- 1NHC Key Laboratory of Nuclear Technology Medical Transformation, Department of Pharmacy, Mianyang Central Hospital, Mianyang, China
- 2Key Laboratory of Molecular Pharmacology and Drug Evaluation, School of Pharmacy, Collaborative Innovation Center of Advanced Drug Delivery System and Biotech Drugs in Universities of Shandong, Yantai University, Yantai, China
- 3School of Pharmacy, Southwest Medical University, Luzhou, China
- 4College of Pharmaceutical Sciences, Institute of Pharmaceutical, Zhejiang University, Hangzhou, China
Objective: To analyze the clinical characteristics of adverse reactions/events based on chemotherapy in cancer patients, and then explore the potential mechanism of Danggui Buxue Decoction (DBD) against chemotherapy-induced bone marrow suppression (BMS).
Methods: Retrospectively collected and evaluated were the clinical data of patients in a hospital who experienced adverse reactions/events brought on by chemotherapeutic medications between 2015 and 2022. We explored the potential mechanism of DBD against BMS using network pharmacology based on the findings of the adverse reactions/events analysis.
Results: 151 instances (72.25%) experienced adverse reactions/events from a single chemotherapy medication. Besides, platinum-based medications produced the most unfavorable effects. The study also found that chemotherapy caused the highest number of cases of BMS, including platinum drugs. Consequently, BMS is the most prevalent adverse reaction disease caused by chemotherapy found in this part. According to network pharmacology findings, DBD can prevent BMS primarily involving 1,510 primary targets and 19 key active ingredients. Based on the enrichment analysis, PI3K-AKT, TNF, MAPK, and IL-17 signaling pathways made up the majority of the DBD-resisting BMS pathways. Molecular docking displayed that kaempferol, the major active ingredient of DBD, had the highest binding energy (−10.08 kJ mol-1) with PTGS2 (a key target of BMS).
Conclusion: Cancer patients who received chemotherapy had a risk to develop BMS. Regular blood tests should be performed while taking medicine; early discovery and treatment can reduce a patient’s risk of experiencing adverse reactions/events. Additionally, this study demonstrated that DBD, through a variety of targets and pathways, may be crucial in avoiding BMS.
1 Introduction
In 2020, China would record 3 million new cancer deaths and 4.56 million new cancer patients, according to a report from the World Health Organization (Sung et al., 2021). Lung, colon, stomach, breast, and liver cancer had the highest incidence rates of all cancers. The top five causes of death were colon, lung, liver, gastric, and esophageal cancer. Furthermore, 3.21 million additional cancer deaths will occur in China in 2022, according to forecasts from the National Cancer Center of China and the Chinese National Cancer Clinical Research Center (Xia et al., 2022). Most tumors are still found most frequently in lung cancer. In contrast, the prevalence of esophageal, liver, and stomach cancer has steadily declined. However, the prevalence of bladder cancer, male prostate cancer, female breast cancer, and colon cancer has steadily climbed. The two main factors driving the rise in cancer fatalities are the population’s aging and the size of the adult population.
According to several studies, the primary methods used to treat tumors at the moment are surgical removal, pharmacological chemotherapy, radiotherapy, targeted therapy, immunotherapy, and traditional Chinese medicine (Bae et al., 2023; Eisenberg et al., 2023; Lazaroff and Bolotin, 2023; Song et al., 2023). Additionally, it is believed that medicinal chemotherapy is still the primary method of tumor treatment, in accordance with a range of different criteria and consensus both domestically and internationally (Chen et al., 2022; Reig et al., 2022; Strazzabosco et al., 2022). However, chemotherapy can kill and stop the growth of tumor cells, but it also has specific toxic and side effects on healthy human cells, which can easily result in some unavoidable adverse reactions/events like liver and kidney dysfunction, nausea, vomiting, headaches, chest tightness, and other reactions (Knezevic and Clarke, 2020; De Francia et al., 2022; Liu et al., 2022). Currently, there are numerous chemotherapy medications on the market today that not only harm the body but also interfere with their therapeutic effects, postponing or worsening the patient’s condition.
We used the data of cancer patients using chemotherapy drugs in a medical hospital from 2015 to 2022 as a sample to study and analyze the severity of adverse reactions/events to more clearly understand the occurrence, severity, and impact on patients of adverse reactions/events in chemotherapy drug antineoplastic treatment. Besides, Danggui Buxue Decoction (DBD), a traditional Chinese medicine, was also used in this study as an intervention method to investigate the potential mechanism of one adverse reaction/event disease brought on by chemotherapy drugs. The disease was based on the previous adverse reactions/events of cancer chemotherapy results, and it caused the most adverse reactions/events. This helped to further reveal the mechanism of DBD in preventing a certain injury disease caused by chemotherapy and provided theoretical support and a scientific foundation for in-depth clinical research and application (Sun et al., 2023; Zhao et al., 2023). Systems biology is the technological theory on which network pharmacology is built, which can be used to do network topology analysis, develop the drug target disease network relationship, discover medications for disease treatment, searche the network database, forecast the drug treatment mechanism, and create a thorough network study of medication effects from various levels and viewpoints starting with a multi-target research method (Hopkins AL., 2007; Li et al., 2022).
2 Materials and methods
2.1 Analysis of adverse drug reactions/events of chemotherapy
2.1.1 Data collection
The clinical data of patients with adverse reactions/events in a hospital from 2015 to 2022 were retrospectively collected. The information on patients with adverse reactions/events was collected through the National Adverse Drug Reaction Monitoring System of China (2015–2017) and the Chinese Hospital Pharmacovigilance System (CHPS) (2018–2022).
2.1.2 Inclusion and exclusion criteria
The criteria for inclusion and exclusion of patients were established as follows: Inclusion criteria: 1) All hospitalized tumor patients who have received a specific diagnosis of their tumor illness are included; 2) Chemotherapy medications are suspected to have been used by the included patients.
Exclusion criteria: 1) Non-cancer patients; 2) Suspected non-chemotherapeutic medication; 3) Radiation therapy was also administered at the time of admission. This criterion was used by two researchers to screen the entire procedure, and when there was a disagreement, a conclusion was taken after speaking with the third researcher.
2.1.3 Content of information collected
The patient’s information was gathered, including 1) General patient data, such as age, gender, and underlying illnesses; 2) Suspected drug data, such as treatment regimen, combined medications, usage, and dosage; 3) Clinical manifestations of adverse reactions/events; and 4) The severity scale for adverse reactions/events.
2.1.4 Evaluation criteria
According to the “Common Adverse Reaction Event Evaluation Standard (CTCAE), version 5.0” published by the US Department of Health and Human Services in 2017 the evaluation of adverse reactions/events was carried out (Freites-Martinez et al., 2021). Two researchers rated and categorized the degree of adverse reactions/events at the same time. In the event of a disagreement, it is settled by debate and bargaining with a third researcher. Grade 1, Grade 2, Grade 3, Grade 4, and Grade 5 were used to categorize the included patients’ adverse reactions/events according to the CTCAE criteria. Grades 1 and 2 are categorized as “general” whereas grades 3, 4, and 5 are categorized as “serious.” Additionally, according to the above criteria, other adverse reactions/events were classified.
2.1.5 Statistical analysis
The data were statistically analyzed using SPSS 24.0 and Excel 2021, and the counting data were expressed in cases (n) and rates (%). Chi-square test was performed to compare the groups, and p < 0.05 denoted statistical significance. In addition, various information distributions (such as admission records, course records, test indicators, etc.) and other influencing factors were analyzed using the electronic case system of the hospital.
2.2 Network pharmacology and molecular docking
2.2.1 DBD and disease target screening
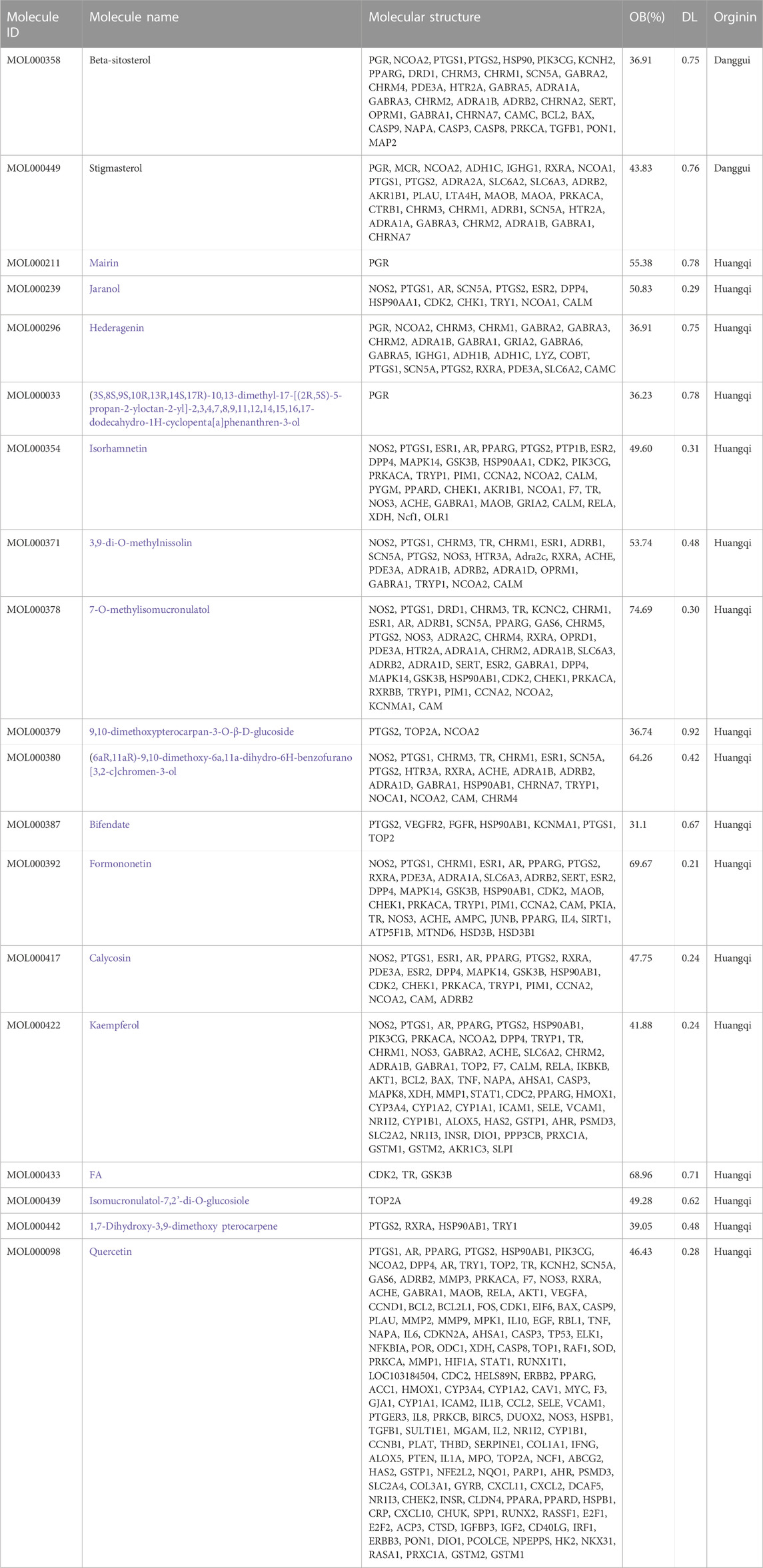
Through the TCMSP platform to obtain the active ingredients of DBD, the keywords “Huangqi” and “Danggui” were searched, and the active ingredients of the compounds that were returned were screened. Moreover, suitable active ingredients were discovered under the screening parameters of OB% > 30% and DL > 0.18 (Wang et al., 2022). The Uniport protein database was utilized to standardize the targets following deduplication and screening.
The keyword “a certain injury disease caused by chemotherapy” was used to search the GeneCards and OMIM databases. Only targets with a score of 10 or higher are extracted from the first database, which is combined with the second database to retrieve related targets by eliminating duplicates. The potential key target is the point at which the associated target of “a certain injury disease caused by chemotherapy” and the target of the DBD active ingredient intersect.
2.2.2 Construction of PPI and construction of an active ingredients-disease-target network
A protein-protein interaction (PPI) network is created by uploading the typical targets of DBD and BMS to the STRING platform and setting the species screening to “Homo sapiens” without completing combined score screening to study the interactions between the target proteins. Creating an active ingredient-disease-target network using Cytoscape and the pertinent target data from DBD and “a certain injury disease caused by chemotherapy.”
2.2.3 GO function and KEGG pathway enrichment analysis
Through the DAVID database, GO analysis (three levels of biological processes, molecular functions, and cellular components) and pathways analysis were performed on the key targets of DBD for the treatment of “a certain injury disease caused by chemotherapy.” The results were saved and sorted out, and the top-ranked biological processes and signaling pathways were screened. Visual analysis was performed through Omicshare.
2.2.4 Molecular docking
To verify the binding of active ingredients and key targets, AutoDock was utilized for the semi-flexible molecular docking of the ligand and receptor, and the AutoDock Vina scoring tool was used to calculate the free binding energy. The presence of a well-docked receptor and ligand was indicated when the free binding energy was less than −5.0 kJ mol-1 and the ability to bind is improved when the value is reduced (Niu et al., 2021). To illustrate the docking outcomes with minimal free binding energy, PyMOL software was employed.
2.2.5 ADMET profiling
Chemical absorption, distribution, metabolism, excretion, and toxicity (ADMET) are particularly important and critical in drug discovery, development, and application. ADMET can assist in the efficient and safe analysis of the properties of discovered drugs. In this aspect, the physicochemical characteristics of the predicted ingredients were identified through two databases (SWISS ADME and admetSAR). They can be utilized to predict the toxicity of potential core ingredients of DBD, such as acute oral toxicity, aes mutation, carcinogenic, hepatotoxicity, and nephrotoxicity.
3 Results and analysis
3.1 Analysis of adverse reactions/events of chemotherapy drugs
3.1.1 Screening of adverse reactions/events in cancer patients
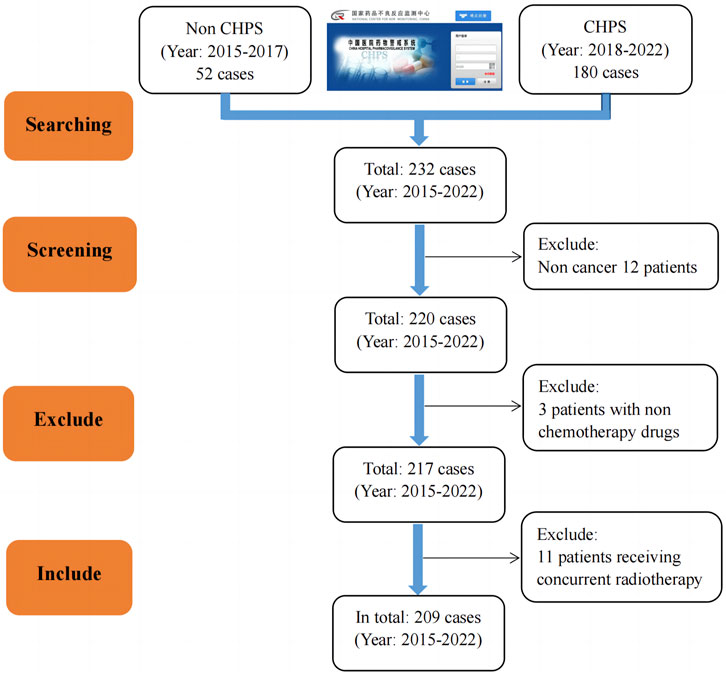
Between 2015 and 2022, the hospital reported a total of 232 patients through the National Adverse Drug Reaction Monitoring System and CHPS. According to the former, the incidence of adverse reactions/events decreased yearly from 2015 to 2017. However, since the CHPS system was launched in 2018, the number of reported adverse reactions/events has increased yearly except for 2020. In addition, out of a total of 232 cases included, 209 adverse reactions/events were ultimately included after screening according to the “1.1.2” criteria. The results are shown in Figure 1.
3.1.2 Basic information and proportion of tumor patients included
In order to better understand the occurrence of adverse reactions/events after chemotherapy in male and female cancer patients at different ages, detailed statistics and analysis were conducted in this study, as shown in Table 1. Non-small cell lung cancer is the most prevalent tumor disease with the highest incidence of adverse reactions/events, accounting for 12.44%. The number of male patients are slightly more than female patients and they are older (over 60 years old). Age and gender among those with NSCLC differ statistically significantly (p < 0.001). The second, which accounts for 11.96%, is colon cancer. Patients who are female are slightly more numerous than patients who are male. Nevertheless, the majority of patients are under 60, and the age and gender differences are statistically significant (p < 0.001). Rectal cancer comes in third place and makes up more than 10% of cases. There are slightly more male patients and the age spans almost the entire age group, while female patients are mostly under the age of 60. Additionally, the ratio of male to female adverse reactions/events for non-Hodgkin’s lymphoma, acute non-lymphocytic leukemia, and gastric cancer is almost 1:1. Males were more likely to develop nasopharyngeal cancer, esophageal cancer, pancreatic cancer, Hodgkin’s lymphoma, and other adverse effects. Women’s attention was focused more on endometrial, breast, ovarian, and cervical cancer at the same time.
3.1.3 Combined medication
Among the 209 cases of adverse reactions/events caused by chemotherapy drugs included in this study, 151 cases were caused by a single use of drugs, 50 cases were caused by a combination of two drugs, 7 cases were caused by a combination of three drugs, and 1 case was caused by a combination of four drugs and above. In this part, single-drug use leads to the most significant proportion of adverse reactions/events in cancer patients. On the contrary, the combined use of drugs reduces the occurrence of adverse reactions/events to a certain extent, which plays an important role in the treatment effect and symptom relief of patients. As a result, when compared to single drug use, combination therapy results in fewer adverse reactions/events with chemotherapy drugs.
3.1.4 Types of chemotherapy drugs
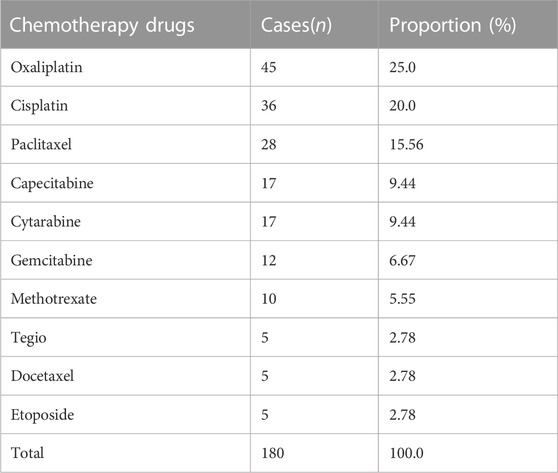
Among the 209 adverse reactions/events included in this study, nearly 30% of the patients had adverse reactions/events due to using 2 or more chemotherapy drugs, so the number of chemotherapy drugs was 271. To further understand the relationship between the types of chemotherapeutic drugs and adverse reactions/events to more clearly define the safety of clinical use of chemotherapeutic drugs, this study classified chemotherapeutic drugs according to the chemical structure and source of drugs. The results are shown in Figure 2. Among them, platinum complexes, plant anti-tumor drugs, and anti-tumor metabolic drugs caused the most adverse reactions/events in cancer patients, accounting for 32.0%, 20.0%, and 21.0%, respectively. At the same time, this study also counts the top 10 chemotherapy drugs that cause adverse reactions/events, with platinum complexes and paclitaxel accounting for the largest proportion. The detailed results are shown in Table 2.
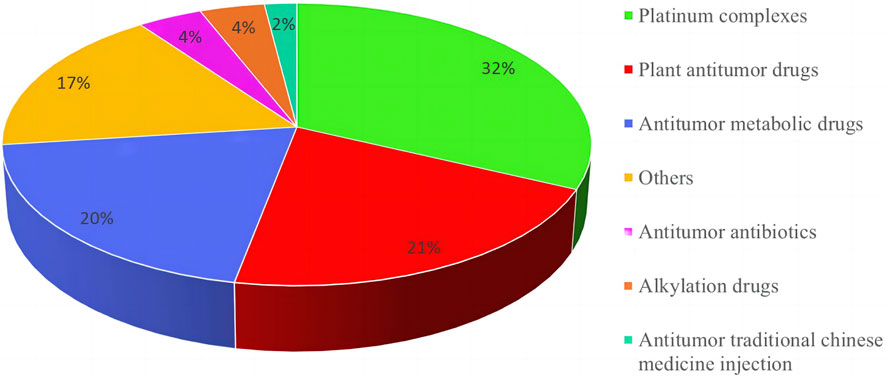
FIGURE 2. Classification and proportion of anti-cancer chemotherapy medications causing adverse reactions/events.
3.1.5 Severity of adverse reactions/events
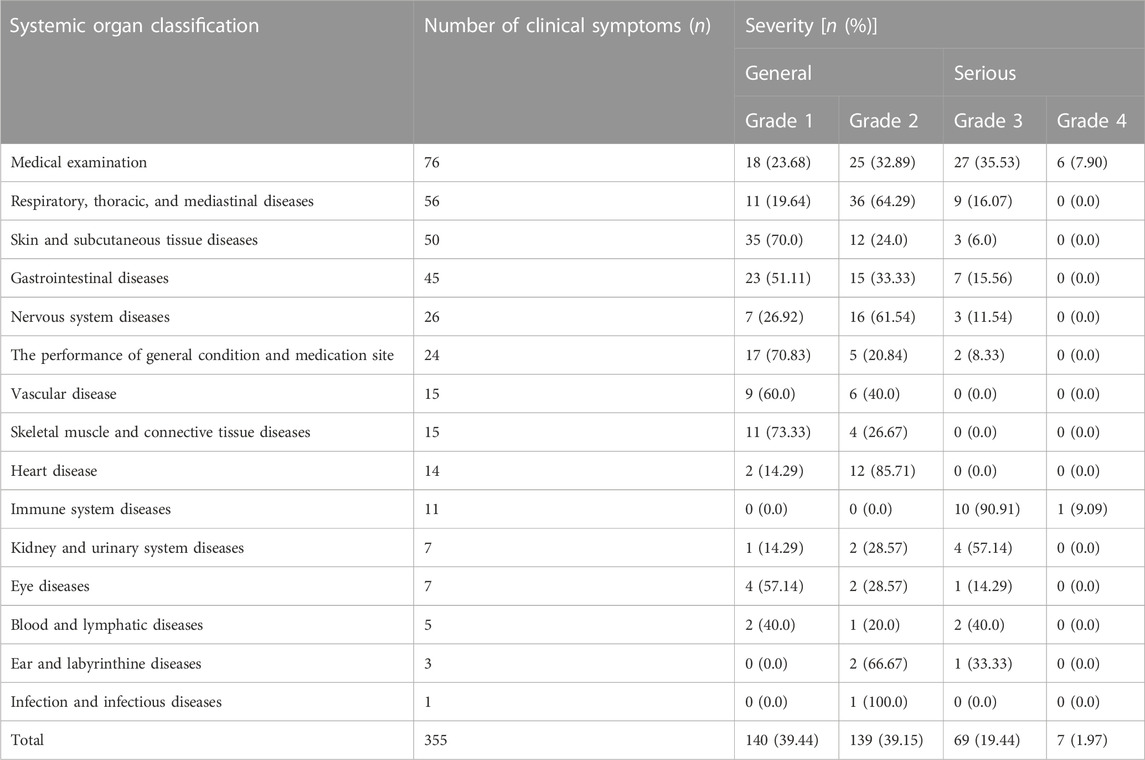
The severity of clinical manifestations of adverse reactions/events in patients was rated based on CTCAE criteria. 76 “serious” adverse reactions/events and 279 “general” adverse reactions/events were recorded. Further investigation revealed that the “medical examination” category had the most cases, with 76 items, including 18 grade 1 and 25 grade 2 items in the “general” category, 27 grade 3 and 6 grade 4 items in the “serious” category, and no grade 5. Besides, the overall proportion of the “genera” category was relatively large, but most patients were tolerable and did not require special treatment.
There are 76 “serious” adverse reactions/events, of which “medical examination” has the highest frequency of clinical symptoms, with 33 items. In addition, there were 11, 9, and 7 “serious” adverse reactions/events related to “immune system diseases,” “respiratory, thoracic, and mediastinal diseases,” and “gastrointestinal diseases,” respectively. Therefore, for patients with “serious” adverse reactions/events, it is not only highly likely to be prolonged hospitalization events, limited activities of daily living, but also potentially disabling and life-threatening. Hence, special attention and care should be paid to “serious” adverse reactions/events. The detail can be seen in Table 3. Meanwhile, we observed that the frequency of BMS was higher in adverse reactions/events in Table 4, which were mostly characterized by a decline in neutrophil, white blood cell, and platelet counts. These findings also revealed that among the expected adverse reactions/events associated with chemotherapeutic medicines, BMS incidence and number were high.
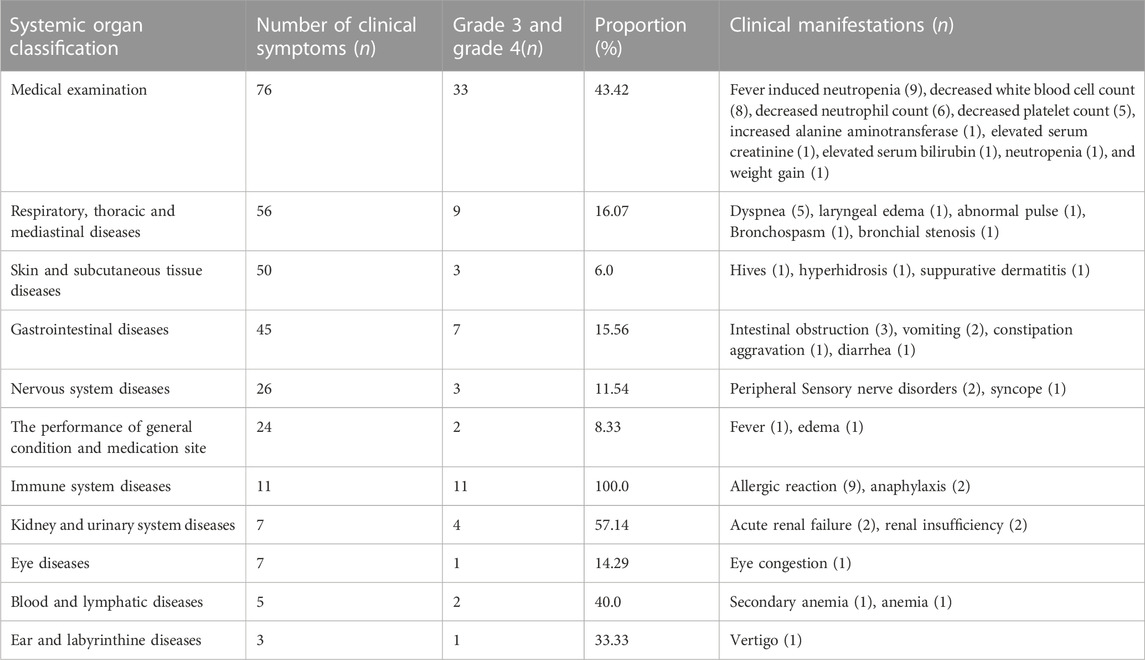
TABLE 4. Proportion and clinical manifestations of severe adverse reactions/events in tumor patients.
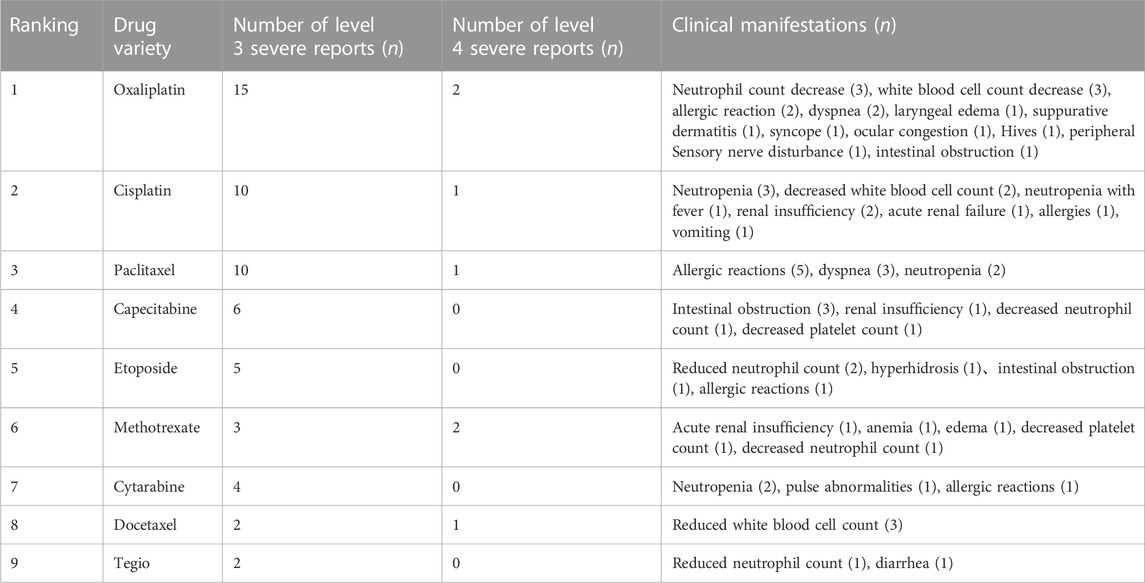
3.1.6 Chemotherapy drugs with serious adverse reactions/events
According to the results of “3.1.4” and “3.1.5,” a total of 76 serious adverse reactions/events were reported. To further clarify which chemotherapy drugs in this study have caused more serious adverse reactions/events, our team analyzed the reports of serious adverse reactions/events caused by the top 10 chemotherapy drugs, Among them, oxaliplatin and cisplatin, the platinum complexes, ranked in the top two places. The clinical manifestations of cancer patients are respectively concentrated in white blood cell count reduction (3 times), neutrophil count reduction (3 times), allergic reactions (2 times), dyspnea (2 times), as well as white blood cell count reduction (3 times), neutrophil count reduction (2 times), and renal insufficiency (2 times), etc. The 11 serious adverse reactions/events caused by paclitaxel were manifested in allergic reactions (5 times), dyspnea (3 times), and neutrophil count reduction (2 times). After paclitaxel, capecitabine was successively ranked, with 6 serious reports. There have also been five serious reports of etoposide. The detailed results can be seen in Table 5, and gemcitabine had no serious adverse reactions/events. In addition, we have also found an interesting phenomenon: in the statistical reports of serious adverse reactions/events caused by the top 10 chemotherapy drugs, almost all of the chemotherapy drugs have a reduction in white blood cells, neutrophils, or platelet counts. These results indicated that these chemotherapy drugs can cause adverse reactions/events in the cancer patient’s blood system, which will lead to the occurrence of BMS. The result was also consistent with Table 4, where the number of cases and incidence of BMS caused by chemotherapy drugs were the highest. DBD has a protective effect on the hematopoietic system of chemotherapy induced BMS, but the specific mechanism is not clear (Kong et al., 2022). Hence, we will utilize the current popular network pharmacology method to explore the potential mechanism of DBD against BMS in the next step.
3.2 Network pharmacology
The second section was based on the BMS caused by chemotherapy, which was a significant and common adverse reaction or event disease, that was discovered in the previous study (“3.1” analysis of adverse reactions/events of chemotherapy drugs).
3.2.1 DBD and disease target screening
Through the TCMSP database and conditional search of DBD, 22 effective components were obtained. Deduplication and exclusion of 3 unrelated target components resulted in a total of 19 active ingredients being included. Among them, Huangqi has 2 active ingredients, and Danggui has 17 active components. After weight removal, there are a total of 229 related targets. The results are shown in Table 6. 8670 BMS-related targets were found in the GeneCards database, but only 1,319 targets with a relevant score of 10 or above were included. Furthermore, 230 BMS targets were obtained from the OMIM database, and 1510 BMS-related targets were included in these two databases’ combination and de-duplication.
3.2.2 Construction of PPI and active ingredient-disease-target network
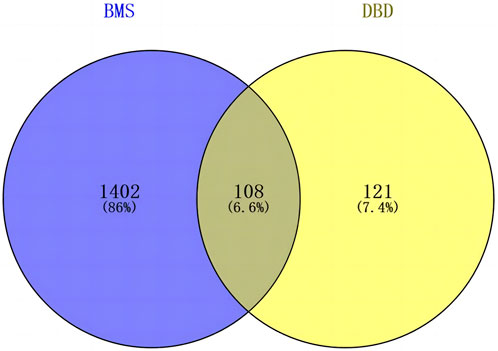
The screened 229 DBD targets and 1510 BMS targets were entered into the Venny online platform, and 108 intersection targets were obtained, accounting for 6.6%. The intersection targets were used as potential targets for DBD treatment of diseases (Figure 3). The potential targets were input into the STRING platform to obtain the PPI network (Figure 4). To understand the interaction between the targets more clearly and see the core targets more intuitively according to the link degree value, the potential intersection targets and other data were imported into Cytascape software to construct the active component-disease-target network (Figure 5).
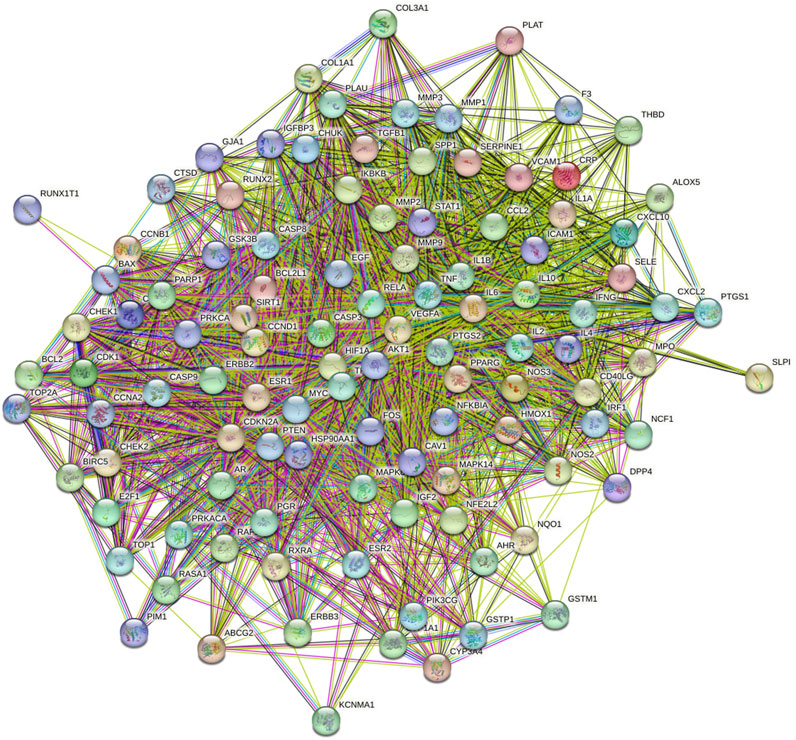
FIGURE 4. PPI network common targets of DBD and BMS. Note: Each node represents a particular protein. The edges of each node showed the innate relationships between the proteins, and the hues from yellow to red signified tiny to big values. A line between proteins served as a visual cue that they were related. The link between the proteins is closer the more lines there are between them.
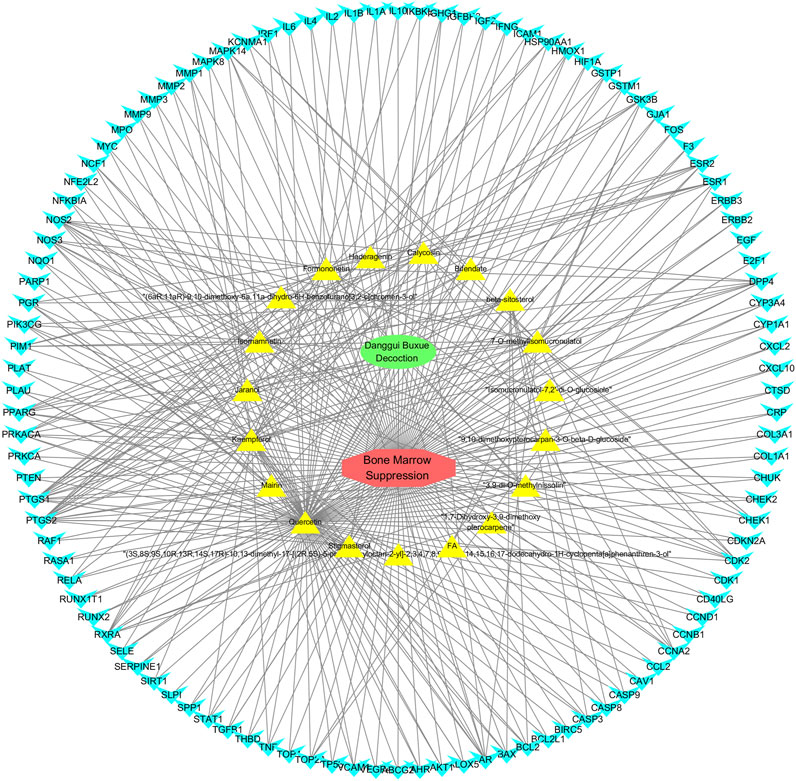
FIGURE 5. Drug-ingredient-target-disease network diagram for DBD against BMS. Note: In the network diagram, green represents Danggui Buxue Decoction (DBD), red represents Bone Marrow Suppression (BMS), yellow represents the active ingredients of DBD, and blue represents their common genes. The more lines, the closer the connection between them.
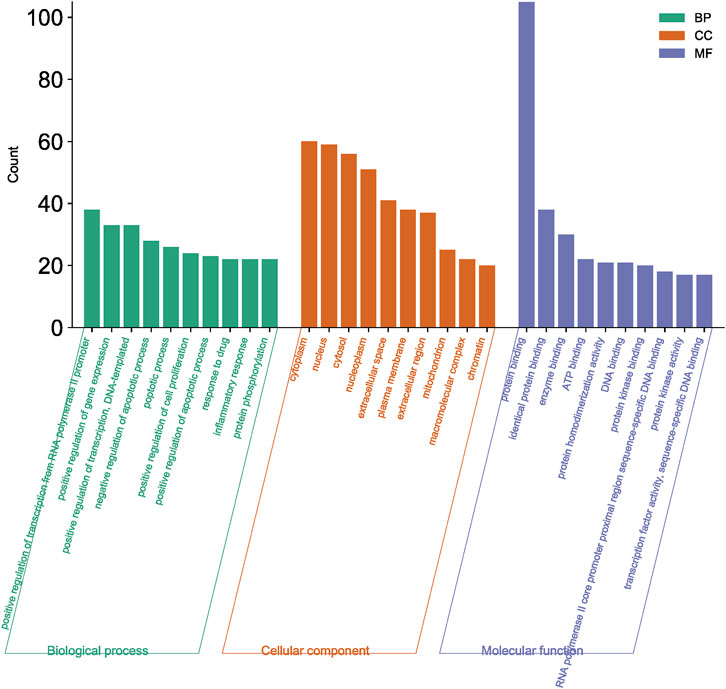
3.2.3 GO function and KEGG pathway enrichment analysis
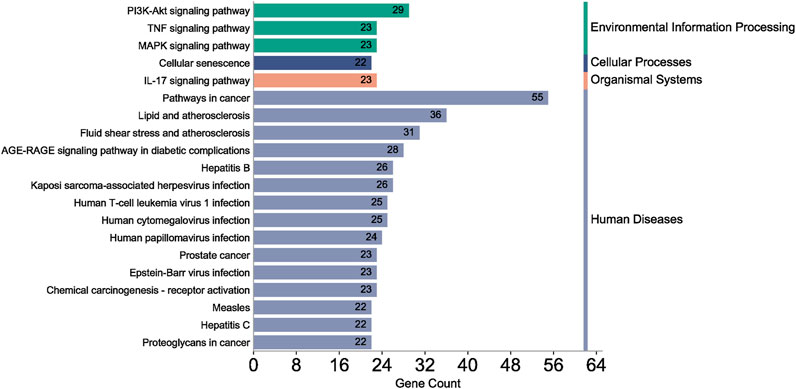
A total of 108 key target protein genes were imported into the DAVID database, and data with p < 0.05 were selected for statistical significance. A total of 570 biological processes, 49 cellular components, 96 molecular functions, and 151 KEGG signaling pathways were involved. The top 20 items based on count were imported into the Bioinformatics platform to draw GO enrichment analysis and KEGG pathway maps. The results can be seen in Figures 6, 7. The GO enrichment analysis map showed that the essential biological process (BP), cellular component (CC) and molecular function (MF) involved in DBD acting on diseases include the positive regulation of RNA polymer II promoter transcription and the positive regulation of gene expression, cytoplasm and nucleus, protein binding and idential protein binding, respectively. From the KEGG pathway enrichment analysis map, the key KEGG pathways were enriched in cancer pathway, AGE-RAGE signaling pathway in diabetic complications, PI3K-AKT signaling pathway, TNF signaling pathway, MAPK signaling pathway, IL-17 signaling pathway, and so on.
3.2.4 Molecular docking
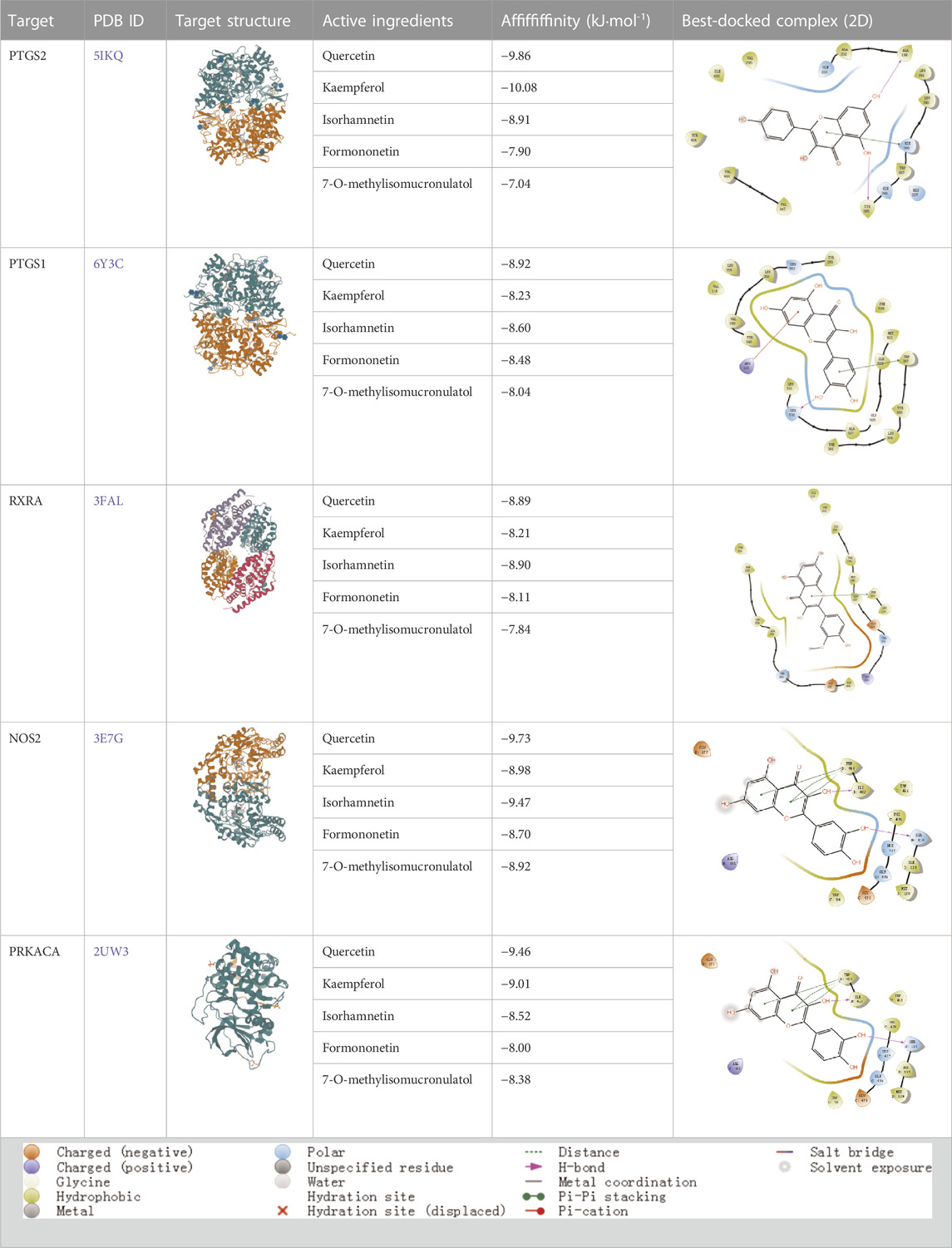
According to the active ingredients-disease-target network, the top five active ingredients and key targets with a degree value are Quercetin, Kaempferol, Isorhamnetin, Formononetin, 7-O-methylisochronotropol, and PTGS2, PTGS1, RXRA, NOS2, as well as PRKACA. The molecular docking results are shown in Table 7, where the binding energy of Kaempferol and PTGS2 is the largest, at −10.08 kJ · mol−1. At the same time, the absolute values of binding energy between Quercetin and PTGS2, NOS2, and PRKACA are all above 9.0 kJ mol−1, with better binding ability. In addition, we found that the absolute values of all the free binding energies obtained were far more significant than 5 kJ mol−1, indicating that the compound had a strong binding activity with the target.
3.2.5 ADMET profiling results
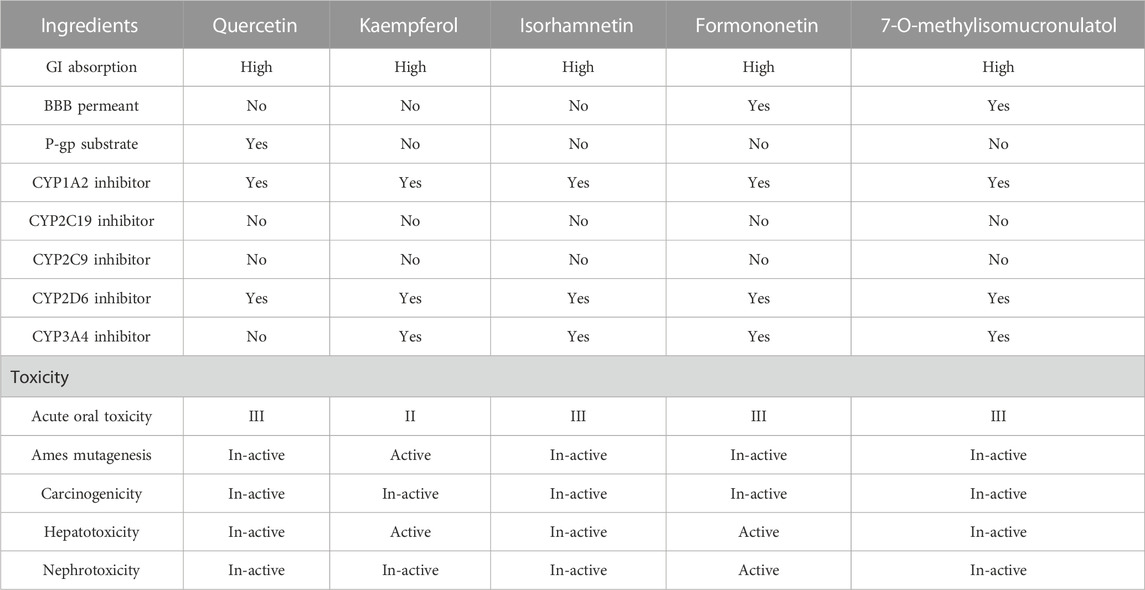
The further development and use of core ingredients in Chinese medicine also require an assessment of the absorption, distribution, metabolism, and excretion (ADME) of different monomer components. The SWISS ADME database and the admetSAR database were used to analyze the ADME analysis of core ingredients, and the results showed that all ingredients had satisfactory pharmacokinetic properties (Table 8). The ADMET analysis results showed that some core active ingredients had slight toxic or no side effects in their pharmacokinetic properties. Quercetin and 7-O-methylisomucronulatol had the least toxic side effects, and there was no liver or kidney toxicity, while kaempferol and formononetin had more toxic side effects. The ADME characteristics of core active ingredients in disparate patterns, such as BBB permeant, P-gp substrate, CYP1A2 inhibitor, CYP2D6 inhibitor, and CYP3A4 inhibitor, showed positive results in some potential ingredients, indicating their ability to serve as candidate drugs, such as quercetin, isorhamnetin, and 7-o-methylisocycloaldol.
4 Discussion
4.1 Analysis of adverse reactions/events of chemotherapy drugs
4.1.1 Screening of adverse reactions/events
The CHPS system and the National Adverse Drug Reaction Monitoring System were used to identify 209 tumor patients who had adverse reactions/events to chemotherapy medicines for this investigation. The hospital recorded fewer adverse reactions/events from 2015 to 2017, however after 2017, they rose. This is primarily because most medical institutions lacked information service systems that could interface with the national monitoring system and all adverse reactions/events reported prior to 2018 were spontaneously conducted by medical institutions and required the use of the National Adverse Drug Reaction Monitoring information system. More importantly, there were problems with non-standard and missing reports, and the entire reporting and filing procedure was complicated, time-consuming, and arduous (Zhao et al., 2021). The previous State Food and Drug Administration of China, consequently, started a pilot project to build adverse drug reaction monitoring outposts in 2016 to address the aforementioned issues. In 2017, the hospital also became a sentinel facility for the monitoring of adverse medication reactions. The number of reported adverse reactions/events increased significantly after the CHPS system was formally established in 2018, and by 2022, there had been approximately 100 occurrences.
4.1.2 Basic information and proportion of tumor patients
Of the 209 cancer patients included, 12.44% had non-small cell lung cancer, and 11.96% had colon cancer of the patients having adverse reactions/events. Additionally, 10.53% of patients had rectal cancer; 9.09% and 7.18% of patients, respectively, had non-Hodgkin’s lymphoma and acute non-lymphocytic leukemia. Furthermore, more than 5% of adverse reactions/events have involved nasopharyngeal carcinoma, cervical malignancy, and acute lymphocytic leukemia. As a result, during the course of clinical treatment, medical professionals should be aware of the possibility of adverse reactions/events, including new ones, brought on by the administration of chemotherapy medications to patients with this particular type of tumor disease.
4.1.3 Combined medication and analysis of types of chemotherapy drugs
In this study, 151 cancer patients had the highest proportion of adverse reactions/events due to single drug use. However, there were still some patients who experienced negative effects or incidents as a result of taking several medications, including 50 patients who took two doses, seven patients who took three doses, and one patient who took four or more doses. Single-use medications increase adverse reactions/events, and chemotherapy drug efficacy will decline when tumor resistance to the therapies rises (Long et al., 2022). Combination chemotherapy, which has been increasingly employed in clinical treatment to accomplish the impact of improving efficacy and reducing toxicity (Pusuluri et al., 2019; Long et al., 2022), is a potential remedy for single chemotherapy resistance. Therefore, in clinical practice, it is crucial to thoroughly understand the indications, take into account the drug interactions and contraindications while using combination chemotherapy, and prevent and treat adverse reactions/events expertly.
The number of cases of chemotherapy drugs used in 209 tumor patients included in this study is 271. Figure 2 was showed that platinum complexes, anti-tumor metabolic drugs, and botanical anti-tumor drug chemotherapy drugs cause the most adverse reactions/events in cancer patients. Anti-tumor drugs are the most likely to cause serious adverse reactions/events in patients, similar to the results of the 2022 National Adverse Drug Reaction Monitoring Annual Report of China (National Medical Products Administration, 2023). The report also pointed out that the proportion of adverse reactions/events caused by cancer drugs is the largest, exceeding 35%. However, it did not provide a detailed description of the specific categories of cancer drugs, only suggesting that clinical needs to strengthen anti-tumor drug risk management. Oxaliplatin, cisplatin, and paclitaxel, three chemotherapeutic medicines, had the largest number and proportion of adverse reactions/events following use, according to Table 2. Platinum complexes such as oxaliplatin, cisplatin, carboplatin, etc. are currently 70%–80% likely to be utilized in clinical chemotherapy or combined chemotherapy for tumor illnesses (Xue et al., 2021; Feng et al., 2023). Moreover, there are many adverse reactions/events caused by chemotherapy drugs for many reasons, including gender, age, underlying disease, dosage, narrow drug treatment window, etc. (Xue et al., 2021). Therefore, in the clinical process of anti-tumor chemotherapy, it is necessary to strictly control and take measures to deal with possible adverse reactions/events to ensure the drug use of tumor patients.
4.1.4 Analysis of clinical manifestations of adverse reactions/events in cancer patients
This study classified the cumulative system organs of adverse reactions/events by CTCAE. It can be seen that “medical examination” accounts for the largest proportion in Table 3. After reviewing electronic cases, it was found that their main clinical manifestations include a decrease in white blood cells and granulocytes and an increase in transaminases, which have caused damage to the body’s blood system and liver function (Ou et al., 2017; Cacabelos et al., 2021). Additionally, the main system organs accumulated by these adverse reactions/events also involve “respiratory, thoracic, and mediastinal diseases,” “skin and subcutaneous tissue diseases,” “gastrointestinal diseases,” etc. However, this result differs from China’s 2022 National Adverse Drug Reaction Monitoring Annual Report. The 2022 monitoring report includes all adverse reactions/events caused by drugs, so there is a difference (National Medical Products Administration, 2023). Moreover, all chemotherapy drugs are highly likely to cause new adverse reactions/events in patients. Therefore, it is equally important to strengthen the rational use of chemotherapy drugs and optimize nursing measures.
4.1.5 Analysis of chemotherapy drugs for serious adverse reactions/events
This study did a statistical analysis of the top 10 chemotherapy medications that resulted in major adverse reactions/events in order to better understand the relationship between these drugs and serious adverse reactions/events. The most severe adverse reactions/events are caused by oxaliplatin and cisplatin, as shown in Tables 2, 5, and their clinical manifestations primarily include decreased neutrophil and leukocyte counts, allergies, and renal impairment. This result is similar to the study by Zhang et al. (2022), where platinum complexes mainly cause significant damage to the blood system, liver, and kidney functions, and is also basically consistent with the prevalent adverse events mentioned in the drug instructions and the results reported in the literature. Meanwhile, paclitaxel can produce a variety of adverse responses or events, the most severe of which are allergic reactions. Allergic reactions to paclitaxel have been known to be fatal, and its harm to the blood system cannot be discounted (Saggam et al., 2022). Apart from that, the primary emphasis of capecitabine and etoposide is on the circulatory system. Consequently, the blood system-particularly granulocytes, white blood cells, and platelets-is affected by the majority of the top 10 chemotherapy medications that induce adverse reactions or occurrences in patients.
After reviewing the CHPS and electronic case system, researchers found that while patients with “general” adverse reactions/events brought on by chemotherapy drugs can get better after drug withdrawal or medical treatment, those with “serious” adverse reactions/events can take much longer to get better, sometimes even after discharge. As a result, “serious” adverse reactions and incidents should receive more attention, and if necessary, combined department meetings should be held.
4.2 Network pharmacology
4.2.1 Brief overview of DBD
The “NeiWaiShangBianHuo Lun” of Li Dongyuan is the source of DBD, which has a 5:1 ratio of Huang Qi and Danggui (Sun et al., 2022). While the former can energize Qi and fortify the exterior, the latter concentrates on activating and replenishing blood. DBD’s main effects include blood production, hematopoiesis promotion, accelerated blood cell maturation, and immune function regulation (Kwan et al., 2019; Tie et al., 2022). Numerous studies have demonstrated that DBD has protective effects on white blood cells and platelets as well as a potential protective effect on chemotherapy-induced BMS, increasing the sensitivity of chemotherapy medications and lowering chemotherapy-induced BMS (Li et al., 2017; Liu et al., 2021; Kong et al., 2022). Several studies on DBD have been conducted both domestically and internationally, but none of them have focused on the molecular mechanisms by which DBD prevents BMS.
4.2.2 The active ingredients of DBD against BMS
In this study, GeneGards and OMIM databases were used to screen disease targets, and the TCMSP platform was used to screen the effective components and targets of DBD and to determine the common targets of the two. The PPI network and the active ingredient-disease-target network were subsequently built, and important targets’ GO and KEGG enrichment analyses were performed using the DAVID database. The effective ingredients of DBD ranked in the top five with the Degree value were quercetin, kaempferol, isorhamnetin, formononetin, and 7-O-methylisochronotropol. According to molecular docking results, these five active ingredients can effectively bind to these five main target proteins, with quercetin, kaempferol, and isorhamnetin having the best binding energy with the target protein. According to recent research, kaempferol-rich non-alcoholic polyphenol concentrate made from Cabernet Sauvignon grapes in Kazakhstan has a healing impact on the hematological system in acute radiation injury (Shulgau et al., 2014). However, the role of kaempferol in the concentration has yet to be determined due to the concentration’s complexity. Additionally, the primary ingredient in the Bushen Jianpi Quyu Formula, kaempferol, can prevent BMS from being alleviated by inhibiting the expression of the PI3K/AKT/NF-B signal pathway (Li et al., 2022). Through mechanisms that control HGF and HIF, quercetin can reduce the BMS that cisplatin causes, likewise, it can lessen DNA oxidative damage brought on by etoposide in rat bone marrow cells and is crucial for bone marrow cell protection (Papiez, 2014; Chuang et al., 2022). Furthermore, the findings in Table 8 also show that quercetin has minimal toxicity, no carcinogenicity, and no mutagenicity, making it a promising active ingredient for future medication development. Isorhamnetin’s function in BMS and hematopoiesis has not been the subject of any studies.
4.2.3 The key targets and major signaling pathways of DBD against BMS
According to the degree ranking in the Drug-ingredient-target-disease network, the key targets are PTGS2, PTGS1, RXRA, NOS2, and PRKACA. PTGS2, also known as COX2, has been found to possess a sublethal or lethal effect on meloxicam, a selective COX2 inhibitor γ-irradiated mice have significant hematopoietic stimulation and survival-enhancing effects (Hofer et al., 2012). These effects are connected to meloxicam’s capacity to raise granulocyte colony-stimulating factor levels in the blood, and it is assumed that blocking the COX2 protein may be a major strategy for preventing BMS. We find that PTGS2 is a key protein in chemotherapy-induced BMS, which is also consistent with the research results of Wang et al. (2020). Besides, the molecular docking studies show that PTGS2 can have the best docking effects with quercetin and kaempferol, and its absolute value of binding energy is about 10 kJ mol−1. In light of these findings, PTGS2 is a potential primary target for the therapy of BMS. We also discovered that ionizing radiation does not require NOS2 to induce BMS and that RXRA ligands are present and dynamically increase in mouse hematopoietic cells (Niu et al., 2017; Li et al., 2018). According to the KEGG pathways analysis, DBD can also reduce BMS by activating the PI3K-AKT, TNF, MAPK, and IL-17 signaling pathways. By controlling the expression of the PI3K-AKT signaling pathway, a variety of pharmacological medicines and components can reduce BMS, enhance hematopoiesis and platelet production, and even play an immunomodulatory effect (Liu et al., 2010; Wang et al., 2011; Chen et al., 2023). In order to shield isolated bone marrow cells from 5-fluorouracil-induced cell death and inflammatory responses, the physiologically active glycoside Martynoside can inhibit the TNF signaling pathway (Hong et al., 2021). At the same time, the TNF signaling system may shield bone marrow cells from the harm caused by carbon ion radiation (Liu et al., 2019). Recent research has found that Specnuezhenide can enhance the hematological and immunological functions of BMS mice, and it is speculated that it can significantly help the hematopoietic and immune functions of tumor patients after chemotherapy and plays a role by increasing the expression levels of key proteins MEK and p-ERK in the MAPK signaling pathway (Han et al., 2023). Leukopenia, thrombocytopenia, erythrocytopenia, and bone marrow cell depletion are caused by radiation therapy associated with hemorrhage, which increases the production of IL-1, IL-6, and IL-17A in the IL-17 signaling pathway and increases BMS (Kiang et al., 2015).
5 Conclusion
The adverse effects/events that chemotherapy medications can have on patients are unavoidable and sometimes ignored in anti-tumor therapy and chemotherapy harm surely lengthens the time it takes for patients to recover. Age, tumor kind, drug type, combination therapy, and other characteristics of tumor patients all have an impact on the likelihood of chemotherapy damage. Among them, we need to focus on the damage of platinum drugs, paclitaxel, and other chemotherapy drugs to the body. At the same time, most chemotherapy drugs will cause BMS in patients. Blood routine function tests should be done on schedule when taking medication, which can improve the prognosis of patients through early detection and treatment. Further, this study has proven that DBD has the effect of preventing BMS after chemotherapy. Through network pharmacology, the effective ingredients of DBD have been analyzed, and their treatment mechanisms have been systematically studied, providing new ideas for the treatment of chemotherapy-induced BMS. The results showed that the key ingredients that increase hematopoiesis and lessen BMS damage through the control of signal pathways such as PI3K-AKT, TNF, MAPK, and IL-17 include quercetin, kaempferol, and isorhamnetin. This research will also serve as a theoretical underpinning and scientific foundation for our subsequent investigations into the effectiveness and molecular basis of self-made drug delivery microspheres for the prevention of chemotherapy-induced BMS.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
The studies involving humans were approved by Biomedical Ethics Committee of Mianyang Central Hospital, affiliated to Mianyang Central Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
BY and HX designed this study. Data collection and analysis were performed by MS, XY, and TL. Investigation, validation were performed by YZ and HN. The manuscript was drafted by BY and XY. HX, HN, and BY revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was Supported by NHC Key Laboratory of Nuclear Technology Medical Transformation (Mianyang Central Hospital) (Grant No. 2022HYX013), and Graduate Innovation Foundation of Yantai University, GIFYTU (No. KGIFYTU2313).
Acknowledgments
The authors would like to thank editors and the reviewers for their valuable comments and suggestions to improve the quality of the paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bae, B. K., Cho, W. K., Lee, J. W., Kim, T. J., Choi, C. H., Lee, Y. Y., et al. (2023). Role of salvage radiotherapy for recurrent ovarian cancer. Int. J. Gynecol. Cancer 33 (1), 66–73. doi:10.1136/ijgc-2022-003834
Cacabelos, R., Naidoo, V., Corzo, L., Cacabelos, N., and Carril, J. C. (2021). Genophenotypic factors and pharmacogenomics in adverse drug reactions. Int. J. Mol. Sci. 22 (24), 13302. doi:10.3390/ijms222413302
Chen, N., Quan, Y., Chen, M., Lu, Y., Yang, L., Wang, S., et al. (2023). Melanocortin/MC5R axis regulates the proliferation of hematopoietic stem cells in mice after ionizing radiation injury. Blood Adv. 15, 3199–3212. doi:10.1182/bloodadvances.2022009249
Chen, P., Liu, Y., Wen, Y., and Zhou, C. (2022). Non-small cell lung cancer in China. Cancer Commun. (Lond) 42 (10), 937–970. doi:10.1002/cac2.12359
Chuang, C. H., Lin, Y. C., Yang, J., Chan, S. T., and Yeh, S. L. (2022). Quercetin supplementation attenuates cisplatin induced myelosuppression in mice through regulation of hematopoietic growth factors and hematopoietic inhibitory factors. J. Nutr. Biochem. 110, 109149. doi:10.1016/j.jnutbio.2022.109149
De Francia, S., Mancardi, D., Berchialla, P., Armando, T., Storto, S., Allegra, S., et al. (2022). Gender-specific side effects of chemotherapy in pancreatic cancer patients. Can. J. Physiol. Pharmacol. 100 (4), 371–377. doi:10.1139/cjpp-2021-0622
Eisenberg, M., Deboever, N., and Antonoff, M. B. (2023). Salvage surgery in lung cancer following definitive therapies. J. Surg. Oncol. 127 (2), 319–328. doi:10.1002/jso.27155
Feng, G., Zhou, X., Chen, J., Li, D., and Chen, L. (2023). Platinum drugs-related safety profile: the latest five-year analysis from FDA adverse event reporting system data. Front. Oncol. 12, 1012093. doi:10.3389/fonc.2022.1012093
Freites-Martinez, A., Santana, N., Arias-Santiago, S., and Viera, A. (2021). Using the common terminology criteria for adverse events (CTCAE - version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr. Engl. Ed. 112 (1), 90–92. doi:10.1016/j.ad.2019.05.009
Han, J., Sun, N., Xing, J., Fei, X., Cai, E., and Su, F. (2023). Effect and mechanism of specnuezhenide on chemotherapy-induced myelosuppression. Comb. Chem. High. Throughput Screen 26, 2393–2400. doi:10.2174/1386207326666230228120608
Hofer, M., Pospíšil, M., Hoferová, Z., Weiterová, L., and Komůrková, D. (2012). Stimulatory action of cyclooxygenase inhibitors on hematopoiesis: a review. Molecules 17 (5), 5615–5625. doi:10.3390/molecules17055615
Hong, M., Chen, D., Hong, Z., Tang, K., Yao, Y., Chen, L., et al. (2021). Ex vivo and in vivo chemoprotective activity and potential mechanism of Martynoside against 5-fluorouracil-induced bone marrow cytotoxicity. Biomed. Pharmacother. 138, 111501. doi:10.1016/j.biopha.2021.111501
Hopkins, A. L. (2007). Network pharmacology. Nat. Biotechnol. 25 (10), 1110–1111. doi:10.1038/nbt1007-1110
Kiang, J. G., Smith, J. T., Anderson, M. N., Swift, J. M., Christensen, C. L., Gupta, P., et al. (2015). Hemorrhage exacerbates radiation effects on survival, leukocytopenia, thrombopenia, erythropenia, bone marrow cell depletion and hematopoiesis, and inflammation-associated microRNAs expression in kidney. PLoS One 10 (9), e0139271. doi:10.1371/journal.pone.0139271
Knezevic, C. E., and Clarke, W. (2020). Cancer chemotherapy: the case for therapeutic drug monitoring. Ther. Drug Monit. 42 (1), 6–19. doi:10.1097/FTD.0000000000000701
Kong, H. M., Su, W. Q., Ye, H., Wang, H., Li, L., Chen, H., et al. (2022). The antiapoptotic effect of danggui buxue tang and its main components on hematopoietic cells in mice with bone marrow suppression. Zhongguo Shi Yan Xue Ye Xue Za Zhi 30 (6), 1679–1687. doi:10.19746/j.cnki.issn.1009-2137.2022.06.009
Kwan, K. K. L., Huang, Y., Leung, K. W., Dong, T. T. X., and Tsim, K. W. K. (2019). Danggui buxue tang, a Chinese herbal decoction containing Astragali Radix and angelicae sinensis radix, Modulates Mitochondrial Bioenergetics in Cultured Cardiomyoblasts. Front. Pharmacol. 10, 614. doi:10.3389/fphar.2019.00614
Lazaroff, J., and Bolotin, D. (2023). Targeted therapy and immunotherapy in melanoma. Dermatol Clin. 41 (1), 65–77. doi:10.1016/j.det.2022.07.007
Li, C., Luo, Y., Shao, L., Meng, A., and Zhou, D. (2018). NOS2 deficiency has no influence on the radiosensitivity of the hematopoietic system. Cell. Biosci. 8, 33. doi:10.1186/s13578-018-0228-0
Li, F., Tang, R., Chen, L. B., Zhang, K. S., Huang, X. P., and Deng, C. Q. (2017). Effects of astragalus combined with angelica on bone marrow hematopoiesis suppression induced by cyclophosphamide in mice. Biol. Pharm. Bull. 40 (5), 598–609. doi:10.1248/bpb.b16-00802
Li, H., Ji, L., Shen, Y., Fu, D., Wu, D., and Ye, B. (2022a). Bushen jianpi quyu formula alleviates myelosuppression of an immune-mediated aplastic anemia mouse model via inhibiting expression of the PI3K/AKT/NF-κB signaling pathway. Evid. Based Complement. Altern. Med. 2022, 9033297. doi:10.1155/2022/9033297
Li, X., Tang, Q., Meng, F., Du, P., and Chen, W. (2022b). Input: an intelligent network pharmacology platform unique for traditional Chinese medicine. Comput. Struct. Biotechnol. J. 20, 1345–1351. doi:10.1016/j.csbj.2022.03.006
Liu, C., Cheng, B., Zhao, G., and Yuan, H. (2022). Process analysis of anthracycline adverse reactions in breast cancer patients with postoperative chemotherapy. J. Investig. Med. 70 (6), 1352–1357. doi:10.1136/jim-2022-002339
Liu, C., Li, J., Meng, F. Y., Liang, S. X., Deng, R., Li, C. K., et al. (2010). Polysaccharides from the root of Angelica sinensis promotes hematopoiesis and thrombopoiesis through the PI3K/AKT pathway. BMC Complement. Altern. Med. 10, 79. doi:10.1186/1472-6882-10-79
Liu, F., Wang, Z., Li, W., and Wei, Y. (2019). Transcriptional response of murine bone marrow cells to total-body carbon-ion irradiation. Mutat. Res. Genet. Toxicol. Environ. Mutagen 839, 49–58. doi:10.1016/j.mrgentox.2019.01.014
Liu, Y., Chang, M., Hu, Z., Xu, X., Wu, W., Ning, M., et al. (2021). Danggui buxue decoction enhances the anticancer activity of gemcitabine and alleviates gemcitabine-induced myelosuppression. J. Ethnopharmacol. 273, 113965. doi:10.1016/j.jep.2021.113965
Long, W., Zhang, L., Wang, Y., Xie, H., Wang, L., and Yu, H. (2022). Research progress and prospects of autophagy in the mechanism of multidrug resistance in tumors. J. Oncol. 2022, 7032614. doi:10.1155/2022/7032614
National Medical Products Administration (2023). National adverse drug reaction monitoring annual report. Beijing: Chin JPharmacov. 20 (06), 712–719. doi:10.19803/j.1672-8629.20230168
Niu, H., Fujiwara, H., diMartino, O., Hadwiger, G., Frederick, T. E., Menéndez-Gutiérrez, M. P., et al. (2017). Endogenous retinoid X receptor ligands in mouse hematopoietic cells. Sci. Signal 10 (503), eaan1011. doi:10.1126/scisignal.aan1011
Niu, W. H., Wu, F., Cao, W. Y., Wu, Z. G., Chao, Y. C., and Liang, C. (2021). Network pharmacology for the identification of phytochemicals in traditional Chinese medicine for COVID-19 that may regulate interleukin-6. Biosci. Rep. 41 (1), BSR20202583. doi:10.1042/BSR20202583
Ou, Z. B., Miao, C. M., Ye, M. X., Xing, D. P., He, K., Li, P. Z., et al. (2017). Investigation for role of tissue factor and blood coagulation system in severe acute pancreatitis and associated liver injury. Biomed. Pharmacother. 85, 380–388. doi:10.1016/j.biopha.2016.11.039
Papież, M. A. (2014). The effect of quercetin on oxidative DNA damage and myelosuppression induced by etoposide in bone marrow cells of rats. Acta Biochim. Pol. 61 (1), 7–11. doi:10.18388/abp.2014_1915
Pusuluri, A., Wu, D., and Mitragotri, S. (2019). Immunological consequences of chemotherapy: single drugs, combination therapies and nanoparticle-based treatments. J. Control Release 305, 130–154. doi:10.1016/j.jconrel.2019.04.020
Reig, M., Forner, A., Rimola, J., Ferrer-Fàbrega, J., Burrel, M., Garcia-Criado, Á., et al. (2022). BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J. Hepatol. 76 (3), 681–693. doi:10.1016/j.jhep.2021.11.018
Saggam, A., Kale, P., Shengule, S., Patil, D., Gautam, M., Tillu, G., et al. (2022). Ayurveda-based botanicals as therapeutic adjuvants in paclitaxel-induced myelosuppression. Front. Pharmacol. 13, 835616. doi:10.3389/fphar.2022.835616
Shulgau, Z., Tritek, V., Gulyaev, A., Adilgozhina, G., and Nurgozhin, T. (2014). Polyphenol concentrate from Kazakhstan cabernet sauvignon collection of grapes. Cent. Asian J. Glob. Health 3, 174. doi:10.5195/cajgh.2014.174
Song, J., Yan, Y., Chen, C., Li, J., Ding, N., Xu, N., et al. (2023). Tumor mutational burden and efficacy of chemotherapy in lung cancer. Clin. Transl. Oncol. 25 (1), 173–184. doi:10.1007/s12094-022-02924-6
Strazzabosco, M., Cabibbo, G., and Colombo, M. (2022). Adjusting barcelona clinic liver cancer staging system to the evolving landscape of hepatocellular carcinoma: a look to the future. Gastroenterology 162 (7):2106–2108. doi:10.1053/j.gastro.2022.01.035
Sun, L., Yang, Z., Zhao, W., Chen, Q., Bai, H., Wang, S., et al. (2022). Integrated lipidomics, transcriptomics and network pharmacology analysis to reveal the mechanisms of Danggui Buxus Decoction in the treatment of diabetic nephropathy in type 2 diabetes mellitus. J. Ethnopharmacol. 283, 114699. doi:10.1016/j.jep.2021.114699
Sun, L., Zhao, M., Li, J., Liu, J., Wang, M., and Zhao, C. (2023). Exploration of the anti-liver injury active components of Shaoyao Gancao decoction by network pharmacology and experiments in vivo. Phytomedicine 112, 154717. doi:10.1016/j.phymed.2023.154717
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Tie, D., Fan, Z., Chen, D., Chen, X., Chen, Q., Chen, J., et al. (2022). Mechanisms of danggui buxue tang on hematopoiesis via multiple targets and multiple components: metabonomics combined with database mining technology. Am. J. Chin. Med. 50 (4), 1155–1171. doi:10.1142/S0192415X22500471
Wang, H., Wang, M., Chen, J., Tang, Y., Dou, J., Yu, J., et al. (2011). A polysaccharide from Strongylocentrotus nudus eggs protects against myelosuppression and immunosuppression in cyclophosphamide-treated mice. Int. Immunopharmacol. 11 (11), 1946–1953. doi:10.1016/j.intimp.2011.06.006
Wang, L., Li, H., Shen, X., Zeng, J., Yue, L., Lin, J., et al. (2020). Elucidation of the molecular mechanism of sanguisorba officinalis L. against leukopenia based on network pharmacology. Biomed. Pharmacother. 132, 110934. doi:10.1016/j.biopha.2020.110934
Wang, M., Yu, B., Wang, J., Wang, Y., and Liang, L. (2022). Exploring the role of Xingren on COVID-19 based on network pharmacology and molecular docking. J. Food Biochem. 46 (10), e14363. doi:10.1111/jfbc.14363
Xia, C., Dong, X., Li, H., Cao, M., Sun, D., He, S., et al. (2022). Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin. Med. J. Engl. 135 (5), 584–590. doi:10.1097/CM9.0000000000002108
Xue, Y., Gao, S., Gou, J., Yin, T., He, H., Wang, Y., et al. (2021). Platinum-based chemotherapy in combination with PD-1/PD-L1 inhibitors: preclinical and clinical studies and mechanism of action. Expert Opin. Drug Deliv. 18 (2), 187–203. doi:10.1080/17425247.2021.1825376
Zhang, C., Xu, C., Gao, X., and Yao, Q. (2022). Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics 12 (5), 2115–2132. doi:10.7150/thno.69424
Zhao, L., Zhang, H., Li, N., Chen, J., Xu, H., Wang, Y., et al. (2023). Network pharmacology, a promising approach to reveal the pharmacology mechanism of Chinese medicine formula. J. Ethnopharmacol. 309, 116306. doi:10.1016/j.jep.2023.116306
Keywords: adverse reactions/events, bone marrow suppression, Danggui Buxue decoction, network pharmacology, molecular docking
Citation: Yu B, Yan X, Zhu Y, Luo T, Sohail M, Ning H and Xu H (2023) Analysis of adverse drug reactions/events of cancer chemotherapy and the potential mechanism of Danggui Buxue decoction against bone marrow suppression induced by chemotherapy. Front. Pharmacol. 14:1227528. doi: 10.3389/fphar.2023.1227528
Received: 01 June 2023; Accepted: 01 August 2023;
Published: 15 August 2023.
Edited by:
Jianqiang Xu, Dalian University of Technology, ChinaReviewed by:
Chunyu Li, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaLiming Jiang, Ningbo University, China
Copyright © 2023 Yu, Yan, Zhu, Luo, Sohail, Ning and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Xu, eHVodWkzM0BzaW5hLmNvbQ==
†These authors have contributed equally to this work
 Bin Yu
Bin Yu Xida Yan3†
Xida Yan3† Muhammad Sohail
Muhammad Sohail Hui Xu
Hui Xu