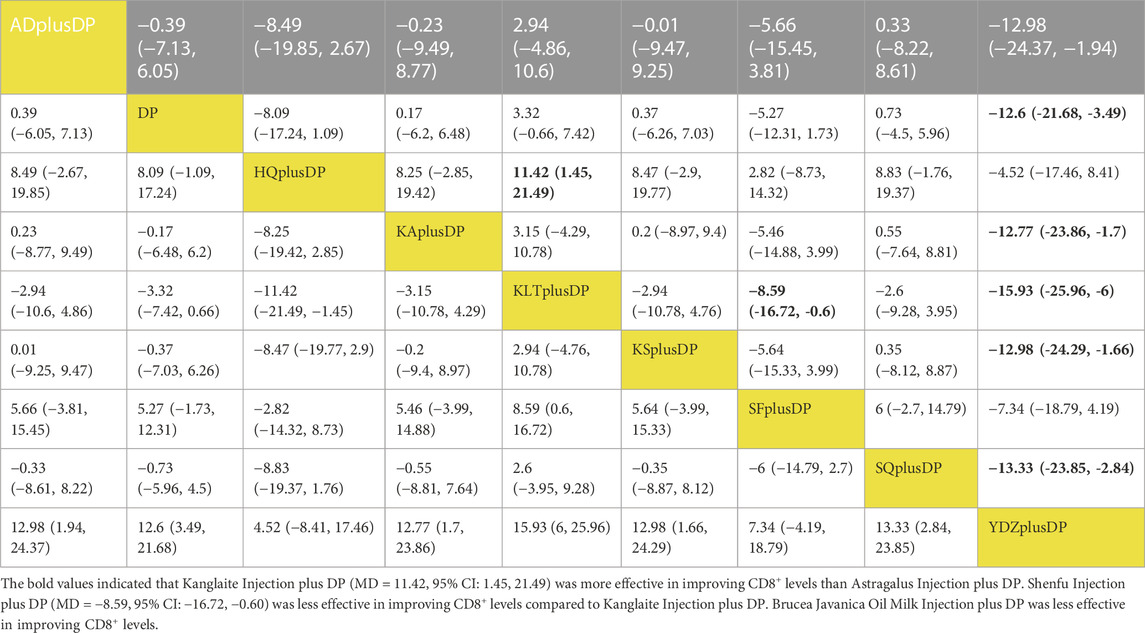
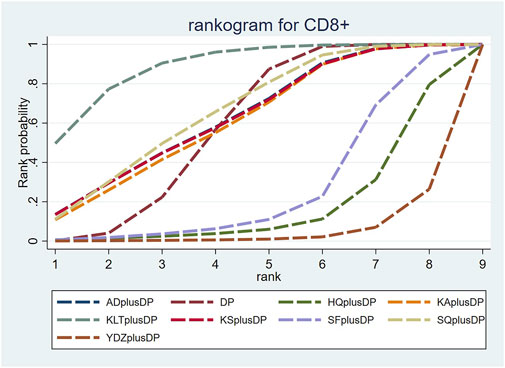
Eighteen studies reported information on CD8+ levels. The results indicated that Kanglaite Injection plus DP (MD = 11.42, 95% CI: 1.45, 21.49) was more effective in improving CD8+ levels than Astragalus Injection plus DP. Shenfu Injection plus DP (MD = −8.59, 95% CI: −16.72, −0.60) was less effective in improving CD8+ levels compared to Kanglaite Injection plus DP. Brucea Javanica Oil Milk Injection plus DP was less effective in improving CD8+ levels compared to Aidi Injection plus DP (MD = −12.98, 95% CI: −24.37, −1.94), DP alone (MD = −12.60, 95% CI: −21.68, −3.49), Kangai Injection plus DP (MD = −12.77, 95% CI: −23.86, −1.70), Kanglaite Injection plus DP (MD = −15.93, 95% CI: −25.96, −6.00), Compound Kushen Injection plus DP (MD = −12.98, 95% CI: −24.29, −1.66), and Shenqi Fuzheng Injection plus DP (MD = −13.33, 95% CI: −23.85, −2.84). Other interventions did not show significant differences in pairwise comparisons (see Table 6). According to the cumulative probability results, Kanglaite Injection plus DP (SUCRAs: 88.96%), Shenqi Fuzheng Injection plus DP (SUCRAs: 66.40%), and Aidi Injection plus DP (SUCRAs: 63.35%) were likely to be the top three measures in improving CD8+ levels (see Figure 8).
3.4.7 CD4+/CD8+
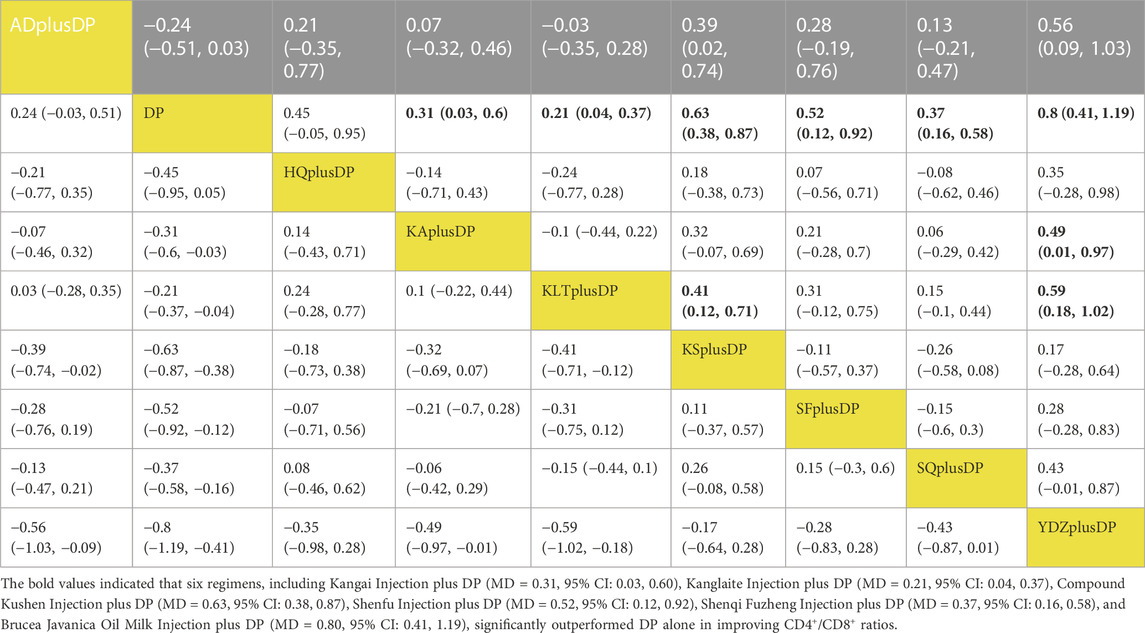
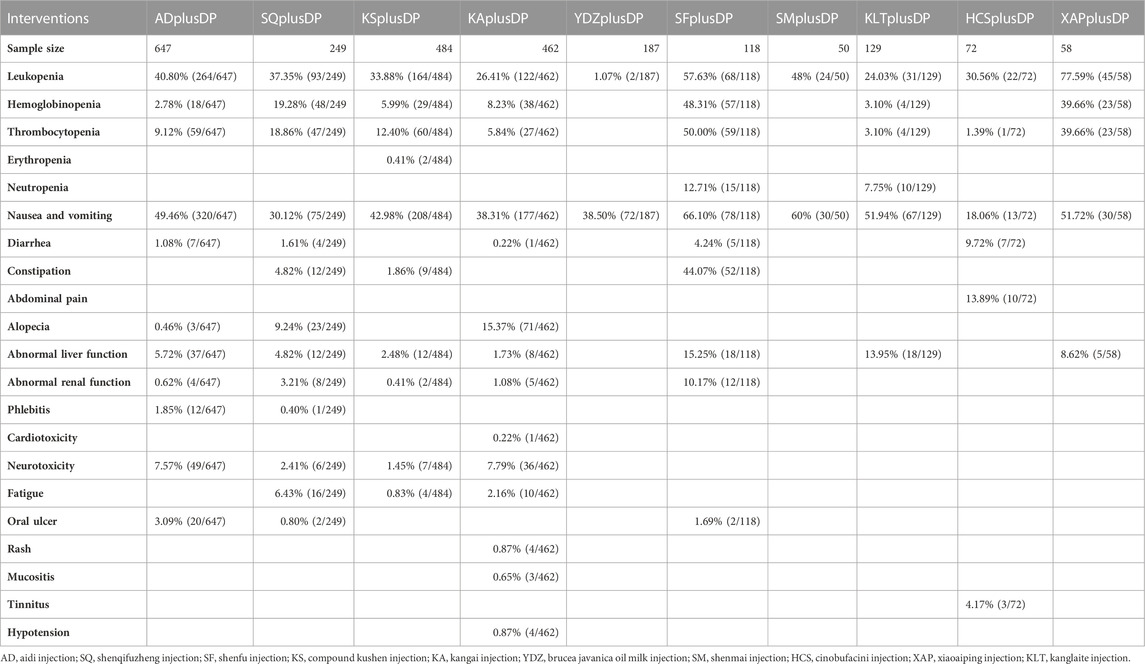
Twenty-one studies reported information on CD4+/CD8+ ratios. The results indicated that six regimens, including Kangai Injection plus DP (MD = 0.31, 95% CI: 0.03, 0.60), Kanglaite Injection plus DP (MD = 0.21, 95% CI: 0.04, 0.37), Compound Kushen Injection plus DP (MD = 0.63, 95% CI: 0.38, 0.87), Shenfu Injection plus DP (MD = 0.52, 95% CI: 0.12, 0.92), Shenqi Fuzheng Injection plus DP (MD = 0.37, 95% CI: 0.16, 0.58), and Brucea Javanica Oil Milk Injection plus DP (MD = 0.80, 95% CI: 0.41, 1.19), significantly outperformed DP alone in improving CD4+/CD8+ ratios, and the differences were statistically significant. Compound Kushen Injection plus DP (MD = 0.41, 95% CI: 0.12, 0.71) was more effective in improving CD4+/CD8+ ratios than Kanglaite Injection plus DP. In comparison to Aidi Injection plus DP (MD = 0.39, 95% CI: 0.02, 0.74), DP alone (MD = 0.80, 95% CI: 0.41, 1.19), Kangai Injection plus DP (MD = 0.49, 95% CI: 0.01, 0.97), and Kanglaite Injection plus DP (MD = 0.59, 95% CI: 0.18, 1.02), Brucea Javanica Oil Milk Injection plus DP was more effective in improving CD4+/CD8+ ratios. Other interventions did not show significant differences in pairwise comparisons (see Table 7). According to the cumulative probability results, Brucea Javanica Oil Milk Injection plus DP (SUCRAs: 93.13%), Compound Kushen Injection plus DP (SUCRAs: 81.25%), and Shenfu Injection plus DP (SUCRAs: 67.75%) were likely to be the top three measures in improving CD4+/CD8+ ratios (see Figure 9).
3.4.8 Adverse reactions
Adverse reactions included leukopenia, hemoglobinopenia, thrombocytopenia, erythropenia, neutropenia, nausea and vomiting, diarrhea, constipation, abdominal pain, alopecia, abnormal liver function, abnormal renal function, phlebitis, cardiotoxicity, neurotoxicity, fatigue, oral ulcer, rash, mucositis, tinnitus, and hypotension. Sixty-four randomized controlled trials recorded the above adverse effects. Overall, Xiaoaiping Injection plus DP had the highest incidence of leukopenia (77.59%); Shenfu Injection plus DP had the highest incidence of hemoglobinopenia (48.31%), thrombocytopenia (50.00%), neutropenia (12.71%), nausea and vomiting (66.10%), constipation (44.07%), abnormal liver function (15.25%), and abnormal renal function (10.17%); Cinobufacini Injection plus DP had the highest incidence of diarrhea (9.72%); Kangai Injection plus DP had the highest incidence of alopecia (15.37%) and neurotoxicity (7.79%); Aidi Injection plus DP had the highest incidence of phlebitis (1.85%) and oral ulcers (3.09%); Shenqi Fuzheng Injection plus DP had the highest incidence of fatigue (6.43%). In addition, the incidence of erythropenia (0.41%), abdominal pain (13.89%), cardiotoxicity (0.22%), rash (0.87%), mucositis (0.65%), tinnitus (4.17%), and hypotension (0.87%) was reported in only one study each, respectively. The results are presented in Table 8 below.
3.5 Cluster analysis
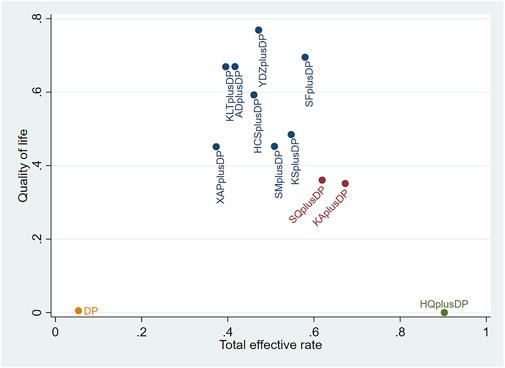
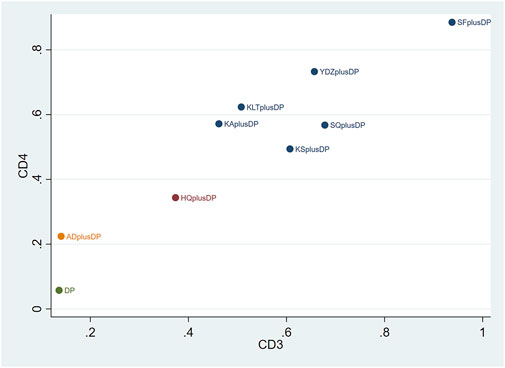
The two-dimensional clustering results showed that Astragalus Injection plus DP was the most effective in improving the response rate; Brucea Javanica Oil Milk Injection plus DP was the most effective in improving QoL, while DP alone ranked the worst overall in improving the response rate and QoL (see Figure 10). Shenfu Injection plus DP was the most effective in improving CD3+ and CD4+ levels, while DP alone ranked the worst overall in increasing CD3+ and CD4+ levels (see Figure 11). Additionally, Kanglaite Injection plus DP was the most effective in improving CD8+ levels; Brucea Javanica Oil Milk Injection plus DP was the most effective in improving CD4+/CD8+ ratios, with DP alone ranking the worst overall in improving CD8+ levels and CD4+/CD8+ ratios (see Figure 12).
3.6 Publication bias
Funnel plots were used to assess the publication bias of all outcome indicators. The funnel plots for the response rate and CD8+ were symmetrical on both sides, indicating no publication bias. However, the funnel plots for QoL, CD3+, CD4+, and CD4+/CD8+ were asymmetrical, suggesting potential publication bias (see Figure 13).
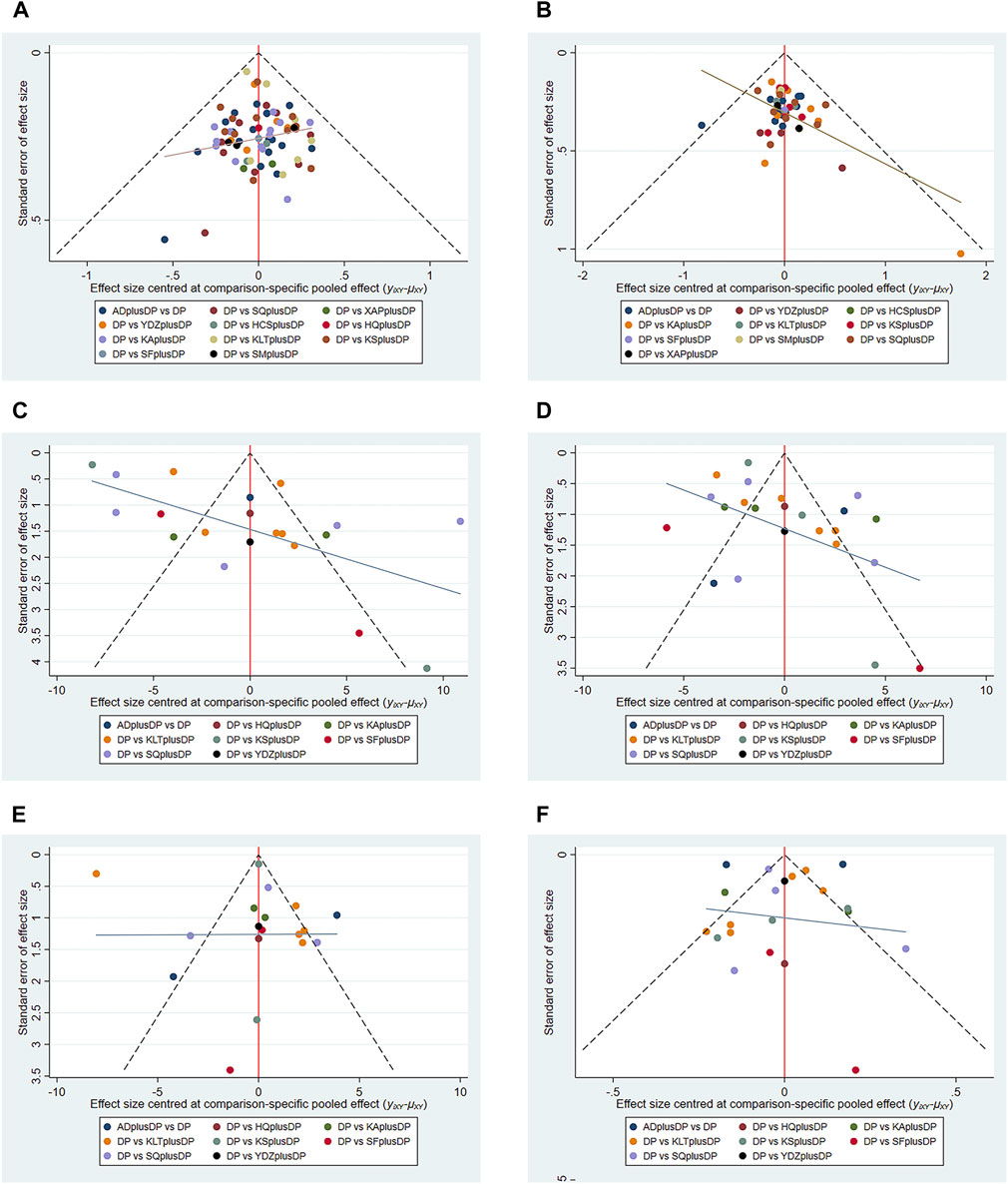
FIGURE 13. Funnel plot. (A) Response rate funnel plot; (B) QoL funnel plot; (C) CD3+ funnel plot; (D) CD4+ funnel plot; (E) CD8+ funnel plot; (F) CD4+/CD8+ funnel plot.
4 Discussion
To the best of our knowledge, this is the first Network Meta-Analysis (NMA) comparing the efficacy and safety of different CHIs in combination with DP for NSCLC. This NMA analyzed the latest data from 85 eligible RCTs. Our results indicate that Astragalus Injection plus DP was the most effective intervention in improving the response rate; Brucea Javanica Oil Milk Injection plus DP was the most effective intervention in enhancing QoL; Shenfu Injection plus DP was the most effective intervention in improving CD3+ and CD4+ levels; Kanglaite Injection plus DP was the most effective intervention in increasing CD8+ levels, and Brucea Javanica Oil Milk Injection plus DP was the most effective intervention in improving CD4+/CD8+ ratios.
In terms of improving RR, Astragalus Injection has a clear advantage. Astragalus Injection is composed of astragalus extract, whose main efficacy components include polysaccharides, saponins and flavonoids (Hui et al., 2002). Modern pharmacological studies show that astragalus polysaccharides can block the liver cancer cell cycle by up-regulating LC3B protein expression and down-regulating LC3A and P62 protein expression in anti-liver cancer cell proliferation experiments, further inducing mitochondrial apoptosis and promoting cell autophagy and apoptosis. Astragalus polysaccharides can inhibit the increase of autophagosomes in xanthine oxidase-induced lung cancer cells during LC3B and P62 protein expression. Astragalus polysaccharides can not only enhance human cell autophagy but also inhibit cancer cell autophagy (Fang and Lijiang, 2020). In addition, AS-IV in astragaloside (AS) can increase the ratio of pro-apoptotic protein (Bax) to anti-apoptotic protein B-cell lymphoma-2 (Bcl-2), upregulate the expression of caspase-3 and caspase-9 in the caspase family, and induce endogenous apoptosis in various types of cancers, including colorectal cancer, breast cancer, lung cancer, hepatocellular carcinoma, etc. (Suying et al., 2016; Lijun et al., 2017; Liwei et al., 2019; SUN et al., 2019; Fang and Lijiang, 2020; Xiang et al., 2020), which is concentration- and time-dependent. Astragalin can increase reduced spleen and thymus indexes caused by Lewis cells and regulate XBP1-mediated endoplasmic reticulum stress response to regulate immunity and inhibit tumor growth (Bing et al., 2019). The latest review shows that there are currently no pharmacokinetic studies on Astragalus Injection (Yixiang et al., 2023). Although it is found that Astragalus Injection is beyond compare in improving the response rate, there is only one article on it. Therefore, the ranking of its results shall be interpreted with caution. It is expected that there will be more RCTs on Astragalus Injection in the future.
Regarding the improvement of QoL and CD4+/CD8+ ratios, Brucea Javanica Oil Milk Injection demonstrated significant effectiveness. The primary medicinal component of Brucea Javanica Oil Milk Injection is Brucea Javanica Oil Milk (Liancui and Xuedong, 2013). A relevant pharmacological study has shown that Brucea Javanica oil emulsion acts as a non-specific anticancer drug affecting various phases of the cell cycle, leading to the killing and inhibition of tumor cells in G0, G1, S, G2, and M phases. It significantly inhibits the synthesis of DNA, RNA, and protein in tumor cells and interferes with the formation of peptide bonds (Peigeng, 2002). Additionally, Zhu Xiangliang et al. confirmed that Brucea Javanica oil inhibited the proliferation and migration of lung cancer A549 cells in a dose-dependent manner, induced the aggregation of green fluorescence of autophagy-related protein (LC)3, and promoted the transformation from LC 3-I to LC 3-II (Xiangliang and Pinhua, 2018). A human pharmacokinetic study showed that the estimated terminal elimination half-life (t1/2) of the concentration of oleic acid in human plasma (the index component in brucea javanica oil milk) was 12.14 ± 6.42 h, time to peak (Tmax) was 1.08 ± 0.19 h, peak concentration (Cmax) was 95.20 ± 29.10 mg L-1, and area under the curve (AUC0-12) was 370.89 ± 70.71 mg h L-1. The recommended clinical dose is 100 mL once daily (Yan, 2009).
For the enhancement of CD3+ and CD4+ levels, Shenfu Injection demonstrates significant advantages. Shenfu Injection is derived from the extract of red ginseng and aconite (black aconite tablet), with its main components being ginsenoside in red ginseng and aconite alkaloids in aconite (Mingyu et al., 2018). A relevant pharmacological study indicates that ginsenoside Rh1 in Shenfu Injection can significantly increase the spleen and thymus indices, enhance macrophage phagocytosis, and promote the proliferation of T lymphocytes in mice. Both ginsenoside Rg1 and Rh1 can stimulate the release of NO and improve macrophage phagocytosis (Yi et al., 2002; Caijun et al., 2009). Aconitum alkaloids in Shenfu Injection can directly inhibit or destroy lung cancer cells (Lew, 2019; Sehouli et al., 2019). Liu Qiang et al. also found that Shenfu Injection can enhance the immune function of patients by increasing the Treg ratio (Qiang et al., 2018). A pharmacokinetic study in rats showed that t1/2 was 8.685 min, terminal elimination rate constant (Ke) was 0.08 min-1, CL was 1.417 L min-1 kg-1, and AUC0-t was 12.63 mg L-1 min-1. The study results showed that the apparent pharmacokinetic process of the plasma concentration of Shenfu Injection injected intravenously into rats was fitted to a one-compartment model (Ting et al., 2012).
Kanglaite Injection clearly demonstrates its benefits in improving CD8+ levels. Kanglaite Injection is primarily composed of coix lachryma-jobi oil extracted from coix lachryma-jobi (Zhiyong and Xuhe, 2009). A related pharmacological study suggests that Kanglaite Injection can prevent tumor cells from entering the G0 and G phases by targeting the G2+M phase and inducing tumor cell apoptosis (Yin and Tingzhang, 2002). Zhang Aiqin et al. investigated the impact of Kanglaite Injection on anti-tumor and immune functions by measuring the activities of TNF-α, IL-1, and IL-6 in the peripheral blood of mice. The results indicated that Kanglaite Injection had a protective effect on the immune organs and immune function of the body while also eliminating tumor cells (Aiqin et al., 2007). A pharmacokinetic study in rats showed that in Kanglaite Injection 10 and 5 mL/kg groups, t1/2α(h) was 0.481 ± 0.168 and 0.322 ± 0.109 respectively; t1/2β(h) was 1.452 ± 0.776 and 1.384 ± 0.404 respectively; Cmax (mmol/L) was 8.532 ± 1.031 and 5.418 ± 0.764 respectively; AUC0-t (mmol/L•h) was 13.248 ± 3.692 and 5.339 ± 1.219 respectively; apparent volume of distribution (Vd) was (1.030 ± 0.131) and (0.756 ± 0.150) L2/(kg·mol) respectively; and CL was (0.838 ± 0.319) and (0.975 ± 0.330)L2/(kg·mol·h) respectively, wherein, t1/2α was 0.135 h and t1/2β was 15.84 h. The study results showed that the pharmacokinetic process of Kanglaite Injection injected intravenously in rats was fitted to a two-compartment open model (Fei et al., 2009).
4.1 Limitations
There are several unavoidable limitations to the current NMA. First, the relatively small number of partial intervention studies included in this NMA may have had some impact on the conclusions. Second, despite the inclusion of randomized controlled studies, some articles lacked blinding, which could have introduced bias. Third, due to limitations in the extracted data from the included studies, it was not possible to perform more detailed subgroup analyses, which may have influenced the final results. Therefore, it is recommended that RCTs be registered in advance to ensure transparency in the timeline and improve the methodological quality. Additionally, RCTs should be conducted in accordance with the latest clinical diagnostic and therapeutic guidelines. Furthermore, RCTs involving cancer patients should focus on long-term and clinically meaningful endpoints. Given the aforementioned limitations, more rigorous, high-quality RCTs are needed to confirm the efficacy of CHIs in combination with DP for the treatment of NSCLC patients.
5 Conclusion
In summary, the current evidence suggests that CHIs in combination with DP may offer more benefits to NSCLC patients compared to DP alone. Among the 11 interventions, Astragalus Injection plus DP, Brucea Javanica Oil Milk Injection plus DP, Shenfu Injection plus DP, and Kanglaite Injection plus DP appear to be the preferred treatment options for NSCLC. However, due to limitations in the number and quality of articles, further research is needed in the form of high-quality, large-scale, double-blind RCTs to confirm the findings of this NMA.
Author contributions
LW: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Writing–original draft, Writing–review and editing. LX: Conceptualization, Data curation, Methodology, Supervision, Writing–original draft, Writing–review and editing. FG: Software, Supervision, Validation, Writing–original draft. SZ: Resources, Software, Visualization, Writing–original draft. TX: Conceptualization, Formal Analysis, Software, Visualization, Writing–original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Research on the cell mechanotransduction mechanism of ultrafine granular powde of Huangqi regulating the biological behavior of lung cancer cells under stress loading through extracellular matrixintegrin aVβ3-cytoskeleton-nuclear skeleton (Project Number: 2023A1515012338) as funded by Traditional Chinese Medicine, Nanfang Hospital, Southern Medical University, Guangdong, China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ai, X., Ge, L., Adalaiti, Y., Tong, Y., ZhangJia, Z., and Zhao, B. (2016). Curative effect of Chinese medicine combined with chemotherapy on advanced non-small cell lung cancer. Liaoning J. Traditional Chin. Med. 43 (8), 1690–1691. doi:10.13192/j.issn.1000-1719.2016.08.044
Aiqin, Z., Shenglin, M., Zaidian, S., and Suzhen, B. (2007). Effect of klt injection on immunological function of rats with Lewis lung cancer. Zhejiang J. Integr. Traditional Chin. West. Med. 17 (4), 199–200. doi:10.3969/j.issn.1005-4561.2007.04.001
Bai, M., Mao, C., Liu, J., Sun, J., Liang, B., and Liang, X. (2017). Shenfu Injection alleviates toxic and side effects of chemotherapy in stage Ⅲ/Ⅳ non-small cell lung cancer. Clin. Res. tcm 9 (29), 29–32.
Bai, M., Wang, X., and Liang, B. (2014). Clinical observation of Shenfu injection combined with DP regimen in treatment of advanced non-small cell lung cancer. Mod. Oncol. 22 (01), 56–58.
Bao, Y., and Jiang, X. (2017). Summary of the experience of Aidi injection in chemotherapy for advanced non-small cell lung cancer. J. Aerosp. Med. 28 (07), 849–850.
Bian, M., Xing, H., Luo, Y., and Xiao, Y. (2006). Evaluation of Aidi injection combined with TP regimen in treatment of 34 cases of advanced non-small cell lung cancer. China Med. Guide (35), 98–99.
Bing, Y., Guihong, Y., Mingyu, L., Huimin, G., Yaping, C., Liang, F., et al. (2019). Mechanism of flavonoid components in Astragali Radix in inhibiting tumor growth and immunoregulation in C57BL/6 tumor bearing mice based on "invigorating Qi for consolidation of exterior. China J. Chin. Materia Medica 44 (23), 5184–5190. doi:10.19540/j.cnki.cjcmm.20191104.401
Bois, F. Y. (2013). Bayesian inference. Methods Mol. Biol. 930, 597–636. doi:10.1007/978-1-62703-059-5_25
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68 (6), 394–424. doi:10.3322/caac.21492
Cai, J., Lv, J., and Liu, H. (2015). Therapeutic effect of brucea javanica oil emulsion injection combined with chemotherapy on advanced non-small cell lung cancer. Chin. J. Clin. Ration. Drug Use (11), 52–53. doi:10.15887/j.cnki.13-1389/r.2015.11.030
Caijun, Z., Min, G., Bo, L., Wenjun, H., and Lin, W. (2009). Immunoregulating effects of ginsenoside Rh1 on immunosuppressive mice model. J. Kunming Med. Univ. 30 (11), 51. doi:10.3969/j.issn.1003-4706.2009.11.012
Cao, J., Zhou, J., Yang, D., and Chu, J. (2016). Effect analysis of cinbufosin injection combined with first-line chemotherapy in the treatment of advanced non-small cell lung cancer. Int. J. Oncol. 43 (10), 741–743. doi:10.3760/cma.j.issn.1673-422X.2016.10.005
Chaimani, A., Higgins, J. P., Mavridis, D., Spyridonos, P., and Salanti, G. (2013). Graphical tools for network meta-analysis in Stata. PLoS One 8 (10), e76654. doi:10.1371/journal.pone.0076654
Chen, J., Hu, Z., Yan, L., Chen, Y., and Jin, J. (2018a). Effect of Shenmai injection combined with chemotherapy on quality of life of advanced non-small cell lung cancer. Shaanxi Tradit. Chin. Med. 39 (03), 298–300.
Chen, H., Zhao, L., Luo, T., Shao, H., Zhang, Z., and Li, S. (2012). Treatment of 34 cases of non-small cell lung cancer with compound matrine injection combined with TP regimen. Chin. Tradit. Med. Mod. distance Educ. 10 (10), 148–149.
Chen, X., Gong, H., and Song, Z. (2007). Clinical study of Aidi injection combined with TP regimen in the treatment of advanced non-small cell lung cancer. Chin. J. Cancer Prev. (18), 1427–1428. doi:10.16073/j.cnki.cjcpt.2007.18.020
Chen, Y., Deng, Y., Liao, Y., and Fu, J. (2018b). Effect of compound matrine injection combined with DP regimen on serum VEGF levels in patients with advanced non-small cell lung cancer. J. Mod. Oncol. 26 (17), 2699–2702. doi:10.3969/j.issn.1672-4992.2018.17.012
Chen, Z. (2017). Clinical value of Kangai combined with chemotherapy in the treatment of advanced non-small cell lung cancer. Wisdom health 3 (07), 114–115. doi:10.19335/j.cnki.2096-1219.2017.07.53
Cheng, Q., Chen, Z., Tang, H., and Chen, F. (2017). Clinical observation of Shenmai Injection combined with DP regimen in the treatment of advanced NSCLC with Qi-Yin deficiency. China Health Care & Nutr. 27 (34), 82–83. doi:10.3969/j.issn.1004-7484.2017.34.108
Cui, H., Niu, Q., Guan, J., Wang, F., and Wang, Y. (2010). Therapeutic effect of brucea javanica oil emulsion injection combined with chemotherapy on advanced non-small cell lung cancer. SICHUAN Med. J. 31 (5), 590–591. doi:10.3969/j.issn.1004-0501.2010.05.016
Dai, L. (2019). Clinical observation and nursing care of Aidi injection combined with chemotherapy in treatment of advanced non-small cell lung cancer. Chin. Tradit. Med. Mod. distance Educ. 17 (19).
Deng, X., Zhao, S., Zhao, H., Yan, Y., Jhe, L., and Zhang, H. (2014). Effect of Conlett injection on immune function in patients with advanced non-small cell lung cancer treated with chemotherapy. Mod. J. Integr. Traditional Chin. West. Med. (34), 3767–3773. doi:10.3969/j.issn.1008-8849.2014.34.003
Dias, S., Sutton, A. J., Welton, N. J., and Ades, A. E. (2012). “Nice decision support unit technical support documents,” in Heterogeneity: subgroups, meta-regression, bias and bias-adjustment (London: National Institute for Health and Care Excellence NICE).
Dias, S., Welton, N. J., Sutton, A. J., Caldwell, D. M., Lu, G., and Ades, A. E. (2013). Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med. Decis. Mak. 33 (5), 641–656. doi:10.1177/0272989x12455847
Ding, J., and Lu, B. (2010). Effect of compound matrine injection combined with docetaxel and cisplatin in the treatment of non-small cell lung cancer. Chin. Community Physician (Medical Spec.) 12 (36), 141.
Dong, Z. (2016). Clinical study of Kanglaite injection combined with chemotherapy in treatment of advanced non-small cell lung cancer. Master, Shandong Traditional Chinese Medicine University.
Du, Z. (2011). Clinical effect of Aidi injection combined with chemotherapy in the treatment of advanced non-small cell lung cancer. Contemp. Med. 17 (1).
Ettinger, D. S., Akerley, W., Borghaei, H., Chang, A. C., Cheney, R. T., Chirieac, L. R., et al. (2013). Non-small cell lung cancer, version 2.2013. J. Natl. Compr. Canc Netw. 11 (6), 645–653; quiz 653. doi:10.6004/jnccn.2013.0084
Fan, X., and Liu, S. (2021). Effects of compound matrine injection combined with chemotherapy on clinical efficacy and adverse reactions in patients with advanced non-small cell lung cancer. Chin. health care · Acad. Ed. 39 (7).
Fang, D., and Lijiang, D. (2020). Mechanism of Astragalus polysaccharide in inhibiting proliferation of human hepatocellular carcinoma cells. West China J. Pharm. Sci. 35 (4), 402–406. doi:10.13375/j.cnki.wcjps.2020.04.012
Fei, Y., Jing, G., Yong, Z., and Cangxiao, L. (2009). Pharmacokinetics of Kanglaite Injection by intravenous administration in rats. Drug Eval. Res. 32 (02), 92–95.
Ferlay, J., Soerjomataram, I., Dikshit, R., Eser, S., Mathers, C., Rebelo, M., et al. (2015). Cancer incidence and mortality worldwide: sources, methods and major patterns in globocan 2012. Int. J. Cancer 136 (5), E359–E386. doi:10.1002/ijc.29210
Fu, H., Feng, X., Peng, W., and Rao, Z. (2018). Clinical study of Kangai injection combined with chemotherapy in the treatment of advanced non-small cell lung cancer. Med. Inf. 31 (24), 115–117. doi:10.3969/j.issn.1006-1959.2018.24.033
Gao, E., and Zhang, F. (2014). Application value of Aidi injection in patients with advanced lung cancer and its influence on quality of life. Chin. Contemp. Med. 21 (25), 107–109.
Gao, Y. (2019). Efficacy and safety analysis of DP chemotherapy combined with Kangai injection in the treatment of advanced non-small cell lung cancer. Chin. Med. Guide 17 (28).
Ge, C., and Luo, Z. (2013). Clinical observation of Aidi injection combined with DP regimen in treatment of 41 cases of non-small cell lung cancer. Guid. J. Traditional Chin. Med. Pharmacol. (10), 47–48. doi:10.3969/j.issn.1672-951X.2013.10.019
Gong, Y. a., Shu, C., Wang, H., and Han, Q. (2019). Clinical observation of Shenqi Fuzheng Injection combined with TP regimen in treatment of non-small cell lung cancer. China Pharm. 28 (22), 46–49. doi:10.3969/j.issn.1006-4931.2019.22.017
Guofu, M., Guohua, N., Min, L., and Mingyue, F. (2020). A study on the clinical efficacy of Aidi injection combined with pp regimen in the treatment of middle and advanced non-small cell lung cancer. J. Hubei Univ. Sci. Technol. Sci. 34 (3), 222–223. doi:10.16751/j.cnki.2095-4646.2020.03.0222
Hamra, G., MacLehose, R., and Richardson, D. (2013). Markov chain Monte Carlo: an introduction for epidemiologists. Int. J. Epidemiol. 42 (2), 627–634. doi:10.1093/ije/dyt043
Han, L., Jin, L., Wang, P., Zhu, X., and Chen, Q. (2015) “Clinical observation of Shenqi Fuzheng injection combined with DP regimen in treatment of non-small cell lung cancer,” in The 15th national association of integrated Chinese and western medicine deficiency and geriatric medicine academic seminar and professional committee change meeting and national continuing teaching project “training course on the diagnosis and treatment of geriatric diseases of traditional Chinese medicine”, 53–56.
He, L., Huang, L., Meng, R., and Yu, X. (2014). Therapeutic effect of Addy combined with chemotherapy on non-small cell lung cancer. Med. Aesthet. Cosmetol. (6), 38–39.
Higgins, J. P. T., and Green, S. (2022). Cochrane handbook for systematic reviews of interventions. John Wiley and Sons Ltd.
Hofseth, L. J., and Wargovich, M. J. (2007). Inflammation, cancer, and targets of ginseng. J. Nutr. 137 (1 Suppl. l), 183S–5s. doi:10.1093/jn/137.1.183S
Huang, Y., Zhen, X., and Liu, H. (2015). Effect of compound matrine injection on the pain of lung cancer with bone metastasis and its effect on Dickkopf1 concentration. World tradit. Chin. Med. 10 (12), 1876–1879.
Hui, D., Tiejun, F., Fan, Z., Zongrong, L., and Lisheng, D. (2002). Chemical composition of Astragalus injection. Nat. Prod. Res. Dev. 14 (6), 14–17. doi:10.3969/j.issn.1001-6880.2002.06.005
Jia, J. (2018). Effects of Kanglaite Injection on immune function and adverse reactions in patients with advanced non-small cell lung cancer treated with chemotherapy. J. Community Med. 16 (5), 11–13.
Jiang, L., Kong, Q., and Hu, X. (2011). Clinical study of Kangai injection combined with DP chemotherapy in the treatment of advanced non-small cell lung cancer. J. Hubei Univ. Chin. Med. 13 (05), 62–63.
Jiang, S. (2012). Observation on the effect of Aidi injection in chemotherapy of non-small cell lung cancer. Mod. DIAGNOSIS Treat. 23 (10), 1628–1629. doi:10.3969/j.issn.1001-8174.2012.10.030
Konkimalla, V. B., and Efferth, T. (2008). Evidence-based Chinese medicine for cancer therapy. J. Ethnopharmacol. 116 (2), 207–210. doi:10.1016/j.jep.2007.12.009
La Fleur, L., Falk-Sörqvist, E., Smeds, P., Berglund, A., Sundström, M., Mattsson, J. S., et al. (2019). Mutation patterns in a population-based non-small cell lung cancer cohort and prognostic impact of concomitant mutations in kras and Tp53 or Stk11. Lung Cancer 130, 50–58. doi:10.1016/j.lungcan.2019.01.003
Lew, R. (2019). Natural history of ovarian function including assessment of ovarian reserve and premature ovarian failure. Best. Pract. Res. Clin. Obstet. Gynaecol. 55, 2–13. doi:10.1016/j.bpobgyn.2018.05.005
Liancui, S., and Xuedong, D. (2013). Experimental study on the long-term toxicity of opuntia oil emulsion injection administered intravenously. Hainan Med. J. 24 (9), 1261–1263. doi:10.3969/j.issn.1003-6350.2013.09.0533
Liang, B., Bai, M., and Liu, J. (2016). Effect of Shenfu injection on quality of life in patients with non-small cell lung cancer after DP chemotherapy. Chin. Disabil. Med. 24 (8), 16–18. doi:10.13214/j.cnki.cjotadm.2016.08.008
Lijun, Y., Tao, H., Jianghan, L., Xiuming, C., Wei, L., Yuan, G., et al. (2017). Effect of astragali radix polysaccharides on proliferation and apoptosis of human colon cancer cell line SW620. Chin. J. Exp. Traditional Med. Formulae 23 (22), 97–101. doi:10.13422/j.cnki.syfjx.2017220097
Lin, Q. (2008). Therapeutic effect of Aidi injection combined with TP regimen on 60 cases of advanced non-small cell lung cancer. Zhejiang Clin. Med. (11), 1464–1465.
Lin, S., Shi, X., Zhang, Y., Wang, P., and Wang, M. (2011). Clinical observation of Aidi injection combined with DP regimen in treatment of advanced non-small cell lung cancer. J. Mod. Chin. West. Integr. Med. 20 (30), 3826–3828.
Liu, H., Wu, G., Peng, W., and Huang, Z. (2014). Effect of Kangai injection combined with chemotherapy on cellular immune function in patients with non-small cell lung cancer. Chin. J. Mod. Drug Appl. 8 (3), 171–172.
Liu, Q., You, S., Wang, P., Luo, X., and Duan, Z. (2021). Clinical effect of TP regimen combined with compound matrine injection on the expression of Foxp3 in peripheral blood of patients with lung cancer. Intern. Med. China 16 (6), 730–733. doi:10.16121/j.cnki.cn45-1347/r.2021.06.06
Liu, S., Mao, W., Qian, W., and Shen, W. (2008). Effects of Shenfu Injection on myelotoxicity and immune function in patients with advanced non-small cell lung cancer treated with chemotherapy. Chin. Tradit. Med. Emerg. 17 (12), 1705–1706.
Liu, Y., and Gao, J. (2011). Shenqi Fuzheng injection combined with docetaxel in treatment of advanced non-small cell lung cancer. Chin. J. Exp. TRADITIONAL Med. FORMULAE 17 (5), 231–232. doi:10.3969/j.issn.1005-9903.2011.05.071
Liwei, J., Dongying, L., Shuang, Z., Zhenyue, W., and Bo, Z. (2019). Astragaloside IV inhibits the progression of non-small cell lung cancer through the Akt/GSK-3β/β-Catenin pathway. Oncol. Res. 27 (4), 503–508. doi:10.3727/096504018X15344989701565
Lu, X., Zhang, Y., Ji, H., Chu, J., and Mao, G. (2016). Effect of Kanglaite injection combined with chemotherapy on the efficacy and immune function of advanced non-small cell lung cancer. Jiangsu Med. 42 (09), 1068–1070. doi:10.19460/j.cnki.0253-3685.2016.09.028
Luo, Y. (2016). Curative effect of Shenqi injection combined with chemotherapy and cisplatin on malignant pleural effusion in patients with non-small cell lung cancer. Master, Lanzhou University.
Ma, C. (2013). Effect of Shenqi Fuzheng Injection on immune function in patients with advanced non-small cell lung cancer treated with chemotherapy. HEILONGJIANG Med. J. 37 (6), 417–418. doi:10.3969/j.issn.1004-5775.2013.06.008
Miller, K. D., Goding Sauer, A., Ortiz, A. P., Fedewa, S. A., Pinheiro, P. S., Tortolero-Luna, G., et al. (2018). Cancer statistics for hispanics/latinos, 2018. CA Cancer J. Clin. 68 (6), 425–445. doi:10.3322/caac.21494
Mills, E. J., Thorlund, K., and Ioannidis, J. P. (2013). Demystifying trial networks and network meta-analysis. Bmj 346, f2914. doi:10.1136/bmj.f2914
Mingyu, M., Dongmei, Y., Jiaxi, X., and Xiaoxia, L. (2018). Retrospective clinical study of the efficacy of Shenfu injection in the adjuvant treatment of severe community-acquired pneumonia. China Pharm. 21 (4), 662–665. doi:10.3969/j.issn.1008-049X.2018.04.026
Mo, Y. (2015). Addy Injection combined with docetaxel and cisplatin in the treatment of non-small cell lung cancer. J. Henan Univ. Sci. Technol. Med. Ed. 33 (04), 277–278. doi:10.15926/j.cnki.issn1672-688x.2015.04.013
Ni, M., Wang, H., Wang, M., Zhou, W., Wu, J., Sun, B., et al. (2020b). Comparative efficacy of Chinese herbal injections combined with paclitaxel plus cisplatin for non-small-cell lung cancer: a multidimensional bayesian network meta-analysis. Evid. Based Complement. Altern. Med. 2020, 1824536. doi:10.1155/2020/1824536
Ni, M., Wang, H., Wang, M., Zhou, W., Zhang, J., Wu, J., et al. (2020a). Investigation on the efficiency of Chinese herbal injections for treating non-small cell lung cancer with vinorelbine and cisplatin based on multidimensional bayesian network meta-analysis. Front. Pharmacol. 11, 631170. doi:10.3389/fphar.2020.631170
Ning, Z. (2014). Clinical observation of Kangai combined with DP regimen in the treatment of advanced non-small cell lung cancer. Guide China Med. (30), 277–278.
Niu, H., Wu, C., Jiang, J., Xu, B., Zhao, J., Zhou, W., et al. (2011). Clinical observation of Brucea brucea oil emulsion injection combined with DP regimen in treatment of advanced non-small cell lung cancer. Chin. J. POSTGRADUATES Med. 34 (22), 13–16. doi:10.3760/cma.j.issn.1673-4904.2011.22.005
Pfister, D. G., Johnson, D. H., Azzoli, C. G., Sause, W., Smith, T. J., Baker, S., et al. (2004). American society of clinical oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J. Clin. Oncol. 22 (2), 330–353. doi:10.1200/jco.2004.09.053
Qi, F., Li, A., Inagaki, Y., Gao, J., Li, J., Kokudo, N., et al. (2010). Chinese herbal medicines as adjuvant treatment during chemo- or radio-therapy for cancer. Biosci. Trends 4 (6), 297–307.
Qiang, L., Zhiwei, W., and Shuwei, S. (2018). Effect of ginseng injection on peripheral blood Cd4+Cd25+Cd127∼(low/-) regulatory T cells in patients with chronic heart failure. Chin. J. Mod. Drug Appl. 12 (06), 126–127. doi:10.14164/j.cnki.cn11-5581/r.2018.06.074
Ren, Z., Ren, X., Zhang, Y., Han, Y., and Zhang, W. (2017). Multi-index efficacy of compound matrine injection combined with chemotherapy in the treatment of advanced non-small cell lung cancer. Chin. J. Surg. Oncol. 9 (06), 370–372.
Rücker, G., and Schwarzer, G. (2015). Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med. Res. Methodol. 15, 58. doi:10.1186/s12874-015-0060-8
Salanti, G., Del Giovane, C., Chaimani, A., Caldwell, D. M., and Higgins, J. P. (2014). Evaluating the quality of evidence from a network meta-analysis. PLoS One 9 (7), e99682. doi:10.1371/journal.pone.0099682
Sehouli, J., Braicu, E. I., Richter, R., Denkert, C., Jank, P., Jurmeister, P. S., et al. (2019). Prognostic significance of ki-67 levels and hormone receptor expression in low-grade serous ovarian carcinoma: an investigation of the tumor bank ovarian cancer network. Hum. Pathol. 85, 299–308. doi:10.1016/j.humpath.2018.10.020
Shan, H., Pan, J., and Zhou, X. (2014). Clinical efficacy of DP combined with Shenqi Fuzheng injection in the treatment of 40 cases of advanced non-small cell lung cancer. Chin. Pharm. Ind. 23 (24), 25–26.
Shaoguang, Z. (2021). Clinical efficacy of kanglet injection combined with dp regimen chemotherapy in the treatment of patients with stage iiib-iv non-small cell lung cancer. Pract. Clin. J. Integr. Traditional Chin. West. Med. 21 (15), 46–47. doi:10.13638/j.issn.1671-4040.2021.15.023
Shen, L., Qiu, W., Lan, C., Yu, X., and Du, L. (2015). Curative effect of Xiaoaiping injection combined with TP chemotherapy on advanced non-small cell lung cancer. Chin. foreign Med. Res. 13 (32), 8–9. doi:10.14033/j.cnki.cfmr.2015.32.004
Shen, W., and Na, X. (2022). Clinical efficacy of Aidi injection combined with chemotherapy in the treatment of advanced non-small cell lung cancer and its influence on immune function. Zhejiang J. Integr. Chin. West. Med. 32 (04), 324–327.
Shuping, S., and Xiaobin, Z. (2022). Clinical efficacy of Shenqi fu zheng injection combined with Np regimen in patients with advanced non-small cell lung cancer. Guizhou Med. J. 46 (5), 764–766.
Sun, P., Liu, Y., Wang, Q., and Zhang, B. (2019). Astragaloside IV inhibits human colorectal cancer cell growth. Front. Biosci. (Landmark Ed. 24 (3), 597–606. doi:10.2741/4738
Sun, T., and Lu, K. (2016). Efficacy and toxicity of Conai adjuvant chemotherapy in the treatment of non-small cell lung cancer. Zhejiang Trauma Surg. Dep. 21 (01), 134–136.
Suying, Z., Fengmeng, T., Pengfei, L., and Feng, G. (2016). Effects of astragaloside on human breast cancer MCF-7 cell line in vitro. Guid. J. Traditional Chin. Med. Pharm. 22 (10), 33–37. doi:10.13862/j.cnki.cn43-1446/r.2016.10.012
Tang, L., Wu, X., Tao, J., and Wang, L. (2011). Clinical observation of Aidi injection combined with chemotherapy in treatment of non-small cell lung cancer. J. Clin. Pulm. Med. 16 (5), 790–791. doi:10.3969/j.issn.1009-6663.2011.05.074
Tian, C., and Yang, J. (2017). Effect of Conlett injection on immune function in patients with advanced non-small cell lung cancer treated with chemotherapy. J. Community Med. 15 (18), 64–65.
Ting, D., Rongjing, S., Guoliang, X., Bingtao, L., Liping, H., and Jia, L. (2012). Study on determining pharmacokinetics parameters of Shenfu injection by pharmacology effect method. Chin. J. Clin. Pharmacol. Ther. 17 (01), 69–72.
Trinquart, L., Attiche, N., Bafeta, A., Porcher, R., and Ravaud, P. (2016). Uncertainty in treatment rankings: reanalysis of network meta-analyses of randomized trials. Ann. Intern Med. 164 (10), 666–673. doi:10.7326/m15-2521
Tu, J. (2012). Clinical study of Kangai injection combined with chemotherapy in the treatment of senile advanced non-small cell lung cancer. J. Clin. Ration. Drug Use 5 (17).
Wang, C. Q., Zheng, X. T., Chen, X. F., Jiang, H., Huang, J., Jiang, Y., et al. (2021). The optimal adjuvant strategy of Aidi injection with gemcitabine and cisplatin in advanced non-small cell lung cancer: a meta-analysis of 70 randomized controlled trials. Front. Pharmacol. 12, 582447. doi:10.3389/fphar.2021.582447
Wang, H., Liao, G., Liu, P., Xie, G., Qu, Y., and Liu, S. (2008). Therapeutic effect of Aidi injection combined with chemotherapy on 80 cases of advanced non-small cell lung cancer. Chin. J. Clin. Oncol. REHABILITATION 15 (1), 53–54. doi:10.3969/j.issn.1005-8664.2008.01.016
Wang, L., Liu, H., Xie, X., Chen, Y., Mai, Z., Wang, W., et al. (2009). Treatment of 44 cases of advanced non-small cell lung cancer with compound matrine injection combined with chemotherapy. Shaanxi Tradit. Chin. Med. 30 (12), 1574–1576.
Wang, R., Ma, L., Liao, Z., Zhang, Y., and Wang, G. (2018). Effects of Astragalus injection on bone marrow system and tumor markers in patients with advanced NSCLC after chemotherapy. World Chin. Med. 13 (11), 2795–2799. doi:10.3969/j.issn.1673-7202.2018.11.033
Wang, T. (2011). Aidi injection combined with chemotherapy for advanced non-small cell lung cancer. Chin. J. Exp. Traditional Med. Formulae 17 (18), 271–273. doi:10.3969/j.issn.1005-9903.2011.18.075
Wang, W., Kong, F., Li, C., Yao, R., Cheng, Y., and Zhang, L. (2011). Clinical observation of Shenqi Fuzheng Injection combined with docetaxel and cisplatin in treatment of advanced non-small cell lung cancer. ANHUI Med. J. 32 (7), 939–941. doi:10.3969/j.issn.1000-0399.2011.07.025
Wang, W., and Xu, J. (2013). Treatment of 46 cases of advanced non-small cell lung cancer with compound matrine injection combined with chemotherapy. CHINA HEALTH CARE & Nutr. 23 (8), 4543–4544. doi:10.3969/j.issn.1004-7484(s).2013.08.526
Wang, X. (2016). Clinical observation of Aidi injection combined with TP regimen in the treatment of advanced NSCLC. Master, Hubei University of Chinese Medicine.
Wang, Y., Wen, Z., Yu, G., Hu, Z., Wang, J., and Wu, S. (2016). Clinical observation and toxicity analysis of Aidi injection combined with TP regimen in the treatment of advanced non-small cell lung cancer. Chin. Tradit. Med. Mod. distance Educ. 14 (23), 94–95.
Wei, H. (2013). Clinical observation of Kangai injection combined with DP regimen in treatment of advanced non-small cell lung cancer. Master, Hebei medical university.
Whegang Youdom, S., Tahar, R., and Basco, L. K. (2017). Comparison of anti-malarial drugs efficacy in the treatment of uncomplicated malaria in african children and adults using network meta-analysis. Malar. J. 16 (1), 311. doi:10.1186/s12936-017-1963-0
Wu, B., Zhang, X., and Yan, G. (2014). Effect of Shenqi Fuzheng Injection on chemotherapy of advanced lung cancer. J. Chengde Med. Coll. 31 (05), 443–444. doi:10.15921/j.cnki.cyxb.2014.05.091
Xiang, C., Xiaoyan, J., Chunshan, W., Yufeng, X., and Guangdong, T. (2020). Astragaloside IV suppresses development of hepatocellular carcinoma by regulating miR-150-5p/β-catenin axis. Environ. Toxicol. Pharmacol. 78, 103397. doi:10.1016/j.etap.2020.103397
Xiangliang, Z., and Pinhua, P. (2018). Effects and mechanisms of opuntia oil emulsion on proliferation, migration and autophagy of human non-small cell lung cancer A549 cells. J. Central South Univ. Med. Sci. 43 (11), 1202–1208. doi:10.11817/j.issn.1672-7347.2018.11.006
Xie, C., Wang, Z., Guo, S., Kang, Z., Huang, P., and He, H. (2015). Effect of Shenfu injection on myelosuppression and gastrointestinal symptoms in patients with non-small cell lung cancer treated with chemotherapy. Jiangxi Med. J. (8), 795–797. doi:10.3969/j.issn.1006-2238.2015.08.027
Xing, L., Qi, C., Bo, P., Jianfeng, F., and Yumeng, H. (2022). Effects of kanglet injection combined with gp regimen on immune function, neoangiogenesis and serum jak2/stat3 signaling pathway in advanced non-small cell lung cancer patients. Prog. Mod. Biomed. 22 (22), 4395–4400. doi:10.13241/j.cnki.pmb.2022.22.038
Xu, J., Ding, R., and Huo, J. (2016). Effect of Shenqi Fuzheng infusion combined with chemotherapy on quality of life in patients with advanced non-small cell lung cancer. World tradit. Chin. Med. 0(B06).
Yan, C. (2009). Clinical pharmacologic study of opuntia oil emulsion drops. J. Nanjing Univ. Traditional Chin. Med. doi:10.7666/d.y1544168
Yang, F., and Cao, P. (2008). Clinical observation of compound matrine injection combined with TP regimen in treatment of non-small cell lung cancer. Guid. J. TRADITIONAL Chin. Med. Pharm. 14 (6), 16–17. doi:10.3969/j.issn.1672-951X.2008.06.008
Yang, Y. (2011). Observation on treatment of 60 cases of non-small cell lung cancer with Aidi injection combined with TP regimen. J. Pract. Chin. Med. 27 (05), 316–317.
Yanlin, L., Qingli, Q., Di, W., Juanjuan, H., Junmei, L., Xiaohong, W., et al. (2019). Effect of Compound bitter ginseng injection combined with tp regimen on the efficacy and prognosis of patients with advanced lung cancer. J. Mod. Oncol. 27 (20), 3624–3627. doi:10.3969/j.issn.1672-4992.2019.20.017
Yao, Y., Hu, M., and Wang, H. (2010). Therapeutic effect of Brucea javanica oil emulsion injection combined with DP chemotherapy on advanced non-small cell lung cancer. J. Pract. Oncol. 25 (1), 74–76.
Ye, L., and Fu, B. (2017). Effect of Xiaoaiping injection on enhancing and reducing toxicity in patients with advanced non-small cell lung cancer treated with TP regimen. Shanxi Med. J. 46 (10), 1189–1191.
Yi, W., Yan, J., Benxiang, W., and Quanyin, Q. (2002). Effects of ginsenoside Rg1 and its intestinal bacterial metabolite Rh1 on immune cell function in mice. Acta Pharm. Sin. 37 (12), 927–929.
Yin, L., and Tingzhang, S. (2002). Mechanisms of kanglaite induced apoptosis in human cancer cells. Chin. J. Clin. Oncol. 29 (12), 869–873.
Yixiang, Y., Dongmei, C., Liqun, J., and Yanni, L. (2023). Antitumor pharmacological mechanism and clinical application of astragali radix in tumor: a review. China J. Chin. Materia Medica 18 (11), 1615–1620. doi:10.3969/j.issn.1673-7202.2023.11.022
Yu, F., Jiang, D., Zhao, Y., Liu, C., and Yang, G. (2018). Effect of DP chemotherapy combined with Kangai injection on advanced non-small cell lung cancer. Chin. J. Public Health Eng. 17 (2), 278–280.
Yu, F., and Li, K. (2007). Clinical observation of Shenqi Fuzheng injection assisted chemotherapy in treatment of non-small cell lung cancer. Chin. J. Integr. Med. (02), 166–167.
Yu, H., Gao, S., and Hao, Y. (2012). Clinical observation of TP regimen combined with cinbufosin in treatment of advanced non-small cell lung cancer. Pract. cancer J. 27 (01), 55–57.
Zhang, L. (2018). Effect of Docetaxel combined with cisplatin and Kangai injection in the treatment of non-small cell lung cancer. Chin. Conval. Med. 27 (9).
Zhang, S. (2013). Clinical observation of brucea javanica oil emulsion combined with chemotherapy in treatment of advanced non-small cell lung cancer. J. Hainan Med. Coll. 19 (01), 58–60. doi:10.13210/j.cnki.jhmu.2013.01.041
Zhang, Y. (2010). Clinical observation of Kangai injection in the treatment of advanced non-small cell lung cancer. Prim. Med. Forum 14 (14), 407–408.
Zhang, Y., and Liu, H. (2014). Clinical efficacy of Brucea javanica oil emulsion injection combined with DP regimen in the treatment of non-small cell lung cancer. Chin. J. Gerontology (17), 4766–4767. doi:10.3969/j.issn.1005-9202.2014.17.022
Zhao, H. (2019). Clinical efficacy and safety analysis of Conlaite injection in patients with advanced non-small cell lung cancer. Chin. foreign women's health Res. 0 (20).
Zhao, J. (2009). Clinical analysis of Kangai injection combined with docetaxel and cisplatin in the treatment of advanced non-small cell lung cancer. China Mod. Dr. (33).
Zhen, J. (2020). Clinical efficacy of Conlaite injection combined with docetaxel cisplatin in the treatment of advanced non-small cell lung cancer. J. North Pharm. 17 (8), 28–29. doi:10.3969/j.issn.1672-8351.2020.08.009
Zhen, Q., Liao, X., and Wang, Y. (2010). Docetaxel plus cisplatin combined with Shenmai injection for treatment of 30 cases of advanced non-small cell lung cancer. Jiangxi J. Tradit. Chin. Med. 41 (01), 57–58.
Zhen, W., Huang, K., and Rao, M. (2016). Effect of Kanglaite injection combined with chemotherapy on the therapeutic effect and immune function of advanced non-small cell lung cancer. China Mod. Med. 23(35), 69–74. doi:10.3969/j.issn.1674-4721.2016.35.022
Zhihao, G. (2016). Observations on the efficacy of carbapenem injection combined with Tc regimen in the treatment of patients with advanced non-small cell lung cancer. Contemp. Med. 22 (25), 153–154. doi:10.3969/j.issn.1009-4393.2016.25.101
Zhiyong, W., and Xuhe, H. (2009). Pharmacologic effects and clinical evaluation of conrad injection. Chin. Traditional Herb. Drugs 40 (07), 1166–1168.
Zhou, J., Fu, P., Han, H., Wang, B., Di, M., and Song, Y. (2021). Effects of Kanglaite Injection combined with chemotherapy on lung function and cancer pain in advanced non-small cell lung cancer patients %J Journal of Hubei University of Traditional Chinese Medicine. J. Hubei Univ. Chin. Med. 23 (4), 34–36. doi:10.3969/j.issn.1008-987x.2021.04.08
Zhu, Q., and Chen, Y. (2006). Aidi injection combined with TP regimen in treatment of advanced non-small cell lung cancer. J. Pharmacoepidemiol. (03), 137–139.
Zhu, Y., and Wu, Y. (2020). Clinical observation of Shenqi Fuzheng injection assisted chemotherapy in the treatment of advanced non-small cell lung cancer. Cap. Med. 27 (12), 68.
Zhu, Y., and Zhang, H. (2010). Curative effect of Kanglaite injection combined with TP regimen on 100 cases of advanced non-small cell lung cancer. J. Mod. Oncol. 18 (8), 1569–1571. doi:10.3969/j.issn.1672-4992.2010.08.39
Keywords: Chinese herbal injection, docetaxel in combination with cisplatin, non-small cell lung cancer, network meta-analysis, systematic evaluation
Citation: Wen L, Xie L, Gong F, Zhang S and Xi T (2023) Efficacy and safety of Chinese medicine injections in combination with docetaxel and cisplatin for non-small cell lung cancer: a network meta-analysis. Front. Pharmacol. 14:1277284. doi: 10.3389/fphar.2023.1277284
Received: 14 August 2023; Accepted: 13 November 2023;
Published: 11 December 2023.
Edited by:
Jadranka Milosevic, Captis Diagnostics Inc., United StatesCopyright © 2023 Wen, Xie, Gong, Zhang and Xi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liangtao Wen, Nzc5MTg2MzE4QHFxLmNvbQ==
†These authors have contributed equally to this work
 Liangtao Wen
Liangtao Wen Lixiang Xie1†
Lixiang Xie1†