- School of Traditional Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
Background: ShenQiWan is commonly used in traditional Chinese medicine for the treatment of diabetic nephropathy, which is closely related to mitochondrial fusion and endoplasmic reticulum stress. This study aimed to investigate the intervention effect and molecular mechanisms of ShenQiWan on renal injury in KKAy mice.
Methods: C57BL/6J mice (11 weeks old) were fed a regular diet upon arrival, while KKAy mice (11 weeks old) were fed a high-fat diet upon arrival. At 12 weeks of age, KKAy mice with random blood glucose ≥13.9 mmol/L were identified as diabetic mice and randomly divided into the model group (n = 30) and the treatment group (n = 30), while C57BL/6J mice of 12 weeks old (n = 30) served as the control group. The treatment group received daily aqueous decoction of ShenQiWan (13.5 g/kg), while the control group and model group received daily equal amounts of saline from 12 weeks old to 24 weeks old. The general status of mice was observed regularly, and fasting blood glucose and 24-hour urine microalbumin were measured. Ten mice were euthanized in each group at the age of 16, 20, and 24 weeks, serum samples were used for biochemical indexes and kidney tissues were used for morphological studies. GRP78, OPA1, MFN1, MFN2 mRNA and protein expression were detected by Real-time PCR, immunohistochemistry and Western blot.
Results: The mice in the model group exhibited symptoms of lethargy, slow movement, obesity, polyuria and proteinuria. Morphological observation revealed pathological changes, including thickening of the glomerular basement membrane and interstitial fibrosis. After treatment with ShenQiWan, the fasting blood glucose level of KKAy mice was significantly reduced, urinary albuminuria was decreased, serum biochemical indexes were improved, renal tissue pathological changes were significantly alleviated. The results also showed a significant reduction in the expression of endoplasmic reticulum stress-related factor GRP78 and an increase in the expression of mitochondrial fusion-related factors OPA1, MFN1 and MFN2 after treatment with ShenQiWan.
Conclusion: ShenQiWan can protect diabetic mice from renal damage by modulating mitochondrial fusion and alleviating endoplasmic reticulum stress, exerting its protective effects.
Introduction
Diabetic Nephropathy (DN) is the most severe diabetic microvascular complication, which is also a significant cause of end-stage renal disease (Umanath and Lewis, 2018; Bonner et al., 2020). Due to the complex development process of DN, its pathogenesis remains incompletely understood, making it a hot topic of research both domestically and internationally. The endoplasmic reticulum (ER) is an important organelle involved in protein synthesis, folding, modification and cell homeostasis. Various pathological factors can disrupt ER homeostasis and trigger endoplasmic reticulum stress (ERS) (Ni et al., 2021; Zhao et al., 2022). Numerous studies have demonstrated that ERS plays an essential role in the occurrence and progression of DN (Fan et al., 2017; Shahzad et al., 2020; Pontes-da-Silva et al., 2022). The kidney contains abundant mitochondria, second only to the heart in terms of quantity. Extensive research has also confirmed the importance of intact mitochondrial structure and function for renal health (Hallan and Sharma, 2016). Impaired mitochondrial function, leading to inadequate ATP supply and excessive generation of reactive oxygen species (ROS), is closely associated with the development of DN (Saxena et al., 2019).
DN falls under the category of “Xiao Ke disease” in traditional Chinese medicine. As one of the representative prescriptions of “Synopsis of the Golden Chamber,” ShenQiWan has the effect of nourishing essence, enriching the marrow, nourishing kidney yin and tonifying kidney yang, and has achieved therapeutic efficacy in the prevention and treatment of diabetic nephropathy (Hu et al., 2021). Previous studies by our research group have also demonstrated that ShenQiWan can alleviate the progression of diabetic nephropathy by influencing endoplasmic reticulum stress (Yumeng et al., 2022; Jiapeng et al., 2023). Currently, research on the treatment of DN with ShenQiWan primarily focuses on clinical efficacy observation, with limited basic research. Furthermore, studies on the mechanism of DN in recent years also have mainly focused on glucose and lipid metabolism disorders and metabolic inflammation, insulin resistance, fibrosis, autophagy and genetics (Thomas et al., 2015; Donate-Correa et al., 2020; Zhongqing et al., 2023), with limited research on endoplasmic reticulum stress and mitochondrial function (Ni and Yuan, 2021). Therefore, it is crucial to clarify the molecular mechanism of ShenQiWan in the treatment of DN for its clinical application. In this study, KKAy mice, a type 2 diabetes model, were fed a high-fat diet to induce severe obesity and diabetes. Our study aims to investigate the specific mechanisms of ShenQiWan in the treatment of DN by observing its effects on renal histopathology in KKAy mice and examining the key factor of endoplasmic reticulum stress, such as Glucose-regulated protein 78 (GRP78), and key factors of mitochondrial fusion, such as Optic atrophy 1 (OPA1), Mitofusin-1 (Mfn1), Mitofusin-2.
Materials and methods
Reagents
ShenQiWan (Radix Rehmanniae 24 g, Rhizoma Dioscoreae 12 g, Fructus Corni 12 g, Rhizoma Alismatis 9 g, Poria 9 g, Cortex Moutan 9 g, Ramulus Cinnamomi 3 g, Radix Aconiti Lateralis 3 g), purchased from Beijing Tongrentang; Modified Masson trichrome staining solution (Beijing Suolaibao Technology Co., Ltd., batch number G1345); Glycogen PAS Staining Kit (Beijing Suo Lai Bao Technology Co., Ltd., batch number G1281); Hexaamine silver staining solution (Beijing Regan Biotechnology Co., Ltd., batch number DG0090); mRNA Reverse Transcription Kit (Thermo Fisher Scientific, USA, batch number 32220); MFN1 Polyclonal antibody (Proteintech Corporation, USA, batch number: 13798-1-AP); MFN2 Polyclonal antibody (Proteintech, USA, batch number: 12186-1-AP); anti-OPA1 antibody (Abcam, USA, lot number: ab157457); anti-GRP78 BiP antibody (Abcam, USA, lot number: ab108613).
Instruments
Blood glucose meter (hanghai Roche Diagnostic Products Co., Ltd.); Automatic biochemical analyzer (Zhongshan Xinrui Medical Equipment Technology Co., Ltd.); Transmission electron microscope (Hitachi, Japan); Synerqy II microplate reader (Bio-Tek, USA); Fluorescence quantitative PCR instrument, vertical electrophoresis system, wet electric transfer printing system (Bole Life Medical Products Co., Ltd.); Gel imager (Protein Simple, USA).
Animals and treatments
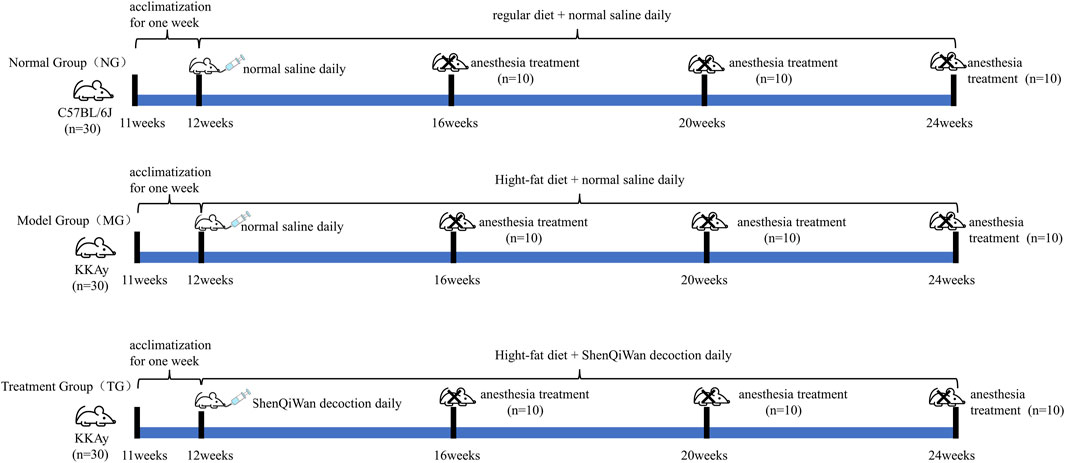
Sixty 11-week-old male KKAy mice, weighing 30–35 g; thirty 11-week-old male C57BL/6J healthy mice, weighing 25–30 g, were purchased from Beijing Huafukang Biotechnology Co., Ltd. (License No.: SCXK (Beijing) 2014-0004). All experimental mice were fed on the animal experimental platform of the Clinical Research Institute of Beijing China-Japan Friendship Hospital [SYXK (Beijing) 2016-0043], with daily free access to water and food, and the feeding environment was an independent ventilation system within the barrier, room temperature 23°C–25°C, relative humidity 50%–60%. During the whole experiment, KKAy mice were allowed access to a high-fat diet, while C57BL/6J mice were fed a regular diet. All mice were adaptively fed for 1 week, that is, at 12 weeks of age, KKAy mice were screened, grouped, and began to be administered. KKAy Mice with random blood glucose ≥13.9 mmol/L were identified as diabetic mice and randomly divided into the model group (n = 30) and the treatment group (n = 30), which continued to be fed a high-fat diet. The C57BL/6J mice served as the control group (n = 30) and were fed a regular diet. The treatment group received daily oral administration of ShenQiWan decoction (13.5 g/kg), while the model group and the control group received an equal volume of normal saline. Throughout the experiment, all groups of animals had free access to water and food. Ten mice from each group were euthanized at the age of 16 weeks, 20 weeks, and 24 weeks (Figure 1).
General state of mice and fasting blood glucose testing
The general conditions of the mice were observed daily, including changes in body weight, fur glossiness, changes in diet and water intake, mental status, and status before and after administration. Fasting blood glucose levels were measured every 2 weeks, and mice were fasted for 8 h before blood collection, and blood glucose concentration was measured via tail vein blood sampling method with a blood glucose meter.
Biochemical analysis
Ten mice were euthanized from each group at 16 weeks, 20 weeks and 24 weeks of age, blood was collected by eyeball blood collection method, and serum and plasma were separated. Total cholesterol (CHOL), triglycerides (TRIG), blood creatinine (CREA), blood urea nitrogen (BUN), and blood uric acid UA in serum were measured by biochemical analyzer, and the level of glycated hemoglobin (HbA1c) in plasma was measured by Elisa kit.
Urine microalbumin analysis
Urine samples of each group were collected respectively at 16 weeks of age, 20 weeks of age and 24 weeks of age over 24 h, and centrifuged at 3,500 r/min for 10 min and the microalbumin concentrations in urine supernatant were detected by Elisa kit.
Renal histological analysis
Kidney tissues were fixed with 4% paraformaldehyde solution, and HE staining, PAS staining, PASM staining and Masson staining were performed respectively after wax embedded and sliced. Some kidney tissues were fixed with 2.5% glutaraldehyde solution, and the paraffin sections were observed under a transmission electron microscope (TEM) after processing for dehydration, embedding, sectioning, and staining with uranyl acetate and lead citrate.
Analysis of GRP78, OPA1, MFN1 and MFN2 mRNA expressions by real-time PCR
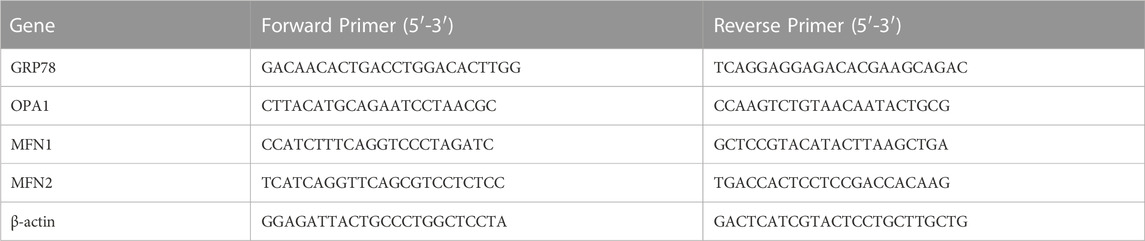
Total RNA was extracted from kidney tissues by using Trizol, and the mRNA expression levels of endoplasmic reticulum stress marker GRP78, mitochondrial fusion key factors OPA1, MFN1 and MFN2 were detected strictly according to the operation steps of Real-time PCR kit with β-actin as the internal reference. Primers for PCR (Table 1) were designed and synthesized by Sangon Biotech (Shanghai) Co., Ltd.
Analysis of GRP78, OPA1, MFN1 and MFN2 protein expressions by Western blot
Total protein was extracted from kidney tissues by ice-cold lysis buffer (RIPA) for 20 min and then centrifuged at 12,000 rpm for 10 min at 4°C. Protein concentrations were collected in a fresh 1.5 mL tube and determined by the bicinchoninic acid assay (BCA) method. SDS-PAGE gels were prepared, and equal amounts of protein samples were loaded for electrophoresis. After that, proteins were transferred to PVDF membranes in an ice-cold buffer via electroblotting for 2 h. The membranes were blocked in 5% skim milk at room temperature for 1 h, and then incubated overnight at 4°C with the primary antibody against GRP78 (dilution 1:5000), OPA1 (dilution 1:5000), MFN1 (dilution 1:5000), MFN2 (dilution 1:5000). After washing with TBST, the membranes were incubated with the secondary antibody (dilution 1:10000)., at room temperature for 1 h. Enhanced chemiluminescence (ECL) was used for imaging and exposure. The images were processed using ImageJ software for grayscale analysis.
Data and statistical analysis
GraphPad Prism9.0 software was used for data analysis. Numerical data were expressed as mean ± standard deviation (X ± SD). Depending on the normal distribution and homogeneity of variances, either one-way analysis of variance (ANOVA) or non-parametric tests were chosen for comparisons between groups. and p-value less than 0.05 (p < 0.05) were considered statistically significant.
Results
Performance status of mice
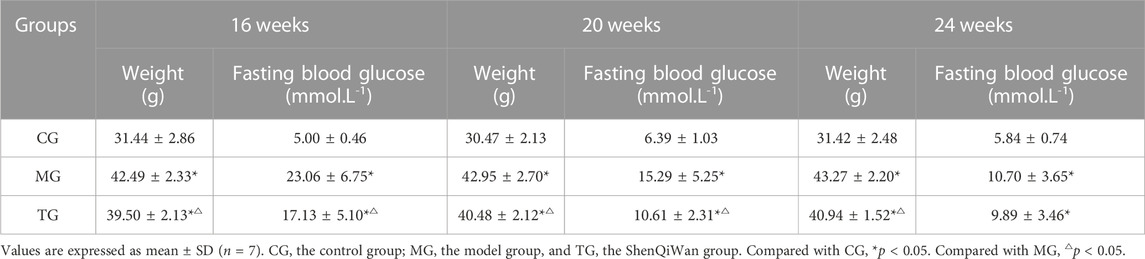
The mice in the control group were bright in coat color, more agile in action, and moved freely. However, the mice in the model group exhibited listlessness, reduced activity, slow movement, and a lackluster coat, and significantly increased water intake and urine volume. Compared with the model group, the above conditions of mice in the treatment group were milder, and the body weights were significantly reduced (Table 2).
Effects of ShenQiWan on fasting blood glucose and glycated hemoglobin (HbA1c) levels
The fasting blood glucose levels were significantly higher in the model group compared with those in the control group at all weeks of age, and the differences were statistically significant (p < 0.05). The fasting blood glucose levels in the treatment group decreased to varying degrees compared with those in the model group, and the differences from the group at the age of 16 weeks, 20 weeks were statistically significant (p < 0.05) (Figure 2A). The results showed that the level of plasma HbA1c in the model group at all weeks of age was significantly higher in comparison with that in the control group, and the level of HbA1c in the treatment group was significantly lower than that in the model group, the differences were statistically significant (p < 0.05) (Figure 2B).
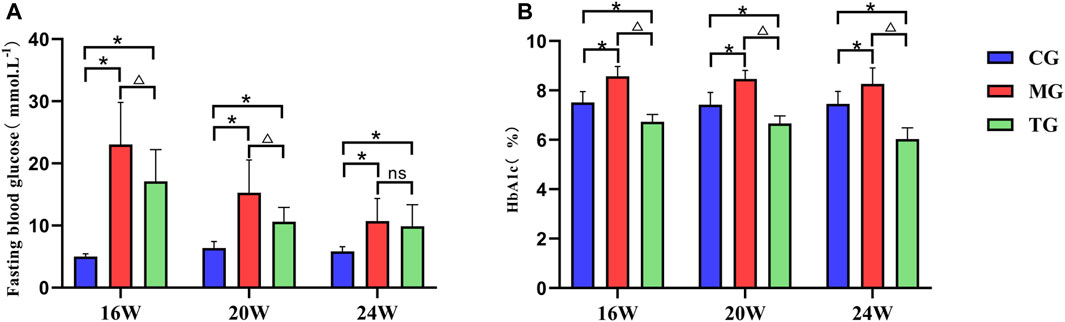
FIGURE 2. ShenQiWan can reduce blood glucose levels and HbA1c levels of diabetic mice at different weeks of age. (A) The change of expression level of fasting blood glucose at different weeks. (B) The change of expression level of HbA1c at different weeks. Values are expressed as mean ± SD (n = 7). CG, the control group; MG, the model group; and TG, the ShenQiWan group. Compared with CG, *p < 0.05. Compared with MG, △p < 0.05.
Effects of ShenQiWan on CHOL, TRIG, CREA, BUN and urinary microalbumin levels
The results showed that the levels of TRIG and CHOL were significantly increased in the model group compared to those in the control group at each week of age (p < 0.05) (Figures 3A, B). The level of TRIG was significantly lower in the treated group than that in the control group at the age of 16 weeks and 20 weeks (p < 0.05) (Figure 3B). While the level of CHOL in the treatment group was not significantly different from that in the model group at each week of age (p > 0.05). (Figure 3A). The level of CREA was significantly increased in the model group compared to that in the control group at each week of age (p < 0.05), while it was significantly lower in the treatment group than that in the model group (p < 0.05) (Figure 3C). The BUN level in the model group at 20 weeks of age was significantly lower than that in the control group (p < 0.05) and had no significant difference compared with that in the treatment group (p > 0.05) (Figure 3D). There was no significant difference in urinary microalbumin expression levels of each group at 16 weeks of age (p > 0.05). Urinary microalbumin levels were significantly higher in the model groups than those in the control group at 20 weeks of age and 24 weeks of age (p < 0.05), while the treatment group showed a significant reduction in urinary microalbumin compared to the model group, with statistically significant differences (p < 0.05) (Figure 3E).
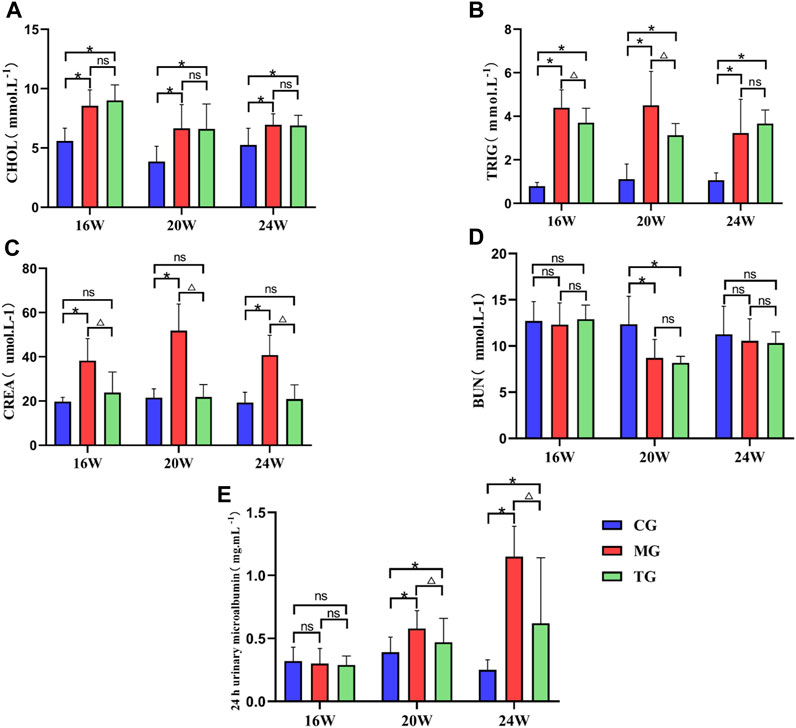
FIGURE 3. ShenQiWan can reduce blood lipid levels and deterioration of renal function. (A) Changes in CHOL levels at different weeks of age. (B) Changes in TRIG levels at different weeks of age. (C) The change of expression level of CREA at different weeks. (D) The change of expression level of BUN at different weeks. (E) The change of expression level of urinary microalbumin at different weeks. Values are expressed as mean ± SD (n = 7). CG, the control group, MG, the model group; and TG, the ShenQiWan group. Compared with CG, *p < 0.05. Compared with MG, △p < 0.05.
Effects of ShenQiWan on morphological changes in kidney tissues of diabetic mice
Compared to the control group, the model group showed various morphological changes in the kidney tissues caused by DN: HE staining revealed narrowed glomerular lumens, thickening of the renal glomerular basement membrane, edema and ballooning of renal tubular epithelial cells (Figure 4A). In addition to the above conditions, PAS staining also revealed collagen fiber proliferation and glycogen deposition in the renal tubular epithelium and stroma (Figure 4B). PASM staining revealed mesangial tissue proliferation and sclerosis, irregular thickening of the capillary wall, partial glomerular sclerosis, significant tubular atrophy and uniform red-stained protein in the lumen of the renal tubule (Figure 4C). Masson staining also showed glomerulosclerosis and interstitial fibrosis (Figure 4D) (Supplementary Figure S1). However, treatment with ShenQiWan reversed these changes to some degree (Figures 4A–D). Transmission electron microscopy (TEM) observation revealed that the control group showed the glomerular endoplasmic reticulum, mitochondria and basement membrane with defined structures and normal foot processes. However, the model group showed mitochondrial fragmentation, endoplasmic reticulum swelling and fracture, irregular thickening of the glomerular basement membrane, effacement of foot processes. After treatment, the severity of all of the morphological changes listed above decreased to varying degrees (Figure 4E).
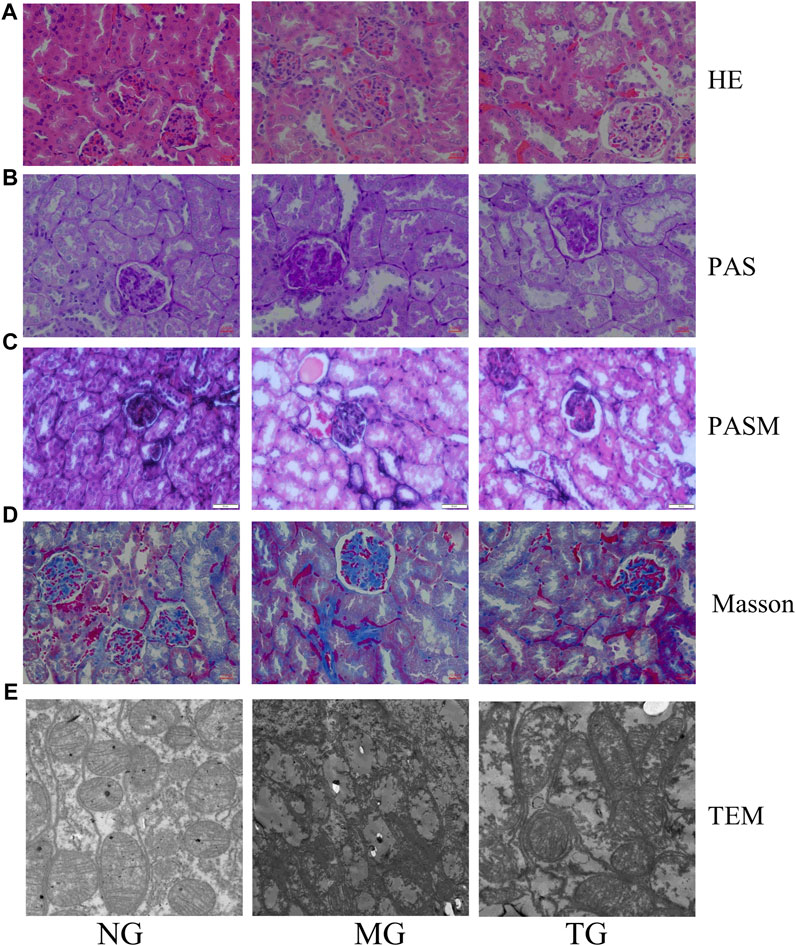
FIGURE 4. Pathological changes of diabetic mice. HE staining (×40), PAS staining (×40), PASM staining (×40), Masson staining (×40). (A) HE staining revealed that ShenQiWan can alleviate changes such as narrowing of renal glomerular tubules, thickening of the renal glomerular basement membrane, swelling of renal tubular epithelial cells, and luminal constriction. (B) PAS staining revealed that ShenQiWan can alleviate changes such as collagen fiber proliferation and glycogen deposition in the renal tubular epithelium and stroma. (C) PASM staining revealed that ShenQiWan can alleviate changes such as mesangial tissue proliferation and sclerosis, irregular thickening of the capillary wall, partial glomerular sclerosis, and significant tubular atrophy. (D) Masson staining revealed that ShenQiWan can also alleviate glomerulosclerosis and interstitial fibrosis. (E) TEM observation revealed that ShenQiWan can reverse mitochondrial fragmentation, endoplasmic reticulum swelling and fracture, irregular thickening of the glomerular basement membrane to some degree.
Effects of ShenQiWan on the mRNA expression levels of GRP78, OPA1, MFN1, MFN2
Real-time PCR results showed that the relative mRNA expression level of GRP78 in the model group was significantly higher compared with that in the control group at all weeks of age (p < 0.05), and the relative mRNA expression level of GRP78 was significantly lower than that in the model group after treatment with ShenQiWan (p < 0.05) (Figure 5A). The relative mRNA expression levels of OPA1, MFN1 and MFN2 in the 20-week-old and 24-week-old model groups were significantly lower than those in the control group (p < 0.05) (Figures 5B–D), however, the relative mRNA expression levels of OPA1, MFN1 and MFN2 increased to varying degrees after treatment with ShenQiWan, with significant differences observed in the 24-week-old group (p < 0.05) (Figures 5B–D).
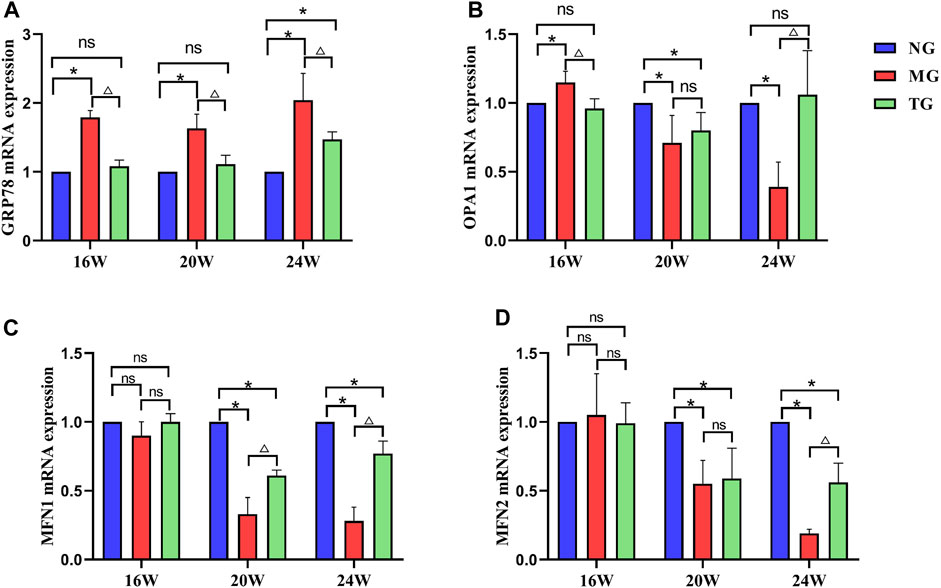
FIGURE 5. ShenQiWan can influence the expression levels of GRP78, OPA1, MFN1, and MFN2 mRNA. (A) ShenQiWan can decrease GRP78 mRNA expression levels. (B) ShenQiWan can increase OPA1 mRNA expression levels. (C) ShenQiWan can increase MFN1 mRNA expression levels. (D) ShenQiWan can increase MFN2 mRNA expression levels. Values are expressed as mean ± SD (n = 3). CG, the control group; MG, the model group; and TG, the ShenQiWan group. Compared with CG, *p < 0.05. Compared with MG, △p < 0.05.
Effects of ShenQiWan on the protein expression level of GRP78, OPA1, MFN1, MFN2
Protein expressions were measured by Western blotting, using β-actin as a housekeeping gene (Figure 6A). Western blot analysis showed the model group displayed significantly higher levels of GRP78 when compared to levels measured in the control group at all weeks of age, treatment with ShenQiWan significantly inhibited the protein expression of GRP78 (p < 0.05) (Figure 6B). The protein expression levels of OPA1 in the 20-week-old and 24-week-old model groups were significantly lower than those in the control group (p < 0.05), however, treatment with ShenQiWan reversed decreases in OPA1 protein to varying degrees, and the difference in the 24-week-old group was significant (p < 0.05) (Figure 6C). The protein expressions of MFN1 and MFN2 in the model group were significantly reduced compared to those in the control group at all weeks of age (p < 0.05), while the treatment group exhibited a significant increase in the protein expression levels of MFN1 and MFN2 compared to those in the model group (p < 0.05) (Figures 6D, E).
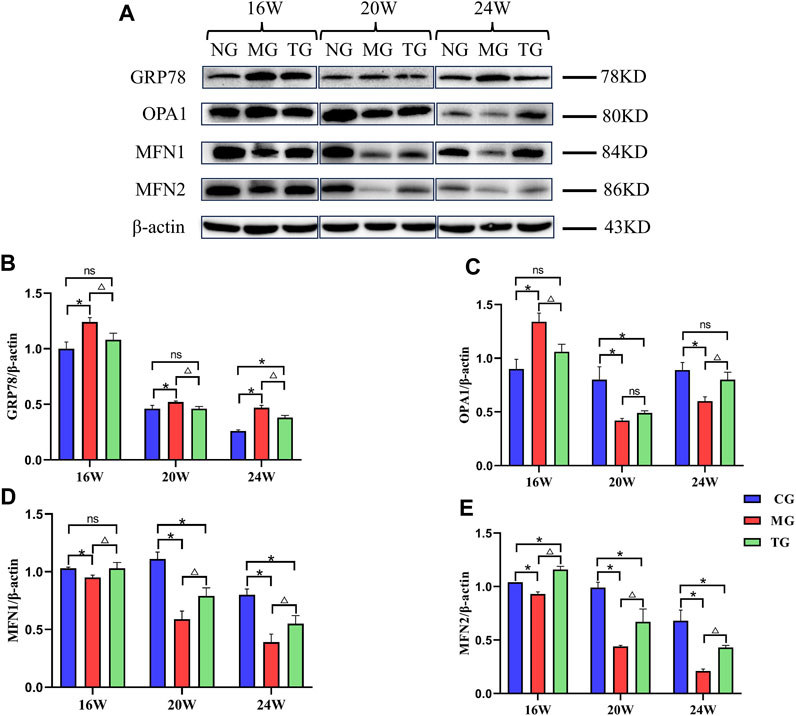
FIGURE 6. ShenQiWan can influence the protein expression levels of GRP78, OPA1, MFN1 and MFN2. (A) Western blot analysis of GRP78, OPA1, MFN1, MFN2. (B) ShenQiWan can decrease the protein expression levels of GRP78. (C) ShenQiWan can increase the protein expression levels of OPA1. (D) ShenQiWan can increase the protein expression levels of MFN1. (E) ShenQiWan can increase the protein expression levels of MFN2. Values are expressed as mean ± SD (n = 3). CG, the control group; MG, the model group; and TG, the ShenQiWan group. Compared with CG, *p < 0.05. Compared with MG, △p < 0.05.
Discussion
Diabetic Nephropathy (DN) is one of the most common chronic microvascular complications of diabetes mellitus, which affects approximately 30% of diabetic patients worldwide and may place a heavy economic burden on patients’ families and society in the future (Ruiz-Ortega et al., 2020). Currently, the pathogenesis of DN is not well understood, and there is also a lack of effective therapeutic drugs specifically targeting DN. Therefore, it is particularly important to seek new therapeutic drugs and deeply explore the pathogenesis of DN. In recent years, traditional Chinese medicine has been widely used in the treatment of diabetic nephropathy (Yulong et al., 2021). In this study, ShenQiWan was used to treat KKAy diabetic mice, and the results showed that ShenQiWan could significantly improve kidney damage in KKAy mice, and its mechanism was closely related to mitochondrial-endoplasmic reticulum coupling.
Diabetes falls under the category of “Xiao Ke” syndrome in traditional Chinese medicine. Zhang Zhongjing first used ShenQiWan to treat diabetes in his book “Synopsis of Golden Chamber.” ShenQiWan is composed of Radix Rehmanniae, Chinese Yam, Fructus Corni, Rhizoma Alismatis, Poria cocos, Cortex Moutan, Ramulus Cinnamomi, Paofuzi and has the effect of invigorating the kidney yang. In modern clinical research, ShenQiWan is also often used to treat various diseases caused by liver-kidney yang deficiency (Zhou et al., 2016). After renal damage occurs in diabetic patients, symptoms such as polyuria and proteinuria will occur in the early stage, dominant proteinuria in the late stage, and finally develop into renal failure. The pathological changes in the early stage are mainly glomerular hypertrophy, basement membrane thickening and accumulation of mesangial extracellular matrix. While the progression to the later stage shows fibrosis of the glomerulus fibrosis and tubulointerstitial fibrosis (Chen et al., 2017). KKAy mice, as a spontaneous animal model of type 2 diabetes, are widely used in the study of type 2 diabetes and its complications. In this study, three time points (16 weeks old, 20 weeks old and 24 weeks old) were selected to observe the general state, body weight, fasting blood glucose, relevant biochemical indexes in serum and urine, pathological changes in kindy tissues, as well as the expression of endoplasmic reticulum stress and mitochondrial fusion key proteins in KKAy diabetic model mice. It aims to investigate the protective mechanism of ShenQiWan against kindy injury in diabetic mice.
Our results showed that ShenQiWan significantly improved symptoms such as mental depression, drowsiness, polyphagia and polyuria in diabetic mice. After treatment with ShenQiWan, the body weight and fasting blood glucose of diabetic mice decreased significantly. There was also an improvement in urine microalbumin levels and relevant serum biochemical indicators (serum creatinine, blood urea nitrogen, etc.) to varying degrees. These positive effects were observed at all three time points, indicating that ShenQiWan plays an important role in the entire treatment of diabetic nephropathy. HE, PAS, PASM and Masson staining showed that ShenQiWan could improve the proliferation of glomerular mesangial cells, thickening of basement membrane, glomerulosclerosis, edema of renal tubular epithelial cells, glycogen deposition in renal tissue and interstitial fibrosis in diabetic mice. These findings suggest that ShenQiWan can protect kindy morphology and alleviate kidney damage in KKAy diabetic mice. Transmission electron microscopy observations also showed that the glomerular basement membrane in the model group was thickened and hardened, the podocyte nucleus was swollen, the foot processes were fused and disappeared, the mitochondria were fragmented, the ridges were broken and disappeared, and the endoplasmic reticulum was swollen and broken. However, these pathological changes were alleviated in the treatment group.
The Endoplasmic reticulum (ER) is an important site for protein secretion, synthesis, processing and modification. Stimuli such as high glucose, high lipids, oxidative stress, ischemia, and hypoxia lead to misfolding of proteins in the endoplasmic reticulum, disrupting endoplasmic reticulum homeostasis, and causing endoplasmic reticulum stress (ERS). GRP78 protein is a hallmark factor of ERS, and its upregulation is specific to endoplasmic reticulum stress (Shu et al., 2008). ERS also had been found to play an important role in the development of DN, and can attenuate endoplasmic reticulum stress-induced apoptosis by reducing the expression of GRP78 and PERK (Xiong et al., 2020). Previous results by our group also indicated that type 2 diabetic mice exhibited enhanced ERS and increased expression of related factors such as GRP78, PERK and EIF2α, and traditional Chinese medicine and its main components can alleviate ERS by downregulating the expression of related factors (Zhi-hui et al., 2015; Da et al., 2017; Shujing et al., 2017). Furthermore, our previous research on another diabetic model, the ZDF rat model, also proved that ShenQiWan may alleviate kidney injury in ZDF rats by potentially affecting endoplasmic reticulum stress (Liu et al., 2022). In this study, we further explored the relationship between ShenQiWan and the key factor of endoplasmic reticulum stress, GRP78. Analysis of GRP78 mRNA and protein expression also showed that the expression levels of GRP78 mRNA and protein in the renal tissue of the model group were significantly higher than those in the control group, and the expression levels were also decreased to varying degrees after treatment with ShenQiWan. This study further confirmed the important role of endoplasmic reticulum stress in diabetic nephropathy and also suggested that ShenQiWan can alleviate renal injury in diabetic mice by alleviating endoplasmic reticulum stress. Our study preliminarily elucidates from the perspective of animal experiments that ShenQiWan can reduce kidney injury by affecting cellular endoplasmic reticulum stress. The direct interaction between ShenQiWan and the key endoplasmic reticulum stress factor GRP78, as well as the specific mechanisms of ShenQiWan about endoplasmic reticulum stress, requires further investigation in vitro experiments.
Mitochondria are the main sites for intracellular oxidative phosphorylation and synthesis of ATP, providing energy for cellular activities. The states of dynamic fusion and division of mitochondria are prerequisites for their self-repair and function. The function of mitochondria is closely related to their structure, and the intact structure is crucial for mitochondrial activities and energy metabolism. The regulation of mitochondrial structure mainly occurs through fusion and fission processes. Mitochondrial outer membrane fusion is regulated by mitofution1 (MFN1) and mitofution2 (MFN2), and mitochondrial inner membrane fusion is regulated by Opticatrophy1 (OPA1) (Rosselin et al., 2017). OPA1 is a key protein of the mitochondrial inner membrane, which is closely related to the fusion of the mitochondrial inner membrane and maintenance of the mitochondrial ridge structure. In this study, we observed mitochondrial fragmentation and mitochondrial cristae deformation and disappearance in the model group by transmission electron microscopy, and the above phenomena were significantly alleviated after treatment with ShenQiWan. We found that the expressions of OPA1 mRNA and protein in the model group were higher than those in the normal group in the early stage of the disease. However, the expression levels of OPA1 decreased significantly again compared to the control group along with the development of the disease, which increased significantly compared to the model group after treatment with ShenQiWan. We speculate that the initial expression of OPA1 in the early stage may be related to the emergence of stress response in cells. However, in general, the expression of OPA1 mRNA and protein showed a time-dependent downregulation with the development of the disease, which also reflected the important role of OPA1 in the mitochondrial ecological balance.
Mitochondrial outer membrane fusion proteins MFN1 and MFN2 have a high degree of homology, which play a role in promoting mitochondrial fusion at different stages and are essential for maintaining the homeostasis of mitochondrial morphology. MFN1 mainly promotes the combination of mitochondria in the early stage of fusion, while MFN2 mainly plays a role in the late stage of mitochondrial fusion. In this study, analysis of MFN1 and MFN2 mRNA and protein expression showed that, in general, the expression levels of MFN1 and MFN2 in the kidney tissues of the model group were significantly lower than those in the control group, and the expression level was significantly increased after treatment with ShenQiWan. The expression of MFN1 protein in the treatment group at 16 weeks of age was significantly increased compared to that in the model group, with no significant difference compared to the control group. The expression of MFN2 in the treatment group at 16 weeks of age was significantly increased compared with the model group and the control group. While the expression levels of MFN1 and MFN2 protein at 20 and 24 weeks of age were significantly higher than those in the model group, but lower than those in the control group. The above results may reflect that ShenQiWan may be better in the early treatment of renal injury. In summary, OPA1, MFN1, MFN2 mRNA and protein expression analysis shows that mitochondrial fusion dysfunction occurs during the development of diabetes, resulting in abnormal mitochondrial morphology, and ShenQiWan can alleviate the renal injury by stabilizing the mitochondrial fusion process.
There is a close association between mitochondria and the endoplasmic reticulum (ER) in terms of structure and function, with the region of their interaction referred to as mitochondria-associated endoplasmic reticulum membranes (MAMs) (van Vliet and Agostinis, 2018). GRP78 and MFN2 proteins are also located in the structural coupling between mitochondria and endoplasmic reticulum. In particular, MFN2 connects the outer mitochondrial membrane and the ER membrane and accumulates at the sites of structural coupling, together with MFN1 supporting the structure of MAM (de Brito and Scorrano, 2008; Leemand and Koh, 2012). Furthermore, studies have demonstrated that MFN2 and MFN1 were not only involved in the process of mitochondrial fusion but also involved in endoplasmic reticulum stress and the interaction between mitochondria and endoplasmic reticulum. (Filadi et al., 2015; McFie et al., 2016; Wang et al., 2021). Our study confirmed that ShenQiWan could alleviate kindy injury in diabetic mice by regulating the expression of endoplasmic reticulum stress key protein GRP78 and mitochondrial fusion proteins MFN1, MFN2 and OPA1. Whether there is a direct interaction between ShenQiWan and the signaling proteins GRP78, MFN1, MFN2, and OPA1, as well as the specific molecular mechanism of ShenQiWan on endoplasmic reticulum stress and mitochondrial fusion, requires further exploration through in vitro cell culture system using silencing or inhibiting specific signaling proteins involved in the cellular damage and mitochondrial function and dynamics. Subsequent studies are necessary to take mitochondria-endoplasmic reticulum structural coupling (MAM) as a point of entry and to further investigate the specific molecular mechanisms of ShenQiWan in ameliorating endoplasmic reticulum stress and mitochondrial dysfunction as well as the key signaling pathways in the process of anti-diabetic kidney injury.
Conclusion
ShenQiWan can significantly reduce body weight, fasting blood glucose level, urinary microalbumin excretion rate and glycosylated hemoglobin level, improve dyslipidemia and alleviate renal structural damage in diabetic mice at different stages of diabetes nephropathy progression. The underlying mechanism of action may be related to maintaining the stability of mitochondrial-endoplasmic reticulum structure coupling by inhibiting endoplasmic reticulum stress and protecting mitochondrial morphology.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the Animal Ethics Committee of the Beijing University of Chinese Medicine, China. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
WX: Data curation, Writing–review and editing, Investigation, Methodology. JF: Writing–review and editing, Data curation, Methodology. YL: Data curation, Methodology, Writing–review and editing. JL: Data curation, Methodology, Writing–review and editing. LF: Data curation, Methodology, Investigation, Software, Writing–review and editing. QW: Supervision, Validation, Writing–review and editing. XL: Data curation, Writing–original draft, Writing–review and editing. SL: Supervision, Validation, Formal Analysis, Writing–review and editing, Funding acquisition.
Funding
This work was supported by the Natural Science Foundation of China (No. 81803941). The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1265551/full#supplementary-material
References
Bonner, R., Albajrami, O., Hudspeth, J., and Upadhyay, A. (2020). Diabetic kidney disease. Prim. Care Clin. Office Pract. 47 (4), 645–659. doi:10.1016/j.pop.2020.08.004
Chen, H., Xu, Y., Yang, Y., Zhou, X., Dai, S., and Li, C. (2017). Shenqiwan ameliorates renal fibrosis in rats by inhibiting TGF-β1/smads signaling pathway. Evidence-Based Complementary Altern. Med. 2017, 7187038. doi:10.1155/2017/7187038
Da, Z., Shuyu, L., Yanei, W., Yi-uan, L., Yuee, Y., Yu-shan, C., et al. (2017). Effects of astragalus injection combined with puerarin injection onprocess of endoplasmic reticulum stress through PERK pathway in dabetic nephropathy mice. Chin. J. Pathophysiol. 33 (01), 166–169+173. doi:10.3969/i.issn.1000-4718.2017.01.028
de Brito, O. M., and Scorrano, L. (2008). Mitofusin 2 tethers endoplasmic reticulum to mitochondria. NATURE 456 (7222), 605–610. doi:10.1038/nature07534
Donate-Correa, J., Luis-Rodríguez, D., Martín-Núñez, E., Tagua, V. G., Hernández-Carballo, C., Ferri, C., et al. (2020). Inflammatory targets in diabetic nephropathy. J. Clin. Med. [Online] 9 (2), 458. doi:10.3390/jcm9020458
Fan, Y., Lee, K., Wang, N. S., and He, J. C. (2017). The role of endoplasmic reticulum stress in diabetic nephropathy. Curr. DIABETES Rep. 17 (3), 17. doi:10.1007/s11892-017-0842-y
Filadi, R., Greotti, E., Turacchio, G., Luini, A., Pozzan, T., and Pizzo, P. (2015). Mitofusin 2 ablation increases endoplasmic reticulum-mitochondria coupling. Proc. Natl. Acad. Sci. U. S. A. 112 (17), E2174–E2181. doi:10.1073/pnas.1504880112
Hallan, S., and Sharma, K. (2016). The role of mitochondria in diabetic kidney disease. Curr. Diabetes Rep. 16 (7), 61. doi:10.1007/s11892-016-0748-0
Hu, Z., Liu, X., and Yang, M. J. F. I. P. (2021). Evidence and potential mechanisms of Jin-Gui Shen-Qi Wan as a treatment for type 2 diabetes mellitus: a systematic review and meta-analysis. Front. Pharmacol. 12, 699932. doi:10.3389/fphar.2021.699932
Jiapeng, L., Jing, F., Yu’e, C., Xiaofeng, Z., Yumeng, L., Qian, W., et al. (2023). The effects of Shenqiwan on renal damage, GRP78, and autophagy-related factors in Zucker diabetic fatty rats with type 2 diabetes mellitus. J. Beijing Univ. Traditional Chin. Med. 46 (02), 224–231.
Leemand, J., and Koh, E. H. (2012). Interaction between mitochondria and the endoplasmic reticulum: implications for the pathogenesis of type 2 diabetes mellitus. Exp. Diabetes Res. 2012, 242984. doi:10.1155/2012/242984
Liu, Y. M., Li, S. Y., and Feng, J. (2022). Effect of ShenQiWan in alleviating renal injury in diabetic ZDF rats. Chin. J. Comp. Med. 32, 8.
McFie, P. J., Ambilwade, P., Vu, H., and Stone, S. J. (2016). Endoplasmic reticulum-mitochondrial interaction mediated by mitofusin-1 or mitofusin-2 is not required for lipid droplet formation or adipocyte differentiation. Biochem. Biophysical Res. Commun. 478 (1), 392–397. doi:10.1016/j.bbrc.2016.07.040
Ni, L., and Yuan, C. (2021). The mitochondrial-associated endoplasmic reticulum membrane and its role in diabetic nephropathy. Oxidative Med. Cell. Longev. 2021, 8054817. doi:10.1155/2021/8054817
Ni, L. H., Yuan, C., and Wu, X. Y. (2021). Endoplasmic reticulum stress in diabetic nephrology: regulation, pathological role, and therapeutic potential. OXIDATIVE Med. Cell. Longev. 2021, 7277966. doi:10.1155/2021/7277966
Pontes-da-Silva, R. M., Marinho, T. D., Cardoso, L. E. D., Mandarim-de-Lacerda, C. A., and Aguila, M. B. (2022). Obese mice weight loss role on nonalcoholic fatty liver disease and endoplasmic reticulum stress treated by a GLP-1 receptor agonist. Int. J. Obes. 46 (1), 21–29. doi:10.1038/s41366-021-00955-7
Rosselin, M., Santo-Domingo, J., Bermont, F., Giacomello, M., and Demaurex, N. (2017). L-OPA1 regulates mitoflash biogenesis independently from membrane fusion. EMBO Rep. 18 (3), 451–463. doi:10.15252/embr.201642931
Ruiz-Ortega, M., Rodrigues-Diez, R. R., Lavoz, C., and Rayego-Mateos, S. (2020). Special issue "diabetic nephropathy: diagnosis, prevention and treatment. J. Clin. Med. 9 (3), 813. doi:10.3390/jcm9030813
Saxena, S., Mathur, A., and Kakkar, P. J. J. O. C. P. (2019). Critical role of mitochondrial dysfunction and impaired mitophagy in diabetic nephropathy. J. Cell. PHYSIOLOGY 234 (11), 19223–19236. doi:10.1002/jcp.28712
Shahzad, K., Ghosh, S., Mathew, A., and Isermann, B. (2020). Methods to detect endoplasmic reticulum stress and apoptosis in diabetic nephropathy. Methods Mol. Biol. 2067, 153–173. doi:10.1007/978-1-4939-9841-8_12
Shu, C. V., Sun, F. C., Cho, J. H., Lin, C. C., Liu, P. F., Chen, P. Y., et al. (2008). GRP78 and Raf-1 cooperatively confer resistance to endoplasmic reticulum stress-induced apoptosis. J. Cell. PHYSIOLOGY 215 (3), 627–635. doi:10.1002/jcp.21340
Shujing, Z., Qian, W., Shuyu, L., Fujian, Z., Yanfei, W., Yushan, G., et al. (2017). Effects of huangqi injection combined with puerarin injection on KKAy micewith diabetic cardiomyopathy on endoplasmic reticulum stress. World Chin. Med. 12 (12), 3050–3054. doi:10.3969/i.issn.1673-7202.2017.12.048
Thomas, M. C., Brownlee, M., Susztak, K., Sharma, K., Jandeleit-Dahm, K. A., Zoungas, S., et al. (2015). Diabetic kidney disease. Nat. Rev. Dis. Prim. 1, 15018. doi:10.1038/nrdp.2015.18
Umanath, K., and Lewis, J. B. (2018). Update on diabetic nephropathy: core curriculum 2018. Am. J. Kidney Dis. 71 (6), 884–895. doi:10.1053/j.ajkd.2017.10.026
van Vliet, A. R., and Agostinis, P. (2018). Mitochondria-associated membranes and ER stress. Curr. Top. Microbiol. Immunol. 414, 73–102. doi:10.1007/82_2017_2
Wang, N., Wang, C., Zhao, H. Y., He, Y. C., Lan, B. W., Sun, L. K., et al. (2021). The MAMs structure and its role in cell death. Cells 10 (3), 657. doi:10.3390/cells10030657
Xiong, S., Han, Y. C., Gao, P., Zhao, H., Jiang, N., and Sun, L. (2020). AdipoRon protects against tubular injury in diabetic nephropathy by inhibiting endoplasmic reticulum stress. OXIDATIVE Med. Cell. Longev. 2020, 6104375. doi:10.1155/2020/6104375
Yulong, G., Fan, D., and Yikun, Z. (2021). Clinical research progress of treating diabetic nephropathy with traditional Chinese medicine. Bachu Med. J. 4 (04), 119–121. doi:10.3969/i.issn.2096-6113.2021.04.026
Yumeng, L., Shuyu, L., Jing, F., Yue, C., Xiaofeng, Z., Jiapeng, L., et al. (2022). Effect of ShenQiWan in alleviating renal injury in diabetic ZDF rats. Chin. J. Comp. Med. 32 (08), 19–26. doi:10.3969/j.issn.1671-7856.2022.08.003
Zhao, H. J., Liu, H. Y., Yang, Y. H., Lan, T. Y., Wang, H. G., and Wu, D. D. (2022). Hydrogen sulfide plays an important role by regulating endoplasmic reticulum stress in diabetes-related diseases. Int. J. Mol. Sci. 23 (13), 7170. doi:10.3390/ijms23137170
Zhi-hui, Z., Shu-yu, L., Yan-fei, W., Da, Z., Ying, W., Yu-shan, G., et al. (2015). Effects of astragalus and puerarin injections on the expression of GRP78 in KKA y mice with diabetic nephropathy. J. Beijing Univ. Traditional Chin. Med. 38 (05), 323–326+362. doi:10.3969/i.issn.1006-2157.2015.05.008
Zhongqing, M., Sifan, S., and Long, C. (2023). Research progress of traditional Chinese medicine in the treatment of diabetic kidney disease. Chin. Foreign Med. Res. 21 (02), 181–184. doi:10.14033/j.cnki.cfmr.2023.02.045
Keywords: diabetic nephropathy, ShenQiWan, type 2 diabetic mice, mitochondrial fusion disorder, endoplasmic reticulum stress
Citation: Xiong W, Feng J, Liu Y, Liu J, Fu L, Wang Q, Li X and Li S (2023) ShenQiWan ameliorates renal injury in type 2 diabetic mice by modulating mitochondrial fusion and endoplasmic reticulum stress. Front. Pharmacol. 14:1265551. doi: 10.3389/fphar.2023.1265551
Received: 04 August 2023; Accepted: 27 October 2023;
Published: 10 November 2023.
Edited by:
Xiaoxin Wang, Georgetown University Medical Center, United StatesReviewed by:
Hewang Lee, George Washington University, United StatesKomuraiah Myakala, Georgetown University Medical Center, United States
Copyright © 2023 Xiong, Feng, Liu, Liu, Fu, Wang, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xia Li, bGl4aWFzZDA3QDE2My5jb20=; Shuyu Li, bGlzaHV5dUBidWNtLmVkdS5jbg==
†These authors have contributed equally to this work
 Wei Xiong
Wei Xiong Jing Feng
Jing Feng Yumeng Liu
Yumeng Liu Jiapeng Liu
Jiapeng Liu Liang Fu
Liang Fu Qian Wang
Qian Wang Xia Li
Xia Li