- The First Affiliated Hospital of Wannan Medical College (Yijishan Hospital of Wannan Medical College), Wuhu, Anhui, China
Objective: The problems and challenges encountered by Chinese medical institutions in implementing the national centralized drug procurement was investigated and analyzed in order to provide reference for the regulatory agencies to formulate policies.
Methods: A questionnaire survey was conducted to collect the problems encountered by 329 Chinese medical institutions in implementing the national centralized drug procurement and the corresponding suggestions provided by relevant experts. Statistical analysis was performed to identify differences in the themes and the number of collected problems, further revealing the relevance to the region in which the medical institutions is located.
Result: 1360 problems and suggestions were collected from 329 Chinese medical institutions that located in North (19.15%), Northeast (5.78%), East (33.43%), Central (10.03%), South (9.73%), Southwest (14.89%), and Northwest China (6.99%). There was statistically significant difference in the number of collected problems and suggestions between regions (p < 0.001). Furthermore, the content of gathered problems and suggestions involves in 15 themes including system construction, organizational system and work responsibilities, reasonable measurement and reporting of procurement volume et al. These themes that these medical institutions are focusing on are mainly centered on the supply guarantee (15%), reasonable measurement and reporting of procurement volume (11.40%) and guarantee measures for clinical priority use (9.48%) of drugs with national centralized procurement. Meanwhile, we found that problems regarding the supply guarantee of drugs with national centralized procurement displayed significant difference between regions (p = 0.0096).
Conclusion: Chinese medical institutions are facing great challenges in implementing the national centralized drug procurement. The scientific study and judgment of the current situation and the construction of corresponding solution require a precise classification of the problems encountered by medical institutions in the process of implementing the national centralized drug procurement policy, which is of great practical significance for deepening the reform of the medical and health system.
Introduction
Increasing pharmaceutical expenditures is plaguing many countries worldwide (Lopez Bastida et al., 2000; Smith.2004). Global medicine spending is projected to increase at 2–5% annually and exceed $1.1 trillion in 2024 (Institute.2020). In China, healthcare expenditures increased rapidly at a nearly 20% annual growth rate (Yuan et al., 2021). In order to reduce the pharmaceutical expenditures and standardize drug circulation, the Chinese government has implemented national centralized drug procurement (NCDP) since 2018. As of February 2022, prices of 234 medicines have been lowered through national centralized procurement, with the average rate of reduction exceeding 53%, and the cumulative savings in drug costs have exceeded 260 billion yuan (Zou et al., 2023). Moreover, national centralized drug procurement is being optimized and improved, gradually including the basic medical insurance drugs with bulk usage and procurement-volume and all types of clinical essential medicines with reliable quality (Chinese Government.2019; 2021).
Public medical institutions are required to participate in the national centralized drug procurement and prioritize the use of drugs with national centralized procurement (DNCP) in accordance with clinical demands (Gong et al., 2021). The application of NCDP has brought significant economic and social benefits and enhanced the rational use of clinical drugs, playing a positive effect on healthcare reform in China (Chen et al., 2020; Yuan et al., 2021). For instance, pharmaceutical expenditures and irrational clinical use rate were reduced comparison before and after NCDP policy (Hu et al., 2022; Wan et al., 2022; Wang et al., 2021). Meanwhile, drug utilization and substitution rate of generic drugs significantly increased after policy intervention (Xie et al., 2021; Zhao et al., 2022). However, some problems have been disclosed in practicing NCDP by medical institutions, which significantly affected the effects of NCDP implementation. For example, lacking scientific estimation system resulted in large discrepancies between procurement volume and usage volume by medical institutions (Xu.2022). Due to the difficulty of securing drug supply in primary medical institutions, some drugs with national centralized procurement are in shortage or even out of supply, thus affecting clinical use of drugs (Guo et al., 2015). In some medical institutions, non-winning drugs are completely discontinued in order to complete the contract dosage, so that the patient’s drug needs are not met, thus aggravating the doctor-patient disputes (Li et al., 2022). Therefore, it is necessary and urgent to comprehensively understand the difficulties encountered by medical institutions in implementing the NCDP and provide corresponding solutions to promote the in-depth implementation of the national centralized drug procurement policy.
To further improve the standardization of NCDP policy at medical institution level, several provinces and autonomous regions have issued expert consensus or recommendations on the implementation of NCDP in Chinese medical institutions (Chen et al., 2022; Ye et al., 2021). Our group as co-leader gathered up 302 multidisciplinary experts from 128 Chinese medical institutions to write the “ Consensus of Chinese expert on the precise management of national centralized drug volume-based procurement in medical institutions” in 2022 (Chinese Pharmacists Association.2023). This study aims to comprehensively reveal the problems encountered by Chinese medical institutions during implementing of NCDP policy and provide relevant recommendations given by experts, which is an important reference for the development of relevant policies.
Methods
Research subjects
This study conducted a targeted survey of 329 Chinese public medical institutions from 22 provinces, 5 autonomous regions and 4 municipalities directly under the Central Government (excluding Hong Kong, Macao and Taiwan). These medical institutions include provincial or university-affiliated hospitals, municipal, county and community health service centers. Meanwhile, 578 participants, which belong to multiple disciplines or departments including pharmacy department, medical department, clinical department, medical insurance department, and medical quality control department, involved in this survey.
Questionnaire design and data collection
After considerable discussion, the outline of the questionnaire was determined by the research committee. As is shown in Supplementary Table S1, the questionnaire contains 6 primary catalogs which further subdivided into 15 secondary catalogs, involved the whole process of implementation of NCDP in medical institutions. Besides, open-ended questions included in each catalog intentionally, mainly for collection of the problems in the implementation of NCDP and corresponding suggestions. Subsequently, the questionnaires were directly conducted to 329 medical institutions. These collected questions are categorized into 15 secondary categories. Regarding the handling of open-ended questions, they will be grouped under the 15 entries mentioned above. However, in case of disputes they will be further discussed by the research committee. Ultimately, these questionnaire results were further analyzed, summarized, and organized using statistical methods.
Statistical methods
IBM 24.0 SPSS statistical software (IBM Corp., Armonk, NY, United States) was used for analysis. Count data were expressed as cases or percentages, and the χ2 test and one-way ANOVA analysis were used for comparison between groups. p < 0.05 was considered a statistically significant difference.
Results
Characteristics of the surveyed medical institutions and respondents
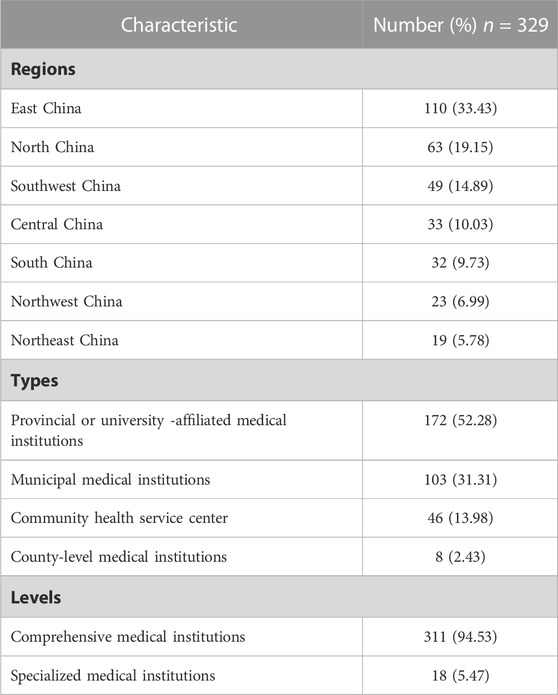
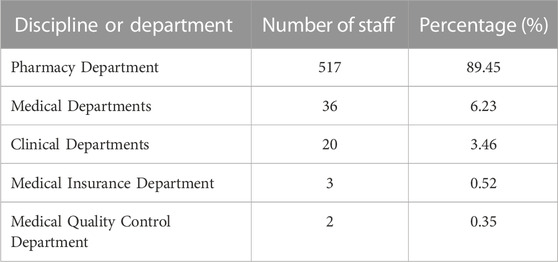
To examine the status of implementing of national centralized drugs procurement in Chinese medical institutions, this study conducted targeted research on 329 Chinese medical institutions. The recall rate and efficiency rate of the questionnaires were 100% and 100%, respectively, attributing to the fact that the research was conducted in a targeted manner. The regional and hierarchical distribution of medical institutions were taken into full consideration. As shown in Table 1, the regions of 329 surveyed medical institutions were located North (19.15%), Northeast (5.78%), East (33.43%), Central (10.03%), South (9.73%), Southwest (14.89%), and Northwest China (6.99%). These investigated institutions contain the provincial or university-affiliated medical institutions (52.28%), municipal medical institutions (31.31%), community health service centers (13.98%), and county-level medical institutions (2.43%). Of note, a total of 578 participants mainly distributed in department of pharmacy, medical, clinical, medical insurance, and medical treatment control, basically covering the essential departments for implementing national centralized drugs procurement. Among of these participants, pharmacy department presented largest number of personnel (89.45%) (Table 2), because they have taken on the primary task of implementing NCDP in medical institutions. Taken together, this research not only reflects the actual situation of implementing of NCDP in different regions and levels of Chinese medical institutions, but also visualizes the problems encountered by the main enforcement departments of NCDP in medical institutions.
Characteristics of problems encountered by Chinese medical institutions in implementing of NCDP
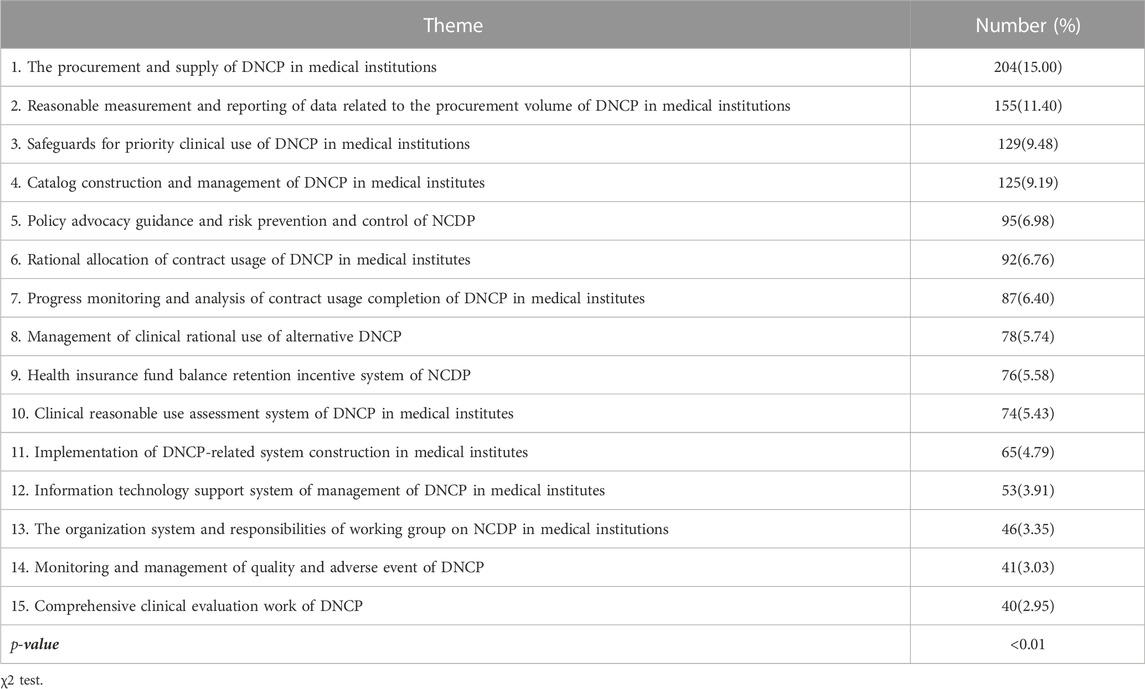
In order to gain an in-depth understanding of the difficulties faced by Chinese medical institutions in implementation of national centralized drug procurement, the 1360 gathered problems and suggestions were further analyzed. As is shown in Table 3, the content of compiled problems and suggestions could be categorized into 15 aspects covering the whole process of implementing NCDP in medical institutions, which revealed the fact that Chinese medical institutions are facing serious challenges in the implementation of NCDP. Statistical analysis revealed significant differences in the number of relevant problems and suggestions between the 15 categories. The gathered opinions and suggestions were relatively focused on the procurement and supply, reporting of procurement volume, and clinical priority use of security measures of drugs with national centralized procurement in Chinese medical institutions, as evidenced by the number of relevant opinions and suggestions accounted for 15%, 11.40%, and 9.48%, respectively. To illustrate the problems and suggestions under the various themes, the detailed enumeration of collected problems and suggestions is displayed in Table 4; Supplementary Table S2.
Correlation between the region of the medical institution and collected problems
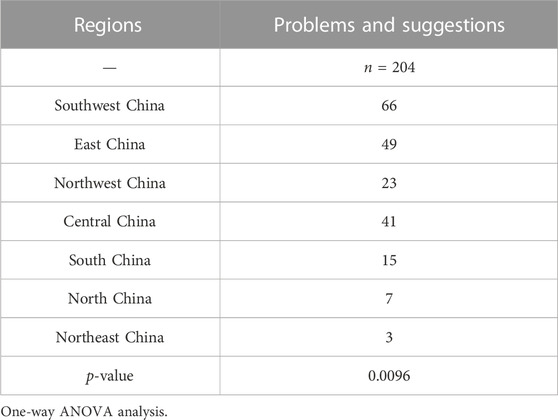
In this study, questionnaires were distributed to 329 Chinese medical institutions and collected 1360 problems and suggestions. As displayed in Table 5, there was statistically significant difference in the number of problems and suggestions between regions (p < 0.001), indicating that the implementation of NCDP by medical institutions is closely related to the region of the medical institution. Besides, the gathered feedback regarding the procurement and supply of drugs with national centralized procurement displayed significant difference (p = 0.0096) between regions (Table 6). Therefore, subsequent policy development and optimization should take regional differences into account.
Discussion
NCDP is a unique form of drug procurement in China, which playing a positive role in Chinese healthcare reform through reducing the prices of medicines and improving the rationality of clinical use of medicines. However, NCDP has gradually highlighted a number of typical problems and contradictions over the years, which have directly led to a reduction in the efficiency of the policy (Gong et al., 2021; Zou et al., 2023). In this study, we aimed to reveal the situation of NCDP in Chinese medical institutions. The questionnaires were distributed to 329 Chinese medical institutions from North, Northeast, East, Central, South, Southwest, and Northwest China, which contains provincial, municipal, and community medical institutions. Meanwhile, this survey focused on multidisciplinary medical staff centered on pharmacy staff, which play a key role in the implementation of NCDP in medical institutions. Therefore, this study provides a more comprehensive and in-depth understanding of the current situation of NCDP in Chinese medical institutions. Furthermore, 329 valid questionnaires and 1360 problems and suggestions were collected. These problems and suggestions cover the whole process of implementation of NCDP, indicating the Chinese medical institutions is facing great challenges in the execution of NCDP.
There was statistically significant difference in the number of problems and suggestions between regions. Of these, the medical institutions coming from Southwest and Northwest regions have the highest average number of opinions and suggestions, probably due to the fact that these regions contain more remote areas, making it more difficult to implement the policy. The most suggested topics involve the clinical rational use of DNCP in medical institutions, including six aspects such as reasonable allocation of contract dosage, monitoring and analysis of completion progress, guarantee measures for clinical priority use, totaling 536 articles (39.41%). In addition, a total of 484 recommendations (35.59%) related to the procurement and supply guarantee of DNCP in medical institutions, including the reasonable measurement and reporting of data related to procurement volume, the construction and management of DNCP catalogs, and procurement and supply, which are basically consistent with those reported in the literature (Chinese Pharmacists Association.2023; Zou et al., 2023). The results demonstrated that the rational clinical use and security of supply are still the most important concerns of medical institutions in the process of implementing the NCDP policy. Among the collected opinions and suggestions, the largest number of problems and suggestions are related to DNCP procurement supply, a total of 204, accounting for 15%, focusing on the shortage of supply of the winning drugs. Some medical institutions reflected that most varieties of the winning drugs were in short supply at the beginning of national centralized drug procurement and 1 to 2 months before the end of the procurement cycle, resulting in discontinuity of clinical treatment, and even some winning drugs were in short supply for a long time, such as acarbose tablets, celecoxib capsules and tenofovir disoproxil fumarate tablets (Xu.2022). Remote areas such as Tibet and Xinjiang lack drug manufacturers, and the vast majority of drugs are transported from the mainland (Li, Yan, Bai, Li and Shao.2022). In addition, icy roads in winter make drugs not available in time, which can further cause local drug shortages and stock-outs. At the same time, some drugs are used in remote areas in small quantities and cannot reach the minimum delivery quantity of the manufacturer, which can also lead to abnormal drug supply. These results are mainly the result of three reasons. First of all, there are problems with the production capacity of manufacturers for winning drugs. Some of winning drug manufacturers failed to fully anticipate the inaccuracy of the medical institutions to report the volume or exceed the agreed procurement volume, resulting in an oversupply (Liu.2023; Sun et al., 2023). Secondly, there are problems with distribution enterprises in the transportation of winning drugs (Yang et al., 2023). According to the relevant documents, only one distribution enterprise is allowed for the winning drugs, but some of the distribution enterprises are small in scale with narrow distribution network, which affects the stability of the drug supply. Third, drug manufacturers control the sale of drugs in quantity and distribution. Some of the selected drug manufacturers who have completed the quantitative procurement of the winning drugs have controlled the distribution of drugs, resulting in tight supply or even shortage of drugs.
In order to avoid the above problems, experts from the participating medical institutions suggest optimizing the management involved in the pre, in and post process. For the pre-event phase, the competent authorities in the tender to fully assess the supply capacity of drug manufacturers. The winning drugs have at least two distribution enterprises for medical institutions to choose. By increasing the supervision of drug distribution enterprises to achieve a smooth interface between the drug manufacturer and the distributor (Tan et al., 2020). Because primary medical institutions in remote areas have less demand for drug use, which inevitably reduces the motivation of enterprises to distribute, the distribution rate of drugs in remote areas can be included as part of the qualification audit of distribution enterprises. In addition, medical institutions can make appropriate reserves of non-winning drugs before the start of a new batch of centralized procurement, leaving a buffer period of 1–2 months between procurement cycles. For the in-event phase, the competent departments regularly supervise drug manufacturers and distribution enterprises. Medical institutions establish contingency plans for shortages of DNCP, and report the production and distribution of the selected enterprises and distribution enterprises to the management. Collective drug procurement platform records the selected drug shortage information and vouchers, while the temporary procurement quantity is combined into the agreed procurement volume. For the post-event phase, the regulatory authorities can strengthen the monitoring and evaluation of the selected production enterprises, and include their production capacity, ability to guarantee supply and integrity into the assessment system. At the same time, it is recommended that specific penalty regulations be formulated at the national level to punish the consequences of default such as malicious bidding or inability to complete the procurement schedule after winning a bid, and failure to guarantee product quality after winning a bid. Increasing penalties at the national level can guide enterprises to bid wisely and strengthen the contractual spirit.
Conclusion
The problems collected in this research cover the whole process of implementing NCDP by medical institutions, and some relatively concentrated problems cannot be solved by medical institutions on their own, which suggests that only continuous summary experience, comparative research and based on national conditions can consolidate and expand the results of NCDP. The deep-seated conflicts in the procurement process should be directly confronted and corresponding solutions proposed in order to achieve meeting the clinical demand for drugs, promoting rational drug management, optimizing hospital management, and forming a market-based price formation mechanism. Only by formulating scientific and reasonable policies, combining and forming synergy from medical insurance, medical treatment, medicine, and patients, can we break through the existing blind spots and blockages, ensure that drug procurement policies are gradually moving on a good and correct track, and realize the reform of the medical and health system to benefit the general public.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
Writing—WZ and QX; Research and data collection—JP, XZ, and LC; Data analysis—YW and KY; Conceptualization and supervision—JL and XL. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by management and service innovation major project of the first affiliated hospital of Wannan medical college (Yijishan hospital of Wannan medical college), grant number CX2022004. Major Research Projects of Universities in Anhui Province, China (Grant no. 2023AH040238).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1233491/full#supplementary-material
References
Chen, L., Yang, Y., Luo, M., Hu, B., Yin, S., and Mao, Z. (2020). The impacts of national centralized drug procurement policy on drug utilization and drug expenditures: the case of shenzhen, China. Int. J. Environ. Res. Public Health 17 (24), 9415. doi:10.3390/ijerph17249415
Chen, Y., Feng, Y., Kong, X., Li, X., Liu, W., Meng, L., et al. (2022). Expert consensus on Beijing`s medical institutions implemented the centralized drug procurement organized by the State. China Med. 17 (11), 1601–1604. doi:10.3760/j.issn.1673-4777.2022.11.001
Chinese Government, General Office of the State Council on promoting the normalization and institutionalization of centralized quantity procurement of drugs [2021Jan 28].Beijing, China Office of the State Council.[cited 2023 May 04] Available from: http://www.gov.cn/zhengce/content/2021-01/28/content_5583305.htm.
Chinese Government, General Office of the State Council on the issuance of a pilot program for the centralized procurement and use of drugs by state organizations [2019 Jan 17].Beijing, China: Office of the State Council.[cited 2023 May 04] Available from: http://www.gov.cn/zhengce/content/2019-01/17/content_5358604.htm.
Chinese Pharmacists Association (2023). Consensus of Chinese experts on the precise management of national centralized drug volume-based procurement in medical institutions. Chin. J. New Drugs 32 (3), 311–322. doi:10.3969/j.issn.1003-3734.2023.03.014
Gong, X., Liu, D., Wei, Y., Yang, H., Chen, S., and He, G. (2021). Problems and countermeasures on the centralized drug procurement in large quantities in public hospitals. Chin. J. Hosp. Adm. 37 (10), 827–830. doi:10.3760/cma.j.cn111325-20210726-00674
Guo, Z., Hong, D., Liu, Y., Guo, N., Wang, B., Han, S., et al. (2015). A novel platform based on immobilized histidine tagged olfactory receptors, for the amperometric detection of an odorant molecule characteristic of boar taint. Chin. J. Health Policy 8 (12), 1–6. doi:10.1016/j.foodchem.2015.03.066
Hu, Q., Xiao, X., Li, C., Meng, X., Liu, S., and Deng, S. (2022). Clinical application of meropenem and rationality of prescriptions before and after centralized procurement of antibacterial agents. Chin. J. Nosocomiology 32 (6), 941–945. doi:10.11816/cn.ni.2022-212404
Institute Report, (2020). Global medicine spending and usage trends outlook to 2024. Beijing, China: The IQVIA Institute.
Li, D., Yan, J., Bai, M., Li, X., and Shao, R. (2022). Research on the supply problems and countermeasures of national centralized drug procurement. Health Econ. Res. 39 (6), 12–16. doi:10.14055/j.cnki.33-1056/f.2022.06.003
Liu, Y. (2023). Problems and discussion on the implementation of national drug centralized procurement policy. China Health Stand. Manag. 14 (2), 70–73. doi:10.3969/j.issn.1674-9316.2023.02.015
Lopez Bastida, J., and Mossialos, E. (2000). Pharmaceutical expenditure in spain: cost and control. Int. J. Health Serv. Plan. Adm. Eval. 30 (3), 597–616. doi:10.2190/YL3J-QK9B-0NMQ-KMVK
Smith, C. (2004). Retail prescription drug spending in the national health accounts. Health Aff. Proj. Hope) 23 (1), 160–167. doi:10.1377/hlthaff.23.1.160
Sun, Q., Jiang, B., and Liu, L. (2023). Investigation on the factors affecting the supply security of bid-winning drugs in national drug volume-based procurement. Chin. J. Pharm. 54 (1), 154–159. doi:10.16522/j.cnki.cjph.2023.01.020
Tan, Z., and Song, Q.(2020). Researches on the legal risks of drug supply in public hospitals under the background of drug volume procurement and expansion and its countermeasures. Chin. Health Serv. Manag. 37 (12), 913–915. doi:10.2147/CEOR.S140063
Wan, Q., Wang, J., and Gu, S. (2022). Influence of the national centralized drug procurement policy on clinical use of three anti-tumor drugs. China Pharm. 31 (16), 15–18. doi:10.3969/j.issn.1006-4931.2022.16.004
Wang, Y., Wang, Q., Su, M., Qi, L., and Yang, L. (2021). Influence of "4+7"quantity purchase policy on the rational use of antiplatelet drugs in a hospital. China Pharm. 30 (21), 8–11. doi:10.3969/j.issn.1006-4931.2021.21.003
Xie, J., Hu, Z., Wang, Y., and Shao, R. (2021). The influences of national centralized drug procurement policy on drug price, cost and generic drug substitution: taking the four municipalities data. Chin. Health Econ. 40, 24–28. doi:10.1101/2021.06.21.21256568
Xu, Y. C., HeSong, M. X. J. J., and Jin, J. C. L. (2022). Supply status of bid-winning drugsin national volume-based procurement and supporting suggestions. Chin. Health Econ. 41 (7), 65–67. doi:10.7664/j.issn.1003-0743.2022.7.zgwsjj202207016
Yang, Z., Xia, W., Luo, Q., Yu, X., Zhou, Y., and Xu, F. (2023). Establishment and application of dynamic evaluation system for drug distribution enterprises guided by drug adminstration management. Chin. J. Hosp. Pharm. 43 (8), 931–935. doi:10.13286/j.1001-5213.2023.08.17
Ye, D., Yang, C., Ji, W., Zheng, J., Zhang, J., Xue, R., et al. (2021). Impact of the expert consensus on carbapenem consumption trends and patterns in public healthcare institutes: an interrupted time series analysis, 2017-2020. Front. Pharmacol. 12, 739960. doi:10.3389/fphar.2021.739960
Yuan, J., Lu, K. L., Xiong, X., and Jiang, B. (2021). Lowering drug prices and enhancing pharmaceutical affordability: an analysis of the national volume-based procurement (NVBP) effect in China. Bmj Glob. Health 6 (9), 5519. doi:10.1136/bmjgh-2021-005519
Zhao, X., Li, M., Wang, H., Xu, X., Wu, X., Sun, Y., et al. (2022). Impact of national centralized drug procurement policy on antiviral utilization and expenditure for hepatitis B in China. J. Clin. Transl. Hepatology 10 (3), 420–428. doi:10.14218/JCTH.2022.00167
Keywords: national centralized drug procurement, medical institutions, problems, challenge, China
Citation: Zhang W, Xu Q, Peng J, Zhang X, Chen L, Wu Y, Yang K, Luan J and Liu X (2023) Problems and challenges encountered by Chinese medical institutions in implementing the national centralized drug procurement. Front. Pharmacol. 14:1233491. doi: 10.3389/fphar.2023.1233491
Received: 02 June 2023; Accepted: 17 August 2023;
Published: 30 August 2023.
Edited by:
Tanveer Ahmed Khan, National Institute of Health, PakistanReviewed by:
Xuedong Jia, The First Affiliated Hospital of Zhengzhou University, ChinaFaiz Ullah Khan, Xi’an Jiaotong University, China
Copyright © 2023 Zhang, Xu, Peng, Zhang, Chen, Wu, Yang, Luan and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyun Liu, MjAxMTEyMjNAd25tYy5lZHUuY24=; Jiajie Luan, bHVhbmppYWppZTc1N0AxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Wen Zhang
Wen Zhang Qingwen Xu†
Qingwen Xu† Yilai Wu
Yilai Wu