- 1Center for Neurodegenerative Diseases and Therapeutics, Rosalind Franklin University of Medicine and Science, North Chicago, IL, United States
- 2Department of Neuroscience, Rosalind Franklin University of Medicine and Science, The Chicago Medical School, North Chicago, IL, United States
Benzodiazepines (BZDs) are anxiolytic drugs that act on GABAa receptors and are used to treat anxiety disorders. However, these drugs come with the detrimental side effect of anterograde amnesia, or the inability to form new memories. In this review we discuss, behavioral paradigms, sex differences and hormonal influences affecting BZD-induced amnesia, molecular manipulations, including the knockout of GABAa receptor subunits, and regional studies utilizing lesion and microinjection techniques targeted to the hippocampus and amygdala. Additionally, the relationship between BZD use and cognitive decline related to Alzheimer’s disease is addressed, as there is a lack of consensus on whether these drugs are involved in inducing or accelerating pathological cognitive deficits. This review aims to inspire new research directions, as there is a gap in knowledge in understanding the cellular and molecular mechanisms behind BZD-induced amnesia. Understanding these mechanisms will allow for the development of alternative treatments and potentially allow BZDs to be used as a novel tool to study Alzheimer’s disease.
1 Introduction
Benzodiazepines (BZDs), a class of anxiolytic drugs, were invented in the mid-1950s by chemist Leo Sternbach. In an effort to create a perfect tranquilizer, Sternbach and his associate Earl Reeder stumbled upon the compound that would soon become the first clinically available BZD. The drug chlordiazepoxide, also known as Librium, was introduced in the 1960s and revolutionized the clinical use of sedatives (Sternbach, 1979). Subsequently, other BZDs like diazepam were released in the early 1960s to improve the efficacy and potency of this new class of drugs (Sternbach, 1979). However, these drugs came with side effects, including impaired motor coordination, vertigo, mood swings, and anterograde amnesia (Griffin et al., 2013).
Although anterograde amnesia is desirable during perioperative surgical periods and times of heightened anxiety, the long-lasting cognitive fog reported by patients needs to be addressed and better understood. Anterograde amnesia, the inability to form new memories, was first observed in 1972 after intravenous injections of 10 mg of diazepam resulted in a reduction of recognition memory in 90% of women (Dundee and Pandit, 1972). This amnesia had a rapid onset of peak incidence 2–3 min after injection, with effects lasting around 1 h (Dundee and Pandit, 1972). More recently, healthy individuals showed impaired recollection of stories encoded immediately after diazepam administration (Segura et al., 2021). Interestingly, this amnesia is similar to the amnesia in patients with severe medial temporal damage (Segura et al., 2021). Acknowledging this observation, studying the relationship between BZD use in aging and Alzheimer’s disease (AD) is of great interest, as AD also results in atrophy of the medial temporal lobe. The cellular and molecular mechanisms underlying BZD-induced anterograde amnesia and cognitive impairment are not fully understood. Understanding these mechanisms could give greater insight into how these drugs might contribute to cognitive decline in aging and AD. This review outlines the historical and current literature surrounding BZDs and anterograde amnesia, intending to inspire new research directions.
2 Behavioral studies to measure anterograde amnesia
Scientists turned to rodent models to understand the cellular and molecular mechanisms underlying BZD-induced anterograde amnesia. Memory tasks used to study the amnestic properties of BZDs include the 1) passive avoidance task (PAT), 2) elevated plus maze (EPM), and 3) contextual fear conditioning (CFC). Early studies using the PAT showed that BZDs induce anterograde amnesia in mice, as mice that received lorazepam before training entered an aversive context significantly quicker than controls during the re-exposure test (Table 1) (E. R. Gamzu, 1988). This result was later confirmed in rats in a dose-dependent manner (Pain et al., 2002). Rats receiving high doses of midazolam before training in a PAT showed decreased latency to enter an aversive context compared to controls during re-exposure, indicating the inability to remember the initial aversive experience (Pain et al., 2002).
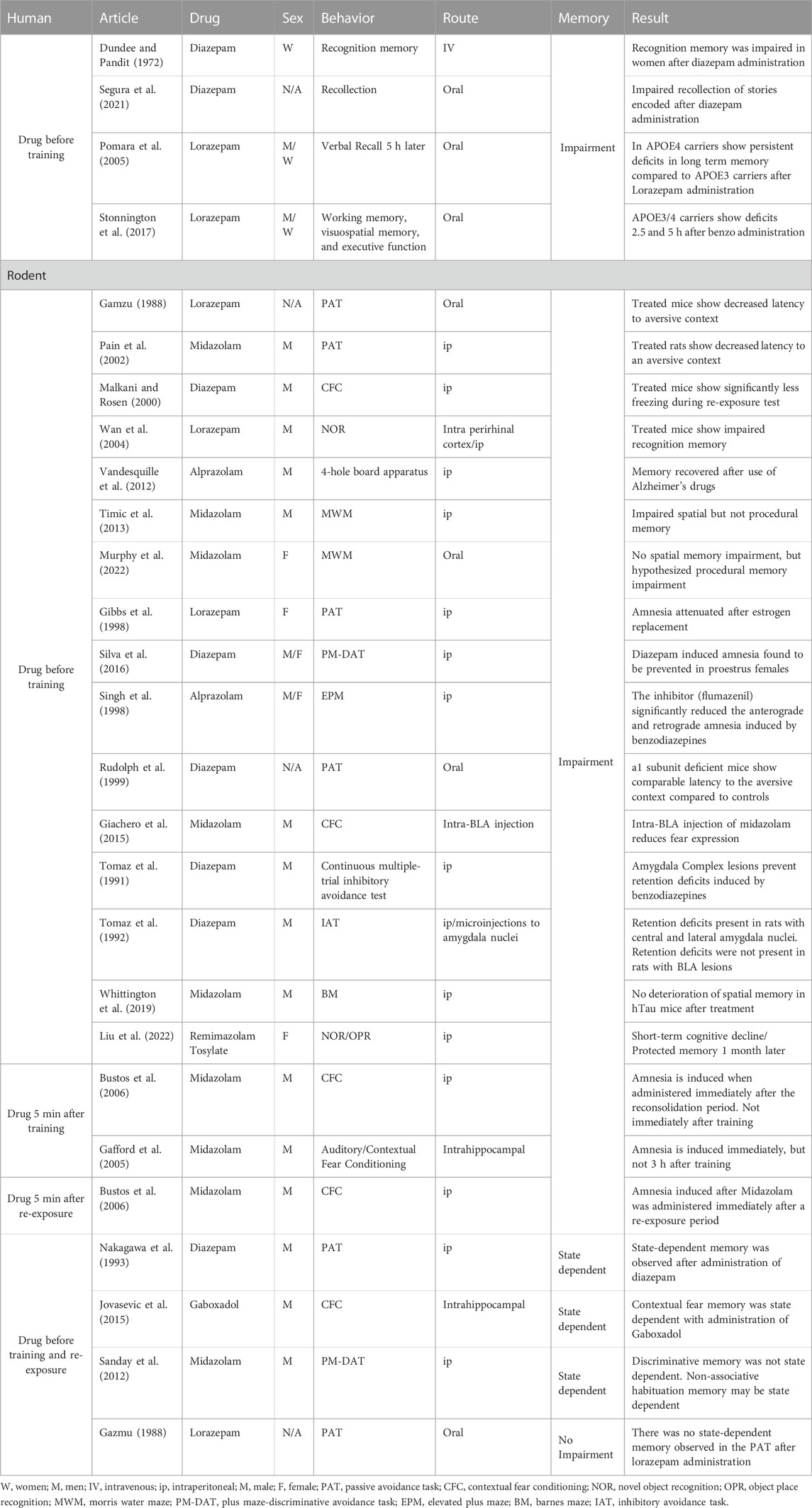
TABLE 1. Amnestic effects of benzodiazepines. Table 1 summarizes changes in memory from all BZD studies throughout the review.
Similar results are observed when diazepam is injected prior to training in a 1-shock aversive CFC paradigm. Freezing behavior during context re-exposure is measured as a proxy for memory. Treated rats freeze significantly less during CFC re-exposure than controls (Malkani and Rosen, 2000). However, different timelines of BZD administration have yielded different results. Midazolam injected 5 min after CFC does not decrease freezing levels during re-exposure (Bustos et al., 2006), but midazolam injected 5 min after the re-exposure session decreases freezing levels in a subsequent test period 24 h later. These results suggest that midazolam impairs the reconsolidation of a memory rather than the initial consolidation phase (Bustos et al., 2006). However, many drugs can also induce state-dependent memory, which further complicates our analysis of memory impairments (Zarrindast and Khakpai, 2020).
2.1 State-dependent memory
State-dependent memory is the retrieval of memory more easily facilitated when the organism is in a similar physiological state to when the memory was first encoded. For example, injection of diazepam induced state-dependent learning in the PAT when given 30 min before training and 30 min before re-exposure, as latency to the aversive chamber was similar to controls in this timeline (Table 1) (Nakagawa et al., 1993). Similarly, intra-hippocampal injections of gamma amino butyric acid (GABA) a agonist, gaboxadol, induced state-dependent learning in a CFC paradigm (Jovasevic et al., 2015).
However, other studies have found that state-dependent memory is not always observed in PAT and Plus Maze-Discriminative Avoidance Task (PM-DAT) paradigms. Contrary to the previous studies, mice receiving oral administration of lorazepam (L) pre-training, regardless of pre-test treatment, entered the aversive context in significantly less time than mice receiving a vehicle (V) before training (V-L or V-V) (E. R. Gamzu, 1988). Similarly, in the PM-DAT, in which one enclosed arm contains aversive stimuli, including a bright light and a loud sound-generating machine, mice pre-treated with both saline and midazolam before training sessions acquired proficiency in the discriminative avoidance task. Upon testing, midazolam-treated groups exhibited a learning impairment as groups receiving saline pre-training (Sal-Sal or Sal-MDZ) spent significantly more time in the non-aversive arms, while groups receiving midazolam pre-training (MDZ-Sal or MDZ-MDZ) did not exhibit significant differences in time spent between the aversive and the non-aversive arms (Sanday et al., 2012). The failure to discriminate between the aversive and the non-aversive areas when midazolam was administered pre-training and pre-test indicates that state-dependent memory was not utilized in this discriminative task.
The differences in results between studies may be attributed to the different drugs used in each study. Different BZDs have various time courses of action and require different dosages to achieve desired effects. Standardizing experiments to use the same drug may result in more precise results. A summary of all behavioral studies and timelines is included in Table 1. To truly understand the relationship between BZDs and state-dependent learning, using other memory tasks could lead to more answers. Examples include consolidation-based memory tests and altering the timelines from training to the test.
2.2 Recognition and spatial memory impairments
Although prior amnestic studies mainly used aversive contextual or avoidance learning, recognition and spatial memory tests may provide clues to determine whether BZDs are truly detrimental to cognition. For example, male rats injected with lorazepam before a novel object acquisition phase exhibited impaired recognition memory during the test day (Table 1) (Wan et al., 2004). This effect was also present when lorazepam was administered locally into the perirhinal cortex via cannula injection (Wan et al., 2004). Drug-induced amnesia is also exhibited in delayed spatial learning and discrimination tasks, including the 4-hole board apparatus where mice search for food. A small dose of 0.1 mg/kg of alprazolam given before the acquisition phase induced amnesia in mice, as alprazolam-treated mice exhibited significantly fewer nose pokes in the previously baited hole than controls (Vandesquille et al., 2012). Surprisingly, mice treated with both alprazolam and AMPA receptor-positive allosteric modulators together did not exhibit BZD-induced amnesia (Vandesquille et al., 2012). These data support the hypothesis that increased GABAergic inhibitory neurotransmission mediates drug-induced amnesic effects. Spatial memory assessed using the Morris Water Maze is also impaired. Specifically, male rats pre-treated with midazolam show impaired acquisition and retention of spatial learning memory but not procedural memory (Timić et al., 2013). A more recent study found the opposite effect in which female rats, pre-treated with midazolam, showed no impairment of spatial working memory but were hypothesized to have impaired procedural memory (Murphy et al., 2022). These contradictory observations may be due to sex differences or opposite circadian cycles between studies (Murphy et al., 2022).
2.3 Sex differences in benzodiazepine research
Although sex differences in drug-related research have been largely ignored, scientists have begun to examine how hormones influence the effects of BZDs. For example, ovariectomized female rats treated with estrogen replacement show attenuation of lorazepam-induced amnesia in the PAT during re-exposure (Gibbs et al., 1998). This suggests that estrogen has a protective effect that can reverse drug-induced amnesia in female rodents. Serum levels of lorazepam in both estrogen-treated and non-estrogen-treated rats were similar, indicating that the attenuation of lorazepam-induced amnesia was not caused by unbalanced drug levels in the blood, further confirming that estrogen is somehow responsible for this effect (Gibbs et al., 1998). More recent studies looking directly at sex differences show that high doses of diazepam (2 mg/kg and 4 mg/kg) impair memory retrieval in both sexes in a PM-DAT (Silva et al., 2016). Upon further examination, results indicate that the estrous cycle phase also influences the amnesic effect in females. Female rats in metestrus, diestrus, and estrus phases showed a significant amnesic effect when injected with 2 and 4 mg/kg of diazepam. Contrarily, female rats in proestrus did not exhibit amnesia at either 1 or 2 mg/kg doses (Silva et al., 2016). Finally, pre-treatment in proestrus female rats with a 5-alpha reductase inhibitor (finasteride) or a progesterone antagonist (mifepristone) restored the amnesic effects induced by 2 mg/kg doses of diazepam (Silva et al., 2016). These results point to sex hormones as mediators that influence BZD-induced amnesia. With limited research analyzing sex differences in this subject, further research should determine whether hormones affect drug-induced amnesia in males, how this changes with age and menopause models, and how this impacts neuronal activity in different brain regions.
It was and is clear that BZD-induced amnesia can be studied in rodents, but the exact injection timeline depends on the scientific questions being asked. Still, many unanswered questions remained- How would chronic administration impact cognition, does age or sex play a role, what brain regions are involved, and could these impairments be reversed by targeting BZD sites?
3 Molecular mechanisms of anterograde amnesia
Because BZDs are classified as positive allosteric modulators of the gamma amino butyric acid (GABA)-a receptor (Griffin et al., 2013), GABA receptor antagonists were used in an attempt to reverse BZD-induced amnesia. While GABA antagonists were ineffective, the BZD binding site competitive antagonist, Ro 15-1788 (Flumazenil), significantly reversed amnesia in the PAT model (E. R. Gamzu, 1988). Additionally, flumazenil significantly reduced the anterograde and retrograde amnesia produced by alprazolam in the EPM, in which transfer latency to a novel region of the maze from day 1–2 was used as a measure of memory (Singh et al., 1998). These early findings suggest that BZD binding sites may play a role in the amnesic effects. However, as GABAa receptors express a variety of other ligand-binding sites specific to ligands such as barbiturates, toxins, and other anesthetics (Sigel and Ernst, 2018), further analysis of how these receptors respond to different ligands may give better insight into how BZDs produce amnesia.
3.1 GABA receptors
GABAa receptors can be categorized as ligand-gated ion channels, permeable to both chloride and bicarbonate anions (Sigel and Ernst, 2018). BZDs bind to separate BZD sites on GABAa receptors, which leads to an increase in both GABA-activated channel openings and ion channel conductance of chloride (Figure 1) (Chebib and Johnston, 2000). This leads to hyperpolarization and reduced excitability of neurons (Griffin et al., 2013). GABAa receptors are comprised of subunits arranged in a pentameric circular structure (Sigel and Ernst, 2018). These receptors can exhibit many different isoforms from a combination of 19 subunits, including alpha (α1-6), beta (β1-3), gamma (γ1-3), ბ, ε, π, θ, and ρ1-3 (Engin et al., 2018). The most expressed GABAa receptor isoform is comprised of two α1, two β2, and one γ2 subunit (α1β2γ2) and accounts for around 60% of all GABAa receptors in the brain (Engin et al., 2018). The BZD binding site is located between the interface of the α and γ subunit and can be characterized as diazepam-sensitive (DS) or diazepam-insensitive (DI) based on the α isoform present within the receptor (Sigel and Ernst, 2018). For example, GABAa receptors containing α1,2,3 & 5 and γ1-3 are considered (DS), while others containing the α4 and 6 subunits are considered (DI) (Sigel and Ernst, 2018). The alpha (α) subunit is of interest, as it is thought to mediate the anxiolytic, sedative, relaxant, and perhaps the amnesic effects of BZDs (Griffin et al., 2013). It will be essential to understand how BZDs affect different GABAa receptor subtypes, as different isoforms of the GABAa receptor have specific subcellular expression patterns, which lead to varying channel-gating actions and unique pharmacological properties (Engin et al., 2018). While GABAa receptors containing α2 and α3 subunits are concentrated more in synaptic regions, α4 and α5 subunits are located more in perisynaptic and extrasynaptic areas (Engin et al., 2018). The localization of GABAa receptors to these areas is specific for the mechanism of inhibition being utilized, as extrasynaptic receptors allow for tonic inhibition, while synaptic receptors mediate phasic inhibition (Engin et al., 2018). Therefore, analysis of the α subunits contributing to BZD-induced amnesia will allow for a better understanding of the mechanism of inhibition that is altered. Most synaptic GABAa receptors are anchored to the inhibitory synapse by Gephyrin scaffolding proteins based on their subunit composition, while others can diffuse laterally through the plasma membrane and be recruited to the synapse (Jacob et al., 2008). Different scaffolding proteins like Radixin anchor extrasynaptic receptors containing the α5 subunit (Jacob et al., 2008). However, these receptors can be recruited to the synapse during times of increased excitation (Hausrat et al., 2015). Both the α1 and α5 subunits are highly expressed in the hippocampus (Hörtnagl et al., 2013), with α5 subunits being largely expressed in hippocampal pyramidal cells (Möhler and Rudolph, 2017). These two subunits are thought to play a role in amnesic side effects, learning, and memory; therefore, they have been heavily researched regarding their role in BZD-induced amnesia.
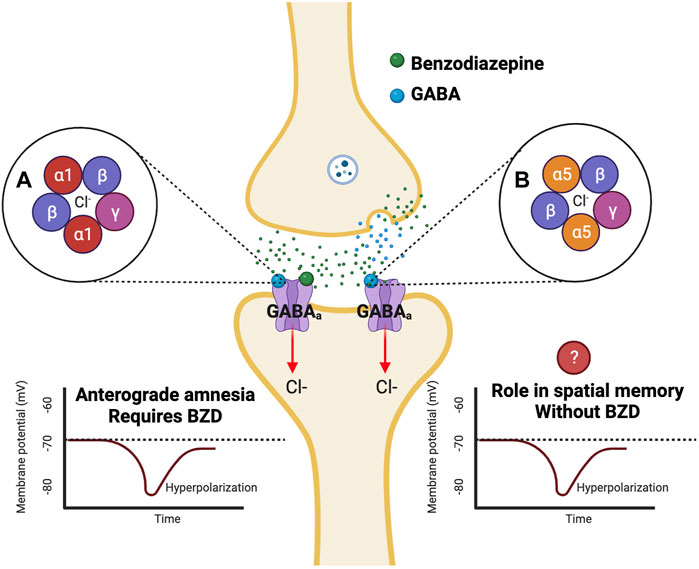
FIGURE 1. Benzodiazepines affect varying isoforms of GABAa receptors which are potentially responsible for anterograde amnesia. Benzodiazepines are positive allosteric modulators that bind to GABAa receptors at a BZD site separate from the GABA binding site. Binding of a benzodiazepine to the GABAa receptor results in an influx of Cl-into the cell leading to hyperpolarization (Griffin et al., 2013). Previous studies have found that α1 knockout mutant mice do not exhibit BZD-induced anterograde amnesia in the PAT test (Rudolph et al., 1999). Additional studies have indicated a role for the α5 subunit in learning and memory in fear conditioning and spatial memory tests (Collinson et al., 2002; Crestani et al., 2002).
To understand the importance of the α1 subunit of GABAa receptors, a point mutation was inserted into a mouse line that replaced a histidine with arginine in the α1 subunit gene, making it insensitive to BZDs (Rudolph et al., 1999). When put through a PAT with diazepam administered 30 min before training, mutant mice showed comparable latency to aversive stimuli compared to controls during recall, meaning they did not experience BZD-induced amnesia, suggesting that the α1 subunit is responsible for the amnestic effects (Rudolph et al., 1999). Comparably, this same mutation was introduced into the α5 subunit gene. Without a BZD injection, freezing levels in CFC and delayed fear conditioning in mutant mice were similar to controls (Crestani et al., 2002). However, mutant mice exhibited a significant increase in freezing during a trace fear conditioning test, indicating that this subunit alone plays a role in learning and memory (Crestani et al., 2002). Additional studies using α5 subunit knockout globally and specific to CA1 cells found similar and contradictory results. Global and CA1 specific α5 knockdown mice show a significant increase in freezing during trace fear conditioning, similar to previous studies (Engin et al., 2020). In contrast to previous findings, mice with global α5 knockdown but not CA1-specific knockdown showed significantly increased freezing in a CFC paradigm (Engin et al., 2020). Acknowledging this result, future studies should analyze if BZD-induced amnesia is present in these α5 knockout mice using CFC.
Recent studies have used the positive allosteric modulator of GABAa receptors, etomidate, known for its memory impairment properties, to understand how α5 subunits on both pyramidal and interneurons affect memory in a CFC paradigm (Zhu et al., 2023). Results indicate that etomidate affects α5 subunits in pyramidal and interneurons to impair memory, as mice with α5 knockouts in both cell types exhibited comparable freezing with and without etomidate treatment, while wild-type controls exhibited significantly less freezing after etomidate treatment (Zhu et al., 2023). While etomidate and BZDs both have memory impairment effects, these compounds have different binding sites on GABAa receptors (Chiara et al., 2013). This further supports the need to conduct additional research using BZDs to see if they impair memory using a similar mechanism. The α5 subunit may also be pertinent for spatial memory, as mice with an α5 knockout mutation perform significantly better in the “matching to place” version of the Morris water maze (Collinson et al., 2002). Interestingly, intrahippocampal knockdown of the α5 subunit using antisense oligonucleotides did not impair auditory contextual fear memory when midazolam was given directly after training (Gafford et al., 2005). While results point to the α1 subunit as a possible mediator for BZD-induced amnesia, the role of the α5 subunit is still ambiguous. Knockout of the α5 subunit has been shown to affect memory; however, these mutations did not affect BZD-induced amnesia. Administration of BZDs in an α5 knockout mutant before the encoding period may further delineate the α5 subunit’s role in memory formation. Further research must decipher how the α5 subunit modulates learning and memory.
3.2 Brain regions involved in anterograde amnesia
Although there have been proposed mechanisms by which BZDs induce anterograde amnesia, no single brain region has been identified as responsible for this effect. With the knowledge that the hippocampus and the amygdala are important regions for fear memory, scientists chose to focus on these areas first. Intrahippocampal injections of midazolam targeting the dorsal hippocampus impairs contextual fear memory when given immediately after training (Gafford et al., 2005). However, the same treatment does not impair fear memory when given 3 hours after the training period (Gafford et al., 2005) or given i.p. immediately after training (Bustos et al., 2006). These results suggest that BZDs impair the ability of the hippocampus to consolidate new memories only after intrahippocampal injection. Future research should focus on the administration route of BZDs. Integration of BZDs into the diet of rodents could mimic the oral administration usually used by humans. Additional research should focus on the relationship between BZD injection and spine density, neuronal projections, and immediate early genes in different hippocampal regions as spine densities decrease in the CA1 and CA3 following chronic diazepam use in young and old mice (Furukawa et al., 2021).
The amygdala processes emotion and has a strong connection with the hippocampus (Maren, 2001). Therefore, the hippocampal-amygdala pathway is worthy of attention to try to understand how BZDs induce amnesia for fear memories. Intra-basolateral amygdala injections of midazolam before stress exposure prevents CA1 structural plasticity and reduces fear expression (Giachero et al., 2015). Previous studies have also deemed the amygdala complex (AC) important for fear memory. Both control and high-dose diazepam-treated rats with bilateral amygdaloid complex lesions showed impaired acquisition in a continuous multiple-trial inhibitory avoidance test (Tomaz et al., 1991). When re-exposed 48 h later, diazepam-treated rats with AC lesions did not show a significant difference in latency to the aversive context compared to controls, indicating that the amygdaloid complex may be crucial for the amnesic effects of BZDs (Tomaz et al., 1991). To further understand which amygdala nuclei were relevant for this amnesia, the central, lateral, and basolateral amygdala were lesioned. Rats with lesions in the central and lateral amygdala exhibited impaired retention after diazepam treatment; however, rats with lesions in the basolateral amygdala did not exhibit retention deficits (Tomaz et al., 1992). Finally, microinjections of diazepam into the basolateral and lateral nuclei of the amygdala induced amnesia in rats (Tomaz et al., 1992). Together, these findings suggest that the basolateral nucleus of the amygdala is crucial for BZD-induced amnesia; however, the affected hippocampal regions are still largely unidentified. Although lesions in the central amygdala did not lead to impaired retention in previous studies, this brain region should still be analyzed. The immediate early gene c-fos, indicative of recent activity, is increased in PKCδ+ neurons in the central amygdala after BZD administration (Griessner et al., 2021). This increase in c-fos has also been correlated with the anxiolytic activity exhibited by mice in the EPM (Griessner et al., 2021). Analysis of the neuronal circuits that include the central amygdala may give further insight into the amnesic effects of BZDs. As previously stated, BZD-induced amnesia shows similarities to the amnesia present in patients with severe damage to the medial temporal lobe, a hallmark of AD. Acknowledging this similarity, research should analyze the relationship between BZDs, aging, and AD.
4 Benzodiazepines and Alzheimer’s disease
4.1 Human studies
As of 2008, 5.2% of the US population aged 18 to 80 reported using BZDs (Olfson et al., 2015). Of the 5.2%, women reported using BZDs almost twice as much as men. Older populations generally report more long-term use than younger populations (Olfson et al., 2015), and BZDs increase adverse drug reactions in elderly patients (Larson et al., 1987). Therefore, understanding how BZD use affects AD patients is needed, as this disease is more prevalent in older populations and women. Studies related to BZDs and how they affect memory in AD patients date back to the late 1980s (Sunderland et al., 1989). While early studies failed to reveal memory deficits between AD patients and age-matched controls, they did indicate a high rate of adverse drug reactions. A population of elderly patients experiencing adverse drug reactions was found to have cognitive impairments associated with long-acting BZD use (Larson et al., 1987). The effects of these drugs on cognitive decline in AD patients have been further analyzed using models such as the Mini-Mental State Exam (MMSE) and the Clinical Dementia Rating Sum of Boxes (CDR-Sum) (Rosenberg et al., 2012; Borda et al., 2021). Constant use of BZDs has been associated with a rapid decline in MMSE and a rapid increase in CDR-Sum (Rosenberg et al., 2012). However, controversy still exists as recent work failed to show an association between BZD use and accelerated cognitive decline in AD patients and elderly patients with normal cognition using these same measures (Zhang et al., 2016; Borda et al., 2021). The lack of consensus on whether BZDs are associated with cognitive decline could be because of different confounding factors within the studied populations. These include the consumption of other drugs and the onset of BZD usage. Controlled longitudinal studies could try to limit these confounding factors to reach a more precise answer.
Studies focusing on the APOE4 gene, an AD risk factor, show deficits in long-term memory and cognitive function after BZD administration (Pomara et al., 2005; Stonnington et al., 2017). Certain patients with the APOE4 gene exhibit persistent deficits in long-term memory compared to controls after lorazepam administration (Pomara et al., 2005). Compared to people with the APOE 3/3 gene, carriers of the APOE 3/4 gene show significantly more cognitive impairments related to working memory, visuospatial memory, and executive function 2.5 and 5 h after administration of lorazepam (Stonnington et al., 2017). These studies suggest that AD risk factor gene carriers are more susceptible to cognitive impairments induced by BZDs. There is no current consensus on whether BZDs increase the risk of developing AD or whether these drugs accelerate cognitive decline in previously diagnosed patients. Future research should look to see if BZDs aggravate AD pathology.
4.2 Rodent studies
Although BZD studies are contradictory in the patient population, we can use AD mouse models to begin answering these complicated questions. Tau phosphorylation, a hallmark of AD, is increased 30 min and 6 h later in wild-type mice injected with midazolam (Whittington et al., 2019). Surprisingly, these same injections did not cause further tau phosphorylation in transgenic hTau mice and did not impair spatial reference memory (Whittington et al., 2019). These results suggest that BZDs may not accelerate pathology but may induce disease hallmarks. Comparably, the injection of a new BZD, remimazolam tosylate, increased tau phosphorylation in certain areas of the frontal cortex in the short term but actually reduced tau phosphorylation in frontal cortex areas over time (Liu et al., 2022). Recognition memory was also impaired short term, but these deficits did not last (Liu et al., 2022). This is similar to other findings indicating that these drugs impair working memory during treatment but do not persist after treatment discontinuation (Carton et al., 2021). Perhaps one of the more interesting findings is that the amnesia produced by BZDs may be similar to the memory deficits observed in AD patients, as Alzheimer’s drugs such as memantine (an NMDA receptor antagonist) and donepezil (an acetylcholinesterase inhibitor) can reverse alprazolam-induced amnesia (Vandesquille et al., 2012). Based on these findings, BZDs could also be used as a novel tool to help understand memory and cognitive impairments produced by AD.
5 Future directions in benzodiazepine research
Even with extensive research exploring how BZDs impair memory, many questions remain unanswered on the behavioral, molecular, and circuit levels. While many studies suggest that BZD binding sites mediates BZD-induced amnesia, other anxiolytic drugs that bind to the BZD binding site do not produce this amnesia (E. R. Gamzu, 1988). These include “anxioselective” drugs such as bretazenil, abecarnil, alpidem, and ocinaplon, which exhibit anxiolytic properties without the side effects of traditional BZDs, including amnesia (Skolnick, 2012). These compounds range in their affinity and selectivity for varying isoforms of GABAa receptors, with some acting as full agonists to receptors containing DS binding sites while others act as partial agonists on receptors with DS and DI binding sites (Figure 2) (Mehta and Shank, 1995; Lippa et al., 2005; Pym et al., 2005; Sieghart and Savic, 2018). Understanding how these anxioselective drugs affect GABAa receptors differently compared to classical BZDs, newer BZD compounds like remimazolam, and other GABAa agonists like gaboxadol could point to new directions regarding how BZDs induce amnesia through different isoforms (Zanettini et al., 2016; Noor et al., 2021; Sente et al., 2022). Evidence strongly suggests that the α1 and α5 subunits of GABAa receptors are also mediators of drug-induced amnesia for different types of memory. New research should look to differentiate which memories are influenced by these subunits. As distinct pharmacological compounds that activate the GABAa receptor may induce different cascades, investigating secondary signaling pathways is essential for future studies. Because sex hormones, such as estrogen and progesterone, protect against drug-induced amnesia, the molecular mechanisms regarding sex differences must also be addressed. Additionally, results have indicated that the BLA of the amygdala mediates anterograde amnesia in BZD-treated rats, but the role of the hippocampus is still unclear. Viral tracing studies and activity-dependent memory tagging mouse lines may give further insight into how these two areas communicate. Finally, the relationship between BZDs and AD is still perplexing. Studies show that BZDs can induce hallmarks of Alzheimer’s but that drug-induced cognitive impairments that mirror AD eventually fade. Therefore, in addition to identifying the mechanism of action behind BZD-induced amnesia, future studies can use BZDs as a novel tool to study AD.
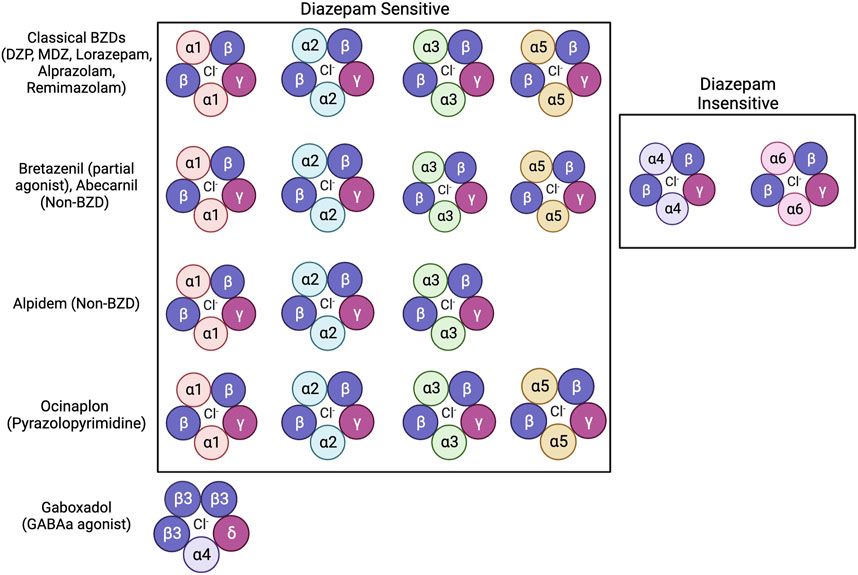
FIGURE 2. Benzodiazepines, non-benzodiazepines, and other anxioselective compounds show affinity for specific isoforms of GABAa receptors. Classical BZDs have high affinity to DS (Diazepam Sensitive) isoforms of GABAa receptors which include α1, α2, α3, and α5 subunits (Sigel and Ernst, 2018). GABAa receptor isoforms containing α4 and α6 subunits are categorized as DI (Diazepam Insensitive) (Sigel and Ernst, 2018). The anxioselective compounds Bretazenil (partial agonist), and Abecarnil (Non-BZD) have affinity to both DS and DI GABAa receptors (Mehta and Shank, 1995; Pym et al., 2005). The anxioselective drug Alpidem (Non-BZD) has affinity to α1, α2, and α3 isoforms, but not the α5 isoform (Sieghart and Savic, 2018). Another anxioselective compound, Ocinaplon (pyrazolopyrimidine) has affinity to all DS isoforms, with higher affinity to α1 compared to α5 (Lippa et al., 2005). The GABAa agonist gaboxadol is known to bind to GABAa receptors containing the α4 and ბ subunits (Zanettini et al., 2016; Sente et al., 2022).
Author contributions
KK: Conceptualization, Investigation, Visualization, Writing–original draft, Writing–review and editing. HH: Funding acquisition, Supervision, Writing–review and editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. KK is supported by HH K99/R00 (R00AG059953) from the NIA and startup funds from RFU.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Borda, M. G., Jaramillo-Jimenez, A., Oesterhus, R., Santacruz, J. M., Tovar-Rios, D. A., Soennesyn, H., et al. (2021). Benzodiazepines and antidepressants: effects on cognitive and functional decline in Alzheimer’s disease and Lewy body dementia. Int. J. Geriatr. Psychiatry 36, 917–925. doi:10.1002/gps.5494
Bustos, S. G., Maldonado, H., and Molina, V. A. (2006). Midazolam disrupts fear memory reconsolidation. Neuroscience 139, 831–842. doi:10.1016/j.neuroscience.2005.12.064
Carton, L., Niot, C., Kyheng, M., Petrault, M., Laloux, C., Potey, C., et al. (2021). Lack of direct involvement of a diazepam long-term treatment in the occurrence of irreversible cognitive impairment: a pre-clinical approach. Transl. Psychiatry 11, 612. doi:10.1038/s41398-021-01718-8
Chebib, M., and Johnston, G. A. R. (2000). GABA-activated ligand gated ion channels: medicinal chemistry and molecular biology. J. Med. Chem. 43, 1427–1447. doi:10.1021/jm9904349
Chiara, D. C., Jayakar, S. S., Zhou, X., Zhang, X., Savechenkov, P. Y., Bruzik, K. S., et al. (2013). Specificity of intersubunit general anesthetic-binding sites in the transmembrane domain of the human α1β3γ2 γ-aminobutyric acid type A (GABAA) receptor. J. Biol. Chem. 288, 19343–19357. doi:10.1074/jbc.M113.479725
Collinson, N., Kuenzi, F. M., Jarolimek, W., Maubach, K. A., Cothliff, R., Sur, C., et al. (2002). Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the 5 subunit of the GABA A receptor. J. Neurosci. 22 (13), 5572–5580. doi:10.1523/JNEUROSCI.22-13-05572.2002
Crestani, F., Keist, R., Fritschy, J.-M., Benke, D., Vogt, K., Prut, L., et al. (2002). Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. PNAS 99, 8980–8985. doi:10.1073/pnas.142288699
Dundee, J. W., and Pandit, S. K. (1972). Anterograde amnesic effects of pethidine, hyoscine and diazepam in adults. Br. J. Pharmacol. 44, 140–144. doi:10.1111/j.1476-5381.1972.tb07245.x
Engin, E., Benham, R. S., and Rudolph, U. (2018). An emerging circuit pharmacology of GABAA receptors. Trends Pharmacol. Sci. 39, 710–732. doi:10.1016/j.tips.2018.04.003
Engin, E., Sigal, M., Benke, D., Zeller, A., and Rudolph, U. (2020). Bidirectional regulation of distinct memory domains by α5-subunit-containing GABAA receptors in CA1 pyramidal neurons. Learn. Mem. 27, 423–428. doi:10.1101/LM.052084.120
Furukawa, T., Nikaido, Y., Shimoyama, S., Masuyama, N., Notoya, A., and Ueno, S. (2021). Impaired cognitive function and hippocampal changes following chronic diazepam treatment in middle-aged mice. Front. Aging Neurosci. 13, 777404. doi:10.3389/fnagi.2021.777404
Gafford, G. M., Parsons, R. G., and Helmstetter, F. J. (2005). Effects of post-training hippocampal injections of midazolam on fear conditioning. Learn. Mem. 12, 573–578. doi:10.1101/lm.51305
Gamzu, E. R. (1988). Animal model studies of benzodiazepine induced amnesia. Psychopharmacol. Ser. 6, 218–229. doi:10.1007/978-3-642-73288-1_16
Giachero, M., Calfa, G. D., and Molina, V. A. (2015). Hippocampal dendritic spines remodeling and fear memory are modulated by GABAergic signaling within the basolateral amygdala complex. Hippocampus 25, 545–555. doi:10.1002/hipo.22409
Gibbs, R. B., Burke, A. M., and Johnson, D. A. (1998). Estrogen replacement attenuates effects of scopolamine and lorazepam on memory acquisition and retention. Horm. Behav. 34 (2), 112–125. doi:10.1006/hbeh.1998.1452
Griessner, J., Pasieka, M., Böhm, V., Grössl, F., Kaczanowska, J., Pliota, P., et al. (2021). Central amygdala circuit dynamics underlying the benzodiazepine anxiolytic effect. Mol. Psychiatry 26, 534–544. doi:10.1038/s41380-018-0310-3
Griffin, C. E., Kaye, A. M., Rivera Bueno, F., and Kaye, A. D. (2013). Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 13, 214–223.
Hausrat, T. J., Muhia, M., Gerrow, K., Thomas, P., Hirdes, W., Tsukita, S., et al. (2015). Radixin regulates synaptic GABA A receptor density and is essential for reversal learning and short-term memory. Nat. Commun. 6, 6872. doi:10.1038/ncomms7872
Hörtnagl, H., Tasan, R. O., Wieselthaler, A., Kirchmair, E., Sieghart, W., and Sperk, G. (2013). Patterns of mRNA and protein expression for 12 GABAA receptor subunits in the mouse brain. Neuroscience 236, 345–372. doi:10.1016/j.neuroscience.2013.01.008
Jacob, T. C., Moss, S. J., and Jurd, R. (2008). GABAA receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat. Rev. Neurosci. 9, 331–343. doi:10.1038/nrn2370
Jovasevic, V., Corcoran, K. A., Leaderbrand, K., Yamawaki, N., Guedea, A. L., Chen, H. J., et al. (2015). GABAergic mechanisms regulated by MIR-33 encode state-dependent fear. Nat. Neurosci. 18, 1265–1271. doi:10.1038/nn.4084
Larson, E. B., Kukull, W. A., Buchner, D., and Reifler, B. V. (1987). Adverse drug reactions associated with global cognitive impairment in elderly persons. Ann. Intern Med. 107, 169–173. doi:10.7326/0003-4819-107-2-169
Lippa, A., Czobor, P., Stark, J., Beer, B., Kostakis, E., Gravielle, M., et al. (2005). Selective anxiolysis produced by ocinaplon, a GABA A receptor modulator. Proc. Natl. Acad. Sci. U. S. A. 102, 7380–7385. doi:10.1073/pnas.0502579102
Liu, X., Guo, L., Duan, B., Wu, J., and Wang, E. (2022). Novel benzodiazepine remimazolam tosylate delays neurodegeneration of aged mice via decreasing tau phosphorylation. Neurotoxicology 92, 156–165. doi:10.1016/j.neuro.2022.08.003
Malkani, S., and Rosen, J. B. (2000). Induction of NGFI-B mRNA following contextual fear conditioning and its blockade by diazepam. Brain Res. Mol. Brain Res. 80, 153–165. doi:10.1016/s0169-328x(00)00130-3
Maren, S. (2001). Neurobiology of pavlovian fear conditioning. Annu. Rev. Neurosci. 24, 897–931. doi:10.1146/annurev.neuro.24.1.897
Mehta, A. K., and Shank, R. P. (1995). Interaction of abecarnil, bretazenil, and Ro 19-8022 with diazepam-sensitive and -insensitive benzodiazepine sites in the rat cerebellum and cerebral cortex. Life Sci. 57, 2215–2222. doi:10.1016/0024-3205(95)02126-4
Möhler, H., and Rudolph, U. (2017). Disinhibition, an emerging pharmacology of learning and memory. F1000Res 6, 101. doi:10.12688/f1000research.9947.1
Murphy, H. M., Kalinina, A. I., and Wideman, C. H. (2022). Effects of chronic oral administration of midazolam on memory and circadian rhythms in rats. Drug Res. 73, 40–45. doi:10.1055/a-1937-9064
Nakagawa, Y., Iwasaki, T., Ishima, T., and Kimura, K. (1993). Interaction between benzodiazepine and GABA-A receptors in state dependent learning. Life Sci. 52, 1935–1945. doi:10.1016/0024-3205(93)90634-f
Noor, N., Legendre, R., Cloutet, A., Chitneni, A., Varrassi, G., and Kaye, A. D. (2021). A comprehensive review of remimazolam for sedation. Health Psychol. Res. 9, 24514. doi:10.52965/001C.24514
Olfson, M., King, M., and Schoenbaum, M. (2015). Benzodiazepine use in the United States. JAMA Psychiatry 72, 136–142. doi:10.1001/jamapsychiatry.2014.1763
Pain, L., Launoy, A., Fouquet, N., and Oberling, P. (2002). Mechanisms of action of midazolam on expression of contextual fear in rats. Br. J. Anaesth. 89, 614–621. doi:10.1093/bja/aef228
Pomara, N., Willoughby, L., Wesnes, K., Greenblatt, D. J., and Sidtis, J. J. (2005). Apolipoprotein E epsilon4 allele and lorazepam effects on memory in high-functioning older adults. Arch. Gen. Psychiatry 62, 209–216. doi:10.1001/archpsyc.62.2.209
Pym, L. J., Cook, S. M., Rosahl, T., McKernan, R. M., and Atack, J. R. (2005). Selective labelling of diazepam-insensitive GABA A receptors in vivo using [ 3H]Ro 15-4513. Br. J. Pharmacol. 146, 817–825. doi:10.1038/sj.bjp.0706392
Rosenberg, P. B., Mielke, M. M., Han, D., Leoutsakos, J. S., Lyketsos, C. G., Rabins, P. V., et al. (2012). The association of psychotropic medication use with the cognitive, functional, and neuropsychiatric trajectory of Alzheimer’s disease. Int. J. Geriatr. Psychiatry 27, 1248–1257. doi:10.1002/gps.3769
Rudolph, U., Crestani, F., Benke, D., Brünig, I., Benson, J. A., Fritschy, J., et al. (1999). Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature 401, 796–800. doi:10.1038/44579
Sanday, L., Zanin, K. A., Patti, C. L., Tufik, S., and Frussa-Filho, R. (2012). Role of state-dependency in memory impairment induced by acute administration of midazolam in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 37, 1–7. doi:10.1016/j.pnpbp.2012.01.013
Segura, I. A., McGhee, J., Della Sala, S., Cowan, N., and Pompéia, S. (2021). A reappraisal of acute doses of benzodiazepines as a model of anterograde amnesia. Hum. Psychopharmacol. 36, e2774. doi:10.1002/hup.2774
Sente, A., Desai, R., Naydenova, K., Malinauskas, T., Jounaidi, Y., Miehling, J., et al. (2022). Differential assembly diversifies GABAA receptor structures and signalling. Nature 604, 190–194. doi:10.1038/s41586-022-04517-3
Sieghart, W., and Savic, M. M. (2018). International union of basic and clinical pharmacology. CVI: GABAA receptor subtype-and function-selective ligands: key issues in translation to humans. Pharmacol. Rev. 70, 836–878. doi:10.1124/PR.117.014449
Sigel, E., and Ernst, M. (2018). The benzodiazepine binding sites of GABAA receptors. Trends Pharmacol. Sci. 39, 659–671. doi:10.1016/j.tips.2018.03.006
Silva, A. F., Sousa, D. S., Medeiros, A. M., Macêdo, P. T., Leão, A. H., Ribeiro, A. M., et al. (2016). Sex and estrous cycle influence diazepam effects on anxiety and memory: possible role of progesterone. Prog. Neuropsychopharmacol. Biol. Psychiatry 70, 68–76. doi:10.1016/j.pnpbp.2016.05.003
Singh, N., Sharma, A., and Singh, M. (1998). Possible mechanism of alprazolam-induced amnesia in mice. Pharmacology 56, 46–50. doi:10.1159/000028181
Skolnick, P. (2012). Anxioselective anxiolytics: on a quest for the holy grail. Trends Pharmacol. Sci. 33, 611–620. doi:10.1016/j.tips.2012.08.003
Stonnington, C. M., Harel, B., Locke, D. E. C., Hentz, J. G., Zhang, N., Maruff, P., et al. (2017). Lorazepam challenge for individuals at varying genetic risk for alzheimer disease. Alzheimer Dis. Assoc. Disord. 31, 271–277. doi:10.1097/WAD.0000000000000200
Sunderland, T., Weingartner, H., Cohen, R. M., Tariot, P. N., Newhouse, P. A., Thompson, K. E., et al. (1989). Low-dose oral lorazepam administration in Alzheimer subjects and age-matched controls. Psychopharmacology 99, 129–133. doi:10.1007/BF00634466
Timić, T., Joksimović, S., Milić, M., Divljaković, J., Batinić, B., and Savić, M. M. (2013). Midazolam impairs acquisition and retrieval, but not consolidation of reference memory in the Morris water maze. Behav. Brain Res. 241, 198–205. doi:10.1016/j.bbr.2012.12.014
Tomaz, C., Dickinson-Anson, H., and Mcgaugh, J. L. (1991). Amygdala lesions block the amnestic effects of diazepam. Brain Res. 568, 85–91. doi:10.1016/0006-8993(91)91382-b
Tomaz, C., Dickinson-Ansontt, H., and Mcgaught, J. L. (1992). Basolateral amygdala lesions block diazepam-induced anterograde amnesia in an inhibitory avoidance task. Proc. Natl. Acad. Sci. U. S. A. 89, 3615–3619. doi:10.1073/pnas.89.8.3615
Vandesquille, M., Carrié, I., Louis, C., Beracochea, D., and Lestage, P. (2012). Effects of positive modulators of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptors in a benzodiazepine-induced deficit of spatial discrimination in mice. J. Psychopharmacol. 26, 845–856. doi:10.1177/0269881111416692
Wan, H., Warburton, E. C., Zhu, X. O., Koder, T. J., Park, Y., Aggleton, J. P., et al. (2004). Benzodiazepine impairment of perirhinal cortical plasticity and recognition memory. Eur. J. Neurosci. 20, 2214–2224. doi:10.1111/j.1460-9568.2004.03688.x
Whittington, R. A., Virág, L., Gratuze, M., Lewkowitz-Shpuntoff, H., Cheheltanan, M., Petry, F., et al. (2019). Administration of the benzodiazepine midazolam increases tau phosphorylation in the mouse brain. Neurobiol. Aging 75, 11–24. doi:10.1016/j.neurobiolaging.2018.10.027
Zanettini, C., Pressly, J. D., Ibarra, M. H., Smith, K. R., and Gerak, L. R. (2016). Comparing the discriminative stimulus effects of modulators of GABAA receptors containing α4-δ subunits with those of gaboxadol in rats. Psychopharmacol. Berl. 233, 2005–2013. doi:10.1007/s00213-016-4243-8
Zarrindast, M. R., and Khakpai, F. (2020). State-dependent memory and its modulation by different brain areas and neurotransmitters. EXCLI J. 19, 1081–1099. doi:10.17179/excli2020-2612
Zhang, Y., Zhou, X. H., Meranus, D. H., Wang, L., and Kukull, W. A. (2016). Benzodiazepine use and cognitive decline in elderly with normal cognition. Alzheimer Dis. Assoc. Disord. 30, 113–117. doi:10.1097/WAD.0000000000000099
Keywords: benzodiazepines, anterograde amnesia, sex differences, Alzheimer’s disease, GABAA, fear conditioning
Citation: Kaplan K and Hunsberger HC (2023) Benzodiazepine-induced anterograde amnesia: detrimental side effect to novel study tool. Front. Pharmacol. 14:1257030. doi: 10.3389/fphar.2023.1257030
Received: 11 July 2023; Accepted: 04 September 2023;
Published: 14 September 2023.
Edited by:
Jacob Raber, Oregon Health and Science University, United StatesReviewed by:
Margot Ernst, Medical University of Vienna, AustriaLuc Ver Donck, Janssen Research and Development, Belgium
Copyright © 2023 Kaplan and Hunsberger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Holly Christian Hunsberger, holly.hunsberger@rosalindfranklin.edu
 Kameron Kaplan
Kameron Kaplan