
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 19 September 2023
Sec. Pharmacology of Anti-Cancer Drugs
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1269863
 Huanhuan Wang1,2
Huanhuan Wang1,2 Zhuangzhuang Zheng1,2
Zhuangzhuang Zheng1,2 Yangyu Zhang3
Yangyu Zhang3 Chenbin Bian1,2
Chenbin Bian1,2 Jindian Bao1,2
Jindian Bao1,2 Ying Xin4,5*
Ying Xin4,5* Xin Jiang1,2*
Xin Jiang1,2*Head and neck squamous cell carcinoma (HNSCC) accounts for approximately 3% of new cancer cases and 3% of all deaths worldwide. Most HNSCC patients are locally advanced (LA) at diagnosis. The combination of radiotherapy (RT), chemotherapy, targeted therapy, and immunotherapy are the primary LA-HNSCC treatment options. Nevertheless, the choice of optimal LA-HNSCC treatment remains controversial. We systematically searched public databases for LA-HNSCC-related studies and assess treatment effectiveness and safety by assessing the objective response rate (ORR), ≥3 adverse events (AEs), overall survival (OS), progression-free survival (PFS), disease-free survival (DFS), local-region control (LRC), and disease-specific survival (DSS). 126 randomized controlled clinical trials (RCTs) were included in this study. We show that concurrent RT with nimotuzumab or conventional concurrent chemo-radiotherapy (CCRT) had significantly better efficacy and long-term survival without increasing AEs than RT alone. Accelerated fractionated radiotherapy (AFRT) showed better efficiency than conventional fractionated RT, although it had higher AEs. In addition, concurrent cetuximab combined with RT failed to show a significant advantage over RT alone.
Trial registration: PROSPERO CRD42022352127.
Question
What is the best treatment for patients with locally advanced head and neck squamous cell carcinoma (LA-HNSCC)?
Findings
In this meta-analysis of 126 clinical trials including 22,726 patients, concurrent RT with nimotuzumab or conventional concurrent chemo-radiotherapy (CCRT) had significantly better efficacy and long-term survival without increasing AEs than RT alone. Accelerated fractionated radiotherapy (AFRT) showed better efficiency than conventional fractionated RT, although it had higher AEs. In addition, concurrent cetuximab combined with RT failed to show a significant advantage over RT alone.
Meaning
Our study provides more precise data on the treatment efficacy and safety of multiple treatment regimens among LA-HNSCC patients which facilitates clinical treatment decision making.
Head and neck cancers (HNCs) are heterogeneous tumors that include oral cavity, oropharynx, hypopharynx, larynx and several other subregions (Wyss et al., 2013; Siegel et al., 2018). Approximately 750,000 new cases and 360,000 deaths occur annually worldwide (Sung et al., 2021). Head and neck squamous cell carcinoma (HNSCC) is the most common type of HNCs. HNSCC accounts for approximately 3% of new cancer cases and 3% of deaths worldwide (Siegel et al., 2020). Approximately 30%–40% of HNSCC patients have early-stage disease (phase I/II) at diagnosis and are usually cured by surgery or radiotherapy (RT) alone (Pfister et al., 2014). More than 60% of HNSCC patients are initially diagnosed as locally advanced (LA) (Argiris et al., 2008; Braakhuis et al., 2012). The American Joint Committee on Cancer (AJCC) 8th edition staging system (2017) defines LA-HNSCC as T3-4 or N1-3 (Sahin et al., 2019). LA-HNSCC has a high risk of local recurrence and poor prognosis and is usually managed with combinations of surgery, RT, and systemic therapy (Pfister et al., 2020).
Previously, surgery combined with postoperative RT are the standard treatment options for LA-HNSCC (Kramer et al., 1987). With the progressive demand of organ function preservation, radical RT plays a crucial role in early and LA disease for maintaining swallowing and speech of LA-HNSCC patients. Compared to RT alone, concurrent RT combined with platinum-based chemotherapy significantly improves patient survival and quality of life, making it the first-line treatment option for LA-HNSCC (Sindhu and Bauman, 2019). However, platinum-based chemotherapy, particularly high-dose cisplatin, may lead to severe adverse events (AEs) in the early treatment stages. Therefore, there is an urgent need for new therapeutic options to improve HNSCC prognosis. Epidermal growth factor receptor (EGFR), a member of the tyrosine kinase receptor family, is closely associated with tumor angiogenesis, cell proliferation, invasion, metastasis, and inhibition of apoptosis (Liang et al., 2020). EGFR is highly expressed in over 90% of HNSCC (Ishitoya et al., 1989; Grandis and Tweardy, 1993) and strongly associated with poor prognosis (Rubin Grandis et al., 1998; Chung et al., 2006). Therefore, EGFR has emerged as an important therapeutic target for HNSCC (Pirker et al., 2012). Cetuximab, a chimeric murine antibody linked to human IgG and EGFR, has been confirmed to enhance the effects of RT in preclinical models (Marur and Forastiere, 2016). The IMCL-9815 study (Bonner et al., 2006) also demonstrated that combining cetuximab with RT resulted in a 13% improvement in local-region control (LRC) and a 10% improvement in overall survival (OS) compared to RT alone. Therefore, cetuximab has been approved by the Food and Drug Administration (FDA) to treat HNSCC. Nimotuzumab, a fully-human antibody against EGFR, increases clinical responses to chemo-radiotherapy (59.5% versus 34.2%), has a lower incidence of AEs, and has also been approved for HNSCC treatment (Rodríguez et al., 2010). In addition, induction chemotherapy (IC) is recommended for some patients, although its benefits remain controversial. In recent years, clinical trials of immunotherapy for LA-HNSCC have demonstrated the successful use of immunotherapy for recurrent/metastatic HNSCC (Seiwert et al., 2016). However, there is no conclusive evidence for application of immunotherapy in LA-HNSCC.
Currently, the combination of multiple antitumor treatment regimens targeting different mechanisms provides treatment options for a larger number of LA-HNSCC patients. Nevertheless, up to 40% of patients eventually develop distant metastases after multimodal therapy (Seiwert and Cohen, 2005; Marur and Forastiere, 2016) and have a poor prognosis (Chow, 2020). Therefore, the best treatment option for LA-HNSCC remains controversial. However, there have been no simultaneous comparisons of multiple treatment options for LA-HNSCC. We performed a systematic review and network meta-analysis to update the existing paradigm of regimen selection. This study summarized and compared multiple treatment options for LA-HNSCC to assess treatment efficacy and safety. We applied multiple indicators such as objective response rate (ORR), OS, and progression-free survival (PFS) to provide recommendations for the best treatment options suitable for LA-HNSCC patients. Notably, nasopharyngeal carcinoma, which is a unique HNSCC subtype that is usually associated with EBV infection. According to National Comprehensive Cancer Network (NCCN) guideline recommendations, the treatment options for nasopharyngeal carcinoma are significantly different compared to other HNSCCs and therefore were not included in this study.
The entire study process, including the original study search, completion of the systematic review, and network meta-analysis, followed the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines (Supplementary Table S1) (Hutton et al., 2015). Before the start of the study, the protocol was registered with PROSPERO (CRD42022352127).
We systematically searched all relevant articles and references published in the PubMed, Embase, Web of Science, and Cochrane Library databases from their establishment to 24 May 2022, using the search strategy designed by experts in the epidemiology laboratory of our institution. No language restrictions were imposed. The search strategy is presented in Supplementary Table S2. To include the most comprehensive research results, we investigated randomized controlled trials (RCTs) published at recent international conferences and searched the reference lists of relevant studies and reviews for additional information.
Studies that met the following criteria were included.
(1) Pathologically confirmed LA-HNSCC;
(2) No surgical treatment option;
(3) Included the outcome indicators considered in this study: ORR, ≥3 AEs, OS, PFS, disease-free survival (DFS), LRC, and disease-specific survival (DSS). ORR defined as the proportion of patients with a complete and partial response to treatment. ≥3 AEs defined as the incidence of level 3 or higher adverse events as defined by the National Cancer Institute’s Generic Term. OS defined as the time from the start of the study to the occurrence of death. PFS defined as the time from the start of the study to the patient’s first tumor progression or death. DFS defined as the time from complete clinical remission following treatment to the reappearance of focal recurrence. LRC defined as the time during which the local tumor shrank consistently or remained stable. DSS defined as the time from the start of the study to the occurrence of death by the specific disease.
(4) The study was an RCT.
In addition, we only included the most recent version that contained appropriate survival data if a study was repeatedly reported. For studies that included overlapping data from other studies, we selected a wider range of studies for analysis.
Two researchers (H. H. W. and Z. Z. Z.) independently reviewed the titles and abstracts according to the inclusion criteria to identify relevant studies for potential inclusion. Studies that potentially met the criteria were carefully reviewed to decide whether to be included in the study. Disputes were resolved by consensus with a third investigator (X. J.). According to predesigned data extraction tables discussed by the group members, two researchers (Y.Y.Z. and C.B.B.) independently extracted basic study characteristics, including study start and end times, sample size, median patient age, intervention protocol, and outcome indicators. Disagreements between the two researchers were resolved through group discussions. We directly extracted the hazard ratios (HR) and 95% confidence intervals (95% CI) of studies and calculated standard errors. For studies that failed to report HR and 95% CI, we used Digitizeit software (V4.1) to extract survival data from the survival curves and to calculate HR and standard errors. For studies with missing data, we attempted to obtain more complete information by contacting the study institution.
The methodological quality of all included studies was assessed independently by two researchers (H. H. W. and Y. Y. Z.) using the Cochrane Risk Assessment Tool (Cumpston et al., 2019). This study focused on seven aspects of the original studies: whether sequence generation was random, the allocation was hidden, participants were blinded, outcome evaluators were blinded, outcome data were selectively reported, outcome data were incomplete, and other biases. Each aspect was assessed as low, high, or uncertain risk. Any disputes were discussed and resolved by a third team member.
To evaluate the effectiveness and safety of the different treatment regimens, all evidence from direct and indirect comparisons was combined. The primary outcome indicators in this study included ORR and ≥3 AEs. The secondary outcome indicators included OS, PFS, DFS, LRC, and DSS. Odds ratios (ORs) and their 95% CIs were used to assess the effectiveness and safety of the two major outcome measures. HR and 95% CI were used as effect measures to assess the survival indicators. Considering the large number of original studies that reported primary outcome indicators, the different treatment regimens were divided into two categories: 1) neoadjuvant chemotherapy combined with concurrent chemo-radiotherapy (CCRT) and 2) systemic treatment with concurrent RT. The Bayesian method was employed to fit the model in this study because this model is more accurate and stable than the traditional classical frequency method. STATA.15 and R 3.6.0 software were applied to analyze the outcome indicators.
First, we pre-processed the data using STAT5 software and plotted a network diagram of ORRs and ≥3 AEs to visualize the direct and indirect comparisons between the different treatment regimens. The circles represent the different treatment regimens. The circle sizes are proportional to the number of patients (Chaimani et al., 2013). The line thickness indicates the number of clinical trials. Study inconsistencies were evaluated, and an effect value was calculated by separately fitting the consistency and inconsistency models. Then, different treatment regimens were ranked according to the cumulative probability ranking calculated using Bayesian analysis. Subsequently, a traditional head-to-head meta-analysis of clinical trials with the same intervention regimen was conducted, and forest plots were drawn. Study heterogeneity was evaluated using Q-tests and I2, with p < 0.05 or I2>50% considered significant consistency (Higgins et al., 2003). Both the fixed and random effects model was chosen based on the heterogeneity results. Finally, funnel plots were drawn to assess publication bias.
The Gemtc and Coda packages in R were applied to pre-process the data, plot survival indicator network, fit the consistency model, and create an effect value matrix (White et al., 2012). Deviance information criteria (DIC) were calculated, and Markov chain Monte Carlo (MCMC) iterations (20,000 times) were performed. Convergence diagnostic plots, trajectory plots, and distribution density plots were also drawn (Supplementary Figure S1). Heterogeneity tests were performed to assess the reliability of the results (Supplementary Figure S2). Node-splitting methods were applied to assess the inconsistency of the results and to suggest stability. Finally, the different treatment regimens were ranked according to surface under the cumulative ranking (SUCRA) values and the stacked ranking chart and individual ranking scale were plotted. All tests were bilateral. Statistical significance was set at p < 0.05.
We found 18,715 articles, 15,542 of which were selected through initial automatic and manual checking. A total of 15,068 articles were removed after browsing the titles and abstracts. Among these articles, 1878 were reviews and meta-analyses, 31 were case reports, 12,731 were unrelated studies, and 428 were trial design protocols. The remaining 126 RCTs were included in this study, comprising a total of 22,726 LA-HNSCC patients. The detailed process of literature search and screening is shown in Figure 1.
This study included 126 RCTs that were conducted in different countries and regions between 1975 and 2021. The basic characteristics of all the original studies included in this study are summarized in Table 1. Because no significant differences were found in the included patient populations, the transferability of this study was considered acceptable.
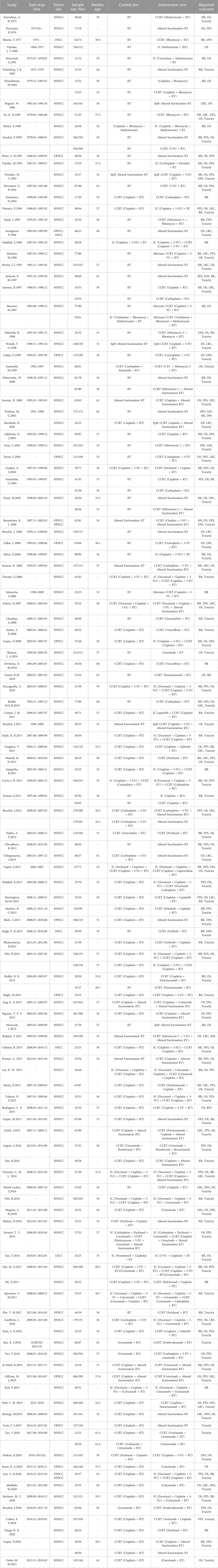
TABLE 1. Baseline characteristics included in a network meta-analysis of the treatment of locally advanced HNSCC patients.
As expected, the methodological quality assessment identified that most of the studies were low risk in terms of random sequence generation and selective reporting. Most studies did not clarify whether blinding and allocation concealment were used. Therefore, we believe that selection bias may exist. The detailed bias risk assessment results are shown in Supplementary Figure S3.
There were 86 RCTs trials reporting ORR, including 12 IC combined with CCRT regimens and 27 systemic treatments with concurrent RT regimens (Figure 2A). The results showed that IC combined with CCRT regimens failed to achieve a significant ORR benefit compared with RT alone (Figure 3A). In contrast, 11 clinical trials compared conventional fractionated RT with accelerated fractionated RT (AFRT). The results showed that AFRT had a better ORR than RT (Figure 3B; Supplementary Figure S4). Similarly, CCRT (cisplatin + AFRT) had a significantly better ORR than CCRT (cisplatin + RT) (Figure 3B). All 12 systemic treatments with concurrent RT regimens were significantly more effective than RT alone (Figure 3B), including CCRT (5-Fluorouracil + RT), CCRT (bleomycin + RT), CCRT (cisplatin+5-Fluorouracil + AFRT), CCRT (cisplatin + AFRT), CCRT (cisplatin + gemcitabine + RT), CCRT (cisplatin + lapatinib + RT), CCRT(cisplatin + nimotuzumab + RT), CCRT (cisplatin + RT), CCRT(erlotinib + cisplatin + RT), CCRT(mitomycin C + bleomycin + RT), CCRT(vinorelbine + RT), CCRT(paclitaxel + RT). Meanwhile, CCRT (cisplatin + nimotuzumab + RT) showed a better ORR than conventional CCRT regimens, such as CCRT (cisplatin + RT), CCRT (gemcitabine + RT), CCRT (mitomycin C + AFRT), CCRT (panitumumab + cisplatin + RT), and so on.
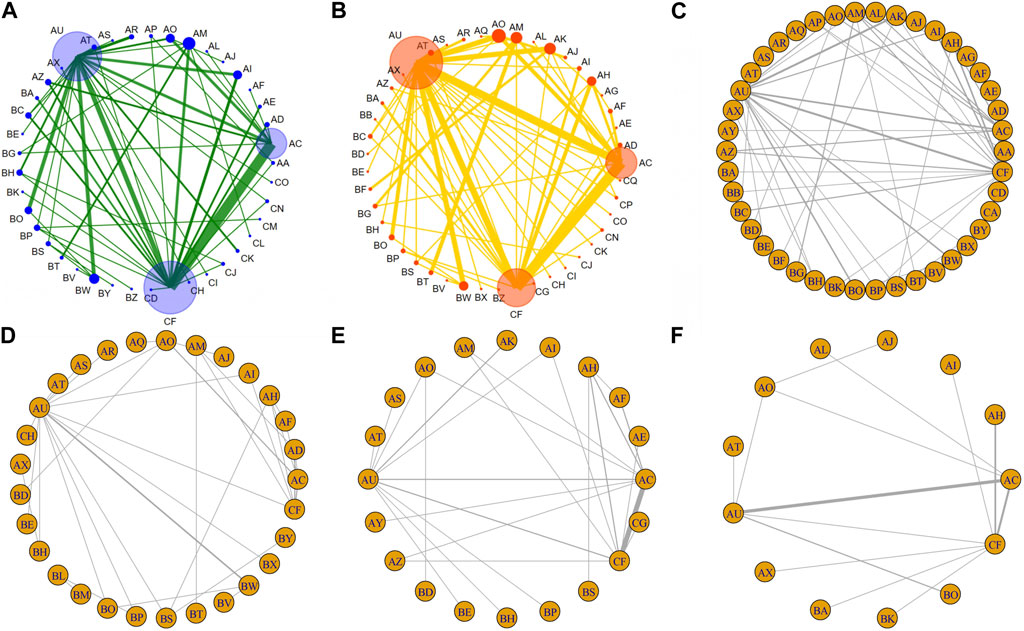
FIGURE 2. Network diagram comparing treatment outcomes of LA-HNSCC patients with different treatment options. (A) ORR. (B) ≥3 AEs. (C) OS. (D) PFS. (E) LRC. (F) DFS. AA: (Cisplatin + Bleomycin), AB: (Cisplatin + Bleomycin + Methotrexate), AC: AFRT, AD: CCRT(5-Fluorouracil + RT), AE: CCRT(Bleomycin + RT), AF: CCRT(Carboplatin+5-Fluorouracil + AFRT), AG: CCRT(Carboplatin+5-Fluorouracil + Cetuximab + RT), AH: CCRT(Carboplatin+5-Fluorouracil + RT), AI: CCRT(Carboplatin + RT), AJ: (Cetuximab + AFRT), AK: (Cetuximab + RT), AL: CCRT(Cisplatin + 5-Fluorouracil + AFRT), AM: CCRT(Cisplatin + 5-Fluorouracil + RT), AN: CCRT(Cisplatin+5-Fluorouracil + RT)/(Cetuximab + RT), AO: CCRT(Cisplatin + AFRT), AP: CCRT(Cisplatin + Bleomycin + RT), AQ: CCRT(Cisplatin + Cetuximab + AFRT), AR: CCRT(Cisplatin + Gemcitabine + RT), AS: CCRT(Cisplatin + Lapatinb + RT), AT: CCRT(Cisplatin + Nimotuzumab + RT), AU: CCRT(Cisplatin + RT), AV: CCRT(Docetaxel + Cisplatin + 5-Fluorouracil + AFRT), AW: CCRT(Docetaxel + Cisplatin + 5-Fluorouracil + RT), AX: CCRT(Gemcitabine + RT), AY: CCRT(Mitomycin + 5-Fluorouracil + AFRT), AZ: CCRT(Mitomycin + AFRT), BA: CCRT(Mitomycin + Bleomycin + RT), BB: CCRT(Methotrexate + RT), BC: CCRT(Nimotuzumab + RT), BD: CCRT(Panitumumab + AFRT), BE: CCRT(Panitumumab + Cisplatin + RT), BF: CCRT(Pemetrexed + RT), BG: CCRT(Paclitaxel + Cisplatin + RT), BH: CCRT(Paclitaxel + RT), BI: CCRT(Vinblastine + Bleomycin + Methotrexate + RT), BJ: IC(5-Fluorouracil + Cisplatin) + RT, BK: IC(Carboplatin + Teganox)+RT, BL: IC(Carboplatin + Paclitaxel + Cetuximab)+CCRT(Cisplatin + Cetuximab + AFRT), BM: IC(Carboplatin + Paclitaxel + Cetuximab)+CCRT(HU+5-Fluorouracil + Cetuximab + AFRT), BN: IC(Cisplatin + 5-Fluorouracil) + CCRT(Carboplatin + RT), BO: IC(Cisplatin + 5-Fluorouracil) + CCRT(Cisplatin + RT), BP: IC(Cisplatin + 5-Fluorouracil) + RT, BQ: IC(Cisplatin + Bleomycin + Methotrexate) + RT, BR: IC(Docetaxel + Cisplatin + 5-Fluorouracil) + CCRT(Carboplatin + RT), BS: IC(Docetaxel + Cisplatin + 5-Fluorouracil)+ (cetuximab + RT), BT: IC(Docetaxel + Cisplatin + 5-Fluorouracil) + CCRT(Cisplatin + 5-Fluorouracil + RT), BU: IC(Docetaxel + Cisplatin+5-Fluorouracil) + CCRT(Cisplatin + 5-Fluorouracil + RT)/(Cetuximab + RT), BV: IC(Docetaxel + Cisplatin+5-Fluorouracil) + CCRT(Cisplatin + AFRT), BW: IC(Docetaxel + Cisplatin + 5-Fluorouracil) + CCRT(Cisplatin + RT), BX: IC(Docetaxel + Cisplatin+5-Fluorouracil) + CCRT(Docetaxel/Carboplatin + RT), BY: IC(Docetaxel + Cisplatin + Cetuximab)+ (cetuximab + RT), BZ: IC(Pemetrexed + Cisplatin)+RT, CA: IC(Methotrexate)+RT, CB: IC(Paclitaxel + Cisplatin) + CCRT(Cisplatin + 5-Fluorouracil + RT), CC: IC(Paclitaxel + Cisplatin) + CCRT(Cisplatin + Capecitabine + RT), CD: IC(Vincristine + Methotrexate)+RT, CE: IC(Vinblastine + Bleomycin + Methotrexate)+RT, CF:RT, CG: CCRT(Mitomycin + RT), CH: CCRT(Erlotinib + Cisplatin + RT), CI: CCRT(Cisplatin + Capecitabine + RT), CJ: CCRT(Gemcitabine + RT), CK: CCRT(Vinorelbine + RT), CL: CCRT(Raloxifen + Cisplatin + RT), CM: IC(Cisplatin + 5-Fluorouracil)+CCRT(Cisplatin + 5-Fluorouracil + RT), CN: IC(Cisplatin)+RT, CO: IC(Lapatinb) + CCRT(Cisplatin + RT), CP: CCRT(Avelumab + Cetuximab + RT), CQ: CCRT(Avelumab + Cisplatin + RT).
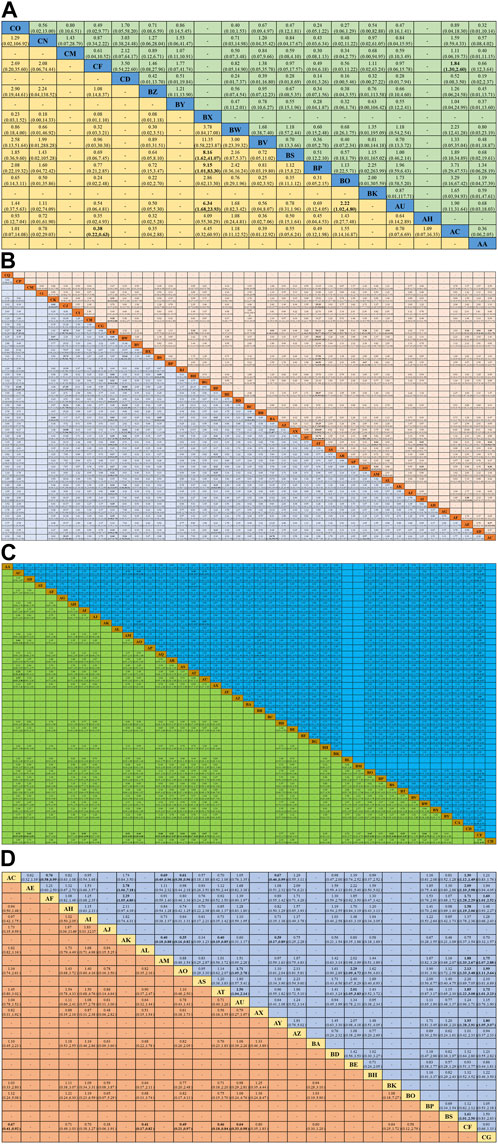
FIGURE 3. Summary of network meta-analysis. (A) Combined odds ratio (95% onfidence interval) of ORR (upper triangle) and ≥3 AEs (lower triangle) for IC combined with CCRT regimens; (B) Combined hazard ratio (95% onfidence interval) of ORR (upper triangle) and ≥3 AEs (lower triangle) for systemic treatment with concurrent RT regimens; (C) Combined hazard ratio (95% confidence interval) for OS (upper triangle) and PFS (lower triangle); (D) Combined hazard ratio (95% confidence interval) for LRC (upper triangle) and DFS (lower triangle). The data in each cell is a risk ratio or hazard ratio (95% confidence interval) that is used to compare the column versus the row. Statistically significant results are shown in bold.
Figures 4A,B; Supplementary Table S3 shows the Bayesian ranking results for the different treatment regimens. SUCRA values were used to rank the different treatment regimens, with higher values indicating higher efficiency (Supplementary Table S4). Similar to the consistency model results, almost all systemic treatments with concurrent RT regimens had better ORR than RT alone. In addition, within the IC combined with CCRT regimens, only IC (docetaxel + cisplatin+5-Fluorouracil) combined with cetuximab and concurrent with RT regimen showed a better ORR compared to RT alone. Meanwhile, CCRT (cisplatin + nimotuzumab + RT) had the highest SUCRA values, while nimotuzumab concurrent with RT regimen was superior to most of CCRT regimens.
FIGURE 4. Bayesian sequencing of the efficacy of different treatment regimens in LA-HNSCC patients. (A) ORR of IC combined with CCRT regimens; (B) ORR of systemic treatment with concurrent RT regimens; (C) ≥ 3AEs of IC combined with CCRT regimens; (D) ≥ 3AEs of systemic treatment with concurrent RT regimens; (E) OS; (F) PFS; (G) LRC; (H) DFS. The sorting graph is described based on bayesian sorting results in Supplementary Table S3.
There were 86 RCT trials reporting ≥3 AEs, including 9 IC combined with CCRT treatment regimens and 35 systemic treatments with concurrent RT regimens (Figure 2B). The results demonstrated that AFRT may occur with higher proportions of ≥3 AEs compared to RT with statistically significant differences (Figure 3B; Supplementary Figure S5). IC (docetaxel + cisplatin+5-Fluorouracil) combined with CCRT (docetaxel/carboplatin + RT) regimen was associated with a higher proportion of ≥3 AEs than IC (docetaxel + cisplatin+5-Fluorouracil) combined with cetuximab concurrent with RT and IC (cisplatin+5-Fluorouracil) combined with RT (Figure 3A). Compared to RT alone, 15 systemic treatments with concurrent RT regimens showed significantly more ≥3 AEs, including CCRT (5-Fluorouracil + RT), CCRT (avelumabe + cetuximab + RT), CCRT (carboplatin+5-Fluorouracil + AFRT), CCRT (carboplatin+5-Fluorouracil + RT), CCRT (cisplatin+5-Fluorouracil + RT), CCRT (cisplatin + AFRT), CCRT (cisplatin + gemcitabine + RT), CCRT (cisplatin + nimotuzumab + RT), CCRT (cisplatin + RT), CCRT (mitomycin C + bleomycin + RT), CCRT (panitumumab + AFRT), CCRT (paclitaxel + cisplatin + RT), CCRT(paclitaxel + RT), and cetuximab concurrent with RT or AFRT regimens. Notably, CCRT (nimotuzumab + RT) showed a statistically significant lower ≥3 AE compared to various common treatment regimens, with statistically significant differences, including CCRT (cisplain + capecitabine + RT), CCRT (mitomycin C + bleomycin + RT) and IC(docetaxel + cisplain+5-Fluorouracil) combined with CCRT(cisplain + RT).
The results of the Bayesian ranking of ≥3 AEs for the different treatment regimens are shown in Figures 4C,D; Supplementary Table S3. SUCRA values were used to rank the different treatment regimens, with higher values indicating lower ≥3 AEs (Supplementary Table S4). Like the consistency model results, almost all systemic treatments with concurrent RT regimens and IC combined with CCRT treatment regimens exhibited higher ≥3 AEs compared with RT alone. The ≥3 AEs of IC (docetaxel + cisplatin+5-Fluorouracil) combined with CCRT (docetaxel/carboplatin + RT) was significantly higher than that of almost all the other treatment regimens. Nimotuzumab concurrent with RT regimen had the highest SUCRA values, followed by RT alone.
There were 93 RCTs reporting OS, including 36 systemic treatments with concurrent RT regimens and 17 IC combined with CCRT regimens (Figure 2C). Of these, 15 treatment regimens showed more favorable OS than RT alone: AFRT, CCRT (5-Fluorouracil + RT), CCRT (carboplatin+5-Fluorouracil + AFRT), CCRT (carboplatin+5-Fluorouracil + RT), CCRT (carboplatin + RT), CCRT (cisplatin+5-Fluorouracil + AFRT), CCRT (cisplatin+5-Fluorouracil + RT), CCRT (cisplatin + AFRT), CCRT (cisplatin + cetuximab + AFRT), CCRT (cisplatin + nimotuzumab + RT), CCRT (cisplatin + RT), CCRT (mitomycin C + AFRT), IC (cisplatin+5-Fluorouracil) combined with CCRT (cisplatin + RT), IC (cisplatin+5-Fluorouracil) combined with RT, and IC (methotrexate) combined with RT. Notably, CCRT (cisplatin + nimotuzumab + RT) showed a statistically significant survival advantage compared to AFRT, CCRT (bleomycin + RT), and cetuximab concurrent with RT regimens. CCRT (cisplatin + RT), a commonly used CCRT regimen recommended by the NCCN guidelines, showed better OS than AFRT and cetuximab concurrent with RT regimens (Figure 3C).
Figure 4E; Supplementary Table S3 shows the Bayesian ranking results of the OS for different treatment regimens. SUCRA values were used to rank the different treatment regimens, with lower scores indicating higher OS (Supplementary Table S4). The results showed that CCRT (cisplatin + nimotuzumab + RT) had the lowest SUCRA scores and the best OS. Platinum-based CCRT regimens such as CCRT (cisplatin + AFRT), CCRT (cisplatin+5-Fluorouracil + RT), CCRT (cisplatin + gemcitabine + RT), and CCRT (cisplatin + RT) may provide better OS. Furthermore, conventional fractionated RT had higher SUCRA values and lower OS than almost all treatment regimens.
PFS was reported in 38 RCTs, including 22 systemic treatments with concurrent RT regimens and 15 IC combined with CCRT regimens (Figure 2D). Consistent with the OS results, 15 combination regimens showed better PFS than RT, including AFRT, CCRT (5-Fluorouracil + RT), CCRT (carboplatin + RT), CCRT (cisplatin+5-Fluorouracil + RT), CCRT (cisplatin + AFRT), CCRT (cisplatin + cetuximab + AFRT), CCRT (cisplatin + gemcitabine + RT), CCRT (cisplatin + lapatinib + RT), CCRT (cisplatin + nimotuzumab + RT), CCRT (cisplatin + RT), CCRT (erlotinib + cisplatin + RT), IC (cisplatin+5-Fluorouracil) combined with CCRT (cisplatin + RT), IC (docetaxel + cisplatin+5-Fluorouracil) combined with CCRT (cisplatin+5-Fluorouracil + RT), IC (docetaxel + cisplatin+5-Fluorouracil) combined with CCRT (cisplatin + RT), IC (docetaxel + cisplatin+5-Fluorouracil) combined with cetuximab concurrent RT,. Furthermore, CCRT (cisplatin + AFRT), CCRT (cisplatin + nimotuzumab + RT), and CCRT (cisplatin + RT) showed significantly better PFS than AFRT (Figure 3C).
Figure 4F; Supplementary Table S3 shows the results of the Bayesian ranking of PFS for different treatment regimens. SUCRA values were used to rank the different treatment regimens, with lower scores indicating higher PFS (Supplementary Table S4). Consistently, CCRT (cisplatin + nimotuzumab + RT) had the lowest SUCRA values and probably the best PFS. Almost all CCRT regimens had better PFS than the RT and AFRT regimens.
There were 32 RCTs reporting LRC, including 22 treatment regimens (Figure 2E). The results showed that AFRT, CCRT (bleomycin + RT), CCRT (carboplatin+5-Fluorouracil + AFRT), CCRT (carboplatin+5-Fluorouracil + RT), CCRT (cisplatin+5-Fluorouracil + RT), CCRT (cisplatin + AFRT), CCRT (mitomycin C+5-Fluorouracil + AFRT), and IC (docetaxel + cisplatin+5-Fluorouracil) combined with cetuximab concurrent with RT regimens showed better local control compared than RT alone. CCRT (mitomycin C + RT), CCRT (carboplatin+5-Fluorouracil + AFRT), CCRT (cisplatin+5-Fluorouracil + RT), CCRT (cisplatin + AFRT), CCRT (cisplatin + nimotuzumab + RT), and CCRT (mitomycin C+5-Fluorouracil + AFRT) showed better LRC. CCRT (cisplatin + AFRT), the gold standard recommended by the NCCN for LA-HNSCC patients, showed better local tumor control than RT, AFRT, CCRT (cisplatin + RT), CCRT (panitumumab + cisplatin + RT), CCRT (mitomycin C + RT), and cetuximab concurrent with RT regimens (Figure 3D).
Figure 4G; Supplementary Table S3 shows the Bayesian ranking results of LRC for different treatment regimens. SUCRA values were used to rank the different treatment regimens, with lower scores indicating better LRC (Supplementary Table S4). It is worth noting that CCRT (cisplatin + AFRT) had the lowest score and was most likely to achieve the highest LRC. In addition, RT and cetuximab concurrent with the RT regimen ranked first.
DFS was reported in 26 RCTs that included 19 treatment regimens (Figure 2F). The results showed that AFRT, CCRT (cisplatin+5-Fluorouracil + AFRT), CCRT (cisplatin + AFRT), CCRT (cisplatin + nimotuzumab + RT), and CCRT (cisplatin + RT) showed statistically significant differences in DFS compared to RT (Figure 3D). Bayesian ranking results showed that CCRT (cisplatin+5-Fluorouracil + AFRT) and CCRT (cisplatin + nimotuzumab + RT) have the lowest DFS and the RT rank first, respectively (Figure 4H; Supplementary Tables S3–S5).
DSS was reported in only 10 RCTs comprised of 9 treatment regimens (Supplementary Figure S6). There were no statistical differences among the treatment regimens after fitting the consistency model (Supplementary Figure S7). Bayesian ranking results showed that concurrent RT and cetuximab with an RT regimen had the lowest possible DSS (Supplementary Figure S8; Supplementary Tables S3–S4). CCRT (cisplatin+5-Fluorouracil + AFRT) and CCRT (cisplatin+5-Fluorouracil + RT) showed the lowest SUCRA values and a better DSS.
In addition, the consistency of results showed that the differences between all the direct and indirect comparisons were not statistically significant, shown in Supplementary Figure S9; Supplementary Figure S10. Further, the consistency model fit satisfactorily. Funnel plots were drawn to assess publication bias (Supplementary Figure S11; Supplementary Figure S12).
In recent years, remarkable progress has been made in LA-HNSCC treatment. Nevertheless, the treatment options for LA-HNSCC patients remain a complex topic. Therefore, we conducted a systematic review and network meta-analysis of multiple treatment regimens for LA-HNSCC. A total of 126 RCTs were included in this network meta-analysis to summarize the evidence on the efficacy and safety of different treatment options.
To date, this is the most comprehensive review of the safety and efficacy of full treatment options for LA-HNSCC patients. The main study findings are summarized as follows: CCRT (cisplatin + nimotuzumab + RT) and nimotuzumab concurrent with RT have significant advantages in both efficacy and long-term survival compared with various conventional LA-HNSCC treatment regimens, including platinum-based CCRT regimens (including cisplatin and carboplatin). At the same time, it was surprising that CCRT (cisplatin + nimotuzumab + RT) and nimotuzumab concurrent with RT did not cause a higher proportion of ≥3 AEs than most combination regimens. This suggests that CCRT (cisplatin + nimotuzumab + RT) and nimotuzumab combined with RT may be the most promising treatment option for LA-HNSCC. The efficacy and safety advantages of CCRT (cisplatin + nimotuzumab + RT) and nimotuzumab concurrent with RT regimens warrant a higher-level recommendation by the NCCN guidelines. Our results provide further directions for using nimotuzumab in LA-HNSCC patients. IC combined with CCRT failed to show a statistically significant difference in efficacy compared to RT alone while incurring a significantly higher risk of AEs, suggesting that IC combined with CCRT regimens should be carefully chosen for LA-HNSCC patients. In addition, AFRT showed higher efficacy than RT, despite being associated with significantly increased AEs. Cetuximab concurrent with RT regimen as an alternative to platinum-based chemotherapy did not show a better advantage in OS, PFS, and DSS. In addition, cetuximab concurrent with RT regimen was associated with a higher rate of ≥3 AEs than RT, suggesting that the recommendation grade in future NCCN guidelines needs to be adjusted or removed.
This study provides a comprehensive analysis of the effectiveness and long-term survival of 78 combination treatment regimens by investigating six outcome indicators, including ORR, OS, PFS, LRC, DFS, and DSS. Before starting this study, an extensive and detailed search strategy was developed. The included data were comprehensive and included results from unofficially published data. The patient population and definition of outcome indicators included in the original studies were assessed to ensure the transferability of the study. Furthermore, an inconsistency examination was conducted to assess whether the direct and indirect comparisons were consistent. More importantly, the generally low I2 values indicated acceptable agreement, suggesting satisfactory confidence in our study.
Nevertheless, systematic reviews and network meta-analyses have certain limitations. First, not all outcome indicators from the original studies were analyzed, leading to the possibility that the strengths of some treatment regimens for a particular indicator may have been overlooked. Second, network meta-analyses simultaneously perform direct and indirect comparisons of multiple treatment regimens, but some studies contained only indirect comparisons between regimens. Indirect comparisons increase uncertainty for results assessment. On the other hand, the validity of indirect comparisons depends on the intrinsic validity and their similarity of the trials involved, so more indirect comparisons may reduce the reliability of the results. Therefore, the indirect comparison results should be cautiously applied in clinical practice. In the future, clinical trials which generated grade 1 evidence should be given increased attention. The SUCRA values obtained from Bayesian analysis reflected the ranking among multiple treatment regimens, but it was impossible to specifically identify the statistically significant differences, which resulted in some uncertainty in the results. Furthermore, most of the included original studies did not clearly articulate the use of blinding and allocation concealment, leading to a decline in methodological quality. Additionally, a total of 126 RCTs from 1975 to 2021 were included in this study, and the updates of tumor staging criteria and treatment regimens during this period increased the heterogeneity of the included studies. In addition, this study did not limit the patient race and tumor subregion from the original studies, which limits the generalizability of the results. Undoubtedly, standardizing the included patients will be vital for comparing and evaluating treatment options in future clinical trials.
This study aimed to determine the best choice of clinical treatment options by providing a comprehensive review of multiple treatment regimens that are currently applied to LA-HNSCC. We also evaluated treatment efficacy and safety by applying seven outcome indicators.
Previous studies confirmed that CCRT increases survival in LA-HNSCC patients, with a 6.5% increase in 5-year survival and significantly reduced local failure rates (Pignon et al., 2009; Lacas et al., 2021). The RTOG91-11 trial also confirmed that CCRT is the most effective method for local control and organ preservation in LA-HNSCC patients (Forastiere et al., 2013). High-dose platinum combined with RT is the accepted standard treatment regimen for improved OS of LA-HNSCC patients (Adelstein et al., 2003). In this study, we demonstrate that nimotuzumab-based CCRT regimens have higher efficacy and are not accompanied by a higher incidence of AEs than conventional CCRT regimens while potentially achieving better long-term survival. Nimotuzumab is safer than cetuximab, which has a higher rate of rash, probably because nimotuzumab is a fully-humanized monoclonal antibody, whereas cetuximab is a human-mouse chimeric antibody (Bonner et al., 2010; Xu et al., 2017). Although this finding needs to be confirmed by higher-quality RCT studies, it may provide a direction for future clinical trials. HNSCC is transforming from uniform intensive treatment focused simply on improving survival to individualized treatment based on a combination of biology, biomarkers, and immunotherapy. In addition, many ongoing RCTs are exploring the efficacy and safety of immunotherapy in LA-HNSCC (Weiss et al., 2020; Zech et al., 2020). Thus, we may obtain more evidence to support the efficacy of immunotherapy in the future.
Previously, IC was reported to reduce the risk of distant metastases (Marta et al., 2015; Li et al., 2021). IC has also been proposed to improve OS in LA-HNSCC patients (Bossi et al., 2014). In patients with unresectable LA-HNSCC, sequential treatment of IC and CCRT has been explored as an intensive treatment approach (Haddad et al., 2013; Cohen et al., 2014). However, the efficacy of IC remains controversial. IC (docetaxel + cisplatin+5-Fluorouracil) combined with CCRT showed no significant improvement in OS compared to CCRT alone (Hitt et al., 2014). This study shows that IC combined with CCRT regimens does not provide a better efficiency and survival benefit than RT alone but is associated with significantly higher AEs. Therefore, the feasibility of IC in LA-HNSCC patients requires further investigation. IC may be reasonably assessed as a screening method, with further options for intensive RT or CCRT depending on the patient’s response to IC (Chen et al., 2017; Marur et al., 2017). As expected, CCRT regimens had higher efficiency and better survival trends compared to RT alone, particularly nimotuzumab-based and conventional platinum-based CCRT regimens. In patients who cannot tolerate platinum-based chemotherapy, cetuximab combined with RT improves survival in LA-HNSCC patients compared with RT alone (Bonner et al., 2006). However, cetuximab concurrent with RT regimen did not show the desired benefit in this study, suggesting that this regimen should be carefully applied in future.
In this systematic review and network meta-analysis, we simultaneously evaluated the effectiveness and safety of various treatment regimens currently used for LA-HNSCC. Thus far, this study is the most comprehensive summary of treatment options available for LA-HNSCC patients. The results present the benefits of nimotuzumab-based systemic therapy regimens combined with RT in both effectiveness and long-term survival, suggesting their potential for LA-HNSCC treatment. This study reinforces the absolute benefits of AFRT in terms of both efficacy and prognosis compared to conventional fractionated RT and provides a reference for further selecting clinical RT regimens. In addition, we observed no significant improvement of cetuximab in combination with RT compared to RT alone. However, cetuximab combined with RT is accompanied by more significant AEs, suggesting that cetuximab concurrent with RT regimen needs to be evaluated in further large-scale trials.
HW: Investigation, Writing–original draft. ZZ: Investigation, Writing–original draft. YZ: Investigation, Resources, Writing–original draft. CB: Resources, Writing–original draft. JB: Resources, Writing–original draft. YX: Conceptualization, Writing–review and editing. XJ: Conceptualization, Funding acquisition, Writing–review and editing.
The authors declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Jilin Provincial Science and Technology Foundations (20210509003RQ and 20210402002GH), the Health Talents Special Project of the Jilin Provincial Finance Department (JLSWSRCZX 2021-065), the program of Changchun Science and Technology Development Plan (21ZY29), and the Achievement Transformation Guiding Foundation of the First Hospital of Jilin University (CGZHYD202012-029).
We would like to thank Editage (www.editage.cn) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1269863/full#supplementary-material
HNCs: Head and neck cancers; LA-HNSCC: Locally advanced head and neck squamous cell carcinoma; RT: radiotherapy; EGFR: epidermal growth factor receptor; FDA: Food and Drug Administration; LRC: local control; OS: overall survival; IC: induction chemotherapy; ORR: objective response rate; PFS: progression-free survival; PRISMA: preferred reporting items for systematic review and meta-analyses, RCTs: randomized controlled trials, DFS: disease-free survival, DSS: disease-specific survival, HR: hazard ratio, CI: confidence interval, OR: odds ratio, CCRT: concurrent radio-chemotherapy, SUCRA: surface under the cumulative ranking, NCCN: National Comprehensive Cancer Network, DIC: deviance information criteria, MCMC: Markov chain Monte Carlo, AE: adverse events.
Adelstein, D. J., Li, Y., Adams, G. L., Wagner, H., Kish, J. A., Ensley, J. F., et al. (2003). An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J. Clin. Oncol. 21 (1), 92–98. doi:10.1200/JCO.2003.01.008
Argiris, A., Karamouzis, M. V., Raben, D., and Ferris, R. L. (2008). Head and neck cancer. Lancet 371 (9625), 1695–1709. doi:10.1016/S0140-6736(08)60728-X
Bonner, J. A., Harari, P. M., Giralt, J., Azarnia, N., Shin, D. M., Cohen, R. B., et al. (2006). Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 354 (6), 567–578. doi:10.1056/NEJMoa053422
Bonner, J. A., Harari, P. M., Giralt, J., Cohen, R. B., Jones, C. U., Sur, R. K., et al. (2010). Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 11 (1), 21–28. doi:10.1016/S1470-2045(09)70311-0
Bossi, P., Lo Vullo, S., Guzzo, M., Mariani, L., Granata, R., Orlandi, E., et al. (2014). Preoperative chemotherapy in advanced resectable OCSCC: Long-term results of a randomized phase III trial. Ann. Oncol. 25 (2), 462–466. doi:10.1093/annonc/mdt555
Braakhuis, B. J., Brakenhoff, R. H., and Leemans, C. R. (2012). Treatment choice for locally advanced head and neck cancers on the basis of risk factors: Biological risk factors. Ann. Oncol. 23 (10), x173–x177. doi:10.1093/annonc/mds299
Chaimani, A., Higgins, J. P. T., Mavridis, D., Spyridonos, P., and Salanti, G. (2013). Graphical tools for network meta-analysis in STATA. PLoS One 8 (10), e76654. doi:10.1371/journal.pone.0076654
Chen, A. M., Felix, C., Wang, P. C., Hsu, S., Basehart, V., Garst, J., et al. (2017). Reduced-dose radiotherapy for human papillomavirus-associated squamous-cell carcinoma of the oropharynx: A single-arm, phase 2 study. Lancet Oncol. 18 (6), 803–811. doi:10.1016/S1470-2045(17)30246-2
Chow, L. Q. M. (2020). Head and neck cancer. N. Engl. J. Med. 382 (1), 60–72. doi:10.1056/NEJMra1715715
Chung, C. H., Ely, K., McGavran, L., Varella-Garcia, M., Parker, J., Parker, N., et al. (2006). Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. J. Clin. Oncol. 24 (25), 4170–4176. doi:10.1200/JCO.2006.07.2587
Cohen, E. E., Karrison, T. G., Kocherginsky, M., Mueller, J., Egan, R., Huang, C. H., et al. (2014). Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J. Clin. Oncol. 32 (25), 2735–2743. doi:10.1200/JCO.2013.54.6309
Cumpston, M., Li, T., Page, M. J., Chandler, J., Welch, V. A., Higgins, J. P., et al. (2019). Updated guidance for trusted systematic reviews: A new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst. Rev. 10, Ed000142. doi:10.1002/14651858.ED000142
Forastiere, A. A., Zhang, Q., Weber, R. S., Maor, M. H., Goepfert, H., Pajak, T. F., et al. (2013). Long-term results of RTOG 91-11: A comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J. Clin. Oncol. 31 (7), 845–852. doi:10.1200/JCO.2012.43.6097
Grandis, J. R., and Tweardy, D. J. (1993). Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. 53 (15), 3579–3584.
Haddad, R., O'Neill, A., Rabinowits, G., Tishler, R., Khuri, F., Adkins, D., et al. (2013). Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): A randomised phase 3 trial. Lancet Oncol. 14 (3), 257–264. doi:10.1016/S1470-2045(13)70011-1
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. Bmj 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Hitt, R., Grau, J. J., López-Pousa, A., Berrocal, A., García-Girón, C., Irigoyen, A., et al. (2014). A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann. Oncol. 25 (1), 216–225. doi:10.1093/annonc/mdt461
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern Med. 162 (11), 777–784. doi:10.7326/M14-2385
Ishitoya, J., Toriyama, M., Oguchi, N., Kitamura, K., Ohshima, M., Asano, K., et al. (1989). Gene amplification and overexpression of EGF receptor in squamous cell carcinomas of the head and neck. Br. J. Cancer 59 (4), 559–562. doi:10.1038/bjc.1989.113
Kramer, S., Gelber, R. D., Snow, J. B., Marcial, V. A., Lowry, L. D., Davis, L. W., et al. (1987). Combined radiation therapy and surgery in the management of advanced head and neck cancer: Final report of study 73-03 of the radiation therapy oncology group. Head. Neck Surg. 10 (1), 19–30. doi:10.1002/hed.2890100105
Lacas, B., Carmel, A., Landais, C., Wong, S. J., Licitra, L., Tobias, J. S., et al. (2021). Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 107 randomized trials and 19,805 patients, on behalf of MACH-NC Group. Radiother. Oncol. 156, 281–293. doi:10.1016/j.radonc.2021.01.013
Li, X., Fang, Q., Du, W., Zhang, X., Dai, L., and Qiao, Y. (2021). Induction chemotherapy combined with immunotherapy in locally advanced head and neck squamous cell carcinoma. BMC Cancer 21 (1), 622. doi:10.1186/s12885-021-08373-8
Liang, Y., Zhang, T., and Zhang, J. (2020). Natural tyrosine kinase inhibitors acting on the epidermal growth factor receptor: Their relevance for cancer therapy. Pharmacol. Res. 161, 105164. doi:10.1016/j.phrs.2020.105164
Marta, G. N., Riera, R., Bossi, P., Zhong, L. p., Licitra, L., Macedo, C. R., et al. (2015). Induction chemotherapy prior to surgery with or without postoperative radiotherapy for oral cavity cancer patients: Systematic review and meta-analysis. Eur. J. Cancer 51 (17), 2596–2603. doi:10.1016/j.ejca.2015.08.007
Marur, S., and Forastiere, A. A. (2016). Head and neck squamous cell carcinoma: Update on epidemiology, diagnosis, and treatment. Mayo Clin. Proc. 91 (3), 386–396. doi:10.1016/j.mayocp.2015.12.017
Marur, S., Li, S., Cmelak, A. J., Gillison, M. L., Zhao, W. J., Ferris, R. L., et al. (2017). E1308: Phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx- ECOG-ACRIN cancer research group. J. Clin. Oncol. 35 (5), 490–497. doi:10.1200/JCO.2016.68.3300
Pfister, D. G., Spencer, S., Adelstein, D., Adkins, D., Anzai, Y., Brizel, D. M., et al. (2020). Head and neck cancers, version 2.2020, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc Netw. 18 (7), 873–898. doi:10.6004/jnccn.2020.0031
Pfister, D. G., Spencer, S., Brizel, D. M., Burtness, B., Busse, P. M., Caudell, J. J., et al. (2014). Head and neck cancers, Version 2.2014. Clinical practice guidelines in oncology. J. Natl. Compr. Canc Netw. 12 (10), 1454–1487. doi:10.6004/jnccn.2014.0142
Pignon, J. P., le Maître, A., Maillard, E., and Bourhis, J.MACH-NC Collaborative Group (2009). Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother. Oncol. 92 (1), 4–14. doi:10.1016/j.radonc.2009.04.014
Pirker, R., Pereira, J. R., von Pawel, J., Krzakowski, M., Ramlau, R., Park, K., et al. (2012). EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: Analysis of data from the phase 3 FLEX study. Lancet Oncol. 13 (1), 33–42. doi:10.1016/S1470-2045(11)70318-7
Rodríguez, M. O., Rivero, T. C., del Castillo Bahi, R., Muchuli, C. R., Bilbao, M. A., Vinageras, E. N., et al. (2010). Nimotuzumab plus radiotherapy for unresectable squamous-cell carcinoma of the head and neck. Cancer Biol. Ther. 9 (5), 343–349. doi:10.4161/cbt.9.5.10981
Rubin Grandis, J., Melhem, M. F., Gooding, W. E., Day, R., Holst, V. A., Wagener, M. M., et al. (1998). Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J. Natl. Cancer Inst. 90 (11), 824–832. doi:10.1093/jnci/90.11.824
Sahin, A. A., Gilligan, T. D., and Caudell, J. J. (2019). Challenges with the 8th edition of the AJCC cancer staging manual for breast, testicular, and head and neck cancers. J. Natl. Compr. Canc Netw. 17 (5), 560–564. doi:10.6004/jnccn.2019.5015
Seiwert, T. Y., Burtness, B., Mehra, R., Weiss, J., Berger, R., Eder, J. P., et al. (2016). Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncol. 17 (7), 956–965. doi:10.1016/S1470-2045(16)30066-3
Seiwert, T. Y., and Cohen, E. E. (2005). State-of-the-art management of locally advanced head and neck cancer. Br. J. Cancer 92 (8), 1341–1348. doi:10.1038/sj.bjc.6602510
Siegel, R. L., Miller, K. D., and Jemal, A. (2018). Cancer statistics. CA Cancer J. Clin. 68 (1), 7–30. doi:10.3322/caac.21442
Siegel, R. L., Miller, K. D., and Jemal, A. (2020). Cancer statistics. CA Cancer J. Clin. 70 (1), 7–30. doi:10.3322/caac.21590
Sindhu, S. K., and Bauman, J. E. (2019). Current concepts in chemotherapy for head and neck cancer. Oral Maxillofac. Surg. Clin. North Am. 31 (1), 145–154. doi:10.1016/j.coms.2018.09.003
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Weiss, J., Sheth, S., Deal, A. M., Grilley Olson, J. E., Patel, S., Hackman, T. G., et al. (2020). Concurrent definitive immunoradiotherapy for patients with stage III-IV head and neck cancer and cisplatin contraindication. Clin. Cancer Res. 26 (16), 4260–4267. doi:10.1158/1078-0432.CCR-20-0230
White, I. R., Barrett, J. K., Jackson, D., and Higgins, J. P. T. (2012). Consistency and inconsistency in network meta-analysis: Model estimation using multivariate meta-regression. Res. Synth. Methods 3 (2), 111–125. doi:10.1002/jrsm.1045
Wyss, A., Hashibe, M., Chuang, S. C., Lee, Y. C. A., Zhang, Z. F., Yu, G. P., et al. (2013). Cigarette, cigar, and pipe smoking and the risk of head and neck cancers: Pooled analysis in the international head and neck cancer epidemiology consortium. Am. J. Epidemiol. 178 (5), 679–690. doi:10.1093/aje/kwt029
Xu, M. J., Johnson, D. E., and Grandis, J. R. (2017). EGFR-targeted therapies in the post-genomic era. Cancer Metastasis Rev. 36 (3), 463–473. doi:10.1007/s10555-017-9687-8
Zech, H. B., Moeckelmann, N., Boettcher, A., Muenscher, A., Binder, M., Vettorazzi, E., et al. (2020). Phase III study of nivolumab alone or combined with ipilimumab as immunotherapy versus standard of care in resectable head and neck squamous cell carcinoma. Future Oncol. 16 (36), 3035–3043. doi:10.2217/fon-2020-0595
Keywords: locally advanced head and neck squamous cell carcinoma, meta-analysis, concurrent chemo-radiotherapy, radiotherapy, accelerated fractionated radiotherapy
Citation: Wang H, Zheng Z, Zhang Y, Bian C, Bao J, Xin Y and Jiang X (2023) Locally advanced head and neck squamous cell carcinoma treatment efficacy and safety: a systematic review and network meta-analysis. Front. Pharmacol. 14:1269863. doi: 10.3389/fphar.2023.1269863
Received: 31 July 2023; Accepted: 04 September 2023;
Published: 19 September 2023.
Edited by:
Zhiyu Zhang, Fourth Affiliated Hospital of China Medical University, ChinaReviewed by:
Ye Zhang, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaCopyright © 2023 Wang, Zheng, Zhang, Bian, Bao, Xin and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Xin, eGlueUBqbHUuZWR1LmNu; Xin Jiang, amlhbmd4QGpsdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.