- 1School of Traditional Chinese Medicine, Inner Mongolia Medical University, Hohhot, Inner Mongolia, China
- 2School of Basic Medicine, Inner Mongolia Medical University, Hohhot, Inner Mongolia, China
- 3School of Water Resources and Hydropower Engineering, Wuhan University, Wuhan, Hubei, China
Background: Long-term maintenance therapy with proton pump inhibitors (PPIs) is a common treatment strategy for acid-related gastrointestinal diseases. However, concerns have been raised about the potential increased risk of gastric cancer and related precancerous lesions with long-term PPI use. This systematic review and meta-analysis aimed to evaluate this potential risk.
Methods: We searched PubMed, Embase, and the Cochrane Central Register of Controlled Trials for randomised controlled trials published before 1 March 2023, with no language restrictions. The primary endpoint was the occurrence and progression of gastric mucosal atrophy, intestinal metaplasia, Enterochromaffin-like (ECL) cell hyperplasia, gastric polyps, and gastric cancer during the trial and follow-up. Data were analysed using a random effects model.
Results: Of the 4,868 identified studies, 10 met the inclusion criteria and were included in our analysis, comprising 27,283 participants. Compared with other treatments, PPI maintenance therapy for more than 6 months was associated with an increased risk of ECL cell hyperplasia (OR 3.01; 95% CI 1.29 to 7.04; p = 0.01). However, no significant increase was found in the risk of gastric mucosal atrophy (OR 1.01; 95% CI 0.55 to 1.85; p = 0.97), intestinal metaplasia (OR 1.14; 95% CI 0.49 to 2.68; p = 0.76), gastric polyps (OR 1.13; 95% CI 0.68 to 1.89; p = 0.64), or gastric cancer (OR 1.06; 95% CI 0.79 to 1.43; p = 0.71).
Conclusion: This systematic review and meta-analysis does not support an increased risk of gastric cancer or related precancerous lesions with long-term PPI maintenance therapy. However, long-term PPI use should be monitored for potential complications such as ECL cell hyperplasia. Further studies are needed to confirm these findings and evaluate the safety of PPI maintenance therapy for acid-related gastrointestinal diseases.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, Identifier: PROSPERO (CRD42022379692).
1 Introduction
Gastric cancer is among the top five causes of death globally (Colquhoun et al., 2015), with a 5-year survival rate of 25%–30% (Allemani et al., 2015). The primary reason for this unfavourable prognosis is the late-stage diagnosis of most gastric cancer patients. Therefore, reducing gastric cancer mortality requires significant attention to managing its pathogenic factors and early signs.
The progression from normal gastric mucosa to early intestinal gastric cancer is typically a sequence of “normal gastric mucosa → chronic gastritis → atrophy → intestinal metaplasia → dysplasia → early gastric cancer” (Correa et al., 1975; Correa, 1992). Atrophy, intestinal metaplasia, and dysplasia are broadly referred to as gastric precancerous lesions due to their potential malignant risk (Piazuelo et al., 2021). Gastric polyps are limited epithelial protrusions of the epithelial mucosa, with fundus glandular polyps (FGPs) and hyperplastic polyps (HPs) being the most common pathological types (Ren et al., 2018). Adenomatous polyps have a high malignant potential and are often associated with gastric adenocarcinoma (Park and Lauwers, 2008). Molecular studies have shown that gastric polyps, excluding adenomatous polyps, also exhibit molecular changes that may lead to tumour progression and may have unknown risks of malignant transformation (Carmack et al., 2009).
Proton pump inhibitors (PPIs), widely used globally for treating acid-related diseases such as gastroesophageal reflux disease (Dilaghi et al., 2022) and peptic ulcer (Malfertheiner et al., 2017), are often used as maintenance therapy to prevent symptom recurrence. Long-term PPI use often results in increased circulating gastrin levels, which can affect gastrointestinal tissue. However, sustained high levels of circulating gastrin can lead to hyperplasia of gastric parietal cells and enterochromaffin-like cells in the gastric mucosa (Lundell et al., 2015), potentially inducing the development of fundic gland polyps (Kim, 2021) and other histopathological changes that may increase the risk of gastric cancer. (Hagiwara et al., 2011). The safety and adverse effects of long-term PPI use are becoming a concern.
The impact of long-term PPI use on gastric cancer-related lesions has been extensively studied, but the results have been inconsistent, leading to controversy about the safety of PPI maintenance therapy (Yadlapati and Kahrilas, 2018). Therefore, a meta-analysis is needed to comprehensively evaluate the association between long-term PPI use and gastric cancer and its related lesions. We conducted a systematic review and meta-analysis of existing randomized controlled trials to assess the adverse effects of long-term PPI use on gastric cancer, gastric mucosal atrophy, intestinal metaplasia, ECL cell hyperplasia, and gastric polyps to elucidate and improve the safety of PPI maintenance therapy.
Previous meta-analyses have shown varying results. Song’s meta-analysis found no clear evidence of an increased risk of gastric mucosal atrophy and intestinal metaplasia but a more significant association with ECL cell hyperplasia (Song et al., 2014). Tran-Duy (Tran-Duy et al., 2016) and Martin (Martin et al., 2016)’s showed an association with an increased risk of fundic gland polyps and possibly an increased risk of gastric cancer. Three recent meta-analyses by Segna (Segna et al., 2021), Guo (Guo et al., 2023) and Peng (Peng et al., 2023) showed that PPI use was significantly associated with gastric cancer, but no duration-dependent effects of PPI with gastric cancer risk were found at < 1 year, 1–3 years, and more than 3 years. This meta-analysis includes more recent high-quality studies related to PPI maintenance therapy and reduced heterogeneity, allowing for a more comprehensive evaluation of the adverse effects on gastric cancer-related lesions.
2 Materials and methods
2.1 Search strategy
We conducted a literature search in PUBMED, EMBASE, and the Cochrane Central Register of Controlled Trials electronic databases to identify all published and unpublished randomised controlled trials up to 1 March 2023. An additional manual search of conference abstracts was performed to ascertain experimental details. Bibliographies of relevant studies were also manually searched. Two investigators independently conducted searches using the keywords “Stomach Neoplasms” and “proton pump inhibitor.” The specific search strategy is detailed in the attached table.
2.2 Selection criteria
The inclusion criteria encompassed randomised controlled trials (RCTs) examining the long-term use of PPIs in adult patients. The study necessitated one group of participants on maintenance therapy with PPIs and a control group using other treatment strategies for an intervention period exceeding 6 months. Participants in both groups underwent upper gastrointestinal endoscopy at the start and conclusion of the trial. They reported the number or proportion of participants with gastric mucosal lesions such as gastric cancer, atrophy, intestinal metaplasia, ECL cell hyperplasia, and gastric polyps. The primary outcome was the study’s ability to provide sufficient data to estimate the odds ratio (OR) between the occurrence and progression of each gastric cancer-related lesion and the use of PPI.
Articles without original data, studies that did not report data related to “gastric cancer, atrophy, intestinal metaplasia, ECL cell hyperplasia, and gastric polyps,” and studies with duplicate reports were excluded.
Two independent investigators screened titles and abstracts to include all potential studies in the search results. They independently screened them in full text to identify eligible studies and document reasons for exclusion. In cases of uncertainty regarding study inclusion, the two review authors discussed to reach a resolution, and if necessary, a third expert was consulted.
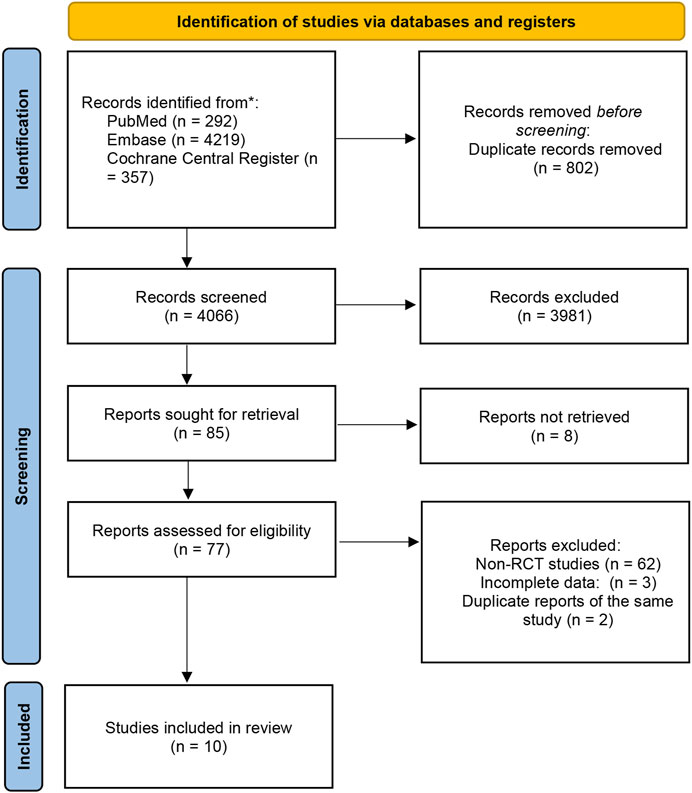
This section was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart (Page et al., 2021), and registered at the International Prospective Register of Systematic Reviews (number CRD42022379692) (Figure 1. PRISMA flowchart illustrating the process of screening and selection of studies).
2.3 Data extraction and quality assessment
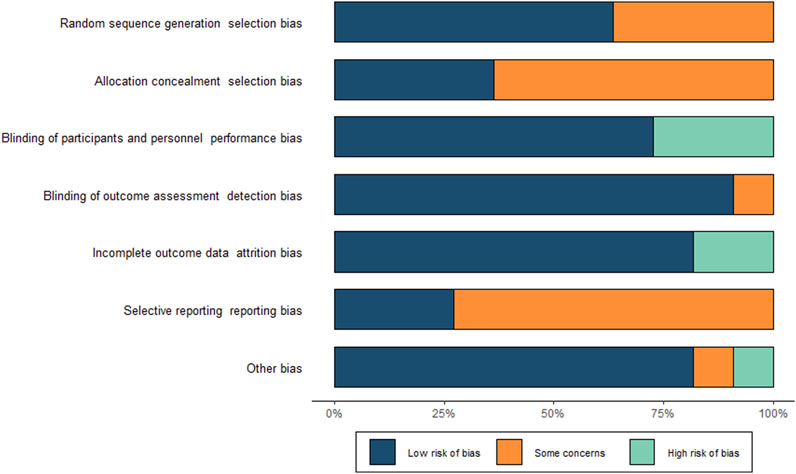
Two independent investigators extracted data from each study using a pre-designed data extraction form. Extracted data included the first author’s surname, year of publication, type of experiment, number of participants, age, sex, baseline diagnosis, inclusion and exclusion criteria, treatment and experimental interventions, duration of intervention, primary outcome, and number of events. For unpublished studies, we referred to their practical design article (Uemura et al., 2018) and extracted data from their most recent conference presentation (Kushima et al., 2022). Two independent investigators assessed the quality of each study using the Cochrane risk of bias tool (Higgins et al., 2011), with any discrepancies resolved by a third investigator.
2.4 Statistical analysis
For dichotomous variables, we assumed that patients who discontinued were negative and that data reporting only the change in the number of events and the possibility of repeated reporting were positive. We used Stata16 software to generate meta-analysis and forest plots. Binary data were analysed into odds ratio (OR) with a 95% confidence interval (CI) using the DerSimonian-Laird random effects model to evaluate the overall sensitivity and specificity of the model, thereby fully accounting for the additional uncertainty related to inter-study differences in the effects of different interventions. We calculated the parameters Q, I2, I2 (≥50%), and P (<0.05) (Higgins and Thompson, 2002; Higgins et al., 2003) to assess heterogeneity of values. If feasible, we planned to conduct a subgroup analysis to evaluate the potential impact of the incident location, Helicobacter pylori infection, PPI dose, and exposure time. In cases of high heterogeneity in the main results, we would conduct a sensitivity analysis to assess the impact of individual studies on aggregate statistics, by excluding one study at a time from the meta-analysis. If the number of included studies was sufficient (≥ 10), we would explore potential publication bias by constructing a funnel plot of the effect size of each trial relative to the standard error, and evaluate the asymmetry of the funnel plot using Begg and Egger tests, defining significant publication bias as a p-value <0.1.
3 Result
Initially, we identified a total of 4,868 studies from electronic databases. Out of these, 802 were duplicates. After screening by title and abstract, we selected 85 studies for further evaluation. However, we could not retrieve 8 reports. Out of the remaining 77 studies, 62 were non-randomized controlled trials (RCTs) or review articles. Additionally, we found 2 reports to be duplicates of the same study, while 3 studies were excluded due to incomplete data. Among the remaining nine studies, one of them (Genta et al., 2003) reported adverse reaction results of two studies (Johnson et al., 2001; Vakil et al., 2001) with identical designs. We found the original texts of these two studies for quality analysis. One study (Haruma et al., 2021) is a meeting abstract for a study that is still in progress. We retrieved the study design (Uemura et al., 2018) for this study and the 4-year study data presented at the meeting in May 2022 (Kushima et al., 2022).
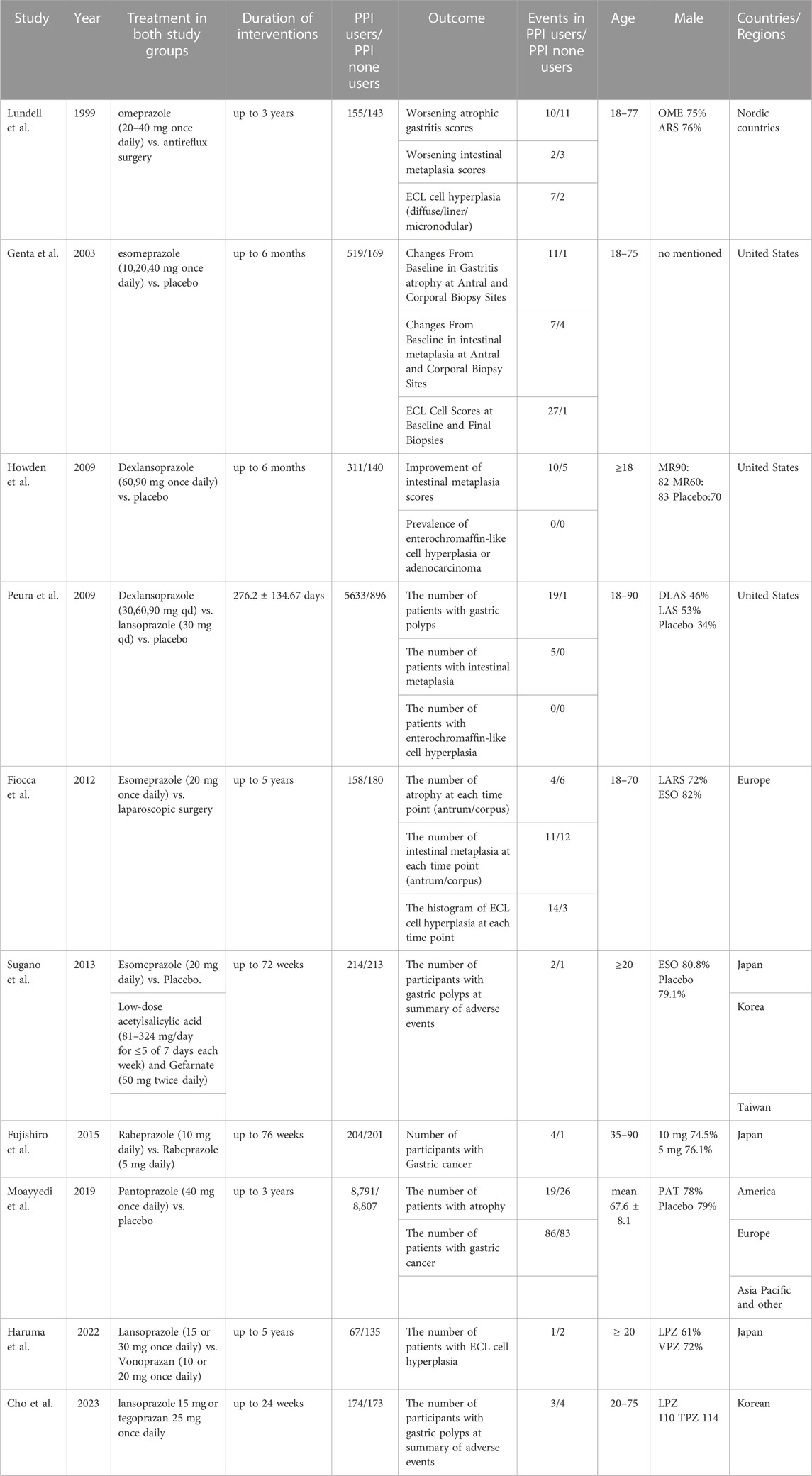
We included ten randomised controlled studies for the effect of proton pump inhibitors on gastric cancer-related lesions, comprising 27,283 subjects (Table 1. Summary of study characteristics) (Figure 2. Risk of bias Figure 3. Risk of bias summary.).
Gastric mucosal atrophy and intestinal metaplasia: Four studies evaluated gastric mucosal atrophy. One study reported the changes in the number of participants with gastric mucosal atrophy and intestinal metaplasia at the endpoint compared to the baseline for each degree of progression (Lundell et al., 1999). Another study reported only the endpoint number of occurrences of gastric mucosal atrophy (Moayyedi et al., 2019). A pooled set (Genta et al., 2003) of adverse effects of two studies (Johnson et al., 2001; Vakil et al., 2001) reported the number of participants whose gastric mucosal atrophy and intestinal metaplasia aggravated and decreased in the antrum and gastric corpus, respectively, compared to the baseline. One study also reported atrophy and intestinal metaplasia at each follow-up time point by dividing the gastric antrum and gastric corpus (Fiocca et al., 2012). However, we could not obtain trends from this study, and we had no way of knowing whether the participants with events in the antrum and gastric corpus overlapped. We first assume that the experimental group using PPI has the most events and the control group has the least events as a premise for including the data in this study. To avoid bias, we subsequently excluded this study and conducted an analysis again to observe whether the direction of the meta-analysis results after exclusion is consistent with that under this extreme condition.
In a pooled analysis of the above four studies, progressive worsening of gastric mucosal atrophy was found in 46 of 9,645 participants treated with PPI maintenance for more than 6 months and in 40 of 9,277 participants in the control group. No statistical difference was found between the groups (OR 1.01; 95% CI 0.55 to 1.85; p-value = 0.97), indicating that most participants showed improvement or no change in the degree of gastric mucosal atrophy (Figure 4. Forest plots of odds ratios for atrophy in participants receiving proton pump inhibitors compared with subjects not receiving proton pump inhibitors.). When re-analysed after excluding studies assuming extreme cases of Fiocca (Fiocca et al., 2012), the results of the meta-analysis were in the same direction (OR 0.84; 95% CI 0.5 to 1.41; p-value = 0.51) and the I2 decreased from 25.8% to 7.48%.
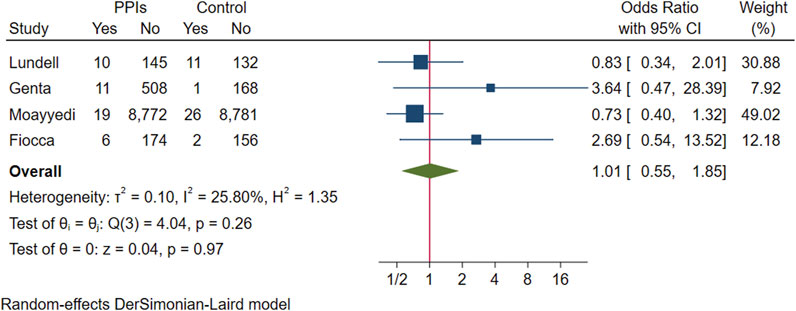
FIGURE 4. Forest plots of odds ratios for atrophy in participants receiving proton pump inhibitors compared with subjects not receiving proton pump inhibitors.
We pooled five RCTs evaluating 8,303 participants, in which one study reported the incidence of intestinal metaplasia in participants with endpoint gastric biopsies (Howden et al., 2009), and another study reported the number of participants who had intestinal metaplasia in gastric biopsies (Peura et al., 2009). Intestinal metaplasia was present on endpoint gastric biopsies in 36 participants in the PPI group and 14 in the control group, with no evidence that intestinal metaplasia was associated with exacerbation and long-term use of PPIs (OR 1.14; 95% CI 0.49 to 2.68; p-value = 0.76) (Figure 5. Forest plots of odds ratios for intestinal metaplasia in participants receiving proton pump inhibitors compared with subjects not receiving proton pump inhibitors.). Likewise, after excluding studies that assumed extreme cases of Fiocca and conducting a subsequent analysis, the meta-analysis results were consistent in direction (OR 0.75; 95% CI 0.36 to 1.55; p-value = 0.44), and I2 decreased from 35.21% to 0.
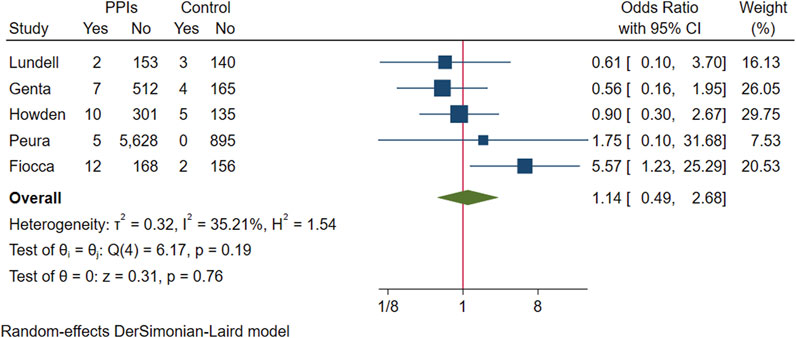
FIGURE 5. Forest plots of odds ratios for intestinal metaplasia in participants receiving proton pump inhibitors compared with subjects not receiving proton pump inhibitors.
Enterochromaffin-like cell hyperplasia: Six studies evaluated changes in ECL cells. Two studies reported no occurrences of ECL cell hyperplasia during the study period (Howden et al., 2009; Peura et al., 2009). One study reported changes in each level of ECL cell abnormalities in participants from baseline to endpoint (Lundell et al., 1999). Another study provided a histogram representation of the number of patients with ECL cell hyperplasia, which we manually entered for measurement (Fiocca et al., 2012). Additionally, one study reported the number of participants with ECL cell hyperplasia on gastric tissue biopsy at the endpoint and the proportion of these participants with participants whose ECL cell scores worsened (Genta et al., 2003). We also retrieved data from one ongoing study with a 5-year duration (Kushima et al., 2022) which presented its fourth-year gastric biopsy data at a conference in May 2022.
The above six studies were pooled and analysed. Among the 6,865 participants who used PPI maintenance therapy for more than 6 months, 49 had deterioration of ECL cell score, and only 8 of the 1,640 participants in the control group had a breakdown of ECL score. This meta-analysis showed that long-term PPI maintenance therapy users had an increased risk of worsening ECL cell scores relative to non-PPI users (OR 3.01; 95% CI 1.29 to 7.04; p-value = 0.01) (Figure 6. Forest plots of odds ratios for ECL cell hyperplastic in participants receiving proton pump inhibitors compared with subjects not receiving proton pump inhibitors.). On this basis, we performed a subgroup analysis regarding the duration of observation and the type of PPI, and the results showed that the negative effect of PPI maintenance treatment on ECL cell proliferation did not seem to be dependent on the duration of observation (<1 year: OR 3.33; 95% CI 0.2 to 54.59; p-value = 0.4, 1–3 years: OR 1.26; 95% CI 0.08 to 20.36; p-value = 0.87, >3 years: OR 3.06; 95% CI 0.89 to 10.45; p-value = 0.07), no trend was observed (Figure 7. Forest plots of the odds of ECL cell proliferation in participants who received proton pump inhibitors compared to those who did not receive proton pump inhibitors by different observation duration.), but it seemed to be related to the type of PPI, and the studies with Esomeprazole (OR 5.4; 95% CI 1.85 to 15.74; p-value = 0) were more significant than those with Lansoprazole (OR 0.9; 95% CI 0.11 to 7.01; p-value = 0.92) or Dexlansoprazole (OR 0.31; 95% CI 0.02 to 4.92; p-value = 0.4) (Figure 8. Forest plots of the odds of ECL cell proliferation in participants who received proton pump inhibitors compared to those who did not receive proton pump inhibitors by using different types of PPIs.).
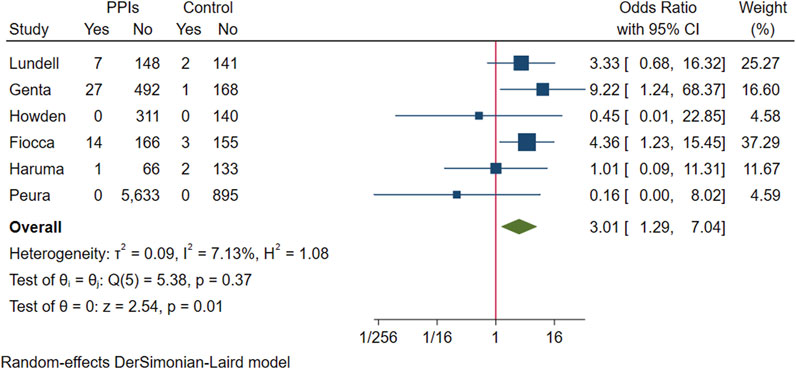
FIGURE 6. Forest plots of odds ratios for ECL cell hyperplastic in participants receiving proton pump inhibitors compared with subjects not receiving proton pump inhibitors.
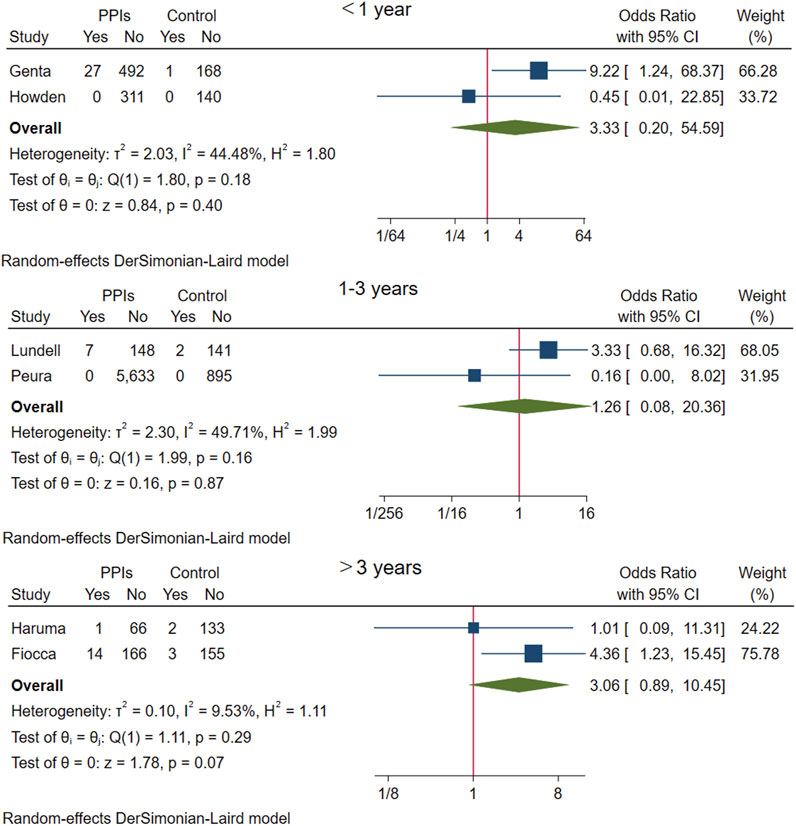
FIGURE 7. Forest plots of the odds of ECL cell proliferation in participants who received proton pump inhibitors compared to those who did not receive proton pump inhibitors by different observation duration.
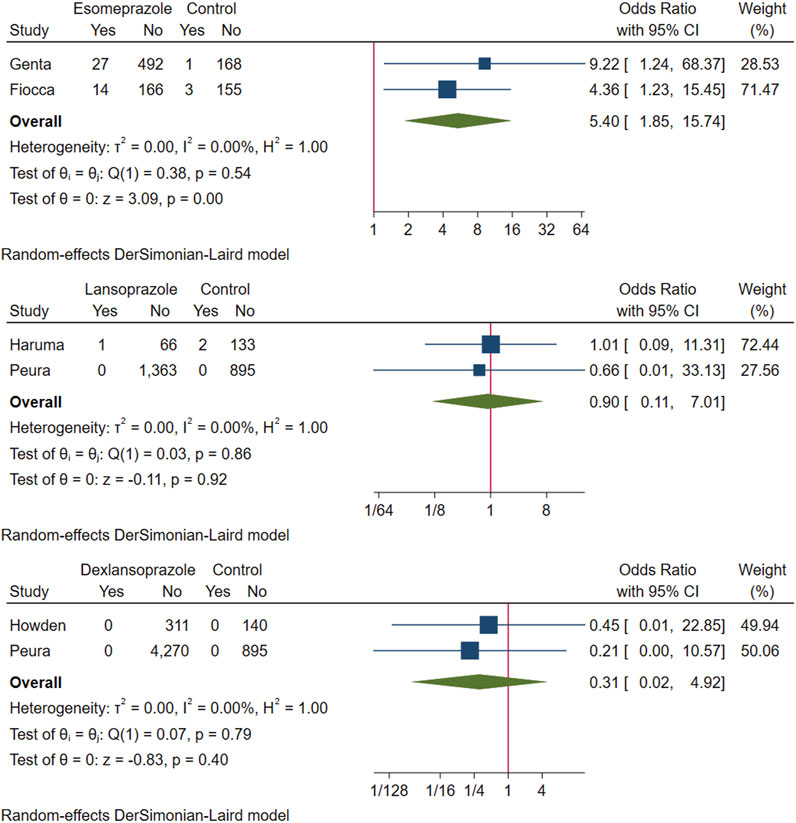
FIGURE 8. Forest plots of the odds of ECL cell proliferation in participants who received proton pump inhibitors compared to those who did not receive proton pump inhibitors by using different types of PPIs.
Gastric polyps and Cancers: Four studies evaluated participants for gastric polyps over the course of the study. One study recorded the number of patients with endoscopic fundic gland polyps at baseline and during each follow-up period (Fiocca et al., 2012). Four other studies reported the number of participants who developed gastric polyps during the study as adverse reactions (Peura et al., 2009; Sugano et al., 2014; Kushima et al., 2022; Cho et al., 2023), but the location of the gastric polyp was not indicated. Three studies clearly described participants with gastrointestinal cancers; one research stated that no patients were found to have cancer during the study (Lundell et al., 1999), and another showed the number of participants with cancer in the adverse effect table (Moayyedi et al., 2019). Two groups of patients in one study used 10 mg and 5 mg of rabeprazole once daily (Fujishiro et al., 2015), respectively, which we included as the experimental and control groups were included in the study.
Pooling the above five studies, 55 of 6,268 participants who received PPI maintenance therapy for more than 6 months developed gastric polyps, gastric polyps were detected in 62 of the 1,575 participants in the control group, and there was no evidence that the presence of gastric polyps was associated with long-term PPI use (OR 1.13; 95% CI 0.68 to 1.89; p-value = 0.64) (Figure 9. Forest plots of odds ratios for gastric polyps in participants receiving proton pump inhibitors compared with subjects not receiving proton pump inhibitors.).
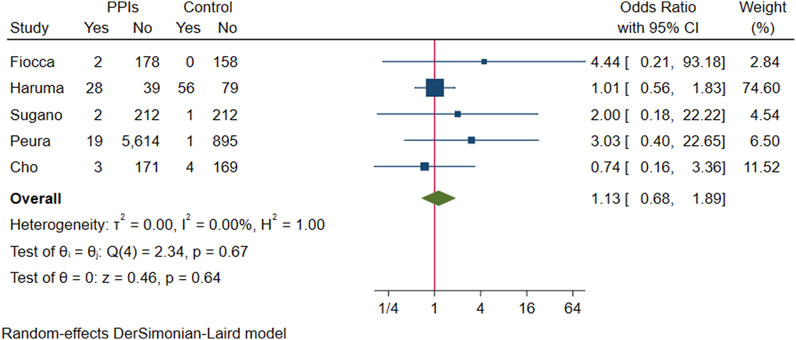
FIGURE 9. Forest plots of odds ratios for gastric polyps in participants receiving proton pump inhibitors compared with subjects not receiving proton pump inhibitors.
In a pooled analysis of three studies with gastric cancer statistics, 90 of the 9,306 participants in the experimental group developed to gastric cancer, and 84 of the 9,148 participants in the control group were found to have gastric cancer. The difference in prevalence between the two groups was not statistically significant (OR 1.06; 95% CI 0.79 to 1.43; p-value = 0.71) (Figure 10. Forest plots of odds ratios for gastric cancer in participants receiving proton pump inhibitors compared with subjects not receiving proton pump inhibitors.).
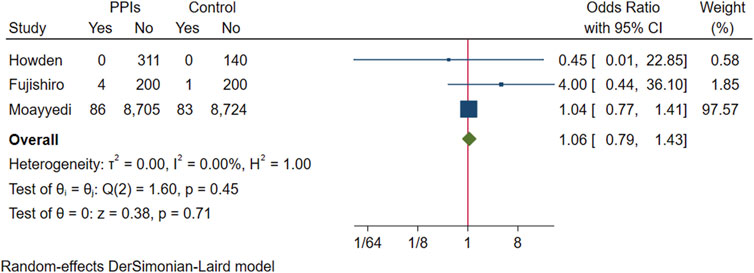
FIGURE 10. Forest plots of odds ratios for gastric cancer in participants receiving proton pump inhibitors compared with subjects not receiving proton pump inhibitors.
4 Discussion
Our analysis found no evidence that long-term maintenance therapy with PPIs causes or exacerbates gastric mucosal atrophy, intestinal metaplasia, gastric polyps, or gastric cancer in users compared to other treatments. However, we did find a correlation between PPI use and ECL cell proliferation, which may depend on the type of PPI used rather than the duration of use. Our results are consistent with Song’s analysis (Song et al., 2014), but incorporate more recent RCT studies to improve the analysis’s quality and minimise bias. Overall, these findings suggest that long-term maintenance therapy with PPIs can be safely used as a therapeutic strategy in clinical practice. However, the potential risk of ECL cell proliferation associated with high gastrin levels over a long time must be considered.
In recent years, concerns have arisen that long-term use of PPIs may increase the risk of gastric cancer-related disease (Salvo et al., 2021), and various hypotheses have been proposed regarding its causes and mechanisms. One hypothesis is that prolonged acid suppression therapy may alter the stomach’s acid environment, allowing colonisation by various bacteria (Yang and Metz, 2010), including H. pylori, which can damage the gastric oxidative glands (Parsons et al., 2017) and potentially trigger malignant transformation, possibly accompanied by a targeted transfer of gastric flora due to profound acid suppression (Malfertheiner et al., 2017). However, two studies (Lundell et al., 1999; Fiocca et al., 2012) found no significant increase in gastric atrophy and intestinal metaplasia in H. pylori-positive patients on long-term PPI maintenance therapy, and the difference in lesions in the gastric body and sinus in the two studies (Genta et al., 2003; Fiocca et al., 2012) was not significant. Although we initially planned to perform a subgroup analysis regarding H. pylori and lesion site, we were unable to do so due to the limited number of relevant trials. Based on the available studies, it appears that acid suppression alone is unlikely to have adverse effects on the human gastric mucosa. Nonetheless, the dysbiosis of the gastrointestinal flora caused by long-term PPI use (Sanduleanu et al., 2001; Lo and Chan, 2013) and the association of H. pylori with gastrointestinal cancers remain areas of concern (Lee et al., 2013; Mera et al., 2018; Yan et al., 2022). Further research is needed to determine the best approach to managing these risks.
Ever since the observation of hypergastrinemia-induced ECL cell hyperplasia, a pre-cancerous condition, in the carcinogenicity study of omeprazole conducted on rats (Havu, 1986), the issue concerning the association between proton pump inhibitors (PPIs) and gastric cancer risk has never ceased. According to another study, when 75% of the acid-producing portion of a rat’s stomach is removed, hypergastrinemia occurs, and over time, there is a proliferation of ECL cells (Mattsson et al., 1991). High-dose, potent, and long-acting acid secretion inhibitors can alter the pH of the gastrointestinal system, affecting acid feedback regulation, leading to an increase in gastrin levels, and subsequently resulting in hypergastrinemia (Håkanson and Sundler, 1990; Veysey-Smith et al., 2021), which has a direct trophic effect on ECL cells (Waldum and Mjønes, 2020). This may be the reason why ECL cell proliferation appears to be related to the long-term use of PPI maintenance therapy. There is no threshold concentration for the trophic effect of gastrin on ECL cells (Peghini et al., 2002), and long-term high gastrin levels will inevitably cause the proliferation of ECL cells (Rais et al., 2022), which may develop into tumour cells, thus increasing the risk of cancer development in the long run, which may be intestinal gastric adenocarcinoma or neuroendocrine cancer (Waldum and Mjønes, 2020; Rais et al., 2022). However, the process of inducing gastric tumours requires a considerable amount of time and may only manifest after several decades. We found that the studies using esomeprazole (Genta et al., 2003; Fiocca et al., 2012) had more ECL cell hyperplasia in participants treated with PPI maintenance therapy compared to those using dexlansoprazole (Howden et al., 2009; Peura et al., 2009) or lansoprazole (Peura et al., 2009; Haruma et al., 2021). In the classification of Proton Pump Inhibitors (PPIs), we categorize them based on their chemical structure and mechanism of action, primarily into benzimidazole and thiazole classes. The benzimidazole class includes omeprazole, lansoprazole, and esomeprazole, while the thiazole class includes pantoprazole and rabeprazole. The majority of PPIs are metabolized primarily through the hepatic cytochrome P450 enzyme system. For instance, omeprazole and lansoprazole are mainly metabolized through CYP2C19 and CYP3A4, while esomeprazole is primarily metabolized through CYP2C19 (Li et al., 2004). There are differences among various PPIs in terms of inhibiting gastric acid secretion, onset time, duration, and protective effects on the gastric mucosa. Generally, the acid-suppressing effect of esomeprazole is considered the strongest (Edwards et al., 2001; Edwards et al., 2006), followed by rabeprazole, then omeprazole, and finally lansoprazole (Miner et al., 2003). This is primarily due to their differing metabolic rates and pathways in the body, which influence their pharmacological effects. Esomeprazole, the S-isomer of omeprazole, has a more stable molecular structure, is more readily absorbed by the human body, and has a longer half-life in gastric acid, enabling it to continuously inhibit gastric acid secretion. Esomeprazole directly acts on the proton pumps of gastric wall cells, inhibiting their production of gastric acid, and its acid-suppressing effect is stronger than other proton pump inhibitors, this may trigger a reflection on the choice of clinical PPI type. The ECL cell density did not increase in many participants, suggesting that not all patients require strong inhibition of gastric acid secretion. Clinically, the appropriate type of PPI and the appropriate dosage should be chosen according to individual needs. The risk of elevated gastrin and consequent ECL cell hyperplasia due to long-term PPI use may lead to gastric neuroendocrine tumours or gastric adenocarcinoma is not negligible (Cavalcoli et al., 2015). However, this may be the result of a combination of causes. The presence of H. pylori and the patient’s original gastric precancerous lesions are also considered to be the causes of elevated gastrin (Veysey-Smith et al., 2021). Therefore, in the long-term management of PPI maintenance therapy, it is important to consider the risk of ECL cell proliferation and manage any H. pylori infection or gastric precancerous lesions.
Regarding gastric polyps and gastric cancer, we did not restrict the location of polyps to include all RCT studies that reported gastric polyps because of the small number of studies addressing fundic gland polyps. Our results reflect some controversy compared to previous pooled analyses by Tran-Duy (Tran-Duy et al., 2016) and F.C. Martin (Martin et al., 2016) which were based on population cohort studies and mostly cross-sectional studies conducted during a specific period. We also found differing conclusions in this area of the literature (Hong et al., 2011; Krishnan et al., 2012; Brusselaers et al., 2020) when we reviewed RCT studies. In gastric cancer, there have also been several meta-analyses (Segna et al., 2021; Guo et al., 2023; Peng et al., 2023) based on case-control studies and retrospective cohort studies in recent years that support the idea that PPI use elevates the risk of gastric cancer, reaching different results than our analysis. The reasons for this may lie mainly in the very limited number of relevant high-quality RCT studies, previous meta-analyses have relied on case-control studies and retrospective cohort studies, which leads to baseline imbalances triggered by inclusion and exclusion criteria, as well as potential difficulties in dosing control and prolonged follow-up, resulting in high heterogeneity. In contrast, our meta-analysis included only existing RCT studies, resulting in lower heterogeneity and different findings from those of recent meta-analyses. Our study offers a unique contribution to the literature by focusing exclusively on high-quality RCT studies, providing a clearer picture of the relationship between PPI use and gastric polyps and cancer. However, we still need more well-designed, high-quality studies to address the current controversy.
In the analyses of gastric atrophy and intestinal metaplasia, after including the data under extreme assumptions, I2 = 25.8% for gastric atrophy and I2 = 35.21% for intestinal metaplasia, and after excluding this data the I2 decreased to 7.48% and 0, respectively, with the same direction of meta-analysis results before and after exclusion, suggesting that the scale of inclusion of data from this study does not affect the final conclusions of the analysis. In the analysis regarding ECL cells, I2 = 7.13%, and I2 = 0 in the rest of the analyses. p > 0.05 was seen in all Q-tests, and the minimum value in several studies was p = 0.19. No significant heterogeneity was observed in our research. This is reassuring. However, there are some limitations in the following aspects. First, the number of pieces of literature included in this study is still relatively limited. We sought as much relevant literature as possible while ensuring the quality of the literature and included trials that had not yet been completed but for which data were published annually; second, since the publication of relevant data differed among the literature, and some literature only published the prevalence rate per year, and we could not identify the trend of change. We did not receive a reply after sending an email to the authors to ask about it. We accumulated this data and included it in the analysis, the lack of response to our email inquiries also resulted in some bias; third, the study had diverse control group intervention modalities, including placebo, surgery, and other therapeutic drugs, the association of the disease with the experimental group might not be stable due to the diverse intervention modalities in the control group; fourth, the primary outcomes of some of the literature did not match the present study. It mainly reported data of interest in the form of secondary results or adverse events found during the primary outcome, and we sought reports of relevant events in the eligible full texts, but there may still be incomplete or non-specific statistics for this part of the data; finally, some of the pieces of the literature may also have factors of bias such as open-label design and pharmaceutical industry funding, and other unidentified biases. This study exclusively incorporated Randomised Controlled Trials (RCTs), without considering real-world cohort studies. This was primarily due to the fact that RCTs are considered the gold standard for assessing intervention effects, given their design which minimizes bias to the greatest extent possible. However, we acknowledge that real-world cohort studies, offering a broader patient population and longer follow-up data, could potentially influence our results. In the literature where cohort studies were included, we found a significant association between long-term use of PPIs and premalignant gastric lesions. This could be attributed to the fact that cohort studies typically encompass a wider patient population, including those who might be excluded in RCTs, such as the elderly and patients with multiple chronic diseases. Furthermore, cohort studies usually have a longer follow-up period, which might allow us to observe the potential impacts of long-term PPI use, a feat quite challenging in RCTs. However, the occurrence of carcinogenesis requires a considerable length of time, possibly spanning decades, hence our results may be biased. Therefore, the conclusion should be that PPI treatment within the follow-up period of the included RCTs, ranging from 6 to 36 weeks, will not induce gastric cancer at the end of treatment, but long-term maintenance treatment with PPIs may increase susceptibility to gastric cancer, making individuals more prone to gastric cancer in their later years. Future research should consider incorporating real-world cohort studies to more comprehensively assess the association between long-term use of PPIs and premalignant gastric lesions.
PPIs are being used more frequently while their application is gradually expanding (Kinoshita et al., 2018), and their relationship with adverse effects such as pneumonia (Zirk-Sadowski et al., 2018) and osteoporosis (Park et al., 2022) has received attention. Despite the extensive use of Proton Pump Inhibitors (PPIs), the influence of long-term maintenance therapy on the risk of developing gastric cancer-related diseases remains uncertain. This necessitates further exploration in high-quality clinical studies, with this as the primary outcome. Nevertheless, our meta-analysis of the existing literature reveals no significant correlation between PPI maintenance therapy and an increased risk of gastric mucosal atrophy, intestinal metaplasia, or gastric polyps within a maximum follow-up period of 3 years. However, it does lead to ECL cell hyperplasia, a consequence of excessive gastrin triggered by strong acid suppression, which heightens the patient’s susceptibility to gastric cancer. Therefore, our findings suggest that PPI therapy can be safely and effectively employed for the treatment of patients with gastrointestinal diseases requiring long-term maintenance therapy. However, caution should be exercised when considering the use of PPIs, and the potential risks and benefits of treatment should be meticulously evaluated for each patient. Not all patients necessitate treatment that strongly suppresses gastric acid secretion, such as the most potent acid-suppressing PPIs or PPIs at effective doses.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
ZZ was responsible for overseeing the entire process from the beginning of the study until the publication of the article. ZZ was accountable for topic selection, literature screening, extraction of mergeable data, and overall study design and manuscript writing. ZL conducted literature screening, performed data extraction, and conducted statistical analysis. YS participated in manuscript writing and proofreading of the language. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1244400/full#supplementary-material
References
Allemani, C., Weir, H. K., Carreira, H., Harewood, R., Spika, D., Wang, X. S., et al. (2015). Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet (London, Engl. 385 (9972), 977–1010. doi:10.1016/S0140-6736(14)62038-9
Brusselaers, N., Sadr-Azodi, O., and Engstrand, L. (2020). Long-term proton pump inhibitor usage and the association with pancreatic cancer in Sweden. J. Gastroenterol. 55 (4), 453–461. doi:10.1007/s00535-019-01652-z
Carmack, S. W., Genta, R. M., Graham, D. Y., and Lauwers, G. Y. (2009). Management of gastric polyps: a pathology-based guide for gastroenterologists. Nat. Rev. Gastroenterology hepatology 6 (6), 331–341. doi:10.1038/nrgastro.2009.70
Cavalcoli, F., Zilli, A., Conte, D., Ciafardini, C., and Massironi, S. (2015). Gastric neuroendocrine neoplasms and proton pump inhibitors: fact or coincidence? Scand. J. Gastroenterol. 50 (11), 1397–1403. doi:10.3109/00365521.2015.1054426
Cho, Y. K., Kim, J. H., Kim, H. S., Kim, T. O., Oh, J. H., Choi, S. C., et al. (2023). Randomised clinical trial: comparison of tegoprazan and lansoprazole as maintenance therapy for healed mild erosive oesophagitis. Aliment. Pharmacol. Ther. 57 (1), 72–80. doi:10.1111/apt.17255
Colquhoun, A., Arnold, M., Ferlay, J., Goodman, K. J., Forman, D., and Soerjomataram, I. (2015). Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut 64 (12), 1881–1888. doi:10.1136/gutjnl-2014-308915
Correa, P., Haenszel, W., Cuello, C., Tannenbaum, S., and Archer, M. (1975). A model for gastric cancer epidemiology. Lancet (London, Engl. 2 (7924), 58–60. doi:10.1016/s0140-6736(75)90498-5
Correa, P. (1992). Human gastric carcinogenesis: a multistep and multifactorial process-first American cancer society award lecture on cancer epidemiology and prevention. Cancer Res. 52 (24), 6735–6740.
Dilaghi, E., Bellisario, M., Esposito, G., Carabotti, M., Annibale, B., and Lahner, E. (2022). The impact of proton pump inhibitors on the development of gastric neoplastic lesions in patients with autoimmune atrophic gastritis. Front. Immunol. 13, 910077. doi:10.3389/fimmu.2022.910077
Edwards, S., Lind, T., and Lundell, L. (2001). Systematic review of proton pump inhibitors for the acute treatment of reflux oesophagitis. Aliment. Pharmacol. Ther. 15 (11), 1729–1736. doi:10.1046/j.1365-2036.2001.01128.x
Edwards, S., Lind, T., and Lundell, L. (2006). Systematic review: proton pump inhibitors (PPIs) for the healing of reflux oesophagitis - a comparison of esomeprazole with other PPIs. Aliment. Pharmacol. Ther. 24 (5), 743–750. doi:10.1111/j.1365-2036.2006.03074.x
Fiocca, R., Mastracci, L., Attwood, S. E., Galmiche, J. P., and Hatlebakk, J. (2012). Gastric exocrine and endocrine cell morphology under prolonged acid inhibition therapy: results of a 5-year follow-up in the LOTUS trial. Alimentary Pharmacol. Ther. 36 (10), 959–971. doi:10.1111/apt.12052
Fujishiro, M., Higuchi, K., Kato, M., Kinoshita, Y., Iwakiri, R., Watanabe, T., et al. (2015). Long-term efficacy and safety of rabeprazole in patients taking low-dose aspirin with a history of peptic ulcers: A phase 2/3, randomized, parallel-group, multicenter, extension clinical trial. J. Clin. Biochem. Nutr. 56 (3), 228–239. doi:10.3164/jcbn.15-1
Genta, R. M., Rindi, G., Fiocca, R., Magner, D. J., D'Amico, D., and Levine, D. S. (2003). Effects of 6-12 months of esomeprazole treatment on the gastric mucosa. Am. J. gastroenterology 98 (6), 1257–1265. doi:10.1111/j.1572-0241.2003.07489.x
Guo, H., Zhang, R., Zhang, P., Chen, Z., Hua, Y., Huang, X., et al. (2023). Association of proton pump inhibitors with gastric and colorectal cancer risk: A systematic review and meta-analysis. Front. Pharmacol. 14, 1129948. doi:10.3389/fphar.2023.1129948
Hagiwara, T., Mukaisho, K., Nakayama, T., Sugihara, H., and Hattori, T. (2011). Long-term proton pump inhibitor administration worsens atrophic corpus gastritis and promotes adenocarcinoma development in Mongolian gerbils infected with Helicobacter pylori. Gut 60 (5), 624–630. doi:10.1136/gut.2010.207662
Håkanson, R., and Sundler, F. (1990). Proposed mechanism of induction of gastric carcinoids: the gastrin hypothesis. Eur. J. Clin. Invest. 20, S65–S71. doi:10.1111/j.1365-2362.1990.tb01780.x
Haruma, K., Uemura, N., Kinoshita, Y., Yao, T., Kushima, R., Akiyama, J., et al. (2021). SA166 3-year interim analysis results of vision trial: a randomized, open-label study to evaluate the long-term safety of vonoprazan as maintenance treatment in patients with erosive esophagitis. Gastroenterology 160 (6), 447. doi:10.1016/s0016-5085(21)01779-0
Havu, N. J. D. (1986). Enterochromaffin-like cell carcinoids of gastric mucosa in rats after life-long inhibition of gastric secretion. Digestion 35, 42–55. doi:10.1159/000199381
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ Clin. Res. ed) 343, d5928. doi:10.1136/bmj.d5928
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ Clin. Res. ed). 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Higgins, J. P., and Thompson, S. G. (2002). Quantifying heterogeneity in a meta-analysis. Statistics Med. 21 (11), 1539–1558. doi:10.1002/sim.1186
Hong, K., Kim, S., Kang, S., and Kim, J. (2011). Effect of long-term use of proton pump inhibitor on atrophic gastritis and gastric cancer: A 5-year longitudinal cohort study in South Korea. Drug Saf. 34 (10), 970.
Howden, C. W., Larsen, L. M., Perez, M. C., Palmer, R., and Atkinson, S. N. (2009). Clinical trial: efficacy and safety of dexlansoprazole MR 60 and 90 mg in healed erosive oesophagitis - maintenance of healing and symptom relief. Alimentary Pharmacol. Ther. 30 (9), 895–907. doi:10.1111/j.1365-2036.2009.04119.x
Johnson, D. A., Benjamin, S. B., Vakil, N. B., Goldstein, J. L., Lamet, M., Whipple, J., et al. (2001). Esomeprazole once daily for 6 months is effective therapy for maintaining healed erosive esophagitis and for controlling gastroesophageal reflux disease symptoms: a randomized, double-blind, placebo-controlled study of efficacy and safety. Am. J. gastroenterology 96 (1), 27–34. doi:10.1111/j.1572-0241.2001.03443.x
Kim, G. H. (2021). Proton pump inhibitor-related gastric mucosal changes. Gut liver 15 (5), 646–652. doi:10.5009/gnl20036
Kinoshita, Y., Ishimura, N., and Ishihara, S. (2018). Advantages and disadvantages of long-term proton pump inhibitor use. J. Neurogastroenterol. Motil. 24 (2), 182–196. doi:10.5056/jnm18001
Krishnan, A., Rajesh Prabu, P., and Jayanthi, V. (2012). Long term acid suppression therapy: it's influence on gastric mucosa. Dis. Esophagus 25, 65A. doi:10.1111/j.1442-2050.2012.01405.x
Kushima, R., Uemura, N., and Kinoshita, Y. (2022). 4-Year interim analysis results of vision trial: a randomized, open-label study to evaluate the long-term safety of vonoprazan as maintenance treatment in patients with erosive esophagitis. Gastroenterology 162 (7)–1066. doi:10.1016/S0016-5085(22)62550-2
Lee, Y. C., Chen, T. H., Chiu, H. M., Shun, C. T., Chiang, H., Liu, T. Y., et al. (2013). The benefit of mass eradication of Helicobacter pylori infection: a community-based study of gastric cancer prevention. Gut 62 (5), 676–682. doi:10.1136/gutjnl-2012-302240
Li, X., Andersson, T., Ahlström, M., Weidolf, L., and Chemicals, D. (2004). Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab. Dispos. 32 (8), 821–827. doi:10.1124/dmd.32.8.821
Lo, W. K., and Chan, W. W. (2013). Proton pump inhibitor use and the risk of small intestinal bacterial overgrowth: a meta-analysis. Clin. gastroenterology hepatology 11 (5), 483–490. doi:10.1016/j.cgh.2012.12.011
Lundell, L., Miettinen, P., Myrvold, H. E., Pedersen, S. A., Thor, K., Andersson, A., et al. (1999). Lack of effect of acid suppression therapy on gastric atrophy. Nordic Gerd Study Group. Gastroenterology. 117 (2), 319–326. doi:10.1053/gast.1999.0029900319
Lundell, L., Vieth, M., Gibson, F., Nagy, P., and Kahrilas, P. J. (2015). Systematic review: the effects of long-term proton pump inhibitor use on serum gastrin levels and gastric histology. Alimentary Pharmacol. Ther. 42 (6), 649–663. doi:10.1111/apt.13324
Malfertheiner, P., Kandulski, A., and Venerito, M. (2017). Proton-pump inhibitors: understanding the complications and risks. Nat. Rev. Gastroenterology hepatology 14 (12), 697–710. doi:10.1038/nrgastro.2017.117
Martin, F. C., Chenevix-Trench, G., and Yeomans, N. D. (2016). Systematic review with meta-analysis: fundic gland polyps and proton pump inhibitors. Alimentary Pharmacol. Ther. 44 (9), 915–925. doi:10.1111/apt.13800
Mattsson, H., Havu, N., Bräutigam, J., Carlsson, K., Lundell, L., and Carlsson, E. J. G. (1991). Partial gastric corpectomy results in hypergastrinemia and development of gastric enterochromaffinlike-cell carcinoids in the rat. Gastroenterology 100 (2), 311–319. doi:10.1016/0016-5085(91)90197-s
Mera, R. M., Bravo, L. E., Camargo, M. C., Bravo, J. C., Delgado, A. G., Romero-Gallo, J., et al. (2018). Dynamics of Helicobacter pylori infection as a determinant of progression of gastric precancerous lesions: 16-year follow-up of an eradication trial. Gut 67 (7), 1239–1246. doi:10.1136/gutjnl-2016-311685
Miner, P., Katz, P., Chen, Y., and Sostek, M. (2003). Gastric acid control with esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole: a five-way crossover study. Am. J. Gastroenterol. 98 (12), 2616–2620. doi:10.1111/j.1572-0241.2003.08783.x
Moayyedi, P., Eikelboom, J. W., Bosch, J., Connolly, S. J., Dyal, L., Shestakovska, O., et al. (2019). Safety of proton pump inhibitors based on a large, multi-year, randomized trial of patients receiving rivaroxaban or aspirin. Gastroenterology 157 (3), 682–691.e2. doi:10.1053/j.gastro.2019.05.056
Page, M. J., Moher, D., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. Bmj 372, n160. doi:10.1136/bmj.n160
Park, D. H., Seo, S. I., Lee, K. J., Kim, J., Kim, Y., Seo, W. W., et al. (2022). Long-term proton pump inhibitor use and risk of osteoporosis and hip fractures: A nationwide population-based and multicenter cohort study using a common data model. J. Gastroenterol. Hepatol. 37 (8), 1534–1543. doi:10.1111/jgh.15879
Park, D. Y., and Lauwers, G. Y. (2008). Gastric polyps: classification and management. Archives pathology laboratory Med. 132 (4), 633–640. doi:10.1043/1543-2165(2008)132[633:GPCAM]2.0.CO;2
Parsons, B. N., Ijaz, U. Z., D'Amore, R., Burkitt, M. D., Eccles, R., Lenzi, L., et al. (2017). Comparison of the human gastric microbiota in hypochlorhydric states arising as a result of Helicobacter pylori-induced atrophic gastritis, autoimmune atrophic gastritis and proton pump inhibitor use. PLoS Pathog. 13 (11), e1006653. doi:10.1371/journal.ppat.1006653
Peghini, P. L., Annibale, B., Azzoni, C., Milione, M., Corleto, V. D., Gibril, F., et al. (2002). Effect of chronic hypergastrinemia on human enterochromaffin-like cells: insights from patients with sporadic gastrinomas. Gastroenterology 123 (1), 68–85. doi:10.1053/gast.2002.34231
Peng, T. R., Wu, T. W., and Li, C. H. (2023). Association between proton-pump inhibitors and the risk of gastric cancer: a systematic review and meta-analysis. Int. J. Clin. Oncol. 28 (1), 99–109. doi:10.1007/s10147-022-02253-2
Peura, D. A., Metz, D. C., Dabholkar, A. H., Paris, M. M., Yu, P., and Atkinson, S. N. (2009). Safety profile of dexlansoprazole MR, a proton pump inhibitor with a novel dual delayed release formulation: global clinical trial experience. Aliment. Pharmacol. Ther. 30 (10), 1010–1021. doi:10.1111/j.1365-2036.2009.04137.x
Piazuelo, M. B., Bravo, L. E., Mera, R. M., Camargo, M. C., Bravo, J. C., Delgado, A. G., et al. (2021). The Colombian chemoprevention trial: 20-Year follow-up of a cohort of patients with gastric precancerous lesions. Gastroenterology 160 (4), 1106–1117.e3. doi:10.1053/j.gastro.2020.11.017
Rais, R., Trikalinos, N. A., Liu, J., and Chatterjee, D. (2022). Enterochromaffin-like cell hyperplasia-associated gastric neuroendocrine tumors may arise in the setting of proton pump inhibitor use. Archives pathology laboratory Med. 146 (3), 366–371. doi:10.5858/arpa.2020-0315-OA
Ren, R., Wang, Z., Sun, H., Gao, X., and Peng, L. (2018). The gastric mucosal-associated microbiome in patients with gastric polyposis. Sci. Rep. 8 (1), 13817. doi:10.1038/s41598-018-31738-2
Salvo, E. M., Ferko, N. C., Cash, S. B., Gonzalez, A., and Kahrilas, P. J. (2021). Umbrella review of 42 systematic reviews with meta-analyses: the safety of proton pump inhibitors. Alimentary Pharmacol. Ther. 54 (2), 129–143. doi:10.1111/apt.16407
Sanduleanu, S., Jonkers, D., De Bruïne, A., Hameeteman, W., and Stockbrügger, R. W. (2001). Double gastric infection with Helicobacter pylori and non-Helicobacter pylori bacteria during acid-suppressive therapy: increase of pro-inflammatory cytokines and development of atrophic gastritis. Alimentary Pharmacol. Ther. 15 (8), 1163–1175. doi:10.1046/j.1365-2036.2001.01029.x
Segna, D., Brusselaers, N., Glaus, D., Krupka, N., and Misselwitz, B. (2021). Association between proton-pump inhibitors and the risk of gastric cancer: a systematic review with meta-analysis. Ther. Adv. Gastroenterol. 14, 17562848211051463. doi:10.1177/17562848211051463
Song, H., Zhu, J., and Lu, D. (2014). Long-term proton pump inhibitor (PPI) use and the development of gastric pre-malignant lesions. Cochrane database Syst. Rev. 2014 (12), Cd010623. doi:10.1002/14651858.CD010623.pub2
Sugano, K., Choi, M. G., Lin, J. T., Goto, S., Okada, Y., Kinoshita, Y., et al. (2014). Multinational, double-blind, randomised, placebocontrolled, prospective study of esomeprazole in the prevention of recurrent peptic ulcer in low-dose acetylsalicylic acid users: the lavender* study. Gut 63 (7), 1061–1068. doi:10.1136/gutjnl-2013-304722
Tran-Duy, A., Spaetgens, B., Hoes, A. W., de Wit, N. J., and Stehouwer, C. D. (2016). Use of proton pump inhibitors and risks of fundic gland polyps and gastric cancer: systematic review and meta-analysis. Clin. gastroenterology hepatology 14 (12), 1706–1719. doi:10.1016/j.cgh.2016.05.018
Uemura, N., Kinoshita, Y., Haruma, K., Yao, T., Kushima, R., and Kanoo, T. (2018). Rationale and design of the VISION study: a randomized, open-label study to evaluate the long-term safety of vonoprazan as maintenance treatment in patients with erosive esophagitis. Clin. Exp. gastroenterology 11, 51–56. doi:10.2147/CEG.S144149
Vakil, N. B., Shaker, R., Johnson, D. A., Kovacs, T., Baerg, R. D., Hwang, C., et al. (2001). The new proton pump inhibitor esomeprazole is effective as a maintenance therapy in GERD patients with healed erosive oesophagitis: a 6-month, randomized, double-blind, placebo-controlled study of efficacy and safety. Alimentary Pharmacol. Ther. 15 (7), 927–935. doi:10.1046/j.1365-2036.2001.01024.x
Veysey-Smith, R., Moore, A. R., Murugesan, S. V., Tiszlavicz, L., Dockray, G. J., Varro, A., et al. (2021). Effects of proton pump inhibitor therapy, H. pylori infection and gastric preneoplastic pathology on fasting serum gastrin concentrations. Front. Endocrinol. 12, 741887. doi:10.3389/fendo.2021.741887
Waldum, H., and Mjønes, P. (2020). Towards understanding of gastric cancer based upon physiological role of gastrin and ECL cells. Cancers 12 (11), 3477. doi:10.3390/cancers12113477
Yadlapati, R., and Kahrilas, P. J. (2018). The "dangers" of chronic proton pump inhibitor use. J. allergy Clin. Immunol. 141 (1), 79–81. doi:10.1016/j.jaci.2017.06.017
Yan, L., Chen, Y., Chen, F., Tao, T., Hu, Z., Wang, J., et al. (2022). Effect of Helicobacter pylori eradication on gastric cancer prevention: updated report from a randomized controlled trial with 26.5 Years of follow-up. Gastroenterology 163 (1), 154–162.e3. doi:10.1053/j.gastro.2022.03.039
Yang, Y. X., and Metz, D. C. (2010). Safety of proton pump inhibitor exposure. Gastroenterology 139 (4), 1115–1127. doi:10.1053/j.gastro.2010.08.023
Keywords: proton pump inhibitors, gastric cancer, meta-analysis, gastric mucosal atrophy, intestinal metaplasia, enterochromaffin-like cell, gastric polyps
Citation: Zheng Z, Lu Z and Song Y (2023) Long-term proton pump inhibitors use and its association with premalignant gastric lesions: a systematic review and meta-analysis. Front. Pharmacol. 14:1244400. doi: 10.3389/fphar.2023.1244400
Received: 22 June 2023; Accepted: 14 August 2023;
Published: 25 August 2023.
Edited by:
Patricia Moriel, State University of Campinas, BrazilReviewed by:
Prince Kasongo Mwila, University of the Witwatersrand, South AfricaXiao Li, Shandong Provincial Qianfoshan Hospital, China
Helge Waldum, Norwegian University of Science and Technology, Norway
Copyright © 2023 Zheng, Lu and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zeyi Zheng, enp5Nzk4MzQzMTA1QG91dGxvb2suY29t
 Zeyi Zheng
Zeyi Zheng Ziyu Lu
Ziyu Lu Yani Song
Yani Song