- 1Department of Emergency Medicine, Taipei Veterans General Hospital, Taipei, Taiwan
- 2Institute of Biomedical Engineering, National Tsing Hua University, Hsinchu, Taiwan
- 3School of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 4Department of Pediatrics and Structural, Congenital Heart and Echocardiography Center, School of Medicine, China Medical University, Taichung, Taiwan
- 5Department of Pediatrics, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
by Chen C-H, Fu Y-C, Lee Y-T, Hsieh K-S, Shen C-F and Cheng C-M (2023). Front. Pharmacol. 14:1166923. doi: 10.3389/fphar.2023.1166923
In the published article, there was an error in the legend for (Figure 4) as published. [(A) Non-respiratory failure group (p = 0.0676): the green line represents the patient who was discharged without experiencing septic shock but required inotropic agents during admission,]. The corrected legend appears below.
[(A) Non-respiratory failure group (p = 0.0676): the green line represents the patient who was discharged without experiencing septic shock nor requiring inotropic agents during admission,]
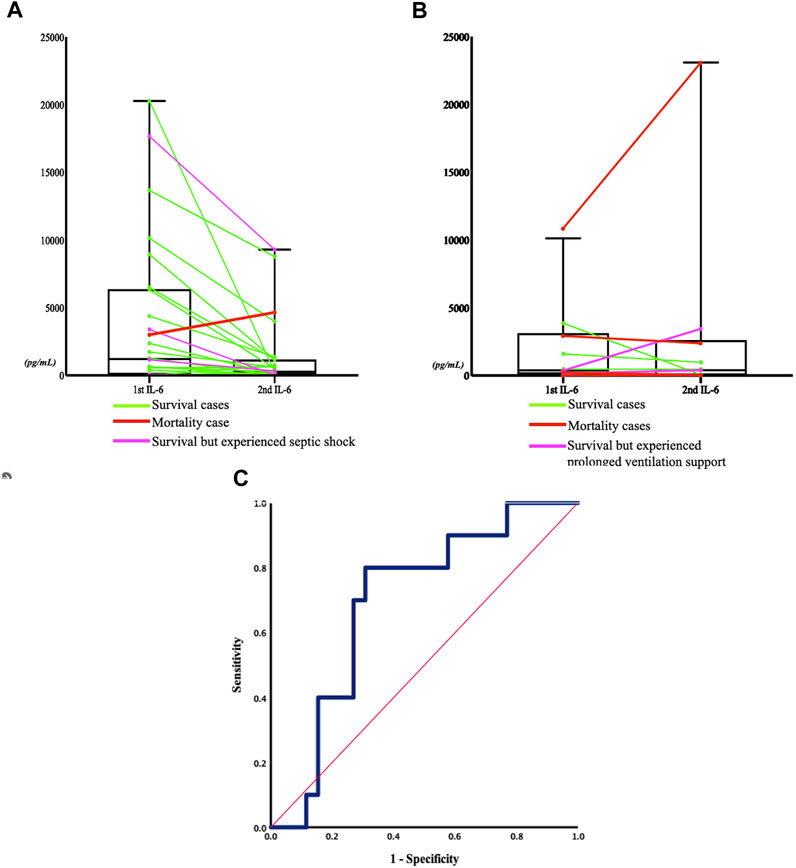
FIGURE 4. Sequential change in IL-6 concentrations between the two severity groups, and the receiver operating characteristic (ROC) curve of sequential IL-6 change and respiratory failure. (A) Non respiratory failure group (P = 0.0676): the green line represents the patient who was discharged without experiencing septic shock nor requiring inotropic agents during admission, the pink line represents the patient who experienced septic shock, and the red line represents the patient who died within 5 days of admission. (B) Respiratory failure group (P = 0.8711): the green line represents the patient who was discharged, the pink line indicates the patient who experienced prolonged ventilation support (more than 21 days (Lone and Walsh, 2011)), and the red line indicates the patient who died within 5 days of admission. (C) The ROC curve (blue line) refers to the relationship between serum IL-6 concentration change after admission and the development of respiratory failure in the later hospitalization course. The area was 0.696 (95% confidential interval 0.515–0.877, P = 0.072). The Youden's index of the ROC curve at a -43% change of the IL-6 concentration indicated that a decrease in IL-6 concentration below this threshold was associated with a higher rate of developing respiratory failure, with a sensitivity of 80% and a specificity of 69.2%. The red line represented the reference line.
In the published article, there was an error in (Table 4) as published.
The Section [21 patients (without septic shock) who required inotropic agents were discharged,] is incorrect. The corrected section is as follows.
[21 patients without septic shock nor required inotropic agents were discharged,]
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: interleukin-6, point-of-care diagnosis, respiratory failure, community-acquired pneumonia, lateral flow immunoassay, elderly pneumonia
Citation: Chen C-H, Fu Y-C, Lee Y-T, Hsieh K-S, Shen C-F and Cheng C-M (2023) Corrigendum: Efficacy of a paper-based interleukin-6 test strip combined with a spectrum-based optical reader for sequential monitoring and early recognition of respiratory failure in elderly pneumonia-a pilot study. Front. Pharmacol. 14:1250358. doi: 10.3389/fphar.2023.1250358
Received: 30 June 2023; Accepted: 25 October 2023;
Published: 13 November 2023.
Edited and reviewed by:
Graeme Barrett Bolger, University of Alabama at Birmingham, United StatesCopyright © 2023 Chen, Fu, Lee, Hsieh, Shen and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ching-Fen Shen, ZHJzaGVuMTExMkBnbWFpbC5jb20=; Chao-Min Cheng, Y2hhb21pbkBteC5udGh1LmVkdS50dw==
 Cheng-Han Chen
Cheng-Han Chen Yi-Chen Fu2
Yi-Chen Fu2 Yi-Tzu Lee
Yi-Tzu Lee Kai-Sheng Hsieh
Kai-Sheng Hsieh Ching-Fen Shen
Ching-Fen Shen Chao-Min Cheng
Chao-Min Cheng