
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pharmacol., 31 August 2023
Sec. Pharmacology of Anti-Cancer Drugs
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1242491
This article is part of the Research TopicAdvances in Drug-induced Diseases Volume IIView all 45 articles
Zanubrutinib is a Bruton tyrosine kinase (BTK) inhibitor used in B cell malignancy treatment and is generally well tolerated in most patients. Zanubrutinib-induced aseptic meningitis is currently not reported. Herein, we present the first case of zanubrutinib-induced aseptic meningitis. A 33-year-old woman was diagnosed with relapsed/refractory follicular lymphoma and subsequently developed aseptic meningitis after receiving zanubrutinib treatment. We reviewed the literature and uncovered the lack of current reports on zanubrutinib or other BTK inhibitor-induced aseptic meningitis. Moreover, we summarized cases on aseptic meningitis induced by common chemotherapy and targeted drugs used for hematological diseases. Drug-induced aseptic meningitis (DIAM) is a drug-induced meningeal inflammation. The possible pathogenesis is the direct stimulation of the meninges via intrathecal injection of chemotherapy drugs and immune hypersensitivity response caused by immunosuppressive drugs. It is more common in women with immune deficiency and mainly manifests as persistent headache and fever. Cerebrospinal fluid examinations mainly demonstrate a significant increase in cells and proteins. DIAM diagnosis needs to exclude bacterial, fungal, viral, and tuberculosis infections; neoplastic meningitis; and systemic diseases involving the meninges. The prognosis of DIAM is usually favorable, and physicians should detect and stop the causative drug. In conclusion, zanubrutinib-induced aseptic meningitis is a rare but serious complication, and physicians should be promptly aware of this adverse event to avoid serious consequences.
Bruton tyrosine kinase (BTK) is a nonreceptor kinase that plays a crucial role in oncogenic signaling for leukemic cell proliferation and survival in multiple B cell malignancies (Pal Singh et al., 2018). BTK inhibitors form a covalent bond with a cysteine residue (Cys-481) in the kinase domain to inactivate BTK, which further restrains the B-cell antigen receptor pathway activation and blocks malignant B-cell proliferation and survival (Kim, 2019). Zanubrutinib is a next-generation BTK inhibitor that has presented promising antitumor activities in both preclinical models and clinical studies (Syed, 2020). Zanubrutinib monotherapy is generally well tolerated in patients with B cell malignancies. A pooled safety analysis of zanubrutinib reports that the common grade of ≥ 3 nonhematologic treatment-emergent adverse events (AEs) (≥ 2%) are pneumonia, hypertension, upper respiratory tract infection, urinary tract infection, sepsis, diarrhea, and musculoskeletal pain (Tam et al., 2022). A grade of ≥ 3 headache is reported in 1% of patients and with no reports of severe AEs of the central nervous system (CNS). Herein, we report the first case of zanubrutinib-induced aseptic meningitis and performed a literature review of drug-induced aseptic meningitis (DIAM) in hematological diseases. We searched PubMed for articles containing the words “zanubrutinib,” “ibrutinib,” “acalabrutinib,” “tirabrutinib,” “orelabrutinib,” “pirtobrutinib,” “nemtabrutinib,” “Bruton tyrosine kinase inhibitors,” “rituximab,” “cytarabine,” “methotrexate,” “apolizumab,” “dasatinib,” “RG7356,” “daratumumab,” “alemtuzumab,” and “aseptic meningitis” from inception up to May 2023 with no language restrictions.
A 33-year-old Chinese woman presented to our department with the chief complaint of headache, fever, tremors, and unstable gait for 10 days in November 2020. She had a known history of relapsed/refractory follicular lymphoma (FL) for 2 years. Two years ago, she presented with multiple lymph node enlargement and splenomegaly a few months after giving birth. She had no symptoms of fever, night sweats, and weight loss. She visited a local hospital. The left armpit lymph node biopsy indicated FL (grade II). Positron emission tomography/computed tomography (PET/CT) revealed lymph node enlargement of ≥3 cm diameter and increased β-2-[18F]-Fluoro-2-deoxy-D-glucose (FDG) uptake in the neck, armpits, mediastinum, abdominopelvic cavity, and groin. Additionally, the FDG uptake was increased in both breasts, spleen, and multiple bones. Therefore, she was diagnosed with FL (stage IV, grade 2, group A, FLIPI 3 points). Considering the history of giving birth, she received four courses of R2 chemotherapy (rituximab and lenalidomide) for 4 months at the local hospital. However, remission was not achieved; thus, she was prescribed four courses of standard R-CHOP chemotherapy (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) for 8 months, and she achieved partial remission. Afterward, she received maintenance therapy with rituximab every 2 months. However, 5 months ago, she experienced recurrent lymphadenopathy and hepatosplenomegaly, and she visited our hospital. The PET/CT confirmed lymphoma relapse. Considering that new targeted drugs, including obinutuzumab and phosphoinositide 3-kinase inhibitors, have not yet been marketed in China and this patient could not be a candidate in the clinical trial of FL at our hospital because of hepatitis B virus infection, she was scheduled to receive zanubrutinib treatment (560 mg per day) after obtaining informed consent. She re-achieved partial remission after taking zanubrutinib for 4 months, and oral zanubrutinib was continued. However, 10 days ago, the patient developed neurological symptoms, including headache, fever, hand tremors, and unsteady gait. The laboratory examination revealed negative results for serum procalcitonin, C-reactive protein (1,3)-β-D-glucan and galactomannan test, tuberculosis antibody, and interferon-gamma release assay. Then, a lumbar puncture was performed, and the opening pressure of cerebrospinal fluid (CSF) was 210 mmH2O. The cell count, protein, and glucose were 300 × 106/L (normal range: 0–10 × 106/L), 690 mg/dL (normal range: 15–45 mg/dL), and 42.84 mg/dL (normal range: 45–79.2 mg/dL), respectively (Figure 1A). The ink stain, herpesvirus type II DNA, mycobacterium tuberculosis DNA, tuberculosis antibody, culture of bacteria, fungi, and mycobacterium, exfoliated cells, metagenome, autoimmune encephalitis-related antibodies, paraneoplastic syndrome-related antibodies, and flow cytometry were negative. Brain magnetic resonance imaging (MRI) enhanced scan illustrated meninges thickening, cistern narrowing, third ventricle and bilateral lateral ventricle enlargement and hydrocephalus, and CSF exudation (Figures 1B, C). Zanubrutinib-induced aseptic meningitis diagnosis was highly suspected based on clinical manifestations and laboratory findings. Therefore, zanubrutinib was stopped and mannitol (125 mL, once every 12 h) was given for dehydration. The patient’s neurological symptoms significantly improved, and headache, fever, hand tremors, and unsteady gait disappeared after stopping zanubrutinib for 2 months. The cell count and protein by the CSF analysis significantly decreased (Figure 1A). Enhanced brain MRI scanning revealed that the enhancement degree of intracranial pia mater and hydrocephalus was reduced (Figures 1D, E). We used another BTK inhibitor orelabrutinib to fight the lymphoma and the patient was well tolerated 4 months later. Up to now, the patient’s lymphoma has been well controlled, and no CNS symptoms have reappeared after 3 years of follow-up. The therapeutic course is summarized in Figure 1F.
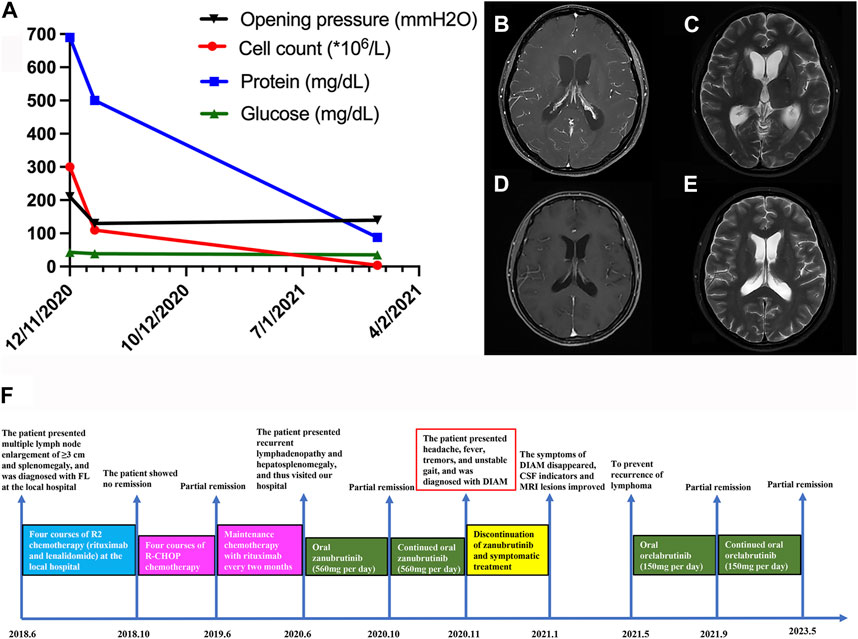
FIGURE 1. (A) Changes in various cerebrospinal fluid indicators before and after zanubrutinib discontinuation in the patient (B–C) Brain MRI of the patient before zanubrutinib discontinuation. (B) T1 enhancement; (C) T2. (D–E) Brain MRI after the patient stopped using zanubrutinib for 4 months. (D) T1 enhancement; (E) T2. (F) Detailed time course of the patient’s clinical course and therapeutic regimen.
Herein, we report a Chinese female patient with relapsed/refractory FL who developed aseptic meningitis after receiving zanubrutinib treatment for 4 months. The diagnosis of zanubrutinib-induced aseptic meningitis was established in our case according to the temporal association between zanubrutinib exposure and the development of CNS symptoms, clinical and laboratory findings, and typical MRI of meningitis. Currently, different BTK inhibitors, including ibrutinib, acalabrutinib, zanubrutinib, tirabrutinib, orelabrutinib, pirtobrutinib, and nemtabrutinib, have been approved or used in the later stage of clinical development for B cell malignancy treatment, such as mantle cell lymphoma, chronic lymphocytic leukemia/small lymphocytic lymphoma, Waldenström’s macroglobulinemia, and FL. Among these BTK inhibitors, except for acalabrutinib, which is prone to causing headaches, other BTK inhibitors rarely cause CNS symptoms (Lipsky and Lamanna, 2020). To the best of our knowledge, no cases of zanubrutinib- or other BTK inhibitor-induced aseptic meningitis have been reported, and this is the first case of zanubrutinib-induced aseptic meningitis. We reviewed the literature on the pathogenesis, clinical manifestations, laboratory test and imaging, diagnosis, differential diagnosis, and management of DIAM.
In DIAM, drug intake history is crucial because no specific characteristics are associated with a specific drug. Nonsteroidal anti-inflammatory drugs, intravenous immunoglobulins, antibiotics, monoclonal antibodies, anticonvulsants, vaccines, and intrathecal drugs are common causes of DIAM (Yelehe-Okouma et al., 2018). To date, no study on the mechanism of BTK inhibitor-induced aseptic meningitis has been published. The mechanisms of aseptic meningitis caused by other drugs may include direct meningeal irritation by intrathecal drug injection and immunologic hypersensitivity reaction caused by systemic administration (Yelehe-Okouma et al., 2018). We speculate that the mechanism of zanubrutinib-induced aseptic meningitis may be the immune hypersensitivity response caused by zanubrutinib that inhibits the activation of the B-cell antigen receptor pathway. Moreover, the patient did not develop DIAM with orelabrutinib; thus, we speculated whether it was related to the differences in CNS permeability between different BTK inhibitors. Currently, no study has compared head-to-head CSF concentrations under treatment with different BTK inhibitors. Three studies have reported that CSF concentrations of three BTK inhibitors (ibrutinib, zanubrutinib, and orelabrutinib) were 1.65, 2.94, and 20.10 ng/mL, respectively (Grommes et al., 2017; Song et al., 2021; Zhang et al., 2021). Therefore, high CSF concentrations of both zanubrutinib and orelabrutinib indicate that DIAM may not be related with the differences in CNS penetration between the different BTK inhibitors. In addition, BTK is the only kinase targeted by orelabrutinib (with >90% inhibition) (Dhillon, 2021), which may explain that aseptic meningitis developed with zanubrutinib rather with orelabrutinib. DIAM symptoms mainly include headache, fever, neck stiffness, nausea, photophobia, disoriented, and short-term memory deficits. The CSF analysis showed high cell and protein and normal glucose levels. MRI of the brain appeared to be normal or displayed signs of meningitis. DIAM is an exclusionary diagnosis, which requires exclusions of bacterial, fungal, viral, and tuberculosis infections, neoplastic meningitis, and systemic diseases involving the meninges (Tattevin et al., 2019). DIAM should be distinguished from these diseases, and we summarized the etiology, clinical features, CSF characteristics, treatment, and prognosis of these diseases in Table 1, which helps the doctors detect and identify DIAM promptly in clinical practice. Overall, no significant difference was found in the clinical symptoms between DIAM and other meningitis. The CSF analysis of DIAM showed high cell count, which may be confused with infectious meningitis and neoplastic meningitis. However, the CSF of DIAM does not present any pathogens or tumor cells, which can be distinguished from infectious meningitis and tumor meningitis. DIAM management mainly includes discontinuing the causative drugs and symptomatic treatments. Most patients can recover after stopping medication for approximately 1 week. Physicians must recognize and accurately discontinue relevant pathogenic drugs. Our patient with FL received immunosuppressive therapy, and CSF examination demonstrated a significant increase in cell and protein levels as previously reported. However, her glucose level was slightly lower than normal, and the brain MRI revealed typical signs of meningitis. In addition, her symptoms significantly improved after discontinuing zanubrutinib for approximately 2 months, which was longer than that previously reported.
Despite the lack of studies reporting aseptic meningitis induced by zanubrutinib and other BTK inhibitors except for our case, BTK inhibitors may cause some other serious and rare CNS AEs, including progressive multifocal encephalopathy (PML) and posterior reversible encephalopathy syndrome (PRES) (Zukas and Schiff, 2018). The mechanism may be associated with impaired cellular immunity caused by BTK inhibitors (Fugate and Rabinstein, 2015; Tattevin et al., 2019; Cortese et al., 2021; Zou et al., 2023). To distinguish aseptic meningitis from other rare complications induced by BTK inhibitors, Table 2 summarizes the pathogenesis, clinical manifestations, imaging features, CSF analysis, diagnostic criteria, treatment strategies, and prognosis of these three rare CNS complications to provide information regarding their management. PML is caused by the reactivation of John Cunningham polyoma virus in the presence of cellular immune impairment. PRES involves perfusion imbalance and reversible vascular edema. DIAM is caused by T cell-mediated immune hypersensitivity and meningeal direct inflammation caused by intrathecal injection of antimetabolic drugs. These three complications occur following immunosuppressive drug therapy, and physicians should promptly identify these rare complications and discontinue relevant suspected immunosuppressive drugs.
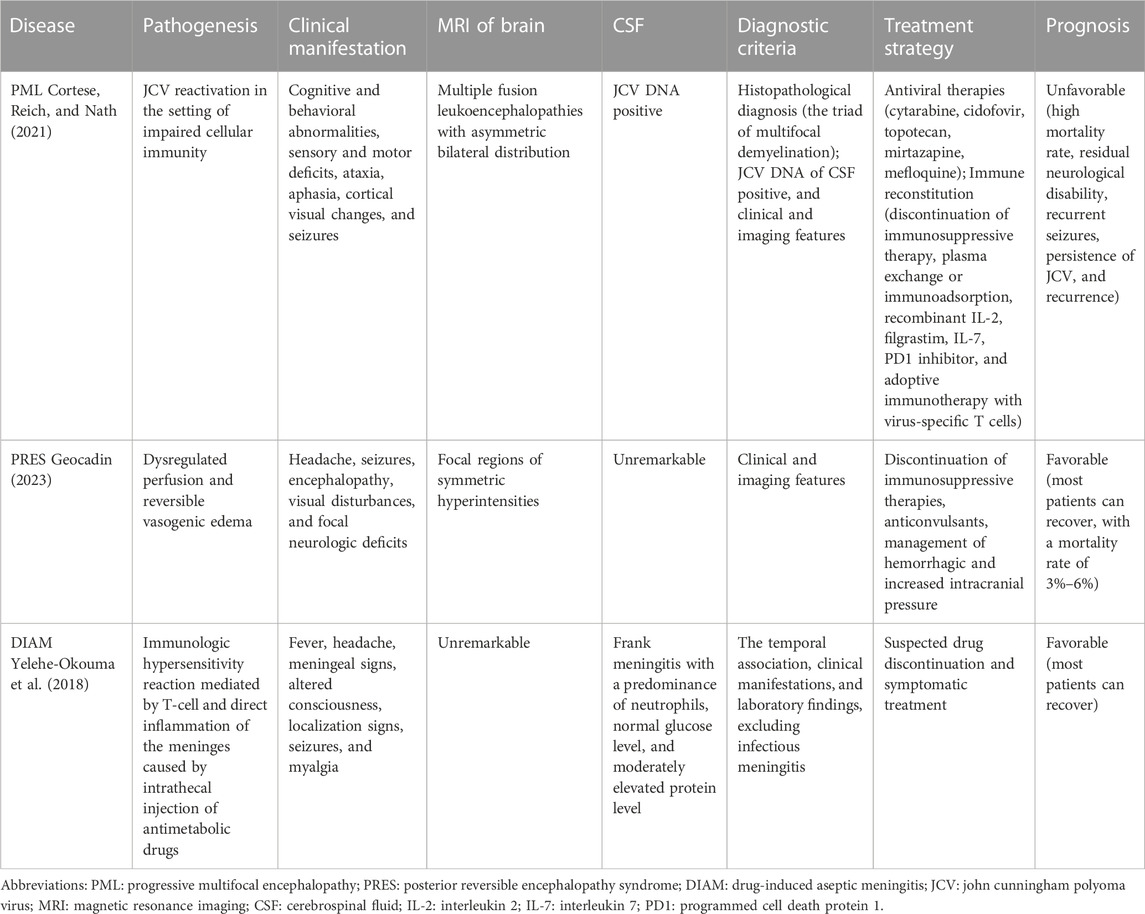
TABLE 2. Clinical characteristics of rare central nervous system complications induced by drugs in hematological malignancies.
In addition, we reviewed the literature on common chemotherapy and targeted drugs that can induce aseptic meningitis in hematological diseases (Table 3). Currently, except for the case reported herein, 10 cases of DIAM have been reported, including a total of 13 patients (Thordarson and Talstad, 1986; Flasshove et al., 1992; van den Berg et al., 2001; Pease et al., 2001; Lin et al., 2009; Imataki et al., 2014; Vey et al., 2016; Reddy et al., 2018; Kako et al., 2019; Beaumont and Suner, 2020). The median patient age is 33 (interquartile range, 20.3–56.8) years, and the female patients are dominant (7/11, 63.6%, sexes of two patients were not reported). The primary diseases mainly include acute lymphoblastic leukemia (6/13, 46.2%), acute myeloid leukemia (3/13, 23.1%), chronic lymphoblastic leukemia (1/13, 7.7%), multiple myeloma (1/13, 7.7%), severe aplastic anemia (1/13, 7.7%), and FL (1/13, 7.7%). The associated drugs include cytarabine, apolizumab, dasatinib, daratumumab, alemtuzumab, methotrexate, and RG7356. The administration routes include intravenous infusion, intrathecal and subcutaneous injections, and oral administration. Hematologists should be vigilant for the occurrence of DIAM when these drugs are administered.
This study has some limitations. First, zanubrutinib was not rechallenged to deeply determine this adverse reaction in our patient. Additionally, this is the first case of zanubrutinib-induced aseptic meningitis, and further observation and the mechanism of zanubrutinib-induced aseptic meningitis should be explored in the future.
Zanubrutinib-induced aseptic meningitis should be considered a potentially serious adverse drug reaction, and whether other BTK inhibitors can cause aseptic meningitis remains unclear. Physicians, especially hematologists, should be aware of this potential AE. Relevant suspicious drugs should be promptly and effectively discontinued when suspected of DIAM because the symptoms of DIAM are severe and most patients can quickly recover after discontinuing the causative drugs.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JY and LW did the literature search and drafted the manuscript. XZ contributed to the imaging analysis. YW and CY conceived the case report and provided guidance for the drafting of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by 1•3•5 project for disciplines of excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University; Sichuan Provincial Academic and Technical Support Funding Project, Grant/Award Number: 2022YFS0191.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Beaumont, A. L., and Suner, L. (2020). Drug-induced aseptic meningitis in a Ph+ ALL patient with meningeal involvement. Blood 136 (4), 520. doi:10.1182/blood.2019004195
Bystritsky, R. J., and Chow, F. C. (2022). Infectious meningitis and encephalitis. Neurol. Clin. 40 (1), 77–91. doi:10.1016/j.ncl.2021.08.006
Cortese, I., Reich, D. S., and Nath, A. (2021). Progressive multifocal leukoencephalopathy and the spectrum of JC virus-related disease. Nat. Rev. Neurol. 17 (1), 37–51. doi:10.1038/s41582-020-00427-y
Dhillon, S. (2021). Orelabrutinib: first approval. Drugs 81 (4), 503–507. doi:10.1007/s40265-021-01482-5
Flasshove, M., Schütte, H. J., Kellner, R., Höffken, K., and Seeber, S. (1992). Meningeal fluid granulocytosis after cytarabine. Eur. J. Cancer 28 (1), 243. doi:10.1016/0959-8049(92)90418-2
Fugate, J. E., and Rabinstein, A. A. (2015). Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 14 (9), 914–925. doi:10.1016/s1474-4422(15)00111-8
Geocadin, R. G. (2023). Posterior reversible encephalopathy syndrome. N. Engl. J. Med. 388 (23), 2171–2178. doi:10.1056/NEJMra2114482
Grommes, C., Pastore, A., Palaskas, N., Tang, S. S., Campos, C., Schartz, D., et al. (2017). Ibrutinib unmasks critical role of Bruton tyrosine kinase in primary CNS lymphoma. Cancer Discov. 7 (9), 1018–1029. doi:10.1158/2159-8290.Cd-17-0613
Gundamraj, V., and Hasbun, R. (2023). Viral meningitis and encephalitis: an update. Curr. Opin. Infect. Dis. 36 (3), 177–185. doi:10.1097/qco.0000000000000922
Hasbun, R. (2022). Progress and challenges in bacterial meningitis: A review. Jama 328 (21), 2147–2154. doi:10.1001/jama.2022.20521
Imataki, O., Arai, T., Yamaoka, G., Matsuoka, A., and Uemura, M. (2014). NKT cell-infiltrating aseptic meningitis on the central nervous system in Philadelphia chromosome-positive acute lymphoblastic leukemia treated with dasatinib. Ann. Hematol. 93 (11), 1935–1936. doi:10.1007/s00277-014-2074-4
Kako, S., Gomyo, A., Akahoshi, Y., Harada, N., Kameda, K., Ugai, T., et al. (2019). Haploidentical transplantation using low-dose alemtuzumab: comparison with haploidentical transplantation using low-dose thymoglobulin. Eur. J. Haematol. 102 (3), 256–264. doi:10.1111/ejh.13204
Kim, H. O. (2019). Development of BTK inhibitors for the treatment of B-cell malignancies. Arch. Pharm. Res. 42 (2), 171–181. doi:10.1007/s12272-019-01124-1
Lin, T. S., Stock, W., Xu, H., Phelps, M. A., Lucas, M. S., Guster, S. K., et al. (2009). A phase I/II dose escalation study of apolizumab (Hu1D10) using a stepped-up dosing schedule in patients with chronic lymphocytic leukemia and acute leukemia. Leuk. Lymphoma 50 (12), 1958–1963. doi:10.3109/10428190903186486
Lipsky, A., and Lamanna, N. (2020). Managing toxicities of Bruton tyrosine kinase inhibitors. Hematol. Am. Soc. Hematol. Educ. Program 2020 (1), 336–345. doi:10.1182/hematology.2020000118
Mount, H. R., and Boyle, S. D. (2017). Aseptic and bacterial meningitis: evaluation, treatment, and prevention. Am. Fam. Physician 96 (5), 314–322.
Pal Singh, S., Dammeijer, F., and Hendriks, R. W. (2018). Role of Bruton's tyrosine kinase in B cells and malignancies. Mol. Cancer 17 (1), 57. doi:10.1186/s12943-018-0779-z
Pease, C. L., Horton, T. M., McClain, K. L., and Kaplan, S. L. (2001). Aseptic meningitis in a child after systemic treatment with high dose cytarabine. Pediatr. Infect. Dis. J. 20 (1), 87–89. doi:10.1097/00006454-200101000-00022
Reddy, K., Htut, M., Krishnan, A., and Dadwal, S. S. (2018). Aseptic meningitis as a complication of daratumumab therapy. Clin. Lymphoma Myeloma Leuk. 18 (8), e333–e335. doi:10.1016/j.clml.2018.05.018
Song, Y., Deng, L., Zhang, B., Luo, H., and Zhao, R. (2021). “Preliminary results of orelabrutinib concentrations in peripheral blood and cerebrospinal fuid in patients with relapsed/refractory primary or secondary CNS lymphoma,” in The 24th national congress of clinical oncology.
Syed, Y. Y. (2020). Zanubrutinib: first approval. Drugs 80 (1), 91–97. doi:10.1007/s40265-019-01252-4
Tam, C. S., Dimopoulos, M., Garcia-Sanz, R., Trotman, J., Opat, S., Roberts, A. W., et al. (2022). Pooled safety analysis of zanubrutinib monotherapy in patients with B-cell malignancies. Blood Adv. 6 (4), 1296–1308. doi:10.1182/bloodadvances.2021005621
Tattevin, P., Tchamgoué, S., Belem, A., Bénézit, F., Pronier, C., and Revest, M. (2019). Aseptic meningitis. Rev. Neurol. Paris. 175 (7-8), 475–480. doi:10.1016/j.neurol.2019.07.005
Thordarson, H., and Talstad, I. (1986). Acute meningitis and cerebellar dysfunction complicating high-dose cytosine arabinoside therapy. Acta Med. Scand. 220 (5), 493–495. doi:10.1111/j.0954-6820.1986.tb02801.x
van den Berg, H., van der Flier, M., and van de Wetering, M. D. (2001). Cytarabine-induced aseptic meningitis. Leukemia 15 (4), 697–699. doi:10.1038/sj.leu.2402063
Vey, N., Delaunay, J., Martinelli, G., Fiedler, W., Raffoux, E., Prebet, T., et al. (2016). Phase I clinical study of RG7356, an anti-CD44 humanized antibody, in patients with acute myeloid leukemia. Oncotarget 7 (22), 32532–32542. doi:10.18632/oncotarget.8687
Yelehe-Okouma, M., Czmil-Garon, J., Pape, E., Petitpain, N., and Gillet, P. (2018). Drug-induced aseptic meningitis: A mini-review. Fundam. Clin. Pharmacol. 32 (3), 252–260. doi:10.1111/fcp.12349
Zhang, Y., Li, Y., Zhuang, Z., Wang, W., Wei, C., Zhao, D., et al. (2021). Preliminary evaluation of zanubrutinib-containing regimens in dlbcl and the cerebrospinal fluid distribution of zanubrutinib: A 13-case series. Front. Oncol. 11, 760405. doi:10.3389/fonc.2021.760405
Zou, X., Zhou, P., Lv, W., Liu, C., and Liu, J. (2023). Posterior reversible encephalopathy syndrome after anlotinib treatment for small cell lung cancer: A case report and literature review. Front. Pharmacol. 14, 1126235. doi:10.3389/fphar.2023.1126235
Keywords: Zanubrutinib, bruton tyrosine kinase, follicular lymphoma, aseptic meningitis, hematological diseases
Citation: Yang J, Wang L, Zhong X, Yang C and Wu Y (2023) Zanubrutinib-induced aseptic meningitis: a case report and literature review. Front. Pharmacol. 14:1242491. doi: 10.3389/fphar.2023.1242491
Received: 19 June 2023; Accepted: 21 August 2023;
Published: 31 August 2023.
Edited by:
Liren Qian, Fifth Medical Center of the PLA General Hospital, ChinaReviewed by:
Yao Liu, Daping Hospital, ChinaCopyright © 2023 Yang, Wang, Zhong, Yang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenlu Yang, NjcyNTE2MzQyQHFxLmNvbQ==; Yu Wu, d3VfeXVAc2N1LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.