
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
POLICY AND PRACTICE REVIEWS article
Front. Pharmacol. , 09 October 2023
Sec. Drugs Outcomes Research and Policies
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1254706
An evidence-based consensus meeting was held with urologists, a pharmacist and a cardiologist to perform a structured benefit-risk analysis of reclassifying tadalafil, a phosphodiesterase type 5 (PDE5) inhibitor for treatment of erectile dysfunction (ED), to be available without prescription in Germany. As per the Brass process endorsed by regulatory authorities, an evidence-based Brass value tree was developed, which identified the incremental benefits and risks that should be considered above the safety and efficacy evidence required for prescription medicines. During the Group Delphi consensus meeting, the expert panel rated the likelihood and clinical impact of each benefit and risk on a scale of 0 (none) to 3 (high). Overall attribute scores were calculated from the product of the mean likelihood and mean clinical impact scores giving a possible score of 0–9. The overall benefit attribute scores ranged from 2.8 to 5.4. The overall risk attribute scores ranged from 0.2 to 2.2 though most were 1.0 or less (3 or more is generally considered to be of concern). On balance, the independent meeting scored the benefits of reclassification of tadalafil higher than the risks and considered the risk mitigation strategies of the packaging label and patient information leaflet (PIL) sufficient.
There is a high prevalence of erectile dysfunction (ED) in Europe, with 42% (53.9 million) of men self-reporting difficulty achieving or maintaining an erection in the previous 5 months (Goldstein et al., 2020). In Germany, the figure is between 33% and 45%, which includes 5% of younger men aged 18 to 39 (Jannini et al., 2014; Briken et al., 2020; Goldstein et al., 2020). Despite the high prevalence, up to 81% of men do not seek treatment, so ED remains under-recognised, underdiagnosed and undertreated (Shabsigh et al., 2004; de Boer et al., 2005; Moreira et al., 2005; Nicolosi and et a l., 2006; Frederick et al., 2014; Jannini et al., 2014).
The phosphodiesterase type 5 (PDE5) inhibitor, tadalafil, is currently only available in Germany via a prescription. The aim of this study was to convene an expert panel to quantify the likelihood of occurrence and the potential clinical impact of each incremental benefit and risk identified for reclassification of tadalafil as a non-prescription medicine.
To ensure a systematic, transparent methodology was followed, a Brass analysis was undertaken (Brass et al., 2013). This structured process has been developed to help in complex decision-making for reclassification of prescription medications and is endorsed by regulatory authorities such as the UK Medicines and Healthcare products Regulatory Agency (MHRA) and the US Federal agency, the Food and Drug Administration (FDA) (Brass et al., 2011; Medicines and Healthcare products Regulatory Agency, 2021).
The Brass analysis began with the development of a Brass value tree, which was informed by a comprehensive evidence review with clinical expert input (Figure 1) (Brass et al., 2011). The Brass value tree is a framework for identifying incremental benefits and risks that might arise in addition to the established efficacy and safety of the product as a prescription medicine according to the European Medicines Agency Article 71 (1) of Directive 2001/83/EC (EMA, 2006). Within each pre-defined major benefit and risk domain that should be considered for any non-prescription drug, specific attributes were identified for tadalafil. The Brass value tree and evidence for each incremental benefit and risk were circulated to an expert clinical panel prior to the consensus meeting.
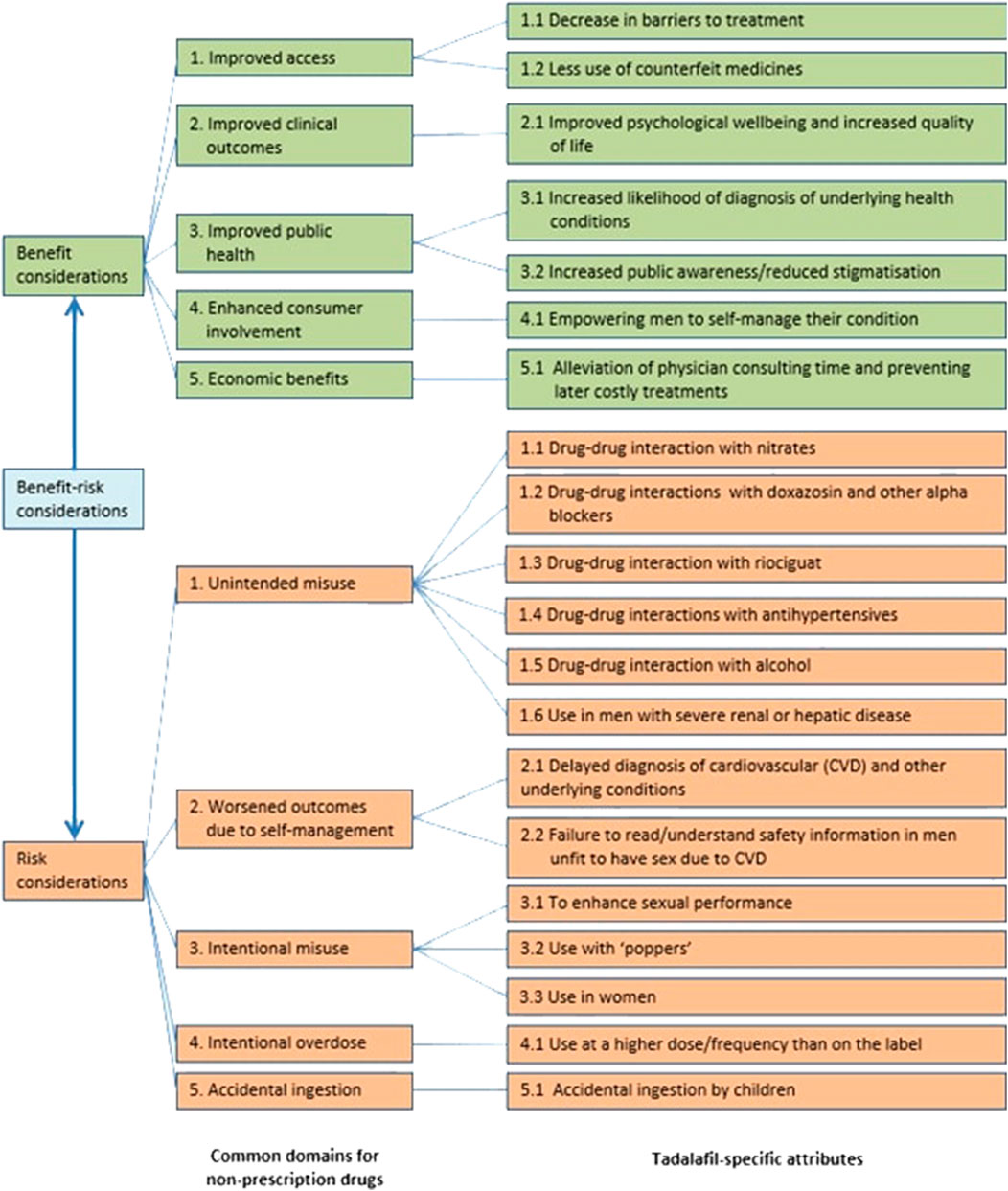
FIGURE 1. Brass value tree of incremental benefits and risks associated with non-prescription tadalafil.
The expert panel comprised four urologists, one of whom chaired the meeting, a cardiologist, a pharmacist, and the speaker was a clinical pharmacologist. This mix of specialties ensured that different important perspectives could be captured with the necessary clinical expertise to critically review the evidence, identify any additional benefits and risks, and understand the clinical impact of making a PDE5 inhibitor for the treatment of ED available without prescription. The objective of the meeting was to achieve an expert consensus on the benefits and risks; hence a Group Delphi technique was utilised. The speaker presented the evidence, it was discussed, and then the panel gave independent ratings on the likelihood and clinical impact of each incremental benefit and risk (Okoli and Pawlowski, 2004; Brass et al., 2013). Both the likelihood and clinical impact were scored from 0 (none) to 3 (high) or a “do not know” option. Results were fed back to the panel. Where consensus was not achieved, defined as scores more than 1 point apart, further open discussion was initiated. The rating was then repeated. Overall scores were calculated from multiplying the mean likelihood of behaviour or event and the mean clinical impact providing an overall benefit or risk score between 0 and 9. A Public Health consultant provided expertise in relation to the health economic and public health benefits but not for the clinical benefits and risks. The speaker and Public Health consultant participated in discussions but did not rate the risks and benefits. The meeting was thus chaired and run by the independent group of experts with no input or participation from pharmaceutical companies.
The evidence for each incremental benefit and risk domain identified in the literature review for tadalafil is summarised below in Tables 1, 2. Post-marketing safety data are reported using periodic safety update reports (PSUR) and periodic benefit-risk evaluation reports (PBRER) for tadalafil that have been submitted to regulatory authorities by Lilly as required by regulations since the first marketing authorisation for tadalafil on 15 October 2002 up to 15 October 2020.
Each incremental benefit was scored in two rounds of ratings, Table 3. Shaded boxes indicate where consensus was reached (all scores within 1 point of each other). According to the second round of scoring, the expert panel considered that all incremental benefits were likely, with the exception of one expert who did not think there would be an increased likelihood of diagnosis of underlying health conditions. Individual scores ranged from low to high with mean likelihood scores of 1.7–2.3 but there was no consensus.
There was consensus of a moderate to high clinical impact if there were less use of counterfeit medicines (2.5). There was also agreement of a moderate clinical impact from empowering men to self-manage their condition (1.8) and for the economic benefits (1.7). For the other incremental benefits, consensus was not reached but the mean clinical impact ranged from 1.7 to 2.3.
Overall attribute scores ranged from 2.8 to 5.4, with the highest scores for a decrease in barriers to treatment (5.4), less use of counterfeit medicines (5.0), improved psychological wellbeing and increased quality of life (4.0), and increased public awareness and reduced stigmatisation (4.0).
There was consensus amongst experts that the risks were low/unlikely to occur and would not have a significant clinical impact. The information on the outer carton was considered to sufficiently manage the risks.
Consensus was reached on how likely 12 out of 14 incremental risks were to occur and of a low clinical impact for 11 of them (Table 4, shaded boxes). The overall attribute scores were much lower than for the benefits, ranging from 0.2 to 2.2, with 11 risks scoring 1.0 or less.
An increase in drug-drug interactions were deemed unlikely for tadalafil with nitrates due to their low and decreasing usage in Germany (0.8); in 2020, there were 53 million defined daily doses (DDD) of long-acting nitrates prescribed (reduced from 233 million DDDs in 2011), 52 million DDDs of molsidomine and 31 million DDDs of glyceryl trinitrate. (Schwabe/Ludwig Hrsg, 2021). In the context of this reducing use, the warning on the package (see below) was considered sufficient mitigation for this risk.
IMPORTANT: Do not use if you are taking either of the following two medicines:
• Nitrate medicine for chest pain (angina pectoris) or heart failure
• Riociguat for high blood pressure in the lungs
The warning messages in the PIL are:
What you need to know before you take *TM* (*TM* is a placeholder for the approved brand name).
Do not take *TM* if you:
Take any medicines called nitrates or nitric oxide donors (such as glyceryl trinitrate, isosorbide mononitrate, isosorbide dinitrate for the relief of chest pain or heart failure, or amyl nitrite also known as “poppers”, nicorandil, molsidomine or sodium nitroprusside).
These are often used for the relief of chest pain (angina pectoris), or heart failure.
Using *TM* with any of these medicines may lead to a dangerous fall in blood pressure.
If it were to occur, the mean clinical impact score was rated low to moderate (1.7). All experts agreed there was very low likelihood of an increase in drug-drug interaction with doxazosin (0.5) or other alpha-blockers (0.7) and that there would be a minimal clinical impact (0.7) as they are not regularly used in Germany for hypertension. In 2020, there were 123 million defined daily doses (DDDs) of alpha blockers used to treat hypertension compared with 2,589 million DDDs of calcium channel blockers and 6,050 million DDDs of ACE inhibitors (Schwabe/Ludwig Hrsg, 2021). Similarly, there was low concern about any increase in interaction with other antihypertensives, with an overall attribute score of 0.4 because of the evidence of extremely few cases of such complications in the post-marketing safety data for 84,674,000 patients who have been exposed to Cialis worldwide from 2002 to 15 October 2020. (EMTEX, 2021).
The expert panel viewed the likelihood of an increase in interaction with riociguat a little higher because of the recent approval for treatment of post thrombotic pulmonary hypertension meaning more men could be at risk. The mean clinical impact score of 1.7 and overall attribute score of 2.2 reflected a moderate level of concern, but being under the care of a specialist and the clear warning messages on the pack (shown above) and in the PIL (see below) were felt to be strong mitigating factors. The warning message in the PIL is:
Do not take *TM* if you are taking a medicine called riociguat (or other medicines of a group called guanylate cyclase stimulators), used to treat pulmonary arterial hypertension (i.e., high blood pressure in the lungs) and chronic thromboembolic pulmonary hypertension (i.e., high blood pressure in the lungs due to blood clots).
There was agreement that the likelihood of alcohol interaction occurring more frequently if tadalafil were available without prescription was low, as was the clinical impact with an overall score of 0.6, reflecting the evidence from clinical pharmacology studies that was reviewed by the experts during the meeting.
The experts also agreed that men may be more likely to use tadalafil to enhance sexual performance (2.3), but that this would have a low clinical impact (0.7) as there was no evidence to suggest that the risks to health from use to enhance clinical performance would be clinically significant. There were mixed opinions on whether intentional use at a higher dose/frequency would increase, but consensus was that this would have a low clinical impact (0.5) according to the evidence and patient reported experiences.
The overall attribute scores reflected consensus of a low likelihood or clinical impact of increased use in men with severe renal or hepatic disease (0.7), failure to read/understand safety information for men unfit to have sex due to cardiovascular disease (CVD) (1.0), use with “poppers” (1.0), use in women (0.2) or accidental ingestion by children (0.4). All considerations of the low clinical impact were based on evidence from published studies and 18 years of post-marketing safety data for patients exposed to Cialis worldwide that were reviewed by the experts in the meeting.
Finally, there were differing views on the likelihood (1.0) and clinical impact (0.8) of delayed diagnosis of CVD and other underlying conditions. If ED were the only early symptom of CVD, it was felt to be unlikely that a man would arrange to see a cardiologist, therefore taking tadalafil would not delay a diagnosis of CVD. However, self-treating ED may reduce the likelihood of seeing an urologist and so could lead to a delay in diagnosis of an underlying urological condition. On balance, the overall attribute of this scenario was scored low (0.7).
If tadalafil were to be reclassified to non-prescription status in Germany, training would be provided to pharmacists highlighting the contraindications, potential drug-drug interactions and conditions where men should first consult their physician. Messages on the carton and patient information leaflet (PIL) would include contraindications, safety information, dosage and side effects. These messages have been tested in men in three separate studies to ensure comprehension. The expert panel felt that these measures were appropriate and sufficient to mitigate against the identified incremental risks and that the results of the studies demonstrated that the information was sufficiently well understood and that consumers would make appropriate self-selection decisions. An additional suggested measure was to add a warning regarding the potential drug-drug interaction with veriziguat which was recently approved for chronic heart failure.
The Brass decision analysis tool worked well in terms of achieving the objectives of the meeting. The identification of the incremental benefits and risks and collation of evidence from a diverse range of information sources including clinical trials, real-world studies and patient safety data was helpful in providing an evidence-based foundation to facilitate a comprehensive and rational assessment of the benefit-risk analysis. The Group Delphi technique provided the opportunity for divergent opinions to be frankly discussed and the experiences and expertise of the different specialists to be presented. In many cases, this led to narrowing of the range of scores where a second round of voting occurred, thereby gaining or moving towards consensus.
The lack of consensus regarding the degree of likelihood and clinical impact of the majority of identified incremental benefits may in part be due to the complex number of factors involved in achieving each benefit. Importantly, the discussion about benefits is hypothetical as there is no PDE5 inhibitor available without prescription in Germany. Data from the UK regarding the experience with Viagra Connect was presented, but this was not felt to be directly applicable because of the differences in healthcare systems. For safety, patient safety update reports and large observational studies were able to provide strong evidence to help quantify the risks and are likely to have contributed to the high level of consensus reached.
It is possible that gaps in evidence or differing opinions on whether evidence from different healthcare systems would be replicable in Germany were factors in the range of scores for the benefits. For instance, some of the evidence for a reduction in barriers to treatment and improved diagnosis of underlying conditions came from the United Kingdom. Germany has substantially more urologists and therefore easier patient access to them. There were also differences of opinion regarding the level of health consultations that take place in pharmacies in Germany compared to the United Kingdom. However, there was general agreement that people are more likely to access services when provided locally, such as the increased take-up of flu vaccinations when provided at pharmacies.
From the patient’s perspective it is more convenient to go straight to the pharmacy to get the medication rather than having to go to the doctor first for a prescription. There was some agreement that men may not want to see a doctor for ED because of embarrassment and therefore prefer to buy PDE5 inhibitors from a pharmacy. Recent TV advertising was believed to have raised awareness of ED and reduced inhibition thresholds to seek treatment. If tadalafil were prescription-free, it was considered likely that the topic of ED would be more publicly discussed, and the feelings of embarrassment reduced.
It was widely agreed that the consumer would be unaware if the product was counterfeited or not, but if it were available to buy in pharmacies, patients would have less reason to go to unreliable internet sources. The New Zealand example of reduced counterfeit packages following reclassification was discussed and considered to be potentially applicable in Germany.
Only three risks scored above 1, the potential drug-drug interaction with riociguat which scored 2.2, potential misuse by men without ED which scored 1.6 and use with nitrates which scored 1.3. Riociguat is a relatively new drug used in the treatment of the rare condition of pulmonary arterial hypertension or more recently chronic thromboembolic pulmonary hypertension. The score reflected the moderate degree of clinical impact such a drug-drug reaction would have, but there was general agreement that this scenario would be unlikely because men would be under specialist clinical supervision and would also see the risk mitigation warning messages on the packaging and PIL.
Potential misuse by men without ED was considered moderately likely to increase if tadalafil were to be available without prescription, but the clinical impact of this overuse was deemed to be low.
The risk of drug-drug interactions with nitrates was considered of low likelihood and to have low clinical impact, largely because they are now rarely used in Germany. The warnings on the package and label were also felt to be sufficient though the panel recommended that men be directed to discuss their medication with a pharmacist or doctor if they are unsure about what they are taking.
Across the domains there were no additional risk mitigation measures considered to be essential beyond the wording on the carton and in the PIL. There were suggestions to include warnings about newer medications for heart failure, such as vericiguat on the packaging and PIL. The discussion highlighted points about drug-drug interactions in general and the balance required in terms of how much information to include on the PIL, such as the trade and generic names. It was agreed that discussion with a GP or pharmacist about any potential interactions with their other medication would be beneficial and that this could be a recommendation in the PIL.
Based on this thorough and systematic assessment of the evidence for the incremental benefits and risks of making tadalafil available without prescription in Germany, the consensus amongst clinical experts was that the incremental risks would be small. They would either be unlikely to occur or, if more likely to occur, would have little clinical impact. There was high level agreement that the risks are sufficiently manageable, so even if there were differing opinions regarding the magnitude of benefits, the average risk evaluation appeared significantly lower than the potential benefits.
KM: Writing–review and editing. UM: Writing–review and editing. W-DB: Writing–review and editing. GH: Writing–review and editing. MB: Writing–review and editing. SF: Writing–review and editing.
The process was sponsored by Sanofi who provided funding to support the extensive literature review, allowed access to their post-marketing safety data, clinical trial data and clinical pharmacology data and provided funding to facilitate the meeting. However, the Group Delphi process and evaluation of the benefits/risks was independent of Sanofi. No members of Sanofi actively participated in the meeting and discussion of the evidence or interacted with the clinical experts. Editorial support was provided by Fiona Hammond and Alison Carr of Hamell Communications, United Kingdom, and was funded by Sanofi.
With thanks to Harald Weigmann and Mohamed Amessou from Sanofi and to the team from Hamell Communications.
Sanofi had no role in the design, execution, or interpretation of the Group Delphi process or in the writing of the paper. The authors received payment from Sanofi for their participation in the group Delphi meeting but no payment was received in relation to the writing or review of the paper.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Althof, S. E., O'Leary, M. P., Cappelleri, J. C., Crowley, A. R., Tseng, L. J., and Collins, S. (2006). Impact of erectile dysfunction on confidence, self-esteem and relationship satisfaction after 9 months of sildenafil citrate treatment. J. Urology 176 (5), p2132–p2137. doi:10.1016/j.juro.2006.07.019
Althof, S. E., Rubio-Aurioles, E., Kingsberg, S., Zeigler, H., Wong, D. G., and Burns, P. (2010). Impact of tadalafil once daily in men with erectile dysfunction--including a report of the partners' evaluation. Urology 75 (6), p1358–p1363. doi:10.1016/j.urology.2009.11.066
Anderson, S. G., Hutchings, D. C., Woodward, M., Rahimi, K., Rutter, M. K., Kirby, M., et al. (2016). Phosphodiesterase type-5 inhibitor use in type 2 diabetes is associated with a reduction in all-cause mortality. Heart 102 (21), p1750–p1756. doi:10.1136/heartjnl-2015-309223
Averbeck, M. A., Colares, C., de Lira, G. H. S., Selbach, T., and Rhoden, E. L. (2012). Evaluation of endothelial function with brachial artery ultrasound in men with or without erectile dysfunction and classified as intermediate risk according to the Framingham Score. J. Sex. Med. 9 (3), p849–p856. doi:10.1111/j.1743-6109.2011.02591.x
Batty, G. D., Li, Q., Czernichow, S., Neal, B., Zoungas, S., Huxley, R., et al. (2010). Erectile dysfunction and later cardiovascular disease in men with type 2 diabetes: Prospective cohort study based on the ADVANCE (action in diabetes and vascular disease: Preterax and diamicron modified-release controlled evaluation) trial. J. Am. Coll. Cardiol. 56 (23), p1908–p1913. doi:10.1016/j.jacc.2010.04.067
Blok-Tip, L., et al. (2005). Counterfeits and imitations of Viagra® and Cialis® tablets: Trends and risks to public health. Available at: https://www.rivm.nl/bibliotheek/rapporten/267041001.pdf (Accessed April 28, 2023).
Brass, E. P., Lofstedt, R., and Renn, O. (2013). A decision-analysis tool for benefit-risk assessment of nonprescription drugs. J. Clin. Pharmacol. 53 (5), p475–p482. doi:10.1002/jcph.22
Brass, E. P., Lofstedt, R., and Renn, O. (2011). Improving the decision-making process for nonprescription drugs: A framework for benefit-risk assessment. Clin. Pharmacol. Ther. 90 (6), p791–p803. doi:10.1038/clpt.2011.231
Breau, R. H., Mcgrath, P. J., and Norman, R. W. (2003). Assessing self-help issues for patients with prostate cancer, interstitial cystitis, erectile dysfunction and urinary diversion. BJU Int. J. 92 (7), p736–p740. doi:10.1046/j.1464-410x.2003.04469.x
Briken, P., Matthiesen, S., Pietras, L., Wiessner, C., Klein, V., Reed, G. M., et al. (2020). Estimating the prevalence of sexual dysfunction using the new ICD-11 guidelines. Dtsch. Ärzteblatt 117 (39), 653–p658. doi:10.3238/arztebl.2020.0653
Campbell, N., Clark, J. P., Stecher, V. J., and Goldstein, I. (2012). Internet-ordered viagra (sildenafil citrate) is rarely genuine. J. Sex. Med. 9 (11), p2943–p2951. doi:10.1111/j.1743-6109.2012.02877.x
Chiang, J., Yafi, F. A., Dorsey, P. J., and Hellstrom, W. J. G. (2017). The dangers of sexual enhancement supplements and counterfeit drugs to “treat” erectile dysfunction. Transl. Androl. Urology 6, p12–p19. doi:10.21037/tau.2016.10.04
Chu, P. L., McFarland, W., Gibson, S., Weide, D., Henne, J., Miller, P., et al. (2003). Viagra use in a community-recruited sample of men who have sex with men, San Francisco. J. Aquired Immune Defic. Syndrome 33 (2), p191–p193. doi:10.1097/00126334-200306010-00012
Corona, G., Maggi, M., and Jannini, E. A. (2018). EDEUS, a real-life study on the users of phosphodiesterase type 5 inhibitors: Prevalence, perceptions, and health care-seeking behavior among European men with a focus on 2nd-generation avanafil. Sex. Med. 6 (1), p15–p23. doi:10.1016/j.esxm.2017.10.003
Crespo-Leiro, M. G., Anker, S. D., Maggioni, A. P., Coats, A. J., Filippatos, G., Ruschitzka, F., et al. (2016). European society of cardiology heart failure long-term registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur. J. Heart Fail. 18 (6), p613–p625. doi:10.1002/ejhf.566
de Boer, B. J., Bots, M. L., Nijeholt, A. A. B. L. A., Moors, J. P. C., and Verheij, T. J. M. (2005). The prevalence of bother, acceptance, and need for help in men with erectile dysfunction. J. Sex. Med. 2 (3), p445–p450. doi:10.1111/j.1743-6109.2005.20364.x
de Silva, D. (2011). The health foundation. Evidence: Helping people help themselves. A review of the evidence considering whether it is worthwhile to support self-management. Available at: http://www.health.org.uk/publications/evidence-helping-people-help-themselves (Accessed April 28, 2023).
Dean, J., de Boer, B. J., Graziottin, A., Hatzichristou, D., Heaton, J., and Tailor, A. (2006). Effective Erectile Dysfunction (ED) Treatment Enables Men to Enjoy Better Sex: The Importance of Erection Hardness, Psychological Well-Being, and Partner Satisfaction. Treat. Enables Men Enjoy Better Sex Importance Erection Hardness, Psychol. Well-Being, Partn. Satisfaction 5, p761–p766. doi:10.1016/j.eursup.2006.06.003
Dong, J. Y., Zhang, Y. H., and Qin, L. Q. (2011). Erectile dysfunction and risk of cardiovascular disease: meta-analysis of prospective cohort studies. J. Am. Coll. Cardiol. 58 (13), p1378–p1385. doi:10.1016/j.jacc.2011.06.024
Ema, (2006). Guideline on legal status for the supply to the patient of centrally authorised medicinal products. European Medicine's Agency. (EMA): Committee for medicinal products for human use (CHMP). [Online]. Available at: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/guideline-legal-status-supply-patient-centrally-authorised-medicinal-products_en.pdf (Accessed April 28, 2023).
European Association of Urology (2020). European survey shows alarmingly low awareness of erectile dysfunction. Available at: https://uroweb.org/european-survey-shows-alarmingly-low-awareness-of-erectile-dysfunction-majority-does-not-know-what-it-is/ (Accessed April 28, 2023).
Frederick, L. R., Cakir, O. O., Arora, H., Helfand, B. T., and McVary, K. T. (2014). Undertreatment of erectile dysfunction: Claims analysis of 6.2 million patients. J. Sex. Med. 11 (10), p2546–p2553. doi:10.1111/jsm.12647
Galiè, N., Humbert, M., Vachiery, J. L., Gibbs, S., Lang, I., Torbicki, A., et al. (2016). 015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the European society of cardiology (ESC) and the European respiratory society (ERS): Endorsed by: Association for European paediatric and congenital cardiology (AEPC), international society for heart and lung transplantation (ISHLT). Eur. Heart J. 37 (1), p67–p119. doi:10.1093/eurheartj/ehv317
Galiè, N., Brundage, B. H., Ghofrani, H. A., Oudiz, R. J., Simonneau, G., Safdar, Z., et al. (2009). Tadalafil therapy for pulmonary arterial hypertension. Circulation 119 (22), p2894–p2903. doi:10.1161/circulationaha.108.839274
Goldfischer, E. .K. J., Clark, Wr, Brady, E., Ma, S., Dgetluck, N., Klise, Sr, et al. (2012). Hemodynamic effects of once-daily tadalafil in men with signs and symptoms of benign prostatic hyperplasia on concomitant α1-adrenergic antagonist therapy: results of a multicenter randomized, double-blind, placebo-controlled trial. Urology 79 (4), p875–p882. doi:10.1016/j.urology.2011.11.040
Goldstein, I., Goren, A., Li, V. W., Tang, W. Y., and Hassan, T. A. (2020). Epidemiology update of erectile dysfunction in eight countries with high burden. Sex. Med. Rev. 8 (1), p48–p58. doi:10.1016/j.sxmr.2019.06.008
Gong, B., Ma, M., Xie, W., Yang, X., Huang, Y., Sun, T., et al. (2017). Direct comparison of tadalafil with sildenafil for the treatment of erectile dysfunction: A systematic review and meta-analysis. Int. Urology Nephrol. 49 (10), p1731–p1740. doi:10.1007/s11255-017-1644-5
Gott, M., Galena, E., Hinchliff, S., and Elford, H. (2004). Opening a can of worms: GP and practice nurse barriers to talking about sexual health in primary care. Fam. Pract. 21 (5), p528–p536. doi:10.1093/fampra/cmh509
Gülpinar, O., Haliloğlu, A. H., Abdulmajed, M. I., Bogga, M. S., and Yaman, O. (2012). Help-Seeking interval in erectile dysfunction: Analysis of attitudes, beliefs, and factors affecting treatment-seeking interval in Turkish men with previously untreated erectile dysfunction. J. Androl. 33 (4), p624–p628. doi:10.2164/jandrol.111.013946
Gupta, B. P., Murad, M. H., Clifton, M. M., Prokop, L., Nehra, A., and Kopecky, S. L. (2011). The effect of lifestyle modification and cardiovascular risk factor reduction on erectile dysfunction: A systematic review and meta-analysis. Archives Intern. Med. 171 (20), p1797–p1803. doi:10.1001/archinternmed.2011.440
H6D-EW-LVFT (2023). Study lvft (H6D-EW-LVFT): A pharmacodynamic study to evaluate the interaction between 20 mg tadalafil and doxazosin, an alpha 1 adrenergic antagonist. healthy male subjects.
H6D-EW-LVAE (2023). Study LVAE (H6D-EW-LVAE): Randomised, placebo-controlled, subject and investigator-blind, four-period cross-over study to investigate the pharmacodynamic interaction between alcohol and tadalafil in healthy volunteers. Sanofi data on file.
H6D-EW-LVAJ (2023). Study LVAJ (H6D-EW-LVAJ): Comparative study on the pharmacokinetics, safety and tolerability of tadalafil, following a single oral dose in patients with mild, moderate or severe renal dysfunction and healthy subjects. Sanofi data on file.
H6D-EW-LVAV (2023). Study lvav (H6D-EW-LVAV): A pharmacodynamic interaction study between tadalafil and a calcium antagonist (amlodipine) in healthy subjects. Sanofi data on file.
H6D-EW-LVAW (2023). Study lvaw (H6D-EW-LVAW): A pharmacodynamic interaction study between tadalafil and a beta blocker (metoprolol) in hypertensive subjects. Sanofi data on file.
H6D-EW-LVAY (2023). Study lvay (H6D-EW-LVAY): A pharmacodynamic study to evaluate the interaction between tadalafil and an alpha 1 adrenergic antagonist (tamsulosin) in healthy subjects. Sanofi data on file.
H6D-EW-LVBC (2023). Study lvbc (H6D-EW-LVBC): A pharmacodynamic interaction study between tadalafil and an ACE inhibitor (enalapril) in hypertensive subjects. Sanofi data on file.
H6D-EW-LVCM (2022). Study lvcm (H6D-EW-LVCM): Tadalafil (LY450190) A pharmacodynamic interaction with sublingual nitroglycerin: A placebo-controlled comparison with sildenafil in healthy subjects.
H6D-EW-LVDN (2022). Study LVDN (H6D-EW-LVDN): Pharmacodynamic drug interaction of 20 mg tadalafil with short-acting nitrates in male subjects.
H6D-EW-LVDO (2023). Study LVDO (H6D-EW-LVDO): Randomised, placebo-controlled, two-period, cross-over study to investigate the pharmacodynamic interaction between alcohol and 20 mg tadalafil in healthy volunteers. Sanofi data on file.
H6D-EW-LVDP (2023). Study lvdp (H6D-EW-LVDP): A pharmacodynamic interaction study between 20 mg tadalafil and a calcium antagonist (amlodipine) in healthy subjects. Sanofi data on file.
H6D-EW-LVDS (2023). Study lvds (H6D-EW-LVDS): A pharmacodynamic interaction study between 20 mg tadalafil and angiotensin II inhibitors in hypertensive subjects. Sanofi data on file.
H6D-EW-LVDT (2023). Study lvdt (H6D-EW-LVDT): A study to investigate the tolerability and pharmacokinetics of tadalafil in subjects on haemodialysis for renal failure. Sanofi data on file.
H6D-EW-LVDV (2023). Study lvdv (H6D-EW-LVDV): A randomised, double blind, placebo controlled, cross-over study to investigate the effect of a single oral dose of 20 mg tadalafil on blood pressure in subjects with hypertension. Sanofi data on file.
H6D-EW-LVFG (2023). Study lvfg (H6D-EW-LVFG): A pharmacodynamic study to evaluate the interaction between 20 mg tadalafil and 8 mg q.d. doxazosin, an alpha 1 adrenergic antagonist. healthy male subjects.
H6D-EW-LVGN (2023). Study lvgn (H6D-EW-LVGN): A pharmacodynamic study to evaluate the interaction between daily dosing of 5 mg tadalafil and addition of concomitant tamsulosin, an alpha 1 adrenergic antagonist. healthy male subjects. Sanofi data on file.
H6D-EW-LVGT (2023). Study lvgt (H6D-EW-LVGT): A pharmacodynamic study to evaluate the interaction between daily dosing of 5 mg tadalafil and concomitant increasing doses of doxazosin, an alpha 1 adrenergic antagonist. healthy male subjects.
H6D-LC-LVAB (2022). Study LVAB (H6D-LC-LVAB): Tadalafil (LY450190): Pharmacodynamic drug interaction with short-acting nitrates.
H6D-LC-LVBY (2022). Study LVBY (H6D-LC-LVBY): Tadalafil (LY450190): Pharmacodynamic drug interaction with short-acting and long-acting nitrates in patients with chronic stable angina.
Haro, J. M., Beardsworth, A., Casariego, J., Gavart, S., Hatzichristou, D., Martin-Morales, A., et al. (2006). Treatment-seeking behavior of erectile dysfunction patients in Europe: Results of the erectile dysfunction observational study. J. Sex. Med. 3 (3), p530–p540. doi:10.1111/j.1743-6109.2006.00250.x
Harte, C. B., and Meston, C. M. (2011). Recreational use of erectile dysfunction medications in undergraduate men in the United States: Characteristics and associated risk factors. Archives Sex. Behav. 40 (3), p597–p606. doi:10.1007/s10508-010-9619-y
Hatzimouratidis, K., Buvat, J., Büttner, H., Vendeira, P. A. S., Moncada, I., Boehmer, M., et al. (2014). Psychosocial outcomes after initial treatment of erectile dysfunction with tadalafil once daily, tadalafil on demand or sildenafil citrate on demand: Results from a randomized, open-label study. Int. J. Impot. Res. 26 (6), p223–p229. doi:10.1038/ijir.2014.15
Jack, A. (2007). Counterfeit medicines. Bitter pills. Br. Med. J. 335, p1120–p1121. doi:10.1136/bmj.39412.431655.AD
Jackson, G., Arver, S., Banks, I., and Stecher, V. J. (2010). Counterfeit phosphodiesterase type 5 inhibitors pose significant safety risks. Int. J. Clin. Pract. 64 (4), p497–p504. doi:10.1111/j.1742-1241.2009.02328.x
Jannini, E. A., Sternbach, N., Limoncin, E., Ciocca, G., Gravina, G. L., Tripodi, F., et al. (2014). Health-related characteristics and unmet needs of men with erectile dysfunction: A survey in five European countries. J. Sex. Med. 11 (1), p40–p50. doi:10.1111/jsm.12344
Kirby, M., Schnetzler, G., Zou, K. H., and Symonds, T. (2011). Prevalence and detection rate of underlying disease in men with erectile dysfunction receiving phosphodiesterase type 5 inhibitors in the United Kingdom: A retrospective database study. Int. J. Clin. Pract. 65 (7), p797–p806. doi:10.1111/j.1742-1241.2011.02693.x
Kloner, R. A., Hutter, A. M., Emmick, J. T., Mitchell, M. I., Denne, J., and Jackson, G. (2003). Time course of the interaction between tadalafil and nitrates. J. Am. Coll. Cardiol. 42 (10), p1855–p1860. doi:10.1016/j.jacc.2003.09.023
Korkes, F., Costa-Matos, A., Gasperini, R., Reginato, P. V., and Perez, M. D. C. (2008). Recreational use of PDE5 inhibitors by young healthy men: Recognizing this issue among medical students. J. Sex. Med. 5 (10), p2414–p2418. doi:10.1111/j.1743-6109.2008.00792.x
Lee, L., Maguire, T., Maculaitis, M., Emir, B., Li, V. W., Jeffress, M., et al. (2021). Increasing access to erectile dysfunction treatment via pharmacies to improve healthcare provider visits and quality of life: Results from a prospective real-world observational study in the United Kingdom. Int. J. Clin. Pract. 75 (4), pe13849, doi:10.1111/ijcp.13849
Levine, G. N., Steinke, E. E., Bakaeen, F. G., Bozkurt, B., Cheitlin, M. D., Conti, J. B., et al. (2012). Sexual activity and cardiovascular disease. Circulation 125 (8), p1058–p1072. doi:10.1161/cir.0b013e3182447787
Li, H. J., Bai, W. J., Dai, Y. T., Xu, W. P., and Wang, C. N. (2016). An analysis of treatment preferences and sexual quality of life outcomes in female partners of Chinese men with erectile dysfunction. Asian J. Androl. 18 (5), p773–p779. doi:10.4103/1008-682X.159719
Lilly, E. (2021). European summary of product characteristics; Cialis. Date of last revision january 2023. Available at: https://www.medicines.org.uk/emc/product/7431/smpc#gref (Accessed April 28, 2023).
Ludman, E. J., Peterson, D., Katon, W. J., Von Korff, M., Ciechanowski, P., et al. (2013). Improving confidence for self care in patients with depression and chronic illnesses. Behav. Med. 39 (1), p1–p6. doi:10.1080/08964289.2012.708682
Medicines and Healthcare products Regulatory Agency (2021). How to change the legal classification of a medicine in the UK. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/949105/Reclassification_guideline_for_20210101_-_final.pdf (Accessed April 28, 2023).
Medicines and Healthcare products Regulatory Agency (2017a). MHRA reclassifies Viagra Connect tablets to a Pharmacy medicine. Available at: https://www.gov.uk/government/news/mhra-reclassifies-viagra-connect-tablets-to-a-pharmacy-medicine (Accessed April 28, 2023).
Medicines and Healthcare products Regulatory Agency (2017b). Public assessment report: Prescription only medicine reclassification. Viagra Connect 50mg film-coated tablets: Sildenafil citrate. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/662968/Viagra_Connect_POM_to_P_PAR_FINAL.pdf (Accessed April 28, 2023).
Montalescot, G., Sechtem, U., Achenbach, S., Andreotti, F., Arden, C., et al. (2013). 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur. Heart J. 34 (38), 2949–3003. doi:10.1093/eurheartj/eht296
Montorsi, F., Briganti, A., Salonia, A., Rigatti, P., Margonato, A., Macchi, A., et al. (2003). Erectile dysfunction prevalence, time of onset and association with risk factors in 300 consecutive patients with acute chest pain and angiographically documented coronary artery disease. Eur. Urol. 44 (3), p360–p364. doi:10.1016/s0302-2838(03)00305-1discussion 364-365
Morales, A. M., Ibáñez, J., Machuca, M., Pol-Yanguas, E., Schnetzler, G., and Renedo, V. P. (2010). The EPIFARM study: An observational study in 574 community pharmacies in Spain characterizing patient profiles of men asking for erectile dysfunction medication. J. Sex. Med. 7 (9), p3153–p3160. doi:10.1111/j.1743-6109.2010.01918.x
Moreira, E. D. J., Brock, G., Glasser, D., Nicolosi, A., Laumann, E., Paik, A., et al. (2005). Help-seeking behaviour for sexual problems: the Global Study of Sexual Attitudes and Behaviors: Help-Seeking Behaviour for Sexual Problems. Int. J. Clin. Pract. 59 (1), p6–p16. doi:10.1111/j.1742-1241.2005.00382.x
NHS Digital (2015). Prescriptions dispensed in the community - statistics for england, 2004-2014. Available at: https://digital.nhs.uk/data-and-information/publications/statistical/prescriptions-dispensed-in-the-community/prescriptions-dispensed-in-the-community-statistics-for-england-2004-2014 (Accessed April 28, 2023).
NICE (2011). Clinical guideline 126. Stable angina: Management. National Institute for Health and Care Excellence.
NICE (2019). Clinical guideline 136. Hypertension in adults: Diagnosis and management. National Institute for Health and Care Excellence.
Nicolosi, A., Buvat, J., Glasser, D. B., Hartmann, U., Laumann, E. O., Gingell, C., et al. (2006). Sexual behaviour, sexual dysfunctions and related help seeking patterns in middle-aged and elderly Europeans: The global study of sexual attitudes and behaviors. World J. Urology 24, p423–p428. doi:10.1007/s00345-006-0088-9
Nunes, A. P., Seeger, J. D., Stewart, A., Gupta, A., and McGraw, T. (2021). Cardiovascular outcome risks in patients with erectile dysfunction Co-prescribed a phosphodiesterase type 5 inhibitor (PDE5i) and a nitrate: A retrospective observational study using electronic health record data in the United States. J. Sex. Med. 18 (9), p1511–p1523. doi:10.1016/j.jsxm.2021.06.010
Okoli, C., and Pawlowski, S. D. (2004). The Delphi method as a research tool: An example, design considerations and applications. Inf. Manag. 42 (1), p15–p29. doi:10.1016/j.im.2003.11.002
Paulsen, L. H., Sørensen Bakke, L., Jarbøl, D. E., Balasubramaniam, K., and Hansen, D. G. (2020). Associations between lifestyle, erectile dysfunction and healthcare seeking: A population-based study. Scand. J. Prim. Health Care 38 (2), p176–p183. doi:10.1080/02813432.2020.1753347
Pike, S. (2019). Healthcare wait time by country. Available at: https://www.carevoyance.com/blog/healthcare-wait-times-by-country (Accessed April 28, 2023).
Rosen, R. C., Fisher, W. A., Eardley, I., Niederberger, C., Nadel, A., and Sand, M. (2004). The multinational Men's Attitudes to Life Events and Sexuality (MALES) study: I. Prevalence oSf erectile dysfunction and related health concerns in the general population. Curr. Med. Res. Opin. 20 (5), p607–p617. doi:10.1185/030079904125003467
Rosenkranz, S., and Erdmann, E. (2004). Cardiovascular safety of sildenafil in the treatment of erectile dysfunction Sildenafil. Birkhauser Verlag.
Rubio-Aurioles, E., Porst, H., Kim, E. D., Montorsi, F., Hackett, G., Morales, A. M., et al. (2012). A randomized open-label trial with a crossover comparison of sexual self-confidence and other treatment outcomes following tadalafil once a day vs. tadalafil or sildenafil on-demand in men with erectile dysfunction. J. Sex. Med. 9 (5), p1418–p1429. doi:10.1111/j.1743-6109.2012.02667.x
Schnetzler, G., Banks, I., Kirby, M., Zou, K. H., and Symonds, T. (2010). Characteristics, behaviors, and attitudes of men bypassing the healthcare system when obtaining phosphodiesterase type 5 inhibitors. J. Sex. Med. 7 (3), p1237–p1246. doi:10.1111/j.1743-6109.2009.01674.x
Seftel, A. D., Buvat, J., Althof, S. E., McMurray, J. G., Zeigler, H. L., Burns, P. R., et al. (2009). Improvements in confidence, sexual relationship and satisfaction measures: Results of a randomized trial of tadalafil 5 mg taken once daily. Int. J. Impot. Res. 21 (4), p240–p248. doi:10.1038/ijir.2009.22
Shabsigh, R., Klein, L. T., Seidman, S., Kaplan, S. A., Lehrhoff, B. J., and Ritter, J. S. (1998). Increased incidence of depressive symptoms in men with erectile dysfunction. Urology 52 (5), p848–p852. doi:10.1016/s0090-4295(98)00292-1
Shabsigh, R., Perelman, M. A., Laumann, E. O., and Lockhart, D. C. (2004). Drivers and barriers to seeking treatment for erectile dysfunction: A comparison of six countries. BJU Int. J. 94 (7), p1055–p1065. doi:10.1111/j.1464-410X.2004.05104.x
Soller, R. W. (1998). Evolution of self-care with over-the-counter medications. Clin. Ther. 20 (3), pC134–140. doi:10.1016/s0149-2918(98)80018-0
Study PDY5734 (2023). Study PDY5734: Hemodynamic effects of a single dose of tadalafil 20 mg administered following seven days treatment with alfuzosin 10 mg once daily in middle-aged healthy volunteers. Sanofi data on file.
Tan, H. M., Tong, S. F., and Ho, C. C. (2012). Men’s health: Sexual dysfunction, physical, and psychological health—is there a link? J. Sex. Med. 9 (3), p663–p671. doi:10.1111/j.1743-6109.2011.02582.x
The Local (2019). Should Sweden make Viagra prescription free? Available at: https://www.thelocal.se/20190825/should-sweden-make-viagra-prescription-free/ (Accessed April 28, 2022).
The Pharmaceutical Journal (2018). Viagra from the pharmacist: Insight from reclassification in New Zealand. Available at: https://pharmaceutical-journal.com/article/opinion/viagra-from-the-pharmacist-insight-from-reclassification-in-new-zealand (Accessed April 28, 2023).
United States Food and Drug Administration FDA (2015). 'All natural' alternatives for erectile dysfunction: A risky proposition. Available at: https://www.fda.gov/consumers/consumer-updates/all-natural-alternatives-erectile-dysfunction-risky-proposition (Accessed April 28, 2023).
Vintura (2021). The health-economic benefits of self-care in Europe. Available at: https://www.vintura.com/wp-content/uploads/2021/03/Report_The-health-economic-benefits-of-self-care-in-Europe_a-collaboration-with-Vintura-and-GSK.pdf (Accessed April 28, 2023).
Keywords: erectile dysfunction, non-prescription, phosphodiesterase type 5 inhibitor (PDE5 inhibitor), reclassification, tadalafil
Citation: Miller K, May U, Beecken W-D, Hatzichristodoulou G, Böhm M and Fink S (2023) Evidence for benefits and risks of tadalafil as a non-prescription medicine: review and evaluation using the Group Delphi technique to achieve consensus amongst clinical experts. Front. Pharmacol. 14:1254706. doi: 10.3389/fphar.2023.1254706
Received: 07 July 2023; Accepted: 19 September 2023;
Published: 09 October 2023.
Edited by:
Gilberto De Nucci, State University of Campinas, BrazilReviewed by:
Adriano Fregonesi, State University of Campinas, BrazilCopyright © 2023 Miller, May, Beecken, Hatzichristodoulou, Böhm and Fink. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kurt Miller, S3VydC5NaWxsZXJAY2hhcml0ZS5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.