
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pharmacol. , 12 December 2023
Sec. Pharmacology of Anti-Cancer Drugs
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1276788
The immune checkpoint inhibitor (ICI), anti-programmed cell death receptor-1 (PD-1) antibody, has gained widespread approval for treating various malignancies. Among the immune-related adverse reactions (irAEs) during ICI treatment, the lichenoid reaction is noteworthy. Sintilimab, a new PD-1 inhibitor, has secured approval in China for treating refractory non-Hodgkin’s lymphoma, and phase I/II clinical trials for other solid tumors are ongoing both domestically and abroad. This paper presents a case of a mucocutaneous lichenoid reaction associated with sintilimab therapy, its diagnosis, and management. Our study, using multiplex immunofluorescence staining, reveals localized infiltration of CD4+ and CD8+ T lymphocytes in the subepithelial lamina propria region with upregulated PD-1 expression, implying an association between PD-1 expression upregulation and lichenoid reactions provoked by PD-1 monoclonal antibody. We provide a summary of clinical characteristics and treatment guidelines for lichenoid reactions induced by ICIs from previous reports, highlighting the success of a combined therapeutic regimen of oral antihistamines and topical corticosteroids in controlling symptoms without interrupting ICI treatment.
Lung cancer remains the leading cause of cancer-related mortality worldwide. The standard treatment protocol involves multimodal therapy, encompassing radiation, chemotherapy, and surgery. Recent advancements, particularly in combination immunotherapy, have significantly improved the 5-year survival rates (Herbst et al., 2022). Additionally, various plant and herbal extracts have demonstrated anticancer effects across multiple cancer cell types (Pathiranage et al., 2020).
At the heart of these advancements are immune checkpoints, a complex array of inhibitory pathways integral to the immune system. These pathways play crucial roles in maintaining self-tolerance and modulating immune responses to prevent collateral tissue damage (Pardoll, 2012). They regulate T-cell activity against tumor cells through mechanisms such as co-inhibition or co-stimulation of signaling pathways. Immune checkpoint inhibitors (ICIs), including agents targeting CTLA-4, PD-1, and PD-L1, have revolutionized the treatment of multiple malignancies (Postow et al., 2018). However, ICIs can also amplify nonspecific immune activities, leading to immune-related adverse events (irAEs) affecting various systems, including dermatologic and mucosal systems (Postow, 2015; Sibaud et al., 2016).
Reports of mucosal involvement in PD-1 and PD-L1 blockade therapies, although sporadic, have highlighted a range of manifestations from lichenoid lesions to Steven Johnson Syndrome/Toxic Epidermal Necrolysis (Saw et al., 2017; Sibaud et al., 2017; Obara et al., 2018; Utsunomiya et al., 2018; Senoo et al., 2020; Shazib et al., 2020; Costedoat et al., 2021). Sintilimab, a fully human IgG4 monoclonal antibody against PD-1, approved by the US FDA and China NMPA for certain cancer treatments, has been implicated in such reactions (Hoy, 2019; Zhang et al., 2022).
This paper presents a novel case of a non-small cell lung adenocarcinoma patient who developed lichenoid reactions following sintilimab therapy. This is, to our knowledge, the first reported instance of lichenoid reaction associated with sintilimab. We examine the underlying mechanisms of this reaction, based on both literature review and detailed analysis of our case. We also propose clinical management strategies for such irAEs, based on the insights gained from this case study. This report aims to contribute to the growing body of knowledge on ICIs’ dermatologic and mucosal side effects, emphasizing the importance of case studies in understanding and managing these complex reactions.
A 38-year-old male patient reported oral mucosa discomfort persisting for 2 weeks to our hospital. Three years prior, the patient had undergone a right lower lung lobectomy due to a diagnosis of non-small cell lung adenocarcinoma. Two years ago, the patient commenced treatment with Camrelizumab, a programmed cell death inhibitor, at a dosage of 200 mg intravenously every 2 weeks (Markham and Keam, 2019). Following the initial injection, the patient developed acute pancreatitis and hyperglycemia. After two additional doses, ketoacidosis ensued, and a diagnosis of type I diabetes was made. Consequently, PD-1 inhibitor therapy was discontinued, and insulin was administered for glycemic control. The patient subsequently received treatment with docetaxel and began a regimen of the PD-1 inhibitor sintilimab every 3 weeks, commencing 3 months ago. After three treatment cycles, the patient developed pruritic skin papules on the palms and soles of the feet. These lesions were marginally improved through the use of rupatadine fumarate tablets and loratadine tablets. Following the fourth sintilimab cycle, the patient reported inner lip irritation and pain when consuming spicy or hot foods, but the skin lesions did not recur. The patient had no history of other systemic diseases or allergies and had no family history of genetic disorders.
Clinical examination exposed irregular pink to purplish flat-topped papules and plaques with hyperpigmentation on the palms, dorsum of the hands, and soles of the feet (Figures 1A–F). Intraoral examination revealed symmetrical papular white streaks with mild erosions and congestion on the oral mucosa and the inner surface of the upper and lower lips. Reticular white streaks, consistent with Wickham’s streaks, were observed on the ventral part of the tongue and lips bilaterally (Figures 1G–L).
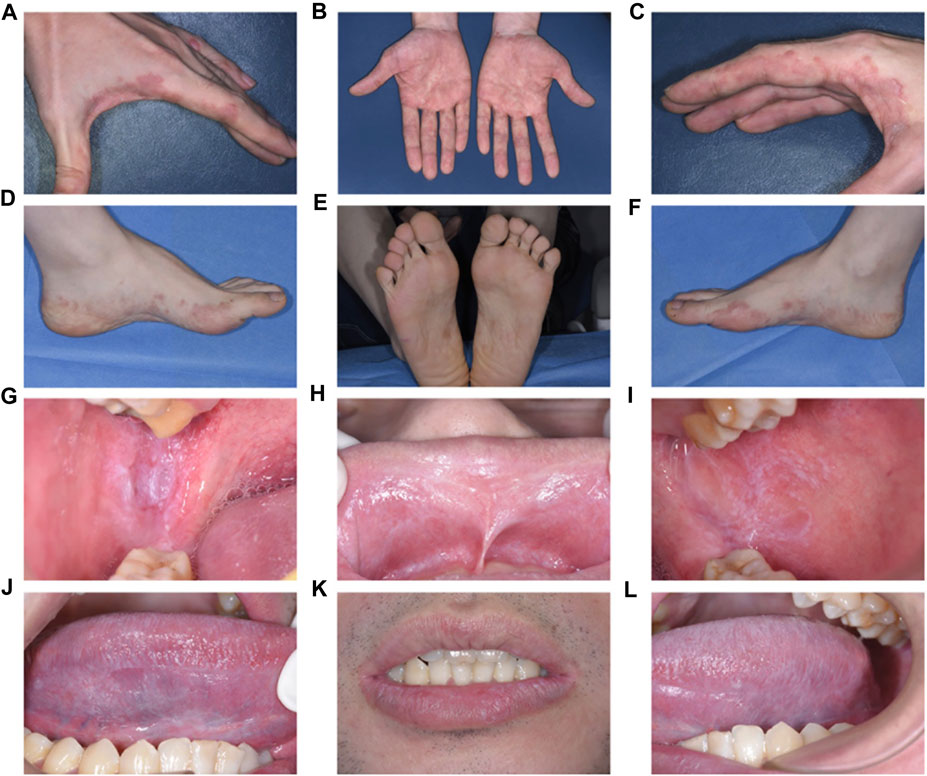
FIGURE 1. Clinical examination and Intraoral examination (A–F). Irregular pink to purplish flat-topped papules and plaques with hyperpigmentation on the palms and dorsum of the hands and soles of the feet (G–L). Symmetrical papular white streaks with mild erosions and congestion on the oral mucosa and the inside of the upper and lower lips, and reticular white streaks consistent with Wickham’s streaks could be seen on the ventral part of the tongue and lips bilaterally.
All routine blood tests, biochemistry analyses, erythrocyte sedimentation rates, coagulation tests, and infection tests yielded negative results. The anti-desmoglein-1 antibody, anti-desmoglein-3 antibody, and anti-BP180 antibody were all negative.
A biopsy was performed on the oral buccal mucosal lesion. Histopathological examination (Figures 2A–C) and IgA, IgG, IgM, and C3 direct immunofluorescence tests yielded negative findings, which indicated a lichenoid lesion. Incorporating the patient’s history, clinical examination, laboratory tests, and histopathological examination, a diagnosis of an oral lichenoid reaction was confirmed.
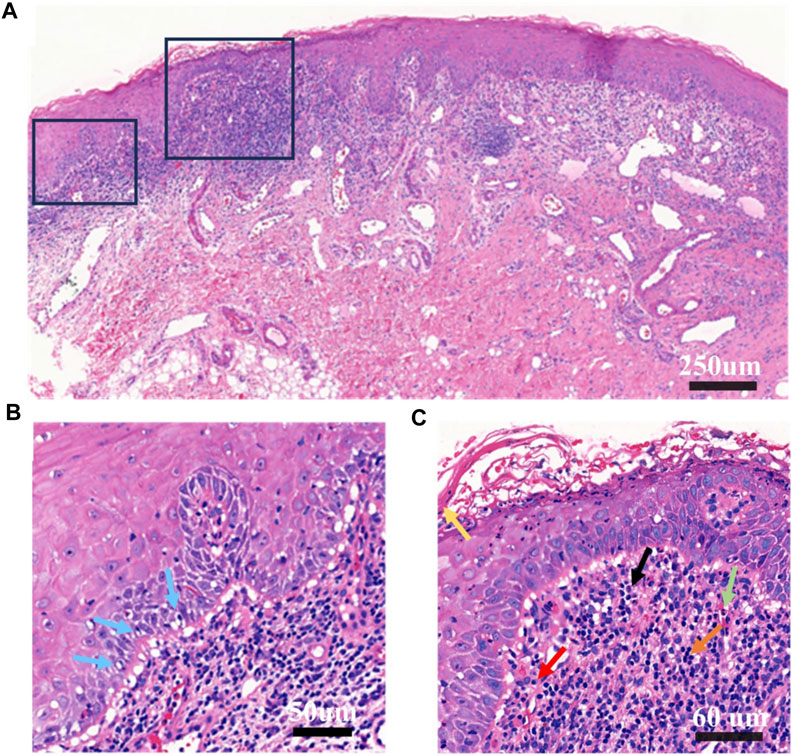
FIGURE 2. Histopathological examination of buccal mucosa (A). Original magnification at ×10 Chronic inflammation; hyperkeratosis of epithelial surface layer; vacuolar degeneration and liquefaction degeneration of some basal cells; capillary proliferation and expansion of lamina propria, more eosinophils, neutrophils, lymphocytes, plasma cells infiltration; proliferation and degeneration of fibrous tissue (B). Original magnification at ×20. This panel highlights vacuolar and liquefaction degeneration of select basal cells, indicated by blue arrows. (C) Original magnification at ×40. The panel features extensive infiltration by eosinophils, neutrophils, lymphocytes, and plasma cells. Yellow arrow points to epithelial hyperkeratosis; the green arrow denotes eosinophils; the black arrow indicates neutrophils; the orange arrow designates lymphocytes; and the red arrow identifies plasma cells.
Further analysis was conducted on the distribution of lymphocyte subtypes and associated biomarkers, including CD4, CD8, and PD-1. This was achieved utilizing the Luminlris™ HyperView multiplex immunostaining kit (IRISKit™MH010101), as shown in Figures 3A–F. Although a banded distribution of lymphocyte infiltration is typically seen in oral lichen planus, our case exhibited a pattern of continuous rather than banded infiltration. The distribution of PD-1 on lymphocytes was evaluated and classified into two zones of patchy and scattered distribution, alongside typical oral lichen planus lesions. Focal infiltration by inflammatory cells was found to coincide with an upregulation in PD-1 expression. Notably, PD-1 expression in the patchy area of inflammatory cells was higher than that in oral lichen planus lesions, while its expression in scattered areas showed no significant differences (Figures 3G, H).
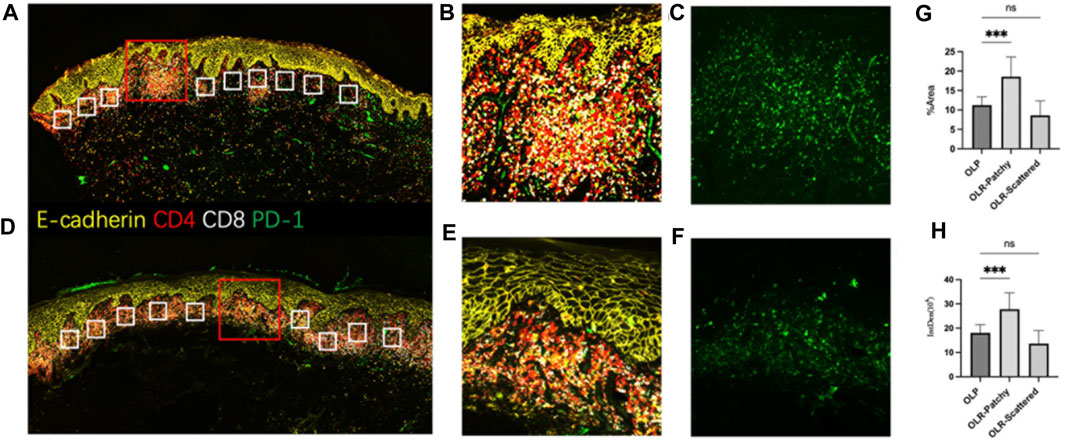
FIGURE 3. The multiplex immunofluorescence staining (A–C). The antigen distribution pattern observed in OLR. (D–F). The antigen distribution pattern observed in OLP. (G,H) Ten areas of equal size were randomly selected from each of the three groups for statistical analysis, including focal infiltration of inflammatory cells in OLR, scattered infiltration of inflammatory cells in OLR and OLP. (G): Comparison of the area% of PD-1 fluorescence-positive expression in OLR and OLP, p < 0.001. (H): Comparison of the integrated density of PD-1 fluorescence-positive expression in OLR and OLP, p <0.001 Note: (A–F) Staining using Luminlris™ HyperView multiplex immunostaining kit (IRISKit™MH010101). Primary antibodies were mouse anti-PD-1(ZSGB-BIO, ZM-0381), mouse anti-CD8(Immunoway, YM6938), and rabbit anti-CD4(HUABIO, ET1609-52).
Following a few days of gargling with a dexamethasone sodium phosphate solution, the patient’s symptoms markedly improved. After 1 month of usage, the majority of the oral lesions began to diminish. Although oral mucosal lesions reappeared regularly during subsequent sintilimab treatments, the skin lesions did not. The oral mucosal symptoms were effectively managed with a dexamethasone sodium phosphate gargle. Following the eighth sintilimab injection, the patient developed hypothermia and experienced worsened oral mucosal symptoms with more extensive and longer-lasting lesions than before. These symptoms were effectively managed with a topical dexamethasone sodium phosphate solution gargle and triamcinolone acetonide dental paste. In accordance with the oncologist’s advice and the patient’s preference, sintilimab treatment was discontinued after the completion of the eighth cycle. Subsequent follow-up assessments were conducted at 3- and 6-month intervals post-discontinuation, revealing no recurrence of intraoral lichenoid reactions.
This study discusses the unique case of a patient with non-small cell lung adenocarcinoma who developed lichenoid lesions after treatment with sintilimab, a phenomenon not previously reported. The characteristic symptoms of immune-related adverse events (irAEs), such as fever, diarrhea, and organ damage, including skin damage, are well-documented in the use of sintilimab (Li et al., 2021; Huang et al., 2022). However, the present case adds to the understanding of potential adverse reactions to this treatment.
The lichenoid lesions observed in our patient differed from traditional oral lichen planus in several key ways. The lesions exhibited reticular white streaks akin to Wickham’s streaks, along with continuous, but not band-like, lymphocyte infiltration adjacent to the basal cell layer. These lesions also presented with a greater infiltration of eosinophils, neutrophils, and plasma cells. The patient responded positively to the administration of topical glucocorticoids, such as dexamethasone and triamcinolone acetonide, which enabled the continuation of sintilimab treatment without interruption.
Oral lichen reactions warrant differentiation from oral lichen planus (OLP) and other lichenoid lesions that may be indicative of bullous diseases. Both direct immunofluorescence and ELISA techniques were employed to rule out bullous conditions (Van Der Meij and Van Der Waal, 2003). The updated diagnostic criteria for OLP, modified from WHO 2003 criteria, were delineated in 2016 (Louisy et al., 2023). These criteria define characteristic clinical features of OLP as well-defined, looping, and intersecting white striae against a backdrop of variable erythema. The lesions typically exhibit a roughly symmetrical distribution. Microscopically, OLP is characterized by hyperparakeratosis, hyperorthokeratosis, or a combination of both, along with cytoid (Civatte) bodies, basal cell hydropic change, and a predominantly lymphocytic infiltrate in the lamina propria (Cheng et al., 2016). In cases where these microscopic features are not clearly exhibited, the term “histopathologically compatible with OLP” is proposed. Under these revised criteria, a definitive diagnosis of OLP necessitates both clinical and histopathological features to be in complete alignment. Any deviation leads to a diagnosis of either oral lichenoid lesion (OLL) or oral lichenoid reaction (OLR) (Cheng et al., 2016). In the present case, clinical manifestations included reticular white patterns in the oral mucosa, while histopathological examination revealed vacuolar and liquefactive degeneration of basal cells, accompanied by infiltration of lymphocytes, eosinophils, and plasma cells in the lamina propria. The patient’s medical history did not indicate mucocutaneous lesions during 2 years of docetaxel treatment, an agent known to interfere with microtubule activity during cell cycle progression (Gupta et al., 2023). Furthermore, a cross-sectional study demonstrated minimal incidence of lichenoid reactions related to chemotherapy (Jena et al., 2022). Interestingly, immune checkpoint inhibitors similar to sintilimab, such as pembrolizumab and nivolumab, have been associated with mucocutaneous lichenoid reactions. In light of the patient’s clinical history and the histopathological findings, there is a strong implication that sintilimab played a pivotal role in the development of oral lichenoid lesions. Consequently, we propose the hypothesis that the oral lichenoid reaction observed in this patient was primarily induced by sintilimab therapy.
The exact pathophysiological mechanisms behind drug eruptions induced by PD-1 monoclonal antibodies have yet to be comprehensively elucidated. Several potential mechanisms are hypothesized, encompassing enhanced T-cell activity against antigens residing in both tumors and healthy tissue, augmentation of pre-existing autoantibodies, and an escalated level of inflammatory cytokines (Postow et al., 2018). The PD-1-PD-L pathway, comprising PD-1 and its ligands PD-L1 (B7-H1) and PD-L2 (B7-DC), has been implicated in these processes. Regarded as a crucial negative regulator of T-cell activation, PD-1 (Okazaki and Honjo, 2006) transmits inhibitory signals that modulate T-cell proliferation, cytokine production, and cytolytic function upon binding with PD-L1 or PD-L2 (Riley, 2009). The blockade of PD-1 or PD-L1 leads to enhanced T-cell activation, consequently inhibiting tumor development. Interestingly, PD-1 inhibitors have been demonstrated to shift antigen-induced cellular reactivity toward a pro-inflammatory Th1/Th17 response. This effect was denoted by the heightened expression of Th1 and Th17 associated markers like interferon γ, interleukin (IL)-2, tumor necrosis factor α, IL-6, and IL-17, while simultaneously suppressing the production of IL-5 and IL-13 cytokines linked to Th2 (Dulos et al., 2012). The B7-H1 blockade also significantly increased the production of IFN-γ and IL-2, akin to the response observed with anti-PD-1 (Zhou et al., 2012). An imbalance in the microenvironmental cytokine profile is closely associated with the onset of lichenoid reactions. The pathogenesis of such reactions is believed to be initiated by the attack and destruction of the basal epidermal layer by autoreactive cytotoxic T cells secreting a TH1-type cytokine profile (Meller et al., 2009). Th1 pro-inflammatory immune response predominance is typically observed in lichenoid dermatitis—irAE (Curry et al., 2019). The involvement of T cells in the pathogenesis of oral lichen planus (OLP) has been extensively studied. Initial evidence for the crucial role of T cells came from a study in which a cytotoxic CD4+ T-cell clone was found to induce oral lesions following localized metastases (Shiohara et al., 1986). Subsequent research has pointed towards cytotoxic CD8+ T cells as primary agents in inducing apoptosis in keratinized cells (Sugerman et al., 2000). This T-cell-mediated etiology of OLP was further refined in 2016 (Wang et al., 2016). Programmed cell death protein 1 (PD-1) is ubiquitously expressed in tumor-infiltrating lymphocytes (TILs) across various tumor types. Elevated PD-1 expression in CD4+ TILs often mirrors heightened PD-1 levels in regulatory T cells (Treg), while an increase in PD-1 expression on CD8+ TILs could indicate anergic or exhausted states (Pardoll, 2012; Farhood et al., 2019). In the context of NSCLC, higher PD-1 levels have been observed on lymphocytes in metastatic lymph nodes (Ma et al., 2017). In the current case, we postulate that the administration of a PD-1 monoclonal antibody led to enhanced local lymphocyte infiltration in the epithelial submucosa, subsequently resulting in mucosal lichenoid lesions. In our investigation, we specifically aimed to evaluate the distribution patterns of CD4+ and CD8+ T cells in oral lichen-like lesions elicited by sintilimab. Our intent was to determine whether these patterns deviate from those documented in prior literature. The observed distribution profiles of CD4+ and CD8+ T cells in the present case not only reinforce the diagnosis of a lichenoid reaction but also align well with previously published studies. Concurrently, PD-1 expression was examined to elucidate the underlying mechanisms contributing to lichenoid reactions caused by anti-PD-1 therapy. Consistent with this, in the present case study, high PD-1 expression was noted in regions harboring inflammatory cells. The enhanced expression and activation of PD-1 negatively regulate T lymphocytes, which are crucial for maintaining immune tolerance in normal organisms and contribute to tumor immune evasion during tumorigenesis. Meanwhile, PD-1 monoclonal antibody heightens T-cell activation by blocking the PD-1/PD-L1 signaling pathway. In this specific case, localized CD4+ and CD8+ T-cell infiltration was observed along with increased PD-1 expression in the subepithelial lamina propria (Figure 3). Based on these findings, we propose that the mucosal lichenoid reaction observed in our patient might be associated with the increased expression of PD-1 in NSCLC patients. The administration of PD-1 antibodies to inhibit receptor binding could result in heightened focal T-cell expression, further leading to the emergence of adverse mucosal reactions. Interestingly, cessation of sintilimab use led to the disappearance of the mucosal adverse effects, substantiating our hypothesis. Further studies are warranted to validate this proposed mechanism and explore its clinical implications.
Immune checkpoint inhibitors (ICIs) can result in adverse effects that inconvenience patients, reduce their quality of life, or, in extreme cases, necessitate the suspension or termination of therapy. However, these adverse events also indicate that ICIs are promoting effective immune activation in patients. Notably, some studies have reported an association between the frequency of adverse events and the efficacy of immunotherapy, suggesting that patients with cutaneous adverse reactions may have a lower risk of tumor progression (Chan et al., 2020; España et al., 2020).
We summarize previously reported cases of mucocutaneous lichenoid reactions to anti-PD-1/PD-L1 agents with well-documented records (Shi et al., 2016; Sibaud et al., 2017; Obara et al., 2018; Enomoto et al., 2019; Kan et al., 2021; Niesert et al., 2021), indicated that most reactions are manageable, allowing continued use of immune checkpoint inhibitors (Table 1). The management of such adverse reactions typically involves the use of topical steroids, with more severe cases requiring oral antihistamines and corticosteroids, or even discontinuation of PD-1 inhibitor therapy.
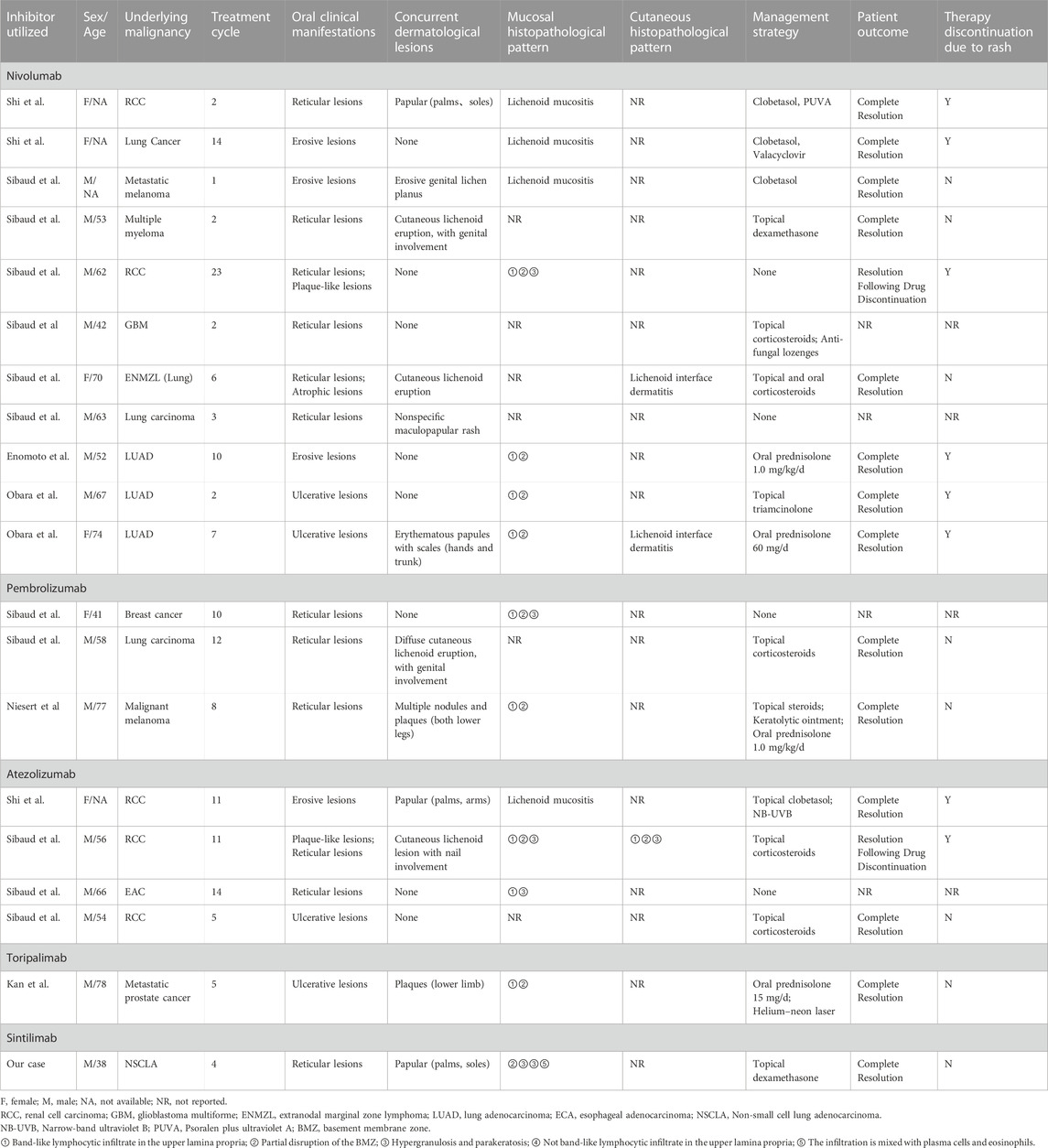
TABLE 1. Mucocutaneous Lichenoid Reactions Induced by Anti-PD-1/PD-L1 Inhibitors: A review of reported cases.
In conclusion, our findings suggest that irAEs induced by ICIs, including sintilimab, can be effectively managed through topical glucocorticoid administration. For severe cases, the combination of oral antihistamines or glucocorticoids may be considered without necessitating drug discontinuation.
As the use of ICIs in cancer treatment expands, the occurrence of irAEs will likely increase. Hence, it is crucial to develop effective strategies for managing irAEs, allowing patients to continue ICI therapy. These strategies should be tailored to each patient’s unique circumstances, taking into account factors such as the severity of irAEs, future cancer treatment plans, and tumor response to ICIs, balancing the risks and benefits of irAE and oncology treatment options. Early recognition and appropriate management of irAEs can significantly improve patients’ symptoms, prevent the cessation of potentially life-saving treatment, and enhance the quality of life of patients.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Medical Ethics Committee of West China Hospital of Stomatology, Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
SZ: Writing–original draft, Conceptualization, Data curation, Formal Analysis, Investigation, Methodology. ZZ: Writing–review and editing, Data curation, Formal Analysis, Investigation. XF: Writing–review and editing, Data curation, Formal Analysis, Investigation. CZ: Writing–review and editing, Data curation, Formal Analysis, Methodology. LJ: Writing–review and editing, Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Validation.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Natural Science Foundation of China (82171809), Clinical and Translational Medicine Research Foundation of Chinese Academy of Medical Sciences (2022-I2M-C&T-B-111), and Research and Develop Program, West China Hospital of Stomatology Sichuan University (RD-03-202307).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Chan, L., Hwang, S. J. E., Byth, K., Kyaw, M., Carlino, M. S., Chou, S., et al. (2020). Survival and prognosis of individuals receiving programmed cell death 1 inhibitor with and without immunologic cutaneous adverse events. J. Am. Acad. Dermatology 82, 311–316. doi:10.1016/j.jaad.2019.06.035
Cheng, Y.-S. L., Gould, A., Kurago, Z., Fantasia, J., and Muller, S. (2016). Diagnosis of oral lichen planus: a position paper of the American Academy of Oral and Maxillofacial Pathology. Oral Surg. Oral Med. Oral Pathology Oral Radiology 122, 332–354. doi:10.1016/j.oooo.2016.05.004
Costedoat, I., Vergara, R., Dousset, L., Gerard, E., Prey, S., Dutriaux, C., et al. (2021). Drug-induced bullous lichenoid eruption: a side effect of nivolumab triggered by the TLR agonist imiquimod. Eur. J. Dermatology 31, 91–92. doi:10.1684/ejd.2020.3951
Curry, J. L., Reuben, A., Szczepaniak-Sloane, R., Ning, J., Milton, D. R., Lee, C. H., et al. (2019). Gene expression profiling of lichenoid dermatitis immune-related adverse event from immune checkpoint inhibitors reveals increased CD14 + and CD16 + monocytes driving an innate immune response. J. Cutan. Pathol. 46, 627–636. doi:10.1111/cup.13454
Dulos, J., Carven, G. J., van Boxtel, S. J., Evers, S., Driessen-Engels, L. J. A., Hobo, W., et al. (2012). PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. J. Immunother. 35, 169–178. doi:10.1097/CJI.0b013e318247a4e7
Enomoto, Y., Nakatani, H., Kondo, S., Kasai, T., and Tsuchiya, Y. (2019). Drug-induced oral lichenoid reaction during nivolumab therapy. Int. J. Oral Maxillofac. Surg. 48, 488–491. doi:10.1016/j.ijom.2018.07.025
España, S., Pérez Montes de Oca, A., Marques-Pamies, M., Cucurull, M., Domenech, M., Velarde, J. M., et al. (2020). Endocrine adverse events related to immune-oncology agents: retrospective experience of a single institution. Transl. Lung Cancer Res. 9, 103–110. doi:10.21037/tlcr.2019.12.17
Farhood, B., Najafi, M., and Mortezaee, K. (2019). CD8 + cytotoxic T lymphocytes in cancer immunotherapy: a review. J. Cell. Physiology 234, 8509–8521. doi:10.1002/jcp.27782
Gupta, R., Kadhim, M. M., Turki Jalil, A., Qasim Alasheqi, M., Alsaikhan, F., Khalimovna Mukhamedova, N., et al. (2023). The interactions of docetaxel with tumor microenvironment. Int. Immunopharmacol. 119, 110214. doi:10.1016/j.intimp.2023.110214
Herbst, R. S., Wang, M., and Chen, L. (2022). When immunotherapy meets surgery in non-small cell lung cancer. Cancer Cell 40, 603–605. doi:10.1016/j.ccell.2022.05.010
Hoy, S. M. (2019). Sintilimab: first global approval. Drugs 79, 341–346. doi:10.1007/s40265-019-1066-z
Huang, Y., Zhu, L., Ma, X., Hong, Y., Su, X., Lai, W., et al. (2022). A case of sintilimab-induced SJS/TEN:Dermatologic adverse reactions associated with programmed cell death protein-1 inhibitors. Dermatol. Ther. 35, e15663. doi:10.1111/dth.15663
Jena, S., Hasan, S., Panigrahi, R., Das, P., Mishra, N., and Saeed, S. (2022). Chemotherapy-associated oral complications in a south Indian population: a cross-sectional study. JMedLife 15, 470–478. doi:10.25122/jml-2021-0342
Kan, S., Ren, H., Gao, Z., Dai, E., Liu, Y., Yang, L., et al. (2021). Lichenoid drug eruption on the lower lip caused by anti-PD-1 monoclonal antibody: a case report and literature review. Immunotherapy 13, 1373–1378. doi:10.2217/imt-2021-0234
Li, X., Qu, L.-X., Ren, Y.-M., and Hu, C. (2021). Case report: a case report and literature review on severe bullous skin reaction induced by anti-PD-1 immunotherapy in a cervical cancer patient. Front. Pharmacol. 12, 707967. doi:10.3389/fphar.2021.707967
Louisy, A., Humbert, E., and Samimi, M. (2023). Oral lichen planus: an update on diagnosis and management. Am. J. Clin. Dermatol. doi:10.1007/s40257-023-00814-3
Ma, H., Mao, G., Zhang, G., and Huang, H. (2017). The expression and clinical signification of PD-1 in lymph nodes of patients with non-small cell lung cancer. Immunol. Investig. 46, 639–646. doi:10.1080/08820139.2017.1341521
Markham, A., and Keam, S. J. (2019). Camrelizumab: first global approval. Drugs 79, 1355–1361. doi:10.1007/s40265-019-01167-0
Meller, S., Gilliet, M., and Homey, B. (2009). Chemokines in the pathogenesis of lichenoid tissue reactions. J. Investigative Dermatology 129, 315–319. doi:10.1038/jid.2008.251
Niesert, A., Guertler, A., Schutti, O., Engels, L., Flaig, M., French, L., et al. (2021). Ulcerated lichen planus after adjuvant use of programmed cell death-1-inhibitor: a case report and systematic review of the literature. Acta Derm. Venereol. 101, adv00472. doi:10.2340/00015555-3840
Obara, K., Masuzawa, M., and Amoh, Y. (2018). Oral lichenoid reaction showing multiple ulcers associated with anti-programmed death cell receptor-1 treatment: a report of two cases and published work review. J. Dermatol 45, 587–591. doi:10.1111/1346-8138.14205
Okazaki, T., and Honjo, T. (2006). The PD-1–PD-L pathway in immunological tolerance. Trends Immunol. 27, 195–201. doi:10.1016/j.it.2006.02.001
Pardoll, D. M. (2012). The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264. doi:10.1038/nrc3239
Pathiranage, V. C., Thabrew, I., Samarakoon, S. R., Tennekoon, K. H., Rajagopalan, U., and Ediriweera, M. K. (2020). Evaluation of anticancer effects of a pharmaceutically viable extract of a traditional polyherbal mixture against non-small-cell lung cancer cells. J. Integr. Med. 18, 242–252. doi:10.1016/j.joim.2020.02.007
Postow, M. A. (2015). Managing immune checkpoint-blocking antibody side effects. Am. Soc. Clin. Oncol. Educ. Book, 76–83. doi:10.14694/EdBook_AM.2015.35.76
Postow, M. A., Sidlow, R., and Hellmann, M. D. (2018). Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 378, 158–168. doi:10.1056/NEJMra1703481
Riley, J. L. (2009). PD-1 signaling in primary T cells. Immunol. Rev. 229, 114–125. doi:10.1111/j.1600-065X.2009.00767.x
Saw, S., Lee, H. Y., and Ng, Q. S. (2017). Pembrolizumab-induced Stevens–Johnson syndrome in non-melanoma patients. Eur. J. Cancer 81, 237–239. doi:10.1016/j.ejca.2017.03.026
Senoo, H., Kawakami, Y., Yokoyama, E., Yamasaki, O., and Morizane, S. (2020). Atezolizumab-induced lichen planus pemphigoides in a patient with metastatic non-small-cell lung cancer. J. Dermatol 47, e121–e122. doi:10.1111/1346-8138.15248
Shazib, M. A., Woo, S., Sroussi, H., Carvo, I., Treister, N., Farag, A., et al. (2020). Oral immune-related adverse events associated with PD-1 inhibitor therapy: a case series. Oral Dis. 26, 325–333. doi:10.1111/odi.13218
Shi, V. J., Rodic, N., Gettinger, S., Leventhal, J. S., Neckman, J. P., Girardi, M., et al. (2016). Clinical and histologic features of lichenoid mucocutaneous eruptions due to anti–programmed cell death 1 and anti–programmed cell death ligand 1 immunotherapy. JAMA Dermatol 152, 1128–1136. doi:10.1001/jamadermatol.2016.2226
Shiohara, T., Moriya, N., Tsuchiya, K., Nagashima, M., and Narimatsu, H. (1986). Lichenoid tissue reaction induced by local transfer of ia-reactive T-cell clones. J. Investigative Dermatology 87, 33–38. doi:10.1111/1523-1747.ep12523539
Sibaud, V., Eid, C., Belum, V. R., Combemale, P., Barres, B., Lamant, L., et al. (2017). Oral lichenoid reactions associated with anti-PD-1/PD-L1 therapies: clinicopathological findings. J. Eur. Acad. Dermatol Venereol. 31, e464–e469. doi:10.1111/jdv.14284
Sibaud, V., Meyer, N., Lamant, L., Vigarios, E., Mazieres, J., and Delord, J. P. (2016). Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr. Opin. Oncol. 28, 254–263. doi:10.1097/CCO.0000000000000290
Sugerman, P. B., Satterwhite, K., and Bigby, M. (2000). Autocytotoxic T-cell clones in lichen planus. Br. J. Dermatology 142, 449–456. doi:10.1046/j.1365-2133.2000.03355.x
Utsunomiya, A., Oyama, N., Iino, S., Baba, N., Chino, T., Utsunomiya, N., et al. (2018). A case of erythema multiforme major developed after sequential use of two immune checkpoint inhibitors, nivolumab and ipilimumab, for advanced melanoma: possible implication of synergistic and/or complementary immunomodulatory effects. Case Rep. Dermatol 10, 1–6. doi:10.1159/000485910
Van Der Meij, E. H., and Van Der Waal, I. (2003). Lack of clinicopathologic correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. J. Oral Pathol. Med. 32, 507–512. doi:10.1034/j.1600-0714.2003.00125.x
Wang, H., Zhang, D., Han, Q., Zhao, X., Zeng, X., Xu, Y., et al. (2016). Role of distinct CD 4 + T helper subset in pathogenesis of oral lichen planus. J. Oral Pathol. Med. 45, 385–393. doi:10.1111/jop.12405
Zhang, L., Lin, W., Tan, F., Li, N., Xue, Q., Gao, S., et al. (2022). Sintilimab for the treatment of non-small cell lung cancer. Biomark. Res. 10, 23. doi:10.1186/s40364-022-00363-7
Keywords: lichenoid reactions, Sintilimab Therapy, immune checkpoint inhibitors (ICIs), PD-1 inhibitor, mucocutaneous reactions, immune-related adverse events (irAEs), non-small cell lung adenocarcinoma
Citation: Zhou S, Zhang Z, Feng X, Zhao C and Jiang L (2023) Lichenoid mucocutaneous reactions associated with sintilimab therapy in a non-small cell lung adenocarcinoma patient: case report and review. Front. Pharmacol. 14:1276788. doi: 10.3389/fphar.2023.1276788
Received: 01 September 2023; Accepted: 20 November 2023;
Published: 12 December 2023.
Edited by:
Graham Robert Leggatt, The University of Queensland, AustraliaReviewed by:
Utpreksha Vaish, University of Alabama at Birmingham, United StatesCopyright © 2023 Zhou, Zhang, Feng, Zhao and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lu Jiang, amlhbmdsdUBzY3UuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.