- 1Department of Pharmacy, The Affiliated Brain Hospital of Guangzhou Medical University, Guangzhou, China
- 2Department of Pharmacy, Affiliated Cancer Hospital and Institute of Guangzhou Medical University, Guangzhou, China
Introduction: Extensive studies indicated that caveolin is a key regulator in multiple cellular processes. Recently, growing evidence demonstrated that caveolin is critically involved in tumor progression. Since no relevant bibliometric study has been published, we performed a bibliometric and visual analysis to depict the knowledge framework of research related to the involvement of caveolin in cancer.
Methods: Relevant studies published in English during 2003–2022 were obtained from the Web of Science Core Collection database. Three programs (VOSviewer, CiteSpace, and R-bibliometrix) and the website of bibliometrics (http://bibliometric.com/) were applied to construct networks based on the analysis of countries, institutions, authors, journals, references, and keywords.
Results: A total of 2,463 documents were extracted and identified. The United States had the greatest number of publications and total citations, and Thomas Jefferson University was the most productive institution. Michael P. Lisanti was the most influential scholar in this research domain. Cell Cycle was the journal with the most publications on this subject. The most local-cited document was the article titled “Caveolin-1 in oncogenic transformation, cancer, and metastasis.” A comprehensive analysis has been conducted based on keywords and cited references. Initially, the research frontiers were predominantly “signal transduction”, “human breast cancer,” “oncogenically transformed cells,” “tumor suppressor gene,” and “fibroblasts.” While in recent years, the research emphasis has shifted to “tumor microenvironment,” “epithelial mesenchymal transition,” “nanoparticles,” and “stem cells.”
Conclusion: Taken together, our bibliometric analysis shows that caveolin continues to be of interest in cancer research. The hotspots and research frontiers have evolved from the regulation of cancer signaling, to potential targets of cancer therapy and novel techniques. These results can provide a data-based reference for the guidance of future research.
Introduction
Cancer is a leading cause of death in the world, and malignant tumors were responsible for approximately 7 million deaths annually (Torre et al., 2016). The global burden of cancer has been further increased due to population aging as well as lifestyles that are known to increase cancer risk, such as alcohol consumption, smoking, poor diet, and obesity (Yang et al., 2018). It is relatively rare for cancer to develop during the average human lifespan because of our intrinsic anti-cancer mechanisms. However, cancer cells can evade defense mechanisms by developing capabilities such as angiogenesis induction, insensitivity to anti-growth signals, drug resistance, and apoptosis resistance (Hanahan and Weinberg, 2000).
Caveolin is a multifunctional scaffolding protein of caveolae, and evidence suggested that caveolin is a key regulator in multiple cellular processes (Williams and Lisanti, 2004; Williams et al., 2005; Volonte and Galbiati, 2020). As a protein family, caveolin has three members: caveolin-1, caveolin-2, and caveolin-3. Caveolin-1 and caveolin-2 are found in all types of cells, and caveolin-3 is mostly expressed in skeletal muscles (Liu et al., 2002; Gupta et al., 2014). Caveolin has been reported to influence various capabilities of cancer cells, supporting the idea that caveolin is a key regulator in the development of cancer (Volonte and Galbiati, 2020). Growing evidence further demonstrated that caveolin contributes significantly in the regulation of multiple cellular processes of cancer, including migration, metastasis, survival, and angiogenesis (Goetz et al., 2008; Ho et al., 2008; Patani et al., 2012; Tang et al., 2012; Faggi et al., 2015; Maiuthed et al., 2018; Yamao et al., 2019).
Since studies related to the involvement of caveolin in cancer have developed rapidly, it is necessary to systematically explore the current status and future trends on this subject. Bibliometrics is a common method for depicting the knowledge structure and frontiers of a specific field (Chen and Song, 2019; Xu et al., 2022). Although many review articles have examined the relationship between caveolin and cancer, bibliometric analysis has not, as yet, been seen in this field. Therefore, in the current study, we conducted a bibliometric and visual analysis to summarize the publications over the past 2 decades. We hope that this study can build a comprehensive picture of research on the links between caveolin and cancer, and provide an effective reference for scholars to better investigate the history and future directions in this research domain.
Materials and methods
Data acquisition
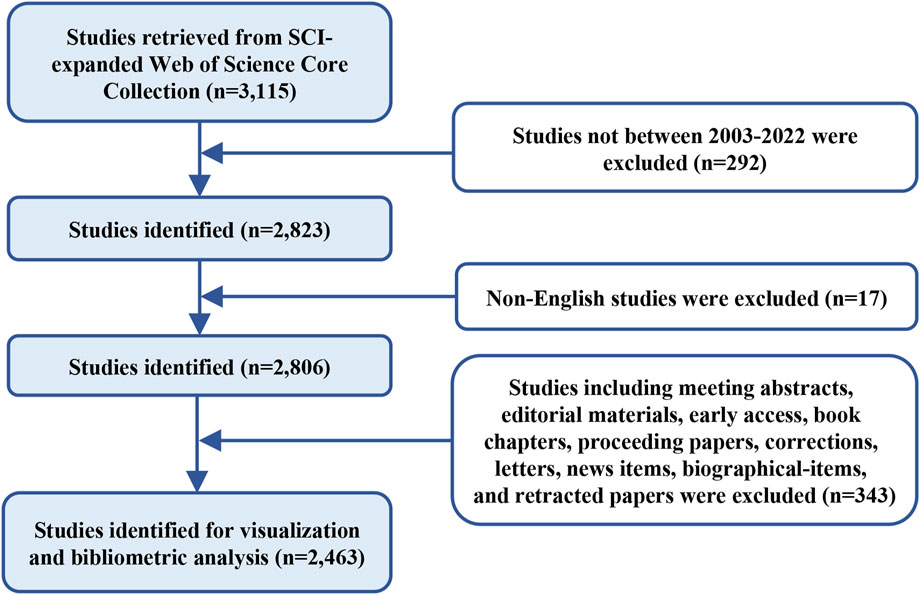
Data retrieval was performed on 22 April 2023 using the Web of Science Core Collection (WOSCC) database, and the search formula was set to TS = (cancer* OR carcinoma* OR neoplasms* OR sarcoma* OR tumor* OR tumour* OR lymphoma* OR leukemia* OR leukaemia* OR malignan*) AND TS = (caveolin*). The time frame was set to 2003–2022 encompassing 20 years, and non-English studies were excluded. Only articles and reviews were included and irrelevant documents (meeting abstracts, biographical-items, editorial materials, early access, letters, book chapters, proceeding papers, corrections, news items, and retracted papers) were excluded (Figure 1).
Data analysis
In our current study, VOSviewer, CiteSpace, R–bibliometrix, and the website of bibliometrics (http://bibliometric.com/), were applied to perform bibliometric and visual analysis.
VOSviewer (version 1.6.18) is a JAVA-based software invented by Professor Nees Jan van Eck and Professor Ludo Waltman (Leiden University, the Netherlands) (van Eck and Waltman, 2010). This software can be applied to analyze the network of countries, institutions, authors, journals, and keywords based on collaborative data or co-occurrence data. In this study, VOSviewer was employed to visualize the institution co-authorship network and keywords co-occurrence map.
CiteSpace (version 6.2.R2) is also a JAVA-based software developed by Professor Chaomei Chen (Drexel University, the United States). It can be employed to combine bibliometric analysis and data mining algorithms for the final visualization (Chen, 2004). In this study, CiteSpace was used to visualize the clusters of cited references by using the LLR (log-likelihood ratio) algorithm. In addition, burst analysis of keywords was applied as an indicator of the research emphasis.
The R–bibliometrix (version 4.1.0) is an R-based package built by Professor Massimo Aria (University of Naples Federico II, Italy) and Professor Corrado Cuccurullo (University of Campania Luigi Vanvitelli, Italy). In the current study, R–bibliometrix was employed to conduct a comprehensive investigation of the countries, institutions, authors, journals, references, and keywords (Aria and Cuccurullo, 2017). The top journals were determined using the Bradford’s law (Brookes, 1985), whereas the h-index and g-index were applied to measure the academic impact of authors (Hirsch, 2005; Ali, 2021).
The website of bibliometrics (http://bibliometric.com/) was applied to present the global cooperation atlas.
Results
Analysis of general trend
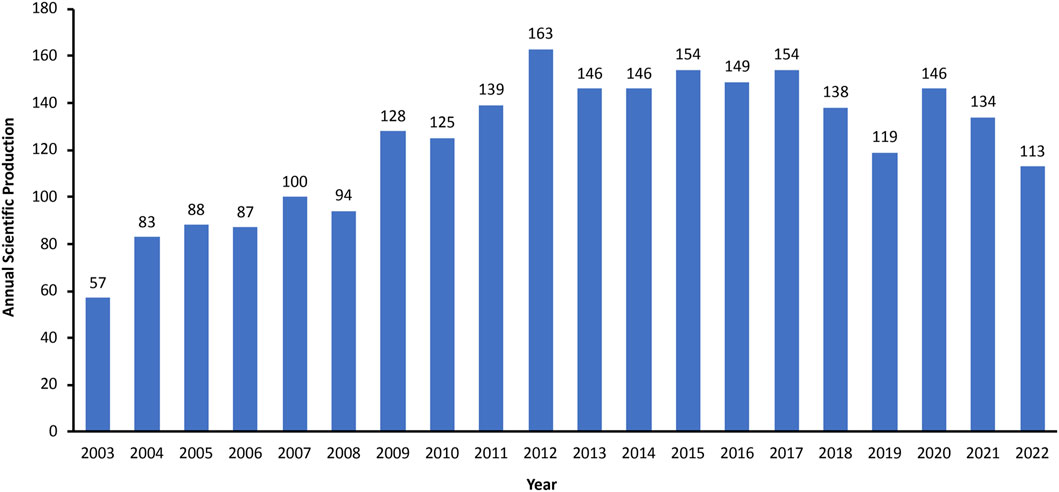
In this study, a total of 2,463 related documents were identified and met the inclusion criteria, and the annual scientific productions showed a general ascending trend (Figure 2). Prior to 2009, research with regard to the links between caveolin and cancer was relatively slow to develop, and none of them had more than 100 annual publications. The annual productions began to increase in 2009 and reached a peak of 163 publications in 2012. After 2012, the number of annual publications stayed relatively stable, fluctuating around 150 for the majority of the period.
Analysis of countries
A total of 65 countries have contributed relevant literature. As shown in Figure 3A, the United States ranked first in terms of publications (2,534 publications, 27.39%), followed by China (2,125 publications, 22.97%) and Italy (529 publications, 5.72%). These three countries contributed approximately 56.08% of total publications in this field from 2003 to 2022. Figure 3B exhibits that the United States, China, and Italy also ranked the top three with regard to the total citations (United States: 44,993 citations, China:130,49 citations, Italy: 6,350 citations). A total of 371 collaborations were identified in the global cooperation networks, and the top three were USA-China (90 collaborations), USA-UK (89 collaborations), and USA-Italy (58 collaborations) (Figure 3C).
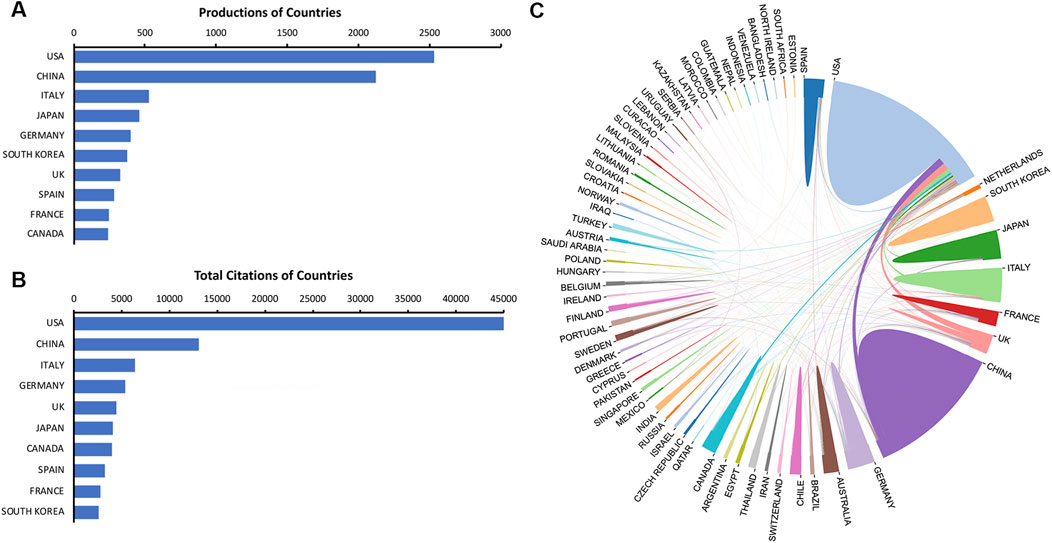
FIGURE 3. Contributions of countries. (A) The top ten countries in publications. (B) The top ten countries in total citations. (C) Cooperation networks of active countries.
Analysis of institutions
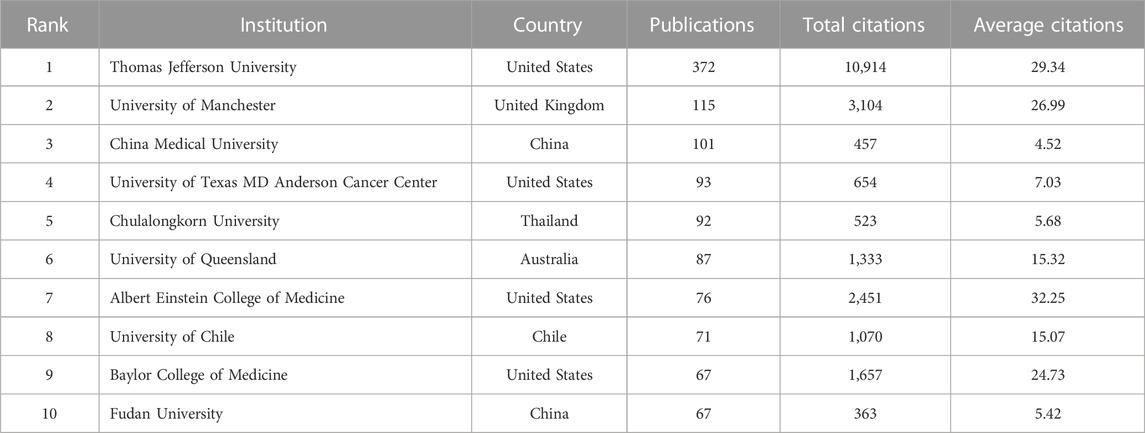
During 2003–2022, a total of 2,438 institutions launched papers on this theme. As listed in Table 1, the top three institutions were Thomas Jefferson University (372 publications), University of Manchester (115 publications), and China Medical University (101 publications). Institutions with 15 or more publications were included in the collaboration network analysis and then visualized by VOSviewer. The clusters are organized in different colors by the frequency of cooperation between each institution (Figure 4A). Thomas Jefferson University was represented by the largest node (with a total link strength of 90), indicating the highest degree of cooperation with other institutions. The strongest link was between Thomas Jefferson University and University of Manchester (with a link strength of 51), and they were thus connected by the thickest line. Figure 4B depicts the timeline visualization for institution co-authorship network. The average publication year of an institution was represented by the color of the node according to the chronological gradient. Harvard University (with an average publication year of 2008.35) was shown in dark blue, indicating that researchers from Harvard University were active during the earlier stage of this research field. Shanghai Jiao Tong University (with an average publication year of 2017.18) was shown in dark red, indicating that this university was more recently active in this field.
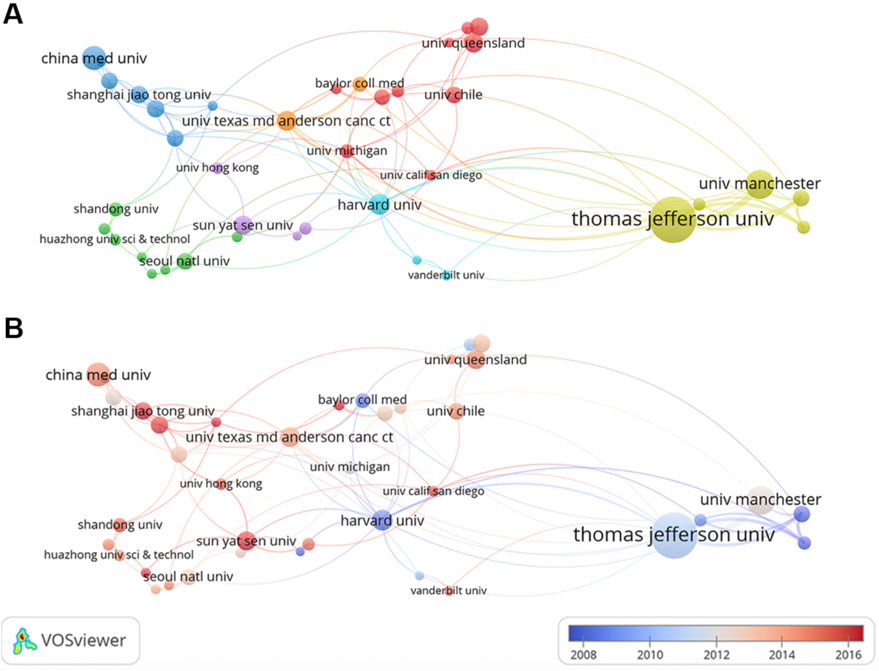
FIGURE 4. Co-authorship analysis of institutions. (A) Cluster visualization for institution co-authorship network. (B) Timeline visualization for institution co-authorship network.
Analysis of authors
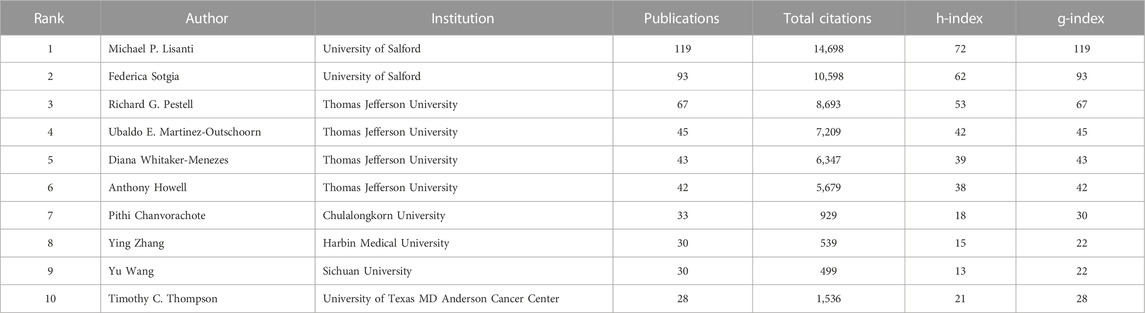
A total of 12,168 authors published relevant papers, and the top ten most fruitful authors are listed in Table 2. Michael P. Lisanti (University of Salford, United Kingdom) was the most influential author with 119 publications and 14,698 total citations, followed by Federica Sotgia (University of Salford, United Kingdom) and Richard G. Pestell (Thomas Jefferson University, United States), whereas the remaining authors had fewer than 50 publications. Michael P. Lisanti, Federica Sotgia, and Richard G. Pestell also ranked the top three authors according to the h-index and g-index values. Figure 5 shows the timeline visualization for the top twenty active researchers and their productions over time.
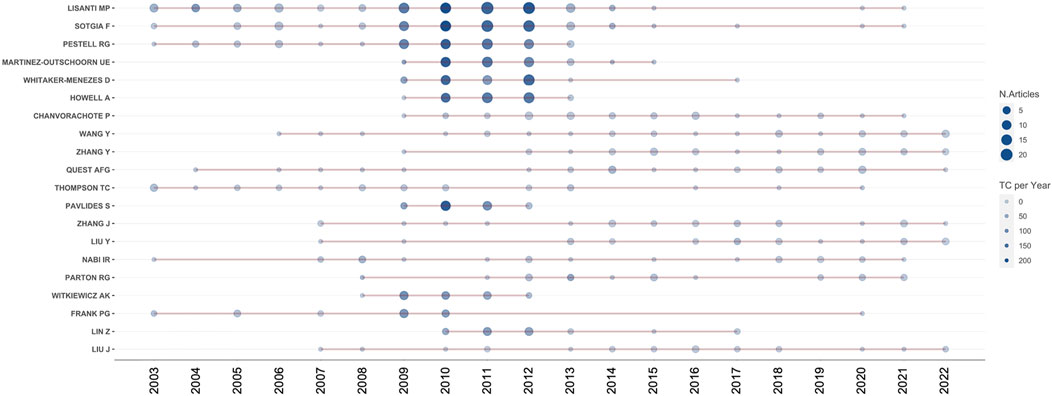
FIGURE 5. Timeline visualization for the top twenty active researchers. The number of publications and citations are represented by the size and color of the node, respectively.
Analysis of journals
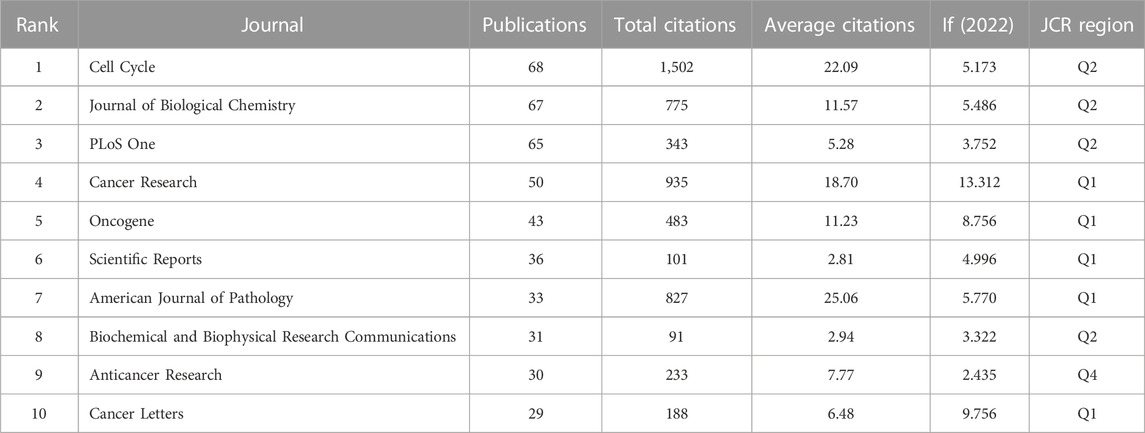
The top ten most relevant journals are listed in Table 3, Cell Cycle (68 publications) was the journal with the most prolific outlet, followed by Journal of Biological Chemistry (67 publications) and PLoS One (65 publications). According to the Journal Citation Reports (JCR) 2022, five journals were located in Q1 region, and four journals were located in Q2 region. The impact factor (IF) ranged from 2.435 in Anticancer Research to 13.312 in Cancer Research among the top ten most relevant journals.
Analysis of references
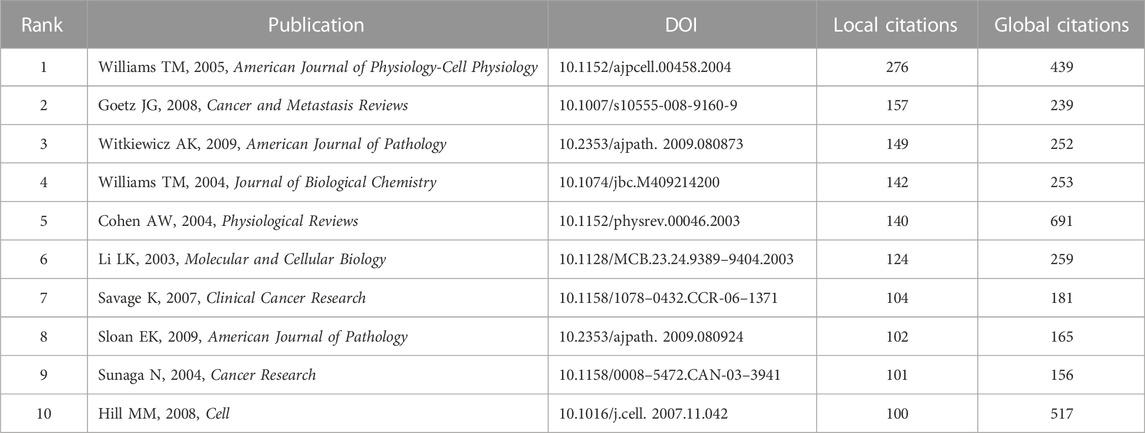
There were two concepts in R-bibliometrix, “local citation” and “global citation”. Local citation represents the citations of a study in the similar fields, while global citation indicates the citations of a study in all fields. A higher local citation value reflects a higher degree of recognition in the peer field (Luo et al., 2022). As listed in Table 4, the literature with the highest value of local citation was the study published in American Journal of Physiology-Cell Physiology titled “Caveolin-1 in oncogenic transformation, cancer, and metastasis” (276 local citations), while the study with the greatest value of global citation was the paper published in Physiological Reviews titled “Role of caveolae and caveolins in health and disease” (691 global citations).
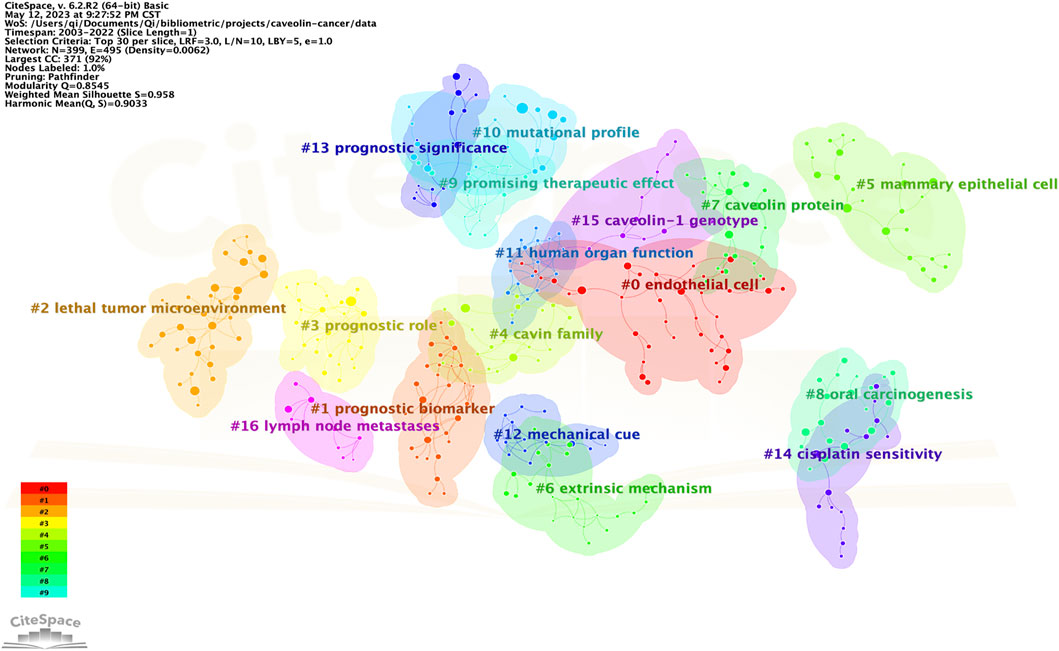
The cluster visualization for cited references was conducted by CiteSpace, and a total of 17 clusters were formed according to the keywords of cited references (Figure 6). The labels of 17 clusters were: #0 endothelial cell, #1 prognosis biomarker, #2 lethal tumor microenvironment, #3 prognostic role, #4 cavin family, #5 mammary endothelial cell, #6 extrinsic mechanism, #7 caveolin protein, #8 oral carcinogenesis, #9 promising therapeutic effect, #10 mutational profile, #11 human organ function, #12 mechanical cue, #13 prognostic significance, #14 cisplatin sensitivity, #15 caveolin-1 genotype, #16 lymph node metastases.
Analysis of keywords
Keywords analysis was conducted by VOSviewer and CiteSpace. The threshold of keywords occurrence was set to 10 considering the readability of the graph, and 446 keywords were included in the analysis.
In Figure 7A, keywords were organized into 6 clusters labeled with different colors. In cluster 1, “caveolae” was the largest node, the other main nodes included lipid rafts, caveolin, gene expression, plasma-membrane, in-vivo, nitric oxide synthase, angiogenesis, inflammation, and signal-transduction. This cluster mainly focused on the structure of caveolin and the underlying mechanisms of cancer signaling. In cluster 2, “caveolin-1” was the largest node, the other main nodes included metastasis, progression, survival, overexpression, prostate-cancer, and adenocarcinoma. This cluster mainly focused on the associations between caveolin-1 overexpression and tumor progression. In cluster 3, “cancer” was the largest node, the other main nodes included activation, receptor, in-vitro, endocytosis, mechanisms, nanoparticles, and therapy. This cluster represented the potential targets and mechanisms for the therapeutics of cancer. In cluster 4, “downregulation” was the largest node, the other main nodes included upregulation, migration, invasion, and tyrosine phosphorylation. This cluster indicated that the regulation of caveolin on tyrosine phosphorylation was widely concerned. In cluster 5, “growth” was the largest node, the other main nodes included oxidative stress, autophagy, stem cells, and fibroblasts. This cluster represented the current research hotspots in cancer therapy. In cluster 6, “protein” was the largest node, the other main nodes included apoptosis, proliferation, and pathway. This cluster focused on proteins in different pathways of cell apoptosis and proliferation.
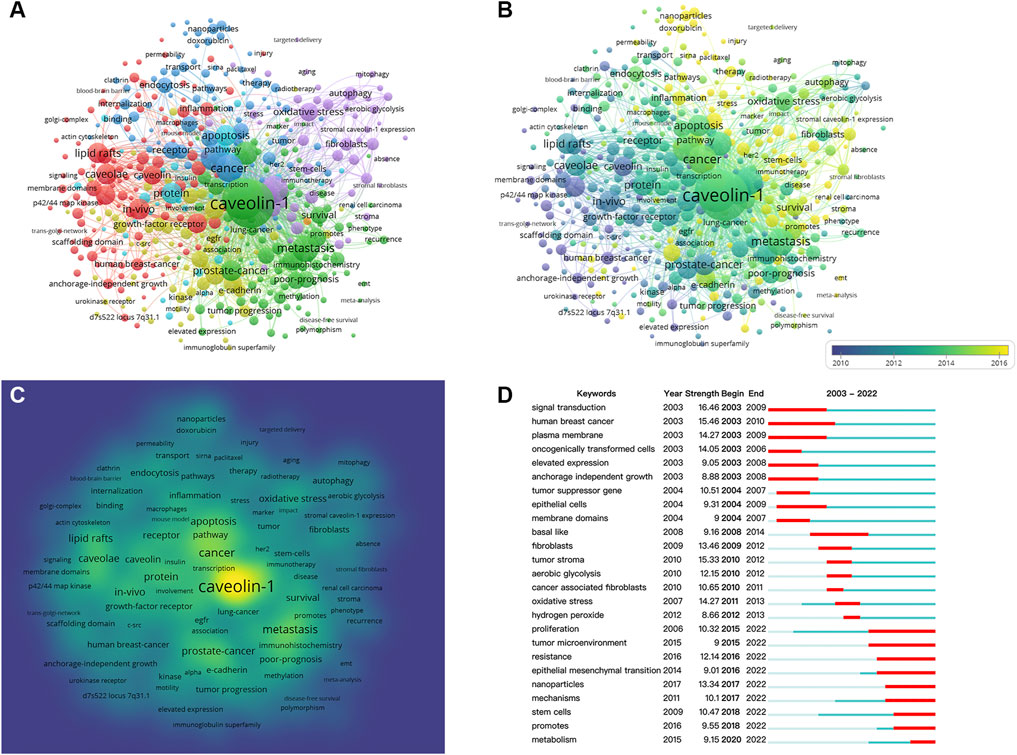
FIGURE 7. Co-occurrence analysis of keywords. (A) Cluster visualization for keywords. (B) Timeline visualization for keywords. (C) Frequency visualization for keywords. (D) Citation bursts of the top twenty-five keywords.
Figure 7B illustrates the timeline visualization for keywords, and the average occurrence time was represented by the color of the node according to the chronological gradient. The keyword “oncogenically transformed-cell” (with an average occurrence time of 2006.98) was shown in blue, revealing an early-stage research emphasis. The keywords “micelles” (with an average occurrence time of 2019.08) was shown in yellow, indicating that this keyword was more recently active in this field.
Figure 7C shows the heatmap of keywords frequency in which the yellow area represents a high frequency of occurrence. The most frequent keyword was “caveolin-1,” other hotspots included “metastasis,” “prostate-cancer,” “apoptosis,” “caveolae” and “lipid rafts.”
Figure 7D presents the citation bursts of the top twenty-five keywords, in which the time interval and duration of the citation burst were marked in blue and red, respectively. It is noticeable that keywords were divided into three stages by the duration of the citation burst. In the early stage (2003–2010), keywords included “signal transduction,” “human breast cancer,” “plasma membrane,” “oncogenically transformed cells,” “elevated expression,” “anchorage independent growth,” “tumor suppressor gene,” “epithelial cells,” and “membrane domains.” Research hotspots during 2008–2014 shifted to “basal like,” “fibroblasts,” “tumor stroma,” “aerobic glycolysis,” “oxidative stress,” and “hydrogen peroxide.” From 2015 to 2022, the main research frontiers were “proliferation,” “tumor microenvironment,” “resistance,” “epithelial mesenchymal transition,” “nanoparticles,” “mechanisms,” “stem cells,” “promotes,” and “metabolism.”
Discussion
General trends and knowledge structure of global publications
The development of a research topic can be reflected by the amount of annual scientific output (Durieux and Gevenois, 2010). As can be observed from the results, the annual publication number in this field showed a fluctuating but generally ascending trend prior to 2013 (with a growth rate of 12.38%), followed by a slightly descending trend during 2013–2022 (with a growth rate of −2.81%). This suggested that this topic had received significant attention in the first decade but entered a period of slow development from 2013, which might indicate that some challenges and bottlenecks appeared in some aspects of this research field and therefore innovation is required in the future investigation.
Our results of the national contributions suggested that the United States had great influence in this field by the leading numbers of publications and total citations. In addition, the United States constituted 33.70% of the amount of publications and 49.50% of the amount of total citations among the top ten most productive countries. Interestingly, although China ranked second in terms of both publications and total citations, we found that the number of publications from China (2,125 publications) was 83.86% of that from the United States (2,534 publications), whereas the number of total citations from China (13,049 citations) was only 29.00% of that from the United States (44,993 citations). This might reflect the global structure that the United States had deeper accumulation and more high-quality publications than other countries. According to our data on the institution distribution, the United States contributed four of the top ten institutions. Moreover, Thomas Jefferson University from the United States was the only institution with more than 300 publications and over 10,000 total citations, indicating the great impact and significant academic accumulation in this domain. Therefore, based on the contributions of countries and institutions, we can assume that the United States is having an absolute leading position in this field.
The h-index and g-index are generally applied to evaluate the academic impact of a researcher (Hirsch, 2005; Ali, 2021). From the perspective of authors, Michael P. Lisanti and Federica Sotgia, both from University of Salford in the United Kingdom, had significant impact in this field by their most prolific output, as well as their high values of h-index and g-index. In particular, Michael P. Lisanti undertook significant research in this field and formed close collaborations with researchers worldwide. As listed in Table 2, the following four active authors, Richard G. Pestell, Ubaldo E. Martinez-Outschoorn, Diana Whitaker-Menezes, and Anthony Howell, were all from Thomas Jefferson University in the United States, and the last four authors on the list were one scholar from Thailand and three scholars from China. This might reflect the late start of Thailand and China in this research field and the last four researchers on the list were therefore not as fruitful as the top six researchers.
Among the top ten most relevant journals, Cell Cycle had the most publications (68 publications), followed by Journal of Biological Chemistry (67 publications) and PLoS One (65 publications). As shown in Table 3, nine of the core journals were located in JCR region Q1 or Q2, and Cancer Research ranked first with the highest IF of 13.312. On the other hand, the top three most cited journals, Cell Cycle (1,502 total citations), Cancer Research (935 total citations), and American Journal of Pathology (827 total citations) and their high IF values (with IF values of 5.173, 13.312, and 5.770, respectively) revealed the strong influence and high quality in this field. Thus, these results will help scholars searching the core journals related to the links between caveolin and cancer.
The research frontiers of a topic can be reflected by the analysis of cited references. Local citation is indicative of the citations of a study in the similar fields, while global citation indicates the citations of a study in all fields. A higher local citation of the study reflects a higher degree of recognition in the peer field (Luo et al., 2022). The literature ranked first in local citation was the study published in American Journal of Physiology-Cell Physiology titled “Caveolin-1 in oncogenic transformation, cancer, and metastasis” (276 local citations), while the study published in Physiological Reviews titled “Role of caveolae and caveolins in health and disease” had the highest number of global citation (691 global citations). These highly cited publications are considered essential references for scholars working in this field. For example, Hill et al. explored the important role of caveolin formation (Hill et al., 2008), Sunaga et al. introduced the opposite effects of caveolin-1 on the progression of non-small cell lung cancer and small cell lung cancer (Sunaga et al., 2004), and Williams et al. summarized the functions of caveolin-1 in cancer migration and metastasis (Williams and Lisanti, 2005). Interestingly, after a comprehensive analysis of authors and cited references, we found that Michael P. Lisanti, as the corresponding author, appeared in four of the top ten highly cited publications. These findings further suggested that he is an accomplished researcher in this field.
Research hotspots of global publications
In bibliometric analysis, keywords co-occurrence analysis can reflect core contents of the literature, and timeline visualization for keywords can reveal the evolution of research frontiers (Liu et al., 2014). Thus, we formed the evolution of keywords into three stages according to the citation bursts of keywords. In the early stage (2003–2010), keywords such as “signal transduction,” “human breast cancer,” “plasma membrane,” “oncogenically transformed cells,” “elevated expression,” “anchorage independent growth,” “tumor suppressor gene,” “epithelial cells,” and “membrane domains,” were mainly associated with cancer-related processes. In the following period (2008–2014), most terms focused on potential targets to the therapeutic of cancer, such as “fibroblasts,” “tumor stroma,” “aerobic glycolysis,” “oxidative stress,” and “hydrogen peroxide.” In recent years (2015–2022), “tumor microenvironment,” “epithelial mesenchymal transition,” “nanoparticles,” “stem cells,” and “metabolism” became the main research frontiers, and these keywords mainly focused on novel technologies and new directions. Interestingly, these data were in line with the timeline visualization (Figure 7B) and frequency visualization of keywords (Figure 7C) that the early-stage emphasis was mainly associated with mechanisms for cancer signaling, then the research hotspots shifted to the application of novel technologies in cancer therapy.
According to the cluster visualization of keywords and cited references, the evolution of studies on the links between caveolin and cancer could be summarized into the following three branches.
1) The general function of caveolin
Caveolae are flask-shaped invagination on the plasma membrane which was initially considered to be a simple membrane structure but later believed to be a more complex organelle related to cellular functions (Parton, 2018). Early in 1955, Yamada first observed caveolae as invaginations of the plasma membrane using electron microscopy (Yamada, 1955). Palade and Bruns subsequently identified caveolae in vascular endothelial cells and suggested its function of delivering molecules across endothelial cells (Palade and Bruns, 1968). Later in 1998, Anderson discovered that caveolae is not just lipid raft with sphingolipids and cholesterol, but more of a platform regulating various signaling activities (Anderson, 1998).
Caveolin as a key component protein of caveolae and has shown to be a critical regulator in multiple cellular processes such as endocytosis, transcytosis, and cellular metabolism (Parton and Richards, 2003; Williams and Lisanti, 2004; Williams et al., 2005; Watanabe et al., 2009). As a protein family, caveolin has three members: caveolin-1, caveolin-2, and caveolin-3. Caveolin-1 and caveolin-2 are found in all types of cells, and caveolin-3 is mostly expressed in skeletal muscles (Liu et al., 2002; Gupta et al., 2014). Particularly, caveolin-1 has been widely investigated and was identified as the main protein in the caveolin family (Bastiani and Parton, 2010). Numerous studies have examined that caveolin-1 is closely associated with the development of various diseases, including pulmonary hypertension, vascular abnormalities, metabolic diseases, and cancer (Razani et al., 2001; Razani et al., 2002; Cohen et al., 2004; Cao et al., 2008; Austin et al., 2012). Recently, with the remarkable development of cancer research, extensive findings have indicated that caveolin-1 is a key factor in the occurrence, progression and prognosis of tumor (Witkiewicz et al., 2009a; Doherty and McMahon, 2009; Nassar et al., 2013; Chanvorachote et al., 2014; Li et al., 2018; Maiuthed et al., 2018). Firstly, abnormal expression of caveolin-1 has been found in several types of malignant tumors, such as gastric cancer, breast cancer, lung cancer, and prostate cancer (Sunaga et al., 2004; Witkiewicz et al., 2009b; Celus et al., 2017; Maiuthed et al., 2018; Lin et al., 2019). Furthermore, many studies demonstrated that caveolin-1 contributes significantly in the modulation of multiple cellular processes of cancer, including migration, metastasis, survival, and angiogenesis (Goetz et al., 2008; Ho et al., 2008; Tang et al., 2012; Faggi et al., 2015; Maiuthed et al., 2018; Yamao et al., 2019). Therefore, caveolin is believed to be a promising therapeutic target as well as an effective prognostic biomarker for cancer treatment.
2) The role of caveolin in carcinogenesis
Since aberrant expression of caveolin was found in several solid tumors, caveolin was considered to be a validated biomarker of cancer prognosis. However, the exact role of caveolin in tumorigenesis remains controversial (Martinez-Outschoorn et al., 2015). In some early studies, caveolin was initially regarded as a tumor suppressor. Insufficient caveolin-1 is able to induce carcinogenesis (Drab et al., 2001; Marx, 2001), and downregulation of caveolin-1 enhances tumor cell invasion and metastasis (Lu et al., 2003; Williams et al., 2004). In more recent studies, Du et al. suggested that complex formation of caveolin-1 in lipid transfer domain contributes tumor suppression (Du et al., 2012), Neofytou et al. reported similar results that the loss of caveolin-1 expression is significantly correlated with the poor prognosis of patients with colorectal cancer (Neofytou et al., 2017), Chatterjee et al. found that overexpression of caveolin enhances the sensitivity of tumor cells to paclitaxel and thus promotes apoptosis of tumor cells (Chatterjee et al., 2017), and Pavlides et al. indicated that stromal caveolin-1 may be a biomarker for human breast cancer such that a low expression of stromal caveolin-1 is associated with tumor recurrence, metastasis, and poor clinical outcome (Pavlides et al., 2009).
On the other hand, caveolin was found to be a tumor promotor. Lobos-González et al. discovered that high level of caveolin-1 expression promotes the invasion and metastasis of melanoma (Lobos-González et al., 2013), Seker et al. discovered that overexpression of caveolin reduced the survival time of patients with gastric cancer (Seker et al., 2017), and Huang et al. indicated that increased expression of caveolin-1 causes malignant transformation of glioma cells (Huang et al., 2018). Interestingly, in lung cancer but between different subtypes, Sunaga et al. found opposite effects of caveolin-1, such that caveolin-1 functions as a tumor suppressor in small cell lung cancer, but a tumor promoter in non-small cell lung cancer (Sunaga et al., 2004).
Although the role of caveolin in carcinogenesis remains to be elucidated, Carver and Schnitzer suggested that caveolin has the role of tumor suppressor mainly in the earlier stages of cancer, while in later stages of cancer, it supports the metastasis and survival of cancer cells due to the biological functions of caveolin in molecule delivery and signal modulation (Carver and Schnitzer, 2003). Therefore, this finding could possibly explain, at least in part, the contradictory results on the role of caveolin in tumorigenesis.
3) The potential caveolin-related targets in cancer therapies
Since caveolin influences multiple processes of tumor and the signal transduction of tumor cells, there are several potential caveolin-related targets in cancer therapies that could be further discussed.
Tumor microenvironment refers to the local stable-state environment which influences the occurrence and metastasis of tumor, and fibroblasts is one of the key element in tumor microenvironment (Shimizu et al., 2017; Yamao et al., 2019). Goetz et al. reported that microenvironment remodeling by caveolin-1 fibroblasts leads to the migration and invasion of cancer cells (Goetz Jacky et al., 2011), Yamao et al. found that downregulated caveolin-1 expression reduces the invasiveness of pancreatic cancer cells (Yamao et al., 2019), and Shimizu et al. discovered that caveolin-1 fibroblasts could induce the growth and metastasis of tumor cells in lung cancer (Shimizu et al., 2017).
Autophagy is a fundamental process for cytoprotection and intracellular homeostasis, and caveolin has been shown to be a critical regulator in autophagy of tumor cells with oxidative stress (Martinez-Outschoorn et al., 2010a; Martinez-Outschoorn et al., 2010b). Liu et al. indicated that caveolin-1 inhibits autophagy of cancer cells in hepatocellular carcinoma, providing potential target for autophagy inhibition as a novel treatment (Liu et al., 2016). More recent studies demonstrated that caveolin overexpression and phosphorylation are positively correlated with autophagy induction, which lead to tumor metastasis and cancer cell survival (Ortiz et al., 2016; Meng et al., 2017; Jiang et al., 2022). Evidence from Luanpitpong et al. revealed that oxidative stress induces the decomposition of tumor-related fibroblasts which further promotes tumor growth, and caveolin-1 has the capability to reverse this process (Luanpitpong et al., 2010). Sotgia et al. further suggested that oxidative stress is a key factor in the loss of stromal caveolin-1 via autophagy, and thus provided guidance for the application of antioxidants in cancer therapy (Sotgia et al., 2012). Another finding of Volonte et al. showed that caveolin-1 reduction inhibits oncogene-induced oxidative stress, thereby blocks the carcinogenesis pathway (Volonte et al., 2018).
In addition, some recent studies have shown that tumor cells with drug resistance exhibited an upregulated expression of caveolin. Wang et al. observed that silencing of caveolin-1 significantly reduces drug resistance of breast cancer cells (Wang et al., 2014), and Yuan et al. introduced the promoting effect of caveolin-1 in drug resistance of human gastric cancer cells (Yuan et al., 2013). Other clinical studies discovered the critical role of caveolin in the effectiveness and drug resistance of trastuzumab, providing promising targets and novel research directions for clinical applications (Pereira et al., 2018; Sung et al., 2018).
With the rapid development of multidiscipline, caveolin has been combined with novel techniques recently. Nanotechnology has been essentially involved in drug delivery and was benefited from endocytosis mediated by caveolin (dos Santos et al., 2011). Shamay et al. found that nanoparticles can selectively target kinase inhibitors in caveolin-dependent cancer models, and this finding would improve the computational modeling of nanomedicine designs (Shamay et al., 2018). Another study using photodynamic therapy reported that photosensitizers and caveolin-mediated endocytosis are critically involved in the precise targeting of cancer photodynamic therapy (Liu et al., 2022). Together, these studies provided promising targets and novel research directions for the therapeutics of cancer.
Conclusion
In summary, our bibliometric analysis shows that caveolin continues to be of interest in the field of cancer research. The United States ranked first with scientific productivity and was closely cooperated with other countries. At the same time, Thomas Jefferson University from the United States have conducted in-depth research in this area. Moreover, Michael P. Lisanti was the most productive author in this domain. The research frontiers of caveolin has undergone a shift from the regulation of cancer signaling to potential targets for cancer therapy and novel techniques. As such, our study conducted a systematic bibliometric analysis of literature on this subject, and provided a data-based reference for the guidance of future investigation in this field.
Limitations
The current study systematically visualized the relationship between caveolin and cancer. However, there are some limitations that should be mentioned as well. First, we only included publications that are written in English, thus potential findings published in other languages may not be covered. Second, only publications retrieved from the WOSCC database were selected, and documents from the Google Scholar database and the Scopus database were not searched. Therefore, some of the relevant studies may have been omitted. Third, we only obtained the published literature during 2003–2022, some of the very recent studies were thus not included in the analysis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
QS and YT co-designed the study. YT conducted the data analysis and generated the figures. QS and YT wrote the manuscript. All authors have read and approved the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Natural Science Foundation of China (No.82104223 and No. 82204372), Guangdong Basic and Applied Basic Research Foundation (No. 2020A1515110008), Science and Technology Program of Guangzhou (No.202102021022 and No. 2023A04J0601), and Guangzhou Municipal Science and Technology Project for Medicine and Healthcare (No.20211A011044).
Acknowledgments
We would like to thank Professor Chaomei Chen, Professor Nees Jan van Eck, Professor Ludo Waltman, Professor Massimo Aria, and Professor Corrado Cuccurullo, for the free use of CiteSpace, VOSviewer, and R-bibliometrix.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ali, M. J. (2021). Understanding the 'g-index' and the 'e-index. Semin. Ophthalmol. 36 (4), 139. doi:10.1080/08820538.2021.1922975
Anderson, R. G. (1998). The caveolae membrane system. Annu. Rev. Biochem. 67, 199–225. doi:10.1146/annurev.biochem.67.1.199
Aria, M., and Cuccurullo, C. (2017). bibliometrix: An R-tool for comprehensive science mapping analysis. J. Inf. 11, 959–975. doi:10.1016/j.joi.2017.08.007
Austin, E. D., Ma, L., LeDuc, C., Berman Rosenzweig, E., Borczuk, A., Phillips, J. A., et al. (2012). Whole exome sequencing to identify a novel gene (caveolin-1) associated with human pulmonary arterial hypertension. Circ. Cardiovasc Genet. 5 (3), 336–343. doi:10.1161/circgenetics.111.961888
Bastiani, M., and Parton, R. G. (2010). Caveolae at a glance. J. Cell. Sci. 123 (22), 3831–3836. doi:10.1242/jcs.070102
Brookes, B. C. (1985). Sources of information on specific subjects" by S.C. Bradford. J. Inf. Sci. 10 (4), 173–175. doi:10.1177/016555158501000406
Cao, H., Alston, L., Ruschman, J., and Hegele, R. A. (2008). Heterozygous CAV1 frameshift mutations (MIM 601047) in patients with atypical partial lipodystrophy and hypertriglyceridemia. Lipids Health Dis. 7, 3. doi:10.1186/1476-511x-7-3
Carver, L. A., and Schnitzer, J. E. (2003). Caveolae: Mining little caves for new cancer targets. Nat. Rev. Cancer 3 (8), 571–581. doi:10.1038/nrc1146
Celus, W., Di Conza, G., Oliveira, A. I., Ehling, M., Costa, B. M., Wenes, M., et al. (2017). Loss of caveolin-1 in metastasis-associated macrophages drives lung metastatic growth through increased angiogenesis. Cell. Rep. 21 (10), 2842–2854. doi:10.1016/j.celrep.2017.11.034
Chanvorachote, P., Pongrakhananon, V., and Halim, H. (2014). Caveolin-1 regulates metastatic behaviors of anoikis resistant lung cancer cells. Mol. Cell. Biochem. 399, 291–302. doi:10.1007/s11010-014-2255-4
Chatterjee, M., Ben-Josef, E., Robb, R., Vedaie, M., Seum, S., Thirumoorthy, K., et al. (2017). Caveolae-mediated endocytosis is critical for albumin cellular uptake and response to albumin-bound chemotherapy. Cancer Res. 77 (21), 5925–5937. doi:10.1158/0008-5472.Can-17-0604
Chen, C., and Song, M. (2019). Visualizing a field of research: A methodology of systematic scientometric reviews. PLoS One 14 (10), e0223994. doi:10.1371/journal.pone.0223994
Chen, C. (2004). Searching for intellectual turning points: Progressive knowledge domain visualization. Proc. Natl. Acad. Sci. 101 (1), 5303–5310. doi:10.1073/pnas.0307513100
Cohen, A. W., Hnasko, R., Schubert, W., and Lisanti, M. P. (2004). Role of caveolae and caveolins in health and disease. Physiol. Rev. 84 (4), 1341–1379. doi:10.1152/physrev.00046.2003
Doherty, G. J., and McMahon, H. T. (2009). Mechanisms of endocytosis. Annu. Rev. Biochem. 78 (1), 857–902. doi:10.1146/annurev.biochem.78.081307.110540
dos Santos, T., Varela, J., Lynch, I., Salvati, A., and Dawson, K. A. (2011). Effects of transport inhibitors on the cellular uptake of carboxylated polystyrene nanoparticles in different cell lines. PLoS One 6 (9), e24438. doi:10.1371/journal.pone.0024438
Drab, M., Verkade, P., Elger, M., Kasper, M., Lohn, M., Lauterbach, B., et al. (2001). Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293 (5539), 2449–2452. doi:10.1126/science.1062688
Du, X., Qian, X., Papageorge, A., Schetter, A. J., Vass, W. C., Liu, X., et al. (2012). Functional interaction of tumor suppressor DLC1 and caveolin-1 in cancer cells. Cancer Res. 72 (17), 4405–4416. doi:10.1158/0008-5472.Can-12-0777
Durieux, V., and Gevenois, P. A. (2010). Bibliometric indicators: Quality measurements of scientific publication. Radiology 255 (2), 342–351. doi:10.1148/radiol.09090626
Faggi, F., Chiarelli, N., Colombi, M., Mitola, S., Ronca, R., Madaro, L., et al. (2015). Cavin-1 and Caveolin-1 are both required to support cell proliferation, migration and anchorage-independent cell growth in rhabdomyosarcoma. Lab. Invest. 95 (6), 585–602. doi:10.1038/labinvest.2015.45
Goetz, J. G., Lajoie, P., Wiseman, S. M., and Nabi, I. R. (2008). Caveolin-1 in tumor progression: The good, the bad and the ugly. Cancer Metastasis Rev. 27 (4), 715–735. doi:10.1007/s10555-008-9160-9
Goetz Jacky, G., Minguet, S., Navarro-Lérida, I., Lazcano Juan, J., Samaniego, R., Calvo, E., et al. (2011). Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell. 146 (1), 148–163. doi:10.1016/j.cell.2011.05.040
Gupta, R., Toufaily, C., and Annabi, B. (2014). Caveolin and cavin family members: Dual roles in cancer. Biochimie 107, 188–202. doi:10.1016/j.biochi.2014.09.010
Hanahan, D., and Weinberg, R. A. (2000). The hallmarks of cancer. Cell. 100 (1), 57–70. doi:10.1016/s0092-8674(00)81683-9
Hill, M. M., Bastiani, M., Luetterforst, R., Kirkham, M., Kirkham, A., Nixon, S. J., et al. (2008). PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell. 132 (1), 113–124. doi:10.1016/j.cell.2007.11.042
Hirsch, J. E. (2005). An index to quantify an individual's scientific research output. Proc. Natl. Acad. Sci. U. S. A. 102 (46), 16569–16572. doi:10.1073/pnas.0507655102
Ho, C. C., Kuo, S. H., Huang, P. H., Huang, H. Y., Yang, C. H., and Yang, P. C. (2008). Caveolin-1 expression is significantly associated with drug resistance and poor prognosis in advanced non-small cell lung cancer patients treated with gemcitabine-based chemotherapy. Lung Cancer 59 (1), 105–110. doi:10.1016/j.lungcan.2007.07.024
Huang, K., Fang, C., Yi, K., Liu, X., Hongzhao, Q., Tan, Y., et al. (2018). The role of PTRF/Cavin1 as a biomarker in both glioma and serum exosomes. Theranostics 8, 1540–1557. doi:10.7150/thno.22952
Jiang, Y., Krantz, S., Qin, X., Li, S., Gunasekara, H., Kim, Y. M., et al. (2022). Caveolin-1 controls mitochondrial damage and ROS production by regulating fission - fusion dynamics and mitophagy. Redox Biol. 52, 102304. doi:10.1016/j.redox.2022.102304
Li, T., Kang, G., Wang, T., and Huang, H. (2018). Tumor angiogenesis and anti-angiogenic gene therapy for cancer. Oncol. Lett. 16 (1), 687–702. doi:10.3892/ol.2018.8733
Lin, C. J., Yun, E. J., Lo, U. G., Tai, Y. L., Deng, S., Hernandez, E., et al. (2019). The paracrine induction of prostate cancer progression by caveolin-1. Cell. Death Dis. 10 (11), 834. doi:10.1038/s41419-019-2066-3
Liu, P., Rudick, M., and Anderson, R. G. (2002). Multiple functions of caveolin-1. J. Biol. Chem. 277 (44), 41295–41298. doi:10.1074/jbc.R200020200
Liu, G., Jiang, R., and Jin, Y. (2014). Sciatic nerve injury repair: A visualized analysis of research fronts and development trends. Neural Regen. Res. 9 (18), 1716–1722. doi:10.4103/1673-5374.141810
Liu, W. R., Jin, L., Tian, M. X., Jiang, X. F., Yang, L. X., Ding, Z. B., et al. (2016). Caveolin-1 promotes tumor growth and metastasis via autophagy inhibition in hepatocellular carcinoma. Clin. Res. Hepatol. Gastroenterol. 40 (2), 169–178. doi:10.1016/j.clinre.2015.06.017
Liu, M., Chen, Y., Guo, Y., Yuan, H., Cui, T., Yao, S., et al. (2022). Golgi apparatus-targeted aggregation-induced emission luminogens for effective cancer photodynamic therapy. Nat. Commun. 13 (1), 2179. doi:10.1038/s41467-022-29872-7
Lobos-González, L., Aguilar, L., Diaz, J., Diaz, N., Urra, H., Torres, V. A., et al. (2013). E-cadherin determines Caveolin-1 tumor suppression or metastasis enhancing function in melanoma cells. Pigment. Cell. Melanoma Res. 26 (4), 555–570. doi:10.1111/pcmr.12085
Lu, Z., Ghosh, S., Wang, Z., and Hunter, T. (2003). Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of β-catenin, and enhanced tumor cell invasion. Cancer Cell. 4, 499–515. doi:10.1016/S1535-6108(03)00304-0
Luanpitpong, S., Talbott, S. J., Rojanasakul, Y., Nimmannit, U., Pongrakhananon, V., Wang, L., et al. (2010). Regulation of lung cancer cell migration and invasion by reactive oxygen species and caveolin-1. J. Biol. Chem. 285 (50), 38832–38840. doi:10.1074/jbc.M110.124958
Luo, D., Liang, W., Ma, B., and Xue, D. (2022). Global trends of indocyanine green fluorescence navigation in laparoscopic cholecystectomy: Bibliometrics and knowledge atlas analysis. Surg. Endosc. 36 (9), 6419–6431. doi:10.1007/s00464-021-08988-9
Maiuthed, A., Bhummaphan, N., Luanpitpong, S., Mutirangura, A., Aporntewan, C., Meeprasert, A., et al. (2018). Nitric oxide promotes cancer cell dedifferentiation by disrupting an oct4:caveolin-1 complex: A new regulatory mechanism for cancer stem cell formation. J. Biol. Chem. 293 (35), 13534–13552. doi:10.1074/jbc.RA117.000287
Martinez-Outschoorn, U. E., Trimmer, C., Lin, Z., Whitaker-Menezes, D., Chiavarina, B., Zhou, J., et al. (2010a). Autophagy in cancer associated fibroblasts promotes tumor cell survival: Role of hypoxia, HIF1 induction and NFκB activation in the tumor stromal microenvironment. Cell. Cycle 9 (17), 3515–3533. doi:10.4161/cc.9.17.12928
Martinez-Outschoorn, U. E., Whitaker-Menezes, D., Pavlides, S., Chiavarina, B., Bonuccelli, G., Casey, T., et al. (2010b). The autophagic tumor stroma model of cancer or "battery-operated tumor growth": A simple solution to the autophagy paradox. Cell. Cycle 9 (21), 4297–4306. doi:10.4161/cc.9.21.13817
Martinez-Outschoorn, U. E., Sotgia, F., and Lisanti, M. P. (2015). Caveolae and signalling in cancer. Nat. Rev. Cancer 15 (4), 225–237. doi:10.1038/nrc3915
Marx, J. (2001). Caveolae: A once-elusive structure gets some respect. Science 294 (5548), 1862–1865. doi:10.1126/science.294.5548.1862
Meng, F., Saxena, S., Liu, Y., Joshi, B., Wong, T. H., Shankar, J., et al. (2017). The phospho-caveolin-1 scaffolding domain dampens force fluctuations in focal adhesions and promotes cancer cell migration. Mol. Biol. Cell. 28 (16), 2190–2201. doi:10.1091/mbc.E17-05-0278
Nassar, Z. D., Hill, M. M., Parton, R. G., and Parat, M. O. (2013). Caveola-forming proteins caveolin-1 and PTRF in prostate cancer. Nat. Rev. Urol. 10 (9), 529–536. doi:10.1038/nrurol.2013.168
Neofytou, K., Pikoulis, E., Petrou, A., Agrogiannis, G., Petrides, C., Papakonstandinou, I., et al. (2017). Weak stromal Caveolin-1 expression in colorectal liver metastases predicts poor prognosis after hepatectomy for liver-only colorectal metastases. Sci. Rep. 7 (1), 2058. doi:10.1038/s41598-017-02251-9
Ortiz, R., Díaz, J., Díaz, N., Lobos-Gonzalez, L., Cárdenas, A., Contreras, P., et al. (2016). Extracellular matrix-specific Caveolin-1 phosphorylation on tyrosine 14 is linked to augmented melanoma metastasis but not tumorigenesis. Oncotarget 7 (26), 40571–40593. doi:10.18632/oncotarget.9738
Palade, G. E., and Bruns, R. R. (1968). Structural modulations of plasmalemmal vesicles. J. Cell. Biol. 37 (3), 633–649. doi:10.1083/jcb.37.3.633
Parton, R. G., and Richards, A. A. (2003). Lipid rafts and caveolae as portals for endocytosis: New insights and common mechanisms. Traffic 4 (11), 724–738. doi:10.1034/j.1600-0854.2003.00128.x
Parton, R. G. (2018). Caveolae: Structure, function, and relationship to disease. Annu. Rev. Cell. Dev. Biol. 34, 111–136. doi:10.1146/annurev-cellbio-100617-062737
Patani, N., Martin, L. A., Reis-Filho, J. S., and Dowsett, M. (2012). The role of caveolin-1 in human breast cancer. Breast Cancer Res. Treat. 131 (1), 1–15. doi:10.1007/s10549-011-1751-4
Pavlides, S., Whitaker-Menezes, D., Castello-Cros, R., Flomenberg, N., Witkiewicz, A. K., Frank, P. G., et al. (2009). The reverse Warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell. Cycle 8 (23), 3984–4001. doi:10.4161/cc.8.23.10238
Pereira, P. M. R., Sharma, S. K., Carter, L. M., Edwards, K. J., Pourat, J., Ragupathi, A., et al. (2018). Caveolin-1 mediates cellular distribution of HER2 and affects trastuzumab binding and therapeutic efficacy. Nat. Commun. 9 (1), 5137. doi:10.1038/s41467-018-07608-w
Razani, B., Engelman, J. A., Wang, X. B., Schubert, W., Zhang, X. L., Marks, C. B., et al. (2001). Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J. Biol. Chem. 276 (41), 38121–38138. doi:10.1074/jbc.M105408200
Razani, B., Combs, T. P., Wang, X. B., Frank, P. G., Park, D. S., Russell, R. G., et al. (2002). Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J. Biol. Chem. 277 (10), 8635–8647. doi:10.1074/jbc.M110970200
Seker, M., Aydin, D., Bilici, A., Yavuzer, D., Ozgun, M. G., Ozcelik, M., et al. (2017). Correlation of caveolin-1 expression with prognosis in patients with gastric cancer after gastrectomy. Oncol. Res. Treat. 40 (4), 185–190. doi:10.1159/000456620
Shamay, Y., Shah, J., Işık, M., Mizrachi, A., Leibold, J., Tschaharganeh, D. F., et al. (2018). Quantitative self-assembly prediction yields targeted nanomedicines. Nat. Mater 17 (4), 361–368. doi:10.1038/s41563-017-0007-z
Shimizu, K., Kirita, K., Aokage, K., Kojima, M., Hishida, T., Kuwata, T., et al. (2017). Clinicopathological significance of caveolin-1 expression by cancer-associated fibroblasts in lung adenocarcinoma. J. Cancer Res. Clin. Oncol. 143 (2), 321–328. doi:10.1007/s00432-016-2285-2
Sotgia, F., Martinez-Outschoorn, U. E., Howell, A., Pestell, R. G., Pavlides, S., and Lisanti, M. P. (2012). Caveolin-1 and cancer metabolism in the tumor microenvironment: Markers, models, and mechanisms. Annu. Rev. Pathol. 7, 423–467. doi:10.1146/annurev-pathol-011811-120856
Sunaga, N., Miyajima, K., Suzuki, M., Sato, M., White, M. A., Ramirez, R. D., et al. (2004). Different roles for caveolin-1 in the development of non-small cell lung cancer versus small cell lung cancer. Cancer Res. 64 (12), 4277–4285. doi:10.1158/0008-5472.Can-03-3941
Sung, M., Tan, X., Lu, B., Golas, J., Hosselet, C., Wang, F., et al. (2018). Caveolae-mediated endocytosis as a novel mechanism of resistance to trastuzumab emtansine (T-DM1). Mol. Cancer Ther. 17 (1), 243–253. doi:10.1158/1535-7163.Mct-17-0403
Tang, Y., Zeng, X., He, F., Liao, Y., Qian, N., and Toi, M. (2012). Caveolin-1 is related to invasion, survival, and poor prognosis in hepatocellular cancer. Med. Oncol. 29 (2), 977–984. doi:10.1007/s12032-011-9900-5
Torre, L. A., Siegel, R. L., Ward, E. M., and Jemal, A. (2016). Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol. Biomarkers Prev. 25 (1), 16–27. doi:10.1158/1055-9965.Epi-15-0578
van Eck, N. J., and Waltman, L. (2010). Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 84 (2), 523–538. doi:10.1007/s11192-009-0146-3
Volonte, D., and Galbiati, F. (2020). Caveolin-1, a master regulator of cellular senescence. Cancer Metastasis Rev. 39 (2), 397–414. doi:10.1007/s10555-020-09875-w
Volonte, D., Vyas, A. R., Chen, C., Dacic, S., Stabile, L. P., Kurland, B. F., et al. (2018). Caveolin-1 promotes the tumor suppressor properties of oncogene-induced cellular senescence. J. Biol. Chem. 293 (5), 1794–1809. doi:10.1074/jbc.M117.815902
Wang, Z., Wang, N., Li, W., Liu, P., Chen, Q., Situ, H., et al. (2014). Caveolin-1 mediates chemoresistance in breast cancer stem cells via β-catenin/ABCG2 signaling pathway. Carcinogenesis 35 (10), 2346–2356. doi:10.1093/carcin/bgu155
Watanabe, M., Yang, G., Cao, G., Tahir, S. A., Naruishi, K., Tabata, K., et al. (2009). Functional analysis of secreted caveolin-1 in mouse models of prostate cancer progression. Mol. Cancer Res. 7 (9), 1446–1455. doi:10.1158/1541-7786.Mcr-09-0071
Williams, T. M., and Lisanti, M. P. (2004). The caveolin genes: From cell biology to medicine. Ann. Med. 36 (8), 584–595. doi:10.1080/07853890410018899
Williams, T. M., and Lisanti, M. P. (2005). Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am. J. Physiol. Cell. Physiol. 288 (3), C494–C506. doi:10.1152/ajpcell.00458.2004
Williams, T. M., Medina, F., Badano, I., Hazan, R. B., Hutchinson, J., Muller, W. J., et al. (2004). Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo. Role of Cav-1 in cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion. J. Biol. Chem. 279 (49), 51630–51646. doi:10.1074/jbc.M409214200
Williams, T. M., Hassan, G. S., Li, J., Cohen, A. W., Medina, F., Frank, P. G., et al. (2005). Caveolin-1 promotes tumor progression in an autochthonous mouse model of prostate cancer: Genetic ablation of cav-1 delays advanced prostate tumor development in tramp mice. J. Biol. Chem. 280 (26), 25134–25145. doi:10.1074/jbc.M501186200
Witkiewicz, A. K., Dasgupta, A., Sotgia, F., Mercier, I., Pestell, R. G., Sabel, M., et al. (2009a). An absence of stromal caveolin-1 expression predicts early tumor recurrence and poor clinical outcome in human breast cancers. Am. J. Pathol. 174 (6), 2023–2034. doi:10.2353/ajpath.2009.080873
Witkiewicz, A. K., Casimiro, M. C., Dasgupta, A., Mercier, I., Wang, C., Bonuccelli, G., et al. (2009b). Towards a new "stromal-based" classification system for human breast cancer prognosis and therapy. Cell. Cycle 8 (11), 1654–1658. doi:10.4161/cc.8.11.8544
Xu, P., Lv, T., Dong, S., Cui, Z., Luo, X., Jia, B., et al. (2022). Association between intestinal microbiome and inflammatory bowel disease: Insights from bibliometric analysis. Comput. Struct. Biotechnol. J. 20, 1716–1725. doi:10.1016/j.csbj.2022.04.006
Yamada, E. (1955). The fine structure of the gall bladder epithelium of the mouse. J. Biophys. Biochem. Cytol. 1 (5), 445–458. doi:10.1083/jcb.1.5.445
Yamao, T., Yamashita, Y. I., Yamamura, K., Nakao, Y., Tsukamoto, M., Nakagawa, S., et al. (2019). Cellular senescence, represented by expression of caveolin-1, in cancer-associated fibroblasts promotes tumor invasion in pancreatic cancer. Ann. Surg. Oncol. 26 (5), 1552–1559. doi:10.1245/s10434-019-07266-2
Yang, X., Huang, B., Deng, L., and Hu, Z. (2018). Progress in gene therapy using oncolytic vaccinia virus as vectors. J. Cancer Res. Clin. Oncol. 144 (12), 2433–2440. doi:10.1007/s00432-018-2762-x
Keywords: bibliometric analysis, visualization, caveolin, cancer, VOSviewer, CiteSpace, R-bibliometrix
Citation: Tan Y and Song Q (2023) Research trends and hotspots on the links between caveolin and cancer: bibliometric and visual analysis from 2003 to 2022. Front. Pharmacol. 14:1237456. doi: 10.3389/fphar.2023.1237456
Received: 09 June 2023; Accepted: 21 July 2023;
Published: 28 July 2023.
Edited by:
Mohammed Abu El-Magd, Kafrelsheikh University, EgyptReviewed by:
Huizhen Nie, Shanghai Cancer Institute, ChinaNeil Bradbury, Rosalind Franklin University of Medicine and Science, United States
Copyright © 2023 Tan and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Song, c29uZ3FpMzE5QGhvdG1haWwuY29t
 Yaqian Tan
Yaqian Tan Qi Song
Qi Song