
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Pharmacol. , 24 August 2023
Sec. Pharmacology of Infectious Diseases
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1228548
This article is part of the Research Topic The Evolution in Pharmacology of Infectious Diseases: 2022 View all 16 articles
Azvudine (FNC) is a synthetic nucleoside analog used to treat adult patients living with human immunodeficiency virus-1 (HIV-1) infection with high viral load. After phosphorylation, Azvudine inhibits RNA-dependent RNA polymerase, leading to the discontinuation of RNA chain synthesis in viruses. In addition, Azvudine is the first dual-target nucleoside oral drug worldwide to simultaneously target reverse transcriptase and viral infectivity factors in the treatment of HIV infection. On 9 August 2022, Azvudine was incorporated into the Guidelines for the Diagnosis and Treatment of Coronavirus Disease 2019 (version ninth) issued by the National Health Commission and the National Administration of Traditional Chinese Medicine. The recommended oral dose of Azvudine for the treatment of moderate coronavirus disease 2019 (COVID-19) is 5 mg once daily, and the duration of Azvudine treatment should not exceed 14 days. Four phase III clinical trials were performed during 2020–2022 to evaluate the efficacy and safety of Azvudine in the treatment of COVID-19. The results revealed that Azvudine could reduce nucleic acid-negative conversion time, viral load, and time to improvement in clinical conditions in patients with moderate COVID-19. In addition, Azvudine exhibited good safety and tolerance. Thereafter, Azvudine was incorporated into the Chinese guidelines and expert consensus for the treatment of COVID-19 and was highly approbated. Furthermore, Azvudine was also included in the Chinese guidelines for HIV infection.
Azvudine (FNC) is a synthetic nucleoside analog used to treat adult patients with plasma human immunodeficiency virus-1 (HIV-1) RNA of more than 100,000 copies/mL through the combination of another nucleoside reverse transcriptase inhibitor (NRTI) or non-nucleoside reverse transcriptase inhibitor (NNRTI). Azvudine inhibits RNA-dependent RNA polymerase (RdRp) after phosphorylation, leading to the discontinuation of RNA chain synthesis in viruses, thereby playing an antiviral role (Henan Genuine Biotech, 2022). On 21 July 2021, Azvudine (Shuangxinaike®, Henan Genuine Biotech Co., Ltd., Pingdingshan, Henan Province, China) was approved by the National Medical Products Administration (NMPA, Beijing, China) for the treatment of adult HIV-1-infected patients (National Medical Products Administration, 2021). In addition, Azvudine is the first dual-target nucleoside oral agent worldwide to simultaneously target both reverse transcriptase and viral infectivity factors for the treatment of HIV infection. As a Chinese homegrown innovative drug for the treatment of acquired immunodeficiency syndrome (AIDS), Azvudine was developed with funding from the National Science and Technology Major Projects for Major New Drug Innovation (Genuine Biotech, 2021). Thereafter, Azvudine was incorporated into the Chinese guidelines for the diagnosis and treatment of AIDS (Acquired Immunodeficiency Syndrome and Hepatitis C Professional Group, Society of Infectious Diseases, Chinese Medical Association and Chinese Center for Disease Control and Prevention, 2021).
The ChemSrc database (https://www.chemsrc.com/en) was used to inquire about the chemical structure of Azvudine. The molecular formula of Azvudine is C9H11FN6O4 with a molecular weight of 286.22, and the chemical name is 4-amino-1-[(2R,3S,4R,5R)-5-azido-3-fluoro-4-hydroxy-5-(hydroxymethyl)tetrahydro-2-furanyl]-2(1H)-pyrimidinone (Supplementary Figure S1).
RdRp is highly conserved in the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and is an excellent target for the development of anti-SARS-CoV-2 agents (Rabie, 2021a; Rabie, 2021b; Rabie, 2021c; Vicenti et al., 2021; Rabie et al., 2023; Zhu, 2023a). Nucleoside analogs were considered candidate drugs for the treatment of coronavirus disease 2019 (COVID-19), and several nucleoside analogs were identified as promising anti-SARS-CoV-2 drugs based on satisfactory results of preclinical trials (Rabie and Abdalla, 2022; Eltayb et al., 2023; Rabie and Abdalla, 2023). Nucleoside analogs targeting RdRp are ideal anti-SARS-CoV-2 candidate drugs in consideration of the broad conservation of RdRp in all SARS-CoV-2 variants (Rabie, 2022a; Rabie, 2022b; Rabie and Abdalla, 2023; Rabie and Eltayb, 2023). Azvudine, as a nucleoside analog targeting highly conserved RdRp, had potential anti-SARS-CoV-2 effects and was selected as a possible effective candidate drug for the treatment of COVID-19 (Yu and Chang, 2020). A preclinical trial revealed that Azvudine was transformed into active Azvudine triphosphate concentrated in the thymus after phosphorylation, inhibited SARS-CoV-2 replication, and improved immunity. The subsequent pilot clinical study of Azvudine exhibited its excellent efficacy and satisfactory safety in the treatment of COVID-19 (Zhang et al., 2021). In addition, Ren et al. (2020) performed a preliminary clinical trial of Azvudine with a randomized, open-label, controlled design. A total of 20 patients with mild-to-moderate COVID-19 were randomly assigned to receive Azvudine or standard antiviral treatment with a ratio of 1:1. The results revealed that Azvudine shortens the time of nucleic acid-negative conversion (NANC) compared to standard antiviral treatment. Moreover, Azvudine displayed satisfactory safety without adverse events occurring during the treatment.
However, the two pilot clinical studies of Azvudine are short of a large sample size. Thereafter, four phase III clinical trials were performed during 2020–2022 to evaluate the efficacy and safety of Azvudine in the treatment of COVID-19. Here, the therapeutic effects of Azvudine on COVID-19 were comprehensively analyzed using the clinical data from four phase III clinical trials.
The four phase III clinical trials were carried out in China, Russia, and Brazil. In China, the phase III clinical trial was registered in the Chinese Clinical Trial Registry (ChiCTR2000032769; registration date: 9 May 2020; website: https://www.chictr.org.cn/showproj.aspx?proj=53368), and the clinical trial was simultaneously registered in the International Clinical Trials Registry Platform (NCT04425772; registration date: 9 May 2020; website: https://trialsearch.who.int/Trial2.aspx?TrialID=NCT04425772). The randomized, double-blinded, parallel-controlled clinical trial was carried out in Beijing Ditan Hospital affiliated to Capital Medical University (Beijing, China) from June 2020 to March 2022. The sponsor was Henan Genuine Biotech Co., Ltd. A total of 348 patients with COVID-19 were enrolled in the study, and the patients were randomly assigned to the Azvudine group or control group with a 1:1 ratio. The patients in the Azvudine group received oral Azvudine tablets (5 mg once daily) plus standard treatment, and the patients in the control group received Azvudine dummy tablets (placebo) and standard treatment. The duration of treatment in both groups was less than 14 days. The primary efficacy outcome was the change (reduction) from baseline in viral load on days 7 and 14. The secondary primary efficacy outcomes included the time of NANC, nucleic acid conversion rate, the proportion of patients changing from mild-or-moderate COVID-19 to severe COVID-19 in the severity of the disease, the proportion of patients changing from severe COVID-19 to critical COVID-19 in severity, time and proportion of improvement in pulmonary imaging, time and rate of improvement in respiratory symptoms and other symptoms, frequency of requirement for supplemental oxygen or non-invasive ventilation, changes in blood oxygen detection index, and time and proportion of temperature return to normal levels. As a result, among the patients with viral load ≥ 3 log10 copies/mL, the reduction from baseline in viral load on days 3, 5, and 7 in the Azvudine group was higher than that in the placebo group. However, only the difference in the reduction from baseline in viral load on day 5 between the Azvudine group and placebo group was statistically significant. This result was similar to that in the patients with viral load ≥ 4 log10 copies/mL. Unfortunately, there were no significant differences in secondary primary efficacy outcomes between the Azvudine and placebo groups. In addition, 341 subjects were involved in the safety analysis. A total of 62 subjects experienced 119 adverse events (AEs) in the Azvudine group, while 76 subjects experienced 175 AEs in the placebo group. Most of the AEs were evaluated as grade 1 or grade 2 according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 of the National Cancer Institute. One patient experienced an AE that was evaluated as grade 3 in the Azvudine group, while three patients experienced three AEs that were evaluated as grade 3 in the placebo group. No AEs were evaluated as grade 4, or no serious AEs occurred. There was no significant difference in the incidence or severity of AEs between the Azvudine and placebo groups (ClinicalTrials, 2022; Zhang, 2022).
In Russia, the phase III clinical trial was approved by the Russian Ministry of Health (http://www.minzdravsoc.ru) in January 2021 and was initiated in June 2021. The results of the two phase III clinical trials performed in China and Russia were disclosed in detail by Fujie Zhang at the 17th National Infectious Diseases Conference, which was hosted by the Chinese Medical Association in Hefei (Anhui, China) during 11-13 August 2022 (Chinese Society of Infectious Diseases and Chinese Medical Association, 2022). Fujie Zhang, a chief physician at Beijing Ditan Hospital affiliated with Capital Medical University (Beijing, China), was also the principal investigator (PI) in the Chinese phase III clinical trial. In addition, the results of the phase III clinical trial performed in Russia were recorded in the package insert of Azvudine tablets, manufactured by Henan Genuine Biotech Co., Ltd., and presented in Chinese language (Henan Genuine Biotech, 2022). A total of 314 patients with moderate COVID-19 were enrolled as the full analysis set (FAS) in the study, and the patients were randomly assigned to the Azvudine group or control group with a 1:1 ratio. The primary efficacy outcome was the median time to improvement in clinical conditions and the proportion of improvement in clinical conditions on day 7. A total of 279 patients were eligible according to inclusion and exclusion criteria and were included in the per-protocol set (PPS), which consisted of 141 subjects in the Azvudine group and 138 subjects in the control group. The mean baseline age was 48 years, with 39% and 61% of subjects falling under age groups 18–45 years (inclusive of 18 years) and 45–65 years (inclusive of 45 years), respectively. Male and female subjects accounted for 43% and 57%, respectively. There was no significant difference in the demographic or clinical characteristics between the Azvudine and control groups. As shown in Table 1, Azvudine significantly elevated the proportion of improvement in clinical conditions on day 7 and reduced the median time to improvement in clinical conditions compared to placebo in both FAS and PPS (Henan Genuine Biotech, 2022). With regard to safety, 34 patients underwent 47 AEs in the Azvudine group, and 35 patients underwent 50 AEs in the placebo group. Most of the AEs were determined as grade 1 or grade 2 according to CTCAE version 4.03. Only one serious AE occurred in the placebo group, and no serious AE occurred in the Azvudine group. There was no statistically significant difference in the frequency or severity of AEs between the Azvudine and placebo groups (ClinicalTrials, 2022; Zhang, 2022).
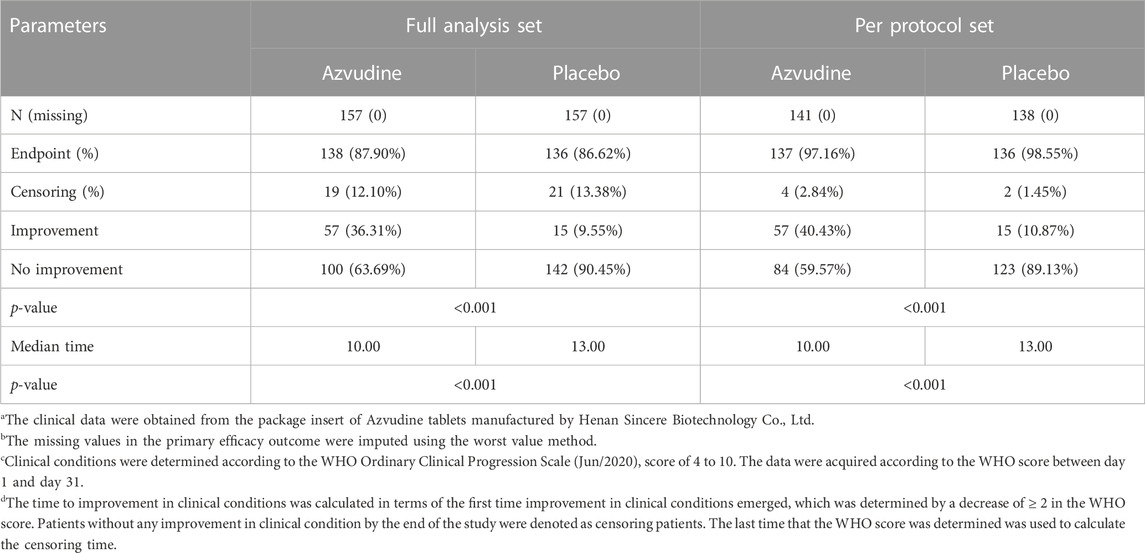
TABLE 1. Median time to improvement in clinical conditions and the proportion of improvement in clinical conditions on day 7 after the administration of Azvudine and placebo.
In Brazil, two phase III clinical trials of Azvudine were registered on the International Clinical Trials Registry Platform (NCT05033145 and NCT04668235). One trial was performed on patients with mild COVID-19, and the other one was performed on patients with moderate COVID-19. In the phase III clinical trial performed on patients with mild COVID-19, the primary outcome was the proportion of patients hospitalized within 4 weeks after randomization. The secondary outcomes involved the negative conversion time of the SARS-CoV-2 viral load, the proportion of patients cured during treatment, the severity and duration of COVID-19 symptoms, and the duration of Azvudine treatment until the second negative conversion. Patients with mild COVID-19 were randomly assigned to receive Azvudine 10 mg once a night or a placebo. Additionally, all the patients received standard treatment. A total of 281 patients with mild COVID-19 were included in the analysis (da Silva et al., 2023). For the phase III clinical trial performed in patients with moderate COVID-19, the primary outcome was the proportion of patients with improved clinical status. The secondary outcomes included the proportion of patients with a clinical outcome of cure after treatment, time to improvement in COVID-19 symptoms, hospital length of stay (LOS), and negative conversion time of the SARS-CoV-2 viral load. Patients with moderate COVID-19 were randomly assigned with a ratio of 1:1 to receive Azvudine 10 mg once a night plus standard treatment or placebo plus standard treatment. A total of 180 eligible patients with moderate COVID-19 were enrolled, of whom 172 completed the study (Cabral et al., 2022). The results of both phase III clinical trials revealed that Azvudine significantly reduced the NANC time and viral load of the patients compared to the placebo group (da Silva et al., 2023; Cabral et al., 2022).
The figures in this paper and Supplementary Material were re-plotted by the author based on the published data, and there is no third-party illustrative material in the report. The clinical data were obtained from package inserts of drugs, news reports, published conference reports, preprints, and published studies, which are publicly available sources.
The State Council of the People’s Republic of China (http://english.www.gov.cn), also known as the Chinese central government, announced 10 new measures for the COVID-19 response on 7 December 2022. Henceforth, the dynamic zero-COVID-19 policy was abrogated (Xinhua, 2022a; Zhu, 2023). Without the restrictions of quarantine measures, SARS-CoV-2 Omicron variants rapidly spread across the Chinese mainland and caused large-scale infections. To date, only two anti-SARS-CoV-2 agents were approved by the NMPA for the treatment of COVID-19, and another one was nirmatrelvir–ritonavir (Paxlovid®, Pfizer, New York, State of New York, USA). NMPA granted conditional approval for the imports of Paxlovid on 11 February 2022 (Xinhua, 2022b). However, the price of Paxlovid was too high relative to Azvudine in the Chinese market. The prices of Paxlovid and Azvudine in medical insurance were 2,300 Yuan per box and 270 Yuan per bottle, respectively. Therefore, the preferred antiviral drug was Azvudine instead of Paxlovid for Chinese people during the COVID-19 pandemic. In this situation, numerous domestic public hospitals introduced Azvudine to cope with the current SARS-CoV-2 Omicron wave. A total of 113 community hospitals admitted Azvudine as a prescription drug in the treatment of COVID-19 on 3 January 2023. Genuine Biotech, together with Shanghai Fosun Pharmaceutical (Group) Co., Ltd., donated 100 million Yuan’s worth of Azvudine to the rural areas of Midwestern China, covering 180 counties, in several phases through the Shanghai Fosun Foundation on 9 January 2023 (Genuine Biotech, 2023a). In fact, patients with COVID-19 in rural and remote areas should be given more attention due to poor medical conditions and health outcomes (Zhu, 2023). Within 48 h of the announcement, the first batch of 6,000 bottles of Azvudine tablets was conveyed to 10 county-/village-level clinics in four provinces through human-powered transportation. As of 28 February 2023, the two pharmaceutical companies had donated nearly 300,000 bottles of Azvudine tablets to Chinese rural areas, covering 250 cities/counties in nine provinces and the Shanghai municipality (Genuine Biotech, 2023b). Thereafter, in the award ceremony for “Henan Socially Responsible Enterprise of 2022 and Entrepreneur with Outstanding Social Contribution,” Genuine Biotech became the winner after several rounds of selection and was honored with the “2022 Annual Award of Henan Socially Responsible Enterprise” (Genuine Biotech, 2023c).
In summary, Azvudine could decrease the time of NANC, viral load, and the median time for improvement in clinical conditions and increase the proportion of improvement in clinical conditions on day 7 in patients with moderate COVID-19. Additionally, Azvudine could reduce body temperature normalization time, fever duration, and hospital LOS. In safety analyses, Azvudine exhibited good safety and tolerance. In summary, Azvudine was effective and well tolerated in the treatment of moderate COVID-19.
However, there were some limitations in the four phase III trials. In the trial performed in China, there were no significant differences between the Azvudine and control groups for most of the clinical outcomes; only the difference in the reduction in viral load from baseline on day 5 between the Azvudine and placebo groups was statistically significant. Azvudine did not display effectiveness in the alleviation of respiratory symptoms and prevention of progression to severe or critical COVID-19. For the phase III clinical trial performed in Brazilian patients with mild COVID-19, there also were no significant differences between Azvudine and placebo for most of the clinical outcomes, especially the primary outcome, and the researchers attributed the failure to the self-limiting nature of mild COVID-19 (da Silva et al., 2023). In the phase III clinical trial performed on Brazilian patients with moderate COVID-19, Azvudine significantly improved the clinical status at the time of discharge and reduced body temperature normalization time, fever duration, and hospital LOS compared to placebo (Cabral et al., 2022). The two phase III clinical trials in Brazil suggested Azvudine exhibited good efficacy in patients with moderate COVID-19 instead of in patients with mild COVID-19. In the four phase III clinical trials, whether Azvudine could reduce COVID-19 mortality or prevent progression to severe/critical COVID-19 was unclear and remained a major concern. Moreover, the sample sizes in the four phase III clinical trials were relatively small as compared to other high-quality phase III clinical trials of anti-SARS-CoV-2 drugs (Hammond et al., 2022; Jayk Bernal et al., 2022; Cao et al., 2023). Nevertheless, the four phase III clinical trials have demonstrated that Azvudine effectively reduced the time of NANC and viral load in patients with COVID-19.
After entering a host cell, the genomic RNA of SARS-CoV-2 acts as a template and is translated to yield two polyproteins by utilizing the host cell protein synthesis system, followed by proteolytic cleavage with two cysteine proteases to form a replication–transcription complex mediating SARS-CoV-2 RNA synthesis, capping, and proofreading. New SARS-CoV-2 genomic RNA is translated to produce additional non-structural proteins, which are used for further RNA synthesis or new virion assembly. Then, SARS-CoV-2 genomic RNA is coated with nucleocapsid proteins to generate nucleocapsid structures. Lipid bilayers consisting of viral spikes, membranes, and envelope proteins are synthesized in the endoplasmic reticulum–Golgi intermediate compartment. A mature progeny SARS-CoV-2 virion emerges after assembly and is released through exocytosis. Eventually, numerous SARS-CoV-2 virions are produced after several cycles of replication, leading to another round of infection (Malone et al., 2022; Yang and Rao, 2021; V'Kovski et al., 2021). The degree of infection is related to the innate antiviral response, and disease severity can promote the enhancement of the antibody response, which is associated with clinical outcomes. The entry of SARS-CoV-2 through the respiratory tract tends to cause the involvement of the upper and lower respiratory tracts, leading to a series of respiratory or systemic symptoms, such as cough, fever, and fatigue (Attaway et al., 2021; Gattinoni et al., 2021) (Figure 1).
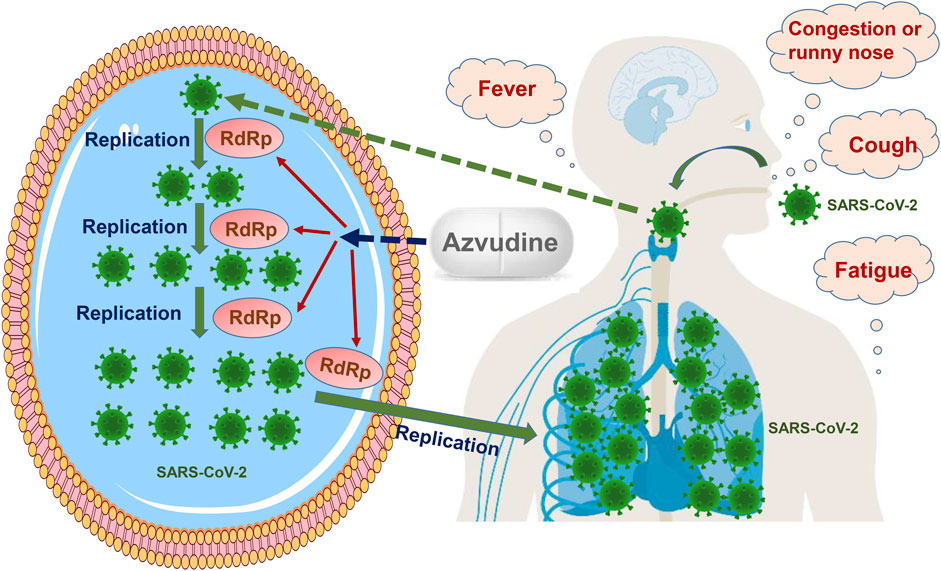
FIGURE 1. Therapeutic effect of Azvudine on COVID-19. Azvudine inhibits RNA-dependent RNA polymerase (RdRp), which is a vital enzyme mediating SARS-CoV-2 replication and transcription. Azvudine plays an anti-SARS-CoV-2 role by blocking virus replication.
RdRp is a vital enzyme that controls SARS-CoV-2 replication and transcription by catalyzing the phosphodiester binding process for RNA synthesis and is identified as a promising molecular target in COVID-19 (Tian et al., 2021; Ng et al., 2022). Azvudine, as an RdRp inhibitor, plays an anti-SARS-CoV-2 role by blocking virus replication (Figure 1). It should be noted that Azvudine has no role in the clearance of SARS-CoV-2, and it might be the fundamental reason that Azvudine did not exhibit effectiveness for several clinical outcomes in the four phase III clinical trials. On the other hand, in order to control viral load and prevent progression to severe COVID-19, Azvudine should be administered as soon as possible after the diagnosis of COVID-19 in accordance with the package insert of Azvudine tablets (Henan Genuine Biotech, 2022).
COVID-19 was added to the Azvudine list of indications in the package insert to resist the COVID-19 Omicron wave, and this application was conditionally approved by the NMPA on 25 July 2022 (National Medical Products Administration, 2022). On 9 August 2022, the National Health Commission and the National Administration of Traditional Chinese Medicine announced the inclusion of Azvudine in the Guidelines for the Diagnosis and Treatment of Coronavirus Disease 2019 (version ninth). The recommended oral dose of Azvudine for the treatment of moderate COVID-19 is 5 mg once daily, and the duration of Azvudine treatment should not exceed 14 days (National Health Commission of the People’s Republic of China and National Administration of Traditional Chinese Medicine, 2022; None, 2022). Azvudine was incorporated into the Guidelines for the Diagnosis and Treatment of Coronavirus Disease 2019 (version 10th) issued by the National Health Commission and National Administration of Traditional Chinese Medicine on 6 January 2023 (General Office of National Health Commission of the People’s Republic of China and General comprehensive affairs department of national administration of traditional Chinese medicine of the People’s Republic of China, 2023). Thereafter, Azvudine was included in the Expert Consensus on Antiviral Therapy for COVID-19, which was compiled by 34 Chinese experts, and was highly approbated (Zhang and Li, 2023). On 18 January 2023, Azvudine was officially included in the National Reimbursement Drug List and was priced at 11.58 Yuan/tablet/3 mg in medical insurance (Genuine Biotech, 2023d).
In patients with moderate COVID-19, Azvudine could reduce NANC time, viral load, and time to improvement in clinical conditions and exhibited good safety and tolerance. Azvudine is a promising anti-SARS-CoV-2 drug. However, randomized controlled trials with large sample sizes or large-scale real-world clinical studies are needed to evaluate the efficacy of Azvudine in preventing disease progression and reducing mortality in patients with COVID-19.
The original contributions presented in the study are included in the article/Supplementary material; further inquiries can be directed to the corresponding author.
K-WZ is the sole author of this paper.
This work is a pooled analysis of the results of four phase III clinical trials, and only introduces the most important results of the four phase III clinical trials. The author thanks all the researchers for conducting the four phase III clinical trials and for disclosing and sharing the results with the public.
Author K-WZ was employed by Guangzhou Baiyunshan Pharmaceutical Holding Co., Ltd. Baiyunshan Pharmaceutical General Factory.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1228548/full#supplementary-material
Acquired Immunodeficiency Syndrome and Hepatitis C Professional Group, Society of Infectious Diseases, Chinese Medical Association, Chinese Center for Disease Control and Prevention (2021). Chinese guidelines for diagnosis and treatment of human immunodeficiency virus infection/acquired immunodeficiency syndrome (2021 edition). Med. J. Peking Union Med. Coll. Hosp. [CNKI] 13 (2), 203–226. doi:10.12290/xhyxzz.2022-0097
Attaway, A. H., Scheraga, R. G., Bhimraj, A., Biehl, M., and Hatipoglu, U. (2021). Severe covid-19 pneumonia: pathogenesis and clinical management. BMJ 372, n436. doi:10.1136/bmj.n436
Cabral, P., de Souza, S., Silva, Rd, Arruda, R., Cabral, S., de Assis, A., et al. Serial viral load analysis by ddpcr to evaluate fnc efficacy and safety in the treatment of moderate cases of covid-19, 21 november 2022, PREPRINT. (Version 1) available at Research Square. doi:10.21203/rs.3.rs-2273657/v1
Cao, Z., Gao, W., Bao, H., Feng, H., Mei, S., Chen, P., et al. (2023). VV116 versus nirmatrelvir-ritonavir for oral treatment of covid-19. N. Engl. J. Med. 388 (5), 406–417. doi:10.1056/NEJMoa2208822
Chinese Society of Infectious Diseases, Chinese Medical Association (2022). The 17th national congress of Chinese society of infectious diseases and the 3rd international forum on infectious diseases and clinical microbiology. Available at: https://csi2022.sciconf.cn/cn/web/index/13482 (accessed June 9, 2022).
ClinicalTrials, (2022). Clinical data and adverse reactions of the oral drug Azvudine for COVID-19. Available at: https://baijiahao.baidu.com/s?id=1753640204148539705&wfr=spider&for=pc (accessed December 30, 2022).
da Silva, R. M., Gebe Abreu Cabral, P., de Souza, S. B., Arruda, R. F., Cabral, S. P. F., de Assis, A., et al. (2023). Serial viral load analysis by DDPCR to evaluate FNC efficacy and safety in the treatment of mild cases of COVID-19. Front. Med. (Lausanne) 10, 1143485. doi:10.3389/fmed.2023.1143485
Eltayb, W. A., Abdalla, M., and Rabie, A. M. (2023). Novel investigational anti-SARS-CoV-2 agent ensitrelvir "S-217622": a very promising potential universal broad-spectrum antiviral at the therapeutic frontline of coronavirus species. ACS Omega 8 (6), 5234–5246. doi:10.1021/acsomega.2c03881
Gattinoni, L., Gattarello, S., Steinberg, I., Busana, M., Palermo, P., Lazzari, S., et al. (2021). COVID-19 pneumonia: pathophysiology and management. Eur. Respir. Rev. 30 (162), 210138. doi:10.1183/16000617.0138-2021
General Office of National Health Commission of the People's Republic of China, General comprehensive affairs department of national administration of traditional Chinese medicine of the People's Republic of China (2023). Guidelines for the Diagnosis and Treatment of Coronavirus Disease 2019 (trial version tenth). China Med. [CNKI] 18 (2), 161–166. doi:10.3760/j.issn.1673-4777.2023.02.001
Genuine Biotech (2021). Genuine Biotech raises $100 million in series B financing, accelerating commercialization of world's first dual-target anti-HIV drug. Available at: http://en.genuine-bio.com/news2021/275.html (accessed August 27, 202).
Genuine Biotech (2023a). Genuine Biotech and Fosun pharma donated 100 million yuan's worth of oral drug for COVID-19, azvudine, to rural areas. Available at: http://en.genuine-bio.com/2023a/359.html (accessed January 9, 2023).
Genuine Biotech (2023b). Genuine Biotech and Fosun pharma donated nearly 300,000 bottles of azvudine tablets to China's rural areas. Available at: http://en.genuine-bio.com/2023a/368.html (accessed March 10, 2023).
Genuine Biotech (2023c). Good news! Genuine Biotech won the 2022 annual award of henan socially responsible Enterprise. Available at: http://en.genuine-bio.com/2023a/371.html (accessed June 2, 2023).
Genuine Biotech (2023d). Good news, azvudine tablets is officially included in the national reimbursement drug list. Available at: http://en.genuine-bio.com/2023a/362.html (accessed January 18, 2023).
Hammond, J., Leister-Tebbe, H., Gardner, A., Abreu, P., Bao, W., Wisemandle, W., et al. (2022). Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19. N. Engl. J. Med. 386 (15), 1397–1408. doi:10.1056/NEJMoa2118542
Henan Genuine Biotech (2022). Package insert of Azifudine tablets. Pingdingshan, China: Henan Genuine Biotech Co.Ltd.
Jayk Bernal, A., Gomes da Silva, M. M., Musungaie, D. B., Kovalchuk, E., Gonzalez, A., Delos Reyes, V., et al. (2022). Molnupiravir for oral treatment of covid-19 in nonhospitalized patients. N. Engl. J. Med. 386 (6), 509–520. doi:10.1056/NEJMoa2116044
Malone, B., Urakova, N., Snijder, E. J., and Campbell, E. A. (2022). Structures and functions of coronavirus replication-transcription complexes and their relevance for SARS-CoV-2 drug design. Nat. Rev. Mol. Cell. Biol. 23 (1), 21–39. doi:10.1038/s41580-021-00432-z
National Health Commission of the People's Republic of China, National Administration of Traditional Chinese Medicine (2022). Guidelines for the diagnosis and treatment of coronavirus disease 2019 (trial version ninth). Chin. J. Viral Dis. [CNKI] 12 (3), 161–169. doi:10.16505/j.2095-0136.2022.0023
National Medical Products Administration (2021). Azvudine was conditionally approved by the national medical products administration. Available at: https://www.nmpa.gov.cn/yaowen/ypjgyw/20210721142223181.html (accessed July 21, 2021).
National Medical Products Administration (2022). The application that coronavirus disease 2019 (COVID-19) was added into the indications of Azvudine was conditionally approved by the National Medical Products Administration. Available at: https://www.nmpa.gov.cn/yaowen/ypjgyw/20220725165620176.html (accessed July 25, 2022).
Ng, T. I., Correia, I., Seagal, J., DeGoey, D. A., Schrimpf, M. R., Hardee, D. J., et al. (2022). Antiviral drug discovery for the treatment of COVID-19 infections. Viruses 14 (5), 961. doi:10.3390/v14050961
None (2022). The national health commission includes azvudine into the guidelines for the diagnosis and treatment of coronavirus disease 2019 (version ninth). Chin. J. Viral Dis. [CNKI] 12 (4), 283. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=ZRYX202204016&DbName=DKFX2022.
Rabie, A. M., and Abdalla, M. (2023). Evaluation of a series of nucleoside analogs as effective anticoronaviral-2 drugs against the Omicron-B.1.1.529/BA.2 subvariant: a repurposing research study. Med. Chem. Res. 32 (2), 326–341. doi:10.1007/s00044-022-02970-3
Rabie, A. M., and Abdalla, M. (2022). Forodesine and riboprine exhibit strong anti-SARS-CoV-2 repurposing potential: in silico and in vitro studies. ACS Bio Med Chem Au 2 (6), 565–585. doi:10.1021/acsbiomedchemau.2c00039
Rabie, A. M., Abdel-Dayem, M. A., and Abdalla, M. (2023). Promising experimental anti-SARS-CoV-2 agent “SLL-0197800”: the prospective universal inhibitory properties against the coming versions of the coronavirus. ACS Omega 8. doi:10.1021/acsomega.2c08073
Rabie, A. M. (2022a). Efficacious preclinical repurposing of the nucleoside analogue didanosine against COVID-19 polymerase and exonuclease. ACS Omega 7 (25), 21385–21396. doi:10.1021/acsomega.1c07095
Rabie, A. M., and Eltayb, W. A. (2023). Potent dual polymerase/exonuclease inhibitory activities of antioxidant aminothiadiazoles against the COVID-19 Omicron virus: a promising in silico/in vitro repositioning research study. Mol. Biotechnol., 1–20. doi:10.1007/s12033-022-00551-8
Rabie, A. M. (2022b). Potent inhibitory activities of the adenosine analogue cordycepin on SARS-CoV-2 replication. ACS Omega 7 (3), 2960–2969. doi:10.1021/acsomega.1c05998
Rabie, A. M. (2021b). Potent toxic effects of Taroxaz-104 on the replication of SARS-CoV-2 particles. Chem. Biol. Interact. 343, 109480. doi:10.1016/j.cbi.2021.109480
Rabie, A. M. (2021c). Teriflunomide: a possible effective drug for the comprehensive treatment of COVID-19. Curr. Res. Pharmacol. Drug Discov. 2, 100055. doi:10.1016/j.crphar.2021.100055
Rabie, A. M. (2021a). Two antioxidant 2,5-disubstituted-1,3,4-oxadiazoles (CoViTris2020 and ChloViD2020): successful repurposing against COVID-19 as the first potent multitarget anti-SARS-CoV-2 drugs. New J. Chem. 45 (2), 761–771. doi:10.1039/d0nj03708g
Ren, Z., Luo, H., Yu, Z., Song, J., Liang, L., Wang, L., et al. (2020). A randomized, open-label, controlled clinical trial of azvudine tablets in the treatment of mild and Common COVID-19, a pilot study. Adv. Sci. (Weinh) 7 (19), e2001435. doi:10.1002/advs.202001435
Tian, L., Qiang, T., Liang, C., Ren, X., Jia, M., Zhang, J., et al. (2021). RNA-dependent RNA polymerase (RdRp) inhibitors: the current landscape and repurposing for the COVID-19 pandemic. Eur. J. Med. Chem. 213, 113201. doi:10.1016/j.ejmech.2021.113201
V'Kovski, P., Kratzel, A., Steiner, S., Stalder, H., and Thiel, V. (2021). Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol. 19 (3), 155–170. doi:10.1038/s41579-020-00468-6
Vicenti, I., Zazzi, M., and Saladini, F. (2021). SARS-CoV-2 RNA-dependent RNA polymerase as a therapeutic target for COVID-19. Expert Opin. Ther. Pat. 31 (4), 325–337. doi:10.1080/13543776.2021.1880568
Xinhua, (2022a). China focus: COVID-19 response further optimized with 10 new measures. Available at: https://english.news.cn/20221207/ca014c043bf24728b8dcbc0198565fdf/c.html (accessed December 7, 2022).
Xinhua (2022b). China grants conditional approval for Pfizer's oral COVID-19 drug. Available at: http://english.nmpa.gov.cn/2022-02/14/c_707085.htm (accessed February 14, 2022).
Yang, H., and Rao, Z. (2021). Structural biology of SARS-CoV-2 and implications for therapeutic development. Nat. Rev. Microbiol. 19 (11), 685–700. doi:10.1038/s41579-021-00630-8
Yu, B., and Chang, J. (2020). Azvudine (FNC): a promising clinical candidate for COVID-19 treatment. Signal Transduct. Target Ther. 5 (1), 236. doi:10.1038/s41392-020-00351-z
F. Zhang (Editor) (2022). “Clinical study of Azfudine against COVID-19,” The 17th national congress of Chinese society of infectious diseases and the 3rd international forum on infectious diseases and clinical microbiology (Anhui, China: Hefei).
Zhang, F., and Li, X. (2023). Interpretation of Expert Consensus on Antiviral Therapy of COVID-19. Chin. J. Clin. Infect. Dis. 16 (1), 21–25. doi:10.3760/cma.j.issn.1674-2397.2023.01.003
Zhang, J. L., Li, Y. H., Wang, L. L., Liu, H. Q., Lu, S. Y., Liu, Y., et al. (2021). Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients. Signal Transduct. Target Ther. 6 (1), 414. doi:10.1038/s41392-021-00835-6
Zhu, K. W. (2023). Why is it necessary to improve COVID-19 vaccination coverage in older people? How to improve the vaccination coverage? Hum. Vaccin Immunother. 19 (2), 2229704. doi:10.1080/21645515.2023.2229704
Keywords: Azvudine (FNC), viral load, severe acute respiratory syndrome coronavirus 2, RNA-dependent RNA polymerase, nucleic acid-negative conversion time
Citation: Zhu K-W (2023) Efficacy and safety evaluation of Azvudine in the prospective treatment of COVID-19 based on four phase III clinical trials. Front. Pharmacol. 14:1228548. doi: 10.3389/fphar.2023.1228548
Received: 25 May 2023; Accepted: 04 August 2023;
Published: 24 August 2023.
Edited by:
Exequiel Oscar Jesus Porta, Durham University, United KingdomReviewed by:
Amgad M. Rabie, Ain Shams University, EgyptCopyright © 2023 Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ke-Wei Zhu, emh1a2V3MjAxOEBvdXRsb29rLmNvbQ==
†ORCID: Ke-Wei Zhu, https://orcid.org/0000-0002-2138-271X
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.