
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 11 January 2024
Sec. Renal Pharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1237583
Introduction: This study aimed to assess the tumor risk of finerenone in individuals with type 2 diabetes mellitus (T2DM) aggravated by chronic kidney disease (CKD).
Methods: A thorough search in the OVID Medline, OVID EMBASE, and Cochrane Library databases from their creation through 2 November 2022 yielded randomized controlled trials (RCTs) reporting on the tumor risks of finerenone in patients with T2DM complicated with CKD. A pair of reviewers selected the relevant studies based on selection criteria, collected data, and assessed the methodological quality of eligible RCTs. The Peto odds ratio (OR) with a 95% confidence interval (CI) was calculated, and subgroup analysis of tumor nature, tumor origin system, tumor origin organ, and follow-up time was performed. Furthermore, Egger’s test was implemented to determine publication bias.
Results: Four RCTs with 14,875 participants who had a low-to-moderate risk of bias were included. Compared with placebo treatment, finerenone did not increase the risk of overall neoplasms (Peto OR = 0.97; 95% CI, 0.83–1.14), malignant neoplasms (Peto OR = 1.03; 95% CI, 0.86–1.23), benign neoplasms (Peto OR = 0.94; 95% CI, 0.50–1.80), or in situ neoplasms (Peto OR = 0.14; 95% CI, 0.01–2.17). Subgroup analysis of the tumor origin system showed that finerenone was associated with an increased risk of malignant neoplasms of urinary tract compared with placebo treatment (Peto OR = 1.69; 95% CI, 1.07–2.67). The results were found to be robust in sensitivity analysis, and there was no indication of publication bias.
Discussion: Finerenone is not associated with an increased risk of overall tumors, but it may be linked to an increased risk of malignant neoplasms in urinary tract. Additional well-planned cohort studies in larger research populations are needed to corroborate these findings.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022374101, Identifier CRD42022374101.
Diabetes mellitus is a major chronic disease worldwide (Author Anyonomus, 2009). According to the latest data from the International Diabetes Federation, in 2021, 537 million adults worldwide aged 20–79 years have diabetes (i.e., approximately one in ten of the global population), and this number is expected to increase to reach 643 million by 2035 and 783 million by 2045 (https://diabetesatlas.org/). Diabetes is characterized by high blood glucose levels that can lead to microvascular and macrovascular diseases, which are the main causes of chronic kidney disease (CKD) (Hardin et al., 1956; Stratton et al., 2000; Sarwar et al., 2010). More importantly, type 2 diabetes mellitus (T2DM) is reported to be associated with an increased risk of cancer (Shlomai et al., 2016), which may be caused by hyperglycemia, hyperinsulinemia, and obesity. Chronically elevated endogenous insulin and/or IGF-1 levels can increase mitogenic signaling and promote tumor growth and metastasis (Renehan et al., 2008; Shlomai et al., 2016), placing a huge economic burden on the patient, their family, and society.
Significant increases in site-specific cancer risk in patients with T2DM have been reported, the most notable of which are risks of breast cancer [risk ratio (RR) = 1.20; 95% confidence interval (CI), 1.12–1.28], intrahepatic bile duct cancer (RR = 1.20; 95% CI, 1.57–2.46), colorectal cancer (RR = 1.27; 95% CI, 1.21–1.34), endometrial cancer (RR = 1.97; 95% CI, 1.71–2.27), hepatocellular carcinoma (RR = 2.43; 95% CI, 1.67–3.35), gallbladder cancer (RR = 1.73; 95% CI, 1.40–2.14), and pancreatic cancer (RR = 1.94; 95% CI, 1.66–2.27), except for the risk of localized prostate cancer (RR = 0.80; 95% CI, 0.70–0.90), which demonstrated the opposite effect (Tsilidis et al., 2015; Pearson-Stuttard et al., 2021). A 10-year prospective cohort study from Korea in 2005 showed that the incidence of cancer increased with blood glucose levels, with the highest corresponding increase being for pancreatic cancer in men [hazard ratio (HR) = 2.09; 95% CI, 1.70–2.58] and cervical cancer in women (HR = 2.20; 95% CI, 1.90–2.54). Fasting plasma glucose (FBG) ≥7.8 mmol/L was associated with higher mortality for all cancers (men: HR = 1.29; 95% CI, 1.22–1.37; women: HR = 1.23; 95% CI, 1.09–1.39) (Jee et al., 2005). Moreover, a duration of diabetes of more than 5 years and FBG ≥10.0 mmol/L were associated with increased cancer risks (HR = 2.35; 95% CI, 1.77–3.13) compared to those with an FBG <6.0 mmol/L (Shen et al., 2023).
Finerenone, a new type of nonsteroidal mineralocorticoid receptor (MR) antagonist, is a naphthyridine derivative developed based on the dihydropyridine structure and has been identified through the high-throughput screening of millions of compounds (Bärfacker et al., 2012). In the human body, 90% of finerenone is metabolized by cytochrome P450 (CYP) 3A4 in the intestinal wall and liver, with the remaining 10% being metabolized by CYP2C8 (Heinig et al., 2016; Gerisch et al., 2018; Heinig et al., 2018). Around 80% of its metabolites are excreted in the urine, while the remainder are excreted in the feces. In vivo studies have shown that most metabolites of finerenone in human plasma are naphthyridine derivatives (48.9% for M1; 21.5% for M2; and 9.0% for M3), and M1, M2, and M3 were not found to have pharmacological activity on human mineralocorticoid receptors (Heinig et al., 2016; Gerisch et al., 2018). Gerisch et al. (2018) found that the excretion rates of M1 were <1.5%, which was negligible, and that elimination of M2 and M3 occurred mainly through the kidneys. Urinary excretion of M2 and M3 decreases with renal function (Heinig et al., 2016). Finerenone is evenly distributed in the heart and kidneys (Kolkhof et al., 2014) and has a stereoscopic structure and side chain; thus, it can bind to the MR more completely and has a stronger MR-antagonistic effect than spironolactone (SPIR) and eplerenone (Bärfacker et al., 2012; Jaisser and Farman, 2016). Additionally, because finerenone has a low affinity for androgen and progesterone receptors, it has no negative effects associated with sex hormones (Bärfacker et al., 2012; Jaisser and Farman, 2016). Finerenone has strong anti-inflammatory and anti-fibrotic effects, inhibiting the progression of CKD and reducing the risk of cardiovascular events (Bakris et al., 2020; Pitt et al., 2021). Finerenone was approved by the US Food and Drug Administration in 2021 for the treatment of adult patients with T2DM compounded by CKD to postpone the ongoing decline in the estimated glomerular filtration rate, and reduce the risk of cardiovascular events (Frampton, 2021). Both the American Heart Association and the American Diabetes Association approved finerenone in 2022 to reduce the risk of cardiovascular events and slow the progression of renal disease, respectively (Draznin et al., 2022; Joseph et al., 2022).
Considering that T2DM is associated with increased cancer risk, there is still no evidence to determine whether treatment with finerenone affects the development of cancer. Therefore, this systematic review and meta-analysis were performed to illustrate the tumor risks of finerenone in patients with T2DM complicated by CKD using currently available evidence from randomized controlled trials (RCTs).
Meta-analysis and systematic review were conducted to assess the risk of tumor development in T2DM patients with CKD who were treated with finerenone (Moher et al., 2009). The study was registered on PROSPERO (CRD42022374101) and adhered to PRISMA guidelines for reporting.
The trial comprised patients (P) with T2DM and CKD, and the intervention (I) was finerenone at any dose or usage. The comparison (C) group could receive any treatment except for finerenone. The outcome (O) of interest was the occurrence of any type of tumor, regardless of its nature. Only RCTs were considered for inclusion in the study (S), while duplicates, letters, abstracts, and studies with irrelevant results were excluded.
OVID Medline, OVID EMBASE, and Cochrane Library databases were searched for relevant studies from their inception to 2 November 2022, using both MeSH terms and keywords with no language restrictions. Among the search terms were “finerenone” and “RCT”. The search strategy is outlined in detail in the supplementary material. Additionally, we manually searched reference lists of included studies and clinicaltrials.gov for potentially eligible studies. Human RCTs were included, while non-randomized trials, studies without control or placebo groups, animal studies, and in vitro studies were excluded. The focus was on human participants.
A team of reviewers (YD, GC, or LG) thoroughly examined the study selection, including preliminary screening of titles and abstracts as well as full-text reading. This was done in accordance with the study selection criteria, and any potentially relevant materials were identified through manual checks of reference lists and unpublished data from clinicaltrials.gov. Any disagreements that arose were resolved by a third reviewer (YC or JL).
Reviewers (YD, GC, or LG) gathered important information such as the registration number of the trial, date of publication or release, trial duration, number of tumors and participants, tumor nature, and location of origin. If there were any discrepancies, a third reviewer (YC or JL) was consulted to resolve the issue.
The Cochrane Collaboration tool was used to assess the risk of bias in the included RCTs (Higgins et al., 2011). Among the evaluation criteria were the generation of a random sequence, participant and personnel blinding, concealment of allocation, blinding of outcome assessment, insufficient outcome data, and selective reporting. To assess each item, a “yes” answer with a detailed description was considered low-risk, a “yes” answer without a detailed description was uncertain, and a “yes” answer with an inappropriate method or non-performance was considered high-risk. This overall evidence was used to determine the risk of bias in the studies. Two reviewers (YD and GC) assessed the risk of bias, and any discrepancies were resolved by a third reviewer (LG).
Data were combined with the RevMan software version 5.4. Considering the very few tumor events, the effect size was estimated using a Peto OR with a 95% CI (Bradburn et al., 2007; AHRQ Methods for Effective Health Care, 2008), because it performs well when dealing with sparse events (<1%) (Bradburn et al., 2007). I2 and heterogeneity p-values were applied to assess clinical diversity at a 0.1 level. The Cochrane Manual recommended that I2 values of 25%, 50%, and 75% indicate low, moderate, and high heterogeneity, respectively (Higgins et al., 2003). The subgroup analyses were carried out in accordance with tumor nature, tumor origin system, tumor origin organ, and follow-up duration, as specified beforehand. The robustness of the findings was examined using sensitivity analysis utilizing the Mantel-Haenszel random-effect model. To assess publication bias, Egger’s test was performed via STATA software, and p < 0.05 was considered significant. The International Classification of Diseases (ICD10) of the World Health Organization, 10th edition, was employed to classify tumor nature and originating system as follows: C00-C97 for malignant neoplasms, D10-D36 for benign neoplasms, D00-D009 for in situ neoplasms, and D37-D48 for neoplasms of uncertain or unknown behavior.
There are no ethical concerns or patient involvement in this systematic review and meta-analysis.
A total of 271 records were searched in electronic databases. After removing duplicates (n = 61), the title and abstract screening excluded unrelated records (n = 196). After reading the full texts, reports with no relevant outcomes (n = 3) or duplicate studies (n = 7) were excluded. Finally, four studies [NCT01807221 (Filippatos et al., 2016), NCT01874431 (Bakris et al., 2015), NCT02540993 (Bakris et al., 2020), and NCT02545049 (Pitt et al., 2021)] involving 14,875 patients with T2DM complicated with CKD were included (Figure 1). The manual review included no additional studies.
The included studies’ follow-up periods ranged from 3 to 40.8 months. The number of participants varied from 821 to 7,352. Approximately 70.75% of participants were men, and the mean patient age was 66.3 years, and the mean estimated glomerular filtration rate (eGFR, calculated with the use of the Chronic Kidney Disease Epidemiology Collaboration formula) of 58.2 mL per minute per 1.73 m2, and the most of CKD stages are 2–3. The common oral dosage of finerenone was 10 or 20 mg once daily (Table 1).
All studies correctly implemented the generation of a random sequence, participant and personnel blinding, concealment of allocation, and blinding of outcome assessment. None of the data were selectively reported in any of the studies. However, all four RCTs included in our study was funded by the same company that produced the drug (finerenone), which may have led to financial conflicts of interest (Figures 2, 3). Overall, there was a low-to-moderate risk of bias in the included trials.
To evaluate the robustness of the results, a sensitivity analysis was carried out using a variety of statistical techniques, such as the Mantel-Haenszel random-effect and Peto fixed-effect models. The results showed no change in the direction of the effect, suggesting that the results were robust. Owing to the relatively short number of included trials, we employed Egger’s test via STATA software to assess publication bias. We found no publication bias results (p > 0.05).
Four published RCTs reported the overall tumor risks of finerenone in patients with T2DM complicated by CKD and enrolled 14,875 patients with 615 tumors. Compared to placebo treatment, finerenone was not associated with an increased overall tumor risk (Peto OR = 0.97; 95% CI, 0.83–1.14; I2 = 0; 306/8,071 cases of cancer among patients treated with finerenone vs. 309/6,804 cases of cancer among those treated with placebo; Figure 4). The longer period of finerenone use did not increase neoplasm risk either (3 months: Peto OR = 3.37; 95% CI, 0.38–30.21; 6/1,561 vs. 0/315; 31.2 months: Peto OR = 1.08; 95% CI, 0.84–1.40; 125/2,827 vs. 116/2,831; 40.8 months: Peto OR = 0.90; 95% CI, 0.73–1.10; 175/3,683 vs. 193/3,658; Figure 5).
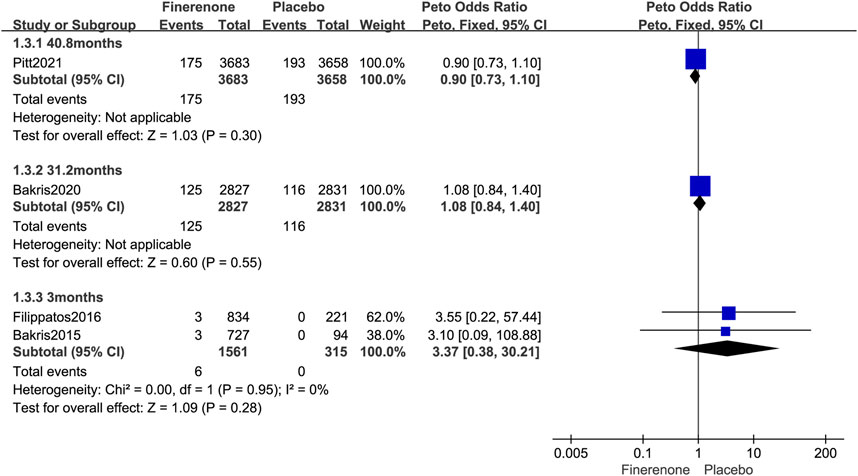
FIGURE 5. Forest plot of overall neoplasms at different follow-up times based on finerenone vs. placebo.
Four published RCTs involving 14,875 patients with T2DM complicated by CKD reported 503 malignancies (Peto OR = 1.03; 95% CI, 0.86–1.23, I2 = 0%; 257/8,071 vs. 246/6,804; Figure 6). Four RCTs reported 13 systems for malignant risks. Finerenone was associated with an increased risk of malignant neoplasms of urinary tract when compared to placebo (Peto OR = 1.69; 95% CI, 1.07–2.67; 47/8,071 vs. 27/6,804; Table 2). Subgroup analysis was performed according to the organ source of the malignancy, there was no statistically significant difference in the risk of malignant neoplasms of the urinary organs between finerenone and placebo (kidney, except renal pelvis: Peto OR = 1.54; 95% CI, 0.68–3.49; 14/8,071 vs. 9/6,804; bladder: Peto OR = 1.72; 95% CI, 0.93–3.16; 27/8,071 vs. 15/6,804; other and unspecified urinary organs: Peto OR = 1.94; 95% CI, 0.52–7.17; 6/8,071 vs. 3/6,804; Figure 8).
Further subgroup analysis was performed based on the follow-up time for malignant neoplasms. The usage of finerenone for a longer period of time did not increase the risk of malignant neoplasm (3 months: Peto OR = 3.33; 95% CI, 0.28–38.98; 5/1,561 vs. 0/315; 31.2 months: Peto OR = 1.13; 95% CI, 0.85–1.50; 103/2,827 vs. 92/2,831; 40.8 months: Peto OR = 0.96; 95% CI, 0.76–1.21; 149/3,683 vs. 154/3,658; Figure 7).
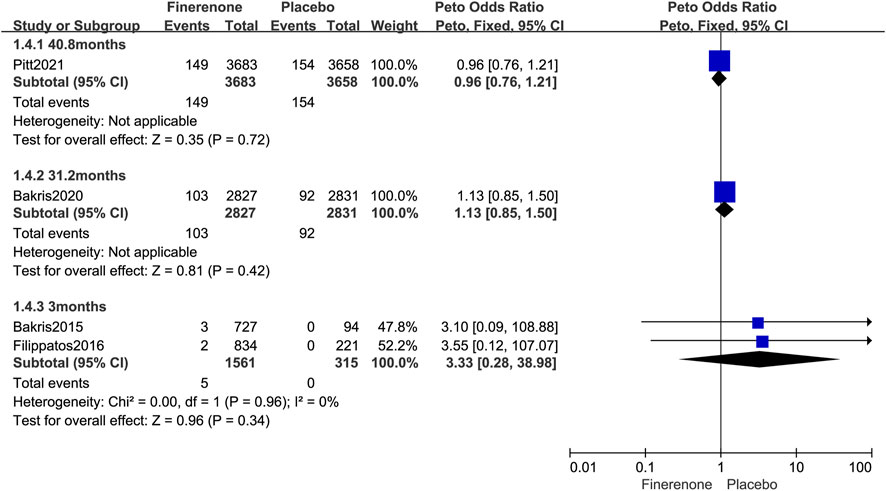
FIGURE 7. Forest plot of malignant neoplasms at different follow-up times based on finerenone vs. placebo.
Two studies (Bakris et al., 2020; Pitt et al., 2021) reported the occurrence of benign neoplasms. Compared with placebo, treatment with finerenone was not correlated with a higher incidence of benign neoplasms (Peto OR = 0.94; 95% CI, 0.50–1.80; I2 = 25%; 18/8,071 vs. 19/6,804; Figure 6).
Three studies (Filippatos et al., 2016; Bakris et al., 2020; Pitt et al., 2021) reported the occurrence of neoplasms with undetermined or unknown dynamics. Compared with placebo, finerenone was not correlated with higher tumor risks of uncertain or unknown behavior (Peto OR = 0.72; 95% CI, 0.46–1.15, I2 = 0%; 31/8,071 vs. 42/6,804; Figure 6).
Only one study (Bakris et al., 2020) reported the occurrence of neoplasms in situ, and the risks of in situ neoplasms were not higher with finerenone than with placebo (Peto OR = 0.14; 95% CI, 0.01–2.17; 0/8,071 vs. 2/6,804; Figure 6).
Our investigation is, as far as we are aware, the first systematic review and meta-analysis to evaluate the tumor risks of finerenone in patients with T2DM exacerbated by CKD. When compared to placebo, finerenone was not associated with increased risks of overall tumor (Peto OR = 0.97; 95% CI, 0.83–1.14), malignant neoplasms (Peto OR = 1.03; 95% CI, 0.86–1.23), benign neoplasms (Peto OR = 0.94; 95% CI, 0.50–1.80), in situ neoplasms (Peto OR = 0.14; 95% CI, 0.01–2.17), or neoplasms of uncertain or unknown behavior compared with placebo treatment (Peto OR = 0.72; 95% CI, 0.46–1.15). Moreover, finerenone had an increased risk of malignant neoplasms of urinary tract (Peto OR = 1.69; 95% CI, 1.07–2.67). Subgroup analysis was conducted based to the organ source of the malignancy. The results showed no statistically significant difference in the risk of malignant neoplasms of the urinary organs between finerenone and placebo. However, it was observed that finerenone increased the risk of malignant neoplasms of the bladder in certain RCTs (Peto OR = 2.44; 95% CI, 1.18–5.07; Figure 8) (Pitt et al., 2021). Further statistical analysis of the data revealed that while individual trial data suggested an increased risk of malignant neoplasms of the bladder with finerenone, the combined data did not show a significant increase in risk, taking into account the limited sample size included.
In people who have arterial hypertension, heart failure, reduced ejection fraction, and CKD, MR blocking has been shown to have definite therapeutic efficacy (Bauersachs et al., 2015). The use of steroid mineralocorticoid receptor antagonists (MRAs) SPIR and eplerenone has been limited due to the risk of hyperkalemia and renal impairment (Svensson et al., 2004; Dinsdale et al., 2005; Vukadinović et al., 2017). Accordingly, scientists have developed a new nonsteroidal MRAs finerenone (Bärfacker et al., 2012), which has higher receptor selectivity (Bärfacker et al., 2012) and a lower risk of hyperkalemia (Pitt et al., 2012) and renal protective effects (Bakris et al., 2015; Bakris et al., 2020), and has been approved to be released in the market in 2021 (Frampton, 2021).
According to published research, SPIR is also associated with a reduced risk of prostate cancer (Mackenzie et al., 2017; Hiebert et al., 2021; Bommareddy et al., 2022). Leung et al. (2013) showed that SPIR inhibited the metastasis of colon cancer cells. Specific mechanisms include reducing the content of cancer stem cells, inhibiting the repair frequency of cancer DNA, and enhancing the sensitivity of cancer cells to chemotherapy (Alekseev et al., 2014; Shahar et al., 2014; Gold et al., 2019). Another MAR, eplerenone, affects the occurrence and development of hepatocellular carcinoma by inhibiting angiogenesis and expression of vascular endothelial growth factor and promoting the apoptosis of cancer cells (Kaji et al., 2010).
Previous studies have shown that naturally derived naphthyridines have anti-infective, anti-cancer, and immune-regulatory effects (Chabowska et al., 2021). The biosynthesis of naphthyridine derivatives also has anti-cancer, anti-microbial, anti-inflammatory, anti-oxidation, immune-regulation, and other effects (Madaan et al., 2015; Lavanya et al., 2021). Finerenone and its metabolites are naphthyridine derivatives (Gerisch et al., 2018; Chabowska et al., 2021). Our research revealed that finerenone was associated with an increased risk of malignant neoplasms of the urinary tract. Our findings are inconsistent with previous studies of the pharmacological effects of naphthyridines derivatives. Further research is needed to explore whether the increased risk of malignant neoplasms of the urinary tract caused by finerenone is related to its excretion pathway or whether finerenone and its metabolites have other pharmacological effects that we have not yet identified.
Kitchlu et al. (2022) found an increased risk of cancer in patients with CKD. Similarly, Kurasawa et al. (2023) reported a U-shaped relationship between estimated glomerular filtration rate and cancer incidence. The FDA has previously warned about the potential risk of medullary thyroid cancer with glucagon-like peptide-1 receptor agonists. García et al. (2021) analyzed cases from the European Pharmacovigilance Database and discovered a higher number of bladder cancer cases among users of sodium-glucose cotransporter-2 inhibitors. Yarmolinsky et al. (2022) studied the cancer risk associated with angiotensin-converting enzyme inhibitors, β-blockers, and thiazide diuretics, finding that long-term use of ACE inhibitors was linked to an increased risk of colorectal cancer. Dąbrowski reported that insulin had a dose-dependent cancer risk, while metformin could reduce cancer incidence and indirectly inhibit tumor growth (Dąbrowski, 2021). Our study focused on patients with T2DM and CKD who received concurrent treatment with antidiabetic, antihypertensive, and diuretic medications. Due to limitations in the trial data, we did not explore variations in tumor risk based on CKD stage or evaluate the impact of concomitant therapy on tumorigenesis.
This systematic review and meta-analysis had certain limitations. The studies analyzed were limited to RCTs, with no cohort or case-control studies involved. While RCTs can address baseline measures, reduce bias, and minimize confounding factors, some studies had small sample sizes and brief follow-up periods, with the minimum follow-up being only 3 months. For rare occurrences like tumors, small sample sizes may not be sufficient to detect meaningful results. Additionally, the number of studies analyzed was limited, with only four RCTs meeting the criteria for inclusion. None of the included studies examined population-specific cancer differences between men and women.
Our findings suggest that finerenone is not correlated with an increased risk of overall tumor development in patients with T2DM complicated with CKD, whereas subgroup analysis by tumor system suggested that finerenone might promote the risk of malignant neoplasms of urinary tract. Nevertheless, due to the small sample size and relatively short follow-up period, further well-planned and larger study populations are warranted.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
YD and YC conceived the study; YD and GC designed the study forms; and YC and JL directed the study. YD searched the literature, and YD, GC, LG, and YC screened the included studies, performed data extraction, and evaluated the methodological quality. YD, GC, and LG collated and analyzed the data. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AHRQ Methods for Effective Health Care (2008). Methods guide for effectiveness and comparative effectiveness reviews (Agency for Healthcare Research and Quality (US)). Available at: https://www.ncbi.nlm.nih.gov/books/NBK47095/
Alekseev, S., Ayadi, M., Brino, L., Egly, J. M., Larsen, A. K., and Coin, F. (2014). A small molecule screen identifies an inhibitor of DNA repair inducing the degradation of TFIIH and the chemosensitization of tumor cells to platinum. Chem. Biol. 21 (3), 398–407. doi:10.1016/j.chembiol.2013.12.014
Author Anyonomus (2009). The global alliance for chronic diseases. Lancet 373 (9681), 2084. doi:10.1016/s0140-6736(09)61129-6
Bakris, G. L., Agarwal, R., Anker, S. D., Pitt, B., Ruilope, L. M., Rossing, P., et al. (2020). Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N. Engl. J. Med. 383 (23), 2219–2229. doi:10.1056/NEJMoa2025845
Bakris, G. L., Agarwal, R., Chan, J. C., Cooper, M. E., Gansevoort, R. T., Haller, H., et al. (2015). Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA 314 (9), 884–894. doi:10.1001/jama.2015.10081
Bärfacker, L., Kuhl, A., Hillisch, A., Grosser, R., Figueroa-Pérez, S., Heckroth, H., et al. (2012). Discovery of BAY 94-8862: a nonsteroidal antagonist of the mineralocorticoid receptor for the treatment of cardiorenal diseases. ChemMedChem 7 (8), 1385–1403. doi:10.1002/cmdc.201200081
Bauersachs, J., Jaisser, F., and Toto, R. (2015). Mineralocorticoid receptor activation and mineralocorticoid receptor antagonist treatment in cardiac and renal diseases. Hypertension 65 (2), 257–263. doi:10.1161/hypertensionaha.114.04488
Bommareddy, K., Hamade, H., Lopez-Olivo, M. A., Wehner, M., Tosh, T., and Barbieri, J. S. (2022). Association of spironolactone use with risk of cancer: a systematic review and meta-analysis. JAMA Dermatol 158 (3), 275–282. doi:10.1001/jamadermatol.2021.5866
Bradburn, M. J., Deeks, J. J., Berlin, J. A., and Russell Localio, A. (2007). Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat. Med. 26 (1), 53–77. doi:10.1002/sim.2528
Chabowska, G., Barg, E., and Wójcicka, A. (2021). Biological activity of naturally derived naphthyridines. Molecules 26 (14). doi:10.3390/molecules26144324
Dąbrowski, M. (2021). Diabetes, antidiabetic medications and cancer risk in type 2 diabetes: focus on SGLT-2 inhibitors. Int. J. Mol. Sci. 22 (4). doi:10.3390/ijms22041680
Dinsdale, C., Wani, M., Steward, J., and O'Mahony, M. S. (2005). Tolerability of spironolactone as adjunctive treatment for heart failure in patients over 75 years of age. Age Ageing 34 (4), 395–398. doi:10.1093/ageing/afi104
Draznin, B., Aroda, V. R., Bakris, G., Benson, G., Brown, F. M., Freeman, R., et al. (2022). 11. Chronic kidney disease and risk management: standards of medical Care in diabetes-2022. Diabetes Care 45 (Suppl. 1), S175–s184. doi:10.2337/dc22-S011
Filippatos, G., Anker, S. D., Böhm, M., Gheorghiade, M., Køber, L., Krum, H., et al. (2016). A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur. Heart J. 37 (27), 2105–2114. doi:10.1093/eurheartj/ehw132
Frampton, J. E. (2021). Finerenone: first approval. Drugs 81 (15), 1787–1794. doi:10.1007/s40265-021-01599-7
García, M., Arteche-Martinez, U., Lertxundi, U., and Aguirre, C. (2021). SGLT2 inhibitors and bladder cancer: analysis of cases reported in the European pharmacovigilance database. J. Clin. Pharmacol. 61 (2), 187–192. doi:10.1002/jcph.1722
Gerisch, M., Heinig, R., Engelen, A., Lang, D., Kolkhof, P., Radtke, M., et al. (2018). Biotransformation of finerenone, a novel nonsteroidal mineralocorticoid receptor antagonist, in dogs, rats, and humans, in vivo and in vitro. Drug Metab. Dispos. 46 (11), 1546–1555. doi:10.1124/dmd.118.083337
Gold, A., Eini, L., Nissim-Rafinia, M., Viner, R., Ezer, S., Erez, K., et al. (2019). Spironolactone inhibits the growth of cancer stem cells by impairing DNA damage response. Oncogene 38 (17), 3103–3118. doi:10.1038/s41388-018-0654-9
Hardin, R. C., Jackson, R. L., Johnston, T. L., and Kelly, H. G. (1956). The development of diabetic retinopathy; effects of duration and control of diabetes. Diabetes 5 (5), 397–405. doi:10.2337/diab.5.5.397
Heinig, R., Gerisch, M., Engelen, A., Nagelschmitz, J., and Loewen, S. (2018). Pharmacokinetics of the novel, selective, non-steroidal mineralocorticoid receptor antagonist finerenone in healthy volunteers: results from an absolute bioavailability study and drug-drug interaction studies in vitro and in vivo. Eur. J. Drug Metab. Pharmacokinet. 43 (6), 715–727. doi:10.1007/s13318-018-0483-9
Heinig, R., Kimmeskamp-Kirschbaum, N., Halabi, A., and Lentini, S. (2016). Pharmacokinetics of the novel nonsteroidal mineralocorticoid receptor antagonist finerenone (BAY 94-8862) in individuals with renal impairment. Clin. Pharmacol. Drug Dev. 5 (6), 488–501. doi:10.1002/cpdd.263
Hiebert, B. M., Janzen, B. W., Sanjanwala, R. M., Ong, A. D., Feldman, R. D., and Kim, J. O. (2021). Impact of spironolactone exposure on prostate cancer incidence amongst men with heart failure: a Pharmacoepidemiological study. Br. J. Clin. Pharmacol. 87 (4), 1801–1813. doi:10.1111/bcp.14568
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj 343, d5928. doi:10.1136/bmj.d5928
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. Bmj 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Jaisser, F., and Farman, N. (2016). Emerging roles of the mineralocorticoid receptor in pathology: toward new paradigms in clinical Pharmacology. Pharmacol. Rev. 68 (1), 49–75. doi:10.1124/pr.115.011106
Jee, S. H., Ohrr, H., Sull, J. W., Yun, J. E., Ji, M., and Samet, J. M. (2005). Fasting serum glucose level and cancer risk in Korean men and women. JAMA 293 (2), 194–202. doi:10.1001/jama.293.2.194
Joseph, J. J., Deedwania, P., Acharya, T., Aguilar, D., Bhatt, D. L., Chyun, D. A., et al. (2022). Comprehensive management of cardiovascular risk factors for adults with type 2 diabetes: a scientific statement from the American heart association. Circulation 145 (9), e722–e759. doi:10.1161/cir.0000000000001040
Kaji, K., Yoshiji, H., Kitade, M., Ikenaka, Y., Noguchi, R., Shirai, Y., et al. (2010). Selective aldosterone blocker, eplerenone, attenuates hepatocellular carcinoma growth and angiogenesis in mice. Hepatol. Res. 40 (5), 540–549. doi:10.1111/j.1872-034X.2010.00636.x
Kitchlu, A., Reid, J., Jeyakumar, N., Dixon, S. N., Munoz, A. M., Silver, S. A., et al. (2022). Cancer risk and mortality in patients with kidney disease: a population-based cohort study. Am. J. Kidney Dis. 80 (4), 436–448. doi:10.1053/j.ajkd.2022.02.020
Kolkhof, P., Delbeck, M., Kretschmer, A., Steinke, W., Hartmann, E., Bärfacker, L., et al. (2014). Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J. Cardiovasc Pharmacol. 64 (1), 69–78. doi:10.1097/fjc.0000000000000091
Kurasawa, S., Imaizumi, T., Maruyama, S., Tanaka, K., Kubo, Y., Nagayoshi, M., et al. (2023). Association of kidney function with cancer incidence and its influence on cancer risk of smoking: the Japan Multi-Institutional Collaborative Cohort Study. Int. J. Cancer 153 (4), 732–741. doi:10.1002/ijc.34554
Lavanya, M., Lin, C., Mao, J., Thirumalai, D., Aabaka, S. R., Yang, X., et al. (2021). Synthesis and anticancer properties of functionalized 1,6-naphthyridines. Top. Curr. Chem. (Cham) 379 (2), 13. doi:10.1007/s41061-020-00314-6
Leung, W. H., Vong, Q. P., Lin, W., Janke, L., Chen, T., and Leung, W. (2013). Modulation of NKG2D ligand expression and metastasis in tumors by spironolactone via RXRγ activation. J. Exp. Med. 210 (12), 2675–2692. doi:10.1084/jem.20122292
Mackenzie, I. S., Morant, S. V., Wei, L., Thompson, A. M., and MacDonald, T. M. (2017). Spironolactone use and risk of incident cancers: a retrospective, matched cohort study. Br. J. Clin. Pharmacol. 83 (3), 653–663. doi:10.1111/bcp.13152
Madaan, A., Verma, R., Kumar, V., Singh, A. T., Jain, S. K., and Jaggi, M. (2015). 1,8-Naphthyridine derivatives: a review of multiple biological activities. Arch. Pharm. Weinh. 348 (12), 837–860. doi:10.1002/ardp.201500237
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj 339, b2535. doi:10.1136/bmj.b2535
Pearson-Stuttard, J., Papadimitriou, N., Markozannes, G., Cividini, S., Kakourou, A., Gill, D., et al. (2021). Type 2 diabetes and cancer: an umbrella review of observational and mendelian randomization studies. Cancer Epidemiol. Biomarkers Prev. 30 (6), 1218–1228. doi:10.1158/1055-9965.Epi-20-1245
Pitt, B., Filippatos, G., Agarwal, R., Anker, S. D., Bakris, G. L., Rossing, P., et al. (2021). Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N. Engl. J. Med. 385 (24), 2252–2263. doi:10.1056/NEJMoa2110956
Pitt, B., Filippatos, G., Gheorghiade, M., Kober, L., Krum, H., Ponikowski, P., et al. (2012). Rationale and design of ARTS: a randomized, double-blind study of BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease. Eur. J. Heart Fail 14 (6), 668–675. doi:10.1093/eurjhf/hfs061
Renehan, A. G., Tyson, M., Egger, M., Heller, R. F., and Zwahlen, M. (2008). Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371 (9612), 569–578. doi:10.1016/s0140-6736(08)60269-x
Sarwar, N., Gao, P., Seshasai, S. R., Gobin, R., Kaptoge, S., Di Angelantonio, E., et al. (2010). Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375 (9733), 2215–2222. doi:10.1016/s0140-6736(10)60484-9
Shahar, O. D., Kalousi, A., Eini, L., Fisher, B., Weiss, A., Darr, J., et al. (2014). A high-throughput chemical screen with FDA approved drugs reveals that the antihypertensive drug Spironolactone impairs cancer cell survival by inhibiting homology directed repair. Nucleic Acids Res. 42 (9), 5689–5701. doi:10.1093/nar/gku217
Shen, B., Li, Y., Sheng, C. S., Liu, L., Hou, T., Xia, N., et al. (2023). Association between age at diabetes onset or diabetes duration and subsequent risk of pancreatic cancer: results from a longitudinal cohort and mendelian randomization study. Lancet Reg. Health West Pac 30, 100596. doi:10.1016/j.lanwpc.2022.100596
Shlomai, G., Neel, B., LeRoith, D., and Gallagher, E. J. (2016). Type 2 diabetes mellitus and cancer: the role of pharmacotherapy. J. Clin. Oncol. 34 (35), 4261–4269. doi:10.1200/jco.2016.67.4044
Stratton, I. M., Adler, A. I., Neil, H. A., Matthews, D. R., Manley, S. E., Cull, C. A., et al. (2000). Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Bmj 321 (7258), 405–412. doi:10.1136/bmj.321.7258.405
Svensson, M., Gustafsson, F., Galatius, S., Hildebrandt, P. R., and Atar, D. (2004). How prevalent is hyperkalemia and renal dysfunction during treatment with spironolactone in patients with congestive heart failure? J. Card. Fail 10 (4), 297–303. doi:10.1016/j.cardfail.2003.10.012
Tsilidis, K. K., Kasimis, J. C., Lopez, D. S., Ntzani, E. E., and Ioannidis, J. P. A. (2015). Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies [Article]. Bmj-British Med. J. 350. Article g7607. doi:10.1136/bmj.g7607
Vukadinović, D., Lavall, D., Vukadinović, A. N., Pitt, B., Wagenpfeil, S., and Böhm, M. (2017). True rate of mineralocorticoid receptor antagonists-related hyperkalemia in placebo-controlled trials: a meta-analysis. Am. Heart J. 188, 99–108. doi:10.1016/j.ahj.2017.03.011
Yarmolinsky, J., Díez-Obrero, V., Richardson, T. G., Pigeyre, M., Sjaarda, J., Paré, G., et al. (2022). Genetically proxied therapeutic inhibition of antihypertensive drug targets and risk of common cancers: a mendelian randomization analysis. PLoS Med. 19 (2), e1003897. doi:10.1371/journal.pmed.1003897
Keywords: finerenone, T2DM, chronic kidney disease, tumor, meta-analysis
Citation: Du Y, Cao G, Gu L, Chen Y and Liu J (2024) Tumor risks of finerenone in patients with type 2 diabetes mellitus complicated with chronic kidney disease: a meta-analysis and systematic review of randomized controlled trials. Front. Pharmacol. 14:1237583. doi: 10.3389/fphar.2023.1237583
Received: 09 June 2023; Accepted: 27 December 2023;
Published: 11 January 2024.
Edited by:
Edgar Jaimes, Memorial Sloan Kettering Cancer Center, United StatesReviewed by:
Piergiorgio Messa, University of Milan, ItalyCopyright © 2024 Du, Cao, Gu, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuehong Chen, Y2hlcnJpc2hjaGVueWhAMTYzLmNvbQ==; Jingyu Liu, bGl1ank5MkAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.