
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
GENERAL COMMENTARY article
Front. Pharmacol. , 16 August 2023
Sec. Pharmacoepidemiology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1241524
This article is a commentary on:
Adverse Event Profiles of PARP Inhibitors: Analysis of Spontaneous Reports Submitted to FAERS
A Commentary on
Adverse event profiles of PARP inhibitors: analysis of spontaneous reports submitted to FAERS
by Tian X, Chen L, Gai D, He S, Jiang X and Zhang N (2022). Front Pharmacol. 13:851246. doi: 10.3389/fphar.2022.851246
Poly(ADP-ribose) polymerase inhibitors (PARPis) are effective treatments in cancers associated with underlying homologous recombination deficiency. Understanding and accurately characterizing their safety profiles is essential to provide comprehensive information for optimal patient care.
Tian et al. endeavored to characterize the safety profiles of four PARPis (niraparib, olaparib, rucaparib, and talazoparib) in a disproportionality analysis using the US FDA’s Adverse Event Reporting System (FAERS) database (Tian et al., 2022). They identified 24,141 FAERS reports listing PARPis as a primary/secondary suspect and calculated reporting odds ratios (RORs) for multiple adverse events (AEs) based on Medical Dictionary for Regulatory Activities (MedDRA) preferred terminology. Duplicate reports were removed based on identification number, and cases/non-cases were represented by AEs mentioning PARPis as suspected versus all other AEs.
As part of our standard review to monitor the safety of our marketed product (niraparib [Zejula]), GSK noted the high ROR between lymphangioleiomyomatosis (LAM) and niraparib (ROR = 471.20) reported in the article by Tian et al. Extreme RORs can be misleading and merit further scrutiny because of the potential volatility of disproportionality scores (A. Bate, PhD, GSK, written communication, 26 April 2023). As such, the high ROR for LAM warranted further investigation, and several limitations should be considered to appropriately contextualize the data. Notably, our investigation identified inadequacies in deduplication efforts, leading to erroneous results.
FAERS is a large, publicly available database of spontaneous AE reports, medication error reports, and product quality complaints designed to support postmarketing safety surveillance (US FDA, 2018; Guo et al., 2022; Khaleel et al., 2022). Reports are based on suspected associations and may name multiple medications (Almenoff et al., 2005). FAERS and other spontaneous-reporting systems have well-known limitations, including incomplete data, report duplication, lack of a denominator to estimate population-based incidence, and lack of a proven, causal relationship between drugs and reported events; therefore, findings from studies leveraging FAERS data may be subject to multiple biases (Almenoff et al., 2005; Sakaeda et al., 2013; US FDA, 2018; Khaleel et al., 2022). Nevertheless, FAERS is a publicly available, well-accepted and essential safety surveillance tool, and implementation of best practices around data integrity, research methodology, and transparency are critical for accuracy and credibility.
FAERS collects reports from healthcare professionals, consumers, and manufacturers (US FDA, 2018). Individuals who have observed, heard about, or suspect they have experienced an adverse drug reaction may provide spontaneous AE reports, and multiple sources may report the same incident (Almenoff et al., 2005). Thus, duplicate reports are a significant limitation of FAERS (Hauben et al., 2007; US FDA, 2018), and thorough deduplication is a prerequisite for all analyses (Khaleel et al., 2022). As deduplication is often complicated by incomplete event records, detailed interrogation is recommended, including visual comparison of data (Hauben et al., 2007; Hauben et al., 2021). Duplicate reports compromise signal detection in disproportionality analyses and may result in an erroneously large signal of disproportionality (Hauben et al., 2021). Tian et al. report excluding duplicates based on identification number. When approached for clarification, the authors responded but declined to share additional information on their findings and deduplication processes. Unfortunately, exclusion based on identification number alone would be insufficient, as demonstrated below.
Tian et al. identified 16 reports of LAM associated with niraparib from the FAERS dataset (December 2014–October 2021), leading to an ROR of 471.20. No cases identified were associated with the other PARPis. LAM is a rare, progressive, systemic disease characterized by cystic lung destruction with a median prevalence of 4.9 per million women across seven countries (Harknett et al., 2011). Because LAM has not previously been associated with PARPis, the finding warranted further investigation.
Compared with the 16 reports identified by Tian et al., our search of the FAERS Public Dashboard identified 14 reports (accessed via the FDA Adverse Event Reporting System [FAERS] Public Dashboard). Following Commonwealth Vigilance Workbench system automated deduplication (CVW Data Mining Build 6.0.2.60) of the FAERS dataset through 1 April 2022, only six reports were identified. Commonwealth uses a quantitative method to identify pairs of duplicate case reports. This method is based on the “hit-miss” statistical algorithm described by Norén et al. in their article about duplicate detection (Norén et al., 2007). To investigate further, we obtained case information for the six FAERS reports through a Freedom of Information Act request (FDA FOIA Request Form). Available data from all six FAERS cases for event date, age, body weight, country, niraparib start/end dates, niraparib lot number, and suspected and concomitant medications were identical, strongly suggesting that all six FAERS cases were duplicates of a single case (Table 1). We also searched the GSK Global Safety Database through 22 July 2022 for reports of patients receiving niraparib that contained the MedDRA preferred term of LAM and found one report. The available FAERS case data match details of the single LAM case reported in the GSK database.
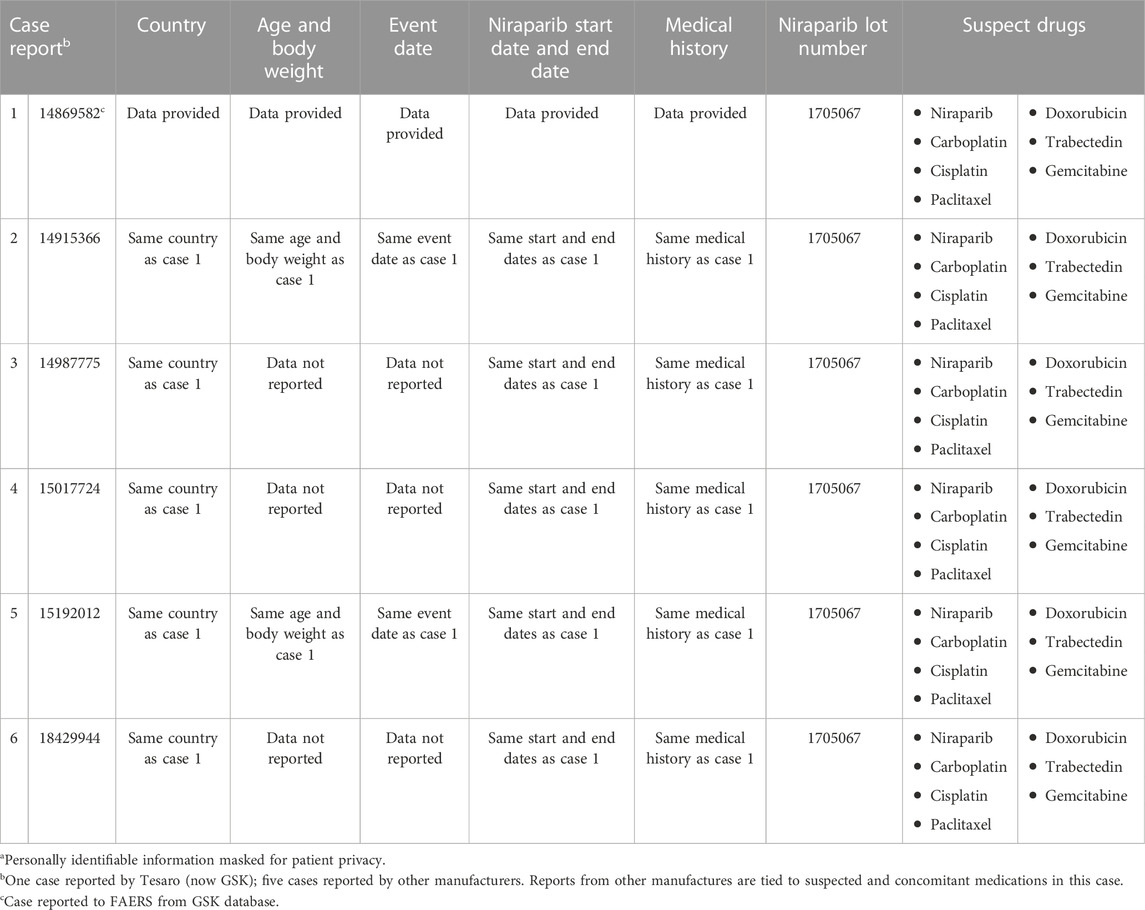
TABLE 1. Potential case duplication: identical data in the six cases of lymphangioleiomyomatosis reported in FAERSa.
We concluded that the ROR calculation from Tian et al. is likely erroneous because the presence of duplicate cases inflated the numerator to 16 instead of 1. With an observed count of 1, disproportionality scores are notoriously volatile, and use of Bayesian statistics are recommended to protect from oversensitivity (DuMouchel, 1999; Bate and Evans, 2009). This example of how duplicate reports can affect disproportionality analyses highlights the importance of medical review and clinical judgment when interpreting FAERS-based analyses. Additional review is particularly relevant with rare events for which deduplication is possible. Such results are best viewed as hypothesis generating (Almenoff et al., 2005; Bate and Evans, 2009).
Tian et al. concluded with a comparison of PARPi AE profiles; however, comparing products based on spontaneous AE data is not recommended (Almenoff et al., 2005; Bate and Evans, 2009). Beyond methodological challenges, variability in the dataset further limits product comparisons. Tian et al. used FAERS data from October 2014 through December 2021. While this period captures entry of multiple PARPis into the postmarketing setting, the length of commercial availability and, thus, the number of patients and duration of treatment, varied substantially between drugs. Reporting practices may also change over time (Bate and Evans, 2009).
Understanding the safety profile of niraparib and other PARPis is crucial for informed decision-making. While we acknowledge the contribution of Tian et al., it is critically important to deduplicate with rigor and interpret findings with caution, considering the limitations of the FAERS database and the methodological approaches. FAERS pharmacovigilance studies can offer important insights into the safety profiles of marketed medicinal products, and conducting these analyses with best practices and transparency is crucial for the generation of rigorous findings that meaningfully impact patients’ lives.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This general commentary article was supported by GSK. GSK was involved in the study design, data collection and analysis, general commentary preparation, and the decision to publish the general commentary.
The authors thank Dr. Ignace Vergote (University Hospitals Leuven, Leuven Cancer Institute, Leuven, Belgium) for his assistance in reviewing drafts of this commentary and Eric Smith (GSK) for his review and analytic insights. Medical writing and editorial assistance, funded by GSK (Waltham, Massachusetts) and coordinated by Hasan Jamal, MSc, and Prudence L. Roaf, MPH, of GSK, were provided by Betsy C. Taylor, PhD, CMPP, and Jennifer Robertson, PhD, of Ashfield MedComms, an Inizio company.
JS, AG, TB, GY, and JK are employees of GSK.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Almenoff, J., Tonning, J. M., Gould, A. L., Szarfman, A., Hauben, M., Ouellet-Hellstrom, R., et al. (2005). Perspectives on the use of data mining in pharmaco-vigilance. Drug Saf. 28 (11), 981–1007. doi:10.2165/00002018-200528110-00002
Bate, A., and Evans, S. J. (2009). Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 18 (6), 427–436. doi:10.1002/pds.1742
DuMouchel, W. (1999). Bayesian data mining in large frequency tables, with an application to the FDA spontaneous reporting system. Am. Stat. 53 (3), 177–190. doi:10.1080/00031305.1999.10474456
Guo, M., Shu, Y., Chen, G., Li, J., and Li, F. (2022). A real-world pharmacovigilance study of FDA adverse event reporting system (FAERS) events for niraparib. Sci. Rep. 12 (1), 20601. doi:10.1038/s41598-022-23726-4
Harknett, E. C., Chang, W. Y., Byrnes, S., Johnson, J., Lazor, R., Cohen, M. M., et al. (2011). Use of variability in national and regional data to estimate the prevalence of lymphangioleiomyomatosis. QJM 104 (11), 971–979. doi:10.1093/qjmed/hcr116
Hauben, M., Reich, L., DeMicco, J., and Kim, K. (2007). Extreme duplication' in the US FDA adverse events reporting system database. Drug Saf. 30 (6), 551–554. doi:10.2165/00002018-200730060-00009
Hauben, M., Zou, C., Bright, S., and Hung, E. (2021). More extreme duplication in the U.S. FDA FAERS database and a suggested check point for disproportionality analysis. Pharmacoepidemiol. Drug Saf. 30 (8), 1140–1141. doi:10.1002/pds.5265
Khaleel, M. A., Khan, A. H., Ghadzi, S. M. S., Adnan, A. S., and Abdallah, Q. M. (2022). A standardized dataset of a spontaneous adverse event reporting system. Healthc. (Basel) 10 (3), 420. doi:10.3390/healthcare10030420
Norén, G. N., Orre, R., Bate, A., and Edwards, I. R. (2007). Duplicate detection in adverse drug reaction surveillance. Data Min. Knowl. Discov. 14, 305–328. doi:10.1007/s10618-006-0052-8
Sakaeda, T., Tamon, A., Kadoyama, K., and Okuno, Y. (2013). Data mining of the public version of the FDA adverse event reporting system. Int. J. Med. Sci. 10 (7), 796–803. doi:10.7150/ijms.6048
Tian, X., Chen, L., Gai, D., He, S., Jiang, X., and Zhang, N. (2022). Adverse event profiles of PARP inhibitors: analysis of spontaneous reports submitted to FAERS. Front. Pharmacol. 13, 851246. doi:10.3389/fphar.2022.851246
US FDA (2018). Questions and answers on FDA's adverse event reporting system (FAERS). Available at: https://www.fda.gov/drugs/surveillance/questions-and-answers-fdas-adverse-event-reporting-system-faers (Accessed January 26, 2022).
Keywords: niraparib, PARP inhibitors, pharmacovigilance, FAERS, lymphangioleiomyomatosis
Citation: Schilder JM, Golembesky A, Boyle TAC, Ye GL and Kuplast J (2023) Commentary: Adverse event profiles of PARP inhibitors: analysis of spontaneous reports submitted to FAERS. Front. Pharmacol. 14:1241524. doi: 10.3389/fphar.2023.1241524
Received: 19 June 2023; Accepted: 04 August 2023;
Published: 16 August 2023.
Edited by:
Emanuel Raschi, University of Bologna, ItalyReviewed by:
Jae Hyun Kim, Jeonbuk National University, Republic of KoreaCopyright © 2023 Schilder, Golembesky, Boyle, Ye and Kuplast. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeanne M. Schilder, amVhbm5lLm0uc2NoaWxkZXJAZ3NrLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.