
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Pharmacol. , 28 July 2023
Sec. Predictive Toxicology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1255471
This article is part of the Research Topic Neurobehavioral Toxicity induced by Environmental Contaminants: Mechanisms of Neurodegeneration and Neuroprotection, Volume II View all 5 articles
Editorial on the Research Topic
Neurobehavioral toxicity induced by environmental contaminants: mechanisms of neurodegeneration and neuroprotection, Volume II
Environmental contaminants pose a significant threat to human health, particularly in the context of neurotoxicity. Heavy metals, herbicides, and persistent organic pollutants have been extensively studied and associated with neurodegeneration and cognitive impairments. The goal of this series of manuscripts, which was published in the scientific journal Frontiers in Toxicology, is to provide a comprehensive understanding on the mechanisms underlying neurotoxicity induced by environmental pollutants. These articles shed light on the complicated processes by which pollutants exert their harmful effects, by investigating the intricate interplay of multiple pathways such as oxidative stress, mitochondrial dysfunction, inflammation, and epigenetic changes.
One of the fundamental mechanisms by which environmental contaminants induce neurotoxicity is through the generation of oxidative stress. Contaminants such as lead, mercury, and cadmium induce reactive oxygen species (ROS), which in turn can trigger lipid peroxidation, DNA damage, and protein oxidation within neural cells. This oxidative stress not only contributes to neuroinflammation, but also activates apoptotic pathways, ultimately leading to neuronal cell death. Further exploration of the specific mechanisms by which contaminants induce oxidative stress and the resulting consequence on neurological function provides valuable insights into putative therapeutic interventions.
Another crucial mechanism underlying neurotoxicity is mitochondrial dysfunction. Many contaminants disrupt mitochondrial function by altering mitochondrial membrane potential, impairing ATP production, and promote mitochondrial ROS generation. Therefore, cellular energy deficits occur, leading to chronic inflammation and neuronal damage. The exploration of how various contaminants interact with mitochondria and the subsequent implication for neuronal health offers insight into the multifaceted nature of neurotoxicity.
Inflammation serves as a significant pathway by which environmental contaminants exert their neurotoxic effects. Pesticides and persistent organic pollutants are known to activate the immune system, resulting in the release of proinflammatory cytokines and chemokines. These inflammatory mediators can disrupt normal neuronal signaling, giving rise to cognitive deficits and neurodegeneration. Unraveling the intricate mechanisms by which contaminants induce inflammation and the subsequent impact on neural function provides crucial insights into the development of targeted therapies.
Emerging evidence suggests that epigenetic modifications play a pivotal role in mediating neurotoxicity induced by environmental contaminants. Contaminants such as bisphenol A and phthalates have been found to alter DNA methylation, histone modifications, and microRNA expression, leading to changes in gene expression that can affect neuronal development and function. Understanding the epigenetic mechanisms involved in neurotoxicity provides novel avenues for interventions that might mitigate the adverse effects of contaminants on the nervous system.
By focusing on oxidative stress, mitochondrial dysfunction, inflammation, and epigenetic modifications, the article Research Topic aims to provide a comprehensive understanding of the complex interplay between contaminants and the nervous system (Figure 1). This knowledge not only expands our understanding of the adverse effects of environmental contaminants on neurobehavioral health, but also opens new avenues for the development of targeted therapeutic strategies.
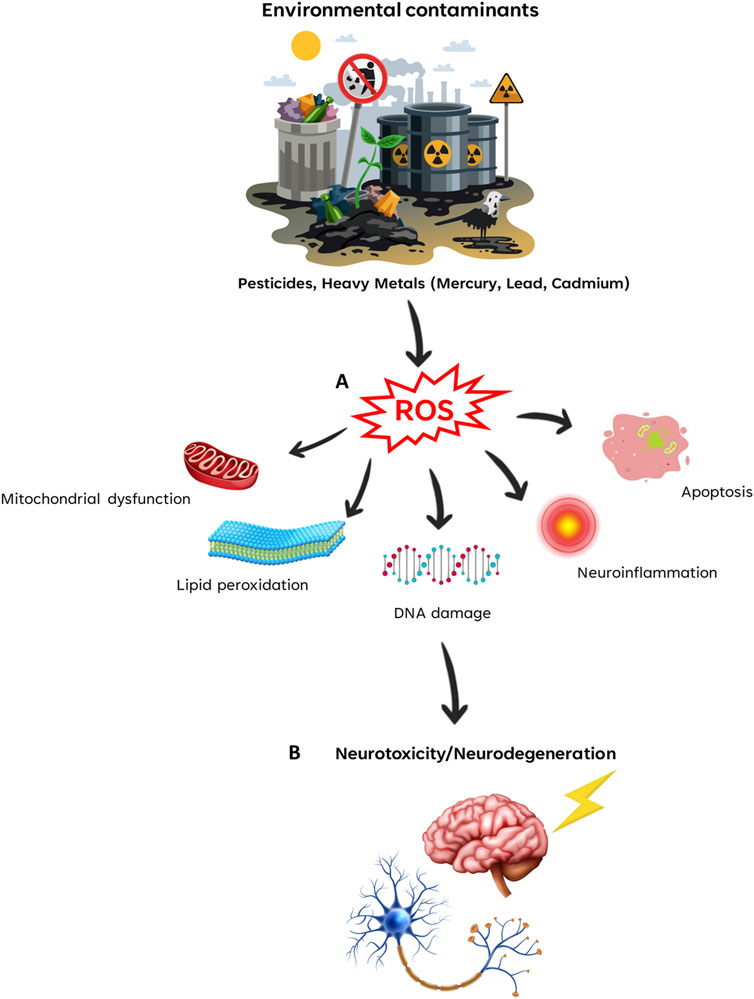
FIGURE 1. Environmental contaminants-induced neurotoxicity. Environmental contaminants induce the production of ROS (Reactive Oxygen Species) (A), which triggers several cellular mechanisms that leads to neurotoxicity and neurodegeneration (B).
The present volume is designed to address contemporary issues in neurodevelopmental neurotoxicity and is comprised of 4 papers which address diverse topics. These include an original research article “Isoimperatorin therapeutic effect against aluminum induced neurotoxicity in albino mice”. The study found that IMP might be a promising treatment option for neurotoxicity and neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease, which are associated with neuro-inflammation and oxidative stress (Rajendran et al.).
A second article intitled: “Neuroprotection of Gastrodia elata polyphenols against H2O2-induced PC12 cell cytotoxicity by reducing oxidative stress” is an original research article that focuses on the potential of Gastrodia elata polyphenols (GPP) for the prevention and treatment of Alzheimer’s disease. The study found that GPP pretreatment had a protective effect by increasing cell viability, reducing lactate dehydrogenase infiltration, decreasing MDA and increasing intracellular antioxidant enzymes, diminishing reactive oxygen species production and decreasing mitochondrial membrane potential, reducing cell inflammation and decreasing apoptosis (Tan et al.).
Another original research published in this volume “Nicotine alleviates MPTP-induced nigrostriatal damage through modulation of JNK and ERK signaling pathways in the mice model of Parkinson’s disease” found that nicotine reduced MPTP-induced behavioral deficits, dopaminergic neuron loss, oxidative stress, inflammation, and apoptosis in the mice. The authors also found that nicotine modulated the activation of JNK and ERK signaling pathways, which are involved in neuronal survival and death, concluding that nicotine may have neuroprotective effects against MPTP-induced nigrostriatal damage by regulating JNK and ERK signaling pathways (Ruan et al.).
Finally, the series includes a review on “Mechanisms of manganese-induced neurotoxicity and the pursuit of neurotherapeutic strategies”. The authors summarize manganese’s toxicodynamic as well as potential molecular targets for treatment and possible neurotherapeutic agents in the search for treatment (Pajarillo et al.).
As Research Topic Editors of this volume, we hope that the articles published here will make a significant contribution to the advancement of research in the field of neurotoxicology.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
We want to thank the authors and all the guest editors who contributed to this Research Topic.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: enviromental contaminats, heavy metals, neurotoxicity, reactive oxygen species (ROS), neurodegenaration
Citation: Dorta DJ, Oliveira DP, Campos AC and Aschner M (2023) Editorial: Neurobehavioral toxicity induced by environmental contaminants: mechanisms of neurodegeneration and neuroprotection, Volume II. Front. Pharmacol. 14:1255471. doi: 10.3389/fphar.2023.1255471
Received: 08 July 2023; Accepted: 17 July 2023;
Published: 28 July 2023.
Edited and reviewed by:
Ursula Gundert-Remy, Charité University Medicine Berlin, GermanyCopyright © 2023 Dorta, Oliveira, Campos and Aschner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel J. Dorta, ZGpkb3J0YUBmZmNscnAudXNwLmJy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.